Abstract
DNA replication in Escherichia coli starts with loading of the replicative helicase, DnaB, onto DNA. This reaction requires the DnaC loader protein, which forms a 6:6 complex with DnaB and opens a channel in the DnaB hexamer through which single-stranded DNA is thought to pass. During replication, replisomes frequently encounter DNA damage and nucleoprotein complexes that can lead to replication fork collapse. Such events require DnaB re-loading onto DNA to allow replication to continue. Replication restart proteins mediate this process by recruiting DnaB6/DnaC6 to abandoned DNA replication forks. Several dnaC mutations that bypass the requirement for replication restart proteins or that block replication restart have been identified in E. coli. To better understand how these DnaC variants function, we have purified and characterized the protein products of several such alleles. Unlike wild-type DnaC, three of the variants (DnaC 809, DnaC 809,820, and DnaC 811) can load DnaB onto replication forks bound by single-stranded DNA-binding protein. DnaC 809 can also load DnaB onto double-stranded DNA. These results suggest that structural changes in the variant DnaB6/DnaC6 complexes expand the range of DNA substrates that can be used for DnaB loading, obviating the need for the existing replication restart pathways. The protein product of dnaC1331, which phenocopies deletion of the priB replication restart gene, blocks loading through the major restart pathway in vitro. Overall, the results of our study highlight the utility of bacterial DnaC variants as tools for probing the regulatory mechanisms that govern replicative helicase loading.
Keywords: ATPase, AAA+ ATPase, DNA, DnaB, DNA helicase, DnaC, DNA helicase loader, DNA replication, DNA replication restart
In bacteria, DNA replication begins with DnaA binding to and melting the origin of replication (1, 2). DnaA then recruits the replicative helicase (DnaB in Gram-negative bacteria) to the origin and DnaB is loaded onto each strand of the single-stranded DNA (ssDNA) bubble (3). DnaB exists as a closed ring-shaped hexamer and ssDNA must bind in the interior of the ring (4, 5). This topological challenge is overcome by a loading factor, DnaC in Escherichia coli, that binds to DnaB and promotes loading (6, 7, 8).
DnaC binding to DnaB causes a large structural rearrangement of the helicase that is thought to be important for loading onto ssDNA. DnaC is comprised of an N-terminal DnaB helicase-binding domain and a C-terminal AAA+ ATPase domain (Fig. 1A) (7). DnaC assembles onto DnaB to form a DnaB6/DnaC6 complex (Fig. 1B) (9). Upon DnaC binding, DnaB’s C-terminal helicase domains adopt a spiral shape within the hexamer, breaking the planar arrangement observed in apo-DnaB structures whereas DnaB’s N-terminal domains lock into a constricted conformation (9). Importantly, DnaC binding also produces an opening, or crack, in the DnaB hexamer that is thought to provide an entry point for ssDNA to load into the interior of the hexameric helicase. After ssDNA loading, DnaC hydrolyzes ATP, triggering DnaC dissociation and closure of the DnaB hexamer around ssDNA (9, 10). DnaB is then competent to unwind parental DNA and recruit other components of the cellular replication machinery (replisome), which begin bidirectional replication around the chromosome (11, 12, 13).
Figure 1.
DnaC mutations map to its AAA+ ATPase domain.A, domain layout of DnaB and DnaC. DnaB contains an N-terminal domain (NTD, blue) and a C-terminal helicase domain (purple) connected by a linker helix (LH). DnaC contains an N-terminal helicase-binding domain (HBD) and a AAA+ ATPase domain. B, structure of DnaB6/DnaC6 bound to ssDNA (9). The DnaC variant sites examined here are mapped onto the AAA+ ATPase domain (insets).
Replisomes routinely encounter impediments such as tightly bound nucleoprotein complexes or damaged DNA that can stall replication or, in severe cases, prematurely eject the replisome from DNA (14, 15). The latter events are frequent in bacteria—it is estimated that each replisome in E. coli synthesizes only a fraction of the chromosome before encountering an impediment that causes it to dissociate (15, 16, 17, 18). Because DnaA loads DnaB in a sequence-dependent manner at the origin of replication, these occurrences require reloading of DnaB by a distinct process mediated by replication restart proteins (RRPs, PriA, PriB, PriC, and DnaT in E. coli) (16). RRPs bind replication forks in a structure-specific manner, remodel the replication fork to expose lagging-strand ssDNA, and recruit DnaB6/DnaC6 to load DnaB onto the lagging strand. Remodeling occurs either through unwinding of the lagging strand when it is duplex or exposure of ssDNA from ssDNA-binding proteins (SSBs) that coat lagging-strand ssDNA (17). The mechanisms by which RRPs recruit and load the DnaB6/DnaC6 complex are not well understood.
In E. coli, there are three genetically defined replication restart pathways (18). Two utilize PriA and either PriB or PriC. A third PriA-independent pathway utilizes PriC and the Rep helicase. The PriA/PriB restart pathway is thought to be the most heavily utilized in cells, and PriA-deficient cells exhibit severe phenotypes including slow growth, rich media sensitivity, SOS induction, filamentation, and ultraviolet light sensitivity (19, 20, 21, 22).
Genetic studies have identified several mutations that suppress replication restart defects in E. coli. Intriguingly, these suppressors are missense mutations that map to dnaC (Fig. 1), suggesting that the mutations produce DnaC variants that can bypass the need for RRPs to load DnaB in vivo (22, 23). Understanding how such DnaC variants function could provide insights into the general DnaB loading process.
The protein product of one ΔpriA suppressor, dnaC809, has been studied previously. Unlike wild-type DnaC, DnaC 809 can load DnaB onto SSB-bound ssDNA without the assistance of RRPs (24). The biochemical activities of additional ΔpriA bypass mutant protein products, including DnaC 809,820 (suppresses a ΔpriA ΔpriC mutant lacking all replication restart pathways) and DnaC 811 (suppresses ΔpriA similarly to dnaC809) (22, 23), have not been examined, making it unclear whether observations made with DnaC 809 explain the functions of all dnaC bypass mutants.
