Abstract
This study aimed to investigate the changes in oxidative stress, adenosine monophosphate-activated protein kinase (AMPK), connexin43 (Cx43), nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) expression, and extracellular matrix (ECM) in the gastric smooth muscle tissues of rats with diabetic gastroparesis (DGP) and high glucose-cultured gastric smooth muscle cells, determine the existence of oxidative stress-AMPK-Cx43-NLRP3 pathway under high glucose condition, and the involvement of this pathway in ECM remodeling in DGP rats. The results showed that with increasing duration of diabetes, oxidation stress levels gradually increased, the AMPK activity decreased first and then increased, NLRP3, CX43 expression, and membrane/cytoplasm ratio of Cx43 expression were increased in the gastric smooth muscle tissues of diabetic rats. Changes in ECM of gastric smooth muscle cells were observed in DGP rats. The DGP group showed higher collagen type I content, increased expression of Caspase-1, transforming growth factor-beta 3 (TGF-β3), and matrix metalloproteinase-2 (MMP-2), decreased tissue inhibitor of metalloproteinase-1 (TIMP-1) expression, and higher interleukin-1 beta content when compared with the control group. For gastric smooth muscle cells cultured under higher glucose, the MMP-2 and TGF-β3 expression was decreased, TGF-β1 and TIMP-1 expression was increased, the interleukin-1 beta content was decreased in cells after inhibition of NLRP3 expression; the NLRP3 and Caspase-1 expression was decreased, and adenosine triphosphate content was lower after inhibition of Cx43; the expression of NLRP3, Caspase-1, P2X7, and the membrane/cytoplasm ratio of CX43 expression was decreased in cells after inhibition of AMPK and oxidative stress, the phospho-AMPK expression was also decreased after suppressing oxidative stress. Our findings suggest that high glucose induced the activation of the AMPK-Cx43-NLRP3 pathway through oxidative stress, and this pathway was involved in the ECM remodeling of gastric smooth muscles in DGP rats by regulating the biological functions of TGF-β3, TGF-β1, MMP-2, and TIMP-1.
Keywords: Diabetic gastroparesis, Rat model, Gastric smooth muscles, Extracellular matrix remodeling, Oxidative stress, AMPK
Introduction
Diabetic gastroparesis (DGP) is one of the common gastrointestinal complications of diabetes, which is mainly induced by high blood glucose and characterized by gastric dysmotility-induced delayed gastric emptying. Gastric motility results from coordinated contractions of smooth muscle cells, and the intercellular relationship between smooth muscle cells is a non-negligible factor that can influence smooth muscle movement. Under normal conditions, the outer surface of gastric smooth muscle cells is surrounded by extracellular matrix (ECM), which can provide mechanical connections, restrict the overextension of muscle cells, and influence intercellular information transfer. Our previous study showed that the amplitude of spontaneous contractions of gastric smooth muscle decreased, and the frequency of spontaneous contractions became slower in DGP rats, indicating that high glucose can reduce the spontaneous contractions of gastric smooth muscle.1 Therefore, we hypothesize that high glucose can cause ECM remodeling of gastric smooth muscle cells, which in turn leads to the disruption of intercellular mechanical connections and delay in information transfer, thus inducing the occurrence of DGP. The nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) is a member of the nucleotide-binding oligomerization domain-like receptor protein superfamily of the innate immune system. It is mainly involved in programmed cell death, chronic inflammation, and immune response, which is a major player in chronic inflammation. Scholars have found that the NLRP3/caspase-1/interleukin-1 beta (IL-1β) pathway was involved in the development of diabetic renal fibrosis,2 and NLRP3 was involved in cardiac fibrosis through the transforming growth factor (TGF)-β1/IL-1β pathway.3 These results suggest that the NLRP3 pathway may be involved in regulating ECM development.
Connexin43 (Cx43) is the most common gap junction (GJ) protein, which is essential for the formation of GJ channels and hemichannels or exists in its monomeric form and plays an important role in cell proliferation, apoptosis, and inflammation. Sun et al.4 found that under high glucose conditions, overexpression of Cx43 inhibited the expression of fibronectin, collagen types I and IV in ECM of normal rat kidney cells NRK-52E, while Cx43 deficiency received opposite results. Additionally, Huang et al.5 found that oxidative stress induced in mouse macrophages promoted NLRP3 activation via regulation of Cx43. Increased Cx43 expression can cause an increase in NLRP3 expression, whereas diminished Cx43 expression can lead to a decrease in NLRP3 expression. These findings suggest that the aberrant expression and distribution of Cx43 in cells may be involved in ECM alterations by regulating NLRP3.
Adenosine monophosphate-activated protein kinase (AMPK) is widely present in various tissues and can be regulated by oxidative stress and reactive oxygen species, which plays an important bioregulatory role in numerous cellular physiopathological processes. High glucose is one of the important triggers of oxidative stress in cells.6 AMPK is activated upon its phosphorylation, and the activated AMPK is closely related to Cx43 expression. Li et al.7 found that the protein expression of Cx43 was reduced in cardiomyocytes under high glucose conditions, apelin administration reversed the reduced expression of Cx43, and silencing of AMPKα can eliminate the effect of apelin on Cx43. Chen et al.8 reported that Apelin-13 reduced Cx43 expression in HL-1 cells (a mouse cardiac cell line) in a concentration-dependent manner by inhibiting angiotensin II production and activating AMPK, suggesting that AMPK exhibits an obvious regulatory effect on Cx43 expression under high glucose conditions. AMPK is also involved in the regulation of ECM secretion and remodeling. Wang et al.9 showed that AMPK activation improved the level of renal tubulointerstitial fibrosis after TGF-β1-mediated renal ischemia-reperfusion injury. Cho et al.10 found that fenofibrate ameliorated TGF-β-induced neurofibrosis in diabetic mice by activating the AMPK-endothelial nitric-oxide synthase pathway.
The changes in oxidative stress, AMPK, Cx43, NLRP3 expression, and ECM remodeling of gastric smooth muscle cells under high glucose conditions, and the underlying mechanisms have not yet been reported. Therefore, in this study, we conducted in vivo animal model and in vitro cell culture experiments to investigate the changes in oxidative stress, AMPK, Cx43, NLRP3 expression, and ECM in the gastric smooth muscle tissues of rats with DGP and high glucose-cultured rat gastric smooth muscle cells, determine the existence of oxidative stress-AMPK-Cx43-NLRP3 pathway under high glucose condition, the involvement of this pathway in remodeling ECM of gastric smooth muscle cells in DGP rats, and explore the underlying mechanisms. Our hope was to elucidate the pathogenesis of DGP and provide an experimental basis for the development of drugs for the prevention and treatment of DGP, as well as other diabetes-associated smooth muscle disorders.
Results
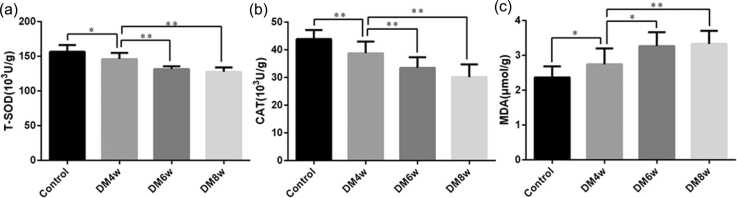
Total superoxide dismutase, catalase activities, and malondialdehyde content in the gastric smooth muscle tissues of diabetic rats
The total superoxide dismutase (T-SOD) activity in the diabetic model 4-week (DM4w) group was lower than in the control group (146.1 ± 2.82 U/g vs. 156.5 ± 3.10 U/g, P = 0.0225), and higher than in the DM6w group (146.1 ± 2.82 U/g vs. 137.1 ± 1.28 U/g, P = 0.0002), and the DM8w group (146.1 ± 2.82 U/g vs. 127.7 ± 1.98 U/g, P = 0.0001, Figure 1(a)).
Fig. 1.
Changes in oxidative stress indicators in the gastric smooth muscle tissues of diabetic tats. (a) Total superoxide dismutase (T-SOD) activity; (b) catalase (CAT) activity; (c) malondialdehyde (MDA) content. Data were expressed as mean ± standard error of the mean, t-test and one-way analysis of variance were used for comparisons between groups, n = 10 per group. *P < 0.05, **P < 0.01.
The catalase (CAT) activity in DM4w group was lower than in the control group (38.78 ± 1.34 U/g vs. 43.91 ± 1.02 U/g, P = 0.007), which was higher than in the DM6w group (38.78 ± 1.34 U/g vs. 33.51 ± 1.20 U/g, P = 0.009) and the DM8w group (38.78 ± 1.34 U/g vs. 30.16 ± 1.45 U/g, P = 0.0004, Figure 1(b)).
