Abstract
Aim:
Increasing venous return during cardiopulmonary resuscitation (CPR) has been shown to improve hemodynamics during CPR and outcomes following cardiac arrest (CA). We hypothesized that a high central venous pressure amplitude (CVP-A), the difference between the maximum and minimum central venous pressure during chest compressions, could serve as a robust predictor of return of spontaneous circulation (ROSC) in addition to traditional measurements of coronary perfusion pressure (CPP) and end-tidal CO2 (etCO2) in a porcine model of CA.
Methods:
After 10 minutes of ventricular fibrillation, 9 anesthetized and intubated female pigs received mechanical chest compressions with active compression/decompression (ACD) and an impedance threshold device (ITD). CPP, CVP-A and etCO2 were measured continuously. All groups received biphasic defibrillation (200J) at minute 4 of CPR and were classified into two groups (ROSC, NO ROSC). Mean values were analyzed over 3 minutes before defibrillation by repeated-measures Analysis of Variance and receiver operating characteristic (ROC).
Results:
Five animals out of 9 experienced ROSC. CVP-A showed a statistically significant difference (p=0.003) between the two groups during 3 minutes of CPR before defibrillation compared to CPP (p=0.056) and etCO2 (p=0.064). Areas-under-the-curve in ROC analysis for CVP-A, CPP and etCO2 were 0.94 (95% Confidence Interval 0.86, 1.00), 0.74 (0.54, 0.95) and 0.78 (0.50, 1.00), respectively.
Conclusion:
In our study, CVP-A was a potentially useful predictor of successful defibrillation and return of spontaneous circulation. Overall, CVP-A could serve as a marker for prediction of ROSC with increased venous return and thereby monitoring the beneficial effects of ACD and ITD.
Introduction
Each year, emergency medical services respond to more than 350,000 adult out-of-hospital cardiac arrests (CA) in the United States with similar numbers in Europe.1,2 Functionally favorable survival is still less than 10% with little demonstrated increase since 2012.3 Modern mechanical adjuncts for cardiopulmonary resuscitation (CPR) aim to improve outcome by targeting and enhancing venous return, such as active compression-decompression (ACD) CPR and the impedance threshold device (ITD).4-8
To date, there is no parameter to quantify or monitor venous return during CPR. Although central venous pressure (CVP) is used to estimate blood volume, studies have shown that high mean CVP values are harmful during CPR and might not reflect venous return.9-11 Further, mean CVP does not reflect the change of direction in venous blood flow during compression and decompression.12,13 Previously, we demonstrated that the CVP amplitude (CVP-A), reflecting the difference between the maximum and minimum central venous pressure (CVPMin) during chest compressions, might reflect changes in blood volume more accurately and serve as a marker of venous return and CPR quality.14
Thus, the objective of this study was to evaluate CVP-A as a marker to predict successful defibrillation and the return of spontaneous circulation (ROSC) compared to coronary perfusion pressure (CPP) and end-tidal CO2 (etCO2) using ACD + ITD CPR in a porcine model of CA.
Methods
This study is a post-hoc analysis of the control group (9 animals) of a previously conducted study (unpublished). We were able to use the data for our exploratory and confirmatory study and, thus, comply with the 3Rs principle of humane animal research. The original study was approved by the Institutional Animal Care and Use Committee of the University of Minnesota (protocol number: 1810-36421A). All animal care was compliant with the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals. This study is reported in accordance with the ARRIVE guidelines.15
Preparatory Phase
Nine Yorkshire female farm-bred pigs were used with an average weight of 42.3 kg. Anesthesia, surgical preparation, data monitoring, and recording procedures used in this study have been described previously.16-18 Briefly, intramuscular ketamine and xylazine were administered as sedation (5 ml of 100 mg/ml dose and 1 to 3 mg/kg, respectively). This was followed by inhaled isoflurane at an end-tidal concentration of 1-1.4 Vol%. Endotracheal intubation was performed with a 7.5-mm endotracheal tube. Animals were ventilated with a tidal volume of 10 ml/kg using an air-oxygen mixture (30% O2, 70% N2) and volume control ventilation (Narkomed, Draeger Medical, Telford, Pennsylvania). The respiratory rate was adjusted to maintain a partial pressure of carbon dioxide (PaCO2) of 40 mmHg as measured by arterial blood gas (Gem 3500, Instrumentation Laboratory, Bedford, Massachusetts). The aortic blood pressure was recorded continuously with a Millar catheter (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) placed through an 8-F left femoral arterial sheath into the descending thoracic aorta. A second Millar catheter was inserted into the right atrium through an 8-F sheath placed into the right external jugular vein to record CVP. All sheaths were placed percutaneously using ultrasound guidance.
