Abstract
Ever since microRNAs (miRNAs) were first recognized as an extensive gene family >20 years ago, a broad community of researchers was drawn to investigate the universe of small regulatory RNAs. Although core features of miRNA biogenesis and function were revealed early on, recent years continue to uncover fundamental information on the structural and molecular dynamics of core miRNA machinery, how miRNA substrates and targets are selected from the transcriptome, new avenues for multi-level regulation of miRNA biogenesis, and mechanisms for miRNA turnover. Many of these latest insights were enabled by recent technological advances, including massively parallel assays, cryogenic electron microscopy, single-molecule imaging and CRISPR–Cas9 screening. Here, we summarize the current understanding of miRNA biogenesis, function and regulation, and outline challenges to address in the future.
Introduction
It has been 30 years since curiosity-driven research into Caenorhabditis elegans development yielded the first insights into microRNAs (miRNAs)1,2, and their general regulatory and targeting principles were evident from Drosophila molecular genetic studies3–5 even before knowledge of miRNAs in this species6. In 2001, miRNAs were recognized as a broad class of small RNAs across higher eukaryotes7–9, triggering intense research into this new regulatory paradigm across scientific disciplines. The next decade revealed major catalogues of conserved miRNA loci, molecular factors central to miRNA biogenesis and function, and their regulatory mechanisms.
miRNAs are ~22 nucleotide (nt) RNAs that derive from longer primary miRNA (pri-miRNA) transcripts, which bear one or more hairpins (Fig. 1a). Most miRNA hairpins derive from non-coding transcripts or introns; few overlap exons of protein-coding genes. Most conserved miRNAs (that is, shared across vertebrates) are generated through a canonical pathway involving two RNase III enzymes10 (Fig. 1a). First, the hairpin base of a pri-miRNA transcript is ‘cropped’ by the nuclear Microprocessor complex, comprising one RNase III Drosha molecule bound to two copies of its partner DGCR8 , releasing a ~55–70 nt precursor miRNA (pre-miRNA) hairpin. The pre-miRNA is exported to the cytoplasm and cleaved near the terminal loop by the RNase III Dicer, yielding a miRNA duplex that can interact with a member of the Argonaute (Ago) protein family. Following discard of the miRNA* species (also known as the passenger strand), a process governed by sequence and structural features of the duplex, the mature single-stranded miRNA guides the Ago complex to repress complementary RNA targets11. Additional non-canonical miRNA pathways exist that are Drosha-independent (Fig. 1b) and/or Dicer-independent (Fig. 1c)12. Knowledge of such atypical pathways enables the design of synthetic substrates that efficiently bypass both RNase III enzymes to generate functional miRNAs13,14.
Figure 1. Canonical and non-canonical miRNA biogenesis pathways.
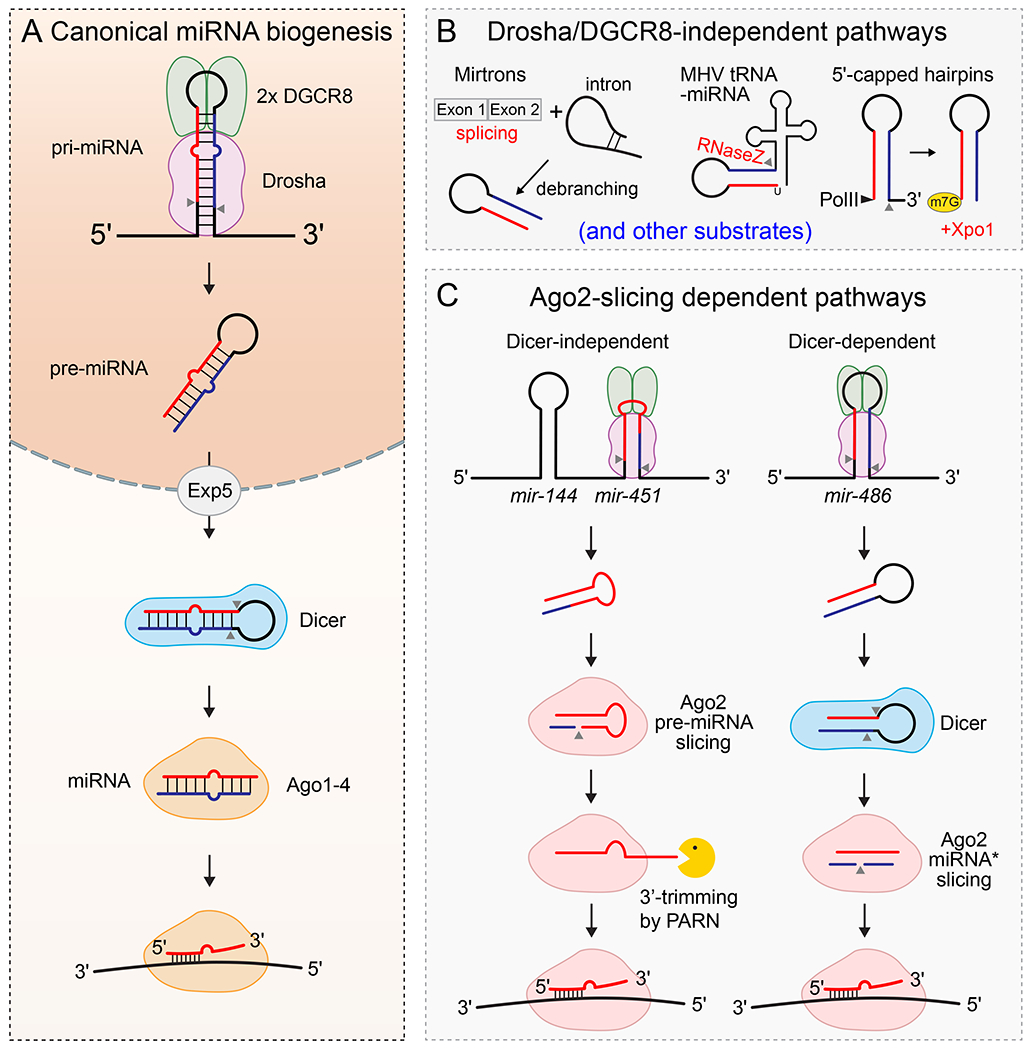
A. Canonical miRNA biogenesis. A primary miRNA (pri-miRNA) transcribed by RNA polymerase II is cleaved in the nucleus by Microprocessor (Drosha-DGCR8). The resulting precursor miRNA (pre-miRNA) is exported to the cytoplasm by Exportin-5 (Exp5), a member of the nuclear transport receptor family. Once there, Dicer cleaves the terminal loop to yield a miRNA duplex that is loaded into an Argonaute (Ago) protein. After removal of the passenger strand (blue), the single-stranded mature miRNA (red) guides the functional Ago effector complex (now termed RNA-induced silencing complex, RISC) to silence complementary RNA targets. Cleavage sites are denoted by grey arrow heads. B. Non-canonical mechanisms of miRNA biogenesis, involving the generation of pre-miRNA hairpins independent of Microprocessor. (Left) Mirtrons are pre-miRNA mimics that are generated by the splicing machinery and intron-debranching enzmes, thereby bypassing Microprocessor215,216. (Middle) MHV (Murine hepatitis virus) tRNA biogenesis via RNase Z (grey arrow head) can liberate functional pre-miRNAs217. (Right) RNA polymerase II (Pol II) transcription can directly yield pre-miRNAs bearing 5’-cap (m7G), which are exported to cytoplasm by XPO1, as exemplified by the mir-320 family. Because Ago binding requires 5’-monophosphate, Dicer cleavage of capped pre-miRNA hairpins yields mature miRNA only from 3’ arms218,219. Additional non-canonical miRNA biogenesis strategies have been described (not shown). C. Ago2-cleavage dependent miRNAs. Most miRNAs do not require catalytic activity of Ago2 for biogenesis, but two conserved exceptions are known. (Left) mir-451 has a short hairpin stem (18 bp) that cannot be cleaved by Dicer. Instead, following cleavage of its primary transcript by Microprocessor, the pre-mir-451 hairpin is directly bound and cleaved (sliced) by Ago2, followed by 3’ resection via poly(A)-specific ribonuclease (PARN) to generate mature miR-451220–222. (Right) mir-486 bears perfect base-pairing in its pre-miRNA stem and requires not only Drosha and Dicer to produce a miRNA duplex, but also cleavage of the passenger strand by Ago2 to yield the mature single-stranded RISC149.
Although various modalities of miRNA–target pairing have been proposed, repression of metazoan mRNAs almost always involves ‘seed’ pairing to nts 2–8 of the miRNA6,15–17. Argonaute–miRNA–target structures reveal a structural basis for such pairing, as the miRNA seed is pre-arranged into A-form conformation that is primed for base-pairing with its target mRNA18–20. Repression of seed-matched targets is mediated by the Ago adapter GW182 (TNRC6), which recruits additional factors for mRNA degradation and/or translational repression. All four mammalian Ago proteins regulate seed-matched targets, but Ago2 uniquely exhibits the ability to use endogenous miRNAs and synthetic small RNA guides to cleave extensively paired targets21,22, an activity that does not require cofactors and mediates experimental RNA interference (RNAi). Under specific circumstances, Ago3 can also cleave targets23,24, although the biological significance of this requires further study.
These core aspects of miRNA biogenesis and function were revealed using traditional biochemistry, molecular genetics and cell biology approaches, along with deep sequencing and X-ray crystallography. Catalysed by recent technical innovations and experimental strategies, including high-throughput substrate assays, cryogenic electron microscopy (cryo-EM), single-molecule approaches and CRISPR screening, the last decade has witnessed the emergence of novel insights into miRNA pathways. Here, we review the application of these techniques to the miRNA pathway (Table 1). Although we focus on recent work in mammalian systems, research across diverse species has and continues to be foundational, and we highlight relevant miRNA research from non-mammalian organisms throughout.
Table 1.
Recent technologies applied to the microRNA (miRNA) pathway
| Methods | General attributes | Recent applications to the miRNA pathway |
|---|---|---|
| High-throughput substrate screening | • Similar to classical in vitro selection assays. • Often involves parallel processing of a large endogenous substrate pool and/or library of designed or randomized variants. • Deep sequencing is used as a readout, to infer functionally relevant features for processing. |
• pri-miRNA features30,31,36 • pre-miRNA features38,54 |
| Cryo-electron microscopy (Cryo-EM) | • Uses an electron beam to image a specimen under cryogenic conditions. • Data are processed into EM densities that can be used to assign atomic coordinates. • Advantages include that Cryo-EM is suitable for proteins or complexes of large molecular weight; relatively small amounts of sample are needed; multiple conformational states can be captured in a single experiment; and no need for crystallization. |
• Mammalian Drosha/DGCR8 complex86,87 • Mammalian Dicer complexes81,90,91 • Drosophila Dicer complexes: Dicer-192 and Dicer-295,96 • Arabidopsis Dicer complexes: DCL193 and DCL397 |
| Single-molecule assays | • Offers real-time dynamics of biological reactions. • In vitro assays usually involve purified materials. • Observation times depend on the reaction kinetics, photostability, and lifetime of fluorophores, but can go down to microseconds. • Detection is generally diffraction limited (~200-300 nm spatial resolution). However, single-molecule Förster resonance energy transfer (smFRET) operates at 1-10 nm, thus resolving inter and intra-molecular motions. • Single-molecule imaging in living cells is typically done with fluorescently-tagged molecules and may utilize multimeric tags or scaffolds to enhance detection. |
• Dynamic interplay of human Dicer and TRBP94 • In vitro target search and interrogation by human Ago2/RISC complexes102–105,107 • Live cell imaging of targeting and regulation by human Ago2108–110 • Assembly and dynamics of Drosophila AGO2/RISC complexes100,101 |
| RNA bind-n-seq (RBNS) | • Yields relative quantitative binding affinities of an RNA binding protein (RBP) across a library of target sites. • Typically, a purified RBP is incubated with a randomized pool of RNAs. Co-purified RBP targets are then analyzed by deep sequencing to identify their features. |
Ago2–miRNA complex binding affinity to target RNAs64,65 |
| CRISPR–Cas9 screening | • Genetic strategies for mutagenesis can be applied to identify factors involved in a cellular process of interest. • miRNA screening often incorporates a specific reporter as a functional readout, enabling cell sorting before deep sequencing of enriched or depleted guide RNAs. |
• ERH/SAFB2 in miRNA cluster assistance153 • ZSWIM8 in target-directed miRNA degradation (TDMD)193,194 |
High-throughput miRNA assays
Until recently, direct experimental tests of miRNA biogenesis and function involved small to medium-scale assays, such as in vitro processing of specific miRNA substrates, and reporter assays of variant miRNA–target pairing configurations. Genomic profiling, such as small RNA sequencing or transcriptome measurements, inherently yields broad information. However, endogenous datasets have limitations for interpretation, including a panoply of indirect effects and the inability to query cell-specific loci, and limited ability to assay synthetic variants. Overcoming these challenges, creatively designed massively parallel substrate processing assays and Ago binding assays (Fig. 2a) have enabled new insights into miRNA biogenesis and target regulation.
Figure 2. Structural and sequence features of miRNA substrates and targets.
