Abstract
Background and Objectives
Anti-IgLON5 disease is an autoimmune neurodegenerative disorder characterized by various phenotypes, notably sleep and movement disorders and tau pathology. Although the disease is known to be associated with the neuronal cell adhesion protein IgLON5, the physiologic function of IgLON5 remains elusive. There are conflicting views on whether autoantibodies cause loss of function, activation of IgLON5, or inflammation-associated neuronal damage, ultimately leading to the disease. We generated IgLON5 knockout (−/−) mice to investigate the functions of IgLON5 and elucidate the pathomechanism of anti-IgLON5 disease.
Methods
IgLON5 knockout (−/−) mice underwent behavioral tests investigating motor function, psychiatric function (notably anxiety and depression), social and exploratory behaviors, spatial learning and memory, and sensory perception. Histologic analysis was conducted to investigate tau aggregation in mice with tauopathy.
Results
IgLON5−/− mice had poorer performance in the wire hang and rotarod tests (which are tests for motor function) than wild-type mice. Moreover, IgLON5−/− mice exhibited decreased anxiety-like behavior and/or hyperactivity in behavior tests, including light/dark transition test and open field test. IgLON5−/− mice also exhibited poorer remote memory in the contextual fear conditioning test. However, neither sleeping disabilities assessed by EEG nor tau aggregation was detected in the knockout mice.
Discussion
These results suggest that IgLON5 is associated with activity, anxiety, motor ability, and contextual fear memory. Comparing the various phenotypes of anti-IgLON5 disease, anti-IgLON5 disease might partially be associated with loss of function of IgLON5; however, other phenotypes, such as sleep disorders and tau aggregation, can be caused by gain of function of IgLON5 and/or neuronal damage due to inflammation. Further studies are needed to elucidate the role of IgLON5 in the pathogenesis of anti-IgLON5 diseases.
Introduction
Anti-IgLON5 disease (an autoimmune neurodegenerative disorder initially discovered in 2014) is characterized by sleep disorders, brainstem dysfunction, and gait disturbance and is associated with autoantibodies against the neuronal cell adhesion protein IgLON5.1
Polysomnographic analyses revealed that the disorder encompasses multifaceted nonrapid eye movement (NREM) sleep parasomnia during sleep initiation, REM sleep behavioral disorder, and obstructive sleep apnea with stridor.2 In addition, other clinical manifestations of the disease include bulbar symptoms, gait abnormalities, oculomotor deficits, cognitive decline, involuntary movement, and psychiatric disorders.3 Reports have shown that the condition can mimic motor neuron disease, evident in progressive dysphagia, limb weakness with muscle atrophy, and extensive denervation in the limb and thoracic paraspinal muscles.4 Notably, neuropathologic findings revealed phosphorylated tau deposits in the hypothalamus and brainstem tegmentum,1,5 indicating that the disease is a novel tauopathy with a correlation between autoimmunity and neurodegeneration. The pathophysiologic mechanisms remain unknown, but a strong association between the disease and the HLA-DRB1*10:01-DQB1*05:01 haplotype supports immune-mediated pathogenesis.6
The exact function of IgLON5 remains unclear. IgLON5 is a member of the IgLON family, a group of cell adhesion molecules that include limbic system-associated membrane proteins (LSAMP), neurotrimin (NTM), opioid-binding proteins, and neuronal growth regulator 1 (NEGR1). Genetic deletion of LSAMP targeted deletion of exon 2 causes novelty induced hyperactivity.7 Genetic deletion of LSAMP also results in reduced hippocampal mineralocorticoid receptor expression and impaired synaptic plasticity.8 Another study reported that LSAMP knockout (KO) mice created by deleting exon 1b exhibited decreased anxiety and alterations in social behavior.9 These studies indicate that LSAMP is involved in anxiety, hyperactivity, and synaptogenesis. However, deletion of the Negr1 gene in mice results in abnormalities in axonal growth in the hippocampus, impairment in social behavior in the three-chamber sociability test, and reversal learning deficits in the Morris water maze.10 NTM-deficient mice demonstrate a deficit in emotional learning in the active avoidance task.11 The NTM KO mouse hippocampal neurons exhibit premature sprouting of neurites with accelerated neurite elongation and branching.12 However, there is a lack of research on the physiologic function of IgLON5.
It is currently unclear whether anti-IgLON5 disease is caused by loss of function, IgLON5 activation, or inflammation-associated neuronal damage. Injection of anti-IgLON5 antibodies from patients into mice causes cognitive deficits and neuroinflammation, supporting the hypothesis that anti-IgLON5 antibodies are pathogenic.13 Moreover, anti-IgLON5 antibodies cause an irreversible reduction in the levels of neuronal cell surface, IgLON5, suggesting that the binding of anti-IgLON5 antibodies induces internalization of IgLON5 clusters.14,15 Introducing anti-IgLON5 antibodies to induced pluripotent stem cell–derived human neurons leads to reductions in IgLON5-positive clusters synaptic proteins, increased phosphorylated tau expression, and eventually cell death, indicating that the autoantibody causes neurodegenerative changes.15 The pathogenicity of anti-IgLON5 antibodies is well-recognized; however, research on the relationship between the internalization of IgLON5 by anti-IgLON5 antibodies and the clinical manifestations of anti-IgLON5 disease is inadequate.
