Significance
The multinucleated architecture of muscle presents unique challenges for AAV-delivered CRISPR/Cas-based gene editing strategies. Because only a small subset of myonuclei are transduced by AAV, and Cas proteins traffic poorly between myonuclear domains, gene editing rates in this tissue have been extremely low. We developed a modular, compact peptide tag that when appended to Cas9 improves propagation across myonuclear domains and enhances gene editing efficiencies. Our strategy is applicable to all nuclear-targeted gene therapy cargoes and highlights the importance of considering the spatial dimension of gene regulation in the context of therapeutic strategies.
Keywords: gene therapy, CRISPR, myonuclear domains, spatial gene regulation, adeno-associated virus
Abstract
Successful CRISPR/Cas9-based gene editing in skeletal muscle is dependent on efficient propagation of Cas9 to all myonuclei in the myofiber. However, nuclear-targeted gene therapy cargos are strongly restricted to their myonuclear domain of origin. By screening nuclear localization signals and nuclear export signals, we identify “Myospreader,” a combination of short peptide sequences that promotes myonuclear propagation. Appending Myospreader to Cas9 enhances protein stability and myonuclear propagation in myoblasts and myofibers. AAV-delivered Myospreader dCas9 better inhibits transcription of toxic RNA in a myotonic dystrophy mouse model. Furthermore, Myospreader Cas9 achieves higher rates of gene editing in CRISPR reporter and Duchenne muscular dystrophy mouse models. Myospreader reveals design principles relevant to all nuclear-targeted gene therapies and highlights the importance of the spatial dimension in therapeutic development.
The application of adeno-associated virus (AAV) to genetic diseases has been moving into clinical practice. However, AAV therapies for muscle face unique challenges due to the syncytial architecture of myofibers. Although a single cytoplasm is shared by hundreds to thousands of myonuclei, gene products remain local to each nucleus by mechanisms of cytoskeletal-dependent RNA transport, protein translation, and protein trafficking (1–4). These principles apply not only to endogenous gene products but also to exogenous therapeutic payloads delivered by AAV. For example, some cytoplasmic proteins readily travel across multiple myonuclear domains, but others are more restricted due to sarcomeric incorporation (5). Given dose-limiting toxicities of AAV, particularly to the liver (6), an important goal is to maximize transgene output per vector genome. While impressive improvements have been made to myotropic capsids (7), another important consideration is to maximize the effective myonuclear domain size of therapeutic transgenes by spreading them throughout the myofiber.
Transduction of a limited number of myonuclei effectively yields chimeric myofibers in which some nuclei express exogenous cargo that must then propagate throughout the syncytium. In particular, CRISPR/Cas-based approaches, which have been applied to many muscle diseases (8–12), must ideally enter as many nuclei as possible. To facilitate nuclear entry, Staphylococcus aureus and Streptococcus pyogenes Cas9 proteins are often tagged with dual nuclear localization sequence (NLS) sequences (13). However, the propagation of nuclear proteins in myofibers is inversely correlated to protein size (14, 15), and each of these proteins is relatively large (127 kDa and 162 kDa, respectively) (16, 17). Indeed, AAV-delivered deactivated Cas9 is restricted to a small number of myonuclei around the transduced myonucleus (12). As a result, gene editing with CRISPR/Cas9 restores dystrophin in a limited number of myonuclear domains (5, 18) and has generally depended on large doses of AAV to achieve low rates of productive editing (8, 9, 19–23).
Numerous endogenous transcription factors and RNA binding proteins use a combination of NLS and nuclear export signals (NESs) to facilitate nucleo-cytoplasmic shuttling (24–26). Here, we test the hypothesis that bidirectional nuclear-cytoplasmic transport can broaden the myonuclear propagation profile of Cas9, resulting in overall improved editing efficiency. We screened combinations of NLS, NES, and other elements to promote propagation and localization to as many nuclei as possible in a syncytial environment. We identify one combination, “Myospreader,” that promotes Cas9 myonuclear propagation across myofibers, enhances protein stability, and increases muscle gene editing efficiency in vivo. This concept is applicable to any gene therapy cargo designed to target myonuclei in the context of sparse delivery and highlights the importance of considering the spatial dimension in gene regulation, editing, and therapy.
Combining Nuclear Import and Export Signals Promotes Protein Shuttling to a Greater Number of Myonuclei in Myotubes and Myofibers
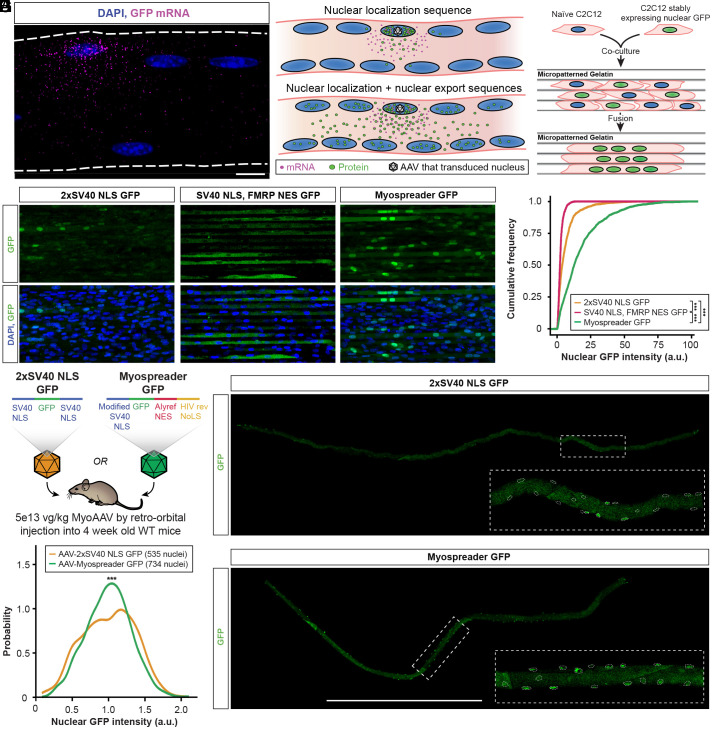
Multiple observations suggest that AAV gene therapies encoding nuclear-targeted cargoes are restricted to transduced myonuclei following mRNA export, translation, and subsequent reimport of the encoded protein (5, 12, 18). Consistent with this, we observed that approximately 10% of myonuclei in WT C57BL/6 mice are functionally transduced by AAV packaged with a GFP transgene (SI Appendix, Fig. S1 A and C), and exogenous mRNAs originating from these myonuclei are restricted to their respective myonuclear domains (Fig. 1A). Inspired by nucleocytoplasmic shuttling of endogenous proteins (24–26), we hypothesized that appending both a NLS and NES onto cargoes may permit escape from AAV transduced nuclei and facilitate propagation to distant myonuclei (Fig. 1B). Using GFP as a reporter of protein localization, we screened multiple NLS/NES combinations (SI Appendix, Table S1) (27) by establishing stable C2C12 myoblasts expressing each reporter and fusing them to naïve C2C12 myoblasts on micropatterned gelatin (28). Chimeric myotubes were then imaged to assess GFP signal in myonuclei (Fig. 1C). The balance of NLS and NES strengths influenced the overall localization pattern and propagation of GFP (Fig. 1D and SI Appendix, Fig. S1D), where stronger overall export behavior resulted in predominantly cytoplasmic localization, while stronger overall import behavior yielded nuclear localization of GFP but a limited number of GFP-positive nuclei. The best candidate, which showed significantly more nuclear and overall GFP signal than all others (Fig. 1E and SI Appendix, Fig. S1 E and F), encoded an attenuated SV40 NLS on the N terminus and both an Alyref NES and HIV rev nucleolar localization signal (NoLS) on the C terminus; we termed this combination Myospreader.
