Abstract
Indenofluorenes are non-benzenoid conjugated hydrocarbons that have received great interest owing to their unusual electronic structure and potential applications in nonlinear optics and photovoltaics. Here we report the generation of unsubstituted indeno[1,2-a]fluorene on various surfaces by the cleavage of two C–H bonds in 7,12-dihydroindeno[1,2-a]fluorene through voltage pulses applied by the tip of a combined scanning tunnelling microscope and atomic force microscope. On bilayer NaCl on Au(111), indeno[1,2-a]fluorene is in the neutral charge state, but it exhibits charge bistability between neutral and anionic states on the lower-workfunction surfaces of bilayer NaCl on Ag(111) and Cu(111). In the neutral state, indeno[1,2-a]fluorene exhibits one of two ground states: an open-shell π-diradical state, predicted to be a triplet by density functional and multireference many-body perturbation theory calculations, or a closed-shell state with a para-quinodimethane moiety in the as-indacene core. We observe switching between open- and closed-shell states of a single molecule by changing its adsorption site on NaCl.
Subject terms: Scanning probe microscopy, Imaging techniques, Structure elucidation, Electronic properties and materials, Magnetic properties and materials
Switching the magnetic state of a polycyclic conjugated hydrocarbon in a reversible and controlled manner is challenging. Now, by means of single-molecule scanning probe microscopy, an indenofluorene isomer on ultrathin NaCl films has been shown to adopt both open- and closed-shell states. Furthermore, bidirectional switching between the two states is achieved by changing the adsorption site of the molecule.
Main
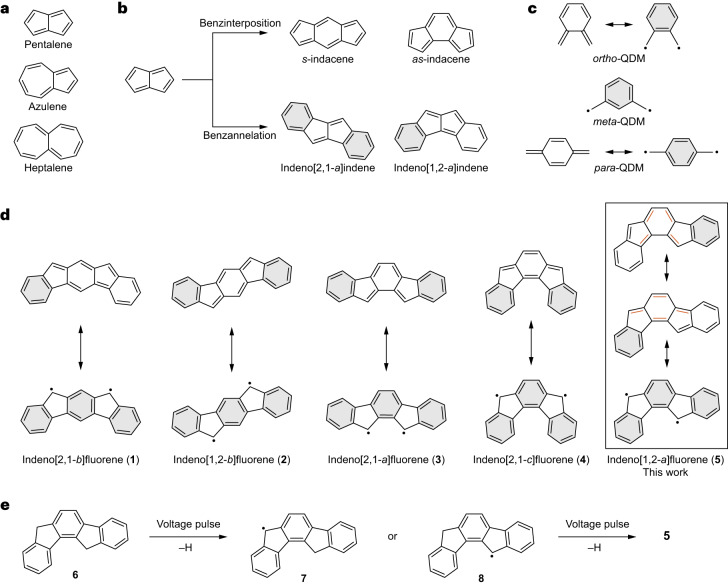
The inclusion of non-benzenoid carbocyclic rings is a viable route to tune the physicochemical properties of polycyclic conjugated hydrocarbons (PCHs)1–3. Non-benzenoid polycycles may lead to local changes in strain, conjugation and aromaticity, and—relevant to the context of the present work—induce an open-shell ground state of the corresponding PCHs4–7. Many non-benzenoid PCHs are also non-alternant, where the presence of odd-membered polycycles breaks the bipartite symmetry of the molecular network8. Figure 1a shows classical examples of non-benzenoid non-alternant PCHs, namely, pentalene, azulene and heptalene. Azulene is a stable PCH exhibiting Hückel aromaticity, but pentalene and heptalene are highly reactive Hückel antiaromatic and non-aromatic compounds, respectively. Benzinterposition of pentalene generates indacenes consisting of two isomers, s-indacene and as-indacene (Fig. 1b). Apart from being antiaromatic, indacenes also contain proaromatic quinodimethane (QDM) moieties (Fig. 1c)9, which endow them with potential open-shell character. Although the parent s-indacene and as-indacene have never been isolated, kinetically and thermodynamically stabilized derivatives of s-indacene have been synthesized10–12. A feasible strategy to isolate congeners of otherwise unstable non-benzenoid non-alternant PCHs is through fusion of benzenoid rings at the ends of the π-system, that is, benzannelation. For example, although the parent pentalene is highly reactive, the benzannelated congener indeno[2,1-a]indene is stable under ambient conditions (Fig. 1b)13. However, the position of benzannelation is crucial for stability: although indeno[2,1-a]indene is stable, its isomer indeno[1,2-a]indene (Fig. 1b) oxidizes under ambient conditions14. Similarly, benzannelation of indacenes gives rise to the family of PCHs known as indenofluorenes (Fig. 1d), which constitute the topic of the present work. Depending on the benzannelation position and the indacene core, five isomers can be constructed, namely, indeno[2,1-b]fluorene (1), indeno[1,2-b]fluorene (2), indeno[2,1-a]fluorene (3), indeno[2,1-c]fluorene (4) and indeno[1,2-a]fluorene (5).
Fig. 1. Non-benzenoid non-alternant polycyclic conjugated hydrocarbons.
a, Classical non-benzenoid non-alternant PCHs: pentalene, azulene and heptalene. b, Generation of indacenes and indenoindenes through benzinterposition and benzannelation of pentalene, respectively. Grey filled rings represent Clar sextets. c, Closed-shell Kekulé (left) and open-shell non-Kekulé (right) resonance structures of QDMs. Note that meta-QDM is a non-Kekulé molecule. All indenofluorene isomers, being derived through benzannelation of indacenes, contain a central QDM moiety. d, Closed-shell Kekulé (top) and open-shell non-Kekulé (bottom) resonance structures of indenofluorenes. Compared to their closed-shell structures, 1 and 5 gain two Clar sextets in the open-shell structure, and 2–4 gain only one Clar sextet in the open-shell structure. The coloured bonds in d highlight the ortho- and para-QDM moieties in the two closed-shell Kekulé structures of 5. e, Scheme of the on-surface generation of 5 (C20H12) by voltage pulse-induced dehydrogenation of 6 (C20H14). Structures 7 and 8 represent the two monoradical species (C20H13).