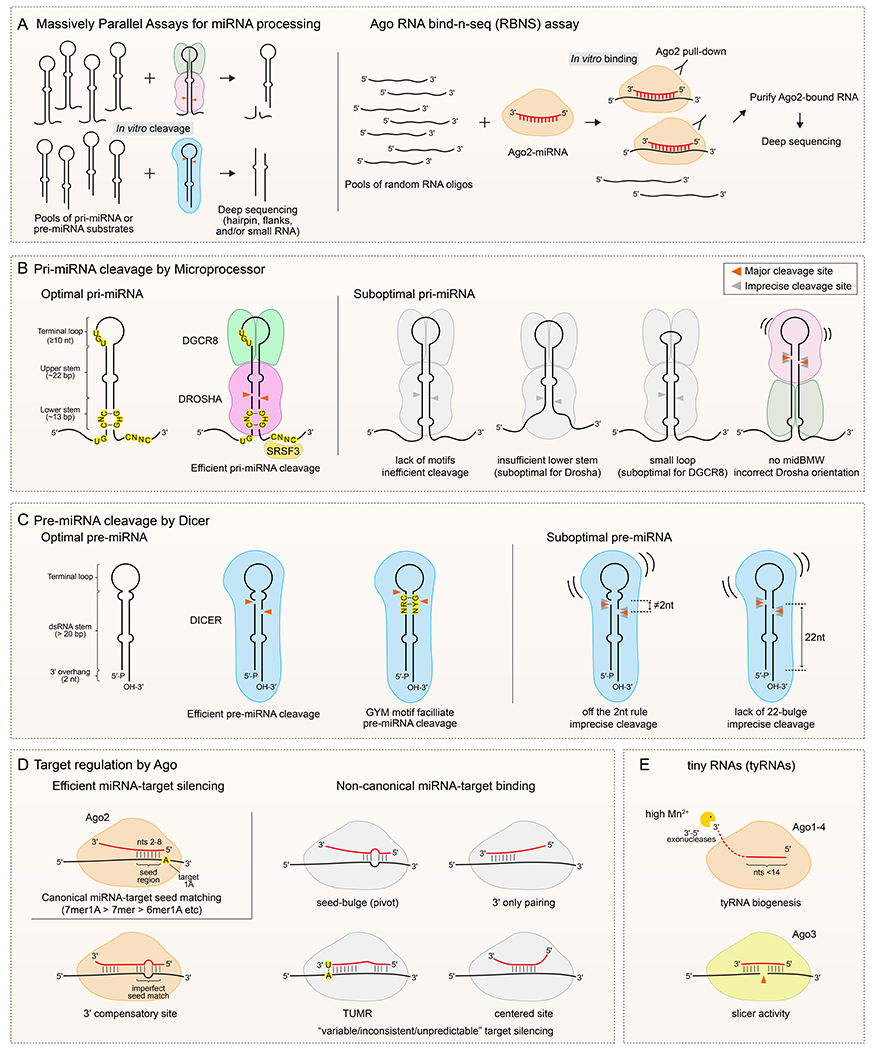
A. Design of massively parallel assays and RNA bind-n-seq (RBNS) assays for identifying miRNA pathway substrates and targets. (Left) A library of pri-miRNA or pre-miRNA substrates is incubated with purified Microprocessor (upper) or Dicer complex (lower) for in vitro cleavage, or for in vivo processing in cells. The reaction products are analysed by deep sequencing to infer structural features and sequence motifs within effective substrates. (Right) Target RNA oligo pools with random sequences are incubated with purified Ago2 loaded with a specific miRNA. Sequencing of bound species permits the relative affinities of different target sequences to be inferred. B. Pri-miRNA features. An optimal pri-miRNA contains characteristic structural features (single-stranded flanking regions, a ~35 bp dsRNA stem and ≥10 nt apical loop), primary motifs (including UG in the basal junction, UGU/GUG in the apical junction and CNNC in the 3’ flanking region), and chimeric structure-motif features such as the mismatched GHG (mGHG) in the lower stem. Drosha recognizes the basal junction, UG motif, and mGHG, and measures ~11 bp from the basal junction to cleave the dsRNA stem (red arrow heads). The DGCR8 dimer recognizes the apical loop and the UGU/GUG motif. Accessory factors can regulate microprocessing of pri-miRNA, including binding of CNNC by SRSF3. Pri-miRNAs with suboptimal features such as missing motifs, unpaired lower stem, and/or a small apical loop, exhibit inefficient and/or imprecise cleavage. Note that a middle bulge, mismatch and wobble base pair (midBMW) in the upper stem can repress unproductive cleavage by Microprocessor from the apical loop side. C. Pre-miRNA features. An optimal pre-miRNA hairpin contains 5’-monophosphate, 2 nt 3’-end overhang, a double-stranded stem of >20 bp and a single-stranded terminal loop. Dicer can measure its cleavage site from both 5’ and 3’ hairpin ends, although not all pre-miRNA substrates are engaged at both termini. A GYM (paired G, paired pyrimidine and mismatched C or A) motif near the Dicer cleavage site promotes pre-miRNA processing. A bulge/mismatch near the dicing sites enhances Dicer cleavage efficiency and/or accuracy, when the first nucleotide of the miRNA-3p species is 2 nt from the bulge/mismatch or when there is a 22-nt bulge in the pre-miRNA 3’ arm. Lack of this feature can cause Dicer to cut poorly and/or imprecisely, yielding multiple miRNA isoforms or lower small RNA levels. D. Ago–miRNA interaction features. (Left, top) The canonical miRNA-target interaction is mediated by seed pairing between nucleotides 2-8 (seed region) of miRNA with target RNA, and is often sufficient for measurable target repression. Shorter seed regions can also be functional, especially when followed by an adenosine opposite the first miRNA position (t1A). 7mer1A: seed 2-8 pairing with t1A; 7mer: seed 2-8 pairing; 6mer1A: seed 2-7 with t1A. (Left, bottom) A special class of target interaction with discontinuous seed pairing is compensated by extensive 3’ pairing. (Right) A variety of miRNA-target interactions lacking canonical seed pairing are found from in vivo Ago2-CLIP assays, in vitro Ago2-RBNS data, and bioinformatics; four types are summarized here. In general, the efficacy of these non-canonical sites is weak or controversial, and they were mostly assayed in vitro or in reporter systems. Unlike the exemplary 3’ compensatory let-7 site in lin-41, whose non-seed pairing is critical for C. elegans development, the impact of other non-canonical sites is mostly unknown. E. Tiny RNAs (tyRNAs). Under in vitro conditions of high manganese, several 3’-5’ exoribonucleases (ISG20, TREX1, and ERI1) can trim Ago-loaded miRNA down to <14 nt, and ISG20-mediated trimmed miRNA activates Ago3 cleavage of target mRNA.
Features of Drosha (pri-miRNA) substrates and cleavage
As gatekeeper for RNA transcripts entering the canonical miRNA pathway (Fig. 1), pri-miRNA surveillance provides the first checkpoint for miRNA substrate selection25. Early assays of pri-miRNA variants demonstrated the importance of general structural features, including a double-stranded stem of ~35 bp, flanking single-stranded sequences, and a single-stranded terminal loop of ≥10 nt26–29 (Fig. 2b). However, these features are insufficient to discriminate genuine miRNA hairpins amongst millions of seemingly comparable hairpins from RNA secondary structure predictions, suggesting other determinants.
High-throughput miRNA processing assays enable systematic discovery of sequence and structural features within functional miRNA substrates. Specific pri-miRNAs can be used as a foundation for large variant pools, which are then subjected to in vitro cleavage using cell lysate or purified Microprocessor (Fig. 2a). The processed products are purified, sequenced and compared to the initial pool to determine preferred features of effective pri-miRNA substrates. Initial assays recapitulated general features of effective pri-miRNA substrates mentioned above and revealed novel attributes such as UG in the 5’ basal junction, UGU/GUG in the apical junction, CNNC in the 3’ flanking region30 and a mismatched GHG (mGHG) motif in the lower stem31 (Fig. 2b). Some of these motifs were suspected to be recognized by Drosha or DGCR8 (see also structural studies below). For example, hemin (an Fe3+-bound porphyrin, distinct from heme which is an Fe2+-bound porphyrin) enhances the interaction between the DGCR8 dimer and the apical loop, especially with the UGU motif, to ensure efficiency and fidelity of pri-miRNA cleavage32,33. Other motifs revealed new molecular players, such as the recognition of 3’ CNNC by SRSF3, which aids proper positioning of Microprocessor30,34,35. Additional features include how mismatches, wobble base pairs and bulges in the upper stem of pri-miRNA hairpins can influence Microprocessor efficiency and accuracy36,37. Notably, the overall hairpin structure remains central to efficient cleavage of pri-miRNAs, and no primary motif seems essential. However, this ‘menu’ of motif features can compensate or enhance miRNA biogenesis when the pri-miRNA lacks optimal structural features31. Reciprocally, knowledge of optimal pri-miRNA backbones can improve designs for highly efficient RNAi vectors31,38.
More recently, comprehensive pri-miRNA processing assays involving a large number of endogenous pri-miRNAs, in vitro39,40 or in cells41, evaluated processing efficiency and accuracy in greater quantitative detail. These studies echoed earlier smaller-scale assays42 in revealing that numerous pri-miRNAs in the miRBase registry43 were not processed above background40,41, and may be false entries44. Still, both in vitro and transfected assays have their own limitations, as robust in vitro microprocessing may not fully recapitulate aspects of in vivo regulation. In addition, assays of transfected plasmids do not mimic normal genomic or chromatin context. An open question from these studies arises from the fact that in vitro cleavage of pri-miRNA hairpins reveals aberrant products, such as nicked hairpins or inverted processing40,45. It remains unclear whether these aberrant products are in vitro artifacts or biochemically valid activities that require suppression in vivo.
Features of Dicer (pre-miRNA) substrates
General features of pre-miRNA hairpins that enable Dicer cleavage emerged from in vitro and in vivo mutational studies of endogenous and synthetic pre-miRNAs. An optimal pre-miRNA usually contains a 5’-end monophosphate, 3’-end 2-nt overhang, a double-stranded stem of >20 bp and a single-stranded terminal loop46. These features are incorporated into several different models of dicing control (Fig. 2c).
Initial studies revealed that Dicer uses its PAZ domain to recognize the 3’-dinucleotide overhang and measure 21~25 nts (depending on the species) to cleave the dsRNA stem and release the small RNA duplex47–51. This so-called 3’-counting rule emphasized ‘ruler’-like activity of Dicer to cut at a prescribed distance from the 3’ substrate terminus. Subsequent work revealed a 5’-counting rule, whereby human Dicer can also recognize the 5’ phosphorylated end of the pre-miRNA hairpin and cleave the hairpin stem ~22 nts from the 5’ end52. Notably, it is not clear how Dicer selects different counting rules for different miRNA substrates. Finally, the distance between 5’ end of miRNA-3p species and the adjacent bulge or loop in the pre-miRNA hairpin influences the Dicer cleavage site, termed the loop-counting rule53. When this distance is 2 nts, Dicer preferentially cuts precisely at the 5’ end of 3p miRNA. Distances other than 2 nts lead to heterogeneous Dicer cleavage, yielding multiple miRNA isoforms (Fig. 2c).
Massively parallel substrate assays confirm these features and reveal new ones. For instance, recent largescale assays of pre-miRNA variants revealed a conserved GYM motif (paired G, paired pyrimidine and mismatched C or A) near the cleavage site of human Dicer. The GYM motif is recognized by dsRBD of Dicer and can override other counting rules for dicing38. Another study of 20,000 pre-miRNA variants showed that a single-nucleotide bulge near the terminal loop on 3’ arm (22-bulge) can promote pre-miRNA dicing and enhance gene silencing54 (Fig. 2c). Ultimately, comprehensive knowledge of combined Microprocessor and Dicer substrate features may permit effective bioinformatic prediction of canonical miRNAs directly from individual genomes, that is, without considering phylogenetic conservation. This remains an enticing challenge, and deep learning methods may bring us closer to the goal of recapitulating in silico what a cell does quite naturally.
Comprehensive analysis of Argonaute targeting
The functional Ago complex, sometimes referred to as the single-stranded guide RNA-induced silencing complex (RISC), identifies targets via base-pairing of loaded RNA (Fig. 2d). The most abundant miRNA target sites (canonical sites) contain 6–7 contiguous base-pairs between the miRNA seed region (preferentially nts 2–8 of the miRNA) and its target mRNA6,16 (Fig. 2d). Such minimal matching to miRNA 5’ ends is necessary and often sufficient for target regulation15,17. Activity of the 7-nt seed match is enhanced by a target adenosine opposite the first miRNA position (t1A). miRNAs usually begin with U, which appears to base-pair with t1A (Fig. 2d). However, as the miRNA 5’ base is bound by the specificity loop in the Ago MID domain55,56, it is not actually available for pairing19,20. Instead, Ago proteins exhibit intrinsic binding preference for miRNA 5’-U, and possess a solvated surface pocket that specifically binds t1A57.
Some canonical sites contain additional base-pairing, although this does not necessarily enhance silencing. However, rare non-canonical sites lacking 6 contiguous seed matches can be compensated by extensive 3’ base-pairing (positions ~13–16), a phenomenon termed supplemental or 3’-compensatory pairing10 (Fig. 2d). Other examples of non-conventional miRNA targeting included extensively ‘centred’ base-pairing58, and certain variations of interrupted seeds59,60 (Fig. 2d). Furthermore, other putative, non-canonical miRNA-target pairing interactions have been reported from Ago-CLIP and reporter studies59–62. In general, it is unclear whether non-seed miRNA–target interactions can be reconciled with structural data of Argonaute regulatory complexes. To address this more systematically, large-scale assays are critical. One study used high-throughput imaging-based assays to assay the binding energies, association, and cleavage rates of 40,000 target site variants for two miRNAs (let-7a and miR-21)63. This work revealed some distinctions in the behavior of individual miRNAs, likely attributable to different G/C content in the seed region. Ago RNA-bind-n-seq (RBNS) assays were also applied64. In such experiments, purified complexes of Ago2 loaded with an individual miRNA were incubated with large random RNA oligo pools, and bound targets were sequenced to infer binding affinities (Fig. 2a). Testing different concentrations of Ago2–miRNA complex with RBNS showed that binding to different site types was dependent on the concentration of the Ago2–miRNA complex, and detected binding idiosyncrasies of specific miRNAs. While expected classes of seed-based matches correlated with binding efficacy and regulation, non-canonical sites were documented with certain miRNAs, although these tended to be of low affinity and did not reliably confer repression in functional sensor assays in cells. Overall, miRNA–target site binding affinity is the major determinant of miRNA-mediated target repression64, but 3’ pairing can have substantial effects depending on the miRNA sequence65.
