Abstract
Tumor-associated macrophages (TAMs) are essential components of the tumor microenvironment (TME) and display phenotypic heterogeneity and plasticity associated with the stimulation of bioactive molecules within the TME. TAMs predominantly exhibit tumor-promoting phenotypes involved in tumor progression, such as tumor angiogenesis, metastasis, immunosuppression and resistance to therapies. In addition, TAMs have the potential to regulate the cytotoxic elimination and phagocytosis of cancer cells and interact with other immune cells to engage in the innate and adaptive immune systems. In this context, targeting TAMs has been a popular area of research in cancer therapy, and a comprehensive understanding of the complex role of TAMs in tumor progression and exploration of macrophage-based therapeutic approaches are essential for future therapeutics against cancers. The present review provided a comprehensive and updated overview of the function of TAMs in tumor progression, summarized recent advances in TAM-targeting therapeutic strategies and discussed the obstacles and perspectives of TAM-targeting therapies for cancers.
Key words: tumor-associated macrophage, tumor microenvironment, tumor progression, targeted therapy
1. Introduction
Accumulating evidence has shown that cancer initiation and progression are determined by genetic mutations, epigenetic modifications and the tumor microenvironment (TME) (1,2). In addition to tumor cells and multiple stromal cells, immune cells, fibroblasts, endothelial cells, mesenchymal stem cells, extracellular matrix and tumor vasculature, various signaling molecules coexist and interact within the TME (3). As a complex and dynamic milieu, the TME is orchestrated by multiple cellular and inflammatory components, and each component within the TME represents a potential therapeutic target that may alter the pattern of cancer treatment.
Although various types of immune cells infiltrate the tumor milieu, macrophages are a prominent group of inflammatory cells, also known as tumor-associated macrophages (TAMs). It is widely accepted that TAMs exert a broad spectrum of biological functions in tumors, depending on their environmental cues (4,5). The contrasting polarization states, classically activated M1 macrophages and alternatively activated M2 macrophages, are two distinct subtypes that are functionally helpful in the context of pro- and anti-cancer characteristics. TAMs serve as double-edge swords, with a dual role in cancer depending on the context. Furthermore, TAMs have tumoricidal potential by regulating the mediator-dependent cytotoxic elimination and phagocytosis of cancer cells. In addition, they interact with other immune cells to engage in the innate and adaptive immune systems (6,7). In comparison, substantial research findings indicate the close association between the high infiltration of TAMs and cancer progression, including tumor angiogenesis, metastasis and immunosuppression, as well as resistance to therapies (4,6-8). Therefore, combined therapies using TAM-targeting strategies with conventional therapeutic treatments for cancer are promising. Therapeutic approaches targeting TAMs range from limiting the recruitment and differentiation of macrophages to reprogramming and promoting the phagocytic activity of macrophages (6,7,9-11).
This review focuses on the latest advances in exploiting TAMs as therapeutic targets for cancer treatment, including the potential role of TAMs in tumor progression, the mechanisms involved and therapeutic strategies targeting TAMs. Furthermore, the challenges and perspectives for TAM-targeted therapeutics for various cancers were discussed.
2. Origin and heterogeneity of TAMs
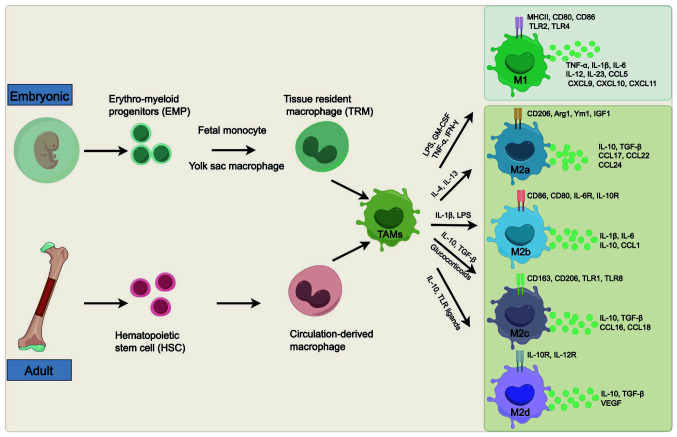
It has long been established that TAMs originate from tissue resident macrophages (TRMs) derived from embryonic precursor (yolk sac or fetal liver) and circulation-derived macrophages differentiated from monocytes that are released from hematopoietic stem cells (HSCs) in the bone marrow (Fig. 1) (12-14). TRMs are present during fetal development and persist in most tissues prenatally (15,16). They can self-renew locally throughout one's lifespan and operate independently of adult hematopoiesis (15-17). Based on available evidence, TRMs are endowed with tissue-specific functions associated with regulating tissue repair, maintaining tissue homeostasis and mediating inflammation (16,18). By contrast, short-lived circulation-derived macrophages require constant replenishment by HSCs-derived circulating monocytes differentiated in response to different signaling molecules. The self-renewal of macrophages correlates with a complex transcriptional network in a tissue-specific manner. Extracellular signal-regulated kinase 1/2 (ERK1/2) is required for the proliferation of macrophages in response to colony-stimulating factor 1 (CSF1), and the activation of ERK1/2 enhances the expression of cyclin-D and c-Myc (19). In addition, CSF1 receptor (CSF1R) promotes the proliferation of macrophages via inducing the MAPK kinase (MEK)5/ERK5 axis, the activation of which supports TAMs proliferation by inhibiting p21 expression (20).
Figure 1.
Origin and heterogeneity of TAMs. Macrophages in tumors are typically produced from bone marrow-derived monocytes or EMP in the yolk sac or fetal liver. Bone marrow-derived monocytes develop from HSC and then differentiate into circulation-derived macrophages, while TRMs originate from EMP in the embryonic yolk sac or fetal liver. Different stimulating factors polarize TAMs towards different subtypes: The M1 and M2 phenotypes. M2 subpopulations occur as a result of specific stimuli. TAMs, tumor-associated macrophages; EMP, erythro-myeloid progenitors; HSC, hematopoietic stem cell; TRMs, tissue-resident macrophages; LPS, lipopolysaccharide; GM-CSF, granulocyte-macrophage colony-stimulating factor; Arg, arginase; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; TGF, transforming growth factor; CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor; MHC, major histocompatibility complex class; IGF, insulin-like growth factor.
Macrophages are recruited and educated by signaling molecules produced by various components of the TME, including CSF1, transforming growth factor-β (TGF-β), cytokines such as interleukin (IL)-4 and IL-10, and chemokines such as C-C motif chemokine ligand (CCL)2 and CCL3 (13,21). The TME also affects the programming of recruited TRMs and circulation-derived macrophages into tumor-specific phenotypes. Within tumor tissues, TRMs exhibit enhanced proliferation ability, and there is increased infiltration of monocytes. Therefore, the macrophages recruited to the tumor site have a crucial role in the regulation of tumor progression.
TAMs exhibit phenotypic heterogeneity and plasticity and can be roughly divided into two contrasting subtypes, each representing a distinct polarization status: The classically activated M1 subtype and the alternatively activated M2 subtype (Fig. 1) (13,22). These two subtypes of macrophages differ in their different inducing factors, gene expression profiles and functions.
Anti-tumor effective M1 macrophages are polarized in response to lipopolysaccharide, interferon-γ (IFN-γ), granulocyte-macrophage CSF and activated Toll-like receptor (TLR). Phenotypically, M1 macrophages typically express high levels of major histocompatibility complex class II (MHC II), CD86 and inducible nitric oxide synthase (iNOS), demonstrating pro-inflammatory and anti-tumor activity (23). In the TME, M1 macrophages secrete proinflammatory cytokines such as IL-1β, IL-6, IL-12, and tumor necrosis factor-α (TNF-α). They also produce reactive oxygen species (ROS) and reactive nitrogen species (RNS), which have the potential to induce DNA damage in cells, thereby exerting an innate immune response and facilitating the elimination of tumor cells (4,24). In addition to their cytotoxic effects, M1 macrophages can phagocytose tumor cells directly and simultaneously exhibit antigen presentation capability (25). Furthermore, research findings also suggest that M1 macrophages could recruit type 1 helper T (Th1) cells and enhance immune responses to kill tumor cells (26,27).
M2 macrophages, also known as anti-inflammatory and tumor-supporting macrophages, are mainly induced by a variety of proteins such as CSF1, TGF-β, IL-4, IL-10 and IL-13, through activation of signal transducer and activator of transcription 6 (STAT6), peroxisome proliferator-activated receptor (PPAR)γ and suppressor of cytokine signaling 2 (28). A recent study has found that tumor-derived exsomal enolase 2 accelerated glycolysis via the glycogen synthase kinase 3β/β-catenin/c-Myc signaling pathway to induce M2 polarization of macrophages (29). Phenotypically, M2 macrophages are characterized by the high expression of arginase 1 (Arg1), scavenger receptor (CD163), mannose receptor (CD206) and vascular endothelial growth factor (VEGF) (30,31). Based on different stimuli and specific functions, M2 macrophages may be further subclassified into M2a, M2b, M2c and M2d. M2a macrophages play a crucial role in promoting cell growth and tissue repair, whereas M2b, M2c and M2d macrophages are involved in inflammatory reactions, phagocytosis and tumor progression, respectively (32-34). Furthermore, M2d macrophages occupy a significant portion of the cellular elements of the TME. Although many researchers tend to regard TAMs as M2 macrophages, particularly M2d macrophages, simply classifying macrophages into M1 and M2 phenotypes oversimplifies the diverse nature of TAMs (35-37). Substantial research findings illustrate that TAMs are characterized by phenotypic heterogeneity and plasticity, with M1 and M2 macrophages being capable of transitioning into each other in response to changes in the TME or therapeutic interventions (8,33,38). Consequently, TAMs can be conceptualized as existing along a spectrum rather than strictly adhering to the M1 or M2 classification, representing a promising target for cancer therapeutic strategies.
3. TAMs and tumor progression
TAMs are a group of heterogeneous and plastic cells with different functional characteristics, exhibiting a dual function of resisting and promoting tumor progression (4,5,39). A significant number of research findings indicate that TAMs play a supportive role in tumor progression, as demonstrated using clinical research and experimental models. As the research further continues and develops, there is an increasing number of studies indicating that TAMs interact with other components of the TME at various stages of tumor progression and then gradually transform from the anti-tumor phenotype into a tumor-supporting phenotype that accelerates tumor progression. A schematic depiction of the role of TAMs in tumor progression is shown in Fig. 2.
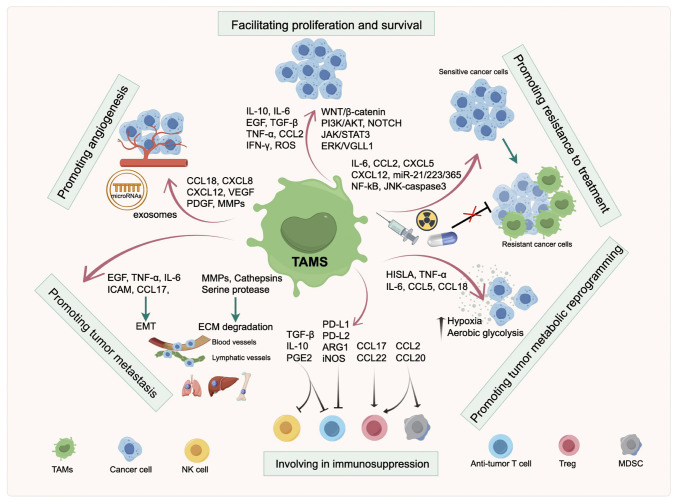
Figure 2.
Role of TAMs in tumor progression. The schematic diagram shows that TAMs play a crucial role in facilitating cancer cell proliferation and survival, promoting angiogenesis, generating resistance to therapy, forming an immunosuppressive microenvironment, and promoting tumor metastasis and metabolic reprogramming. TAMs, tumor-associated macrophages; LPS, lipopolysaccharide; GM-CSF, granulocyte-macrophage colony-stimulating factor; ARG, arginase; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; TGF, transforming growth factor; CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; ROS, reactive oxygen species; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; MMP, matrix metalloproteinase; PD-1, programmed cell death protein 1; HISLA, HIF-1α-stabilizing long noncoding RNA; iNOS, inducible nitric oxide synthase; PDGF, platelet-derived growth factor; ICAM, intercellular adhesion molecule; WNT, wingless/integrated; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; JAK, Janus kinase; STAT, signal transducer of activation; ERK, extracellular signal-regulated kinase; VGLL, vestigial-like protein; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; PGE, prostaglandin E; NK cell, natural killer cell; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell.
Facilitating the proliferation and survival of cancer cells
In contrast to terminally differentiated normal cells, cancer cells retain the ability to re-enter the cell cycle and proliferate unrestrictedly. Furthermore, TAMs can exacerbate this process. In the TME, TAMs interact with cancer cells by secreting various signaling molecules, including pro-inflammatory mediators such as TNF-α, IFN-γ and IL-6, growth factors such as TGF-β and epidermal growth factor. In addition, TAMs release ROS and RNS, which may create a pro-TME, thereby facilitating unrestricted proliferation and stimulating malignant progression (7,40). In prostate cancer, TAMs directly contact cancer cells and promote the proliferation of cancer cells by enhancing γ-secretase activity and elevating mastermind like transcriptional coactivator 2 expression to activate the NOTCH signaling pathway. By contrast, inhibiting TAM recruitment and NOTCH signaling significantly reduces cancer cell proliferation (41). It has been indicated that IL-6 provides survival benefits to various types of cancer. In vitro, previous findings illustrated that TAMs have a critical role in supporting the survival of multiple myeloma cells by activating the IL-6/JAK/STAT3 pathway (40). TAM-derived IL-6 has also been shown to be involved in the induction of genes critical for cancer cell cycle progression, such as cyclin D and p21, and IL-6-induced cancer cell proliferation could be suppressed by inhibiting the activation of STAT3 signals (42,43). In experimental animal models of intrahepatic cholangiocarcinoma, cancer cells polarized macrophages into the M2-TAM phenotype. This phenotype, in turn, promoted cancer cell proliferation through IL-10/STAT3 signaling (44). Azambuja et al (45) observed that the levels of Arg1, an enzyme mainly expressed on the surface of macrophages, were increased in TAM-derived exosomes, potentially promoting glioblastoma cell proliferation. In addition, hypoxic conditions triggered TAMs to secrete C-X-C motif chemokine ligand (CXCL)8, which further induced the proliferation of gastric cancer (GC) cells by activating C-X-C motif chemokine receptor (CXCR)1/2 (46). In addition, TNF-α secreted from macrophages activated TNFR1/ERK/vestigial like family member 1 signaling to support the survival of GC cells (47). The above research findings indicate that TAMs have a critical role in facilitating cancer cell proliferation and survival, providing a theoretical basis for targeting TAMs in cancer treatment.
