Abstract
This study aimed to investigate the effects of different proportions of dietary fermented sweet potato residue (FSPR) supplementation as a substitute for corn on the nutrient digestibility, meat quality, and intestinal microbes of yellow-feathered broilers. Experiment 1 (force-feeding) evaluated the nutrient composition and digestibility of mixtures with different proportions of sweet potato residue (70%, 80%, 90%, and 100%) before and after fermentation. In Experiment 2 (metabolic growth), a total of 420 one-day-old yellow-feathered broilers were randomly allocated to 4 groups and fed corn-soybean meal-based diets with 0, 5%, 8%, and 10% FSPR as a substitute for corn. The force-feeding and metabolic growth experiments were performed for 9 and 70 d, respectively. The treatment of 70% sweet potato residue (after fermentation) had the highest levels of crude protein, ether extract, and crude fiber and improved the digestibility of crude protein and amino acids (P < 0.05). Although dietary FSPR supplementation at different levels had no significant effect on growth performance and intestinal morphology, it improved slaughter rate, half-chamber rate, full clearance rate, and meat color, as well as reduced cooking loss in the breast and thigh muscles (P < 0.05). Dietary supplementation with 8% and 10% FSPR increased the serum immunoglobulin M and immunoglobulin G levels in broilers (P < 0.05). Furthermore, 10% FSPR increased the Shannon index and Ruminococcaceae_UCG-014, Ruminococcaceae_UCG-010 and Romboutsia abundances and decreased Sutterella and Megamonas abundances (P < 0.05). Spearman's correlation analysis showed that meat color was positively correlated with Ruminococcaceae_UCG-014 (P < 0.05) and negatively correlated with Megamonas (P < 0.05). Collectively, 70% sweet potato residue (after fermentation) had the best nutritional value and nutrient digestibility. Dietary supplementation with 8% to 10% FSPR as a substitute for corn can improve the slaughter performance, meat quality, and intestinal microbe profiles of broilers. Our findings suggest that FSPR has the potential to be used as a substitute for corn-soybean meals to improve the meat quality and intestinal health of broilers.
Keywords: Fermented sweet potato residue, Meat quality, Intestinal microbiota, Broiler
1. Introduction
In recent years, antibiotic bans have been implemented, soybean meal and corn prices have been fluctuating, and resources are becoming scarce, resulting in shortages of energy- and protein-based feeds (Chen and Liu, 2020). To address this matter, attention has been refocused to develop and promote unconventional feeds. However, the use of unconventional feeds is limited because they often contain anti-nutritional factors, have lower nutritional value, and are unstable in composition (Sugiharto and Ranjitkar, 2019). Currently, several methods are available to improve the palatability and efficiency of unconventional feeds; including fermentation, comminution, puffing, and microwaving. In particular, fermentation not only reduces toxin levels and anti-nutritional factors in feed but also improves its nutritional value and digestibility (Okeke et al., 2015; Wang et al., 2010). Additionally, fermented feed can improve the growth performance of animals and regulate the balance of the intestinal flora (Gao et al., 2009). Employing fermentation treatments to develop unconventional feed sources for animals can reduce feeding costs and promote the efficient use of agricultural and byproduct resources.
Sweet potato residue (SPR) is a byproduct of the production of sweet potato starch or sweet potato juice concentrate, with starch as the main component and is rich in phenolic compounds, carotenoids, and other nutrients. Most SPR produced during food processing are discarded as fertilizer, leading to the wastage of resources and environmental pollution (Arachchige et al., 2020). Evaluation of the nutrient composition of SPR revealed that its high starch content makes it a good energy source for animals, whereas its high crude fiber (CF) content and low crude protein (CP) content limit its use in feed (Song et al., 2021). However, the nutritional value of SPR can be increased using fermentation technology; for example, Zhao et al. (2015) reported that solid-state fermentation with mixed microbial strains can increase the CP content of SPR. Furthermore, some studies have reported that fermenting SPR with probiotics has improved feeds value for ruminants (Ray et al., 2010). Other studies have demonstrated that dietary fiber isolated from SPR promotes a healthy gut microbiome (Liu et al., 2020). However, few studies have investigated the effects of fermented sweet potato residue (FSPR) on the growth performance and meat quality of chickens. Therefore, this study aimed to evaluate the effects of FSPR on nutrient digestibility, meat quality, and gut microbes in force-feeding and metabolic growth experiments in broilers.
2. Materials and methods
2.1. Animal ethics
The Animal Welfare Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (20220056; Changsha, China) approved all animals used in the study.
2.2. Experimental materials
Sweet potato residue: The test ingredients were provided by Beijing Huaxia Kangyuan Technology Co., Ltd. to get the materials for the SPR group, and the 100%, 90%, 80% and 70% 100% SPRf groups as described in Table 1. SPR and bran were mixed in the ratios of 10:0, 9:1, 8:2 and 7:3, respectively, to get the fermentation materials with different proportions of SPR (100%, 90%, 80% and 70%).
Table 1.
Composition of different proportions of SPR.
| Groups | Materials |
|---|---|
| SPR | Raw materials of SPR |
| 100% SPR/100% SPRf | 100% SPR + strains + enzymes, before/after fermentation |
| 90% SPR/90% SPRf | 90% SPR + 10% bran + strains + enzymes, before/after fermentation |
| 80% SPR/80% SPRf | 80% SPR + 20% bran + strains + enzymes, before/after fermentation |
| 70% SPR/70% SPRf | 70% SPR + 30% bran + strains + enzymes, before/after fermentation |
SPR = sweet potato residue; SPRf = sweet potato residue (after fermentation).
The pre-fermentation samples were dried at 105 °C immediately after the addition of strains and enzymes, and the post-fermentation samples were fermented at 30 °C for 3 d according to the method by Liang et al. (2022). All samples were stored at room temperature until they were mixed into the experimental rations.
Fermentation strains: Pediococcus acidilactici (22.5 × 108 mL−1), Lactobacillus plantarum (15.4 × 108 mL−1), Enterococcus faecalis (11.5 × 108 mL−1), Enterococcus faecium (9.3 × 107 mL−1), Bacillus licheniformis (11.8 × 107 mL−1), Bacillus subtilis (5.1 × 108 mL−1), and Saccharomyces cerevisiae (23.3 × 107 mL−1).
Fermentation enzymes: xylanase (50 U/g), cellulase (5 U/g), amylase (10 U/g), mannanase (1 U/g), acidic protease (150 U/g), and alkaline protease (300 U/g).
2.3. Animals and experimental design
In experiment 1 (Fig. 1), the SPR samples before and after fermentation were crushed with a grinder and mixed well. A mixed sample of 100 g was taken for proximate composition determination. The indicators measured were dry matter (DM), crude protein (CP), crude ash (Ash), crude fiber (CF), ether extract (EE), and gross energy (GE). Based on the results of the nutrient analysis, SPR, 100% SPR (after fermentation) (SPRf), and 70% SPRf were selected for the force-feeding test (GB/T 26437-2010) (China National Standard, 2011). Fifteen adult medium-speed yellow-feathered broilers (90-day-old, 2.74 ± 0.32 kg, purchased from Hunan Xiang Jia husbandry Limited by Share Ltd.) with similar body weight (BW) were selected and randomly divided into 3 groups, with 5 replicates in each group. After 5 d of pre-feeding and 2 d of fasting, each broiler was fed 50 g of feed containing 0.4% TiO2. The excreted products were collected within 48 h and every 12 h. In Exp. 2, 420 healthy yellow-feathered broilers (1-day-old) were randomly divided into 4 groups (CON, 5%FSPR, 8% FSPR and 10% FSPR) of 15 chickens each, with 7 replicates, with consistent average BW (30.97 ± 0.31 g). From the force-feeding test, 70% SPRf was selected to replace corn in the basal diet at different stages of broilers at a certain percentage, respectively. The CON group was fed the basal diet. The other three groups were supplemented with 1%, 3% and 5% FSPR at the chick stage, and with 5%, 8% and 10% FSPR at the growing stage and breeding stage, respectively, in place of corn in the basal diet. The composition and nutritional levels of the diets for each group are given in Table 2. The experiment was performed for 70 d. The metabolizable energy of FSPR was calculated according to the method of Kong and Adeola (2014) and the results of metabolic experiments, and the metabolizable energy of other feed ingredients were referred to the China Feed Database (Feed database in China, 2018) (https://www.chinafeeddata.org.cn/).
Fig. 1.

Experimental design of the force-feeding. Determine the nutrient composition before and after fermentation of different proportions of SPR, select the optimum proportion for force-feeding test to determine the nutrient digestibility of broilers. SPR = sweet potato residue; SPRf = sweet potato residue (after fermentation).
Table 2.
