Significance
Antimicrobial treatments that alter the intestinal microbiota can lead to enteric infections. Intestinal immune cells produce interleukin 22 (IL-22), which stimulates the epithelium to generate antimicrobial peptides thus maintaining a healthy microbiota. IL-22 signaling is reduced by a soluble decoy receptor, IL-22 binding protein (IL-22BP), which is produced by intestinal dendritic cells. In our study, we found that augmentation of IL-22 signaling in IL-22BP–deficient mice altered the bacterial composition of the gut microbiota, conferring resistance to Clostridioides difficile colitis when microbiota components were transferred to wild-type mice. The altered microbiota was associated with enhanced metabolism of dietary fibers as evidenced by the production of acetate. These findings have implications for the development of microbiota-directed therapeutics that fortify resistance against enteric infections.
Keywords: IL-22 signaling, gut microbiota composition, epithelial barrier, Clostridioides difficile colitis, short-chain fatty acids
Abstract
Interleukin 22 (IL-22) promotes intestinal barrier integrity, stimulating epithelial cells to enact defense mechanisms against enteric infections, including the production of antimicrobial peptides. IL-22 binding protein (IL-22BP) is a soluble decoy encoded by the Il22ra2 gene that decreases IL-22 bioavailability, attenuating IL-22 signaling. The impact of IL-22BP on gut microbiota composition and functioning is poorly understood. We found that Il22ra2–/– mice are better protected against Clostridioides difficile and Citrobacter rodentium infections. This protection relied on IL-22-induced antimicrobial mechanisms before the infection occurred, rather than during the infection itself. Indeed, the gut microbiota of Il22ra2–/– mice mitigated infection of wild-type (WT) mice when transferred via cohousing or by cecal microbiota transplantation. Indicator species analysis of WT and Il22ra2–/– mice with and without cohousing disclosed that IL22BP deficiency yields a gut bacterial composition distinct from that of WT mice. Manipulation of dietary fiber content, measurements of intestinal short-chain fatty acids and oral treatment with acetate disclosed that resistance to C. difficile infection is related to increased production of acetate by Il22ra2–/–-associated microbiota. Together, these findings suggest that IL-22BP represents a potential therapeutic target for those at risk for or with already manifest infection with this and perhaps other enteropathogens.
Interleukin 22 (IL-22) is an important promoter of antimicrobial immunity and the integrity of mucosal barriers. IL-22 is produced by group 3 innate lymphoid cells (ILC3s) and T cells, including Th17, Th22, and MAIT cells (1). IL-22 binds to a heterodimeric receptor composed of IL-22R1 and IL-10R2 chains (2). While expression of IL-10R2 is ubiquitous, IL-22R1 expression is restricted to various types of epithelial cells, hepatocytes, pancreatic acinar cells, and endothelial cells (3–6). Engagement of IL-22R1/IL10R2 heterodimers triggers the Janus kinase/Signal Transduction and Transcription Activation (JAK-STAT) signal transduction pathway, activating STAT3, STAT1, and STAT5 (7–9). In the gastrointestinal tract, IL-22 signaling stimulates epithelial cells to produce antimicrobial peptides and secrete mucus and fucosylated proteins on the surface of epithelial cells (9–11). By enhancing these epithelial defense mechanisms, IL-22 prevents colonization of pathogenic bacteria, sustains mucosal barrier integrity, and facilitates tissue repair after injury (12–15).
IL-22 is the only member of the IL-10 family of cytokines with a soluble decoy receptor, the IL-22 binding protein (IL-22BP), which is encoded by the Il22ra2 gene (16). IL-22BP disrupts the signaling and biological effects of IL-22 both in vitro (17–19) and in vivo (20); it does so by sequestering IL-22, with a much higher affinity than its canonical receptor IL-22R1. IL-22BP is constitutively expressed by conventional dendritic cells in numerous tissues, including the gastrointestinal tract, lungs, and skin (17, 21). A few studies have also reported IL-22BP production by eosinophils and T cells (22, 23). Patients with inflammatory bowel disease (IBD) have elevated levels of IL-22BP when compared with healthy controls (23). Conversely, IL-22BP-deficient mice have milder colitis outcomes (22, 23). Thus, sequestration of IL-22 by IL-22BP may have a detrimental role in the context of intestinal inflammation. IL-22BP also modulates tumorigenesis in the intestine (24). Whether and how IL-22BP impacts on the gut microbiota remains unclear (25). In this study, we found that Il22ra2–/– mice harbor a distinct microbiota compared to their wild-type (WT) counterparts, which is enriched in species that increase resistance to enteric infections, at least in part by producing short-chain fatty acids (SCFA).
Results
The Gut Microbiota of Il22ra2–/– Mice Protects from Enteric Infection.
