Abstract
Purpose: Interstitial cystitis/bladder pain syndrome (IC/BPS) is a condition characterized in part by urinary urgency, frequency, and pain. There is a strong interest in gathering more data to compare and assess the differences in characteristics based on the presence of Hunner’s lesions in patients with IC/BPS. Materials and Methods: Using a nationwide crowdsource effort, we collected surveys and urine samples from patients with a history of IC/BPS. Participants completed the Interstitial Cystitis Symptom Index (ICSI) and Problem Index (ICPI), Overactive Bladder questionnaire (OABq SF), and pain scores. In addition, participants reported any co-morbidities and lifestyle modifications. Urinary cytokine levels were measured and compared to symptom severity. Results: 491 participants enrolled: 119 with history of ulcerative Hunner’s lesions (UIC), 372 reported no lesions (NHIC), and 2 unknowns. 96.3% were female, and prevalence of UIC was equal for both genders. Average age was higher for UIC vs. NHIC group (P = 0.011), as was the duration since diagnosis (P < 0.001). Symptom scores were elevated in UIC patients (P < 0.001). Both groups widely implemented lifestyle modifications, with dietary changes being most prevalent (70.1%), followed by prescription medication usage (63.1%). More UIC compared to NHIC participants experienced co-morbidities (P = 0.010). Urine samples were analyzed for GRO, IL-6, IL-8, and MCP-1. MCP-1 levels were significantly higher in UIC patients (P = 0.044). Weak positive correlation was found between cytokines and symptom scores. Conclusions: Patients with UIC and NHIC from across the United States displayed distinct phenotypic and urine biological characteristics. These findings contribute to increased understanding of IC/BPS and may aid in improving our knowledge of the condition.
Keywords: Urine, biomarker, bladder, interstitial cystitis, inflammation
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a complex and chronic syndrome characterized by various symptoms, including bladder pressure, urinary urgency, urinary frequency, and pelvic and genitalia pain [1,2]. The severity of these symptoms can range from mild discomfort to severely debilitating pain. Diagnosing IC/BPS can be challenging since there is no single definitive test, and the exact cause of the syndrome is still unknown. As such, IC/BPS is typically diagnosed by ruling out other potential causes of a patient’s symptoms. This often leads to delays in proper diagnosis and appropriate management of the disease.
Around 10% of IC/BPS patients exhibit observable bladder wall lesions known as Hunner’s lesions, diffuse glomerulations, or ulcerative IC (UIC), which can be detected through cystoscopy [3]. Hunner’s lesions are areas of urothelial denudation with severe inflammation. These patients typically experience more intense symptoms and may find some temporary relief through therapies targeting the bladder. However, most IC/BPS patients do not have visible bladder wall lesions (non-Hunner’s IC; NHIC), adding to the difficulty to come to a rapid and reliable IC/BPS diagnosis [4,5]. Also, it is unknown why some patients develop Hunner’s lesions or if UIC and NHIC have common etiologies. Lack of understanding of IC/BPS prevents effective care of this patient population.
To assess IC/BPS symptoms and evaluate clinical trial endpoints, several validated surveys have been developed, including the O’Leary-Sant IC Symptom Index (ICSI) and IC Problem Index (ICPI). Patient-reported symptoms, particularly pain, are currently important factors in diagnosing IC/BPS [6,7]. As part of a study aimed at developing a urine-based biomarker test using machine learning to identify IC/BPS patients, we performed a nation-wide urine drive using various social media outlets [8]. As part of this study, we collected urine samples from men and women with IC/BPS with and without Hunner’s lesions, which we analyzed for 4 key inflammatory proteins in the urine (IL-6, IL-8, GRO, and MCP-1). These cytokines have previously been associated with bladder disease [9]. Additionally, we considered patient-reported outcomes (PROs) obtained from validated IC/BPS surveys [6,7].
The objective of this study is to describe the characteristics of the IC/BPS patients that participated in the nationwide urine drive and compare the clinical survey and urine cytokine levels based on the presence or absence of Hunner’s lesions.
Materials and methods
Online recruitment
Ethical approval for all study materials was obtained from the Institutional Review Board (IRB# 2019-266), after which participant consent was obtained [8]. Participants were recruited to the study as previously described [8]. An online enrollment survey, designed to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, was created using SurveyMonkey and served the purpose of screening and enrolling participants, as well as collecting shipping addresses to which urine collection kits could be sent.