Composition and nutrient levels of basal diet (air-dry basis, %).
| Item | Chick stage 1–21 d |
Growing stage 22–42 d |
Breeding stage 43–70 d |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | 5% FSPR | 8% FSPR | 10% FSPR | CON | 5% FSPR | 8% FSPR | 10% FSPR | CON | 5% FSPR | 8% FSPR | 10% FSPR | |
| Ingredients | ||||||||||||
| Corn | 63.48 | 61.98 | 59.98 | 57.48 | 69.60 | 63.10 | 59.60 | 57.10 | 74.60 | 68.60 | 64.60 | 62.10 |
| FSPR | 1.00 | 3.00 | 5.00 | 5.00 | 8.00 | 10.00 | 5.00 | 8.00 | 10.00 | |||
| Soybean meal | 31.20 | 31.20 | 31.20 | 31.20 | 25.58 | 25.58 | 25.58 | 25.58 | 20.58 | 20.58 | 20.58 | 20.58 |
| Soybean oil | 1.00 | 1.50 | 1.50 | 2.00 | 0.50 | 2.00 | 2.50 | 3.00 | 0.50 | 1.50 | 2.50 | 3.00 |
| Lysine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Methionine | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Threonine | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| CaHPO4 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 |
| Limestone | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 |
| NaCl | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| Premix1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutrient levels | ||||||||||||
| ME, MJ/kg | 12.10 | 12.15 | 12.04 | 12.02 | 12.15 | 12.18 | 12.11 | 12.10 | 12.28 | 12.21 | 12.24 | 12.23 |
| CP | 20.91 | 20.89 | 20.92 | 20.92 | 18.80 | 18.75 | 18.77 | 18.76 | 16.87 | 16.88 | 16.84 | 16.83 |
| CF | 1.79 | 2.06 | 2.62 | 3.17 | 1.68 | 3.05 | 3.88 | 4.43 | 1.57 | 2.95 | 3.78 | 4.33 |
| Ash | 2.30 | 2.49 | 2.89 | 3.28 | 2.09 | 3.08 | 3.67 | 4.07 | 1.91 | 2.90 | 3.49 | 3.88 |
FSPR = fermented sweet potato residue; ME = metabolizable energy; CP = crude protein; CF = crude fiber; Ash = crude ash.
The premix provided the following per kilogram of diets: vitamin A, 15,000 IU; vitamin B1, 3 mg; vitamin B2, 8 mg; vitamin B12, 0.03 mg; vitamin B6, 7 mg; vitamin E, 20 mg; vitamin K3, 3 mg; vitamin D3, 2500 IU; biotin, 0.1 mg; pantothenic acid, 20 mg; folic acid, 1.5 mg; nicotinic acid, 50 mg; Zn, 110 mg; I, 0.6 mg; Cu, 9 mg; Fe, 100 mg; Se, 0.16 mg; Mn, 100 mg.
2.4. Chemical analysis
The moisture, CP, EE, CF, and Ash contents of the feed and fecal samples were determined according to the methods of National Standards of the People's Republic of China GB/T 6435-2014 (China National Standard, 2014), GB/T 6432-2018 (China National Standard, 2018), GB/T 6433-2006 (China National Standard, 2006), GB/T 6434-2022 (China National Standard, 2022) and GB/T 6438-2007 (China National Standard, 2007) respectively. Moisture content was determined by baking to constant weight at 105 °C in an electric forced ventilation oven (ZXFD-5430, Shanghai Zhicheng Analytical Instrument Manufacturing Ltd., China), and the DM content was further calculated. The GE of all samples was determined using an isothermal auto-calorimeter (5E-AC8018, Changsha Kaide Measurement & Control Instrument Ltd., China). The amino acid (AA) profile was analyzed using an amino acid analyzer (L8900; Hitachi, Tokyo, Japan). The titanium content was determined using an inductively coupled plasma optical emission spectrometer (ICP-OES-5110, Agilent, America). The apparent digestibility of nutrients was calculated as follows:
| Apparent digestibility of nutrients (%) = [1 − (A1/A2) × (F2/F1)] × 100. |
where A1 is the TiO2 content in the feed (%); A2 is the TiO2 content in feces (%); F1 is the nutrient content in the feed (%); and F2 is the nutrient content in feces (%).
2.5. Growth performance
On d 1, 21, 42, and 70 of the feeding periods, the BW and feed intake of broilers in each replicate were recorded to calculate the average daily gain (ADG) and average daily feed intake (ADFI) of each stage, and the feed/gain ratio (F/G) was also calculated.
2.6. Slaughter performance
At d 70, a bird with similar average BW was selected from each replicate for slaughter. The birds were sacrificed by bloodletting the neck and dehaired for slaughter performance determination, comprising slaughter, half-chamber, full clearance, breast muscle, thigh muscle, and abdominal fat rates. For methods, refer to “Terms and Measurement Statistical Methods of Poultry Production Performance (NY/T 823–2020)” (China Agricultural Industry Standard, 2020).
2.7. Meat quality
Samples of the left breast and thigh muscles were collected to determine the meat color, pH, drip loss, cooking loss, and shear force, and this was determined according to a specific determination method by Liang et al. (2022).
2.8. Serum biochemical indicators
Blood samples were collected from the jugular vein of one chicken per replicate at 21, 42, and 70 d of age. After resting and centrifugation, the serum was transferred to a 1.5 mL Eppendorf tube and stored at −20 °C. Serum biochemical indicators were determined using a fully automated biochemical analyzer (Cobas C311, Basel, Switzerland) and reagent kits (Lidman Biotech, Beijing, China), namely total protein (TP), albumin (ALB), urea nitrogen (UN), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), glucose (GLU), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), immunoglobulin G (IgG), and immunoglobulin M (IgM).
2.9. Histological analysis
Intestinal segment samples (approximately 1 cm long) of the duodenum, jejunum, and ileum were collected and fixed in 10% formalin buffer. The fixed samples were processed for sections. Villus height (VH), crypt depth (CD), and villus height/crypt depth (H/D) were determined for the morphological analysis of 6 well-oriented and intact villi selected from the duodenum, jejunum, and ileum. Methods and definitions of intestinal morphology were referred to previous study (He et al., 2017).
2.10. Gut microbiota analysis
The chyme of cecum was collected in sterile tubes and stored at −80 °C prior to analysis. Total microbial DNA was extracted from the samples using a DNA extraction kit, and DNA concentration and purity were determined by 1% agarose gel electrophoresis. DNA was amplified using primers specific for the V3 to V4 region of the 16SrRNA gene, and the purified PCR products were sequenced by Illumina NovaSeq 6000. Sequencing services were performed by Beijing Biomarker Technology Ltd (BMKcloud, Beijing, China). Filtering and denoising the Reads obtained from sequencing were performed to get the final valid data. The taxonomic annotation of the feature sequences was performed using SILVA as a reference database, and the diversity and composition of the microbial communities were further analyzed based on the sequencing results using QIIME and R language.
2.11. Statistical analysis
Data were analyzed and compared using one-way analysis of variance (ANOVA) (SPSS 26.0). The test results were expressed as “mean ± standard error of the mean (SEM)”. P < 0.05 indicated statistical significance.
3. Results
3.1. Effect of fermentation on the nutrient composition and apparent digestibility of SPR
The nutrient composition of the SPR before and after fermentation are presented in Table 3. The highest DM content was found in 90% SPRf, highest ash and GE contents in 80% SPRf, and highest content of other nutrients (namely CP, EE, and CF) in 70% SPRf. A negative CP digestibility was observed in the SPR group (Table 4), indicating that broilers had a negative nitrogen balance, whereas it was significantly higher and reached normal levels in the SPRf group (P < 0.05). In addition, GE digestibility significantly increased in the 100% SPRf group (P < 0.05). The digestibility of the other nutrients was not significantly affected by the fermentation treatment (P > 0.05). Regarding amino acid digestibility (Table 5), almost all amino acids increased to > 80% (P < 0.05). Therefore, 70% SPRf was used for the subsequent experiments.
Table 3.
Nutrient composition of SPR before and after fermentation.
| Item | SPR | 100% SPR | 100% SPRf | 90% SPR | 90% SPRf | 80% SPR | 80% SPRf | 70% SPR | 70% SPRf |
|---|---|---|---|---|---|---|---|---|---|
| DM, % | 89.80 | 90.74 | 98.54 | 86.26 | 98.86 | 89.37 | 98.65 | 80.85 | 98.45 |
| CP, % | 4.01 | 8.05 | 5.90 | 7.85 | 6.57 | 9.04 | 7.93 | 9.51 | 9.87 |
| Ash, % | 3.15 | 4.45 | 4.59 | 4.61 | 4.71 | 4.67 | 4.86 | 4.80 | 4.81 |
| EE, % | 0.39 | 0.46 | 0.81 | 0.65 | 0.84 | 0.82 | 0.75 | 0.89 | 1.15 |
| CF, % | 0.45 | 0.36 | 0.44 | 0.90 | 1.02 | 0.99 | 1.12 | 1.32 | 1.43 |
| GE, MJ/kg | 17.01 | 16.56 | 16.65 | 16.73 | 16.90 | 16.90 | 17.08 | 17.00 | 16.07 |
SPR = sweet potato residue; SPRf = sweet potato residue (after fermentation); DM = dry matter; CP = crude protein; Ash = crude ash; EE = ether extract; CF = crude fiber; GE = gross energy.
