Abstract
Chronic stress disrupts the emotional and energetic balance, which may lead to abnormal behaviors such as binge eating. This overeating behavior alleviating the negative emotions is called emotional eating, which may exacerbate emotional instability and lead to obesity. It is a complex and multifaceted process that has not yet been fully understood. In this study, we constructed an animal model of chronic mild stress (CMS)-induced emotional eating. The emotional eating mice were treated with tryptophan for 21 days to reveal the key role of tryptophan. Furthermore, serum-targeted metabolomics, immunohistochemical staining, qPCR and ELISA were performed. The results showed that CMS led to the binge eating behavior, accompanied by the disturbed intestinal tryptophan-derived serotonin (5-hydroxytryptamine; 5-HT) metabolic pathways. Then we found that tryptophan supplementation improved depression and anxiety-like behaviors as well as abnormal eating behaviors. Tryptophan supplementation improved the abnormal expression of appetite regulators (e.g., AgRP, OX1R, MC4R), and tryptophan supplementation also increased the tryptophan hydroxylase 2 (tph2) and 5-HT receptors in the hypothalamus of CMS mice, which indicates that the 5-HT metabolic pathway influences feeding behavior. In vitro experiments confirmed that 5-HT supplementation ameliorated corticosterone-induced aberrant expression of appetite regulators, such as AgRP and OX1R, in the hypothalamic cell line. In conclusion, our findings revealed that the tryptophan-derived 5-HT pathway plays an important role in emotional eating, especially in providing targeted therapy for stress-induced obesity.
Keywords: Chronic stress, Depression, Emotional eating, Tryptophan, 5-HT, Appetite
Graphical abstract
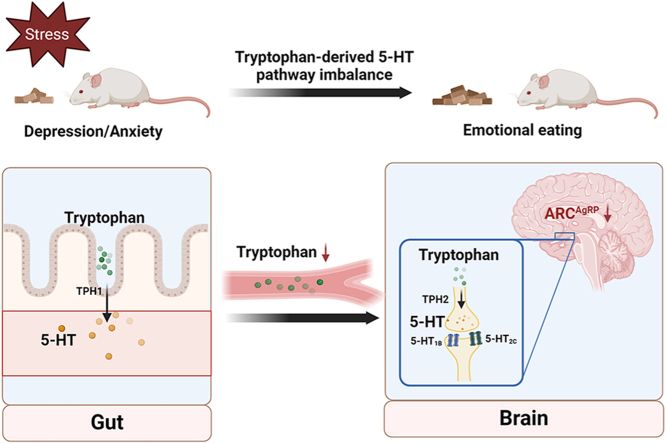
Tryptophan ameliorates chronic stress-induced emotional eating behavior by balancing the tryptophan-derived 5-HT pathway.
Highlights
There are three main highlights of the findings:
-
•
Tryptophan treatment improves emotional eating by balancing the brain's 5-HT pathway.
-
•
5-HT reversed corticosterone-induced abnormal expression of AgRP in GT1-7 cells.
-
•
Tryptophan may treat emotional eating by maintaining central 5-HT and AgRP levels.
1. Introduction
Stress is a physiologic and adaptive response to environmental change, but may also cause pathological changes, such as anxiety, depression, and other mood disorders (McEwen and Morrison, 2013). Recently, several studies have confirmed that stress alters feeding behavior and energy homeostasis (Kuti et al., 2022). Acute or chronic stress affects homeostasis or health, inducing a range of physiological and behavioral responses and altering metabolic and behavioral changes in the body (Dallman et al., 2003); there is a strong link between increased stress-induced negative emotions and food consumption and energy disruption, such as during the Corona Virus Disease 2019, when people commonly eat to relieve stress and loneliness (Ederer et al., 2023). Stress and negative emotions inversely affect appetite, leading to behaviors such as binge eating and anorexia, which are referred to as emotional binge eating and emotional under-eating (Dakanalis et al., 2013, 2014, 2016; Evers et al., 2018).
In recent years, the study of emotional eating has received increasing attention. Emotional eating mainly refers to people who are in low spirits, who will reduce the control of their feeding and consume more food. This behavior is usually considered a poor eating habit and is often associated with several eating disorders, such as bulimia and binge eating disorder (Burmester, Esme & Nicholls, 2021). For a large portion of the population, stress and negative emotions cause them to develop a type of eating known as emotional eating (Buja et al., 2022; Konttinen et al., 2010). Emotional eating is not a separate eating disorder, but rather an eating behavior influenced by behaviors, stress, emotions, and personal feelings related to eating (Dakanalis et al., 2023). Therefore, it is crucial to understand that emotional eating behavior, unlike specific eating disorders, is primarily triggered by stress, resulting in a tendency to eat in response to negative emotions (Godet et al., 2022).
Normal eating behavior is essential for survival and homeostatic control of energy balance. Nevertheless, depression and autism triggered dysphoria and abnormal eating habits (Bremner et al., 2020). It has been shown that chronic stress leads to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which affects eating behavior (Guerrero-Hreins et al., 2021). Appetite disorders may disrupt the metabolic health of the host and lead to various metabolic disorders, including gastrointestinal disorders, schizophrenia, etc. Furthermore, chronic stress may cause over-activation of the brain's reward and emotion regulation regions in response to salient cues from rewarding stimuli (e.g., food and drugs), thereby sensitizing the brain to these stimuli.
The hypothalamus regulates food intake and contains numerous neurons that play a crucial role in maintaining energy balance (Concetti et al., 2023). Many studies have demonstrated that the hypothalamic arcuate nucleus (ARC) integrates circulating signals of hunger and satiety, reflecting energy storage and utilization (Andermann and Lowell, 2017). These neurons in the ARC of the hypothalamus are involved in feeding and food processing (Gao and Horvath, 2007), of which the ARC neuron cluster most closely associated with driving hunger is the AgRP/NPY (Agouti-related protein/Neuropeptide Y) neurons: fasting activates this cluster of neurons (Morton et al., 2006), and inhibition of AgRP neurons reduces overconsumption of food in energy-deficient states (Mandelblat-Cerf et al., 2015; Baver et al., 2014; Chen et al., 2015). In addition, leptin and orexin are appetite-related regulators that have been extensively researched. Among them, leptin, in particular, plays a vital role in the central regulation of food intake and energy homeostasis. It inhibits the activity of AgRP/NPY neurons and AgRP expression, which are responsible for stimulating appetite (Sohn, Elmquist & Williams, 2013). Thus, leptin acts within the hypothalamus to inhibit food intake and increase energy expenditure. Besides, orexin is reported to regulate a variety of physiological functions, including feeding and energy homeostasis, and is distributed throughout the central nervous system (Goforth et al., 2014). It is closely associated with the development of stress-related disorders such as depression and panic disorder (Johnson et al., 2010).
Tryptophan (Trp) metabolism is highly correlated with mood and diet. A study on the effect of dietary tryptophan on affective disorders emphasized that tryptophan-rich diets may reduce depression and improve mood states in individuals (Lindseth, Helland & Caspers, 2015). Serotonin (5-HT) and kynurenine (Kyn), which are metabolites, are known to play a crucial role in depression (Comai et al., 2020). Of these, 5-HT is a neurotransmitter that plays a crucial role in various behaviors such as mood, appetite, and stress response. It is primarily found in the central nervous system (Höglund, Øverli & Winberg, 2019). In addition, the kynurenine pathway, the predominant metabolic pathway of tryptophan, has been strongly with depression, schizophrenia, and other related mental health disorders (Cervenka, Agudelo & Ruas, 2017).