Once potential IC/BPS participants completed the enrollment survey, met the eligibility criteria, and provided consent, we prepared and shipped at-home urine collection kits to them.
Inclusion/exclusion criteria
Individuals with a self-reported physician diagnosis of IC/BPS were included in the study. The diagnostic criteria for IC/BPS are urinary frequency and bladder pain that is relieved with bladder emptying. In addition, other causes of symptoms need to be excluded such as urinary tract infection, bladder cancer and pelvic floor dysfunction. Thus, as part of the IC/BPS diagnosis for this study, patients needed to have suprapubic pain related to bladder filling accompanied by other symptoms such as increased daytime and night time frequency and in the absence of proven urinary infection or other obvious pathology, have had IC symptoms for at least six months, have IC that in the judgment of the investigator has been stable in the previous 30 days, and have IC-related pain defined as a score of > 3 and < 9 on the pain VAS where 0 is no pain and 10 is maximum pain. Exclusion criteria included having received intravesical therapy or bladder hydrodistention within 30 days, having had previous augmentation cystoplasty, cystectomy, or neurectomy, having received bladder botulinum toxin injections within last 3 months, and having had sacral and/or tibial nerve neuromodulation within the last 6 months. In addition, individuals were excluded from the study if they had participated in an IC research trial within 90 days prior to study enrollment, had urinary tract or prostatic infection in the past 90 days before study entry, had active genital herpes or vaginitis, has a uretheral diverticulum or has had a pelvic malignancy within the past five years. Patients with a history of cyclophosphamide or chemical cystitis, tuberculosis or pelvic radiation were also excluded from study participation. Finally, patients were excluded for any condition that in the judgment of the investigator would interfere with the patient’s ability to provide informed consent, comply with study instructions, place the patient at increased risk, or which may confound the interpretation of the study results.
Collection kit and mailing
Urine sample collection kits were shipped to all potential participants that completed the online enrollment survey, met eligibility criteria, and provided consent. Each urine collection kit comprised of information sheets about the study, questionnaires, a biohazard bag with absorbent material, a urine collection cup containing a urine preservative suitable for room temperature storage (Norgen Biotek, Thorold, Ontario, Canada), and a pre-paid United States Postal Service (USPS) return mailing envelope. The urine preservative stabilizes proteins and inactivates viruses and bacteria in the samples. To monitor the shipping conditions, each return box included a non-reversible temperature recording label. This methodology has been previously described [8].
Symptom assessment
Study participants were asked to complete 5 validated questionnaires to document their symptom severity: the Interstitial Cystitis Symptom Index (ICSI), Interstitial Cystitis Problem Index (ICPI), Overactive Bladder questionnaire Short Form (OABq SF) on quality of life and on symptom bother, and the Visual Analogue Scale (VAS) for pain. The ICSI and ICPI each consist of 4 questions on symptom severity and bother respectively related to incontinence, frequency, nocturia and pain. The total score ranges from 0-19 (ICSI) and from 0-16 (ICPI) with the higher score indicating worse symptoms or bother. The OABq SF is divided into two sections on quality of life (QoL) (13 questions) and symptom bother (6 questions) for which the scores range from 1-100, with higher scores indicating worse QoL/bother [6]. Pain scores were assessed using the Visual Analogue Scale on which patients were asked to rank the severity of their pain from 0 (no pain) to 10 (extreme pain).