Table 4.
Nutrient digestibility of SPR before and after fermentation in broilers.
| Item | SPR | 100% SPRf | 70% SPRf | P-value |
|---|---|---|---|---|
| DM, % | 95.43 ± 0.77 | 94.50 ± 0.58 | 93.68 ± 0.70 | 0.239 |
| CP, % | −133.22 ± 23.16b | 53.37 ± 13.13a | 63.67 ± 3.68a | 0.001 |
| Ash, % | 58.24 ± 5.88 | 72.03 ± 1.42 | 68.85 ± 2.92 | 0.065 |
| EE, % | 43.26 ± 3.30 | 40.08 ± 4.70 | 45.73 ± 4.62 | 0.680 |
| CF, % | 66.84 ± 5.21 | 74.01 ± 1.64 | 62.45 ± 9.29 | 0.440 |
| GE, MJ/kg | 78.03 ± 2.64b | 87.89 ± 0.83a | 83.83 ± 2.54ab | 0.023 |
SPR = sweet potato residue; SPRf = sweet potato residue (after fermentation); DM = dry matter; CP = crude protein; Ash = crude ash; EE = ether extract; CF = crude fiber; GE = gross energy.
Data are presented as mean ± SEM (n = 5).
a, b Mean values with different small letter superscripts mean significant difference (P < 0.05).
Table 5.
Amino acid digestibility of SPR before and after fermentation in broilers.
| Item | SPR | 100% SPRf | 70% SPRf | P-value |
|---|---|---|---|---|
| Asp | 64.44 ± 5.57b | 86.94 ± 2.53a | 87.54 ± 1.57a | 0.001 |
| Thr | 48.20 ± 7.79b | 86.25 ± 2.74a | 86.97 ± 1.85a | <0.001 |
| Ser | 49.38 ± 7.25b | 87.51 ± 2.56a | 87.92 ± 1.68a | <0.001 |
| Glu | 18.17 ± 0.81b | 78.84 ± 4.97a | 79.20 ± 2.47a | <0.001 |
| Gly | 32.21 ± 2.12b | 80.10 ± 4.58a | 80.55 ± 2.91a | <0.001 |
| Ala | 44.97 ± 8.02b | 87.70 ± 2.25a | 87.60 ± 1.70a | <0.001 |
| Val | 52.11 ± 6.63b | 89.60 ± 2.07a | 89.95 ± 1.45a | <0.001 |
| Met | 83.55 ± 3.62b | 98.65 ± 0.29a | 99.52 ± 0.26a | <0.001 |
| Ile | 66.77 ± 4.38b | 92.54 ± 1.01a | 91.73 ± 1.69a | <0.001 |
| Leu | 38.44 ± 8.62b | 86.41 ± 2.66a | 86.30 ± 2.28a | <0.001 |
| Tyr | 61.28 ± 5.39b | 92.20 ± 1.25a | 92.31 ± 1.35a | <0.001 |
| Phe | 59.19 ± 5.40b | 91.19 ± 1.67a | 91.35 ± 1.34a | <0.001 |
| Lys | 50.91 ± 7.93b | 91.90 ± 2.15a | 92.43 ± 0.98a | <0.001 |
| His | 80.84 ± 2.69b | 96.00 ± 1.05a | 96.22 ± 0.58a | <0.001 |
| Arg | 59.84 ± 5.93b | 90.18 ± 2.18a | 90.87 ± 0.79a | <0.001 |
| Pro | 52.05 ± 7.50b | 85.89 ± 3.87a | 87.55 ± 1.98a | <0.001 |
SPR = sweet potato residue; SPRf = sweet potato residue (after fermentation).
Data are presented as mean ± SEM (n = 5).
a, b Mean values with different small letter superscripts mean significant difference (P < 0.05).
3.2. Effect of FSPR on growth performance and intestinal morphology of broilers
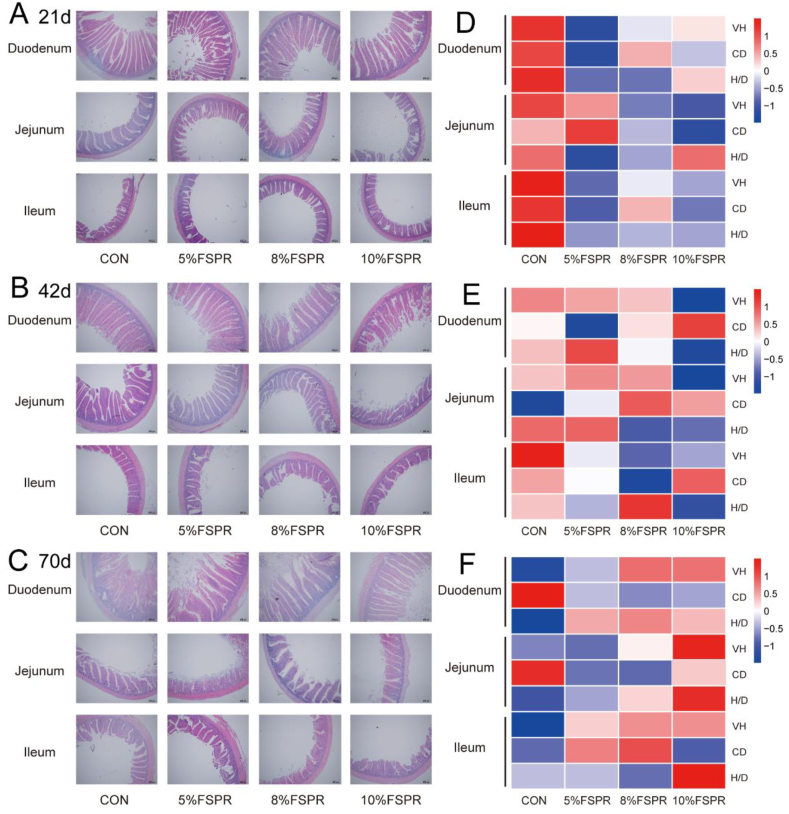
Feeding different levels of FSPR had no significant effect on the BW, ADG, ADFI, and F/G of the broilers; however, compared to the CON group, ADG was 8.93% higher and F/G was 12.71% lower in the 8% FSPR group during 42 to 70 d (Table 6). Morphological results (Fig. 2A–F) revealed that FSPR did not affect the intestinal morphology of broilers. Compared to the CON group, at 42 d of age, the CD of the ileum in the 8% FSPR group was reduced by 31.25% (P > 0.05). At 70 d of age, jejunal VH increased by 45.73% in the 10% FSPR group (P > 0.05); moreover, duodenal H/D increased by 34.15% and 29.64% (P = 0.096) and jejunal H/D increased by 28.27% (P > 0.05) and 51.77% (P = 0.066) in the 8% and 10% FSPR groups, respectively.
Table 6.
Effect of FSPR on growth performance of broilers.
| Item | CON | 5% FSPR | 8% FSPR | 10% FSPR | P-value | |
|---|---|---|---|---|---|---|
| BW, g | 1 d | 31.05 ± 0.09 | 30.89 ± 0.09 | 31.00 ± 0.10 | 30.96 ± 0.21 | 0.715 |
| 21 d | 302.83 ± 4.23 | 301.81 ± 8.45 | 304.20 ± 4.64 | 312.88 ± 7.51 | 0.615 | |
| 42 d | 930.20 ± 20.79 | 858.75 ± 43.36 | 899.27 ± 51.20 | 952.58 ± 31.66 | 0.367 | |
| 70 d | 1801.87 ± 74.41 | 1714.38 ± 108.30 | 1927.27 ± 41.39 | 1819.41 ± 72.59 | 0.419 | |
| ADG, g/d | 1–21 d | 12.94 ± 0.20 | 12.90 ± 0.40 | 13.01 ± 0.22 | 13.43 ± 0.36 | 0.613 |
| 21–42 d | 29.25 ± 1.00 | 26.02 ± 2.00 | 28.34 ± 2.25 | 30.46 ± 1.25 | 0.330 | |
| 42–70 d | 31.13 ± 2.07 | 29.73 ± 2.38 | 33.91 ± 1.18 | 30.96 ± 2.34 | 0.659 | |
| 1–70 d | 25.30 ± 1.06 | 24.05 ± 1.55 | 27.09 ± 0.59 | 25.55 ± 1.04 | 0.420 | |
| ADFI, g/d | 1–21 d | 28.37 ± 0.46 | 29.59 ± 0.63 | 30.02 ± 0.52 | 31.00 ± 1.03 | 0.095 |
| 21–42 d | 64.88 ± 3.46 | 57.94 ± 3.20 | 63.12 ± 3.34 | 64.14 ± 2.89 | 0.437 | |
| 42–70 d | 111.94 ± 6.39 | 105.13 ± 5.87 | 106.71 ± 7.30 | 104.08 ± 6.87 | 0.820 | |
| 1–70 d | 73.00 ± 2.55 | 68.50 ± 3.53 | 72.18 ± 3.34 | 70.17 ± 3.51 | 0.763 | |
| F/G | 1–21 d | 2.20 ± 0.04 | 2.31 ± 0.11 | 2.31 ± 0.06 | 2.32 ± 0.10 | 0.661 |
| 21–42 d | 2.23 ± 0.12 | 2.25 ± 0.08 | 2.26 ± 0.09 | 2.11 ± 0.06 | 0.626 | |
| 42–70 d | 3.62 ± 0.12 | 3.57 ± 0.10 | 3.16 ± 0.24 | 3.46 ± 0.34 | 0.586 | |
| 1–70 d | 2.90 ± 0.08 | 2.86 ± 0.04 | 2.67 ± 0.12 | 2.75 ± 0.12 | 0.378 |
FSPR = fermented sweet potato residue; BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; F/G = feed/gain ratio.