Local features such as flanking AU-rich dinucleotides and structural availability of the miRNA target site can enhance or enable Ago binding. In addition, miRNA-mediated repression is strongly enhanced when sites reside in 3’ untranslated regions (UTRs), which is attributable to dislodging of Ago complexes by translocating ribosomes66,67. However, coding sequence (CDS) sites can be functional, especially in the vicinity of rare codons67 or within CDS repeats68. Another variation was reported for atypical CDS sites lacking seed matches but with 3’-compensatory pairing69. This configuration was concluded to operate in a GW182-independent manner to repress translation by inducing transient ribosome stalling. Although the endogenous regulatory contribution of such sites remains unclear, 3’-only pairing configurations were detected in Ago2-RBNS with select miRNAs64.
miRNA modification can also modulate target binding. The most frequent 3’ non-templated addition of miRNAs is uridine70, which may enhance 3’-supplemental pairing to certain sites. For example, a non-functional miR-27a site with limited seed match is responsive to 3’ uridylated miR-27a, which extends pairing with adenosine in the target71; this phenomenon was termed tail-U-mediated repression (TUMR). This is consistent with evidence that a lengthened miRNA 3’ end can lead to expanded and stronger 3’ supplementary pairing interactions72. Reciprocally, miRNAs can be trimmed from their 3’ ends. A new study reports that under high Mn++ conditions, 3’ exonucleases including ISG20, TREX1 and ERI1/3’hExo can execute extreme trimming of Ago-loaded miRNAs into ~14-nt tinyRNAs (tyRNAs)73 (Fig. 2e). Moreover, certain miRNA-derived tyRNAs can activate cleavage activity of Ago3. Although the in vivo significance of this mechanism remains to be determined, high Mn++ conditions during stress or viral infection could plausibly alter miRNA activity. Finally, the local binding of RNA-binding proteins (RBPs), including HuR, Pumilio, DND1 and potentially many others, can affect miRNA site efficacy74–77.
These scenarios indicate why in vitro affinity of Ago2–miRNA–target ternary complexes might not capture the totality of in vivo Ago-mediated regulation. Conversely, it is important to recognize that many proposed unconventional target sites confer subtle or undetectable regulation. As CRISPR mutations of endogenous miRNA target sites are now straightforward to install, it will be critical to use this approach to evaluate whether atypical sites (as well as canonical seed matches) directly mediate in vivo miRNA silencing and/or phenotypically relevant biology.
Insights from RNase III cryo-EM structures
The typically precise sequences of mature miRNAs that are hewn from long primary transcripts suggest that the stereotypical actions of Drosha and Dicer involve ruler-like measurements. Similarly, the characteristic seed region of miRNAs that is predominantly required for targeting implies specific structural constraints on the ternary Ago–miRNA–target complex. These were inferences from the initial structures of Dicer and Argonaute homologs from protozoan (Giardia intestinalis) and archaeal (Thermus thermophilus and Pyrococcus furiosus) species18,48,78–80. Although small RNA silencing was not well-studied in these organisms, these structures recapitulated expectations from metazoan research. Thus, their mammalian counterparts were presumed to be similar. However, it was many years until structures of full-length human Argonaute19,20 and Dicer81 were solved. In particular, human Dicer was analysed using cryo-EM, which has revolutionized the structural determination of larger proteins and complexes, while providing rich insights into dynamic states. We summarize recent insights into cryo-EM structures of human RNase III complexes during pri/pre-miRNA cropping and dicing (Fig. 3).
Figure 3. Cryo-EM and single-molecule studies of Microprocessor and Dicer complexes.
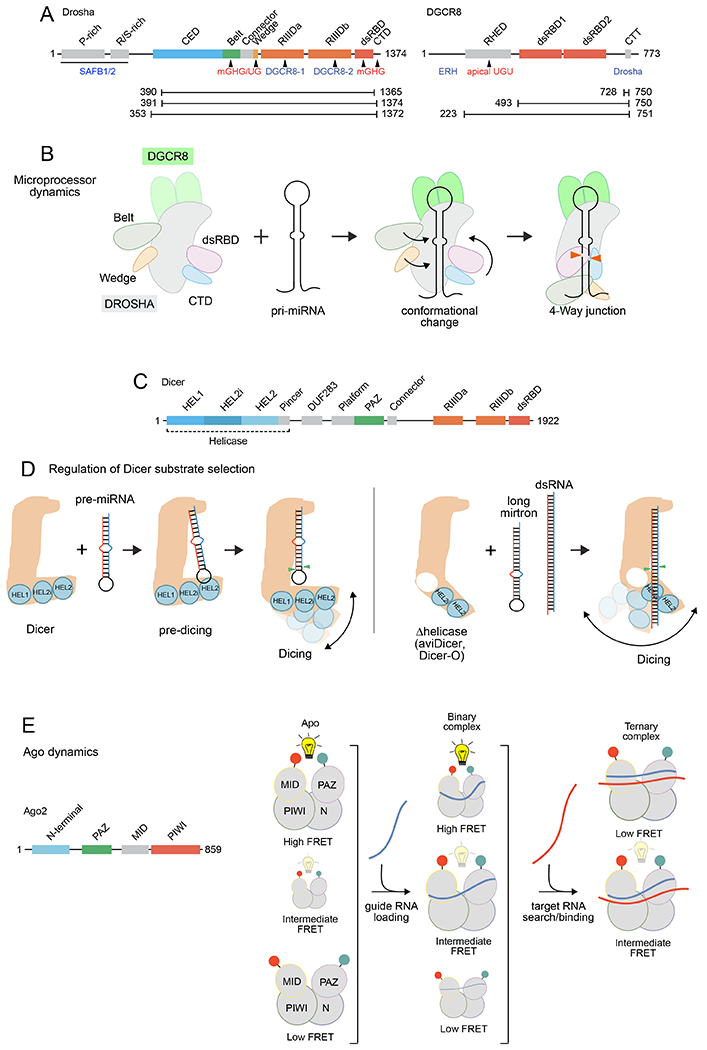
A. Linear domain arrangements of Drosha and DGCR8. The portions that bind other protein factors (blue text) or recognize specific miRNA motifs (red text) are indicated. The different Drosha truncations used in different structural studies are indicated (aa 390-136583, 391-137486, and 353-137287). B. Schematic of structural rearrangements of Microprocessor upon transitioning from Apo state to a partially docked pri-miRNA in the catalytic state. dsRBD, double-stranded RNA binding domain; CTD, C-terminal domain. C. Linear domain arrangement of Dicer. D. Dicer regulation via the N-terminal helicase domain. In mammals, full-length Dicer adopts an L-shape structure and recognizes the 5’ and 3’ termini of pre-miRNAs. (Left) The helicase domain can flexibly interact with the terminal loop region in a pre-dicing state, but when it shifts position, the pre-miRNA can move and align with the main body of Dicer and access the catalytic sites. Due to the overall compact L-shape structure of Dicer during the whole process, only shorter pre-miRNA substrates are efficiently bound and cut by Dicer. (Right) Upon experimental deletion of the helicase domain, or in natural isoforms that remove parts of this region (such as aviDicer and Dicer-O), the Dicer N-terminus remains flexible and does not form a compact L-shape structure. This permits additional substrates to access Dicer for cleavage into small RNAs (such asdsRNA and certain long mirtron hairpins). E. Single-molecule Förster resonance energy transfer (smFRET) visualizes internal structural changes of human Ago2 during target interrogation. (Left) Ago2 domains. (Right) smFRET assays. A FRET donor (green) and an acceptor (red) are incorporated site-specifically into PAZ and MID domains. The energy transfer efficiency (denoted by the brightness of the light bulb above the structures) between the two dyes reports on the physical separation between the two domains, hence the conformational state of Ago2. Three major conformational states of apo Ago2 are indicated by three FRET states, where the protein size corresponds to the relative frequency of each state. Guide strand loading changes the frequencies of conformational states, with only slight changes in their FRET values. However, base pairing with a target reduces the conformational flexibility of Ago2 to only two compact states.
Microprocessor cryo-EM studies
Initial biochemical82 and structural83 analyses utilized truncated Drosha (aa 390–1365) with two copies of short DGCR8 C-terminal peptides (aa 728–750), a minimal functional heterotrimer that is active in Microprocessing in vitro (Fig. 3a). In this model, Drosha alone recognizes the basal junctions and basal UG motif to determine cleavage sites ~11 bp from the basal pri-miRNA junction.
While this minimal complex is active, the original structures did not fully explain microprocessing83. For example, the DGCR8 peptide used lacked the RNA-binding heme domain (RHED) and the dsRNA-binding domains (dsRBDs) (Fig. 3a), and thus does not account for DGCR8-pri-miRNA interactions. Moreover, DGCR8 can dimerize independently of Drosha via its heme-binding RHED domain, which enhances Microprocessor stability as well as pri-miRNA cleavage efficiency and fidelity32,33,84,85. Finally, the crystallized Drosha lacked the N-terminal P-rich and RS-rich regions, whose functions are not fully understood at present (Fig. 3a).
Preparation of full-length recombinant Microprocessor is challenging owing to poor expression and aggregation of full-length proteins. However, cryo-EM enabled analysis of RNA-free, partially RNA-docked and fully RNA-docked structures, revealing dynamic interactions between Microprocessor and pri-miRNA86,87 (Fig. 3b). For example, ~90° rotation of Drosha dsRBD during pri-miRNA loading enables extensive contacts with the pri-miRNA stem in a ravine between dsRBD and RNase IIIa/b domains (Fig. 3b). Moreover, one of the dsRBDs from DGCR8 and dsRBD of Drosha stack into a ruler that measures ~35 bp, providing a molecular basis for pri-miRNA length constraint. The low-resolution electron densities of the DGCR8 RHED domain appear to cap the apical loop87, in line with biochemical data that this region recognizes apical UGU motifs. However, the apical loop electron density lacked RNA in a substantial part of the path, and RHED dimers were not sufficiently resolved. The basal threshold of pri-miRNA is held in place by a four-way junction called the ‘Buckle’, comprised of the Belt, Wedge, dsRBD and C-terminal region of Drosha (Fig. 3b). In addition to fastening the basal pri-miRNA in the fully docked state, the Belt domain acts as a conformational switch. In the RNA-free or partially docked states, the Belt domain occupies the RNA binding cleft, a self-inhibited state of Microprocessor86,87.
The atomic details revealed functionally essential residues and mechanisms for UG and mGHG motif recognition, providing a clear basis for their enrichment in high-throughput screens (Fig. 3a). Finally, these structures reveal insights into the molecular evolution of RNase III enzymes86,87. For example, the structural similarities of human Drosha to Giardia Dicer and human Dicer suggest common evolutionary origins, and features of RNA stem recognition and cleavage are even shared with bacterial RNase III88.
Dicer cryo-EM studies
Dicer RNase III activity is central to miRNA biogenesis, RNAi and antiviral defense89. However, hidden layers of regulation are built into its structures and molecular interactions. Cryo-EM now provides insights into how Dicer utilizes different cofactors, the enigmatic role of its helicase domain (Fig. 3c), selection mechanisms for different small RNAs, and the structural basis for molecular ruler capacity.
Dicer cryo-EM studies from different organisms have recently expanded the rules of pre-miRNA and dsRNA dicing. These include human Dicer and its cofactor TRBP (Transactivation responsive RNA Binding Protein) bound to pre-let-781, human Dicer in a dicing state bound to pre-let-7a-1[GYM]90, mouse Dicer–TRBP bound to pre-mir-15a91, pre-let-7 processing by Drosophila Dicer-1–Loqs-PB complex92 and the structures of Arabidopsis Dicer-like 1 (DCL1) with pri/pre-mir-166f93. Overall, Dicer exhibits a hallmark L-shaped architecture (Fig. 3d). The DExD/H box helicase domain occupies the base; the RNase IIIa/b intramolecular dimer comprises the core, and platform-PAZ domains form the cap. The initial cryo-EM structure of human Dicer exhibits a partially RNA-docked configuration where the helicase, dsRBD, and PAZ helix lock it in a closed state81. Although human TRBP was reported to enhance substrate capture by Dicer94, recent cryo-EM structures indicated that human Dicer can achieve the active dicing state without TRBP90. Transition from pre-dicing to active dicing states involves movement of the pre-miRNA stem into the catalytic centre, aided by conformational changes in the helicase-DUF283, dsRBD, and PAZ helix domains. This is accompanied by sequence-independent electrostatic contacts between PAZ helix and RNA backbone, 5’-phosphate docking into the platform domain, and interactions by dsRBD around the apical junction that include sequence-specific contacts favoring GYM motifs90.
