Abstract
Flowery-like aroma are positive contributors to green tea. Here, the optimal processing conditions for green tea with flowery-like aroma were designed using spreading time, fixation time and drying temperature as three factors designed by response surface methodology (RSM), and the response value of aroma sensory evaluation score. The volatiles in batches of tea samples were analyzed by GC–MS. The optimal quality was obtained with a flowery-like aroma by RSM under a spreading time of 8.97 h, fixation time of 162.3 s, and drying temperature of 103.32 °C. GC-O and odor activity values further revealed floral-like volatiles, including decanal, linalool oxide, β-lonone, geraniol, (Z)-jasmone, linalool, nonanal, and benzeneacetaldehyde. The recombination of these floral volatiles confirmed the consistency with the floral green tea. Furthermore, the extending spreading duration (8–10 h), reducing fixation duration (160–190 s), and increasing drying temperature (100–115 °C) promote their accumulation in green tea. This study provides new perspectives for the precise enhancement of floral odorants for green tea.
Keywords: Floral green tea, Response surface methodology, Gas chromatography–mass spectrometry/olfactometry, Odor activity value, Processing conditions
Highlights
-
•
Optimal processing condition was obtained by RSM and a sensory panel.
-
•
Aroma profiles were detected by SBSE- and HS-SPME-GC–MS.
-
•
GC-O and OAV selected volatiles responsible for floral-like aroma.
-
•
Aroma recombination confirms floral-like volatiles in green tea samples.
-
•
The accumulation patterns of floral-like volatiles during processing were explored.
1. Introduction
Tea is a popular health drink with confirmed health benefits. China ranks first in tea consumption, production and export (Béliveau & Gingras, 2004). The quality of green tea directly shapes the market consumption trend. Aroma is one of the important factors in evaluating the quality of green tea. The aroma profile is determined by key volatiles in a specific concentration ratio (Guo, Schwab, Ho, Song, & Wan, 2021). The key volatiles of green tea have been systematically reported. Ethylbenzene, heptanal, benzaldehyde, 2-pentylfuran, (E,E)-3,5-octadien-2-one, linalool, (Z)-hex-3-en-1-yl hexanoate and trans-β-ionone are aroma-enhancing odorants for green tea with chestnut aroma (Zhu et al., 2018). Recently, there has been a consensus that dimethyl sulfide provides a cooked corn-like flavor to green tea and is one of the potential key volatiles in green tea (Zhai et al., 2022). In our recent study, alcohol, aldehyde, ester, and ketone were the key volatile types in the floral flavored Taiping Houkui green tea (Liu et al., 2023).
The aroma formation of green tea is intimately related to its processing practices. Generally, the processing steps of green tea include spreading, fixation, shaping, and drying (first-drying and second-drying). The volatiles in tea change dynamically under the conditions of dehydration or heat, thus forming products of different aroma types. High-grade green teas usually have an orchid aroma, and suitable processing conditions can contribute to the formation of floral aromas (Feng et al., 2019). Characterization of key volatiles for floral green tea has been reported (Feng et al., 2019; Xie et al., 2023). Linalool, phenylethyl alcohol, geraniol, benzeneacetaldehyde, heptanal, hexanal and trans-β-ionone were selected as the key volatiles of floral green tea. These key volatiles reached high contents at the spreading step (Xie et al., 2023). The purpose of fixation is to inactivate the enzymes in the fresh leaves and stop the oxidation process in the tea to ensure its subsequent quality. Optimization of different fixation methods was compared, and the green tea product obtained by roller-hot air coupling fixation had a strong and persistent chestnut aroma and was more favorable for the formation of 2,6,10,10-tetramethyl-1-oxaspiro [4.5] dec-6-ene, linalool, and 3-methyl-butanal, which have roasted and fruity odors (Wang et al., 2020). Drying is the ultimate process in the production of green tea, and different drying temperatures have a significant effect on the type of aroma of the final finished tea. It has been reported that green tea at drying temperatures of 90 °C, 110 °C, 140 °C and 160 °C produces a clean