Abstract
Introduction
Cleidocranial dysplasia (CCD) is a rare autosomal dominant skeletal dysplasia that presents with abnormalities in the craniofacial region, teeth, and clavicles and is linked to RUNX2 mutation. Prenatal diagnoses of CCD have rarely been reported, and most of these cases have a positive family history. Here we report two prenatally diagnosed CCD cases without a positive family history. We conducted a literature review to summarize the prenatal sonographic findings of CCD.
Case reports
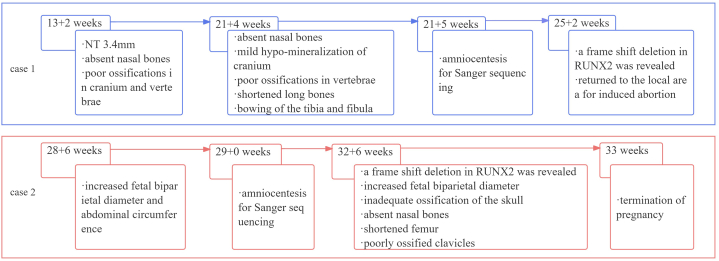
Case 1 (a 26-year-old woman): ultrasound at 13 weeks showed a thickened nuchal translucency with absent nasal bones and poor ossifications in the cranium and vertebrae. Genetic testing confirmed a frame shift deletion of RUNX2. Case 2 (a 27-year-old woman): ultrasound at 32 weeks showed potential fetal skeletal dysplasia, with inadequate skull ossification, mild ossified bilateral clavicles, and RUNX2 frameshift deletion mutation. Both cases were diagnosed with CCD and the parents chose pregnancy termination.
Conclusion
These cases underscore the importance of sonographic examination for prenatal CCD diagnosis with a negative family history. By reviewing previous cases, we concluded that combining NB hypoplasia, clavicle and skull hypoplasia, and shortened long bones may be effective for early screening for CCD. Prenatal diagnosis is crucial for guiding medical decisions.
Keywords: Cleidocranial dysplasia, Nasal bone hypoplasia, Prenatal ultrasound, Case report
1. Introduction
Cleidocranial dysplasia (CCD) is a rare autosomal dominant skeletal dysplasia. The incidence of CCD is estimated to be 1 in 1,000,000 individuals [1]. CCD is often linked to haploinsufficiency or loss-of-function mutations in the RUNX2 gene, located on chromosome 6 at 6p21 [1]. CCD is marked by generalized dysplasia of osseous tissue, particularly in membranous bones like those in the craniofacial region, nasal bones, and clavicles. Patients with CCD show a short stature, poor ossification of affected bones, and a distinctive appearance. CCD is diagnosed on the basis of classic clinical and radiographic findings or confirmation with genetic testing [2]. Prenatal diagnoses have rarely been reported, and most of these cases have a positive family history. Here we report two cases of CCD that were diagnosed prenatally and had a negative family history. We further conducted a literature review to summarize the prenatal sonographic findings of CCD.
2. Case presentation
2.1. Case 1
A 26-year-old Chinese woman, gravida 2, para 0, was referred to our institution for further evaluation due to thickened nuchal translucency (NT) and indistinct fetal nasal bones on the initial ultrasound. The patient had experienced spontaneous abortion during the first pregnancy at 45 days. The current pregnancy was not associated with any maternal complications. There was no pertinent family history in both parents and other family members. The physical examination of the parents was normal with normal shoulder mobility. Previous X-rays showed no skeletal abnormalities. There were no abnormalities in the development of the teeth or in the time of closure of the fontanel.
