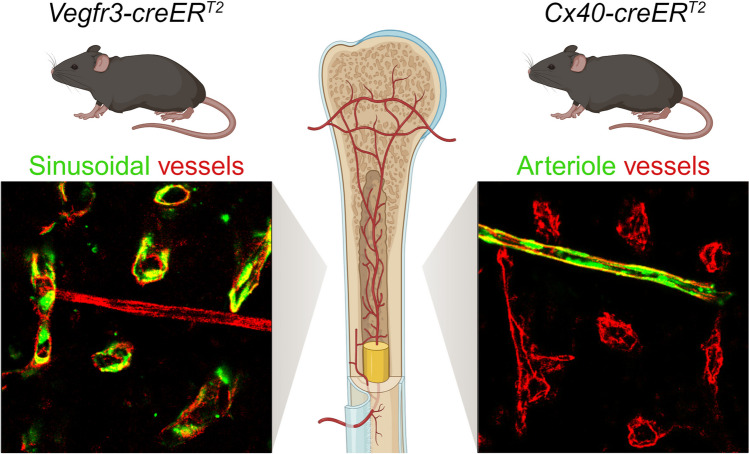
Abstract
In the adult bone marrow (BM), endothelial cells (ECs) are an integral component of the hematopoietic stem cell (HSC)-supportive niche, which modulates HSC activity by producing secreted and membrane-bound paracrine signals. Within the BM, distinct vascular arteriole, transitional, and sinusoidal EC subtypes display unique paracrine expression profiles and create anatomically-discrete microenvironments. However, the relative contributions of vascular endothelial subtypes in supporting hematopoiesis is unclear. Moreover, constitutive expression and off-target activity of currently available endothelial-specific and endothelial-subtype-specific murine cre lines potentially confound data analysis and interpretation. To address this, we describe two tamoxifen-inducible cre-expressing lines, Vegfr3-creERT2 and Cx40-creERT2, that efficiently label sinusoidal/transitional and arteriole endothelium respectively in adult marrow, without off-target activity in hematopoietic or perivascular cells. Utilizing an established mouse model in which cre-dependent recombination constitutively-activates MAPK signaling within adult endothelium, we identify arteriole ECs as the driver of MAPK-mediated hematopoietic dysfunction. These results define complementary tamoxifen-inducible creERT2-expressing mouse lines that label functionally-discrete and non-overlapping sinusoidal/transitional and arteriole EC populations in the adult BM, providing a robust toolset to investigate the differential contributions of vascular subtypes in maintaining hematopoietic homeostasis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12015-024-10703-9.
Keywords: Bone Marrow Niche, Arteriole, Sinusoid, Endothelial Cell, Hematopoietic Stem Cell, Cre Models
Introduction
Hematopoietic stem cells (HSCs) are multipotent precursors that sit atop a hierarchy of hematopoietic progenitor cells (HPCs) responsible for maintaining balanced blood production throughout life [1, 2]. In adults, hematopoietic stem and progenitor cells (HSPCs) are localized to specialized vascularized niches within the bone marrow (BM) that direct stem cell-fate decisions, including quiescence, self-renewal, and restricted progenitor differentiation [3, 4]. Endothelial cells (ECs) are a critical component of the HSC-supportive BM niche, nucleating perivascular stromal and hematopoietic cells to create an instructive multicellular microenvironment through the production of extrinsic cues that maintain hematopoietic homeostasis and regeneration. Within the BM, the vasculature can be subclassified into high-pressure arterioles, branching into transitional vessels located adjacent to trabecular bone in the metaphysis and near cortical bone, before emptying into a low-pressure sinusoidal capillary network in the central marrow [5]. While arteriole, transitional, and sinusoidal vascular microenvironments are anatomically distinct and can be classified by vessel morphology [6, 7], accompanying perivascular stromal and hematopoietic cell association [4], and endothelial immunophenotypic labeling and gene expression signatures [8, 9], the lack of high-fidelity inducible cre systems that allow for targeted genetic manipulations in niche-specific endothelial subtypes have hampered the functional characterization of these vascular subsets.
A diverse array of cre-expressing mouse lines have been successfully used to target pan-endothelial and endothelial subtypes [9–14]. However, existing cre lines have two limitations: (1) Most pan-endothelial cre lines are constitutively expressed and consequently exhibit recombination in HSCs due to their shared developmental ontogeny, and (2) existing cre lines targeting vascular subsets exhibit off-target recombination within BM stromal and hematopoietic subsets. These limitations preclude the investigation of the role of vascular-subtype-specific niches in regulating HSPC activity within the adult BM. In this manuscript, we characterize two inducible cre-expressing murine lines that faithfully identify adult BM endothelial subpopulations to accurately interrogate the mechanisms of endothelial-HSPC instructive function. Herein, we describe inducible and vascular subtype-specific Vegfr3-creERT2 and Cx40-creERT2 mice that respectively target non-overlapping sinusoidal/transitional and arteriole endothelial populations within the adult BM, with no observable off-target stromal or hematopoietic activity. Using a previously described genetic model of MAPK-activation in the BM vascular niche [15], we demonstrate that Vegfr3-creERT2 and Cx40-creERT2 mice are able to faithfully segregate individual sinusoid/transitional and arteriole MAPK-dependent contributions to hematopoietic dysfunction. Taken together, these model systems provide a platform to discriminate endothelial-borne sinusoidal/transitional and arteriole paracrine signals in the adult BM microenvironment.
Materials and Methods
Animals
Murine experiments were performed under the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health (NIH) Office of Laboratory Animal Welfare (OLAW) recommendations, in accordance with the University of Florida Institutional Animal Care and Use Committee (IACUC) guidelines. Mice were maintained in specific-pathogen-free housing in NexGen Individually Ventilated Cages (IVC) with HEPA-filtered air exchange (Allentown, Inc.) and fed on PicoLab Rodent Diet 20 (Lab Diet 5053) and water ad libitum. C57BL/6 J-Tg(Cdh5(PAC)-creERT2) [16, 17] and C57BL/6 J-Tg(Bmx-creERT2)1Rha [18] mice were provided by Dr. Ralf Adams at The Max Planck Institute for Molecular Biomedicine. B6.Cg-Gt(ROSA)26Sortm6(CAG−ZsGreen1)Hze/J (Strain #007906) [19], B6.Cg-Gt(ROSA)26Sortm9(CAG−tdTomato)Hze/J (Strain #007909) [19], C57BL/6 J-Gt(ROSA)26Sortm(Map2k1*EGFP)Rsky/J (Strain #012352) [20], B6.SJL-Ptprca Pepcb/BoyJ (Strain #002014), and C57BL/6 J mice (Strain #000664) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice, with the exception of B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+), were maintained on a C57BL/6 J (CD45.2+) genetic background.
Vegfr3-creERT2 Generation
Vegfr3-creERT2 transgenic animals were generated following a previously published strategy [21]. The Vegfr3-creERT2 bacterial artificial chromosome (BAC)-targeting vector was provided by Jean-Leon Thomas; in short, the described Vegfr3 BAC-targeting vector containing a Venus (YFP) fluorescent cassette [21] was replaced with a tamoxifen-inducible creERT2 cassette in-frame with the Vegfr3 start codon in exon 1. Recombineering bacterial strains (SW102 and SW105) were obtained from the National Cancer Institute at Frederick [22]. The ~ 240 kilobase (kb) murine C57BL/6 Vegfr3-containing BAC (RP23-65D23) was obtained from CHORI (https://bacpacresources.org). Recombineering protocols were followed as detailed [23] and briefly described below. To target the Vegfr3-containing BAC, the creERT2 BAC-targeting vector was digested with PacI/AscI (New England Biolabs) and the resulting 4497 bp fragment was purified from a 0.9% TAE/agarose gel using the QIAquick Gel Extraction Kit (Qiagen) according to the manufacturer’s recommendations. The linearized creERT2 BAC-targeting vector was transformed into RP23-65D23-containing SW102 cells and selected for Kanamycin resistance to identify recombinants (Fig. 1a). Successfully-targeted BACs were purified using the Nucleobond BAC 100 Kit (Takara), stably transformed into SW105 cells by selecting for BAC-specific chloramphenicol resistance (i.e. pBACE3.6 backbone), and the Kanamycin resistance cassette was excised via arabinose-induced FLP-mediated recombination (Fig. 1a). Using replica plating, chloramphenicol+ kanamycin− clones were selected, purified, and transformed into SW102 cells. An ampicillin targeting cassette was amplified from pBluescript using primers with AscI restriction sites and arms of homology flanking the single loxP site located in the pBACE3.6 vector. The ampicillin PCR product was transformed into chloramphenicol+ kanamycin− SW102 cells and successful recombinants with the removed loxP were selected using ampicillin. The finalized creERT2-targeted RP23-65D23 construct was linearized with AscI and fractionated using a Sepharose CL-4B (Sigma-Aldrich) chromatography column (equilibrated to 100 mM NaCl, 10 mM Tris–HCl (pH 7.5), and 0.25 mM EDTA); fractions containing intact targeted-BAC construct at ~ 245 kb were confirmed using pulse-field gel electrophoresis. Transgenic animals were generated at the University of Michigan – Transgenic Animal Core (https://medresearch.umich.edu/office-research/about-office-research/biomedical-research-core-facilities/transgenic-animal-model) via pronuclear injection of linearized creERT2-targeted RP23-65D23 into fertilized C57BL/6 J oocytes. Founders were screened by PCR using Hot Start Taq DNA polymerase (New England BioLabs) with cre-specific primers 5ʹ-atgtccaatttactgaccgtacacca-3ʹ and 5ʹ-acgatgaagcatgtttagctggccca-3ʹ (Integrated DNA Technologies) according to the manufacturer’s recommendations.
