Abstract
Purpose of Review
Fatty acid-binding protein 4 (FABP4) plays a role in lipid metabolism and cardiovascular health. In this paper, we cover FABP4 biology, its implications in atherosclerosis from observational studies, genetic factors affecting FABP4 serum levels, and ongoing drug development to target FABP4 and offer insights into future FABP4 research.
Recent Findings
FABP4 impacts cells through JAK2/STAT2 and c-kit pathways, increasing inflammatory and adhesion-related proteins. In addition, FABP4 induces angiogenesis and vascular smooth muscle cell proliferation and migration. FABP4 is established as a reliable predictive biomarker for cardiovascular disease in specific at-risk groups. Genetic studies robustly link PPARG and FABP4 variants to FABP4 serum levels. Considering the potential effects on atherosclerotic lesion development, drug discovery programs have been initiated in search for potent inhibitors of FABP4.
Summary
Elevated FABP4 levels indicate an increased cardiovascular risk and is causally related to acceleration of atherosclerotic disease, However, clinical trials for FABP4 inhibition are lacking, possibly due to concerns about available compounds’ side effects. Further research on FABP4 genetics and its putative causal role in cardiovascular disease is needed, particularly in aging subgroups.
Keywords: FABP4, Atherosclerosis, Plaque, eQTL, Cardiovascular disease
Introduction
Aging has since long been considered an important factor in the development and progression of atherosclerosis and subsequent events such as myocardial infarction or ischemic stroke [1]. Atherosclerotic plaque size not only increases but also destabilizes with more inflammatory cells and atheromatous lesions that are observed in elderly patients [2]. Although some view(ed) arterial demise due to atherosclerosis as an ‘inevitable destiny,’ just after the Second World War, there was increasing awareness for the social and medical impact of (fatal) atherosclerosis in an aging population and the recognition that more work in the decades to follow was imperative [1]. The decades-old call to study atherosclerosis led to discoveries in the 70 s and 80 s that the activity of the enzymatic proteins prolyl hydroxylase, lysyl oxidase, and collagenase did not change in chickens fed a cholesterol diet compared to controls, rather their enzymatic activity reduced as the chickens grew older [3]. Age-dependent cellular protein activity is now known to influence cell culturing, and cultured vascular smooth muscle cells (SMCs) show reduced low-density lipoprotein (LDL) degradation in cells derived from older donors, and similarly reduced enzymatic activity may modify SMC proliferation and migration [4, 5].
Thus, our vascular proteome changes as we age. Here, we discuss one specific protein, which is equally important for meat quality [6], feed efficiency [7], and milk content variability in livestock [8, 9] as it is for lipid metabolism in health and cardiovascular disease (CVD) in humans: fatty acid-binding protein 4 (FABP4). In this review, we will provide an update on some biology of FABP4, the role of FABP4 in atherosclerotic disease, genetic factors modifying FABP4 levels, drug development, and a future perspective.
The Protein Family of Fatty Acid-Binding Proteins
FABP4, a 132 amino acid protein, is part of a family of fatty acid-binding proteins (FABPs) consisting of nine isoforms, that play a role in fatty acid solubilization, trafficking, and metabolism in different tissues and organs across species, and are named after the first tissue they were discovered in [10]. Fatty acids are hydrophobic, but soluble in the cytosol when bound inside the β-barrel structure, consisting of 10 anti-parallel β-sheets and capped by helix-turn-helix motif of FABPs. The cytoplasmic chaperones FABPs act as local and systemic mediators in intracellular mechanisms behind metabolism, lipid fluxes, intracellular signaling through interaction with membrane and intracellular proteins (for example, peroxisome proliferator-activated receptors (PPARs) and hormone-sensitive lipase (HSL)), and inflammatory processes [11, 12•, 13].
FABP4 Biology
One of the earliest mentions of FABP4 in the context of atherosclerosis dates back to 2002, where FABP4, formerly known as ‘adipocyte lipid binding protein’ (ALBP), ‘adipocyte FABP’ (AFABP), or ‘adipocyte P2’ (aP2), was discovered through a subtractive cDNA library screening in oxidized LDL (oxLDL)-treated and control THP-1 macrophages [14]. As an intracellular, high-affinity, selective lipid-binding protein, FABP4 plays a pivotal role in lipolysis, storage of lipids, and facilitates the transport of fatty acids as a chaperone (Fig. 1A) [15, 16]. It is predominantly expressed in white and brown adipocytes, monocytes and macrophages, and endothelial cells[17] and present in both the nucleus and cytoplasm (Fig. 1B). FABP4 is able to differentially bind ligands, and depending on the type of ligand, a conformational change exposes the nuclear localization signal [18]. Activating ligands, such as linoleic acid and troglitazone, lead to exposure and enable the transport of these ligands from the cytosol to the peroxisome proliferator-activated receptor gamma (PPARγ) in the nucleus, a protein associated with adipocyte differentiation, insulin sensitivity, and macrophage function, enhancing its transcriptional activity [18].
Fig. 1.
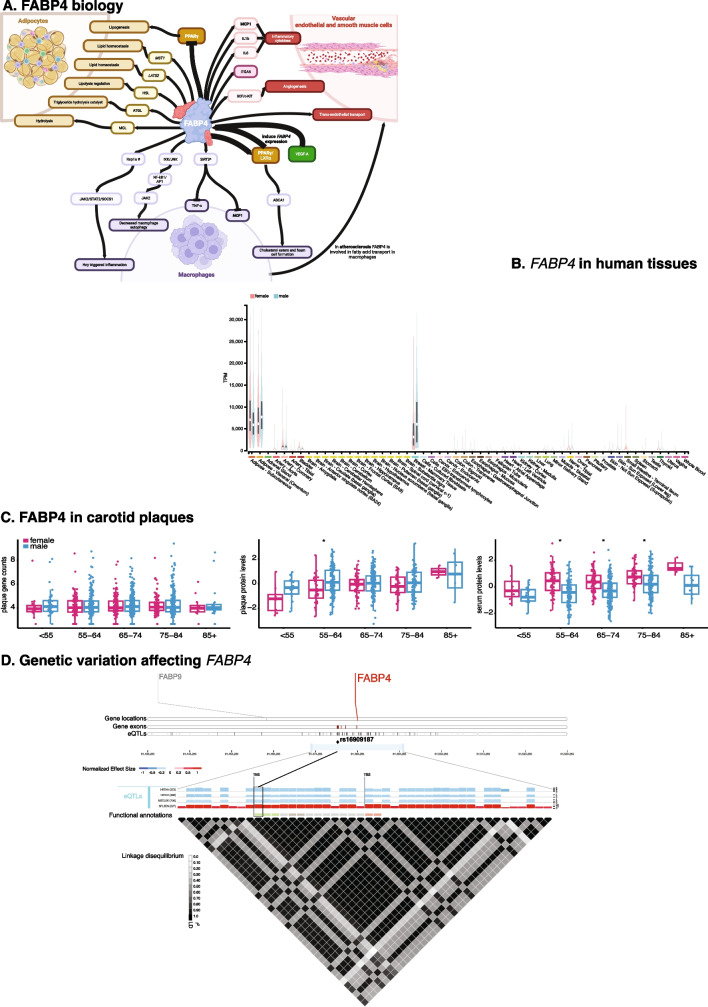
FABP4 in human tissues. A Schematic representation of FABP4 interactions in adipocytes, vascular endothelial cells and smooth muscle cells, and macrophages. PPARγ: peroxisome proliferator-activated receptor gamma; MST1: Mammalian sterile 20-like protein 1; LATS2: large tumor suppressors 1; HSL:Hormone sensitive lipase; ATGL: adipose triglyceride lipase; MGL: monoacylglycerol lipase; MCP1: monocyte chemoattractant protein-1; IL-1b: interleukin 1b; IL6: interleukin 6; ITGA5: integrin subunit alpha 5; VEGF: vascular endothelial growth factor; LXRα: liver X receptor alpha; ABCA1: ATP-binding cassette A1; IKK: IκB kinase; JNK: c-Jun N-terminal kinase; NFkB1: nuclear factor kappa B; AP1: activator protein 1; SIRT3: sirtuin 3; TNFα: tumor necrosis factor alpha; Rap1a: Ras-related protein 1a; JAK2: Janus kinase 2; STAT2: signal transducer and activator of transcription 2; SOCS1: suppressor of cytokine signaling 1; Hcy: homocysteine. Tumor necrosis factor α (TNF-α) and monocyte chemoattractant protein-1 (MCP1) are inflammatory cytokines. Bold letters indicate proteins present in more than one cell type. * = in FABP4-deficient macrophages; # = Hcy treated [12•, 19]. B The FABP4 gene expression in different human tissues stratified by sex. Data were taken from GTEx Portal (http://www.gtexportal.org/) on 2023–10-10 [113]. FABP4 is mostly expressed in adipose and mammary tissues and shows low expression in the circulation. C FABP4 gene expression and FABP4 protein levels in carotid plaques (upper and middle panel), and FABP4 protein levels in serum (lower panel) per age stratum and stratified by gender. No differences are observed for FABP4 expression in carotid plaques per age stratum and gender. Both plaque and serum FABP4 levels rise by age group. However, plaques-derived FABP4 levels are higher in men, whereas serum-derived FABP4 levels are higher in women. Data from the Athero-Express Biobank Study [52, 111, 112], see Methods. D Expression quantitative trait loci (eQTLs) for FABP4 gene expression in human tissues. In this 2000-Kb genomic region on chromosome 8, the FABP4 gene is marked in red. The 4 bars under the gene names show the gene locations, the FABP4 gene exons, and the eQTLs for FABP4. The middle panel shows the normalized expression effects (NES) for eQTLs per tissue, blue is a negative NES, red is a positive NES. The functional annotations are light red) promotor flanking regions, light grey) intronic variant, dark gray) non-coding transcript exon variant, and green) 3′ UTR variant. HRTAA: atrial appendage in the heart; HRTLV: left ventricle in the heart; MSCLSK: skeletal muscle; SPLEEN: spleen; TES: transcription end site; TSS: transcription start site. The yellow diamond and line demarcate rs16909187 at chr_81478482 (b38) with non-effect allele G and effect allele A. The A-allele is associated with lower FABP4 gene expression HRTAA, HRTLV, and MSCLSK, whereas the same allele is associated with higher FABP4 expression in SPLEEN and higher serum FABP4 levels [77••]. The bottom panel shows all the variants, i.e., eQTLs, affecting FABP4 gene expression and the linkage disequilibrium plot.