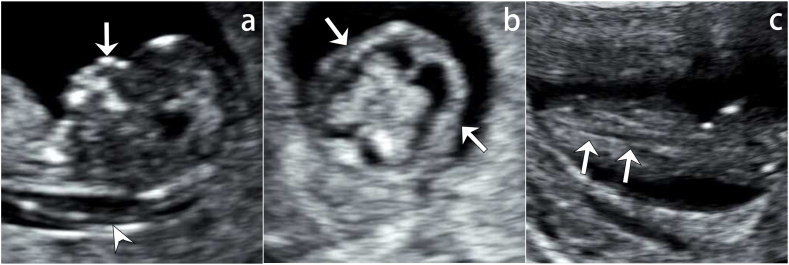
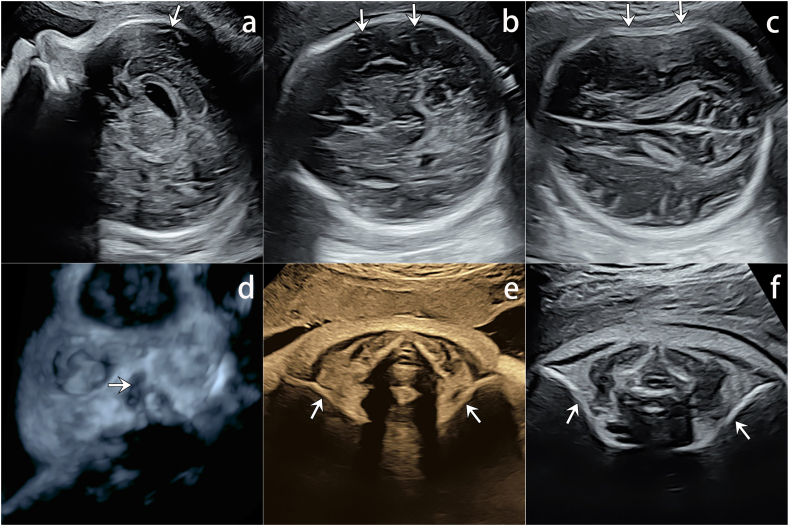
The scan performed at 13 weeks 2 days showed appropriate biometric measurements with thickened NT of 3.4 mm and absence of the nasal bones (Fig. 1a). Additionally, poor ossifications in cranium and vertebrae were observed (Fig. 1b and c). No other anomalies were mentioned.
Fig. 1.
13 weeks 2 days ultrasound scan of Case 1.
a) Sagittal ultrasound shows absent nasal bone and increased nuchal translucency (arrowhead). No echogenic nasal bone below the echogenic skin line (arrow); b) The transverse view showing poorly displayed cranial halo (arrows), suggesting poorly ossified cranium; c) Poorly ossified vertebral spine (arrows) is easily seen as echo-poor, nearly black vertebral bodies.
A subsequent scan was performed at 21 weeks 4 days, but the biometry estimated the gestation at 20 weeks 4 days. While the nasal bones remained unidentifiable, the ossification of the cranium had slightly improved, with mild hypo-mineralization (Fig. 2a and b). The vertebrae remained poorly ossified, especially in the lumbosacral and coccygeal regions (Fig. 2c and d, Videoclip S1). Both the femur length and humerus length were below the tenth percentile, and bowing of the tibia and fibula was observed (Fig. 2e and f, Videoclip S2). Fetal echocardiography was performed, and it showed negative findings.
Fig. 2.
21 weeks 4 days ultrasound scan of Case 1.
a) The profile view in case 1 showed an absent nasal bone. There is no echogenic nasal bone below the echogenic skin line (arrow); b) Cranial halos can be better recognized but cerebral structures (arrows) of the nearfield could be easily identified, suggesting hypo-mineralized cranium; c) Sagittal view of vertebrae. Compared with the thoracic vertebrae, the vertebrae of the lumbosacral segment are smaller and farther apart, especially the L1 vertebrae (arrows), suggesting poor ossification; d) 3D skeletal views show small, irregularly lumbosacral and coccygeal vertebrae, especially the L1 vertebrae (arrows); e-f) long-axis view of left and right tibia and fibula showed congenital bowing.
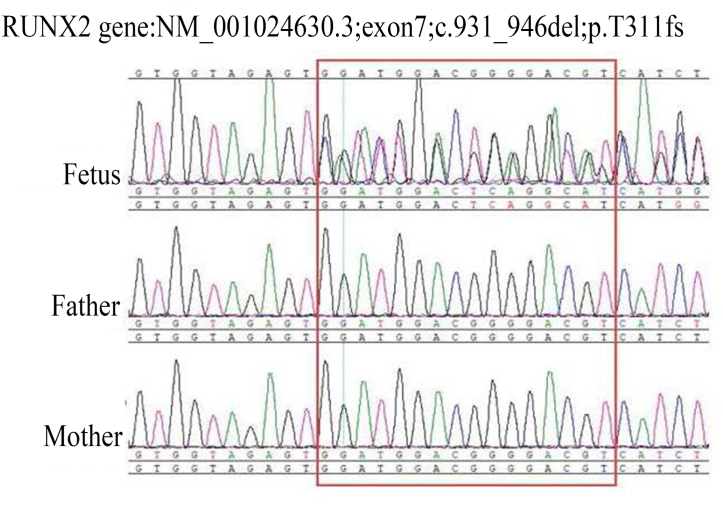
The abnormal sonographic findings prompted further genetic testing. Amniocentesis was performed, and the specimen and peripheral blood samples of the parents were subjected to Sanger sequencing. The results revealed a frame shift deletion in RUNX2 on chromosome 6p21 in the fetus (Fig. 3). CCD was diagnosed based on the sonographic findings and genetic information. The parents consulted with the obstetrician about the disease. The fact is that most individuals are mentally healthy, and their life expectancy is not markedly shortened, but skeleton and dental abnormalities may occur, and the parents requested pregnancy termination. Due to personal reasons, the patients returned to the local area for induced abortion. However, the request to perform postpartum autopsy and imaging studies was denied by the parents.