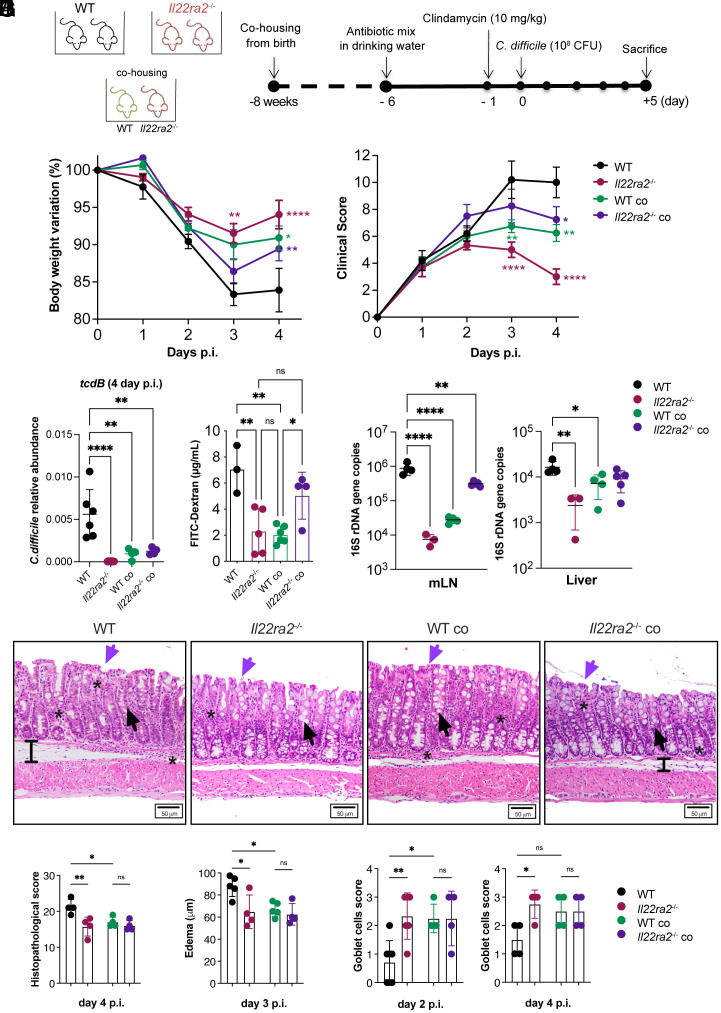
Clostridioides difficile (C. difficile), the most common pathogen associated with antibiotic-induced colitis (26), causes a severe disruption of the intestinal epithelial barrier, bacterial translocation, and morbidity. To investigate the impact of IL-22BP on the host response to C. difficile infection, 6- to 8-wk-old WT and Il22ra2–/– mice were subjected to a 5-d course of antibiotics in their drinking water to induce dysbiosis (27). Subsequently, they were infected with C. difficile VPI-10463 (108 CFU) and killed at 5 d postinfection (p.i.) (Fig. 1A). Both groups of mice exhibited comparable weight loss on days 1 and 2 p.i. However, Il22ra2–/– mice displayed significantly greater resistance to the severe weight loss than WT mice on days 3 and 4 p.i. (Fig. 1B; n = 5 mice/group). Il22ra2–/– mice also manifested a milder clinical score which assesses diarrhea, lethargy, piloerection, and other symptoms outlined in SI Appendix, Table S1 (Fig. 1C). Additionally, Il22ra2–/– mice showed a significantly lower fractional abundance of C. difficile in their feces on day 4 p.i. (Fig. 1D). To assess the permeability of the gut epithelial barrier, mice were orally gavaged with Fluorescein isothiocyanate(FITC)-Dextran on day 4 p.i. and serum accumulation of this compound was measured. WT mice displayed higher serum levels of FITC-Dextran compared to Il22ra2–/– mice (Fig. 1E). Il22ra2–/– mice demonstrated reduced bacterial loads (16S rDNA copy number) in their mesenteric lymph nodes and liver (Fig. 1F).
Fig. 1.
Il22ra2–/– mice provide protection to WT mice against C. difficile infection through cohousing. (A) Schematic representation of C. difficile infection model. Mice received a mixture of antibiotics in their drinking water for 4 d, followed by a single i.p. dose of clindamycin and subsequently were infected with 108 CFU of C. difficile. (B and C) Body weights (B) and clinical scores (C) during C. difficile infection were assessed in comparison to the WT non-cohoused group. The asterisks (*) indicate the statistical significance of the same color group they refer to when compared to the control. (D) Abundance of C. difficile in the feces of infected mice on day 4 p.i. The abundance was calculated by qPCR of the tcdb gene expressed by C. difficile normalized based on the 16S rDNA gene. (E) Intestinal permeability measured by serum FITC-Dextran. (F) Quantification of bacterial 16S rDNA gene copies in mesenteric lymph nodes (mLN) (Left) and liver (Right) on day 4 p.i., normalized by 16S rDNA of E. coli. (G) Representative H&E images of the mid colon of WT and Il22ra2–/– mice reared under different housing conditions on day 4 p.i. (Scale bar, 50 µm.) Black arrows indicate goblet cells; purple arrows indicate epithelial cell damage; black bars show edema; and stars point to areas of immune cell accumulation. (H) Histopathological scoring of colonic sections on day 4 p.i. The total score ranges from 0 to 30 and was calculated as the sum of 10 parameters, each quantified on a scale from 0 (normal) to 3 (severe) as indicated in SI Appendix, Table S2. (I) Submucosa edema on day 3 p.i. Edema was defined as the space between the muscularis mucosae and muscularis externa. The mean value of 20 distinct areas from each mouse is plotted as µm. (J) Goblet cell density defined using the scoring criteria indicated in SI Appendix, Table S3 on day 2 (Left) and 4 (Right) p.i. (A–F) n = 4 to 6; (G–J) n = 4 to 7 mice per group. (B–F) One representative experiment is shown. Data for the second experiment are reported in Dataset S3. Error bars represent mean ± SD. Normality was assessed with D’Agostino–Pearson test; statistical analysis was performed using One-way ANOVA with post hoc Tukey test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns = not significant.
To examine whether microbiota composition influences the increased protection seen in Il22ra2–/– mice during enteric infection, we cohoused WT and Il22ra2–/– mouse mothers during pregnancy, ensuring that their litters shared the same microbiota from birth. When cohoused with Il22ra2–/–mice, WT mice exhibited less weight loss, milder clinical score, decreased C. difficile abundance in the feces, lower intestinal epithelial barrier permeability, and reduced bacterial translocation compared to their non-cohoused WT counterparts (n = 4 to 6 mice/group) (Fig. 1 B–F). Conversely, Il22ra2–/–mice were more susceptible to C. difficile infection when cohoused from birth with WT mice, as compared to non-cohoused Il22ra2–/– mice (Fig. 1 B–F).