Modern-day high-fat diet (HFD)-induced obesity results in expansion of adipose tissue rich in inflammation inducing adipocytes and macrophages and concomitantly increase adipocyte secretion of FABP4 [12•]. Cellular and circulating FABP4 exert different roles in adipocytes, vascular endothelial cells (VECs), VSMCs, and macrophages under healthy and disease conditions.
FABP4 in Adipocytes
A fascinating dynamic exists between PPARG and FABP4. The expression of FABP4 is highly induced during adipocyte differentiation and under transcriptional control of PPARG agonists, fatty acids, insulin, but also cortisone-like medicine such as dexamethasone [19]. On the other hand, FABP4 plays a role in the regulation of enzyme activity by inducing PPARG transcription, while it also triggers PPARγ ubiquitination and subsequent proteasomal degradation [19]. While FABP4 supports the accumulation of surplus fatty acids within lipid droplets in adipocytes, it also possesses a binding site for hormone-sensitive lipase (HSL), a key regulator of lipolysis in adipocytes. While FABP4 lacks the conventional N-terminal signal sequence needed for the classical ER-Golgi-dependent secretion, it is secreted from adipocytes through a series of intracellular triglyceride hydrolysis mediated by adipose triglyceride lipase (ATGL), HSL, and monoacylglycerol lipase (MGL) [19]. In the pursuit of investigating perspective remedies for obesity, human adipose-derived mesenchymal stem cells (hADSCs) were treated with a Boron derivative in conjunction with a pluronic, utilized as a carrier in therapeutic formulations [20]. This treatment resulted in the diminished expression of PPARG and FABP4, as well as a reduction in the intracellular accumulation of lipid-droplet accumulation within hADSCs. Additionally, the two major regulators, LATS2 and MST1, responsible for governing lipid homeostasis in the context of adipocyte differentiation and proliferation, exhibited decreased expression [20]. Post-translational modification, such as phosphorylation (also see ‘FABP4 and the macrophage’), can significantly alter its properties and influences the interactions with other molecules [21]. To explore how modifications may contribute to obesity-induced insulin resistance and type 2 diabetes, the acetyl-lysine proteome profile (acetylome) was studied in subcutaneous and omental adipose tissue [22]. In insulin-resistant subcutaneous fat, FABP4 protein levels were highest, accompanied by a notable increase in acetylation [22]. During adipocyte differentiation, FABP4 protein exhibited a distinctive localization pattern: primarily within the nucleus at early stages, and shifting to the cytosol in later stages [22]. Introducing site-directed mutations targeting acetyl-sites in differentiating adipocytes resulted in substantial alterations in the distribution of FABP4 between the nucleus and the cytosol, reduced secretion of FABP4, changes in intracellular lipid-droplet accumulation, and increased levels of HSL in the mutants compared to the wildtype cells [22].
Effects of FABP4 on Vascular Endothelial and Smooth Muscle Cells
Under normal physiological conditions, FABP4 is expressed in vascular endothelial cells of the capillaries and small veins, but not the arteries. It is involved in trans-endothelial transport of fatty acids, regulated by PPARγ, to fatty acid consuming tissues, such as the heart, skeletal muscle, and kidneys [19, 23]. In vascular endothelial cells, vascular endothelial growth factor A (VEGFA) is a potent regulator of FABP4 which in turn upregulates intracellular signaling pathways, while down-regulation of FABP4 reduces cellular proliferation and angiogenesis in response to VEGFA [24]. FABP4 contributes to endothelial cell proliferation and migration, and through the modulation of the stem cell factor/c-kit pathway acts as a pro-angiogenic agent [24]. Endothelial cell derived FABP4 is also shown to increase pro-inflammatory cytokines, and proliferation and adhesion-related proteins, such as MCP1, IL1b, IL6, and ITGA5, inducing vascular smooth muscle cell proliferation and migration [25]. In patients with coronary artery disease (CAD), there is a remarkable interaction between human coronary arterial cells (HCAECs) and mononuclear cells (MNCs) [26]. The supernatant from MNCs isolated from CAD patients exhibited elevated levels of FABP4, and HCAECs from CAD patients displayed increased adherence to MNCs compared to controls [26]. This effect could be mitigated by the use of a FABP4-antibody [26]. The enhanced cellular adhesion was induced by oxidized-LDL (oxLDL), a modified form of lipids and apolipoprotein B resulting from lipid peroxidation, and a well-known atherogenic agent contributing to the formation of macrophage foam cells [27]. Treatment with oxLDL leads to higher levels of FABP4 proteins and adhesion molecules, including ITGB2, ITGA4, and PSGL1, in the supernatant of CAD patients [26]. However, the use of a FABP4-antibody reduced the expression of these integrins [26].
FABP4 and the Macrophage
It has been known for some time that FABP4 inhibits PPARγ/LXRα and its downstream ABCA1 pathway, which causes the formation of cholesterol esters and foam cells in macrophages [28, 29]. Suppression of FABP4 reduces PPARγ activity, and consequently, foam cell formation by M2 macrophages is impaired [30]. The expression of inflammatory cytokines, including TNF-α and MCP1, is reduced in FABP4-deficient macrophages, and this effect is mediated through SIRT3 [31]. In co-culture experiments of (murine) macrophages and adipocytes, the production of inflammatory cytokines is significantly reduced in the presence of butyrate (which inhibits adipocyte lipolysis), possibly through a pathway involving p38, ERK1/2, JNK1/2, and IκB/NF-κB [32, 33]. FABP4 also activates the IKK/JNK pathway, which leads to inflammatory responses by NF-κB/AP1 pathways, and attenuates Janus kinase 2 (JAK2) activity, which leads to a decrease in macrophage autophagy [34]. Hypomethylation of FABP4 accelerates lipid accumulation in homocysteine-induced (Hcy) atherosclerosis, implying that FABP4 may be involved with foam cell formation and macrophage inflammation [21, 35]. Apolipoprotein E knockout (ApoE−/−) mice treated with Hcy show acceleration of atherosclerosis and activated macrophage inflammation [21]. FABP4 plays a role in promoting macrophage inflammation triggered by Hcy by activating the Janus kinase 2/signal transducer and activator of transcription 2 (JAK2/STAT2) pathway. Ras-related protein (Rap1a) acts as an intermediary in the FABP4-mediated regulation of this pathway during macrophage inflammation.
