Abstract
Background:
Adverse Childhood Experience (ACE) has detrimental impacts on neural development, especially hippocampal morphometry. Mindfulness-Based Interventions (MBI) has been shown to induce adaptive hippocampal changes especially at the subiculum. The present study aims to investigate the effects of MBI on subiculum volumes among ACE survivors, as well as the effects on episodic memory as a probe into hippocampal functionality.
Methods:
We analyzed anatomical MRI data and performance indices from an episodic memory task called the Mnemonic Similarity Task (MST) collected from a randomized controlled longitudinal study that compared an 8-week MBI (N = 20) to an active control condition of Stress Management Education (SME) (N = 19). FreeSurfer 6.0 was used for automated hippocampal subfield segmentation and volumetric estimation.
Results:
Significant group differences were observed with the volumetric changes of the right whole hippocampus and right subiculum. Only the MBI group showed improved pattern separation capability from MST, which was associated with stress reduction and right subiculum volumetric changes.
Limitations:
Modest sample size. MST task was performed outside of MRI.
Conclusions:
These findings suggest beneficial effects of MBI for hippocampal volumes and episodic memory, while highlighting the importance of the subiculum for MBI-induced neural and cognitive changes. The subiculum’s known role in inhibitory control was interpreted as a potential mechanism for it to exhibit MBI-induced volumetric changes, which sheds light on the potential neural underpinnings of mindfulness meditation for reducing stress reactivity among ACE survivors.
Keywords: Meditation, Episodic memory, Subiculum, Cognition, Trauma, Early life stress
1. Introduction
1.1. Childhood adversity is associated with reduced hippocampal volumes
Accumulating research has demonstrated the detrimental impact of Adverse Childhood Experiences (ACEs) on neural development (Teicher et al., 2003), with structural and functional abnormalities persisting into adulthood (Teicher et al., 2016), especially in the hippocampus (Dannlowski et al., 2012; McEwen, 1999; Stein et al., 1997; Teicher et al., 2016; Woon and Hedges, 2008). In particular, left hippocampal subfields CA2-3 and CA4-dentate gyrus, as well as presubicular and subicular components of the hippocampal formation, exhibited reduced gray matter volumes among young adults with ACEs compared to age matched controls (Teicher et al., 2012). Hippocampal subfields CA2-3 and CA4-dentate gyrus are known to be the most stress-sensitive subfields from preclinical and translational studies (McEwen, 2000, 2002). Preclinical studies suggest that early life stress stimulates excessive release of glucocorticoids, which compromises hippocampal integrity by inducing dendritic atrophy of CA3 pyramidal neurons and inhibiting neurogenesis of the granule cells in the dentate gyrus (McEwen, 1999; Mirescu et al., 2004), which are also subfields that showed the robust ACE-related volumetric reduction in human neuroimaging research (Teicher et al., 2012). Similarly, the subiculum also has a high density of glucocorticoid binding sites (Sarrieau et al., 1986) rendering it vulnerable to stress and alterations in corticosterone levels (Zach et al., 2010). Teicher et al (2012) proposed that reduced subiculum volumes in individuals with ACEs may underlie their enhanced stress reactivity given the role of ventral subiculum in inhibiting hypothalamic-pituitary-adrenal (HPA) axis activity to psychological stressors (Herman et al., 1998; Nettles et al., 2000).
Preclinical studies suggest that the detrimental impact of early life stress on hippocampus structures may be reversible. For example, social enrichment was found to increase neuronal proliferation and neurogenesis in the same hippocampal subregions that were reduced by post-weaning social isolation in laboratory rats (Biggio et al., 2019). Environmental enrichment during adolescence has been observed to induce increased spine density in dentate gyrus, while physical enrichment induced increased spine density in CA1 in adult rats (Gabriel et al., 2020). Social enrichment in adult rats was observed to induce increased neurogenesis in the dentate gyrus of the hippocampus accompanied by improved social recognition memory (Monteiro et al., 2014). Such findings on hippocampal plasticity in adulthood suggest that ACE-induced hippocampal abnormality in adult humans may also be reversed through appropriate interventions.
1.2. Impact of ACE on cognitive functioning
ACEs have also been found to be associated with deficits in various cognitive functions (Bremner and Narayan, 1998; Pechtel and Pizzagalli, 2011). Preclinical studies have demonstrated early life stress impairs hippocampus-dependent learning and memory (Karten et al., 2005). Human research has also demonstrated deficiency in memory functioning among ACE survivors (Pechtel and Pizzagalli, 2011). Previous studies have revealed deficits in general intelligence and academic performance in children with a history of institutional care (Pechtel and Pizzagalli, 2011), with particular deficits in visual memory and executive functioning (Bos et al., 2009). College women with a history of sexual abuse were also found to have reduction in short-term, verbal, visual and global memory scores (Navalta et al., 2006). Compromised hippocampal structure and functioning (e.g., pattern separation capability) can account for various information processing distortions among ACE survivors, e.g., compromised ability to accurately recognize one’s emotions (e.g., alexithymia) and ineffective update of internal belief systems based on new information (Herzog et al., 2022).
In the domain of cognitive functioning, compromised pattern separation capability has been speculated to amplify risks for the development of affective, anxiety and psychotic symptoms (Lecei and van Winkel, 2020). Pattern separation is the brain’s ability to distinguish minor differences in similar experiences, which is thought to involve reducing overlap in neuronal activity patterns that represent these experiences (Madar et al., 2019). It is specifically associated with certain cells in the hippocampus, such as the dentate gyrus granule cells and CA3 pyramidal cells (Madar et al., 2019; Yassa and Stark, 2011), and recent research indicates the subiculum also contributes to this process (Nash et al., 2021). Impaired pattern separation capability has been proposed as a candidate mechanistic link to explain the association between compromised hippocampal function and elevated risk for mood and anxiety disorders (Anacker and Hen, 2017; Liberzon and Abelson, 2016). These disorders are characterized by negativity biases (Williams et al., 2009) that favors pattern completion over pattern separation. For example, instead of discerning whether a stimulus is an actual or merely a perceived threat, individuals with severe negativity biases, due to impaired pattern separation capabilities, are more likely to conclude that it is an actual threat and respond as such. Compromised pattern separation capability is particularly evident among ACE survivors (Lecei and van Winkel, 2020), resulting in over-generalization of fear (Lange et al., 2017) and hyper-vigilant threat detection (Lecei and van Winkel, 2020).
1.3. Mindfulness meditation is associated with hippocampal volumetric changes and cognitive improvements
Decades of large-scale population research have demonstrated that ACEs exert a pervasive impact on physical and mental health (Felitti et al., 1998; Finkelhor, 2020; Kalmakis and Chandler, 2015). In particular, ACEs are leading risk factors for a host of psychiatric disorders, which typically have an earlier onset, more severe course and poorer response to traditional treatments such as psychopharmacology or cognitive behavioral therapy in individuals with versus without ACEs (Nanni et al., 2012; Nemeroff et al., 2003). Therefore it has been a pressing matter to identify or develop effective interventions for ACE survivors (Lorenc et al., 2020).
Mindfulness-Based Interventions (MBIs) have been shown to be beneficial for a wide range of psychopathologies, including depression (Hofmann and Gómez, 2017; Teasdale et al., 2000), anxiety (Evans et al., 2008; Hofmann et al., 2010) and PTSD (Boyd et al., 2018), which are some of the most common adverse consequences of ACEs (Nelson et al., 2020). A randomized clinical trial (N = 274) found that Mindfulness Based Cognitive Therapy (MBCT), a MBI designed for recurrent depression, was only more effective than control conditions in preventing relapse of recurrent depression for patients with childhood trauma (Williams et al., 2014). Such findings highlight the clinical benefits of MBIs for ACE survivors, which were further confirmed in later studies (Joss et al., 2019; Kuyken et al., 2015).
Furthermore, an expanding body of neuroimaging research suggests that the clinical benefits of MBIs are substantiated by neural changes (Afonso et al., 2020; Kwak et al., 2019; Yang et al., 2019). Long term meditators have been shown to have larger bilateral hippocampal (Fox et al., 2014; Hölzel et al., 2008; Luders and Kurth, 2019; Luders et al., 2009) and bilateral subiculum (Luders et al., 2013) volumes. Longitudinal studies showed that MBIs led to increase in hippocampal gray matter density among highly stressed adults (Hölzel et al., 2011) and patients with Parkinson’s disease (Pickut et al., 2013), as well as attenuated hippocampal atrophy in elderly individuals with mild cognitive impairments (Wells et al., 2013). Post-MBI increase of subiculum volume was also found to be associated with improved cognitive performance among healthy individuals (Sevinc et al., 2020). Our recent research findings also suggest that MBI can increase hippocampal volumes in adult ACE survivors (Joss et al., 2020), but the prior study did not investigate any particular hippocampal subfields. Based on prior finding on subiculum volumes among long term meditators (Luders et al., 2013) or after an 8-week MBI (Sevinc et al., 2020), the current study is particularly interested in whether MBI can induce subiculum volumetric increases among ACE survivors.
