Summary
Background
Coronary artery disease (CAD) is a prevalent cardiovascular condition, and numerous studies have linked gut bacterial imbalance to CAD. However, the relationship of gut fungi, another essential component of the intestinal microbiota, with CAD remains poorly understood.
Methods
In this cross-sectional study, we analyzed fecal samples from 132 participants, split into 31 healthy controls and 101 CAD patients, further categorized into stable CAD (38), unstable angina (41), and acute myocardial infarction (22) groups. We conducted internal transcribed spacer 1 (ITS1) and 16S sequencing to examine gut fungal and bacterial communities.
Findings
Based on ITS1 analyses, Ascomycota and Basidiomycota were the dominant fungal phyla in all the groups. The α diversity of gut mycobiome remained unaltered among the control group and CAD subgroups; however, the structure and composition of the mycobiota differed significantly with the progression of CAD. The abundances of 15 taxa gradually changed with the occurrence and progression of the disease and were significantly correlated with major CAD risk factor indicators. The mycobiome changes were closely linked to gut microbiome dysbiosis in patients with CAD. Furthermore, disease classifiers based on gut fungi effectively identified subgroups with different degrees of CAD. Finally, the FUNGuild analysis further categorized these fungi into distinct ecological guilds.
Interpretation
In conclusion, the structure and composition of the gut fungal community differed from healthy controls to various subtypes of CAD, revealing key fungi taxa alterations linked to the onset and progression of CAD. Our study highlights the potential role of gut fungi in CAD and may facilitate the development of novel biomarkers and therapeutic targets for CAD.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 82170302, 92168117, 82370432), National clinical key specialty construction project- Cardiovascular Surgery, the Reform and Development Program of Beijing Institute of Respiratory Medicine (No. Ggyfz202417, Ggyfz202308), the Beijing Natural Science Foundation (No. 7222068); and the Clinical Research Incubation Program of Beijing Chaoyang Hospital Affiliated to Capital Medical University (No. CYFH202209).
Keywords: Microbiome, Fungi, Coronary artery disease, Atherosclerosis
Research in context.
Evidence before this study
Prior to this study, substantial research highlighted the strong link between gut bacterial microbiota and coronary artery disease (CAD). However, the role of gut fungi, a crucial part of the intestinal microbiota, in CAD was less understood. Studies indicated a significant association between gut fungi and various human diseases, but specific insights into the relationship between gut fungi and CAD were lacking.
Added value of this study
Our study provides novel insights into the role of the gut mycobiome in CAD. We characterized the gut fungal communities in patients with different stages of CAD and compared them with healthy controls. Our findings reveal significant alterations in the gut mycobiome structure and composition with the progression of CAD. This study not only highlights the potential role of gut fungi in CAD but also establishes a link between specific fungal taxa and CAD risk factors. We demonstrate that gut fungi may serve as potential biomarkers for CAD, paving the way for new diagnostic and therapeutic approaches.
Implications of all the available evidence
Our findings indicate a significant role for the gut mycobiome in CAD progression. This expands our understanding of CAD and opens new paths for diagnosis and treatment, emphasizing microbial interactions in the gut. Future research should focus on the mechanisms by which gut fungi influence CAD.
Introduction
Cardiovascular disease is the most common cause of death globally. In 2020, more than 500 million people had some form of cardiovascular disease, and approximately 19 million deaths were attributable to this type of disease. Annual deaths are projected to rise to 23.3 million by 2030.1 Coronary artery disease (CAD) is the most common type of cardiovascular disease characterized by myocardial dysfunction due to the narrowing of the coronary arteries, which results in inadequate blood supply.
In clinical practice, based on clinical symptoms, ancillary testing and the degree of myocardial damage, CAD can be classified as stable coronary artery disease (SCAD), unstable angina (UA) and acute myocardial infarction (AMI), where UA and AMI are collectively referred to as acute coronary syndrome (ACS).2 Treatment for CAD may require surgical intervention, such as stent replacement, angioplasty, coronary artery bypass graft surgery or off-pump coronary artery bypass surgery. As a chronic and progressive inflammatory disease, the formation and development of coronary atherosclerotic plaque is a dynamic and complex process. Identifying the transformation of atherosclerotic plaque from stable to unstable, and the risk of acute thrombosis after rupture can provide a better basis for clinical treatment.
Previous research has established a strong link between the gut bacterial microbiota and CAD, highlighting that changes in the structure and metabolites of the gut microbiota may influence the development of CAD.3, 4, 5, 6, 7, 8 However, the intestinal microbiota also contains microbial components other than gut bacteria, such as fungi, which are collectively referred to as gut mycobiome. The gut mycobiome exerts crucial roles in intestinal homoeostasis and disease pathogenesis through symbiosis with the gut bacterial microbiome and host immunity.4,5 Additionally, studies have found a strong association between gut fungi and human diseases such as hypertension, alcoholic cirrhosis, autism, inflammatory bowel disease, colon cancer, and rheumatoid arthritis.6, 7, 8 Despite these insights, the specific relationship between gut fungi and CAD has yet to be elucidated.
To investigate the potential association of the gut mycobiome with CAD, we conducted a comprehensive analysis of the gut microbial profile in 101 CAD patients and 31 healthy controls using high-throughput sequencing. We identified gut fungi associated with increased CAD severity and their specific characteristics with coronary risk factors and explored fungal–fungal and fungal-bacterial correlations. Genus-level fungal species-based disease classifiers were constructed to distinguish between healthy controls and different CAD subgroups. Our study revealed an important role of gut fungi in the pathogenesis and development of CAD.
Methods
Patient cohorts
A total of 132 participants were enrolled, including 31 healthy volunteers and 101 hospitalized patients with CAD from the Heart Center of Beijing Chao-Yang Hospital from January 2022 to November 2023. CAD was diagnosed in patients with at least 50% stenosis in one or more major coronary arteries as determined by coronary angiography. The CAD patients were further divided into three subgroups: (1) stable CAD (SCAD, N = 38), (2) unstable angina (UA, N = 41), and (3) acute myocardial infarction (AMI, N = 22), which reflect the onset, progression and severity of CAD.9 The severity of coronary atherosclerosis was assessed using the Gensini score.10 For the control group, we included individuals who had negative findings on coronary computed tomography or coronary angiography or who had no clinical signs and symptoms of CAD.
Exclusion criteria encompassed individuals with gastrointestinal, malignant, autoimmune, or infectious diseases, severe renal impairment (as defined by creatinine levels exceeding 3.0 mg/dL), a recent history of gastrointestinal surgery within the preceding year, or prolonged antibiotic usage exceeding 3 days within the previous month.
Peripheral venous blood was collected from participants before breakfast on the first morning after admission utilizing an Ethylene Diamine Tetraacetic Acid (EDTA) Vacuum Collection Tube and promptly dispatched to the hospital laboratory for testing. Clinical information pertaining to patients, encompassing risk factors for CAD, was acquired through consultation or measurement at admission employing a combination of blood tests, medical history, and physical examination. The study received approval from the local Ethics Committee at Beijing Chao-Yang Hospital (2021-ke-634) and informed consent was obtained from all participants.
