Abstract
A drug molecule is a substance that changes an organism’s mental or physical state. Every approved drug has an indication, which refers to the therapeutic use of that drug for treating a particular medical condition. While the Large Language Model (LLM), a generative Artificial Intelligence (AI) technique, has recently demonstrated effectiveness in translating between molecules and their textual descriptions, there remains a gap in research regarding their application in facilitating the translation between drug molecules and indications (which describes the disease, condition or symptoms for which the drug is used), or vice versa. Addressing this challenge could greatly benefit the drug discovery process. The capability of generating a drug from a given indication would allow for the discovery of drugs targeting specific diseases or targets and ultimately provide patients with better treatments. In this paper, we first propose a new task, the translation between drug molecules and corresponding indications, and then test existing LLMs on this new task. Specifically, we consider nine variations of the T5 LLM and evaluate them on two public datasets obtained from ChEMBL and DrugBank. Our experiments show the early results of using LLMs for this task and provide a perspective on the state-of-the-art. We also emphasize the current limitations and discuss future work that has the potential to improve the performance on this task. The creation of molecules from indications, or vice versa, will allow for more efficient targeting of diseases and significantly reduce the cost of drug discovery, with the potential to revolutionize the field of drug discovery in the era of generative AI.
Keywords: Computer science, Artificial intelligence, Large language models, Drug discovery
Subject terms: Drug discovery, Computational science, Computer science, Scientific data
Introduction
Drug discovery is a costly process1 that identifies chemical entities with the potential to become therapeutic agents2. Due to its clear benefits and significance to health, drug discovery has become an active area of research, with researchers attempting to automate and streamline drug discovery3,4. Approved drugs have indications, which refer to the use of that drug for treating a particular disease, condition, or symptoms5. They specify whether the drug is intended for treatment, prevention, mitigation, cure, relief, or diagnosis of that particular ailment. The creation of molecules from indications, or vice versa, will allow for more efficient targeting of diseases and significantly reduce the cost of drug discovery, with the potential to revolutionize the field.
Large Language Models (LLMs) have become one of the major directions of generative Artificial Intelligence (AI) research, with highly performant models like GPT-36, GPT-47, LLaMA8, and Mixtral9 developed in the recent years and services like ChatGPT reaching over 100 million users10,11. LLMs utilize deep learning methods to perform various Natural Language Processing (NLP) tasks, such as text generation12,13 and neural machine translation14,15. The capabilities of LLMs are due in part to their training on large-scale textual data, making the models familiar with a wide array of topics. LLMs have also demonstrated promising performance in a variety of tasks across different scientific fields16–19. Since LLMs work with textual data, the first step is usually finding a way to express a problem in terms of text or language.
An image or a diagram is a typical way to present a molecule, but methods for obtaining textual representations of molecules do exist. One such method is the Simplified Molecular-Input Line-Entry System (SMILES)20, which is usually considered as a language for describing molecules. As SMILES strings represent drugs in textual form, we can assess the viability of LLMs in translation between drug molecules and their indications. In this paper, we consider two tasks: drug-to-indication and indication-to-drug, where we seek to generate indications from the SMILES strings of drugs, and SMILES strings from possible indications, respectively. Translation between drugs and the corresponding indication will allow for finding a cure for diseases that have no current treatment.
Research efforts have attempted de-novo drug discovery through the use of AI, including graph neural networks21,22 and, more recently, forms of generative AI23. There are numerous existing efforts for molecular design and drug discovery using AI, such as GPT-based models using scaffold SMILES strings accompanied with desired properties of the output molecule24. Others have used T5 architecture for various tasks, such as reaction prediction25 and converting between molecular captions and SMILES strings26. Additional work in the field is centered around the generation of new molecules from gene expression signatures using generative adversarial networks27, training recurrent neural networks on known compounds and their SMILES strings, then fine-tuning for specific agonists of certain receptors28, or using graph neural networks to predict drugs and their corresponding indications from SMILES29. As such, there is an established promise in using AI for drug discovery and molecular design. Efforts to make data more friendly for AI generation of drugs also include the development of the Self-Referencing Embedded Strings (SELFIES)30, which can represent every valid molecule. The reasoning is that such a format will allow generative AI to construct valid molecules while maintaining crucial structural information in the string. The collection of these efforts sets the stage for our attempt at generating drug indications from molecules.
With advancements in medicinal chemistry leading to an increasing number of drugs designed for complex processes, it becomes crucial to comprehend the distinctive characteristics and subtle nuances of each drug. In this direction, researchers have released many resources, including datasets that bridge medicines and chemical ingredients like TCMBank31,32, models for generating high-quality molecular representations to facilitate Computer-Aided Drug Design (CADD)33, and models for drug-drug interactions34,35. This has also led to the development of molecular fingerprints, such as the Morgan fingerprint36 and the MAP4 fingerprint37, which use unique algorithms to vectorize the characteristics of a molecule. Computation of fingerprint representations is rapid, and they maintain much of the features of a molecule38. Molecular fingerprinting methods commonly receive input in the form of SMILES strings, which serve as a linear notation for representing molecules in their structural forms, taking into account the different atoms present, the bonds between atoms, as well as other key characteristics, such as branches, cyclic structures, and aromaticity20. Since SMILES is a universal method of communicating the structure of different molecules, it is appropriate to use SMILES strings for generating fingerprints. Mol2vec39 feeds Morgan fingerprints to the Word2vec40 algorithm by converting molecules into their textual representations. Bidirectional Encoder Representations from Transformers (BERT)41-based models have also been used for obtaining molecular representations, including models like MolBERT42 and ChemBERTa43, which are pretrained BERT instances that take SMILES strings as input and perform downstream tasks on molecular representation and molecular property prediction, respectively. Other efforts in using AI for molecular representations include generating novel molecular graphs through the use of reinforcement learning, decomposition, and reassembly44 and the prediction of 3D representations of small molecules based on their 2D graphical counterparts45.
In this paper, we evaluate the capabilities of MolT5, a T5-based model, in translating between drugs and their indications through the two tasks, drug-to-indication and indication-to-drug, using the drug data from DrugBank and ChEMBL. The drug-to-indication task utilizes SMILES strings for existing drugs as input, with the matching indications of the drug as the target output. The indication-to-drug task takes the set of indications for a drug as input and seeks to generate the corresponding SMILES string for a drug that treats the listed conditions.