One of the metabolites of tryptophan, 5-HT, is a neurotransmitter that plays an important role in the central nervous system, improving mood and appetite abnormalities. Several studies have suggested that stress-induced emotional eating may be associated with diminished central 5-HT neurotransmission (Leibowitz and Alexander, 1998). Brain dysfunction of 5-HT neurological has been associated not only with depressed mood and impaired ability to cope with stress (Jans et al., 2007; Firk and Markus, 2007; Markus, 2008; Gotlib et al., 2008), but also with reduced control over food intake, and consumption of low-calorie, high-fat foods (He et al., 2022). Although 5-HT has been extensively studied as a neurotransmitter, most of 5-HT produced in the body is peripheral. However, 5-HT produced by neurons in the central nervous system plays a crucial role in regulating sleep, mood, and appetite and is also involved in the stress response. Regrettably, it is important to note that 5-HT cannot cross the blood-brain barrier to act on the central nervous system (Martin et al., 2018), whereas its precursor substance, tryptophan, could achieve penetration through the BBB. Thus, the deficiency of 5-HT in the body is compensated by the tryptophan supplementation.
In this study, we evaluated the potential modulatory effects of tryptophan supplementation on feeding and mood by creating a mouse model of chronic stress-induced feeding abnormalities. In addition, we analyzed tryptophan metabolite changes under chronic stress by serum tryptophan-targeted metabolomics. To sum up, this paper aims to investigate the tryptophan/5-HT metabolic pathway in the context of chronic stress. The aim is to shed light on the regulatory role of tryptophan supplementation in addressing the abnormal changes in appetite that occur during chronic stress. Additionally, this study aims to provide some therapeutic measures for tryptophan treatment in individuals who experience abnormal mood or appetite.
2. Materials and methods
2.1. Animal experiments
Forty healthy male balb/c mice (6–8 weeks old, 18–22 g), SPF grade, were purchased from Shanghai Jiesijie Laboratory Animal Co Ltd [Production License No.: SCXK (Shanghai) 2018–0004], and housed under standard conditions with a light/dark cycle of 12 h, a room temperature of 22 ± 1 °C, and a humidity of 65–70%. They were provided with adequate food and water. All animal experiments were conducted by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of the Naval Medical University. The mice were acclimatized for one week and given free access to food and water before the experiments.
The mice were randomly divided into four groups (n = 10/group): (1) Control (Ctrl) group, (2) Chronic Stress (CMS) group, (3) Chronic Stress Tryptophan Low Dose Supplementation group (CMS_Trp-L/100 mg/kg-bw), (4) Chronic Stress Tryptophan High Dose Supplementation group (CMS_Trp-H/300 mg/kg-bw). The experimental cycle lasted for 21 days.
2.1.1. Experimental diets
Some studies have shown that moderate (100 mg/kg-bw) intake of tryptophan may be beneficial, but excessive (500 mg/kg-bw) intake of tryptophan can lead to the accumulation of canine indole metabolites, which can activate the AhR pathway and cause kidney injury. Therefore, tryptophan intake may not always the more the better (Hu et al., 2023).
Four groups of mice were fed a diet based on AIN-93G (3.766 kcal/g) (Nantong TROPHIC Animal Feed High-Tech Co. Ltd, Jiangsu, China) and supplemented with 0.2 ml of tryptophan (Sangon, shanghai) per day in the CMS_Trp-L and CMS_Trp-H groups, and an equal amount of 0.9% saline per day in the Ctrl and CMS groups. All mice were housed in single cages, and body weight and food intake were recorded daily.
2.2. Chronic mild stress process
Mental stress, which mainly induces depression, is always coexisting in daily life with predictable stress, as well as the constant bombardment of chronic unpredictable micro-stressors. Therefore, the CMS procedures were conducted by Qiao et al. and modified (Qiao et al., 2020).
The stress protocol consisted of a stress regimen of 1 h of restraint, 5 min of cage shaking, and 6 h of cold water bath stimulation. Stressors were administered daily at fixed times during a 21-day experiment. This was followed by two consecutive days of behavioral testing, with an open field test (OFT) on the first day and a tail suspension test (TST) on the second day. All tests were conducted in the same environment during the same period (14:00–18:00). After each test, the mice were individually returned to their cages and kept under standard animal facility conditions.
2.3. Behavioral tests
2.3.1. Open-field test
The OFT is commonly used to measure anxiety-like and locomotor behaviors in mice (Belovicova et al., 2017). The open field setup consisted of a 40 cm × 40 cm x 40 cm square black wooden board on which the experiments were conducted. The experimental mice are placed in the same azimuthal corner of the site and allowed to explore freely for 6 min. The movement of each animal is recorded using a video camera directly above the site. A computerized video tracking system is used to obtain mouse location data. The total distance walked, average speed, time spent in the center area of the field, and number of entries into the center area were calculated. Before testing, the mice can acclimatize to the room environment for 1 h. The field was cleaned with 75% alcohol after each test.
2.3.2. Tail suspension test
TST is commonly used to evaluate depressive-like behavior in mice (Belovicova et al., 2017). Tail suspension experiments were performed on a black background, with the tip of the mouse's tail suspended 2 cm from the ground at a distance of approximately 20 cm using adhesive tape. Each animal's locomotor behavior is recorded using a camera directly in front of the site, with mouse positional data obtained from the video using computerized video tracking. The struggle time in the middle 4 min of the 6-min test is counted to assess the degree of desperation of the mice. The mice can acclimate to the room environment for 1 h before the test.
2.4. Immunohistochemical staining
Immunohistochemical analysis was performed using 4% paraformaldehyde-fixed, paraffin-embedded tissue sections. The slides were dried in an oven at 37 °C for 2 h, then deparaffinized with xylene (Sigma-Aldrich) and permeabilized with 0.5% Triton X-100 (ZSGB-BIO). After antigenic repair by boiling the slides in citrate buffer (Beyotime) to extinguish endogenous enzyme activity, then, the sections were closed using goat serum (Beyotime) followed by incubation with primary antibodies c-fos (Abcam), AgRP (Abcam), and LEPR (Abcam) at 4 °C overnight. The sections were stained with biotinylated secondary antibody working solution coupled with horseradish peroxidase complex (ZSGB-BIO), and finally the nuclei were restained using hematoxylin (ZSGB-BIO), and the sections were observed and recorded on a Leica microscope.
2.5. Enzyme-linked immunosorbent (ELISA) assay
The colon tissue was washed with 1 × PBS to remove blood stains, then homogenized and placed at −20 °C overnight. After repeated freeze-thawing for two treatments to destroy the cell membrane, the tissue homogenate was centrifuged at 4 °C and 5000 g for 5 min to obtain the supernatant. An appropriate amount of supernatant was taken and used for the experiment immediately. A 5-HT ELISA kit (Cusabio, China) was used to determine 5-HT content in the mouse colon. The principle is to incubate the 5-HT antibody and HRP marker with the supernatant prepared above at 37 °C for 40 min. The optical density (OD value) of each well was measured with an enzyme marker at 450 nm, and the unit of 5-HT concentration was expressed in ng/ml.