Data are presented as mean ± SEM (n = 7).
Fig. 2.
The effects of FSPR on growth performance and intestinal morphology of broilers. (A-C) Morphology of small intestinal villi in different groups; (D-F) Heatmap of villus height (VH), crypt depth (CD), and ratio of villus height to crypt depth (H/D) in different groups. FSPR = fermented sweet potato residue. Values are presented as mean ± SEM, n = 7.
3.3. Effect of FSPR on slaughter performance and meat quality of broilers
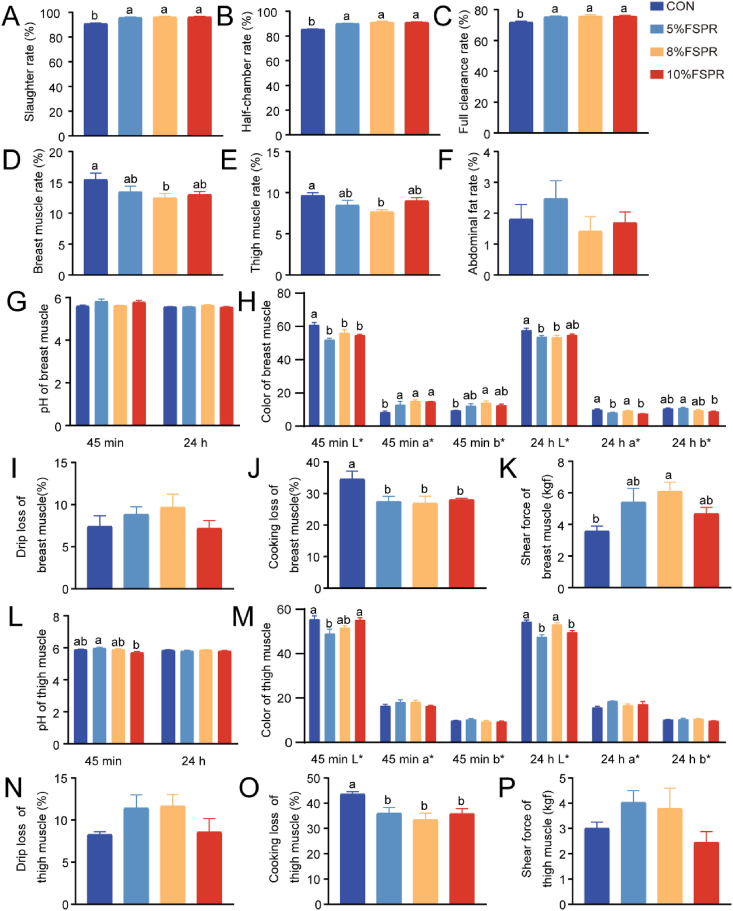
Compared with the CON group, FSPR supplementation significantly increased the slaughter, half-chamber, and full clearance rates of broilers in a dose-dependent manner (P < 0.05). Dietary FSPR supplementation at different levels reduced both breast and thigh muscle rates, but only 8% FSPR achieved a statistical difference (P < 0.05). In terms of meat quality, the pH of the breast muscle was lower than that of the thigh muscle at 45 min and 24 h in the CON group and at 24 h in the 10% FSPR group, whereas this was reversed at 45 min in the 10% FSPR group (Table S1). The addition of FSPR reduced 45 min L∗ and 24 h L∗ of the breast and thigh muscles in a dose-dependent manner (P < 0.05), and increased 45 min a∗ and 45 min b∗ of the breast muscles (P < 0.05). Additionally, the groups supplemented with different levels of FSPR showed a significant reduction in cooking loss of breast and thigh muscles compared to the CON group (P < 0.05), and all groups showed higher cooking loss in the thigh muscle than that in the breast muscle (P < 0.05). However, breast muscle shear force was higher in all FSPR groups than the CON group (Fig. 3K) (P < 0.05).
Fig. 3.
The effects of FSPR on slaughter performance and meat quality of broilers. (A) Slaughter rate (%); (B) Half-chamber rate (%); (C) Full clearance rate (%); (D) Breast muscle rate (%); (E) Thigh muscle rate (%); (F) Abdominal fat rate (%); (G-K) The pH, color, drip loss (%), cooking loss (%) and shear force (kg/f) of breast muscle; (L-P) The pH, color, drip loss (%), cooking loss (%) and shear force (kgf) of thigh muscle. FSPR = fermented sweet potato residue. Values are presented as mean ± SEM, n = 7. a, b Mean values with different small letter superscripts mean significant difference (P < 0.05).
3.4. Effects of FSPR on serum biochemical indexes of broilers
As given in Table 7, Table 8, Table 9, the effects of FSPR on the serum biochemical indices at the 3 stages were different. At the chick stage, the ALB content in the 5% FSPR group was significantly higher than the CON group (P < 0.05). The effect of FSPR on serum GLU levels improved with increasing FSPR substitution levels (P < 0.05). The TG, LDL, and HDL levels in the 5% and 10% FSPR groups were significantly higher than the CON group (P < 0.05). The addition of FSPR also sharply increased serum IgM levels (P < 0.05), especially in the 8% and 10% FSPR groups. In the growing stage, supplementation with fermented feed significantly increased the IgG content (P < 0.05). FSPR supplementation had no significant effect on the serum biochemical indices of broilers during the breeding stage (P > 0.05).
Table 7.
Effects of FSPR on serum biochemical indexes of 21 d old broilers.
| Item | CON | 5% FSPR | 8% FSPR | 10% FSPR | P-value |
|---|---|---|---|---|---|
| TP, g/L | 34.03 ± 2.29 | 42.17 ± 3.17 | 37.20 ± 2.28 | 35.20 ± 2.00 | 0.131 |
| ALB, g/L | 18.35 ± 0.64b | 20.43 ± 0.87a | 17.58 ± 0.38b | 17.98 ± 0.56b | 0.022 |
| ALT, U/L | 5.07 ± 0.31 | 5.68 ± 0.52 | 5.95 ± 0.54 | 5.17 ± 0.29 | 0.433 |
| AST, U/L | 207.67 ± 14.38 | 229.00 ± 11.13 | 212.00 ± 14.33 | 212.50 ± 10.18 | 0.693 |
| ALP, U/L | 4970.67 ± 1132.40 | 4039.17 ± 722.93 | 3048.17 ± 465.14 | 3614.00 ± 468.25 | 0.449 |
| BUN, mmol/L | 0.25 ± 0.02 | 0.23 ± 0.05 | 0.22 ± 0.02 | 0.25 ± 0.05 | 0.910 |
| GLU, mmol/L | 17.12 ± 0.71b | 18.76 ± 0.60b | 19.17 ± 0.55ab | 20.90 ± 0.76a | 0.006 |
| TG, mmol/L | 0.75 ± 0.09b | 1.12 ± 0.06a | 0.98 ± 0.13ab | 1.28 ± 0.12a | 0.014 |
| CHOL, mmol/L | 3.08 ± 0.13 | 3.48 ± 0.69 | 3.24 ± 0.24 | 4.41 ± 0.17 | 0.093 |
| LDL, mmol/L | 0.74 ± 0.04b | 1.06 ± 0.07a | 0.74 ± 0.09b | 1.17 ± 0.12a | 0.008 |
| HDL, mmol/L | 2.15 ± 0.06b | 2.65 ± 0.11a | 2.07 ± 0.12b | 2.74 ± 0.12a | <0.001 |
| IgG, g/L | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.107 |
| IgM, g/L | 0.02 ± 0.00c | 0.04 ± 0.00b | 0.08 ± 0.01a | 0.09 ± 0.00a | <0.001 |
FSPR = fermented sweet potato residue; TP = total protein; ALB = albumin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase; BUN = blood urea nitrogen; GLU = glucose; TG = triglyceride; CHOL = cholesterol; LDL = low-density lipoprotein; HDL = high-density lipoprotein; IgG = immunoglobulin G; IgM = immunoglobulin M.