We employ all available MolT5 model sizes for our experiments and evaluate them separately across the two datasets. Additionally, we perform the experiments under three different configurations:
Evaluation of the baseline models on the entire available dataset
Evaluation of the baseline models on 20% of the dataset
Fine-tuning the models on 80% of the dataset followed by evaluation on the 20% subset
We found that larger MolT5 models outperformed the smaller ones across all configurations and tasks. It should also be noted that fine-tuning MolT5 models has a negative impact on the performance.
Following these preliminary experiments, we train the smallest available MolT5 model from scratch using a custom tokenizer. This custom model performed better on DrugBank data than on ChEMBL data on the drug-to-indication task, perhaps due to a stronger signal between the drug indications and SMILES strings in their dataset, owing to the level of detail in their indication descriptions. Fine-tuning the custom model on 80% of either dataset did not degrade model performance for either task, and some metrics saw improvement due to fine-tuning. Overall, fine-tuning for the indication-to-drug task did not consistently improve the performance, which holds for both ChEMBL and DrugBank datasets.
While the performance of the custom tokenizer approach is still not satisfying, there is promise in using a larger model and having access to more data. If we have a wealth of high-quality data to train models on translation between drugs and their indications, it may become possible to improve performance and facilitate novel drug discovery with LLMs.
In this paper, we make the following contributions:
We introduce a new task: translation between drug molecules and indications.
We conduct various experiments with T5-based LLMs and two datasets (DrugBank and ChEMBL). Our experiments consider 16 evaluation metrics across all experiments. In addition, we discuss the current bottlenecks that, if addressed, have the potential to significantly improve the performance on the task.
Results
Evaluation of MolT5 models
We performed initial experiments using MolT5 models from HuggingFace (GitHub links: https://huggingface.co/laituan245/molt5-small/tree/main, https://huggingface.co/laituan245/molt5-base/tree/main, https://huggingface.co/laituan245/molt5-large/tree/main). MolT5 offers three model sizes and fine-tuned models of each size, which support each task of our experiments. For experiments generating SMILES strings from drug indications (drug-to-indication), we used the fine-tuned models MolT5-smiles-to-caption, and for generating SMILES strings from drug indications (indication-to-drug), we used the models MolT5-caption-to-smiles. For each of our Tables, we use the following flags: FT (denotes experiments where we fine-tuned the models on 80% of the dataset and evaluated on the remaining 20% test subset), SUB (denotes experiments where the models are evaluated solely on the 20% test subset), and FULL (for experiments evaluating the models on the entirety of each dataset).
For evaluating drug-to-indication, we employ the natural language generation metrics BLEU46, ROUGE54–56, and METEOR57, as well as the Text2Mol53 metric, which generates similarities of SMILES-Indication pairs. As for evaluation of indication-to-drug, we measure exact SMILES string matches, Levenshtein distance47, SMILES BLEU scores, the Text2Mol similarity metric, as well as three different molecular fingerprint metrics: MACCS48,49, RDK48,50, and Morgan FTS48,51, where FTS stands for fingerprint Tanimoto similarity48, as well as the proportion of returned SMILES strings that are valid molecules. The final metric for evaluating SMILES generation is FCD, or Fréchet ChemNet Distance, which measures the distance between two distributions of molecules from their SMILES strings52. Table 1 presents both drug-to-indication and indication-to-drug metrics, including their descriptions, values, and supported intervals.
Table 1.
Evaluation metrics used in the experiments.
| Metric | Description | Values | Direction |
|---|---|---|---|
| BLEU46 | Computes similarity as geometric mean of n-gram precisions scaled by brevity penalty | ||
| Exact | Represents whether the string match is exact (1) or not (0) | {0, 1} | |
| Levenshtein47 | Measures Levenshtein edit distance between two strings | ||
| MACCS48,49 | Computes Tanimoto similarity between two molecular MACCS fingerprints | ||
| RDK48,50 | Computes Tanimoto similarity between two molecular RDK fingerprints | ||
| Morgan48,51 | Computes Tanimoto similarity between two molecular Morgan fingerprints | ||
| FCD52 | Measures distance between distributions of real-world and LLM-generated molecules | ||
| Text2Mol53 | Uses pretrained model to compute similarity between SMILES string and text | ||
| Validity | Represents whether the generated SMILES string is syntactically valid (1) or not (0) | ||
| BLEU-246 | Computes cumulative 2-gram BLEU score | ||
| BLEU-446 | Computes cumulative 4-gram BLEU score | ||
| ROUGE-154,55 | Measures overlap of unigrams between the candidate and reference strings | ||
| ROUGE-254,55 | Measures overlap of bigrams between the candidate and reference strings | ||
| ROUGE-L54,56 | Calculates similarity via Longest Common Subsequence (LCS) statistics | ||
| METEOR57 | Computes similarity between two strings via weighted unigram F-score | ||
| Text2Mol53 | Uses a pretrained model to compute similarity between two strings |
: higher values result in higher string similarity.: higher values result in lower string similarity.
Table 2 lists four examples of inputs and our model outputs for both drug-to-indication and indication-to-drug tasks using the large MolT5 model and ChEMBL data. Molecular validity is determined through the use of RDKit (https://www.rdkit.org/docs/index.html), an open-source toolkit for cheminformatics, with the reason for invalidity given. Indication quality is determined by the Text2Mol string similarity between the ground truth and generated indications. We can observe that the proposed model could output valid molecules using SMILES strings for a given indication, and output meaningful indication, such as cancer, for a given molecule. However, there are some misspelling issues in the generated indication due to the small size of T5 model. We hypothesize that LLMs with larger size of parameters could significantly improve the validity of the generated molecules and indications.
Table 2.