2.6. Targeted metabolomics analysis
Targeted metabolism of tryptophan in mouse serum by UPLC-MS/MS. Serum samples were isolated from orbital hemorrhages under anesthesia and tryptophan metabolites were extracted by extraction solution (0.1% formic acid &acetonitrile: IPA = 7:3, pre-cooled at −40 °C, containing 0.1% formic acid and isotopically-labeled internal standard mixture). The master standard tryptophan solution prepared at a concentration of 5.0 mg/ml was diluted and then configured into a series of calibration standard solutions. The analytical columns were prepared on an ACQUITY UPLC BEH C18 column (2.1 × 5 mm) equipped with an ACQUITY UPLC BEH C18 column 1.7 μM VanGuard pre-column (2.1 × 5 mm) and ACQUITY UPLC BEH C18 column 1.7 μM analytical column (2.1 × 100 mm), and after assaying on a 2.0 (ESI-), 1.5 (ESI+) capillary voltage system, the data were processed by MassLynx software (v4.1, Waters, Milford, MA, USA) on the raw data files generated by UPLC-MS/MS were processed for peak integration, calibration and quantification of each metabolite. The self-developed iMAP software (v1.0, Metabo-Profile, Shanghai, China) was used for subsequent statistical analyses such as PCA and OPLS-DA acquisition and processing.
2.7. qRT-PCR analysis
Total RNA from the brain and intestine was extracted using RNAiso Plus reagent (TAKARA, Japan) and reverse transcribed into cDNA using the PrimeScriptTM RT Master Mix (Perfect Real Time) reverse transcription kit (TARAKA, Japan) configured according to the required system. Then, qPCR reactions were performed using the synthesized cDNA as a template according to TB Green® Premix Ex Taq™ (Tli RNaseH Plus) (TAKARA, Japan) real-time fluorescence quantitative kit configuration reaction system, Namely 10 μLTB Green Premix Ex Taq (Tli RNaseH Plus) (2X), 0.4 μL upstream primer, 0.4 μL downstream primer, 0.4 μL ROX Reference Dye II (50X), 2 μl cDNA, 6.8 μl sterilized water, The mixture was configured as a 20 μL reaction system. The reaction conditions were one cycle of 95 °C for 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 20 s, and one cycle of 95 °C for 0 s and 65 °C for 15 s and 95 °C for 0 s. The Ct value of the reaction results was recorded and the relative gene expression was analyzed by the △△Ct method. The △△Ct method was used to calculate the target mRNA relative expression based on the expression of the ACTB reference. As shown in Table 1, the primer sequence was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Table 1.
The real-time PCR primer sequences.
| Genes | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| ACTB | GTGCTATGTTGCTCTAGACTTCG | ATGCCACAGGATTCCATACC |
| AgRP | GGAAGCAGTCACGTGTGGACCC | AGTGGAGCCACGCCCATCTGG |
| OX1R | CAGGCTTTGTGCAAGGTCAT | CTTGAACAACAGTGGGTGGC |
| MC4R | GGTCGGAAACCATCGTCATT | AAAGCAGGCTGCAAATGGAT |
| LEPR | ATGACGCAGGGCTGTATGTC | TGGACTGTTGGGAAGTTGGTAG |
| tph1 | CTAAAGCAGTCTTGCCTGGTCA | CAGTTAACAGCCCCCATGTCT |
| tph2 | CAATCGAGTTCGGCCTTTGC | GCGTCCTGAAAGGTGGTGAT |
| 5-HT1B | AGTCTGTGGCAGCGACTAAA | GCAGTTTTCTTCACCTTGTCCC |
| 5-HT2C | GGGACTACTTGTCATGCCCC | AACTGAAACTCCGGTCCAGC |
2.8. Cell culture and preparation of tryptophan and serotonin
Mouse hypothalamic neuronal GT1-7 cells (Sponsored by Fudan University) were cultured in DMEM high-glucose medium (GIBCO), which contained 10% FBS (GIBCO) and 1% penicillin-streptomycin double-antibody solution (Biosharp), and maintained in a humidified environment at 37 °C with 5% CO2. The GT1-7 cells used in this study were all 3rd-10th generation. The mother liquor was prepared by dissolving 5-HT (MedChemExpress) in dimethyl sulfoxide (DMSO) (Beyotime), and the final concentration of DMSO in the medium was ≤0.1%.5-HT was prepared as follows: 5-HT was dissolved in DMSO to prepare a reserve solution with a final concentration of 2 mM and ultrasonicated in a cold water bath for 5 min and then stored at −80 °C.
2.8.1. Cell viability assay
Cell viability was assessed using the CCK8 kit (Beyotime). Plates were spread in 96-well plates at 3 × 104 cells/well, and the cells were incubated at 37 °C and 5% CO2 for 12 h. After that, the medium of each group was replaced with different concentrations of corticosterone (0.1, 1, 10, 50, 100 μM) solution and 5-HT (0.1, 1, 10, 100 μM) in 100 μL volume and incubated for 24 h, Then, each well was replaced with 100 μL of solution containing 10% CCK-8. After 1 h of incubation, the absorbance was measured at 450 nm.
2.8.2. 5-HT treatment
To investigate the role of 5-HT in corticosterone-induced GT1-7 cytotoxicity, the cells were first pretreated with 0.1, 1, and 10 μM 5-HT for 2 h. After that, they were co-incubated with 10 μM corticosterone and 0.1 μM, 1 μM, and 10 μM 5-HT for 24 h. The study observed the effect of 5-HT on promoting corticosterone-induced cytotoxicity.
2.9. Statistical analysis
The statistical analysis was performed using GraphPad Prism 9.0.1 software. Statistical differences between the two groups were assessed using independent t-tests. Differences between three or more groups were analyzed by one-way ANOVA and Tukey's multiple comparisons. Differences were considered statistically significant at P < 0.05. All data are present as mean ± standard error of the mean (mean ± SEM).