Fig. 1.
Nuclear import and export sequence combinations enhance myonuclear GFP trafficking in myotubes and myofibers. (A) HCR-FISH against GFP mRNA (magenta) in a tibialis anterior (TA) myofiber of a mouse treated with an AAV encoding GFP. (Scale bar: 10 µm.) (B) Model for why protein cargoes with nuclear localization sequences accumulate in certain nuclei and not others. Peptide tags that facilitate both nuclear import and export may improve trafficking of protein cargoes to nontransduced nuclei. (C) Schematic of the C2C12 fusion experiment to identify NLS/NES combinations that improve propagation of GFP across multiple myonuclei. (D) Representative images of GFP fluorescence (green) in chimeric C2C12 myotubes expressing NLS/NES combinations. (Scale bar: 40 µm.) (E) Cumulative distribution functions of nuclear GFP signal for NLS/NES combinations in chimeric C2C12 myotubes. Significance by Kolmogorov–Smirnov test. (F) Schematic of the experiment to assess nuclear propagation of GFP in 4-wk-old WT mice treated systemically with 5E+13vg/kg 2xSV40 NLS GFP AAV or Myospreader GFP AAV. (G) GFP signal (green) in representative TA myofibers isolated from treated mice. Myonuclei borders are indicated with dashed lines. (Scale bar: 1 mm.) (H) Density plot of nuclear GFP signal in TA myofibers of treated mice. Significance by Kolmogorov–Smirnov test (ns = not significant; *P < 0.05; **P < 0.01; ***P < 0.001) (error bars = 95% CI).
To assess the performance of Myospreader in vivo, we packaged CBH promoter-driven 2xSV40 NLS GFP or Myospreader GFP using MyoAAV (7) and systemically administered 5e13 vg/kg virus to 4-wk-old C57BL/6 mice. After 4 wk, TA myofibers were isolated and imaged (Fig. 1F). Myospreader GFP displayed much more uniform localization of GFP across myonuclei of myofibers, in contrast to the patchy, infrequent nuclear GFP signal with the 2xSV40 NLS GFP construct (Fig. 1G). Indeed, the distribution GFP intensity of myonuclei, normalized by average GFP signal per myofiber, showed a more uniform distribution across myonuclei as compared to 2xSV40 NLS GFP (Fig. 1H).
Adding Myospreader to Cas9 Enhances Protein Stability and Localization Patterns in Myoblasts and Myofibers.
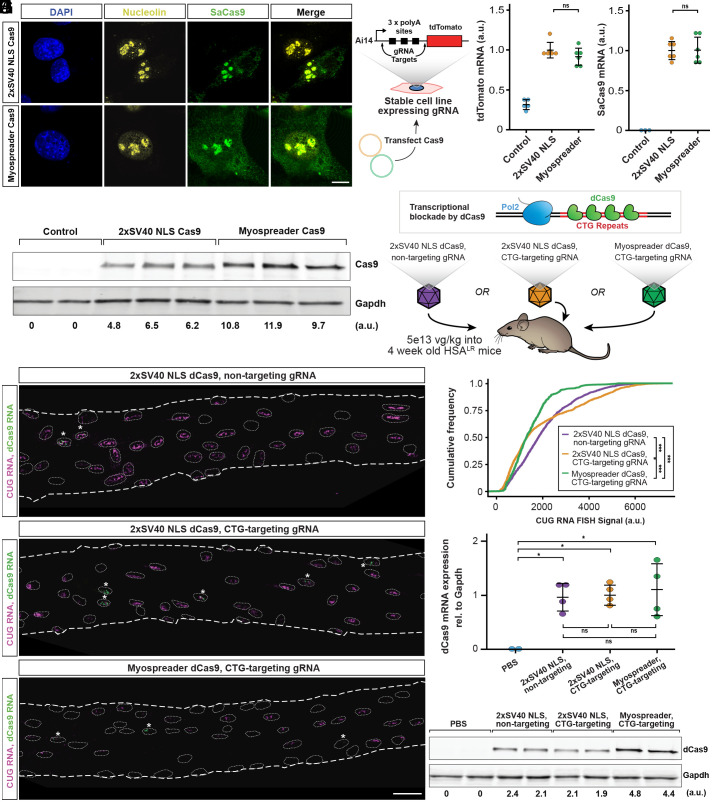
Next, we appended the Myospreader NLS/NES tags to S. aureus Cas9 (SaCas9) and assayed its localization pattern in C2C12 myoblasts. Similar to 2xSV40 NLS Cas9, Myospreader Cas9 maintains nucleolar enrichment, a pattern also observed with other Cas9 variants (29). However, Myospreader Cas9 also showed some cytoplasmic localization (Fig. 2A). To confirm that Myospreader does not reduce Cas9 editing efficiency or stability in mononucleated cells, we transiently transfected both constructs into a C2C12 myoblast line that stably expresses the Ai14 reporter (30) and gRNAs that target the 3 polyadenylation sites upstream of tdTomato; successful excision of this region with a double cut and nonhomologous end joining results in increased tdTomato mRNA and protein expression (Fig. 2B). Both 2xSV40 NLS Cas9 and Myospreader Cas9 showed similar tdTomato and Cas9 mRNA expression levels as assessed by RT-qPCR (Fig. 2 C and D), but Myospreader Cas9 showed greater relative levels of Cas9 protein, as assessed by western blot. This suggests that Myospreader tags also enhance the stability of the protein (Fig. 2E).
Fig. 2.
