Abstract
Fibrodysplasia ossificans progressiva (FOP) is a rare and progressively debilitating disorder affecting 1 in 2 million individuals. It is characterized by the progressive ossification of soft tissues into ectopic bone and congenital malformations of the great toes. FOP leads to significant disability and can result in death due to thoracic insufficiency syndrome. These case reports examine the presentation, diagnosis, and management of FOP, highlighting the diagnostic challenges inherent in managing such rare conditions because of their unique clinical features. They underscore the need for increased awareness among healthcare practitioners to ensure early diagnosis and the implementation of effective management strategies.
Keywords: Fibrodysplasia ossificans progressive (FOP), Great toe malformation, Heterotopic ossification
Introduction
Fibrodysplasia ossificans progressiva (FOP) is a rare, progressively debilitating disorder first described in 1692 by Guy Patin [1]. This disease is characterized by progressive heterotopic ossification that forms normal bone in characteristic extraskeletal sites and congenital malformations of the great toes. Progressive episodes of heterotopic ossification occur in characteristic pattern first at the dorsal, axial, cranial, and proximal part of the body and later in the ventral, appendicular, caudal, and distal part [2]. The heterotopic ossification contributes to a severe restriction of movement, progressing to total immobility, and ultimately leading to mortality from cardiorespiratory complications often by the fourth decade of life [3]. The incidence of FOP is 1 in 2 million individuals, with no observed preference for gender, race, or ethnicity [4].
Most FOP patients have a spontaneous new mutation in the ACVR1 gene, which encodes the bone morphogenic protein type 1 receptor [5]. The mutation results in a form of the receptor that's overly active, even in the absence of its ligand. This results in abnormal activation of osteogenesis at ectopic sites [6]. Though familial cases have been reported, most FOP cases are sporadic [7].
While the genetic cause of FOP is now well established, the clinical course of the disease can vary greatly among patients. An international, natural history study has helped shed light on the debilitating effects and progressive nature of FOP, with the greatest progression noted during childhood and early adulthood [8]. Despite this, the rarity of FOP and its diverse clinical manifestations, as illustrated by a case series reported by Chan et al. in Hong Kong, can result in delayed or incorrect diagnoses [9].
Awareness of FOP among practitioners across a broad spectrum of specialties is key for early diagnosis. Early diagnosis not only slows progression but can help avoid potentially harmful procedures, such as biopsies or surgical interventions, which can exacerbate the condition [10,11].
Case 1
Our first patient is a 9-year-old boy who presented with multiple swellings across his neck and back. These swellings had persisted for 5 years before his arrival. He was born from non-consanguineous parents following an uneventful term pregnancy with no familial history of similar symptoms or conditions.
At the age of 4, he was initially misdiagnosed and managed as a case of multiple exostoses, a condition featuring bony projections capped by cartilage, commonly developing in the metaphysis of long bones. The patient received non-surgical treatment and was unfortunately lost to follow-up.
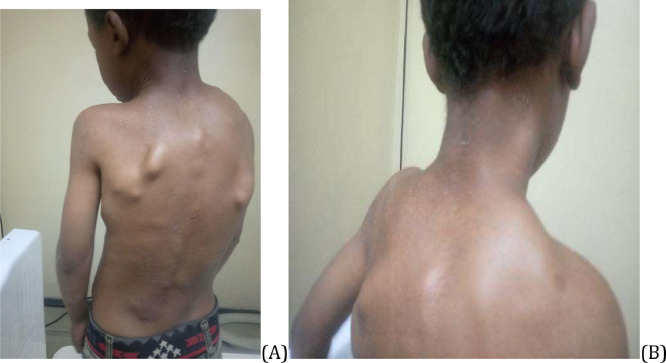
Five years later, the patient returned, reporting increased neck swelling, stiffness, and pain. Physical examination revealed several hard swellings at occipital area, the posterior, and lateral neck region, parascapular, and thoraco-lumbar paraspinal (Fig. 1).
Fig. 1.
(A) and (B) Multifocal swelling at back, lateral chest, and neck in patient with FOP.
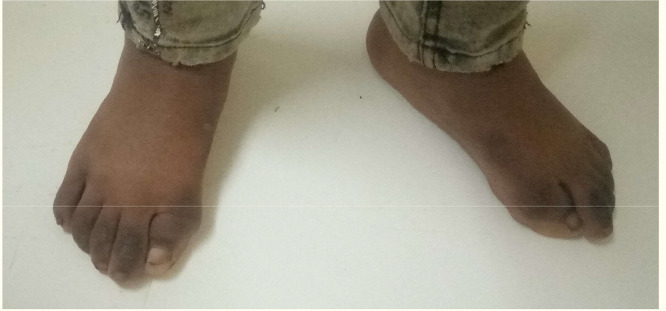
Growths seemed to cause the boy significant discomfort. An additional examination revealed bilateral malformations of his big toes, presented as hallux valgus deformities that had been present since birth, but had never been of concern to either the patient or his parents (Fig. 2).