Fig. 3.
Genome sequencing of parental peripheral blood and amniotic fluid. Red box region shows frame shift deletion of RUNX2 mapped to chromosome 6p21 of the fetus.
Since the fetus was affected with a de novo mutation, the risk of recurrence is low, but we still recommended prenatal counseling if the parents were planning for another pregnancy. The parents concurred with this recommendation.
2.2. Case 2
A 27-year-old Chinese woman, gravida 2, para 1, was referred for further evaluation at 32 weeks due to potential fetal skeletal dysplasia. She had an uncomplicated vaginal delivery to a boy 10 years ago before the second pregnancy. She was otherwise healthy and reported no significant medical history. Both parents reported no family history of genetic diseases or congenital anomalies. There were also no abnormalities on the parents’ physical exams, X-rays, or dental information.
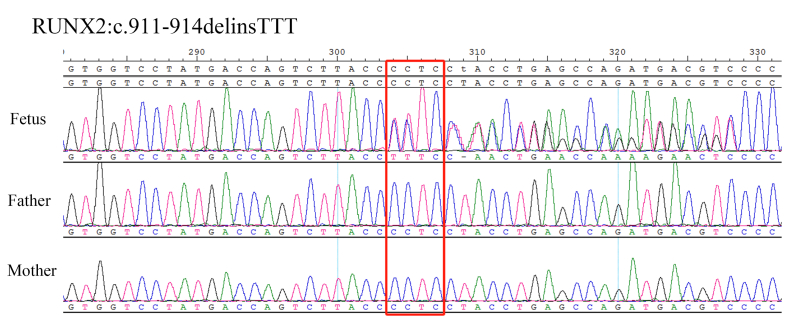
Due to an increased fetal biparietal diameter (BPD), abdominal circumference (AC), and polyhydramnios found on ultrasound examination at 28 weeks 6 days, Sanger sequencing of the amniotic fluid sample and peripheral blood samples of the parents was performed. A frameshift deletion mutation of RUNX2 was confirmed at 32 weeks (Fig. 4).
Fig. 4.
Genome sequencing of case 2. The red box show the detection of the variant sites. The RUNX2:c.911-914delinsTTT heterozygous mutation was detected in the fetus, while both the father and mother harbored the wild-type RUNX2 gene.
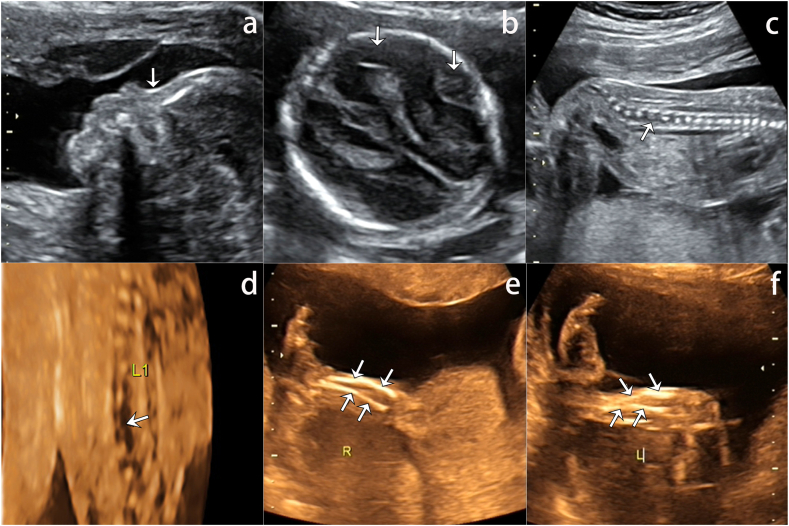
On the 32-week follow-up ultrasound, the BPD was 9.1 cm and the femur length was 5.7 cm. The nasal bones were not prominent (Fig. 5a and d). While the cerebral structures of the near field could be easily identified, skull deformation was observed when light pressure was applied, suggesting inadequate ossification of the skull (Fig. 5b and c, Videoclip S3). Both clavicles were seen; the length of the right clavicle measured 34 mm and the left clavicle measured 36 mm. Additional scanning showed that bilateral clavicles were poorly ossified (Fig. 5e, Videoclip S4). The rest of the fetal anatomy was normal.