Myospreader improves stability and localization of Cas9/dCas9 in myoblasts and myofibers. (A) Representative IF images of C2C12 myoblasts transfected with plasmids encoding 2xSV40 Cas9 or Myospreader Cas9. SaCas9 is shown in green, nucleolin in yellow, and DAPI in blue. (Scale bars: 10 µm.) (B) Schematic of the experiment to assess Myospreader Cas9 stability and editing efficiency in C2C12 myoblasts. (C) tdTomato mRNA expression as assessed by RT-qPCR in AI14 reporter C2C12s following transfection with plasmids encoding 2xSV40 Cas9 or Myospreader Cas9. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (D) Cas9 mRNA expression as assessed by RT-qPCR in AI14 reporter C2C12s following transfection with plasmids encoding 2xSV40 Cas9 or Myospreader Cas9. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (E) Western blot against Cas9 and Gapdh in Ai14 reporter C2C12s following transfection with plasmids encoding 2xSV40 Cas9 or Myospreader Cas9. Relative signal intensity determined by densitometry at the bottom. A.U.: arbitrary unit, normalized to Gapdh. (F) Schematic of dCas9 impeding transcription of toxic CUG RNA and experiment. Each treatment was packaged in a single AAV vector systemically delivered to 4-wk-old HSALR mice at a dose of 5E+13 vg/kg. (G) HCR-FISH to detect CUG RNA foci (magenta) and dCas9 mRNA (green) in myonuclei of representative TA myofibers isolated from treated HSALR mice. Myonuclei borders are indicated with dashed lines. Asterisks indicate dCas9-positive myonuclei. (Scale bar: 30 µm.) (H) Cumulative distribution functions of HCR-FISH CUG RNA signal in myonuclei proximal to dCas9-expressing myonuclei in myofibers isolated from treated HSALR mice. Significance by Kolmogorov–Smirnov test. (I) dCas9 mRNA expression as assessed by RT-qPCR in gastrocnemius muscle of treated HSALR mice. Plotted relative to the mean of the 2xSV40 NLS Cas9 nontargeting group. Significance by Tukey’s HSD. (J) Western blot detecting dCas9 and Gapdh in gastrocnemius muscle of treated mice. Relative signal intensity determined by densitometry at the bottom. A.U.: arbitrary unit, normalized to Gapdh (ns = not significant; *P < 0.05; **P < 0.01; ***P < 0.001) (error bars = 95% CI).
We next sought to investigate how Myospreader might impact the function of Cas9 in muscle in vivo. We first chose to test the binding functions of deactivated Cas9 (dCas9) in the HSALR model of myotonic dystrophy, a setting in which we have previously demonstrated the ability of dCas9 to impede transcription of expanded CTG repeats (12). We packaged 2xSV40 NLS dSaCas9 with a nontargeting gRNA, 2xSV40 NLS dSaCas9 with a CTG-targeting gRNA, or Myospreader dSaCas9 with a CTG-targeting gRNA into MyoAAV, and systemically delivered these constructs (5e13 vg/kg) or PBS to 4-wk-old HSALR mice. After four more weeks, whole muscle and myofibers were harvested (Fig. 2F). HCR-RNA FISH against dCas9 mRNA sequence and CUG repeats revealed which myonuclei were expressing AAV-derived dCas9 and expanded CUG RNA, respectively. As expected, myofibers from mice treated with the 2xSV40 NLS dCas9 with nontargeting gRNA showed no reduction in CUG RNA in myonuclei expressing dCas9 mRNA or in neighboring myonuclei. Myofibers from mice treated with 2xSV40 NLS dCas9 with CTG-targeting gRNA showed reduction of CUG RNA, but the effect was mostly limited to myonuclei expressing dCas9 mRNA. In contrast, Myospreader dCas9 with CTG-targeting gRNA showed reductions in CUG RNA not only in myonuclei expressing dCas9 mRNA but also in more distal myonuclei (Fig. 2G and SI Appendix, Fig. S2A). Quantification of total CUG RNA signal in myonuclei expressing dCas9 mRNA and their neighbors showed a significant reduction of CUG RNA in the Myospreader dCas9 group compared to the CTG-targeting 2xSV40 NLS dCas9 group (Fig. 2H and SI Appendix, Fig. S2A). There was no significant difference in relative dCas9 mRNA expression by RT-qPCR between any of the dCas9 treatment groups (Fig. 2I). However, Myospreader dCas9 showed greater relative protein levels as measured by western blot than either of the 2xSV40 NLS dCas9 groups, again suggesting that Myospreader increases the stability of dCas9 protein in skeletal muscle (Fig. 2J).
Myospreader Improves Cas9 Myonuclear Propagation and Editing Efficiency In Vivo.
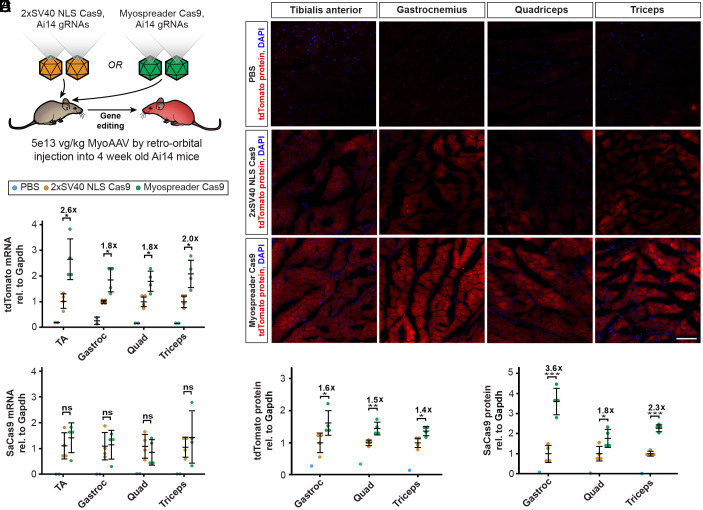
To investigate whether Myospreader improves gene editing performance by Cas9, we turned to Ai14 reporter mice, in which the tdTomato reporter described above has been integrated into the genome (30). We packaged 2xSV40 NLS SaCas9, Myospreader SaCas9, and two Ai14-targeted gRNAs into MyoAAV, and delivered each Cas9-encoding virus paired with the gRNA virus (5e13 vg/kg for SaCas9 virus and 5e13 vg/kg for gRNA virus) systemically into 4-wk-old Ai14 mice. Another 4 wk later, whole muscle and myofibers were isolated (Fig. 3A). Quantification of relative tdTomato mRNA expression by RT-qPCR also showed approximately twofold higher levels with Myospreader as compared to 2xSV40 NLS, despite similar overall levels of Cas9 mRNA (Fig. 3 B and C). Muscle cryosections from mice treated with Myospreader Cas9 had greater tdTomato signal (Fig. 3D). Levels of tdTomato protein were approximately 1.5-fold to twofold higher in muscles of Myospreader-treated mice compared to mice treated with 2xSV40 NLS Cas9 as assessed by western blot (Fig. 3E and SI Appendix, Fig. S3C). Levels of Myospreader Cas9 protein measured by western blot were twofold to fourfold higher than 2xSV40 NLS Cas9 (Fig. 3F and SI Appendix, Fig. S3D), supporting the theory that spreading Cas9 throughout the sarcoplasm and away from the transduced nucleus may extend protein half-life. HCR-FISH-IF in myofibers isolated from TA showed improved myonuclear propagation of Myospreader Cas9 protein as compared to 2xSV40 NLS Cas9 and also increased abundance in the sarcoplasm. TA myofibers from mice treated with Myospreader Cas9 showed tdTomato mRNA spots in and around multiple adjacent myonuclei, suggesting multiple successful editing events originating from a limited number of Cas9-expressing myonuclei (SI Appendix, Fig. S3A). Quantification of tdTomato-positive myonuclei from isolated myofibers showed that Myospreader Cas9 generated significantly more tdTomato mRNA-positive myonuclei relative to the 2xSV40 NLS Cas9 construct (SI Appendix, Fig. S3B).