Assessment of coronary atherosclerosis burden
The Gensini score, used to estimate coronary atherosclerotic burden,10 assigns severity scores based on stenosis degree, location within the coronary vessel, and the affected vessel. Angiography quantifies stenotic plaques, assigning scores (1, 2, 4, 8, 16, 32) for luminal obstructions (25%, 50%, 75%, 90%, 99%, 100%). Additional factors (0.5–5) based on the significance of the coronary segment are applied. The final score is the sum of these scores, adjusted by segment significance.
Stool sample collection and DNA extraction
Upon enrollment, participants were provided with a sterile stool collection container and were instructed on the proper procedure for sample collection. Freshly collected stool samples from each participant were transported to the laboratory within 1 h of collection for aliquot and frozen at −80 °C until analysis. Total genomic Deoxyribonucleic Acid (DNA) in the fecal samples was extracted at Oriental Yeekang (Beijing) Medicine Technology Co., LTD using TIANGEN kit according to the manufacturer's recommendations.
ITS1 rDNA gene sequencing
ITS1 rDNA gene sequencing involved amplifying the ITS1 region of the rDNA gene using specific primers (ITS1-F:5′-GGAAGTAAAAGTCGTAACAAGG-3′; ITS1-R: 5′-GCTGCGTTCTTCATCGATGC-3′) with barcodes. The PCR mix included Phusion® High-Fidelity PCR Master Mix, primers, and target DNA. The cycling protocol started with a 98 °C denaturation step, followed by 30 cycles of varying temperatures, and concluded with a final extension. PCR products underwent electrophoresis, were mixed and purified using the Qiagen Gel Extraction Kit. Sequencing libraries, prepared with the TruSeq® DNA PCR-Free Sample Preparation Kit, were quality-checked using Qubit@ 2.0 Fluorometer and Agilent Bioanalyzer 2100, and sequenced on an Illumina NovaSeq platform for 250 bp paired-end reads.
Fungal ITS sequencing data analysis
Paired-end reads were merged using FLASH (version 1.2.7, http://ccb.jhu.edu/software/FLASH/, 14/12/2024) to obtain Raw Tags.11 Subsequently, the fastp software (Version 0.23.1) was employed for quality filtering of the Raw Tags. The Clean Tags were subsequently compared to the Unite database (https://unite.ut.ee/for ITS, 14/12/2024) using the UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html, 14/12/2024),12 in order to detect chimera sequences, which were subsequently removed to obtain the Effective Tags.
Noise reduction in the data was conducted using DADA2, resulting in the identification of final Amplicon Sequence Variants (ASVs). These ASVs were annotated using QIIME2 software and the Unite Database. QIIME2 was also used for rapid multiple sequence alignment to determine the phylogenetic relationships of the ASVs. Finally, the absolute abundance of ASVs was normalized against the sequence count of the least sequenced sample.
16S rDNA gene sequencing and data analysis
The methodology for bacterial 16S rDNA amplicon sequencing and data analysis is similar to that used for fungi, with the main difference being the targeted 16S v4 region for bacterial amplification. Specific primers used are: forward 5′-GTGCCAGCMGCCGCGGTAA-3′ and reverse 5′-GGACTACHVGGGTWTCTAAT-3′. The Silva Database (http://www.arb-silva.de/, 14/12/2024) is utilized for chimera removal and species annotation in bacterial studies.
Relative abundance and alpha diversity
The distribution histogram of relative abundance in Perl through the Scalable Vector Graphics (SVG) function was plotted by selecting the top 10 taxa at each taxonomic rank (Phylum, Class, Order, Family, Genus, Species) from each sample. To assess the diversity, richness, and uniformity of the communities in the sample, alpha diversity was calculated using three indices (Observed_species, Chao1, and Shannon) in QIIME2.
Beta diversity
Principal Co-ordinates Analysis (PCoA) was conducted in order to acquire and visually represent principal coordinates derived from intricate multidimensional data.13 The previously obtained distance matrix of unweighted UniFrac among samples was converted into a novel set of orthogonal axes, wherein the initial principal coordinate represents the maximum variation factor, and the second principal coordinate represents the subsequent maximum variation factor. The PCoA analysis was visualized using the ade4 package and ggplot2 package within the R software (Version 4.0.3).
Intergroup species difference analysis
The Linear discriminant analysis Effect Size (LEfSe) method was used to identify distinct fungal species abundances across four groups and highlight species with significant differences. MetaStat method calculated P-values for species abundance at various levels.14 Significant species were screened based on q values, adjusted from these P-values. This combination of LEfSe and MetaStat identified different fungal species among the groups.
Construction of random forest models
For the random forest model, fungal species at the genus level were selected as predictors. Samples were split into training (80%) and validation (20%) sets. The model, comprising 10,000 decision trees, identified significant variables for prediction. The Area Under Curve (AUC) values were plotted against the number of variables.
Funguild functional annotation
ITS amplicon analysis with FunGuild effectively identifies and quantifies fungal species in environmental samples, categorizing them by taxonomy and ecological roles. It calculates the abundance of functionalities in Trophic Mode and Guild categories from annotated databases. Heatmaps display the top ten functionalities, including group-specific abundance, based on database annotations.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation, while non-normally distributed data were described as median (interquartile range). One-way Analysis of Variance (ANOVA) was used to analyze continuous variables with normal distributions among the four groups, and the Kruskal–Wallis test was used for data with non-normal distributions. For differences between two groups, t-tests or nonparametric Mann–Whitney U tests were performed when appropriate. Classified data were expressed as numbers and percentages, and statistical analysis was performed using either Chi-square or Fisher's exact test. Kendall's tau-b correlation analysis was employed to examine the correlation between the progression of coronary artery disease and the presence of differentially enriched fungi. IBM SPSS Statistics (version 20, IBM Inc., Armonk, NY, USA) was used for statistical analysis.
To construct the fungal and fungal-bacterial correlation heatmap, we used Spearman correlations and tested for differences using the Kruskal–Wallis rank test. We also determined correlations between the mycobiota, clinical parameters, and serum factors using Spearman correlations with a significance level of P < 0.05.
Role of funders
The funders of this research did not participate in the conception of the study design, the acquisition, analysis, or interpretation of data, nor in the drafting or revising of the manuscript.
Results
Description of the study population
The baseline clinical characteristics of the enrolled patients are shown in Table 1. The ages are higher in the UA and CAD groups. There were no significant differences in gender among the four subgroups. Mean arterial pressure, body mass index (BMI), smoking history, and alcohol consumption were similar among these groups. Among the relevant indicators reflecting the severity of CAD,15 there were differences in Gensini score and cardiac troponin I (cTnI) between the groups. Higher Gensini scores and cTnI value was observed in the severe group. There were also differences in medical treatment, including statins, antihypertensive drugs, and antidiabetic treatment between groups. Regarding laboratory test indicators, except for high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), glycated haemoglobin (HbA1c1), other indicator levels were similar between groups.
Table 1.