The subiculum is a pivotal part of the hippocampal formation with widespread connections to other cortical and subcortical areas (O’Mara et al., 2001). As a result, the subiculum plays a critical role in the process of inhibitory control, in which the subiculum processes incompatible goal-related information and prevents responses towards conflicting goals (McNaughton, 2006). Inhibitory control is a fundamental process in meditation, because a primary goal of meditation is to replace mindless reactivity with mindful responsivity. For example, instead of mindlessly reacting to stressors out of habitual behavioral patterns, meditation practices eventually enable individuals to mindfully recognize and evaluate behavioral options in order to choose wise responses to situations in life (Lacaille et al., 2018). Therefore, the prior findings of enlarged subiculum among meditators were interpreted as major neural substrates for improved inhibitory control as a result of meditation practices (Luders et al., 2013).
Given that ACEs are associated with reduced subiculum volume (Teicher et al., 2012) and that MBI can promote subicular enlargement (Luders et al., 2013; Sevinc et al., 2020), we conducted a randomized control trial to assess whether MBI is more effective than an active control in increasing subiculum volumes among individuals with ACEs. We are specifically focusing on the subiculum as opposed to other stress-susceptible components of the hippocampal formation because MBI has not been reported to increase the volume of other subfields. This study builds on our prior work (Joss et al., 2021, 2020) but represents a significant advancement through the use of an active versus waiting list control, and the use of hippocampal segmentation software rather than voxel-based morphometry to more precisely gauge the effects of MBI on the subiculum (Joss et al., 2020).
Furthermore, research suggests that MBIs are associated with improvement in multiple cognitive domains including attention, memory, executive functioning and higher order processes (Gill et al., 2020; Whitfield et al., 2022). In particular, mindfulness training has been shown to improve the accuracy of discerning positive and negative stimuli (Kiken and Shook, 2011) and for reducing negative interpretations (Gibb et al., 2022). Our recent study using the Mnemonic Similarity Task (MST) also demonstrated that improved performance accuracy was associated with increased hippocampal volumes among ACE survivors (Joss et al., 2020). In the present study we used the pattern separation index from MST (Stark et al., 2013, 2019; Yassa et al., 2010) as a behavioral measurement for pattern separation capability, in order to assess whether MBI is more effective than an active control in enhancing this ability, and whether improvement in pattern separation is associated with hippocampal and subfield volumetric changes.
1.4. Summary of research plans and hypotheses
In summary, the present study aims to investigate the effects of MBI for ACE survivors on hippocampal volumes through a randomized controlled mechanistic clinical trial comparing MBI with an active control condition. In particular, based on prior studies that demonstrated MBI-related subiculum volumetric changes (Luders et al., 2013; Sevinc et al., 2020), we are particularly interested in the volumetric changes of the subiculum. Exploratory analyses will also be conducted to investigate the effect of MBI on other hippocampal subfields. The MST task is utilized to reflect pattern separation capability, and the contribution of subiculum, CA3, and dentate gyrus volumetric changes will be investigated.
2. Methods
2.1. Subject enrollment
This study was approved by the Institutional Review Board (IRB) of Mass General Brigham. Childhood adversity was assessed with the ACE questionnaire (Felitti et al., 1998) and the ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) Scale (Teicher and Parigger, 2015). Clinical profiles were determined through structured clinical interviews based on DSM-IV-TR. Inclusion criteria included: (1) Current age between 21 and 35 years old. (2) Right-handed. (3) ACE exposure with ACE score above 1 or MACE score above 3; (4) Meeting MRI eligibility criteria. Exclusion criteria included psychosis, neurological disorders, use of psychotropic medications and supplements, routine use of illicit drugs during the past month, ongoing psychotherapy, prior participation of systematic meditation or mindfulness programs within the past 2 years or routine meditation practice during the past 6 months . ACE questionnaire (Felitti et al., 1998) is a 10-item questionnaire to inquire existence of 10 scenarios of childhood experiences such as abuse, neglect or household dysfunction (e.g., domestic violence, substance misuse, incarceration). The total number of questions to which a subject answer “Yes” adds up to a score between 0 abd 10. ACE questionnaire is widely utilized but does not capture all forms of childhood maltreatment. MACE is a 75-item questionnaire that includes additional assessments for various types of childhood maltreatment (e.g., non-verbal emotional abuse, peer verbal abuse, peer physical abuse, witnessing siblings abused by parents), but it’s relatively new and not yet as widely utilized. Scoring of MACE is based on the published scoring code (Teicher and Parigger, 2015). The cutoff score of 3 was experimented to be a minimum score to be considered to endorse childhood maltreatment. The reason for using both ACE and MACE for screening purposes was to overcome the possible limitation of a particular instrument and to include sufficient variability in the sample to investigate the potential contribution of childhood maltreatment severity on post-MBI neural changes that was found in our previous research (Joss et al., 2021, 2024). A total of sixty-four subjects were enrolled into the study, with 35 randomly allocated to MBI while the other 29 allocated to an active control condition called Stress Management Education (SME) (Hoge et al., 2013; Holzel et al., 2013; Sevinc et al., 2019) at a ratio of 1.2:1 stratified with gender. Randomization procedure was carried out with the randomization module in RedCap, which implements an allocation table generated in MATLAB with the above specified stratifications. Twenty-one subjects in the MBI group and 19 subjects in the SME control group completed the study. The rest of the subjects dropped out of the study and did not yield any post-intervention data, thus were not included in the analyses. There was no significant difference in the dropout rates between the two groups (40 % vs 38 %, X2= 0.002, p = 0.96).
2.2. Research procedures
2.2.1. Overall procedure
After being determined as eligible, subjects were instructed to fill out online questionnaires and complete an MRI visit within a month before the intervention programs started. The MRI procedures included a 6 min anatomical scan and a 7 min resting state fMRI scan (the resting state fMRI will be reported in a future manuscript). Subjects completed the MST task on a Windows laptop outside the MRI scanner. Then subjects were randomized to attend either MBI or SME. Both intervention programs lasted 8 continuous weeks, which consisted of eight two-hour long in-person weekly meetings plus one 6 h whole day session on a weekend. Within a month after the intervention programs ended, subjects were administered the same research procedures.
2.2.2. MRI parameters
MRIs were acquired on a Siemens 3T Prisma system at the Imaging center of McLean hospital. A 64-channel head coil was used to acquire all MRI images. High resolution anatomical image was acquired using a T1-weighted multi-echo MPRAGE (MEMPRAGE) sequence (van der Kouwe et al., 2008), which acquires 4 separate structural scans with different TE values ranging from 1.69 to 7.27 ms, but in the same time as a conventional scan, and then the 4 separate images were averaged to increase the signal to noise ratio. Voxel size is 1.0 × 1.0 × 1.0 mm, Field of View (FOV) read is 256 mm, base resolution is 256, and there are 176 slices per slab. Phase encoding direction is A>>P. TR=2530 ms, TI=1100 ms, TE-1=1.6 9ms, TE-2=3.55 ms, TE-3=5.41, TE4=7.27 ms. Flip angle =7.0°. MRI anatomical data was visually inspected during data acquisition, any data that showed signs of motion artifact was re-acquired after reiterating to the subjects on the importance of staying still for the duration of MRI data acquisition.
2.3. Interventions
2.3.1. Mindfulness based intervention (MBI)
The MBI program was modeled after the Mindfulness Based Stress Reduction (MBSR) program (Kabat-Zinn, 1982, 1990; Kabat-Zinn et al., 1992), which covered a wide range of topics such as mindfulness and awareness, perception and perspectives, being present, responding vs. reacting to stress, stress coping strategies, dealing with difficult emotions, handling difficult communications, and using mindfulness in everyday life (Santorelli et al., 2017). Based on prior research on specific difficulties faced by trauma survivors (Vallejo and Amaro, 2009), the MBSR program was adapted into a trauma-sensitive MBI with emphasis on empowering the participants by using suggestive instead of directive language, incorporating more mindful movements, implementing more flexibility with homework, and providing options and choices whenever possible (Joss et al., 2019).
2.3.2. Stress Management Education (SME)
The SME program was adapted from our previous studies that used this intervention as the active control condition for MBSR (Hoge et al., 2013; Holzel et al., 2013; Sevinc et al., 2019), it was designed to closely match with the psycho-education component of the MBSR program except for the mindfulness component. The SME protocol closely matched the MBI protocol in the amount of time of weekly meetings and requirements for home practice of skills learned in class. Both MBI and SME groups were taught by licensed independent social workers with prior clinical experience, with the MBI instructor specialized in mindfulness meditation whereas the SME instructor specialized in cognitive behavioral therapy. The SME program includes psychoeducation about stress and stress physiology, sleep hygiene, time management, nutrition and healthy diet, as well as resilience and altruism.
2.4. Questionnaires
All subjects filled out a battery of psychological questionnaires through the REDCap secure online platform (Harris et al., 2009) before and after the intervention programs. Two questionnaires were analyzed in the present study: (1) Perceived Stress Scale (PSS): A brief, validated and widely used psychological instrument for assessing a subject’s perception of stress (Cole, 1999). It consists of 10 questions that measure the degree to which situations in the subject’s life are perceived as stressful, including questions related to how unpredictable or uncontrollable those events are perceived to be. (2) Mindful Attention Awareness Scale (MAAS): A 15 item questionnaire assessing the frequency of mindful states over time with a focus on the presence or absence of attention to and awareness of what is occurring in the present (Brown and Ryan, 2003). Additional questionnaires for comprehensive assessment of psychological symptoms were also administered and have been reported in other manuscripts.