In addition to ΔpriA suppressors, other dnaC alleles have been isolated that suppress or mimic mutations in different RRP genes (25). The dnaC824 allele suppresses the filamentation and SOS induction phenotypes observed in ΔpriB Δrep cells but does not suppress ΔpriA phenotypes to the same extent as dnaC bypass alleles found in the ΔpriA background. Alternatively, dnaC1331 is a loss of function allele that phenocopies deletion of the priB gene in vivo. Although a priB deletion on its own does not exhibit severe phenotypes, it cannot be combined with a priC deletion (18). The biochemical consequences of the dnaC1331 mutation have not been investigated.
In this study, we took a biochemical approach to examine the impact of dnaC mutations in five DnaC variants: DnaC 809 (E176G), DnaC 809,820 (E176G, K178N), DnaC 811 (I184F), DnaC 824 (N255Y) and DnaC 1331 (A84T). The first four variants are the products of dnaC missense mutations that suppress a variety of replication restart-deficient backgrounds whereas the fifth, DnaC 1331, interferes with normal replication restart function in vivo. All five variants were purified and shown to retain the ability to bind DnaB and ssDNA, which was expected given that they all function in vivo. Each variant was also able to load DnaB onto a synthetic replication fork with an ssDNA lagging strand in the absence of SSB. Three variants, DnaC 809, DnaC 809,820, and DnaC 824, were found to also load DnaB onto SSB-coated replication forks, an activity that is not supported by wild-type DnaC. DnaC 809 was unique among the variants in displaying an unexpected ability to load DnaB onto replication forks with duplex lagging strand DNA. While DnaB has been shown to translocate across duplex DNA previously (26, 27), its loading onto duplex DNA has not been observed. Finally, DnaC 1331 blocked the loading of DnaB through the PriA-PriB replication restart pathway in vitro, consistent with its ability to phenocopy priB deletion in vivo. The results collectively suggest that dnaC suppressor bypass functions can arise from DnaC variant loading of DnaB onto a broader spectrum of substrates than is supported by wild-type DnaC and provide new insights into the connections between RRPs and DnaB loading.
Results
DnaC variants retain the ability to bind and load DnaB onto a replication fork that does not require remodeling
We took a biochemical approach to examine how sequence changes in DnaC can either allow cells to bypass the need for RRPs to load DnaB onto DNA or inhibit replication restart. Four DnaC variants that suppress a variety of replication restart deficient backgrounds were purified: DnaC 809 (E176G), DnaC 809,820 (E176G, K178N), DnaC 811 (I184F), and DnaC 824 (N225Y). Additionally, DnaC 1331 (A84T), which mimics priB deletion, was purified. Each variant carried one or two substitutions in the C-terminal AAA+ domain of DnaC (Fig. 1).
The variants were first tested for their ability to bind to DnaB and to ssDNA. We predicted that since each of the variants functions in cells, they would retain interactions with DnaB and DNA. Purified DnaC variants were combined with DnaB in a 1.2:1 (DnaC:DnaB) ratio and complexes were resolved via size exclusion chromatography. Consistent with our prediction, each of the DnaC variants coeluted with DnaB, indicating that they interacted with the helicase (Figs. 2A and S1). Some of the DnaB/DnaC variant peaks were shifted to slightly higher retention volumes or to lower peak heights, potentially indicating that these variants dissociate more readily than wild-type DnaC during the non-equilibrium size exclusion experiment or that the stoichiometry is different for some variants. The ssDNA binding activity of each variant was also very similar to wild-type DnaC, with most variants binding as well as wild-type DNA and only DnaC811 displaying slightly weakened binding (Fig. S2). Thus, the DnaC variants retained interactions with DnaB and DNA, indicating that the sequence changes did not significantly alter folding.
Figure 2.
DnaC variants retain the ability to bind DnaB and load DnaB onto free ssDNA.A, SDS-PAGE of peak fractions from analytical size exclusion chromatography with DnaB and each DnaC variant. Retention volumes (RTs) are reported below each gel. B, helicase loading assay with a replication fork containing 60 bp duplex parental DNA and 38 base ssDNA leading- and lagging-strand arms. Formation of unwound product indicates DnaB has been loaded and unwinds the parental duplex. Unwinding was measured three or more times, with the gel representative of multiple experiments. S = substrate and Δ = boiled substrate. C, bars display the mean fraction of DNA unwound from three or more independent experiments, with the values for each measurement shown as points. Error bars represent standard error.
We next measured the abilities of the DnaC variants to load DnaB onto radiolabeled synthetic DNA replication forks. The first substrate examined was the simplest possible replication fork structure with a parental DNA duplex and single-stranded leading and lagging strands that are free from SSB (Fig. 2, B and C, Table S1). Loading activity was assessed by measuring DnaB-dependent unwinding of parental DNA in the replication fork, which is observed via gel electrophoresis. We predicted that each DnaC variant would retain the ability to load DnaB onto this simple replication fork since each can load DnaB in cells. As reported previously (28), DnaB alone unwinds only a small fraction (∼10%) of the substrate, indicating that the protein could load onto a free replication fork with limited efficiency. The inclusion of wild-type DnaC increased DnaB unwinding efficiency to ∼80%, consistent with its known role as a loader protein for DnaB. Each of the five DnaC variants also stimulated DnaB loading, producing similar levels of unwound product (60–80%) to that observed with wild-type DnaC. Therefore, each of the DnaC variants retained DnaB and ssDNA binding and was able to load DnaB onto a simple DNA replication fork.
Several DnaC bypass variants can load DnaB onto replication fork structures with single-stranded lagging strands in the presence of SSB
We next examined whether the DnaC bypass variants could overcome the need for RRPs in vitro as suggested by their abilities in vivo (18, 22, 25). Two scenarios can prevent DnaC-mediated DnaB loading onto lagging stands in cells. When the lagging strand is single-stranded, SSB coats the strand and prevents DnaB loading (24, 29). Alternatively, when the lagging strand is duplex, DnaC is blocked from loading DnaB (26). DNA replication restart pathways can remodel either substrate to allow DnaC-dependent DnaB loading in these situations, and the reactions have been reconstituted with synthetic replication forks in vitro (29, 30). The RRP suppressor phenotypes of dnaC809, dnaC809,820, dnaC811, and dnaC824 suggest that the variants encoded by these alleles may be able to overcome challenges in loading DnaB onto diverse replication fork substrates.