Moreover, the post-translational phosphorylation of c-Src at Tyr416 appears to be essential for the activation of the pathway. Interestingly, the p-Tyr416 level of c-Src and its membrane localization significantly increased with FABP4, while the knockdown of suppressor of cytokine signaling 1 (SOCS1) led to increased secretion of proinflammatory factors in FABP4-overexpressing macrophages, an effect that was reversed by Rap1a knockdown [21]. It is worth noting that polybrominated diphenyl ethers (PBDEs), which are commonly utilized as flame retardant in numerous industrial and consumer products, possess obesogenic properties and have the ability to bind to PPARγ, thus triggering adipocyte differentiation [36]. PBDEs can also activate PPARγ in THP-1 macrophages, leading to an increase of expression of FABP4 and CD36. CD36, functioning as a scavenger receptor mediating the uptake of free fatty acids and oxLDL in macrophages[37], is upregulated upon PPARγ transactivation through PBDE. This, in turn, promotes the uptake of oxLDL and the accumulation of lipids, ultimately resulting in the formation of foam cells [36].
FABP4 in Animal Studies
Animal studies have clearly shown that FABP4 is associated with atherosclerotic plaque formation and vulnerable plaque features. Mice lacking both ApoE and Fabp4 develop markedly smaller atherosclerotic lesions [38] and contained fewer macrophages than wildtype ApoE-/- mice. In addition, the lack of Fabp4 in donor marrow cells leads to the development of smaller atherosclerotic lesions in the recipient mice, demonstrating the potential role of macrophage expressed Fabp4 in lesion development [39]. Importantly, these studies show that the onset of the metabolic syndrome occurs via expression of Fabp4 in adipocytes, while the initiation of atherosclerotic plaques occurs through Fabp4 expression in macrophages [38, 40, 41]. The Fabp4-deficient macrophages showed alterations in inflammatory cytokine production and a reduced ability to accumulate cholesterol esters when exposed to modified lipoproteins [40]. A drug intervention study in atherosclerotic mice demonstrated that an orally active small-molecule inhibitor of FABP4, BMS309403 (see also “Known drugs and compounds inhibiting FABP4”), was an effective therapeutic agent against severe atherosclerosis and type 2 diabetes in mouse models [42].
Metabolic syndrome describes a multifactorial process of multiple interrelated cardiovascular risk factors, including obesity, hyperlipidemia, and insulin resistance, and increased risk of cardiovascular disease [43]. Indeed, overexpression of a specific microRNA (miR-100) resulted in a reduced weight gain in high-fat fed mice compared to wildtype mice (on the same diet) [44]. These miRNA-100 overexpressing mice show marked decreased expression Cd36, Pparg, and Fabp4 in hepatocytes, reducing hepatic lipid accumulation and inflammation, while demonstrating increased energy expenditure [44]. Thus, hepatic Fabp4 may be an important factor in high-fat diet induced metabolic syndrome and subsequent atherosclerotic disease [44]. Not only selective drugs but also specific nutritional compounds may be able to reduce metabolic syndrome, and in C57BL/6 J mice on a high-fat diet Akebia saponin D, found in plants used in herbal tea, was shown to have beneficial effects on the microbiome [43]. This compound was shown to reduce lipid-induced tight-junction damage in intestinal epithelial cells through the Pparg/Fabp4 pathway, while the use of a PPARγ-inhibitor partially blocked these beneficial effects [43].
Similar to humans (Fig. 1C), FABP4 rises as mice age [45]. Intuitively, FABP4 is also positively correlated with LDL-rich cholesterol and triglycerides, but negatively with HDL-rich cholesterol [45]. When Fabp4 is silenced in these aging mice, they show increased metabolic activity, while at the same time fasting blood glucose and insulin decrease, and reduced hepatic lipid deposition [45]. Hepatic transcriptomic analyses in Fabp4-silenced aging mice reveals a transcriptomic reprogramming evident from reduced oxidative phosphorylation and increased TGF-β signaling [45], while overexpression of Fabp4 induces cellular senescence [45].
Observational Studies of FABP4
A Long History
The association of circulating FABP4 levels with cardiovascular disease has been studied extensively in observational studies. Results from a Japanese population-based study show, that compared to other FABPs, FABP4 levels are highest in the general population, and between the sexes, females have higher FABP4 levels attributed to the higher ratio of body fat in women than men [46]. FABP4 is also associated with known cardiovascular risk factors including body mass index (BMI), blood pressure, high-sensitive CRP, cholesterols, carotid intima-media thickness (cIMT), and negatively correlated to estimated glomerular filtration rate (eGFR) [19, 46, 47]. The latter suggesting that FABP4 is mainly excreted from the body through the renal clearance [19, 46]. Indeed, in the same general population from Japan, urinary FABP4 is correlated to albuminuria and associated with yearly decline in eGFR in the Tanno-Sobetsu Study [48].
In a Chinese population-based study individuals without cardiovascular disease were followed for a median duration of 9.4 years and 182 out of 1847 included developed CVD [49]. Circulating levels of FABP4 were highest in individuals with CVD, and Cox-proportional hazard analyses show FABP4 was associated with incident CVD over a period of 12 years. In another study involving the same cohort FABP4 was shown to correlate with glucose dysregulation, and was predictive for the development of type 2 diabetes in individuals without a previous history of diabetes [50]. In a German study, 1069 patients with previous coronary artery disease were followed for a duration of 10 years and circulating FABP4 levels increased as the number of metabolic syndrome components increased, and more so in females compared to males [51]. Cox regression analyses in the same study show that high FABP4 levels are associated with risk of secondary CVD during follow-up. This aligns with results from two other studies including patients undergoing carotid endarterectomy (CEA), where plaque-derived FABP4 levels were shown to be higher in symptomatic patients than asymptomatic patients [52, 53], and FABP4 gene expression was higher in carotid plaques from symptomatic patients within 1-month preceding CEA [53]. High plaque levels of FABP4 are also associated with a higher risk of secondary cardiovascular events in the three years post-event [52]. In addition, in patients with end-stage renal disease, as well as in patients with type 2 diabetes, FABP4 levels are predictive of cardiovascular death [54, 55]. A similar study in 1316 patients with chronic kidney disease also showed that FABP4 levels, ascertained using the multiplex proximity extension assay (PEA) technique [56], were associated with secondary major cardiovascular events (MACE); however, this could not be replicated in a cohort of 300 patients [57].
FABP4 as a Marker of Disease in At-Risk Populations
These studies are supported by more recent work. In a prospective, population-based cohort study including 5888 individuals aged 65 years and over, circulating FABP4 levels were higher in women than in men[58]. While FABP4 was not associated with incident myocardial infarction or stroke, it was associated with incident cardiovascular death among 4026 individuals free of CVD during follow-up [58]. In 681 patients with prevalent CVD, FABP4 was associated with cardiovascular death even after correction for confounders [58]. In a South African study involving black and white participants FABP4 was measured using the multiplex PEA technique and correlated to several phenotypes of vascular health [59]. FABP4 was associated with cIMT and pulse wave velocity, a marker of arterial stiffness and an independent predictor of cardiovascular risk, but not hypertension [59]. A recent update of the aforementioned Japanese Tanno-Sobetsu Study again confirmed that FABP4 levels are higher in women than men in the general population, and following individuals prospectively for 12 years shows FABP4 levels are predictive of cardiovascular death after adjustment for cardiovascular risk factors [60].
Conversely, a large study involving over 27,000 participants shows that FABP4 is associated with risk of type 2 diabetes, and potentially stroke risk, but not with myocardial infarction [61]. Other data also suggest FABP4 was higher in type 2 diabetes patients compared to healthy individuals [62], and may present a biomarker for cardiovascular disease given its association with arterial stiffness, renal function, adiposity, and hypertriglyceridemia [63, 64]. In high-fat diet fed mice, FABP4 was elevated in plasma and cardiac cells showed higher triglyceride loads [62]. The latter was further investigated in vitro where cardiac cells were challenged with extracellular FABP4 and showed high intracellular lipid content, which could be blocked through inhibition with a FABP4-specific drug [62].