First four rows: example SMILES strings from the indication-to-drug task; Last four rows: example MolT5 indication generations from the drug-to-indication task.
| Input | Ground truth | Output | Validity/similarity |
|---|---|---|---|
| Indication-to-drug | |||
| Diabetes mellitus | COCCOc1cnc(NS(=O)(=O)c2ccccc2)nc1 | O=C([O-])CC(=O)[O-] | Valid |
| Coronary artery disease | CCOC(=O)C(C)=O | CCCCC[C@H](O)CC=CCC=CCCCC(=O)O | Valid |
| Respiratory system disease | CCC1(C)CC(=O)NC(=O)C1 | [H+].C(=O)[O-])[O-] | Syntax Error |
| Hemorrhage | CC1=CC(=O)c2ccccc2C1=O | C(=O)C(=O)O)O.O)O.O)O.O.O | Syntax Error |
| Drug-to-indication | |||
| CN(C)CCOC(c1ccccc1)c1ccccc1 | Allergic disease ... cancer ... eczema ... | ... and cancer ... | 0.2206 |
| CCc1cc(C(N)=S)ccn1 | Multidrug-resistant tuberculosis osteomyelitis ... | ... cancer | 0.2262 |
| O=C([O-])c1ccccc1.[Na+] | Encephalopathy psychosis | Inamideamide protein protein proteinamide. | 0.0183 |
| Clc1ccccc1CN1CCc2sccc2C1 | Internal carotid artery stenosis ... Recurrent thrombophlebitis | Amideamideamide. | 0.0316 |
Tables 3 and 4 show the results of MolT5 drug-to-indication experiments on DrugBank and ChEMBL data, respectively. Larger models tended to perform better across all metrics for each experiment. Across almost all metrics for the drug-to-indication task, on both DrugBank and ChEMBL datasets, the model performed the best on the 20% subset data. At the same time, both the subset and full dataset evaluations yielded better results than fine-tuning experiments. As MolT5 models are trained on molecular captions, fine-tuning using indications could introduce noise and weaken the signal between input and target text. The models performed better on DrugBank data than ChEMBL data, which may be due to the level of detail provided by DrugBank for their drug indications.
Table 3.
DrugBank drug-to-indication results.
| Model | BLEU-2 | BLEU-4 | ROUGE-1 | ROUGE-2 | ROUGE-L | METEOR | Text2Mol |
|---|---|---|---|---|---|---|---|
| FT-small | 0.0013 | 0.0000 | 0.0011 | 0.0000 | 0.0011 | 0.011 | 0.0805 |
| SUB-small | 0.0224 | 0.0053 | 0.0982 | 0.0068 | 0.0809 | 0.1007 | 0.2368 |
| FULL-small | 0.0213 | 0.0036 | 0.0965 | 0.0061 | 0.0801 | 0.0987 | 0.3234 |
| FT-base | 0.0006 | 0.0000 | 0.0004 | 0.0000 | 0.0004 | 0.0092 | 0.0683 |
| SUB-base | 0.0227 | 0.0053 | 0.0973 | 0.0073 | 0.0808 | 0.1020 | 0.3317 |
| FULL-base | 0.0208 | 0.0034 | 0.0966 | 0.0059 | 0.0803 | 0.0992 | 0.3217 |
| FT-large | 0.0006 | 0.0000 | 0.0007 | 0.0000 | 0.0007 | 0.0110 | 0.0532 |
| SUB-large | 0.0298 | 0.0097 | 0.1015 | 0.0115 | 0.0835 | 0.1167 | 0.5001 |
| FULL-large | 0.0281 | 0.0080 | 0.1007 | 0.0098 | 0.0814 | 0.1127 | 0.4864 |
Significant values are in [boldunderlined].
Table 4.
ChEMBL drug-to-indication results.
| Model | BLEU-2 | BLEU-4 | ROUGE-1 | ROUGE-2 | ROUGE-L | METEOR | Text2Mol |
|---|---|---|---|---|---|---|---|
| FT-small | 0.0000 | 0.0000 | 0.0011 | 0.0000 | 0.0011 | 0.0017 | 0.1070 |
| SUB-small | 0.0005 | 0.0000 | 0.0032 | 0.0000 | 0.0029 | 0.0079 | 0.3353 |
| FULL-small | 0.0005 | 0.0000 | 0.0033 | 0.0000 | 0.0032 | 0.0078 | 0.3237 |
| FT-base | 0.0000 | 0.0000 | 0.0012 | 0.0000 | 0.0012 | 0.0026 | 0.0799 |
| SUB-base | 0.0005 | 0.0000 | 0.0033 | 0.0000 | 0.0032 | 0.0076 | 0.3315 |
| FULL-base | 0.0007 | 0.0000 | 0.0034 | 0.0000 | 0.0033 | 0.0078 | 0.3171 |
| FT-large | 0.0000 | 0.0000 | 0.0011 | 0.0000 | 0.0011 | 0.0010 | 0.0917 |
| SUB-large | 0.0021 | 0.0007 | 0.0052 | 0.0007 | 0.0049 | 0.0118 | 0.4903 |
| FULL-large | 0.0019 | 0.0006 | 0.0053 | 0.0007 | 0.0050 | 0.0118 | 0.4830 |
Significant values are in [boldunderlined].
Tables 5 and 6 show the results of MolT5 indication-to-drug experiments on DrugBank and ChEMBL data, respectively. The tables indicate that fine-tuning the models on the new data worsens performance, reflected in FT experiments yielding worse results than SUB or FULL experiments. Also, larger models tend to perform better across all metrics for each experiment.
Table 5.