Fig. 5.
The ultrasound at 32 weeks 6 days of Case 2. a) The profile view showed an absent nasal bone. The cerebral structures (arrow) below the skull was supposed to be obscured by the acoustic shadow, but is clearly identified; b) The cerebral structures (arrow) below the skull was supposed to be obscured by the acoustic shadow, but is clearly identified; c) Skull deformation upon light pressure (arrows), all suggestive of poor cranial ossification; d) Absent nasal bones (arrow) on 3D skeletal views; e) Bilateral hypoplastic clavicles (arrows), stiff and short when compared with normal ones; f) Normal clavicles in a fetus at 32 weeks 2 days.
CCD was diagnosed based on the sonographic findings and genetic information. After consulting with the obstetrician, the parents chose termination of pregnancy and refused the autopsy. The risk of recurrence in this case was similar to that of case 1 and the parents were recommended for prenatal counseling before the next pregnancy.
The history of the clinical timeline of the two cases is shown in Fig. 6.
Fig. 6.
History of the clinical timeline of the two cases.
3. Discussion
While most CCD cases show autosomal dominant inheritance patterns, fetal exposure to certain environmental factors may also cause de novo mutations in RUNX2 [3]. The signs and symptoms of CCD can vary widely and range in severity [4]. The potential characteristics of individuals with CCD are summarized in Table 1 [1,[5], [6], [7]]. The common presentations of CCD are hypermobility of the shoulder joint, unclosed or delayed closure of the anterior fontanelle, and dental complaints including chewing dysfunction, retained deciduous teeth, supernumerary teeth, delayed eruption of permanent teeth, and malocclusion [3,8].
Table 1.
CCD characteristics.
| Anatomical Location | Potential Characteristics |
|---|---|
| Cranium | Hypo-mineralization of the cranial bones; large fontanelles; wide and delayed closure of the cranial sutures; brachycephalic skull; frontal bossing; Wormian bones |
| Face | Dysplasia nasal bones; loss of paranasal sinuses; cleft palate; high-arched palate; hyperplasia of the mastoid bones; hypoplastic maxilla; mandible prognathous; delayed union or non-union of the mandibular symphysis; micrognathia; hypertelorism; underdeveloped malar and lacrimal bones |
| Clavicle | Unilateral/bilateral hypoplastic and hypo-mineralized clavicles; clavicular pseudoarthrosis |
| Teeth | Delayed eruption and shedding of the deciduous teeth and permanent teeth; malocclusion; supernumerary teeth |
| Thorax | Narrow thorax; cone-shaped thorax; rib hypoplasia |
| Vertebrae | Wedged vertebra; cleft vertebrae; scoliosis/kyphosis; spina bifida |
| Pelvis | Underdeveloped vertical hypoplastic iliac wings; narrow pelvis; wide pubic symphysis |
| Limb | Shortened long bones; unequal size of metacarpals; lengthened second metacarpal; short middle phalanges and terminal phalanges; short broad thumbs; flatfoot |
| Other | Increased nuchal translucency thickness; small/poor ossification of scapulae; deformed neck of the femur; fractures |
The possible underlying mechanism of CCD is haploinsufficiency or loss-of-function mutation of the gene encoding the osteoblast-specific transcription factor RUNX2/CBFA1 located at chromosome 6 at 6p21 [5]. RUNX2 has been shown to be associated with the differentiation of osteoblasts and skeletal and dental lamina proliferation [8,9]. Over 90 different RUNX2 mutations have been reported to be associated with CCD, including insertions, deletions, nonsense, and translocations. However, RUNX2 mutations are undetectable in 20–30 % of patients. Thus, the evaluation of RUNX2 mutation should be combined with a review of clinical symptoms and radiological findings to confirm a diagnosis of CCD. While post-natal physical findings might reveal classic signs of CCD, the prenatal diagnosis of the disease is mainly based on clavicular and cranium hypoplasia and is definitely challenging [[10], [11], [12]]. Thus, understanding the sonographic characteristics of CCD plays a vital role in prompting appropriate further evaluation and management plans [13,14]. Given the autosomal dominant inheritance pattern of CCD, a positive family history can be helpful to guide a more detailed prenatal ultrasound examination of musculoskeletal system. However, the two current cases both had a negative family history, posing challenges to the differential diagnoses.