Fig. 3.
Myospreader improves Cas9 editing efficiency in vivo. (A) Schematic of the experiment to measure the efficacy of AAVs containing 2xSV40 NLS Cas9 or Myospreader Cas9, systemically delivered to 4-wk-old Ai14 mice at a dose of 5E+13 vg/kg. (B) tdTomato mRNA expression as assessed by RT-qPCR in muscles of treated Ai14 mice. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (C) Cas9 mRNA expression as assessed by RT-qPCR in muscles of treated Ai14 mice. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (D) Representative images of tdTomato fluorescence (red) and DAPI (blue) in muscles of treated Ai14 mice. (Scale bar: 100 µm.) (E) Quantification of Tdtomato protein as determined by western blot/densitometry, normalized to Gapdh. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (F) Quantification of Cas9 protein as determined by western blot/densitometry, normalized to Gapdh. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test (ns = not significant; *P < 0.05; **P < 0.01; ***P < 0.001) (error bars = 95% CI).
Myospreader Cas9 Increases Myonuclear Propagation and Editing Efficiency in a Mouse Model of Duchenne Muscular Dystrophy.
We further assessed the performance of Myospreader Cas9 in the mdx mouse model of Duchenne muscular dystrophy, which contains a nonsense mutation in exon 23 of the Dmd gene and expresses little to no dystrophin protein (31, 32). Excision of exon 23 with gRNAs targeting the flanking introns can restore reading frame and dystrophin expression (8, 9). We packaged 2xSV40 NLS SaCas9, Myospreader SaCas9, and two Dmd-targeted gRNAs into MyoAAV, and delivered each Cas9-encoding virus paired with the gRNA virus (5e13 vg/kg for SaCas9 virus and 5e13 vg/kg for gRNA virus) by tail vein into 8-wk-old mdx mice. After 4 wk, whole muscle and myofibers were isolated (Fig. 4A). Myospreader Cas9 treatment produced significantly more exon-23 deleted mRNA in all muscles, with levels ranging from approximately 1.5-fold to twofold higher than 2xSV40 NLS Cas9 despite showing similar Cas9 mRNA transcript levels (Fig. 4 B and C). Increases in exon-23 deleted mRNA were supported by Sanger sequencing results showing significantly greater DNA editing in Myospreader Cas9–treated gastrocnemius muscle as compared to 2xSV40 NLS Cas9 (SI Appendix, Fig. S4 A and B). Treatment with Myospreader Cas9 produced significantly higher levels of dystrophin protein and levels of Mysopreader Cas9 protein were also twofold to fourfold higher than 2xSV40 NLS Cas9 when assessed by western blot (Fig. 4 D and E and SI Appendix, Fig. S4 C and D). Immunofluorescence in TA myofibers showed increased abundance and broader myonuclear propagation of Myospreader Cas9 protein as compared to 2xSV40 NLS Cas9, concomitant with increased abundance and broader localization of dystrophin protein (Fig. 4F). Muscles treated with Myospreader Cas9 showed increased dystrophin expression as assessed by immunofluorescence of dystrophin (Fig. 4G). Finally, TA myofibers isolated from mice treated with Myospreader Cas9 displayed more uniform dystrophin expression along the sarcolemma and a decrease in the frequency of centralized myonuclei (Fig. 4 h and I). Quantitation of TA myofiber dystrophin revealed that Myospreader Cas9 restores significantly more dystrophin to the myofiber periphery than 2xSV40 NLS Cas9 (Fig. 4J).
Fig. 4.
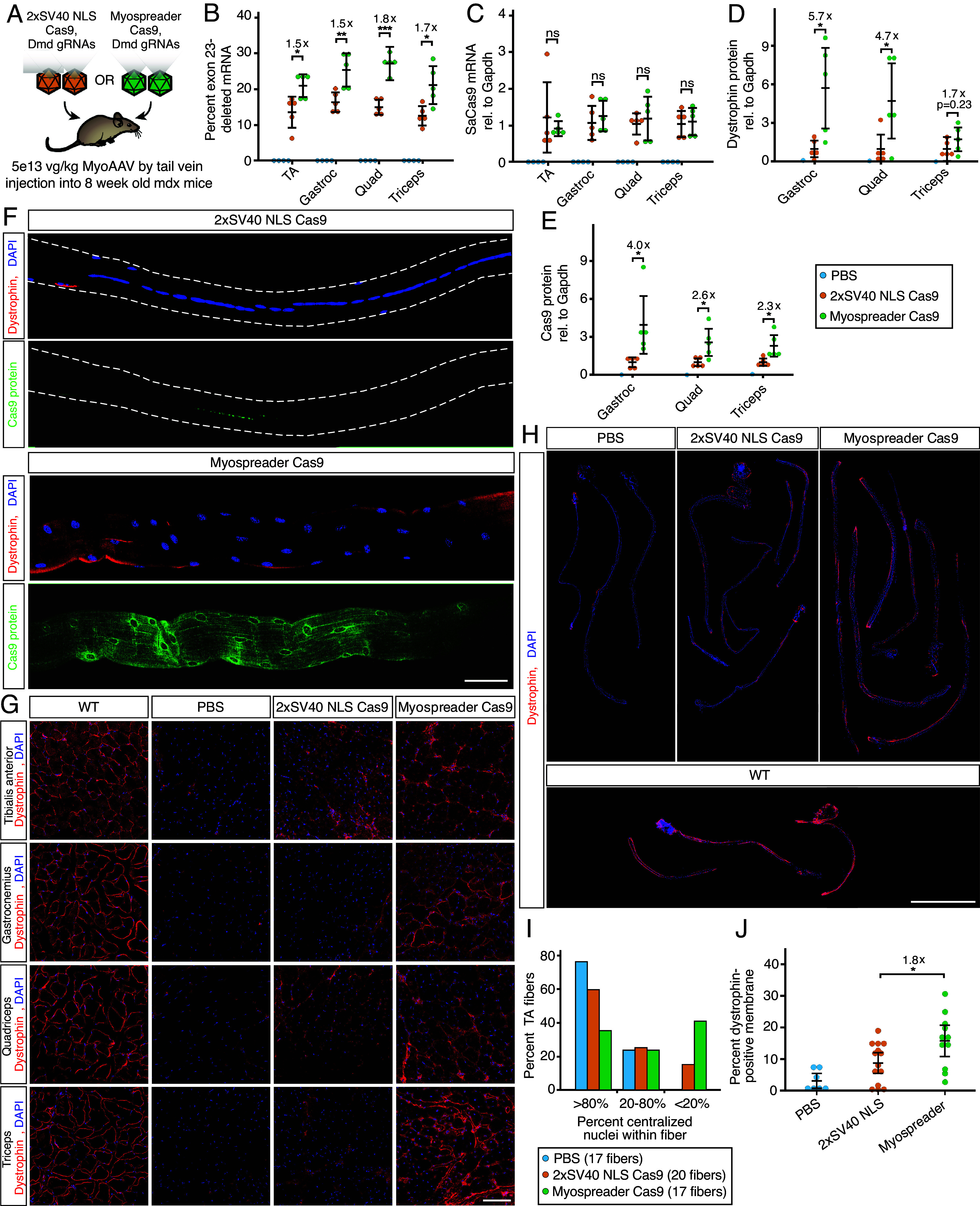
Myospreader improves Cas9 editing efficiency in a Mouse model of Duchenne muscular dystrophy. (A) Schematic of the experiment to measure the efficacy of AAVs containing 2xSV40 NLS Cas9 or Myospreader Cas9, systemically delivered to 4-wk-old mdx mice at a dose of 5E+13 vg/kg. (B) RT-qPCR quantification of exon-23 deleted Dmd mRNA in different muscles of treated mdx mice. Significance by Student’s t test. (C) RT-qPCR quantification of Cas9 mRNA in different muscles of treated mdx mice. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (D) Quantification of dystrophin protein as determined by western blot/densitometry, normalized to Gapdh. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (E) Quantification of Cas9 protein as determined by western blot/densitometry, normalized to Gapdh. Plotted relative to the mean of the 2xSV40 NLS Cas9 group. Significance by Student’s t test. (F) IF to detect dystrophin (red) and Cas9 (green) protein in representative TA myofibers isolated from treated mdx mice. (Scale bar: 50 µm.) (G) Representative images of Dystrophin protein (red) and DAPI (blue) in muscle cryosections of treated mdx mice by IF. (Scale bar: 100 µm.) (H) IF to detect dystrophin protein (red) and DAPI (blue) in whole TA myofibers isolated from treated mdx mice or untreated 8-wk-old WT mice. (Scale bar: 1 mm.) (I) Quantification of centralized nuclei in TA myofibers isolated from treated mdx mice. (J) Quantification of dystrophin protein at the periphery of TA myofibers of treated mdx mice or 8-wk-old WT mice. Significance determined Student’s t test (ns = not significant; *P < 0.05; **P < 0.01; ***P < 0.001) (error bars = 95% CI).