Characteristics of the study cohort.
| Control (n = 31) | SCAD (n = 38) | UA (n = 41) | AMI (n = 22) | CAD (n = 101) | |
|---|---|---|---|---|---|
| Age, years | 57.35 ± 8.20 | 62.53 ± 9.08 | 68.90 ± 9.78b | 60.77 ± 10.09c | 64.73 ± 10.13e |
| Female (%) | 15 (48.39%) | 13 (34.21%) | 17 (41.46%) | 5 (22.73%) | 35 (34.65%) |
| BMI, kg/m2 | 24.88 ± 2.67 | 25.27 ± 3.06 | 24.66 ± 3.62 | 26.76 ± 3.08 | 25.35 ± 3.37 |
| MAP, mmHg | 93.03 ± 10.69 | 97.35 ± 12.20 | 96.14 ± 11.77 | 91.97 ± 13.20 | 95.69 ± 12.30 |
| Smoke (%) | 10 (32.25%) | 20 (52.63%) | 16 (39.02%) | 8 (36.36%) | 44 (43.56%) |
| Drinking (%) | 5 (16.13%) | 10 (26.32%) | 10 (24.39%) | 6 (27.27%) | 26 (25.74%) |
| No. of SV | |||||
| NA | NA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1 | NA | 9 (26.4%) | 6 (16.2%) | 2 (12.5%) | 17 (19.5) |
| 2 | NA | 5 (14.7%) | 6 (16.2%) | 2 (12.5%) | 13 (15.0%) |
| 3 | NA | 16 (47.1%) | 20 (54.1%) | 6 (37.5%) | 42 (48.3%) |
| 4 | NA | 4 (11.8%) | 5 (13.5%) | 6 (37.5%) | 15 (17.2%) |
| Gensini score | NA | 43.00 (19.00, 81.25) | 65.50 (41.00, 103.50)b | 106.00 (84.00, 132.00)c | 70.00 (33.00, 96.00) |
| Syntax score | NA | 15.50 (6.75, 23.75) | 17.00 (11.00, 23.50) | 29.75 (12.00, 32.75) | 18.00 (10.00, 26.50) |
| Medication | |||||
| Statins | 4 (12.90%) | 20 (52.63%)a | 19 (46.34%) | 7 (31.82%) | 46 (45.54%)e |
| Antihypertensive drugs | 7 (22.58%) | 25 (65.79%)a | 32 (78.05%) | 11 (50.00%)d | 70 (69.31%)e |
| Antidiabetic treatment | 2 (6.45%) | 20 (52.63%)a | 11 (26.83%)b | 8 (36.36%) | 39 (38.61%)e |
| Laboratory data | |||||
| TC, mmol/L | 4.32 (3.94, 5.28) | 3.82 (3.30, 4.60) | 3.80 (3.28, 4.42) | 4.01 (3.76, 4.40) | 3.88 (3.39, 4.42) |
| HDL-C, mmol/L | 1.19 ± 0.34 | 1.05 ± 0.33 | 1.00 ± 0.23 | 0.97 ± 0.25 | 1.01 ± 0.27f |
| LDL-C, mmol/L | 2.90 (2.43, 3.60) | 2.07 (1.70, 2.88) | 2.39 (1.76, 2.67) | 2.66 (2.18, 2.98) | 2.39 (1.81, 2.83)f |
| TG, mmol/L | 1.34 (1.05, 1.78) | 1.18 (0.84, 1.89) | 1.20 (0.96, 1.69) | 1.49 (1.13, 1.70) | 1.24 (0.96, 1.70) |
| Lp(a), mg/dL | 13.35 (8.25, 28.00) | 14.40 (7.60, 27.25) | 21.00 (8.10, 39.60) | 13.65 (9.525, 43.43) | 15.70 (9.00, 36.10) |
| hs-CRP, mg/L | 1.13 (0.63, 1.83) | 0.76 (0.41, 1.84) | 0.98 (0.53, 3.08) | 5.42 (0.87, 22.48) | 0.83 (0.49, 3.93) |
| BUN, mmol/L | 2.80 ± 0.83 | 2.66 ± 0.60 | 2.84 ± 0.99 | 2.53 ± 0.78 | 2.70 ± 0.82 |
| Cr., umol/L | 71.50 (51.98, 79.63) | 65.90 (55.70, 77.43) | 68.00 (57.75, 80.05) | 67.15 (59.25, 81.80) | 66.70 (57.35, 79.75) |
| Uric acid, umol/L | 348.5 ± 101.4 | 332.1 ± 83.95 | 341.4 ± 108.2 | 366.8 ± 142.8 | 343.4 ± 108.4 |
| HCY, umol/L | 12.50 (8.75, 14.75) | 13.00 (11.00, 15.00) | 14.00 (11.00, 17.00) | 14.50 (13.25, 21.75) | 14.00 (11.00, 16.00) |
| cTnI, ng/ml | 0.00 (0.00, 0.00) | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.01) | 1.06 (0.23, 5.59)c | 0.01 (0.00, 0.03)e |
| FBG, mmol/L | 4.34 (3.81, 4.62) | 4.77 (4.16, 6.42) | 4.69 (3.91, 5.79) | 6.37 (4.88, 7.87)d | 4.89 (4.20, 6.55)f |
| HbA1c, % | 5.70 (5.53, 5.80) | 6.65 (5.90, 7.38)a | 6.30 (5.73, 6.65) | 6.10 (5.80, 8.00) | 6.30 (5.80, 7.25)e |
| FT3, pg/mL | 3.12 ± 0.56 | 3.30 ± 0.25 | 3.06 ± 0.40 | 2.76 ± 0.66 | 3.09 ± 0.45 |
| FT4, ng/dL | 1.24 (1.11, 1.32) | 1.17 (1.03, 1.29) | 1.20 (1.07, 1.32) | 1.08 (1.03, 1.15) | 1.16 (1.05, 1.30) |
| sTSH, uIU/mL | 2.21 (1.40, 2.54) | 2.14 (1.47, 3.59) | 1.82 (1.25, 2.41) | 2.70 (1.96, 5.98) | 2.16 (1.38, 3.01) |
Data following a normal distribution are presented as mean ± standard deviation; data not following a normal distribution are presented as median (IQR). Gender, Smoke, Drinking, No. of SV, Statins, Antihypertensive drugs and Antidiabetic treatment are shown as percentage (%). The analysis of normally distributed variables among the four groups was conducted using one-way ANOVA. Post hoc multiple comparisons of one-way ANOVA were performed using either the Games–Howell test or the Tukey–Kramer test. The Kruskal–Wallis H test was employed for data that did not follow a normal distribution. The χ 2 test was utilized to compare categorical variables. N/A indicates not applicable.
BMI, body mass index; MAP, mean arterial blood pressure. No. of SV, number of stenosed vessels; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; Lp(a), Lipoprotein(a); hs-CRP, hypersensitive C-reactive protein; BUN, blood urea nitrogen; Cr., creatinine; HCY, homocysteine; cTnI, cardiac troponin I; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; FT3, free triiodothyronine; FT4, free thyroxine; sTSH, serum thyroid-stimulating hormone.
P < 0.01 between CG and SCAD.
P < 0.05 between SCAD and UA.
P < 0.01 between UA and AMI.
P < 0.05 between UA and AMI.
P < 0.01 between CG and CAD.
P < 0.05 between CG and CAD.
Sequence characteristics
Sequencing the fungal ITS1 region and processing with DADA2 identified ASVs. Species-level identification used the Unite database (https://unite.ut.ee/, 15/12/2024), yielding 12,809,567 Effective Tags from 132 patient samples, averaging 97,042 reads each. Analysis revealed 18 phyla, 66 classes, 164 orders, 401 families, 659 genera, 1489 species, and 56,059 ASVs.