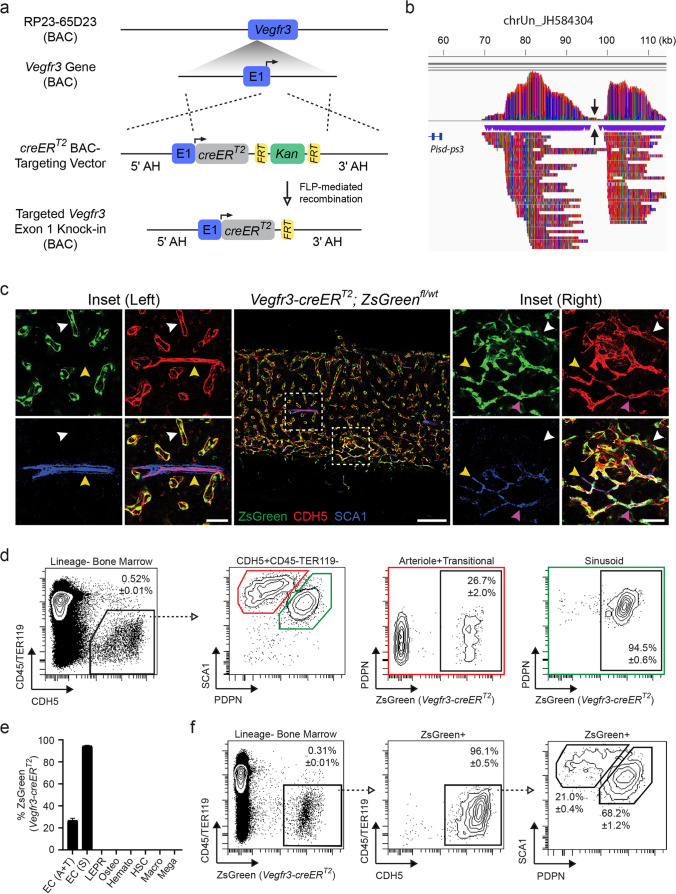
Fig. 1.
Vegfr3-creERT2 activity in the adult BM is restricted to sinusoidal and transitional endothelium. a Schematic of creERT2 BAC-recombineering used to generate Vegfr3-creERT2 transgenic animals. A creERT2 cassette (grey) is knocked-in to exon 1 (E1) of a BAC containing the Vegfr3 gene (blue) at the transcriptional start site (solid arrow) using adjacent arms of homology (AH). The kanamycin selection cassette (green) was removed via FLP-mediated recombination (open arrow) of flanking FRT sites (yellow) prior to pronuclear injection. b Visualization of genomic DNA sequencing reads from Vegfr3-creERT2 transgenic mice mapped to unplaced contig chrUn_JH584304 using Integrative Genome Viewer (IGV). Note: Vegfr3-creERT2 transgene insertion point (inverted black arrows) is approximately 38 kb upstream of the phosphatidylserine decarboxylase - pseudogene 3. c Representative images of Vegfr3-creERT2; ZsGreenfl/wt BM labeled for CDH5 (red), SCA1 (blue), and ZsGreen (green). Insets are denoted by dashed boxes. Arrowheads demarcate vessel type, including sinusoids (white; ZsGreen+SCA1−CDH5+), transitional (magenta; ZsGreen+SCA1+CDH5+), and arterioles (yellow; ZsGreen−SCA1+CDH5+). Scale bars for the central image (200 μm) and insets (50 μm) are noted. d Representative flow plots to quantify Vegfr3-creERT2 activity in BM EC subtypes; arteriole and transitional (red box) and sinusoids (green box) are indicated. Open arrow/dashed line illustrates gating progression. Percentages represent AVE ± SEM; N = 4. e Quantification of Vegfr3-creERT2; ZsGreenfl/wt activity in BM cellular subsets, including arteriole and transitional (EC; A + T) and sinusoidal (EC; S) endothelium, LEPR+ mesenchymal cells, osteoblasts (Osteo), pan-hematopoietic cells (Hemato), hematopoietic stem cells (HSCs), macrophages (Macro), and megakaryocytes (Mega). Percentages represent AVE ± SEM; N = 4. f Representative flow plots assessing endothelial populations in ZsGreen+ cells derived from Vegfr3-creERT2; ZsGreen.fl/wt BM. Open arrow/dashed line illustrates gating progression. Percentages represent AVE ± SEM; N = 4
Vegfr3-creERT2 Transgene Mapping
The Vegfr3-creERT2 BAC genomic insertion site was determined by long-read sequencing of high molecular weight (HMW) leukocyte DNA. In short, HMW DNA was purified from 1 mL of red blood cell (RBC)-lysed peripheral blood from a male Vegfr3-creERT2 heterozygous animal using the Monarch HMW DNA Extraction Kit for Cells & Blood (New England BioLabs) according to the manufacturer’s recommendation. The resulting HMW genomic DNA was sheared to a size of ~ 20 kb using a g-TUBE (Covaris) according to the manufacturer’s suggestions. The sequencing library was prepared using the Ligation Sequencing Kit V14 (Oxford Nanopore Technologies). Briefly, 1 μg of DNA was repaired and end-prepped using the NEBNext Ultra II End Repair/dA-tailing Module (New England Biolabs). Sequencing adapters were ligated to the DNA ends and the adapted library was cleaned to remove fragments shorter than 3 kb. Twenty-two fmol of library DNA was loaded onto an R10.4.1 flow cell (FLO-PRO114M) and sequencing was carried out on a PromethION (Oxford Nanopore Technologies) instrument. Base calling was carried out directly on the device with the MinKNOW software using the high-accuracy setting and a minimum quality score of 8. Long DNA sequencing reads (5.9 M reads, N50 read length = 12,705 bp, average genome coverage = 20X) were split into non-overlapping contiguous 500 bp fragments and independently mapped to the mouse genome (C57BL/6 J; reference version GRCm39) and transgene sequence using minimap2 [24]. The identified 923 long reads containing at least two 500 bp fragments mapping to both the mouse genome and the transgene were mapped back to the mouse genome and custom scripts were used to identify the region with the highest number of aligned reads. The integration site was identified on unplaced contig chrUn_JH584304, representing two contiguous peaks of high coverage. BAM files produced by the alignment of the fragments to the mouse genome were visualized using Integrative Genome Viewer (IGV) [25] to verify the location of the integration site and to generate Fig. 1b.
Cx40-creERT2 Backcross
Outbred Gja5tm2(cre/ERT2,RFP)Lumi (Cx40-creERT2) knock-in mice were generated by and obtained from Dr. Lucile Miquerol at The Developmental Biology Institute of Marseilles [26] and backcrossed to C57BL/6 J for six successive generations using speed congenics. In short, purified genomic DNA from resulting pups were screened between generations using the miniMUGA SNP Array (Neogen) to identify animals with the highest recipient genomic percentage. Selected animals were bred back to C57BL/6 J animals. The congenic C57BL/6 J background in the N6 generation was confirmed (Fig. 2b) prior to generating B6.Cg-Gt(ROSA)26Sortm6(CAG−ZsGreen1)Hze/J or C57BL/6 J-Gt(ROSA)26Sortm(Map2k1*EGFP)Rsky/J models.
