Abstract
Influenza viruses A/PR/8/34 (PR8; H1N1), A/Aichi/68 X-31 (HKx31; H3N2), and A/Beijing/89 X-109 (BJx109; H3N2) show marked differences in their ability to infect murine macrophages, including resident alveolar and peritoneal macrophages as well as the macrophage-derived cell line J774. The hierarchy in infectivity of the viruses (PR8 < HKx31 < BJx109) resembles that of their reactivity with mannose-binding lectins of the collectin family. Since the macrophage mannose receptor recognizes the same spectrum of monosaccharides as the collectins do, we investigated the possible involvement of this receptor in infection of macrophages by influenza virus. In competitive binding studies, the binding of 125I-labeled mannosylated bovine serum albumin to macrophages was inhibited by the purified hemagglutinin and neuraminidase (HANA) glycoproteins of influenza virus but not by HANA that had been treated with periodate to oxidize its oligosaccharide side chains. The inhibitory activity of HANA from the three strains of virus differed markedly and correlated with the infectivity of each virus for macrophages. Infection of macrophages, but not MDCK cells, by influenza virus was inhibited by yeast mannan. A variant line of J774 cells, J774E, which expresses elevated levels of the mannose receptor, was more readily infected than J774, and the sensitivity of J774E cells to infection was greatly reduced by culture in the presence of d-mannose, which down-modulated mannose receptor expression. Together, the data implicate the mannose receptor as a major endocytic receptor in the infectious entry of influenza virus, and perhaps other enveloped viruses, into murine macrophages.
Infection of host cells by influenza virus is mediated by binding of the viral hemagglutinin (HA) to sialylated cell surface molecules, followed by receptor-mediated endocytosis and acid-activated membrane fusion in endosomes (22). Many different sialylated glycoproteins and glycolipids on the cell membrane may function as primary receptors for influenza virus attachment, but not all binding leads to infection (for example, see references 8 and 49). With the exception of recent studies on influenza C virus, little is known about the identity of the functional receptor(s) that initiates the infectious process. Influenza C virus differs from influenza A and B viruses in recognizing the less common N-acetyl-9-O-acetylneuraminic acid, rather than N-acetylneuraminic acid, as its specific receptor determinant. Zimmer et al. (52) have identified a major mucin-type glycoprotein on the surface of Madin-Darby canine kidney type I cells, gp40, that binds influenza C virus and is subject to constitutive endocytosis and that may represent the functional receptor for influenza C virus in this cell type.
In this study we focus on the infectious entry of influenza A virus into macrophages (Mφ). Influenza virus infects Mφ, and viral proteins are expressed, but replication is abortive and little or no infectious virus is produced (38, 46). By acting as a “dead end” for the virus, Mφ play an important role in early host defense against influenza virus infection. Furthermore, infection of Mφ leads to the production of proinflammatory cytokines and alpha/beta interferon (IFN-α/β), which will further act to limit virus spread (31, 32).
We observed a marked difference among three strains of influenza A virus in their ability to infect murine Mφ. Interestingly, the relative infectivity of the viruses for Mφ paralleled their sensitivity to the collectins serum mannose binding lectin (MBL) and lung surfactant protein D (36). These are soluble collagenous Ca2+-dependent (C-type) lectins involved in innate host defense (15), which bind to oligosaccharide moieties on influenza virus glycoproteins and mediate viral aggregation, opsonization, and neutralization of virus infectivity (2, 14). The collectin sensitivity of influenza viruses is related to the level of glycosylation of the viral glycoproteins (36).
The mannose receptor (MR) is an integral membrane protein that is expressed on tissue Mφ and immature dendritic cells and mediates the uptake of glycoproteins terminating in mannose, fucose, or N-acetylglucosamine (34, 39, 41). Since the saccharide specificity of the MR overlaps that of the collectins (15), influenza virus glycoproteins represent potential ligands for this receptor. High-affinity ligand recognition by the MR is effected through clustering of its multiple C-type lectin domains (44). Following endocytosis of the receptor-ligand complex in clathrin-coated pits, bound ligand is released in the acidic environment of the endosome and the MR recycles back to the cell surface to mediate subsequent rounds of internalization (40).
The MR has various functions. It is involved in clearance from the circulation of endogenous proteins bearing high-mannose chains, including lysosomal hydrolases (40) and tissue plasminogen activator (29). It contributes to the acquired immune response by mediating the uptake of mannosylated antigens by dendritic cells for processing and presentation to T lymphocytes (11, 35, 39), and a soluble form of the MR present in serum may be involved in antigen transport and presentation of glycoconjugates to specialized antigen-presenting cells (21). The MR also plays a key role in innate immunity by binding to surface glycans on a wide range of bacterial, fungal, and parasitic pathogens and mediating their uptake by phagocytosis (27, 42). The role of the MR in viral infection is, however, largely unexplored. In this study we investigate the possible involvement of the MR in infectious entry of influenza virus into Mφ.
MATERIALS AND METHODS
Viruses and viral glycoproteins.
The influenza A viruses used in this study were the Mt. Sinai strain of A/PR/8/34 (H1N1) (PR8) and viruses BJx109 (H3N2) and HKx31 (H3N2), which are high-yielding reassortants of PR8 with A/Beijing/353/89 (H3N2) and A/Aichi/68 (H3N2), respectively. In HKx31, all genes except those encoding the hemagglutinin (HA) and neuraminidase (NA) are derived from the PR8 parent (4). BJx109 has not been fully genotyped but is known to carry the HA and NA genes of A/Beijing/353/89 (H3N2) and the M, PA, and PB2 genes of PR8 (Alan Hampson, World Health Organization Collaborating Centre for Reference and Research on Influenza, Melbourne, Australia, personal communication). Viruses were grown in eggs and purified from allantoic fluid as described previously (1). Infectivity titers of allantoic fluids were determined by plaquing on Madin-Darby canine kidney (MDCK) cell in the presence of trypsin (2). Viral HA and NA glycoproteins (hereafter denoted HANA) were prepared by treatment of purified virus with n-octyl-β-d-glucoside, followed by centrifugation to remove the viral cores and dialysis to remove the detergent, as described previously (36).
Periodate treatment of HANA.
BJx109 HANA (350 μg/ml in 50 μl of Tris-buffered saline) was treated with an equal volume of 0.022 M NaIO4 at room temperature for 30 min followed by 1.5 volumes of glycerol (0.44%, wt/vol) to inactivate the NaIO4. For mock-treated samples, the periodate and glycerol were mixed before the addition of HANA.
Mφ.