Sample collection and analysis
Detailed instructions on urine sample collection and return shipping were provided in the collection kits. Participants were guided to collect a midstream urine sample and place it in the biohazard bag along with the absorbent material. They were then instructed to place the sample and the completed survey in the return box and simply put the sealed envelope in their mailbox for return shipment. Urinary cytokine levels were assessed by commercially available Milliplex Human Cytokine Kit (HCTYOMAG-60K; EMD Millipore) following the manufacturer’s protocol and using a Bio-Plex 200 Luminex IS System immunoassay analyzer (Bio-Rad). 25 µl of preserved urine samples were run in duplicate alongside a standard curve and two quality controls. Proteins tested were Interleukin 6 (IL-6), Interleukin 8 (IL-8), Monocyte Chemoattractant Protein-1/C-C Motif Chemokine Ligand 2 (MCP-1/CCL2), and Growth-related oncogene (GRO-α/CXCL-1). Detailed protocol and analysis have been previously described [9]. To assess if urinary cytokine levels were altered based on individual symptom severity, we divided patients into 3 groups depending on severity of urgency, nocturia or frequency as reported on the corresponding questions of the Interstitial Cystitis Symptom Index (ICSI) questionnaire. The ICSI is a validated IC symptom questionnaire that consists of 4 questions, of which two questions are related to urgency and nocturia. The questionnaire asks patients to rank their symptom severity from 0 to 5, with 0 = Not at all and 5 = almost always for urgency and 0 = not at all and 5 = 5 or more times per night for nocturia. Urgency and nocturia were subsequently categorized as: No symptoms = score of 0, Moderate symptoms = score of 1-3 or less than half the time, Severe symptoms = score 4-5 or more than half the time to always. Patients were also categorized based on frequency: no frequency ≤ 10 voids per day, moderate frequency = 11-19 voids per day, severe frequency ≥ 20 voids per day.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 28.0. Categorical data (e.g. gender, complications, and lifestyle modifications) were analyzed using Pearson’s Chi Square test. Continuous data (e.g. cytokine levels, age, years since diagnosis, and survey scores) were analyzed using Student T-test, one- or 2-way ANOVA as appropriate. A statistical significant result with one- or 2-way ANOVA was followed by Tukey’s multiple comparison test. Pearson’s correlation coefficient was calculated to detect any correlation between cytokine levels and symptom scores. Statistical significance was set at P < 0.05.
Results
Patient demographics
We obtained survey responses from a diverse group of 493 participants representing all 50 states of the USA. Among them, 119 participants reported a history of UIC, 372 participants reported to have a diagnosis of NHIC, and the lesion status of 2 participants was unknown. The majority of respondents were female (96.3%), and the prevalence of Hunner’s lesions was similar for both genders (25%; P = 0.724) (Table 1). In terms of demographics, the UIC group had a higher average age (52.7 vs. 47.8; P = 0.011) and a longer duration since diagnosis (14.8 vs. 8.2; P < 0.001) compared to the NHIC group.
Table 1.
IC patient demographics and symptom scores based on presence of Hunner’s lesions
| All samples | NHIC | UIC | p-value | |
|---|---|---|---|---|
| Total | 493 | 372 | 119 | |
| Av Age (range) | 50.29 (20-92) | 47.78 (20-87) | 52.65 (25-84) | 0.011 |
| Av Years since Dx (range) | 10.31 (9.44) | 8.23 (0-42) | 14.83 (0-45) | < 0.001 |
| Gender | ||||
| Female | 473 (96.3%) | 358 (96.2%) | 115 (96.6%) | 0.724 |
| Male | 16 (3.3%) | 12 (3.2%) | 4 (3.4%) | |
| Co-morbidities | N = 294 (59.6%) | N = 209 (56.2%) | N = 83 (69.7%) | 0.010 |
| More than one | 154 (52.4%) | 108 (51.7%) | 44 (53.0%) | 0.106 |
| Female | 287 (60.8%) | 204 (57%) | 83 (72.2%) | 0.004 |
| Male | 5 (25%) | 4 (33.3%) | 0 | 0.182 |
| Types of co-morbidities | ||||
| IBS | 61 (12.4%) | 37 (9.9%) | 24 (20.2%) | 0.003 |
| Migraines | 127 (25.8%) | 89 (23.9%) | 37 (31.1%) | 0.119 |
| Kidney Stones | 52 (10.5%) | 34 (9.1%) | 17 (14.3%) | 0.109 |
| Fibromyalgia | 71 (15%) | 48 (13.4%) | 23 (20%) | 0.085 |
| Vulvodynia | 94 (19.9%) | 71 (19.8%) | 23 (20%) | 0.969 |
| Endometriosis | 107 (22.6%) | 75 (20.9%) | 32 (27.8%) | 0.125 |
| Lifestyle modifications | N = 455 (92.3%) | N = 335 (90.1%) | N = 108 (90.8%) | 0.822 |
| Medication | 287 (63.1%) | 212 (63.3%) | 73 (67.6%) | 0.402 |
| Diet | 319 (70.1%) | 245 (73.1%) | 73 (67.6%) | 0.369 |
| Instillation | 56 (12.3%) | 48 (14.3%) | 17 (15.7%) | 0.699 |
| PFPT | 98 (21.5%) | 75 (22.4%) | 23 (21.3%) | 0.843 |
| Questionnaire scores | N = 488 | N = 371 | N = 117 | |
| ICSI | 16.7 (5.66) | 11.75 (4.32) | 14.14 (4.47) | < 0.001 |
| ICPI | 10.7 (3.6) | 10.13 (3.48) | 11.56 (3.57) | 0.002 |
| OABq SF Symptom bother | 46.44 (21.71) | 43.07 (20.87) | 52.5 (22.22) | < 0.001 |
| OABq SF QoL | 55.97 (26.52) | 53.65 (25.71) | 63.26 (27.99) | < 0.001 |
| VAS | 5.38 (2.077) | 5.28 (2.087) | 5.70 (2.023) | 0.055 |
| Cytokine Levels | ||||
| GRO | 40.81 (76.67) | 34.77 (54.75) | 57.65 (120.14) | 0.102 |
| IL-6 | 1.34 (4.2) | 0.972 (2.46) | 2.39 (7.25) | 0.089 |
| IL-8 | 45.0 (210.77) | 34.03 (99.89) | 77.18 (395.95) | 0.337 |
| MCP-1 | 426.83 (537.42) | 388.3 (498.92) | 546.5 (636.47) | 0.044 |
Statistically significant p-values are bolded.