Discussion
The syncytial nature of myofibers adds complexity to the conventional view of AAV transduction efficiency and its impact on therapeutic development for muscle. In mononucleated cells, although variation in AAV concatemer number can yield different expression levels, AAV transduction success is binary (33). In myofibers, however, successful transduction events occur in a small proportion of myonuclei, leveraging only a fraction of total transgene potential available in the cell. Ultimately, maximum therapeutic benefit will only be achieved if gene therapy cargoes impact all myonuclear domains.
How do size and syncytial nature of myofibers affect AAV transduction? Upon entry into the myofiber, AAV particles may travel along the microtubule network, perhaps visiting several myonuclear domains prior to dynein-dependent recruitment to perinuclear regions and entry into myonuclei (34, 35). Similar to endogenous mRNAs, gene therapy mRNAs are transcribed in successfully transduced nuclei, then exported and dispersed throughout the myonuclear domain (1, 36). Soluble and structural proteins translated from therapeutic mRNAs can then travel further, the latter perhaps being more restricted (18, 37), but nuclear-targeted proteins are trapped close to their original domains (14, 38). Large nuclear-targeted proteins then have no way to passively exit the nuclear pore complex and are degraded within the nucleus over time (15), similar to other nucleocytoplasmic shuttling proteins (39). When combined with low rates of myonuclear transduction, this trapping strongly limits therapeutic efficacy.
By appending an NES, we have facilitated the export of these proteins, extending cytoplasmic residence time and promoting myonuclear propagation. Myospreader, only 29 amino acids longer than 2xSV40 NLS, increases both myonuclear propagation and stability of SaCas9. Indeed, these benefits are probably intertwined; the increased stability of Myospreader Cas9 protein is likely linked to its trafficking behavior. The additional nucleolar localization sequence in Myospreader may help maintain some degree of nucleolar targeting; an intrinsic nucleolar localization signal of SpCas9 was shown to be essential for maintaining protein stability (29). Modifications to the specific NLS and NES utilized as well as their N/C-terminal orientation are potential optimization strategies for Myospreader and other NLS/NES combinations. Overall, the combination of cytoplasmic, nuclear, and nucleolar signals in Myospreader SaCas9 may harmonize stability, trafficking, and editing properties; similar principles may have the potential to improve the effectiveness of AAV- and mRNA-based therapies in mono- and multinucleated cells.
The newest generation of gene editing techniques employs fusions of Cas9 to enzymatic domains to make precise edits to DNA without inducing double-strand breaks. Both base and prime editors are large (>197 kDa) fusions consisting of a Cas9 fused to DNA targeted enzymatic domains (40–43), and additional fusions are being employed for epigenetic editing, transcriptional activation, and transcriptional repression (10, 11, 44–46). Many other myonuclear-targeted cargoes are also under development, such as a DUX4 dominant negative for Facioscapulohumeral dystrophy (47), Pumilio-PIN fusions for myotonic dystrophy (48), and PGC1a for sarcopenia (49). All of these approaches can in principle benefit from the enhanced myonuclear propagation and protein stability provided by Myospreader or other NLS/NES combinations. Ultimately, efforts to develop safe and effective gene therapies in any cell type will benefit from considering the spatial dimension of gene regulation.
Materials and Methods
Mice.
All animal care and experimental procedures were performed in accordance with the University of Florida Animal Care and Use Committee. For the AAV-GFP experiments, 4-wk-old male and female C57BL/6 mice were injected retro-orbitally with AAV. For the AAV-dCas9 experiment, 4-wk-old male and female HSALR mice were injected retro-orbitally with AAV. For the AAV-Cas9-AI14 experiment, 4-wk-old male and female AI14 mice were injected retro-orbitally with AAV. For the AAV-Cas9-mdx experiment, 8-wk-old male mdx mice were injected by tail vein with AAV. Eight-week-old mdx mice were used to avoid confounding effects of widespread myofiber necrosis observed in 4-wk-old male mdx mice.
Cell Lines.
The C2C12 mouse myoblast cell line was obtained from ATCC (CRL-1772).
Constructs.
All plasmids were generated using Gibson assembly and In-Fusion cloning according to manufacturer protocols (Takara, 638920). PB-NCT-GFP plasmids were generated using NCT plasmids donated by Zane Zeier and were flanked by PiggyBac transposon arms. AAV-GFP constructs were generated by cloning the CAG promoter, GFP coding sequence, SV40 PolyA signal into a pAAV plasmid backbone. AAV-dCas9 plasmids were generated from the SaCas9 plasmid pX601-AAVCMV∷NLS-SaCas9-NLS-3xHA-bGHpA;U6∷BsaI-sgRNA (Addgene #61591) by incorporation of NLS/NES elements and a CAG-targeting or nontargeting sgRNA. AAV-Cas9 plasmids were generated from SaCas9 plasmid pX601-AAVCMV∷NLS-SaCas9-NLS-3xHA-bGHpA;U6∷BsaI-sgRNA (Addgene #61591) by incorporation of NLS/NES elements and removal of sgRNA and U6 promoter. AAV-Ai14-sgRNA was generated by cloning dual U6-driven sgRNAs targeting flanking regions of Ai14 SV40 polyA tract into a pAAV backbone. AAV-mdx-sgRNA was generated by cloning dual U6-driven sgRNAs targeting flanking introns of Dmd exon 23 into a pAAV backbone. Target sequences of gRNAs used are listed in SI Appendix, Table S2.