Resident peritoneal and alveolar Mφ from BALB/c mice were cultured in Dulbecco minimal essential medium DMEM/F-12 (Gibco BRL, Grand Island, N.Y.) which had been supplemented with additional folic acid (6 μg/ml), l-asparagine (36 μg/ml), l-arginine (116 μg/ml), NaHCO3 (2 mg/ml), gentamicin (30 μg/ml), 10 mM HEPES, 0.05 mM 2-mercaptoethanol, and 10% fetal calf serum (FCS) and is referred to here as DF-10 medium. Peritoneal cells were obtained by lavage of the peritoneal cavity with 5 ml of cold RPMI 1640 medium (Gibco) supplemented with 30 μg of gentamicin per ml and 10 U of heparin per ml (RPGH). To obtain alveolar cells, lungs were lavaged in situ five times with 1 ml of RPGH by means of a blunted 23-gauge needle inserted into the trachea. Peritoneal and alveolar cells were treated with Tris-NH4Cl (0.14 M NH4Cl in 17 mM Tris [pH 7.2]) to lyse erythrocytes, washed twice, and resuspended in DF-10, and the large Mφ-like cells were counted.
The mouse Mφ lines J774 and J774E were cultured in α-minimal essential medium (Gibco) supplemented with 2 mM glutamine, 2 mM pyruvate, 30 μg of gentamicin per ml, 60 mM thioguanine, and 10% FCS (α-MEM-10). These cell lines were provided by Philip Stahl, Department of Cell Biology and Physiology, Washington University School of Medicine, St. Louis, Mo.
For infection studies, Mφ were seeded in 250 μl into the wells of eight-well glass chamber slides (Lab-Tek; Nunc, Naperville, Ill.) and incubated overnight, and nonadherent cells were removed by washing. The cell density used for seeding was chosen so as to achieve similar densities of adherent cells from the different Mφ populations (2.5 × 105 to 5 × 105 cells per well for resident alveolar and peritoneal Mφ, 6 × 104 cells per well for J774 and J774E).
For binding studies, Mφ were used in suspension. Tissue culture flasks (80 cm2; Nunc, Glostrup, Denmark) were seeded with 2 × 107 peritoneal cells in 10 ml of DF-10 medium, and after 3 to 4 h of incubation at 37°C, nonadherent cells were removed by washing. Adherent cells were cultured overnight, detached from flasks by incubating for 20 min on ice in Hanks balanced salt solution containing 5 mM EDTA, washed, and resuspended in binding buffer (see below) for binding experiments or in DF-10 for microscopy. Microscopic examination of cytocentrifuged samples stained with Diff Quick (Lab Aids, Narrabeen, Victoria, Australia) showed the proportion of Mφ in these preparations to exceed 90%.
Infection of Mφ by influenza virus.
Mφ monolayers in eight-well chamber slides were washed with serum-free medium and incubated for 1 h at 37°C with influenza virus (106 PFU unless otherwise stated) in 300 μl. Unadsorbed virus was removed, and incubation of the cells in serum-free medium was continued for a further 7 to 9 h. The cell monolayers were then washed in phosphate-buffered saline (PBS), fixed in acetone, and stained with a 1/1,000 dilution of a monoclonal antibody (MAb A-3) specific for the nucleoprotein of type A influenza viruses followed by fluorescein isothiocyanate-conjugated sheep anti-mouse immunoglobulin (Silenus, Melbourne, Australia). The wells were viewed under ×128 magnification, fluorescent- and total-cell numbers in four fields were counted (>200 Mφ in total), and the percentage of fluorescent cells was determined. MAb A-3 was provided by Nancy Cox, Influenza Branch, Centers for Disease Control and Prevention, Atlanta, Ga.
To test the effect of mannan on viral infection, cells were preincubated for 20 min at 37°C in 200 μl of medium containing the saccharide at 1.5 times its final concentration, before the addition of 106 PFU of virus in 100 μl. Unless otherwise stated, mannan was included in all washing and culture steps of the experiment.
Assay of virus adsorption.
To assay the adsorption of BJx109 and PR8 viruses to Mφ, peritoneal Mφ in chamber slides were incubated with 3 × 106 PFU virus in 0.1 ml of serum-free medium for 1 h and washed, and the absorbed virus was eluted by incubating the monolayer for 2 h with Vibrio cholerae NA type III (Sigma no. N-7885; 20 mU in 0.1 ml of serum-free medium). All steps were carried out at 4°C to inhibit viral entry. The eluates were removed, 2.5 mM 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DDN; Boehringer, Mannheim, Germany) was added to the eluate samples to inhibit the residual bacterial NA activity, and the titer of infectious virus was determined by plaquing on MDCK cells as described previously (2). Preliminary experiments had shown that the presence of bacterial NA in virus samples had an adverse effect on plaquing efficiency, presumably through destruction of sialylated receptors on the MDCK cells during virus adsorption, and that this effect was reversed by the inclusion of DDN during the adsorption phase.
Radioiodination.
Mannosylated bovine serum albumin (mBSA; 31 mol of mannose per mol of BSA) was purchased from E. Y. Laboratories Inc. (San Mateo, Calif.). Concanavalin A (ConA) was obtained from Boehringer Mannheim Corp., Indianapolis, Ind. ConA, mBSA and purified influenza HANA glycoproteins were labeled with 125I using a modification (18) of the chloramine-T method described by Greenwood (13).
Mφ binding assays. (i) 125I-labeled mBSA.
Binding assays were conducted in Tris-buffered saline (0.05 M Tris-HCl, 0.15 M NaCl [pH 7.2]) supplemented with 20 mM CaCl2 (binding buffer). Mφ suspensions were washed, and aliquots of 5 × 105 cells were resuspended in 90 μl of binding buffer in microcentrifuge tubes in the presence or absence of appropriate inhibitors. The tubes were held on ice for 20 min, and 5 × 105 cpm of 125I-mBSA (1 × 106 to 6 × 106 cpm/μg) was added in a volume of 10 μl or, for determination of saturation binding curves, a range of doses of 125I-mBSA was used. After a further 2 h on ice, cell suspensions were layered over 200 μl of chilled FCS in small, flexible centrifuge tubes (Elkay Products Inc., Shrewsbury, Mass.) and centrifuged in an Eppendorf microcentrifuge at 4°C for 2 min. The tips of the tubes containing the cell pellets were cut off and counted in a γ-counter. The upper parts of the tubes containing supernatants were also counted to confirm that all tubes had received a similar total count of iodinated sample. Assays were performed in duplicate or triplicate. Nonspecific binding of 125I-mBSA was measured in the presence of 2 mg of mannan per ml and was subtracted from total binding to calculate specific binding.