3. Results
3.1. Effect of chronic stress on the metabolic homeostasis of tryptophan in mice
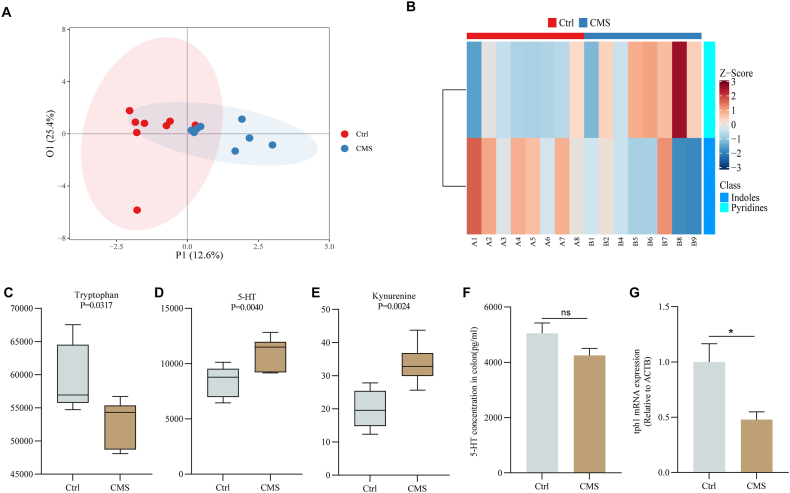
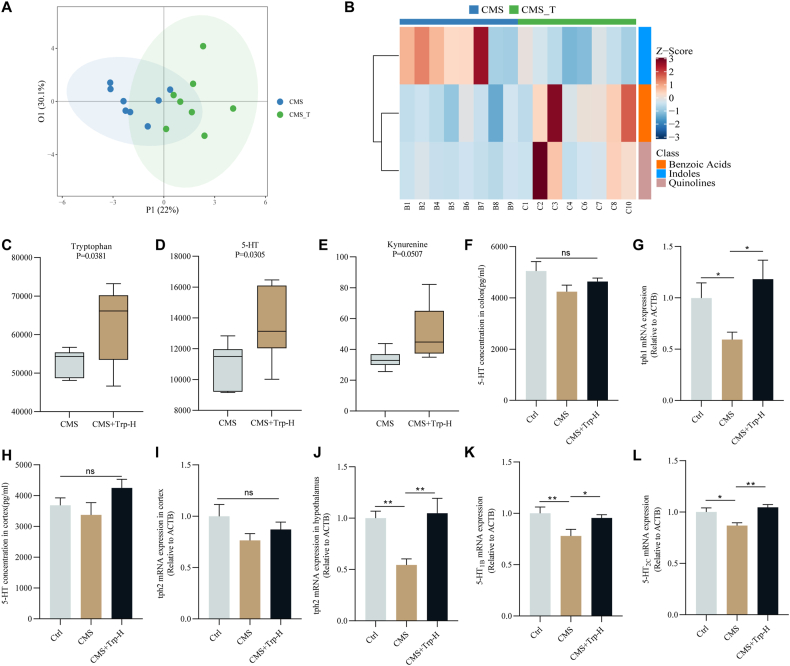
Studies have shown that altered tryptophan metabolism in humans is associated with various neuropsychiatric disorders, such as depression (Kałużna-Czaplińska et al., 2019). Moreover, it has been shown that peripheral serotonin is elevated in patients with neuropsychiatric disorders, but in mouse models, intestinal serotonin levels are reduced, leading to intestinal dysfunctions, such as constipation and reduced intestinal motility (Muller, 2016). In our study, the tryptophan-targeted metabolomic analysis was performed to determine the alteration of tryptophan and its metabolite under chronic stress. The results showed that the degree of sample dispersion between Ctrl and CMS groups is relatively large, which indicated that the chronic stress model was successfully constructed (Fig. 1A). In addition, the Z_score plot indicated that indole substances, such as 5-HIAA, were less abundant in the CMS group compared to the Ctrl group (Fig. 1B). The boxplot showed that the CMS group decreased the level of tryptophan in the serum (P < 0.05) while increasing the level of 5-HT (P < 0.01) and the level of kynurenic acid (P < 0.01) (Fig. 1C–E). However, the results of ELISA showed that colonic 5-HT level in the CMS group was decreased. RT-qPCR showed that the tph1 mRNA level in the mouse colon was significantly decreased (P < 0.01) (Fig. 1F&G). These findings suggested that chronic stress disrupted the tryptophan/5-HT metabolic pathway in the intestine and periphery of mice.
Fig. 1.
Effect of chronic stress on tryptophan metabolic balance in mice
Tryptophan-targeted metabolomics analysis (A) Principal component analysis plot; (B) Z_score plot; Boxplot of serum levels of (C) tryptophan; (D) 5-HT; (E) kynurenic; (F) The content of 5-HT in the mouse colon was detected by ELISA; (G) The mRNA expression of tph1 in the colon of each group.; Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01. Significant differences between means values were determined by t-test.
3.2. Effect of tryptophan on appetite and depression-like behavior in chronically stressed mice
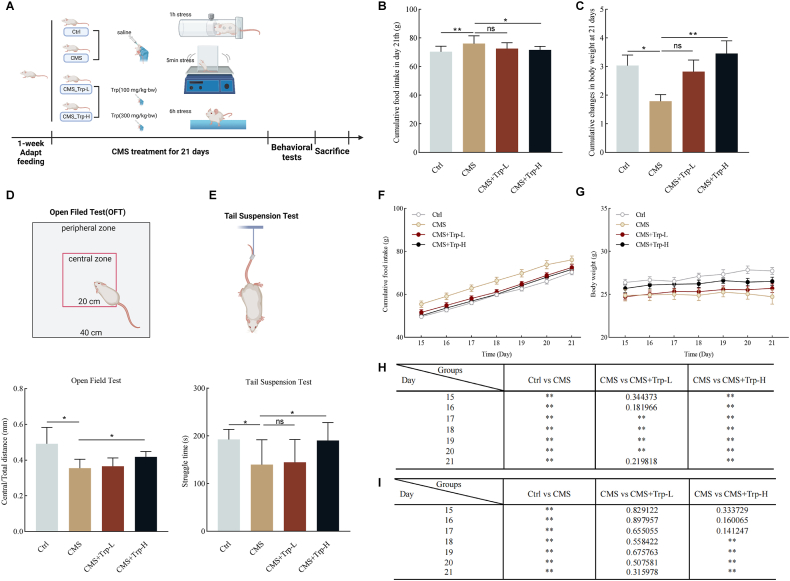
To investigate the ameliorative effects of restoring tryptophan levels on emotional eating, a chronic stress mouse model was constructed. The schedule of the animal model experiment is shown in Fig. 2A. Compared to the Ctrl group, the mice in the CMS group had increased food intake (P < 0.01), but decreased body weight (P < 0.01). Supplementation with high-dose tryptophan restored abnormal food intake (P < 0.05) and ameliorated abnormal body weight loss (P < 0.01) in mice compared to the CMS group. In contrast, no significant changes were observed in the low-dose group (Fig. 2B&C). To assess the effect of tryptophan supplementation on CMS-induced anxiety-depression-like behaviors, we performed the OFT and TST. The results showed that CMS resulted in a decreased desire to explore toward the middle region in the OFT (P < 0.05), and a significant decrease in struggle time in the TST (P < 0.05) compared to the Ctrl group, suggesting that CMS led to anxiety-depression-like behaviors. Whereas, supplementation with high doses of tryptophan improved the autonomous exploration desire of the mice in the open field test (P < 0.05) and significantly improved the desperation behavior of the mice in the tail suspension test (P < 0.05) (Fig. 2D&E), indicating that supplementation of tryptophan reduces the inability to feel pleasure caused by CMS. Moreover, as can be seen from the analysis of cumulative food intake and body weight changes, it was found that supplementation with high-dose tryptophan significantly restored the appetite abnormality in CMS mice on day 15 (P < 0.01) (Fig. 2F&H), while the body weight improvement only appeared to be significantly different on day 18 (P < 0.01), and for four consecutive days thereafter, suggesting that the time of supplementation with high-dose tryptophan was at least about three weeks to significantly improve the abnormal body weight changes in mice (Fig. 2G&I). In summary, the study suggests that supplementation with high doses of tryptophan can ameliorate anxiety-depression-like behaviors and emotional eating behavior in CMS.
Fig. 2.
Effects of tryptophan supplementation on appetite and depression-like behavior in chronic stress mice
(A) Chronic stress experimental procedures and time-course tables related to behavioral tests (n = 10 mice per group); (B) Cumulative food intake at 21 days; (C) Changes in body weight at 21 days; (D) Center/total distance in the open field test (OFT); (C) Struggle time in the tail suspension test (TST); (F) Cumulative food intake; (G) weight; (H) Analysis of differences in cumulative daily food intake; (I) Daily weight variance analysis. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01. Significant differences between means values were determined by t-test.