aroma, chestnut-like aroma, bean-like aroma and high-fired aroma. Floral volatiles such as linalool, benzeneacetaldehyde and heterocyclic compounds increased by 64%, 75% and 83% at 160 °C (Wang et al., 2022). There are many reports on the effects of various processing steps on the aroma of green tea. Most of the current studies focus on the effect of a single step on aroma quality. The enhancement of floral aroma was related to the accumulation of key floral volatiles during processing. The contents of linalool and geraniol were the highest in the spreading step, with 128.92 μg/L, 120.19 μg/L and 126.56 μg/L, and the content gradually decreased as the processing proceeded. The contents of decanal showed an increasing trend from the step of fresh leaves to spreading, and equilibrated in the step of fixation. Aldehydes associated with benzaldehyde, benzeneacetaldehyde and hexanal associated with floral and fruity aroma were the highest in fresh leaves (146.38 μg/L), decreased sharply to 51.61 μg/L in the spreading step, then increased rapidly to 110.39 μg/L in the rolling step, and showed a decreasing trend thereafter ((Xie et al., 2023). The drying process also affected the formation of floral volatiles. The content of nonanal decreased significantly with increasing drying temperature (Yang et al., 2022). (Z)-jasmonate is representative of the unique orchid aroma volatiles, and the high accumulation of this volatile influences the perception of orchid aroma when the tea is consumed. (Z)-jasmonate highly accumulates during fixation and undergoes a certain degree of isomerization during fixation and drying due to high temperatures leading to a decrease in content; therefore, the fixation or drying temperature should not be too high for a long period of time (Wang et al., 2023). Overall, the accumulation of floral volatiles in green tea promotes the formation of floral aroma, and the selection of appropriate processing techniques is essential to improve the quality of green tea floral aroma. However, the different steps interact with each other. The effects of multiple steps on aroma are still unknown. Systematic and integrated effects of multiple steps on aroma are lacking. In addition, the optimized processing conditions of high-quality green teas with perceived floral attributes have not been reported.
The continuous improvement of volatile analysis methods provides a convenient method to deeply understand the contribution of volatile compounds to aroma. Stir bar sorptive extraction (SBSE) is a sample pretreatment technique that uses a polymer coating to adsorb volatile or semivolatile components, and the principle of adsorption is similar to that of SPME (Nogueira, 2012). It was initially applied in the trace detection of drinking water and surface water because of its greater recovery of aroma and higher precision (Habib, Landa, Holbrook, Walker, & Lee, 2023). The combination of SBSE and gas chromatography-olfactometry (GC-O) has been increasingly used. Longjing green tea was identified by SBSE-GC-O, and 14 key volatiles were reported for its aroma (Wang et al., 2020). Recently, it was also reported that the aroma of Dahongpao tea was characterized using SBSE-GC-O. Different preprocesses for sample extraction were used. The results proved that SBSE had a superior extraction effect (Wang et al., 2023). Headspace solid-phase microextraction (HS-SPME) is a method in which a fiber head with the function of adsorbing volatile components is dipped above the liquid, and the adsorption can be completed by extending the fiber head for a period. Similarly, the characteristics of aromatic compounds of Hunan black tea were also deeply explored. Combined with multivariate statistical analysis, it was demonstrated that nonanal, trans-nerol, benzyl alcohol and phenylethanol were positively correlated with the intensity of floral and sweet aromas (Yin et al., 2022). SBSE and HS-SPME are two nonintrusive extraction techniques. In many cases, SBSE is more efficient with solutes that are highly hydrophilic. SPME is less efficient with solutes that are not hydrophilic but prefers gases and low molecular weight volatiles (Lancioni, Castells, Candal, & Tascon, 2022; Nogueira, 2012). Therefore, the combined use of both techniques avoids this shortcoming, and the aroma profile can be presented more completely.