The practical interest in indenofluorenes stems from their low frontier orbital gaps and excellent electrochemical characteristics that render them useful as components in organic electronic devices15. The potential open-shell character of indenofluorenes has led to several theoretical studies on their use as nonlinear optical materials16,17 and as candidates for singlet fission in organic photovoltaics18,19. Recent theoretical work has also shown that indenofluorene-based ladder polymers may exhibit fractionalized excitations20. Fundamentally, indenofluorenes represent model systems to study the interplay between aromaticity and magnetism at the molecular scale17. Motivated by many of these prospects, the past decade has witnessed intensive synthetic efforts towards the realization of indenofluorenes. Derivatives of 1–4 have been realized in solution21–27, and 1–328–31 have also been synthesized on surfaces and characterized using scanning tunnelling microscopy (STM) and atomic force microscopy (AFM), which provide information on the molecular orbital densities32, molecular structure33,34 and oxidation state35,36. With regard to the open-shell character of indenofluorenes, 2–4 are theoretically and experimentally interpreted to be closed-shell, but calculations indicate that 1 and 5 should exhibit open-shell ground states17,28,37. Bulk characterization of mesityl-substituted 1, including X-ray crystallography, temperature-dependent NMR and electron spin resonance spectroscopy, have provided indications of its open-shell ground state21. Electronic characterization of 1 on a Au(111) surface using scanning tunnelling spectroscopy (STS) revealed a low electronic gap of 0.4 eV (ref. 28). However, no experimental proof of an open-shell ground state of 1 on Au(111), such as detection of the orbital densities of singly occupied molecular orbitals (SOMOs)38,39 or spin excitations and correlations due to unpaired electrons40,41, has been shown.
In this Article we report the generation and characterization of unsubstituted 5. Our research is motivated by theoretical calculations that indicate 5 to exhibit the largest diradical character among all indenofluorene isomers37. The same calculations also predict that 5 should possess a triplet ground state. Accordingly, 5 would qualify as a Kekulé triplet, of which only a handful of examples exist42–44. However, the synthesis of 5 has remained a challenge. Previously, Dressler and colleagues have reported the transient isolation of mesityl-substituted 5, but it decomposed both in solution and in the solid state37, and only the structural proof of the corresponding dianion was obtained. On-surface generation of a derivative of 5, starting from truxene as a precursor, was recently reported45,46. STM data on this compound, containing the indeno[1,2-a]fluorene moiety as part of a larger PCH, were interpreted to indicate its open-shell ground state46. However, the results did not imply the ground state of unsubstituted 5. Here we show that on insulating surfaces (ultrathin NaCl films on (111) coinage metal surfaces), 5 can exhibit one of two ground states: an open shell or a closed shell. We infer the existence of these two ground states based on high-resolution AFM imaging with bond-order discrimination34 and STM imaging of molecular orbital densities32. AFM imaging reveals molecules with two different geometries. Characteristic bond-order differences in the two geometries concur with the geometry of either an open- or a closed-shell state. Concurrently, STM images at ionic resonances show molecular orbital densities corresponding to SOMOs for the open-shell geometry, but orbital densities of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) for the closed-shell geometry. Our experimental results are in good agreement with density functional theory (DFT) and multireference perturbation theory calculations. Finally, we observe switching between open- and closed-shell states of a single molecule by changing its adsorption site on the surface.
Results and discussion
Synthetic strategy toward indeno[1,2–a]fluorene
The generation of 5 relies on the solution-phase synthesis of the precursor 7,12-dihydroindeno[1,2-a]fluorene (6). Details on the synthesis and characterization of 6 are reported in Supplementary Figs. 1–3. Single molecules of 6 are deposited on coinage metal (Au(111), Ag(111) and Cu(111)) or insulator surfaces. In our work, insulating surfaces correspond to two-monolayers-thick (denoted as bilayer) NaCl on coinage metal surfaces. Voltage pulses ranging between 4 and 6 V are applied by the tip of a combined STM/AFM system, resulting in cleavage of one C–H bond at each of the pentagonal apices of 6, thereby leading to the generation of 5 (Fig. 1e). In the main text we focus on the generation and characterization of 5 on insulating surfaces. The generation and characterization of 5 on coinage metal surfaces are presented in Supplementary Fig. 4.
Generation and characterization of indeno[1,2-a]fluorene
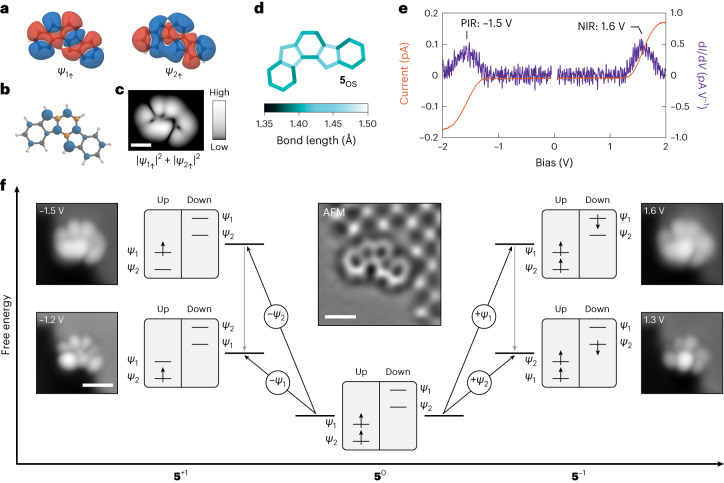
To experimentally explore the electronic structure of 5, we used bilayer NaCl films on coinage metal surfaces to electronically decouple the molecule from the metal surfaces. Before presenting experimental findings, we summarize the results of our theoretical calculations performed on 5 in the neutral charge state (denoted 50). We start by performing DFT calculations on 50 in the gas phase. Geometry optimization performed at the spin-unrestricted UB3LYP/6-31G level of theory leads to one local minimum, 5OS, the geometry of which corresponds to the open-shell resonance structure of 5 (Fig. 1d, Fig. 2d and Supplementary Tables 1–7; the label OS denotes open-shell). The triplet electronic configuration of 5OS is the lowest-energy state, with the open-shell singlet configuration 90 meV higher in energy. Geometry optimization performed at the restricted closed-shell RB3LYP/6-31G level reveals two local minima, 5para and 5ortho, the geometries of which (Fig. 3b) exhibit bond-length alternations in line with the presence of a para- or an ortho-QDM moiety, respectively, in the as-indacene core of the closed-shell resonance structures of 5 (Fig. 1d)37. Relative to 5OS in the triplet configuration, 5para and 5ortho are 0.40 and 0.43 eV higher in energy, respectively. Additional DFT results are shown in Supplementary Fig. 5. To gain more accurate insights into the theoretical electronic structure of 5, we performed multireference perturbation theory calculations (Supplementary Fig. 6) based on quasi-degenerate second-order n-electron valence state perturbation theory (QD-NEVPT2). In so far as the order of the ground and excited states are concerned, the results of QD-NEVPT2 calculations qualitatively match the DFT calculations. For 5OS, the triplet configuration remains the lowest-energy state, with the open-shell singlet configuration 60 meV higher in energy. The energy differences between the open- and closed-shell states are substantially reduced in QD-NEVPT2 calculations, with 5para and 5ortho only 0.11 and 0.21 eV higher in energy, respectively, compared to 5OS in the triplet configuration. We also performed nucleus-independent chemical shift calculations to probe the local aromaticity of 5 in the open- and closed-shell states. Although 5OS in the triplet configuration exhibits local aromaticity at the terminal benzenoid rings, 5OS in the open-shell singlet configuration, 5para and 5ortho all display antiaromaticity (Supplementary Fig. 6).