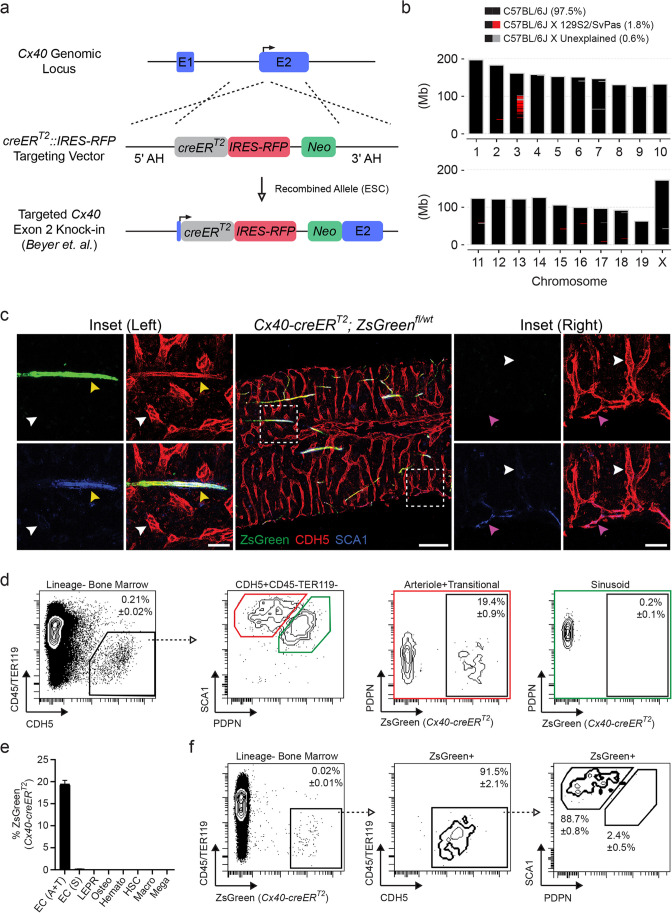
Fig. 2.
Cx40-creERT2 activity in the adult BM is restricted to arteriole endothelium. a Overview of bicistronic creERT2 (grey)::IRES-RFP (red) and PGK-driven Neo (green) embryonic stem cell (ESC) targeting of the endogenous Cx40 gene (blue) at the exon 2 (E2) transcriptional start site (solid arrow) using adjacent arms of homology (AH); schematic adopted from Beyer et al. b Chromosomal Ideogram of Cx40-creERT2 mice following backcrossing to a C57BL/6 J genetic background. Recipient C57BL/6 J congenic (black), ESC-targeted Cx40 allele located on chromosome 3 (red; 129S2/SvPas origin), and unassigned regions (grey) are detailed. Megabase (Mb). c Representative images of Cx40-creERT2; ZsGreenfl/wt BM labeled for CDH5 (red), SCA1 (blue), and ZsGreen (green). Insets are denoted by dashed boxes. Arrowheads demarcate vessel type, including sinusoids (white; ZsGreen−SCA1−CDH5+), transitional (magenta; ZsGreen−SCA1+CDH5+), and arterioles (yellow; ZsGreen+SCA1+CDH5+). Scale bars for the central image (200 μm) and insets (50 μm) are noted. d Representative flow plots to quantify Cx40-creERT2 activity in BM EC subtypes; arteriole and transitional (red box) and sinusoids (green box) are indicated. Open arrow/dashed line illustrates gating progression. Percentages represent AVE ± SEM; N = 4. e Quantification of Cx40-creERT2; ZsGreenfl/wt activity in BM cellular subsets, including arteriole and transitional (EC; A + T) and sinusoidal (EC; S) endothelium, LEPR+ mesenchymal cells, osteoblasts (Osteo), pan-hematopoietic cells (Hemato), hematopoietic stem cells (HSCs), macrophages (Macro), and megakaryocytes (Mega). Percentages represent AVE ± SEM; N = 4. f Representative flow plots assessing endothelial populations in ZsGreen+ cells derived from Cx40-creERT2; ZsGreen.fl/wt BM. Open arrow/dashed line illustrates gating progression. Percentages represent AVE ± SEM; N = 4
Tamoxifen Induction
To induce creERT2-mediated recombination in heterozygous B6.Cg-Gt(ROSA)26Sortm6(CAG−ZsGreen1)Hze /J (ZsGreenfl/wt) or B6.Cg-Gt(ROSA)26Sortm9(CAG−tdTomato)Hze/J (tdTomatofl/wt) reporter animals, adult (8 weeks) Vegfr3-creERT2+; ZsGreenfl/wt, Cx40-creERT2+; ZsGreenfl/wt, and Bmx-creERT2; tdTomatofl/wt mice were fed Custom Teklad PicoLab Rodent Diet 20 (Lab Diet 5053) supplemented with 5% w/w Sucrose and 0.25% Tamoxifen ad libitum for four weeks. Mice were allowed to recover for four weeks prior to analysis. Age- and sex-matched littermate controls (creERT2−; ZsGreenfl/wt or creERT2−; tdTomatofl/wt) that underwent the same Tamoxifen regimen were used as experimental controls.
To induce creERT2-mediated recombination in homozygous C57BL/6 J-Gt(ROSA)26Sortm(Map2k1*EGFP)Rsky/J (Mapkfl/fl) animals, adult (8 weeks) Vegfr3-creERT2+; Mapkfl/fl (R3-MAPK), Cx40-creERT2+; Mapkfl/fl (Cx40-MAPK), and Cdh5-creERT2+; Mapkfl/fl (Cdh5-MAPK) mice were fed Custom Teklad PicoLab Rodent Diet 20 (Lab Diet 5053) supplemented with 5% w/w Sucrose and 0.25% Tamoxifen ad libitum for four weeks. Mice were allowed to recover for four weeks prior to analysis. Age- and sex-matched littermate controls (creERT2−; Mapkfl/fl) that underwent the same Tamoxifen regimen were used as experimental controls.
Microscopy
Endothelium were labeled in situ in tamoxifen-induced adult Vegfr3-creERT2+, Cx40-creERT2+, and Bmx-creERT2+ reporter animals (ZsGreenfl/wt or tdTomatofl/wt) by retro-orbital sinus injections with an αCDH5 antibody (Supplementary Table 1). Mice were euthanized 10 min post-injection and femurs, liver, and spleen samples were collected and fixed overnight with 4% paraformaldehyde (in PBS; pH 7.2) at 4 °C. Femurs were washed three times (15 min/wash) with PBS (pH 7.2) at room temperature, decalcified in 10% EDTA in PBS (pH 7.2) for 72 h at room temperature, and normalized to 30% sucrose (in PBS; pH 7.2) for 72 h at 4 °C. Liver and spleen samples were washed three times (15 min/wash) with PBS (pH 7.2) at room temperature and normalized to 30% sucrose (in PBS; pH 7.2) for 72 h at 4 °C. Tissues were then embedded in 1:1 mixture of Tissue-Tek O.C.T. (Sakura) and 30% sucrose (in PBS; pH 7.2) and snap frozen in N2(l). To expose the marrow cavity for whole mount analysis, femurs were shaved longitudinally using a cryostat (Leica 3050S) and washed three times (5 min/wash) in PBS (pH 7.2) to remove excess O.C.T. Exposed marrow was then permeabilized in PBS (pH 7.2) with 20% (v/v) Normal Goat Serum (Jackson ImmunoResearch) and 0.5% (v/v) Triton X-100 (Sigma-Aldrich) for 2 h at room temperature and stained with an αSCA1 antibody for 48 h at 4 °C (Supplementary Table 1). For liver and spleen, sections were cut (12 μm) using a cryostat (Leica 3050S) and washed three times (5 min/wash) in PBS (pH 7.2) to remove excess O.C.T., and permeabilized in PBS (pH 7.2) with 20% (v/v) Normal Goat Serum (Jackson ImmunoResearch) and 0.5% (v/v) Triton X-100 (Sigma-Aldrich) for 30 min at room temperature. Tissues were washed three times (15 min/wash) in PBS (pH 7.2) and stained with DAPI (Biolegend) at 1 μg/mL in PBS (pH 7.2) for 15 min at room temperature (where applicable). Tissues were mounted with ProLong Gold (ThermoFisher Scientific) and imaged using a Nikon C2 confocal LASER-scanning microscope; 40 μm Z-stack images were acquired and denoised (Denoise.ai) and rendered into a maximum intensity projection using NIS Elements software (Nikon).