The mouse Dicer complexes are structurally similar to human Dicer, including a pre-catalytic state with partially docked pre-miRNA and a catalytic state with fully docked RNA and disordered N-terminal helicase-DUF283 domains91. However, unlike the human studies, mouse TRBP was shown to promote the catalytic state of Dicer (13% of particles). Importantly, a mouse Dicer mutant lacking the Hel1 subdomain had enhanced, aberrant capacity to fully dock pre-miRNAs with long stems and larger loops (including a subset of mirtrons), resulting in enhanced accumulation of these small RNAs in vivo and animal lethality91. Therefore, partially docked structures may represent a kinetic checkpoint selecting genuine pre-miRNAs from the slew of cellular RNA hairpins and dsRNAs (Fig. 3d).
In contrast to mammalian Dicers, the miRNA-processing fly Dicer-1 acquired a catalytic structure by belting the pre-miRNA backbone with Dicer-1 dsRBD and Loqs-PB dsRBD292. In plants, DCL-1 structural studies highlight the unique and conserved structure-function features of Dicer across distant eukaryotes93. Finally, additional contemporary studies report an array of structures for active siRNA processing by Drosophila Dicer-2/R2D295 or Dicer-2/Loqs-PD96 and Arabidopsis Dicer-like 3 (DCL3)97. Collectively, these studies reveal similar domain arrangements to miRNA-processing Dicers, while showing dynamic structural strategies in regulated siRNA production.
Single-molecule imaging of miRNA factors
Despite providing atomic-level information, cryo-EM and X-ray crystallography yield static snapshots that only indirectly inform molecular dynamics. A complementary approach involves real-time single-molecule imaging, which can directly visualize conformational changes, molecular heterogeneities and transient intermediates otherwise lost in ensemble averaging. Building on the strong history of small RNA biochemistry, in vitro single-molecule imaging has recently gained traction in the small RNA field. Efforts include confirming the heterotrimeric structure of Microprocessor82,83,98, coordinated activities of human Dicer and TRBP94, dsRNA processing by fly Dicer-2/Loqs-PD99, assembly and maturation of Ago/RISC100–102, and finally, target interrogation and gene silencing by Ago/RISC103–110.
Human Dicer and TRBP single-molecule imaging studies revealed a rapid and dynamic selection of pre-miRNAs from a sea of other hairpins, which depends on proper 3’-docking into the PAZ domain94. In Drosophila Dicer-2/Loqs-PD studies, dsRNA translocation, RNA cleavage and dynamic selection of different termini were visualized in real-time99. These examples demonstrate the capacity of single-molecule imaging to dissect and resolve highly dynamic processes, which are not amenable to biochemical, genetics or structural studies.
Although Argonaute proteins have been extensively scrutinized using X-ray crystallography111,112, single-molecule imaging has been particularly useful to elucidate dynamic Ago maturation and function. For example, imaging of dynamic assembly of fly RISC complexes showed that Hsp70/90 chaperones activate RISC by stabilizing open Ago2 confirmation100,101. Single-molecule Förster resonance energy transfer (smFRET) experiments extended these studies by directly imaging internal structural motions within human Ago102,106 (Fig. 3e). The Ago2 structural element Helix-7 divides seed pairing by introducing a kink near the 3’ end of the seed (nts 6–8), which interrupts the pre-formed α-helical arrangement of the 5’ seed region. Helix-7 moves away upon productive seed pairing, and this dynamic reshaping of seed pairing enables Ago to reject inappropriate target sites106. smFRET also visualizes continuously dynamic interactions of the Ago PAZ domain with the miRNA 3’ end within ternary Ago–miRNA–target complexes of various pairing configurations102. Such 3’ dynamics seem particularly relevant to expose the miRNA 3’ end in the presence of 3’ supplementary base-pairing102,113 (which triggers Ago degradation, as discussed in the miRNA turnover section).
Fluorescence colocalization and smFRET experiments also provide insights into target searching by Ago/RISC. For example, kinetic and thermodynamic parameters suggest Ago functions by modifying the free energy landscape of RNA hybridization103. Additional smFRET studies using tandem target sites demonstrated repetitive shuttling of a single human Ago2/RISC by 1D facilitated diffusion, enabling rapid target interrogation compared to the 3D collision between targets and the RISC105. Finally, going beyond in vitro reactions, in vivo single-molecule imaging can visualize the dynamic interplay between mRNA translation and gene silencing by Ago/RISC complexes in living cells108–110. Such studies demonstrate that translation facilitates access of Ago2 to mediate target cleavage within coding regions, and that target site exposure limits the rate of mRNA cleavage by RISC.
Overall, single-molecule imaging shines a light on the highly dynamic and multi-step nature of miRNA processing and regulation.
Regulation of miRNA biogenesis
Typical biogenesis schematics imply an unfettered route to generate miRNAs (Fig. 1). Yet, miRNA production is not constitutive but instead tightly regulated at multiple levels. These can promote or inhibit miRNA production, and can occur at various steps of miRNA biogenesis. Investigation of this topic has benefitted from multiple approaches, including detailed analysis of individual miRNA substrates and factors, as well as large-scale genomics and CRISPR screening (Table 1).
One of the first cases of regulated miRNA biogenesis regards the control of let-7 family maturation by Lin-28 RBPs114–117 (Fig. 4a). These recognize a conserved loop motif in pre-let-7 and recruit terminal uridyltransferase (TUTase, TUT4/7) to oligouridylate pre-let-7118,119, which triggers its degradation via Dis3L2120–122. Amongst the large family of mammalian let-7 miRNA loci, those that are inhibited by Lin-28/TUTase/Dis3L2 are classified as Group I members. Other let-7 members bear suboptimal 1-nt 3’ pre-miRNA overhang following Microprocessor cleavage, rendering them poor Dicer substrates (Group II). But in the absence of Lin-28, such pre-let-7 hairpins can be monouridylated by TUT4/7 to restore a 2-nt overhang that enhances Dicer processing123. 3’-tailing of pre-miRNA can also adjust the site of Dicer cleavage, thereby changing either miRNA seed sequence or even switch which miRNA duplex arm is the dominant functional product124.
Figure 4. Strategies for the regulation of miRNA biogenesis.
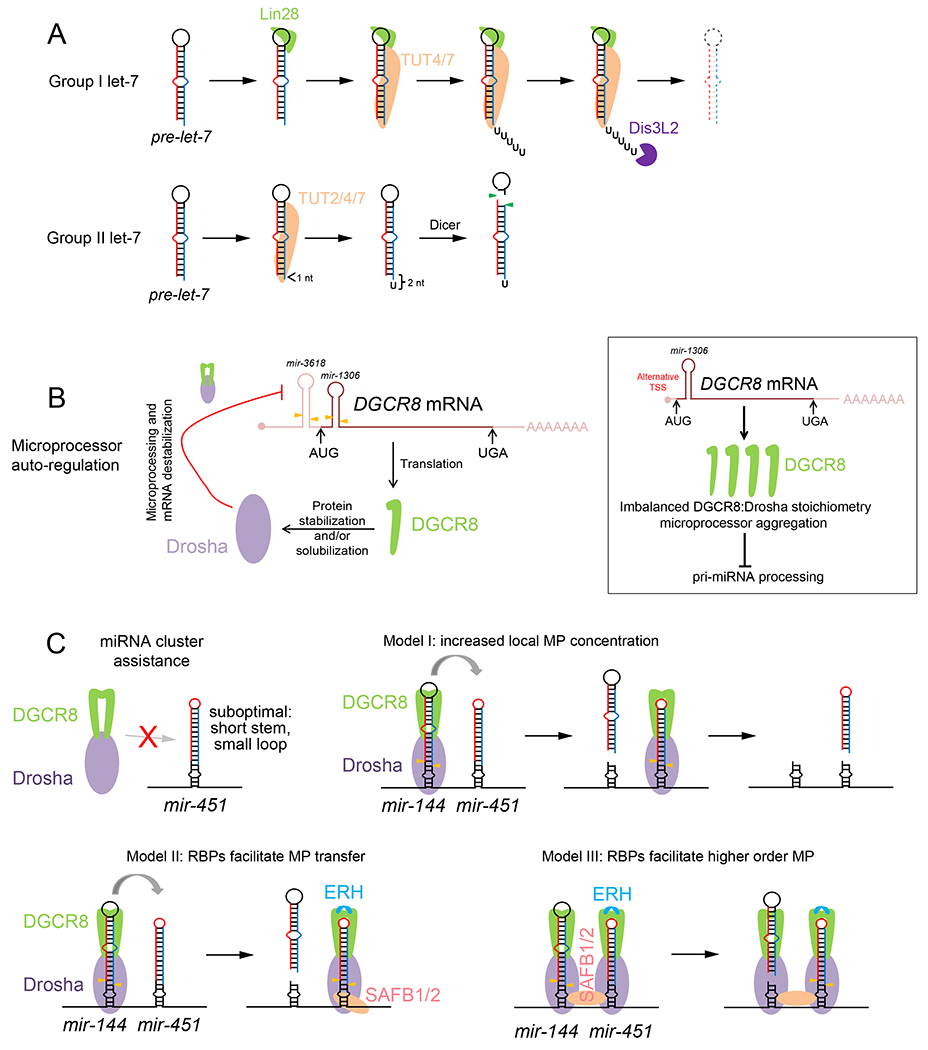
A. Two strategies of regulated maturation of let-7 miRNAs by 3’ tailing. With group I loci, Lin28 binds the terminal loop of pre-let-7 and recruits terminal uridyltransferases (TUT4/7) to oligouridylate its 3’ end, which triggers its degradation via Dis3L2. With group II loci, the initial Drosha-cleaved pre-let-7 only has a 1 nt-3’ overhang, rendering it a suboptimal Dicer substrate. However, this defect can be rescued by 3’-monouridylation via TUT2/4/7 to yield a 2 nt overhang, especially in absence of Lin28. B. Autoregulation of Microprocessor. (Left) The DGCR8 mRNA bears two hairpins within its 5’ UTR and CDS (mir-3618 and mir-1306), which can be cleaved by Drosha (yellow arrowheads). This destabilizes DGCR8 transcripts and reduces protein production. Reciprocally, DGCR8 protein positively regulates Microprocessor by stabilizing or solubilizing Drosha protein. (Right) However, during differentiation of mouse embryonic stem cells, usage of an internal promoter yields a shorter DGCR8 isoform lacking mir-3618, which escapes Microprocessor autoregulation. The resulting accumulation of DGCR8 imbalances DGCR8:Drosha protein stoichiometry, resulting in Microprocessor aggregation and reduced pri-miRNA processing. C. Enhanced biogenesis of suboptimal miRNAs within a genomic cluster. Certain miRNAs that lack optimal Microprocessor features (such as Dicer-independent mir-451) are poorly processed by themselves but are rescued by a neighbouring optimal miRNA (such as the normal context of mir-144/451 operon). Several models remain in play to explain this process, including increased local availability of Microprocessor via the miRNA helper, and various roles involving auxiliary RNA binding proteins (RBPs) such as ERH and SAFB1/2. These might stabilize Microprocessor on a suboptimal hairpin or bridge Microprocessor complexes via dimerization.
The biogenesis of other miRNA precursors is likely to be regulated, as summarized in recent comprehensive reviews125,126. Presumably, the catalogue of miRNA biogenesis regulation will continue to expand, based on several observations. These include that numerous miRNA loci bear conserved loop sequences of unknown functions, but imply regulation127; large-scale CLIP analyses reveal numerous RBP interactions with specific miRNA precursors128; and in vitro pull-down assays of pre-miRNAs with cell lysates also recover many specific RBP and miRNA interactions129. Here, we highlight some recent concepts in regulated miRNA biogenesis.
Cross-regulation and homeostatic control of miRNA pathway factors
miRNA pathway auto-regulation has repeatedly been uncovered in diverse settings. For example, auto-targeting of Dicer by miRNAs is relevant in certain cancers130,131, and other miRNA factors are preferentially targeted by miRNAs132. Another layer of regulation involves control of Ago proteins by miRNA availability, as unloaded Ago proteins are subject to degradation by ubiquitination or autophagy133–136.
A prominent example involves the reciprocal cross-regulation of Drosha and DGCR8 (Fig. 4b). The 5’ UTR and CDS regions of mammalian Dgcr8 bear hairpins (mir-3618 and mir-1306) that are cleaved by Drosha, thereby suppressing DGCR8137. By contrast, DGCR8 stabilizes and solubilizes Drosha through protein–protein interactions. This cross-regulation between Drosha and DGCR8 enables homeostatic control of Microprocessor activity and is conserved in different animal species137–139.
Although the Dgcr8 hairpins are annotated as miRNAs, they do not yield substantial levels of mature miRNA, partly due to nuclear accumulation of the hairpins137. Thus, their main known role is in cis-regulation. Recently, the biological impact of Microprocessor cross-regulation was investigated140. During differentiation of mouse embryonic stem cells, an alternative transcription start site downstream of the first Dgcr8 hairpin yields an isoform that evades Microprocessor auto-regulation and leads to the accumulation of DGCR8 protein. The imbalanced DGCR8:Drosha protein stoichiometry and non-functional Microprocessor aggregation reduced nuclear miRNA biogenesis with an impact on miRNA-mediated repression and embryonic development140.
Regulation of suboptimal miRNAs in genomic clusters
Although most miRNAs are transcribed as single loci, approximately one-third of vertebrate miRNAs reside in genomic clusters, where two or more miRNA hairpins are cleaved from the same primary transcript. The reasons for miRNA clustering are not fully known, but presumed facilitates miRNA co-expression141. miRNA loci from clusters are usually functional when expressed experimentally as solo constructs. Still, cases of miRNA cluster regulation exist, including the mir-17~92 cluster which exhibits stepwise cleavage of different cluster members142,143. Additional studies across different species, and even viruses, reveal that biogenesis of specific miRNAs depends on cluster location144–147.