Symptom severity and life style modifications
The severity of symptoms, as measured by ICSI (P < 0.001), ICPI (P = 0.002), was significantly greater in the UIC group compared to the NHIC group (Table 1). Average VAS scores were higher in UIC patients (5.70) vs. NHIC (5.28), though this did not reach statistical significance (P = 0.055). The quality of life of patients with ulcers was more severely impacted than those without ulcers based on the OABq SF (P < 0.001; Table 1). Both groups widely implemented lifestyle modifications (92.3%) as part of their management strategy, with dietary changes being the most common approach (70.1%), followed by prescription medication usage (63.1%) (Table 1). However, no statistical differences were detected in lifestyle modifications between NHIC and UIC. A higher proportion of UIC participants (69.7%) compared to NHIC participants (56.2%) experienced one or more other health complications, such as inflammatory bowel disease, endometriosis, chronic kidney disease, vulvodynia, fibromyalgia, and endometriosis (P = 0.010), with migraines being the most common co-morbidity. Irritable bowel syndrome was the only co-morbidity that was more prevalent in UIC versus NHIC patients (P = 0.003).
Urinalysis and symptom comparison
For urine analysis, we excluded urine samples that were exposed to temperatures exceeding room temperature as well as those from patients that reported a VAS pain score of zero. A total of 343 urine samples were subsequently analyzed for GRO, IL-6, IL-8 and MCP-1. Among the four cytokines analyzed, MCP-1 levels were significantly higher in patients with Hunner’s lesions (P = 0.044) (Table 1). We subsequently performed an association analysis between symptom scores and cytokine levels. ICSI score showed a weak positive correlation with GRO (ρ = 0.137; P = 0.011), IL-6 (ρ = 0.169; P = 0.002), and IL-8 (ρ = 0.120; P = 0.027) (Table 2). Similarly, GRO exhibited a weak positive correlation with OABq QoL scores (ρ = 0.157; P = 0.004), and IL-8 was positively correlated with VAS pain scores (ρ = 0.122; P = 0.024).
Table 2.
Correlation analysis between validated Symptom/QoL scores and urinary cytokine levels
| Pearson’s correlation | p-value | |
|---|---|---|
| Pain (VAS) | ||
| GRO | 0.088 | 0.105 |
| IL-6 | 0.082 | 0.133 |
| IL-8 | 0.122 | 0.024 |
| MCP-1 | 0.044 | 0.421 |
| OABq SF QoL Score | ||
| GRO | 0.159 | 0.003 |
| IL-6 | 0.172 | 0.001 |
| IL-8 | 0.141 | 0.009 |
| MCP-1 | 0.066 | 0.226 |
| ICSI Score | ||
| GRO | 0.137 | 0.011 |
| IL-6 | 0.169 | 0.002 |
| IL-8 | 0.120 | 0.027 |
| MCP-1 | 0.057 | 0.295 |
Statistically significant p-values are bolded.
Further analysis compared average cytokine levels depending on severity of urgency, nocturia and frequency. GRO and IL-6 urine levels were significantly different among the varying severity groups for urgency, nocturia and frequency (Table 3), while no significant differences were found for IL-8 and MCP-1. Post-hoc analysis subsequently identified which symptom severity groups were significantly different from the others (Figure 1). Furthermore, we assessed cytokine levels strategized for NHIC and UIC. However, statistical analysis indicated that type of IC did not drive altered urinary cytokine levels.