Taiping Houkui is one of the famous green teas produced in Huangshan City, China. It is considered to have distinct floral perceptual attributes under suitable processing conditions (Zhou et al., 2022). However, the effects of these processing steps on aroma quality remain unclear. In addition, the most suitable processing parameters for high-quality tea remain unknown. Therefore, response surface methodology was proposed for the three important processing steps of spreading, fixation, and drying. Sensory-directed flavor analysis was used to reveal the enrichment patterns of floral aroma under 13 batches of different processing conditions. The optimal process parameters for TH green tea were determined, providing a preliminary reference for precise processing of floral-type green teas.
2. Materials and methods
2.1. Chemicals and reagents
The chemical standards of benzeneacetaldehyde, linalool, cis-linalool oxide (pyranoids), geraniol, (Z)-jasmone, β-ionone, (Z)-geraniol, indole, methyl jasmone, nonanal, 2-heptanone, heptanal, 6-methyl-5-hepten-2-one, (E,E)-2,4-heptadienal, dimethyl sulfide, 3-methyl-butanal, β-myrcene, linalool oxide, decanal and naphthalene were purchased from Macklin Biochemistry Co., Ltd. (Shanghai, China) purity. Hexanal was purchased from Aladdin Co., Ltd. (Shanghai, China). (2E,6E)-2,6-Nonadienal was obtained from Bidepharm Co., Ltd. (Shanghai, China). These chemical standards were obtained with a purity ≥90%. Sodium chloride (NaCl) was purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Analytical grade n-alkanes (C5-C25) and ethyl hydroxyacetate were purchased from Sigma Aldrich Trading Co., Ltd. (Shanghai, China). Distilled water was prepared in an ultrapure water machine from Hokee Compony (model HK-DI-10/20/30, Hefei, China). A PDMS rotator (10 mm long, 0.5 mm thick, 24 μL capacity, Gerstel, Germany) and an advanced multipoint 6/15 multipoint magnetic stirrer (Thermo Fisher, USA) were used in the SBSE. Manually sampled fiber scaffolds and carboxen/divinylbenzene/polydimethylsiloxane (CAR-DVB-PDMS; 50/30 μm) used in the HS-SPME operation were all obtained from Supelco (Bellefonte, PA, USA).
2.2. RSM for tea sample preparation
The RSM-Box–Behnken design was employed based on Design-Expert software (Stat-Ease Inc., Minneapolis, MN, USA). The quadratic polynomial regression model is as follows:
here y is the test output, is the coefficient, is the primary term coefficient, and are the quadratic term coefficients (≠), and is the observation error.
The locally harvested Shidacha population containing one bud and two apical leaves was collected on 23 April 2023 from Huangshan City, Anhui Province, China. Subsequently, the fresh leaves were prepared according to the protocol of Fig. 1A and Fig. 1B. First, the picked fresh leaves were spread evenly on the bamboo net according to the programme designed by RSM. The temperature during spreading was 20 ± 1 °C, and the relative humidity was 90%. The temperature of the fixing machine was adjusted to 280 ± 5 °C. After fixation, two workers manually shaped the tea leaves and placed them neatly on the metal screen. The leaves are then pressed with metal rollers, a process known as shaping and flattening. Then, the first drying process was carried out, and the drying time was 15 min. The second drying process was performed at 80 °C for 30 min, after which 13 batches of TH green tea samples were obtained.
