Abstract
Background and Objectives
Chronic systemic inflammation has been hypothesized to be a mechanistic factor leading to post–acute cognitive dysfunction after COVID-19. However, little data exist evaluating longitudinal inflammatory markers.
Methods
We conducted a secondary analysis of data collected from the CONTAIN randomized trial of convalescent plasma in patients hospitalized for COVID-19, including patients who completed an 18-month assessment of cognitive symptoms and PROMIS Global Health questionnaires. Patients with pre–COVID-19 dementia/cognitive abnormalities were excluded. Trajectories of serum cytokine panels, D-dimer, fibrinogen, C-reactive peptide (CRP), ferritin, lactate dehydrogenase (LDH), and absolute neutrophil counts (ANCs) were evaluated over 18 months using repeated measures and Friedman nonparametric tests. The relationships between the area under the curve (AUC) for each inflammatory marker and 18-month cognitive and global health outcomes were assessed.
Results
A total of 279 patients (N = 140 received plasma, N = 139 received placebo) were included. At 18 months, 76/279 (27%) reported cognitive abnormalities and 78/279 (28%) reported fair or poor overall health. PROMIS Global Mental and Physical Health T-scores were 0.5 standard deviations below normal in 24% and 51% of patients, respectively. Inflammatory marker levels declined significantly from hospitalization to 18 months for all markers (IL-2, IL-2R, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, INFγ, TNFα, D-dimer, fibrinogen, ferritin, LDH, CRP, neutrophils; all p < 0.05), with the exception of IL-1β, which remained stable over time. There were no significant associations between the AUC for any inflammatory marker and 18-month cognitive symptoms, any neurologic symptom, or PROMIS Global Physical or Mental health T-scores. Receipt of convalescent plasma was not associated with any outcome measure.
Discussion
At 18 months posthospitalization for COVID-19, cognitive abnormalities were reported in 27% of patients, and below average PROMIS Global Mental and Physical Health scores occurred in 24% and 51%, respectively. However, there were no associations with measured inflammatory markers, which decreased over time.
Introduction
Cognitive impairment (“brain fog”) is one of the most prevalent neurologic postacute sequelae of COVID-19 (PASC) and has been reported in up to 50% of previously healthy, cognitively normal patients at 6 months and 12 months post-COVID hospitalization.1-3 However, the mechanisms that contribute to post–COVID-19 cognitive disorders remain unclear. Some have posited that ongoing inflammation,4-7 cerebrovascular endothelial dysfunction,8-12 and viral persistence13,14 may play a role in post–COVID-19 symptoms.
In previous work, we identified elevations in serum inflammatory markers (IL-6, D-dimer, C-reactive peptide [CRP], and ferritin) and neurodegenerative biomarkers (tau, ptau181, GFAP, UCHL1, and NFL) during acute COVID-19. We found that neurodegenerative biomarkers were elevated to levels even higher than those observed in non-COVID Alzheimer patients.15 These inflammatory and neurodegenerative makers correlated with both severity of index COVID-19 illness and with the occurrence of new neurologic events during hospitalization, most notably toxic metabolic encephalopathy.15 It remains unknown, however, whether elevations in these biomarkers are transient (indicating a monophasic insult) or sustained (suggestive of ongoing inflammation). Similarly, the predictive value of inflammatory biomarkers in regards to cognitive PASC symptoms and global health outcomes remains unknown.
Using data prospectively collected from the CONTAIN COVID-19 randomized clinical trial (CONTAIN-RCT), which investigated the effects of convalescent plasma in adults hospitalized with acute COVID-19, and data from the CONTAIN-Extend study, a longitudinal follow-up of CONTAIN-RCT participants conducted 18 months postrandomization, we aimed to examine the trajectories of serum cytokine and inflammatory markers over time. In addition, we aimed to identify any associations between these markers and outcomes observed at 18 months, including self-reported cognitive abnormalities and PROMIS Global Physical and Mental health scores.
Methods
Study Design and Patient Population
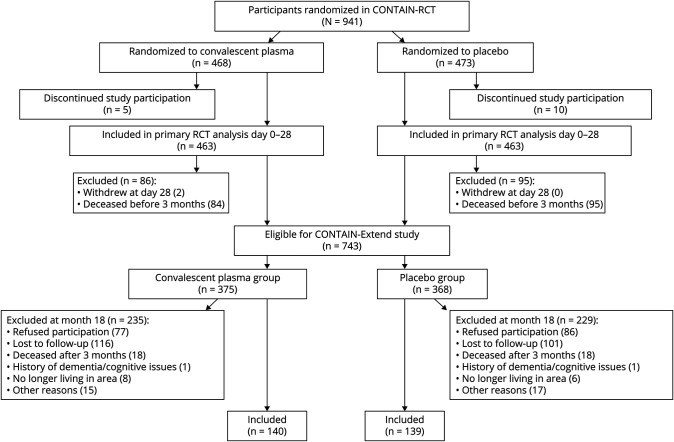
We conducted a retrospective secondary analysis of data collected from patients enrolled in both the CONTAIN-RCT16 and the CONTAIN-Extend study. The CONTAIN COVID-19 randomized, double-blind, placebo control trial (CONTAIN-RCT) was conducted between April 17, 2020, through March 15, 2021, at 21 hospitals throughout the United States.17 Hospitalized patients aged ≥18 years with laboratory-confirmed COVID-19 who required noninvasive oxygen supplementation were randomized to receive either one unit (∼250 mL) of COVID-19 convalescent plasma or an equivalent volume of normal saline (placebo). A total of 941 patients were randomized (468 to convalescent plasma and 473 to placebo), and there were no significant differences in either the primary or secondary outcomes (11-point World Health Organization Ordinal Scale for Clinical Improvement on day 14 or 28 after randomization), and adverse events occurred in 9.4% of patients in the convalescent plasma group vs 8.2% in the placebo group (p = 0.57). Of 21 sites that participated in the CONTAIN-RCT, 16 agreed to participate in the CONTAIN-Extend Study, including sites in Manhattan, the Bronx, Brooklyn, and Long Island (New York), New Haven (Connecticut), Miami (Florida), and Houston and Tyler (Texas). Participants previously randomized in CONTAIN-RCT who had not withdrawn from the study and had survived 3 months were eligible and recruited for an extended 18-month visit posthospitalization (CONTAIN-Extend study). Conducted from November 2021 to October 2022, CONTAIN-Extend included a symptom survey, global health assessments using the PROMIS-10 survey (version 1.2) and biospecimen collection. Patients with a pre–COVID-19 history of dementia or cognitive impairment (as documented at the time of randomization in the CONTAIN-RCT) were excluded.