3.3. Effects of tryptophan supplementation on hypothalamic feeding neurons and appetite regulators in chronically stressed mice
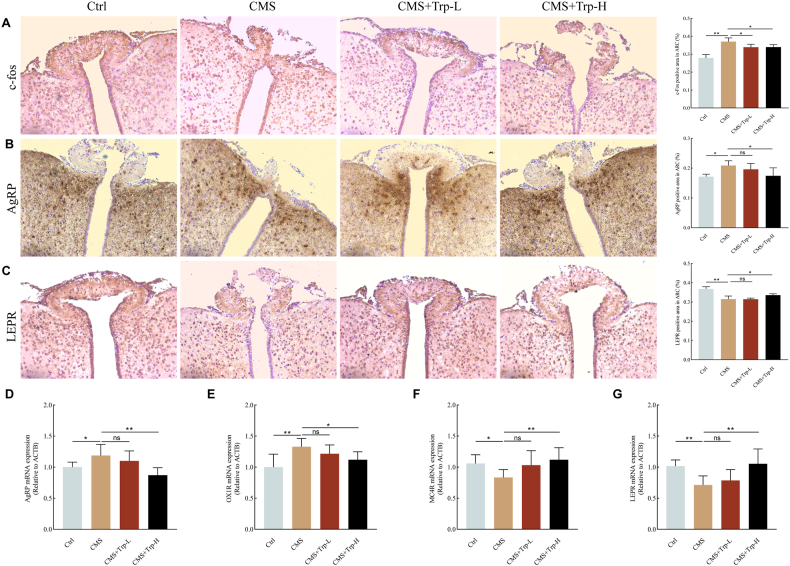
The central nervous system has a key role in feeding regulation. Therefore, to investigate the effect of tryptophan supplementation on feeding-related nuclei in CMS mice, first we performed c-fos immunohistochemical staining. As shown in Fig. 3A, c-fos expression was significantly elevated in the region of the ARC of the hypothalamus in the CMS group compared with the Ctrl group (P < 0.01), whereas supplementation with tryptophan significantly alleviated the abnormal expression of neuronal activity (P < 0.05). The effects of tryptophan supplementation on hypothalamic AgRP and LEPR proteins in CMS mice were then explored. Immunohistochemical staining results showed that AgRP positive expression in the region of the ARC of the hypothalamus increased (P < 0.05) as well as LEPR positive expression decreased (P < 0.01) in the CMS group compared to the Ctrl group, and that after supplementation with high-dose tryptophan, the AgRP expression decreased (P < 0.05) as well as the LEPR expression increased (P < 0.05) (Fig. 3B&C).
Fig. 3.
Effects of tryptophan supplementation on hypothalamic feeding neurons and appetite regulators in chronic stress mice
(A-C) Representative images of Immunohistochemical of c-fos, AgRP, and LEPR in the hypothalamus of each group; (D–G) The mRNA expression of AgRP, OX1R, MC4R, and LEPR in the hypothalamus of each group. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01. Significant differences between means were determined by t-test.
To further explore the level of neurophagic expression in CMS-treated mice, RT-qPCR was used to detect mRNA expression of appetite regulators in the hypothalamus. The results showed that the mRNA expression of AgRP and OX1R in the hypothalamic tissues of mice in the CMS group was significantly higher (P < 0.01), while the mRNA expression of MC4R and LEPR was significantly lower (P < 0.05) compared with that of the Ctrl group. After tryptophan intervention, the mRNA expression of AgRP (P < 0.01) and OX1R (P < 0.05) in the high-dose group of mice was significantly reduced compared with that in the CMS group, and the mRNA expression of MC4R as well as LEPR was significantly elevated (P < 0.01) (Fig. 3D–G). Above experiments suggest that restoring tryptophan levels improves chronic stress-activated AgRP neurons in the ARC, and restores the abnormal expression of hypothalamic appetite regulators AgRP, OX1R, LEPR, and MC4R. Above results suggest that tryptophan supplementation has a significant improvement effect on emotional eating.
3.4. Tryptophan to chronic stress the influence of the hypothalamus 5-HT metabolic pathway in mice
Studies have shown that in addition to the regulation of feeding behavior by the central nervous system, the gut endocrine factor 5-HT, intestinal nerve, and stretch can induce the vagal afferent nerve to mediate the activity of AgRP neurons, and then regulate the feeding behavior of the body (Milani et al., 2017). Therefore, to investigate the effects of tryptophan supplementation on 5-HT metabolic pathways in the hypothalamus of CMS mice, we analyzed the effects of tryptophan and metabolites after tryptophan supplementation under chronic stress conditions by targeted metabolomics. The results showed that the degree of sample dispersion between Ctrl and CMS groups was relatively large, which indicated that the chronic stress model was successfully constructed (Fig. 4A). In addition, by Z_score figure can be seen, compared with the CMS group, indoles material content in the CMS + Trp_H group more (Fig. 4B). The boxplot showed that the levels of tryptophan and 5-HT in the serum of mice were significantly increased after tryptophan supplementation (P < 0.05), and the level of kynurenic acid was increased (Fig. 4C–E). Then, the changes in 5-HT content in the cerebral colon and cortex were detected by enzyme-linked immunosorbent assay (ELISA), respectively, and the results showed that there was no significant difference in 5-HT content between the cerebral cortex and the colon (Fig. 4F&H). The mRNA levels of tph1 in the colon and tph2 in the hypothalamus and cerebral cortex of mice were detected by RT-qPCR, and the results showed that tryptophan supplementation significantly increased the expression of tph1 in the colon (P < 0.05), and furthermore, tph2 was significantly decreased in the hypothalamus of mice in the chronic stress group only (P < 0.01), which could be reversed by supplementation of tryptophan (Fig. 4G, H-J). In addition, mRNA levels of 5-HT receptors 5-HT1B(P < 0.01) and 5-HT2C(P < 0.05) detected in CMS conditions were significantly decreased, and their expression was increased by tryptophan supplementation (Fig. 4K&L). Therefore, the restoration of tryptophan levels promotes the hypothalamic tryptophan 5-HT metabolic pathway, which contributes to the treatment of emotional eating.
Fig. 4.
Effect of tryptophan on 5-HT metabolic pathway in the hypothalamus of chronically stressed mice
Tryptophan-targeted metabolomics analysis (A) PCA plot; (B) Z_score plot; Serum box plots of (C) tryptophan; (D) 5-hydroxytryptophan; (E) kynurenine; (F) The content of 5-HT in the mice colon was detected by ELISA; (G) RT-qPCR was used to detect the expression of tph1 in the mice colon, (H) The content of 5-HT in the mice cortex was detected by ELISA; (I–L) RT-qPCR was used to detect the expression of tph2 in the mouse cortex, and tph2、5-HT1B and 5-HT2C mRNA in the mice hypothalamus. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01. Significant differences between means were determined by t-test or one-way ANOVA and Tukey's multiple comparison test.