We further assessed colonic tissue sampled at different time points after the infection, using a histopathologic scoring index that included measurements of ulceration, mucus depletion, infiltration of inflammatory cells, and other factors detailed in SI Appendix, Table S2. Additionally, we measured submucosa edema and the numbers of mucus-producing goblet cells as described in SI Appendix, Table S3. WT mice exhibited more tissue alterations (Fig. 1 G and H), more submucosal edema (Fig. 1 G and I), and less goblet cells (Fig. 1 G and J) than Il22ra2–/– mice on day 4 p.i. (n = 4 mice/group). Cohoused mice showed no statistically significant differences in these parameters compared to non-cohoused Il22ra2–/– controls.
Il22ra2–/– and WT mice cohoused with Il22ra2–/– mice were also more resistant than WT mice to a model of repeated acute infection, in which mice were challenged again with C. difficile 2 wk after the first infection. Parameters measured included weight and the clinical score as shown in SI Appendix, Table S1 (n = 6 to 9 mice/ group; SI Appendix, Fig. S1 A and B) (28). This experiment further supported the notion that the gut microbiota of Il22ra2–/– mice plays a role in protecting WT mice from C. difficile infection, whether the host response is mediated by innate responses in the context of a primary infection or involves recall adaptive responses to repeated infections.
The ability of microbiota of Il22ra2–/– mice to enhance the control of pathogenic infection was not specific to C. difficile. Citrobacter rodentium (C. rodentium) shares several pathogenic mechanisms with human strains of enteropathogenic Escherichia coli and enterohemorrhagic E. coli (29). Il22ra2–/– mice exhibited greater resistance to C. rodentium, as manifested by significantly less weight loss, lower levels of fecal lipocalin-2 at 18 p.i., and reduced fecal C. rodentium CFUs compared to their WT counterparts (SI Appendix, Fig. S2 A–D). Cohousing of WT and Il22ra2–/– mice from birth reduced the severity of C. rodentium infection in WT mice, but also partially diminished protection in Il22ra2–/– mice. Indeed, cohoused mice showed weight loss, lipocalin-2 levels and C. rodentium CFUs counts in the feces that were neither as low as those in the non-cohoused Il22ra2–/– mice nor as high as those in the non-cohoused WT mice (SI Appendix, Fig. S2 B–D). Overall, these results provided additional evidence that the gut microbiota of Il22ra2–/– mice is associated with reduced severity of enteropathogen infection.
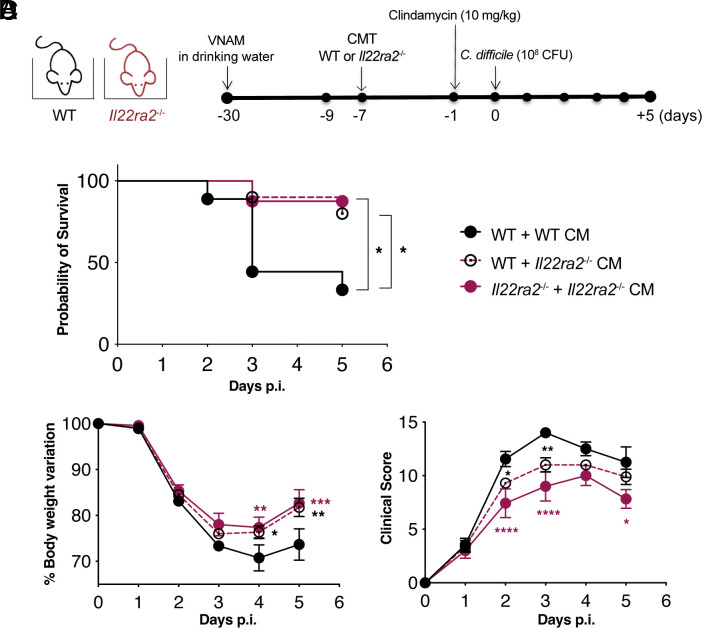
To directly demonstrate the role of microbiota in the enhanced protection observed in WT mice cohoused with Il22ra2–/– mice during enteric infection, we conducted a cecal microbiota transplantation (CMT) experiment. WT mice were treated with an antibiotic cocktail to deplete their intestinal microbiota, followed by reconstitution with either WT or Il22ra2–/– microbiota. Il22ra2–/– mice reconstituted with the cecal microbiota (CM) of Il22ra2–/– mice were used as controls. A week after CMT, all mice were infected with C. difficile, as previously outlined, and monitored for 5 d (Fig. 2A). WT mice reconstituted with Il22ra2–/– microbiota exhibited significantly increased survival, significantly reduced weight loss, and a significantly improved clinical score compared to mice receiving WT microbiota (Fig. 2 B–D).
Fig. 2.
Transplantation of Il22ra2–/– CM provides protection to WT mice against C. difficile infection. (A) Schematic representation of antibiotic treatment (VNAM: vancomycin, neomycin, ampicillin, and metronidazole), cecal microbiota transplantation (CMT), and C. difficile infection. (B–D) After CMT, C. difficile-infected mice were monitored for survival (B), body weight variation (C), and clinical score (D). All parameters were assessed in comparison to the WT + WT CM group (n = 3 to 5 mice per group). Two independent experiments are combined. Error bars represent mean ± SEM. Normality was assessed with D’Agostino–Pearson test; statistical analysis was performed using One-way ANOVA with post hoc Tukey test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
The Resistance of Il22ra2–/– Mice to Infection Is Independent of IL-22.