For case 1, the sonographer had more than 10 years of obstetric ultrasound experience, but she did not assess the clavicles. The clavicle is the first bone to ossify with two primary ossification centers at approximately 5–6 weeks of gestation [15]. Abnormal development of clavicles is a major feature of CCD and can manifest as complete absence of both clavicles or a small unilateral defect [14]. Notably, routine detailed ultrasound examination of fetal clavicles is not standard practice, making clavicle abnormalities easy to be missed [16]. Many sonographers are not familiar with the prenatal ultrasonography of clavicles, including the length, shape, and ossification characteristics. Therefore, it is challenging for sonographers to diagnose CCD through poorly ossified clavicles, especially in patients with a negative family history.
Another important prenatal finding of CCD is the poorly ossified fetal skull, which can present with clear display of the near field cerebral structures, deformation of the skull, Wormian bones, irregular bone contours, and widening of the sutures [17]. Stewart et al. reported a case of CCD diagnosed prenatally; the authors noticed a significant skull developmental delay as well as poor ossifications [12]. Hermann et al. also mentioned large fontanelles in their case, indicating possible underlying insufficient cranial ossification [18]. Similarly, both of our cases showed skull changes and signs of poor ossifications, implying that impaired skull ossification should be taken into consideration when making a prenatal diagnosis of CCD.
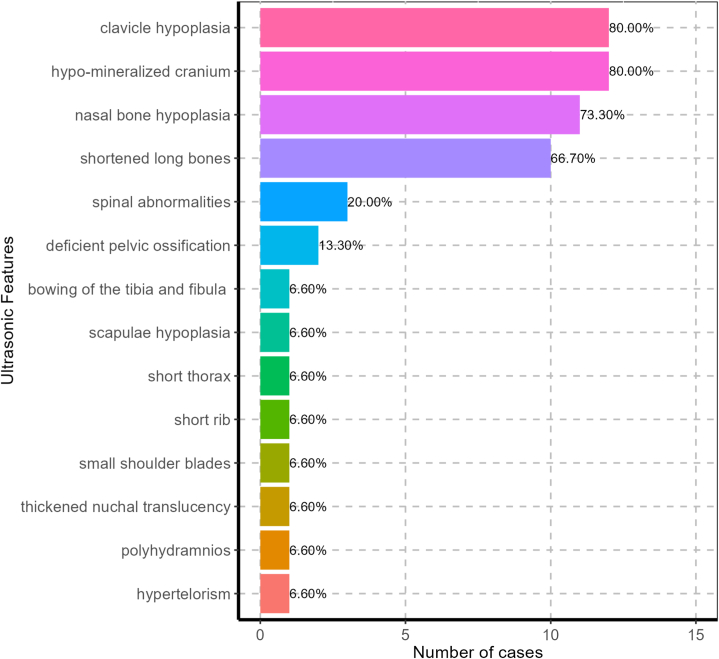
To better understand the prenatal sonographic findings of CCD, we conducted a comprehensive literature search using PubMed, Web of Science, and Springer databases. The keywords used were cleidocranial dysplasia ± cleidocranial dysostosis and prenatal; a total of 332 studies were initially identified. After review, 13 studies associated with prenatal ultrasound findings and images were summarized. The two current cases from our center were included, leading to a total of 15 cases for analysis. The average gestational age for ultrasound examination was 20.3 ± 6.0 weeks, with the earliest one of 13 weeks 3 days and the latest one at 37 gestational weeks. The prenatal sonographic manifestations are summarized in Table 2. Clavicle hypoplasia and skull hypo-mineralization were the most common manifestations on prenatal ultrasound and were observed in 12 (80.0 %) patients. Furthermore, 11 (73.3 %) patients had nasal bone hypoplasia and 10 (66.7 %) showed shortened long bones (Fig. 7). It is worth noting that first-trimester sonographic assessment of the NB was introduced into clinical practice for more than 20 years. This structure is conventionally observed by prenatal US and is visible sonographically in 99.8 % of fetuses as early as 10–14 weeks [19]. Once nasal bone hypoplasia is detected early in pregnancy, a comprehensive examination of the skull, clavicles, and long bones should be conducted when evaluating the possibility of CCD via ultrasound. Thus, the prenatal diagnostic rate of CCD may potentially be improved.
Table 2.
