Abstract
Objectives
To assess the daily function of children with anti-N-methyl-d-aspartate receptor encephalitis (NMDARe) after a minimal follow-up of 5 years.
Methods
Patients 18 years and younger by the time of disease onset, whose serum and CSF were studied in our center between 2013 and 2017, were included in the study. Patients' daily life function was assessed by their physicians using a 15-domain question format (Liverpool Outcome Score).
Results
Of 76 patients, 8 (11%) died and 68 were followed for a mean of 7.1 years (SD 1.5 years, range: 5.0–10.1). Three outcome patterns were identified: full recovery (50; 73%); behavioral and school/working deficits (12; 18%); and multidomain deficits (6; 9%) involving self-care ability, behavioral-cognitive impairment, and seizures. Younger age of disease onset was significantly associated with multidomain deficits (OR 1.6, 95% CI 1.02–2.4, p = 0.04), particularly in children younger than 6 years, among whom 8 of 23 (35%) remained sociofamiliar dependent.
Discussion
After a minimal follow-up of 5 years, most children with NMDARe had substantial or full functional recovery, but approximately one-fifth remained with behavioral and school/working deficits. The younger the patient at disease onset, the more probable it was to remain with multidomain deficits and dependent on sociofamiliar support.
Introduction
Most patients with anti-N-methyl-d-aspartate receptor encephalitis (NMDARe) recover from motor disabilities,1 but residual cognitive deficits are frequent.2,3 Previous reports examining the short and middle-term outcomes of children with NMDARe found that approximately 45% remained with cognitive deficits and 36%–70% with schooling or academic difficulties.2,3 However, the long-term prognosis regarding how disease burden affects patient's integrated daily function has not been explored. A key issue in disease burden is to assess how the disability is likely to make a child dependent on others. To this end, we have applied an easy to use 15-question format score (Liverpool Outcome Score [LOS])4 to assess pediatric patients with NMDARe after a minimal follow-up of 5 years.
Methods
Patients 18 years and younger by the time of NMDARe onset, whose serum or CSF was studied in our center between January 1, 2013, and December 31, 2017, and with follow-up ≥5 years were included in the study. Patients with NMDARe after herpes simplex encephalitis were excluded.
Acute-phase symptoms were reviewed from information obtained by the time of NMDARe diagnosis. Primary care physicians were contacted with several rounds of emails and invited to provide follow-up information through a REDCap system.5
For each patient, a clinical information questionnaire (symptoms, treatment, tumor association, relapses) and LOS4 were obtained. LOS domains are explored with 15 questions that assess sitting, standing up, walking, raising arms over the head, picking up objects, hearing, bladder/bowel control, feeding, dressing, recognizing people, being left alone, seizure, school/working, behavior, and speech/communication (eTable 1).4
Statistics
Demographics and acute-phase symptoms of included and lost to follow-up patients were compared using t-test and Fisher exact test. Outcome patterns were clustered based on the 15 LOS domain scores using the K-modes analysis, an unbiased nonparametric approach to deriving clusters from categorical data.6 Number of clusters was determined by the elbow method.7 The associations between outcome patterns and age of disease onset, sex, number of core symptoms of NMDARe (eMethods),8 admission to intensive care, modified Rankin scale (mRS) at nadir (eMethods), use of second-line immunotherapy (rituximab, cyclophosphamide), and relapses were examined by multinomial logistic regression analysis. Age was first examined as a continuous variable and validated with a cutoff (6 years). The significance level was set at p < 0.05 for 2-sided hypothesis tests. Statistical analyses were performed using GraphPad Prism 10 and R software 4.0.2, package “klaR”.9
Standard Protocol Approvals and Consents
This study was approved by the Ethical Board of Hospital Clínic de Barcelona. Written consents were obtained from patients or proxies.
Data Availability
Anonymized data are available by request from qualified investigators.