In N-methyl-d-aspartate (NMDA) receptor encephalopathy (the most common form of autoimmune encephalitis), there is evidence of internalization and loss of NMDA receptors. PET studies have shown a mean 30% regional reduction in the density of NMDA receptors in patients.16 Moreover, the titer of the autoantibody directly correlates with the reduction of NMDA receptors, suggesting that the clinical manifestations of this disease are directly related to the intensity of antibody-mediated loss of NMDA receptors.17 Therefore, it is speculated that anti-IgLON5 shares a similar pathomechanism (that is loss of targeting protein function) with NMDA receptor encephalopathy.
To elucidate the function of IgLON5 in the context of anti-IgLON5 disease, we generated IgLON5−/− mice and conducted comparative analyses of their behavior, omics, and tau accumulation.
Methods
Generation of IgLON5−/− Mice
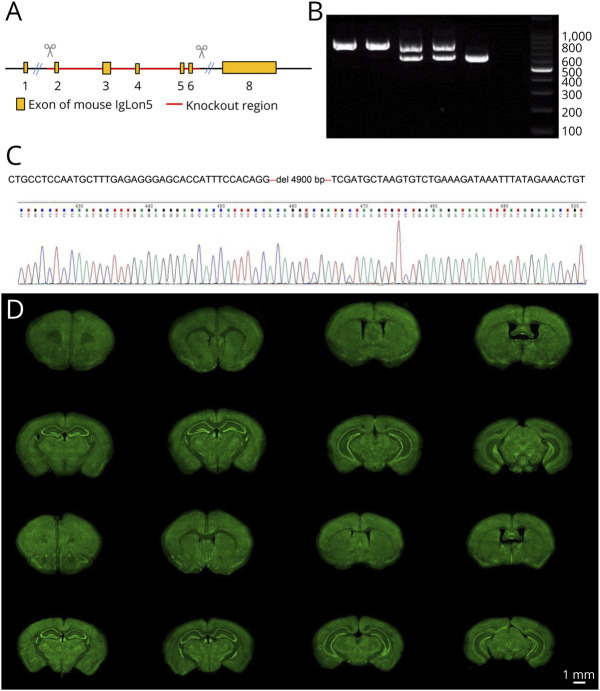
Mice were generated by Cyagen Biosciences18 using CRISPR/Cas9. KO was performed in exon 2–6 of the IgLON5 gene using the following guide RNAs (gRNA): gRNA1 (matching the reverse strand of the gene): ATTGGACTAATATTCCCCTGTGG and gRNA2 (matching the forward strand of the gene): CACCAAAGATCTGGGGTCGAAGG (Figure 1A). The genotypes of the mice were determined using PCR, followed by Sanger sequencing (Figure 1, B and C). Three generations of heterozygous mice were crossed with the C57BL/6N mice to establish a strain with a clear and identical background. The last generation was intercrossed to establish homozygous KO and wild-type (WT) mice with identical backgrounds. WT mice were used as controls.
Figure 1. Generation of IgLON5-Deficient Mice (IgLON5−/−).
(A) Targeted disruption of the IgLON5 gene by knockout exons 2–6 by CRISPR/Cas9. (B) A PCR-based genotyping assay amplifies 602 bp fragment from the WT allele and a 767 bp from the targeted allele. Lanes 1 and 2 show the genotyping results of the IgLON5−/− mice. NTC = no template control. (C) Sanger sequencing results. (D) NeuroTrace staining of brains shows no distinctive differences in the anatomic structure and neuron distribution between WT mice and IgLON5−/− mice. Scale bar, 1 mm. WT = wild-type.
Mutant human tau transgenic mice (B6-Tg(Thy1-MAPT*P301S)2541), also known as hTau.P301S (Tg2541) mice,19 were purchased from Jackson Laboratories and were backcrossed over 5 generations to a C57BL/6J background to obtain homozygous hTau.P301S+/+ animals. Homozygous hTau.P301S+/+ and WT or IgLON5−/− mice were paired to obtain WT: hTau.P301S+/+ and IgLON5−/−: hTau.P301S+/+ mice. A total of 100 mice were used in this study. Mice were housed on ventilated racks in a specific pathogen-free barrier facility under a 12-hour light/dark cycle. Mice were group-housed with their littermates to a maximum of 4 mice per cage and were given free access to food and water. All animal experiments were approved by the Ethics Committee of Keio University and conducted according to the Animal Experimentation Guidelines of Keio University School of Medicine and the guidelines of the International Committee of Medical Journal Editors.
Behavioral Tests
Male IgLON5−/− and WT 9-week-old mice were subjected to a battery of behavioral tests in the following sequence: general health and neurologic screening, light/dark transition test, open field test, elevated plus maze test, hot plate test, social interaction test, rotarod test, three-chamber social approach test, acoustic startle response/prepulse inhibition test, Porsolt forced swim test, balance beam test, gait analysis, T-maze spontaneous alternation test, Barnes maze test, tail suspension test, fear conditioning test, and home cage social interaction test. After each test, the floors and walls of the test apparatus were cleaned with 70% ethanol solution and hypochlorous acid water to prevent bias based on olfactory cues. Behavioral tests were performed between 9:00 and 17:00 as previously described.20-22 Detailed methods are available in the Supplementary eMethods.
General Health and Neurologic Screening
Physical characteristics including body weight and rectal temperature were recorded. Neuromuscular strength was assessed using the grip strength and wire hang tests as previously described.21 Forelimb grip strength was measured using a grip strength meter (O'Hara & Co., Tokyo, Japan). The mice were lifted by their tails to grasp a wire grid with their forelimbs and then gently pulled backward until they released the grid. The peak force exerted by the forelimbs of each mouse was recorded in Newtons. In the wire hang test, the mice were placed on a wire mesh (O'Hara & Co., Tokyo, Japan), which was then gently inverted so that they could grasp the wire. The latency to fall from the wire was recorded with a 60-second cutoff time.