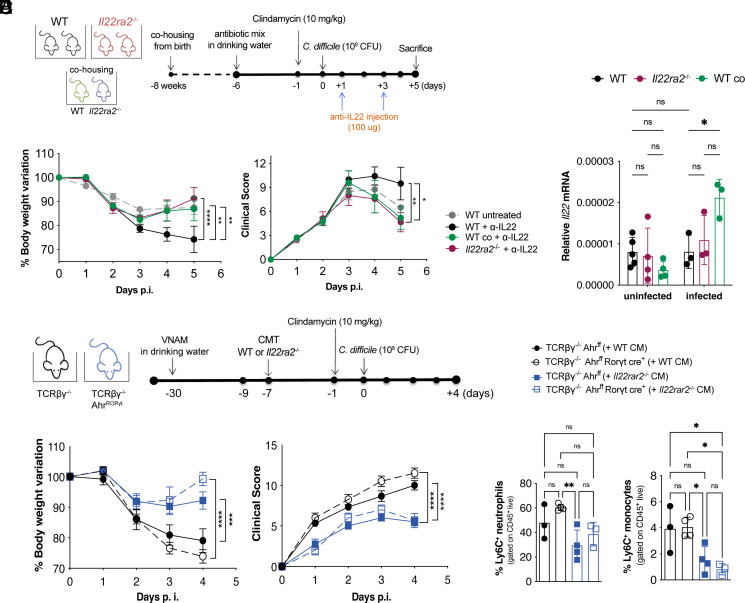
Since IL-22BP reduces the availability of IL-22 that promotes epithelial defense mechanisms, we asked whether the resistance of Il22ra2–/– mice to infection depends on enhanced IL-22 signaling in the course of infection. To address this question, WT mice and Il22ra2–/– mice, either separated or cohoused from birth, were infected with C. difficile and then treated with an antibody that neutralizes IL-22 at day 1 and 3 p.i. (Fig. 3A). Notably, Il22ra2–/– mice and WT mice cohoused with Il22ra2–/– mice remained more resistant to C. difficile infection than non-cohoused WT controls despite the neutralization of IL-22 (Fig. 3B). WT and Il22ra2–/– mice, whether separated or cohoused, had comparable levels of Il22 mRNA in their proximal colon (Fig. 3C), suggesting that deficiency of Il22ra2 has no impact on IL-22 production during infection. Thus, the greater protection observed in the Il22ra2–/– mice, and in WT mice cohoused with Il22ra2–/– mice compared with non-cohoused WT mice during C. difficile infection is independent, at least in part, on the concomitant production of IL-22, pointing to an independent contribution of the gut microbiota shaped by the absence of IL-22BP. We further observed that even though Il22ra2 expression decreased in infected WT mice compared to uninfected ones (SI Appendix, Fig. S3), WT mice were still more susceptible to infection than Il22ra2–/– mice. This corroborates that Il22ra2–/– mice’s resistance to infection is not due to heightened IL-22 signaling during the infection.
Fig. 3.
Production of IL-22 during C. difficile infection is not required to protect Il22ra2–/– mice. (A) Schematic representation of C. difficile infection and IL-22 blockade by i.p. injection of anti–IL-22 neutralizing antibody on days 1 and 3 p.i. (B) Body weights (Left) and clinical scores (Right) of WT and Il22ra2–/– mice. (C) Relative expression of Il22 mRNA in total proximal colon of naïve WT and Il22ra2–/– mice, whether cohoused or not since birth, normalized by Gapdh. (D) Schematic representation of CMT from Il22ra2–/– into TCRβδ–/– Ahrfl/fl (T cell-deficient) and TCRβδ–/– Ahrfl/fl RORγt-Cre (T cell- and ILC3-deficient) mice. (E) Body weights (Left) and clinical scores (Right) of TCRβδ–/– Ahrfl/fl and TCRβδ–/– Ahrfl/fl RORγt-Cre mice that received WT or Il22ra2–/– CM before C. difficile infection. (F) FACS quantification of CD11b+ Ly6G+ neutrophils (Left) and CD11b+ Ly6C+ inflammatory monocytes (Right) in the colonic lamina propria of mice in (E) on day 4 p.i. (A and B) n = 5, (C) n = 6, and (D and E) n = 3 to 4 mice per group. (B and E) Two independent experiments are combined. Error bars represent mean ± SEM. Normality was assessed by D’Agostino–Pearson test; statistical analysis was performed using One-way ANOVA with post hoc Tukey test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns = not significant.
We also asked whether the resistance of Il22ra2–/– mice and WT mice cohoused with Il22ra2–/– mice could be explained by changes in intestinal immune cells that produce IL-22, such as ILC3s and T cells, or other cells that have been implicated in host defense against C. difficile. The absolute number of ILC3s in the colonic lamina propria was comparable between Il22ra2–/– and WT mice on day 2 p.i. regardless of the housing condition (SI Appendix, Fig. S4A). The colonic lamina propria of infected Il22ra2–/– and WT mice showed no statistically significant differences in the representation of ILC1s, ILC2s (SI Appendix, Fig. S4A), CCR6+, NCR+, CCR6–NCR– ILC3 subsets (SI Appendix, Fig. S4B), T cells, neutrophils, Ly6C+ inflammatory monocytes, macrophages, and CD11b+ dendritic cells (SI Appendix, Fig. S4C). Intraepithelial lymphocytes were also equivalent among all groups (SI Appendix, Fig. S4D). In addition, there were no significant differences in ILC3s, ILC1s, T cells, neutrophils, Ly6C+ monocytes, and macrophages on day 4 p.i. (SI Appendix, Fig. S5 A and B).
To directly address whether ILC3s and/or T cells contributed to host resistance to C. difficile independently of the Il22ra2–/– microbiota, we transplanted Il22ra2–/– or WT CM into RORγt-Cre x Ahrfl/fl x TCRβδ–/– mice, which lack ILC3s and T cells and have severely reduced levels of IL-22 in gut (30). Mice were subsequently infected with C. difficile and monitored as described above (Fig. 3D). The transfer of Il22ra2–/– microbiota had a prominent protective effect against C. difficile infection independent of the presence or absence of T cells and ILC3s (Fig. 3 E and F). These findings support the notion that the beneficial effect of IL-22BP-deficiency on host susceptibility to enteric infection is due to gut microbiota modulation rather than enhanced IL-22 signaling during the course of the infection.