Recent studies reveal that Microprocessing of suboptimal mammalian miRNA hairpins is enhanced by an optimal miRNA neighbour (Fig. 4c). An archetype for this is mir-451, whose short stem and small terminal loop are integral to its unusual Dicer-independent, Ago2-dependent, biogenesis strategy148 (Fig. 1c). Although these features render it an extremely suboptimal Microprocessor substrate, miR-451 is nonetheless a highly abundant erythroid miRNA149,150 whose nuclear processing is strongly enhanced by proximity to its operon neighbour mir-144151,152. Mechanistically, following efficient recruitment and cleavage of pri-mir-144 by Microprocessor, the released complex is in proximity to cleave nearby pri-mir-451151,152. The identity and relative location of the neighbouring miRNA is not critical for this, and maturation of the neighbouring miRNA can be uncoupled from enhancement of pri-mir-451 cleavage. In particular, substituting the miRNA neighbour with synthetic sites that directly recruit DGCR8 can still enhance miR-451 biogenesis151.
Parallel work on the mir-15a/16 cluster utilized CRISPR–Cas9 screening to identify factors that are selectively required for suboptimal miR-15a. A dual genetic screen was conducted using cells that harbour GFP and RFP transgenes whose 3’ UTRs bear multiple sites for miR-15a and miR-16, respectively153. In this background, CRISPR–Cas9 hits that selectively derepress the reporter for suboptimal miR-15a, while maintaining repression of the miR-16 reporter, comprise candidates for miRNA neighbour enhancement. Amongst several such hits, SAFB1/2 and ERH were confirmed as positive factors in the processing of suboptimal miRNAs152,153.
A pressing question regards the molecular mechanisms by which these factors promote Microprocessor activity. ERH binds DGCR8 directly, and ERH dimerization may assist DGCR8 dimerization within Microprocessor154. A specific domain of SAFB2 interacts with the N-terminal region of Drosha and is sufficient to recapitulate cluster assistance153; however, another study found that the N-terminal region of Drosha is dispensable for cluster assistance152. The discrepancy remains to be resolved. At present, several models seem compatible with the available data (Fig. 4c). In one scenario, after recruitment and release of Microprocessor from the optimal hairpin, Microprocessor can identify the suboptimal hairpin, but its interaction is unstable. In that model, ERH and SAFB1/2 may help to stabilize the complex and activate cleavage of the suboptimal miRNA hairpin. Alternatively, ERH and SAFB1/2 might facilitate dimerization of Microprocessor units to promote processing of suboptimal miRNA hairpins.
Curiously, although mir-144 knockout in K562 human lymphoblast cells illustrates a strong reliance of mir-451 biogenesis on its canonical partner151,152, maturation of miR-451 is only subtly affected in zebrafish mir-144 knockouts150, suggesting that other strategies can enhance nuclear processing of mir-451. Although these remain to be elucidated, it is worth noting that nuclear processing of miRNAs is co-transcriptional155–157, and transcription and chromatin retention can affect Microprocessor activity on pri-miRNA transcripts158–160. Their impact on suboptimal miRNAs deserves further investigation.
Regulation of Dicer activity via its N-terminus/helicase domain
Dicer was initially isolated via cleavage activity on long dsRNA substrates161 and subsequently realized to process pre-miRNA hairpins162. Plants and arthropods encode multiple Dicers, which have distinct preferences for miRNAs and dsRNAs. Although mammals harbour only one Dicer gene, its isoforms exhibit functional diversity. For example, an intronic promoter derived from a rodent-specific retrotransposon insertion yields Dicer-O, an N-terminally truncated Dicer isoform expressed specifically in oocytes163. This isoform lacks part of its helix domain and has enhanced activity to process long dsRNA into endogenous siRNAs. Indeed, mouse oocytes are a unique mammalian setting where siRNAs are not only abundant and functional164,165 but may play a dominant regulatory role instead of miRNAs or piwi-interacting RNAs (piRNAs)166–168. However, as the canonical Dicer isoform is prevented from processing long dsRNA through its N-terminal helix domain, this raises questions as to its involvement in antiviral defense, especially as long dsRNA activates the interferon pathway as a dominant antiviral response169.
Interestingly, experimental deletion of the Dicer N-terminus enhances its capacity to cleave viral dsRNA during infection170. Recently, an endogenous N-terminally truncated Dicer isoform (aviDicer) lacking the helix domain was identified171. aviDicer is generated by alternative splicing and is apparently of low abundance; nevertheless, it is detected in certain stem cells, proposed as settings of antiviral activity by mammalian RNAi172,173. Loss of the helicase domain unlocks Dicer and expands its processing capacity on substrates bearing extended dsRNA stems (Fig. 3d)91. Thus, there may be cell-specific requirements to regulate substrate selection by mammalian Dicer, for endogenous regulatory purposes or to enable restriction of foreign nucleic acids.
Regulation of miRNA turnover
The lifetime of different RNA molecules varies tremendously, and the same RNA can have distinct dynamics in different settings or conditions. Such observations indicate distinct regulatory pathways for RNA decay. However, for many years, this was not thought to be a substantial route for miRNA regulation, as most miRNAs seemed extremely stable. For example, while the average mRNA half-life is 2–4 hours (with some decayed within minutes), the half-life of many miRNAs is days174 to weeks175,176. The stability of mature miRNAs is attributed to their tight association with Ago proteins, which protects them from exoribonucleases. Eventual dissociation of the Ago–miRNA complex results in turnover of unloaded Ago133,135,177 via the Iruka E3 ubiquitin ligase134, along with exoribonucleolytic degradation of free miRNAs (Fig. 5a).
Figure 5. Regulation of miRNA turnover.
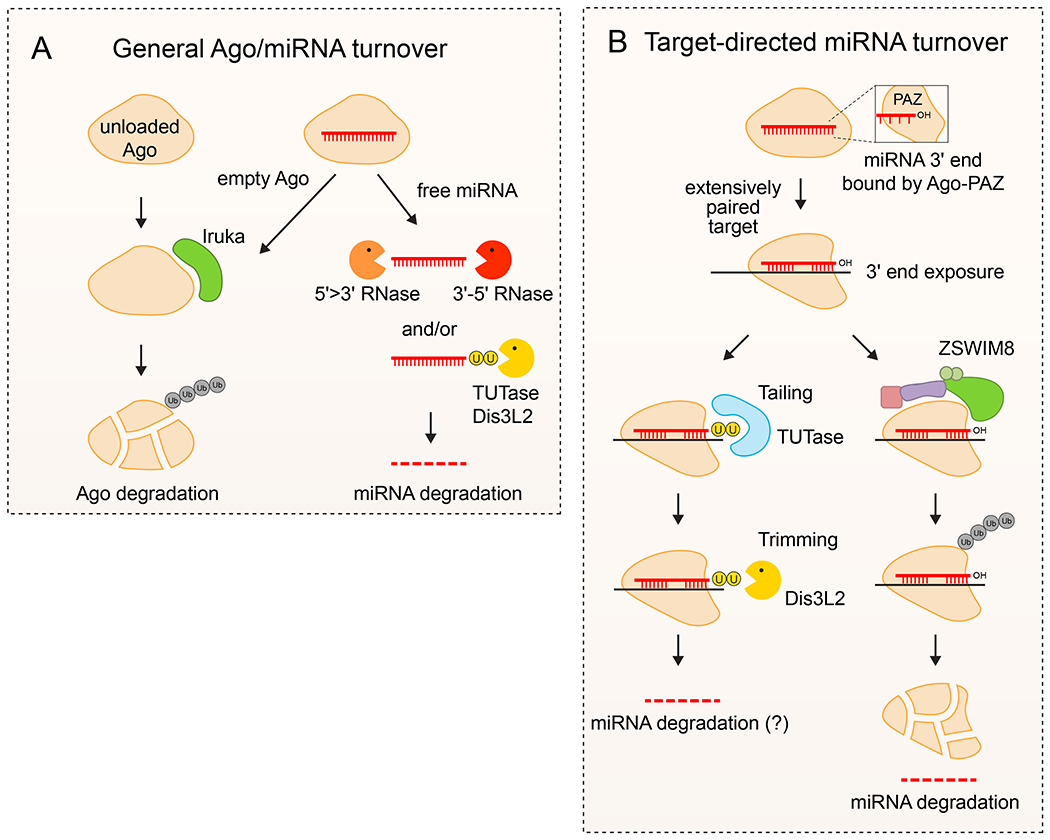
A. General turnover of Ago-miRNA complexes. Ago proteins that are not associated with small RNAs are usually unstable and selectively degraded across species. In principle, these exist prior to miRNA loading, or after ejection of the mature miRNA. Empty Ago proteins are selectively tagged by the E3 ubiquitin ligase Iruka, which signals its degradation by the proteasome. In concert, miRNAs are generally stable within Ago complexes. However, when not shielded by Ago protein, miRNAs are generally unstable since they lack 5’ cap and 3’ polyA tails. Such free miRNAs may be degraded directly by various exoribonucleolytic pathways or may be subject to 3’ tailing by TUTases and degradation by Dis3L2 3’ exoribonuclease. B. Target-directed miRNA degradation (TDMD) is a sequence-specific process by which individual designated miRNAs are degraded upon interaction with highly complementary trigger RNAs. Normally, the miRNA 3’-end is bound by the Ago-PAZ domain. However, when engaged in extensive base-pairing with a TDMD trigger, the miRNA 3’-end is exposed. This provides access to modification by TUTase and potential degradation by Dis3L2; however, tailing is not generally coupled to turnover. Instead, the dominant mechanism for TDMD involves a conformational change of Ago that allows it to be selectively recognized by the ZSWIM8, which recruits a Cullin-RING E3 ubiquitin ligase complex to degrade Ago. The exposed miRNA is then presumably subject to turnover by general exoribonucleases.
Introduction of nucleoside analogues into cells enables temporally-resolved characterization of transcript dynamics. This approach validated the general stability of mature miRNAs and rapid degradation of their duplex partner passenger strands, which are not retained in Ago/RISC176,178. However, these studies also reveal substantial variation in the stability of mature miRNAs, implying strategies for their regulated turnover. In addition, analysis of in vivo settings revealed differences from cultured cells, such as that miRNA turnover is accelerated in neurons179. Several nucleases were indeed reported to degrade miRNAs in different organisms180–184, although the mechanisms that underlie their specificity and action are often not well-understood. Moreover, a dozen years ago, the viral transcripts HSUR1 and m169 were found to specifically degrade cognate miRNAs with extensive complementarity185,186. Later, it was shown mRNA can also serve as triggers for miRNA degradation187. This process became known as target RNA-directed miRNA degradation (TDMD) and was demonstrated to compete with miRNA-directed decay of the mRNA target188.
miRNA tailing
Early studies indicated that engagement of miRNA with a highly complementary target can induce miRNA tailing or trimming188,189. As mentioned above, literature on the inhibitory consequences of tailing and trimming on pre-miRNA, along with studies that connected 3’ untemplated modifications with miRNA dynamics70,190, led to the notion that tailing and trimming processes underlie TDMD. However, careful molecular genetic studies indicate that 3’-untemplated modifications constitute a parallel regulatory pathway largely separate from miRNA decay176. This conclusion was echoed with the analysis of cells that coordinately delete the major known miRNA tailing enzymes (TENT2, TUT4 and TUT7) as well as Dis3L2191,192, in which TDMD triggers retain capacity for miRNA downregulation. Thus, other regulatory mechanisms for TDMD must exist.
TDMD: ZSWIM8 mediates ubiquitination of Ago2 engaged with highly complementary target
Two recent studies used CRISPR screening to elucidate the mechanism of TDMD193,194. Both exploited the endogenous TDMD trigger - the non-coding RNA Cyrano - which is highly complementary and abundantly bound to miR-7195,196. Cyrano mutants strongly upregulate miR-7197, setting the stage for genetic screens for other mutants that similarly upregulate miR-7, recovered via decreased fluorescence from a GFP-miR-7 sensor. Surprisingly, such screens reveal that factors involved in protein turnover, rather than RNA turnover, mediate TDMD193,194. A central factor is ZSWIM8, which binds Ago2 and recruits a multiprotein ElonginB–C-Cul3 ubiquitin ligase complex to tag Ago2 protein for proteasome-mediated turnover (Fig. 5b).
A protein-based mechanism for selective miRNA turnover was unexpected but is in retrospect an appealing strategy. Pairing to the TDMD target releases the miRNA 3’-end, accompanied by structural rearrangement of Ago113 (Fig. 5b). Selective decay of Ago2, which requires surface-exposed lysines193,194, would release the free miRNA for degradation. A future challenge will be to elucidate how ZSWIM8 selectively recognizes the form of Ago2 engaged in TDMD. In addition, a new study concluded that TDMD in C. elegans does not necessarily require 3’-end pairing198. Thus, additional mechanistic surprises for TDMD may await.
The elucidation of TDMD paves the way for understanding its biological impact. TDMD requires extensive base-pairing between target RNA and miRNA sequences in the seed region and 3’ end, but not the central region. This knowledge can be used to prioritize TDMD candidates through bioinformatics or experimental approaches such as Ago-CLASH, where miRNAs are directly ligated to targets199. More importantly, the discovery of the TDMD-specific factor ZSWIM8 opens a more direct path into TDMD networks, since zswim8 mutants across a broad range of species upregulate select cohorts of miRNAs193. Such data pinpoint miRNAs with greatest suppression via TDMD and provide a gauge for the functional efficacy of individual TDMD targets.