Other studies show association of FABP4 levels with elongated QT intervals in patients with stable angina and kidney disease [65], but also with rapid renal functional decline in patients with diabetes [66]. A study of 973 patients undergoing coronary intervention shows FABP4 associated with cardiovascular outcome [67]. In type 2 diabetes patients, FABP4 levels were positively associated with peripheral artery disease [68], similar to a study involving hemodialysis patients [69]. A study in 777 Europeans applied the same PEA technique to measure FABP4 and found a strong correlation with leptin, a marker of the amount of adipose tissue and the development of CVD, both in diabetic patients and the general population [70], and especially in men. Longitudinal analyses showed that the association of leptin with risk of MACE was slightly attenuated by adding FABP4 to the model, suggesting a potential mediation effect [70].
Genetics of FABP4 in Humans
The FABP4 gene, encoding the FABP4 protein, is located on chromosome 8q.21.13 and evolutionarily conserved with a four-exon organization and 4 known isoforms. Several candidate gene studies reported on associations of arbitrarily chosen common DNA sequence variations in or near FABP4 with its expression or FABP4 protein levels across differing tissues and ancestral diverse populations. One common variant (rs77878271, T-87C), discovered through resequencing in 98 individuals [71], is located in the promoter region of FABP4. Genotyping of 7900 individuals from two large prospective population studies associated this variant with significantly lower triglycerides levels, lower risk of coronary heart disease, and diabetes [71]. The same variant was also reported to associate with lower prevalence of carotid plaque, reduced carotid intima-media thickness and total cholesterol levels, and lower odds of myocardial infarction [72]. Conversely, in a large population study, this variant was not associated with diabetes risk in ancestry-specific and pooled analyses [73]. Other variants, most in linkage disequilibrium to some extent, were also associated with FABP4 levels and fasting glucose, but not insulin, in the circulation of older individuals. More recently, this variant was shown to decrease FABP4 expression in epicardial adipose tissue [74] and associates with risk for cardiovascular disease in type 1 diabetes patients.
Large-Scale Genetics Discoveries
Most of these studies predate, or were on the cusp of, the era of genome-wide association studies and lacked robust study designs or methods, potentially leading to under-powered studies and irreproducible associations. Only a few GWAS were executed and reported on agnostically discovered common variants associated with FABP4 circulating levels [75, 76]. Of course, biologically, it makes sense that common variants near or in any given gene, its transcription start site, or its enhancer regions, should modulate expression in the context of the Central Dogma where DNA encodes RNA that is translated to protein. The large-scale Genotype-Tissue Expression (GTEx) Project includes over 50 different tissues in which gene expression was measured through RNA sequencing and the samples were genotyped (Fig. 1D). Analyses within this dataset robustly show that FABP4 is expressed in most tissues, showing few sex-specific differences, and highest expression in adipose and mammary tissues. Confirming earlier reports, FABP4 is expressed in atherosclerotic plaques, but no apparent sex differences can be seen, whereas FABP4 protein levels do differ between the sexes in both plaque and serum. Many common variants in and near FABP4 modulate its expression in some, but not all, tissues, specifically in heart tissues, skeletal muscle, and spleen (Fig. 1C, D). Most recently, a large-scale meta-analysis including over 30,000 individuals looked at the genetics of circulating levels of 90 proteins, including FABP4 [77••]. Mainly focused on the discovery of druggable targets, it did discover two loci, one near FABP4 and one near PPARG, showing the strongest association with FABP4 levels in the blood [77••]. Using a different methodology to measure FABP4, the INTERVAL study showed a positive and causal correlation of FABP4 with BMI in 2737 healthy participants [78]. Another study provided evidence of a large polygenic component to FABP4 protein levels, and genetic correlation analyses shows that variants affecting FABP4 protein levels are also positively correlated with coronary artery disease, type 2 diabetes, waist-hip-ratio, and creatinine [79].
Known Drugs and Compounds Inhibiting FABP4
There are 12 different chemical classes that are known to have an inhibitory effect on FABPs: pyrazole derivatives, oxazole derivatives, imidazole derivatives, indole derivatives, benzimidazole derivatives, thiophene and thiazole derivatives, pyrimidine, bicyclic pyridine and quinoxaline derivatives, urea and carbamoyl derivatives, sulfonamide derivatives, triazole derivatives, 2-aminobenzoic acid derivatives, and other compounds like natural compounds or FDA-approved drugs [80].
Drugs or compounds known for inhibition of FABP4 affect either the initiation of expression (indirect effect) or block the fatty acid binding site of the protein (direct effect). The best known is BMS309403, a small molecule that competitively inhibits fatty acid binding sites and thus directly has an effect on FABP4 activity [42, 81]. Another compound with similar functionality is HTS01037 [82]. The downside of these compounds is the relative high affinity for FABP3, or heart FABP, which can lead to cardiotoxicity given its high expression in cardiac tissue [19]. Currently, there are no clinical trials involving FABP4 inhibitors due to this side effect, along with metabolic issues and drug resistance [80].
The search for the most optimal compound is ongoing. A class of biphenyl scaffold molecules are shown to have promising inhibitory properties [83••]. To make the most optimal inhibitor with best specificity, compound 10g was developed and with a dose of 25 μM this compound inhibits FABP4 92% and FABP3 26% [84]. The inhibition by BMS309403 is higher for FABP4 (98%), but this is also the case for FABP3 (53%). Although the selectivity of BMS309403 for FABP4 is better, the unintended side effects are considerable due to high selectivity for FABP3, which leads to cardiotoxicity [84]. The inhibitory constant for BMS309403 and 10g are similar for FABP4 (respectively 0.36 μM and 0.51 μM), but for FABP3, 10g is more favorable (respectively, 30 μM and 33 μM) [84]. Other compounds resulting from tweaking this inhibitory protein are 16dk, 16do, and 16du [84].
The expression of FABP4 can be indirectly affected by inhibition of other pathways. Metformin, for example, is a hypoglycemic drug with an effect on macrophage-induced inflammation [85]. It is known to inhibit inflammation, reduce oxidative stress, lower foam cell formation, and induce apoptosis of macrophages in type 2 diabetes patients. The expression of FABP4. is indirectly reduced via inhibition of FOXO1-induced FABP4 transcription in macrophages by metformin [85].
Most drugs or compounds have been tested and researched in vitro with murine 3T3-L1 adipocytes, human monocytic leukemia THP-1 macrophages, but were also tested in vivo with leptin receptor-deficient and high-fat diet-induced mouse models [30, 43, 86]. There are some studies conducted into the effect of antidiabetic drug (Sitagliptin) or LDL-cholesterol lowering drug (Anagliptin, a DPP-4 inhibitor) on the gene expression or protein serum levels of FABP4 [87]. The latter, anagliptin, showed reduced serum FABP4 levels independent of HbA1c or LDL-cholesterol levels.
By combining machine learning and molecular docking on the approximately 2600 compounds in the FDA-approved drug library (https://go.drugbank.com), four different compounds (cobimetinib, larotrectinib, pantoprazole, and vildagliptin) were identified [88]. Cobimetinib, a mitogen-activated protein kinase (MEK)-specific small molecule inhibitor, is a drug used in melanoma (cancer) research and out of the four the most effective inhibitor of FABP4 [89, 90]. The compound is thought to inhibit the phosphorylation of JNK/c-Jun by FABP4, which might lead to a decrease in inflammatory response factors.
Future Perspective on FABP4 Research
Clearly considering FABP4 as an adipocyte-only-FABP is oversimplifying matters. Preclinical work has shown that FABP4 is critical for intracellular fatty acid transportation in the endothelium, affecting cellular adherence and altering cytokine expression [25]; stimulation through VEGFA and PPARγ induces endothelial cell proliferation and angiogenesis [91]. Ectopic endothelial expression of FABP4 induces an inflammatory response from VSMCs and increase proliferation and migration [25]. In macrophages, FABP4 is critical to the inflammatory response, oxLDL uptake, intracellular lipid accumulation, and concomitant foam cell formation [21]. In both mice and men, FABP4 expression and protein levels, in lipid-rich macrophages in early and advanced atherosclerotic plaques, is elevated [52, 92–94]. Results from observational studies convincingly position serum FABP4 as a biomarker for cardiovascular disease, specifically in at-risk populations with type 2 diabetes or kidney disease. In point of fact, a potent FABP4 inhibitor was shown to reduce atherosclerosis and reduces inflammation while increasing insulin sensitivity of adipose tissues in obese and diabetic mice [42].