Standard Protocol Approvals, Registrations, and Patient Consents
All patients or their surrogates provided consent to participate in both CONTAIN COVID-19 and CONTAIN-Extend, and both studies were approved by institutional review boards at each center that participated.16 Oversight for the initial CONTAIN-RCT was conducted by the New York University CONTAIN Coordinating Center and Data Safety Monitoring Board. This retrospective analysis of anonymized data was not considered to be human subjects research according to the NYU IRB and was thereby exempt from IRB review.
Data Collection
Baseline demographics, comorbidities, concomitant medications (at the time of randomization), hospital course, and COVID-19 vaccination status at 18 months were collected. Admission COVID-19 severity was measured by a prerandomization WHO Ordinal Scale for Clinical Improvement (WHO scale).18 Adverse events including transfusion-related events (e.g., transfusion-related acute lung injury and transfusion-associated circulatory overload), arterial thromboembolism, venous thromboembolism, infection, and bleeding were systematically collected. A serum cytokine panel (Cytokine Panel,19 ARUP laboratories, Salt Lake City, UT) was assessed at baseline; days 1, 7, 14, 28, and 90 postrandomization; and at 18-month follow-up. The panel included the following cytokines: interleukin (IL)-1β, IL-2, IL-2 soluble receptor (IL-2R), IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, INFγ, and TNFα. Other inflammatory markers including CRP, ferritin, fibrinogen, lactate dehydrogenase (LDH), and D-dimer were evaluated at baseline and days 1, 3, 7, and 14 postrandomization and at 18-month follow-up. Absolute neutrophil cell counts were assessed at baseline and day 3 and 18 months postrandomization. The choice of cytokine panel and inflammatory markers was based on routine clinical availability, with the intent of promoting generalizability and limiting the amount of blood sample required. The value representing lower limit of detection was entered when laboratory values were reported as less than or below the lower limit. No imputation of missing data was performed. As such, serial measurements were evaluated at time points with the least missing data to maximize the number of patients included in analyses.
Outcomes
Participants were interviewed by phone or in-person and completed a symptom questionnaire developed by the CONTAIN investigators and based on the literature at the time of protocol development in August 2021 (eTable 1).20 The primary outcome was new cognitive dysfunction reported at the 18-month follow-up, which was defined as new symptoms of memory, concentration, or attention impairment present within the week before completing the symptom questionnaire. Secondary outcomes included (1) any neurologic symptom at 18 months (choices included headache, myalgia, anosmia, dysgeusia, numbness/tingling, dizziness, lightheadedness, cognitive, fainting, seizure, imbalance, speech, tremor, fatigue, sleep disorder, anxiety, depression); (2) 18-month PROMIS Global Health 10 Physical Health21; and (3) 18-month PROMIS Global Health 10 Mental Health21 scores. The PROMIS Global Health inventory consists of 10 items, 4 of which are used to score the PROMIS Global Mental Health T-score, another 4 items are used to score the Global Physical Health T-score, and 2 items represent overall health (eTable 2).22 PROMIS Global Physical Health questions examine physical activities such as walking, climbing stairs, carrying groceries, fatigue, and pain. PROMIS Global Mental Health items focus on quality of life, mood, ability to think, satisfaction with social activities and relationships, and feelings of anxiety, depression, or irritability. Scores were collected as a 5–10 point Likert scale. Raw scores for Global Mental and Global Physical health were converted into T-scores with a mean of 50 and SD of 10.21 Lower Physical and Mental Health T-scores indicate more severe impairment. Scores were dichotomized at 0.5 standard deviations below the mean (e.g., T-score <45) based on data suggesting that this is a clinically meaningful threshold for dichotomization.23,24
Statistical Analyses
Demographics, comorbidities, concurrent medications, and COVID-19 vaccination status were compared among those with and without 18-month cognitive symptoms and among those with or without any neurologic symptom, and those with and without PROMIS Global Health Physical or Mental health T-scores <45 using Mann-Whitney U nonparametric tests, χ2, and Fisher exact tests, as appropriate. Baseline and 18-month cytokine and inflammatory laboratory data were compared between those with and without 18-month cognitive symptoms using the Mann-Whitney U nonparametric tests. One-way repeated measures ANOVA and nonparametric related-samples Friedman 2-way analysis of variance by ranks were used, as appropriate, to assess the relationship of cytokine levels over time (baseline, day 1 postrandomization, and at 18 months) and other inflammatory laboratory values (CRP, ferritin, fibrinogen, LDH, D-dimer, neutrophil count) over time (baseline, day 3, day 7, and 18 months). Patients with missing data at any time point were excluded from analysis, and no data imputation was performed. In addition, an area under the curve (AUC) was computed for each cytokine and inflammatory laboratory value (using the above time points), and AUC values were compared among those with and without the following 18-month outcomes: cognitive symptoms, any neurologic symptom, Global Physical health T-score <45, and Global Mental health T-score <45. AUC values were also compared among those who received convalescent plasma vs those who received placebo. Plots of estimated marginal means at each time point were constructed for each cytokine and inflammatory laboratory value. Bonferroni corrections were made for multiple comparisons. All analyses were conducted using IBM SPSS Statistics for Mac version 25 (IBM Corp., Armonk, NY).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Patient Characteristics
A total of 281 patients completed 18-month follow-up as part of CONTAIN-Extend. Two patients with a pre-COVID-19 history of dementia were excluded, leaving 279 patients for the final analysis (Figure 1). The median age at randomization was 59 years (interquartile range [IQR] 49–67), 157/279 (56%) were male, 119/279 (43%) were Hispanic, 140/279 (50%) received convalescent plasma, and 139/279 (50%) received placebo. At enrollment, 79% of patients were a 5 on the WHO severity of illness scale (hospitalized, requiring supplemental oxygen), and 21% were WHO 6 (hospitalized requiring noninvasive ventilation or high-flow nasal cannula). At the time of randomization, 230/279 (82%) received steroids, and 170/279 (61%) received remdesivir.
Figure 1. Flow Sheet of Enrollment From the CONTAIN-RCT Through the CONTAIN-Extend Study.