We first examined DnaC-mediated DnaB loading and unwinding of a replication fork with SSB bound to ssDNA leading and lagging strands (Fig. 3). SSB blocked wild-type DnaC-mediated DnaB loading and unwinding of this simple replication fork as expected (Fig. 3A, compare lanes 3 and 4, and 3B). As a positive control, PriC, an RRP that loads DnaB onto replication forks with ssDNA ends (29, 31), was included in the assay, resulting in DnaB loading in the presence of SSB and unwinding of ∼10% of the substrate (Fig. 3A, lane 5, and B).
Figure 3.
DnaC variants load DnaB onto an SSB-coated “simple” replication fork.A, helicase loading assay with replication fork described in Figure 2. The formation of unwound products indicates DnaB has been loaded and unwinds the parental duplex. Unwinding was measured three times, with the gel representative of multiple experiments. S = substrate and Δ = boiled substrate (expected product). B, bars display the mean fraction DNA unwound from three independent experiments, with the values for each measurement shown as points. Error bars represent standard error. N.D. = no product detected.
The DnaB-loading activity of each of the DnaC variants was examined to determine if they had gained the ability to load DnaB onto the SSB-coated simple fork. DnaC 809 efficiently loaded DnaB onto the fork structure in the presence of SSB (Fig. 3A, lane 6, and B). This result is similar to that observed in a prior study (24). Interestingly, DnaC 809,820, and DnaC 811 were also able to load DnaB onto an SSB-coated simple fork at levels similar to that measured with DnaC 809 (Fig. 3A, lanes 7 and 8, and B). Neither DnaC 824 nor DnaC 1331 were capable of loading DnaB onto an SSB-coated simple fork, suggesting these variants do not have the same activity as the other three variants. The lack of activity for DnaC 824 contrasts with its bypass activity in cells, suggesting that the in vitro reloading assay does not fully recapitulate DnaC 824 action in cells or that the bypass mechanism used by DnaC 824 differs from other DnaC variants. DnaC 1331, which is not a bypass variant, was not expected to load DnaB in the presence of SSB.
Next, we measured DnaC-mediated loading of DnaB onto a fork with a nascent leading strand (Fig. 4, Table S1). This substrate mimics replication forks with unprimed lagging strands and D-loop DNA structures that are formed as intermediates in double-strand DNA break repair. The PriA/PriB replication restart pathway, which relies on PriA, PriB, and DnaT proteins, loads DnaB onto such forks (29, 30). As observed with the simple fork, wild-type DnaC loading of DnaB onto the replication fork was blocked by SSB (Fig. 4A, lanes 3 and 4, and B). As a positive control, PriA, PriB, and DnaT were included in the assay, resulting in DnaB loading in the presence of SSB and unwinding of ∼10% of the substrate (Fig. 4A, lane 5, and B). Similar DnaB loading/unwinding levels have been previously reported for the reconstituted PriA/PriB pathway in vitro (29, 32).
Figure 4.
DnaC variants load DnaB onto SSB-coated replication fork with a nascent leading strand.A, helicase loading assay with a replication fork containing 60 bp duplex parental DNA, a 38 base ssDNA lagging strand arm, and a 38 base leading strand arm with a 33 base annealed nascent leading strand (leaving a 5 base ssDNA gap at the fork junction). The formation of unwound product indicates DnaB has been loaded and unwinds the parental duplex. Unwinding was measured three times, with the gel representative of multiple experiments. S = substrate and Δ = boiled substrate (expected product). B, bars display the mean fraction DNA unwound from three independent experiments, with the values for each measurement shown as points. Error bars represent standard error. N.D. = no product detected. C, titrations of DnaC 809,820 (0, 50, 100, 200, 400, 800 nM) and DnaC 811 (0, 50, 100, 200, 400, 800 nM) in the reconstituted reaction. Unwinding was measured three times, with the gel representative of multiple experiments.
Interestingly, the DnaC 809 and DnaC 809,820 bypass variants were able to load DnaB onto this replication fork without the assistance of RRPs (Fig. 4A, lanes 6 and 7, and B). However, DnaC 809,820 loaded DnaB less efficiently than DnaC 809. Titrations of variants were performed to test whether the two proteins loaded DnaB at different optimal DnaC:DnaB ratios. DnaC 809,820 was more efficient at loading DnaB at DnaC concentrations up to 400 nM. At the highest concentration tested (800 nM), DnaC 809,820 loading of DnaB is less efficient (Fig. 4C). DnaC 811 was also able to load DnaB, but only at the highest concentration tested. Wild-type DnaC, DnaC 824, and DnaC 1331 did not load DnaB onto SSB-coated forks with a nascent leading strand under any conditions tested (Fig. S3).
DnaC 809 loads DnaB onto replication forks with dsDNA lagging strands
We next assessed the ability of each DnaC variant to load DnaB onto a synthetic DNA fork that contains a nascent lagging strand. DnaC loads DnaB onto single-stranded lagging strand DNA, positioning DnaB to unwind parental DNA in a 5′ to 3′ direction. To overcome duplex lagging strands in the PriA/PriB replication restart pathway, PriA helicase first unwinds the lagging strand to prepare ssDNA for DnaB loading. PriA then acts with PriB and DnaT to mediate DnaC-dependent DnaB loading onto the replication fork (16). As expected, wild-type DnaC failed to load DnaB onto this replication fork on its own as evidenced by the absence of DnaB-dependent unwinding (Fig. 5A, lane 4). However, the inclusion of PriA, PriB, and DnaT led to the unwinding of the lagging strand by PriA and the unwinding of the parental DNA by DnaB (Fig. 5A, lane 5). Additionally, the inclusion of PriA and PriC allows for PriA unwinding of the lagging strand followed by PriC-mediated DnaB loading, as reported previously (33) (Fig. 5A, lane 6).