Fig. 2.
Malformed short great toes and hallux valgus.
Plain radiographic assessment as well as cross-sectional imaging confirmed the extraskeletal ossification of the soft tissues in the neck, parascapular, thoracolumbar regions, and validated the bilateral hallux valgus deformities (Fig. 3, Fig. 4, Fig. 5, Fig. 6). A significant part of the diagnostic process included ruling out other conditions with similar presentations, such as multiple exostoses, and other forms of heterotopic ossification.
Fig. 3.
Chest radiograph showing bilateral lateral chest (blue arrow), left paravertebral (black arrow) and right lateral neck (white arrow) extraskeletal bone formation.
Fig. 4.
AP radiography of the feet demonstrating bilateral big toe hallux valgus deformity, medially deviated first metatarsal with malformed head, left monophalangism with enlarged epiphysis of the remaining phalanx and deformed proximal phalanx on the right toe.
Fig. 5.
Precontrast Sagittal (A) and Coronal (B) bone window images demonstrating extraskeletal bone formation.
Fig. 6.
Three-dimensional reconstructed computed tomography (CT) scan of the back of showing extensive heterotopic ossification.
Case 2
Our second patient is a 1-year-and-5-month-old female child who was previously in good health until approximately 1 year ago, when she began to develop a swelling on her scalp. Initially soft, the swelling became hard and fixed over time. She was evaluated at a local health center, but without subsequent management, her family discontinued seeking medical advice.
Four months prior, a similar swelling appeared in the neck area, which subsequently became hard and immobile, restricting her neck's movement. The swelling extended to the back, lateral chest, and thigh following an intramuscular vaccine administration.
The patient was born to a 38-year-old primipara mother following a 9-month period of amenorrhea. The mother received regular antenatal care, with baseline investigations reported as normal. Delivery was by cesarean section due to prolonged labor. The infant cried immediately post-delivery, with no neonatal intensive care unit admission required.
The child was exclusively breastfed until 6 months of age, after which complementary feeding was introduced with mashed potatoes and porridge made from various cereals. She is currently eating a family diet 4–5 times daily.
Physical examination revealed multiple non-tender masses over the neck, shoulder, and scapula area, the largest measuring 4 × 5 cm. The masses were hard and fixed, with no discernible underlying structure, limiting neck, and shoulder movement. There was no change in overlying skin color or temperature. Similar masses were observed on the left thigh area. Bilateral hallux valgus deformity was present on both feet (Fig. 7, Fig. 8).
Fig. 7.
Multifocal swellings over the back (A) and lateral chest wall (B) in a patient with FOP.
Fig. 8.
Hallux valgus deformities bilaterally.
Radiological investigations, including chest and lower limb X-rays, indicated bone-like calcification around the left thigh and bilaterally on the lateral chest as well as along the left paravertebral region (Fig. 9).
Fig. 9.
Chest xray (A) and bilateral lower limb (B) radiographs showing bilateral lateral chest (blue arrows) and left paravertebral (green arrow) extra skeletal bone formation. This is also observed along the left thigh (white arrow head).
Based on the clinical presentation, physical examination, radiographic assessments, and after excluding other differential diagnoses, these 2 patients were diagnosed with FOP. The unique combination of extraskeletal new bone formation and great toe abnormalities were critical in arriving at this diagnosis. The cases showcase the importance of thorough patient examination and the utility of the hallux valgus deformity as an early clinical indicator of FOP [7,9,10]. The diagnostic journey of these patients demonstrates that, despite the rarity of FOP, its distinctive clinical features can lead clinicians to a correct diagnosis and highlight the necessity of avoiding potentially harmful diagnostic procedures [11,12].
Discussion
FOP is a rare condition marked by unique clinical features such as extraskeletal new bone formation and great toe abnormalities. Although FOP is rare, the clinical manifestations are distinct and unambiguous, which can aid in its early diagnosis [7,10,11]. However, the variability in its clinical presentation, as highlighted by the case series from Hong Kong, emphasizes the importance of comprehensive clinical examination, detailed patient history, and a high index of suspicion [9].