Although the field is young, several studies identified endogenous TDMD triggers whose miRNA binding sites induce substantial miRNA degradation. In mouse brain, knockout of the lncRNA and TDMD trigger Cyrano, or even specific knockout of the Cyrano miR-7 binding site, induces miR-7 levels 40-50-fold197. However, Cyrano knockout mice have only subtly increased repression of miR-7 targets and appear phenotypically normal. Disrupting the TDMD site of miR-29b in the 3’-UTR of mouse NREP increases miR-29b levels and impairs coordination and motor learning200. Finally, in D. melanogaster, multiple functional TDMD targets were validated, for which mutation of endogenous highly complementary target sites upregulates the cognate miRNAs201. Notably, disruption of a TDMD trigger (the lncRNA Marge) for miR-310 family miRNAs results in defective embryonic cuticle formation201, demonstrating biological significance.
Although ZSWIM8 clearly mediates TDMD restriction of miRNA accumulation, this is not to say that tailing or trimming does not impact cellular miRNA pools. For example, 3’-adenylation can stabilize certain miRNAs202,203, but has also been suggested to induce miRNA degradation181. Recently, it was reported that mutation of the miRNA deadenylase USB1 in the disease poikiloderma with neutropenia (PN) leads to miRNA downregulation and derepression of miRNA targets204. Overall, as discussed with miRNA uridylation, the impact of miRNA adenylation on their in vivo accumulation is not straightforward to predict, and may not necessarily affect levels205. Further study of the functional impacts of tailing enzymes are needed for a comprehensive understanding of their positive and negative roles in shaping miRNA pools206,207.
Conclusions and future challenges
The application of new techniques to the miRNA field continues to yield fundamental insights into the operation of core miRNA factors and substrates, as well as new layers of regulated miRNA processing and function. For most of the experimental strategies discussed, there are clear next steps to follow. For example, only a few miRNAs were assessed in structural studies of miRNA biogenesis factors (Fig. 3a–d). As endogenous miRNAs vary widely in their biogenesis efficiency (Fig. 2a–c), including diverse miRNAs in future cryo-EM studies will be informative for both Microprocessor and Dicer substrates. This may yield insights into how Microprocessor cofactors such as ERH and SAFB contribute to the biogenesis of suboptimal and/or clustered miRNAs (Fig. 4c).
Single-molecule imaging uniquely provides direct visualization of dynamic miRNA processing and regulatory complexes. However, it is notable that in vitro studies of Microprocessor have been notably lacking (compared to Dicer and Ago), while in vivo assays have mostly focused on Ago (Fig. 3e) with little application to miRNA biogenesis factors thus far. In particular, the subcellular contexts of Microprocessor156–160 and Dicer208,209 are functionally relevant and should be explored further in the future.
As mentioned, miRNA biogenesis is often not a straight shot from primary transcript to mature small RNA (Fig. 1) but can be highly regulated (Fig. 4). There is surely much more to learn, given the existence of numerous phylogenetically conserved miRNA hairpin loops127 and extensive catalogues of specific associations between RBPs and miRNA precursors128,129, which generally remain to be studied. For instance, recent work shows that mir-144, the canonical partner of Dicer-independent mir-451, is itself highly regulated. This involves structural rearrangement of the pre-mir-144 stem into a Dicer-competent structure, mediated by a conserved loop motif and the dsRBD factor ILF3210. Undoubtedly, additional mechanisms of positive and negative regulation in miRNA biogenesis will be elucidated in the future.
Finally, we must not lose sight of the implications of miRNA biology, such as whether the regulation of defined miRNA targets truly mediate organismal phenotypes. To date, most studies of miRNA–target biology remain correlative, and the mere fact that numerous derepressed targets can be detected upon miRNA loss does not necessarily mean they all contribute functionally to miRNA phenotypes. Recently, CRISPR mutagenesis has validated a growing number of TDMD target sites that genuinely restrict miRNA abundance197,200,201 (Fig. 5), as well as demonstrated strong phenotypic impact of target mRNAs via defined miRNA sites211–214. Ultimately, achieving such direct connections between target site-mediated gene regulation and in vivo phenotype are required to fully understand miRNA function in development and disease.
Acknowledgements
The authors thank Gaspare La Rocca, Ben Kleaveland, and Leemor Joshua-Tor for critical reading, and the referees for informative comments. S.L. was supported by a training award from the NYSTEM contract #C32559GG and the Center for Stem Cell Biology at MSK. Work in E.C.L.’s group was supported by the National Institutes of Health (R01-GM083300) and MSK Core Grant P30-CA008748. We apologize to those whose work is not included owing to space constraints.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Lee RC, Feinbaum RL & Ambros V The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Wightman B, Ha I & Ruvkun G Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Leviten MW, Lai EC & Posakony JW The Drosophila gene Bearded encodes a novel small protein and shares 3’ UTR sequence motifs with multiple Enhancer of split Complex genes. Development 124, 4039–4051 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Lai EC & Posakony JW The Bearded box, a novel 3’ UTR sequence motif, mediates negative post-transcriptional regulation of Bearded and Enhancer of split Complex gene expression. Development 124, 4847–4856 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Lai EC, Burks C & Posakony JW The K box, a conserved 3’ UTR sequence motif, negatively regulates accumulation of Enhancer of split Complex transcripts. Development 125, 4077–4088 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Lai EC microRNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nature genetics 30, 363–364 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Lau N, Lim L, Weinstein E & Bartel DP An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858–862 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Lee RC & Ambros V An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Lagos-Quintana M, Rauhut R, Lendeckel W & Tuschl T Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP Metazoan MicroRNAs. Cell 173, 20–51, doi: 10.1016/j.cell.2018.03.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czech B & Hannon GJ Small RNA sorting: matchmaking for Argonautes. Nature reviews. Genetics 12, 19–31, doi:nrg2916 [pii]: 10.1038/nrg2916 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JS & Lai EC Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Molecular cell 43, 892–903 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurin T, Cazalla D, Yang JS, Bortolamiol-Becet D & Lai EC RNase III-independent microRNA biogenesis in mammalian cells. RNA 18, 2166–2173, doi: 10.1261/rna.036194.112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang R et al. Ribozyme-enhanced single-stranded Ago2-processed interfering RNA triggers efficient gene silencing with fewer off-target effects. Nature communications 6, 8430, doi: 10.1038/ncomms9430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai EC, Tam B & Rubin GM Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs. Genes & development 19, 1067–1080 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BP, Burge CB & Bartel DP Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Brennecke J, Stark A, Russell RB & Cohen SM Principles of MicroRNA-Target Recognition. PLoS biology 3, e85 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Sheng G, Juranek S, Tuschl T & Patel DJ Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schirle NT & MacRae IJ The crystal structure of human Argonaute2. Science 336, 1037–1040, doi: 10.1126/science.1221551 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkayam E et al. The Structure of Human Argonaute-2 in Complex with miR-20a. Cell 150, 100–110, doi: 10.1016/j.cell.2012.05.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Meister G et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Molecular cell 15, 185–197 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Park MS et al. Human Argonaute3 has slicer activity. Nucleic acids research 45, 11867–11877, doi: 10.1093/nar/gkx916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park MS, Sim G, Kehling AC & Nakanishi K Human Argonaute2 and Argonaute3 are catalytically activated by different lengths of guide RNA. Proceedings of the National Academy of Sciences of the United States of America 117, 28576–28578, doi: 10.1073/pnas.2015026117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HM, Nguyen TD, Nguyen TL & Nguyen TA Orientation of Human Microprocessor on Primary MicroRNAs. Biochemistry 58, 189–198, doi: 10.1021/acs.biochem.8b00944 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Han J et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Zeng Y & Cullen BR Efficient processing of primary microRNA hairpins by Drosha requires flanking non-structured RNA sequences. The Journal of biological chemistry (2005). [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Yi R & Cullen BR Recognition and cleavage of primary microRNA precursors by the nuclear processing enzyme Drosha. The EMBO journal 24, 138–148 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Wu Y, Choi JG & Wu H Lower and upper stem-single-stranded RNA junctions together determine the Drosha cleavage site. Proceedings of the National Academy of Sciences of the United States of America 110, 20687–20692, doi: 10.1073/pnas.1311639110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auyeung VC, Ulitsky I, McGeary SE & Bartel DP Beyond Secondary Structure: Primary-Sequence Determinants License Pri-miRNA Hairpins for Processing. Cell 152, 844–858, doi: 10.1016/j.cell.2013.01.031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang W & Bartel DP The Menu of Features that Define Primary MicroRNAs and Enable De Novo Design of MicroRNA Genes. Molecular cell 60, 131–145, doi: 10.1016/j.molcel.2015.08.015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partin AC et al. Heme enables proper positioning of Drosha and DGCR8 on primary microRNAs. Nature communications 8, 1737, doi: 10.1038/s41467-017-01713-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TA, Park J, Dang TL, Choi YG & Kim VN Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic acids research 46, 5726–5736, doi: 10.1093/nar/gky248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Nguyen TD, Li S & Nguyen TA SRSF3 recruits DROSHA to the basal junction of primary microRNAs. RNA 24, 892–898, doi: 10.1261/rna.065862.118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez N et al. Genetic variation and RNA structure regulate microRNA biogenesis. Nature communications 8, 15114, doi: 10.1038/ncomms15114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Nguyen TD, Nguyen TL & Nguyen TA Mismatched and wobble base pairs govern primary microRNA processing by human Microprocessor. Nature communications 11, 1926, doi: 10.1038/s41467-020-15674-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Le TN, Nguyen TD, Trinh TA & Nguyen TA Bulges control pri-miRNA processing in a position and strand-dependent manner. RNA biology 18, 1716–1726, doi: 10.1080/15476286.2020.1868139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YY, Kim H & Kim VN Sequence determinant of small RNA production by DICER. Nature 615, 323–330, doi: 10.1038/s41586-023-05722-4 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Rice GM, Shivashankar V, Ma EJ, Baryza JL & Nutiu R Functional Atlas of Primary miRNA Maturation by the Microprocessor. Molecular cell 80, 892–902 e894, doi: 10.1016/j.molcel.2020.10.028 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Kim K et al. A quantitative map of human primary microRNA processing sites. Molecular cell 81, 3422–3439 e3411, doi: 10.1016/j.molcel.2021.07.002 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Kang W et al. MapToCleave: High-throughput profiling of microRNA biogenesis in living cells. Cell reports 37, 110015, doi: 10.1016/j.celrep.2021.110015 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Chiang HR et al. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes & development 24, 992–1009, doi:gad.1884710 [pii] 10.1101/gad.1884710 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consortium R RNAcentral 2021: secondary structure integration, improved sequence search and new member databases. Nucleic acids research 49, D212–D220, doi: 10.1093/nar/gkaa921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fromm B, Zhong X, Tarbier M, Friedlander MR & Hackenberg M The limits of human microRNA annotation have been met. RNA, doi: 10.1261/rna.079098.122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen TL, Nguyen TD, Bao S, Li S & Nguyen TA The internal loops in the lower stem of primary microRNA transcripts facilitate single cleavage of human Microprocessor. Nucleic acids research 48, 2579–2593, doi: 10.1093/nar/gkaa018 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo QJ et al. RNA structure probing reveals the structural basis of Dicer binding and cleavage. Nature communications 12, 3397, doi: 10.1038/s41467-021-23607-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Kolb FA, Jaskiewicz L, Westhof E & Filipowicz W Single processing center models for human Dicer and bacterial RNase III. Cell 118, 57–68 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Macrae IJ et al. Structural basis for double-stranded RNA processing by Dicer. Science 311, 195–198, doi:311/5758/195 [pii] 10.1126/science.1121638 (2006). [DOI] [PubMed] [Google Scholar]