DrugBank indication-to-drug results.
| Model | BLEU | Exact | Levenshtein | MACCS | RDK | Morgan | FCD | Text2Mol | Validity |
|---|---|---|---|---|---|---|---|---|---|
| FT-small | 0.0020 | 0.0000 | 77.0375 | 0.0408 | 0.0023 | 0.0241 | 0.0000 | 0.0000 | 0.0017 |
| SUB-small | 0.1524 | 0.0000 | 89.3278 | 0.2747 | 0.1729 | 0.1026 | 0.0000 | 0.1663 | 0.3661 |
| FULL-small | 0.1627 | 0.0003 | 87.0366 | 0.2822 | 0.1644 | 0.0992 | 11.2862 | 0.0645 | 0.3628 |
| FT-base | 0.0002 | 0.0000 | 640.9418 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0017 |
| SUB-base | 0.1563 | 0.0000 | 92.9151 | 0.3147 | 0.1898 | 0.1214 | 0.0000 | 0.1220 | 0.3278 |
| FULL-base | 0.1614 | 0.0003 | 95.0343 | 0.3145 | 0.1933 | 0.1177 | 11.2079 | 0.1472 | 0.3106 |
| FT-large | 0.0000 | 0.0000 | 1315.0585 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.1472 | 0.0000 |
| SUB-large | 0.1314 | 0.0166 | 113.3877 | 0.3907 | 0.2758 | 0.1673 | 0.0000 | 0.2972 | 0.5655 |
| FULL-large | 0.1375 | 0.0163 | 114.6298 | 0.3982 | 0.2819 | 0.1709 | 5.5990 | 0.2516 | 0.5462 |
Significant values are in [boldunderlined].
Table 6.
ChEMBL indication-to-drug results.
| Model | BLEU | Exact | Levenshtein | MACCS | RDK | Morgan | FCD | Text2Mol | Validity |
|---|---|---|---|---|---|---|---|---|---|
| FT-small | 0.0401 | 0.0000 | 84.3199 | 0.0571 | 0.0094 | 0.0070 | 0.0000 | 0.0000 | 0.0142 |
| SUB-small | 0.1190 | 0.0000 | 126.1835 | 0.2387 | 0.1162 | 0.0629 | 0.0000 | 0.0395 | 0.3246 |
| FULL-small | 0.1114 | 0.0000 | 132.5282 | 0.2442 | 0.1247 | 0.0656 | 19.6213 | 0.0219 | 0.3199 |
| FT-base | 0.0203 | 0.0000 | 516.0212 | 0.1235 | 0.0237 | 0.0325 | 0.0000 | 0.0000 | 0.0098 |
| SUB-base | 0.1956 | 0.0000 | 76.7455 | 0.2997 | 0.1878 | 0.0945 | 0.0000 | 0.0566 | 0.3662 |
| FULL-base | 0.1935 | 0.0000 | 77.3259 | 0.2996 | 0.1924 | 0.0922 | 19.6774 | 0.0620 | 0.3404 |
| FT-large | 0.0115 | 0.0000 | 339.3972 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0620 | 0.0000 |
| SUB-large | 0.0699 | 0.0000 | 276.5310 | 0.3590 | 0.2613 | 0.0851 | 0.0000 | 0.1934 | 0.3140 |
| FULL-large | 0.0684 | 0.0000 | 280.9910 | 0.3559 | 0.2626 | 0.0830 | 16.3108 | 0.0482 | 0.3199 |
Significant values are in [boldunderlined].
In our drug-to-indication and indication-to-drug experiments, we see that fine-tuning the models causes the models to perform worse across all metrics. Additionally, larger models perform better on our tasks. However, in our custom tokenizer experiments, we pretrain MolT5-Small without the added layers of SMILES-to-caption and caption-to-SMILES. By fine-tuning the custom pretrained model on our data for drug-to-indication and indication-to-drug tasks, we aim to see improved results.
Evaluation of custom tokenizer
Tables 7 and 8 show the evaluation of MolT5 pretrained with the custom tokenizer on the drug-to-indication and indication-to-drug tasks, respectively. For drug-to-indication, the model performed better on the DrugBank dataset, reflected across all metrics. This performance difference may be due to a stronger signal between drug indication and SMILES strings in the DrugBank dataset, as the drug indication contains more details. Fine-tuning the model on 80% of either of the datasets did not worsen the performance for drug-to-indication as it did in the baseline results, and some metrics showed improved results. The results for indication-to-drug are more mixed. The model does not consistently perform better across either dataset and fine-tuning the model affects the evaluation metrics inconsistently.
Table 7.
Results for MolT5 augmented with custom tokenizer, drug-to-indication.
| Model | BLEU-2 | BLEU-4 | ROUGE-1 | ROUGE-2 | ROUGE-L | METEOR | Text2Mol |
|---|---|---|---|---|---|---|---|
| FT-DrugBank | 0.0006 | 0.0000 | 0.0013 | 0.0000 | 0.0013 | 0.0141 | 0.0706 |
| FT-ChEMBL | 0.0000 | 0.0000 | 0.0011 | 0.0000 | 0.0011 | 0.0017 | 0.0699 |
| SUB-DrugBank | 0.0008 | 0.0000 | 0.0014 | 0.0000 | 0.0013 | 0.0137 | 0.0811 |
| SUB-ChEMBL | 0.0000 | 0.0000 | 0.0012 | 0.0000 | 0.0012 | 0.0012 | 0.0836 |
| FULL-DrugBank | 0.0010 | 0.0000 | 0.0014 | 0.0000 | 0.0014 | 0.0133 | 0.0787 |
| FULL-ChEMBL | 0.0000 | 0.0000 | 0.0016 | 0.0000 | 0.0016 | 0.0014 | 0.0868 |
Significant values are in [boldunderlined].
Table 8.
Results for MolT5 augmented with custom tokenizer, indication-to-drug.
| Model | BLEU | Exact | Levenshtein | MACCS | RDK | Morgan | FCD | Text2Mol | Validity |
|---|---|---|---|---|---|---|---|---|---|
| FT-DrugBank | 0.0154 | 0.0000 | 174.0865 | 0.0440 | 0.0354 | 0.0513 | 0.0000 | 0.0000 | 0.0050 |
| FT-ChEMBL | 0.0136 | 0.0000 | 454.1142 | 0.1455 | 0.0233 | 0.0327 | 0.0000 | 0.0000 | 0.0073 |
| SUB-DrugBank | 0.0175 | 0.0000 | 170.0050 | 0.0452 | 0.0140 | 0.0532 | 0.0000 | 0.0000 | 0.0067 |
| SUB-ChEMBL | 0.0252 | 0.0000 | 281.2072 | 0.0605 | 0.0239 | 0.0493 | 0.0000 | 0.1989 | 0.0090 |
| FULL-DrugBank | 0.0174 | 0.0000 | 175.9574 | 0.0825 | 0.0552 | 0.0532 | 0.0000 | 0.0728 | 0.0087 |
| FULL-ChEMBL | 0.0234 | 0.0000 | 286.5869 | 0.1180 | 0.0449 | 0.0356 | 0.0000 | 0.2707 | 0.0072 |
Significant values are in [boldunderlined].
