Abstract
Orange carotenoid proteins (OCPs) are unique photoreceptors that are critical for cyanobacterial photoprotection. Upon exposure to blue-green light, OCPs are activated from a stable orange form, OCPO, to an active red form, OCPR, which binds to phycobilisomes (PBSs) and performs photoprotective non-photochemical quenching (NPQ). OCPs can be divided into three main families: the most abundant and best studied OCP1, and two others, OCP2 and OCP3, which have different activation and quenching properties and are yet underexplored. Crystal structures have been acquired for the three OCP clades, providing a glimpse into the conformational underpinnings of their light-absorption and energy dissipation attributes. Recently, the structure of the PBS-OCPR complex has been obtained allowing for an unprecedented insight into the photoprotective action of OCPs. Here, we review the latest findings in the field that have substantially improved our understanding of how cyanobacteria protect themselves from the toxic consequences of excess light absorption. Furthermore, current research is applying the structure of OCPs to bio-inspired optogenetic tools, to function as carotenoid delivery devices, as well as engineering the NPQ mechanism of cyanobacteria to enhance their photosynthetic biomass production.
Keywords: Orange carotenoid protein, OCP, Non-photochemical quenching, NPQ, Photoprotection, Cyanobacteria, Phycobilisome, Fluorescence recovery protein
Graphical abstract
Highlights
-
•
The Orange Carotenoid Protein (OCP) is responsible of the Non-Photochemical quenching in cyanobacteria.
-
•
OCP is a water-soluble protein composed of two discrete domains, the N-terminal domain and the C-terminal domain, with a carotenoid as a chromophore spanning both domains.
-
•
Multiple families of OCP have been revealed: OCP1, OCP2, and OCPx(3).
-
•
Each family presents different photoactivation, oligomerization, and functional properties.
1. Introduction
Since the dawn of life, approximately 1039–1040 cells have existed on Earth, with cyanobacteria standing as the most abundant organism (Crockford et al., 2023). Cyanobacteria are a phylum of gram-negative bacteria having the characteristic ability to perform oxygenic photosynthesis (Flores and Herrero, 2005). Their photosynthetic activity plays a central role in the global production of oxygen and organic matter, and thus, they are the most important primary producers on Earth (Sanchez-Baracaldo et al., 2022).
To carry out photosynthesis, photons are absorbed by pigments within phycobilisomes (PBSs), major light-harvesting complexes in cyanobacteria (Arteni et al., 2009; Bryant et al., 1979; Dominguez-Martin et al., 2022; Glazer, 1989; Glazer et al., 1983). The harvested light energy is transferred to the chlorophyll pigment P680 in photosystem II, where it is converted into chemical energy by promoting charge separation (Vinyard et al., 2013). This chemical energy eventually leads to the production of the reductant NADPH and the energy currency ATP which are used for the carbon fixation reactions (Nikkanen et al., 2021).
Although light is essential for photosynthesis, an excess of it can be detrimental. When a photosynthetic pigment molecule is excited, there are multiple possible pathways to return to the ground state. Under normal photosynthetic operation, the energy is transferred to the photosynthetic reaction center. However, when the reaction center cannot accept incoming light energy, for example in high-light conditions, in which light-harvesting outpaces the kinetics of photosynthetic downstream reactions, the energy can be emitted as fluorescence. Still, there is also a chance that it can be transferred onto oxygen leading to the creation of reactive oxygen species (ROS). This can cause repairable dysfunction of the photosynthetic machinery (photoinhibition), permanent damage (photoinactivation), or even cell death (Derks et al., 2015; Melis, 1999; Rastogi et al., 2014) (Fig. 1A). Like other photosynthetic organisms, cyanobacteria have evolved mechanisms to protect themselves against the toxic consequences of excess light absorption. These range from enzymatic repair of photodamage, ROS scavenging and non-photochemical quenching (NPQ) (Fig. 1A–D). (Derks et al., 2015; Muller et al., 2001; Pathak J et al., 2019; Rastogi et al., 2014). NPQ is the conversion of excitation energy into heat and thereby prevents the possibility of ROS formation under high-light conditions (Pathak J et al., 2019). In cyanobacteria, NPQ is facilitated by the orange carotenoid protein (OCP) (Wilson et al., 2006) (Fig. 1C–D). As the name suggests, it contains a single carotenoid, typically a ketocarotenoid, giving it its characteristic orange color in the inactive resting form (OCPO). Upon absorption of strong blue-green light, OCP transitions from OCPO to its red active conformation (OCPR) (Fig. 1C). In this state, OCPR binds to the PBS, and the light energy harvested by the antenna pigments is transferred to the ketocarotenoid within OCP, where it is converted into heat (Dominguez-Martin et al., 2022; Liguori et al., 2022). Once the light intensity decreases and OCP-mediated excitation energy quenching is no longer needed, the 13 kDa fluorescence recovery protein (FRP) (Boulay et al., 2010; Sutter et al., 2013) back-converts OCPR into inactive OCPO, which again allows for normal energy flow from antenna pigments to PSII to operate photosynthesis (Boulay et al., 2010; Sluchanko et al., 2018) (Fig. 1D)
Fig. 1.