Promoting angiogenesis
Due to the unrestrictedly rapid proliferation and expansion of the tumor mass, the TME in which cancer cells reside often experiences hypoxia and nutrient deprivation. Angiogenesis, the process by which a network of blood vessels grows and delivers oxygen and nutrients to the tumor area, becomes crucial under such conditions (48). Studies have indicated a significant increase in the number of macrophages in anoxic areas compared to normal tissues. Furthermore, TAMs are recognized as significant contributors to the angiogenesis process, and the infiltration of TAMs is closely associated with vascular density (49-52). In addition, the depletion of TAMs was demonstrated to delay the angiogenic process (53,54).
Hypoxia-inducible factor (HIF)-1α is involved in the stimulation of neovascularization and induces cancer cells to produce proangiogenic factors in hypoxic areas. In response to the hypoxic environment, TAMs activate proangiogenic programs and upregulate several transcription factors, including HIF-1α, which regulate various genes to facilitate angiogenesis (55). In line with this finding, Du et al (56) indicated that HIF-1α could upregulate VEGF expression in hypoxic glioma to promote tumor angiogenesis. Furthermore, TAMs secrete various proangiogenic factors, such as VEGF, platelet-derived growth factor, thymidine phosphorylase and angiogenic chemokines (54,57-59). In addition to producing proangiogenic factors, TAMs also express matrix metalloproteinase (MMPs), which can degrade the extracellular matrix (ECM), thereby further facilitating the release of proangiogenic factors to govern tumor angiogenesis (60).
In the field of research associated with tumor angiogenesis, emerging studies have shifted their focus from soluble signaling molecules to exosomes, which are small cellular vesicles that originate from cells and carry genetic information. Yang et al (61) found that TAM-derived exosomes carried microRNA (miR)-155-5p and miR-221-5, which they transported to bind to E2F2 in endothelial cells, resulting in the promotion of angiogenesis in pancreatic ductal adenocarcinoma (PDAC). Similarly, exosomes originating from TAMs act as carriers that transport miR-501-3p to increase the expression of the angiogenesis-related factor VEGF in PDAC (62). These research findings indicate that targeting TAM-induced angiogenesis may be a potential strategy for cancer treatment.
Generating resistance to treatment
One of the biggest obstacles to achieving a satisfactory therapeutic effect in cancer is the development of resistance to treatment. Accumulating evidence has illustrated that resistance to anti-cancer therapy is determined by the inherent ability of cancer cells and by the reciprocal interaction between cancer cells and nonmalignant cells within the TME, including TAMs (2,7,63). Chemotherapy and radiotherapy are common strategies for cancer treatment, and the critical roles of TAMs in these therapies have been widely researched. TAM-mediated chemoresistance was initially demonstrated in a xenograft mouse model experiment, where CSF1 inhibition was able to reverse chemoresistance in breast cancer (64). Subsequently, research extended this initial observation and confirmed higher infiltration of CD45+CD11b+CD14+ macrophages in breast cancer biopsy samples from patients who received neoadjuvant chemotherapy compared to those who had surgery alone. In the same study, inhibiting the recruitment of macrophages with CSF1R-signaling antagonists improved the cells' chemosensitivity to paclitaxel and slowed primary tumor progression (65). In the prostate, the combined treatment with docetaxel and androgen deprivation led to the recruitment of macrophages into the TME and induced TAMs to release CXCL12. This release, in turn, mediated chemoresistance via CXCR4 activation (66). In colorectal cancer (CRC), TAM activation during 5-fluorouracil (5-FU) treatment led to the excretion of putrescine, a polyamine, protecting CRC cells from 5-FU-induced apoptosis by suppressing the JNK-caspase-3 pathway (67). Similarly, drug-resistant GC cells facilitate M2 polarization of macrophages, leading to the release of CXCL5 by TAMs, promoting chemoresistance of GC cells via activation of the PI3K/AKT/mTOR pathway (68). Exosomes have also been demonstrated to contain chemoresistance-related factors and transfer them to the TME, thereby increasing chemoresistance. For instance, in one study, TAM-derived exosomes containing miR-223 were found to mediate drug resistance in epithelial ovarian cancer through the phosphatase and tensin homolog/PI3K/AKT signaling pathway (69). Furthermore, Binenbaum et al (70) also revealed that the exosomal transfer of miR-365 upregulated pyrimidine metabolism and increased triphosphate nucleotide levels to inhibit the effect of gemcitabine on PDAC. These studies suggest that TAMs are potent mediators of chemoresistance and can serve as potential targets to improve chemotherapy sensitivity in cancer patients.
Consistent with chemotherapy, radiotherapy influences the TME in a dynamic and complex manner, impacting its efficacy. Targeting TAMs using CSF1R inhibitors has been confirmed to improve the treatment responses of glioblastomas to radiotherapy (71). In inflammatory breast cancer, co-culturing cancer cells with M2-polarized macrophages promoted resistance to radiotherapy, whereas the inhibition of M2 polarization using phosphopeptide mimetic prodrugs protected against TAM-mediated radioresistance (72). In addition, several other studies reported and validated that TAMs play critical roles in shaping the TME and radioresistance (73-76).
Over the past decades, immunotherapy based on immune checkpoint inhibitors (ICIs) has shown revolutionary benefits in prolonging the survival of patients with cancers. ICIs eliminate immune suppression by binding to cytotoxic T lymphocyte antigen 4 (CTLA-4) or programmed cell death protein 1 (PD-1) and its ligand PD-L1, which serve as critical targets related to the activation or exhaustion of T lymphocytes (77,78). However, TAMs have been demonstrated to contribute to the dysfunction and exhaustion of T lymphocytes through the release of cytokines or metabolites, and the high infiltration of TAMs often correlates with resistance to ICIs (79-82).
Involvement in immunosuppression
As mentioned above, TAMs can induce immunosuppression and promote tumor immune escape via various mechanisms. Research findings have demonstrated that TAMs modify immune cells by inhibiting the activation and/or function of anti-tumor immune cells while increasing the presence of immunosuppressive cells (83). CD8+ T cells are cytotoxic T cells that serve as effector cells and have a critical role in the anti-tumor immune response. Arginine metabolism has been demonstrated to be involved in the activation of T cells and immune response regulation, and T cells with increased levels of L-arginine exert improved anti-tumor activity by bolstering survival capacity, metabolic adaptations and T-cell memory phenotypes (84). However, TAMs can inhibit the activity of T cells by secreting Arg1, which metabolizes L-arginine into L-ornithine and urea. Consequently, T cells become unresponsive to tumor antigens (84). Furthermore, L-arginine serves as a substrate for iNOS, and TAM-derived iNOS can mediate the L-arginine catabolic process and lead to T-cell suppression (85). In addition to Arg1 and iNOS, oxygen radicals and RNS derived from TAMs can also inhibit the activation of T cells (86,87).
Numerous studies have indicated that TAMs suppress the functions of CD8+ T cells, CD4+ T cells and natural killer (NK) cells by secreting an array of immunosuppressive cytokines. High expression levels of IL-10, TGF-β and prostaglandin E2 influence the immunosuppressive microenvironment by directly inhibiting the effector functions of anti-tumor T cells and NK cells, thereby inducing the expansion of regulatory T (Treg) cells and creating an immunosuppressive TME (88-90). In addition, Smith et al (91) found that IL-10 enhanced N-glycan branching and reduced the co-localization of CD8 with T-cell receptor, ultimately increasing the antigenic threshold required for the activation of T cells. Furthermore, CCL22 derived from TAMs facilitated Treg recruitment into the TME, resulting in the suppression of cytotoxic T-cell responses (92).
The TAM-induced immunosuppressive TME is also regulated by the expression of inhibitory receptors on TAMs. MHC-I molecules have a pivotal role in antigen presentation to T cells and can be divided into either the classical group, which includes human leukocyte antigen-C (HLA-C), or the nonclassical group, exemplified by HLA-E and HLA-G. Of note, the nonclassical group inhibits the activation of NK cells and T cells by interacting with CD94 and leukocyte immunoglobulin-like receptor B 1 (LILRB1), respectively (93,94). TAMs also express T-cell immune checkpoint ligands such as PD-L1, PD-L2, CD86 and CD80, which bind to the inhibitory receptors PD-1 and CTLA-4 to suppress the function of immune effector cells (95,96). The above findings indicate that TAMs serve as a crucial driver of the immunosuppressive TME and promote tumor progression by inhibiting the immune response and facilitating immune evasion.
Promoting tumor metastasis
Metastasis of cancer cells is an outstanding characteristic of all malignancies and the leading cause of tumor-related deaths, representing a significant challenge in cancer treatment. Tumor metastasis begins with the detachment of cancer cells from the primary site, followed by their invasion through blood or lymphatic vessels, ultimately resulting in the growth of secondary tumors with the same pathological features as the primary site (97). It is within this context that the phenomenon of epithelial-mesenchymal transition (EMT) emerges. EMT denotes the process of morphological transformation in which epithelial cells acquire mesenchymal features and malignant biological properties, including enhanced invasion ability and cancer stem cell-specific characteristics (98). Furthermore, a growing body of research studies highlighted the critical role of TAMs in regulating the EMT process of tumor cells and facilitating invasion from the basement membrane into the surrounding stroma (37,99-101).
In a model of CRC, research demonstrated that TAM-derived IL-6 induced EMT in cancer cells by regulating the JAK2/STAT3/miR-506-3p/forkhead box Q1 axis, which in turn contributed to the release of CCL2 and thereby facilitated the recruitment of macrophages, ultimately resulting in the promotion of CRC metastasis (99). Furthermore, CCL17 secreted from TAMs regulated the TGF-β1 and Wnt/β-catenin signaling pathway to promote the EMT and stemness of hepatocellular carcinoma (HCC) cells (102). In addition, TAMs orchestrated the TME by secreting various cytokines, such as TNF-α, IL-6 and ICAM-1, thereby modulating the EMT of intrahepatic cholangiocarcinoma cells through the AKT3/PRAS40 signaling pathway (103).
The ECM constitutes a complex network of macromolecules with cellular regulatory and structural roles and serves as a scaffold and surrounding barrier for cancer-cell invasion. Therefore, the degradation of the ECM contributes to the formation of cleavages through which cancer cells can metastasize (104,105). It has been demonstrated that TAMs are capable of mediating ECM degradation and ECM-cell crosstalk by upregulating proteolytic enzymes, including MMP7, MMP9, cathepsins and serine proteases (37,57,99,104,106). In addition, TAM-secreted chitinase 3-like protein 1 interacts with IL-13 receptor α2 chain on the plasma membranes of cancer cells. This interaction activates the MAPK signaling pathway, contributing to the upregulation of MMP genes (107).
The tumor vasculature represents a primary avenue for the metastasis of various malignancies, attracting cancer cells after detachment from the primary tumor site. When cancer cells invade blood vessels, they must avoid being recognized and eliminated by the immune system to reach distant organs and grow up to a certain size. Research findings have indicated that TAMs protect cancer cells against cytotoxic T-cells by contributing to an immunosuppressive TME and promoting the extravasation of metastatic cancer cells from blood vessels (108-110). In addition, earlier studies have demonstrated that TAMs can remodel the vasculature into a leaky and tortuous form, which could facilitate the metastasis of cancer cells (111,112). Furthermore, TAMs are also involved in the process of lymphangiogenesis, which serves as a significant pathway for cancer cells to metastasize to regional lymph nodes and distant organs (59,113,114).
Promoting tumor metabolic reprogramming
Metabolic reprogramming is one of the hallmarks of malignancies, during which the features of metabolic enzymes, regulatory molecules and metabolic products are modified (115). A series of research work has indicated that abnormal metabolites involved in glucose, lipid and amino acid metabolism pathways induce tumor-related metabolic reprogramming (116,117). These metabolites are transferred and accumulated in the TME and affect the metabolism of recipient cells to promote tumor progression (118). In particular, metabolites deriving from cancer cells, mast cells, T cells, adipocytes and cancer-associated fibroblasts can be ingested by TAMs and affect their polarization and function (4,119). In turn, TAMs are capable of promoting tumor progression via reprogramming tumor metabolism. A study indicated that TAMs transmitted an extracellular vesicle-packaged long noncoding (lnc)RNA, HIF-1α-stabilizing lncRNA (HISLA), to breast cancer cells, and subsequently enhanced their ability of aerobic glycolysis and apoptotic resistance. In terms of the mechanism, HISLA inhibited the hydroxylation and degradation of HIF-1α by blocking the interaction between HIF-1α and PHD2 (120). Tumor hypoxia and aerobic glycolysis have been demonstrated to promote resistance to anti-tumor treatment (121,122). Jeong et al (123) found that TAM-derived TNF-α promoted the glycolysis of non-small cell lung cancer (NSCLC) cells and facilitated tumor hypoxia by increasing AMP-activated protein kinase and PPAR-γ coactivator 1-α. On the contrary, depleting TAMs could abrogate tumor hypoxia and aerobic glycolysis, thereby resulting in an improved therapeutic effect of PD-L1 (123). Furthermore, several other studies indicated that TAMs released cytokines with metabolic function, including IL-6, CCL5 and CCL18, to alter tumor metabolism (124-126). The blockade of metabolic pathways involved in TAMs can be used for drug discovery and tumor treatment.
4. Therapeutic strategies targeting TAMs
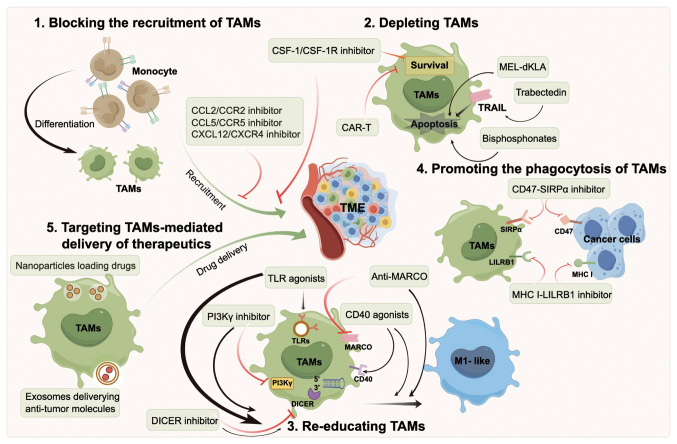
Therapeutic strategies targeting TAMs show promising potential for tumor treatment. The current therapeutic strategies targeting TAMs can be roughly divided into five types (Fig. 3), including depleting TAMs, blocking the recruitment of TAMs, re-educating TAMs, promoting the phagocytosis of TAMs and targeting TAMs-mediated delivery of therapeutics. Therapeutic strategies targeting TAMs in selected clinical trials are listed in Table I.