Data are presented as mean ± SEM (n = 7).
a, b, c Mean values with different small letter superscripts mean significant difference (P < 0.05).
Table 8.
Effects of FSPR on serum biochemical indexes of 42 d old broilers.
| Item | CON | 5% FSPR | 8% FSPR | 10% FSPR | P-value |
|---|---|---|---|---|---|
| TP, g/L | 33.72 ± 2.56 | 35.43 ± 2.42 | 39.20 ± 2.96 | 34.03 ± 1.67 | 0.390 |
| ALB, g/L | 17.52 ± 1.78 | 18.33 ± 1.56 | 17.67 ± 1.61 | 15.18 ± 1.00 | 0.497 |
| ALT, U/L | 3.07 ± 0.57 | 3.02 ± 0.64 | 2.12 ± 0.47 | 3.28 ± 0.35 | 0.420 |
| AST, U/L | 183.50 ± 10.57 | 206.17 ± 20.47 | 221.83 ± 16.77 | 194.67 ± 9.34 | 0.337 |
| ALP, U/L | 1551.00 ± 173.33 | 1367.33 ± 80.65 | 1859.17 ± 408.04 | 1276.33 ± 155.04 | 0.510 |
| BUN, mmol/L | 0.18 ± 0.05 | 0.28 ± 0.12 | 0.33 ± 0.13 | 0.17 ± 0.03 | 0.566 |
| GLU, mmol/L | 15.67 ± 0.86 | 15.42 ± 0.54 | 14.90 ± 0.78 | 16.03 ± 0.88 | 0.772 |
| TG, mmol/L | 0.82 ± 0.09 | 0.55 ± 0.04 | 0.83 ± 0.13 | 0.97 ± 0.16 | 0.106 |
| CHOL, mmol/L | 3.34 ± 0.33 | 3.29 ± 0.40 | 3.43 ± 0.39 | 3.03 ± 0.33 | 0.880 |
| LDL, mmol/L | 0.78 ± 0.14 | 0.88 ± 0.26 | 1.22 ± 0.26 | 0.63 ± 0.07 | 0.229 |
| HDL, mmol/L | 2.16 ± 0.14 | 2.26 ± 0.20 | 2.04 ± 0.14 | 2.06 ± 0.17 | 0.767 |
| IgG, g/L | 0.04 ± 0.00b | 0.05 ± 0.00a | 0.05 ± 0.00a | 0.05 ± 0.00a | 0.004 |
| IgM, g/L | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.279 |
FSPR = fermented sweet potato residue; TP = total protein; ALB = albumin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase; BUN = blood urea nitrogen; GLU = glucose; TG = triglyceride; CHOL = cholesterol; LDL = low-density lipoprotein; HDL = high-density lipoprotein; IgG = immunoglobulin G; IgM = immunoglobulin M.
Data are presented as mean ± SEM (n = 7).
a, b Mean values with different small letter superscripts mean significant difference (P < 0.05).
Table 9.
Effects of FSPR on serum biochemical indexes of 70 d old broilers.
| Item | CON | 5% FSPR | 8% FSPR | 10% FSPR | P-value |
|---|---|---|---|---|---|
| TP, g/L | 36.02 ± 2.47 | 42.08 ± 2.43 | 37.75 ± 2.19 | 35.55 ± 3.47 | 0.325 |
| ALB, g/L | 17.50 ± 1.19 | 21.28 ± 1.96 | 19.27 ± 1.52 | 18.60 ± 1.71 | 0.427 |
| ALT, U/L | 3.25 ± 0.37 | 3.37 ± 0.57 | 3.47 ± 0.49 | 3.45 ± 0.26 | 0.984 |
| AST, U/L | 238.33 ± 15.37 | 272.00 ± 20.9 | 262.50 ± 20.21 | 217.50 ± 19.89 | 0.213 |
| ALP, U/L | 1117.83 ± 103.64 | 1184.17 ± 367.66 | 1315.17 ± 323.57 | 949.67 ± 31.34 | 0.777 |
| BUN, mmol/L | 0.33 ± 0.07 | 0.24 ± 0.05 | 0.16 ± 0.04 | 0.18 ± 0.09 | 0.300 |
| GLU, mmol/L | 13.80 ± 1.28 | 16.15 ± 0.51 | 16.33 ± 0.69 | 16.08 ± 0.85 | 0.169 |
| TG, mmol/L | 0.88 ± 0.10 | 0.95 ± 0.17 | 0.62 ± 0.11 | 0.65 ± 0.09 | 0.161 |
| CHOL, mmol/L | 3.31 ± 0.20 | 4.10 ± 0.25 | 3.49 ± 0.31 | 3.47 ± 0.39 | 0.288 |
| LDL, mmol/L | 1.21 ± 0.17 | 1.36 ± 0.24 | 1.11 ± 0.19 | 0.91 ± 0.20 | 0.454 |
| HDL, mmol/L | 1.86 ± 0.12 | 2.36 ± 0.17 | 2.20 ± 0.18 | 2.24 ± 0.17 | 0.182 |
| IgG, g/L | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.801 |
| IgM, g/L | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.09 ± 0.00 | 0.359 |
FSPR = fermented sweet potato residue; TP = total protein; ALB = albumin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase; BUN = blood urea nitrogen; GLU = glucose; TG = triglyceride; CHOL = cholesterol; LDL = low-density lipoprotein; HDL = high-density lipoprotein; IgG = immunoglobulin G; IgM = immunoglobulin M.
Data are presented as mean ± SEM (n = 7).
3.5. Effects of FSPR on intestinal flora composition of broilers
Based on the above results, the optimal supplemental level of FSPR to replace corn is 8% to 10%. Therefore, the CON and 10% FSPR groups were selected for intestinal microbial analysis. As shown in Fig. 4A, there was no significant difference in the ACE, Chao1, and Simpson indices between the 2 groups (P > 0.05), whereas the Shannon index in the 10% FSPR group was significantly higher than that in the CON group (P < 0.05). This indicated that the addition of FSPR could improve the diversity of the intestinal flora. Principal coordinate analysis (PCoA) based on the binary Jaccard distance revealed that the gut microbiota samples from the 10% FSPR group were clustered together and clearly separated from those from the CON group. The test statistic (R) for the analysis of similarity (ANOSIM) was 0.463 (P = 0.003), indicating that samples within groups were more similar to each other than those between groups. Based on the results of species annotation, the top 10 species were selected with the greatest abundance at the phylum (Fig. 4C) and genus levels (Fig. 4D) to generate pie charts of species relative abundance. The analysis showed that the bacteria at the phylum level were mainly Firmicutes, Bacteroidetes, and Proteobacteria. The relative abundance of Proteobacteria in the 10% FSPR group was significantly lower than that in the CON group (P < 0.05) (Fig. 4E). At the genus level, bacteria in the CON and 10% FSPR groups were almost completely different. Using linear discriminant analysis effect size (LEfSe) to identify statistically significant biomarkers in each group in relation to feed additives, 27 microorganisms were recorded and differed between the 2 groups at 5 taxonomic levels (Fig. 4K), indicating that feed treatment changed the gut microbial composition of the broilers. A rank-sum test of the top 30 biomarkers in terms of abundance at the genus level revealed that dietary 10% FSPR supplementation significantly increased the relative abundance of Ruminococcaceae_UCG-014, Ruminococcaceae_UCG-010, and Romboutsia (P < 0.05) (Fig. 4F–H), but significantly decreased the abundance of Megamonas and Sutterella (P < 0.05) (Fig. 4I and J). Analysis on the relationship between gut microbes and differential indicators in the 2 groups (Fig. 4L) showed that Ruminococcaceae_UCG-010 and Ruminococcaceae_UCG-014 were negatively correlated at 24 h L∗ and cooking loss in the thigh muscle and positively correlated with 45 min a∗ in the breast muscle (P < 0.05). This correlation was completely reversed in Megamonas, which also showed a highly significant positive correlation with cooking loss in the breast muscle (P < 0.01). Additionally, Romboutsia was negatively correlated with cooking loss in the thigh muscle (P < 0.01).
Fig. 4.
The effects of FSPR on intestinal flora composition of broilers. (A) ACE index, Chao1 index, Shannon index and Simpson index; (B) Principal coordinate analysis (PCoA) based on the binary Jaccard distance on samples; (C) Pie charts of the relative abundance of the top 10 species at the phylum level; (D) Pie charts of the relative abundance of the top 10 species at the genus level; (E) The relative abundance of Proteobacteria at the phylum; (F) The relative abundance of Ruminococcaceae_UCG-014 at the genus; (G) The relative abundance of Ruminococcaceae_UCG-010 at the genus; (H) The relative abundance of Romboutsia at the genus; (I) The relative abundance of Megamonas at the genus; (J) The relative abundance of Sutterella at the genus; (K) Linear discriminant analysis effect size (LEfSe) analysis of differential cecum microorganisms; (L) Heatmap of correlation between differential microorganisms and differential indicators. FSPR = fermented sweet potato residue. Values are presented as mean ± SEM, n = 6. ∗ Means significant difference (P < 0.05), ∗∗ means extremely significant difference (P < 0.01), and ns means no significance (P > 0.05).