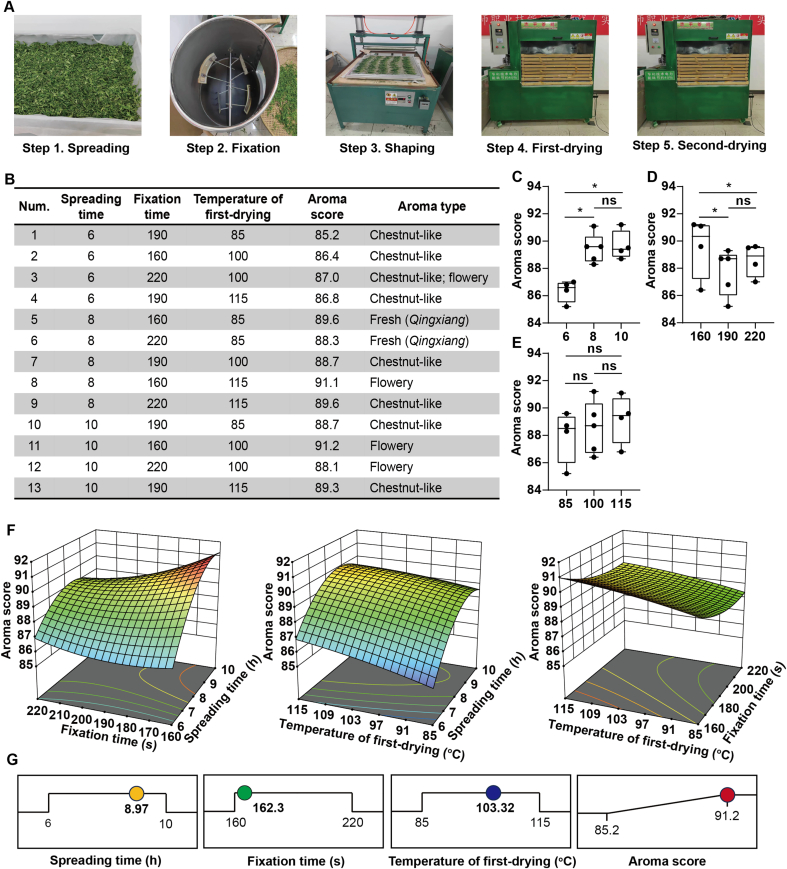
Fig. 1.
The results of RSM for the production of Taiping Houkui green tea. (A) Processing flows of Taiping Houkui green tea; (B) Design results of the RSM; (C-E) Box plots of the effect of individual independent variables on the overall aroma scores, (C) spreading time; (D) fixation time; (E) first-drying temperature; (F) 3D plots of the RSM results, (G) Plots of the optimal conditions and aroma sensory scores predicted by the RSM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. Determination of volatiles in tea samples
2.3.1. Extraction of volatiles using SBSE
Three grams of tea leaves were weighed into a 250-mL conical flask, and 150 mL of purified water at a temperature of 100 °C was added to the conical flask for 4 min. The tea broth was then cooled in ice water. After cooling, the tea broth was filtered, and then 10 mL of tea broth was pipetted. Three grams of NaCl was transferred to a 20-mL headspace flask. Three microliters of ethyl decanoate at a concentration of 5 ppm was added to the tea broth as an internal standard. The PDMS twister magnetic stirring material with polydimethylsiloxane was immersed in the liquid. The headspace vial was equilibrated in a thermostatic water bath at 60 °C for 15 min at a speed of 1100 r/min. After 45 min of adsorption, the PDMS twister was withdrawn from the vial with a magnetic stick, washed in distilled water and blotted dry with micro paper. The PDMS twister was transferred to a thermal desorption glass tube and placed in a Twister tray (Gerstel, Germany). Autosampling was performed by means of a multipurpose sampler (MPS 2XL, Gerstel) with the desorption procedure as follows: the thermal desorption unit (TDU, Gerstel, Germany) was operated at an initial temperature of 40 °C for 1 min, ramped up to 240 °C at a rate of 100 °C/min, and then held for 5 min in splitless mode. The 99% pure liquid nitrogen was fed into the cooling injection unit (CIS, Gerstel, Germany), where the liquid nitrogen was cooled to −100 °C, equilibrated for 1 min and then ramped up to 280 °C at a rate of 12 °C/s and held for 3 min.
2.3.2. Extraction of volatiles using HS-SPME
The SPME method is consistent with the method of our previous study.5 Briefly, 3 g of tea was weighed into a 250-mL conical flask, and 150 mL of purified water at a temperature of 100 °C was added to the conical flask for 4 min. Subsequently, the tea broth was cooled in ice water. After cooling, the tea broth was filtered through gauze, and 10 mL of tea broth and 3 g NaCl were added to a 20-mL headspace vial. Then, 3 μL of ethyl decanoate at a concentration of 5 ppm was added to the tea broth as an internal standard. The headspace flask was placed in a water bath at 60 °C and equilibrated for 15 min. The fiber tip probe was pushed out 1.5 cm above the surface of the tea broth and extracted with magnetic stirring for 45 min. The fiber tip was withdrawn at the end of the extraction.