3.5. Effect of 5-HT on glucocorticoid-induced expression of appetite regulatory factors in GT1-7 neuronal cells
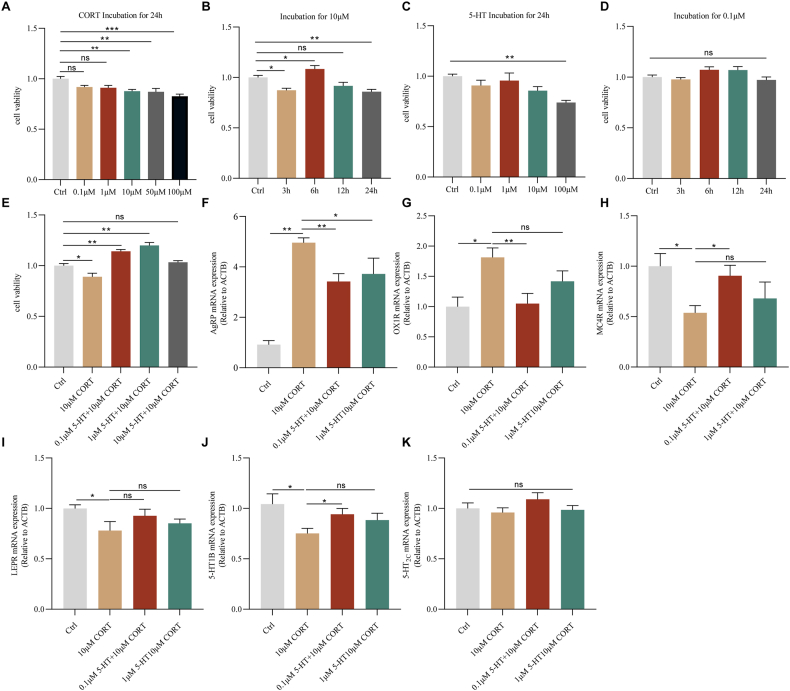
GT1-7 cells, a hypothalamic neuronal cell line derived from the mouse hypothalamus, are capable of expressing anorexigenic POMC-derived peptide, orexigenic peptide (NPY), AgRP, and other appetite regulators (Deng et al., 2017). So cell energy metabolism is often used in the hypothalamus and related research appetite (Cortes-Campos et al., 2013). CORT is a kind of glucocorticoid (GC), which is the end product of the HPA axis. A significant increase in CORT concentration leads to neuronal damage and depression-like behavior (Monaghan, Bridges & Cotman, 1989). Therefore, the use of corticosterone to induce an in vitro cellular model is reliable. Firstly, to analyze the effect of corticosterone on cell viability, GT1-7 cells were incubated with increasing concentrations of corticosterone (0.1 μM/1 μM/10 μM/50 μM/100 μM) for 24 h, respectively. Then the cell viability was assessed by CCK8 assay. As shown in Fig. 5A, with increasing corticosterone concentration, cell viability was impaired. Among them, the viability of GT1-7 cells was significantly reduced at 10, 50, and 100 μM concentrations (P < 0.01). Secondly, in parallel experiments, we investigated the time course of corticosterone-induced cytotoxicity. GT1-7 cells incubated at 10 μM corticosterone concentration for 3, 6, 12, and 24 h showed a promotive effect at 6 h (P < 0.05), as well as no toxic effect on the cells at 12 h (P < 0.05) (Fig. 5B).
Fig. 5.
The effects of tryptophan and 5-HT on hypothalamic agouti-related proteins were studied by the GT1-7 cell line
Dose-response (A) and time course (B) of cell activity after in vitro administration of corticosterone; Dose-response (C) and time course (D) of cell viability after in vitro administration of 5-HT; (E) Incubation of GT1-7 cells with 5-HT (0.1, 1 μM) for 2 h before co-incubation with 10 μM corticosterone for 24 h prevented the cytotoxic effects of corticosterone. At the end of the treatment, cell viability was measured by the CCK8 assay. (F–K) In vitro administration of 5-HT prevented the mRNA expression of AgRP, OX1R, MC4R, LEPR, 5-HT1B, and 5-HT2C after corticosterone-induced cytotoxicity. Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01. Significant differences between means were determined by t-test.
Similarly, to test the effect of 5-HT on cell viability, GT1-7 cells were incubated with increasing concentrations of 5-HT (0.1 μM/1 μM/10 μM/100 μM) for 24 h, respectively, then cell viability was detected by the CCK8 assay. The results showed that 0.1, 1, and 10 μM of 5-HT did not change the cell viability. However, incubation with 100 μM 5-HT reduced the viability of GT1-7 cells by nearly 70% (P < 0.01) (Fig. 5C). We also measured the changes in cell viability after incubation with 0.1 μM 5-HT at different time points (3\6\12\24 h). Statistical analysis showed that 5-HT had no significant effect on cell viability at different time points (Fig. 5D).
The cells were incubated with 5-HT (0.1, 1 μM) before co-incubation with 10 μM corticosterone for 2 h. The results showed that both 0.1 and 1 μM 5-HT reversed corticosterone-induced cytotoxicity (P < 0.01) (Fig. 5E). GT1-7 cells were incubated with 5-HT (0.1、1 μM) for 2 h before co-incubation with 10 μM corticosterone for 24 h. The cells were incubated with 5-HT (0.1, 1 μM) for 2 h before co-incubation with 10 μM corticosterone. The results showed that the aberrant expression of mRNAs of AgRP (P < 0.01), OX1R, MC4R (P < 0.05), LEPR, 5-HT1B, and 5-HT2C was ameliorated under the condition of 0.1 μM 5-HT concentration (Fig. 5F–K).
4. Discussion
In this study, we established a mouse of emotional eating induced by CMS. We found that supplementation with a high dose of tryptophan was effective in reversing the abnormal increase in appetite and abnormal weight loss, and improving abnormal behaviors such as depression and anxiety in CMS mice compared with a low dose of tryptophan. Tryptophan treatment inhibited the secretion of appetite-promoting factors such as AgRP and OX1R, and promoted the secretion of appetite-suppressing factors such as LEPR, MC4R, and 5HT1B in the brain of CMS mice. Among them, 5-HT, a tryptophan metabolite, plays a crucial role in feeding regulation in the central nervous system mainly by its two receptors, 5-HT1B and 5-HT2C, and further acts as an appetite suppressor through its downstream substance MC4R. Our experiments revealed that supplementation with high doses of tryptophan ameliorated the CMS-induced reduction of 5-HT1B and 5-HT2C. Therefore, we concluded that tryptophan and its metabolites are important in improving appetite and emotional function in CMS mice.
Tryptophan is an amino acid essential for the growth and health of animals and humans that can only be obtained from the diet and is a precursor of 5-HT. Increasing evidence suggests that primarily tryptophan metabolism is associated with a variety of human diseases, including neuropsychiatric disorders, irritable bowel syndrome, inflammatory bowel disease, metabolic syndrome, and so on (Agus, Planchais & Sokol, 2018). The mouse model of stress-induced emotional eating is also accompanied by tryptophan metabolism disorder, suggesting that stress disrupts tryptophan metabolic pathways. Studies have shown that the tryptophan metabolic pathway shifts mainly to the kynurenine pathway under stress (Platten et al., 2019). Similarly, in our study, serum tryptophan targeted metabolomics analysis revealed an upregulation of the kynurenine pathway and the 5-HT pathway, while a decrease in tryptophan content in the serum of the CMS group. In addition, CMS resulted in a trend of decreased 5-HT content in the colon, but tph1 expression was significantly decreased. In contrast, after supplementation of tryptophan, the mRNA expression of tph1 in the colon also increased significantly, and the content of 5-HT also showed a rising trend. In other words, supplementation with high doses of tryptophan restored the peripheral tryptophan metabolic pathway. We also found that the mRNA expression of tph1 in the colon was also significantly increased after tryptophan supplementation. Furthermore, in the central nervous system, the direction of tryptophan metabolism is biased toward the 5-HT pathway, consistent with the elevated mRNA levels of tph2 that we measured.