Methods describing the image analysis, EEG/electromyogram (EEG/EMG) studies, bulk mRNA-sequence transcriptome analysis, and proteomic analysis are available in the Supplementary eMethods.
Immunohistochemical Analysis
Frozen brain sections fixed with 4% paraformaldehyde were sliced 40 μm coronally. The slides were incubated with Mouse on Mouse (MOM) Blocking Reagent (MKB-2213-1, Vector Laboratorie) for 1 hour. For the first treatment, the slides were washed with 0.1% phosphate buffered saline and then a diluted solution of MOM. protein concentrate was prepared, and the slides were incubated with this solution for 5 minutes. The primary antibody targeting AT8 (Dako), Iba1 (Wako), and vesicular glutamate transporter 1 (VGLUT1) (Synaptic Systems) was diluted with this solution, and the slides were incubated overnight with the primary antibody at 4°C. After incubation with primary antibodies, slides were incubated for 1 hour at room temperature with secondary antibodies conjugated to Alexa Fluor 488 or 555 (Thermo Fisher Scientific) diluted in blocking solution. After washing, the slides were mounted with ProLong Diamond (Life Technologies). Neurotrace 435/455 (Invitrogen) was used to stain the Nissl substance characteristic of neurons. Black Gold II Myelin staining (Biosensis) was performed as described by the manufacturer. Images were captured using Axioscan Z1 (Zeiss) or Keyence BZ-X800. To assess the tau burden, the intensity of the immunohistochemical images was quantified using ImageJ software (NIH, Bethesda, MD).
Statistics Analyses
In the behavioral analysis, all t-tests performed were two-tailed. The Welch t-test and subsequent q-value calculation by Benjamini-Hochberg adjustment were performed using JMP, version 17 (SAS Institute Inc., Cary, NC) and Perseus (version 1.6.14.0).23 Statistical significance was set at p < 0.05.
Data Availability
The data sets generated and/or analyzed in this study are available from the corresponding author on reasonable request.
Results
Initial Characterization of IgLON5−/− Mice
The IgLON5 gene was successfully deleted after targeting exon 2–6 (Figure 1A), which was confirmed using a PCR-based genotyping assay (Figure 1B). Genotyping results show that homogenic IgLON5−/− mice exhibit a specific band of 767 bp, WT mice exhibit 1 band of 602 bp, and heterogenic mice have both, proving that homogenic IgLON5−/− mice was generated. Sequencing confirmed a large deletion in the IgLON5 gene (Figure 1C). NeuroTrace, a histologic test for visualizing neurons, showed no distinctive difference in light microscopy between IgLON5−/− and WT mice in terms of the anatomic structure and distribution of neurons (Figure 1D).
To assess the influence of IgLON5 on synaptic formation, frozen sections of brains of IgLON5−/− and WT mice were fluorescent stained with VGLUT1, a marker of synaptic vesicles, and the intensity of signals in the molecular layer of dentate gyrus and granular layer of the cerebellum was analyzed. No obvious differences were identified between the intensity of signals between WT mice and knockout mice (eFigure 1), indicating that IgLON5 does not have a major effect on VGLUT1-positive synaptic formation.
As all IgLON members were expressed in myelinating oligodendrocytes,24 we examined the effects on myelination by performing Black-Gold myelin staining and analyzed the intensity of the staining. No significant differences in the anatomic morphology and intensity of the corpus callosum were observed between WT and IgLON5−/− mice (eFigure 2), indicating that myelination did not show major changes in Black-Gold staining.
General health test and neurologic screening were also performed, and no significant differences between IgLON5−/− mice and WT mice were observed in terms of body weight (eFigure 3A) and rectal temperature (eFigure 3B), indicating that IgLON5−/− and WT mice broadly share similar health conditions.
Decreased Muscular Strength and Slight Motor Deficits in IgLON5−/− Mice
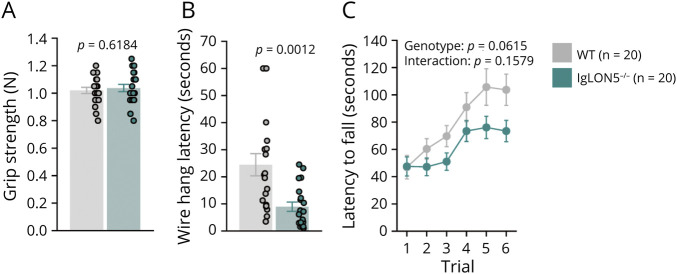
IgLON5−/− and WT mice showed similar grip strength (Figure 2A); however, IgLON5−/− mice could maintain hanging on a wire for a shorter time than WT mice (Figure 2B), indicating that IgLON5−/− mice have a lower muscular endurance. Despite being statistically insignificant, IgLON5−/− mice showed a trend toward shorter latencies to fall in the rotarod test (Figure 2C). By contrast, balance perception analysis in the beam test and gait analysis showed no significant differences between IgLON5−/− and WT mice (eFigures 4 and 5). Collectively, the motor testing results suggested that IgLON5−/− mice have normal balance and gait but may have decreased muscular strength and slight motor deficits.
Figure 2. Muscular and Motor Functions of IgLON5−/− Mice.