Yet, although literature on FABP4 points to a clear predictive value of elevated serum FABP4 for atherosclerotic disease and cardiovascular death [60], clinical interventional studies inhibiting protein expression are lacking. This is surprising because studies of human atherosclerotic plaques show that FABP4 can be recognized as a marker that is strongly associated with vulnerable plaque characteristics. High local plaque FABP4 expression levels are strongly associated with an inflammatory unstable plaque composition and with symptomatic lesions [52]. In addition to these cross-sectional observations in human samples, plaque FABP4 protein levels are strongly predictive for systemic cardiovascular outcome during 3-year follow-up [52]. Still, despite these results being promising, FABP4 inhibitors are not available for clinical use, which may be associated with unsatisfactory efficacy and physicochemical properties [83••].There are a few compounds, some of which are approved drugs for type 2 diabetes, that directly affect FABP4, or its downstream pathways. Future studies should focus on the adverse and therapeutic effects of these drugs and specifically address effects on FABP4 in the context of atherosclerosis. Additionally, as post-translational modification appears to be critical for the properties and the cellular localization of FABP4, the development of site-directed compounds, that for instance alter acetylation or phosphorylation, could be of interest.
Another factor in play, is the central role FABP4 has in lipid homeostasis in both health and at-risk individuals with obesity, decreased kidney function, and/or type 2 diabetes. Large-scale genetic studies have robustly associated common variants near FABP4 and PPARγ with FABP4 serum levels. Interestingly, the same alleles that affect FABP4 in serum one direction may affect its gene expression differently in other tissues (Fig. 1), but how these affect expression in macrophages in the vessel is unknown. Comprehensive molecular QTL analyses to investigate how genetic variation impacts protein levels and gene expression in both adipocytes and vascular cells are needed, while being considerate on the different measurement platforms used [95]. These studies should encompass samples from both healthy individuals and those with evident atherosclerosis to unveil genetic effects specific to the disease.
Because of the invariant nature of the human genome and the random distribution of alleles from parents to offspring at conception, the effects of genetic variation should be free of confounding by traditional risk factors and not influenced by disease status (reverse causality) [96]. In essence, we are all part of a natural clinical trial, in which genetic risk is randomly distributed and unaffected by confounding (Mendelian randomization (MR)). MR studies are a powerful means to gauge the causal relation between a putative biomarker (and implicit druggable target) and disease [97–99]. However, both observational studies and genetic correlation analyses show profound positive correlations of FABP4 serum levels and genetic variation with markers of cardiometabolic health, including BMI, kidney function, serum lipid levels, and type 2 diabetes. This makes inference of whether FABP4 is causal to CVD challenging as collider bias, where FABP4 (exposure) and cardiovascular disease (outcome) are both influenced by other factors, may muddy the water [100, 101]. Additional research should focus on specific at-risk subgroups and apply strategies to address collider bias by other factors—in this case kidney function, lipid levels, BMI, and type 2 diabetes.
Prenatal malnutrition can have a profound impact on health in the human offspring [102]. DNA methylation acts as a mediator between transient adverse environmental factors in early life and metabolic health risk in adulthood [103]. In humans, FABP4 has been shown to vary during lactation and shows strong correlations with adiponectin and leptin in breast milk [104], and maternal adiposity and breast milk composition may be linked to infant growth [105]. At the same time, weight trajectories in individuals with different polygenic risks for obesity start to diverge in early life [106], and the effects of an obesogenic environment on health are most pronounced in those with a genetic predisposition [106, 107].
It is intriguing to hypothesize how FABP4 may be epigenetically influenced by maternal adiposity and breast milk composition and to study what the mediating effects of FABP4 may be in those genetically predisposed for obesity and concomitant poorer cardiovascular health. Likewise, other environmental factors, including smoking, are known to affect DNA methylation of the transcriptome in atherosclerosis [108]. As technology evolved, we are now able to partially disassociate chronological age from biological age and show how biological age may result in mesenchymal reprogramming [109]. It will be intriguing to further dissect the molecular mechanisms through which natural or man-made environmental factors change the FABP4-associated methylome and thereby influence atherogenesis. Specifically, it would be of interest to investigate whether FABP4 is involved in cellular phenotype switching through reprogramming of macrophages and vascular endothelial and smooth muscle cells.
Curing disease and prolonging life has been a grand human endeavor since the age of Aristotle. Whether FABP4 will be a means to an end will hopefully crystallize in the next decade.
Methods
Literature Search
We used PubMed to collect the cumulative knowledge on FABP4 in literature, 6619 articles in Dutch or English involved the keyword “FABP4” (October 2023) in animals, including humans. We narrowed the search parameters based on the query below and found 356 articles on FABP4 in atherosclerotic disease. These were supplemented with 4 additional articles covering a genetic study of circulating protein levels [77••, 79, 110]. We used this set as the starting point for this review:
(“fabp4” OR “aP2” OR “A-FABP” OR “AFABP” OR “ALBP”) and (“cardiovascular disease” OR “coronary artery disease” OR atherosclerosis OR “atherosclerotic disease” OR “atherosclerotic plaque”)
We downloaded this dataset as a.nbib file from PubMed and collected the abstracts and checked accessibility through PaperPile (www.paperpile.com).
Statistical Analyses, Data, and Code Availability
We used data from the Athero-Express Biobank Study, an ongoing study including patients undergoing carotid endarterectomy in The Netherlands [111]. Routinely plaque material with associated clinical data and blood samples are collected. The local medical ethics committee approved of the study, all patients provided written consent, and the study adheres to the guidelines of the Helsinki Declaration.
For the purposes of this review, we compared the expression of FABP4 in plaque [112], and the FABP4 protein levels in plaques and the circulation [52]. We previously described the collection of carotid plaques for the transcriptomic measurements, and after quality control, we normalized and log-transformed expression [112]. The protein levels were normalized by subtracting the mean from each value and divided this by the standard deviation. To test for significant differences between groups, we applied Kruskal–Wallis tests. All data are available through DataverseNL (doi.org/10.34894/4IKE3T) and codes are available through GitHub (github.com/CirculatoryHealth/Review_FABP4).
Acknowledgements
The research for this contribution was made possible by the AI for Health working group of the EWUU alliance (https://aiforhealth.ewuu.nl/).
Author Contribution
EMvdA and SWvdL wrote the initial version. MVP and GP provided critical feedback and edits to the final version.
Funding
Dr. Sander W. van der Laan is funded through the HealthHolland PPP Allowance ‘Getting the Perfect Image’. We are thankful for the support of the Netherlands CardioVascular Research Initiative of the Netherlands Heart Foundation (CVON 2011/B019 and CVON 2017–20: Generating the best evidence-based pharmaceutical targets for atherosclerosis (GENIUS I&II)), the ERA-CVD program ‘druggable-MI-targets’ (grant number: 01KL1802), the Leducq Fondation ‘PlaqOmics’, EU HORIZON MIRACLE (grant number: 101115381), EU HORIZON NextGen (grant number: 101136962), and EU H2020 TO_AITION (grant number: 848146).
Declarations
Conflict of Interest
Dr. Sander W. van der Laan and prof. dr. Gerard Pasterkamp have received Roche funding for unrelated work. Roche had no part in this study, neither in the conception, design, and execution of this study, nor in the preparation and contents of this manuscript. The other authors have no disclosures to report.