(ii) 125I-labeled HANA.
Binding of 125I-HANA to murine Mφ was assayed similarly to 125I-mBSA binding, except that 5 × 104 cpm of 125I-HANA (1 × 106 cpm/μg) was added to suspensions of 5 × 105 cells and no correction was made for nonspecific binding.
Sialidase treatment of Mφ.
To examine the effect of sialidase treatment on the binding of 125I-mBSA and 125I-HANA and on infection of Mφ by influenza virus, 9 × 106 peritoneal Mφ in suspension were treated with 300 mU of V. cholerae NA in 1.5 ml of serum-free DF-10 medium for 1 h at 37°C. Mock-treated cells were incubated similarly in serum-free medium alone. The cells were then washed three times and resuspended in binding buffer for binding studies (see above) or in serum-free medium for infection studies. For infection, 106 sialidase- or mock-treated Mφ were incubated for 30 min at 4°C with 6 × 106 PFU of BJx109 virus in 0.5 ml, after which the cells were pelleted by centrifugation, washed, and incubated in serum-free medium in Teflon pots (Savillex, Minnetonka, Minn.) for 7 h. The cells were then cytocentrifuged, fixed in acetone, and stained by immunofluorescence with anti-NP MAb A-3.
Binding of 125I-labeled ConA to influenza virus.
Wells of a polyvinyl microtiter tray were coated overnight with 50 μl of a series of concentrations of purified influenza virus in PBS and then blocked for 1 h with BSA (10 mg/ml). The wells were washed with PBS containing 0.05% Tween 20 (PBST) and then incubated for 3 h with 2 × 105 cpm of 125I-ConA in PBST containing 5 mg of BSA per ml. The wells were washed again, and the radioactivity associated with individual wells was determined in a γ-counter.
To confirm that the different viruses (BJx109, HKx31, and PR8) bound to the plastic wells with similar efficiency, additional sets of virus-coated and blocked wells were incubated for 2 h with either (i) MAb 165, a carbohydrate-specific MAb that recognizes the cross-reactive host antigen common to all egg-grown influenza viruses (36) or (ii) MAb A-3, which recognizes the NP of type A influenza viruses. After being washed, the wells were incubated with 2 × 105 cpm of 125I-labeled rabbit anti-mouse immunoglobulins and processed as above.
RESULTS
Infection of murine Mφ by different strains of influenza virus.
We observed a marked difference among three strains of influenza A virus, BJx109, HKx31 and PR8, in their ability to infect murine Mφ, as assessed by immunofluorescence microscopy at 8 to 10 h postinfection. This difference in infectivity was observed with resident peritoneal and alveolar Mφ from BALB/c mice and with the murine Mφ cell line J774 (Table 1), as well as with peritoneal Mφ from C57BL/10 and CBA mice (data not shown). BJx109 infected each of the Mφ populations most efficiently, HKx31 gave intermediate levels of infection, and PR8 infected only a small percentage of cells. For PR8 virus, immunofluorescent staining at 24 and 48 h postinfection revealed no further increase in infection and minimal cytopathic effect was observed. In contrast, Mφ infected with BJx109 and HKx31 viruses showed extensive cytopathic effect by 24 h postinfection. Assay of Mφ culture supernatants for infectious virus by plaquing on MDCK cells in the presence of trypsin revealed no increase in virus titer at 24 h postinfection compared to 2 h, with the latter titer representing virus inoculum that had spontaneously eluted from the cells (data not shown). These observations are consistent with the reports of others that influenza virus infection of Mφ is abortive (38, 46).
TABLE 1.
Differences in the ability of influenza virus strains to infect murine Mφ
| Virusa | % of infected cellsb
|
||
|---|---|---|---|
| Peritoneal Mφ | Alveolar Mφ | J774 Mφ cell line | |
| BJx109 | 59 ± 11 | 71 ± 6 | 53 ± 7 |
| HKx31 | 35 ± 7 | 42 ± 9 | 37 ± 12 |
| PR8 | 3 ± 1 | 8 ± 1 | 3 ± 1 |
Mφ monolayers in chamber slides were infected for 1 h with 106 PFU of influenza virus. At 8 to 10 h postinfection, cells were stained by immunofluorescence for expression of influenza virus NP.
Data represent the mean percent infection ± 1 standard error of the mean from three experiments. MDCK cells infected under the same conditions showed >95% infection by all three viruses.
The reassortant HKx31 (H3N2) virus is known to derive all of its genes for internal components from A/PR/8/34 (H1N1) virus (4); hence, the difference in ability of these two viruses to infect murine Mφ most probably reflects a difference in their surface glycoproteins. The low infectivity of PR8 for Mφ was not typical of other H1N1 subtype viruses, however, since A/USSR/77 (H1N1) and A/Brazil/78 (H1N1) viruses infected Mφ with high efficiency (data not shown). Furthermore, the low infectivity was not due to failure of PR8 to bind to Mφ, since the quantity of infectious virus that could be eluted from Mφ monolayers with V. cholerae NA following adsorption of virus for 1 h at 4°C was shown to be very similar for PR8 and BJx109 viruses (1.8 × 104 to 7.5 × 104 and 3.1 × 104 to 17.1 × 104 PFU, respectively, in three experiments).
A particular feature of the HA molecule of PR8 (Mt. Sinai) is the absence of carbohydrate from the globular head of the molecule and its overall lack of high-mannose-type glycans (9, 25). In contrast, BJx109 and HKx31 viruses carry 4 and 2 potential glycosylation sites on the head of HA, respectively (37, 45). In a previous study we have shown that differences in glycosylation of the HA molecules of influenza viruses markedly affect their interaction with collectins, the collagenous mannan-binding C-type lectins that are present in serum and pulmonary fluids (36). We observed here that the hierarchy in the ability of the three viruses to infect Mφ (BJx109 > HKx31 > PR8) paralleled their sensitivity to collectins. Since the Mφ MR recognizes the same spectrum of monosaccharides as the collectins do (15) and functions in both endocytosis and phagocytosis (41), we investigated a possible role for the MR in infection of Mφ by influenza virus.
Interaction of influenza virus glycoproteins with the Mφ MR.