At 18 months, 76/279 (27%) described their overall health as fair to poor, and 92/279 (33%) described their overall health as very good/excellent (Likert scale choices: poor, fair, good, very good, excellent). The median 18-month PROMIS Global Physical Health T-score was 44.9 (IQR 39.8–54.1) and 51% had a T-score <45. The median 18-month PROMIS Global Mental Health T-score was 50.8 (IQR 45.8–56.0), and 24% had a T-score <45. Overall, 76/279 (27%) of patients noted cognitive abnormalities at 18 months, and 160/279 (57%) had at least one neurologic symptom (eFigure 1). There were no significant differences in neurologic symptoms among those who received convalescent plasma during hospitalization compared with those who received placebo (all p > 0.05).
Predictors of Primary and Secondary Outcomes
Predictors of 18-month cognitive symptoms and 18-month secondary outcomes (any neurologic symptom, PROMIS Global Physical Health T-score <45, PROMIS Global Mental Health T-score <45) are shown in Table 1. Those with 18-month cognitive abnormalities tended to be younger and were more often women. Female sex was also related to worse PROMIS Global Physical and Mental Health T-scores. The receipt of convalescent plasma was not related to any outcome, and there were no consistent relationships between COVID-19 vaccination (90% were vaccinated) and any primary or secondary outcome. There were several medical history comorbidities associated with 18-month neurologic symptoms or poor PROMIS Global Health Physical and Mental scores, but all were nonsignificant after Bonferroni correction for multiple comparisons.
Table 1.
Relationship of Demographics, Comorbidities, Hospital Factors, Concurrent Medications, and COVID-19 Vaccination With 18-Month Outcomes, Including Cognitive Symptoms, Any Neurologic Symptoms, and Low PROMIS Global Physical and Mental Health Scores
| 18-mo cognitive symptoms (N = 76) | No 18-mo cognitive symptoms (N = 203) |
p Value 18-mo cognitive symptom |
p Value 18-mo any neurologic symptom |
p Value Global health physical T-score <45 |
p Value Global health mental T-score <45 |
|
| Demographics | ||||||
| Age (median, IQR) | 57 (47–64) | 59 (52–69) | 0.035 | 0.315 | 0.378 | 0.426 |
| Sex (female), N (%) | 43/76 (57%) | 179/203 (39%) | 0.008 | 0.003 | 0.001 | <0.001 |
| Race (White), N (%) | 40/76 (53%) | 103/203 (51%) | 0.778 | 0.227 | 0.394 | 0.061 |
| Hispanic, N (%) | 26/76 (34%) | 93/203 (46%) | 0.081 | 0.244 | 0.008 | 0.354 |
| BMI (median, IQR) | 32.7 (25.7–38.6) | 31.5 (27.4–35.9) | 0.613 | 0.601 | <0.001 | 0.232 |
| Medical history | ||||||
| Hypertension, N (%) | 39/76 (51%) | 120/203 (59%) | 0.187 | 0.045 | 0.009 | 0.252 |
| Diabetes, N (%) | 27/76 (36%) | 69/199 (35%) | 0.894 | 0.464 | 0.110 | 0.021 |
| Hyperlipidemia, N (%) | 941/76 (54%) | 3/203 (46%) | 0.262 | 0.065 | 0.563 | 0.495 |
| CHF, N (%) | 4/76 (5%) | 10/203 (5%) | 0.466 | 0.058 | 0.004 | 0.040 |
| Peripheral vascular disease, N (%) | 4/76 (5%) | 9/203 (4%) | 0.452 | 0.063 | 0.438 | 0.059 |
| Stroke, N (%) | 7/76 (9%) | 7/203 (3%) | 0.073 | 0.038 | 0.069 | 0.139 |
| COPD/asthma/bronchitis, N (%) | 23/76 (30%) | 45/199 (23%) | 0.189 | 0.330 | 0.003 | 0.172 |
| Cancer, N (%) | 7/76 (9%) | 28/203 (14%) | 0.259 | 0.041 | 0.318 | 0.341 |
| CAD/MI, N (%) | 2/76 (3%) | 6/203 (3%) | 0.461 | 0.041 | 0.070 | 0.361 |
| Valvular heart disease, N (%) | 2/76 (3%) | 5/203 (3%) | 0.467 | 0.046 | 0.020 | 0.505 |
| Chronic kidney disease, N (%) | 10/76 (13%) | 23/199 (12%) | 0.715 | 0.408 | 0.199 | 0.361 |
| Transplant, N (%) | 2/76 (3%) | 8/203 (4%) | 0.402 | 0.057 | 0.562 | 0.481 |
| Autoimmune disease, N (%) | 6/76 (8%) | 9/203 (4%) | 0.254 | 0.005 | 0.282 | 0.378 |
| HIV, N (%) | 0/76 (0%) | 5/203 (3%) | 0.175 | 0.046 | 0.491 | 0.504 |
| Immunodeficiency, N (%) | 0/76 (0%) | 5/203 (3%) | 0.175 | 0.065 | 0.529 | 0.223 |
| Smoking, N (%) | 14/76 (18%) | 57/203 (28%) | 0.099 | 0.937 | 0.008 | 0.173 |
| Hospital factors | ||||||
| Received convalescent plasma, N (%) | 41/76 (54%) | 99/203 (49%) | 0.441 | 0.678 | 0.631 | 0.699 |
| Admission WHO, (median, IQR) | 5 (5–5) | 5 (5–6) | 0.661 | 0.862 | 0.689 | 0.763 |