Measurements
Hemodynamic data, electrocardiograms, as well as end-tidal CO2 (etCO2) (Cardiocap/5, Datex-Ohmeda, Louisville, Colorado) were continuously recorded (250 Hz, Labview 2015, National Instruments, Austin, Texas). The measurements from both Millar catheters were used for acquisition of diastolic blood pressure (DBP), CVP, CVP-A and CVP at maximal decompression (CVPMin). Coronary perfusion pressure (CPP) was calculated as the difference of DBP and CVPMin during CPR.19 Compression force, rate and depth were also continuously recorded throughout all experiments and controlled during CPR to assure all animals received identical CPR quality. For post-hoc analysis, all data blocks were converted and merged into LabChart 8 (ADInstruments, Colorado Springs, CO).
Experimental Protocol
After the surgical preparation and a short stabilization period, baseline values were recorded (Figure 1). Ventricular fibrillation (VF) was induced by delivering direct intracardiac current with a pacing wire through the right jugular vein and confirmed by electrocardiogram (ECG). At VF initiation, mechanical ventilation was suspended. After 10 minutes of untreated VF, basic life support (BLS) with ACD + ITD CPR and mechanical ventilations (30% O2, 70% N2) at a rate of 10 respirations per minute was initiated for all animals. The depth of chest compressions was slowly increased over the first minute until a depth of 20% of the anteroposterior diameter was achieved. All other mechanical CPR parameters were kept constant with a rate of 100 compressions per minute and a compression/decompression duty cycle of 50%. Three minutes after the start of chest compressions, all animals received an intravenous bolus of epinephrine 0.5 mg, 25 mg amiodarone and 50 mEq bicarbonate per protocol. After 4 minutes of CPR, animals in VF confirmed by ECG were defibrillated with up to 8 200-J biphasic shocks with 2 min of CPR interspersed. ROSC was defined as a mean arterial blood pressure >60 mmHg for at least 3 minutes. Animals that needed more than 2 shocks or additional chest compressions were classified as NO ROSC. Further treatment after ROSC was not part of this study.
Figure 1. Experimental Protocol.
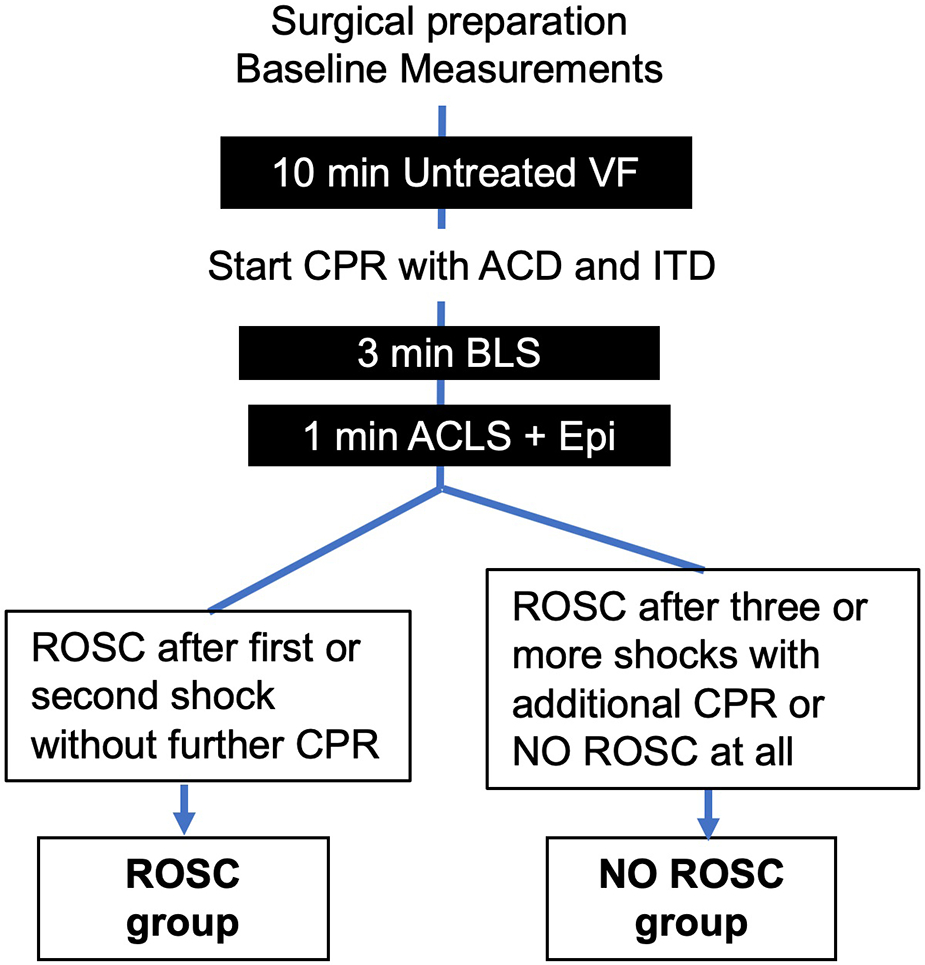