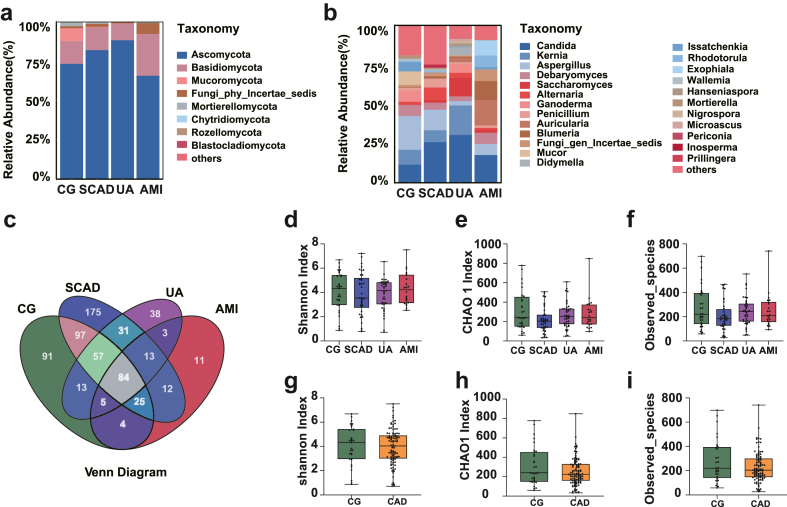
Next, we evaluated the taxonomic abundance of fecal fungi in different stages of CAD patients and the control group at the phylum and genus level. As shown in Fig. 1a, Ascomycota and Basidiomycota were the dominant fungal phyla, accounting for more than 90% of the total abundance. At the genus level, Candida, Kernia and Aspergillus were the most abundant fungal genera (Fig. 1b).
Fig. 1.
Fungal ecological characteristics in control and coronary artery disease (CAD) subgroups: (a) Phylum-level relative abundance in various groups. (b) Genus-level relative abundance in various groups. (c) Genera overlap and distinctiveness across the four groups. (d–f) Comparative analysis of the Shannon index (d), Chao 1 index (e), and observed species counts (f) among control, stable coronary artery disease (SCAD), unstable angina (UA), and acute myocardial infarction (AMI) groups. (g–i) Comparative analysis of the Shannon index (g), Chao 1 index (h), and observed species counts (i) between control group (CG) and coronary artery disease (CAD) group. Data representation: Median, 25th, and 75th percentiles as box plots. Statistical notes: All P-values >0.05. Differences among the four subgroups assessed using the Kruskal–Wallis test; Mann–Whitney test applied for P-value determination between CG and CAD groups. In Figure (a), only phyla representing an abundance of 1% or greater in at least one sample were depicted. Similarly, Figure (b) exclusively showcased genera that exhibited an abundance of 10% or more in at least one sample. Species that have not been described were collectively categorized under “Other”.
The Venn diagram depicted the intersection and distinctiveness of genera within the four groups. Among a total of 659 genera, 82 were found to be common to all four groups, whereas the CG, SCAD, UA, and AMI groups exhibited 91, 175, 38, and 11 unique genera, respectively (Fig. 1c).
Ecological features of the fecal fungal flora in CAD subgroups
To assess the ecological attributes of the fecal flora, we employed the α diversity index. Shannon, Chao 1, and observed_species metrics were computed for each group to evaluate the abundance and diversity of all samples. The obtained alpha diversity index did not exhibit any noteworthy disparity between each subgroup of CAD and the control group (Fig. 1d–f). Additionally, no significant distinction was observed between the control and CAD group (Fig. 1g–i), suggesting comparable levels of diversity and richness within the fungal communities of these groups.
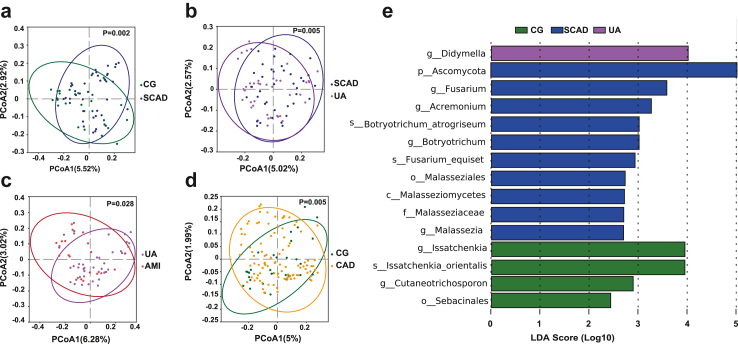
To assess overall mycobiome community differences between CAD subgroups and controls, PCoA was performed. PCoA based on unweighted UniFrac distance showed significant differences in the composition of gut fungi between the CG vs SCAD group (P = 0.002), SCAD vs UA group (P = 0.005), UA vs AMI group (P = 0.028), and CG vs. CAD group (P = 0.005) (Fig. 2a–d), suggesting that the structure and composition of the mycobiota change with the progression of CAD.
Fig. 2.
Comparative analysis of fungal diversity and discriminant factors in CAD. (a–d) Principal Co-ordinates Analysis (PCoA) plots illustrating unweighted UniFrac distances among groups: Control (CG) vs. Stable Coronary Artery Disease (SCAD) (a), SCAD vs. Unstable Angina (UA) (b), UA vs. Acute Myocardial Infarction (AMI) (c), CG vs. Coronary Artery Disease (CAD) (d). Beta diversity variances evaluated using PERMANOVA. Ellipses denote 95% confidence intervals for each group. (e) Linear discriminant analysis (LDA) on effect size (LEfSe) across different fungal taxa levels (LDA>2, P < 0.05).
To further explore potential confounding factors within our study, additional PCoA analyses were performed to evaluate the influence of age, gender, and the use of medications such as antihypertensive, lipid-lowering, and antidiabetic agents on the mycobiome composition. The results, illustrated in Fig. S1, indicate that these medications do not significantly differentiate participant groups (P = 0.711 for age, P = 0.169 for gender, P = 0.836 for antihypertensive medication, P = 0.179 for antidiabetic medication, and P = 0.184 for lipid-lowering medication), indicating a minimal influence of these factors on our study outcomes.
Altered mycobiome taxonomic compositions in CAD subgroups
To identify taxa with significant differences at each classification level among different groups, we conducted LEfSe. This analysis revealed a total of 15 taxa at various taxonomic levels: one phylum, one class, two orders, one family, seven genera, and three species. These taxa were distributed among the CG (4), SCAD (10), and UA (1) groups, respectively (LDA >2.0; P < 0.05; Fig. 2e).