Figure 3.
Treatment strategies for TAMs-directed antitumor therapy. Strategies that target TAMs for cancer treatment mainly fall into five groups: 1) Blocking the recruitment of TAMs into the TME. 2) Directly depleting TAMs. 3) Re-educating TAMs to the M1-like phenotype with anti-tumor activity. 4) Promoting phagocytosis of TAMs to cancer cells. 5) TAMs-mediated delivery of therapeutics. TAMs, tumor-associated macrophages; TME, tumor microenvironment; CSF-1, colony-stimulating factor-1; CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; TLR, toll-like receptor; MARCO, macrophage receptor with collagenous structure; MHC, major histocompatibility complex class; SIRP, signal regulatory protein; LILRB, leukocyte immunoglobulin-like receptor B; CAR-T, chimeric antigen receptor-T cell; TRAIL, TNF-related apoptosis-inducing ligand; PI3K, phosphoinositide 3-kinase.
Table I.
Selected clinical trials targeting TAMs in cancers.
| Treatment strategy | Target | Agent | Combination | Tumor type | Phase | Clinical trial number |
|---|---|---|---|---|---|---|
| Blocking TAMs recruitment | CSF-1 | MCS110 | Carboplatin Gemcitabine | TNBC | II | NCT02435680 |
| CSF-1 | MCS110 | Dabrafenib Trametinib | Melanoma | I/II | NCT03455764 | |
| CSF-1R | IMC-CS4 | NA | Solid tumors | I | NCT01346358 | |
| CSF-1R | IMC-CS4 | Pembrolizumab Cyclophosphamide GVAX | PC | I | NCT03153410 | |
| CSF-1R | PLX3397 | Eribulin | MBC | I/II | NCT01596751 | |
| CSF-1R | PLX3397 | NA | Melanoma | II | NCT02071940 | |
| CSF-1R | BLZ945 | PDR001 | Solid tumors | I/II | NCT02829723 | |
| CSF-1R | ARRY382 | Pembrolizumab | Solid tumors | I/II | NCT02880371 | |
| CCR2/5 | BMS813160 | Nivolumab Paclitaxel | CRC/PC | I/II | NCT03184870 | |
| CCR2/5 | BMS813160 | Nivolumab | NSCLC HCC | II | NCT04123379 | |
| CXCR4 | BL8040 | G-CSF | Multiple myeloma | III | NCT03246529 | |
| CXCR4 | X4P-001 | Axitinib | RCC | I/II | NCT02667886 | |
| Depleting TAMs | NA | Zoledronate | IL-2 | Kidney cancer | II | NCT00582790 |
| Caspase 8 | Trabectedin | Olaparib | Sarcoma | II | NCT04076579 | |
| Caspase 8 | Trabectedin | NA | MPM | II | NCT02194231 | |
| Caspase 8 | Trabectedin | Durvalumab | Ovarian carcinoma | I | NCT03085225 | |
| Re-educating TAMs | TLR7/8 | NKTR-262 | Nivolumab | TNBC, MCC, Melanoma, HNSCC | I/II | NCT03435640 |
| TLR7/8 | MEDI-9197 | Durvalumab | Solid tumors | I | NCT02556463 | |
| TLR4 | GSK1795091 | Pembrolizumab | Neoplasm | I | NCT03447314 | |
| TLR9 | CMP-001 | Nivolumab | Melanoma | II | NCT03618641 | |
| CD40 | APX005M | Pembrolizumab | Melanoma | I/II | NCT02706353 | |
| CD40 | SEA-CD40 | Pembrolizumab Pemetrexed Carboplatin | NSCLC | II | NCT04993677 | |
| CD40 | RO7009789 | Paclitaxel Gemcitabine | PC | I | NCT02588443 | |
| PI3Kγ | IPI-549 | Nivolumab | Solid tumors | I | NCT02637531 | |
| PI3Kγ/δ | Duvelisib | Pembrolizumab | HNSCC | I/II | NCT04193293 | |
| Promoting | CD47 | Hu5F9-G4 | NA | Solid tumors | I | NCT02216409 |
| phagocytosis | CD47 | Hu5F9-G4 | Avelumab | Ovarian cancer | I | NCT03558139 |
| SIRPα | ALX148 | Pembrolizumab Trastuzumab | Solid tumors Lymphoma | I | NCT03013218 | |
| SIRPα | TTI-621 | Nivolumab Rituximab | Hematologic and solid tumors | I | NCT02663518 | |
| CD47/SIRPα | RRx-001 | Platinum chemotherapy | SCLC | III | NCT05566041 | |
| LILRB2 | MK-4830 | Pembrolizumab | Solid tumors | I | NCT03564691 |
TAMs, tumor-associated macrophages; NA, not available; TNBC, triple-negative breast cancer; PC, pancreatic cancer; MBC, metastatic breast cancer; CRC, colorectal cancer; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; TRAIL, TNF-related apoptosis-inducing ligand; MPM, malignant pleural mesothelioma; TLR, toll-like receptors; MCC, Merkel cell carcinoma; HNSCC, head and neck squamous cell carcinoma; LILRB2, leukocyte immunoglobulin-like receptor B2; SIRPα, signal regulatory protein α; PI3K, phosphoinositide 3-kinase; CCR, C-C motif chemokine receptor; CXCR, C-X-C motif chemokine receptor; CSF-1, colony-stimulating factor-1.
Blocking the recruitment of TAMs
TAM replenishment in the TME is primarily mediated by macrophage recruitment and differentiation, a process in which many cytokines and chemokines have critical roles. Blocking the recruitment of TAMs into the TME to alleviate their pro-tumor effects holds promise as a strategy for anti-tumor targeting. CSF1R, a member of the tyrosine kinase receptor family, undergoes homodimerization and activated receptor signaling by binding to its ligands CSF1 and IL-34 (127). This CSF1/CSF1R axis facilitates the recruitment of TAMs to the TME and promotes the acquisition of a protumor phenotype. Targeting CSF1/CSF1R signaling has been extensively investigated to prevent TAM accumulation in tumors (128). As reviewed elsewhere, preclinical research studies indicated that blocking the CSF1/CSF1R axis reduces macrophage recruitment (95,128,129). Furthermore, with the development of antibody antagonists and small molecules that restrain receptor dimerization, current clinical studies focus on abrogating ligand binding and signaling activation. The tyrosine kinase inhibitor PLX3397 (pexidartinib), a small molecular CSF1R inhibitor, can significantly reduce TAM infiltration and shows potent anti-tumor effects in multiple models, including breast cancers, lung cancers and gliomas (130-132). In particular, a clinical phase III trial demonstrated the good tolerance and meaningful clinical activity of PLX3379 in patients with tenosynovial giant cell tumor (TSGCT), leading to Food and Drug Administration (FDA) approval for TSGCT treatment (133). Several other small molecules, such as PLX7486, BLZ945 and ARRY-382, and monoclonal antibodies such as MCS110 and LY3022855, have been designed to block the CSF1/CSF1R axis, albeit with mixed results (134-138). Furthermore, apart from preventing TAM recruitment, blocking the CSF1/CSF1R axis may also increase the ratio of CD8+/CD4+ T cells within the TME (139).
Research studies also indicate that CCL2 has a potent chemotactic effect on immune cells, including monocytes, NK cells and T cells, and the interaction between CCL2 and its receptor CCR2 has a crucial role in the replenishment and accumulation of TAMs, as well as the recruitment of other immune cells (140,141). Targeting CCL2/CCR2 signaling blocks the recruitment of monocytes into the TME, ultimately reducing the infiltration of TAMs and consequently exerting anti-tumor effects (142). CNTO888, also known as carlumab, is a monoclonal antibody that binds with CCL2, thereby competing for the CCR2 binding site. A phase I clinical trial demonstrated that carlumab was well tolerated by patients with advanced solid tumors, showing preliminary anti-tumor activity with evidence of a transient decrease in CCL2 levels and maintenance of stable disease in several patients (143). However, in a phase II study of carlumab in patients with drug-resistant metastatic prostate cancer previously treated with docetaxel, single carlumab treatment did not result in complete or partial remission, and only 34% of patients maintained a stable disease status for >3 months (144). By contrast, CCX872, a CCR2 antagonist, has shown the ability to enhance the median survival when administered as a monotherapy in glioma-bearing animals and further increase the median survival and overall survival when administered in combination with immunotherapy. Of note, examination of tumor-infiltrating immune cells indicated a decrease in myeloid-derived suppressor cells (MDSCs), which have the potential to convert into TAMs within the TME (145).
The CXCL12/CXCR4 axis has also been demonstrated to be correlated with macrophage recruitment and blocking CXCL12/CXCR4 signaling can suppress TAM recruitment into the TME (146-148). Mavorixafor (X4P-001) is an oral, allosteric CXCR4 inhibitor that restricts the recruitment of immunosuppressive cells. A phase Ib clinical trial showed the potential anti-tumor activity and well-tolerated profile of mavorixafor in combination with nivolumab treatment for metastatic clear cell renal cell carcinoma (149). In addition, the results of a phase II, open-label, two-cohort study indicated that the combination of the CXCR4 antagonist BL-8040 and pembrolizumab enhanced the therapeutic efficacy of chemotherapy for patients with PDAC (150). Furthermore, inhibiting protein neddylation decreases the recruitment of macrophages, and targeting neddylation modification serves as a promising therapeutic anti-TAM strategy in lung cancer (151,152).
Depleting TAMs
In addition to blocking the recruitment of macrophages, the direct depletion of TAMs within the TME by inducing apoptosis has also been explored as a strategy for tumor treatment. Bisphosphonates are a family of antiresorptive regents that have traditionally been applied in the treatment of osteoporosis and bone metastasis. Of note, bisphosphonates can also evoke apoptosis of TAMs. Clodronate, the first generation of bisphosphonates, has been demonstrated to have an inhibitory effect on the development of tumors in animal models by depleting macrophages and reducing TAM infiltration (153-155). Zoledronate or zoledronic acid, the latest generation of bisphosphonates, participates in immune regulation by attacking TAMs. Furthermore, in efforts to enhance the efficacy of zoledronic acid in targeting TAMs, Zang et al (156) developed lipid-coated calcium zoledronate nanoparticles that could effectively induce the apoptosis of TAMs, and consequently decrease TAM-related angiogenesis and immunosuppression in tumor-bearing mouse models. In addition, a recent study highlighted the use of a nanoliposome encapsulating zoledronic acid, which effectively remodeled the TME by targeting the depletion of TAMs. Consequently, this approach led to the effective inhibition of tumor progression (157). In terms of function, zoledronic acid not only directly eliminates TAMs but also increases the infiltration of cytotoxic CD8+ T cells and promotes tumor inflammation when administered in combination with thymosin α1 (158).
Trabectedin, a tetrahydroisoquinoline alkylating agent, is recognized as an anti-neoplastic drug used in clinical settings for the second-line treatment of advanced soft tissue sarcoma and relapsed platinum-sensitive ovarian cancers (159,160). Of note, besides its direct elimination of cancer cells by inducing DNA double-strand breaks, another remarkable characteristic of trabectedin is its ability to induce monocyte/macrophage apoptosis through the TNF-related apoptosis-inducing ligand-dependent pathway, thereby blocking the release of certain pro-metastatic cytokines, including VEGF, IL-6 and CCL2 (161,162). A prospective study evaluated the pro-apoptotic effect of trabectedin, revealing that 19 out of 34 patients suffering from soft tissue sarcoma experienced a reduction in monocytes, ranging from 30-77% (161). Furthermore, a study demonstrated that trabectedin reduced TAM infiltration and tumor blood vessel density to restrict melanoma growth and metastasis (163). However, despite the potential of these agents to deplete TAMs, they may also deplete anti-tumor immune cells, leading to adverse effects. Hence, it needs to be further verified whether the complete deletion strategy is feasible or not.
A hybrid peptide comprised of melittin (MEL) and the pro-apoptotic peptide d (dKLA) (MEL-dKLA) binds to TAMs and induces mitochondrial death after cell membrane penetration, contributing to the apoptosis of TAMs. Research studies have also shown that MEL-dKLA is capable of selectively binding to CD206+ M2-type TAMs while protecting the function of anti-tumor immune cells (164). Furthermore, Sánchez-Paulete et al (165) used chimeric antigen receptor T (CAR-T) cells targeting F4/80 to effectively eliminate TAMs, leading to the expansion of tumor antigen-related endogenous CD8+ T cells and facilitating the anti-tumor immune response (165). They also found that the anti-tumor effect of CAR-T was present in TAM-rich PDAC and ovarian cancer models, resulting in significant tumor growth inhibition.
Re-educating TAMs
The phenotypes and function of TAMs are determined by macrophages' response to various extracellular factors within the TME (24). Despite shifting from M1 to M2 macrophages at various stages of tumor progression and being generally tumor-promoting, TAMs have the potential to play tumoricidal roles and inhibit tumor growth by manipulating environmental stimuli and re-educating macrophages from the M2 to M1 phenotype, a process known as TAM reprogramming.
Damage-associated molecular patterns (DAMPs) released from dying tumor cells can activate the immune system by interacting with pattern recognition receptors (PRRs) (166). TLRs, a family of proteins and essential PRRs expressed by immune cells, have critical roles in innate immunity by recognizing DAMPs (167). Activation of TLRs can induce macrophage polarization into the pro-inflammatory phenotype and promote an inflammatory response within the TME. Consequently, TLR agonists have been investigated in cancer research to evaluate their potential in modulating TAM polarization towards a tumoricidal phenotype (168,169). TLR7 and TLR8 have a high degree of sequence homology and display similarity in structure; agonists of TLR7 and TLR8 have demonstrated the most promising anti-tumor effect among all the TLR agonists (170,171). Of note, TLR7/8 agonists have shown significant potential in reversing oxaliplatin resistance in CRC by inducing MDSCs to differentiate into tumoricidal phenotypes (172). Figueiredo et al (173) developed lignin-based nanoparticles that carried TLR7/8 agonist (resiquimod, R848) to convert TAMs from M2 to M1 phenotype, thereby enhancing the anti-tumor effect of vinblastine in triple-negative breast cancer (TNBC). In addition, the TLR7/8 agonist MEDI9197 has been demonstrated to induce both innate and adaptive immune response, as evidenced by the release of IL-12, IFN-γ and IFN-α. These cytokines can polarize TAMs towards a tumoricidal phenotype and activate NK and CD8+ cells (174). These results suggest the potential of utilizing TLR7/8 agonists in combination with other therapies. In particular, IMO-2055, a TLR9 agonist, showed good tolerability and possible anti-tumor effects when administered in combination with bevacizumab and erlotinib for the treatment of advanced or metastatic NSCLC (175). Furthermore, BCG, one of the FDA-approved TLR agonists used for bladder cancer, activates the TLR2 and TLR4 signaling pathways, leading to the conversion of TAMs towards anti-tumor phenotypes. This process enhances the cytotoxicity of macrophages against cancer cells (176,177).