ACD = active compression-decompression; ACLS = advanced life support; BLS = basic life support; CPR = cardiopulmonary resuscitation; CVP = central venous pressure; Epi = epinephrine; ITD = impedance threshold device; ROSC = return of spontaneous circulation; VF = ventricular fibrillation.
Statistical Analysis
The first minute of CPR was excluded from statistical analysis and the 3 minutes before defibrillation were analyzed in 8-second intervals. Unless otherwise indicated, all parametric values are expressed as a mean ± standard error of the mean (SEM), all non-parametric as median (interquartile range). Differences between groups in baseline values were assessed using unpaired Student’s t-test or Mann-Whitney U test, according to parametric vs non-parametric data distribution, respectively.
For continuous hemodynamic data, a repeated-measures analysis of variance (two-way ANOVA) with Bonferroni’s multiple comparison test was employed for group comparisons over 3 minutes. Differences were considered statistically significant when p<0.05 (two-tailed). Receiver operating characteristic (ROC) curves with area-under-the-curve (AUC) analyses were used to prove the ability of CVP-A, etCO2 and CPP to discriminate between ROSC vs. NO ROSC. The Youden index was calculated to determine the cutoff values with the optimal balance of sensitivity and specificity.20 Since we have used multiple test results from the same pig, we adjusted ROC and AUC for intra-cluster correlations by using an additional analysis in R (R Core Team [2021], Vienna, Austria) with a clustered ROC R Package.21-23 All other calculations and graphics were performed using Graphpad Prism (Prism 9, GraphPad Software, LA Jolla, CA, USA).
Results
Nine animals were instrumented without complications. After successful induction of VF and 4 minutes of CPR, all animals were still in VF and defibrillated. Four animals achieved ROSC with 1 shock and another animal with 2 shocks; these animals required no further chest compressions (ROSC group). Two animals achieved ROSC after prolonged resuscitation requiring additional medications and >2 defibrillations, and 2 animals never achieved ROSC after 8 defibrillations (NO ROSC group). There were no significant differences in baseline values between the ROSC and NO-ROSC groups in either group (Table 1), as well as in the measurement of compression depth and force during CPR (supplemental Figure 1).
Table 1.