Figure 5.
DnaC 809 loads DnaB onto dsDNA.A, helicase loading assay with a replication fork containing 60 bp duplex parental DNA, a 38 base ssDNA leading strand arm, and a 38 bp lagging strand duplex arm. For positive controls, PriA unwinds the nascent lagging strand of the substrate (fork 1) to form fork 2. PriA, PriB, DnaT or PriC then load DnaB onto fork 2 to form fork 4. Fork 3 is formed when DnaC 809 loads DnaB. No helicase is present to unwind the nascent lagging strand, so it remains duplex. S = substrate and Δ = boiled substrate. B, helicase loading assay with a replication fork containing a 60 bp duplex parental DNA, a 38 bp duplex lagging strand arm, and a 38 base leading strand arm with a 33 base annealed nascent leading strand (leaving a 5 base ssDNA gap at the fork junction). For positive controls, PriA unwinds the nascent lagging strand of fork 1 to form fork 2. When PriB and DnaT are also added, DnaB is loaded onto fork 2 to form product 4. C, titrations of DnaC 809 (0, 50, 100, 200, 400, 800 nM) with the replication fork from (B). Unwinding was measured three times, with the gels representative of multiple experiments.
Substitution of the DnaC variants in the assay showed that, unexpectedly, DnaC 809 loads DnaB onto the replication fork in the absence of RRPs (Fig. 5A, lane 7). Parental duplex unwinding occurred without lagging strand unwinding, indicating that DnaC 809 loaded DnaB onto duplex DNA. None of the other DnaC variants were able to load DnaB onto the fork with a nascent lagging strand.
Finally, we assessed the ability of each variant to load DnaB onto a four-stranded replication fork, with both nascent leading and lagging strands. Wild-type DnaC required PriA, PriB, and DnaT to load DnaB onto the four-stranded fork (Fig. 5B, lanes 3 and 5). PriA alone was able to unwind the nascent lagging strand as expected, but this activity was insufficient for loading DnaB by wild-type DnaC (Fig. 5B, lane 4). Consistent with the result observed with the previous replication fork, DnaC 809 was able to load DnaB onto the four-stranded replication fork in the absence of all RRPs, leading to unwinding of the parental duplex without lagging strand DNA winding. This further supports to notion that DnaC 809 can bypass the requirement for an ssDNA lagging strand for DnaB loading, loading the helicase directly onto duplex lagging-strand DNA. At higher DnaC 809:DnaB ratios, DnaC 809 loads DnaB onto the four-stranded replication fork even more efficiently (Fig. 5C). None of the other DnaC variants were capable of loading DnaB onto the four-stranded replication fork (Fig. 5B).
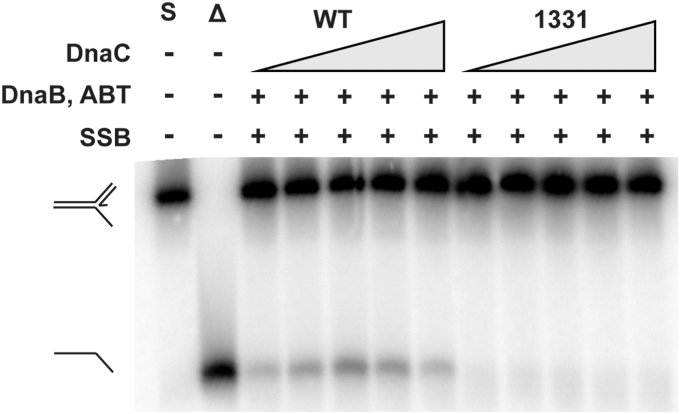
DnaC 1331 blocks PriA/PriB pathway DnaB loading
DnaC 1331 is unique among the DnaC variants in this study in that it is not an RRP deficiency suppressor. Instead, dnaC1331 phenocopies ΔpriB in vivo, suggesting that DnaC 1331 could block the PriA/PriB replication restart pathway (22). DnaC 1331 can support DnaB loading onto ssDNA in the absence of SSB but not when SSB is included (Figs. 2 and 3), which parallels wild-type DnaC activity. To test whether DnaC 1331 alters replication restart, we measured the ability of DnaC 1331 to load DnaB onto an SSB-bound replication fork via the PriA/PriB pathway. With wild-type DnaC in this assay, DnaB loading and unwinding are observed when PriA, PriB, and DnaT are present (Fig. 6, lanes 3–7). However, substituting DnaC 1331 in the assay blocks DnaB loading and unwinding is not observed (Fig. 6, lanes 8–12). Thus, DnaC 1331 impairs loading by the PriA/PriB restart assay in vitro, which parallels dnaC1331 acting as a ΔpriB phenocopy in vivo (25).
Figure 6.
DnaC 1331 blocks DnaB loading through the PriA/B pathway. Helicase loading assay with a fork containing 60 bp duplex parental DNA, a 38 base ssDNA lagging strand arm, and a 38 base leading strand arm with a 33 base annealed nascent leading strand (leaving a 5 base ssDNA gap at the fork junction). Formation of unwound product indicates DnaB has been loaded and unwinds the parental duplex. S = substrate and Δ = boiled substrate. DnaC and DnaC 1331 were titrated (50, 100, 200, 400, 800 nM). “ABT” indicates that PriA, PriB and DnaT have been included. Unwinding was measured three times, with the gel representative of multiple experiments.