The malondialdehyde (MDA) content in the DM4w group was higher than in the control group (2.75 ± 0.14 µmol/g vs. 2.37 ± 0.10 µmol/g, P = 0.0435), which was lower than in the DM6w group (2.75 ± 0.14 µmol/g vs. 3.27 ± 0.13 µmol/g, P = 0.0136), and the DM8w group (2.75 ± 0.14 µmol/g vs. 3.33 ± 0.12 µmol/g, P = 0.0054, Figure 1(c)).
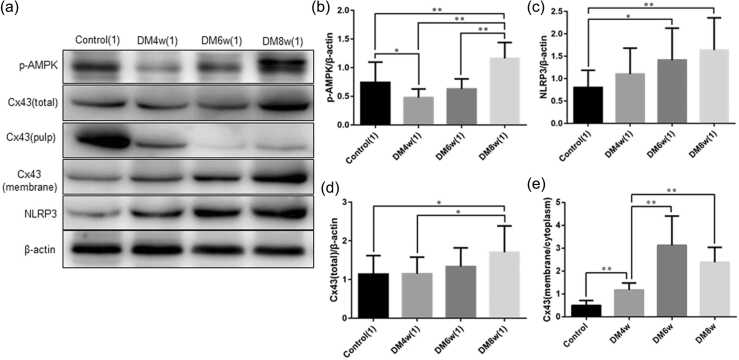
The expression of phospho-adenosine monophosphate-activated protein kinase, NLRP3,and CX43in the gastric smooth muscle tissues of diabetic rats
Phospho-adenosine monophosphate-activated protein kinase (p-AMPK), NLRP3, and Cx43 expression in the gastric smooth muscle tissues of diabetic rats was detected using Western blot analysis (Figure 2(a)). The expression of p-AMPK expression was decreased in the DM4w group than in the control group (0.48 ± 0.05 vs. 0.74 ± 0.11, P = 0.0442), whereas p-AMPK expression was higher in the DM8w group when compared with the control (1.16 ± 0.09 vs. 0.74 ± 0.11, P = 0.0084), DM4w (1.16 ± 0.09 vs. 0.48 ± 0.05, P = 0.0001), and DM6w groups (1.16 ± 0.09 vs. 0.63 ± 0.06, P = 0.0001, Figure 2(b)).
Fig. 2.
The protein expression of p-AMPK, NLRP3, and CX43 in the gastric smooth muscle tissues of diabetic rats detected by western blot analysis. (a) Representative western blot bands of proteins; (b) p-AMPK expression; (c) NLRP3 expression; (d) total protein expression of CX43; (e) the membrane/cytoplasm (M/C) ratio of Cx43 expression. Data were expressed as mean ± standard error of the mean, t-test and one-way analysis of variance were used for comparisons between groups, n = 10 per group. *P < 0.05, **P < 0.01. Abbreviations used: Cx43, Connexin43; DM4w, diabetic model 4-week; DM6w, diabetic model 6-week; DM8w, diabetic model 8-week; NLRP3, nucleotide-binding oligomerization domain-like receptor 3; p-AMPK, phospho-adenosine monophosphate-activated protein kinase.
Compared with the control group, NLRP3 expression was increased in the DM6w (0.80 ± 0.12 vs. 1.41 ± 0.23, P = 0.028), and DM8w groups (0.80 ± 0.12 vs. 1.64 ± 0.23, P = 0.0044, Figure 2(c)).
CX43 expression was increased in the DM8w group than in the control (1.70 ± 0.22 vs. 1.14 ± 0.15, P = 0.0469) and DM4w groups (1.70 ± 0.22 vs. 1.15 ± 0.14, P = 0.0433, Figure 2(d)). Additionally, we detected the membrane and cytoplasmic expression of Cx43 and calculated the membrane/cytoplasm (M/C ratio), the results showed that the M/C ratio of Cx43 expression in the DM4w group was higher than in the control group (1.18 ± 0.10 vs. 0.50 ± 0.07, P = 0.0001), which was lower than in the DM6w (1.18 ± 0.10 vs. 3.13 ± 0.40, P = 0.0002), and DM8w groups (1.18 ± 0.10 vs. 2.39 ± 0.21, P = 0.0001, Figure 2(e)).
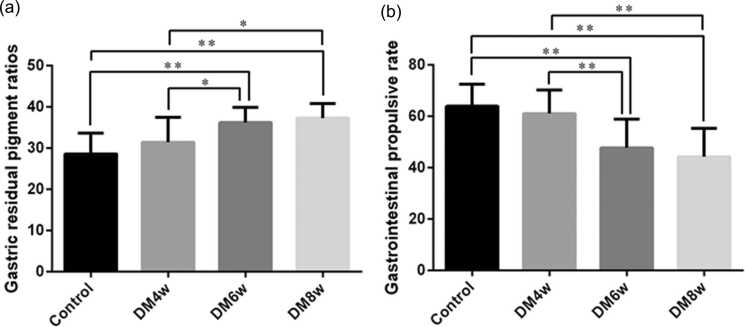
Gastric residual pigment ratio and gastrointestinal propulsion rate of diabetic rats
Compared with the control group, the gastric residual pigment ratio was higher in the DM6w (28.61 ± 1.59 vs. 36.24 ± 1.15, P = 0.0001) and DM8w groups (28.61 ± 1.59 vs. 37.3 ± 1.11, P = 0.0003). The gastric residual pigment ratio was also higher in the DM6w (36.24 ± 1.15 vs. 31.44 ± 1.92, P = 0.0457) and DM8w groups (37.3 ± 1.11 vs. 31.44 ± 1.92, P = 0.0164) than in the DM4w group (Figure 3(a)).
Fig. 3.
Gastric residual pigment ratio and gastrointestinal propulsion rate of diabetic rats. (a) Gastric residual pigment ratio; (b) Gastrointestinal propulsion rate. Data were expressed as mean ± standard error of the mean, t-test and one-way analysis of variance were used for comparisons between groups, n = 10 per group. *P < 0.05, **P < 0.01. Abbreviations used: DM4w, diabetic model 4-week; DM6w, diabetic model 6-week; DM8w, diabetic model 8-week.
The DM6w and DM8w groups showed lower gastrointestinal propulsion rate compared with the control (47.72 ± 3.54 vs. 63.93 ± 2.69, P = 0.0019; 44.24 ± 3.49 vs. 63.93 ± 2.69, P = 0.0003, respectively) and DM4w groups (47.72 ± 3.54 vs. 61.03 ± 2.90, P = 0.0094; 44.24 ± 3.49 vs. 61.03 ± 2.90, P = 0.0016, respectively, Figure 3(b)).
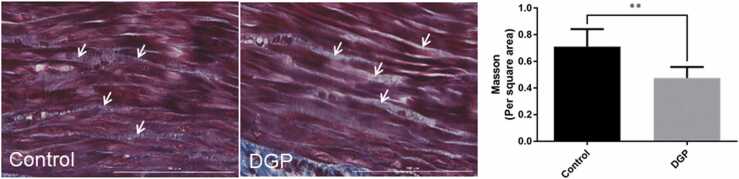
Changes in ECM of gastric smooth muscle cells in DGP rats
Results from Masson's trichome staining showed that the space between the gastric smooth muscle cells showed a blue color in the control group, which showed an appearance of light blue or white in color in the DGP group (Figure 4). Furthermore, the space between the gastric smooth muscle cells was enlarged in the DGP group. ECM content was higher in the control group compared with the DGP group (0.71 ± 0.04 vs. 0.48 ± 0.03, P = 0.0001, Figure 4).
Fig. 4.
Changes in extracellular matrix of gastric smooth muscles of DGP rats analyzed using Masson's trichome staining. Scale bar = 100 µm. Data were expressed as mean ± standard error of the mean, t-test and one-way analysis of variance were used for comparisons between groups, n = 10 per group. **P < 0.01. Abbreviation used: DGP, diabetic gastroparesis.