Overview of photoprotection mechanism in cyanobacteria. A. Strategies to cope with excess light and its effects. High light intensity can cause adverse effects in cyanobacteria, either directly or indirectly through the production of ROS. Some of these damages can lead to mutations, photoinhibition, and photoinactivation, or even cell death. Photoprotective mechanisms that cyanobacteria possess include light avoidance (through movement, synthesis of various compounds, etc.), electron sinks, antioxidant enzymes or compounds, and finally NPQ. B. The PBS and its role in NPQ. The PBS is a complex structure that acts as a light-harvesting antenna for cyanobacteria. Under normal light conditions, the pigments in this structure transfer the harvested light energy to the reaction center of PSII (Top). When light is received in excess photodamage, and ROS formation can occur (Bottom). C. Schematic of the OCP activation process under high-light conditions. In cyanobacteria, NPQ is mainly carried out by OCP, a photoactive protein that is activated when it receives a certain amount of blue-green light. OCP then transforms from its inactive state, OCPO, to its active state, OCPR. D. Scheme of the NQP mechanism and its recovery. Under high-light conditions, OCP is activated, as the carotenoid absorbs a blue-green light quantum. In its active conformation, a carotenoid that normally resides symmetrically within the N-terminal domain (NTD) and C-terminal domain (CTD) is partially exposed and pulled toward the NTD. This red active form of OCP associates with a PBS. This leads to effective quenching of harvested light-energy as heat, which reduces the energy reaching the reaction center of PSII to prevent photodamage and ROS formation. When the light intensity drops, FRP dimers bind to OCPR, causing its dissociation from PBS and back-conversion into OCPO. Additionally, FRP is also able to act on free OCPR, which is not bound to a PBS (Bao et al., 2017; Sluchanko et al., 2018; Sutter et al., 2013). The asterisk panel shows a cartoon of the OCPR bound to the core of the PBS (Dominguez-Martin et al., 2022): T means Top and B1 and B2 bottom cylinders. To simplify the scheme the interaction of OCP is shown in one of the cylinders. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In this graphical review, we illustrate the structure and function of the families of OCPs and highlight that the photoprotection mechanism in cyanobacteria is not as simplistic as it might seem.
2. Phylogenetic distribution of OCP paralogs
Phylogenomic analysis has revealed that there are at least three paralogous families of OCP: OCP1, OCP2, and OCPx (or OCP3) (you can find both names in the literature, hereafter OCPx(3)) (Bao et al., 2017). Among these phylogenetically distinct groups, the well-characterized OCP from Synechocystis PCC 6803 is the most widespread and is referred to as OCP1. Members of this family are found in almost every phylogenetic subclade of cyanobacteria (with the exception of Prochlorococcus), and always co-occur with FRP (Fig. 2). In contrast, OCP2-containing genomes never contain a gene encoding FRP unless an OCP1 gene is also present, indicating that FRP is not required to inactivate OCP2 (Bao et al., 2017). A potential third clade of OCPs most distantly related to OCP1 is OCPx(3). Members of this family rarely co-occur with OCP1 and occasionally co-occur with OCP2. OCPx(3) can be divided into at least three subfamilies (OCPx(3)a, OCPx(3)b, OCPx(3)c). It has been shown that OCPx(3)a from the most ancient cyanobacteria group, Gleobacter is the most primitive and it has been hypothesized that it is most closely related to the common OCP ancestor (Slonimskiy et al., 2022).
Fig. 2.