Stable C2C12 Cell Line Production.
A wild-type C2C12 myoblast line was cotransfected with PB-NCT-GFP constructs and an mPB plasmid expressing a PiggyBac transposase. Piggybac facilitates stable integration into the host genome (50). Forty-eight hours after transfection, C2C12s were selected with 5 µg/mL puromycin for 1 wk. All transfections were performed using TransIT X2 (Mirus, MIR6003) according to the manufacturer’s instructions.
Gelatin Hydrogels.
Gelatin (8% weight/volume, Sigma-Aldrich, G1890) and chloroform (0.02% volume/volume) were dissolved in autoclaved water at 65 °C. 10% Microbial transglutaminase (mTG) solution was prepared using the heated gelatin solution. mTG gelatin solution was added dropwise onto glass coverslips previously activated using 100 mM NaOH, 0.5% (3-Aminopropyl) trimethoxysilane, and 0.5% glutaraldehyde as described in ref. 51. Polydimethylsiloxane (PDMS) stamps were then pressed onto the gelatin and incubated overnight at 37 °C. With stamps attached, gelatin was rehydrated with PBS for 1 h. PDMS stamps were carefully removed from gelatin, and the hydrogel was incubated in cell culture media until plating. Cells on hydrogels were fixed with 4% PFA for 10 min before imaging.
Cell Culture.
Mouse C2C12 myoblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified incubator kept at 37 °C and 5% CO2. Once cells reached 70% confluency, they were serum restricted with differentiation media consisting of DMEM supplemented with 2% horse serum and 1% penicillin–streptomycin. Myotubes were imaged approximately 4 d after introduction of differentiation media. Transient transfections were performed using TransIT X2 (Mirus, MIR6003) according to the manufacturer’s instructions. For experiments involving fusion of GFP stably expressing C2C12s with WT C2C12s, cells were plated together at a 1:1 ratio.
AAV Vectors.
All AAV plasmids contained AAV2 ITRs. All AAVs were packaged using MyoAAV 2A, with the exception of experiments for Fig. 1 F–H, which were packaged using MyoAAV 3A. The vector used in Fig. 1A was a gift from Sharif Tabebordbar. All other vectors were packaged at SignaGen Laboratories and retitered by qPCR after digestion with Turbonuclease (BPS Bioscience, 50310) for 1 h at 37 °C and then proteinase K for 2 h at 50 °C. iTaq Universal Probes Supermix (Bio-Rad, 1725130) was utilized for qPCR. qPCR primers used for titering are listed in SI Appendix, Table S3.
Western Blots.
Protein lysates from tissue samples and cells were prepared in HEPES lysis buffer [20 mM HEPES, 100 mM KCl, pH8.0, 0.1% Igepal, and 1× protease inhibitor (Sigma-Aldrich, P8340)]. Protein lysates were centrifuged to remove debris. Total protein concentrations were determined using the Pierce 660 protein assay reagent (Thermo Fisher, 22660) and measured on the Nanodrop 2000 (Thermo Fisher). Equal amounts of total protein were loaded per well in each western blot.
Proteins were fractionated in 4 to 12% Tris-Glycine gel (XP04120BOX, Thermo Fisher). For dystrophin western blots, the first two lanes contain different percentages of total protein isolated from WT mouse muscle diluted in total protein isolated from the same muscle of an untreated mdx mouse. Protein samples were run with NuPAGE™ LDS Sample Buffer (Thermo Fisher, NP0008) and National Diagnostics 10X TRIS-GLYCINE-SDS PAGE Running BUFFER (Fisher, 50-899-90103). All gels were transferred to PVDF membranes using Trans-Blot Turbo RTA Mini LF PVDF Transfer Kits (Bio-Rad, 1704274).
Membranes were blocked for 1 h in Intercept® Blocking Buffer (LI-COR, 927-60001). All membranes were then incubated for 16 h at 4 °C in primary antibody diluted in blocking buffer. Primary antibodies used were against HA-tag (Cell Signaling Technology 3724S), tdTomato (Takara Bio, 632496), Dystrophin (DSHB, MANEX1011B(1C7), MANEX1011C(4F9), MANHINGE1B clone 10F9), and GAPDH (Cell Signaling Technology, 2118L) (Abcam, ab83956). For dystrophin western, three primary antibodies were used simultaneously. Membranes were washed three times in TBST and then incubated with a secondary antibody in blocking buffer for 1 h at room temperature. Secondary antibodies used were IRDye® 800CW Donkey anti-Mouse IgG (LI-COR, 926-32212), IRDye® 800CW Donkey anti-Rabbit IgG (LI-COR, 926-32213), IRDye® 680RD Donkey anti-Chicken IgG(LI-COR, 926-68075), and IRDye® 680RD Donkey anti-Rabbit IgG (LI-COR, 926-68073). Membranes were washed three times in TBST after secondary antibody incubation. Membranes were imaged on LI-COR Odyssey imager.
Cryosectioning.
Dissected muscles were flash-frozen for 10 s in an isopentane bath cooled by liquid nitrogen and stored at −80 °C until sectioning. Cryosectioning was performed on a Leica CM3050 S cryostat microtome. Ten-micrometer sections were adhered to microscope slides and stored at 4 °C until fixation.
TA Myofiber Isolation.
TA muscle was dissected from 8- or 12-wk-old mice (4 wk postinjection) of both sexes (only male mdx mice). Muscle was digested with prefiltered 0.02% collagenase in DMEM at 37 °C for 1 h. Digested TAs were agitated by pipetting in prewarmed collagenase DMEM in horse serum–coated plates under microscopy to dissociate individual fibers. Fibers were collected in horse-serum coated plates containing DMEM and stored in 37 °C, 5% CO2 tissue culture incubator for no more than 1 h before fixation.
HCR RNA smFISH and Immunofluorescence.