2.5. MST Episodic memory task
The MST episodic memory task (Stark et al., 2019) is an explicit 3-alternative forced choice task, in which participants view novel (new), repeated (old) and lure (similar) stimuli. Stimuli are color photographs of common everyday objects. Each run consists of 16 similar pairs, 16 identical pairs and 44 unrelated novel items (foils), fully randomized throughout the run. Each stimulus is presented for 2000 ms with a 500 ms inter-stimulus-interval. Participants are instructed to make a judgment as to whether the object seen was new (i.e., novel items), old (i.e., repeated items) or similar but not identical (i.e., lure items). The whole task included 768 trials that were equally divided into 8 blocks, with 576 foil trials for which “new” was the correct answer, 96 target trials for which “old” was the correct answer, and 96 lure trials for which “similar” was the correct answer. Of critical interest are the participants’ responses on the lure items. A response of “old” to a lure (i.e., similar) item would suggest that the participant was more biased towards pattern completion, whereas an accurate response of “similar” to a lure would suggest a bias towards pattern separation instead. The Lure Discrimination Index (LDI) (Stark et al., 2019) was calculated as:
and the Corrected Recognition Memory (REC) score (Stark et al., 2019) was calculated as:
2.6. Data analysis
2.6.1. Statistical analysis of questionnaire scores
We used Linear Mixed Effects Models (LME) (Lindstrom and Bates, 1988) to evaluate the treatment effects on PSS and MAAS scores. LME analyses were conducted with the “nlme” and “MuMIn” packages in statistical software R. The scores of each questionnaire were used as the dependent variable for each model, with “group” (i.e., MBI or SME) and “time point” (i.e., before or after the intervention) and group by time interaction as independent variables, with age, sex, race, and time-interval between the two measurements as covariates. Separate variance for each group and time point was used, and “REML” method was chosen to maximize the restricted log-likelihood.
2.6.2. Analyses of hippocampal subfields volumes
All anatomical MRI data was visually inspected for motion artifacts; for subjects that needed re-acquisition due to motion artifact, only the data without motion artifact was used for further analyses. Anatomical MRI data of each subject was analyzed with the longitudinal module of the FreeSurfer 6.0 software, which performs automated hippocampal subfield segmentation using a probabilistic atlas built with ultra-high-resolution ex vivo MRI data (Iglesias et al., 2015, 2016). A total of 12 hippocampal subfields (CA1, CA3, CA4, molecular layer, hippocampus tail, hippocampal fissure, para-subiculum, hippocampus-amygdala-transitional-area, pre-subiculum, fimbria, subiculum, dentate gyrus) were automatically segmented for each hippocampus with estimated volumes, but only the subiculum was included in hypothesis-driven group statistics.
Volumetric changes were further calculated as Symmetrized Percent Change (SPC) (Reuter et al., 2012), which is the rate of change with respect to the average of Baseline Volume (BV) and Post-Intervention Volume (PV), as outlined in Eq. (1). SPC is a more robust measure than the traditional calculation of Percent Change (PC) in Eq. (2), because the denominator is the average of BV and PV instead of BV alone, thereby SPC reduces the influence of BV on the quantification of post-intervention change. SPC also takes into the consideration of the individual variability with time interval between the two MRI measurements in number of days.
| (1) |
| (2) |
LME analyses were conducted for group statistics, with SPC of the right whole hippocampus and subiculum as dependent variables, and “group” (MBI or SME) as independent variable, with Total Intracranial Volume (TIV), age, sex and race as demographic covariates.
Exploratory two-sample t-tests were conducted with the SPC values of other hippocampal subfields. To investigate whether maltreatment severity affects the effects of MBI on hippocampal volumes, exploratory Pearson correlation analyses were conducted, with pooled data from both groups, and separately with data from MBI group alone, between maltreatment measures including scores of ACE, MACE-number of types of maltreatment and MACE-total severity scores, and SPC of bilateral hippocampus and subiculum. Correction for multiple comparison was conducted with the “p.adjust” function in R using the Benjamini & Hochberg method (Benjamini and Hochberg, 1995).
In order to identify major factors contributing to hippocampal and subiculum volumetric changes, post hoc Variance Decomposition Analyses (VDA) were conducted with data from the MBI group using the R package “relaimpo”. VDA is a widely utilized statistical technique to partition the total variance in an outcome variable into components of interest in order to quantify the amount of contribution of each regressor and to identify regressors that explain the most amount of variance in the regression model (Callen and Segal, 2004; Isakin and Ngo, 2020; Zaefarian et al., 2022). VDA analysis in this study started with a linear regression then utilized the “calc.relimp” R function to generate an estimated percentage of the amount of variance explained by each independent variable. The algorithm implemented in this R function (Lindeman, 1980) particularly takes into consideration of the correlations among regressors to more accurately gauge the relative importance of each regressor. The same algorithm was used in our previous cross-sectional study to quantify the amount of variance in hippocampal subfield volumes explained by ACE scores (Teicher et al., 2012). For VDA analyses in the present study, dependent variables in the linear regression models were SPCs of the right hippocampus and right subiculum volumes, with regressors included scores of the ACE questionnaire, total hours of intervention session attendance, and score changes of MAAS and score changes of PSS.
2.6.3. Analyses of performance from the MST episodic memory task
LME models were used to analyze group statistics of MST task performance. LDI or REC were used as dependent variables, with scores of the ACE questionnaire, pre-intervention and post-intervention scores of MAAS and pre-intervention and post-intervention scores of PSS, as well as group, time point (i.e., pre- or post-intervention) and group by time interaction as independent variables, with age, sex, race, and time-interval between the two measurements as covariates.
In order to quantify the amount of contributions of neural changes to changes of LDI and REC, post hoc VDA were conducted with data from the MBI group with the same procedure as the VDA analyses described in Section 2.6.2. Dependent variables were score changes of LDI or REC, with predictors include SPC values of bilateral CA3, dentate gyrus and subiculum based on exiting MST literature (Bakker et al., 2012; Nash et al., 2021; Stark et al., 2013).
3. Results
3.1. Demographics and intervention compliance
The two groups did not differ significantly in the distribution of age, sex, race, childhood adversity, lifetime DSM diagnoses, or amount of intervention attendance (Table 1). The percentages of subjects with ACE scores of 0, 1, 2, 3, 4, and ≥5 are: 25 %, 20 %, 15 %, 10 %, 12.5 % and 17.5 %. As measured by MACE, the percentages of subjects having 0, 1, 2, 3, 4, and ≥5 types of childhood maltreatment are: 37.5 %, 12.5 %, 10 %, 10 %, 5 % and 25 %. One subject in the MBI group was excluded from further analyses due to incidental finding of neurological abnormality in MRI. A total of 20 subjects in MBI and 19 subjects in SME group were included in the rest of analyses.
Table 1.
Subject demographic and clinical information.
| Mindfulness | Control | Group Difference |
|
|---|---|---|---|
| Sample Size (N) | 21 | 19 | |
| Sex: Female(F); Male(M) | F: 17; M: 4 | F: 14; M: 5 | Fisher’s exact test: p = 0.71 |
| Average Age (in years) (SE, range) | 26.71 (0.88), 22-23 | 25.58 (0.74), 22-34 | t= 0.97, p = 0.34 |
| Race (Frequencies) | |||
| White | 10 | 8 | Fisher’s exact |
| Black/African American | 0 | 3 | test: p = 0.21 |
| Asian | 8 | 8 | |
| Hispanic | 2 | 0 | |
| Unknown | 1 | 0 | |
| Childhood Maltreatment Assessments (Mean (SE), range) | One-Way ANOVA | ||
| ACE Scores | 2.14 (0.48), 0-9 | 2.42 (0.46), 0-5 | F = 0.17, p = 0.68 |
| MACE-number of types of maltreatment | 2.00 (0.58), 0-10 | 2.95 (0.66), 0-8 | F = 1.17, p = 0.29 |
| MACE-total severity scores | 21.10 (3.69), 0-61 | 27.16 (4.06), 2-55 | F = 1.22, p = 0.28 |
| DSM-IV-TR lifetime diagnosis (Frequencies) | Fisher’s exact test | ||
| Depressive Disorders | 10 | 13 | p = 0.21 |
| Anxiety Disorders | 15 | 15 | p = 0.72 |
| Average total hours of intervention class attendance (SE, range) | 16.64 (1.01), 4-22 | 14.43 (0.84), 8-20 | t= 1.68, p = 0.10 |
3.2. Self-report questionnaires
LME analyses of PSS and MAAS scores demonstrated a significant effect of time (p < 0.0001) with no significant group by time interaction. Post hoc t-tests showed that both groups had reduced PSS scores (p < 0.01) and increased MAAS scores (p < 0.05). Score changes were calculated as post-intervention scores minus pre-intervention scores, and there was no significant group difference with MAAS or PSS score changes.