2.3.3. Detection of volatiles by GC–MS
The volatiles extracted by SBSE and HS-SPME were analyzed using an Agilent system equipped with a 7890B GC and a 5975B MS. The running procedure of HS-SPME-GC–MS was consistent with our previous study (Liu et al., 2023). The running procedure of SBSE-GC–MS was as follows: the initial temperature was 40 °C, held for 5 min, and then turned to 70 °C. The temperature was increased to 100 °C at a rate of 3 °C/min, 130 °C at a rate of 2 °C/min, 250 °C at a rate of 10 °C/min, and held for 5 min. The mass spectrometry detector was operated in positron ionization mode with a scanning current of 70 eV, and mass spectra were recorded at a mass scan range of 30 to 350 m/z. The identification of the volatiles was based on mass spectra and retention indices (RIs). RIs were calculated based on n-alkane (C5-C25) standards and compared with the RIs of volatiles reported by the National Institute of Standards and Technology (Liu et al., 2023).
2.3.4. Quantification of volatiles by chemical standards
In addition to using public datasets, available chemical standards were used for the identification of volatiles. The identified volatiles were relatively quantified by the internal standard (ethyl decanoate), as previously reported (Liu et al., 2023). The relative odor activity value (ROAV) of each volatile was then calculated. Volatiles with ROAV >0.5 were then quantified absolutely by means of the standard curves constructed by the chemical standards.
2.4. Identification of the key active volatiles
2.4.1. Sensory intensity of volatiles by GC-O
GC-O was performed by an Agilent system (Santa Clara, CA, USA) equipped with sniff port ODP4 (Gerstel, Germany), a 7890B gas chromatograph and a 5975B mass spectrometer in 1:1 splitless mode, with the same running procedure as in Section 2.3. The sniffing panel consisted of 5 persons (3 females and 2 males, aged 20–30 years). GC-O was performed by an Agilent system (Santa Clara, CA, USA) equipped with sniff port ODP4 (Gerstel, Germany), a 7890B gas chromatograph and a 5975B mass spectrometer in 1:1 splitless mode, with the same running procedure as in Section 2.3. The sniffing panel consisted of 5 persons (3 females and 2 males, aged 20–30 years). The sniffing team members had all received >30 h of sniffing training prior to the test. Aroma intensity was recorded and characterized by the sniffing team using a mobile resistor. Aroma intensity was expressed using numbers 1–4 to indicate the magnitude of aroma intensity in order (1 = weak, 2 = moderate, 3 = strong, 4 = extreme). The presence of the volatiles was finally determined by retention time, Ris, and the presence of the same odor profile sniffed by the sniffing panel. All samples were taken in triplicate, and aroma strengths were averaged. The sniffing team members had all received >30 h of sniffing training prior to the test. Aroma intensity was recorded and characterized by the sniffing team using a mobile resistor. Aroma intensity was expressed using numbers 1–4 to indicate the magnitude of aroma intensity in order (1 = weak, 2 = moderate, 3 = strong, 4 = extreme). The presence of the volatiles was finally determined by retention time, Ris, and the presence of the same odor profile sniffed by the sniffing panel. All samples were taken in triplicate, and aroma strengths were averaged.
2.4.2. Calculation of odor activity value (OAV)
The OAV was obtained from the ratio of the concentration of each volatile to its perception threshold in water (Wang, Ma, et al., 2020). Volatiles with OAVs >1 are considered to contribute significantly to the overall aroma of the sample (Wang, Ma, et al., 2020;Wei et al., 2024; Zhang et al., 2023).