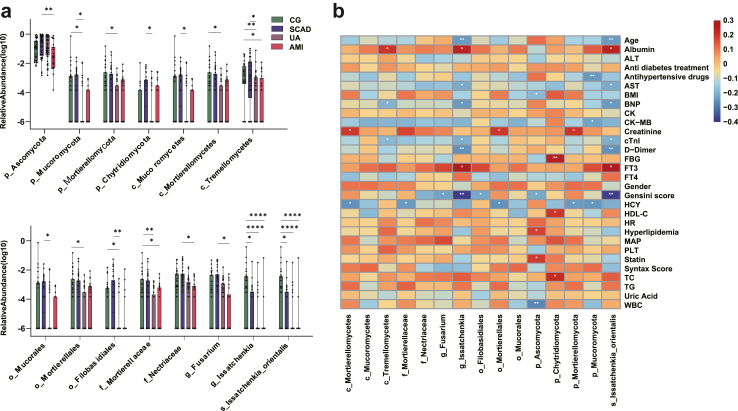
Further, we employed the MetaStat method to assess taxa richness at various levels between two groups, with the aim of identifying species showing significant differences in relation to the progression of CAD. By integrating the findings from both LEfSe and MetaStat analyses, we focused on species that were prevalent in at least 30% of the samples. This combined analysis approach enabled us to identify 15 taxa with differential abundances (Fig. 3a). These taxa were distributed across multiple taxonomic levels, including four phyla (p_Ascomycota, p_Mucoromycota, p_Mortierellomycota, p_Chytridiomycota), three classes (c_Mucoromycetes, c_Mortierellomycetes, c_Tremellomycetes), three orders (o_Mucorales, o_Mortierellales, o_Filobasidiales), two families (f_Mortierellaceae, f_Nectriaceae), two genera (g_Fusarium, g_Issatchenkia), and one species (s_Issatchenkia_orientalis). While no single taxon showed significant differences between any two adjacent groups, Kendall's tau-b test indicated that 13 of these taxa were significantly correlated with the severity of CAD (Table 2).
Fig. 3.
Major fungal taxa associated with CAD and correlation with major CAD risk factors. (a) Identification of the major fungal taxa of different levels associated with the onset and development of CAD. The box plot shows that the fungal taxa significantly changed between different groups. ∗P value < 0.05, ∗∗P value < 0.01. P values are from the Kruskal–Wallis test, boxes represent the inter-quartile ranges, and lines inside the boxes denote medians, (b) Spearman correlations between fungal taxa and major CAD risk factor indicators from the groups. The rows display major CAD risk factors, the columns represent the fungal taxa at the different levels. Red and blue represents the positive and negative correlations, respectively. The intensity of the colors indicates the degree of correlation between the fungal taxa abundances and the levels of CAD risk factor. ∗P < 0.05; ∗∗P < 0.01; cTnI, cardiac troponin I; Albumin, serum albumin; ALT, alanine transaminase; AST, aspartate transaminase; BNP, brain natriuretic peptide; CK, creatine kinase; CK-MB, creatine kinase isoenzymes-MB; Cr, creatinine; FT3, free triiodothyronine; FT4, free thyroxine; FBG, fasting blood glucose; HCY, homocysteine; PLT, platelet; WBC, white blood cell; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; BMI, body mass index; MAP, mean arterial blood pressure.
Table 2.
Correlation between differentially enriched taxa and severity of CAD.a
| Taxonomy | Kendall's tau-b correlation coefficient | P value |
|---|---|---|
| p_Ascomycota | −0.093 | 0.156 |
| p_Mucoromycota | −0.242 | 0.001 |
| p_Mortierellomycota | −0.17 | 0.017 |
| p_Chytridiomycota | 0.004 | 0.956 |
| c_Mucoromycetes | −0.169 | 0.023 |
| c_Mortierellomycetes | −0.175 | 0.014 |
| c_Tremellomycetes | −0.27 | <0.001 |
| o_Mucorales | −0.169 | 0.023 |
| o_Mortierellales | −0.175 | 0.014 |
| o_Filobasidiales | −0.243 | 0.001 |
| f_Mortierellaceae | −0.217 | 0.002 |
| f_Nectriaceae | −0.193 | 0.005 |
| g_Fusarium | −0.2 | 0.004 |
| g_Issatchenkia | −0.381 | <0.001 |
| s_Issatchenkia_orientalis | −0.381 | <0.001 |
Kendall's tau-b test.
The ratio of Ascomycota to Basidiomycetes (A/B) is often used to describe the characteristics of gut mycobiota.16 Thus, in this study, we investigated the A/B ratio in our cohort. The results revealed that, although there were no statistical differences, the UA group exhibited the highest A/B ratio. Furthermore, the analysis indicated no significant statistical variations either among the four subgroups or between the CG and CAD groups (Fig. S2).
These results suggested that the intestinal mycobiome differed significantly in CAD patients compared with healthy controls, and that the levels of specific intestinal fungal taxa may further shift with CAD severity.
Correlation analysis between mycobiota features and CAD risk factors
To reveal the potential interaction between gut fungal communities and CAD risk factors, as well as to explore the potential role of specific fungi in the onset and development of CAD, we performed correlation analyses between the aforementioned differentially enriched fungal components and host clinical characteristics, as well as circulating serum factors. We assessed a comprehensive set of 30 CAD risk factors associated with mycobiota features and found that the fungal communities exhibited strong correlations with these risk factors. For example, g__Issatchenkia, and s__Issatchenkia_orientali showed negative correlations with age, while p_Ascomycota negatively correlated with BMI. Regarding the indicators of CAD severity, g_Fusarium, o_Filobasidiales, p_Ascomycota, g__Issatchenkia, and s__Issatchenkia_orientali showed significantly negative correlations with Gensini score, consistenting with the observed differences in abundance of these fungi between different groups. Interestingly, no differentially enriched fungal taxa significantly correlated with Syntax score (Fig. 3b). The reason may be that Syntax score does not reflect the severity of atherosclerosis as the Gensini score.
With regard to circulating serum factors, we identified differential fungi that correlated with factors such as albumin, aspartate transaminase (AST), cTnI, Brain Natriuretic Peptic (BNP), Creatine Kinase MB (CK-MB), Creatinine, D-Dimer, FBG, Free Triiodothyronine (FT3), Homocysteine (HCY), and Total Cholesterol (TC). For example, c_Tremellomycetes, g_Issatchenkia, s_Issatchenkia_orientalis were found to have a negative correlation with BNP. Additionally, c_Mortierellomycetes, f_Mortierellaceae, o_Mortierellales, p_Mortierellomycota, and p_Mucoromycota showed negative correlations with HCY (Fig. 3b), a widely recognized risk factor for CAD, in accordance with the discovery that these taxa were significantly enriched in the control group and their abundance decreased with the severity of CAD (Fig. 3a). Furthermore, p_Chytridiomycota was found to be positively correlated with HDL-C, and TC (Fig. 3b). These findings suggest that intestinal fungi may serve as potential biomarkers and therapeutic targets for CAD.
Interactions between fungi–fungi and fungi-bacteria
The gut microbiome functions as an ecosystem wherein intricate microbial interactions govern the dynamics and homeostasis of the entire community.5 Notably, the interactions of intra-kingdom or interkingdom between gut fungi and bacteria underlie both the pathogenesis and the progression of diseases.17,18 For example, Sokol et al. found that in inflammatory bowel disease, gut fungal-bacterial correlations were significantly increased in patients with ulcerative colitis, whereas no such correlation was observed in patients with Crohn's disease.19 In contrast, a high-fat diet significantly reduced gut fungal-bacterial correlations in mice.20
To investigate relationships between fungi–fungi and fungi-bacteria in CAD patients, the gut bacterial profiles were analyzed by 16S rRNA sequencing. In addition, Spearman correlation analysis was performed at different taxonomic levels. Consistent with previous reports, the bacterial communities were different in the following groups: CG vs. CAD, CG vs. SCAD, SCAD vs. UA and UA vs. AMI in the PCoA plot (Fig. S3, all p < 0.05, by PERMANOVA test). Using LEfse analysis, 38 differentially enriched taxa of bacteria were found, including two phylum, three classes, five orders, eight families, thirteen genera and seven species (Fig. S4).