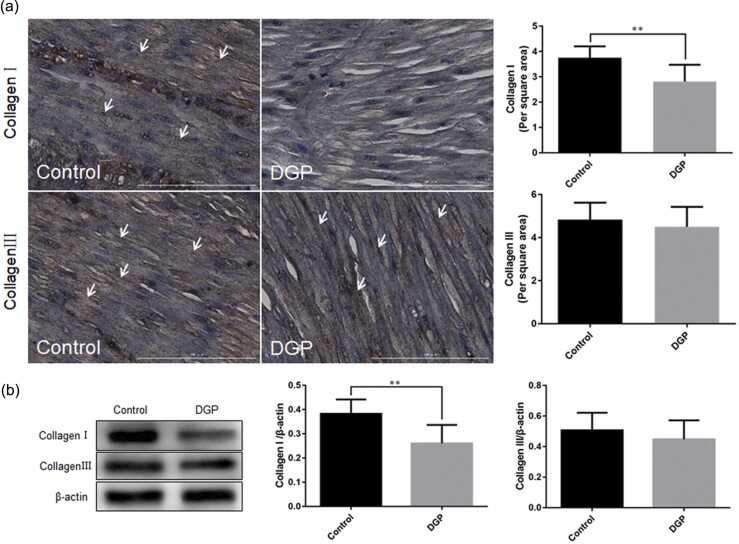
Changes in collagen types I and III in the gastric smooth muscle tissues of DGP rats
Results from immunohistochemical staining showed that in the control group, collagen type I appeared as brownish-yellow flocculent streaks and was uniformly distributed in the gastric smooth muscle tissues. In the DGP group, the brownish-yellow flocculent streaks were obviously reduced, with uneven distribution. The collagen type I content was higher in the control group compared with the DGP group (3.76 ± 0.14 vs. 2.82 ± 0.21, P = 0.0016, Figure 5(a)).
Fig. 5.
The expression changes of collagen types I and III in the gastric smooth muscle tissues of DGP rats. (a) Immunohistochemical staining for type I and III collagen, scale bar = 100 µm; (b) Western blot analysis of types I and III collagen. Data were expressed as mean ± standard error of the mean, t-test and one-way analysis of variance were used for comparisons between groups, n = 10 per group. **P < 0.01. Abbreviation used: DGP, diabetic gastroparesis.
Collagen type III appeared as brownish-yellow flocculent streaks and was uniformly distributed in the gastric smooth muscle tissues in both the control and DGP group. No significant difference was found in the collagen type III content between the control and DGP groups (4.84 ± 0.25 vs. 4.50 ± 0.29, P = 0.3925, Figure 5(a)).
Western blot analysis showed that the expression of collagen type I was higher in the control group than in the DGP group (0.38 ± 0.02 vs. 0.26 ± 0.03, P = 0.0031), while collagen type III expression did not differ significantly between the two groups (0.51 ± 0.04 vs. 0.45 ± 0.04, P = 0.3489, Figure 5(b)).
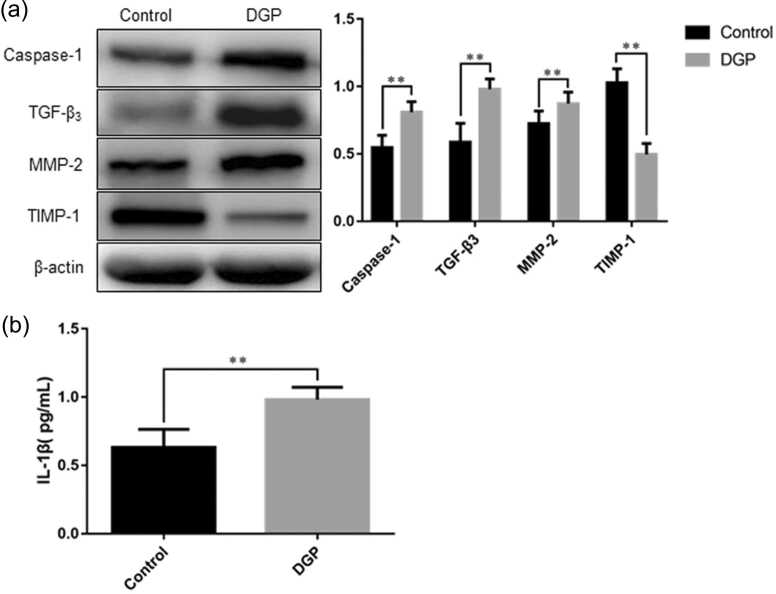
The expression of Caspase-1 TGF-β3, MMP-2, TIMP-1 and IL-1β content in the gastric smooth muscle tissues of DGP rats
The DGP group showed increased expression of Caspase-1, TGF-β3, matrix metalloproteinase-2 (MMP-2) (Caspase-1, 0.87 ± 0.02 vs. 0.55 ± 0.03, P = 0.0001; TGF-β3, 0.98 ± 0.02 vs. 0.59 ± 0.04, P = 0.0001; MMP-2, 0.87 ± 0.03 vs. 0.73 ± 0.03, P = 0.0016, Figure 6(a)), decreased tissue inhibitor of metalloproteinase-1 (TIMP-1) expression (0.50 ± 0.03 vs. 1.03 ± 0.03, P = 0.0001, Figure 6(a)), and higher IL-1β content (0.98 ± 0.03 pg/mL vs. 0.63 ± 0.04 pg/mL, P = 0.0001) when compared with the control group (Figure 6(b)).
Fig. 6.
The protein expression of Caspase-1, TGF-β3, MMP-2, TIMP-1, and IL-1β content in the gastric smooth muscle tissues of DGP rats. (a) Western blot analysis of Caspase-1, TGF-β3, MMP-2, and TIMP-1 expression; (b) Enzyme-linked immunosorbent assay detection of IL-1β content. Data are expressed as mean ± standard error of the mean, t-test and one-way ANOVA were used for comparisons between groups, n = 10 per group. **P < 0.01. Abbreviations used: DGP, diabetic gastroparesis; IL-1β, interleukin-1 beta; MMP-2, matrix metalloproteinase-2; TGF-β3, transforming growth factor-β3; TIMP-1, tissue inhibitor of metalloproteinase-1.
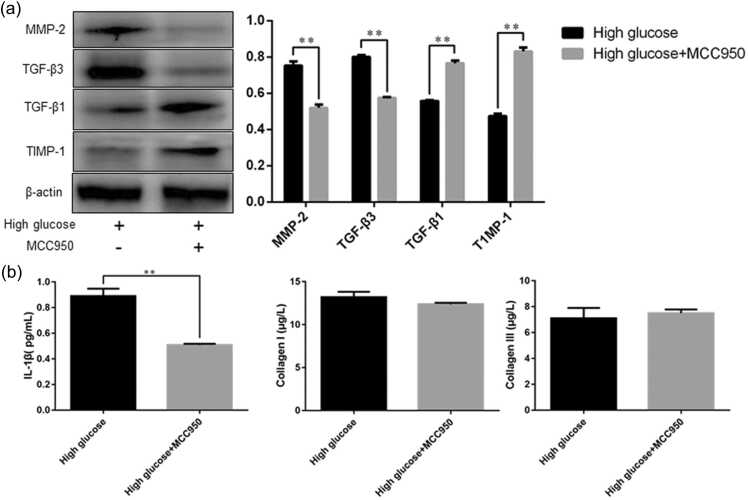
MMP-2 TGF-β3, TGF-β1, TIMP-1 expression, IL-1β, and the contents of collagen types I and III in rat gastric smooth muscle cells after inhibition of NLRP3 expression
Results from western blot analysis showed that after the cells were treated with NLRP3 inhibitor MCC950, the expression of MMP-2 and TGF-β3 in rat gastric smooth muscle cells was decreased in the high glucose + MCC950 group compared with the high glucose group (0.52 ± 0.02 vs. 0.75 ± 0.03, P = 0.0013; 0.57 ± 0.004 vs. 0.80 ± 0.01, P = 0.0001, respectively), whereas the expression of TGF-β1 and TIMP-1 was increased in the high glucose + MCC950 group compared with the high glucose group (0.77 ± 0.02 vs. 0.56 ± 0.01, P = 0.0002; 0.83 ± 0.02 vs. 0.47 ± 0.01, P = 0.0001, respectively, Figure 7(a)).
Fig. 7.
The protein expression of MMP-2, TGF-β3, TGF-β1, and TIMP-1 in rat gastric smooth muscle cells and the contents of IL-1β, collagen types I and III in the cell culture supernatants. (a) Western blot analysis of MMP-2, TGF-β3, TGF-β1, and TIMP-1 expression; (b)EEnzyme-linked immunosorbent assay detection of IL-1β, collagen types I and III content. Data are expressed as mean ± standard error of the mean, t-test and one-way ANOVA were used for comparisons between groups, n = 3 per group. **P < 0.01. Abbreviations used: IL-1β, interleukin-1 beta; MMP-2, matrix metalloproteinase-2; TGF-β3, transforming growth factor-β3; TGF-β1, transforming growth factor-β1; TIMP-1, tissue inhibitor of metalloproteinase-1.