IL-22BP-Deficient Mice Harbor a Distinct Gut Microbiota.
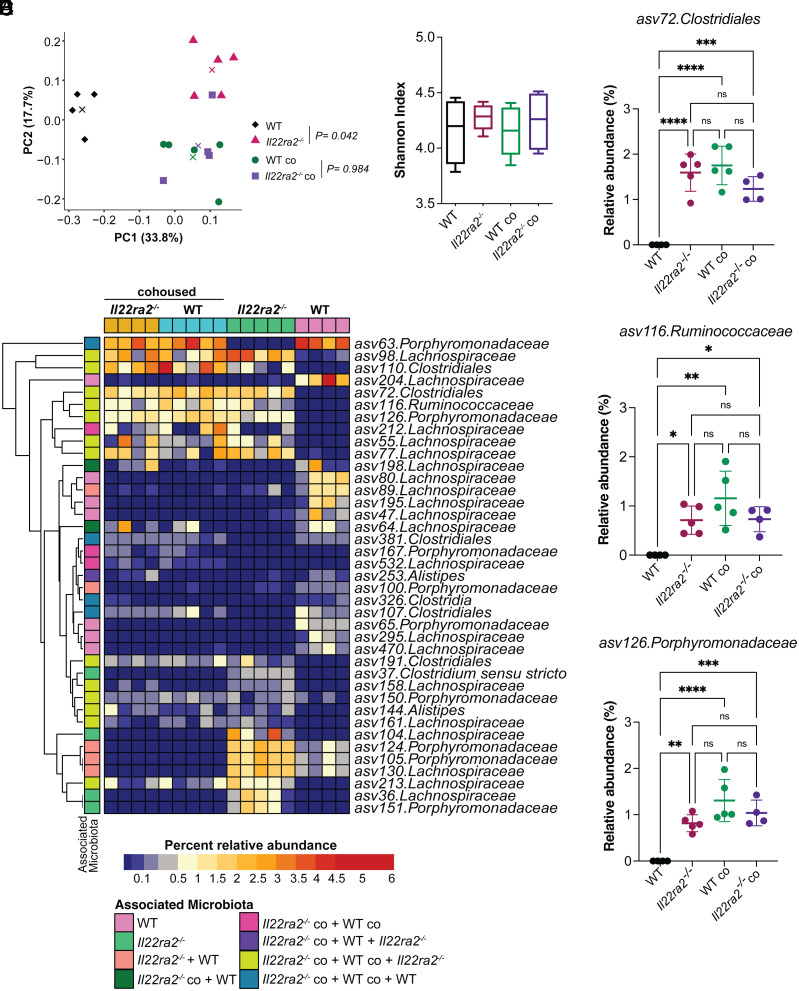
To identify bacterial taxa that enhanced protection to pathogen infection in WT mice cohoused with Il22ra2–/– mice, we performed V4 16S rDNA sequencing of cecal contents collected from WT and Il22ra2–/– mice that were either kept in separate cages (WT and Il22ra2–/–) or cohoused (WTco and Il22ra2–/–co). Principal Coordinate Analysis (PCoA) analysis showed that the microbiota of WT mice clustered separately from that of WTco (P = 0.04; PERMANOVA), which, instead, had similar composition to that of Il22ra2–/–co mice (P = 0.98; PERMANOVA; Fig. 4A). No statistically significant differences in alpha-diversity between the four microbial communities were found, as measured by the Shannon index (Fig. 4B).
Fig. 4.
Il22ra2–/– mice harbor a different microbiota compared to their WT counterparts. (A) PCoA plot showing beta-diversity analysis, based on Bray–Curtis dissimilarity’s metric, performed on the CM of WT (black), Il22ra2–/– (bright pink), WTco (green) and Il22ra2–/–co (purple) mice before infection. Centroids are indicated by “x” for each group. PERMANOVA analysis indicates that WTco and Il22ra2–/–co mice have the most similar bacterial composition in their microbial communities (P = 0.940) while WT and Il22ra2–/– are significantly different (P = 0.042). (B) Alpha-diversity of the microbiota of the experimental groups in (A) as defined by Shannon index. (C) Heatmap of indicator species analysis comparing the microbiota composition of WT, Il22ra2–/–, WTco, and Il22ra2–/–co mice. Each column represents a different mouse and each row a different asv significantly associated with one or more microbiota, as indicated. (D–F) Relative abundance of asv72.Clostridiales (D), asv116. Ruminococcaceae (E), and Porphyromonadaceae (F) families in WT and Il22ra2–/– mice. (A–F) n = 4 to 5 mice per group. Error bars represent mean ± SD. Normality of the samples was assessed with D’Agostino–Pearson normality test; statistical analysis was then performed using One-way ANOVA with post hoc Tukey test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns = not significant.