A major challenge in managing FOP is the delay in diagnosis, as evidenced in our case, where the patient was initially misdiagnosed with multiple exostoses. A multi-country survey by Kitterman et al. showed that approximately 90% of FOP patients initially received erroneous diagnoses, with 67% undergoing invasive diagnostic procedures that might exacerbate the disease [12]. These findings underscore the necessity of raising awareness about FOP among healthcare practitioners to facilitate early diagnosis and to prevent harmful diagnostic or therapeutic interventions.
Biopsy is particularly contraindicated in FOP due to the risk of provoking explosive new bone formation [12]. Disease flare-ups can be triggered by trauma, including intramuscular injections, lesion biopsies, and nerve blocks, especially around the temporomandibular joint [4,12]. The need for extreme caution during invasive procedures is evident from the catastrophic consequences seen in patients who have had biopsies, emphasizing the importance of non-invasive diagnostic strategies [8].
While no cure is currently available for FOP, advancements in understanding the pathophysiology of the disease offer hope for future therapeutic options [5,13]. Current management strategies involve the use of non-steroidal anti-inflammatory drugs and short courses of corticosteroids at the onset of flare-ups [13]. Recent studies have shown promise in targeted therapeutics, with a particular focus on inhibiting the hyperactive ACVR1 receptor that drives the disease [6].
The case report adds to the existing body of evidence that highlights the need for continued research into FOP to better understand its pathophysiology, improve diagnostic accuracy, and explore effective therapeutic interventions. The need for such research is especially urgent given the devastating impact of FOP on patients' quality of life [8,9].
Conclusion
Fibrodysplasia ossificans progressiva is a rare disabling disorder which can be diagnosed by physical examination. Presence of malformed great toes and heterotopic ossification should alert practitioner about conditions. Through early diagnosis and proactive medical care, the debilitating impact of FOP can be significantly mitigated. Continued research is essential to devise effective therapeutic interventions, improving patient outcomes, and quality of life [8,9,13].
Patient consent
We have received written informed consent from the patient's parents to publish this case report and related images.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6(1):1–6. doi: 10.1186/1750-1172-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS Age-and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clinical Orthopaedics and Related Research®. 1994;301:243–248. [PubMed] [Google Scholar]
- 3.Hino K, Keya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, et al. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci. 2015;112(50):15438–15443. doi: 10.1073/pnas.1510540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees-Shepard JB, Stoessel SJ, Chandler JT, Bouchard K, Bento P, Apuzzo LN, et al. An anti-ACVR1 antibody exacerbates heterotopic ossification by fibro-adipogenic progenitors in fibrodysplasia ossificans progressiva mice. J Clin Invest. 2022;132(12) doi: 10.1172/JCI153795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H, Wang Q, Wen J, Liu S, Gao X, Cheng J, et al. ACVR1, a therapeutic target of fibrodysplasia ossificans progressiva, is negatively regulated by miR-148a. Int J Mol Sci. 2012;13(2):2063–2077. doi: 10.3390/ijms13022063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, et al. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284(11):7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nature Rev Rheumatol. 2010;6(9):518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignolo RJ, Baujat G, Brown MA, De Cunto C, Hsiao EC, Keen R, et al. The natural history of fibrodysplasia ossificans progressiva: a prospective, global 36-month study. Genet Med. 2022;24(12):2422–2433. doi: 10.1016/j.gim.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Chan JCK, Kuong EE, Chan JPK, Luk HM, Fung JLF, Tung JY-l, et al. Fibrodysplasia ossificans progressiva in Hong Kong: a case report series. Front Pediatr. 2023;11:1152731. doi: 10.3389/fped.2023.1152731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan L, Liu Y, McGuire TL, Berger DMP, Awatramani RB, Dymecki SM, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27(1):150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo K, Chavez RD, Barruet E. Hsiao EC Inflammation in fibrodysplasia ossificans progressiva and other forms of heterotopic ossification. Curr Osteoporosis Rep. 2019;17:387–394. doi: 10.1007/s11914-019-00541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haupt J, Stanley A, McLeod CM, Cosgrove BD, Culbert AL, Wang L, et al. ACVR1R206H FOP mutation alters mechanosensing and tissue stiffness during heterotopic ossification. Mol Biol Cell. 2019;30(1):17–29. doi: 10.1091/mbc.E18-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barruet E, Morales BM, Lwin W, White MP, Theodoris CV, Kim H, et al. The ACVR1 R206H mutation found in fibrodysplasia ossificans progressiva increases human induced pluripotent stem cell-derived endothelial cell formation and collagen production through BMP-mediated SMAD1/5/8 signaling. Stem Cell Res Ther. 2016;7:1–13. doi: 10.1186/s13287-016-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]