2.4.3. QDA and aroma recombination
The QDA of floral intensity was needed before aroma recombination. The evaluation panel consists of 12 persons (6 males and 6 females, aged 20–30 years) with at least 3 months of professionally trained odor training for the panel members. β-Ionone was used as a reference for the floral odor standard. β-Ionone was dissolved in ethanol and then diluted with water 100 times higher than the common threshold for volatiles (Zhang et al., 2023). All samples were brewed, and 25 mL of tea broth was poured into a 50-mL brown sniff bottle. Subsequently, the panelists assigned the floral intensity against the standard solution with the original tea broth.
For aroma recombination, the concentration of floral-like volatiles (OAV > 1) was used as a standard for our detection of 13 groups of samples (Wei et al., 2024; Zhang et al., 2023). All volatiles were first dissolved in ethanol, and then the volatiles were sequentially transferred to a 50-mL colorless sniffing bottle according to the quantitative concentration. The final volume was determined to be 25 mL. The original tea broth was brewed according to the tea-water ratio at 1:50, and 25 mL was transferred to a brown bottle after the tea was separated and pipetted. Finally, the recombined solution and the original tea broth were placed into a water bath at a constant temperature of 60 °C. The intensities were then evaluated. All the ratings were scored according to 0–10 (0–2 = very weak; 2–4 = weak; 4–6 = moderate; 6–8 = strong; 8–10 = very strong).
2.5. Statistical analysis
All experiments and samples analyzed were replicated three times, and the content of volatiles was expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed using SPSS software (IBM, Armonk, NY, USA), with p values <0.05 considered significant.
3. Results and discussion
3.1. Optimized processing conditions of green tea by RSM
RSM was used to obtain the optimal processing conditions. The aroma sensory score was used as the response value, whereas spreading time (1), fixation time (2), and temperature of first-drying (3) were the three independent variables. The protocol designed by Box–Behnken is presented in Fig. 1B, where 13 batches of samples were obtained. Although having the same processing steps, green tea with different aroma qualities can be obtained under different processing parameters (Fig. 1B). The aroma scores were then used to build a mathematical model with multiple quadratic regression equations (Table S1). The regression model with p < 0.0001 (highly significant) and the misfit term with p = 0.9645 > 0.05 (nonsignificant) indicate that the model was well fitted. Finally, the quadratic polynomial method regression equation was obtained as follows:
The 3D plots of RSM showed the effects of spreading time, fixation time, and temperature of first-drying on the aroma of TH green tea under the interaction of the three steps, as shown in Fig. 1F. The steepness was obvious under the interaction of spreading time (8–10 h) and fixation time (160–190 s). Proper spreading of fresh leaves after harvesting can promote the accumulation of aroma substances and accelerate the dissipation of water from the leaves (Huang, Lu, Deng, & Ning, 2022; Qiao et al., 2023). The temperature of the first-drying and spreading time interaction also had significant positive effects on aroma. In particular, the interaction between the temperature of first-drying (105–115 °C) and spreading time (8–10 h) could reach high aroma scores. Different drying temperatures affect the quality of green tea, which may be due to the biochemical effects of volatile and nonvolatile compounds at specific temperatures (Wang et al., 2022). However, the interaction of fixation time and temperature of first-drying did not have a significant effect on the aroma, presenting curved surfaces with smooth and flat shapes (Fig. 1F). The volatile content equilibrated or decreased during the fixation and drying steps, which explains the non-significant enhancement of aroma by the interaction of the two (Xie et al., 2023). Finally, the RSM model predicted the optimal conditions as follows: spreading time (8.97 h), fixation time (162.3 s), and temperature of first-drying (103.32 °C). The highest aroma score for sensory evaluation predicted in this condition was 91.26 (Fig. 1G).