To determine whether influenza viruses interact with the MR, we examined the ability of purified HANA viral glycoproteins to inhibit the binding of a known ligand of this receptor, 125I-labeled mBSA, to peritoneal Mφ. We established in a separate experiment that 125I-mBSA and HANA do not themselves interact, by demonstrating the failure of 125I-mBSA to bind to HANA-coated microtiter wells under conditions where the binding of specific antibody to such wells and the binding of 125I-mBSA to wells coated with the collectin MBL were readily demonstrated (data not shown). Any inhibition of binding of 125I-mBSA to Mφ by HANA should therefore indicate direct interaction of HANA with the MR.
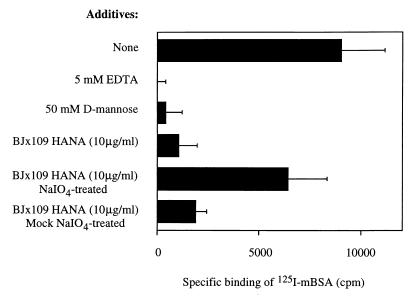
Specific binding of 125I-mBSA to macrophages was inhibited by EDTA and by d-mannose, as expected (Fig. 1). Binding was also strongly inhibited by the HANA glycoproteins of BJx109 virus, and this inhibitory capacity was lost if the HANA was treated with periodate to oxidize viral carbohydrate.
FIG. 1.
Binding of 125I-mBSA to peritoneal Mφ. Mφ were preincubated on ice for 20 min with binding buffer alone or supplemented with additives as indicated, before the addition of 5 × 105 cpm of 125I-mBSA. Results are expressed as the mean specific binding (and 1 standard error) of 125I-mBSA in triplicate samples as described in Materials and Methods.
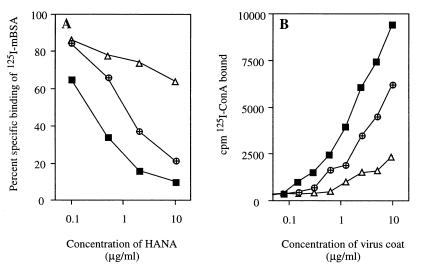
When HANA preparations of the three viruses were compared, marked differences in their apparent avidity for the MR were evident: inhibition of 125I-mBSA binding was strongest with BJx109, intermediate with HKx31, and weakest with PR8 HANA (Fig. 2A). The inhibitory activities of the three HANA preparations correlated directly with their content of high-mannose or hybrid-type glycans, as indicated by binding of 125I-labeled ConA to purified viruses of the three strains (Fig. 2B) (3, 28), and paralleled the efficiency with which the respective viruses infect Mφ (Table 1). Taken together with the effect of periodate treatment mentioned above, these data imply a direct interaction of influenza virus HANA glycoproteins, through their carbohydrate, with the lectin domain(s) of the MR and are consistent with an involvement of the MR in infection of Mφ by influenza virus.
FIG. 2.
Relationship between the relative binding avidity of the virus HANA glycoproteins for the MR on peritoneal Mφ and the content of high-mannose and hybrid-type oligosaccharide on influenza virus as determined by 125I-ConA binding. The virus strains used were BJx109 (■), HKx31 (⊕), and PR8 (▵). (A) Ability of HANA preparations of the three viruses to inhibit the binding of 125I-mBSA to peritoneal Mφ. (B) Binding of 125I-ConA to microtiter wells coated with increasing concentrations of purified influenza viruses. Binding was completely inhibited in the presence of 100 mM α-methylmannoside (data not shown). Coating levels of the three viruses were confirmed to be similar by using MAbs to the viral NP and to the host-derived carbohydrate antigen common to all egg-grown strains of influenza virus, as described in Materials and Methods (data not shown).
Effect of mannan on infection of Mφ by influenza virus.
Since binding of mannosylated ligands to the MR is inhibited by yeast mannan, it was of interest to investigate the effect of mannan on infection of macrophages by influenza virus. Mφ monolayers in chamber slides were incubated for 1 h with 106 PFU of influenza virus in the presence or absence of mannan (5 mg/ml). Following removal of unbound virus, the cells were washed and incubated for a further 8 h, again in the presence or absence of mannan, and infection was assessed by immunofluorescence.
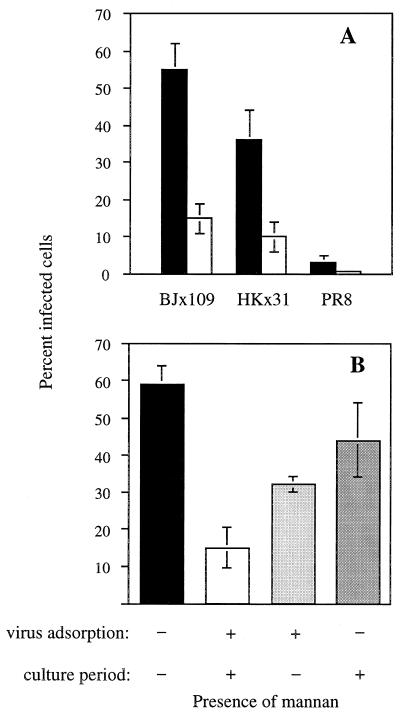
The presence of mannan throughout the experiment led to marked inhibition of infection of Mφ by each of the three viruses (Fig. 3A). Under the same conditions, mannan had no effect on the ability of these viruses to infect MDCK cells, which lack an MR (data not shown). When mannan was included only for the first hour of the experiment (i.e., during the virus adsorption and early-entry phase), it was less effective at inhibiting Mφ infection (Fig. 3B), suggesting that virus adsorption to sialylated receptors was not blocked by this treatment and that the process of infection could resume once mannan was removed. Consistent with this finding, mannan had no effect on the binding of 125I-labeled HANA glycoproteins of BJx109 to peritoneal Mφ (data not shown). Mannan added after 1 h had little inhibitory effect on infection of Mφ by BJx109, indicating that it does not block postentry stages of influenza virus replication or gene expression in Mφ. Together, these results point to the effect of mannan on infection being mediated at the stage of virus entry, possibly through an effect on the MR.
FIG. 3.
Mannan inhibits infection of peritoneal Mφ by influenza virus. (A) Infection of peritoneal Mφ by 106 PFU of influenza viruses BJx109, HKx31, and PR8 in the absence (■) or presence (□) of 5 mg of mannan per ml. Mannan was present both at the time of virus adsorption and during subsequent culture of the Mφ. (B) Effect of the time of addition of mannan on inhibition of infection of Mφ by BJx109 virus. Results are presented as the mean percent infection (and 1 standard error) from three experiments.
Effect of different levels of MR expression on infection of Mφ by influenza virus.