HCR v3.0 RNA FISH probes, amplifier, and buffers for each target were purchased from molecular instruments. Isolated myofibers, C2C12 myoblasts/myotubes, and muscle sections were processed identically. Samples were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 10 min at room temperature, then washed three times for 5 min with PBS at room temperature. Samples were permeabilized with 1% Triton x-100 for 10 min at room temperature. Immunofluorescence was performed before HCR FISH. Samples were blocked in 1% ultrapure RNase-free BSA (Sigma), 1 U/µL NxGen RNase inhibitor (Lucigen), and 0.1% Tween 20 (blocking buffer) for 1 h at RT. Samples were then incubated in primary antibody diluted in blocking buffer for 1 h at room temperature. Primary antibodies used were against HA-tag [Cell Signaling Technology 3724S, Dystrophin (DSHB, MANEX1011B(1C7)], nucleolin (Bethyl Laboratories, A300-711A-T), and Aly (Thermo Scientific, MA1-26754). Samples were washed three times for 5 min with PBS + 0.1% Tween. Samples were incubated with secondary antibodies for 1 h at room temperature. Secondary antibodies used were Goat Anti-Rabbit IgG H&L Alexa Fluor® 647 (Abcam, ab150079), Goat Anti-Rabbit IgG H&L Alexa Fluor® 555 (Abcam, ab150086), and Goat Anti-Rabbit IgG H&L Alexa Fluor® 488 (Abcam, 150081). Samples were washed three times for 5 min with PBS + 0.1% Tween. If HCR FISH was performed, samples would be fixed with 4% PFA for 10 min followed by three washes for 5 min with PBS and one wash for 5 min with 2× saline-sodium citrate (SSC) buffer at room temperature. Samples were incubated in prewarmed HCR hybridization buffer for 30 min at 37 °C in a humidified chamber. Primary HCR FISH probes were aliquoted into PCR tubes, heated to 95 °C for 90 s, and then diluted to 1 nM in hybridization buffer and kept at 37 °C. Sample hybridization buffer was replaced with hybridization buffer containing probes, and samples were incubated overnight in a humidified chamber at 37 °C. Samples were washed five times for 10 min with prewarmer HCR wash buffer and washed two times for 5 min with 5xSSC + 0.1% Tween (SSCT) and incubated in HCR amplification buffer for 30 min at room temperature. During incubation, HCR amplifiers were aliquoted into PCR tubes and incubated at 95 °C for 90 s and then allowed to cool at room temperature protected from light for 30 min. Amplifiers were diluted to 60 nM in HCR amplification buffer. Sample amplification buffer was replaced with amplification buffer containing amplifiers and incubated at room temperature for 3 h. Samples were washed five times for 10 min with SSCT and one time for 5 min with PBS containing 0.1 µg/mL DAPI and then mounted on slides.
RT-qPCR.
RNA was isolated from cell and tissue samples using TRI Reagent (Zymo, R2050-1-200) and Direct-zol RNA MiniPrep kit (Zymo, R2052). cDNA was made using the iScript cDNA synthesis kit (Bio-Rad, 1708890). A TaqMan probe assay against the exon 4-5 junction was used for quantification of total Dmd transcripts. A TaqMan probe assay against exon 22-24 was used to quantify exon 23-deleted transcripts. TaqMan probe assays utilized iTaq Universal Probes Supermix (Bio-Rad, 1725130). Sacas9, tdtomato, and dsacas9 transcripts were quantified using SYBR green PCR assays with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, 1725270). Mouse Gapdh mRNA was used as the housekeeping control for quantification of Sacas9, tdtomato, and dsacas9 expression. Standard curves were generated for all RT-qPCR experiments by amplifying varying amounts of the target sequence in each run. Quantification was done based on the standard curves. RT-qPCR primers are listed in SI Appendix, Table S3.
Sanger Sequencing.
gDNA was isolated from mouse gastrocnemius using Quick-DNA Microprep Plus Kit (Zymo D4074). gDNA was amplified using CloneAmp™ HiFi PCR Premix (Takara 639298) and dmd Sanger sequencing primers. Sanger Sequencing was performed by Genewiz (Azenta). Inference of CRISPR Edits (ICE) tool (Synthego) was used to obtain ICE-Discordance values for each sample sequenced. The sequencing result from a PBS-treated mdx mouse was used as a control to obtain ICE-discordance values. The ICE-Discordance algorithm accounts for large deletions and multiguide editing events. Given high-quality alignment upstream of the cut site, the ICE-Discordance algorithm considers any downstream signal discordant with the control trace as evidence of editing (52).
Imaging of Fixed Samples.
Myoblasts, myotubes, and myofibers were imaged on a Zeiss LSM 880 AxioObserver microscope with Airyscan using a Plan-Apochromat 0.8 NA ×20 Objective (Fig. 4), a Plan-Apochromat 1.3 NA ×40 oil objective (Figs. 1, 3, and 4 and SI Appendix, Fig. S1), a Plan-Apochromat 1.4 NA ×63 oil objective (Figs. 2–4), or a Plan-Apochromat 1.4 NA ×100 oil objective (Fig. 2). Airyscan processing was performed on all images using the Zeiss ZEN Black software. HSALR myofibers used in transcription impeding analysis (Fig. 2H) were imaged on an Optical Biosystems Stellarvision instrument.
Image Analysis Pipeline.
All code is written in R and Python 3. Image analysis was also done in ImageJ. For each image, a maximum intensity z-projection array was generated from 3D Airyscan confocal stacks in CZI format. To detect myonuclei, the DAPI channel was thresholded using Li’s or Otsu’s method for automatic threshold selection (53, 54). Total and maximum signal intensity of other channels in myonuclei was measured. For Fig. 1H, GFP signal for each myonucleus was normalized to the mean GFP signal of all myonuclei of that fiber. For SI Appendix, Fig. S1F, total GFP/DAPI is the sum of both cytoplasmic and nuclear GFP signal divided by the total DAPI signal of each image. For Fig. 2G, only myonuclei that were within the same 470 µm × 470 µm FOV as dCas9 mRNA positive (identified by dCas9 mRNA channel signal) nuclei were included in the analysis. For Fig. 4J, dystrophin signal was measured on a segmented line along the perimeter of each fiber, and the 10th percentile signal intensity was subtracted from all signal values to account for varying background signal between fibers. Percent Dystrophin positive membrane was calculated based on the percent of signal readings above 60 AU for each fiber.
Statistical Analysis.
All statistical analysis was performed in R. All data are presented as mean ± 95% CI. For datasets with only two groups compared at once, an unpaired two-tailed Student’s t test was performed. Equal variance and normality were assumed. For Figs. 1 E and G and 2G, the Kolmogorov–Smirnov test was performed. For Figs. 2I and 4J, Tukey’s HSD test was performed.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Sharif Tabebordbar for multiple discussions related to the efficiency of gene editing in muscle over the years and for the eMyoAAV-GFP virus used for experiments in Fig. 1A. We thank Ann Kennedey for the mouse illustration used in the figures (10.5281/zenodo.3925921). We thank Karyn Esser and members of the Wang lab for comments and suggestions related to this work. This work was funded by NIH grant R01 AG058636 (E.T.W.), Chan-Zuckerberg Initiative Ben Barres Early Career Acceleration Award (E.T.W.), and NIH grant P50-AR052646 (H.L.S.).
Author contributions
K.K.P., h.L.S., and E.T.W. designed research; K.K.P., M.C.V., D.R.M., L.M.A., h.C., and Y.i.L. performed research; K.K.P., N.S.A., and Z.Z. contributed new reagents/analytic tools; K.K.P. analyzed data; and K.K.P. and E.T.W. wrote the paper.
Competing interests
E.T.W. is a co-founder and consultant to Kate Therapeutics. K.K.P. and E.T.W. are inventors on a patent related to this work.