Prenatal sonographic findings and summary of previously reported cases and the cases in the current report.
| Number | Publication year | Journal | Author | Title | Paper type | GA at diagnosis (weeks) | Skull | Clavicle | Nasal bone | Long bone | Others | Family history | RUNX2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1993 | Obstetrics and Gynecology | Hamner LH 3rd | Prenatal diagnosis of cleidocranial dysostosis | case report | 15 + 4 | NR | left clavicle normal, right clavicle absent | NR | NR | NR | + | NR |
| 20 + 5, 30 | left clavicle normal, right clavicle short and poorly mineralized | ||||||||||||
| 2 | 2002 | Ultrasound in Obstet Gyne | Paladini D | Cleidocranial dysostosis Prenatal ultrasound diagnosis of a late onset | letters to the editor | 37 | hypo-mineralization cranial bones, unusually obvious intracranial structure, parietal bone deformation under pressure, fronto-parietal bossing | moderately hypoplastic | low nasal bridge | 22 weeks normal, 30 weeks on the 5th centile, 32 and 37 weeks below 5th centile | NR | + | NR |
| 3 | 2002 | Ultrasound in Obstet Gyne | Stewart PA | Early prenatal ultrasound diagnosis of cleidocranial dysplasia | case report | 14 + 4, 21 + 2 | less well ossified | short and hypoplastic | NR | normal | NR | + | NR |
| 4 | 2003 | Ultrasound in Obstet Gyne | Winer N | Prenatal diagnosis of a cleidocranial dysplasia-like phenotype associated with a de novo balanced t(2q; 6q)(q36; q16) translocation | case report, letters to the editor | 24 | very poor ossification of the frontal and parietal bones, fronto-parieto-occipital bossing, brachycephaly | poor ossification | low nasal bridge | below the 3rd centile |
poor ossification of the scapulae, short thorax, coronal cleft vertebrae, deficient pelvic ossification with hypoplasia of the iliac wings |
– | + |
| 5 | 2004 | Prenatal Diagnosis | Chen CP | Second-trimester nasal bone hypoplasia/aplasia associated with cleidocranial dysplasia | letters to the editor | 20, 22, 31 | NR | NR | absent | at the 10th centile | NR | – | NR |
| 6 | 2006 | Ultrasound in Obstet Gyne | Soto E | Three-dimensional ultrasound in the prenatal diagnosis of cleidocranial dysplasia associated with B-cell immunodeficiency | case report | 18 + 3 | hypo-mineralization skull bones, well defined brain structures in the nearfield, absence of the squamous portion of the temporal bone, poor ossification of the occipital bone | NR | absent | normal | NR | – | – |
| 21 + 3 | Right clavicle fractured | + | |||||||||||
| 7 | 2008 | Fetal Diagnosis and Therapy | Hove HD | An Echo-Poor Spine at 13 Weeks: An Early Sign of Cleidocranial Dysplasia | case report | 13 + 6 | severe delay in calvarial ossification | the clavicles were barely seen, lacking the typical S shape | NR | short femurs | severely delayed ossification of the vertebral spine | + | NR |
| 8 | 2009 | Fetal Diagnosis and Therapy | Hermann NV | Prenatal 3D Ultrasound Diagnostics in Cleidocranial Dysplasia | case report | 15 + 4, 20 + 5, 24 + 5 | severe delay in calvarial ossification, large fontanelles | short and without the typical S form | absent | at the 5th centile | normal NT thickness | + | NR |
| 9 | 2017 | Ultrasound in Obstet Gyne | Waelti S | Absent nasal bone without other features of Trisomy 21 in pregnant Caucasian women: check the clavicles for cleidocranial dysplasia | conference paper | 24 | fontanelles were large with wide sutures, the brain parenchyma was unusually clearly visible | hypoplastic | absent | normal | NR | + | + |
| 10 | 2019 | Chin J Med Genet | Jiao ZH | Prenatal diagnosis for two families affected cleidocranial dysplasia due to novel RUNY2 variants | sporadic case | 24 | hypo-mineralization skull bones | NR | NR | at the 3rd centile | NR | + | + |
| 11 | 2021 | Ultrasound in Obstet | Delgado, TB | Pregnant women and fetus affected by cleidocranial dysplasia: a case report | conference paper | 20 | widening of the coronal suture, a large anterior fontanelle, a prominent forehead, brachycephaly | hypoplastic clavicles with agenesis at the acromial extremity |
absent | normal | – | + | NR |
| 33 + 5 | mild polyhydramnios | ||||||||||||
| 12 | 2022 | J. Clin. Med | Moczulska H | Fetal Nasal Bone Hypoplasia in the Second Trimester as a Marker of Multiple Genetic Syndromes | sporadic case | 19 | NR | significantly shortened | hypoplasia | shortening of long bones | hypertelorism, small shoulder blades | NR | + |
| 13 | 2022 | Prenatal Diagnosis | Dufke A | A single center experience of prenatal parent-fetus trio exome sequencing for pregnancies with congenital anomalies | sporadic case | 23 | frontal bossing, enlarged suturae | bilateral short claviculae | absent | short femurs | short ribs | NR | + |
| 14 | 2023 | Case 1 (current report) | case report | 13 + 3 | hypo-mineralization skull bones, well demonstrated cerebral structures of the near field | NR | absent | normal | poor ossification of spine and pelvis, thickened nuchal translucency, congenital bowing of the tibia and fibula | – | + | ||
| 20 + 4 | below the 3rd centile | ||||||||||||
| 15 | 2023 | Case 2 (current report) | case report | 32 + 3 | hypo-mineralization skull bones, clearly identified cerebral structures of the near field, skull deformation, prominent parietal and frontal bones | short and hypoplastic | absent | below the 10th centile | – | – | + |
Fig. 7.