Spearman correlation analysis among 15 differentially enriched fungal taxa and 38 bacterial taxa showed extensive correlations between fungi–fungi and fungi-bacteria in control and CAD patients. Most of the results showed more positive correlations between fungi–fungi (Fig. 4). In the correlation analysis between fungi and bacteria at different levels, both positive and negative correlations were observed (Fig. 4). Notably, at the species level, there were negative correlations between s__Issatchenkia_orientalis and bacteria s_Bacteroides_massiliensis, s_Bacteroides_stercoris, and s_Parabacteroides_merdae. These results suggest that intestinal fungi may exert important roles in the onset and development of CAD by inter-kingdom or intra-kingdom interactions with bacteria.
Fig. 4.
Correlations at various taxonomic levels between fungi and between fungi and bacteria. (a) Correlations at the phylum level, (b) Correlations at the class level, (c) Correlations at the order level, (d) Correlations at the family level, (e) Correlations at the genus level, (f) Correlations at the species level. On the right side of each graph, the correlations among fungi at the respective level are depicted, while the left side illustrates the correlations between fungi and bacteria at the same taxonomic level. Red squares signify positive correlations, whereas blue squares indicate negative correlations. The direction of the correlations was determined using Spearman's method. ∗P < 0.05; ∗∗P < 0.01.
Subgroup identification and prediction based on gut fungi
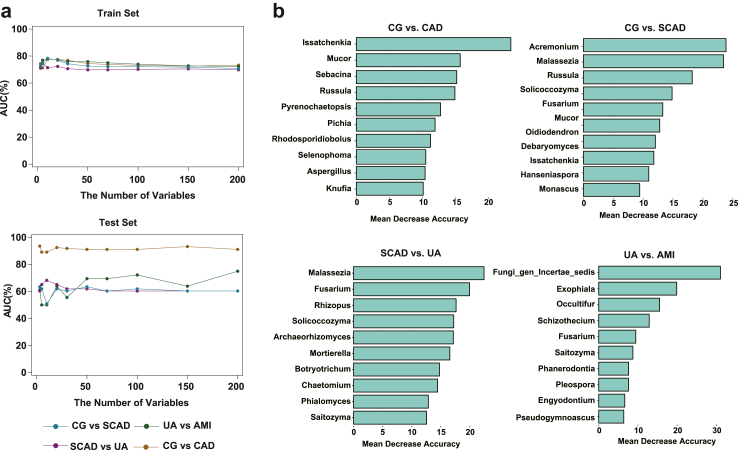
To determine whether gut fungi could serve as biomarkers to distinguish different stages of CAD, random forest models were constructed based on explanatory variables of the fungi at the genus level. Four distinct models were constructed, each predicated on the fungal profiles at the genus level.
In the train set, the maximum AUC values of the classifiers for CG vs. CAD, CG vs. SCAD, SCAD vs. UA, and UA vs. AMI were 77.55%, 78.30%, 74.54%, and 78.30% respectively. In the validation set, the maximum AUC values of the classifiers for CG vs. CAD, CG vs. SCAD, SCAD vs. UA, and UA vs. AMI were 93.54%, 63.49%, 68.25%, and 75.00% respectively. The random forest model was generally effective in discriminating between different severities of CAD, especially for the ability to discriminate CG vs. CAD, with an AUC of 93.54% in the validation set (Fig. 5a). We also examined the most critical predictor variables in different classifiers, with genus-level species of Issatchenkia, Mucor, Russula, Malassezia, Solicoccozyma, Fusarium and Saitozyma playing an important role in different classifiers (Fig. 5b). In particular, Issatchenkia was derived from one of the 15 taxa that differed between subgroups, as mentioned above (Fig. 3a). Moreover, the optimal number of variables was determined through Receiver Operating Characteristic (ROC) curve analysis, and the accuracy of predictions on both the test and validation sets was illustrated in Fig. S4.
Fig. 5.
The classifiers to identify CAD patients of different severity and controls. (a) Random forest models of the train and test sets are constructed using explanatory variables from Fungi, the AUC shows the classification of CG vs. CAD, CG vs. SCAD, SCAD vs. UA, and UA vs. AMI as the numbers of variables increase. (b) The detailed explanatory variables from the random forest model based on fungi. The lengths of the bars in the histogram represent the mean decrease accuracy, which indicates the importance of the species for classification.
Overall, the fungal signature associated with CAD captured by the random forest model provides further evidence for gut myobiota dysbiosis in CAD and highlights its great potential for detecting and diagnosing of CAD.
Ecological guilds of sampled taxa
Utilizing relative abundances of ASVs, FUNGuild was applied for the functional classification and predictive analysis of differential microbiota profiles across various stages of CAD. This methodology, involving ITS amplicon sequencing, enabled the categorization of fungal species according to potential ecological roles. Consequently, 61 distinct ecological guilds within the fungal taxa were identified (Table S1). The top ten of these guilds, including those with the highest diversity, are depicted in Fig. 6a, with ‘unassigned’ and ‘undefined saprotrophs' being the most diverse categories.
Fig. 6.
Ecological guilds of sampled taxa. (a–b) Guild and trophic mode function abundance histogram of the CG and CAD subgroups. (c) Functional abundance guild heatmap-guild. (d) Functional abundance guild heatmap-trophic.
Further analysis differentiated the fungal taxa into ten distinct trophic types (Table S2). Among these types, the categories ‘Unassigned’ and ‘Saprotroph’ demonstrated significant diversity (Fig. 6b). Heatmaps were created to visualize these classifications, displaying the functional predictions based on the analyses (Fig. 6c–d). In the examination of different CAD patient cohorts (CG, SCAD, UA, and AMI), variations in the enrichment of certain guilds were observed. Specifically, the most enriched guilds in these cohorts were Plant_Pathogen−Wood_Saprotroph in CG, Ectomycorrhizal in SCAD, Plant_Pathogen−Undefined_Saprotroph in UA, and Plant_Pathogen in AMI. In terms of trophic modes, the dominant types in the CG, SCAD, UA, and AMI groups were identified as Saprotroph−Symbiotroph, Symbiotroph, Pathotroph−Saprotroph, and Pathotroph, respectively.
Discussion
In the present study, we demonstrated that the structure and composition of gut fungal communities change with the onset and progression of CAD. Spearman correlation analysis suggest that specific gut fungi are markedly correlated with CAD risk factors such as Gensini score, AST, HCY, etc., and there were universal correlations between intestinal fungi–fungi as well as fungi-bacterial. Furthermore, gut fungi might be used independently as biomarkers for CAD and CAD sub-type diagnosis. The FUNGuild analysis further categorized these fungi into distinct ecological guilds, revealing a diverse range of functional roles within the gut mycobiome.
Regarding the composition of fungal taxa, Ascomycota and Basidiomycota were the dominant phyla, accounting for over 90% of the total abundance in different groups. Candida, Kernia and Aspergillus were the most abundant genera at the genus level. Interestingly, this composition was similar to that of the healthy control group in previous studies of Clostridium difficile infection,21,22 emphasizing their potential importance in human health and disease.
The ecological analysis of the fecal fungal flora in CAD revealed that while α diversity indices show no significant changes, indicating stable richness and diversity of the mycobiome in CAD, the PCoA based on unweighted UniFrac distances uncovered significant compositional differences among the CAD subgroups and controls. This suggests that specific changes in the gut mycobiome's structure, rather than its overall diversity, could be more relevant to CAD progression, potentially reflecting shifts in the microbial environment or host immune responses.