- 49.MacRae IJ, Zhou K & Doudna JA Structural determinants of RNA recognition and cleavage by Dicer. Nature structural & molecular biology 14, 934–940, doi:nsmb1293 [pii] 10.1038/nsmb1293 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Vermeulen A et al. The contributions of dsRNA structure to Dicer specificity and efficiency. RNA 11, 674–682, doi: 10.1261/rna.7272305 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Kolb FA, Brondani V, Billy E & Filipowicz W Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. The EMBO journal 21, 5875–5885, doi: 10.1093/emboj/cdf582 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JE et al. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 475, 201–205, doi: 10.1038/nature10198:nature10198 [pii] (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu S et al. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell 151, 900–911, doi: 10.1016/j.cell.2012.09.042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen TD, Trinh TA, Bao S & Nguyen TA Secondary structure RNA elements control the cleavage activity of DICER. Nature communications 13, 2138, doi: 10.1038/s41467-022-29822-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank F, Sonenberg N & Nagar B Structural basis for 5’-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822, doi:nature09039 [pii]: 10.1038/nature09039 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Boland A, Tritschler F, Heimstadt S, Izaurralde E & Weichenrieder O Crystal structure and ligand binding of the MID domain of a eukaryotic Argonaute protein. EMBO reports 11, 522–527, doi:embor201081 [pii]: 10.1038/embor.2010.81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schirle NT, Sheu-Gruttadauria J, Chandradoss SD, Joo C & MacRae IJ Water-mediated recognition of t1-adenosine anchors Argonaute2 to microRNA targets. eLife 4, doi: 10.7554/eLife.07646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin C et al. Expanding the MicroRNA Targeting Code: Functional Sites with Centered Pairing. Molecular cell 38, 789–802, doi:S1097-2765(10)00446-6 [pii]: 10.1016/j.molcel.2010.06.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi SW, Hannon GJ & Darnell RB An alternative mode of microRNA target recognition. Nature structural & molecular biology 19, 321–327, doi: 10.1038/nsmb.2230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lal A et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3’UTR microRNA recognition elements. Molecular cell 35, 610–625, doi:S1097-2765(09)00600-5 [pii]: 10.1016/j.molcel.2009.08.020 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loeb GB et al. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Molecular cell 48, 760–770, doi: 10.1016/j.molcel.2012.10.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hafner M et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141, doi:S0092-8674(10)00245-X [pii]: 10.1016/j.cell.2010.03.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker WR et al. High-Throughput Analysis Reveals Rules for Target RNA Binding and Cleavage by AGO2. Molecular cell 75, 741–755 e711, doi: 10.1016/j.molcel.2019.06.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGeary SE et al. The biochemical basis of microRNA targeting efficacy. Science 366, doi: 10.1126/science.aav1741 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGeary SE, Bisaria N, Pham TM, Wang PY & Bartel DP MicroRNA 3’-compensatory pairing occurs through two binding modes, with affinity shaped by nucleotide identity and position. eLife 11, doi: 10.7554/eLife.69803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimson A et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell 27, 91–105 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu S, Jin L, Zhang F, Sarnow P & Kay MA Biological basis for restriction of microRNA targets to the 3’ untranslated region in mammalian mRNAs. Nature structural & molecular biology 16, 144–150 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnall-Levin M et al. Unusually effective microRNA targeting within repeat-rich coding regions of mammalian mRNAs. Genome research 21, 1395–1403, doi:gr.121210.111 [pii]: 10.1101/gr.121210.111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang K et al. A novel class of microRNA-recognition elements that function only within open reading frames. Nature structural & molecular biology 25, 1019–1027, doi: 10.1038/s41594-018-0136-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu S & Kim VN A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing. Nature reviews. Molecular cell biology, doi: 10.1038/s41580-020-0246-8 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Yang A et al. 3’ Uridylation Confers miRNAs with Non-canonical Target Repertoires. Molecular cell 75, 511–522 e514, doi: 10.1016/j.molcel.2019.05.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheu-Gruttadauria J, Xiao Y, Gebert LF & MacRae IJ Beyond the seed: structural basis for supplementary microRNA targeting by human Argonaute2. The EMBO journal 38, e101153, doi: 10.15252/embj.2018101153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sim G et al. Manganese-dependent microRNA trimming by 3’-->5’ exonucleases generates 14-nucleotide or shorter tiny RNAs. Proceedings of the National Academy of Sciences of the United States of America 119, e2214335119, doi: 10.1073/pnas.2214335119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim HH et al. HuR recruits let-7/RISC to repress c-Myc expression. Genes & development 23, 1743–1748, doi: 10.1101/gad.1812509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sternburg EL, Estep JA, Nguyen DK, Li Y & Karginov FV Antagonistic and cooperative AGO2-PUM interactions in regulating mRNAs. Scientific reports 8, 15316, doi: 10.1038/s41598-018-33596-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kedde M et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Kim S et al. The regulatory impact of RNA-binding proteins on microRNA targeting. Nature communications 12, 5057, doi: 10.1038/s41467-021-25078-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y et al. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song JJ, Smith SK, Hannon GJ & Joshua-Tor L Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305, 1434–1437, doi: 10.1126/science.1102514: 1102514 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 81.Liu Z et al. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-miRNA Substrate. Cell 173, 1191–1203 e1112, doi: 10.1016/j.cell.2018.03.080 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Nguyen TA et al. Functional Anatomy of the Human Microprocessor. Cell 161, 1374–1387, doi: 10.1016/j.cell.2015.05.010 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Kwon SC et al. Structure of Human DROSHA. Cell 164, 81–90, doi: 10.1016/j.cell.2015.12.019 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Barr I et al. DiGeorge critical region 8 (DGCR8) is a double-cysteine-ligated heme protein. The Journal of biological chemistry 286, 16716–16725, doi: 10.1074/jbc.M110.180844 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quick-Cleveland J et al. The DGCR8 RNA-binding heme domain recognizes primary microRNAs by clamping the hairpin. Cell reports 7, 1994–2005, doi: 10.1016/j.celrep.2014.05.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin W, Wang J, Liu CP, Wang HW & Xu RM Structural Basis for pri-miRNA Recognition by Drosha. Molecular cell 78, 423–433 e425, doi: 10.1016/j.molcel.2020.02.024 (2020). [DOI] [PubMed] [Google Scholar]
- 87.Partin AC et al. Cryo-EM Structures of Human Drosha and DGCR8 in Complex with Primary MicroRNA. Molecular cell 78, 411–422 e414, doi: 10.1016/j.molcel.2020.02.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Z, Nicholson RH, Jaggi R & Nicholson AW Characterization of Aquifex aeolicus ribonuclease III and the reactivity epitopes of its pre-ribosomal RNA substrates. Nucleic acids research 39, 2756–2768, doi: 10.1093/nar/gkq1030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carmell MA & Hannon GJ RNase III enzymes and the initiation of gene silencing. Nature structural & molecular biology 11, 214–218 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Lee YY, Lee H, Kim H, Kim VN & Roh SH Structure of the human DICER-pre-miRNA complex in a dicing state. Nature 615, 331–338, doi: 10.1038/s41586-023-05723-3 (2023). [DOI] [PubMed] [Google Scholar]
- 91.Zapletal D et al. Structural and functional basis of mammalian microRNA biogenesis by Dicer. Molecular cell 82, 4064–4079 e4013, doi: 10.1016/j.molcel.2022.10.010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jouravleva K et al. Structural basis of microRNA biogenesis by Dicer-1 and its partner protein Loqs-PB. Molecular cell 82, 4049–4063 e4046, doi: 10.1016/j.molcel.2022.09.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei X et al. Structural basis of microRNA processing by Dicer-like 1. Nat Plants 7, 1389–1396, doi: 10.1038/s41477-021-01000-1 (2021). [DOI] [PubMed] [Google Scholar]
- 94.Fareh M et al. TRBP ensures efficient Dicer processing of precursor microRNA in RNA-crowded environments. Nature communications 7, 13694, doi: 10.1038/ncomms13694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamaguchi S et al. Structure of the Dicer-2-R2D2 heterodimer bound to a small RNA duplex. Nature 607, 393–398, doi: 10.1038/s41586-022-04790-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su S et al. Structural insights into dsRNA processing by Drosophila Dicer-2-Loqs-PD. Nature 607, 399–406, doi: 10.1038/s41586-022-04911-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Q et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science 374, 1152–1157, doi: 10.1126/science.abl4546 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herbert KM et al. A heterotrimer model of the complete Microprocessor complex revealed by single-molecule subunit counting. RNA 22, 175–183, doi: 10.1261/rna.054684.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naganuma M, Tadakuma H & Tomari Y Single-molecule analysis of processive double-stranded RNA cleavage by Drosophila Dicer-2. Nature communications 12, 4268, doi: 10.1038/s41467-021-24555-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iwasaki S et al. Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature 521, 533–536, doi: 10.1038/nature14254 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Tsuboyama K, Tadakuma H & Tomari Y Conformational Activation of Argonaute by Distinct yet Coordinated Actions of the Hsp70 and Hsp90 Chaperone Systems. Molecular cell 70, 722–729 e724, doi: 10.1016/j.molcel.2018.04.010 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Willkomm S et al. Single-molecule FRET uncovers hidden conformations and dynamics of human Argonaute 2. Nature communications 13, 3825, doi: 10.1038/s41467-022-31480-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salomon WE, Jolly SM, Moore MJ, Zamore PD & Serebrov V Single-Molecule Imaging Reveals that Argonaute Reshapes the Binding Properties of Its Nucleic Acid Guides. Cell 162, 84–95, doi: 10.1016/j.cell.2015.06.029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao C, Sasaki HM, Ueda T, Tomari Y & Tadakuma H Single-Molecule Analysis of the Target Cleavage Reaction by the Drosophila RNAi Enzyme Complex. Molecular cell 59, 125–132, doi: 10.1016/j.molcel.2015.05.015 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Chandradoss SD, Schirle NT, Szczepaniak M, MacRae IJ & Joo C A Dynamic Search Process Underlies MicroRNA Targeting. Cell 162, 96–107, doi: 10.1016/j.cell.2015.06.032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klum SM, Chandradoss SD, Schirle NT, Joo C & MacRae IJ Helix-7 in Argonaute2 shapes the microRNA seed region for rapid target recognition. The EMBO journal 37, 75–88, doi: 10.15252/embj.201796474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cui TJ et al. Argonaute bypasses cellular obstacles without hindrance during target search. Nature communications 10, 4390, doi: 10.1038/s41467-019-12415-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruijtenberg S et al. mRNA structural dynamics shape Argonaute-target interactions. Nature structural & molecular biology 27, 790–801, doi: 10.1038/s41594-020-0461-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cialek CA et al. Imaging translational control by Argonaute with single-molecule resolution in live cells. Nature communications 13, 3345, doi: 10.1038/s41467-022-30976-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kobayashi H & Singer RH Single-molecule imaging of microRNA-mediated gene silencing in cells. Nature communications 13, 1435, doi: 10.1038/s41467-022-29046-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sheu-Gruttadauria J & MacRae IJ Structural Foundations of RNA Silencing by Argonaute. Journal of molecular biology 429, 2619–2639, doi: 10.1016/j.jmb.2017.07.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakanishi K Anatomy of four human Argonaute proteins. Nucleic acids research 50, 6618–6638, doi: 10.1093/nar/gkac519 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheu-Gruttadauria J et al. Structural Basis for Target-Directed MicroRNA Degradation. Molecular cell, doi: 10.1016/j.molcel.2019.06.019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Newman MA, Thomson JM & Hammond SM Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heo I et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Molecular cell 32, 276–284 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Viswanathan SR, Daley GQ & Gregory RI Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rybak A et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature cell biology 10, 987–993 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Heo I et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Thornton JE, Chang HM, Piskounova E & Gregory RI Lin28-mediated control of let-7 microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). RNA 18, 1875–1885, doi: 10.1261/rna.034538.112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang HM, Triboulet R, Thornton JE & Gregory RI A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature, doi: 10.1038/nature12119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Faehnle CR, Walleshauser J & Joshua-Tor L Mechanism of Dis3l2 substrate recognition in the Lin28-let-7 pathway. Nature 514, 252–256, doi: 10.1038/nature13553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ustianenko D et al. Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs. RNA 19, 1632–1638, doi: 10.1261/rna.040055.113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heo I et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell 151, 521–532, doi: 10.1016/j.cell.2012.09.022 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Kim H et al. A Mechanism for microRNA Arm Switching Regulated by Uridylation. Molecular cell 78, 1224–1236 e1225, doi: 10.1016/j.molcel.2020.04.030 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Michlewski G & Caceres JF Post-transcriptional control of miRNA biogenesis. RNA 25, 1–16, doi: 10.1261/rna.068692.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Treiber T, Treiber N & Meister G Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nature reviews. Molecular cell biology 20, 5–20, doi: 10.1038/s41580-018-0059-1 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Michlewski G, Guil S, Semple CA & Caceres JF Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Molecular cell 32, 383–393, doi:S1097-2765(08)00728-4 [pii]: 10.1016/j.molcel.2008.10.013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nussbacher JK & Yeo GW Systematic Discovery of RNA Binding Proteins that Regulate MicroRNA Levels. Molecular cell 69, 1005–1016 e1007, doi: 10.1016/j.molcel.2018.02.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Treiber T et al. A Compendium of RNA-Binding Proteins that Regulate MicroRNA Biogenesis. Molecular cell 66, 270–284 e213, doi: 10.1016/j.molcel.2017.03.014 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Tokumaru S, Suzuki M, Yamada H, Nagino M & Takahashi T let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis 29, 2073–2077, doi: 10.1093/carcin/bgn187 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Martello G et al. A MicroRNA targeting dicer for metastasis control. Cell 141, 1195–1207, doi:S0092-8674(10)00553-2 [pii]: 10.1016/j.cell.2010.05.017 (2010). [DOI] [PubMed] [Google Scholar]