The prenatal US manifestations of 15 CCD cases.
The management plan for a pregnancy with a confirmed fetal diagnosis of CCD can vary; some individuals choose to continue pregnancy and delivery, and some opt for termination. Several cases of CCD with neonatal intracranial hemorrhage (IH) have been reported in the literature; all the cases followed a spontaneous vaginal delivery [6]. Clinical symptoms of IH in patients with CCD were heterogeneous, and poor outcomes were observed in some cases. Notably, family history was unknown before delivery in half of the cases. That's one of the reasons why exploring more effective ways for prenatal diagnosis of cases with negative family histories is important. In terms of delivery plans, a clear prenatal diagnosis could be helpful in the management of the mode of delivery to reduce the risks of intracranial hemorrhage via vaginal delivery. For individuals who choose termination, adequate patient education and genetic counseling should be offered to help the parents better understand the medical decisions and prognoses.
4. Conclusion
This report discusses two cases of prenatal diagnosis of CCD with a negative family history. Since it is challenging for sonographers to diagnose CCD through poorly ossified clavicles, especially in patients without a positive family history, the literature review emphasized the significance of comprehensive prenatal evaluations involving nasal bones, the skull, clavicles and long bones when suspecting CCD. Once nasal bone hypoplasia is detected early in pregnancy, it is important to bear in mind the evaluation of the other three findings, as this may enhance the prenatal diagnostic rate of CCD. However, due to the small sample size and retrospective nature of the analysis, further validation is needed.
Ethics statement
This study was approved by the ethics committee of the Third Affiliated Hospital of Zhengzhou University. Prior to the examination, the parents of the cases involved in the study provided written informed consent for the use of the relevant clinical data for research purposes. All study methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Written consent for publication was obtained from the parents. There are no identifying images or other personal or clinical details of the patients that compromise anonymity in this manuscript.
Data availability statement
The data of the study will be available upon reasonable request after the approval of the corresponding author (Hezhou Li, email: lihezhou67@126.com).
Funding
This work was supported by the Promotion Project of Henan Appropriate Medical Technology (SYJS2020080) and the Joint Construction Project of Henan Medical Education Research (Wjlx2020091).
CRediT authorship contribution statement
Ruizheng Han: Writing – original draft, Formal analysis. Chunshuang Zhang: Visualization, Data curation. Xiling Fu: Visualization, Data curation. Zhengfeng Zhu: Visualization, Formal analysis. Xinxia Wang: Writing – review & editing, Visualization, Conceptualization. Hezhou Li: Writing – review & editing, Visualization, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Medjaden Inc. for its assistance in the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29816.
Contributor Information
Xinxia Wang, Email: wangxinxia83@126.com.
Hezhou Li, Email: lihezhou67@126.com.