Results
Of 217 children diagnosed with NMDARe between 2013 and 2017, the questionnaires of 76 were returned by their physicians; for the remaining patients, their physicians could not be reached or did not answer (n = 116) or the follow-up was <5 years (n = 25). During the acute stage, the clinical features of patients with follow-up were similar to those without follow-up (eTable 2).
Among 76 patients with follow-up, 32 (42%) were from middle/south America, 20 (26%) from Asia, 17 (23%) from Europe, and 7 (9%) from North America. The mean age by the time of disease onset was 9.7 years (range 1.9–18.1 years), and 52 (68%) were female. Information about treatment was available from 73 patients (96%); of these, 32 (44%) received first-line immunotherapy only (steroids, immunoglobulin, or plasma exchange), 39 (53%) first and second-line immunotherapy (24 rituximab, 5 cyclophosphamide, 10 both), and 2 (3%) did not receive immunotherapy. Eight of 76 patients (11%) died, 5 during the acute stage and 3 later (1 neuroblastoma, 1 immature teratoma, and 1 severe combined immunodeficiency). The mean follow-up of the 68 survivors was 7.1 years (SD: 1.5 years, range: 5.0–10.1), and 6 (9%) had a relapse of NMDARe.
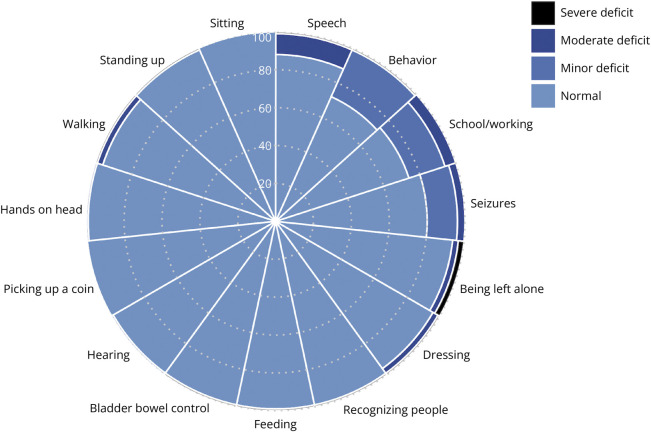
The LOS domain scores of these 68 patients demonstrated well-preserved motor function and self-care ability, but the domains related to cognitive deficits were frequently involved, including behavioral problems in 19 patients (28%), school/working difficulties in 18 (26%), and impairment of speech/communication in 8 (12%) (Figure 1). At the last visit, 3 patients (4%) had seizures and 14 (20%) remained on antiseizure medication.
Figure 1. Functional Outcomes Using the Liverpool Outcome Score (LOS).
Percentages of normal function and deficits according to the 15 LOS domain scores. Domains with deficits affecting more than 1 patient include (1) walking: severe 1 (1%), moderate 2 (3%); (2) dressing: severe 1 (1%), moderate 2 (3%); (3) being left alone: severe 2 (3%), moderate 2 (3%); (4) seizures: moderate 3 (4%), minor 11 (16%); (5) school/working: moderate 4 (6%), minor 14 (20%); (6) behavior: severe 1 (1%), minor 18 (27%) including inattention/impulsivity 10, mood problem 7, anxiety 3, autism spectrum disorder 2, depression 1, and unspecified psychiatric disorder 1; and (7) speech: severe 1 (1%), moderate 7 (11%).