- 132.Wang D et al. Uncovering the cellular capacity for intensive and specific feedback self-control of the argonautes and MicroRNA targeting activity. Nucleic acids research 48, 4681–4697, doi: 10.1093/nar/gkaa209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smibert P, Yang JS, Azzam G, Liu JL & Lai EC Homeostatic control of Argonaute stability by microRNA availability. Nature structural & molecular biology 20, 789–795, doi: 10.1038/nsmb.2606 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kobayashi H, Shoji K, Kiyokawa K, Negishi L & Tomari Y Iruka Eliminates Dysfunctional Argonaute by Selective Ubiquitination of Its Empty State. Molecular cell 73, 119–129 e115, doi: 10.1016/j.molcel.2018.10.033 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Martinez NJ & Gregory RI Argonaute2 expression is post-transcriptionally coupled to microRNA abundance. RNA 19, 605–612, doi: 10.1261/rna.036434.112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Derrien B et al. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proceedings of the National Academy of Sciences of the United States of America 109, 15942–15946, doi: 10.1073/pnas.1209487109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Han J et al. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 136, 75–84 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kadener S et al. Genome-wide identification of targets of the drosha-pasha/DGCR8 complex. RNA 15, 537–545, doi:rna.1319309 [pii]: 10.1261/rna.1319309 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smibert P et al. A Drosophila genetic screen yields allelic series of core microRNA biogenesis factors and reveals post-developmental roles for microRNAs. RNA 17, 1997–2010, doi:rna.2983511 [pii]: 10.1261/rna.2983511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cui Y et al. Global miRNA dosage control of embryonic germ layer specification. Nature 593, 602–606, doi: 10.1038/s41586-021-03524-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baskerville S & Bartel DP Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11, 241–247 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du P, Wang L, Sliz P & Gregory RI A Biogenesis Step Upstream of Microprocessor Controls miR-17 approximately 92 Expression. Cell 162, 885–899, doi: 10.1016/j.cell.2015.07.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Donayo AO et al. Oncogenic Biogenesis of pri-miR-17-92 Reveals Hierarchy and Competition among Polycistronic MicroRNAs. Molecular cell 75, 340–356, doi: 10.1016/j.molcel.2019.05.033 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Truscott M, Islam AB & Frolov MV Novel regulation and functional interaction of polycistronic miRNAs. RNA 22, 129–138, doi: 10.1261/rna.053264.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lataniotis L et al. CRISPR/Cas9 editing reveals novel mechanisms of clustered microRNA regulation and function. Scientific reports 7, 8585, doi: 10.1038/s41598-017-09268-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haar J et al. The expression of a viral microRNA is regulated by clustering to allow optimal B cell transformation. Nucleic acids research 44, 1326–1341, doi: 10.1093/nar/gkv1330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vilimova M et al. Cis regulation within a cluster of viral microRNAs. Nucleic acids research 49, 10018–10033, doi: 10.1093/nar/gkab731 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang JS & Lai EC Dicer-independent, Ago2-mediated microRNA biogenesis in vertebrates. Cell cycle 9, 4455–4460, doi:13958 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jee D et al. Dual Strategies for Argonaute2-Mediated Biogenesis of Erythroid miRNAs Underlie Conserved Requirements for Slicing in Mammals. Molecular cell 69, 265–278 e266, doi: 10.1016/j.molcel.2017.12.027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kretov DA et al. Ago2-Dependent Processing Allows miR-451 to Evade the Global MicroRNA Turnover Elicited during Erythropoiesis. Molecular cell 78, 317–328 e316, doi: 10.1016/j.molcel.2020.02.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shang R et al. Genomic Clustering Facilitates Nuclear Processing of Suboptimal Pri-miRNA Loci. Molecular cell 78, 303–316 e304, doi: 10.1016/j.molcel.2020.02.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fang W & Bartel DP MicroRNA Clustering Assists Processing of Suboptimal MicroRNA Hairpins through the Action of the ERH Protein. Molecular cell 78, 289–302 e286, doi: 10.1016/j.molcel.2020.01.026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hutter K et al. SAFB2 Enables the Processing of Suboptimal Stem-Loop Structures in Clustered Primary miRNA Transcripts. Molecular cell 78, 876–889, doi: 10.1016/j.molcel.2020.05.011 (2020). [DOI] [PubMed] [Google Scholar]
- 154.Kwon SC et al. ERH facilitates microRNA maturation through the interaction with the N-terminus of DGCR8. Nucleic acids research 48, 11097–11112, doi: 10.1093/nar/gkaa827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim YK & Kim VN Processing of intronic microRNAs. The EMBO journal 26, 775–783 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ballarino M et al. Coupled RNA processing and transcription of intergenic primary microRNAs. Molecular and cellular biology 29, 5632–5638, doi:MCB.00664-09 [pii]: 10.1128/MCB.00664-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morlando M et al. Primary microRNA transcripts are processed co-transcriptionally. Nature structural & molecular biology 15, 902–909 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu H et al. HP1BP3, a Chromatin Retention Factor for Co-transcriptional MicroRNA Processing. Molecular cell 63, 420–432, doi: 10.1016/j.molcel.2016.06.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Church VA et al. Microprocessor Recruitment to Elongating RNA Polymerase II Is Required for Differential Expression of MicroRNAs. Cell reports 20, 3123–3134, doi: 10.1016/j.celrep.2017.09.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pawlicki JM & Steitz JA Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. The Journal of cell biology 182, 61–76 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bernstein E, Caudy A, Hammond S & Hannon G Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001). [DOI] [PubMed] [Google Scholar]
- 162.Hutvagner G et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001). [DOI] [PubMed] [Google Scholar]
- 163.Flemr M et al. A retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 155, 807–816, doi: 10.1016/j.cell.2013.10.001 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Watanabe T et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543, doi: 10.1038/nature06908 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Tam OH et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453, 534–538, doi: 10.1038/nature06904 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Suh N et al. MicroRNA Function Is Globally Suppressed in Mouse Oocytes and Early Embryos. Current Biology 20, 271–277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Murchison EP et al. Critical roles for Dicer in the female germline. Genes & development 21, 682–693 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kataruka S et al. MicroRNA dilution during oocyte growth disables the microRNA pathway in mammalian oocytes. Nucleic acids research 48, 8050–8062, doi: 10.1093/nar/gkaa543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schuster S, Miesen P & van Rij RP Antiviral RNAi in Insects and Mammals: Parallels and Differences. Viruses 11, doi: 10.3390/v11050448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kennedy EM et al. Production of functional small interfering RNAs by an amino-terminal deletion mutant of human Dicer. Proceedings of the National Academy of Sciences of the United States of America 112, E6945–6954, doi: 10.1073/pnas.1513421112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Poirier EZ et al. An isoform of Dicer protects mammalian stem cells against multiple RNA viruses. Science 373, 231–236, doi: 10.1126/science.abg2264 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Y, Lu J, Han Y, Fan X & Ding SW RNA interference functions as an antiviral immunity mechanism in mammals. Science 342, 231–234, doi: 10.1126/science.1241911 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maillard PV et al. Antiviral RNA interference in mammalian cells. Science 342, 235–238, doi: 10.1126/science.1241930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gantier MP et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic acids research 39, 5692–5703, doi: 10.1093/nar/gkr148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Rooij E et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316, 575–579 (2007). [DOI] [PubMed] [Google Scholar]
- 176.Kingston ER & Bartel DP Global analyses of the dynamics of mammalian microRNA metabolism. Genome research 29, 1777–1790, doi: 10.1101/gr.251421.119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gibbings D et al. Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nature cell biology 14, 1314–1321, doi: 10.1038/ncb2611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reichholf B et al. Time-Resolved Small RNA Sequencing Unravels the Molecular Principles of MicroRNA Homeostasis. Molecular cell 75, 756–768 e757, doi: 10.1016/j.molcel.2019.06.018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Krol J et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631, doi:S0092-8674(10)00357-0 [pii]: 10.1016/j.cell.2010.03.039 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Ramachandran V & Chen X Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321, 1490–1492 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shukla S, Bjerke GA, Muhlrad D, Yi R & Parker R The RNase PARN Controls the Levels of Specific miRNAs that Contribute to p53 Regulation. Molecular cell 73, 1204–1216 e1204, doi: 10.1016/j.molcel.2019.01.010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu Y et al. ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLoS biology 15, e2001272, doi: 10.1371/journal.pbio.2001272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ibrahim F et al. Uridylation of mature miRNAs and siRNAs by the MUT68 nucleotidyltransferase promotes their degradation in Chlamydomonas. Proceedings of the National Academy of Sciences of the United States of America 107, 3906–3911, doi: 10.1073/pnas.0912632107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chatterjee S & Grosshans H Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature 461, 546–549, doi:nature08349 [pii]: 10.1038/nature08349 (2009). [DOI] [PubMed] [Google Scholar]
- 185.Cazalla D, Yario T & Steitz JA Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 328, 1563–1566, doi:328/5985/1563 [pii]: 10.1126/science.1187197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marcinowski L et al. Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS pathogens 8, e1002510, doi: 10.1371/journal.ppat.1002510 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghini F et al. Endogenous transcripts control miRNA levels and activity in mammalian cells by target-directed miRNA degradation. Nature communications 9, 3119, doi: 10.1038/s41467-018-05182-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de la Mata M et al. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO reports, doi: 10.15252/embr.201540078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ameres SL et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539, doi:328/5985/1534 [pii]: 10.1126/science.1187058 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ameres SL & Zamore PD Diversifying microRNA sequence and function. Nature reviews. Molecular cell biology 14, 475–488, doi: 10.1038/nrm3611 (2013). [DOI] [PubMed] [Google Scholar]
- 191.Yang A et al. AGO-bound mature miRNAs are oligouridylated by TUTs and subsequently degraded by DIS3L2. Nature communications 11, 2765, doi: 10.1038/s41467-020-16533-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang A et al. TENT2, TUT4, and TUT7 selectively regulate miRNA sequence and abundance. Nature communications 13, 5260, doi: 10.1038/s41467-022-32969-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shi CY et al. The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science 370, doi: 10.1126/science.abc9359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Han J et al. A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science 370, doi: 10.1126/science.abc9546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Piwecka M et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357, doi: 10.1126/science.aam8526 (2017). [DOI] [PubMed] [Google Scholar]
- 196.Ulitsky I, Shkumatava A, Jan CH, Sive H & Bartel DP Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell 147, 1537–1550, doi: 10.1016/j.cell.2011.11.055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kleaveland B, Shi CY, Stefano J & Bartel DP A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 174, 350–362 e317, doi: 10.1016/j.cell.2018.05.022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Donnelly BF et al. The developmentally timed decay of an essential microRNA family is seed-sequence dependent. Cell reports 40, 111154, doi: 10.1016/j.celrep.2022.111154 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li L et al. Widespread microRNA degradation elements in target mRNAs can assist the encoded proteins. Genes & development 35, 1595–1609, doi: 10.1101/gad.348874.121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bitetti A et al. MicroRNA degradation by a conserved target RNA regulates animal behavior. Nature structural & molecular biology 25, 244–251, doi: 10.1038/s41594-018-0032-x (2018). [DOI] [PubMed] [Google Scholar]
- 201.Kingston ER, Blodgett LW & Bartel DP Endogenous transcripts direct microRNA degradation in Drosophila, and this targeted degradation is required for proper embryonic development. Molecular cell 82, 3872–3884 e3879, doi: 10.1016/j.molcel.2022.08.029 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Katoh T et al. Selective stabilization of mammalian microRNAs by 3’ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes & development 23, 433–438, doi:23/4/433 [pii]: 10.1101/gad.1761509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.D’Ambrogio A, Gu W, Udagawa T, Mello CC & Richter JD Specific miRNA Stabilization by Gld2-Catalyzed Monoadenylation. Cell reports, doi: 10.1016/j.celrep.2012.10.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jeong HC et al. USB1 is a miRNA deadenylase that regulates hematopoietic development. Science 379, 901–907, doi: 10.1126/science.abj8379 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mansur F et al. Gld2-catalyzed 3’ monoadenylation of miRNAs in the hippocampus has no detectable effect on their stability or on animal behavior. RNA 22, 1492–1499, doi: 10.1261/rna.056937.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vieux KF et al. Screening by deep sequencing reveals mediators of microRNA tailing in C. elegans. Nucleic acids research 49, 11167–11180, doi: 10.1093/nar/gkab840 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee S et al. Promiscuous splicing-derived hairpins are dominant substrates of tailing-mediated defense of miRNA biogenesis in mammals. Cell reports 42, 112111, doi: 10.1016/j.celrep.2023.112111 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nishida KM et al. Roles of R2D2, a cytoplasmic D2 body component, in the endogenous siRNA pathway in Drosophila. Molecular cell 49, 680–691, doi: 10.1016/j.molcel.2012.12.024 (2013). [DOI] [PubMed] [Google Scholar]
- 209.Drake M et al. A Requirement for ERK dependent Dicer Phosphorylation in Coordinating Oocyte-to- Embryo Transition in Caenorhabditis elegans. Developmental cell in press (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shang R et al. Regulated dicing of pre-mir-144 via reshaping of its terminal loop. Nucleic acids research 50, 7637–7654, doi: 10.1093/nar/gkac568 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gutierrez-Perez P et al. miR-1 sustains muscle physiology by controlling V-ATPase complex assembly. Sci Adv 7, eabh1434, doi: 10.1126/sciadv.abh1434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang B, Schwartz M & McJunkin K In vivo CRISPR screening for phenotypic targets of the mir-35-42 family in C. elegans. Genes & development 34, 1227–1238, doi: 10.1101/gad.339333.120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ecsedi M, Rausch M & Grosshans H The let-7 microRNA Directs Vulval Development through a Single Target. Developmental cell 32, 335–344, doi: 10.1016/j.devcel.2014.12.018 (2015). [DOI] [PubMed] [Google Scholar]
- 214.Garaulet DL, Zhang B, Wei L, Li E & Lai EC miRNAs and Neural Alternative Polyadenylation Specify the Virgin Behavioral State. Developmental cell 54, 410–423, doi: 10.1016/j.devcel.2020.06.004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Okamura K, Hagen JW, Duan H, Tyler DM & Lai EC The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 130, 89–100 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ruby JG, Jan CH & Bartel DP Intronic microRNA precursors that bypass Drosha processing. Nature 448, 83–86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bogerd HP et al. A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral MicroRNAs. Molecular cell 37, 135–142, doi:S1097-2765(09)00920-4 [pii]: 10.1016/j.molcel.2009.12.016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xie M et al. Mammalian 5’-capped microRNA precursors that generate a single microRNA. Cell 155, 1568–1580, doi: 10.1016/j.cell.2013.11.027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zamudio JR, Kelly TJ & Sharp PA Argonaute-bound small RNAs from promoter-proximal RNA polymerase II. Cell 156, 920–934, doi: 10.1016/j.cell.2014.01.041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cheloufi S, Dos Santos CO, Chong MM & Hannon GJ A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589, doi:nature09092 [pii]: 10.1038/nature09092 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cifuentes D et al. A Novel miRNA Processing Pathway Independent of Dicer Requires Argonaute2 Catalytic Activity. Science 328, 1694–1698, doi:science.1190809 [pii]: 10.1126/science.1190809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang JS et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proceedings of the National Academy of Sciences of the United States of America 107, 15163–15168, doi: 10.1073/pnas.1006432107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