Grip strength test (A), wire hang test (B), and rotarod test (C). Notably, the latency of the wire hang test to fall for IgLON5−/− mice (n = 20) was significantly shorter than that of WT mice (n = 20). WT = wild-type.
Decreased Anxiety-Like Behavior and Increased Locomotor Activity in IgLON5−/− Mice
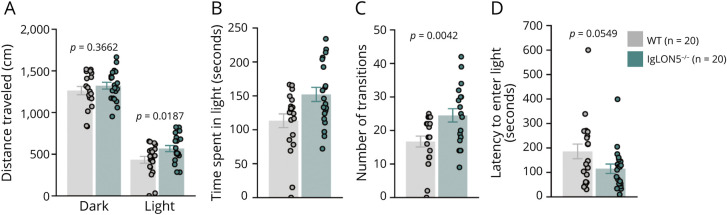
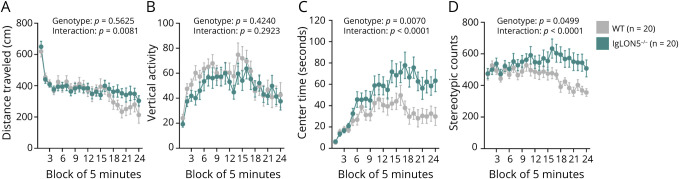
Although IgLON5−/− and WT mice performed similarly in the elevated plus maze test (eFigure 6) in terms of distance traveled and time spent in open arms, the effect of illumination on exploratory behavior in the light/dark transition test (Figure 3) showed that IgLON5−/− mice spent significantly more time in the light than WT mice (Figure 3C). Similar observations were made in the open field test (Figure 4), showing IgLON5−/− mice having reduced anxiety behaviors and/or hyperactivity. IgLON5−/− mice spent significantly more time in the center (Figure 4C) than WT mice, indicating lower anxiety levels. Generally, anxiety tests results suggested that IgLON5−/− mice showed reduced anxiety behaviors and/or hyperactivity compared with WT mice.
Figure 3. Behavioral Tests for Assessing Anxiety by Light/Dark Transition Test.
(A) IgLON5−/− mice traveled further in the dark than WT mice. Moreover, the IgLON5−/− mice spent more time in the light chamber (B) and had more transitions between the light and dark chambers (C) than WT mice. (D) IgLON5−/− mice spent less time entering the light than WT mice, although this difference was not significant. WT = wild-type.
Figure 4. Behavioral Tests for Assessing Anxiety by Open Field Test.
In the open field test, there were no differences between IgLON5−/− and WT mice vertical activity (B), although distance traveled was significantly higher in the IgLON5−/− mice in the second half of the test (A). However, IgLON5−/− mice spent more time in the center of the field (C) and had higher stereotypic counts than WT mice (D). WT = wild-type.
Depression-Related Behavior
Regarding depressive behaviors, the behaviors of IgLON5−/− mice were similar to those of WT mice in the Porsolt forced swim test and tail suspension test. These data are presented in eFigure 7.
Social Behavior Test in IgLON5−/− Mice
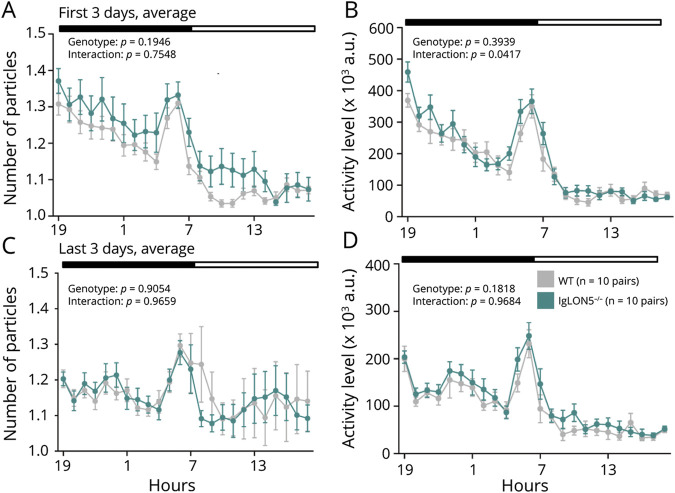
IgLON5−/− and WT mice behaved similarly in the social interaction and three-chamber tests (eFigure 8). In addition, both genotypes displayed similar exploratory behaviors in the T-maze spontaneous alteration test (eFigure 9). However, in the home cage social interaction test, over 1 week (Figure 5, eFigure 9), IgLON5−/− mice were more active for the first 3 days (Figure 5, A and B) but not the last 3 days (Figure 5, C and D), indicating that low anxiety-like behavior and/or hyperactivity are elicited in novel situations.
Figure 5. Social Behavior in IgLON5−/− Mice.
Sociability was measured using a home cage social interaction test. (A and B) Number of particles and activity levels of the 2 mice in the same cage for the first 3 days of analysis. (C and D) Number of particles and activity levels of the 2 mice in the same cage during the last 3 days of analysis.
Impaired Context-Dependent Memory in IgLON5−/− Mice
In the contextual and cued fear conditioning tests (eFigure 10), only the conditioning phase (eFigure 10, A and F) showed significantly decreased freezing time and increased distance traveled in IgLON5−/− mice compared with WT mice. This finding supports the hypothesis that the mouse exhibits hyperactivity elicited in novel situations, as described above. Moreover, compared with day 2 (eFigure 10, B and G), the difference in the freezing time and distance traveled was more significant on day 29 (eFigure 10, D and I) between IgLON5−/− and WT mice, indicating that IgLON5−/− mice have a deficit in remote contextual fear memory.