The current study presents a supplementary tryptophan that can prevent stress and lead to emotional eating. For the relationship between stress, emotional eating, and 5-HT transmission, the effects of tryptophan treatment on hypothalamus 5-HT synthesis and release, and the ability of hypothalamus 5-HT to control appetite are consistent with previous studies (Markus, 2012). Stress conditions, especially prone to emotional eating, and increased 5-HT in the brain may help reduce stress-related emotional eating (Markus et al., 2012). Emotional eating, in response to negative emotions such as nervousness, irritability, and depression, is strongly associated with binge eating in adult (clinical) samples (Racine et al., 2009) and is characterized by a lack of physiological control over appetite and emotional disturbances, including depression and anxiety. Many factors are involved in appetite disorders, and neuroleptin may be the key molecule. Many studies have shown that changes in central and peripheral neuroleptic signals are accompanied by disorders regulating appetite, body weight, and mood (Fetissov et al., 2008). The hypothalamic arcuate nucleus acts as a major hub integrating nutrition-related information from all peripheral organs and is regulated through metabolite, hormonal, and neural pathways (Lenard and Berthoud, 2008). In addition, serotonergic neurotransmission in the hypothalamus is a component of the energy homeostasis network that receives satiety-related signals from the periphery to regulate appropriate central responses (Heisler et al., 2006). In addition, its related receptors, such as 5-HT1B and 5-HT2C, are located on AgRP, POMC, and orexin neurons. The weakened serotonin transmission in the lateral hypothalamus and peri cortical regions is related to obesity and changes in eating, which emphasizes the complex role of hypothalamic serotonin in energy balance (Romanova et al., 2018).
As mentioned above, in recent decades, 5-HT, as a monoamine neurotransmitter, is an important signaling molecule in the central nervous system and plays an important role in the periphery (Matthes and Bader, 2018). Therefore, the hypothalamic GT1-7 cell line is an excellent in vitro model for studying the regulation of appetite regulators and their metabolic functions by hypothalamic neurons. A corticosterone-induced gradient stress injury model of GT1-7 cells was established. It was found that incubation of GT1-7 cells at 10 μM corticosterone concentration for 12 h was the optimal intervention condition for the stress injury cell model. The optimal conditions for 5-HT intervention were further screened: incubation at 0.1 μM and 1 μM 5-HT concentration for 24 h. The effects of the tryptophan metabolite 5-HT on the expression of AgRP, OX1R and other appetite regulators in the hypothalamus were investigated using the GT1-7 cell line. Supplementation with the tryptophan metabolite 5-HT was found to improve the aberrant expression of hypothalamic appetite regulators such as AgRP and OX1R. In this study, we investigated the effects of tryptophan and its metabolite 5-HT on the expression of hypothalamic agonist-associated proteins and other appetite regulators in the GT1-7 cell line.
In conclusion, chronic stress can lead to emotional disturbance and emotional eating behaviors accompanied by tryptophan metabolism disorder in mice. Tryptophan supplementation ameliorated anxiety and depression-like behaviors in CMS mice. Tryptophan treatment also improved the abnormal appetite caused by stress, as well as reversed the abnormal expression of appetite regulators in the hypothalamus, which was verified by in vivo experiments. This study provides a theoretical basis for exploring an effective way to improve emotional eating by targeting tryptophan and its metabolites. Although the potential effects of tryptophan on the gut microbiome and its metabolites require further investigation, the present study provides a theoretical basis for understanding the mechanism of tryptophan on emotional eating.
Funding
This work was supported by grants from Sci-Tech Innovation (2030) Brain Science and Brain-Like Intelligence Technology Project (grant number: 2022ZD0208100), and the Project of 2023 Foundation for Basic Medicine of Naval Medical University (grant number: 2023QN013).
Ethical statement
The experimental animal studies involved in this article. 40 healthy male balb/c mice (6–8 weeks old, 18–22 g), SPF grade, were purchased from Shanghai Jiesijie Laboratory Animal Co. [Production License No.: SCXK (Shanghai) 2018–0004]. In addition, all animal experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Naval Medical University.
Declaration of competing interest
The author state that they have no known competitive economic interest or personal relationship which may affect the work reported in this paper.
Acknowledgments
Qing Liu, Lifeng Yin, and Ying Zhu conceived and designed this study. Ying Zhu and Lifeng Yin participated in the animal experiments. Ying Zhu and Lifeng Yin participated in the cell experiments. Ying Zhu and Lifeng Yin analyzed and interpreted the data. Yaoxing Guan and Shuang Nie reviewed the data, Ying Zhu wrote the first draft of this study, and Qing Liu revised the first draft. Fengfeng Mo and Yongheng Zhu undertook the funding and design of the project as well as the writing and checking of the article. All authors agreed with the findings and approved the final study.
Handling Editor: Dr. Yeonhwa Park
Contributor Information
Yongheng Zhu, Email: yh-zhu@shou.edu.cn.
Fengfeng Mo, Email: mofengfeng@smmu.edu.cn.
Abbreviations
- CMS
Chronic mild stress
- 5-HT
5-hydroxytryptamine;
- HPA
Hypothalamic-pituitary-adrenal (HPA) axis
- tph2
Tryptophan hydroxylase 2
- ARC
Arcuate nucleus
- AgRP/NPY
Agouti-related protein/Neuropeptide Y
- OX1R
Orexin Receptor 1
- MC4R
Melanocortin 4 Receptor
- LEPR
Leptin Receptor
- POMC
Pro-opiomelanocortin
- Trp
Tryptophan
- Kyn
Kynurenine;
- 5-HT1B
5-hydroxytryptamine/serotonin Receptor 1 B
- 5-HT2C
5-hydroxytryptamine Receptor 2C
- OFT
Open field test
- TST
Tail-suspension test
- ELISA
Enzyme-Linked Immunosorbent
Data availability
Data will be made available on request.