List of abbreviations
- CCD
cleidocranial dysplasia
- NT
nuchal translucency
- NB
nasal bone
- US
ultrasound
- IH
intracranial hemorrhage
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Tsuji M., Suzuki H., Suzuki S., Moriyama K. Three-dimensional evaluation of morphology and position of impacted supernumerary teeth in cases of cleidocranial dysplasia. Congenit Anom (Kyoto) 2020;60:106–114. doi: 10.1111/cga.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machol K., Mendoza-Londono R., Lee B. In: GeneReviews(®). Seattle (WA): University of Washington, Seattle Copyright © 1993-2024, University of Washington, Seattle. Adam M.P., Feldman J., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., et al., editors. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.; 1993. Cleidocranial dysplasia spectrum disorder. [Google Scholar]
- 3.Mundlos S., Otto F., Mundlos C., Mulliken J.B., Aylsworth A.S., Albright S., Lindhout D., Cole W.G., Henn W., Knoll J.H., Owen M.J., Mertelsmann R., Zabel B.U., Olsen B.R. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 4.Nawalkha P.L., Saxena M.N., Sharda P., Sharda D.C. Cleidocranial dysostosis. A report of three cases. Indian J. Pediatr. 1969;36:175–177. doi: 10.1007/bf02749326. [DOI] [PubMed] [Google Scholar]
- 5.Pan C.Y., Tseng Y.C., Lan T.H., Chang H.P. Craniofacial features of cleidocranial dysplasia. J. Dent. Sci. 2017;12:313–318. doi: 10.1016/j.jds.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagasaka S., Suzuki K., Saito T., Tanaka K., Yamamoto J. Posterior fossa subdural hematoma in a neonate with cleidocranial dysostosis after a spontaneous vaginal delivery: a case report. Childs Nerv Syst. 2021;37:683–686. doi: 10.1007/s00381-020-04689-1. [DOI] [PubMed] [Google Scholar]
- 7.Farrow E., Nicot R., Wiss A., Laborde A., Ferri J. Cleidocranial dysplasia: a review of clinical, radiological, genetic implications and a guidelines proposal. J. Craniofac. Surg. 2018;29:382–389. doi: 10.1097/scs.0000000000004200. [DOI] [PubMed] [Google Scholar]
- 8.Dinçsoy Bir F., Dinçkan N., Güven Y., Baş F., Altunoğlu U., Kuvvetli S.S., Poyrazoğlu Ş., Toksoy G., Kayserili H., Uyguner Z.O. Cleidocranial dysplasia: clinical, endocrinologic and molecular findings in 15 patients from 11 families. Eur. J. Med. Genet. 2017;60:163–168. doi: 10.1016/j.ejmg.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 9.S. A . 2006. Genetic and Functional Characterization of RUNX2. PhD Dissertation. [Google Scholar]
- 10.Ayub N., Hamzah S.H., Ahmad M.S., Hussein A.S., Rajali A. A case report of cleidocranial dysplasia: a noninvasive approach. Spec Care Dentist. 2021;41:111–117. doi: 10.1111/scd.12532. [DOI] [PubMed] [Google Scholar]
- 11.Paladini D., Lamberti A., Agangi A., Martinelli P., dysostosis Cleidocranial. Prenatal ultrasound diagnosis of a late onset form. Ultrasound Obstet. Gynecol. 2000;16:100–101. doi: 10.1046/j.1469-0705.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- 12.Stewart P.A., Wallerstein R., Moran E., Lee M.J. Early prenatal ultrasound diagnosis of cleidocranial dysplasia. Ultrasound Obstet. Gynecol. 2000;15:154–156. doi: 10.1046/j.1469-0705.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.Noel A.E., Brown R.N. Advances in evaluating the fetal skeleton. Int J Womens Health. 2014;6:489–500. doi: 10.2147/ijwh.s47073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyer C.E., Tatevosyants N.G., Cortez S.C., Hornstein A., Wallach M. Cleidocranial dysplasia with neonatal death due to central nervous system injury in utero: case report and literature review. Pediatr. Dev. Pathol. 1998;1:314–318. doi: 10.1007/s100249900045. [DOI] [PubMed] [Google Scholar]
- 15.Dey D., Ghosh S.K., Raja S.W., Dolui K.B., Ak Evaluation of fetal clavicular length as a sonological parameter for the estimation of gestational age. Sch J App Med Sci. 2021;9 [Google Scholar]
- 16.Sherer D.M., Sokolovski M., Dalloul M., Khoury-Collado F., Osho J.A., Lamarque M.D., Abulafia O. Fetal clavicle length throughout gestation: a nomogram. Ultrasound Obstet. Gynecol. 2006;27:306–310. doi: 10.1002/uog.2706. [DOI] [PubMed] [Google Scholar]
- 17.Pekçevik Y., Hasbay E., Pekçevik R. Three-dimensional CT imaging in pediatric calvarial pathologies. Diagn Interv Radiol. 2013;19:488–494. doi: 10.5152/dir.2013.13140. [DOI] [PubMed] [Google Scholar]
- 18.Hermann N.V., Hove H.D., Jørgensen C., Larsen P., Darvann T.A., Kreiborg S., Sundberg K. Prenatal 3D ultrasound diagnostics in cleidocranial dysplasia. Fetal Diagn. Ther. 2009;25:36–39. doi: 10.1159/000195634. [DOI] [PubMed] [Google Scholar]
- 19.Cicero S., Curcio P., Papageorghiou A., Sonek J., Nicolaides K. Absence of nasal bone in fetuses with trisomy 21 at 11-14 weeks of gestation: an observational study. Lancet. 2001;358:1665–1667. doi: 10.1016/s0140-6736(01)06709-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the study will be available upon reasonable request after the approval of the corresponding author (Hezhou Li, email: lihezhou67@126.com).