3.3. Hippocampal volumetric changes
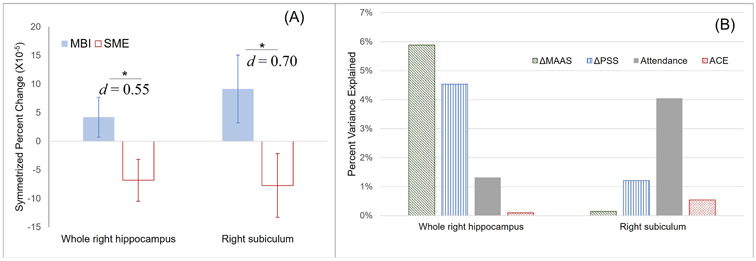
LME analyses of SPC values revealed significant group effects with the right whole hippocampus (F = 5.43, p = 0.027, effect size Cohen’s d = 0.55) and the right subiculum (F = 4.56, p = 0.041, d = 0.70) (Fig. 1A). TIV was not a significant predictor for SPC of whole right hippocampus (F = 1.01, p = 0.32) or the right subiculum (F = 0.68, p = 0.42).
Fig. 1.
Findings on hippocampal volumetric changes. (A) Significant group differences (p < 0.05, medium effect sizes) with symmetrized percent change of the volumes of the whole right hippocampus and right subiculum. (B) Quantification of contributing factors to hippocampus and subiculum volumetric changes in the MBI group.
Exploratory two-sample t-test analyses were conducted with the SPC values of other hippocampal subfields, and no significant group difference was found. No significant correlations were found from exploratory analyses between measures of maltreatment severity and SPC values of bilateral hippocampus or subiculum.
Post hoc VDA analysis demonstrated that score changes (Δ) of MAAS (6 %) and score changes of PSS (5 %) were major contributors to SPC of the whole right hippocampus, whereas amount of intervention session attendance (4 %) was a major contributor to right subiculum SPC (Fig.1B).
3.4. Episodic memory performance changes and contributing factors
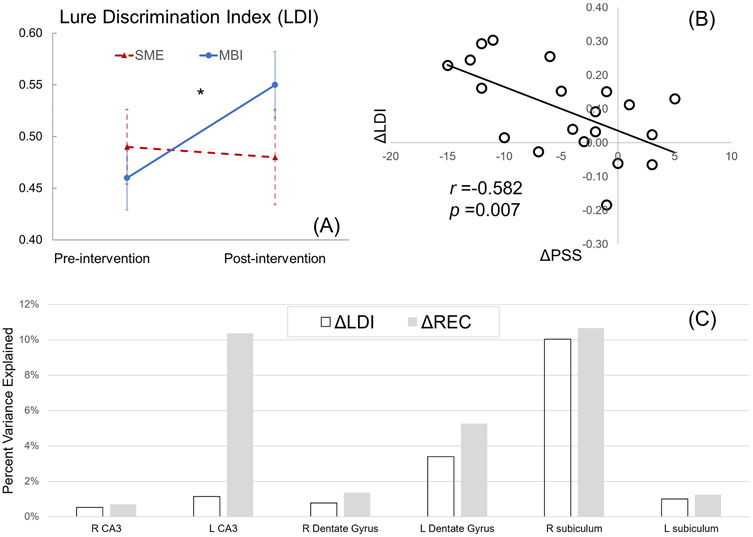
LME analysis demonstrated a significant group by time interaction effect for LDI (p < 0.05) with PSS score as a significant predictor (p < 0.01). Post hoc analysis with paired t-test showed that the MBI group had increased LDI (p < 0.01) while the SME group had no significant change (Fig. 2A). There was no significant group by time interaction effect for REC and neither group had significant change. Post hoc analysis with Pearson correlation revealed a significant correlation between LDI changes (ΔLDI) and PSS score changes (ΔPSS) in the MBI group (r = −0.58, p < 0.01, N = 20, Fig. 2B).
Fig. 2.
Findings from the MST episodic memory task. (A): Significant group by time interaction effect with LDI (reflecting pattern separation capability), with only the MBI group showing improvement. (B) Significant negative correlation between PSS score change and LDI change (r = −0.33, p < 0.05), suggesting stress reduction was associated with improvement of pattern separation capability.
VDA shows that right subiculum SPC explained 10.05 % of the individual variability in ΔLDI in the MBI group, whereas the individual variability in ΔREC was mostly explained by SPC of the right subiculum (10.66 %), left CA3 (10.37 %), and left dentate gyrus (5.26 %) (Fig. 2C).
4. Discussion
Major findings from the present study include significant group differences with volumetric changes of the right whole hippocampus and right subiculum, as well as significant post-MBI improvement in pattern separation capability as reflected from LDI of the MST task.
4.1. Beneficial effects of MBI for hippocampal volumes
Our findings add to the growing body of literature demonstrating morphometric changes in the hippocampus following mindfulness training (Fotuhi et al., 2016; Hölzel et al., 2011; Joss et al., 2020; Pickut et al., 2013), in addition to cross-sectional findings of larger hippocampal volumes among long term meditators (Fox et al., 2014; Hölzel et al., 2008; Luders and Kurth, 2019; Luders et al., 2009). The longitudinal studies in particular have provided insights on the possible causality between MBI and hippocampus morphometric changes. For example, one VMB study identified a left hippocampus cluster that showed increased gray matter concentration after MBSR while the waiting list control had no significant change (Hölzel et al., 2011). Another study with a 12-week “brain fitness program” that included meditation training reported volumetric growth of the hippocampus in elderly with mild cognitive impairment (Fotuhi et al., 2016). A randomized controlled trial on MBI for patients with Parkinson’s disease (Pickut et al., 2013) showed increased gray matter density in bilateral hippocampus. Our prior study with ACE survivors also identified a right hippocampal cluster with post-MBI volumetric increase that was associated with amounts of stress reduction (Joss et al., 2020). Consistently, the present study also found group differences of volumetric changes of the right whole hippocampus and right subiculum. A recent study with healthy adults did not find significant post-MBI hippocampal volumetric changes (Kral et al., 2022), however there were multiple methodological issues such as overly extended data collection periods before and after MBI that might have compromised the sensitivity of neuroimaging discovery. Post-MBI hippocampal volumetric changes were also found to be associated with memory improvement (Greenberg et al., 2019). Our finding also revealed that improvement in mindfulness (ΔMAAS) and perceived stress (ΔPSS) contributed to the overall volumetric change of the whole right hippocampus, demonstrating a brain-behavior association with regard to the psychological and neural effects of MBI.
Very few prior studies investigated meditation related volumetric changes of specific hippocampal subfields, among which the subiculum in particular was identified to have morphological changes among long-term meditators (Luders et al., 2013) or in response to an 8-week MBI (Sevinc et al., 2020). The current study not only revealed a significant group difference with subiculum volumetric changes, but also found that the amount of intervention attendance specifically contributed to the volumetric changes of the right subiculum in the MBI group. Detailed discussions in the following paragraphs will comprehensively review the function of the subiculum and potential mechanisms for its involvement in MBIs.
One reason for the subiculum to be particularly responsive to MBI may be related to its critical role in inhibitory control (McNaughton, 2006) and latent inhibition (Weiner and Feldon, 1997) (Mingote et al., 2019), which is the essence of meditation practices (Cásedas et al.,2020). Inhibitory control refers to the conscious effort for inhibiting impulses, e.g., as measured by “go-no-go” cognitive tasks (Durston et al., 2002; Schall et al., 2017; Williams et al., 1999), whereas “latent inhibition” refers to the ability to ignore irrelevant stimuli, a concept originated from schizophrenia research to describe the inability to ignore non-salient stimuli (Lubow, 1973; Lubow and Gewirtz, 1995; Weiner and Feldon, 1997). Despite the subtle differences of these two concepts from respective literature background, both inhibitory processes are practiced during meditation, e.g., inhibiting the impulses to stop meditating (e.g., the urge to get up from the cushion) and practicing the ability to ignore any stimuli that’s not the prespecified focus of awareness (e.g., ignoring the sound of traffic from outside and focus the awareness on breathing). Another example is the cognitive processes during the MST task in this study, e.g., because 75 % trials in MST was foil (for which “new” was the correct answer), subjects had to inhibit the impulse to answer “new” to the less frequent target and lure trials, which is an example of inhibitory control. Because it was a lengthy task (~40 min), in order to stay focused, subjects had to ignore internal and external distractors, such as fatigue, boredom, ruminative thoughts or background noise from the external environment, which is an example of latent inhibition.