References
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Andermann M.L., Lowell B.B. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95(4):757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baver S.B., Hope K., Guyot S., Bjorbaek C., Kaczorowski C., O'Connell K.M. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014;34(16):5486–5496. doi: 10.1523/JNEUROSCI.4861-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belovicova Kristina, et al. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscipl. Toxicol. 2017;10(1):40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Moazzami K., Wittbrodt M.T., Nye J.A., Lima B.B., Gillespie C.F., Rapaport M.H., Pearce B.D., Shah A.J., Vaccarino V. Diet, stress and mental health. Nutrients. 2020;12(8):24–28. doi: 10.3390/nu12082428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja A., Manfredi M., Zampieri C., Minnicelli A., Bolda R., Brocadello F., Gatti M., Baldovin T., Baldo V. Is emotional eating associated with behavioral traits and Mediterranean diet in children? A cross-sectional study. BMC Publ. Health. 2022;22(1):1794. doi: 10.1186/s12889-022-14192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester V., Esme G., Nicholls D. Physiological, emotional and neural responses to visual stimuli in eating disorders: a review. Journal of eating disorders. 2021;9(1):23. doi: 10.1186/s40337-021-00372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349) doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lin Y.C., Kuo T.W., Knight Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai S., Bertazzo A., Brughera M., Crotti S. Tryptophan in health and disease. Adv. Clin. Chem. 2020;95:165–218. doi: 10.1016/bs.acc.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Concetti C., Peleg-Raibstein D., Burdakov D. Hypothalamic MCH neurons: from feeding to cognitive control. Function (Oxford, England) 2023;5(1) doi: 10.1093/function/zqad059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Campos C., Elizondo R., Claudio C., et al. MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakanalis A., Zanetti M.A., Clerici M., Madeddu F., Riva G., Caccialanza R. Italian version of the Dutch Eating Behavior Questionnaire. Psychometric proprieties and measurement invariance across sex, BMI-status and age. Appetite. 2013;71:187–195. doi: 10.1016/j.appet.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Dakanalis A., Timko C.A., Carrà G., Clerici M., Zanetti M.A., Riva G., Caccialanza R. Testing the original and the extended dual-pathway model of lack of control over eating in adolescent girls. A two-year longitudinal study. Appetite. 2014;82:180–193. doi: 10.1016/j.appet.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Dakanalis A., Clerici M., Caslini M., Gaudio S., Serino S., Riva G., Carrà G. Predictors of initiation and persistence of recurrent binge eating and inappropriate weight compensatory behaviors in college men. Int. J. Eat. Disord. 2016;49(6):581–590. doi: 10.1002/eat.22535. [DOI] [PubMed] [Google Scholar]
- Dakanalis A., Mentzelou M., Papadopoulou S.K., Papandreou D., Spanoudaki M., Vasios G.K., Pavlidou E., Mantzorou M., Giaginis C. The association of emotional eating with overweight/obesity, depression, anxiety/stress, and dietary p atterns: a review of the current clinical evidence. Nutrients. 2023;15(5):1173. doi: 10.3390/nu15051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M.F., Pecoraro N., Akana S.F., Fleur Sel, Gomez F., Houshyar H., Bell M.E., Bhatnagar S., Laugero K.D., Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc. Natl. Acad. Sci. U.S.A. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Yuan F., Guo Y., et al. Deletion of ATF4 in AgRP neurons promotes fat loss mainly via increasing energy expenditure. Diabetes. 2017;66(3):640–650. doi: 10.2337/db16-0954. [DOI] [PubMed] [Google Scholar]
- Ederer D.J., Lee S.H., Belay B., Boutelle K., Park S. Associations between comfort eating and weight change during the COVID-19 pandemic among U.S. adults. Human nutrition & metabolism. 2023;33 doi: 10.1016/j.hnm.2023.200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers C., Dingemans A., Junghans A.F., Boevé A. Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence. Neurosci. Biobehav. Rev. 2018;92:195–208. doi: 10.1016/j.neubiorev.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Fetissov Sergueï O., et al. Emerging role of autoantibodies against appetite-regulating neuropeptides in eating disorders. Nutrition. 2008;24(9):854–859. doi: 10.1016/j.nut.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Firk C., Markus C.R. Review: serotonin by stress interaction: a susceptibility factor for the development of depression? J. Psychopharmacol. 2007;21(5):538–544. doi: 10.1177/0269881106075588. [DOI] [PubMed] [Google Scholar]
- Gao Q., Horvath T.L. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Godet A., Fortier A., Bannier E., Coquery N., Val-Laillet D. Interactions between emotions and eating behaviors: main issues, neuroimaging contributions, and innovative preventive or corrective strategies. Rev. Endocr. Metab. Disord. 2022;23(4):807–831. doi: 10.1007/s11154-021-09700-x. [DOI] [PubMed] [Google Scholar]
- Goforth P.B., Leinninger G.M., Patterson C.M., Satin L.S., Myers Jr MG. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J. Neurosci. 2014;34(34):11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J., Minor K.L., Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatr. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Hreins E., Goldstone A.P., Brown R.M., Sumithran P. The therapeutic potential of GLP-1 analogues for stress-related eating and role of GLP-1 in stress, emotion and mood: a review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;110 doi: 10.1016/j.pnpbp.2021.110303. [DOI] [PubMed] [Google Scholar]
- He Y., Brouwers B., Liu H., et al. Human loss-of-function variants in the serotonin 2C receptor associated with obesity and maladaptive behavior. Nat. Med. 2022;28(12):2537–2546. doi: 10.1038/s41591-022-02106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler L.K., Jobst E.E., Sutton G.M., Zhou L., Borok E., Thornton-Jones Z., Cowley M.A. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Höglund E., Øverli Ø., Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front. Endocrinol. 2019;10:158. doi: 10.3389/fendo.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.M., Liu J.Y., Yu Wl, Li Ch, Huang L.H., Mao W., Lu Z.Y. Tryptophan intake, not always the more the better. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans L.A., Riedel W.J., Markus C.R., Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatr. 2007;12(6):522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Johnson P.L., Truitt W., Fitz S.D., Minick P.E., Dietrich A., Sanghani S., Traskman-Bendz L., Goddard A.W., et al. A key role for orexin in panic anxiety. Nat. Med. 2010;16(1):111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kałużna-Czaplińska J., Gatarek P., Chirumbolo S., Chartrand M.S., Bjorklund G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019;59(1):72–88. doi: 10.1080/10408398.2017.1357534. [DOI] [PubMed] [Google Scholar]
- Konttinen H., Männistö S., Sarlio-Lähteenkorva S., Silventoinen K., Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54(3):473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kuti D., Winkler Z., Horváth K., Juhász B., Szilvásy-Szabó A., Fekete C., Ferenczi S., Kovács K.J. The metabolic stress response: adaptation to acute-, repeated- and chronic challenges in mice. iScience. 2022;25(8) doi: 10.1016/j.isci.2022.104693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz S.F., Alexander J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatr. 1998;44(9):851–864. doi: 10.1016/s0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Lenard N.R., Berthoud Hans-Rudolf. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity. 2008;3(Suppl. 3):S11–S22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindseth G., Helland B., Caspers J. The effects of dietary tryptophan on affective disorders. Arch. Psychiatr. Nurs. 2015;29(2):102–107. doi: 10.1016/j.apnu.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y., Ramesh R.N., Burgess C.R., Patella P., Yang Z.F., Lowell B.B., et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple time scales. Elife. 2015;4 doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus C.R. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. NeuroMolecular Med. 2008;10(4):247–258. doi: 10.1007/s12017-008-8039-9. [DOI] [PubMed] [Google Scholar]
- Markus C Rob, et al. Differential effect of the 5-HTT gene-linked polymorphic region on emotional eating during stress exposure following tryptophan challenge. J. Nutr. Biochem. 2012;23(4):410–416. doi: 10.1016/j.jnutbio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Martin C.R., Osadchiy V., Kalani A. Mayer EA. “The brain-gut-microbiome Axis.”. Cellular and molecular gastroenterology and hepatology. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes S., Bader M. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol. Sci. 2018;39(6):560–572. doi: 10.1016/j.tips.2018.03.004. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani Christian, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017;81(4) doi: 10.1128/MMBR.00036-17. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D.T., Bridges R.J., Cotman C.W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Muller C.L., et al. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience. 2016;321:24–41. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten Michael, et al. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- Qiao Y.J., Zhao J.B., Li C., et al. Effect of combined chronic predictable and unpredictable stress on depression-like symptoms in mice. Ann. Transl. Med. 2020;8(15):942. doi: 10.21037/atm-20-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine S.E., Culbert K.M., Larsen C.L., Klump K.L. The possible influence of impulsivity and dietary restraint on associations between serotonin genes and binge eating. J. Psychiatr. Res. 2009;43(16):1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova I.V., Derkach K.V., Mikhrina A.L., Sukhov I.B., Mikhailova E.V., Shpakov A.O. The leptin, dopamine and serotonin receptors in hypothalamic POMC-neurons of normal and obese rodents. Neurochem. Res. 2018;43(4):821–837. doi: 10.1007/s11064-018-2485-z. [DOI] [PubMed] [Google Scholar]
- Sohn J.-W., Elmquist J.K., Williams K.W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36(9):504–512. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.