Fig. 2. Characterization of open-shell indeno[1,2-a]fluorene on bilayer NaCl/Au(111).
a, DFT-calculated wavefunctions of the frontier orbitals of 5OS in the triplet configuration for the spin up (occupied) levels (isovalue, 0.002 e− Å−3). Blue and red colours represent opposite phases of the wavefunction. Orbital densities (wavefunctions squared) are presented in Supplementary Fig. 12. b, Corresponding DFT-calculated spin density of 5OS (isovalue, 0.01 e− Å−3). Blue and orange colours represent spin up and spin down densities, respectively. c, Mean-field Hubbard local density of states map of the superposition of the SOMOs of 5OS, calculated at a height of 7 Å above the molecular plane. d, DFT-calculated bond lengths of 5OS. e, Constant-height I(V) spectra acquired on a species of 5 assigned as 5OS, along with the corresponding dI/dV(V) spectra. Open feedback parameters: V = −2 V, I = 0.17 pA (negative bias side) and V = 2 V, I = 0.17 pA (positive bias side). The acquisition position of the spectra is shown in Supplementary Fig. 7. f, Scheme of many-body transitions associated with the measured ionic resonances of 5OS, together with STM images of assigned 5OS at biases where the corresponding transitions become accessible. Scanning parameters: I = 0.3 pA (V = −1.2 V and −1.5 V) and 0.2 pA (V = 1.3 V and 1.6 V). Centre inset: Laplace-filtered AFM image of assigned 5OS. STM setpoint: V = 0.2 V, I = 0.5 pA on bilayer NaCl, Δz = −0.3 Å. The tip-height offset, Δz, is provided with respect to the STM setpoint, and positive (negative) values of Δz denote tip approach (retraction) from the STM setpoint. The STM and AFM images shown in f are of the same molecule at the same adsorption site, which is next to a third-layer NaCl island. The bright and dark features in the third-layer NaCl island in the AFM image correspond to Cl− and Na+ ions, respectively. The STS data shown in e were acquired on the molecule shown in f. Scale bars, 5 Å (simulated local density of states map and AFM image) and 10 Å (STM images).
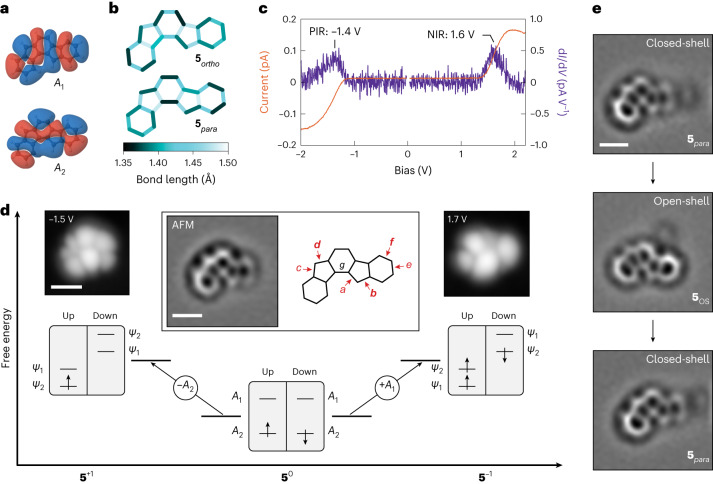
Fig. 3. Characterization of closed-shell indeno[1,2-a]fluorene on bilayer NaCl/Au(111).
a, DFT-calculated wavefunctions of the frontier orbitals of closed-shell 50 (isovalue, 0.002 e− Å−3). The wavefunctions shown here are calculated for the 5para geometry. Orbital densities (wavefunctions squared) are presented in Supplementary Fig. 12. b, DFT-calculated bond lengths of 5ortho (top) and 5para (bottom). c, Constant-height I(V) spectra acquired on a species of 5 assigned as 5para, along with the corresponding dI/dV(V) spectra. Open feedback parameters: V = −2 V, I = 0.15 pA (negative bias side) and V = 2.2 V, I = 0.15 pA (positive bias side). The acquisition position of the spectra is shown in Supplementary Fig. 7. d, Scheme of many-body transitions associated with the measured ionic resonances of 5para, together with STM images of assigned 5para at biases where the corresponding transitions become accessible. Scanning parameters: I = 0.15 pA (V = −1.5 V) and 0.2 pA (V = 1.7 V). Centre inset: Laplace-filtered AFM image of assigned 5para. STM setpoint: V = 0.2 V, I = 0.5 pA on bilayer NaCl, Δz = −0.7 Å. Here, the molecule is adsorbed on top of a defect on the surface. For an example of a 5para species adsorbed adjacent to a third-layer NaCl island, see Supplementary Fig. 15. Also shown in the inset are seven bonds labelled a–g to highlight the bond-order differences between 5para and 5ortho. For the bond pairs a/b, c/d and e/f, the bonds labelled in bold exhibit a higher bond order than their neighbouring labelled bonds in 5para. e, Laplace-filtered AFM images of 5 on bilayer NaCl/Cu(111) showing switching between 5OS and 5para as the molecule changes its adsorption position. Switching from 5para to 5OS was induced by scanning at 1.1 V, while switching from 5OS back to 5para took place by scanning at −2.2 V. The faint protrusion adjacent to 5 is a defect that stabilizes the adsorption of 5. STM setpoint: V = 0.2 V, I = 0.5 pA on bilayer NaCl, Δz = −0.3 Å. STS and STM data in c and d, respectively, are acquired on the same molecule, whereas the AFM image in d is acquired on a different molecule. Scale bars, 5 Å (AFM images) and 10 Å (STM images).
The choice of the insulating surface determines the charge state of 5: whereas 5 adopts a neutral charge state on the high-workfunction bilayer NaCl/Au(111) surface (irrespective of its open- or closed-shell state; Supplementary Fig. 7), it exhibits charge bistability between 50 and the anionic state 5−1 on the lower-workfunction bilayer NaCl/Ag(111) and Cu(111) surfaces (Supplementary Figs. 8 and 9). In the main text, we focus on the characterization of 5 on bilayer NaCl/Au(111). Characterization of charge bistable 5 is reported in Supplementary Figs. 10 and 11. We first describe experiments on 5 on bilayer NaCl/Au(111), where 5 exhibits a geometry corresponding to the calculated 5OS geometry, and an open-shell electronic configuration. We compare the experimental data on this species to calculations on 5OS with a triplet configuration, as theory predicts a triplet ground state for 5OS. For 5OS, the calculated frontier orbitals correspond to the SOMOs ψ1 and ψ2 (Fig. 2a–c and Supplementary Fig. 12), whose spin up levels are occupied but the spin down levels are empty. Figure 2d shows the DFT-calculated bond lengths of 5OS, where the two salient features, namely the small difference in bond lengths within each ring and the notably longer bond lengths in the pentagonal rings, agree with the open-shell resonance structure of 5 (Fig. 1d).