Meditation is essentially an inhibitory process. Meditation training includes the instruction to remain still for the duration of the meditation period, thus requiring repeated inhibition of urges to adjust posture, stretch, or get up and do something else (Kabat-Zinn, 2003). Furthermore, meditation is not a thought-free state, but rather a dynamic process during which “mind wandering” spontaneously occur (Hasenkamp et al., 2012) while the meditator repeatedly makes a conscious effort to bring awareness back to pre-specified focal point of attention such as breathing or body sensations (Feruglio et al., 2021; Hasenkamp et al., 2012). In other words, the meditator consciously inhibits the impulse to engage with the content of mind wandering (e.g., ruminating about the past or actively planning for the future). Prior meditation research have demonstrated enhanced response inhibition among experienced meditators (Andreu et al., 2019) or after intensive meditation training (Sahdra et al., 2011), or after several weeks of MBI (Greenberg et al., 2019). Such practice of inhibitory control may be particularly beneficial for trauma survivors, who are not only vulnerable to spontaneous mind wandering (Brosowsky et al., 2022; Nayda and Takarangi, 2021; Takarangi et al., 2014) but also susceptible to negative emotional reactivity in response to mind wandering (Green et al., 2016; Marcusson-Clavertz et al., 2017).
Anatomical research has demonstrated that the subiculum has widespread cortical and subcortical connectivity, enabling it to influence neural activity in disparate brain regions (O’Mara et al., 2001; Quintero et al., 2011), thereby substantiating its role in inhibitory control. For example, the subiculum has been found to have functional connectivity with the precuneus and posterior cingulate cortex of the Default Mode Network (DMN) (de Flores et al., 2017; Vos de Wael et al., 2018), in particular, a recent study found the subiculum has stronger functional connectivity with DMN than other hippocampal subfields (Katsumi et al., 2023). DMN is heavily involved in mind wandering (Mittner et al., 2016; Poerio et al., 2017), while numerous studies have shown mindfulness meditation modulates the DMN (Brewer and Garrison, 2014; Garrison et al., 2015) as well as its connectivity with other neural circuitries (Bremer et al., 2022; Rahrig et al., 2022). These findings further indicate that during meditation practices, the inhibitory control against mind wandering may extensively utilize the subiculum due to its connectivity with the DMN.
The subiculum’s role in latent inhibition is another possible mechanism for its involvement in MBI. When impulses are effortfully inhibited during meditation (e.g., noticing being lost in mind wandering and bring attention back to focal point), it mostly engages the behavioral inhibition system in which the subiculum plays a pivotal role in resolving conflicts between incompatible goals hence achieving inhibitory control (McNaughton, 2006). Another mechanism is for meditators to ignore distraction by allowing the spontaneous thoughts from mind wandering to pass without engaging with them, which involves the process of latent inhibition (Weiner and Feldon, 1997), which refers to inhibited neural reactivity to non-salient stimuli and reflects the ability to ignore irrelevant stimuli (Weiner and Feldon, 1997). Preclinical and lesion studies have demonstrated that latent inhibition relies on input from the ventral subiculum to the nucleus accumbens (Quintero et al., 2011; Weiner and Feldon, 1997). As a major neural substrate for latent inhibition, the ventral subiculum acts as an interface between the hippocampus circuitry for contextual information processing and the ventral striatum of the motivation system, and modulates the dopamine levels in the mesolimbic system thereby affecting the expression of latent inhibition (Quintero et al., 2011).
Therefore, due to its pivotal role in the inhibitory control and latent inhibition processes that are heavily utilized in meditation practices, the observed post-MBI subiculum volumetric changes could be resulted from the increased utilization of this structure (Luders et al., 2013; Sevinc et al., 2020). Gray matter increases have been observed in other brain areas following repeated concentrated utilization of these brain regions during skill acquisition and expertise development. For example, motor skill learning (Sampaio-Baptista et al., 2014) and music training (Hyde et al., 2009) have been shown to lead to increased gray matter in motor (Sampaio-Baptista et al., 2014) and auditory cortices (Hyde et al., 2009). Hippocampal morphological changes can be induced by various physiological mechanisms. Preclinical studies have demonstrated that social (Biggio et al., 2019), physical (Gabriel et al., 2020) or environmental enrichment (Monteiro et al., 2014) can stimulate neurogenesis of rodent hippocampus through restoring BDNF, NGF and Arc gene expression, increasing the density and morphology of dendritic spines, neuronal tree arborisation in granule cells (Biggio et al., 2019), or decreasing dopamine D2 receptor expression (Gabriel et al., 2020). The beneficial effect of MBI for hippocampal volumes likely works through similar processes, the exact cellular and molecular mechanisms of which are worth further investigation.
From a computational neuroscience perspective, the inhibitory control practiced during meditation interrupts existing habitual thought-feeling-behavior associations and rebuild new associations for new behavioral response patterns, which is a learning process that the hippocampus plays a pivotal role in Aitken and Kok (2022). A recent fMRI study showed the hippocampus engages in retrieval of existing associations at the beginning of the learning process and preferentially encodes unexpected stimuli according to the old framework, and as the learning of new association progresses and completes, the hippocampus switches to encode stimuli as predicted by the newly learned associations; in particular, in this process, the subiculum relays predictions from the hippocampal circuitry to the sensory cortex for processing the visual and auditory stimuli (Aitken and Kok, 2022). Such “belief updating” computational models (Nassar et al., 2010) are also endorsed in the meditation research field where the inhibition process in meditation practices facilitates the update of prior internal prediction model in response to unexpected sensory input to reduce prediction error, leading to adaptive behaviors well-adjusted to new contexts (Barrett, 2017; Barrett and Simmons, 2015; Farb et al., 2015; Kirk et al., 2019; Laukkonen and Slagter, 2021).
Although multiple subfields of the left hippocampus (the left CA2-3, CA4-dentate gyrus, pre-subiculum and subiculum) were found with smaller volumes among young adults with ACE compared to controls in the previous study (Teicher et al., 2012), our exploratory analyses of these subfields did not identify significant group differences with their SPC values. This could be attributed to multiple reasons: (1) the developmental impairment of these subfields might have compromised their ability for neural changes in response to MBI, or perhaps longer training periods are required to achieve observable effects. Therefore the current findings of post-MBI volumetric change of the right hippocampus could reflect a neural compensatory mechanism (Martins et al., 2015), e.g., working through areas with less developmental impairment to achieve behavioral improvements. (2) It is also possible that MBI does not influence all hippocampal subfields equally, and may particularly have selective effects on the subiculum (Luders et al., 2013; Sevinc et al., 2020) due to its pivotal role in inhibitory control and latent inhibition as extensively discussed above. Nevertheless the fact that the present study only observed significant group difference with the right, but not the left subiculum is still intriguing since prior meditation study has found effects with either bilateral (Luders et al., 2013) or left (Sevinc et al., 2020) subiculum. This could be due to the detrimental impact of ACE on the left subiculum (Teicher et al., 2012).
4.2. MBI improved pattern separation capability
The present study found that the MBI group, but not the SME group, had improved “pattern separation” capability of episodic memory as reflected from the LDI score from MST (Stark et al., 2019). Traumatic experience often results in compromised ability to discern perceived threats from actual threats (Miller, 2015), leading to the “hypervigilance” (Kimble et al., 2014) typically seen among trauma survivors (Dalgleish et al., 2001). The present study found that post-MBI reduction of perceived stress was significantly associated with LDI improvement (Fig. 2B), which is consistent with a recent finding that LDI was predicted by individual variability in PSS scores (Grupe et al., 2022). Such association is consistent with prior knowledge about the detrimental effects of stress on cognitive function, especially memory formation (McEwen and Sapolsky, 1995; Sandi, 2013). The finding from this longitudinal MBI study highlights the malleability of LDI in response to changes in stress levels, thus has potential clinical implications for treatments of stress-related disorders.
MST is known to reflect hippocampal functionality, particularly that of the CA3 and dentate gyrus (Bakker et al., 2012; Stark et al., 2013), as well as the subiculum (Nash et al., 2021). In the present study, the individual variability with volumetric changes of the right subiculum was a major contributor to the individual variability of post-MBI LDI changes (Fig. 2C), which elucidated the possibility of subiculum volumetric increase as potential neural substrates for post-MBI improvement in pattern separation capabilities. Post-MBI improvement in pattern separation capability is consistent with prior findings on the effect of mindfulness training for improving sensory acuity such as visual acuity (García-Martin et al., 2016; Langer et al., 2010) or tactile acuity (Kerr et al., 2008), which collectively reflect mindfulness-induced improvements in multiple domains such as attention control (Moore et al., 2012) and somatosensory awareness (Kerr et al., 2013). From the perspective of fundamental hippocampal function, the subiculum plays a key role in coordinating hippocampal-cortical interactions (O’Mara et al., 2001). A recent fMRI study identified right subiculum activation as well as right subiculum functional connectivity with DMN structures and temporal cortices during the pattern separation process of the MST experiment (Nash et al., 2021). Another fMRI study found post-MBI subiculum volumetric increase was associated with hippocampal-cortical functional connectivity during a fear conditioning task (Sevinc et al., 2020). Therefore, the observed contribution of subiculum volumetric change to the individual variability in post-MBI LDI change sheds light on potential neural mechanisms of MBI-induced pattern separation cognitive ability improvements in which the subiculum might play a critical role.