Discussion
DnaC loads the replicative helicase, DnaB, onto DNA to initiate replication and replication restart. To better understand how mutations of dnaC impact replication and replication restart, we purified a panel of DnaC variants with altered cellular functions and tested their DnaB loading abilities in vitro. Four of the DnaC variants act as suppressors that can bypass normal DNA replication restart processes in cells whereas a fifth phenocopies deletion of the priB replication restart gene. All five DnaC variants were able to load DnaB onto ssDNA in the absence of SSB. Interestingly, three of the bypass variants, DnaC 809, DnaC 809,820 and DnaC 811, were also able to load DnaB onto SSB-coated replication forks in a reconstituted helicase loading assay, which represents a gain of function relative to wild-type DnaC. DnaC 809 also had the unexpected ability to load DnaB onto replication forks with a duplex lagging strand. These results point to a loosening of substrate fidelity (and broadening use of diverse replication fork substrates) as an important aspect of the DnaC variants’ ability to bypass DNA replication restart pathways in vivo. DnaC 1331 blocks replication restart through the PriA/PriB replication restart pathway, which aligns with the observation that the dnaC1331 allele phenocopies a ΔpriB mutation in vivo. Taken together, our results suggest that DnaC plays important roles in selecting replication fork substrates onto which DnaB can be loaded and that sequence changes in DnaC can alter coordination with RRPs in a manner that allows bypass or blockage of restart in E. coli.
In our analysis, we found that DnaC bypass variants differ in the breadth of their activities, with most loading DnaB onto a wider range of substrates than wild-type DnaC. The results point to possible structural or regulatory impacts of the sequence changes present in the variants. A comparison of DnaC 809 and DnaC 809,820 potentially offers insights into the differences among the DnaC bypass variants. DnaC 811 behaved quite similarly to DnaC 809,820 with respect to the breadth of substrates it can use for DnaB loading, albeit with lower apparent activity levels than DnaC 809,820.
DnaC 809 displayed the broadest activity profile observed among our panel of DnaC variants, loading DnaB onto replication forks with single-stranded, SSB-bound, and duplex lagging-strand DNA. DnaC 809,820 also loads DnaB onto more substrates than wild type DnaC, but it is restricted to ssDNA and SSB/ssDNA lagging strand substrates. dnaC809 was originally isolated in a priA-deficient strain and the mutation suppresses most phenotypes associated with ΔpriA but it requires PriC and Rep in vivo (22, 23). dnaC809,820 was isolated in a ΔpriB ΔpriC dnaC809 strain and can suppress lethality caused by deletion of priA and priC or rep (22, 23). The results presented here show that DnaC 809 and DnaC 809,820 can both load DnaB onto replication forks that can be processed by the PriA/PriB and PriC pathways. PriA/PriB is highly active on substrates with little or no ssDNA gap in the nascent leading strand while PriC operates best on substrates that contain long gaps in the nascent leading strand (29). Also, prior studies have shown that DnaC 809 can bypass the need for RRPs to initiate DNA replication on D-loop DNA structures, which have little or no ssDNA leading strand gaps (34). Consistent with these prior studies, our results suggest that most PriA/PriB and PriC replication forks can serve as substrates for DnaB loading by either DnaC 809 or DnaC 809,820 in vivo.
The dnaC809 mutation encodes for an E176G substitution. E176 lies near the ssDNA entry channel on DnaC, and it is adjacent to the side chain of K178 (the residue altered in dnaC820) in the DnaC/DnaC interface (Fig. 1) (9). Two highly conserved residues adjacent to the DnaC 809 mutation site, S177 and Y179, are directly involved in binding to ssDNA in the DnaB6/DnaC6 structure (9). While we did not detect a change in ssDNA binding affinity with DnaC 809, mutations near the ssDNA channel may alter how DnaB6/DnaC6 loads onto DNA or interacts with various DNA substrates.
The dnaC820 mutation encodes for a K178N substitution. Although decreasing the positive charge near the ssDNA pore doesn’t appear to impact ssDNA affinity when combined with the dnaC809 mutation, it could significantly alter the DnaC/DnaC interface (Fig. 1). These differences may contribute to the lower activity of DnaC 809,820 compared to DnaC 809 on some replication fork substrates examined in our study (Figs. 4 and 5). Why dnaC809,820 suppresses more phenotypes in vivo is a mystery. One possibility is that indiscriminate DnaB loading by DnaC 809 is a liability to cell growth whereas the additional mutation in DnaC 809,820 alleviates some promiscuity making it more advantageous in vivo. This may also explain why dnaC809 is dependent upon priC in vivo while dnaC809,820 is not. Our results suggest that DnaC 809,820 may be better able to recognize proper substrates for DnaB loading than DnaC 809.
DnaB loading is typically restricted to ssDNA, loading onto the lagging strand where it then proceeds in the 5′ to 3′ direction to unwind parental duplex DNA (26, 35, 36). Although DnaB loading onto duplex DNA has never before been observed, DnaB has been shown to be able to translocate over duplexes, which requires the DnaB pore to expand to accommodate duplex DNA (26, 27). We have shown that DnaC 809 can load DnaB onto replication forks that have duplex DNA on the lagging strand. How DnaC 809 does this remains unknown. We speculate that the crack in the DnaB6/DnaC 8096 complex could possibly expand wider than that in wild-type DnaB6/DnaC6 to accommodate duplex DNA. The E176G mutation may lead to greater flexibility in the DnaB6/DnaC6 complex, which expands the DNA entry channel for DnaB loading.
DnaC 824 was not found to bypass RRPs in our reconstituted replication restart assays. This was unexpected since the dnaC824 mutations suppress the phenotypes of ΔpriB priA300 (priA300 encodes an ATPase-deficient PriA missense variant (25, 37)), Δrep priA300, and ΔpriB ΔpriC strains (25, 37)). In the ΔpriB ΔpriC context, all three replication restart pathways are inactive. In contrast to the other dnaC suppressor mutations, dnaC824 can only weakly suppress ΔpriA phenotypes, indicating it retains some dependence upon PriA. However, DnaC 824 remained inactive in our replication restart systems even when PriA was included (and with other replication restart proteins omitted). It is therefore likely that our in vitro system does not entirely recapitulate the reactions stimulated by DnaC 824 in vivo. Moreover, the distinct behavior of DnaC 824 suggests that while most DnaC bypass variants appear to suppress RRP deficiencies by expanding their activity on diverse DNA replication forks, at least one does not. This argues that there are likely multiple physical mechanisms by which RRP bypass can occur.