To gain further insights into the bacterial taxa that contribute to the enhanced protective phenotype documented during C. difficile infection, we conducted an indicator species analysis by comparing the microbiota of WT and Il22ra2–/– animals maintained under different housing conditions (Fig. 4C). This analysis revealed 13 amplicon sequence variants (asv) whose abundances were associated with Il22ra2–/–, WTco, and Il22ra2–/–co microbiota but not with WT microbiota (Dataset S1). Among these asv, only three (asv72 Clostridiales, asv116 Ruminococcaceae, and asv126 Porphyromonadaceae) displayed a higher relative abundance in the microbiota associated with Il22ra2–/–, WTco, and Il22ra2–/–co mice compared with that associated with WT mice (Fig. 4 D–F). Given that our initial taxonomic assignment was limited to the Family level, we further aligned these three asv against the NCBI 16S rDNA database to achieve a more detailed classification (Dataset S2). Asv72, asv116, and asv126 were aligned with Ruminococcus champanellensis 18P13 (31), Intestinimonas butyriciproducens strain SRB-521-5-I (32), and Muribaculum intestinale strain YL27 (33), respectively. These bacterial taxa were transmitted from Il22ra2–/– to WT mice with cohousing (Fig. 4 C–F). Moreover, we observed unique expression patterns of various antimicrobial peptides (encoded by Defa1, Defa2, Defa21, Defa-rs1, and Defb3, Reg3g, S100a8, S100a9) and enzymes related to antimicrobial functions, like fucosyltransferase 2 (Fut2) and nitric oxide synthase 2 (Nos2), in the ileal and colonic regions of both uninfected Il22ra2–/– and WT mice, as shown in SI Appendix, Fig. S6. Specifically, in the ileum of Il22ra2–/– mice, there was an upregulation of Defa1, Defa2, Defb3, and Fut2 (>onefold compared to WT). Meanwhile, the colon exhibited higher expression levels of Defa2, Defa21, Defa-rs1, and Reg3g (>onefold compared to WT), though there was a notable decrease in Defb3. These findings highlight a distinct antimicrobial signature in the gut of Il22ra2–/– mice, potentially influencing their specific intestinal microbial composition under normal conditions. Together, our data indicate that i) naive WT and Il22ra2–/– have distinct gut microbiota, ii) WT mice cohoused with Il22ra2–/– mice acquire bacterial taxa from Il22ra2–/– mice, and iii) the distinctive microbiota observed in Il22ra2–/– mice may be influenced over time by the heightened IL-22 signaling.
The Microbiota in Il22ra2–/– Mice Is Efficient at Producing SCFA.
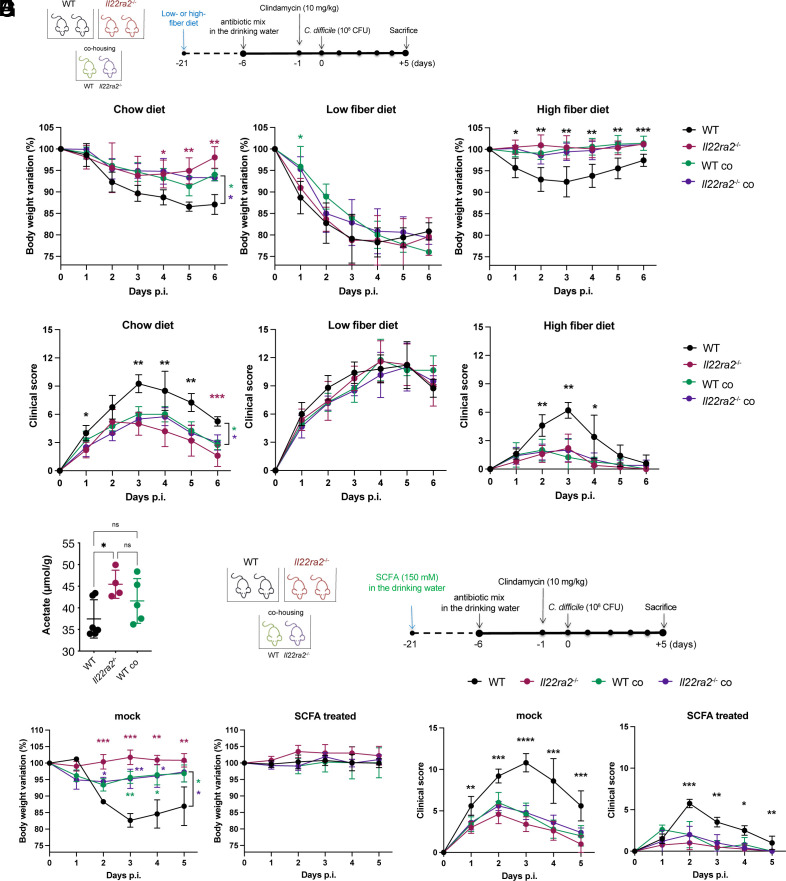
We noted that the Il22ra2–/– gut microbiota showed increased abundance of anaerobic species normally associated with the fermentation of dietary fibers leading to the generation of SCFA (34). Therefore, we hypothesized that Il22ra2–/– gut microbiota may exert its protective function in part by producing SCFA more efficiently than WT microbiota. To test this hypothesis, WT and Il22ra2–/– mice both in non-cohoused and cohoused settings were fed either a polysaccharide-rich conventional mouse chow, a diet containing only insoluble fibers (low-fiber) or a diet supplemented with 10% inulin (high-fiber). After 4 wk, all groups were infected with C. difficile (Fig. 5A). Consistent with our earlier results, Il22ra2–/– mice fed a standard chow diet showed reduced weight loss and clinical scores compared to WT mice, while WTco and Il22ra2–/–co mice showed an intermediate phenotype. Il22ra2–/–, WTco and Il22ra2–/–co mice fed the low-fiber diet all exhibited a reduction in their heightened resistance compared to WT mice (Fig. 5 B and C). WTco and Il22ra2–/–co mice fed an inulin-enriched diet became as resistant to infection as Il22ra2–/– mice (Fig. 5 B and C) while WT mice showed improvement in their resistance to infection, albeit they remained more susceptible than WTco and Il22ra2–/–co animals, suggesting a positive impact of the inulin-enriched diet on overall infection resistance.
Fig. 5.