The inset of Fig. 2f shows an AFM image of 5 adsorbed on bilayer NaCl/Au(111) that we assign as 5OS, where the bond-order differences qualitatively correspond to the calculated 5OS geometry (discussed and compared to the closed-shell state below). Differential conductance spectra (dI/dV(V), where I and V denote the tunnelling current and bias voltage, respectively) acquired on assigned 5OS exhibit two peaks centred at −1.5 V and 1.6 V (Fig. 2e), which we assign to the positive and negative ion resonances (PIR and NIR), respectively. Figure 2f shows the corresponding STM images acquired at the onset (V = −1.2 V/1.3 V) and peak (V = −1.5 V/1.6 V) of the ionic resonances. To draw a correspondence between the STM images and the molecular orbital densities, we consider tunnelling events as many-body electronic transitions between different charge states of 5OS (Fig. 2f). Within this framework, the PIR corresponds to transitions between 50 and the cationic state 5+1. At the onset of the PIR at −1.2 V, an electron can only be detached from the SOMO ψ1, and the corresponding STM image at −1.2 V shows the orbital density of ψ1. Increasing the bias to the peak of the PIR at −1.5 V, it becomes possible to also empty the SOMO ψ2, such that the corresponding STM image shows the superposition of ψ1 and ψ2, that is, |ψ1|2 + |ψ2|2 (ref. 39). Similarly, the NIR corresponds to transitions between 50 and 5–1. At the NIR onset of 1.3 V, only electron attachment to ψ2 is energetically possible. At 1.6 V, electron attachment to ψ1 also becomes possible, and the corresponding STM image shows the superposition of ψ1 and ψ2. The observation of the orbital densities of SOMOs, and not the hybridized HOMO and LUMO, proves the open-shell ground state of assigned 5OS. Measurements of the monoradical species with a doublet ground state are shown in Supplementary Fig. 13.
Unexpectedly, another species of 5 was also experimentally observed that exhibited a closed-shell ground state. In contrast to 5OS, where the frontier orbitals correspond to the SOMOs ψ1 and ψ2, DFT calculations predict orbitals of different shapes and symmetries for 5para and 5ortho, denoted as A1 and A2 and shown in Fig. 3a and Supplementary Fig. 12. For 5ortho, A1 and A2 correspond to HOMO and LUMO, respectively. The orbitals are inverted in energy and occupation for 5para, where A2 is the HOMO and A1 is the LUMO. The inset of Fig. 3d shows an AFM image of 5 that we assign as 5para. We experimentally infer its closed-shell state first by using qualitative bond-order discrimination by AFM. In high-resolution AFM imaging, chemical bonds with higher bond order are imaged more brightly (that is, with higher frequency shift, Δf) due to stronger repulsive forces, and they appear shorter34,47,48. In the inset of Fig. 3d, we also show seven labelled bonds whose bond orders show notable qualitative differences in the calculated 5ortho, 5para (Fig. 3b) and 5OS (Fig. 2d) geometries. In 5para, bonds b and d exhibit a higher bond order than a and c, respectively. This pattern is reversed for 5ortho, whereas the bond orders of bonds a–d are all similar and small for 5OS. Furthermore, in 5para, bond f exhibits a higher bond order than e, whereas in 5ortho and 5OS, bonds e and f exhibit similar bond order (because they belong to Clar sextets). Finally, the bond labelled g shows a higher bond order in 5para than in 5ortho and 5OS. The AFM image of assigned 5para shown in the inset of Fig. 3d indicates higher bond orders of the bonds b, d and f compared to a, c and e, respectively. In addition, bond g appears almost point-like, and with enhanced Δf contrast compared to its neighbouring bonds, indicative of a high bond order (see Supplementary Fig. 14 for height-dependent measurements). These observations concur with the calculated 5para geometry (Fig. 3b). Importantly, all these distinguishing bond-order differences are distinctly different in the AFM image of 5OS (Fig. 2f, inset), which is consistent with the calculated 5OS geometry (Fig. 2d). In the AFM images of 5OS (Fig. 2f, inset and Supplementary Fig. 10), bonds a–d at the pentagon apices appear with similar contrast and apparent bond length. Bonds e and f, at one of the terminal benzenoid rings, also exhibit a similar contrast and apparent bond length, and the central bond g appears longer compared to assigned 5para.
Further compelling evidence for the closed-shell state of assigned 5para is obtained by STM and STS. dI/dV(V) spectra acquired on an assigned 5para species exhibit two peaks centred at −1.4 V (PIR) and 1.6 V (NIR) (Fig. 3c). STM images acquired at these biases (Fig. 3d) show orbital densities of A2 (PIR) and A1 (NIR). First, the observation of A1 and A2 (and not the SOMOs) as the frontier orbitals of this species strongly indicates its closed-shell state. Second, consistent with the AFM measurements, which indicate good correspondence to the calculated 5para geometry, we observe A2 as the HOMO and A1 as the LUMO. For 5ortho, A1 should be observed as the HOMO and A2 as the LUMO. We did not observe molecules with the signatures of 5ortho in our experiments.
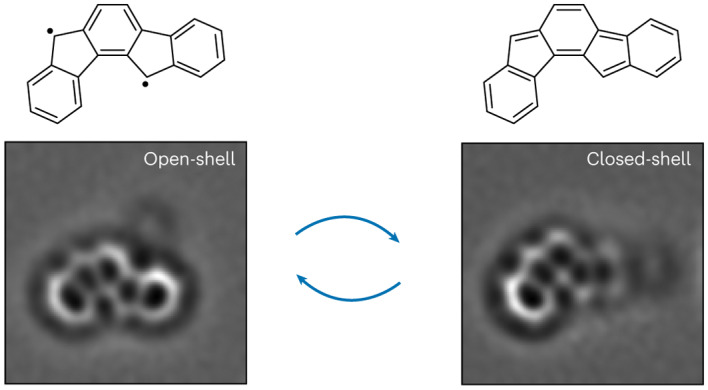
We observed molecules in open-shell (5OS; Fig. 2) and closed-shell (5para; Fig. 3) states in a similar occurrence after their generation from 6 on the surface (of 47 molecules, 23 and 24 molecules corresponded to 5OS and 5para, respectively). We could also switch individual molecules between the open- and closed-shell states, as shown in Fig. 3e and Supplementary Fig. 15. To this end, a change in the adsorption site of a molecule (whether 5OS or 5para) was induced by STM imaging at either of the ionic resonances, which often resulted in movement of the molecule. The example presented in Fig. 3e shows a molecule that was switched from 5para to 5OS, and back to 5para. The switching is not directed; that is, we cannot choose which of the two species will be formed when changing the adsorption site. Out of 22 instances where the molecules moved, 14 resulted in switching between 5OS and 5para, and in eight instances there was no switching of the ground state. Furthermore, we observed 5OS and 5para in equal yields upon changing the adsorption site. The molecule in the inset of Fig. 3d is adsorbed on top of a defect that stabilizes its adsorption geometry on bilayer NaCl. At defect-free adsorption sites on bilayer NaCl, that is, without a third-layer NaCl island or atomic defects in the vicinity of the molecule, 5 could be stably imaged neither by AFM nor by STM at ionic resonances (Supplementary Fig. 9). Without changing the adsorption site, the state of 5 (open or closed shell) never changed, including in the experiments on bilayer NaCl/Ag(111) and Cu(111), on which the charge state of 5 could be switched (Supplementary Figs. 8 and 9). Also on these lower-workfunction surfaces, both open- and closed-shell species were observed for 50, and both showed charge bistability36 between 50 (5OS or 5para) and 5−1 (Supplementary Figs. 10 and 11). The geometrical structure of 5−1 probed by AFM, and its electronic structure probed by STM imaging at the NIR (corresponding to transitions between 5−1 and the dianionic state 5−2), are identical within measurement accuracy for the charged species of both 5OS and 5para. When cycling the charge state of 5 between 50 and 5−1 several times, we always observed the same state (5OS or 5para) when returning to 50, provided the molecule did not move during the charging/discharging process. For a discussion pertaining to the stabilization of and switching between the open- and closed-shell states of 5, see Supplementary Note 1, and Supplementary Figs. 16 and 17.