Finally, the DnaC 1331 variant blocked restart through the PriA/PriB pathway in vitro. DnaC has not been shown to directly interact with PriA, PriB, or DnaT, although DnaB6/DnaC6 most likely interacts with at least one protein within the system to stimulate DnaB loading. It is possible that the DnaC 1331 mutation (A84Y) directly or indirectly alters a surface that is critical for interaction with one of the RRPs in the PriA/PriB pathway and that disruption of this interface blocks replication restart.
Overall, our results show that variants of DnaC derived from genetic studies offer novel insights into the mechanisms that govern replication fork selection by DnaB6/DnaC6 and the interplay between DnaB6/DnaC6 and RRPs. Sequence changes in DnaC confer expanded substrate utilization for DnaB loading, which, in most cases, allows the bypass of RRPs in vivo (37). All the mutations described in our study lie within the C-terminal AAA+ domain of DnaC, pointing to an important role for the domain in regulating DnaB6/DnaC6 interactions with replication fork substrates and RRPs. In addition to offering insights into the general DnaC-mediated DnaB loading process, the DnaC variants described here may prove useful for molecular biology applications in which DnaB loading onto duplex DNA would be beneficial.
Experimental procedures
Protein purification
6XHis-MBP tagged E. coli dnaC and dnaC mutant genes were cloned into pET28b-derived plasmids. Wild-type DnaC was purified as previously described (28). Briefly, DnaC cultures of C43 E. coli cells (Lucigen) carrying the DnaC-encoding plasmids were grown by shaking at 37 °C. DnaC variant expression was induced at the mid-log phase (OD600 ∼0.4) by the addition of 1 mM isopropyl B-D-1-thiogalactopyranoside (IPTG). After induction, cells were grown for an additional 2.5 h. Cultures were then clarified via centrifugation and cell pellets were resuspended in Lysis Buffer supplemented with protease inhibitors (50 mM HEPES-KOH pH 7.5, 1 M KCl, 10% glycerol, 30 mM imidazole, 10 mM MgCl2, 0.1 mM ATP, 1 mM β-mercaptoethanol (BME), 2 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM benzamide, half protease inhibitor tablet (Sigma)). Resuspended cells were stored at −80 °C until purification.
Cells were thawed on ice, lysed by sonication, and clarified via centrifugation. Clarified lysates were loaded onto a 5 ml HisTrap FF crude FPLC column (GE) equilibrated in Lysis Buffer. The column was washed with 10 ml of Wash Buffer (50 mM HEPES-KOH, pH 7.5, 0.5 M KCl, 10% (v/v) glycerol, 20 mM imidazole, 10 mM MgCl2, 0.05 mM ATP, 1 mM BME and protease inhibitors) then DnaC was eluted with 30 ml of Elution Buffer (50 mM HEPES-KOH pH 7.5, 0.5 M KCl, 10% glycerol, 500 mM imidazole, 10 mM MgCl2, 0.1 mM ATP, 1 mM BME and protease inhibitors). The eluate was concentrated to 2 ml and applied to a HiPrep S100 FPLC column (GE) equilibrated in 50 mM HEPES-KOH pH 7.5, 500 mM KCl, 10% glycerol, 10 mM MgCl2, 0.1 mM ATP, 1 mM BME and protease inhibitors). Peak fractions were concentrated to 1 ml and incubated overnight at 4 °C with Tobacco Etch Virus protease overnight to remove the 6XHis-MBP tag. The cleaved protein was diluted with Dilution Buffer (30 mM Tris-HCl pH 7.5, 10% glycerol, 10 mM MgCl2, 0.1 mM ATP, 1 mM BME and protease inhibitors) and loaded onto a HiPrep Q Sepharose fast flow (QFF) FPLC column (GE) equilibrated in QFF buffer (30 mM Tris-HCl pH 7.5, 20 mM KCl, 10% glycerol, 10 mM MgCl2, 0.1 mM ATP, 1 mM BME and protease inhibitors). DnaC was eluted with a linear KCl gradient. Eluted protein was concentrated and dialyzed overnight against storage buffer (20 mM HEPES-KOH pH 7.5, 500 mM KCl, and 50% glycerol).
For the DnaC variants, overexpression led to significantly slowed cell growth. To overcome this problem, a strategy was used to target the variants to the periplasm. Wild-type dnaC was cloned as a 6XHis-MBP fusion with an N-terminal periplasmic localization signal and the dnaC point mutations were introduced via site-directed mutagenesis. All constructs were sequenced to verify the mutations. Variants were expressed in arabinose-inducible BL21 cells by induction at mid-log phase (OD600 ∼0.6) with 0.5 mM IPTG and 0.1% arabinose overnight at 16 °C. Purification followed the wild-type DnaC protocol, except that an additional maltose affinity gravity column was used to further separate MBP from DnaC variants following ion exchange purification.
E. coli wild-type DnaB, PriA, PriB, PriC, SSB, and DnaT were purified as previously described (32, 38).
Construction of synthetic replication fork substrates
Synthetic DNA replication forks were generated as previously described (29). Briefly, oligonucleotide 3L-98 was 5′-radiolabeled with ATP-[γ-32P] using T4 polynucleotide kinase and annealed to the complementary strand (1b-98) with or without nascent leading (11b-38) and lagging strands (b-33) (Table S1). Forks were purified via PAGE electrophoresis. The resulting substrates have a 60-bp parental duplex, with either a 38-base ssDNA or a 38-bp leading strand duplex DNA, and either a 38-base ssDNA or a 33-base pair lagging strand duplex DNA with a 5-base ssDNA gap at the fork junction.
Analytical size exclusion chromatography
10 nmol of DnaB and 12 nmol of DnaC were diluted into 5 ml of S6 Buffer (20 mM Tris-HCl pH 8.5, 0.2 M NaCl, 5 mM MgCl2, 1 mM BME, 1 mM ATP) and concentrated to 0.5 ml. Samples were loaded onto a Superdex 600 Increase 10/300 Gl analytical size exclusion FPLC column (Cytiva) and eluted with S6 buffer. Peak fractions were analyzed via PAGE (4–15% gel) for the presence of DnaB and DnaC.