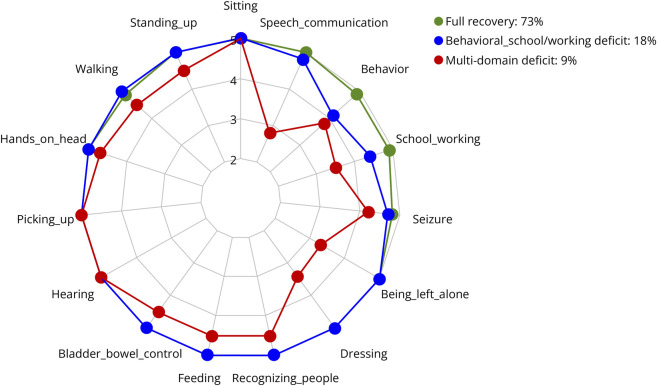
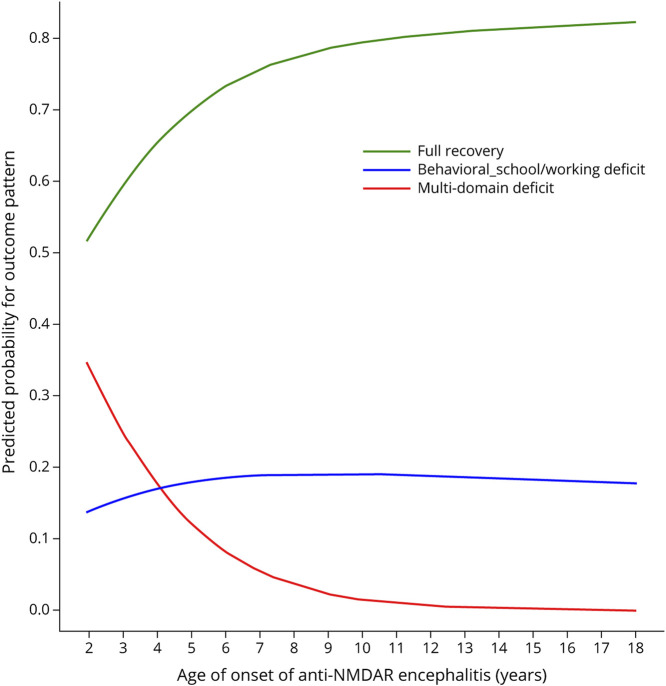
The K-modes analysis identified 3 patterns of long-term outcome according to the 15 LOS domain scores (Figure 2). The first included 50 patients (73%) who had total or substantial recovery of all paradigms; the second, 12 patients (18%) who had minor deficits in behavior and school/working performance; and the third, 6 patients (9%) with extensive multidomain deficits (eTable 3). Younger age of disease onset was associated with multidomain deficits (odds ratio [OR] 1.6, 95% confidence interval 1.02–2.4, p = 0.04) (eTable 4 and Figure 3) particularly in children younger than 6 years (OR 11.7, 1.3–108.6, p = 0.03). However, behavioral and school/working deficits were invariant to age (OR 1.01, 0.9–1.1, p = 0.8). Among the 23 children younger than 6 years, 8 (35%) became sociofamiliar dependent including 5 with multidomain deficits and 3 with behavioral-school/working problems. Sex, number of core symptoms, admission to intensive care, mRS at nadir, second-line immunotherapy, and clinical relapse were not associated with outcome patterns (eTable 4).
Figure 2. Outcome Patterns Identified by the K-Modes Analysis.
Radar plot showing the mean scores of the 15 Liverpool Outcome Score (LOS) domain questions according to the 3 indicated patterns. 5 = full recovery, 4 = minor deficit, 3 = moderate deficit, and 2 = severe deficit.
Figure 3. Predicted Probabilities for Outcome Patterns by Age of Onset of Anti-NMDA Receptor Encephalitis.
The probability for developing multidomain deficits (red) increases as the age of disease onset decreases; children younger than 6 years show higher probability. The probability for full recovery (green) approximates 80% in schoolers and >80% in teenagers. The probability for behavioral and school/working deficits (blue) remains consistent at ∼20% across age.
Discussion
In this long-term follow-up study, most children with NMDARe had functional recovery, but one-fifth remained with behavioral alterations and school/working deficits and one-tenth had multidomain alterations. Children younger than 6 years by the time of disease onset were more likely to have multidomain deficits.