CD40, a receptor that belongs to the TNF receptor superfamily, is broadly expressed on macrophages and other antigen-presenting cells. The interaction between CD40 and its natural ligand CD40L helps support the anti-tumor activity of T cells and facilitates the polarization of macrophages into the M1 phenotype (178,179). Studies have shown that CD40 agonists can promote the infiltration of macrophages and induce their polarization into a pro-inflammatory phenotype. Furthermore, CD40 agonists have demonstrated a combinational effect in pancreatic carcinoma when used alongside the chemotherapeutic agent gemcitabine, resulting in tumor regression and prolonged patient survival (180,181). The combination of CD40 agonists and anti-CSF1R antibodies has also been demonstrated to reprogram TAMs before their depletion, creating a pro-inflammatory TME to enhance the anti-tumor response (182,183). In addition, it has been shown that MEK inhibitors improve the anti-tumor efficacy of CD40 agonists by inhibiting the immunosuppressive activity of M2 TAMs, Tregs and MDSCs, and increasing the tumoricidal immune response (184). Furthermore, according to the study by Leblond et al (185), resistance to anti-PD1 therapy was attenuated by the combination of CD40 agonists, resulting in a solid anti-tumor immune response. In terms of the mechanism, the combination contributed to the recruitment of CD8+ cells and induced IFNγ-independent repolarization into M1 TAMs (185). Agonistic CD40 antibodies and recombinant CD40 ligands such as CD-870, 893, APX005M, ADC-1013, dacetuzumab and SEA-CD40, are currently being evaluated in early-phase clinical trials as single agents or in combination with chemotherapy, immunotherapy and tumor vaccines (129,186).
Macrophage receptor with collagenous structure (MARCO) is a pattern recognition receptor belonging to the class A scavenger receptor family. Research has recently indicated that MARCO plays a critical role in regulating macrophage polarization and that MARCO+ TAMs are a subgroup of macrophages with strong immunosuppressive capabilities that are negatively associated with patient prognosis (187-189). Therefore, inhibiting MARCO is expected to reprogram the phenotype of TAMs. In an animal model of melanoma, inhibiting MARCO alleviated the inhibitory action of TAMs on NK cells, and the anti-MARCO antibody synergized with T cell-directed immunotherapy, such as PD-1/PD-L1, to increase the efficacy of tumor eradication (190). Furthermore, several preclinical models have demonstrated that anti-MARCO antibodies restrict the progression of tumors by remodeling MARCO+ TAMs from the M2 to M1 phenotype and reducing the levels of Tregs (187). In prostate cancer, MARCO-neutralizing antibody hindered lipid accumulation in TAMs and reprogrammed macrophages, restricting cancer growth and invasiveness. Of note, anti-MARCO treatment also improved the response to docetaxel in prostate cancer models (191). In a different study, Georgoudaki et al (187) developed an anti-MARCO monoclonal antibody that exerted anti-tumor effects in breast and colon cancer models. This antibody effectively reprogrammed TAMs into a pro-inflammatory phenotype while also enhancing the efficacy of immune checkpoint therapy (187). While research on inhibitors against MARCO remains in its early stages, inhibitors against other class A scavenger receptors, such as the scavenger receptor B class type 1 and lectin-like oxidized low-density lipoprotein receptor-1, are also available for investigation (192,193).
Other strategies to reprogram TAMs for anti-tumor therapy include the targeting of PI3Kγ, which is a critical regulator of tumor immune suppression induced by TAMs. Activation of PI3Kγ signaling facilitates immunosuppressive transcriptional programming in TAMs and then inhibits the adaptive immune response. By contrast, suppression of PI3Kγ using genetic and pharmacological inhibitors results in macrophage reprogramming, resulting in increased anti-tumor TAM infiltration and T-cell response, while reducing pro-tumor TAMs (194,195). In head and neck squamous cell carcinoma, inhibiting PI3Kγ in macrophages indirectly facilitated both the cytotoxic and Th1 adaptive immune response, synergizing with T cell-targeted therapy to enhance the anti-tumor immune response and restrain tumor progression, suggesting the potential therapeutic target of PI3Kγ (195). In particular, IPI-549 (eganelisib), a PI3Kγ inhibitor, was evaluated for its anti-tumor efficacy alone or in combination with PD-1/PD-L1 inhibitors in a clinical trial (NCT02637531). In a phase 1/1b trial, the safety and tolerability of IPI-549 were investigated, with doses of 30 and 40 mg administered once daily as part of a phase 2 study (196). In addition, Giurisato et al (197) found that ERK5 was a determinant of macrophage polarization, and the inactivation of ERK5 specifically decreased the relative percentage of M2 tumor-supportive macrophages. Furthermore, increasing evidence indicates that miRNA serves as a key modulator in macrophage polarization. The inhibition of DICER, an RNase-III enzyme that regulates the maturation of miRNA, reprograms TAMs into an anti-tumor phenotype and promotes tumor regression (7,198). Overall, these findings have prompted investigation into targeting miRNAs to reprogram macrophages.
Promoting the phagocytosis of TAMs
Phagocytic activity is a key characteristic of macrophages to exert anti-tumor effects. However, macrophage phagocytosis is largely inhibited by 'don't eat me' signals. Limiting 'don't eat me' signals and improving the phagocytic activity of TAMs represents a promising strategy for cancer treatment.
Signal regulatory protein α (SIRPα) is an inhibitory receptor expressed on myeloid cells, including monocytes, macrophages, and dendritic cells. SIRPα recognizes the ligand CD47, which is widely overexpressed on various cancer cells and acts as a 'don't eat me' signal to restrict innate immunity (199-201). The CD47-SIRPα axis enables cancer cells to evade phagocytosis and escape from immune surveillance, blocking either CD47 or SIRPα using monoclonal antibodies, fusion proteins or bispecific antibodies can trigger cellular cytotoxicity/phagocytosis of cancer cells by TAMs (202,203). Therapeutic agents targeting the CD47-SIRPα axis have been evaluated in preclinical and clinical trials. CD47-SIRPα axis blockade using SIRPα-Fc increases TAM-triggered phagocytosis of glioblastoma cells and enhances the response of cytotoxic CD8+ T cells to tumor cells (204). Research based on CD47 antibodies found that treatment with CD47 monoclonal antibodies enhances macrophages phagocytosis of HCC cells and increases infiltration of proinflammatory macrophages in tumor tissue to inhibit tumor progression in xenograft models (205). A phase I clinical study (NCT02216409) evaluated the safety, pharmacokinetics and pharmacodynamics of Hu5F9-G4, a humanized IgG4 antibody targeting CD47, and the results indicated that blocking CD47 is a promising strategy for cancer treatment (206). Enhancing the capability of macrophage's phagocytosis has also been found to contribute to the induction of an effective immune response against cancer cells, and blockade of the CD47-SIRPα axis in combination with ICIs increases the efficiency of anti-tumor immunotherapy (207,208). An ongoing phase 1b trial (NCT03558139) of Hu5F9-G4 in combination with Avelumab in participants with advanced solid tumors that have progressed within 6 months after receiving platinum-based chemotherapy is aiming to investigate the safety and tolerability of this combination and evaluate the anti-tumor effects. ALX148, a novel CD47-SIRPα axis-blocking protein generated by fusing a modified SIRPα N-terminal D1 domain to an inactive IgG Fc region, is currently in a phase I clinical trial (NCT03013218), being used as a single-agent therapy or in combination with Pembrolizumab or Trastuzumab for solid tumors (209). The SIRPα-Fc fusion protein TTI-621, another therapeutic agent that targets the CD47-SIRPα axis, has been found to facilitate macrophage-mediated phagocytosis of cancer cells (210). TTI-621 in combination with Nivolumab is being evaluated in subjects with relapsed hematologic malignancies and selected solid tumors in an ongoing phase I clinical study (NCT02663518).
LILRB is a family of transmembrane glycoproteins, including LILRB1 and LILRB2, which have been known to inhibit immune activation. Like the CD47-SIRPα axis, the interaction between MHC-I and LILRB1 also serves as a 'don't eat me' signal and blocking this interaction has shown efficacy in numerous cancer models (211). Furthermore, simultaneous blockade of CD47 and MHC-I produces a synergistic effect on tumor suppression (212). However, the anti-tumor effect of cytotoxic T cells is dependent on the antigen presentation of MHC-I. Thus, specifically blocking the β2-microglobulin subunit of MHC-I or LILRB1 seems to be a promising innate immune targeting strategy. Preclinical data have indicated that LILRB2 antagonism effectively polarized TAMs to the proinflammatory phenotype and enhanced phagocytosis, resulting in an increased anti-tumor immunity response (213). MK-4830, a novel human IgG4 monoclonal antibody targeting LILRB2, is under investigation for safety and tolerability alone or in combination with pembrolizumab in a phase I clinical trial (NCT03564691) (214). Their value and the mechanisms need to be further verified in more clinical trials.
Targeting TAM-mediated delivery of therapeutics
Although monoclonal antibodies, agonists and pharmacological inhibitors have been developed and evaluated for TAM-targeting therapy, the difficulty of penetrating biological barriers and the lack of specific targeting properties, as well as the side effects, largely limit the therapeutic effect. Therefore, a novel strategy of drug delivery mediated by live cell is on the rise as the conditions require. As one of the most abundant types of circulating cells, macrophages have received much interest as a drug-loading/drug-releasing carrier, for their high phagocytic capability, non-immunogenicity, long blood-circulation time and ability to infiltrate tumors (215,216). Macrophages cannot directly load most anti-tumor agents due to their cytotoxicity, whereas the progress in the engineering of nanoparticles has made it feasible to load nanomedicines into macrophages and release drugs in the bulk of the tumor. For instance, it has been reported that one sort of genetically engineered cell membrane-coated magnetic nanoparticle may be used to promote the repolarization of M2 TAMs, as well as the systemic circulation and accumulation of the loaded drugs in the tumor. In addition, the magnetic nanoparticles significantly prolonged overall survival by inhibiting tumor growth and metastasis in animal models (217). Zhang et al (218) developed a biomimetic macrophage membrane-coated nanoparticle with loaded paclitaxel (cskc-PPiP/PTX@Ma) for the treatment of breast cancer, and cskc-PPiP/PTX@ Ma was highly accumulated in the tumor site and represented an effective drug delivery system tailored to the TME. Although macrophages are capable of delivering the active nanomedicine into tumor sites, this field remains in its infancy and there are still numerous challenges for their application in the clinic. Among the major reasons are the pro-tumor and anti-tumor activities of macrophages within the TME, and inducing and maintaining the anti-tumor phenotype of macrophages to further maximize the effect of macrophage-delivered nanomedicines is important.
In addition, the use of exosomes for cancer treatment has drawn the attention of investigators for their good biocompatibility, natural capacity to deliver molecules and nanoscale size (215,219). Given these properties, exosomes have shown great potential to be an excellent tool for the delivery of anti-tumor drugs. Furthermore, exosomes can be preferentially sequestered by macrophages and may represent an attractive carrier for transporting cytotoxic agents into the TME (216). For example, a study has indicated that exosomes isolated from breast cancer cells were capable of delivering miR-33 to M2 TAMs and covert M2 into M1 phenotypes, which was crucial for inhibiting tumor progression (220). Of note, the major challenge for the application of an exosomal delivery system may be the isolation of exosomes (221).
5. Combinations of TAM-targeted and conventional therapies
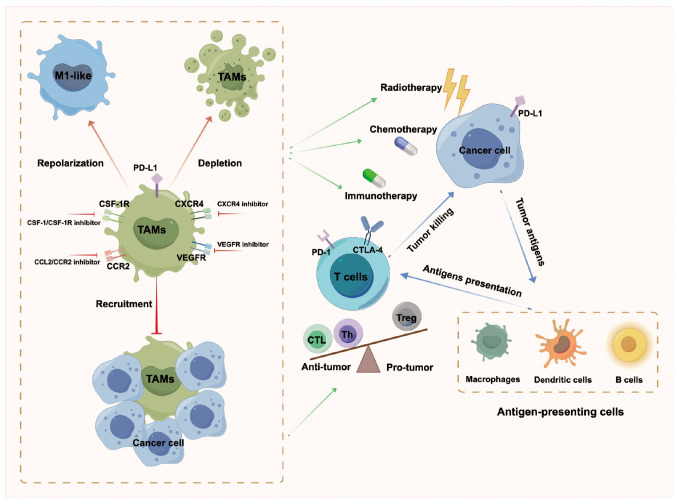
The essential conventional non-operative strategies for cancer treatment include chemotherapy, radiotherapy and immunotherapy. The chapters above elaborated on the crucial role of TAMs in promoting tumor progression and summarized recent advances in TAM-targeting therapeutic strategies. Combined therapies are likely to improve the clinical outcome for cancer patients and be one of the megatrends of cancer treatment (Fig. 4).
Figure 4.
Combination of TAMs-targeted therapy with chemotherapy, radiotherapy, or immunotherapy. Chemotherapy and radiotherapy induce cancer cell death and the release of tumor-associated antigens. Tumor-associated antigens are administered to T cells and activate immune response through antigen-presenting cells. TAMs-targeted therapy improves the efficacy of chemotherapy and radiotherapy, activates anti-tumor T cells and enhances the sensitivity to immunotherapy. TAMs, tumor-associated macrophages; CSF-1, colony-stimulating factor-1; CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; CXCR, C-X-C motif chemokine receptor; PD-1, programmed cell death protein-1; CTLA, cytotoxic T lymphocyte antigen; VEGFR, vascular endothelial growth factor receptor; CTL, cytotoxic T lymphocyte; Th, helper T cell; Treg, regulatory T cell.