Footnotes
This article is a PNAS Direct Submission.
Preprint Servers: BioRxiv(https://www.biorxiv.org/content/10.1101/2023.11.06.565807v1) CC-BY-NC-ND 4. International license.
Data, Materials, and Software Availability
Code is available at https://github.com/kirilpoukalov/Myospreader.git (55). All data is included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Denes L. T., Kelley C. P., Wang E. T., Microtubule-based transport is essential to distribute RNA and nascent protein in skeletal muscle. Nat. Commun. 12, 6079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlie J. P., Sanes J. R., Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature 317, 66–68 (1985). [DOI] [PubMed] [Google Scholar]
- 3.Fontaine B., Sassoon D., Buckingham M., Changeux J. P., Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 7, 603–609 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlath G. K., Rich K., Webster S. G., h. Blau M., Localization of muscle gene products in nuclear domains. Nature 337, 570–573 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Morin A., et al. , Dystrophin myonuclear domain restoration governs treatment efficacy in dystrophic muscle. Proc. Natl. Acad. Sci. U.S.A. 120, e2206324120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertl H. C. J., Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 13, 975803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabebordbar M., et al. , Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938.e22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabebordbar M., et al. , In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson C. E., et al. , In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porquet F., et al. , Specific DMPK-promoter targeting by CRISPRi reverses myotonic dystrophy type 1-associated defects in patient muscle cells. Mol. Ther. Nucleic Acids 32, 857–871 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himeda C. L., Jones T. I., Jones P. L., Targeted epigenetic repression by CRISPR/dSaCas9 suppresses pathogenic DUX4-fl expression in FSHD. Mol. Ther. Methods Clin. Dev. 20, 298–311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto B. S., et al. , Impeding transcription of expanded microsatellite repeats by deactivated Cas9. Mol. Cell 68, 479–490.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shui S., Wang S., Liu J., Systematic investigation of the effects of multiple SV40 nuclear localization signal fusion on the genome editing activity of purified SpCas9. Bioengineering (Basel) 9, 83 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor-Weiner H., et al. , Modeling the transport of nuclear proteins along single skeletal muscle cells. Proc. Natl. Acad. Sci. U.S.A. 117, 2978–2986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R., Brattain M. G., The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 581, 3164 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinek M., et al. , A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ran F. A., et al. , In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson B., et al. , Non-uniform dystrophin re-expression after CRISPR-mediated exon excision in the dystrophin/utrophin double-knockout mouse model of DMD. Mol. Ther. Nucleic Acids 30, 379–397 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amoasii L., et al. , Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretti A., et al. , Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat. Med. 26, 207–214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L., et al. , Efficient precise in vivo base editing in adult dystrophic mice. Nat. Commun. 12, 3719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemello F., et al. , Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 7, eabg4910 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis J. R., et al. , Efficient prime editing in mouse brain, liver and heart with dual AAVs. Nat. Biotechnol. 42, 253–264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X. C., Steitz J. A., HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. U.S.A. 95, 15293–15298 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Keefe K., Li h., Zhang Y., Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol. Cell Biol. 23, 6396–6405 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavolieri M. V., Droppelmann C. A., Campos-Melo D., Volkening K., Strong M. J., A novel overlapping NLS/NES region within the PH domain of Rho Guanine Nucleotide Exchange Factor (RGNEF) regulates its nuclear-cytoplasmic localization. Eur. J. Cell Biol. 98, 27–35 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Ramic M., et al. , Epigenetic small molecules rescue nucleocytoplasmic transport and DNA damage phenotypes in C9ORF72 ALS/FTD. Brain Sci. 11, 1543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denes L. T., et al. , Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet Muscle 9, 17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan R., et al. , Nucleolus localization of SpyCas9 affects its stability and interferes with host protein translation in mammalian cells. Genes Dis. 9, 731–740 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madisen L., et al. , A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulfield G., Siller W. G., Wight P. A., Moore K. J., X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. U.S.A. 81, 1189–1192 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicinski P., et al. , The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science 244, 1578–1580 (1989). [DOI] [PubMed] [Google Scholar]
- 33.Dhungel B. P., Bailey C. G., Rasko J. E. J., Journey to the center of the cell: Tracing the path of AAV transduction. Trends Mol. Med. 27, 172–184 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Castle M. J., Perlson E., Holzbaur E. L., Wolfe J. h., Long-distance axonal transport of AAV9 is driven by Dynein and Kinesin-2 And is trafficked in a highly motile Rab7-positive compartment. Mol. Ther. 22, 554–566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P.-J., Mitchell A. M., Huang L., Li C., Samulski R. J., Disruption of microtubules post-virus entry enhances adeno-associated virus vector transduction. Hum. Gene Ther. 27, 309–324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro H., et al. , mRNA distribution in skeletal muscle is associated with mRNA size. J. Cell Sci. 134, jcs256388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva Lopes K., Pietas A., Radke M. h., Gotthardt M., Titin visualization in real time reveals an unexpected level of mobility within and between sarcomeres. J. Cell Biol. 193, 785–798 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralston E., Hall Z. W., Transfer of a protein encoded by a single nucleus to nearby nuclei in multinucleated myotubes. Science 244, 1066–1069 (1989). [DOI] [PubMed] [Google Scholar]
- 39.Lahaye F., et al. , hMSH5 is a nucleocytoplasmic shuttling protein whose stability depends on its subcellular localization. Nucleic Acids Res. 38, 3655–3671 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komor A. C., Kim Y. B., Packer M. S., Zuris J. A., Liu D. R., Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaudelli N. M., et al. , Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anzalone A. V., Koblan L. W., Liu D. R., Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Anzalone A. V., et al. , Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao H.-K., et al. , In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 171, 1495–1507.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi L. S., et al. , Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert L. A., et al. , CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsuhashi H., et al. , Functional domains of the FSHD-associated DUX4 protein. Biol. Open 7, bio033977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W., et al. , Treatment of Type 1 myotonic dystrophy by engineering site-specific RNA endonucleases that target (CUG)n repeats. Mol. Ther. 22, 312–320 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo M., et al. , AAV-Mediated nuclear localized PGC1α4 delivery in muscle ameliorates sarcopenia and aging-associated metabolic dysfunctions. Aging Cell 22, e13961 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., et al. , piggyBac transposase tools for genome engineering. Proc. Natl. Acad. Sci. U.S.A. 110, E2279–E2287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bettadapur A., et al. , Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels. Sci. Rep. 6, 28855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conant D., et al. , Inference of CRISPR edits from sanger trace data. CRISPR J 5, 123–130 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Otsu N., A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979). [Google Scholar]
- 54.Li C. h., Tam P. K. S., An iterative algorithm for minimum cross entropy thresholding. Pattern Recognit. Lett. 19, 771–776 (1998). [Google Scholar]
- 55.Poukalov K., Myospreader. GitHub. https://github.com/kirilpoukalov/Myospreader. Deposited 31 October 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Code is available at https://github.com/kirilpoukalov/Myospreader.git (55). All data is included in the manuscript and/or SI Appendix.