Our LEfSe and MetaStat analyses identified 15 distinct fungal taxa at various taxonomic levels that were differentially abundant among the groups. While no single taxon showed significant differences between any two adjacent groups, Kendall's tau-b test indicated that 13 of these taxa were significantly correlated with the severity of CAD. Currently, there is limited information about the role of these taxa in human health and disease. Each taxon has the potential to affect CAD through various mechanisms, such as influencing the host's immunity, metabolic processes, or systemic inflammation.23,24 The variety of these taxa and their association with CAD severity highlight the intricate connection between the gut mycobiome and cardiovascular health, providing new insights into CAD development and potential treatment approaches.
In the context of CAD severity indicators, certain fungal taxa, including g_Fusarium, o_Filobasidiales, p_Ascomycota, g_Issatchenkia, and s_Issatchenkia_orientalis, showed significant negative correlations with the Gensini score, a measure indicating the extent of plaque lesions in CAD.10 This suggests that the abundance of these taxa changes with the severity of the lesions. However, these fungi did not correlate significantly with the SYNTAX score, possibly due to its different focus, which may not capture the extent of plaque lesions as effectively as the Gensini score. This highlights the varied responses of the gut mycobiome to different aspects of CAD pathology, particularly in relation to plaque development and severity.
Several previous studies have reported an association between enteric fungi and serum factors.5,25,26 In our study, we identified serum factors including albumin, AST, cTnI, BNP, CK-MB, Creatinine, D-Dimer, FBG, FT3, HCY, and TC. In particular, c_Mortierellomycetes, f_Mortierellaceae, o_Mortierellales, p_Mortierellomycota, and p_Mucoromycota were inversely associated with HCY, which is widely recognized as risk factors for CAD and associated with thrombosis, oxidative stress, and endothelial dysfunction.27,28 These findings suggest that these fungi may influence the initiation and development of CAD by participating in the metabolism of substances. Therefore, these specific differential fungi may be targeted for future CAD preventive treatments. Still, further research is needed to elucidate the underlying mechanisms and to validate these findings.
Changes in the gut fungal community can significantly affect host health and are associated with several pathological conditions.17 The dynamic balance of fungal and bacterial biomes in the gut is critical for protecting the host from disease.29 Previous studies have shown that specific fungi can affect the bacterial community and that dysregulated bacterial flora, tissue damage and an inflammatory environment may contribute to intestinal fungal overgrowth.24,30 Our study observed positive correlations between fungi at different levels, except for negative correlations at the species level, which suggests a greater overall coordination between fungi. In the correlation analysis between fungi and bacteria, we observed both positive and negative correlations at different levels. However, at the species level, s__Issatchenkia_orientalis was only negatively correlated with bacteria, indicating a closer relationship between s__Issatchenkia_orientalis and the bacterial community. The fungal and bacterial microbiota interact with each other and play important roles in the pathogenesis of CAD.
The random forest model constructed by gut fungi effectively identified different CAD subgroups and controls, suggesting to some extent that gut fungi, which account for a small proportion of gut microbes, have a vital role in detecting CAD. The different severities of CAD respond pathologically to the development and rupture of atherosclerotic plaques.31,32 The identification of gut fungi as predictors of controls and different severity of CAD subgroups remained better compared to the bacterial models in previous studies,15 suggesting that gut fungi, may exert an essential role in developing and rupturing coronary atherosclerotic plaques. Thus, further studies are needed to investigate the specific mechanisms of the critical microbiota identified in our research, in the progression of CAD.
The functional analysis of the mycobiome, using tools like FUNGuild, provided insights into the ecological roles of different fungal taxa. The shifts in ecological guilds and trophic types across different stages of CAD could reflect changes in the gut environment and host–microbe interactions. Understanding these functional aspects of the mycobiome could lead to novel therapeutic strategies targeting the gut microbiota.
Conclusions
In conclusion, we characterized the disordered profiles of intestinal fungal microbiota in human patients with different stages of CAD. We correlated CAD risk factors with specific fungal taxa and explored fungal–fungal and fungal-bacterial interactions. Our findings highlight the potential role of gut fungal-bacterial interaction as a novel etiology for CAD. Furthermore, gut fungi may serve as a potential diagnostic tool in the future to identify CAD patients and assess disease severity.
Strength and limitations
Strengths: Despite evidence for a close association between the gut bacterial dysbiosis and CAD, the relationship of gut fungi, another essential component of the intestinal microbiota, with CAD remains poorly understood. This study fills this gap in knowledge and demonstrates the gut mycobiome alters with the severity of CAD. The data suggest that uniquely altered mycobiome may play a role in CAD pathophysiology and thus presents novel target for both CAD diagnosis and therapy.
Limitations: This study presents several limitations. The cross-sectional design, coupled with a small sample size from a restricted geographical area, limits the generalizability of our findings. Additionally, while the use of a random forest model-based ROC curve is effective for predicting CAD in a small cohort, its broader validity needs confirmation through a well-powered clinical trial with an independent test cohort. Unanswered questions remain about the origins of the altered fungal community, the mechanisms by which gut fungal-derived signals influence atherosclerosis progression, and the causal links between these changes and CAD development. It's speculated that factors such as environmental influences and genetic predispositions may contribute to gut dysbiosis and increased intestinal permeability, potentially leading to atherosclerosis and CAD through vascular inflammation and infection. Furthermore, the absence of external validation of our findings limits our ability to assess the reproducibility and applicability of our results across different populations, emphasizing the need for further research to validate and extend our findings in diverse cohorts and longitudinal studies.
Contributors
JZ, PS, and XL designed the study, secured funding, and supervised the project. KA, YJ, BX, and JG executed experiments and managed data. XL and KA handled analysis and visualization. KA, YC, BX, JG, and WY collected samples. KA wrote the initial draft, with XL, YJ, BX, and PS revised it. All authors have accessed and verified the data, and collectively decided to submit the manuscript.
Data sharing statement
The Amplicon Sequencing data sets for all samples have been deposited in the Sequence Read Archive (SRA) database under the accession code PRJNA984163 for fungi and code PRJNA984626 for bacteria. The authors declare that all the data supporting the findings of this study is available within the paper or from the corresponding authors upon request.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We want to thank all the participants who volunteered to participate in our study.
This work was supported by the grants from the National Natural Science Foundation of China (No. 82170302, 92168117, 82370432), National clinical key specialty construction project- Cardiovascular Surgery, Reform and Development Program of Beijing Institute of Respiratory Medicine (No. Ggyfz202417, Ggyfz202308), the Beijing Natural Science Foundation (No. 7222068); and the Clinical Research Incubation Program of Beijing Chaoyang Hospital Affiliated to Capital Medical University (No. CYFH202209).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105137.
Contributor Information
Jiuchang Zhong, Email: jczhong@sina.com.
Pixiong Su, Email: supixiong1130@163.com.
Xiaoyan Liu, Email: lxycyyy@mail.ccmu.edu.cn.
Appendix A. Supplementary data
Principal Co-ordinates Analysis (PCoA) plots depicting gut fungi variation. These plots are based on unweighted UniFrac distances, highlighting the impacts of different factors: (a) age, (b) gender, and various medications including antihypertensive (c), antidiabetic (d), and lipid-lowering drugs (e), on the composition of gut fungi. Differences in beta diversity were tested by PERMANOVA test. The ellipses represent 95% confidence regions for each group.