Whole Bone Marrow Isolation
Individual femurs were disassociated using a mortar and pestle in PBS (pH 7.2) + 0.5% BSA (w/v) + 2 mM EDTA and filtered (40 μm; Corning) to ensure a single cell suspension. Cell counts were determined using a Hemocytometer (Reichert Bright-Line; Hausser) and Trypan Blue (ThermoFisher Scientific) according to the manufacturer’s recommendations. For HSPC analysis by flow cytometry, whole bone marrow (WBM) suspensions were depleted of terminally-differentiated hematopoietic cells using the murine-specific Lineage Cell-Depletion Kit (MiltenyiBiotec) according to the manufacturer’s recommendations.
Bone Marrow Digestion
Individual femurs were disrupted using a mortar and pestle in Hanks Balanced Salt Solution (Corning) + 10 mM HEPES (pH 7.2) and enzymatically disassociated under gentle agitation with 2.5 mg/mL Collagenase A (Roche) and 1 Unit/mL Dispase II (Roche) for 20 min at 37 °C. Resulting cell suspensions were filtered (40 μm; Corning) and washed using ten times digestion volume with PBS (pH 7.2) + 0.5% BSA (w/v) + 2 mM EDTA. Cell counts were determined using a Hemocytometer (Reichert Bright-Line; Hausser) and Trypan Blue (ThermoFisher Scientific) according to the manufacturer’s recommendations. To enrich for EC, stromal cell, and HSPC fractions, digested BM was depleted of terminally-differentiated hematopoietic cells using the murine-specific Lineage Cell-Depletion Kit (MiltenyiBiotec) according to the manufacturer’s recommendations.
Liver/Spleen Digestion
Individual liver or spleen samples were minced to ~ 1mm3 using sterile scalpels and enzymatically disassociated under gentle agitation in Hanks Balanced Salt Solution (Corning) + 10 mM HEPES (pH 7.2) with 2.5 mg/mL Collagenase A (Roche) and 1 Unit/mL Dispase II (Roche) for 30 min at 37 °C. Resulting cell suspensions were filtered (40 μm; Corning) and washed using ten times digestion volume with PBS (pH 7.2) + 0.5% BSA (w/v) + 2 mM EDTA. Cell counts were determined using a Hemocytometer (Reichert Bright-Line; Hausser) and Trypan Blue (ThermoFisher Scientific) according to the manufacturer’s recommendations.
Flow Cytometry
For all flow cytometry, 2 × 106 total cells were blocked with αCD16/CD32 (Supplementary Table 1) in 200 μL PBS (pH 7.2) + 0.5% BSA (w/v) + 2 mM EDTA for 10 min at 4 °C and stained with the appropriate antibodies for 45 min at 4 °C. Stained cells were washed with 1 mL PBS (pH 7.2) + 0.5% BSA (w/v) + 2 mM EDTA and fixed in in PBS (pH 7.2) + 2 mM EDTA with 1% paraformaldehyde. All samples were analyzed on a BD LSRFortessa SORP using BD FACSDiva Software (v9.0).
Recombination Quantification
To quantify creERT2-mediated recombination in tamoxifen-induced adult Vegfr3-creERT2+, Cx40-creERT2+, and Bmx-creERT2+ reporter animals (ZsGreenfl/wt or tdTomatofl/wt), WBM (i.e. hematopoietic cells), digested WBM (i.e. LEPR+ cells/osteoblasts), digested/lineage-depleted BM cells (i.e. endothelium/HSPCs), and digested liver/spleen cells were stained with antibodies described in Supplementary Table 1 and analyzed by flow cytometry.
HSPC Analysis
HSPC populations were quantified by flow cytometry from WBM stained with antibodies described in Supplementary Table 1.
Colony Forming Assays
Based on Hemocytometer counts, 7.5 × 104 total WBM cells in 300 μL Low-Glucose DMEM (ThermoFisher Scientific) were added to 3 mL MethoCult GF M3434 (StemCell Technologies) and plated in duplicate (2.5 × 104 cells/well) on low-adherent 6-well plates. Cells were incubated at 37 °C 5% CO2 and scored for hematopoietic progenitor colony-forming units ten days post-plating using an SZX16 Stereo Microscope (Olympus) according to the manufacturer’s guidelines.
Peripheral Blood
To examine multilineage donor engraftment or complete blood counts, mice were bled via the retro-orbital sinus using 75 mm heparinized capillary tubes (Kimble-Chase) into microfuge tubes with PBS (pH 7.2) + 10 mM EDTA and analyzed as described.
Complete Blood Counts
Complete blood counts were quantified using an Element HT5 (Heska) veterinary hematological analyzer according to the manufacturer’s recommendations.
Competitive Transplantation
For competitive transplantations, 5 × 105 CD45.2+ donor BM cells + 5 × 105 CD45.1+ competitor BM cells isolated from 16-week-old mice were intravenously injected into adult (12 weeks) CD45.1+ recipient mice pre-conditioned with split-dose total body irradiation (2 × 475 cGy; RadSource RS2000 Small Animal X-Ray Irradiator). For long-term (16 weeks post-transplantation) multilineage engraftment quantification by flow cytometry, peripheral blood was depleted of red blood cells using RBC lysis buffer (Biolegend) according to the manufacturer’s recommendations, stained with antibodies described in Supplementary Table 1, and analyzed by flow cytometry.
Hematopoietic Recovery
For myelosuppression, control (creERT2−; Mapkfl/fl) and experimental (creERT2+; Mapkfl/fl) mice were subjected to single-dose total body irradiation (450 cGy; RadSource RS2000 Small Animal X-Ray Irradiator) and bled weekly to determine complete blood count recovery kinetics. Non-irradiated baseline counts were determined two weeks prior to myelosuppression.
Statistical Analysis
Experimental significance was determined using Prism 9.5.1 Software (Graphpad). Statistical analysis and parameters are indicated in individual figure legends.
Results
Vegfr3-creERT2 Targets Sinusoidal and Transitional Endothelium in the Bone Marrow
To generate an inducible sinusoid-specific cre-expressing mouse model, we utilized a C57BL/6 J-derived bacterial artificial chromosome (BAC) containing the Vegfr3 gene with approximately 130 kb upstream and 60 kb downstream genomic DNA sequence. This BAC was previously used to generate Vegfr3-Yfp reporter mice [21] that discriminate sinusoidal endothelium from arterioles in adult BM [27]. Using bacterial recombineering, a creERT2 cassette was introduced in-frame downstream of the Vegfr3 exon 1 start codon (Fig. 1a). The residual Kanamycin selection cassette was removed via FLP-mediated recombination prior to targeted-BAC linearization and pronuclear injection into fertilized C57BL/6 J zygotes. Resulting Vegfr3-creERT2+ offspring were maintained on a C57BL/6 J background. The transgene insertion site was mapped to unplaced scaffold chromosome chrUN_JH584304 approximately 40 kb upstream of phosphatidylserine decarboxylase - pseudogene 3 (Pisd-ps3) (Fig. 1b), avoiding the disruption of any known protein-coding genes. We next sought to evaluate the fidelity of Vegfr3-creERT2+ mice to mark sinusoidal endothelium in the adult BM microenvironment.