Long-term observational studies are important in assessing the outcome of pediatric NMDARe because functional evaluations are difficult at younger age. A study that included neuropsychological assessment 2.5 years after NMDARe onset excluded patients younger than 4 years at follow-up because of limitations in assessment tools.2 Our findings confirm the frequent presence of protracted cognitive deficits, which was lower compared with other reports.2,3 A study of 81 children with a 3-year follow-up described academic difficulties in 70% and cognitive deficits in 45%.3 Another study showed impaired sustained attention and school difficulties in 63% and 36% of children, respectively, after a follow-up of 2.5 years.2 The lower prevalence of cognitive-behavioral deficits in our study might reflect the longer follow-up,10,11 and the absence of neuropsychological evaluations more sensitive to capture deficits even if these are less impactful for daily functional activities.
The association between age at disease onset and outcome is inconsistent in the literature. A 4-year follow-up study reported lower adaptive function in children younger than 12 years compared with adolescents/adults.12 Other studies did not find an association between age and neuropsychological outcomes.2,3 These controversies highlight the value of our LOS-related outcome categorization, suggesting that the pattern of cognitive-behavioral deficits is invariant to age, whereas multidomain deficits predominate in toddlers/preschoolers, and full recovery is more expected in schoolers/teenagers.
NMDAR has multiple roles in synapse formation.13 The blockade of NMDAR in developing mice brains leads to imprecise callosal projections to the somatosensory cortex, showing an increasing aberrancy with time that illustrates the long-term impact of early-life NMDAR hypofunction.13
Our study has several limitations. First, it has a cross-sectional design; thus, the long interval between diagnosis and last follow-up may have contributed to the loss of cases and selection or information bias in some assessments. However, patients included in the study had comparable demographics and disease severity with those without follow-up. Second, because the questionnaire did not include neuropsychological tests, the specific cognitive phenotypes were not available. Third, the generalizability of our findings may vary by geographic area. By contrast, our cluster analysis captured the heterogeneity of outcomes, facilitating risk factor determination.
Overall, our findings show that the long-term outcome of survivors of the acute phase of NMDARe is good regarding functional recovery, but approximately 27% (35% if younger than 6 years at disease onset) remain sociofamiliar dependent.
Acknowledgment
The authors thank the patients and parents who collaborated with our laboratory in search of neural antibodies in children with autoimmune encephalitis and the physicians who continuously devote to the diagnosis, treatment, and follow-up of children with autoimmune encephalitis. The authors also thank Dr. Mar Guasp for the critical review of the manuscript and Maria Rodés, Esther Aguilar, Mercè Alba, and Eva Caballero for their excellent technical support.
Appendix 1. Authors
| Name | Location | Contribution |
| Li-Wen Chen, MD, PhD | Group of Experimental Neuroimmunology Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Caixa Research Institute, Barcelona, Spain; Department of Pediatrics, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Gemma Olivé-Cirera, MD | Group of Experimental Neuroimmunology, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Caixa Research Institute, Barcelona; Pediatric Neurology Unit, Hospital Parc Taulí de Sabadell, Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Elianet G. Fonseca, MD, PhD | Group of Experimental Neuroimmunology Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Caixa Research Institute, Barcelona; Neurology Department, Hospital Clínic de Barcelona; Pediatric Neuroimmunology Unit, Neurology Department, Sant Joan de Déu Children's Hospital, Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Mateus Mistieri Simabukuro, MD, PhD | Division of Neurology, Hospital das Clinicas (HCFMUSP), Faculdade de Medicina, University of São Paulo, Brazil | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Takahiro Iizuka, MD, PhD | Department of Neurology, Kitasato University School of Medicine, Sagamihara, Japan | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Thais Armangue, MD, PhD | Group of Experimental Neuroimmunology, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Caixa Research Institute, Barcelona; Pediatric Neuroimmunology Unit, Neurology Department, Sant Joan de Déu Children's Hospital, Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Josep Dalmau, MD, PhD | Group of Experimental Neuroimmunology, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Caixa Research Institute, Barcelona; Neurology Department, Hospital Clínic de Barcelona; Centro de Investigación Biomédica en Red, Enfermedades Raras (CIBERER), Spain; Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia (USA) | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Appendix 2. Coinvestigators
| Coinvestigators are listed at Neurology.org. |
Study Funding
This study was supported in part by the Mutua Madrileña Foundation (AP162572016, TA); Plan Nacional de I+D+I and co-financed by the ISCIII - Subdirección General de Evaluación y Formento de la Investigación Sanitaria - and the Fondo Europeo de Desarrollo Regional (ISCIII-FEDER; PI20/00197 to JD, PI20/00280 and PI21/00316 to TA); Pla estratègic de recerca i innovació en salut (PERIS), Departament de Salut, Generalitat de Catalunya (SLT006/17/00362, TA); Marato TV3 Foundation (37/C/2021, TA), the Pablove Foundation (689368, TA), and Torrons Vicenç Foundation (PFNR0144, TA); Spanish Pediatric Association (AEP) (PI047351, TA); La Caixa Foundation Health research grants (HR-22-00221) to TA and JD. Dr. G. Olivé-Cirera is recipient of a Rio Hortega grant (CM22/00066) from the Instituto de Salud Carlos III (ISCIII), Spain, co-financed by Fondo Social Europeo Plus (FSE+).
Disclosure
J. Dalmau holds patents for the use of Ma2, NMDAR, GABABR, GABAAR, DPPX and IgLON5 as autoantibody tests. J. Dalmau receives royalties related to autoantibody tests from Euroimmun, Inc. The rest of the authors (L.W.C., G.O.C., E.F., M.S., T.I., and T.A.) have no conflicts of interest related to the submitted work. Go to Neurology.org/NN for full disclosures.
References
- 1.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. doi: 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bruijn MAAM, Aarsen FK, van Oosterhout MP, et al. Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology. 2018;90(22):e1997-e2005. doi: 10.1212/WNL.0000000000005605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flet-Berliac L, Tchitchek N, Lépine A, et al. Long-term outcome of paediatric anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2023;65(5):691-700. doi: 10.1111/dmcn.15429 [DOI] [PubMed] [Google Scholar]
- 4.Lewthwaite P, Begum A, Ooi MH, et al. Disability after encephalitis: development and validation of a new outcome score. Bull World Health Organ. 2010;88(8):584-592. doi: 10.2471/BLT.09.071357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z. Extensions to the k-means algorithm for clustering large data sets with categorical values. Data Min Knowl Discov. 1998;2:283-304. [Google Scholar]
- 7.Thorndike RL. Who belongs in the family?. Psychometrika. 1953;18(4):267-276. doi: 10.1007/bf02289263 [DOI] [Google Scholar]
- 8.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weihs C, Ligges U, Luebke K, Raabe N. klaR analyzing German business cycles. In: Baier D, Decker R, Schmidt-Thieme L, eds. Data Analysis and Decision Support. Springer-Verlag; 2005:335-343. [Google Scholar]
- 10.Matricardi S, Patrini M, Freri E, et al. Cognitive and neuropsychological evolution in children with anti-NMDAR encephalitis. J Neurol. 2016;263(4):765-771. doi: 10.1007/s00415-016-8056-9 [DOI] [PubMed] [Google Scholar]
- 11.Heine J, Kopp UA, Klag J, Ploner CJ, Prüss H, Finke C. Long-term cognitive outcome in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2021;90(6):949-961. doi: 10.1002/ana.26241 [DOI] [PubMed] [Google Scholar]
- 12.Yeshokumar A, Gordon-Lipkin E, Arenivas A, et al. Younger age at onset is associated with worse long-term behavioral outcomes in anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2022;9(5):e200013. doi: 10.1212/NXI.0000000000200013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Lin Y, Huynh T, Noguchi H, Bush JO, Pleasure SJ. NMDA receptors control development of somatosensory callosal axonal projections. Elife. 2021;10:e59612. doi: 10.7554/eLife.59612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available by request from qualified investigators.