| Adverse event: TRALI, TACO, N (%) | 0 | 0 | — | — | — | — |
| Adverse event: arterial thromboembolism, N (%) | 0/76 (0%) | 4/203 (2%) | 0.218 | 0.188 | 0.038 | 0.253 |
| Adverse event: venous thromboembolism, N (%) | 6/76 (8%) | 10/203 (5%) | 0.342 | 0.342 | 0.250 | 0.255 |
| Adverse event: infection, N (%) | 15/76 (20%) | 38/203 (19%) | 0.847 | 0.042 | 0.026 | 0.631 |
| Adverse event: bleeding, N (%) | 7/76 (9%) | 8/203 (4%) | 0.131 | 0.594 | 0.599 | 0.211 |
| Concurrent medications at the time of randomization | ||||||
| Remdesivir, N (%) | 40/76 (53%) | 130/203 (64%) | 0.082 | 0.537 | 0.258 | 0.707 |
| NSAIDs, N (%) | 52/76 (68%) | 116/203 (57%) | 0.087 | 0.682 | 0.445 | 0.551 |
| Steroids, N (%) | 63/76 (83%) | 173/203 (85%) | 0.632 | 0.578 | 0.588 | 0.929 |
| Statins, N (%) | 26/76 (34%) | 52/203 (26%) | 0.154 | 0.844 | 0.047 | 0.157 |
| Therapeutic anticoagulation, N (%) | 65/76 (86%) | 161/203 (79%) | 0.239 | 0.667 | 0.933 | 0.764 |
| Antiplatelets, N (%) | 20/76 (26%) | 41/203 (20%) | 0.271 | 0.766 | 0.971 | 0.367 |
| Hydroxychloroquine, N (%) | 7/76 (9%) | 7/203 (3%) | 0.050 | 0.590 | 0.911 | 0.367 |
| ACEI, N (%) | 3/76 (4%) | 21/203 (10%) | 0.090 | 0.446 | 0.481 | 0.760 |
| Discharge WHO (median, IQR) | 2 (2–3) | 2 (2–3) | 0.178 | 0.260 | 0.141 | 0.623 |
| COVID vaccination after hospitalization | ||||||
| Any vaccine, N (%) | 71/76 (93%) | 180/203 (89%) | 0.240 | 0.042 | 0.326 | 0.522 |
| Pfizer, N (%) | 35/76 (46%) | 105/203 (52%) | 0.399 | 0.580 | 0.541 | 0.337 |
| Moderna, N (%) | 33/76 (43%) | 61/203 (30%) | 0.035 | 0.020 | 0.148 | 0.981 |
| Jansen, N (%) | 3/76 (4%) | 14/203 (7%) | 0.359 | 0.376 | 0.708 | 0.212 |
| Number of doses (median, IQR) | 3 (2–3) | 3 (2–3) | 0.472 | 0.974 | 0.202 | 0.303 |
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor; BMI = body mass index; CAD/MI = coronary artery disease/myocardial infarction; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; NSAIDs = nonsteroidal anti-inflammatory drugs; TACO = transfusion-associated circulatory overload; TRALI = transfusion-related acute lung injury; WHO = World Health Organization.
Bold indicates significance after Bonferroni correction for multiple comparisons (p ≤ 0.001).
Cytokine and Inflammatory Laboratory Studies Over Time
Cytokine levels across multiple time points (baseline, day 1 and 18-month) were available in N = 123 patients. Patients with available cytokine data came primarily from 3 of 16 enrolling sites (eTable 3). Those included in cytokine analyses were of similar age, sex, and race as those with missing cytokine data. Admission and discharge severity of illness (as measured by WHO score) and receipt of convalescent plasma vs placebo were also similar between those with cytokine data compared with those with missing data. However, the proportion of Hispanic patients included in the cytokine analysis was significantly less compared with those with missing data (30% vs 51%, p = 0.002, eTable 3).
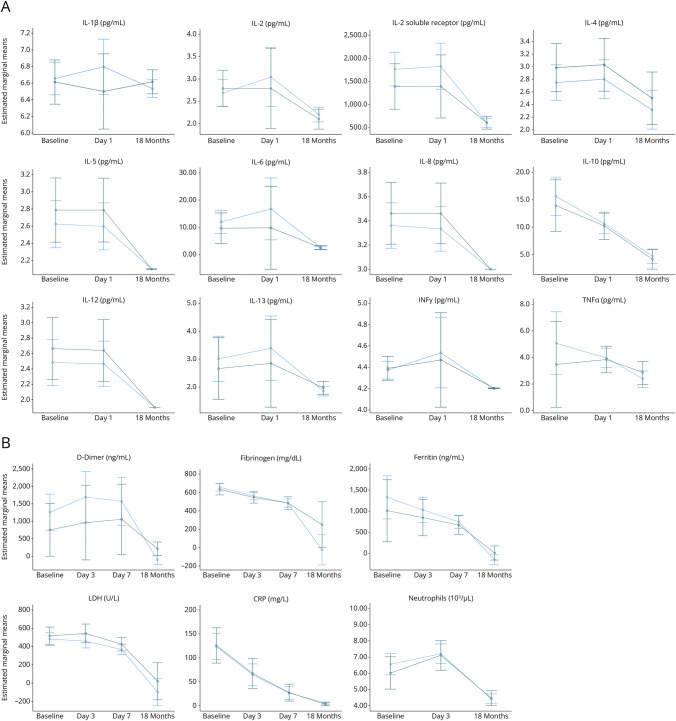
Among the 123 patients included in analysis, all cytokine levels declined significantly over time from baseline, to day 1 to 18 months (all Friedman p < 0.001), with the exception of IL-1β, which remained unchanged over time (Friedman p = 0.738, Figures 2A and 3A., eFigures 2A and 3A). Men had significantly higher ferritin AUC values than women (median AUC 264268 vs 105,150, p < 0.001); however, no other differences in cytokine or inflammatory AUC values were observed.
Figure 2. Serum Cytokine (N = 123) (A) and Inflammatory Laboratory (N = 270) (B) Levels (Estimated Marginal Means) Over Time Compared Between Those With and Without a Cognitive Symptoms at 18 Months.
The dark green line represents patients with cognitive symptoms at 18 months, and the blue line represents those without cognitive symptoms at 18 months (with error bars). All cytokine and inflammatory laboratory levels significantly declined over time (all Friedman p < 0.001), with the exception of IL-1ß (Friedman p = 0.738). There were no significant differences in the areas under the curves of any cytokine or inflammatory laboratory measure when comparing those with or without 18-month cognitive symptoms (all p > 0.05).