Comparison of Ascomycota to Basidiomycota Ratios in Gut Fungi Across Subgroups. (a) The ratio between the control group (CG) and coronary artery disease (CAD) subgroups. (b) The ratio between CG and CAD. Box plots indicate the median, 25th, and 75th percentiles. Statistical analysis revealed no significant differences, with all P-values exceeding 0.05.
Principal Co-ordinates Analysis (PCoA) of bacterial communities across different Groups. These plots are based on unweighted UniFrac distances, comparing: (a) Control (CG) versus Stable Coronary Artery Disease (SCAD) group, (b) SCAD versus Unstable Angina (UA) group, (c) UA versus Acute Myocardial Infarction (AMI) group, and (d) Control versus overall CAD. Beta diversity differences among these groups were assessed using the PERMANOVA test. The ellipses in each plot represent the 95% confidence regions for the respective groups.
Prediction accuracy as determined by the optimal number of variables selected through ROC curve analysis. This figure displays the accuracy of predictions for both the test and validation sets, corresponding to the variables identified as providing the highest diagnostic performance.
Visualization of Linear Discriminant Analysis Effect Size (LEfSe) for bacterial taxa across four subgroups at various taxonomic levels. Displayed are only those taxa that surpass a significant LDA threshold of >3.0 and exhibit P-values <0.05 in the Kruskal-Wallis rank-sum test.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147(8) doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visseren F.L.J., Mach F., Smulders Y.M., et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 3.Ramírez-Macías I., Orenes-Piñero E., Camelo-Castillo A., Rivera-Caravaca J.M., López-García C., Marín F. Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit Rev Food Sci Nutr. 2022;62(14):3738–3750. doi: 10.1080/10408398.2020.1868397. [DOI] [PubMed] [Google Scholar]
- 4.Hallen-Adams H.E., Suhr M.J. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8(3):352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F., Aschenbrenner D., Yoo J.Y., Zuo T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe. 2022;3(12):e969–e983. doi: 10.1016/S2666-5247(22)00203-8. [DOI] [PubMed] [Google Scholar]
- 6.Yang A.-M., Inamine T., Hochrath K., et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Investig. 2017;127(7):2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou R., Wang Y., Duan M., Guo M., Zhang Q., Zheng H. Dysbiosis of gut fungal microbiota in children with autism spectrum disorders. J Autism Dev Disord. 2021;51(1):267–275. doi: 10.1007/s10803-020-04543-y. [DOI] [PubMed] [Google Scholar]
- 8.Sun X., Wang Y., Li X., et al. Alterations of gut fungal microbiota in patients with rheumatoid arthritis. PeerJ. 2022;10 doi: 10.7717/peerj.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fihn S.D., Gardin J.M., Abrams J., et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology foundation/American heart association task force on practice guidelines, and the American college of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Gensini G.G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 11.Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minchin P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio. 1987;69(1/3) [Google Scholar]
- 14.White J.R., Nagarajan N., Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5(4) doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Chen X., Hu X., et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. 2019;7(1):68. doi: 10.1186/s40168-019-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y., Wang L., Ke S., et al. Ergotamine in the acute treatment of migraine: a review and European consensushort. Microbiol Spectr. 2022;10(4) [Google Scholar]
- 17.Santus W., Devlin J.R., Behnsen J. Crossing kingdoms: how the mycobiota and fungal-bacterial interactions impact host health and disease. Infect Immun. 2021;89(4) doi: 10.1128/IAI.00648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krüger W., Vielreicher S., Kapitan M., Jacobsen I.D., Niemiec M.J. Fungal-bacterial interactions in health and disease. Pathogens. 2019;8(2) doi: 10.3390/pathogens8020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokol H., Leducq V., Aschard H., et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heisel T., Montassier E., Johnson A., et al. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. mSphere. 2017;2(5) doi: 10.1128/mSphere.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y., Wang L., Ke S., et al. Fecal mycobiota combined with host immune factors distinguish clostridioides difficile infection from asymptomatic carriage. Gastroenterology. 2021;160(7) doi: 10.1053/j.gastro.2021.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y., Wang L., Ke S., et al. Analysis of intestinal mycobiota of patients with clostridioides difficile infection among a prospective inpatient cohort. Microbiol Spectr. 2022;10(4) doi: 10.1128/spectrum.01362-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogler G., Rosano G. The heart and the gut. Eur Heart J. 2014;35(7):426–430. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 24.Liguori G., Lamas B., Richard M.L., et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn's disease patients. J Crohns Colitis. 2016;10(3):296–305. doi: 10.1093/ecco-jcc/jjv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang S., Duan Y., Liu J., et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71(2):522–538. doi: 10.1002/hep.30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mogilnicka I., Ufnal M. Gut mycobiota and fungal metabolites in human homeostasis. Curr Drug Targets. 2019;20(2):232–240. doi: 10.2174/1389450119666180724125020. [DOI] [PubMed] [Google Scholar]
- 27.Schaffer A., Verdoia M., Cassetti E., Marino P., Suryapranata H., De Luca G. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014;134(2):288–293. doi: 10.1016/j.thromres.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Reiner Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. 2017;14(7):401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 29.Sommer F., Anderson J.M., Bharti R., Raes J., Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15(10):630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 30.Cao D., Liu W., Lyu N., et al. Gut mycobiota dysbiosis in pulmonary tuberculosis patients undergoing anti-tuberculosis treatment. Microbiol Spectr. 2021;9(3) doi: 10.1128/spectrum.00615-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 32.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Principal Co-ordinates Analysis (PCoA) plots depicting gut fungi variation. These plots are based on unweighted UniFrac distances, highlighting the impacts of different factors: (a) age, (b) gender, and various medications including antihypertensive (c), antidiabetic (d), and lipid-lowering drugs (e), on the composition of gut fungi. Differences in beta diversity were tested by PERMANOVA test. The ellipses represent 95% confidence regions for each group.
Comparison of Ascomycota to Basidiomycota Ratios in Gut Fungi Across Subgroups. (a) The ratio between the control group (CG) and coronary artery disease (CAD) subgroups. (b) The ratio between CG and CAD. Box plots indicate the median, 25th, and 75th percentiles. Statistical analysis revealed no significant differences, with all P-values exceeding 0.05.
Principal Co-ordinates Analysis (PCoA) of bacterial communities across different Groups. These plots are based on unweighted UniFrac distances, comparing: (a) Control (CG) versus Stable Coronary Artery Disease (SCAD) group, (b) SCAD versus Unstable Angina (UA) group, (c) UA versus Acute Myocardial Infarction (AMI) group, and (d) Control versus overall CAD. Beta diversity differences among these groups were assessed using the PERMANOVA test. The ellipses in each plot represent the 95% confidence regions for the respective groups.
Prediction accuracy as determined by the optimal number of variables selected through ROC curve analysis. This figure displays the accuracy of predictions for both the test and validation sets, corresponding to the variables identified as providing the highest diagnostic performance.
Visualization of Linear Discriminant Analysis Effect Size (LEfSe) for bacterial taxa across four subgroups at various taxonomic levels. Displayed are only those taxa that surpass a significant LDA threshold of >3.0 and exhibit P-values <0.05 in the Kruskal-Wallis rank-sum test.