Conclusions
Based on our experimental observations, we conclude that indeno[1,2-a]fluorene (5) can be stabilized in and switched between an open-shell (5OS) and a closed-shell (5para) state on NaCl. For the former, both DFT and QD-NEVPT2 calculations predict a triplet electronic configuration. Therefore, 5 can be considered to exhibit the spin-crossover effect, involving magnetic switching between high-spin (5OS) and low-spin (5para) states, coupled with a reversible structural transformation. So far, the spin-crossover effect has mainly been observed in transition-metal-based coordination compounds with near-octahedral geometry49, with relatively few examples of PCHs exhibiting the effect50. The observation that the switching between open- and closed-shell states is related to changes in the adsorption site but is not achieved by charge-state cycling alone, indicates that the NaCl surface and local defects facilitate different electronic configurations of 5 depending on the adsorption site. Gas-phase QD-NEVPT2 calculations predict that 5OS is the ground state, and the closed-shell 5para and 5ortho states are 0.11 and 0.21 eV higher in energy. The experiments, showing bidirectional switching between 5OS and 5para, indicate that a change in the adsorption site can induce sufficient change in the geometry of 5 (leading to a corresponding change in the ground-state electronic configuration) and thus induce switching. Switching between open- and closed-shell states in 5 does not require the formation or dissociation of covalent bonds51, but a change of adsorption site on NaCl where the molecule is physisorbed.
Our results should have implications for single-molecule devices, capitalizing on the altered electronic and chemical properties of a system in π-diradical open-shell and closed-shell states such as frontier orbital and singlet–triplet gaps, and chemical reactivity. For possible future applications as a single-molecule switch, it might be possible to also switch between open- and closed-shell states by changing the local electric field, such as by using chargeable adsorbates52.
Methods
Scanning probe microscopy measurements and sample preparation
STM and AFM measurements were performed in a home-built system operating at base pressures below 1 × 10−10 mbar and a base temperature of 5 K. Bias voltages are provided with respect to the sample. All STM, AFM and spectroscopy measurements were performed with carbon monoxide (CO)-functionalized tips. AFM measurements were performed in non-contact mode with a qPlus sensor53. The sensor was operated in frequency modulation mode54 with a constant oscillation amplitude of 0.5 Å. STM measurements were performed in constant-current mode, AFM measurements were performed in constant-height mode with V = 0 V, and I(V) and Δf(V) spectra were acquired in constant-height mode. Positive (negative) values of the tip-height offset, Δz, represent tip approach (retraction) from the STM setpoint. All dI/dV(V) spectra were obtained by numerical differentiation of the corresponding I(V) spectra. STM and AFM images, and spectroscopy curves, were post-processed using Gaussian low-pass filters.
Au(111), Ag(111) and Cu(111) surfaces were cleaned by iterative cycles of sputtering with Ne+ ions and annealing up to 800 K. NaCl was thermally evaporated on Au(111), Ag(111) and Cu(111) surfaces held at 323 K, 303 K and 283 K, respectively. This protocol results in the growth of predominantly bilayer (100)-terminated islands, with a minority of third-layer islands. Submonolayer coverage of 6 on the surfaces was obtained by flashing an oxidized silicon wafer containing the precursor molecules in front of the cold sample in the microscope. CO molecules for tip functionalization were dosed from the gas phase on the cold sample.
Mean-field Hubbard calculations
Tight-binding/mean-field Hubbard calculations were performed by numerically solving the mean-field Hubbard Hamiltonian with nearest-neighbour hopping:
| 1 |
where and denote the spin selective ( with ) creation and annihilation operator at neighbouring sites i and j, t = 2.7 eV is the nearest-neighbour hopping parameter, U = 3.5 eV is the on-site Coulomb repulsion, and ni, σ and 〈ni, σ〉 denote the number operator and mean occupation number at site i, respectively. Orbital electron densities, ρ, of the nth eigenstate with energy En have been simulated from the corresponding state vector an, i, σ by
| 2 |
where is the Slater 2pz orbital for carbon.
DFT calculations
Gas-phase DFT was employed using the PSI4 program package55. All molecules with different charge (neutral and anionic) and electronic (open- and closed-shell) states were independently investigated. The B3LYP exchange-correlation functional with 6-31G basis set was employed for structural relaxation and single-point energy calculations. Convergence criteria were set to 3 × 10−4 eV Å−1 for the total forces and 10−6 eV for the total energies.
For the on-surface DFT calculations shown in Supplementary Fig. 16, we employed the FHI-aims56 package. Molecules in the open- and closed-shell states were first independently investigated in the gas phase. The optimized molecular geometries were then optimized on a 9 × 9 bilayer NaCl slab in a cluster-type calculation. Molecular geometries in the gas phase were optimized with the really tight basis defaults. For the on-surface calculations we used light basis for NaCl atoms, and really tight basis defaults for atoms in the molecule. For structural relaxation, we employed the B3LYP exchange-correlation functional using the Vosko–Wilk–Nusair57 local-density approximation, as implemented in the FHI-aims package. In addition, we used the van der Waals scheme by Tkatchenko and Scheffler58. The convergence criteria for on-surface calculations were set to 10−3 eV Å−1 for the total forces and 10−2 eV for the total energies. For the NaCl slab, we constrained the atoms at the edges of the slab, while the atoms located in the top NaCl layer away from the edges were allowed to relax.