Targeting TA Ms combined with chemotherapy
Chemotherapeutic drugs are mainly applied to selectively eradicate tumor cells or suppress tumor growth. However, TAMs have been verified to reduce the chemotherapeutic efficacy and indue tumor recurrence, which is closely associated with chemotherapy resistance (27). Combining TAM-targeted therapy with chemotherapy has been indicated to achieve excellent antitumor effects. The blockade of macrophage recruitment with CSF1R-signaling antagonists was found to improve chemosensitivity to paclitaxel, suppress primary tumor progression and reduce pulmonary metastasis in mammary tumor-bearing mice (65). Besides, targeting TAMs by CSF1R blockade activated intratumoral type I interferon signaling in breast cancer and consequently increased the antitumor efficacy of platinum-based chemotherapeutics (222). The infiltration of TAMs is frequently associated with the density of tumor vessels due to their secretion and response to angiogenic growth factors, particularly VEGF (6,95,223). Combination of chemotherapy and TAM elimination was found to decrease the density of tumor vessels by 50%. Depleting TAMs in a tumor mass skews perivascular TAMs from their pro-angiogenic to their angiostatic properties, which contributes to the increase of blood flow and the delivery of chemotherapeutic drugs to malignant lesions, contributing to enhanced efficacy of chemotherapy (224). Furthermore, Alishekevitz et al (114) provided evidence that TAMs could contribute to lymphangiogenesis and subsequent metastasis in a VEGFR3-dependent manner. Blockade of the VEGF-C/VEGFR3 axis inhibited lymphangiogenesis and blocked the pro-metastatic activity of TAMs in PTX-treated mice (114). Furthermore, Duhamel et al (225) demonstrated a therapeutic strategy of combining PTX and proprotein convertase 1/3 inhibitor to induce TAM polarization towards the antitumor phenotype in glioma. The anti-inflammatory pathway STAT3 was inhibited in proprotein convertase 1/3 knockdown TAMs, and more proinflammatory cytokines were secreted to inhibit tumor growth (225).
Targeting TAMs combined with radiotherapy
Radiotherapy is widely used in controlling local tumors, and ionizing radiation exerts major effects on tumor cells by inducing DNA damage, cell apoptosis, autophagy, mitotic catastrophe and necrosis to facilitate tumor regression (226). Previous studies indicated that ionizing radiation could affect antitumor immune response, including the recruitment of TAMs (227,228). TAMs accumulate in the irradiated tumor lesions and stimulate the resumption of blood flow, thereby facilitating the recurrence of tumors. Blocking the key chemokine pathway, the stromal cell-derived factor-1/CXCR4 axis that leads to the accumulation of TAMs enhances tumor response to radiotherapy and protects the irradiated normal tissues (229). Akkari et al (71) found that targeting TAMs using CSF1R inhibitor combined with ionizing radiation enhanced the efficacy of radiotherapy in gliomas and prolonged the survival of preclinical models.
In addition, radiotherapy has controversial effects on the polarization of macrophages. Certain studies indicated that low-dose irradiation (2 Gy) or short-course radiotherapy induced the repolarization of M2 phenotype macrophages into the M1 phenotype and subsequently enhanced the antitumor effect (230,231). On the contrary, other studies reported that irradiation contributed to the increased infiltration of CD68+CD163+ M2 phenotype macrophages (228). The release of ATP caused by irradiation-induced cancer cell death, which could be decomposed into adenosine, results in the accumulation of extracellular adenosine and thereby induces the polarization of TAMs to M2 phenotype (232,233). It is hypothesized that the effect of radiotherapy on TAMs depends on irradiation dose and tumor histotype. The combination of targeting TAMs and radiotherapy needs to be further explored to achieve more individualized applications and better antitumor effects.
Targeting TAMs combined with immunotherapy
Immune escape has been indicated to be one of the main hallmarks of malignancies, and immunotherapy is intended to reverse the immunosuppressive state of the TME by activating the immune system against cancer cells. ICIs binding to CTLA-4, PD-1 and its ligand PD-L1, the key inhibitory signals of T-cell activation, is the representative strategy for consolidating immune surveillance that yields survival benefits for patients with malignancies (78,234). However, certain studies have indicated that only a subset of patients could achieve complete response and in numerous patients, the benefit was limited and they even experienced recurrence after a period of remission (235,236). As mentioned earlier, TAMs can decrease immunotherapy efficacy by suppressing the activation of T cells or secreting anti-inflammatory cytokines (83-86). Therefore, targeting TAMs is of great significance to improve the efficacy of immunotherapy, and the combination of targeting TAMs and immunotherapy should be carefully considered.
The CCL2/CCR2 axis plays a crucial role in the replenishment and recruitment of M2-like TAMs to induce immune suppression, making it a promising TAM-targeted therapy (140,141). The combination of anti-PD-L1 and CCR2 antagonists that deplete TAMs shows a synergetic effect on tumor eradication associated with the activation of CD8+ T cells (237). Certain preclinical studies on different types of malignancies have also indicated that either depleting CCL2 or disrupting the CCL2/CCR2 axis could enhance the antitumor effect of immune agents (238,239). Similarly, the CSF1/CSF1R axis facilitates the recruitment of TAMs to the TME and promotes the acquisition of a protumor phenotype, suggesting that CSF1R antagonists can be an alternative target. Combining CSF1R antagonist and anti-PD-L1 shows potent antitumor effects through inhibiting TAM recruitment, increasing CD8+ T-cell infiltration and maintaining the Th1/Th2 cytokine balance in mouse models of HCC (240). In addition, depleting TAMs enhances the efficacy of immunotherapy. Li et al (241) constructed a biocompatible alginate-based hydrogel loaded with PLX, which was gradually released at the tumor site to deplete TAMs, and consequently established a favorable milieu for the delivery of anti-PD-1 antibody-conjugated platelets and the infiltration of T cells into tumor lesions. CTLA-4 has an inhibitory effect on the activation of T cells and humanized anti-CTLA-4 antibody has doubled the 10-year survival rates of patients with metastatic melanoma. Of note, TAMs express the ligands of CTLA-4 and play an important role in T cell-mediated immune response (242).
As described above, TAMs demonstrated an important role in regulating immunotherapy. Targeting TAMs in combination with immune checkpoint inhibition significantly improves the therapeutic effect, offering a promising strategy for tumor treatment.
6. Conclusion and perspective
In light of the growing understanding of the critical role of TAMs in tumor progression, targeting TAMs has emerged as a novel approach to cancer therapy. As described above, the present review focuses on the latest advances in exploiting TAMs as therapeutic targets for cancer treatment and provides a comprehensive and updated overview of the function of TAMs in tumor progression, including facilitating cancer cell proliferation and survival, contributing to angiogenesis, triggering treatment resistance and immunosuppression, promoting tumor metastasis and reprogramming tumor metabolism. Recent advances in therapeutic strategies targeting TAMs were also summarized, including the blockade of TAM recruitment, TAM depletion and modulation of anti-tumor polarization of TAMs, particularly the augmentation of the phagocytic activity of TAMs and enhancement of TAM-mediated delivery of therapeutics, which have hardly ever been summarized by previous reports. In addition, combinations of TAM-targeted and conventional therapies were summarized and described, which may be a novel strategy for comprehensive treatments targeting TAMs.
Despite the strengths of the findings above, there are certain limitations that remain to be addressed. The present review put forward the challenges and perspectives for TAM-targeted therapeutics for various cancers based on the understanding of the whole subject. First, the specific characteristics of TAMs have a significant role in the development of personalized TAM-targeting strategies. Although TAMs are conventionally categorized as the M2 phenotype, they constitute a complex heterogeneous cell group, exhibiting both tumoricidal M1 and pro-tumoral M2 attributes (13,22,37). In addition, how TAMs transform from an anti-tumor phenotype into a tumor-supporting phenotype throughout tumor progression remains incompletely understood. However, the extensive use of sequencing technologies, mass cytometry techniques and metabolomics will contribute to a comprehensive interpretation of the mechanisms underlying the polarization of tumoricidal and pro-tumoral macrophages, localization of macrophage subtypes, phenotype switching of TAMs during tumor progression and the genetic constitution involved in the secretory factors within the TME. Furthermore, a more detailed classification of macrophages and an in-depth illustration of the characteristics of various macrophage subtypes may lead to the development of more appropriate and effective strategies for targeting TAMs. Secondly, given the rapid development of TAM-targeting therapeutics and the extensive evaluation of various antibodies, antagonists or agonists in preclinical and clinical studies, delivering these small molecules into TAMs effectively and selectively while minimizing the off-target effects may be the problem-resolving key. In this context, nanoparticle development offers a promising strategy for drug loading and delivery; however, more research is needed to optimize TAM-targeted cancer treatment, mainly focusing on improving the efficiency and accuracy of nanoparticles and drug delivery. In addition, it is important to address the intricacies of the TME. The TME is a complex and dynamic milieu consisting of multiple stromal cells, ECMs, tumor vasculature and signaling molecules (3). Numerous preclinical studies targeting TAMs overlook the versatility and intricacy of the TME, leading to ineffective therapeutic outcomes in clinical studies. Therefore, digging deep into the roles of various components of the TME and modeling the intricate interactions involved in tumor progression may be the focus of future research. As such, TAM-targeting therapies affect TAMs and renovate and reconstruct the TME, which is expected to improve conventional cancer treatment and lead to favorable clinical results. Ultimately, combining TAM-targeted therapies with immunotherapy, chemotherapy and nanotechnology-based treatments may become a promising trend in the future.
Certain limitations of the present review article should also be mentioned. As tumor-infiltrating myeloid cells affecting tumor progression, the activities of TAMs can be influenced by microenvironmental characteristics such as nutrition availability, hypoxia and fibrosis. In addition, given the complexity of the TME, there is growing awareness that the crosstalk between TAMs and T cells, NK cells and dendritic cells may affect the function of TAMs. Furthermore, increasing evidence has shown that the interaction between microbiota and TAMs can affect immunomodulatory activities. These topics were not described in the present review article and these contents will be discussed in a subsequent article by our group.
In conclusion, this review provides a comprehensive and updated overview of the function of TAMs in tumor progression and summarizes the recent advances in TAM-targeting therapeutic strategies. TAMs represent an attractive and promising target that may innovate the landscape of future cancer treatments; however, numerous obstacles remain to be addressed.
Acknowledgments
Not applicable.
Abbreviations
- TAMs
tumor-associated macrophages
- TME
tumor microenvironment
- TRMs
tissue resident macrophages
- CSF1
colony-stimulating factor 1
- TGF-β
transforming growth factor-β
- IL
interleukin
- CCL
C-C motif chemokine ligand
- CXCL
C-X-C motif chemokine ligand
- LPS
lipopolysaccharide
- IFN-γ
interferon-γ
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- TLRs
Toll-like receptors
- MHC
major histocompatibility complex class
- iNOS
inducible nitric oxide synthase
- TNF-α
tumor necrosis factor-α
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- Th
helper T cells
- Treg
regulatory T cells
- Arg
arginase
- CD163
scavenger receptor
- CD206
mannose receptor
- VEGF
vascular endothelial growth factor
- EMP
erythro-myeloid progenitors
- HSC
hematopoietic stem cell
- EGF
epidermal growth factor
- HIF-1α
hypoxia-inducible factor 1α
- PDGF
platelet-derived growth factor
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- PDAC
pancreatic ductal adenocarcinoma
- CRC
colorectal cancer
- GC
gastric cancer
- ICIs
immune checkpoint inhibitors
- CTLA-4
cytotoxic T lymphocyte antigen 4
- PD-1
programmed cell death protein 1
- LILRB
leukocyte immunoglobulin-like receptor B
- EMT
epithelial-mesenchymal transition
- HCC
hepatocellular carcinoma
- NK cells
natural killer cells
- TSGCT
tenosynovial giant cell tumor
- MDSCs
myeloid-derived suppressor cells
- CAR-T
chimeric antigen receptor T cell
- PTX
paclitaxel
- DAMPs
damage-associated molecular patterns
- MARCO
macrophage receptor with collagenous structure
- SIRPα
signal regulatory protein α
- NA
not available
- NSCLC
non-small cell lung cancer
Funding Statement
The present study was supported by grants from the Key Medical Science and Technology Project of Zhejiang Province (grant no. WKJ-ZJ-2201), the Key Projects of Zhejiang Provincial Science and Technology (grant no. 2022C03099), Zhejiang Provincial Science and Technology Program of Traditional Chinese Medicine (grant nos. GZY-ZJ-KJ-24056 and 2023ZL252), the Zhejiang Provincial Medical and Health Technology Program (grant no. 2023KY517) and the Key Project of Laboratory Research in Hangzhou Medical College (grant no. KYZD2023010).
Availability of data and materials
Not applicable.