Acetate production by Il22ra2–/– microbiota promotes protection against C. difficile infection. (A) Schematic representation of WT and Il22ra2–/– mice treatment with different types of fiber diet. Mice were fed a conventional rodent diet or a low-fiber or 10% inulin (high-fiber) diet for 3 wk. Mice were subsequently returned to a conventional rodent diet, received antibiotics, and were infected with C. difficile. (B and C) Mice were monitored during infection for body weight (B) and clinical score (C), and comparisons were calculated against the WT group. (D) GC-MS quantification of acetate levels in proximal colonic contents. (E) Schematic representation of SCFA treatment of WT and Il22ra2–/– mice before C. difficile infection. Mice received acetate (150 mM) in the drinking water for 3 wk. SCFA supplementation was subsequently discontinued and mice received antibiotics for 4 d followed by infection with C. difficile on day 0. (F and G) Mice were monitored daily after infection for changes in body weight (F) and clinical scores (G). Comparisons were conducted against the WT group. (A–C) n = 10; (D) n = 4 to 5; and (E–G) n = 8 to 9 mice per group. (B, C, F, and G) One representative experiment is shown. Data for the second experiment are reported in Dataset S3. The asterisks (*) indicate the statistical significance of the same color group they refer to when compared to the control. Black only (*) indicates that the significance of all groups is the same compared to the control. Error bars represent mean ± SD. Normality was assessed by D’Agostino–Pearson test. Statistical analysis was performed using a one-way ANOVA with post hoc Tukey test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns = not significant.
We next evaluated the concentration of SCFA in the proximal colons of naive uninfected WT and Il22ra2–/– mice fed the polysaccharide-rich conventional mouse chow but maintained under different housing conditions. Gas chromatography coupled with mass spectrometry (GC-MS) disclosed that levels of acetate were significantly increased in uninfected Il22ra2–/– mice compared to their WT counterparts and showed a trend to an increase in cohoused WT mice (Fig. 5D); the latter group of mice also exhibited the highest levels of butyrate (SI Appendix, Fig. S7). There were no discernible differences in propionate, succinate, or lactate levels between the groups (SI Appendix, Fig. S7).
To substantiate a functional role for acetate, WT and Il22ra2–/– mice in different housing conditions were treated with acetate for 4 wk in the drinking water prior to C. difficile infection (Fig. 5E). Cohoused WT and Il22ra2–/– mice treated with acetate were significantly more resistant to infection as defined by their weight loss and clinical score (Fig. 5 F and G). WTco and Il22ra2–/–co mice receiving acetate became as resistant to infection as Il22ra2–/– mice (Fig. 5 F and G); WT mice also showed improvement, although to a lesser extent than WTco and Il22ra2–/–co. Together, these findings support the beneficial effect of acetate in overall infection resistance in this model.
Discussion
This study reveals that Il22ra2–/– mice exhibit resistance to C. difficile infection due in part to their microbiota. This microbiota exhibits a distinctive composition that is associated with enhanced protection against infection when transferred to WT mice, either through cohousing or CM transplantation. IL-22 is known to trigger various defense mechanisms in intestinal epithelial cells against enteric pathogens, such as the production of antimicrobial peptides, mucus, and neutrophil chemoattractants. Consequently, the constitutive deficiency of IL-22BP in Il22ra2–/– mice, leading to an increase in IL-22 bioavailability, was expected to mitigate enteric infections.
Surprisingly, in our settings, enhanced IL-22 signaling does not appear to be crucial during infection but rather plays a vital role in shaping a protective microbiota in the steady state before infections occur. This conclusion is based on experiments where Il22ra2–/– mice, either separated from WT animals or cohoused with them from birth, were infected with C. difficile and then treated with an antibody that neutralizes IL-22; Il22ra2–/– mice and WT mice cohoused with Il22ra2–/– mice remained more resistant to C. difficile infection than non-cohoused WT controls despite the neutralization of IL-22. Moreover, WT and Il22ra2–/– mice, whether separated or cohoused, had comparable colonic levels of Il22 mRNA suggesting that deficiency of Il22ra2 does not impact IL-22 production during infection. The protective effect of this microbiota is not unique to C. difficile; it extends to C. rodentium and persists, remaining active even when mice are challenged a second time with the same pathogen. Although a previous report found no impact of IL-22BP deficiency on microbial community composition (25), experiments were performed only in WT and Il22ra2–/– mice that were cohoused.
How does the microbiota of Il22ra2–/– mice provide protection? When RORγt-Cre x Ahrfl/fl x TCRβδ–/– mice, which lack ILC3s and T cells and have severely reduced levels of IL-22 in gut were subsequently infected with C. difficile, transfer of Il22ra2–/– microbiota had a prominent protective effect against C. difficile infection and colitis—a finding consistent with the beneficial effect of IL-22BP-deficiency on host susceptibility to infection largely reflecting gut microbiota modulation rather than enhanced IL-22 signaling.
Indicator species analyses conducted on cohoused mice and non-cohoused controls identified taxa enriched in the microbiota of Il22ra2–/– mice that are known to produce SCFA from dietary fibers. We documented distinct profiles of antimicrobial peptides in the intestines of Il22ra2–/–compared to WT mice consistent with the notion that the distinctive microbiota observed in Il22ra2–/– mice is influenced by intestinal factors activated from heightened IL-22 signaling. The protective activity of Il22ra2–/– microbiota was abolished when animals were fed a diet deficient in fibers. Additionally, the cecal luminal contents of Il22ra2–/– mice had increased levels of acetate compared to WT mice. Administering acetate to WT mice mimicked the beneficial effect observed with Il22ra2–/– gut microbiota transplantation. Acetate also plays a pivotal role in the function of intestinal epithelial cells (35, 36), providing a significant energy source for the generation of ATP, promotion of cell proliferation and differentiation, and contributing to mucosal maintenance and repair. Thus, the microbiota of Il22ra2–/– mice may act, at least in part, by increasing the basal levels of acetate available for epithelial cells. While the protective phenotype associated with the Il22ra2–/– microbiota and its basal production of acetate was independent of IL-22 levels during the infection, it should be noted that acute oral supplementation of acetate during C. difficile infection does facilitate epithelial healing through neutrophil production of IL-1β, which boosts ILC3-mediated IL-22 production (37). Thus, acetate may exert a protective function through various mechanisms depending on the context.