Cyanobacterial species containing orange carotenoid proteins (OCPs) and the fluorescence recovery protein (FRP). The data were obtained from the supplementary information of (Bao et al., 2017) filtered only for OCP and FRP content. The gene content is divided into all morphological subsections with available cyanobacterial genome sequences: Subsection I (Chroococcales), Subsection II (Pleurocapsales), Subsection III (Oscillatoriales), Subsection IV (Nostocales), Subsection V (Stigonematales) (Shih et al., 2013). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. The structural modularity of the OCP
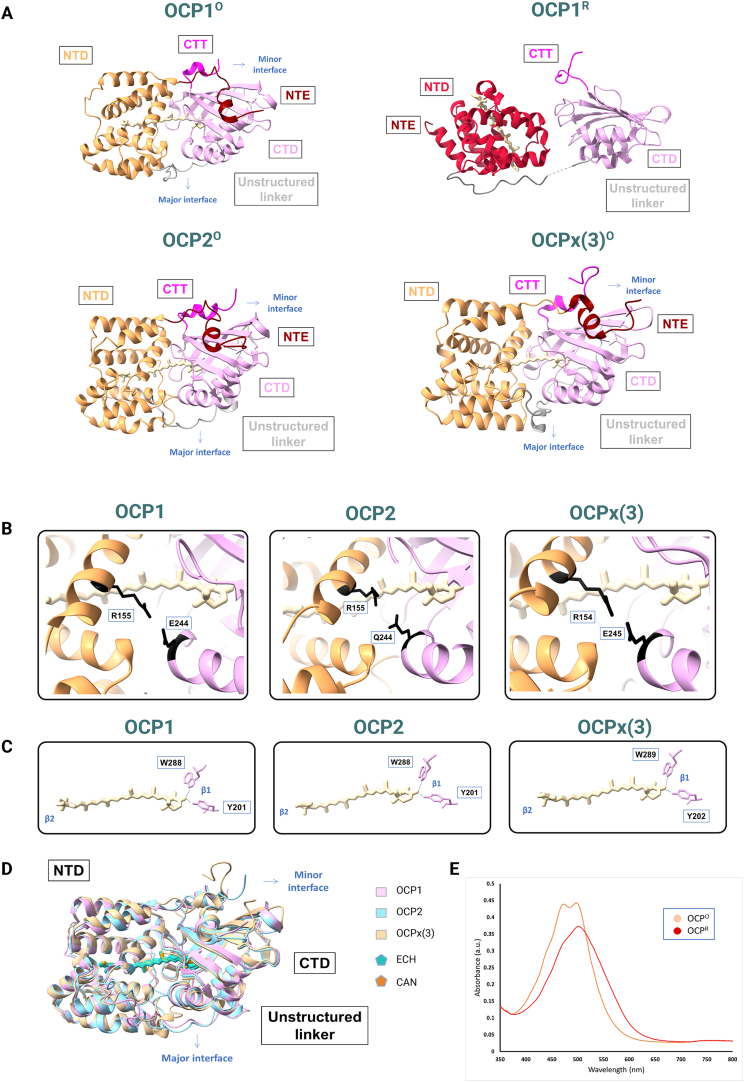
OCPs are structurally and functionally modular proteins organized into two domains: an all-helical N-terminal domain (NTD) is connected with an α/β C-terminal domain (CTD) by a flexible linker of about 25 amino acids (Fig. 3A). While the fold of the NTD (Pfam09150), consisting of two discontinuous four-helix bundles, is unique to cyanobacteria, the CTD (Pfam02136) consists of a structure similar to the nuclear transport factor 2 (NTF2)-like superfamily found in a wide range of organisms (Kerfeld et al., 2003; Wilson et al., 2010). A non-covalently bound ketocarotenoid spans the two domains (Fig. 3) which functions as a light-sensor. The light absorption properties and thereby photoactivation and NPQ function of OCPs are tuned by the precise carotenoid molecule it carries, which can be canthaxanthin, echinenone or 3-hydroxyechinenone (Fig. 3D) (Punginelli et al., 2009). The NTD is the effector domain capable of performing NPQ, while the CTD seems to act as a regulator for photoactivation. This has been demonstrated by expressing the NTD separately, without the CTD, which was able to quench phycobilisome fluorescence (Leverenz et al., 2014).
Fig. 3.
A. Structural features of the OCP family. Upper-left: modular structure of OCP1O from Synechocystis sp. PCC 6803 (PDB: 4XB5) containing canthaxanthin as the carotenoid chromophore. Upper-right: structure of OCP1R from Synechocystis sp. PCC 6803 (PDB: 7SCC), containing canthaxanthin. bottom-left: structure of OCP2 from Crinalium epipsammum PCC 9333 (PDB: 8PYH), containing echinenone. Bottom-right: structure of OCPx(3) from Gloeobacter kilaueensis JS1 (PDB: 8A0H), containing echinenone. The NTD is shown in sandy brown (except for OCP1R, in which it is shown in red), the NTE is colored in maroon, the CTD is purple, the CTT is in magenta, and the linker is colored in grey. B. Residues involved in the salt bridge of the major interface for each OCP clade. The residues are highlighted in black, and the number of the residues for each OCP has been indicated. C. Residues involved in the H-bonds between the OCP apoprotein and the keto group of the carotenoid. The conserved Tyr and Trp are highlighted in black, and the number of the residues for each OCP has been indicated. The H-bonds between the apoprotein and the carotenoid are shown as blue lines. D. Superposition of the 3 different OCP clades. The color for each OCP has been indicated on the right side of the panel as well as the color for each carotenoid. E. U-vis spectra of the inactive form of OCP1 (OCPO) and the active form of OCP1 (OCPR). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The inactive OCPO form is stabilized by NTD/CTD interactions at the major interdomain groove, including the salt bridge between Arg155 and Glu244 (Synechocystis PCC 6803 numbering) in the NTD and CTD respectively (Fig. 3B). Further stabilization is caused by the N-terminal extension (NTE) attached to the CTD β-sheet (Kerfeld et al., 2003) (Fig. 3A). Additionally, a short helix on the C-terminus, the C-terminal tail (CTT), was identified as a potentially important control element that docks on the C-terminal domain β-sheet in the OCPO state (Harris et al., 2018) (Fig. 3A). The carotenoid keto group is coordinated by H-bonds with the side chains of the conserved Tyr201 and Trp288 residues (Synechocystis PCC 6803 numbering) in the CTD (Kerfeld et al., 2003) in the resting form OCPO (Fig. 3C).