Baseline values of animals before cardiac arrest
| ROSC group (n=5) |
NO ROSC group (n=4) |
p | |
|---|---|---|---|
| Weight (kg) | 42.1 (40.9, 47.5) | 43.2 (40.8, 44.2) | 0.90 |
| Heart rate (beats/min) | 99.7 ± 4.8 | 98.7 ± 3.7 | 0.88 |
| Mean Arterial Pressure (mmHg) | 87.9 ± 6.9 | 84.6 ± 8.0 | 0.77 |
| Oxygen Saturation (%) | 98.5 (95.0, 99.6) | 99.8 (94.6, 99.9) | 0.28 |
| End-tidal CO2 (mmHg) | 40.0 ± 0.3 | 41.3 ± 2.9 | 0.64 |
All parametric values are mean ± SEM, non-parametric values are median (25th, 75th quartile). ROSC: return of spontaneous circulation.
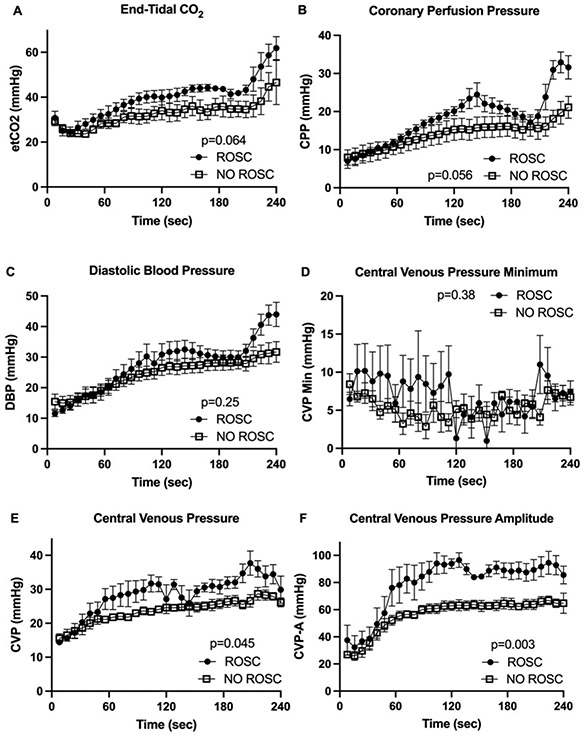
The average of all values over three minutes of CPR showed a significant difference between ROSC and NO ROSC group (Table 2). Further analysis of continuous hemodynamic data by two-way ANOVA revealed that CVP-A (p=0.003) showed a statistically significant discriminating ability between ROSC and NO ROSC in comparison to CVP (p=0.045), CPP (p=0.056), etCO2 (p=0.064), DBP (p=0.250), and CVPMin (p=0.380) (Figure 2).
Table 2.
Hemodynamic measurements averaged over 3 minutes
| ROSC group (n=5) |
NO ROSC group (n=4) |
p | |
|---|---|---|---|
| Central Venous Amplitude (mmHg) | 88.56 ± 1.37 | 62.40 ± 0.74 | <0.001 |
| End-tidal CO2 (mmHg) | 43.19 ± 0.86 | 34.55 ± 0.90 | <0.001 |
| Coronary Perfusion Pressure (mmHg) | 21.32 ± 0.86 | 15.44 ± 0.55 | <0.001 |
| Central Venous Pressure (mmHg) | 30.91 ± 0.66 | 25.03 ± 0.30 | <0.001 |
| Central Venous Pressure Minimum (mmHg) | 6.54 ± 0.63 | 5.06 ± 0.28 | 0.01 |
| Diastolic Blood Pressure (mmHg) | 31.26 ± 0.76 | 26.78 ± 0.54 | 0.002 |
All parametric values are mean ± SEM. ROSC: return of spontaneous circulation.
Figure 2. Hemodynamic Data.
(A) End-tidal CO2 (etCO2), (B) coronary perfusion pressure (CPP), (C) diastolic blood pressure (DBP), (D) central venous pressure minimum (CVPMin), (E) mean central venous pressure (CVP), (F) central venous pressure amplitude (CVP-A) over the 4 min of cardiopulmonary resuscitation (CPR) (mean ± SEM, p values for two-way ANOVA ROSC vs. NO ROSC).