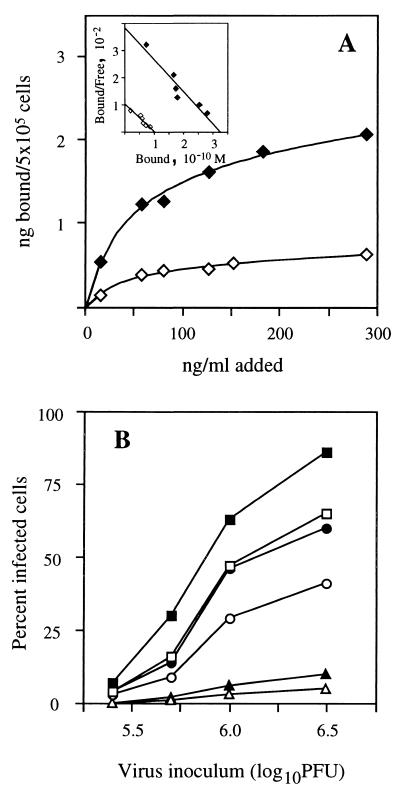
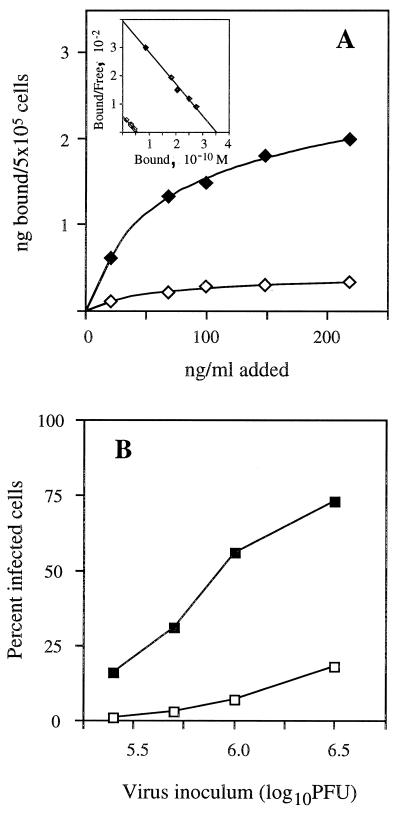
To further assess the role of the MR in influenza virus infection, we compared the sensitivities of two murine Mφ lines, J774 and J774E, to infection by influenza virus. J774E is a variant line of J774 that was selected on the basis of its increased expression of the MR (10). Binding studies with 125I-mBSA confirmed the differing MR expression of the two cell lines (Fig. 4A), with binding to each cell line being saturable and being three- to fourfold higher for J774E than for J774. Scatchard analysis of the data yielded similar dissociation constants for binding of ligand to the two cell lines (Kd = 10 and 11.5 nM for J774 and J774E, respectively), indicating that the different levels of binding reflect a difference in receptor number rather than receptor affinity.
FIG. 4.
Comparison of J774 and J774E cells for MR expression and susceptibility to influenza virus infection. (A) Binding of 125I-mBSA to J774 (◊) and J774E (⧫). Scatchard plots of the data (inset) yielded Kd values of 10 and 11.5 nM for J774 and J774E cells, respectively. For these calculations, the molecular mass of mBSA (31 mol of mannose per mol of BSA) was taken to be 73,000 kDa. (B) Infection of J774 (open symbols) and J774E (solid symbols) by BJx109 (□, ■), HKx31 (○, ●), and PR8 (▵, ▴). The greater susceptibility of J774E cells to influenza virus infection was observed in multiple experiments.
In infection studies, each of the three strains of influenza virus showed higher infectivity for J774E than for J774 cells (Fig. 4B). Although we cannot rule out the possibility that J774 and J774E differ in ways other than MR expression, the higher infectivity of influenza viruses for J774E is consistent with involvement of MR in the infectious process.
The effect of downregulation of MR expression on J774E cells was also examined. Culture of J774E cells in 25 mM d-mannose for 2 h resulted in a marked (five- to sixfold) reduction in MR expression as assessed by binding of 125I-mBSA to the cells after they had been washed free of mannose (Fig. 5A). The sensitivity of J774E cells to infection by BJx109 virus was likewise markedly reduced (Fig. 5B); in this experiment, 25 mM d-mannose was present throughout the infection and subsequent culture period. Under the same experimental conditions, d-mannose had no effect on infection of MDCK cells by BJx109 virus (data not shown), arguing against a nonspecific effect of this sugar on cell susceptibility to influenza virus or on influenza virus replication per se. Taken together, the results of this and the previous experiment indicate a close association between levels of MR expression and sensitivity to influenza virus infection of the J774 and J774E Mφ cell lines.
FIG. 5.
Downregulation of MR in J774E cells is associated with reduced susceptibility to influenza virus infection. (A) J774E cells were cultured for 2 h in the presence (◊) or absence (⧫) of 25 mM d-mannose, then washed free of the sugar and assayed for 125I-mBSA binding. Scatchard plots of the data (inset) yielded Kd values of 10 and 11.1 nM for cells cultured in the presence or absence of mannose, respectively. (B) J774E cell monolayers in chamber slides were incubated for 2 h in the presence (□) or absence (■) of 25 mM d-mannose and then infected for 1 h with increasing doses of BJx109. The presence or absence of mannose was maintained, as appropriate, throughout all stages of the infection assay including virus adsorption, washing, and subsequent culture steps. The results given are from one of two similar experiments.
Sialic acid requirements for interaction of influenza virus with the MR and for viral infection.
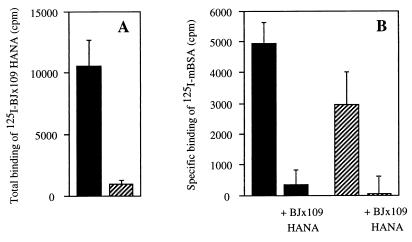
As described above, the blocking of 125I-mBSA binding to Mφ by HANA glycoproteins from the three strains of virus suggested a direct interaction of HANA with the MR mediated through the viral carbohydrate. Since the primary receptor for influenza virus is sialic acid and since the MR itself is a sialylated glycoprotein (19), it was also of interest to examine the sialic acid dependence or otherwise of the interaction of HANA glycoproteins with the MR. Peritoneal Mφ were treated with V. cholerae NA or mock treated and then tested for binding of 125I-mBSA in the presence or absence of BJx109 HANA. They were also tested for their ability to be infected by BJx109 virus. The effectiveness of the sialidase treatment was monitored by comparing the binding of 125I-labeled BJx109 HANA to treated and mock-treated Mφ; as shown in Fig. 6A, binding of 125I-labeled HANA was reduced by >90% following sialidase treatment.