4. Discussion
Recent studies on fermented feeds have concluded that, after fermentation, nutrition is often optimized and is better suited for use in feed than in raw materials (Khempaka et al., 2014; Nan et al., 2022; Sugiharto et al., 2015). In this study, the nutrient composition of SPR changed after fermentation, which mainly affected the CP and CF content. However, unlike most studies, the CP content of SPR after fermentation was lower than that before, possibly because the bacteria used in the fermentation process took advantage of the protein in the feed or metabolized it into small molecules, such as amino acids, small peptides, and bioactive compounds. Because the CP content in bran was much higher than that in the SPR raw material, the CP content increased gradually with a reduction in the proportion of SPR, as did the EE content. In the present study, FSPR increased the digestibility of certain nutrients, suggesting that FSPR may be conducive to reduce the content of toxins and anti-nutritional factors in the feed and then to improve the ability of nutrient uptake and absorption (Liu et al., 2021). The CP digestibility value calculated from the feeding SPR raw materials was negative. Negative protein digestibility, also known as negative nitrogen balance, may be due to the low CP and high CF contents of SPR, leading to insufficient protein intake by the broilers. FSPR feeding can change this adverse effect owing to the fermentation of microorganisms and the breakdown of digestive enzymes, such as amylase and cellulase (Alshelmani et al., 2021; Selle et al., 2009), resulting in better digestion and absorption by broilers. Lipases produced by microbial fermentation can also improve EE digestibility. In addition, the significantly increased amino acid digestibility indicated that feeding FSPR could improve the utilization of amino acids and better meet the nutritional requirements (Ding et al., 2022). Our study showed that the optimal proportion of FSPR was 70% because of its high comprehensive nutritional value and improved nutrient digestibility.
Previous studies have suggested that fermented feeds may have either an improved or an inhibiting effect on growth performance, depending on the proportion. For example, Nan et al. (2022) found that the addition of 2%, 4%, and 6% fermented grape seed meal to the diet increased ADG and reduced FCR of broilers. In contrast, another study showed that adding 3% and 6% fermented cottonseed meal had no effect on the growth performance of broilers but significantly reduced ADG and ADFI when administered at 9% (Niu et al., 2021). In the present study, the addition of 5%, 8%, and 10% FSPR to the feed had no significant effect on growth performance, suggesting 5% to 10% FSPR was a reasonable range of addition level for the growth of broiler chickens. This may be due to the differences in feed composition, level of addition, and specific fermentation methods. Furthermore, the growth performance of animals was affected by feed intake, digestive function, and state of intestinal health, and different fermented feeds have different effects on these factors, which ultimately affect growth performance. The integrity of intestinal morphology is crucial for reflecting the intestinal absorption capacity and developmental status of broilers. In the present study, dietary FSPR supplementation at different levels had no adverse effects on the intestinal morphology of broilers, and the replacement ratio of 8% to 10% improved the intestinal morphology to some extent. This improved effect may be due to the increased abundance of beneficial bacteria in the intestine, whose metabolites (lactic acid, succinic acid, short-chain fatty acids [SCFAs], etc.) provided an acidic environment, an increased source of energy for the gastrointestinal epithelium, and promote the development of the intestinal villi (Nicolas and Chang, 2019).
Slaughter performance is an important index that reflects the body composition of livestock, as well as the proportion of edible parts. Our results showed that dietary FSPR supplementation at different levels (5%, 8% and 10%) improved the slaughter and full clearance rates, indicating its potential to improve broiler production performance. This may be the result of amino acids, small peptides, and other fermentation products that enhance the absorption and deposition of nutrients by broilers. Additionally, it was found that broilers supplemented with FSPR showed varying degrees of reduction in breast and thigh muscle rates. This may be attributed to the low protein content in FSPR and inadequate protein intake of broilers, resulting in reduced protein deposition in the muscle. Studies have shown that diets containing yeast probiotics inhibit lipid synthesis and reduce fat deposition in broilers (Homma and Shinohara, 2004). In addition, small peptides produced during fermentation can inhibit fat absorption and promote lipid metabolism (Niu et al., 2021). Therefore, it was suggested that dietary supplementation at 8% and 10% FSPR may influence lipid metabolism through probiotics utilized by fermentation and the metabolites produced by fermentation, thereby reducing the abdominal fat rate. It has been reported that the pH of breast muscle is lower than that of thigh muscle because breast muscle consists of type IIB fibers with high glycogen content and has higher post-mortem lactic acid accumulation than thigh muscle (Ahmed et al., 2014). Our results showed that the pH of chicken breast muscle in the 10% FSPR group was slightly higher than that of the thigh muscle at 45 min after slaughter, and at 24 h this change returned to normal. This may be attributed to the addition of FSPR altering the fiber composition of the thigh muscle and promoting anaerobic glycolysis in the muscle, resulting in a rapid decrease in pH. Meat color directly reflects the appearance and quality of meat and is usually evaluated using L∗, a∗, and b∗. Fleming et al. (1991) found that the thigh muscle had higher myoglobin content and, therefore, higher a∗ values and lower b∗ values than the breast tissue of broilers, which was observed in this study with similar results. The results also showed that the addition of FSPR significantly improved the color quality of broilers and reduced cooking loss, suggesting that feeding FSPR can improve the myoglobin content, thermal stability, and water-holding capacity of broiler muscles, thereby improving meat quality. This improvement can be attributed to the presence of antioxidant substances, such as carotenoids and flavonoids in FSPR, which prevent lipid peroxidation and slow down the oxidation of myoglobin (Tang et al., 2020), thus improving meat color. In contrast to previous results (Panpipat et al., 2022), our results showed higher cooking loss and lower shear force in the thigh muscle than in the breast muscle for either treatment, which may be explained by differences in the water-binding capacity and collagen content in the muscles due to differences in the breed and age of the chickens.
Serum biochemical indicators can directly reflect the nutritional and metabolic functions and health status of the body. The study showed that the effect of FSPR on serum biochemical parameters in broilers was mainly at the chick stage, probably because young broilers are still incompletely developed, and their health status is more susceptible to the influence of feed (Jiang et al., 2020). Addition of 5% FSPR significantly increased the ALB content, which was consistent with the effect of fermented cottonseed meal on the serum ALB content of yellow-feathered broilers (Zhang et al., 2016). Elevated serum ALB levels indicate strong protein metabolism, as well as increased absorption and utilization of amino acids and proteins. Glucose, the main source of energy in animals, is broken down to produce energy for normal body metabolism. Our results demonstrate that dietary FSPR supplementation at different levels could increase serum GLU content owing to its rich starch content, which could be converted into GLU to meet energy requirements more adequately. Serum TG, CHOL, HDL, and LDL levels are important indicators of lipid metabolism in animals, and their levels were higher in the groups supplemented with 5% and 8% FSPR than those in the other groups. Increased HDL and LDL levels indicate vigorous lipid metabolism and increased cholesterol transport efficiency. Cholesterol is an important component of biological membranes and a precursor for the synthesis of bile acids and various hormones, and its increased levels can maintain the normal growth and metabolism of the organism. Triglyceride is an important form of energy storage in the body, mainly synthesized by the liver, and its increased content indicates that dietary FSPR supplementation can improve the lipid synthesis capacity of the liver and store more energy in broilers. Our study also found that the addition of 8% to 10% FSPR obtained results consistent with those reported in other studies (Tang et al., 2012; Xu et al., 2012), which was to increase the levels of IgM and IgG in the serum, suggesting an improvement in immune function in broilers. Studies have reported that an increase in immunoglobulins may be associated with the formation of small peptides during fermentation (Xu et al., 2012), improvement of gut bacteria (Missotten et al., 2013) and increases in SCFAs (Canibe and Jensen, 2003). This was further confirmed by our results, which showed that FSPR improved nutrient digestibility and increased the abundance of SCFA-producing bacteria in the gut.