Human Rights and Informed Consent
Some data reported in this review are derived from the Athero-Express Biobank Study, an ongoing multi-center cohort study as described before [111]. The local medical ethics committee of the respective hospitals approved of this study, all patients provided written consent, and the study adheres to the guidelines of the Helsinki Declaration.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Waife SO. Recent advances in the study of arteriosclerosis. Ann Intern Med. 1949;30:635–645. doi: 10.7326/0003-4819-30-3-635. [DOI] [PubMed] [Google Scholar]
- 2.van Oostrom O, Velema E, Schoneveld AH, et al. Age-related changes in plaque composition: a study in patients suffering from carotid artery stenosis. Cardiovasc Pathol. 2005;14:126–134. doi: 10.1016/j.carpath.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Chvapil M, Stith PL, Tillema LM, Carlson EC, Campbell JB, Eskelson CD. Early changes in the arterial wall of chickens fed a cholesterol diet. Atherosclerosis. 1976;24:393–405. doi: 10.1016/0021-9150(76)90132-5. [DOI] [PubMed] [Google Scholar]
- 4.Bierman EL, Albers JJ, Chait A. Effect of donor age on the binding and degradation of low density lipoproteins by cultured human arterial smooth muscle cells. J Gerontol. 1979;34:483–488. doi: 10.1093/geronj/34.4.483. [DOI] [PubMed] [Google Scholar]
- 5.Chajara A, Delpech B, Courel M-N, Leroy M, Basuyau J-P, Lévesque H. Effect of aging on neointima formation and hyaluronan, hyaluronidase and hyaluronectin production in injured rat aorta. Atherosclerosis. 1998;138:53–64. doi: 10.1016/S0021-9150(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 6.Wibowo TA, Gaskins CT, Newberry RC, Thorgaard GH, Michal JJ, Jiang Z. Genome assembly anchored QTL map of bovine chromosome 14. Int J Biol Sci. 2008;4:406–414. doi: 10.7150/ijbs.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Zinder M, Lipkin E, Shor-Shimoni E, Ben-Meir Y, Agmon R, Asher A, Miron J, Shabtay A. FABP4gene has a very large effect on feed efficiency in lactating Israeli Holstein cows. Physiol Genomics. 2019;51:481–487. doi: 10.1152/physiolgenomics.00051.2019. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Cheng L, Azimu W, Hodge S, Edwards GR, Hickford JGH. Variation in the bovine FABP4 gene affects milk yield and milk protein content in dairy cows. Sci Rep. 2015. [DOI] [PMC free article] [PubMed]
- 9.Li Y, Zhou H, Cheng L, Hodge M, Zhao J, Tung R, Edwards G, Hickford J. Effects of FABP4 variation on milk fatty-acid composition for dairy cattle grazed on pasture in late lactation. J Dairy Res. 2020;87:32–36. doi: 10.1017/S0022029920000011. [DOI] [PubMed] [Google Scholar]
- 10.Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J Lipid Res. 2009;50(Suppl):S126–S131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat Rev Endocrinol. 2015;11:592–605. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Hao J, Zeng J, Sauter ER. SnapShot: FABP Functions. Cell. 2020;182:1066–1066.e1. doi: 10.1016/j.cell.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smathers RL, Petersen DR. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics. 2011;5:170–191. doi: 10.1186/1479-7364-5-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165:259–269. doi: 10.1016/S0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 15.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res. 1999;40:967–972. doi: 10.1016/S0022-2275(20)32133-7. [DOI] [PubMed] [Google Scholar]
- 16.Sha RS, Kane CD, Xu Z, Banaszak LJ, Bernlohr DA. Modulation of ligand binding affinity of the adipocyte lipid-binding protein by selective mutation. Analysis in vitro and in situ. J Biol Chem. 1993;268:7885–7892. doi: 10.1016/S0021-9258(18)53040-4. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Diolintzi A, Storch J. Fatty acid-binding proteins. Curr Opin Clin Nutr Metab Care. 2019;22:407–412. doi: 10.1097/MCO.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol. 2007;372:1246–1260. doi: 10.1016/j.jmb.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2019;26:216–232. doi: 10.5551/jat.48710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz AB, Tapsin S, Elbasan EB, Kayhan HD, Sahin F, Turkel N. Suppressor effects of sodium pentaborate pentahydrate and pluronic F68 on adipogenic differentiation and fat accumulation. Biol Trace Elem Res. 2020;193:390–399. doi: 10.1007/s12011-019-01738-y. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Zhang H, Wang Y, Yang A, Dong X, Gu L, Liu D, Ding N, Jiang Y. FABP4 activates the JAK2/STAT2 pathway via Rap1a in the homocysteine-induced macrophage inflammatory response in ApoE-/- mice atherosclerosis. Lab Invest. 2022;102:25–37. doi: 10.1038/s41374-021-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro-Ruiz MDC, López-Alcalá J, Díaz-Ruiz A, Moral SDD, Tercero-Alcázar C, Nieto-Calonge A, López-Miranda J, Tinahones FJ, Malagón MM, Guzmán-Ruiz R. Understanding the adipose tissue acetylome in obesity and insulin resistance. Transl Res. 2022;246:15–32. doi: 10.1016/j.trsl.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Goto K, Iso T, Hanaoka H, et al. Peroxisome proliferator-activated receptor-γ in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J Am Heart Assoc. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289:23168–23176. doi: 10.1074/jbc.M114.576512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuseya T, Furuhashi M, Matsumoto M, Watanabe Y, Hoshina K, Mita T, Ishimura S, Tanaka M, Miura T. Ectopic fatty acid-binding protein 4 expression in the vascular endothelium is involved in neointima formation after vascular injury. J Am Heart Assoc. 2017. [DOI] [PMC free article] [PubMed]
- 26.Wu Y-W, Chang T-T, Chang C-C, Chen J-W. Fatty-acid-binding protein 4 as a novel contributor to mononuclear cell activation and endothelial cell dysfunction in atherosclerosis. Int J Mol Sci. 2020. [DOI] [PMC free article] [PubMed]
- 27.Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev. 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Hui X, Li H, Zhou Z, Lam KSL, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH 2-terminal kinases and activator protein-1. J Biol Chem. 2010;285:10273–10280. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boss M, Kemmerer M, Brüne B, Namgaladze D. FABP4 inhibition suppresses PPARγ activity and VLDL-induced foam cell formation in IL-4-polarized human macrophages. Atherosclerosis. 2015;240:424–430. doi: 10.1016/j.atherosclerosis.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Hertzel AV, Steen KA, Bernlohr DA. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Mol Endocrinol. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohira H, Fujioka Y, Katagiri C, et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. 2013;20:425–442. doi: 10.5551/jat.15065. [DOI] [PubMed] [Google Scholar]
- 33.Lázaro I, Ferré R, Masana L, Cabré A. Akt and ERK/Nrf2 activation by PUFA oxidation-derived aldehydes upregulates FABP4 expression in human macrophages. Atherosclerosis. 2013;230:216–222. doi: 10.1016/j.atherosclerosis.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Lee C-H, Lui DTW, Lam KSL. Adipocyte fatty acid-binding protein, cardiovascular diseases and mortality. Front Immunol. 2021;12:589206. doi: 10.3389/fimmu.2021.589206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Ma S, Zhang H, et al. FABP4-mediated homocysteine-induced cholesterol accumulation in THP-1 monocyte-derived macrophages and the potential epigenetic mechanism. Mol Med Rep. 2016;14:969–976. doi: 10.3892/mmr.2016.5315. [DOI] [PubMed] [Google Scholar]
- 36.Ren Q, Xie X, Zhao C, Wen Q, Pan R, Du Y. 2,2’,4,4’-Tetrabromodiphenyl ether (PBDE 47) selectively stimulates proatherogenic PPARγ signatures in human THP-1 macrophages to contribute to foam cell formation. Chem Res Toxicol. 2022;35:1023–1035. doi: 10.1021/acs.chemrestox.2c00043. [DOI] [PubMed] [Google Scholar]
- 37.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layne MD, Patel A, Chen YH, et al. Role of macrophage-expressed adipocyte fatty acid binding protein in the development of accelerated atherosclerosis in hypercholesterolemic mice. FASEB J. 2001;15:2733–2735. doi: 10.1096/fj.01-0374fje. [DOI] [PubMed] [Google Scholar]
- 40.Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majchrzak K, Piotrowska M, Krajewska J, Fichna J. Adipocyte fatty acid binding protein (A-FABP) as a potential new therapeutic target for the treatment of obesity - associated cancers. Curr Drug Targets. 2022;23:597–605. doi: 10.2174/1389450122666210712193654. [DOI] [PubMed] [Google Scholar]