Spatial Learning and Memory
Both genotypes displayed spatial learning and memory capabilities without significant differences in the T-maze spontaneous alternation and Barnes maze tests. These data are presented in eFigures 11 and 12.
Sensory and Perception Capabilities
The reactivity to thermal and auditory stimuli was measured using the hot plate test and acoustic startle response/prepulse inhibition tests, respectively. Both genotypes displayed capabilities to receive sensory signals without significant differences (eFigure 13).
Evaluation of Sleep and Wake Cycles
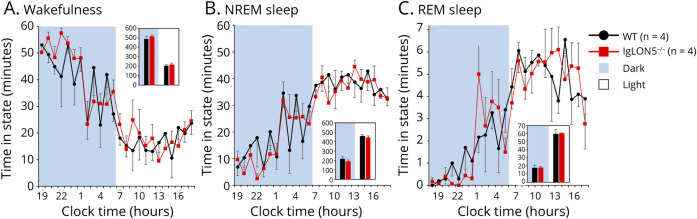
Electroencephalograms and hypnograms were used to check the sleep and wake cycles of IgLON5−/− and WT mice. The EEG showed that the KO genotypes had normal hourly amounts of wakefulness, NREM sleep, and REM sleep (Figure 6, eFigure 14). No distinctive differences were found between the 2 genotypes in the sleep and wake cycles, indicating that neither genotype showed a prominent difference in the sleep-wake cycles.
Figure 6. IgLON5−/− Mice Exhibit Nearly Normal Amounts of Wake and Sleep on a 12-Hour Dark and Light Cycle.
IgLON5−/− mice at 9-mo-old have normal hourly amounts of waking (A), NREM (B), and REM (C) sleep. Black indicates WT mice (n = 4) and red indicates IgLON5−/− mice (n = 4). NERM = nonrapid eye movement; WT = wild-type.
Omics Analysis in IgLON5−/− Mice
RNA-seq analysis was performed to investigate the gene expression in the brains of 11-month-old IgLON5−/− and WT mice, and volcano plots (eFigure 15) were constructed to identify the genes that are upregulated or downregulated. Among the genes of known functions, growth hormone was downregulated in the cerebral cortex (eFigure 15A) and hippocampus (eFigure 15B), whereas prolactin was downregulated in the hippocampus (eFigure 15B). However, no significant differences were found between IgLON5−/− and WT mice in the plasma concentrations of growth hormone and prolactin by ELISA (eFigure 16).
In the proteomic LC–MS analysis, 6,658 proteins were identified and quantified. Of these, 5,925 proteins were quantified with N ≥ 3. With the exception of the IgLON5 protein, no significantly different proteins were found using both the permutation-based FDR24 cutoff and the Benjamini-Hochberg p-value correction (adj. p-value <0.05) (eFigure 17).
Tau Pathology in IgLON5−/− Mice in a P301S Tau Transgenic Background
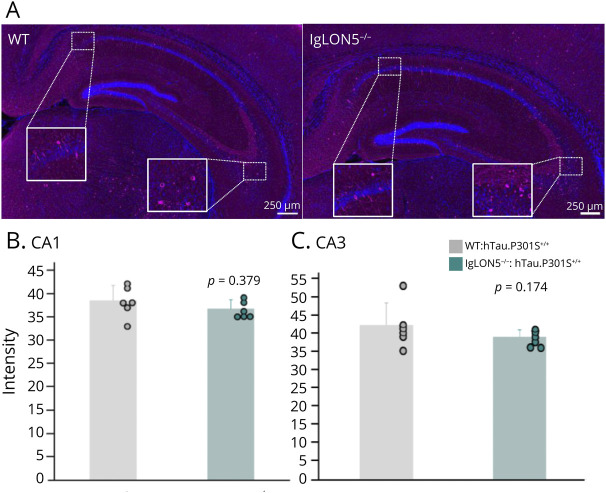
To assess the influence of IgLON5 on tau pathology, a characteristic neuropathologic feature of anti-IgLON5 disease, we examined the forebrain of IgLON5−/−mice in a P301S tau transgenic background.19 Immunofluorescent staining of frozen sections of brains of WT:hTau.P301S+/+ and IgLON5−/−:hTau.P301S+/+ mice with hyperphosphorylated tau protein (AT8) was performed (Figure 7, A–C), and the intensity of the signals was analyzed. No significant differences in CA1 and CA3 intensity were observed between mice backcrossed with IgLON5−/− mice and those backcrossed with WT mice (Figure 7, B and C), indicating that the loss of IgLON5 does not affect hyperphosphorylated tau deposits in this mouse model. Moreover, in the anterior horns of spinal cord, in which tau accumulation appears from an early stage, no significant differences were found between 5-month-old and 8-month-old WT:hTau.P301S+/+ and IgLON5−/−:hTau.P301S+/+ mice (eFigure 18), indicating that age-dependent effects on tau pathology are unlikely.
Figure 7. Immunohistochemical Results of Hippocampal Tau Deposits in IgLON5−/−: P hTau.P301S+/+ Mice.