The REC index from MST reflects recognition memory for repeated items in the task, thus reflecting the tendency for pattern completion. Studies from the aging literature suggest that LDI declines with age whereas REC remains fairly stable (Dillon et al., 2017; Stark et al., 2019). Although the present study did not find significant longitudinal changes with REC at the group level, VDA analysis revealed that the individual variability in post-intervention REC changes were mostly driven by hippocampal subfield volumetric changes including that of CA3, dentate gyrus and subiculum. Such neural correlates are consistent with existing knowledge about the critical role of CA3 for identical object recognition (Dillon et al., 2017) and its activation during the pattern completion process of the MST task (Kirwan and Stark, 2007; Lee et al., 2004). Although the dentate gyrus (Bakker et al., 2012; Stark et al., 2013) and subiculum (Nash et al., 2021) were previously reported to primarily involve in the pattern separation process, their contribution to the individual variability in REC in the present study may reflect their potential role in substantiating MBI-related changes in episodic memory functioning.
4.3. Limitations and future directions
There are several major limitations with the present study that shall be addressed in future research: (1) The sample size is modest due to budget limitations, although it is comparable to several prior MBI neuroscience studies, e.g., N = 26 of the previous report on MBI-induced amygdala volumetric changes (Hölzel et al., 2010), N = 27 of an MRI study on the neural effects of MBI for Parkinson’s disease (Pickut et al., 2013) as well as N = 34 of our previous study on a similar topic (Joss et al., 2020). Nevertheless, a larger sample is still desirable for yielding more robust findings. (2) This study included subjects with a wide range of severity and types of childhood maltreatment for the purpose of enabling investigation of the possible influence of childhood maltreatment severity on post-MBI neural changes. Such variability enabled our recent discovery of maltreatment severity modulating post-MBI amygdala volumetric changes (Joss et al., 2024). Nevertheless, this study did not find maltreatment severity significantly affecting post-MBI hippocampal volumetric changes. In future studies, an alternative approach would be purposeful selection of a relatively homogenous patient population, which can be more advantageous for illuminating MBI’s effects for healing specific neural impacts of specific childhood adversity experiences with specific current psychopathology (e.g., target patient population of childhood sexual abuse survivors with current diagnosis of PTSD). (3) Despite our speculation on the involvement of the subiculum during the inhibitory control process in meditation, the current study did not have an fMRI experiment to directly observe such effects. Future studies with well-designed fMRI paradigms may shed light on real-time subiculum engagement during meditation. (4) The MST task was originally planned to be implemented as an fMRI task, but due to budget limitation, the task was administered outside the MRI scanner. It would have been more informative to directly investigate the hippocampal subfield activations during the MST task. (5) The interpretation for the role of subiculum in meditation practices is highly speculative due to lack of operationalized measures for inhibitory control or latent inhibition. To obtain further understanding on this topic, future research shall develop quantitative measures to probe the engagement of subiculum during these inhibitory processes in the context of meditation practices during fMRI experiments. (6) Human neuroimaging studies on the impact of ACE on hippocampal morphometry is fundamentally limited by the methodological limitations of MRI. Despite the tremendous progress with MRI technology and analyses algorithms during the past decades, there are still limited accuracy and precision for making definitive conclusions, and the investigation is constrained by the resolution of MRI signal and data analyses algorithms. Prior research also demonstrated different neural impacts of childhood maltreatment depending on the gender as well as the types and timing of the maltreatment (Teicher et al., 2018), therefore, depending on the sample composition of a particular study, the effects on a particular hippocampal subfield may not be significant. Similarly, investigation of neural effects of MBI is also constrained by the methodological limitations of MRI technology.
Acknowledgments
This study was funded by the National Center for Complementary and Integrative Health (Grant No.: 5K01AT009085). The funding source played no role in the study design, data collection, data analysis or manuscript writing. We thank Lauri Klein and Colette Coleman for teaching the intervention classes. We thank Junjie Lu for helping with data organization. We thank all research subjects for participating in this study.
Footnotes
CRediT authorship contribution statement
Diane Joss: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Martin H. Teicher: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Data curation, Funding acquisition, Conceptualization, Methodology, Visualization. Sara W. Lazar: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization, Data curation, Formal analysis, Visualization.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DJ and SWL are practitioners of mindfulness meditation.
References
- Afonso RF, Kraft I, Aratanha MA, Kozasa EH, 2020. Neural correlates of meditation: a review of structural and functional MRI studies. Front. Biosci 12, 92–115. [DOI] [PubMed] [Google Scholar]
- Aitken F, Kok P, 2022. Hippocampal representations switch from errors to predictions during acquisition of predictive associations. Nat. Commun 13, 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Hen R, 2017. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu CI, Palacios I, Moënne-Loccoz C, López V, Franken IH, Cosmelli D, Slagter HA, 2019. Enhanced response inhibition and reduced midfrontal theta activity in experienced Vipassana meditators. Sci. Rep 9, 13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M, 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci 12, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK, 2015. Interoceptive predictions in the brain. Nat. Rev. Neurosci 16, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. [Google Scholar]
- Biggio F, Mostallino M, Talani G, Locci V, Mostallino R, Calandra G, Sanna E, Biggio G, 2019. Social enrichment reverses the isolation-induced deficits of neuronal plasticity in the hippocampus of male rats. Neuropharmacology 151, 45–54. [DOI] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, Nelson Iii CA, 2009. Effects of early psychosocial deprivation on the development of memory and executive function. Front. Behav. Neurosci 3, el6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JE, Lanius RA, McKinnon MC, 2018. Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J. Psychiatry Neurosci 43, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer B, Wu Q, Mora Álvarez MG, Hölzel BK, Wilhelm M, Hell E, Tavacioglu EE, Torske A, Koch K, 2022. Mindfulness meditation increases default mode, salience, and central executive network connectivity. Sci. Rep 12, 13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, 1998. The effects of stress on memory and the hippocampus throughout the life cycle: implications for childhood development and aging. Dev. Psychopathol 10, 871–885. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, 2014. The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Ann. N. Y. Acad. Sci 1307, 19–27. [DOI] [PubMed] [Google Scholar]
- Brosowsky NP, Smith AC, Smilek D, Seli P, 2022. On the relation between mind wandering, PTSD symptomology, and self-control. Conscious. Cogn 99, 103288. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, 2003. The benefits of being present: mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol 84, 822. [DOI] [PubMed] [Google Scholar]
- Callen JL, Segal D, 2004. Do accruals drive firm-level stock returns? A variance decomposition analysis. J. Account. Res 42, 527–560. [Google Scholar]
- Cásedas L, Pirruccio V, Vadillo MA, Lupiáñez J, 2020. Does mindfulness meditation training enhance executive control? A systematic review and meta-analysis of randomized controlled trials in adults. Mindfulness 11, 411–424. [Google Scholar]
- Cole SR, 1999. Assessment of differential item functioning in the Perceived Stress Scale-10. J. Epidemiol. Community Health 53, 319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T, Moradi A, Taghavi M, Neshat-Doost H, Yule W, 2001. An experimental investigation of hypervigilance for threat in children and adolescents with post-traumatic stress disorder. Psychol. Med 31, 541–547. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 71, 286–293. [DOI] [PubMed] [Google Scholar]
- de Flores R, Mutlu J, Bejanin A, Gonneaud J, Landeau B, Tomadesso C, Mézenge F, de La Sayette V, Eustache F, Chételat G, 2017. Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum. Brain Mapp 38, 4922–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SE, Tsivos D, Knight M, McCann B, Pennington C, Shiel AI, Conway ME, Newson MA, Kauppinen RA, Coulthard EJ, 2017. The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Sci. Rep 7, 14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey B, 2002. A neural basis for the development of inhibitory control. Dev. Sci 5, F9–F16. [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D, 2008. Mindfulness-based cognitive therapy for generalized anxiety disorder. J. Anxiety Disord 22, 716–721. [DOI] [PubMed] [Google Scholar]
- Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, Klein AC, Paulus MP, Mehling WE, 2015. Interoception, contemplative practice, and health. Front. Psychol 6, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Feruglio S, Matiz A, Pagnoni G, Fabbro F, Crescentini C, 2021. The impact of mindfulness meditation on the wandering mind: a systematic review. Neurosci. Biobehav. Rev 131, 313–330. [DOI] [PubMed] [Google Scholar]
- Finkelhor D., 2020. Trends in adverse childhood experiences (ACEs) in the United States. Child Abuse Negl. 108, 104641. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Lubinski B, Trullinger M, Hausterman N, Riloff T, Hadadi M, Raji C, 2016. A personalized 12-week “brain fitness program” for improving cognitive function and increasing the volume of hippocampus in elderly with mild cognitive impairment. J. Prev. Alzheimer’s Dis 3, 133–137. [DOI] [PubMed] [Google Scholar]
- Fox KC, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Sedlmeier P, Christoff K, 2014. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev 43, 48–73. [DOI] [PubMed] [Google Scholar]