FIG. 6.
Role of sialic acid in the interaction of influenza virus glycoproteins with the MR. Peritoneal Mφ were incubated with V. cholerae type III NA (▨) or in medium alone (mock treated) (■) for 60 min at 37°C, washed, and used in binding assays with 125I-BJx109 HANA (A) and 125I-mBSA (B). Specific binding of 125I-mBSA was determined in the absence or presence of 10 μg of unlabeled BJx109 HANA per ml. Results are expressed as mean cpm bound (and 1 standard error) from triplicate samples and are from one of two similar experiments.
Sialidase-treated Mφ retained the capacity for specific binding of 125I-mBSA (Fig. 6B), an observation consistent with the finding of Pontow et al. (33) that inhibition of sialylation of the glycans on newly synthesized MR did not affect the lectin activity of the receptor. Furthermore, BJx109 HANA blocked the binding of 125I-mBSA to sialidase-treated Mφ and control Mφ to a similar extent (Fig. 6B), indicating that interaction of HANA with the MR does not require sialic acid on the latter and can occur, as with other MR ligands, through direct binding of viral carbohydrate to the lectin domains of the MR. Infection of Mφ by BJx109 virus, however, was highly sialic acid dependent, in that 74% of mock-treated Mφ and only 10% of sialidase-treated Mφ became infected following incubation with BJx109 virus for 30 min at a multiplicity of infection of 3. The carbohydrate-mediated interaction of influenza virus glycoproteins with lectin domains of the MR is thus, on its own, not sufficient to mediate infection of Mφ by influenza virus in the absence of sialic acid.
DISCUSSION
The results of the present study point strongly to involvement of the MR in infection of Mφ by influenza A virus. The study was prompted by our observation that the efficiency of infection of murine Mφ by three strains of influenza virus, BJx109, HKx31, and PR8, differed markedly and paralleled the sensitivity of the viruses to C-type lectins of the collectin family (36), whose carbohydrate specificity is similar to that of the MR. Evidence for a direct interaction of viral HANA glycoproteins with MR on the Mφ surface was obtained from competitive binding experiments with 125I-mBSA, and the avidity of HANA for the MR correlated with the efficiency of infection of Mφ by the three viruses in question. The efficiency of infection by influenza virus also correlated with the level of expression of MR on the Mφ. Furthermore, infection of Mφ was inhibited by yeast mannan, a known ligand of the MR, at a stage subsequent to virus adsorption. Given the known endocytic activity of the MR and the fact that uptake of influenza virus into an endosome following adsorption is an obligatory step in the infectious process, the present results suggest that uptake via the MR represents a major endocytic route for influenza virus into Mφ.
Since the MR is both sialylated and a lectin, interaction of influenza virus with this receptor might occur in two ways: by binding of the viral HA through its receptor binding site to sialic acid on the MR, or by binding of glycans on the HA and NA glycoproteins to the lectin domains of the MR. The competition experiments with 125I-mBSA indicated binding by the latter mechanism. Thus, (i) treatment of HANA glycoproteins with periodate destroyed their ability to inhibit the binding of 125I-mBSA to Mφ; (ii) the avidity of HANA preparations from the three viruses for the MR correlated directly with their high mannose and/or hybrid glycan content, as indicated by the ability of the viruses to bind ConA (3, 28); and (iii) HANA could block binding of 125I-mBSA to Mφ that had been extensively desialylated. We conclude that binding of the viral glycoproteins to the MR occurs predominantly through the viral carbohydrate and does not require interaction through sialic acid, although HA binding to sialic acid on the MR under normal circumstances is not excluded.
As observed by others (26, 43) and confirmed in this study, infection of Mφ by influenza virus is sialic acid dependent, as it is for other cell types. Interaction of the virus through its carbohydrate with the MR is thus clearly not sufficient to mediate infectious entry of the virus, even though, by analogy to other MR ligands, uptake of the virus into endosomes under these circumstances might be expected. Receptor binding by the HA, however, is now recognized to be required not only for binding and subsequent endocytosis of the virus by the host cell but also for efficient fusion of host and viral membranes in the endosome to bring about the entry of the viral nucleocapsid into the cytoplasm (23, 30). The latter requirement is thought to reflect the need for close apposition of viral and endosomal membranes and correct orientation of the HA at the time of the acid-induced conformational change in HA and exposure of the fusion peptide. Under normal circumstances, this apposition is mediated by binding of the HA to sialic acid on the endosomal membrane. Since the MR dissociates from its ligand at the pH of the endosome, it would be unable to substitute for sialic acid in providing this link in desialylated cells: virus particles that during endocytosis were bound only to the lectin domains of the MR would be released from the host membrane in the endosome and membrane fusion would not occur. The situation can be contrasted with the ability of influenza virus to infect desialylated Mφ in the presence of subneutralizing levels of antiviral antibody (26, 43). In that case, antibody bound to Fc receptors on the Mφ can act as a surrogate receptor for the virus, the antigen-antibody association being stable in the acidic environment of the endosome.
The dual dependence on sialic acid and the MR for infection of Mφ by influenza virus suggests the following model. Following, or coincident with, primary binding of virus to sialylated glycoprotein or glycolipid receptors on the cell surface, virus particles bind through their oligosaccharide moieties to the lectin domains of the MR. The avidity of the latter interaction, and hence the efficiency of endocytosis, will be determined by the nature and density of glycosylation of the HA and NA glycoproteins of the virus in question (16). Since the MR is itself sialylated, the sialic acid-binding requirement for infection may be met by the MR also, although whether sialic acid on the MR is accessible, of an appropriate type, and present in the correct linkage or conformation to be recognized by influenza virus is not known at present. Alternatively, neighboring sialylated receptors that are bound by the virus may be taken into the endosome along with the virus and the MR.
The finding that the HANA glycoproteins of PR8 (Mt. Sinai) virus interact poorly with the MR is consistent with the known paucity of high-mannose or hybrid-type glycans on its surface glycoproteins (Fig. 2B) (25) and the overall lack of glycosylation sites on the head of its HA molecule (9), and in the proposed model this finding accounts for the low level of infection of Mφ by PR8 that we have observed here. Interestingly, we have found the Cambridge strain of PR8 virus to infect Mφ with three- to fivefold higher efficiency than the Mt. Sinai strain does. PR8 (Cambridge) carries a potential glycosylation site on the head of HA (at residue 131 in the H3 numbering) which is lacking in PR8 (Mt. Sinai) (9, 50). A difference in electrophoretic mobility of the HA molecules of the two viruses indicated that this site is glycosylated in the Cambridge strain, and PR8 (Cambridge) was shown to be more sensitive than PR8 (Mt. Sinai) to hemagglutination inhibition by the collectin MBL in mouse serum (G. Selvaraj, J. L. Miller, and E. M. Anders, unpublished data). These findings further implicate glycosylation as a factor in the infectivity of influenza virus for Mφ.