We also studied 5 in the gas phase and adsorbed on bilayer NaCl in a 5 × 5 surface cell using periodic, plane-wave DFT calculations with VASP59,60. We employed the optB86b version of the van der Waals density functional61–64, a plane-wave energy cutoff of 600 eV, and a 2 × 2 Monkhorst–Pack k-point mesh for the surface cell. The NaCl slab was constructed with a bulk lattice constant of 5.64 Å. Structural relaxations of molecule and top NaCl layer (with fixed bottom layer) were performed until residual forces were below 10–2 eV Å–1. The VASP-calculated adsorption sites and their qualitative differences in energy for the open- and closed-shell states were found to be consistent with the other DFT calculations shown in the main text and the Supplementary Information.
Multireference calculations
Multireference calculations were performed on the DFT-optimized geometries using the QD-NEVPT2 level of theory65,66, with three singlet roots and one triplet root included in the state-averaged calculation. A (10, 10) active space (that is, 10 electrons in 10 orbitals) was used along with the def2-TZVP basis set67. Increasing either the active-space size or expanding the basis set resulted in changes of ~50 meV in the relative energies of the singlet and triplet states. These calculations were performed using the ORCA package68.
Nucleus-independent chemical shift calculations
Isotropic nucleus-independent chemical shift values were evaluated at the centre of each ring using the B3LYP exchange-correlation functional with def2-TZVP basis set using the Gaussian 16 software package69.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-023-01431-7.
Supplementary information
Supplementary Figs. 1–17, Tables 1–7, Note 1 and Discussion.
Source data for Supplementary Figs. 5–8, 10 and 11.
Source data
Source data for scanning tunnelling spectroscopy measurements shown in Fig. 2e.
Source data for scanning tunnelling spectroscopy measurements shown in Fig. 3c.
Acknowledgements
We thank H. L. Anderson, K. Eimre, M. Solà and G. Giedke for discussions. This work was supported financially by the European Union project SPRING (grant no. 863098; T.F., D.P. and L.G.), the European Research Council Synergy grant MolDAM (grant no. 951519; D.P. and L.G.), the H2020-MSCA-ITN ULTIMATE (grant no. 813036; L.-A.L. and L.G.), the Spanish Agencia Estatal de Investigación (PID2019-107338RB-C62; D.P. and PID2020-115406GB-I00; R.O. and T.F.), Xunta de Galicia (Centro de Investigación de Galicia accreditation 2019–2022, ED431G 2019/03; D.P.), the European Regional Development Fund, UK Research and Innovation (project ElDelPath, EP/X030075/1; I.R.) and the KAUST Office of Sponsored Research (award no. OSR-CRG2022-5038; S.F.). This work used the Cirrus UK National Tier-2 high-performance computing service at EPCC (http://www.cirrus.ac.uk), funded by the University of Edinburgh and the Engineering and Physical Sciences Research Council (EP/P020267/1; I.R.) and the Supercomputing Laboratory at KAUST.
Author contributions
S.M. and L.G. performed the on-surface synthesis and scanning probe microscopy measurements. M.V.-V. and D.P. synthesized and characterized the precursor molecule in solution. L.-A.L., S.F., I.R. and T.F. performed the DFT calculations. I.R. performed the NICS calculations. S.M., R.O. and T.F. performed the tight-binding calculations. R.O., I.R. and T.F. performed the multireference calculations. S.M. drafted the first version of the manuscript, and all authors contributed to discussing the results and writing the manuscript.
Peer review
Peer review information
Nature Chemistry thanks Ganna Gryn’ova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The data that support the findings of this study are available in the paper and its Supplementary Information, which contains materials and methods, solution synthesis and characterization of 6, additional STM and AFM data of 5, STM and AFM data of monoradical species, analysis of Δf(V) spectra, and additional calculations. Output files of DFT and multireference calculations are available at 10.5281/zenodo.8234159. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shantanu Mishra, Email: SHM@zurich.ibm.com.
Diego Peña, Email: diego.pena@usc.es.
Leo Gross, Email: LGR@zurich.ibm.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-023-01431-7.
References
- 1.Banhart F, Kotakoski J, Krasheninnikov AV. Structural defects in graphene. ACS Nano. 2011;5:26–41. doi: 10.1021/nn102598m. [DOI] [PubMed] [Google Scholar]
- 2.Chaolumen, Stepek IA, Yamada KE, Ito H, Itami K. Construction of heptagon-containing molecular nanocarbons. Angew. Chem. Int. Ed. 2021;60:23508–23532. doi: 10.1002/anie.202100260. [DOI] [PubMed] [Google Scholar]
- 3.Fei Y, Liu J. Synthesis of defective nanographenes containing joined pentagons and heptagons. Adv. Sci. 2022;9:2201000. doi: 10.1002/advs.202201000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudebusch GE, et al. Diindeno-fusion of an anthracene as a design strategy for stable organic biradicals. Nat. Chem. 2016;8:753–759. doi: 10.1038/nchem.2518. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S, et al. Tailoring bond topologies in open-shell graphene nanostructures. ACS Nano. 2018;12:11917–11927. doi: 10.1021/acsnano.8b07225. [DOI] [PubMed] [Google Scholar]
- 6.Konishi A, et al. Open-shell and antiaromatic character induced by the highly symmetric geometry of the planar heptalene structure: synthesis and characterization of a nonalternant isomer of bisanthene. J. Am. Chem. Soc. 2019;141:10165–10170. doi: 10.1021/jacs.9b04080. [DOI] [PubMed] [Google Scholar]
- 7.Moles Quintero S, Haley MM, Kertesz M, Casado J. Polycyclic hydrocarbons from [4n]annulenes: correlation versus hybridization forces in the formation of diradicaloids. Angew. Chem. Int. Ed. 2022;61:e202209138. doi: 10.1002/anie.202209138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobe Y. Non-alternant non-benzenoid aromatic compounds: past, present and future. Chem. Rec. 2015;15:86–96. doi: 10.1002/tcr.201402077. [DOI] [PubMed] [Google Scholar]
- 9.Konishi A, Kubo T. Benzenoid quinodimethanes. Top. Curr. Chem. 2017;375:83. doi: 10.1007/s41061-017-0171-2. [DOI] [PubMed] [Google Scholar]