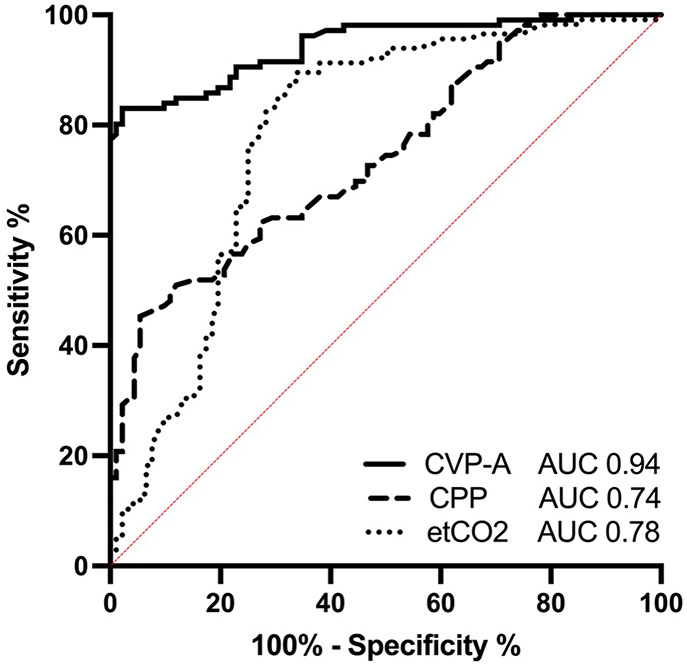
For ROC, the AUC of CVP-A was 0.94 (95% Confidence Interval [CI] 0.86, 1.00) vs. CPP 0.74 (0.54, 0.95) vs. etCO2 0.78 (0.50, 1.00) (Table 3, Figure 3). The optimal threshold to discriminate between ROSC and NO ROSC evaluated with the Youden Index identified a CVP-A >77.2 mmHg (Sensitivity 80.19 [CI 71.60, 86.66] %, Specificity 98.91 [94.10, 99.94] %), etCO2 >33.1 mmHg (Sensitivity 89.57 [82.64, 93.93] %, Specificity 66.30 [56.17, 75.14] %) and CPP >21.1 mmHg (Sensitivity 45.28 [36.14, 54.76] %, Specificity 94.57 [87.90, 97.66] %) to be associated with a higher likelihood of ROSC.
Table 3.
Receiver Operating Characteristic Statistic
| AUC | CI | |
| etCO2 | 0.78 | 0.50, 1.00 |
| CPP | 0.74 | 0.54, 0.95 |
| CVP-A | 0.94 | 0.86, 1.00 |
Receiver operating characteristics (ROC) curves with area-under-the-curve (AUC) analyses were used to compare the ability of end-tidal CO2 (etCO2), coronary perfusion pressure (CPP) and central venous pressure amplitude (CVP-A) to discriminate between ROSC and NO ROSC. CI: 95% Confidence Interval
Figure 3. Receiver Operating Characteristic Curves.
ROC curves evaluating the ability of end-tidal CO2 (etCO2), coronary perfusion pressure (CPP) and central venous pressure amplitude (CVP-A) to discriminate between ROSC and NO ROSC after 3 min of CPR and defibrillation. Area under the curve (AUC) of CVP-A: 0.94, 95% CI: (0.86, 1.00); CPP: 0.74, 95% CI: (0.54, 0.95); etCO2: 0.78, 95% CI: (0.50, 1.00).
Discussion
Our study evaluated the role of CVP-A during chest compressions in a porcine model of CA and ACD + ITD CPR and suggests this variable as a potentially useful marker for predicting ROSC. Compared with other existing markers of blood flow during CPR and predictors of successful defibrillation, we not only introduced CVP-A as a new and at least equivalent parameter but also demonstrated better discriminatory power compared with CVP and etCO2.