Discussion
In this paper, we proposed a novel task of translating between drugs and indications, considering both drug-to-indication and indication-to-drug subtasks. We focus on generating indications from the SMILES strings of existing drugs and generating SMILES strings from sets of indications. Our experiments are the first attempt at tackling this problem. After conducting experiments with various model configurations and two datasets, we hypothesized potential issues that need further work. We believe that properly addressing these issues could significantly improve the performance of the proposed tasks.
The signal between SMILES strings and indications is poor. In the original MolT5 task (translation between molecules and their textual descriptions), “similar” SMILES strings often had similar textual descriptions. In the case of drug-to-indication and indication-to-drug tasks, similar SMILES strings might have completely different textual descriptions as they are different drugs, and their indications also differ. One could also make a similar observation about SMILES strings that are different: drug indications may be similar. Having no direct relationships between drugs and indications makes it hard to achieve high performance on proposed tasks. We hypothesize that having an intermediate representation that drugs (or indications) map to may improve the performance. As an example, mapping a SMILES string to its caption (MolT5 task) and then caption to indication may be a potential future direction of research.
The signal between drugs and indications is not the only issue: the data is also scarce. Since we do not consider random molecules and their textual descriptions but drugs and their indications, the available data is limited by the number of drugs. In the case of both ChEMBL and DrugBank datasets, the number of drug-indication pairs was under 10000, with the combined size also being under 10000. Finding ways to enrich data may help establish a signal between SMILES strings and indications and could be a potential future avenue for exploration.
Overall, the takeaway from our experiments is that larger models tend to perform better. By using a larger model and having more data (or data that has a stronger signal between drug indications and SMILES strings), we may be able to successfully translate between drug indications and molecules (i.e., SMILES strings) and ultimately facilitate novel drug discovery.
We note that our experiments did not involve human evaluation of the generated indications and relied entirely on automated metrics. We acknowledge that such metrics may not correlate well with human judgment58–60. At the same time, manually reviewing thousands of indications would have been expensive and would involve a lot of human labor. Future work could potentially consider incorporating humans in the loop or using LLMs to assess the quality of generated indications.
Experiments with other models and model architectures can be another avenue for exploration. Some potential benefits may include better performance, lower latency, and improved computational complexity. As an example, our current method uses the transformer architecture, which has the overall time complexity of (where is the sequence length and is the embedding dimension), with being the time complexity of the attention layer alone. On the other hand, State Space Models (SSMs), such as Mamba61, scale linearly with the sequence length.
Methods
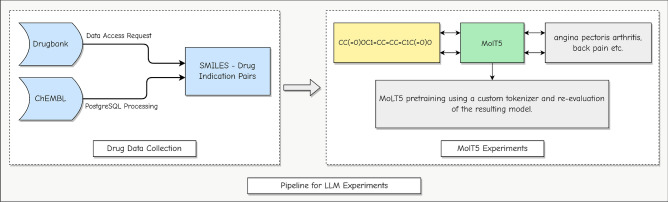
This section describes the dataset, analysis methods, ML models, and feature extraction techniques used in this study. Figure 1 shows the flowchart of the process. We adjust the workflow of existing models for generating molecular captions to instead generate indications for drugs. By training LLMs on the translation between SMILES strings and drug indications, we endeavor to one day be able to create novel drugs that treat medical conditions.
Figure 1.
Overview of the methodology of the experiments: drug data is compiled from ChEMBL and DrugBank and utilized as input for MolT5. Our experiments involved two tasks: drug-to-indication and indication-to-drug. For the drug-to-indication task, SMILES strings of existing drugs were used as input, producing drug indications as output. Conversely, for the indication-to-drug task, drug indications of the same set of drugs were the input, resulting in SMILES strings as output. Additionally, we augmented MolT5 with a custom tokenizer in pretraining and evaluated the resulting model on the same tasks.
Data
Our data comes from two databases, DrugBank62 and ChEMBL63, which we selected due to the different ways they represent drug indications. DrugBank gives in-depth descriptions of how each drug treats patients, while ChEMBL provides a list of medical conditions each drug treats. Table 9 outlines the size of each dataset, as well as the length of the SMILES and indication data. In the case of DrugBank, we had to request access to use the drug indication and SMILES data. The ChEMBL data was available without request but required establishing a database locally to query and parse the drug indication and SMILES strings into a workable format. Finally, we prepared a pickle file for both databases to allow for metric calculation following the steps presented in MolT526.
Table 9.
Dataset Details.
| Dataset statistic | DrugBank | ChEMBL |
|---|---|---|
| Number of drug-Indication pairs | 3004 | 6127 |
| Minimum indication length (characters) | 19 | 34 |
| Minimum SMILES length (characters) | 1 | 1 |
| Average indication length (characters) | 259 | 114 |
| Average SMILES length (characters) | 59 | 67 |
| Maximum indication length (characters) | 3517 | 524 |
| Maximum SMILES length (characters) | 710 | 1486 |
Models
We conducted initial experiments using the MolT5 model, based on the T5 architecture26. The T5 basis of the model gives it textual modality from pretraining on the natural language text dataset Colossal Clean Crawled Corpus (C4)64, and the pretraining on 100 million SMILES strings from the ZINC-15 dataset65 gives the model molecular modality.
In our experiments, we utilized fine-tuned versions of the available MolT5 models: SMILES-to-caption, fine-tuned for generating molecular captions from SMILES strings, and caption-to-SMILES, fine-tuned for generating SMILES strings from molecular captions. However, we seek to evaluate the model’s capacity to translate between drug indications and SMILES strings. Thus, we use drug indications in the place of molecular captions, yielding our two tasks: drug-to-indication and indication-to-drug.
The process of our experiments begins with evaluating the baseline MolT5 model for each task on the entirety of the available data (3004 pairs for DrugBank, 6127 pairs for ChEMBL), on a 20% subset of the data (601 pairs for DrugBank, 1225 pairs for ChEMBL), and then fine-tuning the model on 80% (2403 pairs for DrugBank, 4902 pairs for ChEMBL) of the data and evaluating on that same 20% subset.