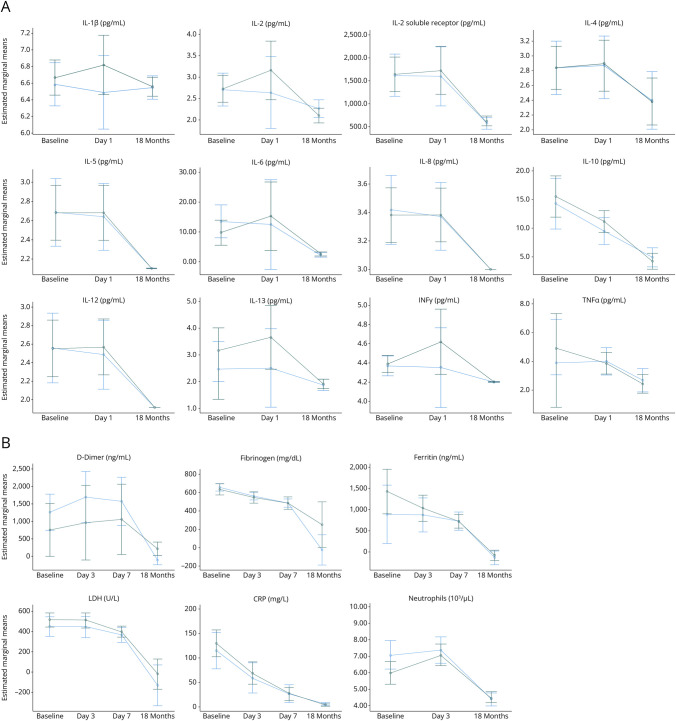
Figure 3. Serum Cytokine (N = 123) (A) and Inflammatory Laboratory (N = 270) (B) Levels (Estimated Marginal Means) Over Time Compared Between Those With and Without Any Neurologic Symptom at 18 Months.
Neurologic symptoms included headache, myalgia, anosmia, dysgeusia, numbness/tingling, dizziness, lightheadedness, cognitive, fainting, seizure, imbalance, speech, tremor, fatigue, sleep disorder, anxiety, depression. The dark green line represents patients with any neurologic symptom at 18 months, and the blue line represents those without a neurologic symptom at 18 months (with error bars). All cytokine and inflammatory laboratory levels significantly declined over time (all Friedman p < 0.001).
D-dimer, LDH, ferritin, fibrinogen, CRP, and neutrophil values were available at all time points (baseline, day 3, day 7, and 18 months) in 195/279 (70%) of patients. Similarly, all these measures significantly declined from baseline to days 3, 7, and 18 months postrandomization (all Friedman p < 0.001, Figures 2B and 3B, eFigures 2B and 3B).
Association of Cytokine and Inflammatory Laboratory Levels With Primary and Secondary Outcomes
No significant relationships were identified between baseline (prerandomization) cytokine or inflammatory laboratory values and 18-month cognitive or neurologic symptoms (all p > 0.05), with the exception of baseline D-dimer levels, which were lower in those without 18-month cognitive or neurologic complaints (336 ng/mL in those without cognitive symptoms vs 595 ng/mL in those with symptoms). The areas under the curve for cytokines (measured at baseline, day 1 and 18 months) and inflammatory laboratory markers (measured at baseline, hospital day 3, hospital day 7 and 18 months) over time did not vary significantly among those with or without cognitive symptoms at 18 months (Table 2, Figure 2, A and B), nor were there any differences when evaluating the secondary outcomes of any neurologic symptom at 18 months (Figures 3, A and B), 18-month PROMIS Global Physical Health T-score<45 (all p > 0.05, eFigures 2 and 3), or WHO severity of illness scores at 18 months Similarly, there were no significant correlations between PROMIS questionnaire items pertaining specifically to ability to participate in regular leisure, family, or work activities and any cytokine or inflammatory markers. However, patients with worse 18-month Global Mental Health T-scores (<45) tended to have higher neutrophil count AUCs (Table 2), though these differences did not remain significant after Bonferroni correction for multiple comparisons. The results depicted in Table 2 did not change when analysis was stratified by sex. There were no significant differences in AUC values for any cytokine or inflammatory laboratory measure among those who received convalescent plasma during hospitalization compared with those who received placebo (all p > 0.05).
Table 2.
Relationship of Outcomes at 18 Months With Cytokine Levels and Inflammatory Markers Over Time
| 18-mo cognitive symptom present (N = 76) | 18-mo cognitive symptom absent (N = 203) | p Value | 18-mo any neurologic symptom present (N = 160) | No 18-mo neurologic symptoms (N = 119) | p Value | 18-mo PROMIS physical health T-score<45 (N = 141) | 18-mo PROMIS physical health T-score≥45 (N = 133) | p Value | 18-mo PROMIS mental health T-score<45 (N = 66) | 18-mo PROMIS mental health T-score≥45 (N = 205) | p Value | |
| Cytokine area under the curve (baseline, day 1, 18 months) | ||||||||||||
| IL-1β (pg/mL) | 3,267 | 3,267 | 0.706 | 3,627 | 3,627 | 0.924 | 3,627 | 3,627 | 0.831 | 3,627 | 3,627 | 0.232 |
| IL-10 (pg/mL) | 3,491 | 3,701 | 0.711 | 2,628 | 3,775 | 0.903 | 2,909 | 3,775 | 0.276 | 2,848 | 3,812 | 0.255 |
| IL-12 (pg/mL) | 1,060 | 1,060 | 0.522 | 1,060 | 1,060 | 0.691 | 1,060 | 1,060 | 0.928 | 1,060 | 1,060 | 0.619 |
| IL-13 (pg/mL) | 949 | 949 | 0.352 | 949 | 949 | 0.839 | 949 | 949 | 0.925 | 949 | 949 | 0.643 |
| IL-2 (pg/mL) | 1,172 | 1,172 | 0.955 | 1,172 | 1,172 | 0.736 | 1,172 | 1,172 | 0.632 | 1,172 | 1,172 | 0.535 |
| IL-2sR (pg/mL) | 467,704 | 524,280 | 0.348 | 471,933 | 527,632 | 0.293 | 475,484 | 520,929 | 0.700 | 401,731 | 526,589 | 0.050 |
| IL-4 (pg/mL) | 1,228 | 1,228 | 0.602 | 1,228 | 1,228 | 0.597 | 1,228 | 1,228 | 0.495 | 1,228 | 1,228 | 0.548 |
| IL-5 (pg/mL) | 1,172 | 1,172 | 0.572 | 1,172 | 1,172 | 0.819 | 1,172 | 1,172 | 0.965 | 1,172 | 1,172 | 0.786 |
| IL-6 (pg/mL) | 1786 | 1,479 | 0.883 | 1,398 | 1955 | 0.656 | 1761 | 1,401 | 0.581 | 1,398 | 1,508 | 0.650 |
| IL-8 (pg/mL) | 1,674 | 1,674 | 0.549 | 1,674 | 1,674 | 0.922 | 1,674 | 1,674 | 0.922 | 1,674 | 1,674 | 0.782 |
| INF-γ (pg/mL) | 2,344 | 2,344 | 0.607 | 2,344 | 2,344 | 0.776 | 2,344 | 2,344 | 0.915 | 2,344 | 2,344 | 0.745 |
| TNF-α (pg/mL) | 1,450 | 1,367 | 0.841 | 1,369 | 1,367 | 0.466 | 1,284 | 1,534 | 0.489 | 1,088 | 1,453 | 0.366 |
| Inflammatory laboratory area under the curve (baseline, day 3, day 7, 18 months) | ||||||||||||
| D-dimer (ng/mL) | 219,887 | 203,875 | 0.806 | 232,739 | 188,342 | 0.308 | 208,350 | 193,120 | 0.963 | 253,614 | 194,160 | 0.334 |