4. OCP function
4.1. OCP photoactivation
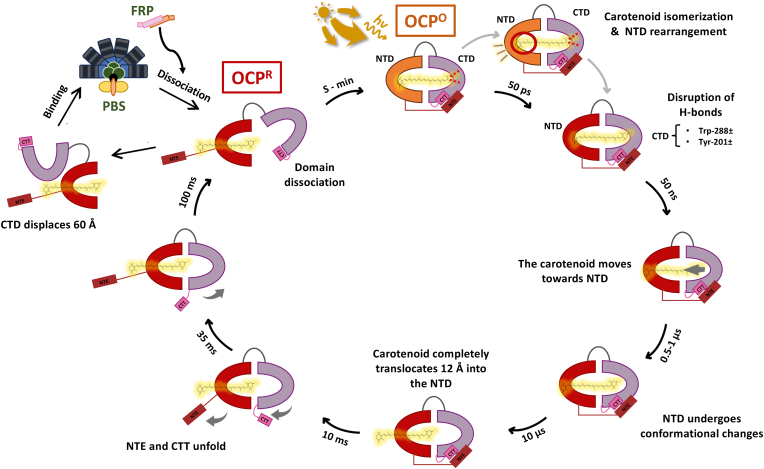
The photocycle commences upon absorption of strong blue-green light (450–560 nm) (Fig. 4). This leads to isomerization of the carotenoid C9′-C8′and C7′-C6′single bonds, inducing structural changes in the NTD, which propagate to the CTD, causing disruption of the H-bonds between the carotenoid β1 ring keto group and the CTD amino acids Trp288 and Tyr201 (Chukhutsina et al., 2022). After this event, several red intermediate states occur between the ps to second time-scaled, the carotenoid translocates 12 Å into the NTD, then the NTE and CTT unfold, and the two domains, NTD and CTD fully dissociate (Gupta et al., 2015; Konold et al., 2019; Leverenz et al., 2015) (Fig. 4).
Fig. 4.
Scheme of the multi-step reaction of the photocycle of the OCP. Upon exposure to light, OCPO undergoes several changes that transform this inactive orange form into its active red version, OCPR. After the carotenoid isomerization and NTD rearrangement step, the two hydrogen bonds between the CTD and the carotenoid molecule break. After this disruption, the carotenoid moves towards the NTD, while some conformational changes occur in this domain. The complete repositioning of the carotenoid ends when the molecule has moved a total of 12 Å towards the NTD. After the translocation of the carotenoid, further conformational changes occur in the OCP, starting with the movement of the NTE and CTT. Finally, the NTD and CTD dissociate, and the protein is in its red active form, OCPR. OCPR has the ability to bind to the PBS (when the CTD is displaced 60 Å) and quench the excess energy of the antenna pigments. This energy is transferred to the ketocarotenoid and later converted to heat by vibrational relaxation. OCPR dissociates from the PBS upon the interaction with the active dimeric form of FRP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Notably, comparing the active form of the structure of the OCPR-PBS complex with the inactive OCP, it showed a 60 Å displacement of the CTD. This exposes the surface of the NTD that binds to the PBS (β1 surface), enabling the carotenoid to convert the incoming light energy into a harmless heat (Dominguez-Martin et al., 2022). The CTD dimerizes with a CTD from a second activated OCP, forming a PBS bound OCP dimer (Dominguez-Martin et al., 2022). However, it is yet unclear if OCPR dimerization occurs prior to PBS binding, after, or simultaneously.
4.2. Interaction of the OCP with the PBS
The role of OCP binding to the PBS was shown almost two decades ago (Wilson et al., 2006); however, the specific site of the binding and the stoichiometry have been a dispute topic in the field (Gwizdala et al., 2011; Harris et al., 2016; Jallet et al., 2012; Stadnichuk et al., 2012; Tian et al., 2011). Recently, the structure of the OCPR-PBS complex from the model organism, Synechocystis PCC 6803 has been elucidated (Dominguez-Martin et al., 2022) and showed, that four OCPR molecules arranged as two dimers bind to the PBS core in the quenched complex. This is in contrast to previous hypotheses suggesting only 1 or 2 OCPR per PBS (Gwizdala et al., 2011; Squires et al., 2019). Likewise, there were several mutually exclusive models about where OCPR binds to the PBS (Jallet et al., 2012; Tian et al., 2012; Tian et al., 2011). However, the structure of PBS in complex with OCPR revealed that OCPR interacts mostly with the outermost ApcA and ApcB disc in the Top cylinder. On the bottom cylinder, OCPR is bound to the two ApcA and ApcB trimers with only a minor interaction with an ApcB from the ApcABEF trimer. Furthermore, the structure showed that OCPR interacts with the CpcG1 rod–linker.