To evaluate Vegfr3-creERT2 activity in the BM, adult Vegfr3-creERT2+; ZsGreenfl/wt reporter mice were induced with tamoxifen-supplemented feed and assessed for ZsGreen expression within the BM by immunofluorescence (IF) imaging and flow cytometry. To discriminate patent vasculature, pan-endothelium were labeled in vivo by intravital αCDH5 staining that excludes lymphatic vessels [28]. In combination with αCDH5 staining, high expression levels of Sca1 on arteriole endothelium make it an effective marker to delineate arteriole from sinusoidal vasculature in the BM [29, 30]. IF analysis revealed that Vegfr3-creERT2 mice labeled ZsGreen+CDH5+SCA1− sinusoidal endothelium (Fig. 1c; left inset-white arrowhead) that were distinct from ZsGreen−CDH5+SCA1+ arteriole endothelium (Fig. 1c; left inset-yellow arrowhead). In addition to sinusoids, Vegfr3-creERT2 mice marked ZsGreen+CDH5+SCA1+ transitional endothelium (Fig. 1c; right inset-magenta arrowhead). Transitional endothelium are distinct from ZsGreen−CDH5+SCA1+ arterioles (Fig. 1c; right inset-yellow arrowhead) and ZsGreen+CDH5+SCA1− sinusoids (Fig. 1c; right inset-white arrowhead). We confirmed Vegfr3-creERT2 specificity in BM cellular niche constituents and hematopoietic cell populations by flow cytometry. Using PDPN and SCA1 to delineate EC subtypes in enzymatically-dissociated WBM [9], Vegfr3-creERT2 efficiently labeled 94.5% of immunophenotypic PDPN+SCA1dim sinusoidal endothelium within the pan-endothelial CDH5+CD45−TER119− population (Fig. 1d, e). Interestingly, Vegfr3-creERT2 activity was also observed in 26.7% of the PDPN−/dimSCA1bright ECs (Fig. 1d, e), revealing that the previously described PDPN−/dimSCA1bright “arterioles” described by flow cytometry contain both arterioles and transitional endothelium identified by imaging (Fig. 1c). Gating on total ZsGreen+CD45−TER119− WBM cells confirmed Vegfr3-creERT2 vascular endothelial specificity, in which 96.1% of ZsGreen+ cells were CD45−TER119−CDH5+ (Fig. 1f). Additionally, ZsGreen+CDH5+ mark transitional endothelium as a subset within PDPN−/dimSCA1bright endothelial population (Fig. 1d, f). Vegfr3-creERT2 activity is notably absent in immunophenotypic LEPR+ mesenchymal stem cells (MSCs), CD51+SCA1− osteoblasts, CD45+ pan-hematopoietic cells, lineage−cKIT+SCA1+CD48−CD150+ HSCs, GR1Low/−F4/80+CD115− macrophages, and CD41+ megakaryocytes (Fig. 1e and Supplementary Fig. 1a-f). In addition to the BM, Vegfr3-creERT2 activity is also observed in CDH5+ liver and spleen sinusoidal endothelium (Supplementary Fig. 2a and Supplementary Fig. 3a). Moreover, Vegfr3-creERT2 activity is restricted to the vasculature and not observed in either hematopoietic or stromal compartments (Supplementary Fig. 2b and Supplementary Fig. 3b).
Bmx-creERT2 Labels Arteriole Endothelium and LEPR+ Cells in the Bone Marrow
We next evaluated Bmx-creERT2 transgenic animals [18], an established inducible creERT2 model system used to label BM arterioles [9, 31–33]. To confirm their specificity, we examined BM from tamoxifen-induced adult Bmx-creERT2+; tdTomatofl/wt reporter mice. Bmx-creERT2 mice efficiently labeled BM arteriole endothelium (Supplementary Fig. 4a; right inset-white arrowhead), but also marked CDH5− non-endothelial perisinusoidal cells (Supplementary Fig. 4a; right inset-yellow arrowhead) and interstitial cells (Supplementary Fig. 4a; left inset-magenta arrowhead). While flow cytometry confirmed that Bmx-creERT2 labeled a subset of vascular endothelium in the BM (Supplementary Fig. 4b), the vast majority of tdTomato+ cells (~ 80%) were non-endothelial (Supplementary Fig. 4c). Because the perisinusoidal and interstitial tdTomato+CDH5− staining pattern is reminiscent of previously described Lepr-cre activity in the BM [34–36], we examined Bmx-creERT2 activity in LEPR+ cells by flow cytometry. While Bmx-creERT2 activity was observed in only 7.1% of LEPR+CD45−TER119−CDH5− MSCs (Supplementary Fig. 4d), the vast majority of tdTomato+CD45−TER119−CDH5− cells (i.e. non-endothelial and non-hematopoietic) were LEPR+ (Supplementary Fig. 4e). Alongside vascular endothelium in the BM microenvironment, LEPR+ MSCs cooperatively support HSPC function and niche integrity through the expression of hematopoietic-supportive factors [34, 36–44]. While Bmx-creERT2 efficiently labels BM arterioles, the potential for off-target effects in LEPR+ cells should be taken into consideration during experimental design and data interpretation.
Cx40-creERT2 Activity is Restricted to Arteriole Endothelium in the Bone Marrow
To identify an arteriole-specific expression pattern within the BM, we analyzed previously published RNA sequencing data of sort-purified BM arteriole and sinusoidal ECs by Xu et. al., revealing Gja5 (Connexin40/Cx40) as a candidate gene [9]. Cx40 is a member of the connexin family of transmembrane gap junction proteins that mediate intercellular crosstalk and coordinate multicellular functionality through ion exchange [45–47]. In the murine vasculature, Cx40 is expressed in large artery and arteriolar endothelium [48, 49]. To establish a BM arteriole-specific model system, we utilized a mouse line previously published by Beyer et. al. in which a creERT2::IRES-RFP was knocked-in the endogenous Cx40 gene in-frame with the canonical translational start site located in exon 2 (Fig. 2a) [26]. Relatively weak RFP signal in Cx40-creERT2::IRES-RFP mice does not interfere with immunohistochemistry or flow cytometry readouts in the red channel and requires an RFP-specific antibody for detection (data not shown) [26]. Outbred Cx40-creERT2 mice were backcrossed to C57BL/6 J recipients using a speed congenics approach for six generations; mating pair selection and backcrosses were confirmed using the miniMUGA array-based platform (Fig. 2b) [50]. To evaluate Cx40-creERT2 activity in the BM, adult Cx40-creERT2+; ZsGreenfl/wt reporter mice were induced with tamoxifen-supplemented feed and assessed for ZsGreen expression by imaging and flow cytometry. BM IF analysis demonstrated that Cx40-creERT2 specifically mark ZsGreen+CDH5+SCA1+ arteriole endothelium (Fig. 2c; left inset-yellow arrowhead), with no observable activity in the sinusoidal compartment (Fig. 2c; left inset-white arrowhead). Notably, Cx40-creERT2 activity was not observed in transitional endothelium (Fig. 2c; right inset-magenta arrowhead), making Vegfr3-creERT2 and Cx40-creERT2 mice compatible systems to label discrete endothelial subtypes in the adult BM. Furthermore, quantification of Cx40-creERT2 activity of enzymatically-dissociated WBM using flow cytometry revealed ~ 20% ZsGreen+ arteriole staining within the PDPN−/dimSCA1bright gate containing both arteriole and transitional ECs, while no signal was detected in the PDPN+SCA1dim sinusoidal population (Fig. 2d, e). Among total ZsGreen+CD45−TER119− BM cells, ZsGreen+ cells primarily fell within the CDH5+ pan-endothelial population and subsequently the PDPN−/dimSCA1bright gate arteriole/transitional endothelial gate (Fig. 2f). Cx40-creERT2 activity was not detected in immunophenotypically-defined LEPR+ MSCs, CD51+SCA1− osteoblasts, CD45+ pan-hematopoietic cells, lineage−cKIT+SCA1+CD48−CD150+ HSCs, GR1Low/−F4/80+CD115− macrophages, or CD41+ megakaryocytes (Fig. 2e and Supplementary Fig. 5a-f). Cx40-creERT2 activity is also detected in CDH5+ liver and spleen arteriole endothelium (Supplementary Fig. 2a and Supplementary Fig. 3a). Cx40-creERT2 activity is vascular-specific and not observed in either hematopoietic or stromal compartments in the liver or spleen (Supplementary Fig. 2c and Supplementary Fig. 3c). Moreover, direct comparison of Cx40-creERT2 and Vegfr3-creERT2 liver and spleen activity reveals anatomically-discrete vascular labeling, with Cx40-creERT2 marking typical large arteries (Supplementary Fig. 2a) in the liver and arteriole-enriched white pulp in the spleen (Supplementary Fig. 3a), while Vegfr3-creERT2 identifies characteristic sinusoidal vascular of the liver (Supplementary Fig. 2a) and sinus-enriched red pulp of the spleen (Supplementary Fig. 3a).