- 42.Furuhashi M, Tuncman G, Görgün CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Hu T, Liu H, Lv Y-L, Zhang W, Li H, Xuan L, Gong L-L, Liu L-H. Akebia saponin D ameliorates metabolic syndrome (MetS) via remodeling gut microbiota and attenuating intestinal barrier injury. Biomed Pharmacother. 2021;138:111441. doi: 10.1016/j.biopha.2021.111441. [DOI] [PubMed] [Google Scholar]
- 44.Smolka C, Schlösser D, Hohnloser C, et al. MiR-100 overexpression attenuates high fat diet induced weight gain, liver steatosis, hypertriglyceridemia and development of metabolic syndrome in mice. Mol Med. 2021;27:101. doi: 10.1186/s10020-021-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lv J, Hu Y, Li L, et al. Targeting FABP4 in elderly mice rejuvenates liver metabolism and ameliorates aging-associated metabolic disorders. Metabolism. 2023;142:155528. doi: 10.1016/j.metabol.2023.155528. [DOI] [PubMed] [Google Scholar]
- 46.Ishimura S, Furuhashi M, Watanabe Y, et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE. 2013;8:e81318. doi: 10.1371/journal.pone.0081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeung DCY, Xu A, Cheung CWS, Wat NMS, Yau MH, Fong CHY, Chau MT, Lam KSL. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1796–1802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- 48.Okazaki Y, Furuhashi M, Tanaka M, et al. Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS ONE. 2014;9:e115429. doi: 10.1371/journal.pone.0115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow WS, Tso AWK, Xu A, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc. 2013;2:e004176–e004176. doi: 10.1161/JAHA.112.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tso AWK, Xu A, Sham PC, Wat NMS, Wang Y, Fong CHY, Cheung BMY, Janus ED, Lam KSL. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30:2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 51.von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. 2012;32:2327–2335. doi: 10.1161/ATVBAHA.112.248609. [DOI] [PubMed] [Google Scholar]
- 52.Peeters W, de Kleijn DPV, Vink A, van de Weg S, Schoneveld AH, Sze SK, van der Spek PJ, de Vries J-PPM, Moll FL, Pasterkamp G. Adipocyte fatty acid binding protein in atherosclerotic plaques is associated with local vulnerability and is predictive for the occurrence of adverse cardiovascular events. Eur Heart J. 2011;32:1758–1768. doi: 10.1093/eurheartj/ehq387. [DOI] [PubMed] [Google Scholar]
- 53.Holm S, Ueland T, Dahl TB, et al. Fatty acid binding protein 4 is associated with carotid atherosclerosis and outcome in patients with acute ischemic stroke. PLoS ONE. 2011;6:e28785. doi: 10.1371/journal.pone.0028785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, Shimamoto K, Hotamisligil GS, Miura T. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS ONE. 2011;6:e27356. doi: 10.1371/journal.pone.0027356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu G, Ding M, Chiuve SE, Rimm EB, Franks PW, Meigs JB, Hu FB, Sun Q. Plasma levels of fatty acid–binding protein 4, retinol-binding protein 4, high-molecular-weight adiponectin, and cardiovascular mortality among men with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2016;36:2259–2267. doi: 10.1161/ATVBAHA.116.308320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldreich T, Nowak C, Carlsson AC, et al. The association between plasma proteomics and incident cardiovascular disease identifies MMP-12 as a promising cardiovascular risk marker in patients with chronic kidney disease. Atherosclerosis. 2020;307:11–15. doi: 10.1016/j.atherosclerosis.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Egbuche O, Biggs ML, Ix JH, Kizer JR, Lyles MF, Siscovick DS, Djoussé L, Mukamal KJ. Fatty acid binding protein-4 and risk of cardiovascular disease: the Cardiovascular Health Study. J Am Heart Assoc. 2020;9:e014070. doi: 10.1161/JAHA.119.014070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieden A, Malan L, Mels CMC, Lammertyn L, Wentzel A, Nilsson PM, Gudmundsson P, Jujic A, Magnusson M. Exploring biomarkers associated with deteriorating vascular health using a targeted proteomics chip: The SABPA study. Medicine. 2021;100:e25936. doi: 10.1097/MD.0000000000025936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saito N, Furuhashi M, Koyama M, et al. Elevated circulating FABP4 concentration predicts cardiovascular death in a general population: a 12-year prospective study. Sci Rep. 2021;11:4008. doi: 10.1038/s41598-021-83494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aleksandrova K, Drogan D, Weikert C, Schulze MB, Fritsche A, Boeing H, Pischon T. Fatty acid-binding protein 4 and risk of type 2 diabetes, myocardial infarction, and stroke: a prospective cohort study. J Clin Endocrinol Metab. 2019;104:5991–6002. doi: 10.1210/jc.2019-00477. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Calvo R, Girona J, Rodríguez M, et al. Fatty acid binding protein 4 (FABP4) as a potential biomarker reflecting myocardial lipid storage in type 2 diabetes. Metabolism. 2019;96:12–21. doi: 10.1016/j.metabol.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Tseng P-W, Hou J-S, Wu D-A, Hsu B-G. High serum adipocyte fatty acid binding protein concentration linked with increased aortic arterial stiffness in patients with type 2 diabetes. Clin Chim Acta. 2019;495:35–39. doi: 10.1016/j.cca.2019.03.1629. [DOI] [PubMed] [Google Scholar]
- 64.Furuhashi M, Sakuma I, Morimoto T, et al. Independent and distinct associations of FABP4 and FABP5 with metabolic parameters in type 2 diabetes mellitus. Front Endocrinol. 2020;11:575557. doi: 10.3389/fendo.2020.575557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C-P, Hsu C-C, Hung W-C, et al. Plasma fatty acid-binding protein 4 (FABP4) level is associated with abnormal QTc interval in patients with stable angina and chronic kidney disease. BMC Cardiovasc Disord. 2019;19:153. doi: 10.1186/s12872-019-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo DH, Nam M, Jung M, Suh YJ, Ahn SH, Hong S, Kim SH. Serum levels of adipocyte fatty acid-binding protein are associated with rapid renal function decline in patients with type 2 diabetes mellitus and preserved renal function. Diabetes Metab J. 2020;44:875–886. doi: 10.4093/dmj.2019.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai H-Y, Wu Y-W, Tseng W-K, et al. Circulating fatty-acid binding-protein 4 levels predict CV events in patients after coronary interventions. J Formos Med Assoc. 2021;120:728–736. doi: 10.1016/j.jfma.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Hsu B-G, Mah C-Y, Wu D-A, Chen M-C. Serum adipocyte fatty-acid binding protein as an independent marker of peripheral artery disease in patients with type-2 diabetes mellitus. Int J Environ Res Public Health. 2022. [DOI] [PMC free article] [PubMed]
- 69.Lai Y-H, Lin Y-L, Wang C-H, Kuo C-H, Hsu B-G. Positive Association of serum adipocyte fatty acid binding protein level with peripheral artery disease in hemodialysis patients. Ther Apher Dial. 2020;24:300–306. doi: 10.1111/1744-9987.13431. [DOI] [PubMed] [Google Scholar]
- 70.Vavruch C, Nowak C, Feldreich T, Östgren CJ, Sundström J, Söderberg S, Lind L, Nyström F, Ärnlöv J. Using proximity extension proteomics assay to discover novel biomarkers associated with circulating leptin levels in patients with type 2 diabetes. Sci Rep. 2020;10:13097. doi: 10.1038/s41598-020-69473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saksi J, Ijäs P, Mäyränpää MI, et al. Low-expression variant of fatty acid-binding protein 4 favors reduced manifestations of atherosclerotic disease and increased plaque stability. Circ Cardiovasc Genet. 2014;7:588–598. doi: 10.1161/CIRCGENETICS.113.000499. [DOI] [PubMed] [Google Scholar]
- 73.Chan K-HK, Song Y, Hsu Y-H, You N-CY, Tinker LF, Liu S. Common genetic variants in fatty acid-binding protein-4 (FABP4) and clinical diabetes risk in the Women’s Health Initiative Observational Study. Obesity. 2010;18:1812–1820. doi: 10.1038/oby.2009.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gormez S, Erdim R, Akan G, Caynak B, Duran C, Gunay D, Sozer V, Atalar F. Relationships between visceral/subcutaneous adipose tissue FABP4 expression and coronary atherosclerosis in patients with metabolic syndrome. Cardiovasc Pathol. 2020;46:107192. doi: 10.1016/j.carpath.2019.107192. [DOI] [PubMed] [Google Scholar]
- 75.Kaess BM, Enserro DM, McManus DD, et al. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab. 2012;97:E1943–E1947. doi: 10.1210/jc.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tönjes A, Kralisch S, Lössner U, Kovacs P, Blüher M, Stumvoll M, Fasshauer M. Metabolic and genetic predictors of circulating adipocyte fatty acid-binding protein. Int J Obes. 2012;36:766–773. doi: 10.1038/ijo.2011.162. [DOI] [PubMed] [Google Scholar]