- Gabriel P, Mastracchio TA, Bordner K, Jeffrey R, 2020. Impact of enriched environment during adolescence on adult social behavior, hippocampal synaptic density and dopamine D2 receptor expression in rats. Physiol. Behav 226, 113133. [DOI] [PubMed] [Google Scholar]
- García-Martin E, Ruiz-de-Gopegui E, Otin S, Blasco A, Larrosa J, Polo V, Pablo L, Demarzo M, Garcia-Campayo J, 2016. Assessment of visual function and structural retinal changes in zen meditators: potential effect of mindfulness on visual ability. Mindfulness 7, 979–987. [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, Brewer JA, 2015. Meditation leads to reduced default mode network activity beyond an active task. Cogn. Affect. Behav. Neurosci 15, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A, Wilson JM, Ford C, Shook NJ, 2022. Does mindfulness reduce negative interpretation bias? Cogn. Emot 36, 284–299. [DOI] [PubMed] [Google Scholar]
- Gill LN, Renault R, Campbell E, Rainville P, Khoury B, 2020. Mindfulness induction and cognition: a systematic review and meta-analysis. Conscious. Cogn 84, 102991. [DOI] [PubMed] [Google Scholar]
- Green DM, Strange D, Lindsay DS, Takarangi MK, 2016. Trauma-related versus positive involuntary thoughts with and without meta-awareness. Conscious. Cogn 46, 163–172. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Romero VL, Elkin-Frankston S, Bezdek MA, Schumacher EH, Lazar SW, 2019. Reduced interference in working memory following mindfulness training is associated with increases in hippocampal volume. Brain Imaging Behav. 13, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Barnes AL, Gresham L, Kirvin-Quamme A, Nord E, Alexander AL, Abercrombie HC, Schaefer SM, Davidson RJ, 2022. Perceived stress associations with hippocampal-dependent behavior and hippocampal subfield volume. Neurobiol. Stress 19, 100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW, 2012. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 59, 750–760. [DOI] [PubMed] [Google Scholar]
- Herman J, Dolgas C, Carlson S, 1998. Ventral subiculum regulates hypothalamo–pituitary–adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86, 449–459. [DOI] [PubMed] [Google Scholar]
- Herzog P, Kube T, Fassbinder E, 2022. How childhood maltreatment alters perception and cognition–the predictive processing account of borderline personality disorder. Psychol. Med 52, 2899–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Gómez AF, 2017. Mindfulness-based interventions for anxiety and depression. Psychiatr. Clin. N. Am 40, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D, 2010. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol 78, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, Worthington JJ, Pollack MH, Simon NM, 2013. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry 74, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW, 2010. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci 5, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW, 2011. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. Neuroimaging 191, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, Barrett LF, Schwartz C, Vaitl D, Lazar SW, 2013. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D, 2008. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci 3, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G, 2009. Musical training shapes structural brain development. J. Neurosci 29, 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, 2015. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Van Leemput K, Augustinack J, Insausti R, Fischl B, Reuter M, For the Alzheimer’s Disease Neuroimaging Initiative, 2016. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage 141, 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakin M, Ngo PV, 2020. Variance decomposition analysis for nonlinear economic models 1. Oxf. Bull. Econ. Stat 82, 1362–1374. [Google Scholar]
- Joss D, Khan A, Lazar SW, Teicher MH, 2019. Effects of a mindfulness-based intervention on self-compassion and psychological health among young adults with a history of childhood maltreatment. Front. Psychol 10, 2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Khan A, Lazar SW, Teicher MH, 2021. A pilot study on amygdala volumetric changes among young adults with childhood maltreatment histories after a mindfulness intervention. Behav. Brain Res 399, 113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Lazar SW, Teicher MH, 2020. Effects of a mindfulness based behavioral intervention for young adults with childhood maltreatment history on hippocampal morphometry: a pilot MRI study with voxel-based morphometry. Psychiatry Res. Neuroimaging 301, 111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss D, Lu J, Teicher MH, Lazar SW, 2024. Childhood adversity severity modulates the associations between adaptive psychological changes and amygdala volumetric changes in response to behavioral interventions. J. Affect. Disord. Rep 15, 100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J., 1982. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 4, 33–47. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J., 1990. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. Delta. [Google Scholar]
- Kabat-Zinn J., 2003. Mindfulness-based interventions in context: past, present, and future. Clin. Psychol. Sci. Pract 10, 144–156. [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, et al. , 1992. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am. J. Psychiatry 149, 936–943. [DOI] [PubMed] [Google Scholar]
- Kalmakis KA, Chandler GE, 2015. Health consequences of adverse childhood experiences: a systematic review. J. Am. Assoc. Nurse Pract 27, 457–465. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Olariu A, Cameron HA, 2005. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci. 28, 171–172. [DOI] [PubMed] [Google Scholar]
- Katsumi Y, Zhang J, Chen D, Kamona N, Bunce JG, Hutchinson JB, Yarossi M, Tunik E, Dickerson BC, Quigley KS, Barrett LF, 2023. Correspondence of functional connectivity gradients across human isocortex, cerebellum, and hippocampus. Commun. Biol 6, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR, 2013. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front. Hum. Neurosci 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CE, Shaw JR, Wasserman RH, Chen VW, Kanojia A, Bayer T, Kelley JM, 2008. Tactile acuity in experienced Tai Chi practitioners: evidence for use dependent plasticity as an effect of sensory-attentional training. Exp. Brain Res 188, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiken LG, Shook NJ, 2011. Looking up: mindfulness increases positive judgments and reduces negativity bias. Soc. Psychol. Pers. Sci 2, 425–431. [Google Scholar]
- Kimble M, Boxwala M, Bean W, Maletsky K, Halper J, Spollen K, Fleming K, 2014. The impact of hypervigilance: evidence for a forward feedback loop. J. Anxiety Disord 28, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U, Pagnoni G, Hétu S, Montague R, 2019. Short-term mindfulness practice attenuates reward prediction errors signals in the brain. Sci. Rep 9, 6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE, 2007. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem 14, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krai TR, Davis K, Korponay C, Hirshberg MJ, Hoel R, Tello LY, Goldman RI, Rosenkranz MA, Lutz A, Davidson RJ, 2022. Absence of structural brain changes from mindfulness-based stress reduction: two combined randomized controlled trials. Sci. Adv 8, eabk3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S, 2015. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet 62222–62224. [DOI] [PubMed] [Google Scholar]
- Kwak S, Lee TY, Jung WH, Hur JW, Bae D, Hwang WJ, Cho KIK, Lim KO, Kim SY, Park HY, 2019. The immediate and sustained positive effects of meditation on resilience are mediated by changes in the resting brain. Front. Hum. Neurosci 13, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J, Sadikaj G, Nishioka M, Carrière K, Flanders J, Knäuper B, 2018. Daily mindful responding mediates the effect of meditation practice on stress and mood: the role of practice duration and adherence. J. Clin. Psychol 74, 109–122. [DOI] [PubMed] [Google Scholar]
- Lange I, Goossens L, Michielse S, Bakker J, Lissek S, Papalini S, Verhagen S, Leibold N, Marcelis M, Wichers M, 2017. Behavioral pattern separation and its link to the neural mechanisms of fear generalization. Soc. Cogn. Affect. Neurosci 12, 1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer E, Djikic M, Pirson M, Madenci A, Donohue R, 2010. Believing is seeing: using mindlessness (mindfully) to improve visual acuity. Psychol. Sci 21, 661–666. [DOI] [PubMed] [Google Scholar]
- Laukkonen RE, Slagter HA, 2021. From many to (n) one: meditation and the plasticity of the predictive mind. Neurosci. Biobehav. Rev 128, 199–217. [DOI] [PubMed] [Google Scholar]
- Lecei A, van Winkel R, 2020. Hippocampal pattern separation of emotional information determining risk or resilience in individuals exposed to childhood trauma: linking exposure to neurodevelopmental alterations and threat anticipation. Neurosci. Biobehav. Rev 108, 160–170. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ, 2004. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature 430, 456–459. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, 2016. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 92, 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RH, 1980. Introduction to bivariate and multivariate analysis. Vol 4. Scott, Foresman, Glenview, IL. [Google Scholar]
- Lindstrom MJ, Bates DM, 1988. Newton—raphson and EM algorithms for linear mixedeffects models for repeated-measures data. J. Am. Stat. Assoc 83, 1014–1022. [Google Scholar]
- Lorenc T, Lester S, Sutcliffe K, Stansfield C, Thomas J, 2020. Interventions to support people exposed to adverse childhood experiences: systematic review of systematic reviews. BMC Public Health 20, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow R., 1973. Latent inhibition. Psychol. Bull 79, 398–407. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Gewirtz JC, 1995. Latent inhibition in humans: data, theory, and implications for schizophrenia. Psychol. Bull 117, 87. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, 2019. The neuroanatomy of long-term meditators. Curr. Opin. Psychol 28, 172–178. [DOI] [PubMed] [Google Scholar]
- Luders E, Kurth F, Toga AW, Narr KL, Gaser C, 2013. Meditation effects within the hippocampal complex revealed by voxel-based morphometry and cytoarchitectonic probabilistic mapping. Front. Psychol 4, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C, 2009. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage 45, 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madar AD, Ewell LA, Jones MV, 2019. Pattern separation of spiketrains in hippocampal neurons. Sci. Rep 9, 5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson-Clavertz D, Gušić S, Bengtsson H, Jacobsen H, Cardeña E, 2017. The relation of dissociation and mind wandering to unresolved/disorganized attachment: an experience sampling study. Attach. Hum. Dev 19, 170–190. [DOI] [PubMed] [Google Scholar]