The effect of the three steps on the aroma scores of green teas was further explored. The highest aroma scores were achieved at a spreading time of 8 h (Fig. 1C). Spreading is the process of slow conversion of volatiles and their high accumulation (Xie et al., 2023). The aroma scores at 8 and 10 h were significantly higher (p < 0.05) than those at 6 h. This indicates that a moderate spreading time improves the overall aroma of TH green tea, which is consistent with what has been reported (Qiao et al., 2023). For fixation time, the aroma scores of 160 s were significantly higher (p < 0.05) than those of 190 and 220 s (Fig. 1D). Fixation is a thermal degradation process in which volatiles and Maillard reaction products with carotenoids and lipids as precursors generate alcohols, ketones, aldehydes, alkenes, etc. at high temperatures (Ho, Zheng, & Li, 2015; Wang, Hua, et al., 2020). Proper fixation accumulates a large number of secondary products for the formation of more volatiles in the next step (Ho et al., 2015). Volatiles with floral, fruity and sweet aromas were widely formed in green tea samples with fixation time of 160 s. As shown in Fig. 1E, the three temperatures (85 °C, 100 °C, 115 °C) in the first drying step did not significantly (p > 0.05) improve the aroma of green tea. However, their contributions to the aroma scores were all above 88, showing a trend of increasing aroma scores with increasing drying temperatures. Maillard reaction has been reported to occur readily and produce many aroma volatiles at high temperatures during the fixation (200–300 °C) and drying (90–120 °C) step of green tea processing (Chen et al., 2019). We hypothesized that it might be related to the high drying temperature (85–115 °C) used for green tea. Nevertheless, there was a lesser increase in the sensory evaluation scores as the drying temperatures increased, indicating that higher drying temperatures contributed to the overall aroma. Multiple steps of continuous processing also had an effect on the aroma evaluation scores. Fixation and drying after 6 h of staging yielded samples that were evaluated as chestnut-like (LX) regardless of how the fixation and drying parameters were varied, with aroma scores ranging from 85.2 to 87.0. We hypothesized that this was related to the short spreading time, and that the volatiles were not properly transformed at the shorter spreading time (Xie et al., 2023). The samples that were spread for 8 h were processed and after a shorter fixation time (160 s) using different drying temperatures (115 °C and 85 °C) florwary-like (HX) and fresh-like (QX) samples were obtained with aroma scores of 91.1 and 89.6. In contrast, extended fixation times (190–220 s) using different drying temperatures (85–115 °C) obtained QX and LX with low or equal aroma scores compared to shorter fixation times (160 s). The extension of fixation time may inactivate polyphenol oxidase and delay the volatilization of low-boiling point compounds (Xie et al., 2023; Yin et al., 2022). As the spreading time was extended to 10 h, HX was more likely to be present in the samples after fixation and drying compared to the samples after 6-h spreading, and the highest aroma score (91.2) was also found in this processing combination. We hypothesized that the extended spreading time allowed sufficient transformation and accumulation of volatiles for further processing (Xie et al., 2023). Whether the differences in aroma of these samples are related to the optimized conditions and differences in volatiles still needs to be further confirmed.
3.2. Volatile profiles in tea samples
The SBSE was used as the lead and supplemented by HS-SPME to achieve the complete aroma profile of TH green tea. Eighty-three and 76 volatiles were detected in these samples by SBSE-GC–MS and HS-SPME-GC–MS, respectively (Fig. 2A, B; Tables S2, S3). These volatiles were classified into seven classes, including 21 alcohols, 15 aldehydes, 15 ketones, 11 esters, 6 alkanes, 2 heterocyclics, and 13 others (Fig. 2A, B). More volatiles were detected by SBSE-GC–MS than HS-SPME-GC–MS, with 37 volatiles only detected by SBSE and 30 only by HS-SPME (Fig. 2C). In particular, floral-like volatiles, including cis-linalool oxide (pyranoid), 4-acetyltoluene, methyl anthranilate, and isoeugenol, were detected individually by SBSE-GC–MS, while linalool oxide, linalool oxide pyranoside, (E)-β-damascone, and α-ionone were detected only by HS-SPME-GC–MS. The quantities of volatiles adsorbed by different extraction methods also differed (Gamero, Wesselink, & de Jong, 2013).