After compiling the results of the preliminary experiments, we decided to use a custom tokenizer with the MolT5 model architecture. While the default tokenizer leverages the T5 pretraining, the reason is that treating SMILES strings as a form of natural language and tokenizing it accordingly into its component bonds and molecules could improve model understanding of SMILES strings and thus improve performance.
MolT5 with custom tokenizer
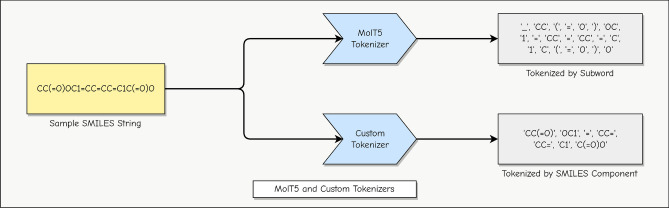
The tokenizer for custom pretraining of MolT5 that we selected came from previous work on adapting transformers for SMILES strings66. This tokenizer separates SMILES strings into individual bonds and molecules. Figure 2 illustrates the behavior of both MolT5 and custom tokenizers. Due to computational limits, we only performed custom pretraining of the smallest available MolT5 model, with 77 million parameters. Our pretraining approach utilized the model configuration of MolT5 and JAX (https://jax.readthedocs.io/en/latest/index.html) / Flax (https://github.com/google/flax) to execute the span-masked language model objective on the ZINC dataset64. Following pretraining, we assessed model performance on both datasets. The experiments comprised three conditions: fine-tuning on 80% (2403 pairs for DrugBank, 4902 pairs for ChEMBL) of the data and evaluating on the remaining 20% (601 pairs for DrugBank, 1225 pairs for ChEMBL), evaluating on 20% of the data without fine-tuning, and evaluating on 100% (3004 pairs for DrugBank, 6127 pairs for ChEMBL) of the data.
Figure 2.
MolT5 and custom tokenizers: MolT5 tokenizer uses the default English language tokenization and splits the input text into subwords. The intuition is that SMILES strings are composed of characters typically found in English text, and pretraining on large-scale English corpora may be helpful. On the other hand, the custom tokenizer method utilizes the grammar of SMILES and decomposes the input into grammatically valid components.
Acknowledgements
This work used Bridges-2 at Pittsburgh Supercomputing Center through allocation from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296.
Author contributions
D.O. led the study, designed the experiments, helped conduct the experiments, analyzed the results, and wrote, reviewed, and revised the paper. J.H. contributed to the study design, conducted the experiments, analyzed the results, and wrote, reviewed, and revised the paper. C.Z. shared resources, contributed to discussions, and reviewed the paper. J.W. helped streamline the idea and provided guidance from the perspective of chemistry. L.C. shared resources and contributed to discussions. J.Z. contributed to discussions. Y.W. conceptualized and led the study, designed the experiments, and reviewed and revised the paper.
Data availability
ChEMBL and DrugBank datasets are publicly available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: David Oniani and Jordan Hilsman.
References
- 1.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA. 2020;323:844–853. doi: 10.1001/jama.2020.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decker, S. & Sausville, E. A. Chapter 28: Drug discovery. in Principles of Clinical Pharmacology (Second Edition) (eds Atkinson, A. J., Abernethy, D. R., Daniels, C. E., Dedrick, R. L. & Markey, S. P.) (Academic Press, 2007), editionsecond edition edn. 439–447. 10.1016/B978-012369417-1/50068-7
- 3.Schneider G. Automating drug discovery. Nat. Rev. Drug Discov. 2018;17:97–113. doi: 10.1038/nrd.2017.232. [DOI] [PubMed] [Google Scholar]
- 4.Sadybekov AV, Katritch V. Computational approaches streamlining drug discovery. Nature. 2023;616:673–685. doi: 10.1038/s41586-023-05905-z. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SS. Commercializing Successful Biomedical Technologies. Cambridge, England: PublisherCambridge University Press; 2008. [Google Scholar]
- 6.Brown T, et al. Language models are few-shot learners. In: Larochelle H, Ranzato M, Hadsell R, Balcan M, Lin H, et al., editors. Advances in Neural Information Processing Systems. Curran Associates Inc; 2020. pp. 1877–1901. [Google Scholar]
- 7.OpenAI et al. Gpt-4 technical report (2023). arXiv: 2303.08774
- 8.Touvron, H. et al. Llama: Open and efficient foundation language models (2023). arXiv: 2302.13971
- 9.Jiang, A. Q. et al. Mixtral of experts (2024). arXiv: 2401.04088
- 10.Porter, J. Chatgpt continues to be one of the fastest-growing services ever. https://www.theverge.com/2023/11/6/23948386/chatgpt-active-user-count-openai-developer-conference (2023). Accessed 31 Jan 2024.
- 11.Hu, K. Chatgpt sets record for fastest-growing user base: Analyst note. https://www.reuters.com/technology/chatgpt-sets-record-fastest-growing-user-base-analyst-note-2023-02-01/ (2023). Accessed 31 Jan 2024.
- 12.Chung, J., Kamar, E. & Amershi, S. Increasing diversity while maintaining accuracy: Text data generation with large language models and human interventions. in Proceedings of the 61st Annual Meeting of the Association for Computational Linguistics (Volume 1: Long Papers) (eds Rogers, A., Boyd-Graber, J. & Okazaki, N.) (Association for Computational Linguistics, 2023) 575–593. 10.18653/v1/2023.acl-long.34
- 13.Lee N, et al. et al. Factuality enhanced language models for open-ended text generation. In: Koyejo S, et al.et al., editors. Advances in Neural Information Processing Systems. Curran Associates Inc; 2022. pp. 34586–34599. [Google Scholar]
- 14.Moslem, Y., Haque, R., Kelleher, J. D. & Way, A. Adaptive machine translation with large language models. in Proceedings of the 24th Annual Conference of the European Association for Machine Translation, (eds Nurminen, M. et al.) 227–237 (European Association for Machine Translation, 2023).