- 77.Folkersen L, Gustafsson S, Wang Q, et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab. 2020;2:1135–1148. doi: 10.1038/s42255-020-00287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goudswaard LJ, Bell JA, Hughes DA, et al. Effects of adiposity on the human plasma proteome: observational and Mendelian randomisation estimates. Int J Obes. 2021;45:2221–2229. doi: 10.1038/s41366-021-00896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macdonald-Dunlop E, Klarić L, Folkersen L, et al. Mapping genetic determinants of 184 circulating proteins in 26,494 individuals to connect proteins and diseases. medRxiv 2021;08.03.21261494
- 80.Floresta G, Patamia V, Zagni C, Rescifina A. Adipocyte fatty acid binding protein 4 (FABP4) inhibitors. An update from 2017 to early 2022. Eur J Med Chem. 2022;240:114604. doi: 10.1016/j.ejmech.2022.114604. [DOI] [PubMed] [Google Scholar]
- 81.Sulsky R, Magnin DR, Huang Y, et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP) Bioorg Med Chem Lett. 2007;17:3511–3515. doi: 10.1016/j.bmcl.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 82.Hertzel AV, Hellberg K, Reynolds JM, Kruse AC, Juhlmann BE, Smith AJ, Sanders MA, Ohlendorf DH, Suttles J, Bernlohr DA. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J Med Chem. 2009;52:6024–6031. doi: 10.1021/jm900720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Y, Li S, Zhu Y, Wang Y, Chen Y, Zhang D, Wang H, Li Y. Optimization of potent, selective and orally bioavailable biphenyl scaffold as FABP4 inhibitors for anti-inflammation. Eur J Med Chem. 2023;253:115319. doi: 10.1016/j.ejmech.2023.115319. [DOI] [PubMed] [Google Scholar]
- 84.Gao D-D, Dou H-X, Su H-X, et al. From hit to lead: Structure-based discovery of naphthalene-1-sulfonamide derivatives as potent and selective inhibitors of fatty acid binding protein 4. Eur J Med Chem. 2018;154:44–59. doi: 10.1016/j.ejmech.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Feng X, Chen W, Ni X, Little PJ, Xu S, Tang L, Weng J. Metformin, macrophage dysfunction and atherosclerosis. Front Immunol. 2021;12:682853. doi: 10.3389/fimmu.2021.682853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai H-Y, Wang T, Zhao J-C, Sun P, Yan G-R, Ding H-P, Li Y-X, Wang H-Y, Zhu W-L, Chen K-X. Benzbromarone, an old uricosuric drug, inhibits human fatty acid binding protein 4 in vitro and lowers the blood glucose level in db/db mice. Acta Pharmacol Sin. 2013;34:1397–1402. doi: 10.1038/aps.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Furuhashi M, Sakuma I, Morimoto T, et al. Treatment with anagliptin, a DPP-4 inhibitor, decreases FABP4 concentration in patients with type 2 diabetes mellitus at a high risk for cardiovascular disease who are receiving statin therapy. Cardiovasc Diabetol. 2020;19:89. doi: 10.1186/s12933-020-01061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang S, Li S, Chang J. Discovery of Cobimetinib as a novel A-FABP inhibitor using machine learning and molecular docking-based virtual screening. RSC Adv. 2022;12:13500–13510. doi: 10.1039/D2RA01057G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh A, Ruan Y, Tippett T, Narendran A. Targeted inhibition of MEK1 by cobimetinib leads to differentiation and apoptosis in neuroblastoma cells. J Exp Clin Cancer Res. 2015;34:104. doi: 10.1186/s13046-015-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seipel K, Marques MAT, Sidler C, Mueller BU, Pabst T. The cellular p53 inhibitor MDM2 and the growth factor receptor FLT3 as biomarkers for treatment responses to the MDM2-inhibitor idasanutlin and the MEK1 inhibitor cobimetinib in acute myeloid leukemia. Cancers (Basel). 2018. [DOI] [PMC free article] [PubMed]
- 91.Liu B, Dai Z. Fatty acid metabolism in endothelial cell. Genes. 2022. [DOI] [PMC free article] [PubMed]
- 92.Agardh HE, Folkersen L, Ekstrand J, Marcus D, Swedenborg J, Hedin U, Gabrielsen A, Paulsson-Berne G. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med. 2011;269:200–210. doi: 10.1111/j.1365-2796.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 93.Umbarawan Y, Enoura A, Ogura H, et al. FABP5 is a sensitive marker for lipid-rich macrophages in the luminal side of atherosclerotic lesions. Int Heart J. 2021;62:666–676. doi: 10.1536/ihj.20-676. [DOI] [PubMed] [Google Scholar]
- 94.Lee K, Santibanez-Koref M, Polvikoski T, Birchall D, Mendelow AD, Keavney B. Increased expression of fatty acid binding protein 4 and leptin in resident macrophages characterises atherosclerotic plaque rupture. Atherosclerosis. 2013;226:74–81. doi: 10.1016/j.atherosclerosis.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eldjarn GH, Ferkingstad E, Lund SH, et al. Large-scale plasma proteomics comparisons through genetics and disease associations. Nature. 2023;622:348–358. doi: 10.1038/s41586-023-06563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Laan SW, Fall T, Soumaré A, et al. Cystatin C and cardiovascular disease: a mendelian randomization study. J Am Coll Cardiol. 2016;68:934–945. doi: 10.1016/j.jacc.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarwar N, Butterworth AS, IL6R Genetics Consortium Emerging Risk Factors Collaboration et al. Interleukin-6 receptor pathways in coronary heart disease a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holmberg MJ, Andersen LW. Collider bias. JAMA. 2022;327:1282. doi: 10.1001/jama.2022.1820. [DOI] [PubMed] [Google Scholar]
- 101.Mitchell RE, Hartley AE, Walker VM, Gkatzionis A, Yarmolinsky J, Bell JA, Chong AHW, Paternoster L, Tilling K, Smith GD. Strategies to investigate and mitigate collider bias in genetic and Mendelian randomisation studies of disease progression. PLoS Genet. 2023;19:e1010596. doi: 10.1371/journal.pgen.1010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ekamper P, van Poppel F, Stein AD, Lumey LH. Independent and additive association of prenatal famine exposure and intermediary life conditions with adult mortality between age 18–63 years. Soc Sci Med. 2014;119:232–239. doi: 10.1016/j.socscimed.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tobi EW, Slieker RC, Luijk R, et al. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci Adv. 2018;4:eaao4364. doi: 10.1126/sciadv.aao4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bronsky J, Mitrova K, Karpisek M, Mazoch J, Durilova M, Fisarkova B, Stechova K, Prusa R, Nevoral J. Adiponectin, AFABP, and Leptin in human breast milk during 12 months of lactation. J Pediatr Gastroenterol Nutr. 2011;52:474–477. doi: 10.1097/MPG.0b013e3182062fcc. [DOI] [PubMed] [Google Scholar]
- 105.Christensen SH, Lewis JI, Larnkjær A, Frøkiær H, Allen LH, Mølgaard C, Michaelsen KF. Associations between maternal adiposity and appetite-regulating hormones in human milk are mediated through maternal circulating concentrations and might affect infant outcomes. Front Nutr. 2022. [DOI] [PMC free article] [PubMed]
- 106.Khera AV, Chaffin M, Wade KH, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177:587–596.e9. doi: 10.1016/j.cell.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tyrrell J, Wood AR, Ames RM, et al. Gene-obesogenic environment interactions in the UK Biobank study. Int J Epidemiol. 2017;46:559–575. doi: 10.1093/ije/dyw337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siemelink MA, van der Laan SW, Haitjema S, et al. Smoking is associated to DNA methylation in atherosclerotic carotid lesions. Circ Genom Precis Med. 2018;11:e002030. doi: 10.1161/CIRCGEN.117.002030. [DOI] [PubMed] [Google Scholar]
- 109.Hartman RJG, Benavente ED, Slenders L, et al. Atherosclerotic plaque epigenetic age acceleration is characterized by mesenchymal reprogramming and poor prognosis. medRxiv 2023;02.16.23286067. 10.1101/2023.02.16.23286067
- 110.Folkersen L, Fauman E, Sabater-Lleal M, et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 2017;13:e1006706. doi: 10.1371/journal.pgen.1006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verhoeven BAN, Velema E, Schoneveld AH, et al. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design Eur J Epidemiol. 2004;19:1127–1133. doi: 10.1007/s10564-004-2304-6. [DOI] [PubMed] [Google Scholar]
- 112.Mokry M, Boltjes A, Slenders L, et al. Transcriptomic-based clustering of human atherosclerotic plaques identifies subgroups with different underlying biology and clinical presentation. Nature Cardiovascular Research. 2022;1:1140–1155. doi: 10.1038/s44161-022-00171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]