Table 3.
Association between urinary cytokine levels and symptom severity
| None | Moderate | Severe | p-value | |
|---|---|---|---|---|
| Urgency | N = 27 | N = 214 | N = 101 | |
| GRO | 23.67 (33.28) | 32.37 (46.44) | 62.63 (120.28) | 0.002 |
| IL-6 | 0.35 (0.49) | 0.88 (1.56) | 2.41 (7.06) | 0.031 |
| IL-8 | 10.67 (27.49) | 31.1 (95.37) | 83.3 (360.75) | 0.083 |
| MCP-1 | 239.9 (190.79) | 438.75 (595.97) | 454.32 (458.79) | 0.163 |
| Nocturia | N = 24 | N = 188 | N = 124 | |
| GRO | 32.32 (60.1) | 30.21 (44.2) | 55.66 (106.81) | 0.012 |
| IL-6 | 0.90 (1.24) | 0.85 (2.26) | 2.08 (6.11) | 0.029 |
| IL-8 | 55.54 (146.65) | 25.53 (68.13) | 72.07 (333.13) | 0.162 |
| MCP-1 | 456.71 (505.47) | 406.92 (545.9) | 460.89 (544.05) | 0.670 |
| Frequency | N = 133 | N = 131 | N = 30 | |
| GRO | 33.85 (55.38) | 40.31 (61.08) | 79.77 (175.85) | 0.015 |
| IL-6 | 0.75 (1.5) | 1.52 (3.57) | 3.32 (10.6) | 0.01 |
| IL-8 | 27.34 (77.0) | 41.9 (111.13) | 45.50 (111.38) | 0.403 |
| MCP-1 | 445.84 (617.67) | 441.91 (508.9) | 444.77 (507.30) | 0.998 |
Values are average cytokine levels in pg/ml with standard deviations. Statistical analysis performed using one-way ANOVA. Statistically significant p-values are bolded.
Figure 1.
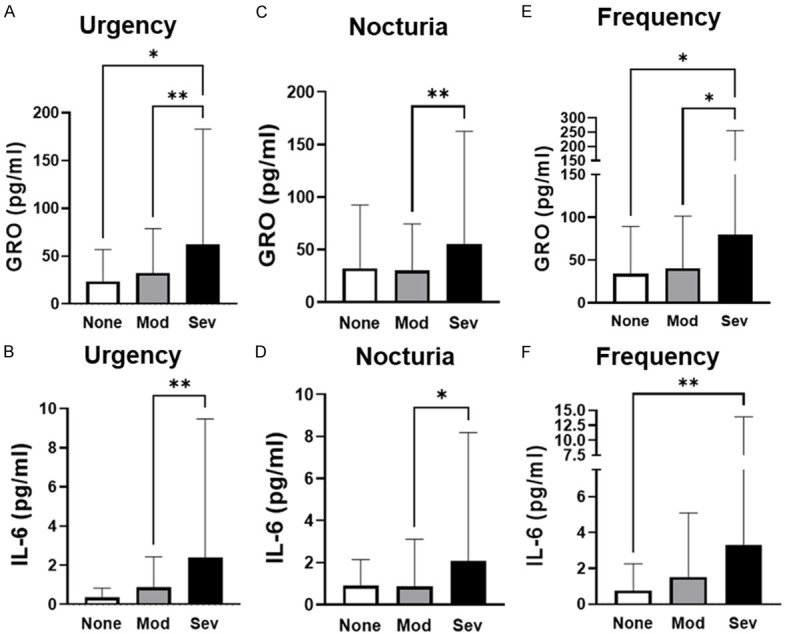
GRO and IL-6 urinary levels are elevated with increasing symptom severity. Statistical analysis performed with one-way ANOVA followed by Tukey’s multiple comparison test. None = no symptoms; Mod = moderate symptoms; Sev = severe symptoms; *P < 0.05; **P < 0.01.