- 15.Mu Y, et al. Augmenting large language model translators via translation memories. In: Rogers A, Boyd-Graber J, Okazaki N, et al., editors. Findings of the Association for Computational Linguistics: ACL 2023, 10287–10299, (Association for Computational Linguistics. Toronto: Canada; 2023. [Google Scholar]
- 16.Singhal K, et al. Large language models encode clinical knowledge. Nature. 2023;620:172–180. doi: 10.1038/s41586-023-06291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu, X., Chen, Z. & Lu, Y. Harnessing LLMs for temporal data: A study on explainable financial time series forecasting. in Proceedings of the 2023 Conference on Empirical Methods in Natural Language Processing: Industry Track (eds Wang, M. & Zitouni, I.) 739–753 (Association for Computational Linguistics, 2023). 10.18653/v1/2023.emnlp-industry.69
- 18.Gomez-Rodriguez C, Williams P. A confederacy of models: A comprehensive evaluation of LLMs on creative writing. In: Bouamor H, Pino J, Bali K, editors. Findings of the Association for Computational Linguistics: EMNLP 2023, 14504–14528. Singapore: Association for Computational Linguistics; 2023. [Google Scholar]
- 19.Boiko DA, MacKnight R, Kline B, Gomes G. Autonomous chemical research with large language models. Nature. 2023;624:570–578. doi: 10.1038/s41586-023-06792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weininger D. Smiles, A chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988;28:31–36. doi: 10.1021/ci00057a005. [DOI] [Google Scholar]
- 21.Lv Q, Chen G, Yang Z, Zhong W, Chen CY-C. Meta learning with graph attention networks for low-data drug discovery. IEEE Trans. Neural Netw. Learn. Syst. 2023 doi: 10.1109/TNNLS.2023.3250324. [DOI] [PubMed] [Google Scholar]
- 22.Lv Q, Chen G, Yang Z, Zhong W, Chen CY-C. Meta-molnet: A cross-domain benchmark for few examples drug discovery. IEEE Trans. Neural Netw. Learn. Syst. 2024 doi: 10.1109/TNNLS.2024.3359657. [DOI] [PubMed] [Google Scholar]
- 23.Paul D, et al. Artificial intelligence in drug discovery and development. Drug Discov. Today. 2021;26:80–93. doi: 10.1016/j.drudis.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagal V, Aggarwal R, Vinod PK, Priyakumar UD. Molgpt: Molecular generation using a transformer-decoder model. J. Chem. Inf. Model. 2022;62:2064–2076. doi: 10.1021/acs.jcim.1c00600. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Zhang Y. Unified deep learning model for multitask reaction predictions with explanation. J. Chem. Inf. Model. 2022;62:1376–1387. doi: 10.1021/acs.jcim.1c01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards, C. et al. Translation between molecules and natural language. in Proceedings of the 2022 Conference on Empirical Methods in Natural Language Processing10.18653/v1/2022.emnlp-main.26 (2022).
- 27.Méndez-Lucio O, Baillif B, Clevert D-A, Rouquié D, Wichard J. De. novo generation of hit-like molecules from gene expression signatures using artificial intelligence. Nat. Commun. 2020 doi: 10.1038/s41467-019-13807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merk D, Friedrich L, Grisoni F, Schneider G. De. novo design of bioactive small molecules by artificial intelligence. Mol. Inform. 2018 doi: 10.1002/minf.201700153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X, Xie R, Li X, Li J. Smilegnn: Drug–drug interaction prediction based on the smiles and graph neural network. Life. 2022;12:319. doi: 10.3390/life12020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krenn M, Häse F, Nigam A, Friederich P, Aspuru-Guzik A. Self-referencing embedded strings (selfies): A 100% robust molecular string representation. Mach. Learn.: Sci. Technol. 2020;1:045024. doi: 10.1088/2632-2153/aba947. [DOI] [Google Scholar]
- 31.Lv Q, et al. Tcmbank: Bridges between the largest herbal medicines, chemical ingredients, target proteins, and associated diseases with intelligence text mining. Chem. Sci. 2023;14:10684–10701. doi: 10.1039/d3sc02139d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv Q, et al. Tcmbank-the largest tcm database provides deep learning-based Chinese-western medicine exclusion prediction. Sign. Transduct. Target. Ther. 2023 doi: 10.1038/s41392-023-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv Q, Chen G, Zhao L, Zhong W, Yu-Chian Chen C. Mol2Context-vec: Learning molecular representation from context awareness for drug discovery. Brief. Bioinform. 2021;22:bbab317. doi: 10.1093/bib/bbab317. [DOI] [PubMed] [Google Scholar]
- 34.Lv Q, Zhou J, Yang Z, He H, Chen CY-C. 3d graph neural network with few-shot learning for predicting drug-drug interactions in scaffold-based cold start scenario. Neural Netw. 2023;165:94–105. doi: 10.1016/j.neunet.2023.05.039. [DOI] [PubMed] [Google Scholar]
- 35.Luo H, et al. Drug-drug interactions prediction based on deep learning and knowledge graph: A review. iScience. 2024;27:109148. doi: 10.1016/j.isci.2024.109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan HL. The generation of a unique machine description for chemical structures-a technique developed at chemical abstracts service. J. Chem. Document. 1965;5:107–113. doi: 10.1021/c160017a018. [DOI] [Google Scholar]
- 37.Capecchi A, Probst D, Reymond J-L. One molecular fingerprint to rule them all: Drugs, biomolecules, and the metabolome. J. Cheminform. 2020 doi: 10.1186/s13321-020-00445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wigh DS, Goodman JM, Lapkin AA. A review of molecular representation in the age of machine learning. WIREs Comput. Mol. Sci. 2022;12:e1603. doi: 10.1002/wcms.1603. [DOI] [Google Scholar]
- 39.Jaeger S, Fulle S, Turk S. Mol2vec: Unsupervised machine learning approach with chemical intuition. J. Chem. Inf. Model. 2018;58:27–35. doi: 10.1021/acs.jcim.7b00616. [DOI] [PubMed] [Google Scholar]
- 40.Mikolov, T., Chen, K., Corrado, G. & Dean, J. Efficient estimation of word representations in vector space. in International Conference on Learning Representations (2013).