The majority of OCPR residues involved in the interaction with PBS are within the β1 face of the NTD. This includes the conserved positively charged Arg155 in the center of the interaction surface next to the carotenoid confirming its crucial role in binding OCP to the PBS (Dominguez-Martin et al., 2022, Wilson et al., 2012).
Furthermore, it is shown that mutants of the cyanobacteria Synechocystis PCC 6803 lacking OCP are more sensitive to high light or white light (Wilson et al., 2006).
4.3. OCP and singlet O2 quenching
Besides being essential for the NPQ process, OCP is able to quench 1O2 (Kerfeld et al., 2003; Sedoud et al., 2014). This quenching activity is retained even in OCP mutants which prevent photoactivation and/or bind non-keto-carotenoids (such as zeaxanthin) (Sedoud et al., 2014). Quenching of singlet oxygen is substantial when catalyzed by either the OCPO or OCPR form, as both showed activity largely exceeding that of smaller 1O2 quenchers, such as ascorbate, histidine or Trolox (a soluble carotenoid derivative (Sedoud et al., 2014)), and in the same range as other antioxidant proteins, such as superoxide dismutase (Kerfeld et al., 2003). Thus, OCP not only prevents ROS formation by inducing NPQ but can also scavenge ROS, making it a multifunctional protein in the photoprotection process of cyanobacteria.
4.4. The role of individual residues in the OCP function
There are several studies showing the specific roles or functional associations of amino acids in the light activation process, carotenoid selectivity, or NPQ mechanisms (Fig. 5). Using mutagenesis, the important role of Arg155 was elucidated before the structure of the OCP-PBS complex was obtained (Wilson et al., 2012; Wilson et al., 2010) (Fig. 5C). This method also revealed that Tyr44 is essential for the photoconversion of OCP (Wilson et al., 2010). Other site-directed mutagenesis studies demonstrated the importance of the Trp288 and the Tyr201, when they are substitute with Histidine or Serine, the OCP lost the ability to photoconvert and also to quench the PBS (Wilson et al., 2011). Interestingly, when Trp288 is substituted with Alanine, OCP becomes constantly active and the visual color is purple (Sluchanko et al., 2017). The role of the conserved residues Cys84 and Tyr129 was also shown when they were mutated to Alanine and Phenylalanine, respectively. These OCP mutants showed a faster dark recovery, and the PBS quenching efficiency was lower (Leverenz et al., 2015). Mutagenesis studies have focused on OCP1, and while some of the key residues in OCP1 are conserved in OCP2 and OCPx(3), others are not. For instance, the Arg155 is substituted in OCP2 with a Gln155. Thus, it is intriguing if these differences might critically modify absorption, activation, activity, or back-conversion properties in OCP2 and OCPx(3).
Fig. 5.
Summary of the OCP1 variants. Each panel shows the mutated residues and the effect on the OCP1 function. Residues in green are conserved among all the OCP families; residues in red are not conserved. This figure summarizes the work from (Leverenz et al., 2015; Lou et al., 2020; Sluchanko et al., 2017; Thurotte et al., 2015; Wilson et al., 2010, 2012; Wilson et al., 2011). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5. Structural and functional comparison of the different OCPs
Currently, OCP1 is the best characterized OCP. However, a few studies have begun to elucidate the functional and structural features of the new OCP families (Bao et al., 2017, Muzzopappa et al., 2019; Slonimskiy et al., 2022; Sluchanko et al., 2024). OCPx(3) and OCP2 present fast deactivation and interact weakly with the PBS. In OCP1 the deactivation is slower, and the interaction with the antenna is stronger, requiring FRP to detach from it (Bao et al., 2017; Muzzopappa et al., 2019; Slonimskiy et al., 2022; Sluchanko et al., 2024). The oligomeric state of OCP remains controversial, OCP1 from Synechocystis PCC 6803 has been described either as a monomer or a dimer. The reports suggest that the oligomeric state is an OCP family feature, with OCP1 and OCPx(3) being dimeric and OCP2 being monomeric (Bao et al., 2017; Muzzopappa et al., 2019).
The overall architecture of the OCP1, OCP2 and OCPx(3) is similar (Fig. 3A and D). The CTT can adopt an unusual conformation in one of the OCP2 structures solved. The NTE is connected similarly in all of them, and the interdomain linker is the most variable part in OCP, contributing to the functionality of the different OCPs.
6. Concluding remarks
In recent years, considerable progress has been made in understanding the mechanisms of OCP-mediated photoprotection in cyanobacteria. However, many questions remain unanswered, and new ones have emerged. For example, we don’t understand the structural basis of how FRP acts to deactivate OCP, or even what triggers FRP action. In addition, the discovery of several OCP families suggests that there are slightly different photoprotection mechanisms. It can be hypothesized that these mechanisms are presumably linked to the light-conditions that the cyanobacteria are encountering in the ecological niche they are inhabiting. For example, surface-dwelling marine cyanobacteria are exposed to much more rapidly changing light intensities than deep-dwelling species, and therefore require different NPQ activation and deactivation kinetics. However, more research needs to be done to fully understand the properties of the different OCP families and if/how they are linked to the habitat of cyanobacteria species.