DnaC ssDNA binding assays
Fluorescence anisotropy assays were carried out as previously described (9) with minor modifications. DnaC (0–50 μM) was diluted into 50 mM HEPES-KOH, pH 7.5, 125 mM KCl, 10% glycerol, 5 mM MgCl2, 2 mM ATP, 2 mM DTT, 0.1 mg/ml BSA and 10 nM fluorescein-labeled dT20 oligonucleotide. Samples were incubated at 37 °C for 15 min then fluorescence anisotropy values were recorded at 25 °C using a Beacon 2000 fluorescence polarization system. Fluorescence anisotropy values were normalized to a no-DnaC control. Data points are the mean from three independent measurements, with error bars representing standard error. Due to limitations in the highest concentrations of DnaC and DnaC variants that could be tested, binding saturation was not reached, and interpretations were restricted to qualitative comparisons.
DnaB loading assay
DnaB loading assays were carried out as previously described (29). Briefly, 1 nM (molecules) synthetic DNA replication fork was incubated with either 0 nM or 60 nM SSB (simple fork) or 120 nM SSB (leading, lagging, full forks) in reaction buffer (50 mM HEPES pH 7.0, 0.04 mg/ml BSA, 2 mM DTT, 2 mM ATP, 4 mM Magnesium acetate). Loading reactions were initiated by the addition of 240 nM DnaB and 200 nM DnaC variants unless otherwise indicated. Where indicated, 10 nM PriA, 100 nM PriB, and 960 nM DnaT or 40 nM of PriC were also added. The reactions were incubated at 37 °C for 30 min and terminated with 20 mM EDTA, 0.5% SDS, and 0.2 g/L protease K. DNA loading buffer was added, and the samples were resolved by electrophoresis using a 10% TBE-PAGE gel. Gels were quantified using ImageQuant and analyzed with the GraphPad Prism software. Data in graphs are represented as fractions unwound from three independent experiments, with the value for each independent measurement shown. Error bars represent standard error.
Data availability
All data described in this study are presented in the article and accompanying supporting information. All original data are freely available through the Dryad repository (https://doi.org/10.5061/dryad.qrfj6q5pg).
Supporting information
This article contains supporting information (33).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Steven Sandler, Alex Duckworth, and members of the Keck laboratory for critical reading of the manuscript.
Author contributions
S. D. M. and J. L. K. conceptualization; S. D. M. and J. L. K. methodology; S. D. M. investigation; S. D. M. data curation; S. D. M. writing–original draft; S. D. M. and J. L. K. writing–review & editing; J. L. K. project administration; J. L. K. funding acquisition.
Funding and additional information
This work was supported by the National Institutes of Health (grant number: R01GM098885) to J. L. K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Patrick Sung
Supporting information
References
- 1.Bramhill D., Kornberg A. Duplex opening by DnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988;52:743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade J.E., Torgue J.C., McGarry K.C., Rozgaja T., Enloe S.T., Leonard A.C. Mutational analysis reveals Escherichia coli oriC interacts with both DnaA-ATP and DnaA-ADP during pre-RC assembly. Mol. Microbiol. 2007;66:428–439. doi: 10.1111/j.1365-2958.2007.05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seitz H., Weigel C., Messer W. The interaction domains of the DnaA and DnaB replication proteins of Escherichia coli. Mol. Microbiol. 2000;37:1270–1279. doi: 10.1046/j.1365-2958.2000.02096.x. [DOI] [PubMed] [Google Scholar]
- 4.LeBowitz J.H., McMacken R. The Escherichia coli DnaB replication protein is a DNA helicase. J. Biol. Chem. 1986;261:4738–4748. [PubMed] [Google Scholar]
- 5.Fass D., Bogden C.E., Berger J.M. Crystal structure of the N-terminal domain of the DnaB hexameric helicase. Structure. 1999;7:691–698. doi: 10.1016/s0969-2126(99)80090-2. [DOI] [PubMed] [Google Scholar]
- 6.Chodavarapu S., Jones A.D., Feig M., Kaguni J.M. DnaC traps DnaB as an open ring and remodels the domain that binds primase. Nucleic Acids Res. 2016;44:210–220. doi: 10.1093/nar/gkv961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludlam A.V., McNatt M.W., Carr K.M., Kaguni J.M. Essential amino acids of Escherichia coli DnaC Protein in an N-terminal domain interact with DnaB helicase. J. Biol. Chem. 2001;276:27345–27353. doi: 10.1074/jbc.M101940200. [DOI] [PubMed] [Google Scholar]
- 8.Arias-Palomo E., O’Shea V.L., Hood I.V., Berger J.M. The bacterial DnaC helicase loader is a DnaB ring breaker. Cell. 2013;153:438–448. doi: 10.1016/j.cell.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias-Palomo E., Puri N., O’Shea Murray V.L., Yan Q., Berger J.M. Physical basis for the loading of a bacterial replicative helicase onto DNA. Mol. Cell. 2019;74:173–184. doi: 10.1016/j.molcel.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makowska-Grzyska M., Kaguni J.M. Primase directs the release of DnaC from DnaB. Mol. Cell. 2010;37:90–101. doi: 10.1016/j.molcel.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S., Dallmann H.G., Mchenry C.S., Marians K.J. Coupling of a replicative polymerase and helicase: a τ-DnaB interaction mediates rapid replication fork movement. Cell. 1996;84:643–650. doi: 10.1016/s0092-8674(00)81039-9. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y.B., Ratnakar P.V., Mohanty B.K., Bastia D. Direct physical interaction between DnaG primase and DnaB helicase of Escherichia coli is necessary for optimal synthesis of primer RNA. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12902–12907. doi: 10.1073/pnas.93.23.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funnells B.E., Baker T.A., Kornberg A. In vitro assembly of a prepriming complex at the origin of the Escherichia coli chromosome. J. Biol. Chem. 1987;262:10327–10334. [PubMed] [Google Scholar]