Authors' contributions
PS and WJ conceived and designed the study; PS drafted the manuscript; OL, KK, ZJ and JW prepared the figures and contributed to the literature collection and analysis, and to the editing of the manuscript; YW, YM and WJ revised the manuscript and provided critical comments. All authors have read and agreed to the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
References
- 1.Xiao Y, Yu DH. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumari S, Advani D, Sharma S, Ambasta RK, Kumar P. Combinatorial therapy in tumor microenvironment: Where do we stand? Biochim Biophys Acta Rev Cancer. 2021;1876:188585. doi: 10.1016/j.bbcan.2021.188585. [DOI] [PubMed] [Google Scholar]
- 3.Wang HG, Yung MMH, Ngan HY, Chan KKL, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. 2021;22:6560. doi: 10.3390/ijms22126560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian Z, Gong Y, Huang T, Lee CZW, Bian L, Bai Z, Shi H, Zeng Y, Liu C, He J, et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582:571–576. doi: 10.1038/s41586-020-2316-7. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassetta L, Pollard JW. Targeting macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 8.Guo S, Chen X, Guo C, Wang W. Tumor-associated macrophages heterogeneity drives resistance to clinical therapy. Expert Rev Mol Med. 2022;24:e17. doi: 10.1017/erm.2022.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, Ju R, Lu Y, Wang H, Wang L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. 2021;11:2892–2916. doi: 10.7150/thno.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68. doi: 10.1186/s13046-022-02272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Song Y, Du W, Gong L, Chang H, Zhou Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreejit G, Fleetwood AJ, Murphy AJ, Nagareddy PR. Origins and diversity of macrophages in health and disease. Clin Transl Immunology. 2020;9:e1222. doi: 10.1002/cti2.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hourani T, Holden JA, Li W, Lenzo JC, Hadjigol S, O'Brien-Simpson NM. Tumor associated macrophages: Origin, recruitment, phenotypic diversity, and targeting. Front Oncol. 2021;11:788365. doi: 10.3389/fonc.2021.788365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazarov T, Juarez-Carre ño S, Cox N, Geissmann F. Physiology and diseases of tissue-resident macrophages. Nature. 2023;618:698–707. doi: 10.1038/s41586-023-06002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casanova-Acebes M, Dalla E, Leader AM, LeBerichel J, Nikolic J, Morales BM, Brown M, Chang C, Troncoso L, Chen ST, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595:578–584. doi: 10.1038/s41586-021-03651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu X, Li Y, Fan GC. Tissue-resident macrophages in the control of infection and resolution of inflammation. Shock. 2021;55:14–23. doi: 10.1097/SHK.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Zhang X. Pivotal regulators of tissue homeostasis and cancer: Macrophages. Exp Hematol Oncol. 2017;6:23. doi: 10.1186/s40164-017-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filiberti S, Russo M, Lonardi S, Bugatti M, Vermi W, Tournier C, Giurisato E. Self-renewal of acrophages: Tumor-released factors and signaling pathways. Biomedicines. 2022;10:2709. doi: 10.3390/biomedicines10112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giurisato E, Lonardi S, Telfer B, Lussoso S, Risa-Ebrí B, Zhang J, Russo I, Wang J, Santucci A, Finegan KG, et al. Extracellular-regulated protein kinase 5-mediated control of p21 expression promotes macrophage proliferation associated with tumor growth and metastasis. Cancer Res. 2020;80:3319–3330. doi: 10.1158/0008-5472.CAN-19-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins EJ, Cervantes-Silva MP, Timmons GA, O'Siorain JR, Curtis AM, Hurley JM. Post-transcriptional circadian regulation in macrophages organizes temporally distinct immunometabolic states. Genome Res. 2021;31:171–185. doi: 10.1101/gr.263814.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan R, Li S, Geng H, Wang X, Guan Q, Li X, Ren C, Yuan X. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol. 2017;49:30–37. doi: 10.1016/j.intimp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Mantuano NR, Oliveira-Nunes MC, Alisson-Silva F, Dias WB, Todeschini AR. Emerging role of glycosylation in the polarization of tumor-associated macrophages. Pharmacol Res. 2019;146:104285. doi: 10.1016/j.phrs.2019.104285. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Liang YZ, Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. 2022;13:888713. doi: 10.3389/fimmu.2022.888713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YL, Yang F, Huang ZQ, Li YY, Shi HY, Sun Q, Ma Y, Wang Y, Zhang Y, Yang S, et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front Immunol. 2023;14:1199173. doi: 10.3389/fimmu.2023.1199173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Sun J, Zeng Z, Liu Z, Ma M, Zheng Z, He Y, Kang W. Tumor-associated macrophages in gastric cancer: From function and mechanism to application. Clin Transl Med. 2023;13:e1386. doi: 10.1002/ctm2.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8:1596004. doi: 10.1080/2162402X.2019.1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: Potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9:e001341. doi: 10.1136/jitc-2020-001341. Senior Correspondence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao R, Liu C, Xue R, Deng X, Liu L, Song C, Xie J, Tang H, Liu W. Tumor-derived exosomal ENO2 modulates polarization of tumor-associated macrophages through reprogramming glycolysis to promote progression of diffuse large B-cell lymphoma. Int J Biol Sci. 2024;20:848–863. doi: 10.7150/ijbs.91154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Gharib SA, McMahan RS, Eddy WE, Long ME, Parks WC, Aitken ML, Manicone AM. Transcriptional and functional diversity of human macrophage repolarization. J Allergy Clin Immunol. 2019;143:1536–1548. doi: 10.1016/j.jaci.2018.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang S, Kumanogoh A. The spectrum of macrophage activation by immunometabolism. Int Immunol. 2020;32:467–473. doi: 10.1093/intimm/dxaa017. [DOI] [PubMed] [Google Scholar]
- 34.Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cell Mol Biol Lett. 2022;27:83. doi: 10.1186/s11658-022-00384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 37.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Liu R, Yu Q, Dong L, Bi Y, Liu G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019;452:14–22. doi: 10.1016/j.canlet.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Kwak T, Wang F, Deng H, Condamine T, Kumar V, Perego M, Kossenkov A, Montaner LJ, Xu X, Xu W, et al. Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep. 2020;33:108571. doi: 10.1016/j.celrep.2020.108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Park C, Guenthner N, Gurley S, Zhang L, Lubben B, Adebayo O, Bash H, Chen Y, Maksimos M, et al. Tumorassociated macrophages in multiple myeloma: Advances in biology and therapy. J Immunother Cancer. 2022;10:e003975. doi: 10.1136/jitc-2021-003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi F, Sun MH, Zhou Z, Wu L, Zhu Z, Xia SJ, Han BM, Zhao YY, Jing YF, Cui D. Tumor-associated macrophages in direct contact with prostate cancer cells promote malignant proliferation and metastasis through NOTCH1 pathway. Int J Biol Sci. 2022;18:5994–6007. doi: 10.7150/ijbs.73141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao Q, Zeng Z, Guo X, Li X, Wei F, Zhang W, Li X, Chen P, Liang F, Xiang B, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the stat3 activation. Oncogene. 2014;33:2098–2109. doi: 10.1038/onc.2013.161. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Q, Fang Y, Lai Q, Wang S, He C, Li A, Liu S, Yan Q. CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling. J Exp Clin Cancer Res. 2020;39:132. doi: 10.1186/s13046-020-01637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan H, Lin Z, Liu Y, Jiang Y, Liu K, Tu M, Yao N, Qu C, Hong J. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness. Cancer Cell Int. 2020;20:586. doi: 10.1186/s12935-020-01687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azambuja JH, Ludwig N, Yerneni SS, Braganhol E, Whiteside TL. Arginase-1+ exosomes from reprogrammed macrophages promote glioblastoma progression. Int J Mol Sci. 2020;21:3990. doi: 10.3390/ijms21113990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piao H, Fu L, Wang Y, Liu Y, Wang Y, Meng X, Yang D, Xiao X, Zhang J. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J Exp Clin Cancer Res. 2022;41:174. doi: 10.1186/s13046-022-02366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang MA, Won M, Im JY, Kang MJ, Kweon DH, Kim BK. TNF-α secreted from macrophages increases the expression of prometastatic integrin αV in gastric cancer. Int J Mol Sci. 2022;24:376. doi: 10.3390/ijms24010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, Zhang H, Shan B, Zhang C, Duan C. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol. 2020;13:19. doi: 10.1186/s13045-020-00858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, Prenen H, Ghesquière B, Carmeliet P, Mazzone M. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 2016;24:701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Cowman SJ, Fuja DG, Liu XD, Tidwell RSS, Kandula N, Sirohi D, Agarwal AM, Emerson LL, Tripp SR, Mohlman JS, et al. Macrophage HIF-1alpha is an independent prognostic indicator in kidney cancer. Clin Cancer Res. 2020;26:4970–4982. doi: 10.1158/1078-0432.CCR-19-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Do MH, Shi W, Ji L, Ladewig E, Zhang X, Srivastava RM, Capistrano KJ, Edwards C, Malik I, Nixon BG, et al. Reprogramming tumor-associated macrophages to outcompete endovascular endothelial progenitor cells and suppress tumor neoangiogenesis. Immunity. 2023;56:2555–2569. doi: 10.1016/j.immuni.2023.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godet I, Shin YJ, Ju JA, Ye IC, Wang G, Gilkes DM. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun. 2019;10:4862. doi: 10.1038/s41467-019-12412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu M, Liu L, Song Y, Li W, Xu L. Targeting macrophages: A novel treatment strategy in solid tumors. J Transl Mel. 2022;20:586. doi: 10.1186/s12967-022-03813-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu T, Yu S, Zhang J, Wu S. Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers. J Hematol Oncol. 2021;14:181. doi: 10.1186/s13045-021-01198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Zhang Y. Tumor-associated macrophages: From basic research to clinical application. J Hematol Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen JL, Mohamadzadeh M. Macrophages and chemokines as mediators of angiogenesis. Front Physiol. 2013;4:159. doi: 10.3389/fphys.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119. doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, Zhang K, Teng BW, Wu W, Cao P, et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. 2021;29:1226–1238. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan J, Zou Y, Chen S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res. 2019;38:310. doi: 10.1186/s13046-019-1313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii13–viii15. doi: 10.1093/annonc/mdx446. [DOI] [PubMed] [Google Scholar]
- 64.Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–4356. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 65.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan W, Li F, Zhao Z, Zhang Z, Hu J, Zhang Y. Tumor-associated macrophage promotes the survival of cancer cells upon docetaxel chemotherapy via the CSF1/CSF1R-CXCL12/CXCR4 axis in castration-resistant prostate cancer. Genes (Basel) 2021;12:773. doi: 10.3390/genes12050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Chen Y, Hao L, Hou A, Chen X, Li Y, Wang R, Luo P, Ruan Z, Ou J, et al. Macrophages induce resistance to 5-fuorouracil chemotherapy in colorectal cancer through the release of putrescine. Cancer Lett. 2016;381:305–313. doi: 10.1016/j.canlet.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Su P, Jiang L, Zhang Y, Yu T, Kang W, Liu Y, Yu J. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 2022;22:290. doi: 10.1186/s12935-022-02717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, Feng F, Liu Y, Xu W, Li Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, Shlomi T, Gil Z. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 71.Akkari L, Bowman RL, Tessier J, Klemm F, Handgraaf SM, de Groot M, Quail DF, Tillard L, Gadiot J, Huse JT, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12:eaaw7843. doi: 10.1126/scitranslmed.aaw7843. [DOI] [PubMed] [Google Scholar]
- 72.Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S, Jimenez C, Zhang D, Horton J, Reuben JM, McMurray JS, Woodward WA. Blocking interleukin (IL)4and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100:1034–1043. doi: 10.1016/j.ijrobp.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 73.Lee HL, Tsai YC, Pikatan NW, Yeh CT, Yadav VK, Chen MY, Tsai JT. Tumor-associated macrophages affect the tumor microenvironment and radioresistance via the Upregulation of CXCL6/CXCR2 in hepatocellular carcinoma. Biomedicines. 2023;11:2081. doi: 10.3390/biomedicines11072081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Feng Z, Liu J, Li H, Su Q, Zhang J, Huang P, Wang W, Liu J. Polarization of tumor-associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact Mater. 2022;16:359–371. doi: 10.1016/j.bioactmat.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao F, Tian H, Wang Y, Zhang J, Liu F, Fu L. LINC01004-SPI1 axis-activated SIGLEC9 in tumor-associated macrophages induces radioresistance and the formation of immunosuppressive tumor microenvironment in esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2023;72:1835–1851. doi: 10.1007/s00262-022-03364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu X, Shi Y, Dong M, Jiang L, Yang J, Liu Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021;12:818. doi: 10.1038/s41419-021-04087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang YS, Chen M, Nie H, Yuan YY. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111–1122. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren D, Hua Y, Yu B, Ye X, He Z, Li C, Wang J, Mo Y, Wei X, Chen Y, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19:19. doi: 10.1186/s12943-020-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quaranta V, Rainer C, Nielsen SR, Raymant ML, Ahmed MS, Engle DD, Taylor A, Murray T, Campbell F, Palmer DH, et al. Macrophage-derived granulin drives resistance to immune checkpoint inhibition in metastatic pancreatic cancer. Cancer Res. 2018;78:4253–4269. doi: 10.1158/0008-5472.CAN-17-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, Brioschi S, Bugatti M, Omodei AS, Ricci B, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182:886–900.e17. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, Wu F, Zhao S, Shi P, Wang S, Cui D. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 2022;67:49–57. doi: 10.1016/j.cytogfr.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Pich-Bavastro C, Yerly L, Di Domizio J, Tissot-Renaud S, Gilliet M, Kuonen F. Activin A-mediated polarization of cancer-associated fibroblasts and macrophages confers resistance to checkpoint immunotherapy in skin cancer. Clin Cancer Res. 2023;29:3498–3513. doi: 10.1158/1078-0432.CCR-23-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu KX, Joshi S. 'Re-educating' tumor associated macrophages as a novel immunotherapy strategy for neuroblastoma. Front Immunol. 2020;11:1947. doi: 10.3389/fimmu.2020.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 86.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baesselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C (high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 87.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Delivery Rev. 2016;99(Pt B):180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 91.Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, Butler NS, Bruneau J, Shoukry NH, Krawczyk CM, Richer MJ. Interleukin-10 directly inhibits CD8(+) T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity. 2018;48:299–312.e5. doi: 10.1016/j.immuni.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang D, Yang L, Yue D, Cao L, Li L, Wang D, Ping Y, Shen Z, Zheng Y, Wang L, Zhang Y. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019;452:244–253. doi: 10.1016/j.canlet.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 93.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morandi F, Pistoia V. Interactions between HLA-G and HLA-E in physiological and pathological conditions. Front Immunol. 2014;5:394. doi: 10.3389/fimmu.2014.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:31. doi: 10.1186/s13045-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ganesh K, Massagué J. Targeting metastatic cancer. Nat Med. 2021;27:34–44. doi: 10.1038/s41591-020-01195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pastushenko L, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lim GJ, Kang S, Lee JY. Novel invasion indices quantify the feed forward facilitation of tumor invasion by macrophages. Sci Rep. 2020;10:718–727. doi: 10.1038/s41598-020-57517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li X, Chen L, Peng X, Zhan X. Progress of tumor-associated macrophages in the epithelial-mesenchymal transition of tumor. Front Oncol. 2022;12:911410. doi: 10.3389/fonc.2022.911410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu F, Li X, Chen S, Zeng Q, Zhao Y, Luo F. Tumorassociated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med Oncol. 2016;33:17. doi: 10.1007/s12032-016-0729-9. [DOI] [PubMed] [Google Scholar]