We showed that increased CVP-A with values greater than 77 mmHg over 3 minutes of CPR in our model are associated with a higher chance of successful defibrillation and ROSC. In contrast to our study, a high CVP-A during CPR has not always been reported as beneficial during resuscitation from CA. Hilty et al., for example, noted that venous pulsation during chest compressions reflects retrograde venous blood flow in the direction of the brain and periphery.12 In particular, simultaneous arterial and venous pressure towards the brain causes venous congestion with the potential of additional brain injury during CPR.24 Magliocca et al. attributed a higher CVP-A to increased intrathoracic pressure and showed that higher intrathoracic pressure swings, along with a high CVP-A, were associated with lung injury and reduced oxygenation in a CPR swine model;25 this study also revealed that a higher CVP-A (around 50 mmHg) in animals with mechanical CPR (LUCAS® 3.0) was significantly associated with pulmonary edema, increased lung weight, and decreased respiratory compliance compared to a lower CVP-A during manual CPR (around 30 mmHg). In comparison to those findings, our study shows an even higher CVP-A (at least 80 mmHg and higher) using ACD CPR and, importantly, also an ITD to avoid constantly elevated intrathoracic pressures during decompression. Studies using similar forms of CPR have also reported elevated CVP peaks together with a low CVPMin during compression and decompression. Although these studies showed a higher venous return, CVP-A was not calculated or addressed.26-29
Unfortunately, these observations from other studies do not provide a physiological explanation for the improved rate of successful defibrillation with high CVP-A in our study or the adverse effects of high CVP-A in other studies. In addition, we also demonstrated a higher mean CVP in animals with ROSC that had been described as detrimental for defibrillation success in other studies,9,10,30,31 which ascribed worse outcome with insufficient chest recoil and leaning during chest decompressions, in particular, to lead to higher intrathoracic pressures and CVPMin during decompression. This could cause a lower CPP, which is detrimental for defibrillation success.32,33 Since CVPMin was not statistically different between ROSC and NO ROSC, we could conclude that the CPP solely depended on the DBP in our study and that high mean CVP and CVP-A do not influence CPP. This independence of CVP-A to other vital parameters was also seen in a porcine CPR study of Lee et al. who were able to show that changes in right atrial pressure are correlated to the mechanical power and effects of chest compressions and not to the condition of the animal.34
However, the present study still indicates a relation of CVP-A and CPP with potential defibrillation success, since animals of the ROSC group, with a higher CVP-A early on, not only show a trend towards higher CPP and etCO2, but also show higher CPP and DBP values after epinephrine administration. This observation contributes to our hypothesis that higher CVP-A reflects achievement of a critical threshold of blood flow during CPR with ACD and ITD and a higher venous return that leads to an improved effect of epinephrine.
Furthermore, all values of CPP and etCO2 in our study were higher than the cutoff values for ROSC described in other studies, highlighting improved discrimination of CVP-A for predicting ROSC.32,35-39
Limitations
This initial retrospective analysis of experiments with a limited number of animals will need to be confirmed by a prospective study. In particular, the outcome under the different methods of CPR (e.g. standard CPR, ACD only, ITD only) will need to be analyzed to elucidate a possible influence of either of these.
Despite demonstrating significant differences of vital parameters for animals with and without ROSC, this study did not evaluate the cause of those differences in the two groups. Since baseline values and treatment were not different, we can only attribute those findings to anatomic differences causing a different response to the same treatment. Most importantly, the study was performed in young healthy swine and electrically induced VF, in contrast to the typical human CA patient with the burden of cardiovascular and ischemic heart diseases. This study did not investigate short- or longer-term survival or neurological outcome which will require prospective validation.
Conclusion
In this porcine model of CA and high-quality CPR, CVP-A was a potential predictor for successful defibrillation and ROSC and as least as reliable as etCO2 or CPP. Overall, CVP-A could serve as a robust marker for prediction of ROSC with increased venous return and thereby monitor the beneficial effects of ACD and ITD.