Vegfr3-creERT2 and Cx40-creERT2 Activity Does Not Alter Hematopoietic Engraftment
To evaluate whether creERT2 induction in Vegfr3-creERT2 and Cx40-creERT2 mice have a deleterious effect on HSC-niche function, we performed a 1:1 competitive transplantation of WBM from tamoxifen-induced adult Vegfr3-creERT2 or Cx40-creERT2 mice (CD45.2+) with competitor WBM (CD45.1+) into lethally-irradiated CD45.1 recipients to examine HSC repopulation activity. Tamoxifen-induced C57BL/6 J wild type and pan-endothelial expressing Cdh5-creERT2 mice (CD45.2+) were used as controls for comparison. WBM cells derived from all three creERT2 lines displayed robust long-term multilineage hematopoietic reconstitution (Supplementary Fig. 6a, b), with no discernible off-target effects when compared to controls.
Arteriole-specific MAPK-activation Drives HSC and Hematopoietic Dysfunction
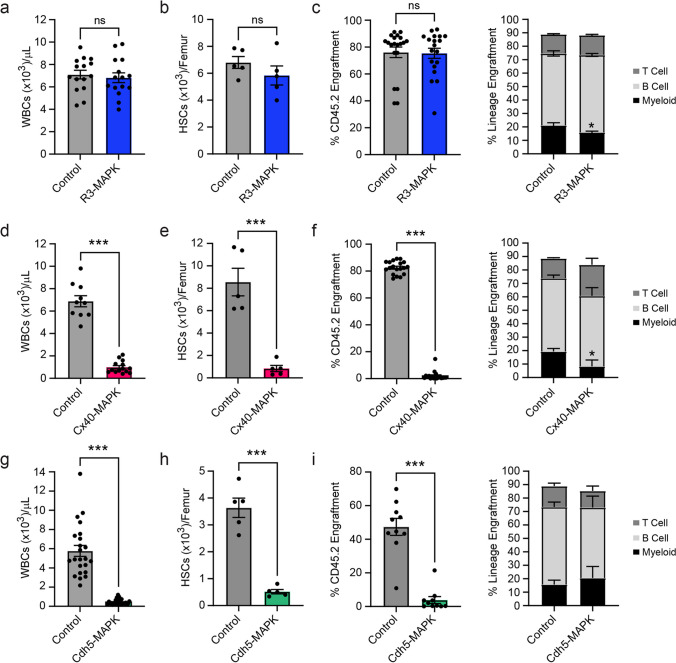
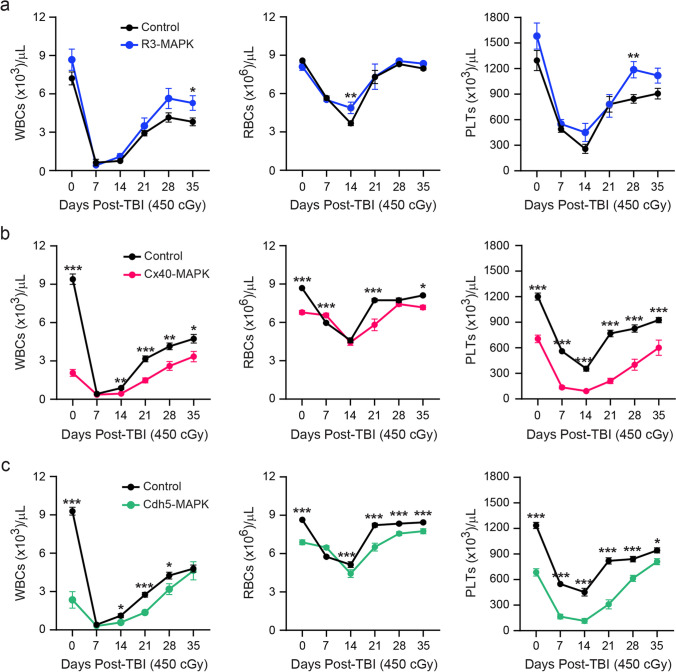
To validate the experimental utility of complementary BM sinusoidal/transitional and arteriole vascular-specific creERT2 mice, we generated Vegfr3-creERT2; Mapkfl/fl (R3-MAPK) and Cx40-creERT2; Mapkfl/fl (Cx40-MAPK) models in which cre-driven recombination of an upstream loxP-flanked stop cassette at the Rosa26 locus induces constitutively-active Map2k1 (S218D/S222D) signaling [20]. Chronic activation of the MAPK-ERK pathway in endothelium has been shown to drive vascular inflammation and HSC dysfunction in the BM microenvironment [15]. Adult creERT2; Mapkfl/fl mice were induced with tamoxifen-supplemented feed and assessed for their respective contributions to endothelial-driven hematopoietic dysfunction in previously described Cdh5-creERT2; Mapkfl/fl (Cdh5-MAPK) mice [15]. Arteriole-specific Cx40-MAPK mice developed a robust peripheral pancytopenia that phenocopied the previously observed dysfunction in Cdh5-MAPK mice, including a loss in white blood cells (WBCs), RBCs, and platelets (Fig. 3d, g and Supplementary Fig. 7b, c). Interestingly, sinusoidal/transitional-specific R3-MAPK mice showed no overt changes in peripheral blood counts (Fig. 3a and Supplementary Fig. 7a). Because pancytopenia is indicative of impaired HSC function in Cdh5-MAPK mice, we examined HSPC parameters in R3-MAPK and Cx40-MAPK models. Cx40-MAPK and Cdh5-MAPK mice displayed a significant decrease in both absolute numbers (Fig. 3e, h) and frequency (Supplementary Fig. 8d, g) of immunophenotypically-defined HSCs (lineage−cKIT+SCA1+CD48−CD150+) and HSPCs (lineage−cKIT+SCA1+) (Supplementary Fig. 8e, h), while R3-MAPK mice showed no changes (Fig. 3b and Supplementary Fig. 8a, b). Impaired HSC function in Cx40-MAPK mice was confirmed following competitive WBM transplantation; Cx40-MAPK mice displayed a significant decrease in multilineage engraftment (Fig. 3f), comparable to Cdh5-MAPK (Fig. 3i), while R3-MAPK engraftment was unaffected (Fig. 3c). Impaired HSPC activity in a semi-solid methyl cellulose assay was also observed in Cx40-MAPK, but not R3-MAPK mice (Supplementary Fig. 8c, f, i). Furthermore, Cx40-MAPK mice demonstrated a comparable delay in hematopoietic recovery to Cdh5-MAPK mice following a myelosuppressive dose of total body irradiation (Fig. 4b, c), while R3-MAPK mice were largely unaffected (Fig. 4a).
Fig. 3.
Hematopoietic defects observed in adult Cdh5-MAPK mice are driven by arteriole MAPK-activation. Adult Mapkfl/fl mice crossed with sinusoid/transitional-specific Vegfr3-creERT2 (R3-MAPK; blue), arteriole-specific Cx40-creERT2 (Cx40-MAPK; red), or pan-endothelial Cdh5-creERT2 (Cdh5-MAPK; green) were induced with tamoxifen to activate MAPK signaling in BM endothelial subpopulations and assessed for hematopoietic dysfunction. Peripheral WBC counts in (a) R3-MAPK, (d) Cx40-MAPK, and (g) Cdh5-MAPK mice. Quantification of immunophenotypic HSCs in (b) R3-MAPK, (e) Cx40-MAPK, and (h) Cdh5-MAPK femurs. Total hematopoietic and lineage engraftment in competitive BM transplantation recipients from (c) R3-MAPK, (f) Cx40-MAPK, and (i) Cdh5-MAPK CD45.2+ donors. Tamoxifen-treated littermate Mapkfl/fl mice serve as controls. Individual biological replicates are indicated per bar graph. Data is presented as AVE ± SEM. Significance is established using a Student’s t-test with P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***); ns = not significant
Fig. 4.