- 10.Hafner K, Krimmer H-P. Synthesis of carbocyclic and heterocyclic π-electron systems with pentafulvenoid chloroformamidinium chlorides. Angew. Chem. Int. Ed. 1980;19:199–201. doi: 10.1002/anie.198001992. [DOI] [Google Scholar]
- 11.Hafner K, et al. Synthesis and properties of 1,3,5,7-tetra-tert-butyl-s-indacene. Angew. Chem. Int. Ed. 1986;25:630–632. doi: 10.1002/anie.198606301. [DOI] [Google Scholar]
- 12.Jhang S-J, et al. s-Indacene revisited: modular synthesis and modulation of structures and molecular orbitals of hexaaryl derivatives. J. Am. Chem. Soc. 2023;145:4716–4729. doi: 10.1021/jacs.2c13159. [DOI] [PubMed] [Google Scholar]
- 13.Hopf H. Pentalenes—from highly reactive antiaromatics to substrates for material science. Angew. Chem. Int. Ed. 2013;52:12224–12226. doi: 10.1002/anie.201307162. [DOI] [PubMed] [Google Scholar]
- 14.Konishi A, et al. Synthesis and characterization of dibenzo[a,f]pentalene: harmonization of the antiaromatic and singlet biradical character. J. Am. Chem. Soc. 2017;139:15284–15287. doi: 10.1021/jacs.7b05709. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, J. L. & Haley, M. M. in Organic Redox Systems (ed. Nishinaga, T.) 311–358 (Wiley, 2016); 10.1002/9781118858981.ch10
- 16.Thomas S, Kim KS. Linear and nonlinear optical properties of indeno[2,1-b]fluorene and its structural isomers. Phys. Chem. Chem. Phys. 2014;16:24592–24597. doi: 10.1039/C4CP03169E. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K, Nagami T, Fujiyoshi J, Nakano M. Interplay between open-shell character, aromaticity and second hyperpolarizabilities in indenofluorenes. J. Phys. Chem. A. 2015;119:10620–10627. doi: 10.1021/acs.jpca.5b08520. [DOI] [PubMed] [Google Scholar]
- 18.Minami T, Nakano M. Diradical character view of singlet fission. J. Phys. Chem. Lett. 2012;3:145–150. doi: 10.1021/jz2015346. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Minami T, Nakano M. Diradical character based design for singlet fission of condensed-ring systems with 4nπ electrons. J. Phys. Chem. C. 2012;116:19729–19736. doi: 10.1021/jp3072684. [DOI] [Google Scholar]
- 20.Ortiz R, Giedke G, Frederiksen T. Magnetic frustration and fractionalization in oligo(indenoindenes) Phys. Rev. B. 2023;107:L100416. doi: 10.1103/PhysRevB.107.L100416. [DOI] [Google Scholar]
- 21.Shimizu A, et al. Indeno[2,1-b]fluorene: a 20-π-electron hydrocarbon with very low-energy light absorption. Angew. Chem. Int. Ed. 2013;52:6076–6079. doi: 10.1002/anie.201302091. [DOI] [PubMed] [Google Scholar]
- 22.Chase DT, Rose BD, McClintock SP, Zakharov LN, Haley MM. Indeno[1,2-b]fluorenes: fully conjugated antiaromatic analogues of acenes. Angew. Chem. Int. Ed. 2011;50:1127–1130. doi: 10.1002/anie.201006312. [DOI] [PubMed] [Google Scholar]
- 23.Chase DT, et al. Electron-accepting 6,12-diethynylindeno[1,2-b]fluorenes: synthesis, crystal structures and photophysical properties. Angew. Chem. Int. Ed. 2011;50:11103–11106. doi: 10.1002/anie.201104797. [DOI] [PubMed] [Google Scholar]
- 24.Nishida J, Tsukaguchi S, Yamashita Y. Synthesis, crystal structures and properties of 6,12-diaryl-substituted indeno[1,2-b]fluorenes. Chem. Eur. J. 2012;18:8964–8970. doi: 10.1002/chem.201200591. [DOI] [PubMed] [Google Scholar]
- 25.Chase DT, et al. 6,12-Diarylindeno[1,2-b]fluorenes: syntheses, photophysics and ambipolar OFETs. J. Am. Chem. Soc. 2012;134:10349–10352. doi: 10.1021/ja303402p. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu A, Tobe Y. Indeno[2,1-a]fluorene: an air-stable ortho-quinodimethane derivative. Angew. Chem. Int. Ed. 2011;50:6906–6910. doi: 10.1002/anie.201101950. [DOI] [PubMed] [Google Scholar]
- 27.Fix AG, et al. Indeno[2,1-c]fluorene: a new electron-accepting scaffold for organic electronics. Org. Lett. 2013;15:1362–1365. doi: 10.1021/ol400318z. [DOI] [PubMed] [Google Scholar]
- 28.Di Giovannantonio M, et al. On-surface synthesis of antiaromatic and open-shell indeno[2,1-b]fluorene polymers and their lateral fusion into porous ribbons. J. Am. Chem. Soc. 2019;141:12346–12354. doi: 10.1021/jacs.9b05335. [DOI] [PubMed] [Google Scholar]
- 29.Majzik Z, et al. Studying an antiaromatic polycyclic hydrocarbon adsorbed on different surfaces. Nat. Commun. 2018;9:1198. doi: 10.1038/s41467-018-03368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giovannantonio M, et al. On-surface synthesis of indenofluorene polymers by oxidative five-membered ring formation. J. Am. Chem. Soc. 2018;140:3532–3536. doi: 10.1021/jacs.8b00587. [DOI] [PubMed] [Google Scholar]
- 31.Di Giovannantonio M, et al. On-surface synthesis of oligo(indenoindene) J. Am. Chem. Soc. 2020;142:12925–12929. doi: 10.1021/jacs.0c05701. [DOI] [PubMed] [Google Scholar]
- 32.Repp J, Meyer G, Stojković SM, Gourdon A, Joachim C. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 2005;94:026803. doi: 10.1103/PhysRevLett.94.026803. [DOI] [PubMed] [Google Scholar]
- 33.Gross L, Mohn F, Moll N, Liljeroth P, Meyer G. The chemical structure of a molecule resolved by atomic force microscopy. Science. 2009;325:1110–1114. doi: 10.1126/science.1176210. [DOI] [PubMed] [Google Scholar]
- 34.Gross L, et al. Bond-order discrimination by atomic force microscopy. Science. 2012;337:1326–1329. doi: 10.1126/science.1225621. [DOI] [PubMed] [Google Scholar]
- 35.Gross L, et al. Measuring the charge state of an adatom with noncontact atomic force microscopy. Science. 2009;324:1428–1431. doi: 10.1126/science.1172273. [DOI] [PubMed] [Google Scholar]
- 36.Swart I, Sonnleitner T, Repp J. Charge state control of molecules reveals modification of the tunneling barrier with intramolecular contrast. Nano Lett. 2011;11:1580–1584. doi: 10.1021/nl104452x. [DOI] [PubMed] [Google Scholar]
- 37.Dressler JJ, et al. Synthesis of the unknown indeno[1,2-a]fluorene regioisomer: crystallographic characterization of its dianion. Angew. Chem. Int. Ed. 2017;56:15363–15367. doi: 10.1002/anie.201709282. [DOI] [PubMed] [Google Scholar]
- 38.Repp J, Meyer G, Paavilainen S, Olsson FE, Persson M. Imaging bond formation between a gold atom and pentacene on an insulating surface. Science. 2006;312:1196–1199. doi: 10.1126/science.1126073. [DOI] [PubMed] [Google Scholar]
- 39.Pavliček N, et al. Synthesis and characterization of triangulene. Nat. Nanotechnol. 2017;12:308–311. doi: 10.1038/nnano.2016.305. [DOI] [PubMed] [Google Scholar]