Supplementary Material
Supplemental Figure 1 Metrics of Chest Compression Device
(A) Compression Depth (mm) and (B) Compression Force (N) during 4 min of CPR (mean ± SEM).
Acknowledgements
The authors thank Dr. Andreas Leha (Department of Medical Statistics, University of Goettingen, Germany) for statistical consulting on this project and Dr. Franz J. Baudenbacher (Department of Biomedical Engineering, Vanderbilt University, Nashville, Tennessee, USA) and Dr. Robert W. Neumar (Department of Emergency Medicine, University of Michigan, Ann Arbor, Michigan, USA) for their technical advice.
Dr. Balzer was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project BA 6287/1-1. Drs. Yannopoulos, Aufderheide and Riess were supported by NIH grant 5R01 HL123227. Additional unrelated support was provided by institutional funds, a Merit Review Award (I01 BX003482) from the U.S. Department of Veteran Affairs Biomedical Laboratory R&D Service, and a Transformative Project Award (962204) from the American Heart Association awarded to Dr. Riess.
Footnotes
Institutional protocol number 1810-36421A
Conflicts of interest
None
References
- 1.Gräsner J-T, Lefering R, Koster RW, et al. EuReCa ONE—27 Nations, ONE Europe, ONE Registry: A prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366–S468. [DOI] [PubMed] [Google Scholar]
- 4.Lurie KG, Lindo C, Chin J. CPR: the P stands for plumber’s helper. JAMA. 1990;264(13):1661. [DOI] [PubMed] [Google Scholar]
- 5.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. [DOI] [PubMed] [Google Scholar]
- 6.Lurie KG, Voelckel WG, Zielinski T, et al. Improving standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve in a porcine model of cardiac arrest. Anesth Analg. 2001;93(3):649–655. [DOI] [PubMed] [Google Scholar]
- 7.Cohen TJ, Tucker KJ, Lurie KG, et al. Active compression-decompression. A new method of cardiopulmonary resuscitation. Cardiopulmonary Resuscitation Working Group. JAMA. 1992;267(21):2916–2923. [DOI] [PubMed] [Google Scholar]
- 8.Riess ML, Balzer C. Mechanical adjuncts for cardiocerebral resuscitation. Expert Rev Med Devices. 2019;16(9):771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamrick JL, Hamrick JT, Lee JK, Lee BH, Koehler RC, Shaffner DH. Efficacy of chest compressions directed by end-tidal CO2 feedback in a pediatric resuscitation model of basic life support. J Am Heart Assoc. 2014;3(2):e000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niles DE, Sutton RM, Nadkarni VM, et al. Prevalence and hemodynamic effects of leaning during CPR. Resuscitation. 2011;82 Suppl 2:S23–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yannopoulos D, McKnite S, Aufderheide TP, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64(3):363–372. [DOI] [PubMed] [Google Scholar]
- 12.Hilty WM, Hudson PA, Levitt MA, Hall JB. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29(3):331–6–discussion337. [DOI] [PubMed] [Google Scholar]
- 13.Koyama Y, Matsuyama T, Inoue Y. Blood flow forward into the artery and backward into the vein during chest compression in out-of-hospital cardiac arrest. Resuscitation. 2019;137:244–245. [DOI] [PubMed] [Google Scholar]
- 14.Lefevre RJ, Balzer C, Baudenbacher FJ, Riess ML, Hernandez A, Eagle SS. Venous Waveform Analysis Correlates With Echocardiography in Detecting Hypovolemia in a Rat Hemorrhage Model. Semin Cardiothorac Vasc Anesth. 2020;61:1089253220960894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripeckyj A, Kosmopoulos M, Shekar K, et al. Sodium Nitroprusside-Enhanced Cardiopulmonary Resuscitation Improves Blood Flow by Pulmonary Vasodilation Leading to Higher Oxygen Requirements. JACC: Basic to Translational Science. 2020;5(2):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal N, Matsuura T, Caldwell E, et al. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83(11):1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riess ML, Matsuura TR, Bartos JA, et al. Anaesthetic Postconditioning at the Initiation of CPR Improves Myocardial and Mitochondrial Function in a Pig Model of Prolonged Untreated Ventricular Fibrillation. Resuscitation. 2014;85(12):1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otlewski MP, Geddes LA, Pargett M, Babbs CF. Methods for calculating coronary perfusion pressure during CPR. Cardiovasc Eng. 2009;9(3):98–103. [DOI] [PubMed] [Google Scholar]
- 20.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50(3):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53(2):567–578. [PubMed] [Google Scholar]
- 22.Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio. Clustered ROC. R package. https://www.lerner.ccf.org/qhs/software/lib/funcs_clusteredROC.R (accessed on 21 January 2021).