Hematopoietic recovery is inhibited in arteriole-specific Cx40-MAPK mice. Adult (a) sinusoid/transitional-specific R3-MAPK (blue), (b) arteriole-specific Cx40-MAPK (red), or (c) pan-endothelial Cdh5-MAPK (green) mice were subjected to myelosuppressive irradiation and assessed for peripheral hematopoietic recovery. Tamoxifen-treated littermate Mapkfl/fl mice serve as controls. Cohort size for individual groups (N = Control mice/N = Experimental mice): R3-MAPK (14/10), Cx40-MAPK (18/9), Cdh5-MAPK (24/11). Data is presented as AVE ± SEM. Significance is established using a Student’s t-test at individual timepoints with P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***); ns = not significant
Discussion
Vascular-directed cre mice are a vital tool to unravel the complex BM EC-instructive mechanisms that modulate HSPC function in vivo. However, model-specific differences in cre activity can have a profound impact on the interpretation of experimental phenotypes [10, 51]. In this study, we set out to characterize congenic C57BL/6 J cre-expressing mice models that (1) allow for inducible cre-mediated recombination in adult BM EC subsets, bypassing the potential pitfalls of embryonic HSC involvement, (2) avoid off-target cre activity in HSPC-supportive BM perivascular niche cells, and (3) define non-overlapping arteriole, transitional, and sinusoidal BM EC cre activity. In reporter mice, tamoxifen-treated Vegfr3-creERT2 and Cx40-creERT2 efficiently labeled discrete adult BM sinusoidal/transitional and arteriole EC populations, respectively, while avoiding hematopoietic and stromal involvement. Vegfr3-creERT2 and Cx40-creERT2 also specifically label discrete sinusoidal and arteriole endothelium in secondary hematopoietic tissues, including the liver and spleen, with no detectable activity in non-endothelial populations. Functionally, we demonstrated that Vegfr3-creERT2 and Cx40-creERT2 mice were able to phenotypically identify and segregate arteriole-specific activation of MAPK signaling as the source of hematopoietic dysfunction previously reported using a vascular-specific pan-endothelial Cdh5-creERT2 driver in adult animals [15].
While the identified Vegfr3-creERT2 BAC-transgene insertion point was mapped to an unplaced genomic segment and does not appear to directly disrupt any protein-coding genes, Cx40-creERT2 knock-in animals [26] disrupt the endogenous Cx40 allele. However, the cardiovascular system in Cx40+/- knockout mice display no reported gross differences when compared with wild type littermates [52–54]. Nonetheless, Cx40-creERT2 and Vegfr3-creERT2 mice should be maintained as heterozygotes to avoid potential complications due to excessive cre activity or loss of gene function at the transgene insertion loci. Because cre recombinase activity in murine model systems have been implicated in loxP-independent cellular cytotoxicity [55, 56], we also examined the potential effect of BM EC subset-specific creERT2 induction on HSC activity. Competitive transplantation of WBM from Vegfr3-creERT2+ or Cx40-creERT2+ tamoxifen-inducted animals demonstrated that creERT2 activity in these models does not impair hematopoietic reconstitution.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge Thomas L. Saunders, Elizabeth Hughes, Wanda Filipiak, Galina Gavrilina, and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities for the production of the Vegfr3-creERT2 BAC (#2725) transgenic mice. Research reported in this publication was supported by the University of Michigan Transgenic Animal Model Core and the Biomedical Research Core Facilities. We would also like to acknowledge the University of Florida ICBR Facilities, including Diansy Zincke and NextGen DNA Sequencing Core (RRID:SCR_019152) and Alberto Riva and the Bioinformatics Core (RRID:SCR_019120) for DNA sequencing and insertion site determination of the Vegfr3-creERT2 transgene.
Author Contributions
MGP and JMB conceived and designed the experimental approach. MGP, PR, AW, MCG, LK, and CC conducted all experiments. LPF, AE, and JLT provided the creERT2 BAC-targeting construct. LM provided outcrossed Cx40-creERT2 mice. MGP analyzed data and wrote the report. All authors have approved the experimental approach and results.
Funding
JMB is funded by R01s (R01CA204308, R01HL133021, R01AG065436, and R01HL166512) and is a Lymphoma and Leukemia Society Scholar. MGP was supported by The American Cancer Society Young Investigator Award, through Georgetown University and the Lombardi Comprehensive Cancer Center Institutional Research Grant.
Data Availability
All primary data generated in this study are available from the corresponding authors upon reasonable request.
Code Availability
Not applicable.
Declarations
Ethics Approval
All animal experiments were performed under AAALAC and NIH OLAW recommendations, in accordance with the University of Florida IACUC guidelines.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publish.
Competing Interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y, Gao S, Xia J, Liu F. Hematopoietic hierarchy - An updated roadmap. Trends in Cell Biology. 2018;28(12):976–986. doi: 10.1016/j.tcb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Kasbekar M, Mitchell CA, Proven MA, Passegue E. Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands. Cell Stem Cell. 2023;30(11):1403–1420. doi: 10.1016/j.stem.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam P, Butler JM, Poulos MG. Vascular regulation of hematopoietic stem cell homeostasis, regeneration, and aging. Current Stem Cell Reports. 2021;7(4):194–203. doi: 10.1007/s40778-021-00198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Developmental Cell. 2021;56(13):1848–1860. doi: 10.1016/j.devcel.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramasamy SK. Structure and functions of blood vessels and vascular niches in bone. Stem Cells International. 2017;2017:5046953. doi: 10.1155/2017/5046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson, E. C., & Adams, R. H. (2018). Biology of bone: The vasculature of the skeletal system. Cold Spring Harbor Perspectives in Medicine, 8(7). 10.1101/cshperspect.a031559 [DOI] [PMC free article] [PubMed]
- 7.Taylor, A. M., & Bordoni, B. (2023). Histology, blood vascular system.In StatPearls. https://www.ncbi.nlm.nih.gov/pubmed/31985998. Accessed 8 Jan 2024. [PubMed]
- 8.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Gao X, Wei Q, Nakahara F, Zimmerman SE, Mar J, Frenette PS. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nature Communications. 2018;9(1):2449. doi: 10.1038/s41467-018-04726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne S, De Val S, Neal A. Endothelial-specific cre mouse models. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(11):2550–2561. doi: 10.1161/ATVBAHA.118.309669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao WP, Uetzmann L, Burtscher I, Lickert H. Generation of a mouse line expressing Sox17-driven Cre recombinase with specific activity in arteries. Genesis. 2009;47(7):476–483. doi: 10.1002/dvg.20520. [DOI] [PubMed] [Google Scholar]
- 12.Ichise T, Yoshida N, Ichise H. H-, N- and Kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice. Development. 2010;137(6):1003–1013. doi: 10.1242/dev.043489. [DOI] [PubMed] [Google Scholar]
- 13.Geraud, C., Koch, P. S., Zierow, J., Klapproth, K., Busch, K., Olsavszky, V., Leibing, T., Demory, A., Ulbrich, F., Diett, M., Singh, S., Sticht, C., Breitkopf-Heinlein, K., Richter, K., Karppinen, S. M., Pihlajaniemi, T., Arnold, B., Rodewald, H. R., Augustin, H. G., ... Goerdt, S. (2017). GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. Journal of Clinical Investigation,127(3), 1099–1114. 10.1172/JCI90086 [DOI] [PMC free article] [PubMed]
- 14.Heil, J., Olsavszky, V., Busch, K., Klapproth, K., de la Torre, C., Sticht, C., Sandorski, K., Hoffmann, J., Schonhaber, H., Zierow, J., Winkler, M., Schmid, C. D., Staniczek, T., Daniels, D. E., Frayne, J., Metzgeroth, G., Nowak, D., Schneider, S., Neumaier, M., ... Koch, P. S. (2021). Bone marrow sinusoidal endothelium controls terminal erythroid differentiation and reticulocyte maturation. Nature Communications, 12(1), 6963. 10.1038/s41467-021-27161-3 [DOI] [PMC free article] [PubMed]