(A) Representative staining of AT8 at WT and IgLON5−/− mice in the P301S tau transgenic background. Staining of AT8 at mice backcrossed with P301S-mutant human tau transgenic mice (hTau.P301S+/+) and IgLON5−/− mice. Magenta represents AT8 while blue represents DAPI. Scale bar: 250 μm. (B and C) Comparison of intensity of AT8 signal between 8-mo-old WT: hTau.P301S+/+ (n = 6) and IgLON5−/−: hTau.P301S+/+ mice (n = 6) in CA1 (B), and CA3(C). WT = wild-type.
Finally, to assess the influence of IgLON5 on the inflammatory process, a process in anti-IgLON5 disease pathology, we examined the forebrain of this mouse model. Immunofluorescent staining of frozen sections of brains of WT:hTau.P301S+/+ and IgLON5−/−:hTau.P301S+/+ mice was performed with Iba1 antibody, a marker of inflammation,25,26 and the intensity of the signals was analyzed (eFigure 19A). No significant differences in signal intensity were observed between mice backcrossed with IgLON5−/− mice and WT mice (eFigure 19B-C) in CA1 and CA3, indicating that loss of IgLON5 does not affect the inflammatory process in response to tau deposition in this model mouse.
Discussion
In this study, we established a mouse model to determine the functions of IgLON5 by deleting the IgLON5 gene to mimic the symptoms of anti-IgLON5 disease. Behavioral, biochemical, and histologic tests were performed, and IgLON5 functions were examined by comparing the performance of IgLON5−/− mice with that of WT mice. The tests assessing anxiety-like behavior (light/dark transition test, open field test, and home cage social interaction test) suggested that IgLON5−/− mice exhibited reduced anxiety-like behavior and/or hyperactivity compared with WT mice. Moreover, IgLON5 mice showed a larger difference in freezing time and distance traveled on day 29 of the contextual fear conditioning tests than on day 2, suggesting that IgLON5 is related to poorer remote contextual fear memory. In addition, motor testing results showed that IgLON5−/− mice had poor motor performance in the wire hang and rotarod tests but had normal balance and gait. While tests regarding bulbar symptoms were not conducted, no body weight loss was observed in IgLON5−/− mice, indicating that their bulbar function was probably not affected. EEG also showed no abnormalities during sleep. Collectively, although the deletion of mouse IgLON5 is associated with partial clinical features (psychiatric disorders, motor deficits, and remote memory), other symptoms may not result from the loss of function of IgLON5. We postulated that the main features (sleep disorders and bulbar dysfunction) of anti-IgLON5 disease result from the activation of IgLON5 and/or inflammation-associated neuronal damage to the brainstem and hypothalamus.27
The IgLON family of proteins is involved in neurite outgrowth and synaptic plasticity. Polymorphisms in IgLON genes are associated with major depressive disorder, panic disorder, and schizophrenia,27 indicating that the IgLON family is associated with psychiatric disorders by aberrant neuronal connectivity. In a rodent model, LSAMP KO mice displayed hyperactivity in a novel arena in the open field test, elevated plus maze, and y-maze test.7 By contrast, Innos et al. reported that different LSAMP KO mice lines showed a similar behavioral pattern in the elevated plus maze, although the authors concluded that the mice displayed reduced anxiety in detail experiment using illuminated/dim conditions and anxiolytic drug diazepam.9 Negr1 and Ntm-deficient mice showed no differences in anxiety and locomotor activity.10,11 IgLON5−/− mice did not show any differences in the elevated plus maze; however, low anxiety-like behavior and/or hyperactivity were observed in the open field test, light/dark transition test, and conditioning phase in the contextual fear conditioning tests. Notably, the home cage social interaction test also showed high activity in the first 3 days, indicating that low anxiety-like behavior and/or hyperactivity were elicited in novel situations. Therefore, IgLON5−/− mouse showed similar phenotypes to that of LSAMP KO mice. Considering the similar expression pattern of LSAMP and IgLON5,28 both proteins may have overlapping roles.
The amygdala contributes to contextual fear conditioning and anxiety.28-30 Inflammatory cell infiltration and tau accumulation at the same sites have also been reported in anti-IgLON5 disease,27,31 indicating that IgLON5 in the amygdala is a target for autoantibodies. Therefore, the phenotype of IgLON5−/− mice may reflect one of the physiologic functions of IgLON5 in the amygdala.
As phosphorylated tau accumulation was not evident in IgLON5−/− mice in the P301S tau transgenic background (Figure 7), IgLON5 loss of function alone was insufficient to induce tau pathology. Recent research has shown that tau accumulation is not present in all cases of anti-IgLON5 disease; therefore, it may be a secondary neuropathologic change due to anti-IgLON5 antibody toxicity and is a hallmark of the irreversible stage.27 In a study on Alzheimer disease, it was observed that the nucleotide-binding domain (NOD)–like receptor protein 3 inflammasome leads to tauopathies by microglia.32 The tauopathy after subacute sclerotic panencephalitis was considered to be the result of diffuse brain inflammation triggered by measles.33 Another possibility is that tau pathology reflects a nonspecific reaction to repeated neuronal excitation by autoantibodies. The frequent presence of tau pathology in surgically resected temporal lobes from patients with refractory epilepsy could support this hypothesis.34 Further neuropathologic studies are needed to understand tau deposition in anti-IgLON5 disease cases. Our findings suggest that tau pathology in anti-IgLON5 disease may be related to secondary phenomena, inflammation, and/or hyperexcitation in the hypothalamus and brainstem but not directly related to IgLON5 loss-of-function.