- 103.Sun D, Luo T, Dong P, Zhang N, Chen J, Zhang S, Dong L, Janssen HLA, Zhang S. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J Cell Biochem. 2020;121:2828–2838. doi: 10.1002/jcb.29514. [DOI] [PubMed] [Google Scholar]
- 104.Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES, Koh M, Lim HK, Jung J, Park SY, Moon A. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-alpha and MMP-9. Cancer Lett. 2018;437:25–34. doi: 10.1016/j.canlet.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 105.Paolillo M, Schinelli S. Extracellular matrix alterations in metastatic processes. Int J Mol Sci. 2019;20:4947. doi: 10.3390/ijms20194947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10:36. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan Y, Wang M, Zhang Y, Ge S, Zhong F, Xia G, Sun C. Tumor-associated macrophages: A potential target for cancer therapy. Front Oncol. 2021;11:693517. doi: 10.3389/fonc.2021.693517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li JF, Pollard JW. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, Carragher N, Pollard JW. Monocytes differentiate to immune suppressive precursors of metastasis associated macrophages in mouse models of metastatic breast cancer. Front Immunol. 2018;8:2004. doi: 10.3389/fimmu.2017.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 112.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 113.Cao R, Ji H, Yang Y, Cao Y. Collaborative effects between the TNFα-TNFR1-macrophage axis and the VEGF-C-VEGFR3 signaling in lymphangiogenesis and metastasis. Oncoimmunology. 2015;4:e989777. doi: 10.4161/2162402X.2014.989777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, Raviv Z, Merquiol E, Ben-Nun Y, Miller V, Rachman-Tzemah C, et al. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep. 2016;17:1344–1356. doi: 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877–919. doi: 10.1007/s13238-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21:363–381. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 117.Ringel AE, Drijvers JM, Baker GJ, Catozzi A, Garcia-Canaveras JC, Gassaway BM, Miller BC, Juneja VR, Nguyen TH, Joshi S, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–1866. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen D, Zhang X, Li Z, Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics. 2021;11:1016–1030. doi: 10.7150/thno.51777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Netea-Maier RT, Smit JWA, Netea MG. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018;413:102–109. doi: 10.1016/j.canlet.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 120.Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al. Extracellular vesicle-packaged HIF-1α-tabilizing lncRNA from tumor-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21:498–510. doi: 10.1038/s41556-019-0299-0. [DOI] [PubMed] [Google Scholar]
- 121.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 122.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 2019;79:795–806. doi: 10.1158/0008-5472.CAN-18-2545. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai G, Liu R, Gao H, Tao B, Li W, et al. Macrophage-associated PGK1 phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol Cell. 2018;71:201–215.e7. doi: 10.1016/j.molcel.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 125.Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li H, Zhang L, Yu J, Yuan S. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: A positive metabolic feedback loop. Oncotarget. 2017;8:110426–110443. doi: 10.18632/oncotarget.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye H, Zhou Q, Zheng S, Li G, Lin Q, Wei L, Fu Z, Zhang B, Liu Y, Li Z, Chen R. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:453. doi: 10.1038/s41419-018-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ishida Y, Kuninaka Y, Yamamoto Y, Nosaka M, Kimura A, Furukawa F, Mukaida N, Kondo T. Pivotal involvement of the CX3CL1-CX3CR1 axis for the recruitment of M2 tumor-associated macrophages in skin carcinogenesis. J Invest Dermatol. 2020;140:1951–1961. doi: 10.1016/j.jid.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 128.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Kielbassa K, Vegna S, Ramirez C, Akkari L. Understanding the origin and diversity of macrophages to tailor their targeting in solid cancers. Front Immunol. 2019;10:2215. doi: 10.3389/fimmu.2019.02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fujiwara T, Yakoub MA, Chandler A, Christ AB, Yang G, Ouerfelli O, Rajasekhar VK, Yoshida A, Kondo H, Hata T, et al. CSF1/CSF1R signaling inhibitor pexidartinib (PLX3397) reprograms tumor-associated macrophages and stimulates T-cell infltration in the sarcoma microenvironment. Mol Cancer Ther. 2021;20:1388–1399. doi: 10.1158/1535-7163.MCT-20-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wesolowski R, Sharma N, Reebel L, Rodal MB, Peck A, West BL, Marimuthu A, Severson P, Karlin DA, Dowlati A, et al. Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther Adv Med Oncol. 2019;11:1758835919854238. doi: 10.1177/1758835919854238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, Alcindor T, Ganjoo K, Martín-Broto J, Ryan CW, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): A randomised phase 3 trial. Lancet. 2019;394:478–487. doi: 10.1016/S0140-6736(19)30764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58–66. doi: 10.1016/j.pharmthera.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 135.Thongchot S, Duangkaew S, Yotchai W, Maungsomboon S, Phimolsarnti R, Asavamongkolkul A, Thuwajit P, Thuwajit C, Chandhanayingyong C. Novel CSF1R-positive tenosynovial giant cell tumor cell lines and their pexidartinib (PLX3397) and sotuletinib (BLZ945)-induced apoptosis. Hum Cell. 2023;36:456–467. doi: 10.1007/s13577-022-00823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnson M, Dudek AZ, Sukari A, Call J, Kunk PR, Lewis K, Gainor JF, Sarantopoulos J, Lee P, Golden A, et al. ARRY-382 in combination with pembrolizumab in patients with advanced solid tumors: Results from a phase 1b/2 study. Clin Cancer Res. 2022;28:2517–2526. doi: 10.1158/1078-0432.CCR-21-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuemmel S, Campone M, Loirat D, Lopez RL, Beck JT, De Laurentiis M, Im SA, Kim SB, Kwong A, Steger GG, et al. A randomized phase II study of anti-CSF1 monoclonal antibody lacnotuzumab (MCS110) combined with gemcitabine and carboplatin in advanced triple-negative breast cancer. Clin Cancer Res. 2022;28:106–115. doi: 10.1158/1078-0432.CCR-20-3955. [DOI] [PubMed] [Google Scholar]
- 138.Autio KA, Klebanoff CA, Schaer D, Kauh JSW, Slovin SF, Adamow M, Blinder VS, Brahmachary M, Carlsen M, Comen E, et al. Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: A phase I study. Clin Cancer Res. 2020;26:5609–5620. doi: 10.1158/1078-0432.CCR-20-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Uddin MN, Wang XS. Identifcation of key tumor stroma associated transcriptional. signatures correlated with survival prognosis and tumor progression in breast cancer. Breast Cancer. 2022;29:541–561. doi: 10.1007/s12282-022-01332-6. [DOI] [PubMed] [Google Scholar]
- 140.Kadomoto S, Izumi K, Mizokami A. Roles of CCL2-CCR2 axis in the tumor microenvironment. Int J Mol Sci. 2021;22:8530. doi: 10.3390/ijms22168530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020;18:82. doi: 10.1186/s12964-020-00589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 143.Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F, et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 144.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalsko TA, Takimoto C, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 145.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M, Jain RK, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci USA. 2020;117:1129–1138. doi: 10.1073/pnas.1910856117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sleightholm RL, Neilsen BK, Li J, Steele MM, Singh RK, Hollingsworth MA, Oupicky D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol Ther. 2017;179:158–170. doi: 10.1016/j.pharmthera.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 147.Tang C, Lei X, Xiong L, Hu Z, Tang B. HMGA1B/2 transcriptionally activated-POU1F1 facilitates gastric carcinoma metastasis via CXCL12/CXCR4 axis-mediated macrophage polarization. Cell Death Dis. 2021;12:422. doi: 10.1038/s41419-021-03703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi T, Li X, Zheng J, Duan Z, Ooi YY, Gao Y, Wang Q, Yang J, Wang L, Yao L. Increased SPRY1 expression activates NF-κB signaling and promotes pancreatic cancer progression by recruiting neutrophils and macrophages through CXCL12-CXCR4 axis. Cell Oncol (Dordr) 2023;46:969–985. doi: 10.1007/s13402-023-00791-z. [DOI] [PubMed] [Google Scholar]
- 149.Choueiri TK, Atkins MB, Rose TL, Alter RS, Ju Y, Niland K, Wang Y, Arbeit R, Parasuraman S, Gan L, McDermott DF. A phase 1b trial of the CXCR4 inhibitor mavorixafor and nivolumab in advanced renal cell carcinoma patients with no prior response to nivolumab monotherapy. Invest New Drugs. 2021;39:1019–1027. doi: 10.1007/s10637-020-01058-2. [DOI] [PubMed] [Google Scholar]
- 150.Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878–885. doi: 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- 151.Jiang Y, Liang Y, Li L, Zhou L, Cheng W, Yang X, Yang X, Qi H, Yu J, Jeong LS, et al. Targeting neddylation inhibits intravascular survival and extravasation of cancer cells to prevent lung-cancer metastasis. Cell Biol Toxicol. 2019;35:233–245. doi: 10.1007/s10565-019-09472-w. [DOI] [PubMed] [Google Scholar]
- 152.Zhou L, Jiang Y, Liu X, Li L, Yang X, Dong C, Liu X, Lin Y, Li Y, Yu J, et al. Promotion of tumor-associated macrophages infltration by elevated neddylation pathway via NF-κB-CCL2 signaling in lung cancer. Oncogene. 2019;38:5792–5804. doi: 10.1038/s41388-019-0840-4. [DOI] [PubMed] [Google Scholar]
- 153.Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, Shin MG, Chung IJ, Hong Y, Bom HS, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 154.Wu X, Schulte BC, Zhou Y, Haribhai D, Mackinnon AC, Plaza JA, Williams CB, Hwang ST. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. J Invest Dermatol. 2014;134:2814–2822. doi: 10.1038/jid.2014.206. [DOI] [PubMed] [Google Scholar]
- 155.Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I, Schorn F, Marinaccio C, Murgia D, Daga A, et al. A novel liposomal Clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: anti-angiogenic and anti-tumor effects. J Control Release. 2016;223:165–177. doi: 10.1016/j.jconrel.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 156.Zang X, Zhang X, Hu H, Qiao M, Zhao X, Deng Y, Chen D. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol Pharm. 2019;16:2249–2258. doi: 10.1021/acs.molpharmaceut.9b00261. [DOI] [PubMed] [Google Scholar]
- 157.Cao Y, Qiao B, Chen Q, Xie Z, Dou X, Xu L, Ran H, Zhang L, Wang Z. Tumor microenvironment remodeling via targeted depletion of M2-like tumor-associated macrophages for cancer immunotherapy. Acta Biomater. 2023;160:239–251. doi: 10.1016/j.actbio.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 158.Wang S, Huang M, Chen M, Sun Z, Jiao Y, Ye G, Pan J, Ye W, Zhao J, Zhang D. Zoledronic acid and thymosin α1 elicit antitumor immunity against prostate cancer by enhancing tumor inflammation and cytotoxic T cells. J Immunother Cancer. 2023;11:e006381. doi: 10.1136/jitc-2022-006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grignani G, D'Ambrosio L, Pignochino Y, Palmerini E, Zucchetti M, Boccone P, Aliberti S, Stacchiotti S, Bertulli R, Piana R, et al. Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): An open-label, phase 1b study from the Italian Sarcoma Group. Lancet Oncol. 2018;19:1360–1371. doi: 10.1016/S1470-2045(18)30438-8. [DOI] [PubMed] [Google Scholar]
- 160.Povo-Retana A, Fariñas M, Landauro-Vera R, Mojena M, Alvarez-Lucena C, Fernández-Moreno MA, Castrillo A, de la Rosa Medina JV, Sánchez-García S, Foguet C, et al. Immunometabolic actions of trabectedin and lurbinectedin on human macrophages: Relevance for their anti-tumor activity. Front Immunol. 2023;14:1211068. doi: 10.3389/fimmu.2023.1211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 162.D'Incalci M, Zambelli A. Trabectedin for the treatment of breast cancer. Expert Opin Investig Drugs. 2016;25:105–115. doi: 10.1517/13543784.2016.1124086. [DOI] [PubMed] [Google Scholar]
- 163.Carminati L, Pinessi D, Borsotti P, Minoli L, Giavazzi R, D'Incalci M, Belotti D, Taraboletti G. Antimetastatic and antiangiogenic activity of trabectedin in cutaneous melanoma. Carcinogenesis. 2019;40:303–312. doi: 10.1093/carcin/bgy177. [DOI] [PubMed] [Google Scholar]
- 164.Lee C, Jeong H, Bae Y, Shin K, Kang S, Kim H, Oh J, Bae H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J Immunother Cancer. 2019;7:147. doi: 10.1186/s40425-019-0610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sánchez-Paulete AR, Mateus-Tique J, Mollaoglu G, Nielsen SR, Marks A, Lakshmi A, Khan JA, Wilk CM, Pia L, Baccarini A, et al. Targeting macrophages with CAR T cells delays solid tumor progression and enhances antitumor immunity. Cancer Immunol Res. 2022;10:1354–1369. doi: 10.1158/2326-6066.CIR-21-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yanai H, Hangai S, Taniguchi T. Damage-associated molecular patterns and Toll-like receptors in the tumor immune microenvironment. Int Immunol. 2021;33:841–846. doi: 10.1093/intimm/dxab050. [DOI] [PubMed] [Google Scholar]
- 167.Rameshbabu S, Labadie BW, Argulian A, Patnaik A. Targeting innate immunity in cancer therapy. Vaccines (Basel) 2021;9:138. doi: 10.3390/vaccines9020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Urban-Wojciuk Z, Khan MM, Oyler BL, Fahraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR, Goodlett DR. The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol. 2019;10:2388. doi: 10.3389/fimmu.2019.02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK, Agrewala JN. TLR-3 stimulation skews M2 macrophages to M1 through IFN-αβ signaling and restricts tumor progression. Front Immunol. 2018;9:1650. doi: 10.3389/fimmu.2018.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McGowan DC. Latest advances in small molecule TLR7/8 agonist drug research. Curr Top Med Chem. 2019;19:2228–2238. doi: 10.2174/1568026619666191009165418. [DOI] [PubMed] [Google Scholar]