| Fibrinogen (mg/dL) | 250,470 | 137,879 | 0.388 | 246,023 | 238,233 | 0.860 | 241,575 | 245,021 | 0.223 | 229,673 | 254,218 | 0.657 |
| LDH (U/L) | 175,220 | 150,614 | 0.040 | 164,552 | 144,299 | 0.076 | 150,614 | 265,339 | 0.187 | 170,585 | 162,408 | 0.239 |
| Ferritin (ng/mL) | 240,887 | 194,511 | 0.360 | 227,210 | 194,511 | 0.406 | 175,579 | 240,333 | 0.086 | 182,854 | 215,155 | 0.484 |
| CRP (mg/L) | 4,327 | 4,136 | 0.727 | 4,143 | 4,452 | 0.336 | 4,615 | 4,279 | 0.604 | 2,681 | 4,452 | 0.107 |
| Neutrophil counta (103/µL) | 3,114 | 3,046 | 0.842 | 3,092 | 3,098 | 0.650 | 3,046 | 3,092 | 0.494 | 3,611 | 3,012 | 0.022 |
Median Area under the curve (AUC) for each serum cytokine (measured at baseline, day 1, and 18 months post-randomization), and serum inflammatory marker (measured at baseline, day 3, day 7, and 18 months post-randomization) compared between those with or without secondary outcomes of 18-mo neurologic symptoms, PROMIS Global Physical Health T-score<45, and PROMIS Global Mental Health T-score<45.
Measured at baseline, 3 days postrandomization and 18 months postrandomization; LDH = lactate dehydrogenase; CRP = C-reactive peptide. Significance after Bonferroni correction for multiple comparisons was calculated as p ≤ 0.003.
Discussion
In this secondary analysis of longitudinal data collected from the randomized CONTAIN-RCT and CONTAIN-Extend trials, we found that cognitive and neurologic symptoms in general were common at 18 months post–COVID-19 hospitalization. However, we did not identify any relationships between the measured cytokine or inflammatory marker levels or trajectories over time and these PASC neurologic outcomes. The repeated measures of cytokine and inflammatory laboratory data used in our study provide a more complete picture of the inflammatory milieu over time. Indeed, several inflammatory markers appeared to increase after randomization (e.g., INF-γ, D-dimer, neutrophil count), before declining at the 18-month mark. It is possible that patients may have experienced further increases in inflammatory markers or different PASC symptoms at other time points (e.g., 1 month, 3 months, or 6 months postinfection); however, limited data were available at these intervals. Although some have hypothesized that persistent inflammation because of either residual viral reservoirs or autoantigens is a prime driver of PASC,5,14 we found that, in aggregate, cytokine and inflammatory markers significantly declined over time and did not discriminate between those with or without PASC neurologic symptoms.
We did, however, identify a possible relationship between baseline D-dimer levels, elevated neutrophil AUC, and worse 18-month neurologic symptoms and PROMIS Global Mental Health scores, respectively. Both D-dimer and elevated neutrophil levels have been associated with activation of the coagulation cascade and thrombosis among acutely ill patients with COVID-19.25,26 Furthermore, increased neutrophil activity and signatures of clotting cascade activity (e.g., elevated D-dimer, LDH, ferritin) have been shown to be related to PASC in several studies.6,7,27,28 One prevailing hypothesis for PASC neurologic impairment is based on neuropathologic data among patients who died in the acute phase after SARS-CoV-2 infection. Several autopsy studies demonstrated endothelial cell abnormalities, ischemia, microhemorrhages, disruption of the microvasculature basal lamina, and extravasation of fibrinogen into brain parenchyma, suggestive of blood-brain barrier disruption, inflammation, and microthrombosis.11,29,30 While we did not detect significant differences in inflammatory markers suggestive of coagulation cascade activation among PASC patients, we did not examine specific markers of blood-brain barrier disruption, and it is possible that serum inflammatory markers may not adequately reflect CNS inflammation. Indeed, CSF studies have shown differences in immunophenotyping of PASC patients compared with healthy controls that were not detected in matching serum samples,31 indicating that further research is warranted.
We also identified female sex as a risk factor not only for 18-month cognitive and neurologic symptoms but also for worse PROMIS Global Physical and Mental Health scores. These findings complement those of several other studies that have identified female sex as a risk factor of PASC.32-34 While the mechanisms underlying this association remain obscure, some have posited that higher rates of autoimmune disorders among women and higher basal levels of immunoglobulins and more robust immunologic responses to both infections and vaccines, with increased cytokine production and T-cell response, may, in part, be responsible for this relationship.35-37 However, in our study, female sex was not significantly associated with higher AUC values for any cytokine or inflammatory marker, nor did female sex modify the relationship between these measures and 18-month outcomes. These results suggest that factors other than inflammation may play a role in the female predominance of PASC symptoms.