A difference in the substrain of PR8 virus used may in part explain the fact that other researchers studying the interaction of influenza virus with Mφ have not found the infectivity of PR8 virus to be particularly low (31). Another important difference may lie in the Mφ populations used. Alternative or additional routes of infectious entry of influenza virus may exist in Mφ at different stages of differentiation or activation from those used here, as they clearly do in cell types that lack the MR. Expression of the MR itself is downregulated on Mφ activation (12, 17). For the Mφ populations used here, however, which included murine resident alveolar and peritoneal Mφ and J774 cells, the MR appears to represent an important endocytic route of virus entry into the cell.
Involvement of lectin-like cell receptors in viral binding or entry has been described previously for certain other enveloped viruses. Thus, for Sendai virus, the hepatic asialoglycoprotein receptor (ASGPR) was shown to represent an alternative route into HepG2 hepatoma cells under conditions where involvement of sialylated receptors was bypassed, either by use of a mutant virus with a temperature-sensitive HN glycoprotein (20) or by use of wild-type virus with desialylated cells (6). Viral attachment was mediated through recognition by the ASGPR of galactose-terminated glycans on the viral fusion (F) glycoprotein, and viral entry occurred by the usual mode, i.e., membrane fusion at the cell surface. Interaction of Marburg virus with the ASGPR has also been documented, a finding which may explain the marked hepatotropism of the virus (5). Another lectin, the mannose-6-phosphate receptor, has been implicated in infection of Vero cells by herpes simplex virus (7) and of human embryonic lung fibroblasts by varicella-zoster virus (51). Whether the MR facilitates infection of Mφ by other enveloped viruses has yet to be determined.
Marked differences between influenza virus strains in their ability to infect Mφ through the MR may have biological consequences. In particular, since infection of Mφ by influenza virus represents a “dead end” for incoming virions, with no infectious progeny being released (38, 46), and also stimulates the production of the antiviral cytokines tumor necrosis factor alpha and IFN-α/β (31, 32), evasion of Mφ entry by PR8 virus in the early stages of infection may enhance its survival in the respiratory tract and contribute to the virulence this virus displays for mice. In studies to be reported elsewhere, we have indeed found that both induction of Mφ cytokines in vitro and the early inflammatory response in vivo induced by PR8 (Mt. Sinai) are substantially weaker than the responses induced by BJx109 virus (P. C. Reading, J. L. Miller, and E. M. Anders, unpublished data). Furthermore, recent studies by Wijburg et al. (47, 48) indicated a differential effect of alveolar Mφ depletion on replication in mouse lung of PR8 compared with Mem71 influenza virus, a strain that interacts with the MR and infects Mφ readily in vitro (Reading et al., unpublished). Following treatment with dichloromethylene diphosphonate-loaded liposomes to deplete alveolar Mφ, mice infected with Mem71 virus had significantly higher titers of virus in the lungs at 4 days postinfection than did normal infected mice (48), whereas for PR8 the treatment had little effect on virus yield (47). These observations are consistent with (i) a role for alveolar Mφ in early containment of Mem71 infection and (ii) evasion of Mφ by PR8 in vivo and suggest, by inference, that the MR-mediated route of entry of influenza virus into Mφ is biologically significant.
The MR is expressed not only by Mφ but also by dendritic cells, where it facilitates the capture of mannosylated antigens for processing and presentation to T cells (11). In addition, the MR has been implicated as a receptor for nonspecific recognition of enveloped viruses leading to IFN-α production by peripheral blood dendritic cells (24). Studies looking for differences between influenza virus strains in their interaction with dendritic cells that might relate to their interaction with the MR are under way in this laboratory.
ACKNOWLEDGMENTS
This work was supported by grant 970283 from the National Health and Medical Research Council of Australia.
We thank Sharon Feigl for technical assistance.
REFERENCES
- 1.Anders E M, Hartley C A, Jackson D C. Bovine and mouse serum β inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci USA. 1990;87:4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders E M, Hartley C A, Reading P C, Ezekowitz R A. Complement-dependent neutralization of influenza virus by a serum mannose-binding lectin. J Gen Virol. 1994;75:615–622. doi: 10.1099/0022-1317-75-3-615. [DOI] [PubMed] [Google Scholar]
- 3.Baenziger J U, Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979;254:2400–2407. [PubMed] [Google Scholar]
- 4.Baez M, Palese P, Kilbourne E D. Gene composition of high-yielding influenza vaccine strains obtained by recombination. J Infect Dis. 1980;141:362–365. doi: 10.1093/infdis/141.3.362. [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Spiess M, Klenk H D. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J Gen Virol. 1995;76:393–399. doi: 10.1099/0022-1317-76-2-393. [DOI] [PubMed] [Google Scholar]
- 6.Bitzer M, Lauer U, Baumann C, Spiegel M, Gregor M, Neubert W J. Sendai virus efficiently infects cells via the asialoglycoprotein receptor and requires the presence of cleaved F0 precursor proteins for this alternative route of cell entry. J Virol. 1997;71:5481–5486. doi: 10.1128/jvi.71.7.5481-5486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunetti C R, Burke R L, Hoflack B, Ludwig T, Dingwell K S, Johnson D C. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J Virol. 1995;69:3517–3528. doi: 10.1128/jvi.69.6.3517-3528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll S M, Paulson J C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 9.Caton A J, Brownlee G G, Yewdell J W, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 10.Diment S, Leech M S, Stahl P D. Generation of macrophage variants with 5-azacytidine: selection for mannose receptor expression. J Leukoc Biol. 1987;42:485–490. doi: 10.1002/jlb.42.5.485. [DOI] [PubMed] [Google Scholar]
- 11.Engering A J, Cella M, Fluitsma D, Brockhaus M, Hoefsmit E C, Lanzavecchia A, Pieters J. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 12.Ezekowitz R A, Austyn J, Stahl P D, Gordon S. Surface properties of bacillus Calmette-Guerin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981;154:60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood F C. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartshorn K L, White M R, Shepherd V, Reid K, Jensenius J C, Crouch E C. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol. 1997;273:L1156–1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- 15.Holmskov U, Malhotra R, Sim R B, Jensenius J C. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoppe C A, Lee Y C. The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. Effect of the sugar density. J Biol Chem. 1983;258:14193–14199. [PubMed] [Google Scholar]