The enzyme-linked immunosorbent assay (ELISA) assay results showed that the high glucose + MCC950 group had lower IL-1β content in the cell supernatants when compared with the high glucose group (0.51 ± 0.01 pg/mL vs. 0.89 ± 0.03 pg/mL, P = 0.0004), while no significant difference was found in the contents of collagen types I and III between the two groups (Figure 7(b)).
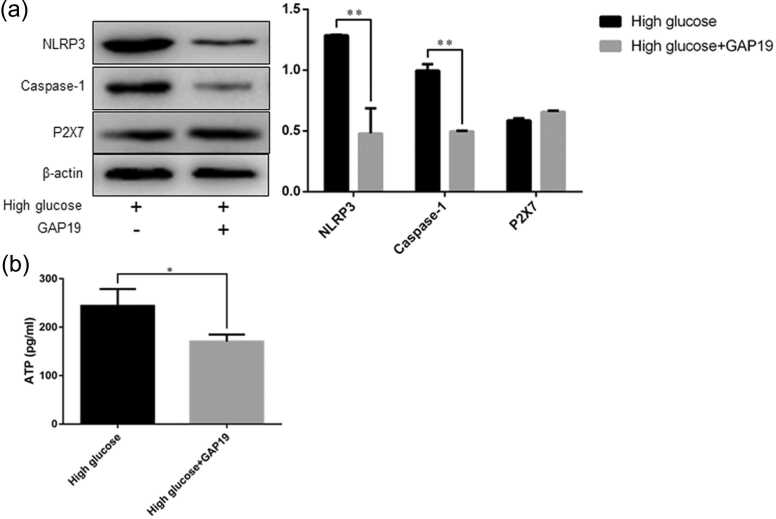
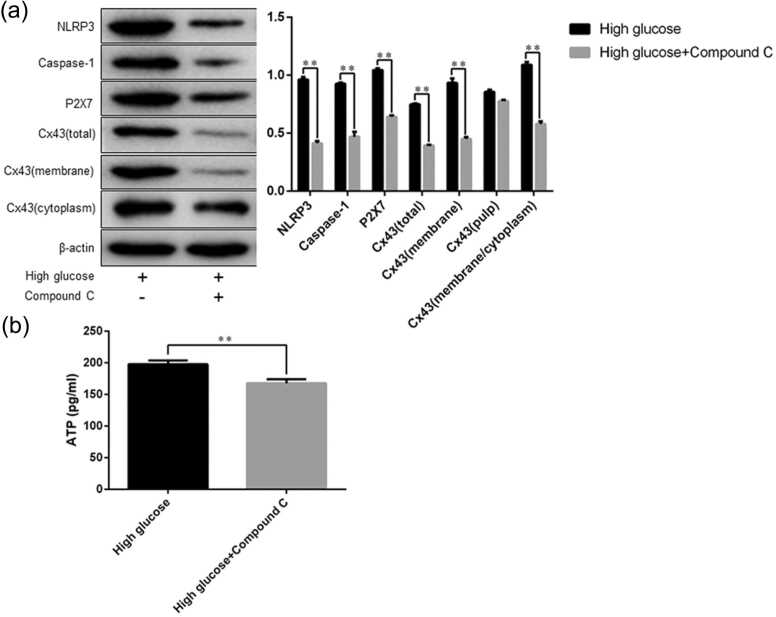
NLRP3 Caspase-1, P2X7 expression and adenosine triphosphate content in rat gastric smooth muscle cells after inhibition of Cx43 expression
Western blot analysis showed that after the cells were treated with Cx43 inhibitor growth-associated protein (GAP)1, NLRP3 and Caspase-1 expression was decreased in the high glucose + GAP19 group than in the high glucose group (NLRP3, 0.69 ± 0.01 vs. 1.29 ± 0.01, P = 0.0001; Caspase-1, 0.50 ± 0.01 vs. 1.00 ± 0.05, P = 0.0008), whereas no significant difference was found in P2X7 expression between the two groups (Figure 8(a)).
Fig. 8.
The protein expression of NLRP3, Caspase-1, and P2X7 in rat gastric smooth muscle cells, and ATP content in the cell culture supernatants. (a) Western blot analysis of NLRP3, Caspase-1, and P2X7 expression; (b) ELISA detection of ATP content. Data are expressed as mean ± standard error of the mean, t-test and one-way ANOVA were used for comparisons between groups, n = 3 per group. *P < 0.05, **P < 0.01. Abbreviations used: ATP, adenosine triphosphate; NLRP3, nucleotide-binding oligomerization domain-like receptor 3.
The ELISA assay results showed that the adenosine triphosphate (ATP) content was lower in the high glucose + GAP19 group than in the high glucose group (170.40 ± 8.41 pg/mL vs. 244.00 ± 20.14 pg/mL, P = 0.028, Figure 8(b)).
NLRP3 Caspase-1, P2X7, Cx43 expression, and ATP content in rat gastric smooth muscle cells after AMPK inhibition
Western blot analysis showed that after the cells were treated with AMPK inhibitor compound C, the total protein expression of NLRP3, Caspase-1, P2X7, Cx43, membrane expression of Cx43, as well as the M/C ratio of Cx43 expression were both lower in the high glucose + compound C than in the high glucose group (NLRP3, 0.41 ± 0.02 vs. 0.96 ± 0.02, P = 0.0001; Caspase-1, 0.47 ± 0.04 vs. 0.93 ± 0.01, P = 0.0005; P2X7, 0.64 ± 0.01 vs. 1.05 ± 0.02, P = 0.0001; Cx43, 0.40 ± 0.004 vs. 0.75 ± 0.01, P = 0.0001; membrane expression of Cx43, 0.45 ± 0.02 vs. 0.93 ± 0.04, P = 0.0003; M/C ratio of Cx43 expression, 0.58 ± 0.02 vs. 1.09 ± 0.02, P = 0.0001, Figure 9(a)).
Fig. 9.
The total protein expression of NLRP3, Caspase-1, P2X7, and Cx43, membrane and cytoplasmic expression of Cx43 in rat gastric smooth muscle cells, and ATP content in the cell culture supernatants. (a) Western blot analysis of NLRP3, Caspase-1, P2X7, and Cx43 expression; (b) ELISA detection of ATP content. Data are expressed as mean ± standard error of the mean, t-test and one-way ANOVA were used for comparisons between groups, n = 3 per group. **P < 0.01. Abbreviations used: ATP, adenosine triphosphate; Cx43, Connexin43; NLRP3, nucleotide-binding oligomerization domain-like receptor 3.
ELISA assay results showed that the ATP content was lower in the high glucose + compound C group than in the high glucose group (167.90 ± 3.73 pg/mL vs. 197.80 ± 3.40 pg/mL, P = 0.004, Figure 9(b)).
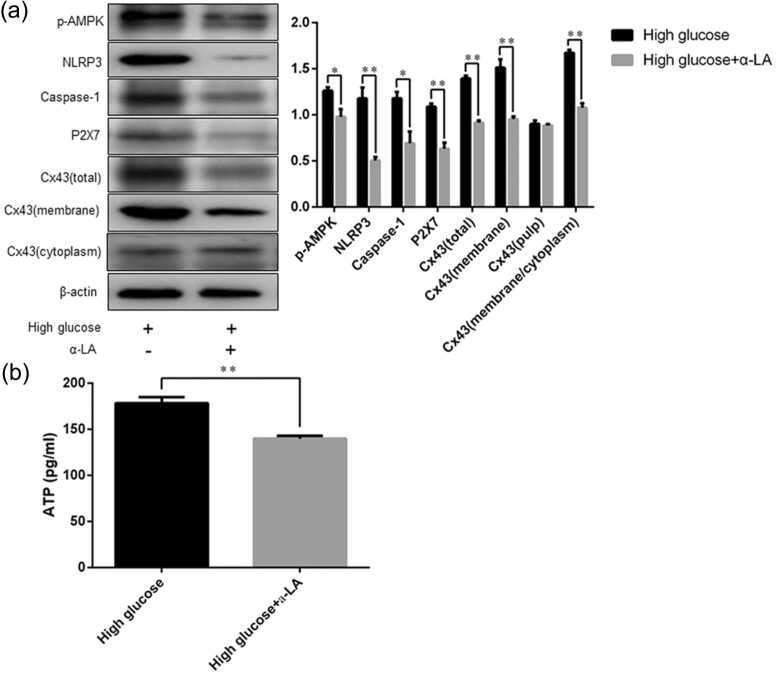
The expression of p-AMPK, NLRP3 Caspase-1, P2X7, and Cx43 in rat gastric smooth muscle cells after suppressing oxidative stress
Western blot analysis showed that after the cells were treated with antioxidant alpha-linolenic acid (α-LA), the p-AMPK, NLRP3, Caspase-1, P2X7, and Cx43 expression in rat gastric smooth muscle cells was higher in the high glucose group than in the high glucose + α-LA group (p-AMPK, 1.26 ± 0.04 vs. 0.99 ± 0.08, P = 0.0367; NLRP3, 1.18 ± 0.12 vs. 0.51 ± 0.04, P = 0.0052; Caspase-1, 1.18 ± 0.07 vs. 0.69 ± 0.13, P = 0.0287; P2X7, 1.09 ± 0.04 vs. 0.63 ± 0.07, P = 0.0042; Cx43, 1.40 ± 0.03 vs. 0.92 ± 0.02, P = 0.0003). The membrane expression of Cx43 and the M/C ratio of Cx43 expression were also higher in the high glucose group than in the high glucose + α-LA group (1.51 ± 0.10 vs. 0.95 ± 0.03, P = 0.004; 1.67 ± 0.03 vs. 1.08 ± 0.05, P = 0.0005, respectively, Figure 10(a)).