The complex microbial composition of the chicken gastrointestinal tract is of great significance to chicken health and production performance, and the stability of the bacterial community can maintain the digestion and absorption of nutrients and immune defense. Many studies have shown that the addition of fermented feed affects the intestinal microbial composition of chickens, and this effect varies with feed type (Kim and Kang, 2016; Loh et al., 2007; Missotten et al., 2013). The results of the alpha and beta diversity analyses showed that fermentation significantly increased the intestinal species diversity of broilers, with significant differences in composition. This may be attributed to the addition of probiotics to the fermented feed that regulates the balance of intestinal microbes and enriches their composition of intestinal bacteria. For example, Lactobacillus plantarum and Bacillus licheniformis, which are used in fermentation, have been reported to alter the composition of intestinal microorganisms (Hang et al., 2022; Pan et al., 2022). Proteobacteria have been reported to increase in abundance as a characteristic of a disturbed gut microbiota, and most Proteobacteria are facultative anaerobes that can utilize SCFAs to compete with the host for nutrients. In the present study, dietary 10% FSPR supplementation decreased Proteobacteria, suggesting its effect in improving intestinal health. At the genus level, our results showed that dietary supplementation with 10% FSPR enriched Ruminococcaceae_UCG-014, Ruminococcaceae_UCG-010 and Romboutsia, whereas the abundances of Sutterella and Megamonas were reduced. Ruminococcaceae is a representative member of Firmicutes (Whelan et al., 2019) and its relative abundance increased in line with that of Firmicutes. Studies have shown that Ruminococcaceae_UCG-010 and Ruminococcaceae_UCG-014 can promote the use of nutrients by degrading fibers (Ma et al., 2022) and producing SCFAs (Zhuge et al., 2022) to provide energy for the epithelial cells, promote lipolysis in the body, and also reduce fat deposition. Romboutsia is a bacterium capable of using mono- and disaccharides to produce SCFAs, and is commonly found in the mucosa of healthy people (Song et al., 2022). Sutterella has been reported to have the ability to degrade IgA, potentially impairing the intestinal antimicrobial immune response (Kaakoush, 2020). Megamonas was found to be enriched in the gut of vaccine recipients with fewer adverse events following COVID-19 vaccination, suggesting that it may play an anti-inflammatory role in the host immune response (Ng et al., 2022). However, Megamonas was also found to be significantly increased in the gut of obese individuals (Chiu et al., 2014); therefore, we cannot draw conclusions regarding its role. These findings suggest that 10% FSPR can improve the gut microbiota by increasing the abundance of SCFA-producing bacteria and reducing the presence of harmful bacteria. Furthermore, the positive effects of Ruminococcaceae on meat quality may be mediated by amino acids (e.g., arginine, isoleucine, etc.). Some studies have shown a strong positive correlation between Ruminococcaceae and the biosynthesis of most amino acids (Kim et al., 2023), and that amino acids can reduce cooking loss and improve meat color by increasing the antioxidant status of chicken meat (Hu et al., 2020; Zeitz et al., 2020). Megamonas was found to be negatively correlated with flavonoids (Feng et al., 2019); therefore, we speculate that Megamonas may affect flesh color by reducing flavonoids and inhibiting their antioxidant capacity. This further suggests that FSPR may improve meat quality by regulating the intestinal flora.
5. Conclusions
The results from the study showed that 70% SPR (after fermentation) had the highest nutritional value and nutrient digestibility. Dietary FSPR supplementation at different levels had no significant effect on growth performance or intestinal morphology; however, dietary 8% to 10% FSPR supplementation improved slaughter performance, meat quality, and immunity in broilers. Moreover, the administration of 10% FSPR remarkably regulated the composition of intestinal microbes. Hence, the recommended level of FSPR supplementation to replace corn in the broiler chicken diets was 8% to 10%. This was the first report demonstrating the beneficial effects of FSPR in broilers, suggesting that FSPR, as an unconventional feed, has the potential to be used as a substitute for corn to improve meat quality and intestinal health. However, the molecular mechanisms underlying the regulation of meat quality and gut microbiota profiles by FSPR remain unclear.
Author contributions
Ting Yao: formal analysis, methodology, visualization, and writing - original draft; Chenyu Wang: methodology and investigation; Lifen Liang: methodology and investigation; Xuan Xiang: methodology; Hui Zhou: methodology; Wentao Zhou: methodology; Ruoxin Hou: methodology; Tianli Wang: visualization; Liuqin He: visualization and writing - review & editing; Shiyu Bin: project administration, supervision; Yulong Yin: project administration, supervision, and writing - review & editing; Tiejun Li: project administration, supervision, and writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Key R&D Program (2022YFD1300905), Guangxi Natural Science Foundation Project (2022JJA130333), Hunan science and technology innovation leading Talent Support Program (2023RC1054), Shandong Province Taishan Industry Leading Talents Project Blue Talents Project, China Agriculture Research System of MOF and MARA (CARS-35), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-038).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2024.03.007.
Contributor Information
Liuqin He, Email: heliuqin@hunnu.edu.cn.
Tiejun Li, Email: tjli@isa.ac.cn.
Appendix. Supplementary data
The following is the Supplementary data to this article.
References
- Ahmed S.T., Mun H.S., Islam M.M., Kim S.S., Hwang J.A., Kim Y.J., Yang C.J. British Poultry science. 2014. Effects of citrus junos by-products fermented with multistrain probiotics on growth performance, immunity, caecal microbiology and meat oxidative stability in broilers; pp. 540–547. [DOI] [PubMed] [Google Scholar]
- Alshelmani M.I., Kaka U., Abdalla E.A., Humam A.M., Zamani H.U. Effect of feeding fermented and non-fermented palm kernel cake on the performance of broiler chickens: a review. World Poultry Sci J. 2021;77:377–388. [Google Scholar]
- Arachchige M.P.M., Mu T.H., Ma M.M. Structural, physicochemical and emulsifying properties of sweet potato pectin treated by high hydrostatic pressure and/or pectinase: a comparative study. J Sci Food Agric. 2020;100:4911–4920. doi: 10.1002/jsfa.10552. [DOI] [PubMed] [Google Scholar]
- Canibe N., Jensen B.B. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J Anim Sci. 2003;81(8):2019–2031. doi: 10.2527/2003.8182019x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Liu G. Research progress in development and utilization of poultry feed resources. Chin J Animal Nutr. 2020;32:4646–4658. [Google Scholar]
- China Agricultural Industry Standard . China Agriculture Press; Beijing: 2020. Performance terminology and measurements for poultry No. NY/T 823-2020. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2006. Determination of crude fat in feeds No. GB/T 6433-2006. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2007. Animal feeding stuffs - determination of crude ash No. GB/T 6438-2007. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2011. Feed efficacy and safety evaluation - guidelines for the determination of apparent metabolizable energy for chickens by the force feeding method No. GB/T 26437-2010. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2014. Determination of moisture in feedstuffs No. GB/T 6435-2014. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2018. Determination of crude protein in feeds—Kjeldahl method No. GB/T 6432-2018. [Google Scholar]
- China National Standard . Standards Press of China; Beijing: 2022. Determination of crude fiber content in feeds No. GB/T 6434-2022. [Google Scholar]
- Chiu C.M., Huang W.C., Weng S.L., Tseng H.C., Liang C., Wang W.C., Yang T., Yang T.L., Weng C.T., Chang T.H., Huang H.D. Systematic analysis of the association between gut flora and obesity through high-throughput sequencing and bioinformatics approaches. BioMed Res Int. 2014;2014 doi: 10.1155/2014/906168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., He L., Li T., Yin Y. Research progress on the function of the amino acid transporter slc1a3 and its regulation mechanism of action in the nervous system and mitochondria. Chin Sci Bull. 2022;67:3005–3013. [Google Scholar]
- Feed database in China . 2018. Tables of feed composition and nutritive values in China.https://www.chinafeeddata.org.cn/admin/Login/slcfb (in Chinese) [Google Scholar]
- Feng J., Zhao F., Sun J., Lin B., Zhao L., Liu Y., Jin Y., Li S., Li A., Wei Y. Alterations in the gut microbiota and metabolite profiles of thyroid carcinoma patients. Int J Cancer. 2019;144:2728–2745. doi: 10.1002/ijc.32007. [DOI] [PubMed] [Google Scholar]
- Fleming B.K., Froning G.W., Yang T.S. Heme pigment levels in chicken broilers chilled in ice slush and air1. Poultry Sci. 1991;70:2197–2200. [Google Scholar]
- Gao J., Zhang H.J., Wu S.G., Yu S.H., Yoon I., Moore D., Gao Y.P., Yan H.J., Qi G.H. Effect of saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with eimeria tenella. Poultry Sci. 2009;88:2141–2151. doi: 10.3382/ps.2009-00151. [DOI] [PubMed] [Google Scholar]