- 171.Wang Z, Gao Y, He L, Sun S, Xia T, Hu L, Yao L, Wang L, Li D, Shi H, Liao X. Structure-based design of highly potent toll-like receptor 7/8 dual agonists for cancer immunotherapy. J Med Chem. 2021;64:7507–7532. doi: 10.1021/acs.jmedchem.1c00179. [DOI] [PubMed] [Google Scholar]
- 172.Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173–185. doi: 10.1016/j.canlet.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 173.Figueiredo P, Lepland A, Scodeller P, Fontana F, Torrieri G, Tiboni M, Shahbazi MA, Casettari L, Kostiainen MA, Hirvonen J, et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2021;133:231–243. doi: 10.1016/j.actbio.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 174.Mullins SR, Vasilakos JP, Deschler K, Grigsby I, Gillis P, John J, Elder MJ, Swales J, Timosenko E, Cooper Z, et al. Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies. J Immunother Cancer. 2019;7:244. doi: 10.1186/s40425-019-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smith DA, Conkling P, Richards DA, Nemunaitis JJ, Boyd TE, Mita AC, de La Bourdonnaye G, Wages D, Bexon AS. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother. 2014;63:787–796. doi: 10.1007/s00262-014-1547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ji N, Mukherjee N, Morales EE, Tomasini ME, Hurez V, Curiel TJ, Abate G, Hoft DF, Zhao XR, Gelfond J, et al. Percutaneous BCG enhances innate effector antitumor cytotoxicity during treatment of bladder cancer: A translational clinical trial. Oncoimmunology. 2019;8:1614857. doi: 10.1080/2162402X.2019.1614857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ji N, Mukherjee N, Reyes RM, Gelfond J, Javors M, Meeks JJ, McConey DJ, Shu ZJ, Ramamurthy C, Dennett R, et al. Rapamycin enhances BCG-specifc γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: A randomized, double-blind study. J Immunother Cancer. 2021;9:e001941. doi: 10.1136/jitc-2020-001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Takada YK, Yu J, Shimoda M, Takada Y. Integrin binding to the trimeric interface of CD40L plays a critical role in CD40/CD40L signaling. J Immunol. 2019;203:1383–1391. doi: 10.4049/jimmunol.1801630. [DOI] [PubMed] [Google Scholar]
- 179.Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med. 2020;71:47–58. doi: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 180.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nanda S. Cancer: CD40 agonists-a promising new treatment for pancreatic cancer? Nat Rev Gastroenterol Hepatol. 2011;8:300. doi: 10.1038/nrgastro.2011.65. [DOI] [PubMed] [Google Scholar]
- 182.Hoves S, Ooi CH, Wolter C, Sade H, Bissinger S, Schmittnaegel M, Ast O, Giusti AM, Wartha K, Runza V, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 2018;215:859–876. doi: 10.1084/jem.20171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, Quigley M, Verona RI. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 184.Baumann D, Hägele T, Mochayedi J, Drebant J, Vent C, Blobner S, Noll JH, Nickel I, Schumacher C, Boos SL, et al. Proimmunogenic impact of MEK inhibition synergizes with agonist anti-CD40 immunostimulatory antibodies in tumor therapy. Nat Commun. 2020;11:2176. doi: 10.1038/s41467-020-15979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Leblond MM, Tillé L, Nassiri S, Gilfillan CB, Imbratta C, Schmittnaegel M, Ries CH, Speiser DE, Verdeil G. CD40 agonist restores the antitumor efficacy of anti-PD1 therapy in muscle-invasive bladder cancer in an IFN I/II-mediated manner. Cancer Immunol Res. 2020;8:1180–1192. doi: 10.1158/2326-6066.CIR-19-0826. [DOI] [PubMed] [Google Scholar]
- 186.Djureinovic D, Wang M, Kluger HM. Agonistic CD40 antibodies in cancer treatment. Cancers (Basel) 2021;13:1302. doi: 10.3390/cancers13061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 188.Ding L, Qian J, Yu X, Wu Q, Mao J, Liu X, Wang Y, Guo D, Su R, Xie H, et al. Blocking MARCO+ tumor-associated macrophages improves anti-PD-L1 therapy of hepatocellular carcinoma by promoting the activation of STING-IFN type I pathway. Cancer Lett. 2024;582:216568. doi: 10.1016/j.canlet.2023.216568. [DOI] [PubMed] [Google Scholar]
- 189.Dong Q, Zhang S, Zhang H, Sun J, Lu J, Wang G, Wang X. MARCO is a potential prognostic and immunotherapy biomarker. Int Immunopharmacol. 2023;116:109783. doi: 10.1016/j.intimp.2023.109783. [DOI] [PubMed] [Google Scholar]
- 190.Eisinger S, Sarhan D, Boura VF, Ibarlucea-Benitez I, Tyystjärvi S, Oliynyk G, Arsenian-Henriksson M, Lane D, Wikström SL, Kiessling R, et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci USA. 2020;117:32005–32016. doi: 10.1073/pnas.2015343117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masetti M, Carriero R, Portale F, Marelli G, Morina N, Pandini M, Iovino M, Partini B, Erreni M, Ponzetta A, et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J Exp Med. 2022;219:e20210564. doi: 10.1084/jem.20210564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toma VA, Tigu AB, Farcaș AD, Sevastre B, Taulescu M, Gherman AMR, Roman I, Fischer-Fodor E, Pârvu M. New aspects towards a molecular understanding of the allicin immunostimulatory mechanism via Colec12, MARCO, and SCARB1 receptors. Int J Mol Sci. 2019;20:3627. doi: 10.3390/ijms20153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu B, Li L, Xiu B, Zhang Y, Zhou Y, Yang Q, Qi W, Wu W, Wang L, Gu J, Xie J. C-terminus of heat shock protein 60 can activate macrophages by lectin-like oxidized low-density lipoprotein receptor 1. Biochem Biophys Res Commun. 2019;508:1113–1119. doi: 10.1016/j.bbrc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 194.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, Schmid MC, Sun P, Mose E, Bouvet M, et al. Macrophage PI3Kγ drives pancreatic ductal adenocarcinoma progression. Cancer Discov. 2016;6:870–885. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hong DS, Postow M, Chmielowski B, Sullivan R, Patnaik A, Cohen EEW, Shapiro G, Steuer C, Gutierrez M, Yeckes-Rodin H, et al. Eganelisib, a first-in-class PI3Kγ inhibitor, in patients with advanced solid tumors: results of the phase 1/1b MARIO-1 trial. Clin Cancer Res. 2023;29:2210–2219. doi: 10.1158/1078-0432.CCR-22-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Giurisato E, Xu Q, Lonardi S, Telfer B, Russo I, Pearson A, Finegan KG, Wang W, Wang J, Gray NS, et al. Myeloid ERK5 deficiency suppresses tumor growth by blocking protumor macrophage polarization via STAT3 inhibition. Proc Natl Acad Sci USA. 2018;115:E2801–E2810. doi: 10.1073/pnas.1707929115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, Hoves S, Ries CH, Ooi CH, De Palma M. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat Cell Biol. 2016;18:790–802. doi: 10.1038/ncb3371. [DOI] [PubMed] [Google Scholar]
- 199.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y, Zhao C, Liu Y, Wang C, Jiang H, Hu Y, Wu J. Recent advances of tumor therapy based on the CD47-SIRPα axis. Mol Pharm. 2022;19:1273–1293. doi: 10.1021/acs.molpharmaceut.2c00073. [DOI] [PubMed] [Google Scholar]
- 201.Grottoli M, Carrega P, Zullo L, Dellepiane C, Rossi G, Parisi F, Barletta G, Zinoli L, Coco S, Alama A, et al. Immune checkpoint blockade: A strategy to unleash the potential of natural killer cells in the anti-cancer therapy. Cancers (Basel) 2022;14:5046. doi: 10.3390/cancers14205046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47: Role in the immune system and application to cancer therapy. Cell Oncol (Dordr) 2020;43:19–30. doi: 10.1007/s13402-019-00469-5. [DOI] [PubMed] [Google Scholar]
- 203.Eladl E, Tremblay-LeMay R, Rastgoo N, Musani R, Chen W, Liu A, Chang H. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13:96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang X, Chen W, Fan J, Wang S, Xian Z, Luan J, Li Y, Wang Y, Nan Y, Luo M, et al. Disrupting CD47-SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. 2018;39:689–699. doi: 10.1093/carcin/bgy041. [DOI] [PubMed] [Google Scholar]
- 205.Xiao Z, Chung H, Banan B, Manning PT, Ott KC, Lin S, Capoccia BJ, Subramanian V, Hiebsch RR, Upadhya GA, et al. Antibody mediated therapy targeting CD47 inhibits tumor progression of hepatocellular carcinoma. Cancer Lett. 2015;360:302–309. doi: 10.1016/j.canlet.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sikic BI, Lakhani N, Patnaik A, Shah SA, Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S, Papadopoulos K, et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Su S, Zhao J, Xing Y, Zhang X, Liu J, Ouyang Q, Chen J, Su F, Liu Q, Song E. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell. 2018;175:442–457.e23. doi: 10.1016/j.cell.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 208.Liu J, Xavy S, Mihardja S, Chen S, Sompalli K, Feng D, Choi T, Agoram B, Majeti R, Weissman IL, Volkmer JP. Targeting macrophage checkpoint inhibitor SIRPα for anticancer therapy. JCI Insight. 2020;5:e134728. doi: 10.1172/jci.insight.134728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lakhani NJ, Chow LQM, Gainor JF, LoRusso P, Lee KW, Chung HC, Lee J, Bang YJ, Hodi FS, Kim WS, et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): A first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1740–1751. doi: 10.1016/S1470-2045(21)00584-2. [DOI] [PubMed] [Google Scholar]
- 210.Oronsky B, Carter C, Reid T, Brinkhaus F, Knox SJ. Just eat it: A review of CD47 and SIRP-α antagonism. Semin Oncol. 2020;47:117–124. doi: 10.1053/j.seminoncol.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 211.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen HM, van der Touw W, Wang YS, Kang K, Mai S, Zhang J, Alsina-Beauchamp D, Duty JA, Mungamuri SK, Zhang B, et al. Blocking immunoinhibitory receptor LILRB2 reprograms tumor associated myeloid cells and promotes antitumor immunity. J Clin Invest. 2018;128:5647–5662. doi: 10.1172/JCI97570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Siu LL, Wang D, Hilton J, Geva R, Rasco D, Perets R, Abraham AK, Wilson DC, Markensohn JF, Lunceford J, et al. First-in-class anti-immunoglobulin-like transcript 4 myeloid-specific antibody MK-4830 abrogates a PD-1 resistance mechanism in patients with advanced solid tumors. Clin Cancer Res. 2022;28:57–70. doi: 10.1158/1078-0432.CCR-21-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32:e2002054. doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 216.Wang N, Wang S, Wang X, Zheng Y, Yang B, Zhang J, Pan B, Gao J, Wang Z. Research trends in pharmacological modulation of tumor-associated macrophages. Clin Transl Med. 2021;11:e288. doi: 10.1002/ctm2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, Sun Y, Kang F, Yang Z, He L, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32:e2004853. doi: 10.1002/adma.202004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X, Liu L, Zhang Y, Lu Y, Chen X, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18:1908–1915. doi: 10.1021/acs.nanolett.7b05263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhu S, Li S, Yi M, Li N, Wu K. Roles of microvesicles in tumor progression and clinical applications. Int J Nanomedicine. 2021;16:7071–7090. doi: 10.2147/IJN.S325448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moradi-Chaleshtori M, Bandehpour M, Heidari N, Mohammadi-Yeganeh S, Mahmoud Hashemi S. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int Immunopharmacol. 2021;90:107198. doi: 10.1016/j.intimp.2020.107198. [DOI] [PubMed] [Google Scholar]
- 221.Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 222.Salvagno C, Ciampricotti M, Tuit S, Hau CS, van Weverwijk A, Coffelt SB, Kersten K, Vrijland K, Kos K, Ulas T, et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol. 2019;21:511–521. doi: 10.1038/s41556-019-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lapenna A, De Palma M, Lewis CE. Perivascular macrophages in health and disease. Nat Rev Immunol. 2018;18:689–702. doi: 10.1038/s41577-018-0056-9. [DOI] [PubMed] [Google Scholar]
- 224.De Palma M, Lewis CE. Macrophages limit chemotherapy. Cancer Discov. 2011;1:54–67. [Google Scholar]
- 225.Duhamel M, Rose M, Rodet F, Murgoci AN, Zografidou L, Régnier-Vigouroux A, Vanden Abeele F, Kobeissy F, Nataf S, Pays L, et al. Paclitaxel treatment and PC1/3 knockdown in macrophages is a promising anti-glioma strategy as revealed by proteomics and cytotoxicity studies. Mol Cell Proteomics. 2018;17:1126–1143. doi: 10.1074/mcp.RA117.000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.P ra kash H, K lug F, Nadella V, Ma zumda r V, Schmitz-Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: Lesson from insulinoma. Carcinogenesis. 2016;37:301–313. doi: 10.1093/carcin/bgw007. [DOI] [PubMed] [Google Scholar]
- 227.Choi SH, Kim AR, Nam JK, Kim JM, Kim JY, Seo HR, Lee HJ, Cho J, Lee YJ. Tumor-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6+ cancer cell and macrophage polarization. Nat Commun. 2018;9:5108. doi: 10.1038/s41467-018-07470-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Genard G, Lucas S, Michiels C. Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemoand immunotherapies. Front Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Brown JM, Thomas R, Nagpal S, Recht L. Macrophage exclusion after radiation therapy (MERT): A new and efective way to increase the therapeutic ratio of radiotherapy. Radiother Oncol. 2019;144:159–164. doi: 10.1016/j.radonc.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 230.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 231.Stary V, Wolf B, Unterleuthner D, List J, Talic M, Laengle J, Beer A, Strobl J, Stary G, Dolznig H, Bergmann M. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J Immunother Cancer. 2020;8:e000667. doi: 10.1136/jitc-2020-000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lv M, Zhuang X, Shao S, Li X, Cheng Y, Wu D, Wang X, Qiao T. Myeloid-derived suppressor cells and CD68+CD163+ M2-like macrophages as therapeutic response biomarkers are associated with plasma inflammatory cytokines: A preliminary study for non-small cell lung cancer patients in radiotherapy. J Immunol Res. 2022;2022:3621496. doi: 10.1155/2022/3621496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 235.Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, Sridhara R, Goldberg KB, Chuk MK, Chi DC, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229–239. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75. doi: 10.1038/s41392-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wu X, Singh R, Hsu DK, Zhou Y, Yu S, Han D, Shi ZR, Huynh M, Campbell JJ, Hwang ST. A small molecule CCR2 antagonist depletes tumor macrophages and synergizes with anti-PD1 in a murine model of cutaneous T cell lymphoma (CTCL) J Invest Dermatol. 2020;140:1390–1400.e4. doi: 10.1016/j.jid.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 238.Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, et al. Blocking the CCL2-CCR2 axis using CCL2-neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther. 2017;16:312–322. doi: 10.1158/1535-7163.MCT-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H. A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, Li CW, Lim SO, Sheng YY, Zhang Y, et al. Disruption of tumor-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signaling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653–1666. doi: 10.1136/gutjnl-2019-318419. [DOI] [PubMed] [Google Scholar]
- 241.Li Z, Ding Y, Liu J, Wang J, Mo F, Wang Y, Chen-Mayfield TJ, Sondel PM, Hong S, Hu Q. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. 2022;13:1845. doi: 10.1038/s41467-022-29388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.