Fig. 10.
The total protein expression of p-AMPK, NLRP3, Caspase-1, P2X7, and Cx43, membrane and cytoplasmic expression of Cx43 in rat gastric smooth muscle cells, and ATP content in the cell culture supernatants. (a) Western blot analysis of p-AMPK, NLRP3, Caspase-1, P2X7, and Cx43 expression; (b) ELISA detection of ATP content. Data are expressed as mean ± standard error of the mean, t-test and one-way ANOVA were used for comparisons between groups, n = 3 per group. *P < 0.05, **P < 0.01. Abbreviations used: α-LA, alpha-linolenic acid; ATP, adenosine triphosphate; Cx43, Connexin43; NLRP3, nucleotide-binding oligomerization domain-like receptor 3; p-AMPK, phospho-adenosine monophosphate-activated protein kinase.
ELISA assay results showed that the ATP content in the cell culture supernatants was lower in the high glucose + α-LA group than in the high glucose group (140.00 ± 1.74 pg/mL vs. 178.50 ± 3.76 pg/mL, P = 0.0008, Figure 10(b)).
Discussion
According to the characteristics of the spread of excitation, gastric smooth muscles belong to single-unit smooth muscle, which can spontaneously contract without nervous system or hormonal stimulation. The reason is that some of these smooth muscle cells are pacemaker cells. The excitation of pacemaker cells can spread to the surrounding smooth muscle cells through the widespread, abundant GJ, which can make many smooth muscle cells move along with the pacemaker cells, thus forming a functional unit. In addition to accomplishing contraction and dilation functions, smooth muscle cells can also synthesize and secrete collagen, elastin, proteoglycans, and ECM. ECM is one of the factors that maintain the microenvironment of cell physiological activities and undergoes dynamic processes of constant synthesis and degradation. ECM not only provides mechanical support and connection for cells but also acts as a bridge for cell-to-cell signaling, which is involved in a variety of physiological and pathological processes of cells. Since the outer surface of gastric smooth muscle cells is surrounded by ECM, ECM remodeling could definitely cause GJ dysfunction. The synthesis and degradation of ECM are regulated by various factors. It has been reported that oxidation stress, AMPK, CX43, and NLRP3 are mechanisms that regulate ECM synthesis and degradation. Therefore, in the present study, we studied the dynamic changes in the oxidation stress indicators and the expression of p-AMPK, CX43, and NLRP3 in the gastric smooth muscle tissues of diabetic rats. The results showed that with increasing duration of diabetes, oxidation stress levels gradually increased, the AMPK activity decreased first and then increased, however, the specific mechanism behind the changes in AMPK activity is unclear and needs to be further studied. Additionally, NLRP3 expression and total expression of CX43 were increased in the gastric smooth muscle tissues of diabetic rats. The membrane expression of CX43 was also increased, whereas the cytoplasmic expression of CX43 was decreased, indicating a higher M/C ratio of Cx43 expression. The M/C ratio of Cx43 expression can reflect the open rate of GJ hemichannels, and directly determine the material and information transfer between cells, thus regulating the changes in the microenvironment by increasing intercellular material transport and information transfer.11 These findings suggest that oxidation stress, AMPK activity, CX43, and NLRP3 are involved in the pathophysiologic changes in gastric smooth muscles of diabetic rats. Interestingly, Thakur et al.12 concluded that high glucose can mediate a decrease in Cx43 expression in human cardiomyocytes lacking mitochondrial DNA. This is contrary to our results, which may be related to mitochondrial DNA. Further studies are needed to investigate the exact mechanisms underlying these conditions.
In order to verify our hypothesis that ECM remodeling exists in the gastric smooth muscle of DGP rats, we detected gastric residual pigment ratio and gastrointestinal propulsion rate at different time periods after induction of diabetes to confirm the occurrence of DGP and observed the changes in ECM of gastric smooth muscle cells of DGP rats by using Masson's trichrome staining. The results showed that the space between the gastric smooth muscle cells was enlarged, and showed an appearance of a light blue or white color. Collagen types I and III are the major protein components of ECM, so we detected the changes in the contents of collagen types I and III in the gastric smooth muscle tissues of DGP rats using immunohistochemical staining and western blot analysis. The results revealed increased collagen type I content in the gastric smooth muscle tissues of DGP rats, with no changes in collagen type III content being observed. The findings suggest that prolonged exposure to high glucose can alter ECM composition in the gastric smooth muscle tissues, leading to ECM remodeling. These results also verify our hypothesis that ECM remodeling is present in the gastric smooth muscle tissues of DGP rats.
The mechanism behind the high glucose-induced ECM remodeling is unknown. TGF-β has a role in regulating ECM synthesis and degradation. It is also an important downstream factor of NLRP3. A previous study has shown that NLRP3 can stimulate the release of downstream inflammatory substances such as Caspase-1, IL-1β, IL-18, and is involved in several cell behaviors.13 Additionally, TGF-β plays a key role in cell growth, differentiation, apoptosis and ECM remodeling.14 The MMPs are downstream targets of TGF-β, and neutral endopeptidases that can promote ECM degradation. MMP-2 can digest collagen types I, II, and III. TIMPs are specific inhibitors that participate in controlling the local activities of MMPs. TIMPs can bind MMPs in a 1:1 stoichiometry to form TIMP/MMP complexes, thereby suppressing MMP activity. TIMP-1 is the most effective inhibitor of MMPs.15 In the present study, we used western blot analysis and ELISA method to detect the expression of Caspase-1, TGF-β3, MMP-2, TIMP-1, and IL-1β content in the gastric smooth muscle tissues of DGP rats. The results revealed an increase in protein expression of Caspase-1, TGF-β3, MMP-2, and IL-1β content, and a decrease in TIMP-1 expression in the gastric smooth muscle tissues of DGP rats. The results indicate that prolonged exposure to high glucose can downregulate TIMP-1 expression, increase MMP-2 activity by upregulating TGF-β3 expression, which in turn participates in ECM remodeling by downregulating collagen type I content in the gastric smooth muscle tissues of DGP rats. The reasons why there were no changes in collagen type Ⅲ content need to be further investigated.
From the results mentioned above, we can see that AMPK was activated, and CX43 and NLRP3 expression was highly expressed in the gastric smooth muscle tissues of DGP rats. In order to further determine whether the oxidative stress-AMPK-Cx43-NLRP3 pathway exists and is involved in ECM remodeling of gastric smooth muscle cells in DGP rats, we subsequently conducted in vitro experiments, i.e., rat gastric smooth muscle cells were cultured under high glucose condition and treated with MCC950 (NLRP3 inhibitor), GAP1 (Cx43-hemichannel inhibitor), compound C (AMPK inhibitor), and α-LA (antioxidant), respectively. The results showed that after inhibition of NLRP3 expression in cells cultured under high glucose conditions, the expression of its downstream factors MMP-2 and TGF-β3 was decreased, TGF-β1 and TIMP-1 expression was increased in rat gastric smooth muscle cells, and the IL-1β content was decreased in the cell culture supernatants when compared to the high glucose group, but no changes were found in the contents of collagen types I and III. A previous study reported that TGF-β3 inhibited ECM synthesis by inhibiting the functions of TGF-β1,16 and NLRP3 inflammasome activation is the prerequisite for the release of IL-1β.17 These findings suggest that under high glucose, NLRP3 can downregulate TGF-β1 expression by upregulating TGF-β3 expression and further downregulate TIMP-1 expression by upregulating MMP-2 expression, thus regulating the secretion of collagen type I from rat gastric smooth muscle cells and participating in ECM remodeling.