- 23.R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. https://www.R-project.org (accessed on 21 January 2021) [Google Scholar]
- 24.Debaty G, Shin SD, Metzger A, et al. Tilting for perfusion: head-up position during cardiopulmonary resuscitation improves brain flow in a porcine model of cardiac arrest. Resuscitation. 2015;87:38–43. [DOI] [PubMed] [Google Scholar]
- 25.Magliocca A, Rezoagli E, Zani D, et al. Cardiopulmonary Resuscitation-Associated Lung Edema (CRALE) - A Translational Study. Am J Respir Crit Care Med. September 2020:rccm.201912–2454OC–66. [DOI] [PubMed] [Google Scholar]
- 26.Moore JC, Salverda B, Rojas-Salvador C, Lick M, Debaty G, G Lurie K. Controlled sequential elevation of the head and thorax combined with active compression decompression cardiopulmonary resuscitation and an impedance threshold device improves neurological survival in a porcine model of cardiac arrest. Resuscitation. October 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg MT, Olsen J-A, Eriksen M, et al. Haemodynamic outcomes during piston-based mechanical CPR with or without active decompression in a porcine model of cardiac arrest. April 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner H, Madsen Hardig B, Steen S, Sjoberg T, Harnek J, Olivecrona GK. Evaluation of coronary blood flow velocity during cardiac arrest with circulation maintained through mechanical chest compressions in a porcine model. BMC Cardiovasc Disord. 2011;11(1):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debaty G, Moore J, Duhem H, et al. Relationship between hemodynamic parameters and cerebral blood flow during cardiopulmonary resuscitation. Resuscitation. 2020;153:20–27. [DOI] [PubMed] [Google Scholar]
- 30.Yannopoulos D, McKnite S, Aufderheide TP, et al. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64(3):363–372. [DOI] [PubMed] [Google Scholar]
- 31.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary Perfusion Pressure and Return of Spontaneous Circulation after Prolonged Cardiac Arrest. Prehosp Emerg Care. 2009;14(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 34.Lee D-Y, Kang S-M, Choi S-W. Utility of CPR Machine Power and Change in Right Atrial Pressure for Estimating CPR Quality. Sci Rep. 2019:25;9(1):9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paiva EF, Paxton JH, O’Neil BJ. The use of end-tidal carbon dioxide (ETCO2) measurement to guide management of cardiac arrest: A systematic review. Resuscitation. 2018;123:1–7. [DOI] [PubMed] [Google Scholar]
- 36.Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality during both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–154. [DOI] [PubMed] [Google Scholar]
- 37.Javaudin F, Her S, Le Bastard Q, et al. Maximum Value of End-Tidal Carbon Dioxide Concentrations during Resuscitation as an Indicator of Return of Spontaneous Circulation in out-of-Hospital Cardiac Arrest. Prehosp Emerg Care. 2020;24(4):478–484. [DOI] [PubMed] [Google Scholar]
- 38.Chicote B, Aramendi E, Irusta U, Owens P, Daya M, Idris A. Value of capnography to predict defibrillation success in out-of-hospital cardiac arrest. Resuscitation. 2019;138:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RW, French B, Kilbaugh TJ, et al. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation. 2016;104:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Metrics of Chest Compression Device
(A) Compression Depth (mm) and (B) Compression Force (N) during 4 min of CPR (mean ± SEM).