- 17.Imber M J, Pizzo S V, Johnson W J, Adams D O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982;257:5129–5135. [PubMed] [Google Scholar]
- 18.Jackson D C. Some effects of chloramin T induced radioiodination on the physiochemical properties of oligomeric proteins. J Immunol Methods. 1980;34:253–260. [Google Scholar]
- 19.Lennartz M R, Cole F S, Stahl P D. Biosynthesis and processing of the mannose receptor in human macrophages. J Biol Chem. 1989;264:2385–2390. [PubMed] [Google Scholar]
- 20.Markwell M A, Portner A, Schwartz A L. An alternative route of infection for viruses: entry by means of the asialoglycoprotein receptor of a Sendai virus mutant lacking its attachment protein. Proc Natl Acad Sci USA. 1985;82:978–982. doi: 10.1073/pnas.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Pomares L, Gordon S. Potential role of the mannose receptor in antigen transport. Immunol Lett. 1999;65:9–13. doi: 10.1016/s0165-2478(98)00117-5. [DOI] [PubMed] [Google Scholar]
- 22.Matlin K S, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millar B M, Calder L J, Skehel J J, Wiley D C. Membrane fusion by surrogate receptor-bound influenza haemagglutinin. Virology. 1999;257:415–423. doi: 10.1006/viro.1999.9624. [DOI] [PubMed] [Google Scholar]
- 24.Milone M C, Fitzgerald-Bocarsly P. The mannose receptor mediates induction of IFN-alpha in peripheral blood dendritic cells by enveloped RNA and DNA viruses. J Immunol. 1998;161:2391–2399. [PubMed] [Google Scholar]
- 25.Nakamura K, Compans R W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979;95:8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- 26.Ochiai H, Kurokawa M, Hayashi K, Niwayama S. Antibody-mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J Virol. 1988;62:20–26. doi: 10.1128/jvi.62.1.20-26.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 28.Ogata S, Muramatsu T, Kobata A. Fractionation of glycopeptides by affinity column chromatography on concanavalin A-Sepharose. J Biochem. 1975;78:687–696. doi: 10.1093/oxfordjournals.jbchem.a130956. [DOI] [PubMed] [Google Scholar]
- 29.Otter M, Barrett-Bergshoeff M M, Rijken D C. Binding of tissue-type plasminogen activator by the mannose receptor. J Biol Chem. 1991;266:13931–13935. [PubMed] [Google Scholar]
- 30.Pedroso de Lima M C, Ramalho-Santos J, Flasher D, Slepushkin V A, Nir S, Duzgunes N. Target cell membrane sialic acid modulates both binding and fusion activity of influenza virus. Biochim Biophys Acta. 1995;1236:323–330. doi: 10.1016/0005-2736(95)00067-d. [DOI] [PubMed] [Google Scholar]
- 31.Peschke T, Bender A, Nain M, Gemsa D. Role of macrophage cytokines in influenza A virus infections. Immunobiology. 1993;189:340–355. doi: 10.1016/s0171-2985(11)80365-7. [DOI] [PubMed] [Google Scholar]
- 32.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 33.Pontow S E, Blum J S, Stahl P D. Delayed activation of the mannose receptor following synthesis. Requirement for exit from the endoplasmic reticulum. J Biol Chem. 1996;271:30736–30740. doi: 10.1074/jbc.271.48.30736. [DOI] [PubMed] [Google Scholar]
- 34.Pontow S E, Kery V, Stahl P D. Mannose receptor. Int Rev Cytol. 1992;137B:221–244. doi: 10.1016/s0074-7696(08)62606-6. [DOI] [PubMed] [Google Scholar]
- 35.Prigozy T I, Sieling P A, Clemens D, Stewart P L, Behar S M, Porcelli S A, Brenner M B, Modlin R L, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 36.Reading P C, Morey L S, Crouch E C, Anders E M. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocha E P, Xu X, Hall H E, Allen J R, Regnery H L, Cox N J. Comparison of 10 influenza A (H1N1 and H3N2) haemagglutinin sequences obtained directly from clinical specimens to those of MDCK cell- and egg-grown viruses. J Gen Virol. 1993;74:2513–2518. doi: 10.1099/0022-1317-74-11-2513. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers B, Mims C A. Interaction of influenza virus with mouse macrophages. Infect Immun. 1981;31:751–757. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahl P, Schlesinger P H, Sigardson E, Rodman J S, Lee Y C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 41.Stahl P D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990;2:317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- 42.Stahl P D, Ezekowitz R A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol. 1998;10:50–55. doi: 10.1016/s0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 43.Tamura M, Webster R G, Ennis F A. Antibodies to HA and NA augment uptake of influenza A viruses into cells via Fc receptor entry. Virology. 1991;182:211–219. doi: 10.1016/0042-6822(91)90664-w. [DOI] [PubMed] [Google Scholar]
- 44.Taylor M E, Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J Biol Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 45.Verhoeyen M, Fang R, Min Jou W, Devos R, Huylebroeck D, Saman E, Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature (London) 1980;2286:771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- 46.Wells M, Albrecht P, Daniel S, Ennis F A. Host defense mechanisms against influenza virus: Interaction of influenza virus with murine macrophages in vitro. Infect Immun. 1978;22:758–762. doi: 10.1128/iai.22.3.758-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijburg O C L. Macrophages in viral immunity. Implications for vaccine development. Ph.D. thesis. Amsterdam, The Netherlands: Free University; 1997. [Google Scholar]
- 48.Wijburg O C L, DiNatale S, Vadolas J, van Rooijen N, Strugnell R A. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J Virol. 1997;71:9450–9457. doi: 10.1128/jvi.71.12.9450-9457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams S P, Robertson J R. Analysis of the restriction of the growth of nonegg-adapted human influenza virus in eggs. Virology. 1993;196:660–665. doi: 10.1006/viro.1993.1522. [DOI] [PubMed] [Google Scholar]
- 50.Winter G, Fields S, Brownlee G G. Nucleotide sequence of the haemagglutinin gene of a human influenza virus of the H1 subtype. Nature (London) 1981;292:72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z, Gershon M D, Ambron R, Gabel C, Gershon A A. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci USA. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmer G, Klenk H D, Herrler G. Identification of a 40-kDa cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J Biol Chem. 1995;270:17815–17822. doi: 10.1074/jbc.270.30.17815. [DOI] [PubMed] [Google Scholar]