In order to explore the role of Cx43 in the regulation of the function of NLRP3, we inhibited Cx43 expression in cells cultured under high glucose conditions. The results showed that after inhibition of Cx43 hemichannels in cells, the expression of its downstream factors NLRP3 and Caspase-1 were decreased, but P2X7 expression was not changed in cells, and ATP content was decreased in the cell culture supernatants when compared to the high glucose group. Purinergic ligand-gated ion channel 7 receptor is an ATP-gated ion channel receptor, which can regulate a variety of physiological functions, including cell death, inflammatory processes.18 When the cells are damaged, large amounts of ATP can be released into the extracellular environment. The extracellular ATP can bind to the P2X7 on the surface of the cell membrane that opens the channel pore, thus inducing CA2+ influx and K+ efflux. Intracellular K+ efflux can promote NLRP3 expression, NLRP3 inflammasome assembly, and the release of IL-1β and IL-18.19 These findings indicate that under high glucose conditions, CX43 can enhance the binding of ATP to P2X7 by upregulating the release of ATP from gastric smooth muscle cells into the extracellular space, thus promoting intracellular K+ efflux, increasing NLRP3 expression, and promoting NLRP3 inflammasome activation. Further studies are needed to explore whether Cx43-hemichannel opening is involved in K+ efflux.
Conclusion
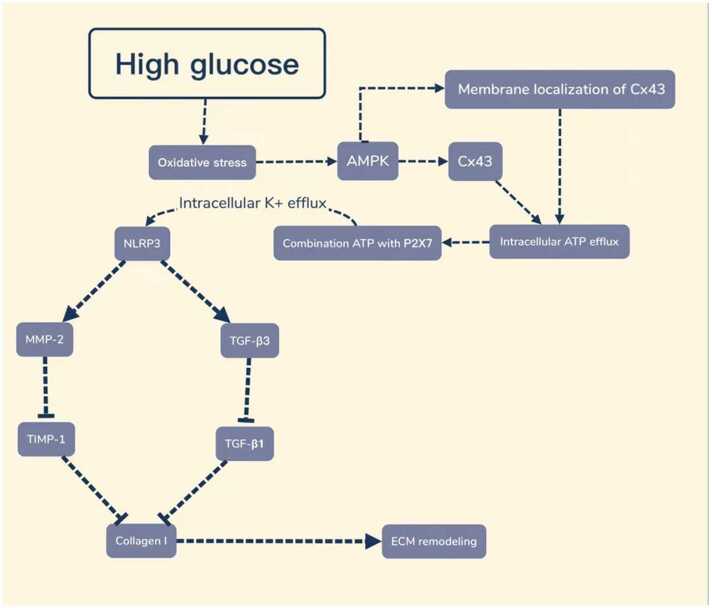
High glucose can induce oxidative stress, and oxidative stress is an upstream factor of AMPK activation. In order to explore the regulatory effect of oxidative stress and AMPK on the expression of CX43 and NLRP3, the cells cultured under high glucose conditions were treated with an antioxidant and AMPK inhibitor, respectively, the expression of p-AMPK and NLRP3, Caspase-1, P2X7, and CX43 in cells was detected. The results showed that after AMPK inhibition, the expression of CX43, NLRP3, Caspase-1, and P2X7 was both decreased, and the M/C ratio of CX43 expression was also decreased compared with the high-glucose group. After the cells were treated with antioxidants, the expression of p-AMPK was decreased, and the changes in Caspase-1, CX43, NLRP3, P2X7, and M-C ratio of CX43 expression were the same as after AMPK inhibition in cells. These results suggest that high glucose can regulate AMPK activity through oxidative stress, thus affecting the expression of downstream factors of AMPK, including Cx43 and NLRP3, as well as the M/C ratio of Cx43 expression (Figure 11). This also proves the presence of oxidative stress-AMPK-Cx43-NLRP3 pathway under high glucose conditions.
Fig. 11.
The oxidative stress-AMPK-Cx43-NLRP3 pathway is involved in extracellular matrix remodeling of gastric smooth muscle cells in rats with diabetic gastroparesis. Abbreviations used: AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; Cx43, connexin43; ECM, extracellular matrix; MMP-2, matrix metalloproteinase-2; NLRP3, nucleotide-binding oligomerization domain-like receptor protein 3; TGF-β1, transforming growth factor-beta 1; TGF-β3, transforming growth factor-beta 3; TIMP-1, tissue inhibitor of metalloproteinases-1.
In conclusion, our findings suggest that high glucose can induce the activation of the AMPK-Cx43-NLRP3 pathway through oxidative stress, which is involved in the ECM remodeling of gastric smooth muscles in DGP rats by regulating the biological functions of TGF-β3, TGF-β1, MMP-2, and TIMP-1.
Materials and methods
Materials
Streptozotocin (STZ, S0130, Sigma, MO, USA); GAP-19 (HY-P1136A, MedChemExpress, NJ, USA); MCC950 (HY-12815A, MedChemExpress, NJ, USA); compound C (HY-13418A, MedChemExpress, NJ, USA); collagen type III antibody (M00788, Wuhan Boster Biological Technology, Ltd., Wuhan, China); collagen type I antibody (bs-10423R, Bioss, Beijing, China); Cx43 antibody (3512S, Cell Signaling Technology, MA, USA); NLRP3 antibody (Cat No. ab214185, Abcam, Cambridge, UK); Caspase-1 antibody (A0964, ABclonal, MA, USA); MMP-2 antibody (ab92536, Abcam, Cambridge, UK); TGF-β3 antibody (A8460, ABclonal, MA, USA); TIMP-1 antibody (SC-21734, Santa Cruz Biotechnology, TX, USA); phospho (p)-AMPK antibody (Cat No. ab133448, Abcam, Cambridge, UK); P2X7 antibody (ab109054, Abcam, Cambridge, UK); TGF-β1 antibody (ab215715, Abcam, Cambridge, UK); β-actin antibody (Cat no. A5316, Sigma, MO, USA); α-LA (STADA pharmaceuticals); horseradish peroxidase-labeled goat anti-rabbit or goat anti-mouse IgG secondary antibody (Sigma, MO, USA); rat IL-1β ELISA kit (ml037361, Mlbio, Shanghai, China); collagen type I ELISA kit (SAB4500362, Merk, MA, USA); collagen type III ELISA kit (AB7579, Merk, MA, USA); rat ATP ELISA kit (JM-10331R1, Jiangsu Jingmei Biotechnology Co., Ltd, Jiangsu, China). MDA assay kit (thiobarbituric acid method, A003-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China); T-SOD assay kit (Hydroxylamine method, A001-1-2, Nanjing Jiancheng Bioengineering Institute); CAT assay kit (Visible light method, A007-1-1, Nanjing Jiancheng Bioengineering Institute).
Animals and establishment of the diabetic rat model
A total of 120 adult male Wistar rats (weighing 200 ± 20 g) were provided by Yanbian University Laboratory Animal Center, and randomly divided into control (n = 30) and DM (n = 90) groups. And according to different time points after model establishment, rats in the DM group were further divided into three subgroups: 4-week (DM4w, n = 30), 6-week (DM6w, n = 30), 8-week (DM8w, n = 30) groups. These time points were selected based on our previous studies.1, 20 All rats were acclimatized for 1 week, and were housed in single cages at a relative humidity of 50–80%, and room temperature of 18–25 °C.
All rats in the model group received an injection of 0.5% STZ to establish a diabetic rat model. Exactly 0.5% STZ solution was prepared by dissolution in 0.1 mol/L citrate buffer (pH 4.0). After rats were fasted but allowed free access to water for 12 h, and weighed, a single dose of STZ (65 mg/kg) was injected intraperitoneally. After injection, all rats were housed under the same condition. At 7 days after the administration of STZ, blood from the tail vein was collected. A blood glucose level of >350 mg/dL indicated that the diabetic rat model was successfully established. The modeling was successful in all rats of the model group, no rats died during model establishment.
All experimental procedures involving animals were performed in accordance with Institutional Animal Care and Use Committee guidelines, and approved by the Animal Care and Use Committee of Medical College of Yanbian University (approval number SYXK (JI) 2020-0009).
Detection of oxidative stress indicators in the gastric smooth muscle tissues of diabetic rats
Ten rats from each group were euthanized at the determined time points. The whole stomach was quickly dissected, and the gastric smooth muscle tissues were peeled off. A portion of the gastric smooth muscle tissues was weighed, mechanically triturated in saline into chyle-like shapes, and centrifuged at 4000 r/min for 15 min, the supernatants were then collected. The MDA content, T-SOD, and CAT activities were detected using the MDA assay kit (thiobarbituric acid method), T-SOD assay kit (Hydroxylamine method), and CAT assay kit (Visible light method), respectively, according to the manufacturer’s instructions. The optical density (OD) was measured at 532 nm, 550 nm, 405 nm, respectively, using a UV spectrophotometer. The remaining gastric smooth muscle tissues were stored in liquid nitrogen for future use.