Discussion
IC/BPS is a complex condition that is typically diagnosed by excluding other conditions with overlapping symptoms, such as urinary tract infections, pelvic floor dysfunction or bladder cancer. Patients with IC/BPS can have varying degrees of symptom severity and types of symptoms, raising the question whether IC/BPS consists of multiple subtypes. Currently, IC/BPS can be classified into those with and those without ulcers, also called Hunner’s lesions. These lesions are identified through cystoscopy, but there’s been an ongoing effort to develop non-invasive means of diagnosing patients. The goal of this study was to use urine samples and bladder surveys collected through a nationwide urine drive to identify differences between IC/BPS patients with and without Hunner’s lesions based on general health, lifestyle modifications, and urinary cytokine levels. Understanding the demographic characteristics of patients with this specific subtype is crucial for better disease management and patient care.
Age has been studied and compared between UIC and NHIC [10-14]. While the average age of UIC was significantly higher than NHIC (P = 0.011), participant ages in both groups ranged from 20s to 80s. Time since diagnosis was also significantly higher in UIC versus NHIC group (P < 0.001) though both ranged anywhere from 6 months to 45 years (Table 1). We did not find a correlation between ICSI symptom score and age, indicating that this chronic condition can impact all ages and that symptom severity is not age dependent. Pediatric cases are relatively rare but have been documented as well. Gender Distribution with UIC and NUIC both predominantly affects females with a female-to-male ratio of approximately 9:1. In our study, over 96% of participants were female, though this may be skewed by the nature of our data collection.
Patients with UIC often present with comorbidities such as: Fibromyalgia, irritable bowel syndrome (IBS), endometriosis, other chronic pain disorders, anxiety and depression [11,12]. We had similar findings in our study. Overall, 59.6% of participants reported having other co-morbidities, with significantly higher number of UIC patients reporting co-morbidities than NHIC patients. Migraines was the most common co-morbidity, followed by endometriosis and vulvodynia in women. However, irritable bowel syndrome was the only one that was more prevalent in IUC than NHIC participants. A previous study has shown that patients with IBS are at increased risk for developing IC/BPS indicating there may be a cause-effect relationship or a common denominator between the 2 conditions [6,15]. We did not identify a difference in cytokine concentrations between patients with and without IBS, not even when stratifying for the presence of ulcers. Further studies are needed to help us understand this relationship.
The strongest differences we observed between UIC and NHIC were symptom and bother scores (Table 1). Both ICSI and ICPI scores were significantly higher in the UIC population versus NHIC, as were the OABq SF QoL and bother scores. Surprisingly, pain scores, although higher in UIC patients, were not statistically different, which may be explained by the subjective nature of pain sensation. These findings indicate that patients with UIC experience more severe urinary symptoms compared to those without Hunner’s lesions.
Several cytokines have been implemented in IC/BPS, including GRO, IL-6, IL-8, and MCP-1 [16,17]. When comparing urinary cytokine levels between NHIC and UIC, only MCP-1 was significantly higher in patients with ulcers. This could indicate that MCPI-1 plays a more pronounced role in UIC or that it is more easily released in the urine through ulcers. Thus, MCP-1 may be a marker to help identify the presence of ulcers. The other three cytokines, GRO, IL-6 and IL-8 were weakly associated with symptom scores, and only IL-8 was weekly associated with pain scores. The lack of association between VAS pain scores and cytokines and lack of difference between pain scores and IC/BPS subtype may be explained by the subjective nature of pain scores. When stratifying based on individual symptom severity, GRO and IL-6 were significantly different depending on severity of urgency, nocturia or frequency (Table 3). Post-hoc analysis identified that overall, patient with severe symptoms had higher levels of GRO and IL-6 than patients with moderate or no symptoms (Figure 1). Furthermore, we observed a weak positive correlation between the ICSI and OABq scores and the levels of GRO, IL-6, and IL-8. These findings suggest that certain cytokines, particularly GRO and IL-6, may provide an indication of symptom severity and QoL. As there is a need for a reliable biomarker test for IC/BPS to aid in rapid diagnosis, future studies could focus on comparing the concentration of additional urinary cytokines to patient symptoms. In addition, with the growing interest in implementing artificial intelligence or Machine Learning, these techniques could be used here to develop an algorithm based on multiple urinary cytokines (instead of a single analyte) to diagnose patients.
Current understanding is that the demographic characteristics of patients with UIC reveal a higher prevalence in middle-aged females. Comorbidities, including chronic pain disorders and psychological conditions, are common among affected individuals. Geographic variations in disease prevalence further highlight the complex nature of this condition. Understanding the demographics of UIC is essential for tailored management strategies, improved patient outcomes, and future research.