- 40.Li J, et al. Single spin localization and manipulation in graphene open-shell nanostructures. Nat. Commun. 2019;10:200. doi: 10.1038/s41467-018-08060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra S, et al. Topological frustration induces unconventional magnetism in a nanographene. Nat. Nanotechnol. 2020;15:22–28. doi: 10.1038/s41565-019-0577-9. [DOI] [PubMed] [Google Scholar]
- 42.Liu C, et al. Macrocyclic polyradicaloids with unusual super-ring structure and global aromaticity. Chem. 2018;4:1586–1595. doi: 10.1016/j.chempr.2018.03.020. [DOI] [Google Scholar]
- 43.Zeng Z, et al. Stable tetrabenzo-chichibabin’s hydrocarbons: tunable ground state and unusual transition between their closed-shell and open-shell resonance forms. J. Am. Chem. Soc. 2012;134:14513–14525. doi: 10.1021/ja3050579. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu A, et al. Synthesis and isolation of a Kekulé hydrocarbon with a triplet ground state. Angew. Chem. Int. Ed. 2022;61:e202205729. doi: 10.1002/anie.202205729. [DOI] [PubMed] [Google Scholar]
- 45.Mishra S, et al. Nonbenzenoid high-spin polycyclic hydrocarbons generated by atom manipulation. ACS Nano. 2022;16:3264–3271. doi: 10.1021/acsnano.1c11157. [DOI] [PubMed] [Google Scholar]
- 46.Li C, et al. Topological defects induced high-spin quartet state in truxene-based molecular graphenoids. CCS Chem. 2022;5:695–703. doi: 10.31635/ccschem.022.202201895. [DOI] [Google Scholar]
- 47.de Oteyza DG, et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science. 2013;340:1434–1437. doi: 10.1126/science.1238187. [DOI] [PubMed] [Google Scholar]
- 48.Kawai S, et al. Competing annulene and radialene structures in a single anti-aromatic molecule studied by high-resolution atomic force microscopy. ACS Nano. 2017;11:8122–8130. doi: 10.1021/acsnano.7b02973. [DOI] [PubMed] [Google Scholar]
- 49.Kipgen L, Bernien M, Tuczek F, Kuch W. Spin-crossover molecules on surfaces: from isolated molecules to ultrathin films. Adv. Mater. 2021;33:2008141. doi: 10.1002/adma.202008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gryn’ova G, Coote ML, Corminboeuf C. Theory and practice of uncommon molecular electronic configurations. WIREs Comput. Mol. Sci. 2015;5:440–459. doi: 10.1002/wcms.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuler B, et al. Reversible Bergman cyclization by atomic manipulation. Nat. Chem. 2016;8:220–224. doi: 10.1038/nchem.2438. [DOI] [PubMed] [Google Scholar]
- 52.Uhlmann C, Swart I, Repp J. Controlling the orbital sequence in individual Cu-phthalocyanine molecules. Nano Lett. 2013;13:777–780. doi: 10.1021/nl304483h. [DOI] [PubMed] [Google Scholar]
- 53.Giessibl FJ. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 1998;73:3956–3958. doi: 10.1063/1.122948. [DOI] [Google Scholar]
- 54.Albrecht TR, Grütter P, Horne D, Rugar D. Frequency modulation detection using high‐Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 1991;69:668–673. doi: 10.1063/1.347347. [DOI] [Google Scholar]
- 55.Smith DGA, et al. PSI4 1.4: open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020;152:184108. doi: 10.1063/5.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blum V, et al. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 2009;180:2175–2196. doi: 10.1016/j.cpc.2009.06.022. [DOI] [Google Scholar]
- 57.Scuseria, G. E. & Staroverov, V. N. in Theory and Applications of Computational Chemistry (eds Dykstra, C. E. et al.) 669–724 (Elsevier, 2005); 10.1016/B978-044451719-7/50067-6
- 58.Tkatchenko A, Scheffler M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009;102:073005. doi: 10.1103/PhysRevLett.102.073005. [DOI] [PubMed] [Google Scholar]
- 59.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 60.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 61.Klimeš J, Bowler DR, Michaelides A. Van der Waals density functionals applied to solids. Phys. Rev. B. 2011;83:195131. doi: 10.1103/PhysRevB.83.195131. [DOI] [Google Scholar]
- 62.Dion M, Rydberg H, Schröder E, Langreth DC, Lundqvist BI. Van der Waals density functional for general geometries. Phys. Rev. Lett. 2004;92:246401. doi: 10.1103/PhysRevLett.92.246401. [DOI] [PubMed] [Google Scholar]
- 63.Thonhauser T, et al. Van der Waals density functional: self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B. 2007;76:125112. doi: 10.1103/PhysRevB.76.125112. [DOI] [Google Scholar]
- 64.Román-Pérez G, Soler JM. Efficient implementation of a van der Waals density functional: application to double-wall carbon nanotubes. Phys. Rev. Lett. 2009;103:096102. doi: 10.1103/PhysRevLett.103.096102. [DOI] [PubMed] [Google Scholar]
- 65.Angeli C, Borini S, Cestari M, Cimiraglia R. A quasidegenerate formulation of the second order n-electron valence state perturbation theory approach. J. Chem. Phys. 2004;121:4043–4049. doi: 10.1063/1.1778711. [DOI] [PubMed] [Google Scholar]
- 66.Lang L, Sivalingam K, Neese F. The combination of multipartitioning of the Hamiltonian with canonical Van Vleck perturbation theory leads to a Hermitian variant of quasidegenerate N-electron valence perturbation theory. J. Chem. Phys. 2020;152:014109. doi: 10.1063/1.5133746. [DOI] [PubMed] [Google Scholar]
- 67.Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]
- 68.Neese F, Wennmohs F, Becker U, Riplinger C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020;152:224108. doi: 10.1063/5.0004608. [DOI] [PubMed] [Google Scholar]
- 69.Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–17, Tables 1–7, Note 1 and Discussion.
Source data for Supplementary Figs. 5–8, 10 and 11.
Source data for scanning tunnelling spectroscopy measurements shown in Fig. 2e.
Source data for scanning tunnelling spectroscopy measurements shown in Fig. 3c.
Data Availability Statement
The data that support the findings of this study are available in the paper and its Supplementary Information, which contains materials and methods, solution synthesis and characterization of 6, additional STM and AFM data of 5, STM and AFM data of monoradical species, analysis of Δf(V) spectra, and additional calculations. Output files of DFT and multireference calculations are available at 10.5281/zenodo.8234159. Source data are provided with this paper.