CRediT authorship contribution statement
Teresa M. García-Oneto: Writing, preparing figures, review and editing. Claudia Moyano-Bellido: Writing, preparing figures, review and editing. M. Agustina Domínguez-Martín: Conceptualization, writing, preparing figures, review and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are supported by grant CNS2022-136043 funded by MCIN/AEI/10.13039/501100011033 and, by EuropeanUnion NextGenerationEU/PRTR. C.M.B. is supported by the Fellowship “Semillero de Investigación” from the University of Córdoba. The authors thank all the scientists that have been involved in the study of the NPQ-OCP mechanism in cyanobacteria, for their contributions to this amazing field.
Handling Editor: Dr. N Strynadka
Glossary
- PSII
Photosystem II
- ApcA/ApcB
Allophycocyanin A/ B
- OCP
Orange Carotenoid Protein
- NPQ
Non-photochemical quenching
- PBS
phycobilisome
- FRP
Fluorescence recovery protein
- CTD
C-terminal domain of OCP
- NTD
N-terminal domain of OCP
- NTE
N-terminal extension of OCP
- CTT
C-terminal tail of OCP
- ROS
Reactive Oxygen species
- OCPO
Orange Carotenoid protein, inactive form
- OCPR
Orange Carotenoid protein, active form
- OCPR-PBS
complex of the Orange Carotenoid protein, active form, and the phycobilisome
Data availability
Data will be made available on request.
References
- Arteni A.A., Ajlani G., Boekema E.J. Structural organisation of phycobilisomes from Synechocystis sp. strain PCC 6803 and their interaction with the membrane. Biochim. Biophys. Acta. 2009;1787:272–279. doi: 10.1016/j.bbabio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Bao H., Melnicki M.R., Pawlowski E.G., Sutter M., Agostoni M., Lechno-Yossef S., Cai F., Montgomery B.L., Kerfeld C.A. Additional families of orange carotenoid proteins in the photoprotective system of cyanobacteria. Nat. Plants. 2017;3 doi: 10.1038/nplants.2017.89. [DOI] [PubMed] [Google Scholar]
- Boulay C., Wilson A., D'Haene S., Kirilovsky D. Identification of a protein required for recovery of full antenna capacity in OCP-related photoprotective mechanism in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 2010;107:11620–11625. doi: 10.1073/pnas.1002912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D.A., Guglielmi G., Tandeau de Marsac N., Castets A., Cohen-bazire G. The structure of cyanobacterial phycobilisomes: a model. Arch. Microbiol. 1979;123:113–127. [Google Scholar]
- Chukhutsina V.U., Baxter J.M., Fadini A., Morgan R.M., Pope M.A., Maghlaoui K., Orr C.M., Wagner A., van Thor J.J. Light activation of Orange Carotenoid Protein reveals bicycle-pedal single-bond isomerization. Nat. Commun. 2022;13:6420. doi: 10.1038/s41467-022-34137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford P.W., Bar On YM., Ward L.M., Milo R., Halevy I. The geologic history of primary productivity. Curr. Biol. 2023;33:4741–4750 e4745. doi: 10.1016/j.cub.2023.09.040. [DOI] [PubMed] [Google Scholar]
- Derks A., Schaven K., Bruce D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta. 2015;1847:468–485. doi: 10.1016/j.bbabio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Dominguez-Martin M.A., Sauer P.V., Kirst H., Sutter M., Bina D., Greber B.J., Nogales E., Polivka T., Kerfeld C.A. Structures of a phycobilisome in light-harvesting and photoprotected states. Nature. 609. 2022;7928:835–845. doi: 10.1038/s41586-022-05156-4. [DOI] [PubMed] [Google Scholar]
- Flores E., Herrero A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005;33:164–167. doi: 10.1042/BST0330164. [DOI] [PubMed] [Google Scholar]
- Glazer A.N. Light guides. Directional energy transfer in a photosynthetic antenna. J. Biol. Chem. 1989;264:1–4. [PubMed] [Google Scholar]
- Glazer A.N., Lundell D.J., Yamanaka G., Williams R.C. 134B. Ann Microbiol; 1983. pp. 159–180. (The Structure of a "simple" Phycobilisome). (Paris) [DOI] [PubMed] [Google Scholar]
- Gupta S., Guttman M., Leverenz R.L., Zhumadilova K., Pawlowski E.G., Petzold C.J., Lee K.K., Ralston C.Y., Kerfeld C.A. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E5567–E5574. doi: 10.1073/pnas.1512240112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwizdala M., Wilson A., Kirilovsky D. In vitro reconstitution of the cyanobacterial photoprotective mechanism mediated by the Orange Carotenoid Protein in Synechocystis PCC 6803. Plant Cell. 2011;23:2631–2643. doi: 10.1105/tpc.111.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D., Tal O., Jallet D., Wilson A., Kirilovsky D., Adir N. Orange carotenoid protein burrows into the phycobilisome to provide photoprotection. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E1655–E1662. doi: 10.1073/pnas.1523680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D., Wilson A., Muzzopappa F., Sluchanko N.N., Friedrich T., Maksimov E.G., Kirilovsky D., Adir N. Structural rearrangements in the C-terminal domain homolog of Orange Carotenoid Protein are crucial for carotenoid transfer. Commun. Biol. 2018;1:125. doi: 10.1038/s42003-018-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallet D., Gwizdala M., Kirilovsky D. ApcD, ApcF and ApcE are not required for the Orange Carotenoid Protein related phycobilisome fluorescence quenching in the cyanobacterium Synechocystis PCC 6803. Biochim. Biophys. Acta. 2012;1817:1418–1427. doi: 10.1016/j.bbabio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Kerfeld C.A., Sawaya M.R., Brahmandam V., Cascio D., Ho K.K., Trevithick-Sutton C.C., Krogmann D.W., Yeates T.O. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure. 2003;11:55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- Konold P.E., van Stokkum I.H.M., Muzzopappa F., Wilson A., Groot M.L., Kirilovsky D., Kennis J.T.M. Photoactivation mechanism, timing of protein secondary structure dynamics and carotenoid translocation in the orange carotenoid protein. J. Am. Chem. Soc. 2019;141:520–530. doi: 10.1021/jacs.8b11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz R.L., Jallet D., Li M.D., Mathies R.A., Kirilovsky D., Kerfeld C.A. Structural and functional modularity of the orange carotenoid protein: distinct roles for the N- and C-terminal domains in cyanobacterial photoprotection. Plant Cell. 2014;26:426–437. doi: 10.1105/tpc.113.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverenz R.L., Sutter M., Wilson A., Gupta S., Thurotte A., Bourcier de Carbon C., Petzold C.J., Ralston C., Perreau F., Kirilovsky D., et al. PHOTOSYNTHESIS. A 12 A carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science. 2015;348:1463–1466. doi: 10.1126/science.aaa7234. [DOI] [PubMed] [Google Scholar]
- Liguori Nicoletta, van Stokkum Ivo, Muzzopappa Fernando, Kennis John, Kirilovsky Diana, Croce R. The molecular origin of the OCP-dependent non-photochemical quenching mechanism in cyanobacteria. ChemRxiv. 2022 [Google Scholar]
- Lou W., Niedzwiedzki D.M., Jiang R.J., Blankenship R.E., Liu H. Binding of red form of Orange Carotenoid Protein (OCP) to phycobilisome is not sufficient for quenching. Biochim. Biophys. Acta Bioenerg. 2020;1861 doi: 10.1016/j.bbabio.2020.148155. [DOI] [PubMed] [Google Scholar]
- Melis A. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage. Trends Plant Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Muller P., Li X.P., Niyogi K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzopappa F., Wilson A., Kirilovsky D. Interdomain interactions reveal the molecular evolution of the orange carotenoid protein. Nat. Plants. 2019;5:1076–1086. doi: 10.1038/s41477-019-0514-9. [DOI] [PubMed] [Google Scholar]
- Nikkanen L., Solymosi D., Jokel M., Allahverdiyeva Y. Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: recent advances and biotechnological prospects. Physiol. Plantarum. 2021;173:514–525. doi: 10.1111/ppl.13404. [DOI] [PubMed] [Google Scholar]
- Pathak J., Ahmed H., Singh P.R., Singh S.P., Häder D.P., Rp S. Cyanobacteria: from Basic Science To Applications, Academic Press. 2019. Chapter 7- Mechanisms of photoprotection in cyanobacteria. [Google Scholar]
- Punginelli C., Wilson A., Routaboul J.M., Kirilovsky D. Influence of zeaxanthin and echinenone binding on the activity of the orange carotenoid protein. Biochim. Biophys. Acta. 2009;1787:280–288. doi: 10.1016/j.bbabio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Rastogi R.P., Sinha R.P., Moh S.H., Lee T.K., Kottuparambil S., Kim Y.J., Rhee J.S., Choi E.M., Brown M.T., Hader D.P., et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol., B. 2014;141:154–169. doi: 10.1016/j.jphotobiol.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Sanchez-Baracaldo P., Bianchini G., Wilson J.D., Knoll A.H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2022;30:143–157. doi: 10.1016/j.tim.2021.05.008. [DOI] [PubMed] [Google Scholar]
- Sedoud A., Lopez-Igual R., Ur Rehman A., Wilson A., Perreau F., Boulay C., Vass I., Krieger-Liszkay A., Kirilovsky D. The cyanobacterial photoactive orange carotenoid protein is an excellent singlet oxygen quencher. Plant Cell. 2014;26:1781–1791. doi: 10.1105/tpc.114.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P.M., Wu D., Latifi A., Axen S.D., Fewer D.P., Talla E., Calteau A., Cai F., Tandeau de Marsac N., Rippka R., et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonimskiy Y.B., Zupnik A.O., Varfolomeeva L.A., Boyko K.M., Maksimov E.G., Sluchanko N.N. A primordial Orange Carotenoid Protein: structure, photoswitching activity and evolutionary aspects. Int. J. Biol. Macromol. 2022;222:167–180. doi: 10.1016/j.ijbiomac.2022.09.131. [DOI] [PubMed] [Google Scholar]