A prior investigation, which focused on the impact of C. difficile in patients with IBD, demonstrated that colonization resistance to C. difficile can be reinstated by administering the succinate-consuming bacteria Phascolarctobacterium in a gnotobiotic mouse model (38). Moreover, the expansion of this microorganism was sustained by IL-22-mediated changes in intestinal mucus composition during inflammation (38). In this mouse model, Phascolarctobacterium successfully countered C. difficile colonization by competing for succinate availability in the intestinal lumen. Along this line, our study underscores how a deficiency in IL-22BP may prevent intestinal inflammation by averting dysbiosis and C. difficile colonization. Consequently, targeting IL-22BP emerges as a potential therapeutic intervention in those at risk for or with already manifest infection with this and perhaps other enteropathogens.
Materials and Methods
Experimental details related to infection models, assessment of intestinal epithelial permeability, histopathological and clinical scoring, cecal microbiota transplantation, in vivo neutralization of IL-22, gene expression analysis, measurement of lipocalin-2 and SCFA as well as flow cytometry, 16S rDNA sequencing, dietary manipulations, and statistical analyses are described in detail in SI Appendix, Material and Methods.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Acknowledgments
We would like to thank T.E. Ohara for help in histological analyses; Genentech for the generous gift of anti-IL-22 antibody; the Flow Cytometry & Fluorescence Activated Cell Sorting Core, the Digestive Diseases Research Core Center, the Molecular Microbiology Imaging Facility and the Immunomonitoring Laboratory at the Bursky Center for Human Immunology and Immunotherapy Programs at Washington University School of Medicine (supported by the Rheumatic Diseases Core Center, NIH WLC6313040077). This study was supported by NIH (1R01DK126969, R01DK132327, R01DK30292), the Pew Charitable Thrust (00035299), and the São Paulo Research Foundation, Brazil (FAPESP 2017/06577-9, 2018/15313-8).
Author contributions
M.A.V. and M. Colonna designed research; J.L.F., B.D.L., S.G., H.-W.C., C.S., and J.C. performed research; B.D.L., H.-W.C., M. Cella, M.A.V., and J.I.G. analyzed data; S.G. prepared the colonies of co-housed and separated mice; and J.L.F., B.D.L., and M. Colonna wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: M.R., Humanitas University; and T.N., Oregon Health & Science University.
Data, Materials, and Software Availability
Bacterial V4-16S rDNA amplicon sequencing data in raw format prior to postprocessing and data analyses have been deposited at the NCBI SRA, under accession no. PRJNA1084021.
Supporting Information
References
- 1.Sabat R., Ouyang W., Wolk K., Therapeutic opportunities of the IL-22–IL-22R1 system. Nat. Rev. Drug Discov. 13, 21–38 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Pestka S., et al. , Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22, 929–979 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Wolk K., et al. , IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Radaeva S., Sun R., Pan H., Hong F., Gao B., Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S., Xie M.-H., Maruoka M., Foster J., Gurney A. L., Acinar cells of the pancreas are a target of interleukin-22. J. Interferon Cytokine Res. 21, 1047–1053 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Giannou A. D., et al. , Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 56, 125–142.e12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumoutier L., Louahed J., Renauld J.-C., Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and Inducible by IL-9. J. Immunol. 164, 1814–1819 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Lejeune D., et al. , Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. J. Biol. Chem. 277, 33676–33682 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Guo X., et al. , Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y., et al. , Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Tumanov A. V., et al. , Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 10, 44–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickard J. M., et al. , Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 514, 638–641 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabihi M., Böttcher M., Pelczar P., Huber S., Microbiota-dependent effects of IL-22. Cells 9, 2205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenewicz L. A., et al. , IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190, 5306–5312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickert G., et al. , STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenewicz L. A., IL-22 binding protein (IL-22BP) in the regulation of IL-22 biology. Front. Immunol. 12, 766586 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumoutier L., Lejeune D., Colau D., Renauld J.-C., Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Kotenko S. V., et al. , Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 166, 7096–7103 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Xu W., et al. , A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl. Acad. Sci. U.S.A. 98, 9511–9516 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimoto K., et al. , IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J. C., et al. , Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 7, 101–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin J. C., et al. , IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 9, 539–549 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Pelczar P., et al. , A pathogenic role for T cell–derived IL-22BP in inflammatory bowel disease. Science 1979, 358–362 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Huber S., et al. , IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinnohara T., et al. , IL-22BP dictates characteristics of Peyer’s patch follicle-associated epithelium for antigen uptake. J. Exp. Med. 214, 1607–1618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnizlein M. K., Young V. B., Capturing the environment of the Clostridioides difficile infection cycle. Nat. Rev. Gastroenterol. Hepatol. 19, 508–520 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Chen X., et al. , A mouse model of Clostridium difficile–Associated disease. Gastroenterology 135, 1984–1992 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Sun X., et al. , Mouse relapse model of Clostridium difficile infection. Infect. Immun. 79, 2856–2864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins J. W., et al. , Citrobacter rodentium: Infection, inflammation and the microbiota. Nat. Rev. Microbiol. 12, 612–623 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Song C., et al. , Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chassard C., Delmas E., Robert C., Lawson P. A., Bernalier-Donadille A., Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int. J. Syst. Evol. Microbiol. 62, 138–143 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Kläring K., et al. , Intestinimonas butyriciproducens gen. nov., sp. nov., a butyrate-producing bacterium from the mouse intestine. Int. J. Syst. Evol. Microbiol. 63, 4606–4612 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Smith B. J., et al. , Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 19, 130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parada Venegas D., et al. , Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makki K., Deehan E. C., Walter J., Bäckhed F., The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Corrêa-Oliveira R., Fachi J. L., Vieira A., Sato F. T., Vinolo M. A. R., Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5, e73 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fachi J. L., et al. , Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J. Exp. Med. 217, jem.20190489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagao-Kitamoto H., et al. , Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 26, 608–617 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Data Availability Statement
Bacterial V4-16S rDNA amplicon sequencing data in raw format prior to postprocessing and data analyses have been deposited at the NCBI SRA, under accession no. PRJNA1084021.