Western blot analysis of p-AMPK, NLRP3 Cx43 expression in the gastric smooth muscle tissues of diabetic rats
The gastric smooth muscle tissues stored in liquid nitrogen were taken and homogenized to extract total protein, membrane and cytoplasmic proteins. The protein concentration was determined with a full-wavelength spectrophotometer. The protein samples were boiled for 2 min, 40 μg of protein was then loaded per lane, which was subjected to electrophoretic separation by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes using a semi-dry transfer method. After the PVDF membrane was blocked with 5% skim milk powder in Tris Buffered Saline with Tween 20 buffer (25 mmol/L Tris, 150 mmol/L NaCl, 1% Tween 20, pH 7.5) and washed, the membrane was incubated with p-AMPK Thr172 antibody (1:1000), NLRP3 antibody (1:1000), and Cx43 antibody (1:1, 000) overnight at 4 °C. After washing, the membrane was incubated with secondary IgG antibody (1:1000) for 1 h at room temperature, and washed. A gel imaging analysis system was used to acquire and analyze images. Using β-actin (1:500) as an internal reference, the total expression of p-AMPK, NLRP3, Cx43, membrane and cytoplasmic expression of Cx43 were measured. The M/C ratio of Cx43 expression was calculated.
Detection of gastric residual pigment ratio and gastrointestinal propulsion rate of diabetic rats
Gastric residual pigment ratio
At 4, 6, and 8 weeks after model establishment, 10 rats from each group were taken. After they were fasted with free access to water for 24 h, 0.4 mL of methylene blue solution (1 mg/mL) was administered by oral gavage, and rats were euthanized 30 min later. Then, the whole stomach was dissected, and the gastric residuals were collected quickly and rinsed with saline. The rinsate was collected and centrifuged at 3500 rpm/min for 15 min, and the supernatants were collected. The OD value was measured at 640 nm using a spectrophotometer to determine gastric residual pigment ratio:1 gastric residual pigment ratio (%) = OD value of detection tube/OD value of standard tube × 100%.
Gastrointestinal propulsion rate
At 4, 6, and 8 weeks after model establishment, 10 rats from each group were taken. After they were fasted with free access to water for 24 h, rats were administered with 1 mL/0.1 kg of carbon powder suspension (activated carbon 10%, gum arabic 10%) by gavage, and euthanized 30 min later. The intestine from the pylorus to the ileocecal region was rapidly dissected, and the distance from the anterior end of the carbon powder to the pyloric sphincter, and the distance from the pyloric sphincter to the end of the small intestine were measured to calculate the gastrointestinal propulsion rate:1 Gastrointestinal propulsion rate (%) = distance from the anterior end of the carbon powder to the pyloric sphincter (cm)/distance from the pyloric sphincter to the end of the small intestine (cm) × 100%.
Determination of the successful establishment of DGP rat model
The differences in the gastric residual pigment ratio and gastrointestinal propulsion rate between the DM and NC groups were analyzed, a P < 0.05 or P < 0.01 indicated the successful establishment of the DGP rat model. Ten rats from the DM8W group were used as the DGP group, and 10 normal rats were used as control.
Morphological observations of the gastric smooth muscle tissues of DGP rats
After rats in the control and DGP groups were euthanized, the whole stomach was dissected, and the gastric antral wall was removed. The samples were fixed in Bouin's solution (25 mL of 40% formaldehyde, 75 mL of saturated aqueous picric acid, 5 mL of glacial acetic acid), dehydrated in gradient ethanol, embedded in paraffin, and cut into tissue sections at 5 µm. The muscle tissues were isolated from the remaining gastric wall, and stored in liquid nitrogen for future use.
Masson's trichrome staining
After hydration, the sections were stained with Regaud's hematoxylin (1 g hematoxylin, 10 mL of 95% alcohol, 10 mL of glycerol, 80 mL of distilled water) for 8 min. After rinsing in distilled water, the sections were stained with ponceau acid fuchsin solution (0.7 g ponceau S, 0.3 g acid fuchsin, 99 mL distilled water, 1 mL glacial acetic acid) for 8 min. The sections were subsequently immersed in 0.2% glacial acetic acid for 15 s, placed in 1% phosphomolybdic acid aqueous solution for 4 min, and counterstained with aniline blue solution (2 g aniline blue, 98 mL distilled water, 2 mL glacial acetic acid) for 5 min, followed by soaking in 0.2% glacial acetic acid solution again for 1 min. Subsequently, the sections were dehydrated, cleared, and mounted. The Image-Pro Plus 6.0 software was used to examine the sections under double-blind conditions to observe the changes in ECM of gastric smooth muscles in DGP rats.
Immunohistochemical staining
After hydration, the sections were rinsed 3 times for 2 min each with phosphate-buffered saline (PBS), soaked in citrate buffer (PH6.0), heated to boiling by a microwave oven for 20 min, and then powered off. After a 3-minute interval, the microwave power was turned on. These steps were repeated four times. The top of the slides should be covered completely with the citrate buffer to avoid drying out of the sections due to evaporation. After rinsing 3 times for 5 min each with PBS, sections were incubated with 3% H2O2 at room temperature for 20 min and washed 3 times for 5 min each with PBS. Subsequently, the sections were blocked with normal goat serum for 40 min at room temperature and incubated with collagen type III antibody (1:200 dilution) and collagen type I antibody (1:200 dilution) at 4 ℃ overnight. After rewarming sections for 1 h, they were rinsed 3 times for 5 min each with PBS, and incubated with biotinylated secondary antibody working solution for 40 min at 37 ℃, followed by washing with PBS 3 times for 5 min each. Then the sections were incubated with horseradish peroxidase-labeled streptavidin working solution at 37 ℃ for 40 min, rinsed with PBS 3 times for 5 min each. Color development was performed using diaminobenzidine. The sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted. The Image-Pro Plus 6.0 software was used to examine the sections under double-blind conditions to observe the changes in collagen types I and III in the gastric smooth muscle tissues of DGP rats.
Western blot analysis of gastric smooth muscle tissues of DGP rats
The gastric smooth muscle tissues stored in liquid nitrogen were taken and homogenized to extract total protein. The protein concentration was determined with a full-wavelength spectrophotometer. The protein samples were boiled for 2 min, 40 μg of protein was then loaded per lane, which was subjected to electrophoretic separation by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to PVDF membranes using a semi-dry transfer method. After the PVDF membrane was blocked with 5% skim milk powder in TBS-T buffer (25 mmol/L Tris, 150 mmol/L NaCl, 1% Tween 20, pH 7.5) and washed, the membrane was incubated with collagen type III antibody (1:1000), collagen type I antibody (1:1000), Caspase-1 antibody (1:500), MMP-2 antibody (1:1000), TGF-β3 antibody (1:500), TIMP-1 antibody (1:500), and β-actin (1:500) overnight at 4 °C. After washing, the membrane was incubated with secondary IgG antibody (1:1000) for 1 h at room temperature, and washed. A gel imaging analysis system was used to acquire and analyze images. Using β-actin as an internal reference, the relative expression levels of proteins were calculated.
Detection of IL-1β content in the gastric smooth muscle tissues of DGP rats using ELISA
The gastric smooth muscle tissues stored in liquid nitrogen were taken, homogenized, and centrifuged at 1000 g for 10 min; the supernatants were collected. ELISA was performed according to the manufacturers' instructions. Then the OD value was measured at 450 nm by using a microplate reader.
Cell resuscitation and passaging
Frozen rat primary gastric smooth muscle cell line (iCell Bioscience Inc., Shanghai, China) was taken and thawed at 37 ℃. The cell suspension was aspirated into centrifuge tubes and centrifuged at 1000 rpm for 10 min. After the supernatants were discarded, 1 mL of culture medium was added to the precipitate, which was then gently blown, and recentrifuged for 10 min. Subsequently, the supernatants were discarded, the appropriate amount of medium was added, and the cells were transferred to a culture flask for culture at 37 ℃. The cell status was observed with an inverted microscope. After the culture medium was sucked out, the cells were washed for one time by adding 5 mL PBS. PBS was then removed by aspiration, 1.5 mL of trypsin was added, and the cells were digested for 1 min at 37 ℃, followed by adding fresh medium and transferring to culture flasks. Then the appropriate amount of fresh medium was added to the cell, which was then split into 2–3 culture flasks, with an appropriate amount of medium being added to each flask. Then, the flasks were placed in a 37 °C incubator for culture. The cell density was adjusted to 1 × 106 cells/mL for subsequent use.