- Hang S., Zeng L., Han J., Zhang Z., Zhou Q., Meng X., Gu Q., Li P. Lactobacillus plantarum zj316 improves the quality of stachys sieboldii miq. Pickle by inhibiting harmful bacteria growth, degrading nitrite and promoting the gut microbiota health in vitro. Food Funct. 2022;13:1551–1562. doi: 10.1039/d1fo03025f. [DOI] [PubMed] [Google Scholar]
- He L., Huang N., Li H., Tian J., Zhou X., Li T., Yao K., Wu G., Yin Y. Ampk/α-ketoglutarate axis regulates intestinal water and ion homeostasis in young pigs. J Agric Food Chem. 2017;65:2287–2298. doi: 10.1021/acs.jafc.7b00324. [DOI] [PubMed] [Google Scholar]
- Homma H., Shinohara T. Effects of probiotic bacillus cereus toyoi on abdominal fat accumulation in the Japanese quail (coturnix japonica) Anim Sci J. 2004;75:37–41. [Google Scholar]
- Hu H., Chen L., Dai S., Li J., Bai X. Effect of glutamine on antioxidant capacity and lipid peroxidation in the breast muscle of heat-stressed broilers via antioxidant genes and hsp70 pathway. Animals. 2020;10 doi: 10.3390/ani10030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Li L., Gou Z., Wang Y., Lin X., Jiang Z. Research progress of nutrient requirements for yellow-feathered chickens. Chin J Animal Nutr. 2020;32:4577–4591. [Google Scholar]
- Kaakoush N.O. Sutterella species, iga-degrading bacteria in ulcerative colitis. Trends Microbiol. 2020;28:519–522. doi: 10.1016/j.tim.2020.02.018. [DOI] [PubMed] [Google Scholar]
- Khempaka S., Thongkratok R., Okrathok S., Molee W. An evaluation of cassava pulp feedstuff fermented with a. Oryzae, on growth performance, nutrient digestibility and carcass quality of broilers. J Poultry Sci. 2014;51:71–79. [Google Scholar]
- Kim C.H., Kang H.K. Effects of fermented barley or wheat as feed supplement on growth performance, gut health and meat quality of broilers. Eur Poult Sci. 2016;80 [Google Scholar]
- Kim J.H., Ku B.H., Ko G.P., Kang M.J., Son K.H., Bang M.A., Park H.Y. Enzyme feed additive with arazyme improve growth performance, meat quality, and gut microbiome of pigs. Animals. 2023;13 doi: 10.3390/ani13030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C., Adeola O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas J Anim Sci. 2014;27:917–925. doi: 10.5713/ajas.2014.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Sun T., Li T., Bin S., Hou Z. Effects of fermented siraitia grosvenorii residue on growth performance, serum biochemical indexes and meat quality of yellow-feathered broilers. Chin J Animal Nutr. 2022;34:6514–6526. [Google Scholar]
- Liu M., Li X., Zhou S., Wang T.T.Y., Zhou S., Yang K., Li Y., Tian J., Wang J. Dietary fiber isolated from sweet potato residues promotes a healthy gut microbiome profile. Food Funct. 2020;11:689–699. doi: 10.1039/c9fo01009b. [DOI] [PubMed] [Google Scholar]
- Liu Y., Feng J., Wang Y., Lv J., Li J., Guo L., Min Y. Fermented corn-soybean meal mixed feed modulates intestinal morphology, barrier functions and cecal microbiota in laying hens. Animals. 2021;11 doi: 10.3390/ani11113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh T.C., Law F.L., Foo H.L., Goh Y.M., Zulkifli I. Effects of feeding a fermented product on egg production, faecal microflora and faecal ph in laying hens. J Anim Feed Sci. 2007;16:452–462. [Google Scholar]
- Ma Y., Deng X., Yang X., Wang J., Li T., Hua G., Han D., Da L., Li R., Rong W., Deng X. Characteristics of bacterial microbiota in different intestinal segments of aohan fine-wool sheep. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.874536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missotten J.A., Michiels J., Dierick N., Ovyn A., Akbarian A., De Smet S. Effect of fermented moist feed on performance, gut bacteria and gut histo-morphology in broilers. Br Poultry Sci. 2013;54:627–634. doi: 10.1080/00071668.2013.811718. [DOI] [PubMed] [Google Scholar]
- Nan S., Yao M., Zhang X., Wang H., Li J., Niu J., Chen C., Zhang W., Nie C. Fermented grape seed meal promotes broiler growth and reduces abdominal fat deposition through intestinal microorganisms. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.994033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.C., Peng Y., Zhang L., Mok C.K., Zhao S., Li A., Ching J.Y., Liu Y., Yan S., Chan D.L.S., Zhu J., Chen C., Fung A.C., Wong K.K., Hui D.S., Chan F.K., Tun H.M. Gut microbiota composition is associated with sars-cov-2 vaccine immunogenicity and adverse events. Gut. 2022;71:1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G.R., Chang P.V. Deciphering the chemical lexicon of host–gut microbiota interactions. Trends Pharmacol Sci. 2019;40:430–445. doi: 10.1016/j.tips.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J.L., Wei L.Q., Luo Y.Q., Yang W.T., Lu Q.C., Zheng X.X., Niu Y.J., Sheng W., Cheng H., Zhang W.J., Nie C.X. Fermented cottonseed meal improves production performance and reduces fat deposition in broiler chickens. Anim Biosci. 2021;34:680–691. doi: 10.5713/ajas.20.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke C.A., Ezekiel C.N., Nwangburuka C.C., Sulyok M., Ezeamagu C.O., Adeleke R.A., Dike S.K., Krska R. Bacterial diversity and mycotoxin reduction during maize fermentation (steeping) for ogi production. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Cai Y.L., Kong L.L., Xiao C.P., Zhu Q.D., Song Z.G. Probiotic effects of bacillus licheniformis dsm5749 on growth performance and intestinal microecological balance of laying hens. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.868093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panpipat W., Chaijan M., Karnjanapratum S., Keawtong P., Tansakul P., Panya A., Phonsatta N., Aoumtes K., Quan T.H., Petcharat T. Quality characterization of different parts of broiler and ligor hybrid chickens. Foods. 2022;11 doi: 10.3390/foods11131929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.C., Naskar S.K., Tomlins K.I. 2010. Bio-processing of sweet potato into food, feed and bio-ethanol. [Google Scholar]
- Selle P.H., Ravindran V., Partridge G.G. Beneficial effects of xylanase and/or phytase inclusions on ileal amino acid digestibility, energy utilisation, mineral retention and growth performance in wheat-based broiler diets. Anim Feed Sci Technol. 2009;153:303–313. [Google Scholar]
- Song B., Li P., Yan S., Liu Y., Gao M., Lv H., Lv Z., Guo Y. Effects of dietary astragalus polysaccharide supplementation on the th17/treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.781934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q.H., Meng Q.X., Wu H., Yi S.M., Zhou Z.M. Evaluation and comparative analysis of the nutritional value of potato pulp and sweet potato pulp in part of northern China. China Animal Husbandry & Veterinary Medicine. 2021;48:1222–1228. [Google Scholar]
- Sugiharto S., Ranjitkar S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: a review. Animal Nutrition. 2019;5:1–10. doi: 10.1016/j.aninu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I. Functional properties of filamentous fungi isolated from the Indonesian fermented dried cassava, with particular application on poultry. Mycobiology. 2015;43:415–422. doi: 10.5941/MYCO.2015.43.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Sun H., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Australas J Anim Sci. 2012;25:393–400. doi: 10.5713/ajas.2011.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Wu J., Jin S., He L., Lin Q., Luo F., He X., Feng Y., He B., Bing P., Li T., Yin Y. Glutamate and aspartate alleviate testicular/epididymal oxidative stress by supporting antioxidant enzymes and immune defense systems in boars. Sci China Life Sci. 2020;63:116–124. doi: 10.1007/s11427-018-9492-8. [DOI] [PubMed] [Google Scholar]
- Wang T.Y., Wu Y.H., Jiang C.Y., Liu Y. Solid state fermented potato pulp can be used as poultry feed. Br Poult Sci. 2010;51:229–234. doi: 10.1080/00071661003781864. [DOI] [PubMed] [Google Scholar]
- Whelan R.A., Doranalli K., Rinttila T., Vienola K., Jurgens G., Apajalahti J. The impact of bacillus subtilis dsm 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poultry Sci. 2019;98:3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.Z., Zeng X.G., Ding X.L. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian Australas J Anim Sci. 2012;25:1734–1741. doi: 10.5713/ajas.2012.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz J.O., Fleischmann A., Ehbrecht T., Most E., Friedrichs S., Whelan R., Gessner D.K., Failing K., Lütjohann D., Eder K. Effects of supplementation of dl-methionine on tissue and plasma antioxidant status during heat-induced oxidative stress in broilers. Poultry Sci. 2020;99:6837–6847. doi: 10.1016/j.psj.2020.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Zhang W., Liu J., Yang L. Effects of cottonseed meal fermented by lactobacillus acidophilus on growth performance, slaughter performance and biochemical indexes in serum of yellow-feathered broilers. Chin J Animal Nutr. 2016;28:3885–3893. [Google Scholar]
- Zhao H., Wang X., Tang J., Tang X., Jia G., Liu G., Chen X., Long D., Wang K. Nutritional improvement of sweet potato residue by solid-state fermentation with mixed microbe strains. Chin J Animal Nutr. 2015;27:1191–1198. [Google Scholar]
- Zhuge A., Li S., Lou P., Wu W., Wang K., Yuan Y., Xia J., Li B., Li L. Longitudinal 16s rrna sequencing reveals relationships among alterations of gut microbiota and nonalcoholic fatty liver disease progression in mice. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.00047-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.