- Sluchanko N.N., Klementiev K.E., Shirshin E.A., Tsoraev G.V., Friedrich T., Maksimov E.G. The purple Trp288Ala mutant of Synechocystis OCP persistently quenches phycobilisome fluorescence and tightly interacts with FRP. Biochim. Biophys. Acta Bioenerg. 2017;1858:1–11. doi: 10.1016/j.bbabio.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Sluchanko N.N., Slonimskiy Y.B., Shirshin E.A., Moldenhauer M., Friedrich T., Maksimov E.G. OCP-FRP protein complex topologies suggest a mechanism for controlling high light tolerance in cyanobacteria. Nat. Commun. 2018;9:3869. doi: 10.1038/s41467-018-06195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluchanko N.N., Maksimov E.G., Slonimskiy Y.B., Varfolomeeva L.A., Bukhanko A.Y., Egorkin N.A., Tsoraev G.V., Khrenova M.G., Ge B., Qin S., et al. Structural framework for the understanding spectroscopic and functional signatures of the cyanobacterial Orange Carotenoid Protein families. Int. J. Biol. Macromol. 2024;254 doi: 10.1016/j.ijbiomac.2023.127874. [DOI] [PubMed] [Google Scholar]
- Squires A.H., Dahlberg P.D., Liu H.J., Magdaong N.C.M., Blankenship R.E., Moerner W.E. Single-molecule trapping and spectroscopy reveals photophysical heterogeneity of phycobilisomes quenched by Orange Carotenoid Protein. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnichuk I.N., Yanyushin M.F., Maksimov E.G., Lukashev E.P., Zharmukhamedov S.K., Elanskaya I.V., Paschenko V.Z. Site of non-photochemical quenching of the phycobilisome by orange carotenoid protein in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta. 2012;1817:1436–1445. doi: 10.1016/j.bbabio.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Sutter M., Wilson A., Leverenz R.L., Lopez-Igual R., Thurotte A., Salmeen A.E., Kirilovsky D., Kerfeld C.A. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 2013;110:10022–10027. doi: 10.1073/pnas.1303673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurotte A., Lopez-Igual R., Wilson A., Comolet L., Bourcier de Carbon C., Xiao F., Kirilovsky D. Regulation of orange carotenoid protein activity in cyanobacterial photoprotection. Plant Physiol. 2015;169:737–747. doi: 10.1104/pp.15.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., van Stokkum I.H., Koehorst R.B., Jongerius A., Kirilovsky D., van Amerongen H. Site, rate, and mechanism of photoprotective quenching in cyanobacteria. J. Am. Chem. Soc. 2011;133:18304–18311. doi: 10.1021/ja206414m. [DOI] [PubMed] [Google Scholar]
- Tian L., Gwizdala M., van Stokkum I.H., Koehorst R.B., Kirilovsky D., van Amerongen H. Picosecond kinetics of light harvesting and photoprotective quenching in wild-type and mutant phycobilisomes isolated from the cyanobacterium Synechocystis PCC 6803. Biophys. J. 2012;102:1692–1700. doi: 10.1016/j.bpj.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinyard D.J., Ananyev G.M., Dismukes G.C. Photosystem II: the reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 2013;82:577–606. doi: 10.1146/annurev-biochem-070511-100425. [DOI] [PubMed] [Google Scholar]
- Wilson A., Ajlani G., Verbavatz J.M., Vass I., Kerfeld C.A., Kirilovsky D. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell. 2006;18:992–1007. doi: 10.1105/tpc.105.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Gwizdala M., Mezzetti A., Alexandre M., Kerfeld C.A., Kirilovsky D. The essential role of the N-terminal domain of the orange carotenoid protein in cyanobacterial photoprotection: importance of a positive charge for phycobilisome binding. Plant Cell. 2012;24:1972–1983. doi: 10.1105/tpc.112.096909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Kinney J.N., Zwart P.H., Punginelli C., D'Haene S., Perreau F., Klein M.G., Kirilovsky D., Kerfeld C.A. Structural determinants underlying photoprotection in the photoactive orange carotenoid protein of cyanobacteria. J. Biol. Chem. 2010;285:18364–18375. doi: 10.1074/jbc.M110.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Punginelli C., Couturier M., Perreau F., Kirilovsky D. Essential role of two tyrosines and two tryptophans on the photoprotection activity of the Orange Carotenoid Protein. Biochim. Biophys. Acta. 2011;1807:293–301. doi: 10.1016/j.bbabio.2010.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.