This study has several limitations. First, we did not use conditional KO mice. Other members of the IgLON family may compensate for IgLON5 function during development. In addition, previous studies suggested that LSAMP and NTM have combined effects on neuronal development and behavior. Further studies are needed to investigate the interactions between IgLON5 and other members of the IgLON family, and their role in the pathogenesis of anti-IgLON5 diseases. Time-dependent conditional KO is also useful to reveal the role of IgLON5 since adolescence. Second, anti-IgLON5 disease occurs in older patients. As we have only studied young adult KO mice, it is important to analyze whether there are any age-dependent effects in the loss of IgLON5. Third, the expression site of the IgLON5 protein is unknown because of the lack of reliable antibodies. Histologic studies are required to determine whether the expression sites in the brain are the same in humans and rodents. Fourth, bulk RNA-seq analysis could not identify specific differentially expressed genes in this study. In the future, single-cell RNA-seq will reveal cell type-specific molecular and genetic associations with disease phenotypes. Finally, we have not evaluated the effect of patient antibodies. It is important to examine differences in behavioral and neuropathologic changes13 between WT and KO mice after the administration of anti-IgLON5 antibodies from patients.
In summary, this IgLON5−/− study evaluates the functions of IgLON5 and its role in the pathology of anti-IgLON5 disease. Low anxiety-like behavior and hyperactivity in IgLON5−/− mouse line may provide a good model for studying the molecular mechanisms underlying several psychiatric disorders. We propose that IgLON5 is associated with anxiety-like behavior, partial motor impairment, and remote contextual fear memory and that IgLON5 loss of function is not related to other clinical manifestations observed in anti-IgLON5 disease, notably sleeping disorders, brain stem abnormalities, and tau accumulation. Although our data do not exclude the possibility that IgLON5 loss of function modulates the disease process and influences disease onset and severity in patients with anti-IgLON5 disease, critical symptoms could be induced by other factors, including the activation of IgLON5 or inflammation/hyperexcitation-associated neuronal damage.
Acknowledgment
The authors thank the Collaborative Research Resources, School of Medicine, Keio University, for technical assistance and Mr. Takehiro Maki, Mr.Yoshinobu Iwaki, Mr. Keita Sakai, in Sleep Science Laboratory, Tsukuba Research Center, HAMRI Co., Ltd. for EEG/EMG studies.
Glossary
- KO
knockout
- LOC
locomotor activity
- LSAMP
limbic system-associated membrane protein
- NEGR1
neuronal growth regulator 1
- NMDA
N-methyl-d-aspartate
- NTM
neurotrimin
- NREM
nonrapid eye movement
- WT
wild-type
Appendix. Authors
| Name | Location | Contribution |
| Sin Yi Lee, MS | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Hirotaka Shoji, PhD | Division of Systems Medical Science, Center for Medical Science, Fujita Health University, Toyoake, Japan | Major role in the acquisition of data |
| Aki Shimozawa, PhD | Eisai-Keio Innovation Laboratory for Dementia, Human Biology Integration, DHBL, Eisai Co., Ltd., Shinjuku-ku, Japan | Major role in the acquisition of data |
| Hirofumi Aoyagi, PhD | Eisai-Keio Innovation Laboratory for Dementia, Human Biology Integration, DHBL, Eisai Co., Ltd., Shinjuku-ku, Japan | Major role in the acquisition of data |
| Yoshiaki Sato, PhD | Eisai-Keio Innovation Laboratory for Dementia, Human Biology Integration, DHBL, Eisai Co., Ltd., Shinjuku-ku, Japan | Major role in the acquisition of data |
| Kazuya Tsumagari, PhD | Proteome Homeostasis Research Unit, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan | Major role in the acquisition of data |
| Mika Terumitsu, PhD | Eisai-Keio Innovation Laboratory for Dementia, Human Biology Integration, DHBL, Eisai Co., Ltd., Shinjuku-ku, Japan | Major role in the acquisition of data |
| Haruhiko Motegi, MD | Department of Neurology, Keio University School of Medicine; Department of Neurology, The Jikei University School of Medicine, Tokyo, Japan | Major role in the acquisition of data |
| Kensuke Okada, MD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Major role in the acquisition of data |
| Koji Sekiguchi, MD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Major role in the acquisition of data |
| Junro Kuromitsu, PhD | Eisai-Keio Innovation Laboratory for Dementia, Human Biology Integration, DHBL, Eisai Co., Ltd., Shinjuku-ku, Japan | Study concept or design; analysis or interpretation of data |
| Jin Nakahara, MD, PhD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Study concept or design |
| Tsuyoshi Miyakawa, PhD | Division of Systems Medical Science, Center for Medical Science, Fujita Health University, Toyoake, Japan | Study concept or design; analysis or interpretation of data |
| Daisuke Ito, MD, PhD | Department of Physiology/Memory center, Keio University School of Medicine, Tokyo, Japan | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grant 21H02812, JSPS KAKENHI grant JP 22H04922 (AdAMS), MEXT Promotion of Distinctive Joint Research Center Program grant FY2021-2023 JPMXP0621467949 and Japan Agency for Medical Research and Development under grant JP17pc0101006.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575-586. doi: 10.1016/S1474-4422(14)70051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaig C, Iranzo A, Santamaria J, Graus F. The sleep disorder in anti-lgLON5 disease. Curr Neurol Neurosci Rep. 2018;18(7):41. doi: 10.1007/s11910-018-0848-0 [DOI] [PubMed] [Google Scholar]