- Martins R, Joanette Y, Monchi O, 2015. The implications of age-related neurofunctional compensatory mechanisms in executive function and language processing including the new temporal hypothesis for compensation. Front. Hum. Neurosci 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1999. Stress and hippocampal plasticity. Annu. Rev. Neurosci 22, 105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2000. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 886, 172–189. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2002. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging 23, 921–939. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM, 1995. Stress and cognitive function. Curr. Opin. Neurobiol 5, 205–216. [DOI] [PubMed] [Google Scholar]
- McNaughton N., 2006. The role of the subiculum within the behavioural inhibition system. Behav. Brain Res 174, 232–250. [DOI] [PubMed] [Google Scholar]
- Miller LE, 2015. Perceived threat in childhood: a review of research and implications for children living in violent households. Trauma Violence Abuse 16, 153–168. [DOI] [PubMed] [Google Scholar]
- Mingote S, Amsellem A, Kempf A, Rayport S, Chuhma N, 2019. Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochem. Int 129, 104482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E, 2004. Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci 7, 841–846. [DOI] [PubMed] [Google Scholar]
- Mittner M, Hawkins GE, Boekel W, Forstmann BU, 2016. A neural model of mind wandering. Trends Cogn. Sci 20, 570–578. [DOI] [PubMed] [Google Scholar]
- Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS, 2014. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus 24, 239–248. [DOI] [PubMed] [Google Scholar]
- Moore AW, Gruber T, Derose J, Malinowski P, 2012. Regular, brief mindfulness meditation practice improves electrophysiological markers of attentional control. Front. Hum. Neurosci 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A, 2012. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169, 141–151. [DOI] [PubMed] [Google Scholar]
- Nash MI, Hodges CB, Muncy NM, Kirwan CB, 2021. Pattern separation beyond the hippocampus: a high-resolution whole-brain investigation of mnemonic discrimination in healthy adults. Hippocampus 31, 408–421. [DOI] [PubMed] [Google Scholar]
- Nassar MR, Wilson RC, Heasly B, Gold JI, 2010. An approximately Bayesian delta-rule model explains the dynamics of belief updating in a changing environment. J. Neurosci 30, 12366–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH, 2006. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. J. Neuropsychiatry Clin. Neurosci 18, 45–53. [DOI] [PubMed] [Google Scholar]
- Nayda DM, Takarangi MK, 2021. The cost of being absent: is meta-awareness of mind-wandering related to depression symptom severity, rumination tendencies and trauma intrusions? J. Affect. Disord 292, 131–138. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M, 2020. Adversity in childhood is linked to mental and physical health throughout life. BMJ 371, m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP Jr, Weiss PM, Dunner DL, 2003. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl. Acad. Sci 100, 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB, 2000. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain Res. 858, 181–190. [DOI] [PubMed] [Google Scholar]
- O’Mara SM, Commins S, Anderson M, Gigg J, 2001. The subiculum: a review of form, physiology and function. Prog. Neurobiol 64, 129–155. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA, 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickut BA, Van Hecke W, Kerckhofs E, Mariën P, Vanneste S, Cras P, Parizel PM, 2013. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin. Neurol. Neurosurg 115, 2419–2425. [DOI] [PubMed] [Google Scholar]
- Poerio GL, Sormaz M, Wang HT, Margulies D, Jefferies E, Smallwood J, 2017. The role of the default mode network in component processes underlying the wandering mind. Soc. Cogn. Affect. Neurosci 12, 1047–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero E, Díaz E, Vargas JP, de la Casa G, López JC, 2011. Ventral subiculum involvement in latent inhibition context specificity. Physiol. Behav 102, 414–420. [DOI] [PubMed] [Google Scholar]
- Rahrig H, Vago DR, Passarelli MA, Auten A, Lynn NA, Brown KW, 2022. Meta-analytic evidence that mindfulness training alters resting state default mode network connectivity. Sci. Rep 12, 12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B, 2012. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahdra BK, MacLean KA, Ferrer E, Shaver PR, Rosenberg EL, Jacobs TL, Zanesco AP, King BG, Aichele SR, Bridwell DA, 2011. Enhanced response inhibition during intensive meditation training predicts improvements in self-reported adaptive socioemotional functioning. Emotion 11, 299. [DOI] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Scholz J, Jenkinson M, Thomas AG, Filippini N, Smit G, Douaud G, Johansen-Berg H, 2014. Gray matter volume is associated with rate of subsequent skill learning after a long term training intervention. Neuroimage 96, 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., 2013. Stress and cognition. Wiley Interdiscip. Rev. Cogn. Sci 4, 245–261. [DOI] [PubMed] [Google Scholar]
- Santorelli SF, Kabat-Zinn J, Blacker M, Meleo-Meyer F, Koerbel L, 2017. Mindfulness-based stress reduction (MBSR) authorized curriculum guide. Cent. Mindfulness Med. Heal. Care, Soc. (CFM). Univ. Massachusetts Med. Sch [Google Scholar]
- Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W, 1986. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J. Steroid. Biochem 25, 717–721. [DOI] [PubMed] [Google Scholar]
- Schall JD, Palmeri TJ, Logan GD, 2017. Models of inhibitory control. Philos. Trans. R. Soc. Lond. B Biol. Sci 372, 20160193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevinc G, Greenberg J, Hölzel BK, Gard T, Calahan T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, 2020. Hippocampal circuits underlie improvements in self-reported anxiety following mindfulness training. Brain Behav. 10, e01766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevinc G, Hölzel BK, Greenberg J, Gard T, Brunsch V, Hashmi JA, Vangel M, Orr SP, Milad MR, Lazar SW, 2019. Strengthened hippocampal circuits underlie enhanced retrieval of extinguished fear memories following mindfulness training. Biol. Psychiatry 86, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Kirwan CB, Stark CE, 2019. Mnemonic similarity task: a tool for assessing hippocampal integrity. Trends Cogn. Sci 23, 938–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CE, 2013. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 51, 2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, MeClarty B, 1997. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med 27, 951–959. [DOI] [PubMed] [Google Scholar]
- Takarangi MK, Strange D, Lindsay DS, 2014. Self-report may underestimate trauma intrusions. Conscious. Cogn 27, 297–305. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA, 2000. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol 68, 615–623. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Km DM, 2003. The neurobiologieal consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Khan A, McGreenery CE, Bolger EA, Rohan ML, Vitaliano GD, 2018. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 169, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci 109, E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Parigger A, 2015. The ‘Maltreatment and Abuse Chronology of Exposure’(MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS One 10, eOl17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Vallejo Z, Amaro H, 2009. Adaptation of mindfulness-based stress reduction program for addiction relapse prevention. The Humanistic Psychologist 37, 192–206. [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B, 2008. Brain morphometry with multiecho MPRAGE. Neuroimage 40, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos de Wael R, Larivière S, Caldairou B, Hong SJ, Margulies DS, Jefferies E, Bernasconi A, Smallwood J, Bernasconi N, Bernhardt BC, 2018. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc. Natl. Acad. Sci 115, 10154–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, Feldon J, 1997. The switching model of latent inhibition: an update of neural substrates. Behav. Brain Res 88, 11–25. [DOI] [PubMed] [Google Scholar]
- Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, Spaeth R, Wall RB, Walsh J, Kaptchuk TJ, 2013. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neurosci. Lett 556, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield T, Barnhofer T, Acabchuk R, Cohen A, Lee M, Schlosser M, Arenaza-Urquijo EM, Böttcher A, Britton W, Coll-Padros N, 2022. The effect of mindfulness-based programs on cognitive function in adults: a systematic review and meta-analysis. Neuropsychol. Rev 32, 677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Ponesse J, Schachar R, Logan G, Tannock R, 1999. Development of inhibitory control across the life span. Dev. Psychol 35, 205–213. [DOI] [PubMed] [Google Scholar]
- Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Hackmann A, Krusche A, Muse K, Von Rohr IR, Shah D, Crane RS, Eames C, Jones M, Radford S, Silverton S, Sun Y, Weatherley-Jones E, Whitaker CJ, Russell D, Russell IT, 2014. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J. Consult. Clin. Psychol 82, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E, 2009. Negativity bias’ in risk for depression and anxiety: brain–body fear circuitry correlates, 5-HTT-LPR and early life stress. Neuroimage 47, 804–814. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW, 2008. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus 18, 729–736. [DOI] [PubMed] [Google Scholar]
- Yang CC, Barrós-Loscertales A, Li M, Pinazo D, Borchardt V, Ávila C, Walter M, 2019. Alterations in brain structure and amplitude of low-frequency after 8 weeks of mindfulness meditation training in meditation-naïve subjects. Sci. Rep 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE, 2011. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL, 2010. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 51, 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zach P, Mrzílková J, Řezáčová L, Stuchlík A, Valeš K, 2010. Delayed effects of elevated corticosterone level on volume of hippocampal formation in laboratory rat. Physiol. Res 59, 985–996. [DOI] [PubMed] [Google Scholar]
- Zaefarian G, Iurkov V, Koval M, 2022. Variance decomposition analysis: what is it and how to perform it – A complete guide for B2B researchers. Ind. Mark. Manag 107, 315–322. [Google Scholar]