One strength of this study is that it represents one of the largest collections of urine samples from IC/BPS patients with and without Hunner’s lesions. However, a limitation is that online recruitment may exclude individuals without internet access or those who are not techsavvy, potentially disproportionately affecting underserved minorities. In addition, while patients were required to have a physician diagnosis of IC/BPS to participate, we had no means of verifying this. This highlights the need for further unbiased research in this area.
The novel method used in our study has potential broad application and could be extended to other urological diseases, such as overactive bladder, benign prostatic hyperplasia, prostate cancer, and bladder cancer, by leveraging existing social media and disease interest groups to spread awareness of the study. The availability of room-temperature urine preservatives allows researchers to analyze a large volume of samples that would traditionally be challenging to acquire due to geographic limitations.
In conclusion, our survey results provide valuable insights into the characteristics of UIC and NHIC patients, highlighting differences in phenotypic traits, urine biological characteristics, and symptom severity. These findings contribute to a better understanding of IC/BPS and may have implications for improving diagnosis and management strategies for IC/BPS patients, both with and without Hunner’s lesions.
Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Technology/Therapeutic Development Research Program under Award No. W81XWH-19-1-0288. The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Disclosure of conflict of interest
MBC has intellectual property associated with methods for diagnosing interstitial cystitis.
References
- 1.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–145. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanno PM, Erickson D, Moldwin R, Faraday MM American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 4.Chancellor MB, Bartolone SN, Veerecke A, Lamb LE. Crowdsourcing disease biomarker discovery research: the IP4IC study. J Urol. 2018;199:1344–1350. doi: 10.1016/j.juro.2017.09.167. [DOI] [PubMed] [Google Scholar]
- 5.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ MAPP Research Network Study Group. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyne KS, Thompson CL, Lai JS, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015;34:255–263. doi: 10.1002/nau.22559. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(Suppl):58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 8.Ward EP, Bartolone SN, Sharma P, Chancellor MB, Lamb LE. Using social media to crowdsource collection of urine samples during a national pandemic. Int Urol Nephrol. 2022;54:493–498. doi: 10.1007/s11255-022-03108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb LE, Janicki JJ, Bartolone SN, Peters KM, Chancellor MB. Development of an interstitial cystitis risk score for bladder permeability. PLoS One. 2017;12:e0185686. doi: 10.1371/journal.pone.0185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama Y, Luo Y, Hanno PM, Maeda D, Homma Y. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol. 2020;27:491–503. doi: 10.1111/iju.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2022;208:34–42. doi: 10.1097/JU.0000000000002756. [DOI] [PubMed] [Google Scholar]
- 12.Lai HH, Pickersgill NA, Vetter JM. Hunner lesion phenotype in interstitial cystitis/bladder pain syndrome: a systematic review and meta-analysis. J Urol. 2020;204:518–523. doi: 10.1097/JU.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe D, Akiyama Y, Niimi A, Nomiya A, Yamada Y, Sato Y, Nakamura M, Kawai T, Yamada D, Suzuki M, Igawa Y, Kume H, Homma Y. Clinical characterization of interstitial cystitis/bladder pain syndrome in women based on the presence or absence of Hunner lesions and glomerulations. Low Urin Tract Symptoms. 2021;13:139–143. doi: 10.1111/luts.12344. [DOI] [PubMed] [Google Scholar]
- 14.Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int J Urol. 2019;26(Suppl 1):26–34. doi: 10.1111/iju.13971. [DOI] [PubMed] [Google Scholar]
- 15.Chang KM, Lee MH, Lin HH, Wu SL, Wu HC. Does irritable bowel syndrome increase the risk of interstitial cystitis/bladder pain syndrome? A cohort study of long term follow-up. Int Urogynecol J. 2021;32:1307–1312. doi: 10.1007/s00192-021-04711-3. [DOI] [PubMed] [Google Scholar]
- 16.Jiang YH, Jhang JF, Hsu YH, Kuo HC. Usefulness of urinary biomarkers for assessing bladder condition and histopathology in patients with interstitial cystitis/bladder pain syndrome. Int J Mol Sci. 2022;23:12044. doi: 10.3390/ijms231912044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamale LM, Lutgendorf SK, Zimmerman MB, Kreder KJ. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology. 2006;68:702–706. doi: 10.1016/j.urology.2006.04.033. [DOI] [PubMed] [Google Scholar]