- 14.Cox M.M., Goodman M.F., Kreuzer K.N., Sherratt D.J., Sandlerk S.J., Marians K.J. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 15.Mangiameli S.M., Merrikh C.N., Wiggins P.A., Merrikh H. Transcription leads to pervasive replisome instability in bacteria. Elife. 2017;6:1–27. doi: 10.7554/eLife.19848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windgassen T.A., Wessel S.R., Bhattacharyya B., Keck J.L. Mechanisms of bacterial DNA replication restart. Nucleic Acids Res. 2018;46:504–519. doi: 10.1093/nar/gkx1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bujalowski W., Lohman T.M. Escherichia coli single-strand binding protein forms multiple, distinct complexes with single-stranded DNA. Biochemistry. 1986;25:7799–7802. doi: 10.1021/bi00372a003. [DOI] [PubMed] [Google Scholar]
- 18.Sandler S.J. Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics. 2000;155:487–497. doi: 10.1093/genetics/155.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E.H., Kornberg A. Replication deficiencies in priA mutants of Escherichia coli lacking the primosomal replication n’ protein. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3029–3032. doi: 10.1073/pnas.88.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurse P., Zavitz K.H., Marians K.J. Inactivation of the Escherichia coli PriA DNA replication protein induces the SOS response. J. Bacteriol. 1991;173:6686–6693. doi: 10.1128/jb.173.21.6686-6693.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masai H., Asai T., Kubota Y., Arai K.I., Kogoma T. Escherichia coli PriA protein is essential for inducible and constitutive stable DNA replication. EMBO J. 1994;13:5338–5345. doi: 10.1002/j.1460-2075.1994.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandler S.J., Samra H.S., Clark A.J. Differential suppression priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA and dnaC. Genetics. 1996;143:5–13. doi: 10.1093/genetics/143.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandler S.J., Marians K.J., Zavitz K.H., Coutu J., Parent M.A., Clark A.J. dnaC mutations suppress defects in DNA replication- and recombination-associated functions in priB and priC double mutants in Escherichia coli K-12. Mol. Microbiol. 1999;34:91–101. doi: 10.1046/j.1365-2958.1999.01576.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu L., Marians K.J. Purification and characterization of DnaC810, a primosomal protein capable of bypassing PriA function. J. Biol. Chem. 2000;275:8196–8205. doi: 10.1074/jbc.275.11.8196. [DOI] [PubMed] [Google Scholar]
- 25.Boonsombat R., Yeh S., Milne A., Sandler S.J. A novel dnaC mutation that suppresses priB rep mutant phenotypes in Escherichia coli K-12. Mol. Microbiol. 2006;60:973–983. doi: 10.1111/j.1365-2958.2006.05147.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan D.L. The 3’-tail of a forked-duplex sterically determines whether one or two DNA strands pass through the central channel of a replication-fork helicase. J. Mol. Biol. 2000;301:285–299. doi: 10.1006/jmbi.2000.3965. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan D.L., Donnell M.O. DnaB drives DNA branch migration and dislodges proteins while encircling two DNA strands. J. Mol. Biol. 2002;10:647–657. doi: 10.1016/s1097-2765(02)00642-1. [DOI] [PubMed] [Google Scholar]
- 28.Puri N., Fernandez A.J., O’Shea Murray V.L., McMillan S., Keck J.L., Berger J.M. The molecular coupling between substrate recognition and ATP turnover in a AAA+ hexameric helicase loader. Elife. 2021;10 doi: 10.7554/eLife.64232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heller R.C., Marians K.J. The disposition of nascent strands at stalled replication forks dictates the pathway of replisome loading during restart. Mol. Cell. 2005;17:733–743. doi: 10.1016/j.molcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Manhart C.M., McHenry C.S. The PriA replication restart protein blocks replicase access prior to helicase assembly and directs template specificity through its ATPase activity. J. Biol. Chem. 2013;288:3989–3999. doi: 10.1074/jbc.M112.435966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wessel S.R., Cornilescu C.C., Cornilescu G., Metz A., Leroux M., Hu K., et al. Structure and function of the PriC DNA replication restart. J. Biol. Chem. 2016;291:18384–18396. doi: 10.1074/jbc.M116.738781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duckworth A.T., Ducos P.L., McMillan S.D., Satyshur K.A., Blumenthal K.H., Deorio H.R., et al. Replication fork binding triggers structural changes in the PriA helicase that govern DNA replication restart in E. coli. Nat. Commun. 2023;14:2725. doi: 10.1038/s41467-023-38144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heller R.C., Marians K.J. Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J. Biol. Chem. 2005;280:34143–34151. doi: 10.1074/jbc.M507224200. [DOI] [PubMed] [Google Scholar]
- 34.Liu J., Xu L., Sandler S.J., Marians K.J. Replication fork assembly at recombination intermediates is required for bacterial growth. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3552–3555. doi: 10.1073/pnas.96.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arai K., Yasuda S., Kornberg A. Mechanism of DnaB protein action. J. Biol. Chem. 1981;256:5247–5252. [PubMed] [Google Scholar]
- 36.Wiegand T., Cadalbert R., Lacabanne D., Timmins J., Terradot L., Böckmann A., et al. The conformational changes coupling ATP hydrolysis and translocation in a bacterial DnaB helicase. Nat. Commun. 2019;10:31. doi: 10.1038/s41467-018-07968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavitz K.H., Marians K.J. ATPase-deficient mutants of the Escherichia coli DNA replication protein priA are capable of catalyzing the assembly of active primosomes. J. Biol. Chem. 1992;267:6933–6940. [PubMed] [Google Scholar]
- 38.Duckworth A.T., Windgassen T.A., Keck J.L. Examination of the roles of a conserved motif in the PriA helicase in structure-specific DNA unwinding and processivity. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data described in this study are presented in the article and accompanying supporting information. All original data are freely available through the Dryad repository (https://doi.org/10.5061/dryad.qrfj6q5pg).