- 3.Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736-1743. doi: 10.1212/wnl.0000000000003887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao QQ, Wei Q, Song SJ, Yin XZ. Motor neuron disease-like phenotype associated with anti-IgLON5 disease. CNS Neurosci Ther. 2018;24(12):1305-1308. doi: 10.1111/cns.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelpi E, Hoftberger R, Graus F, et al. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol. 2016;132(4):531-543. doi: 10.1007/s00401-016-1591-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaig C, Ercilla G, Daura X, et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e605. doi: 10.1212/nxi.0000000000000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catania EH, Pimenta A, Levitt P. Genetic deletion of Lsamp causes exaggerated behavioral activation in novel environments. Behav Brain Res. 2008;188(2):380-390. doi: 10.1016/j.bbr.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu S, Champagne DL, Peters M, et al. Loss of limbic system-associated membrane protein leads to reduced hippocampal mineralocorticoid receptor expression, impaired synaptic plasticity, and spatial memory deficit. Biol Psychiatry. 2010;68(2):197-204. doi: 10.1016/j.biopsych.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innos J, Philips MA, Leidmaa E, et al. Lower anxiety and a decrease in agonistic behaviour in Lsamp-deficient mice. Behav Brain Res. 2011;217(1):21-31. doi: 10.1016/j.bbr.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 10.Singh K, Loreth D, Pottker B, et al. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease-associated Negr1 gene. Front Mol Neurosci. 2018;11:30. doi: 10.3389/fnmol.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazitov T, Bregin A, Philips MA, Innos J, Vasar E. Deficit in emotional learning in neurotrimin knockout mice. Behav Brain Res. 2017;317:311-318. doi: 10.1016/j.bbr.2016.09.064 [DOI] [PubMed] [Google Scholar]
- 12.Singh K, Lillevali K, Gilbert SF, et al. The combined impact of IgLON family proteins Lsamp and Neurotrimin on developing neurons and behavioral profiles in mouse. Brain Res Bull. 2018;140:5-18. doi: 10.1016/j.brainresbull.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Ni Y, Feng Y, Shen D, et al. Anti-IgLON5 antibodies cause progressive behavioral and neuropathological changes in mice. J Neuroinflammation. 2022;19(1):140. doi: 10.1186/s12974-022-02520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabater L, Planaguma J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflammation. 2016;13(1):226. doi: 10.1186/s12974-016-0689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryding M, Gamre M, Nissen MS, et al. Neurodegeneration induced by anti-IgLON5 antibodies studied in induced pluripotent stem cell-derived human neurons. Cells. 2021;10(4):837. doi: 10.3390/cells10040837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galovic M, Al-Diwani A, Vivekananda U, et al. In vivo N-methyl-d-aspartate receptor (NMDAR) density as assessed using positron emission tomography during recovery from NMDAR-antibody encephalitis. JAMA Neurol. 2023;80(2):211-213. doi: 10.1001/jamaneurol.2022.4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74. doi: 10.1016/S1474-4422(10)70253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cyagen. Accessed April 13, 2024. cyagen.com
- 19.Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22(21):9340-9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:104. doi: 10.3791/104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoji H, Ikeda K, Miyakawa T. Behavioral phenotype, intestinal microbiome, and brain neuronal activity of male serotonin transporter knockout mice. Mol Brain. 2023;16(1):32. doi: 10.1186/s13041-023-01020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoji H, Takao K, Hattori S, Miyakawa T. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014;85:50871. doi: 10.3791/50871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyanova S, Temu T, Sinitcyn P, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016;13(9):731-740. doi: 10.1038/nmeth.3901 [DOI] [PubMed] [Google Scholar]
- 24.Kubick N, Brösamle D, Mickael ME. Molecular evolution and functional divergence of the IgLON family. Evol Bioinform Online. 2018;14:1176934318775081. doi: 10.1177/1176934318775081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57(1):1-9. doi: 10.1016/s0169-328x(98)00040-0 [DOI] [PubMed] [Google Scholar]
- 26.Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32(5):1208-1215. doi: 10.1161/01.str.32.5.1208 [DOI] [PubMed] [Google Scholar]
- 27.Erro ME, Sabater L, Martinez L, et al. Anti-IGLON5 disease: a new case without neuropathologic evidence of brainstem tauopathy. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e651. doi: 10.1212/NXI.0000000000000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanaveski T, Singh K, Narvik J, et al. Promoter-specific expression and genomic structure of IgLON family genes in mouse. Front Neurosci. 2017;11:38. doi: 10.3389/fnins.2017.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274-285. doi: 10.1037//0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- 30.Tye KM, Prakash R, Kim SY, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358-362. doi: 10.1038/nature09820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cagnin A, Mariotto S, Fiorini M, et al. Microglial and neuronal TDP-43 pathology in anti-IgLON5-related tauopathy. J Alzheimers Dis. 2017;59(1):13-20. doi: 10.3233/jad-170189 [DOI] [PubMed] [Google Scholar]
- 32.Ising C, Venegas C, Zhang S, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575(7784):669-673. doi: 10.1038/s41586-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bancher C, Leitner H, Jellinger K, et al. On the relationship between measles virus and Alzheimer neurofibrillary tangles in subacute sclerosing panencephalitis. Neurobiol Aging. 1996;17(4):527-533. doi: 10.1016/0197-4580(96)00069-3 [DOI] [PubMed] [Google Scholar]
- 34.Tai XY, Koepp M, Duncan JS, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain. 2016;139(Pt 9):2441-2455. doi: 10.1093/brain/aww187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed in this study are available from the corresponding author on reasonable request.