- 15.Ramalingam P, Poulos MG, Lazzari E, Gutkin MC, Lopez D, Kloss CC, Crowley MJ, Katsnelson L, Freire AG, Greenblatt MB, Park CY, Butler JM. Chronic activation of endothelial MAPK disrupts hematopoiesis via NFKB dependent inflammatory stress reversible by SCGF. Nature Communications. 2020;11(1):666. doi: 10.1038/s41467-020-14478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 18.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140(14):3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 19.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo, C. F., Fontaine, R. H., Soueid, J., Tammela, T., Makinen, T., Alfaro-Cervello, C., Bonnaud, F., Miguez, A., Benhaim, L., Xu, Y., Barallobre, M. J., Moutkine, I., Lyytikka, J., Tatlisumak, T., Pytowski, B., Zalc, B., Richardson, W., Kessaris, N., Garcia-Verdugo, J. M., ... Thomas, J. L. (2011). Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes & Development, 25(8), 831–844. 10.1101/gad.615311 [DOI] [PMC free article] [PubMed]
- 22.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Research. 2005;33(4):e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharan SK, Thomason LC, Kuznetsov SG, Court DL. Recombineering: A homologous recombination-based method of genetic engineering. Nature Protocols. 2009;4(2):206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nature Biotechnology. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer S, Kelly RG, Miquerol L. Inducible Cx40-Cre expression in the cardiac conduction system and arterial endothelial cells. Genesis. 2011;49(2):83–91. doi: 10.1002/dvg.20687. [DOI] [PubMed] [Google Scholar]
- 27.Poulos MG, Crowley MJP, Gutkin MC, Ramalingam P, Schachterle W, Thomas JL, Elemento O, Butler JM. Vascular platform to define hematopoietic stem cell factors and enhance regenerative hematopoiesis. Stem Cell Reports. 2015;5(5):881–894. doi: 10.1016/j.stemcr.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental Cell. 2013;26(2):204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp HG, Hooper AT, Avecilla ST, Rafii S. Functional heterogeneity of the bone marrow vascular niche. Annals of the New York Academy of Sciences. 2009;1176:47–54. doi: 10.1111/j.1749-6632.2009.04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Liu Y, Jeong HW, Stehling M, Dinh VV, Zhou B, Adams RH. Apelin(+) endothelial niche cells control hematopoiesis and mediate vascular regeneration after myeloablative injury. Cell Stem Cell. 2019;25(6):768–783 e766. doi: 10.1016/j.stem.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emoto, T., Lu, J., Sivasubramaniyam, T., Maan, H., Khan, A. B., Abow, A. A., Schroer, S. A., Hyduk, S. J., Althagafi, M. G., McKee, T. D., Fu, F., Shabro, S., Ulndreaj, A., Chiu, F., Paneda, E., Pacheco, S., Wang, T., Li, A., Jiang, J. X., ... Robbins, C. S. (2022). Colony stimulating factor-1 producing endothelial cells and mesenchymal stromal cells maintain monocytes within a perivascular bone marrow niche. Immunity, 55(5), 862–878 e868. 10.1016/j.immuni.2022.04.005 [DOI] [PubMed]
- 33.Liu Y, Chen Q, Jeong HW, Koh BI, Watson EC, Xu C, Stehling M, Zhou B, Adams RH. A specialized bone marrow microenvironment for fetal haematopoiesis. Nature Communications. 2022;13(1):1327. doi: 10.1038/s41467-022-28775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nature Cell Biology. 2017;19(8):891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes & Development. 2018;32(5–6):359–372. doi: 10.1101/gad.311068.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato Y, Hou LB, Miyagi S, Nitta E, Aoyama K, Shinoda D, Yamazaki S, Kuribayashi W, Isshiki Y, Koide S, Si S, Saraya A, Matsuzaki Y, van Lohuizen M, Iwama A. Bmi1 restricts the adipogenic differentiation of bone marrow stromal cells to maintain the integrity of the hematopoietic stem cell niche. Experimental Hematology. 2019;76:24–37. doi: 10.1016/j.exphem.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Fang S, Chen S, Nurmi H, Leppanen VM, Jeltsch M, Scadden D, Silberstein L, Mikkola H, Alitalo K. VEGF-C protects the integrity of the bone marrow perivascular niche in mice. Blood. 2020;136(16):1871–1883. doi: 10.1182/blood.2020005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramalingam P, Gutkin MC, Poulos MG, Tillery T, Doughty C, Winiarski A, Freire AG, Rafii S, Redmond D, Butler JM. Restoring bone marrow niche function rejuvenates aged hematopoietic stem cells by reactivating the DNA Damage Response. Nature Communications. 2023;14(1):2018. doi: 10.1038/s41467-023-37783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L, Lin Q, Chatla S, Amarachintha S, Wilson AF, Atale N, Gao ZJ, Joseph J, Wolff EV, Du W. LepR+ niche cell-derived AREG compromises hematopoietic stem cell maintenance under conditions of DNA repair deficiency and aging. Blood. 2023;142(18):1529–1542. doi: 10.1182/blood.2022018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kara N, Xue Y, Zhao Z, Murphy MM, Comazzetto S, Lesser A, Du L, Morrison SJ. Endothelial and leptin receptor(+) cells promote the maintenance of stem cells and hematopoiesis in early postnatal murine bone marrow. Developmental Cell. 2023;58(5):348–360 e346. doi: 10.1016/j.devcel.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao X, Murphy MM, Peyer JG, Ni Y, Yang M, Zhang Y, Guo J, Kara N, Embree C, Tasdogan A, Ubellacker JM, Crane GM, Fang S, Zhao Z, Shen B, Morrison SJ. Leptin receptor(+) cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nature Cell Biology. 2023;25(12):1746–1757. doi: 10.1038/s41556-023-01284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hautefort A, Pfenniger A, Kwak BR. Endothelial connexins in vascular function. Vascular Biology. 2019;1(1):H117–H124. doi: 10.1530/VB-19-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pohl U. Connexins: Key players in the control of vascular plasticity and function. Physiological Reviews. 2020;100(2):525–572. doi: 10.1152/physrev.00010.2019. [DOI] [PubMed] [Google Scholar]
- 47.Marquez M, Munoz M, Cordova A, Puebla M, Figueroa XF. Connexin 40-mediated regulation of systemic circulation and arterial blood pressure. Journal of Vascular Research. 2023;60(2):87–100. doi: 10.1159/000531035. [DOI] [PubMed] [Google Scholar]
- 48.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: Electrotonic versus regenerative conduction. American Journal of Physiology Heart and Circulatory Physiology. 2008;295(5):H2001–2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leybaert L, Lampe PD, Dhein S, Kwak BR, Ferdinandy P, Beyer EC, Laird DW, Naus CC, Green CR, Schulz R. Connexins in cardiovascular and neurovascular health and disease: Pharmacological implications. Pharmacological Reviews. 2017;69(4):396–478. doi: 10.1124/pr.115.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigmon, J. S., Blanchard, M. W., Baric, R. S., Bell, T. A., Brennan, J., Brockmann, G. A., Burks, A. W., Calabrese, J. M., Caron, K. M., Cheney, R. E., Ciavatta, D., Conlon, F., Darr, D. B., Faber, J., Franklin, C., Gershon, T. R., Gralinski, L., Gu, B., Gaines, C. H., ... Manuel de Villena, F. P. (2020). Content and performance of the MiniMUGA Genotyping Array: A new tool to improve rigor and reproducibility in mouse research. Genetics, 216(4), 905–930. 10.1534/genetics.120.303596 [DOI] [PMC free article] [PubMed]
- 51.Song AJ, Palmiter RD. Detecting and avoiding problems when using the Cre-lox system. Trends in Genetics. 2018;34(5):333–340. doi: 10.1016/j.tig.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Current Biology. 1998;8(5):295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 53.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney International. 2007;72(7):814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 54.Kim KH, Rosen A, Hussein SM, Puviindran V, Korogyi AS, Chiarello C, Nagy A, Hui CC, Backx PH. Irx3 is required for postnatal maturation of the mouse ventricular conduction system. Science and Reports. 2016;6:19197. doi: 10.1038/srep19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loonstra, A., Vooijs, M., Beverloo, H. B., Allak, B. A., van Drunen, E., Kanaar, R., Berns, A., & Jonkers, J. (2001). Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proceedings of the National Academy of Sciences,98(16), 9209-9214. 10.1073/pnas.161269798 [DOI] [PMC free article] [PubMed]
- 56.Pepin G, Ferrand J, Honing K, Jayasekara WSN, Cain JE, Behlke MA, Gough DJ, Williams BR, Hornung V, Gantier MP. Cre-dependent DNA recombination activates a STING-dependent innate immune response. Nucleic Acids Research . 2016;44(11):5356–5364. doi: 10.1093/nar/gkw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All primary data generated in this study are available from the corresponding authors upon reasonable request.
Not applicable.