Western blot analysis and ELISA of rat gastric smooth muscle cells after inhibition of NLRP3expression
Cells were divided into the high glucose (35 mmol/L glucose) group and the high glucose + MCC950 (15 nM) group. After 48 h of culture, total protein was extracted from cells. The protein concentration was determined with a full-wavelength spectrophotometer. The primary antibodies were MMP-2 antibody (1:1000), TGF-β3 antibody (1:500), and TIMP-1 antibody (1:500). The remaining procedures were the same as mentioned above.
For ELISA, gastric smooth muscle cells were taken from each group, and the supernatants were collected. The contents of IL-1β, collagen types I, and III were detected using IL-1β, collagen Ⅰ, and Ⅲ ELISA kits according to the manufacturer's instructions. The OD value was measured at 450 nm using a microplate reader. The experiments were repeated three times.
Western blot analysis and ELISA of rat gastric smooth muscle cells after inhibition of Cx43expression
Cells were divided into the high glucose (35 mmol/L glucose) group and high glucose + GAP19 (100 μM) group. After 48 h of culture, total protein was extracted from cells. The protein concentration was determined with a full-wavelength spectrophotometer. The primary antibodies were NLRP3 antibody (1:1000), Caspase-1 antibody (1:500), and P2X7 antibody (1:1000). The remaining procedures were the same as mentioned above.
For the ELISA, gastric smooth muscle cells were taken from each group, and the supernatants were collected. The ATP content was detected using an ATP ELISA kit according to the manufacturer's instructions. The OD value was measured at 450 nm using a microplate reader. The experiments were repeated three times.
Western blot analysis and ELISA assay of rat gastric smooth muscle cells after AMPK inhibition
Cells were divided into the high glucose (35 mmol/L glucose) group and high glucose + compound C (10 μM) group. After 48 h of culture, total protein, membrane and cytoplasmic proteins were extracted from cells. The protein concentration was determined with a full-wavelength spectrophotometer. The primary antibodies were Cx43 antibody (1:1000), NLRP3 antibody (1:1000), Caspase-1 antibody (1:500), and P2X7 antibody (1:1000). The remaining procedures were the same as mentioned above. The total protein expression of Cx43, NLRP3, Caspase-1, and P2X7, as well as membrane and cytoplasmic expression of Cx43 in cells were detected.
ELISA assay was performed to measure ATP content in the cell culture supernatants. The procedures were the same as mentioned above. The experiments were repeated three times.
Western blot analysis and ELISA assay of rat gastric smooth muscle cells after suppressing oxidative stress
Cells were divided into the high glucose (35 mmol/L glucose) group and high glucose + α-ALA (100 μM) group. After 48 h of culture, total protein, membrane and cytoplasmic proteins were extracted from cells. The protein concentration was determined with a full-wavelength spectrophotometer. The primary antibodies were Cx43 antibody (1:1000), p-AMPK Thr172 antibody (1:1000), NLRP3 antibody (1:1000), Caspase-1 antibody (1:500), and P2X7 antibody (1:1000). The remaining procedures were the same as mentioned above. The total protein expression of Cx43, p-AMPK, NLRP3, Caspase-1, and P2X7, as well as membrane and cytoplasmic expression of Cx43 in cells, were detected.
ELISA assay was performed to measure ATP content in the cell culture supernatants. The procedures were the same as mentioned above. The experiments were repeated three times.
Statistical analysis
GraphPad Prism 5 was used for statistical analysis and graphing. Continuous data were expressed as mean ± standard error of the mean. t-Test and one-way analysis of variance were used for comparisons between groups. P < 0.05 was considered as statistically significant.
Declarations of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding and support
The work was supported by the National Natural Science Foundation of China (82060154), the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS147), and the Science and Technology Project of the Education Department of Jilin Province (JJKH20221351KJ).
Data availability statement
Data will be made available on request.
NA (Original data) (NA)
References
- 1.Zhang M.H., Fang X.S., Guo J.Y., Jin Z. Effects of AMPK on apoptosis and energy metabolism of gastric smooth muscle cells in rats with diabetic gastroparesis. Cell Biochem Biophys. 2019;77:165–177. doi: 10.1007/s12013-019-00870-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang S., Li Y., Fan J., et al. Interleukin-22 ameliorated renal injury and fibrosis in diabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cáceres F.T., Gaspari T.A., Samuel C.S., Pinar A.A. Serelaxin inhibits the profibrotic TGF-β1/IL-1β axis by targeting TLR-4 and the NLRP3 inflammasome in cardiac myofibroblasts. FASEB J. 2019;33:14717–14733. doi: 10.1096/fj.201901079RR. [DOI] [PubMed] [Google Scholar]
- 4.Sun X., Huang K., Haiming X., et al. Connexin 43 prevents the progression of diabetic renal tubulointerstitial fibrosis by regulating the SIRT1-HIF-1α signaling pathway. Clin Sci. 2020;134:1573–1592. doi: 10.1042/CS20200171. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y., Mao Z., Zhang Z., et al. Connexin43 contributes to inflammasome activation and lipopolysaccharide-initiated acute renal injury via modulation of intracellular oxidative status. Antioxid Redox Signal. 2019;31:1194–1212. doi: 10.1089/ars.2018.7636. [DOI] [PubMed] [Google Scholar]
- 6.Xie X., Chen Y., Liu J., et al. High glucose induced endothelial cell reactive oxygen species via OGG1/PKC/NADPH oxidase pathway. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117886. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Yu L., Gao J., et al. Apelin Ameliorates high glucose-induced downregulation of Connexin 43 via AMPK-dependent pathway in neonatal rat cardiomyocytes. Aging Dis. 2018;9:66–76. doi: 10.14336/AD.2017.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Qiao X., Zhang L., Li X., Liu Q. Apelin-13 regulates angiotensin ii-induced Cx43 downregulation and autophagy via the AMPK/mTOR signaling pathway in HL-1 cells. Physiol Res. 2020;69:813–822. doi: 10.33549/physiolres.934488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Weng X., Guo J., Chen Z., Jiang G., Liu X. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats. Ren Fail. 2016;38:614–621. doi: 10.3109/0886022X.2016.1149770. [DOI] [PubMed] [Google Scholar]
- 10.Cho Y.R., Lim J.H., Kim M.Y., et al. Therapeutic effects of fenofibrate on diabetic peripheral neuropathy by improving endothelial and neural survival in db/db mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boengler K., Rohrbach S., Weissmann N., Schulz R. Importance of Cx43 for right ventricular function. Int J Mol Sci. 2021;22:987. doi: 10.3390/ijms22030987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakur V., Alcoreza N., Cazares J., Chattopadhyay M. Changes in stress-mediated markers in a human cardiomyocyte cell line under hyperglycemia. Int J Mol Sci. 2021;22:10802. doi: 10.3390/ijms221910802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Hauenstein A.V. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76 doi: 10.1016/j.mam.2020.100889. [DOI] [PubMed] [Google Scholar]
- 14.Peng D., Fu M., Wang M., Wei Y., Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T.H., Wei Y., Dong X.L., et al. The dual roles of three MMPs and TIMP in innate immunity and metamorphosis in the silkworm, Bombyx mori. FEBS J. 2022;289:2828–2846. doi: 10.1111/febs.16313. [DOI] [PubMed] [Google Scholar]
- 16.Wilson S.E. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp Eye Res. 2021;207 doi: 10.1016/j.exer.2021.108594. [DOI] [PubMed] [Google Scholar]
- 17.Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 2013;65:872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani A.L., Sarti A.C., Falzoni S., Di Virgilio F. The P2×7 receptor-interleukin-1 Liaison. Front Pharmacol. 2017;123 doi: 10.3389/fphar.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koumangoye R. The role of Cl- and K+ efflux in NLRP3 inflammasome and innate immune response activation. Am J Physiol Cell Physiol. 2022;322:C645–C652. doi: 10.1152/ajpcell.00421.2021. [DOI] [PubMed] [Google Scholar]
- 20.Yan S., Zheng Y.R., Jin Z., Zhang M.H., Cui X.S. Involvement of Rictor/mTORC2/Akt/GLUT4 pathway in the regulation of energy metabolism in the gastric smooth muscle of diabetic rats. Acta Biochim Pol. 2023;70:233–238. doi: 10.18388/abp.2020_5652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
NA (Original data) (NA)