While some cross-sectional proteomic and molecular studies have identified increased markers of chronic inflammation among PASC patients, including increased IL-12/INF-γ and IL-6 expression and perturbation in natural killer cells,6,38 others have found that proinflammatory cytokines, such as IL-6, IL-8, and IL-1ß, do not robustly discriminate between patients who do or do not develop PASC.7 Similarly, there has been conflicting literature regarding the relationships between PASC and circulating immune cell populations, the role of SARS-CoV-2 adaptive immunity (including T-cell responses), and the impact of levels of antibodies to SARS-CoV-2, with significant relationships identified in some cohorts, but not others.39,40 Discrepancies in these results may be ascribed to differences in the severity of index COVID-19, the variety of PASC symptoms assessed, and the time frames of symptom evaluation and biomarker measurement across studies, all of which make direct comparisons difficult. Indeed, several studies attempting to identify molecular-clinical correlates for PASC have found substantial heterogeneity across patients, implying that a large number of participants are likely needed to identify biological signatures of PASC.6,7,38 In addition, utilization of PASC cognitive symptoms as a primary outcome is problematic since subjective cognitive complaints do not appear to correlate well with objective cognitive testing.41
Strengths of this study include its use of a multicentered sample of hospitalized patients followed longitudinally for 18 months with serial blood sampling. This is, to our knowledge, one of the largest cohort of patients providing serial blood samples for such an extensive duration of time postindex SARS-CoV-2 infection. There are limitations that should be mentioned as well. First, the timing of blood sampling and follow-up interviews may miss stochastic windows of inflammation or symptomatology. Some patients may have had PASC symptoms but improved by the 18-month follow-up. Furthermore, subjective PASC symptoms or patient-reported outcomes may miss abnormalities that can only be detected by objective testing.42-44 Although we evaluated quantitatively assessed patient-reported outcomes using the PROMIS-10 Global Health questionnaire, this still represents subjective data. Second, because we aimed to evaluate trajectories of laboratory data, the number of patients with data at each time point was limited, thereby reducing statistical power to detect differences between groups. In addition, fewer Hispanics had cytokine data available compared with the overall study population. This appeared to be due to the fact that sites that were more likely to enroll Hispanic patients also contributed less cytokine data. Furthermore, this data set included only hospitalized patients with COVID-19. Therefore, generalizability to nonhospitalized patients with COVID-19 is limited. Last, while we evaluated 17 cytokines and inflammatory markers, this was not an exhaustive assessment of all possible cytokines/chemokines. Indeed, a metabolomics study suggested that type I interferons (e.g., INFα and INFß, which we did not assess) reduce systemic serotonin levels, which in turn impair vagal nerve signaling, contributing to PASC cognitive symptoms.45 While serotonin levels were lower in some clinical cohorts assessed in that study, the link between type 1 INF and serotonin levels was established in mouse models.45 Hence, further study of serial type 1 INF levels in humans and its impact on both subjective and objective cognitive impairment is warranted.
Among hospitalized patients with COVID-19 followed longitudinally, more than 25% reported cognitive symptoms at 18 months, although there was no significant relationship with any serially measured serum inflammatory marker. While there has been a great deal of interest in determining whether chronic inflammation is an underlying cause of post-COVID cognitive impairment, these data do not support this mechanism, but rather suggest that commercially available cytokine panels and other routine inflammatory laboratory measures are unlikely to serve as robust biomarkers for neurologic PASC symptoms. While PROMIS Global Mental Health and neurologic symptom outcomes appear to be associated with elevated neutrophil and D-dimer levels, respectively, more research with larger cohorts of patients would be needed to confirm this relationship. The RECOVER (Researching COVID to Enhance Recovery) observational study,46 pathophysiology studies, and RECOVER clinical trials47 should offer additional insight into underlying mechanisms of neurologic PASC, potential biomarkers, and therapeutic strategies.
Acknowledgment
The authors would like to thank the patients who participated in this trial, as well as the COVID-Extend team that supported this study including: Gia Cobb, MA, Department of Medicine, NYU Grossman School of Medicine, New York, NY; Bruce N. Cronstein, MD, NYC Health and Hospitals Corporation Clinical and Translational Science Institute, NYU Grossman School of Medicine, New York, NY, Division of Rheumatology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; Mahalia Desruisseaux, MD, Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT; Judith S. Hochman, MD, NYC Health and Hospitals Corporation Clinical and Translational Science Institute, NYU Grossman School of Medicine, New York, NY, Leon H. Charney Division of Cardiology, Department of Medicine, NYU Grossman School of Medicine, New York, NY; Dushyantha T. Jayaweera, MD, MRCOG (UK), FACP, Division of Infectious Disease, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, Miami Clinical and Translational Science Institute, University of Miami Miller School of Medicine Miami, Florida FL; Julie V. Philley, MD, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, The University of Texas Health Science Center at Tyler, UTHealth East Texas, Tyler; Luis Ostrosky-Zeichner, MD, Division of Infectious Disease, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston; Caroline-Sturm Reganato, RN, BS, BA, Department of Medicine, NYU Grossman School of Medicine, New York, NY.
Glossary
- ANCs
absolute neutrophil counts
- AUC
area under the curve
- CRP
C-reactive peptide
- IQR
interquartile range
- LDH
ferritin, lactate dehydrogenase
- PASC
postacute sequelae of COVID-19
Appendix. Authors
| Name | Location | Contribution |
| Jennifer A. Frontera, MD | Department of Neurology, New York University Grossman School of Medicine | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Rebecca A. Betensky, PhD | Department of Biostatistics, NYU, New York | Analysis or interpretation of data |
| Liise-anne Pirofski, MD | Division of Infectious Disease, Department of Medicine, Montefiore Medical Center; Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York | Major role in the acquisition of data |
| Thomas Wisniewski, MD | Department of Neurology, New York University Grossman School of Medicine | Study concept or design |
| Hyunah Yoon, MD | Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Mila B. Ortigoza, MD, PhD | Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
Study Funding
This project was supported by a grant from NCATS/NIH Centers for Translational Science Awards (CTSA) to New York University (UL1-TR001445-06-A1-S1) to JAF, RB, and MBO.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96(4):e575–e586. doi: 10.1212/WNL.0000000000010979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frontera JA, Yang D, Lewis A, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontera JA, Simon NM. Bridging knowledge gaps in the diagnosis and management of neuropsychiatric sequelae of COVID-19. JAMA Psychiatry. 2022;79(8):811-817. doi: 10.1001/jamapsychiatry.2022.1616 [DOI] [PubMed] [Google Scholar]