- 41.Devlin, J., Chang, M.-W., Lee, K. & Toutanova, K. BERT: Pre-training of deep bidirectional transformers for language understanding. In Proceedings of the 2019 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies, Volume 1 (Long and Short Papers), 4171–4186 (Association for Computational Linguistics, 2019). 10.18653/v1/N19-1423
- 42.Fabian, B. et al. Molecular representation learning with language models and domain-relevant auxiliary tasks. in Machine Learning for Molecules (2020).
- 43.Chithrananda, S., Grand, G. & Ramsundar, B. Large-scale self-supervised pretraining for molecular property prediction, Chemberta (2020).
- 44.Yamada M, Sugiyama M. Molecular graph generation by decomposition and reassembling. ACS Omega. 2023;8:19575–19586. doi: 10.1021/acsomega.3c01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganea O, et al. Geomol: Torsional geometric generation of molecular 3d conformer ensembles. In: Ranzato M, Beygelzimer A, Dauphin Y, Liang P, Vaughan JW, et al., editors. Advances in Neural Information Processing Systems. Curran Associates Inc; 2021. pp. 13757–13769. [Google Scholar]
- 46.Papineni, K., Roukos, S., Ward, T. & Zhu, W.-J. Bleu: A method for automatic evaluation of machine translation. in Proceedings of the 40th Annual Meeting on Association for Computational Linguistics, ACL ’02, 311-318 (Association for Computational Linguistics, 2002). 10.3115/1073083.1073135
- 47.Miller, F. P., Vandome, A. F. & McBrewster, J. Levenshtein Distance: Information Theory, Computer Science, String (Computer Science), String Metric, Damerau?Levenshtein Distance (Hamming Distance (Alpha Press, Spell Checker, 2009).
- 48.Tanimoto T. An Elementary Mathematical Theory of Classification and Prediction. International Business Machines Corporation; 1958. [Google Scholar]
- 49.Durant JL, Leland BA, Henry DR, Nourse JG. Reoptimization of mdl keys for use in drug discovery. J. Chem. Inf. Comput. Sci. 2002;42:1273–1280. doi: 10.1021/ci010132r. [DOI] [PubMed] [Google Scholar]
- 50.Schneider N, Sayle RA, Landrum GA. Get your atoms in order-an open-source implementation of a novel and robust molecular canonicalization algorithm. J. Chem. Inf. Model. 2015;55:2111–2120. doi: 10.1021/acs.jcim.5b00543. [DOI] [PubMed] [Google Scholar]
- 51.Rogers D, Hahn M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 2010;50:742–754. doi: 10.1021/ci100050t. [DOI] [PubMed] [Google Scholar]
- 52.Preuer K, Renz P, Unterthiner T, Hochreiter S, Klambauer G. Fréchet chemnet distance: A metric for generative models for molecules in drug discovery. J. Chem. Inf. Model. 2018;58:1736–1741. doi: 10.1021/acs.jcim.8b00234. [DOI] [PubMed] [Google Scholar]
- 53.Edwards, C., Zhai, C. & Ji, H. Text2Mol: Cross-modal molecule retrieval with natural language queries. in Proceedings of the 2021 Conference on Empirical Methods in Natural Language Processing (eds Moens, M.-F., Huang, X., Specia, L. & Yih, S. W.-T.) 595–607 (Association for Computational Linguistics, Online and Punta Cana, 2021). 10.18653/v1/2021.emnlp-main.47
- 54.Lin, C.-Y. ROUGE: A package for automatic evaluation of summaries. In Text Summarization Branches Out, 74–81 (Association for Computational Linguistics, 2004).
- 55.Lin, C.-Y. & Hovy, E. Automatic evaluation of summaries using n-gram co-occurrence statistics. In Proceedings of the 2003 Human Language Technology Conference of the North American Chapter of the Association for Computational Linguistics, 150–157 (2003).
- 56.Lin, C.-Y. & Och, F. J. Automatic evaluation of machine translation quality using longest common subsequence and skip-bigram statistics. in Proceedings of the 42nd Annual Meeting of the Association for Computational Linguistics (ACL-04), 605–612 (2004). 10.3115/1218955.1219032
- 57.Banerjee, S. & Lavie, A. METEOR: An automatic metric for MT evaluation with improved correlation with human judgments. in Proceedings of the ACL Workshop on Intrinsic and Extrinsic Evaluation Measures for Machine Translation and/or Summarization (eds Goldstein, J., Lavie, A., Lin, C.-Y. & Voss, C.) 65–72 (Association for Computational Linguistics, 2005).
- 58.Thoppilan, R. et al. Lamda: Language models for dialog applications (2022). arXiv: 2201.08239.
- 59.Liu, C.-W. et al. How NOT to evaluate your dialogue system: An empirical study of unsupervised evaluation metrics for dialogue response generation. in Proceedings of the 2016 Conference on Empirical Methods in Natural Language Processing (eds Su, J., Duh, K. & Carreras, X.) 2122–2132 (Association for Computational Linguistics, 2016). 10.18653/v1/D16-1230
- 60.Abbasian, M. et al. Foundation metrics for evaluating effectiveness of healthcare conversations powered by generative ai (2024). arXiv: 2309.12444. [DOI] [PMC free article] [PubMed]
- 61.Gu, A. & Dao, T. Mamba: Linear-time sequence modeling with selective state spaces (2023). arXiv: 2312.00752.
- 62.Wishart DS. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006 doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies M, et al. Chembl web services: Streamlining access to drug discovery data and utilities. Nucleic Acids Res. 2015 doi: 10.1093/nar/gkv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raffel C, et al. Exploring the limits of transfer learning with a unified text-to-text transformer. J. Mach. Learn. Res. 2020;21:1–67. [Google Scholar]
- 65.Sterling T, Irwin JJ. Zinc 15- ligand discovery for everyone. J. Chem. Inf. Model. 2015;55:2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adilov, S. Generative pre-training from molecules. ChemRxiv10.33774/chemrxiv-2021-5fwjd (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ChEMBL and DrugBank datasets are publicly available.