- 4.Nicolai L, Kaiser R, Stark K. Thromboinflammation in long COVID-the elusive key to postinfection sequelae? J Thromb Haemost. 2023;21(8):2020-2031. doi: 10.1016/j.jtha.2023.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newell KL, Waickman AT. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infectionImmunity and inflammaion in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol. 2022;77:102228. doi: 10.1016/j.coi.2022.102228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talla A, Vasaikar SV, Szeto GL, et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat Commun. 2023;14(1):3417. doi: 10.1038/s41467-023-38682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff MC, Bonham KS, Anam FA, et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat Commun. 2023;14(1):4201. doi: 10.1038/s41467-023-40012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu SW, Ilyas I, Weng JP. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44(4):695-709. doi: 10.1038/s41401-022-00998-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaventura A, Vecchie A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319-329. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279-292. doi: 10.1159/000079408 [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384(5):481-483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MH, Perl DP, Steiner J, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145(7):2555-2568. doi: 10.1093/brain/awac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB, RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12:e86002. doi: 10.7554/eLife.86002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76(3):e487-e490. doi: 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frontera JA, Boutajangout A, Masurkar A, et al. Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment or Alzheimer's dementia. Alzheimers Dement. 2021;18(5):899-910. doi: 10.1002/alz.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu LN, Chen D, Xu D, Tan G, Wang HJ, Liu L. Newer antiepileptic drugs compared to levetiracetam as adjunctive treatments for uncontrolled focal epilepsy: an indirect comparison. Seizure. 2017;51:121-132. doi: 10.1016/j.seizure.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 17.Ortigoza MB, Yoon H, Goldfeld KS, et al. Efficacy and safety of COVID-19 Convalescent plasma in hospitalized patients: a randomized clinical trial. JAMA Intern Med 2022;182(2):115-126. doi: 10.1001/jamainternmed.2021.6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ARUP. Cytokine Panel 13, Serum. Accessed November 12, 2023. ltd.aruplab.com/Tests/Pub/0051394 [Google Scholar]
- 20.WHO. Global COVID-19 Clinical Platform Caser Report Form (CRF) for Post COVID Condition. Accessed November 12, 2023. who.int/publications/i/item/global-covid-19-clinical-platform-case-report-form-(crf)-for-post-covid-conditions-(post-covid-19-crf-) [Google Scholar]
- 21.NIH. PROMIS Global Health Item Bank; 2023. Accessed November 12, 2023. commondataelements.ninds.nih.gov/report-viewer/130328/PROMIS%20Item%20Bank%20v1.2%20-%20Global%20Health [Google Scholar]
- 22.PROMIS. PROMIS Global Health Scoring Manual; 2023. Accessed November 12, 2023. healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20Global%20Health%20Scoring%20Manual_22June2023.pdf [Google Scholar]
- 23.PROMIS. Meaningful Change for PROMIS. 2022. Accessed November 12, 2023. healthmeasures.net/score-and-interpret/interpret-scores/promis/meaningful-change [Google Scholar]
- 24.Terwee CB, Peipert JD, Chapman R, et al. Minimal important change (MIC): a conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual Life Res. 2021;30(10):2729-2754. doi: 10.1007/s11136-021-02925-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna E, Wubben R, Isaza-Correa JM, et al. Neutrophils in COVID-19: not innocent bystanders. Front Immunol. 2022;13:864387. doi: 10.3389/fimmu.2022.864387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galland J, Thoreau B, Delrue M, et al. White blood count, D-dimers, and ferritin levels as predictive factors of pulmonary embolism suspected upon admission in noncritically ill COVID-19 patients: the French multicenter CLOTVID retrospective study. Eur J Haematol. 2021;107(2):190-201. doi: 10.1111/ejh.13638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. doi: 10.1186/s12933-021-01359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eddins DJ, Yang J, Kosters A, et al. Transcriptional reprogramming of infiltrating neutrophils drives lung pathology in severe COVID-19 despite low viral load. Blood Adv. 2023;7(5):778-799. doi: 10.1182/bloodadvances.2022008834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144(9):2696-2708. doi: 10.1093/brain/awab148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383(10):989-992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mina Y, Enose-Akahata Y, Hammoud DA, et al. Deep phenotyping of neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2023;10(4):e200097. doi: 10.1212/NXI.0000000000200097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun. 2022;13(1):1957. doi: 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611 e9-e611 e16. doi: 10.1016/j.cmi.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rainville JR, Hodes GE. Inflaming sex differences in mood disorders. Neuropsychopharmacology. 2019;44(1):184-199. doi: 10.1038/s41386-018-0124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, tamoxifen and raloxifene, impair dendritic cell differentiation and activation. J Immunol. 2005;175(4):2666-2675. doi: 10.4049/jimmunol.175.4.2666 [DOI] [PubMed] [Google Scholar]
- 37.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411-423. doi: 10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- 38.Ruffieux H, Hanson AL, Lodge S, et al. A patient-centric modeling framework captures recovery from SARS-CoV-2 infection. Nat Immunol. 2023;24(2):349-358. doi: 10.1038/s41590-022-01380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altmann DM, Reynolds CJ, Joy G, et al. Persistent symptoms after COVID-19 are not associated with differential SARS-CoV-2 antibody or T cell immunity. Nat Commun. 2023;14(1):5139. doi: 10.1038/s41467-023-40460-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein J, Wood J, Jaycox J, et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139-148. doi: 10.1038/s41586-023-06651-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez Giraldo GS, Ali ST, Kang AK, et al. Neurologic manifestations of long COVID Differ based on acute COVID-19 severity. Ann Neurol. 2023;94(1):146-159. doi: 10.1002/ana.26649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frontera JA, Thorpe LE, Simon NM, et al. Post-acute sequelae of COVID-19 symptom phenotypes and therapeutic strategies: a prospective, observational study. PLoS One. 2022;17(9):e0275274. doi: 10.1371/journal.pone.0275274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frontera JA, Yang D, Medicherla C, et al. Trajectories of neurologic recovery 12 months after hospitalization for COVID-19: a prospective longitudinal study. Neurology. 2022;99(1):e33-e45. doi: 10.1212/WNL.0000000000200356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez Giraldo GS, Ali ST, Kang AK, et al. Neurologic manifestations of long COVID differ based on acute COVID-19 severity. Ann Neurol. 2023;94(1):146-159. doi: 10.1002/ana.26649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong AC, Devason AS, Umana IC, et al. Serotonin reduction in post-acute sequelae of viral infection. Cell. 2023;186(22):4851-4867.e20. doi: 10.1016/j.cell.2023.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RECOVER. RECOVER: Researching COVID to Enhance Recovery. Accessed November 12, 2023. recovercovid.org/ [Google Scholar]
- 47.RECOVER. RECOVER in Action: Status of Clinical Trial Protocols. Accessed November 12, 2023. rethinkingclinicaltrials.org/news/grand-rounds-april-14-2023-recover-in-action-status-of-clinical-trial-protocols-kanecia-zimmerman-phd-md-mph/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.