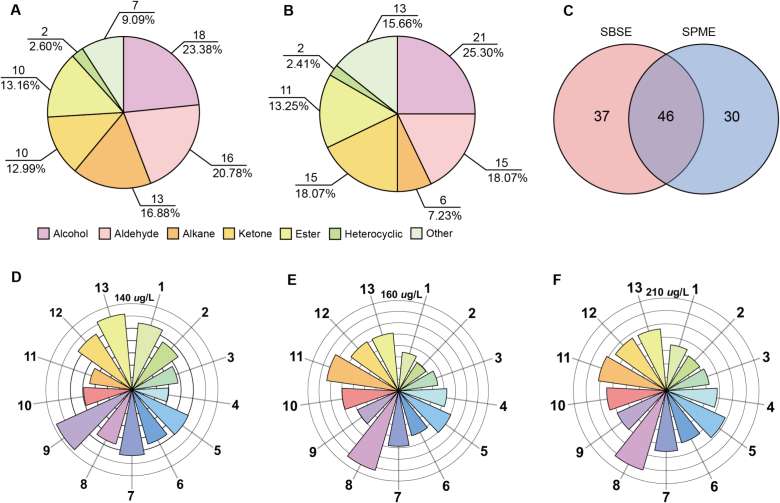
Fig. 2.
The volatile profiles of Taiping Houkui green tea samples detected by HS-SPME-GC–MS and SBSE-GC–MS. (A) The volatile profiles detected by HS-SPME; (B) The volatile profiles detected by SBSE; (C) Venn diagrams of volatiles detected by HS-SPME and SBSE; (D) Total volatile content of 13 groups of tea samples detected by HS-SPME; (E) Total volatile content of 13 groups of tea samples detected by SBSE; (F) Total volatile content of 13 groups of tea samples detected by HS-SPME and SBSE. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The total contents of volatiles extracted by SBSE and HS-SPME in these 13 batches of samples are shown in Fig. 2D and Fig. 2E, respectively. Different extraction methods show different content totals. For SBSE, a higher total content was shown in samples 9, 13, 1, and 12. Samples 8 and 11 showed higher total content under HS-SPME than the other samples. This result shows the difference between SBSE and HS-SPME. Therefore, the combination of the two methods may be more beneficial to obtain the global volatile profile. When the two methods were combined, samples 8, 11, 5, and 12 showed high total volatile content (Fig. 2F). Among them, samples 8, 11, and 12 are floral in sensory and have higher aroma scores (Fig. 1B). These results confirmed the positive correlation between the content of volatiles and the aroma scores.
3.3. Aroma-active volatiles in tea samples by GC-O
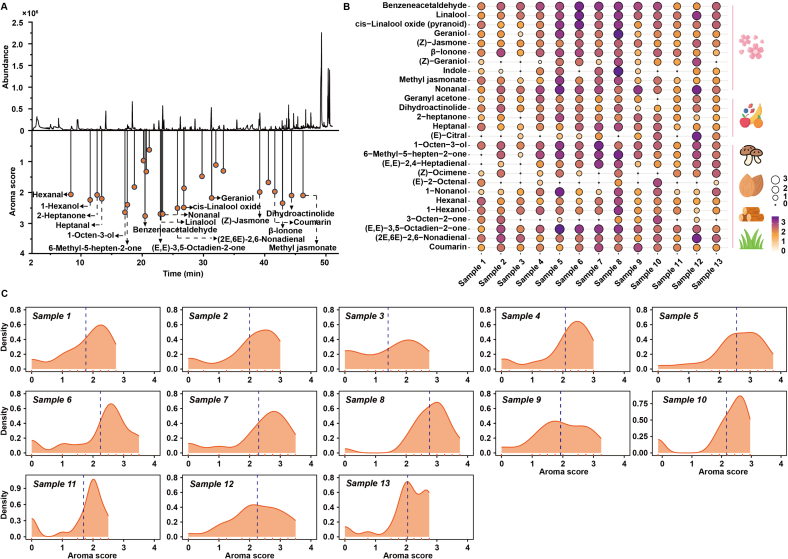
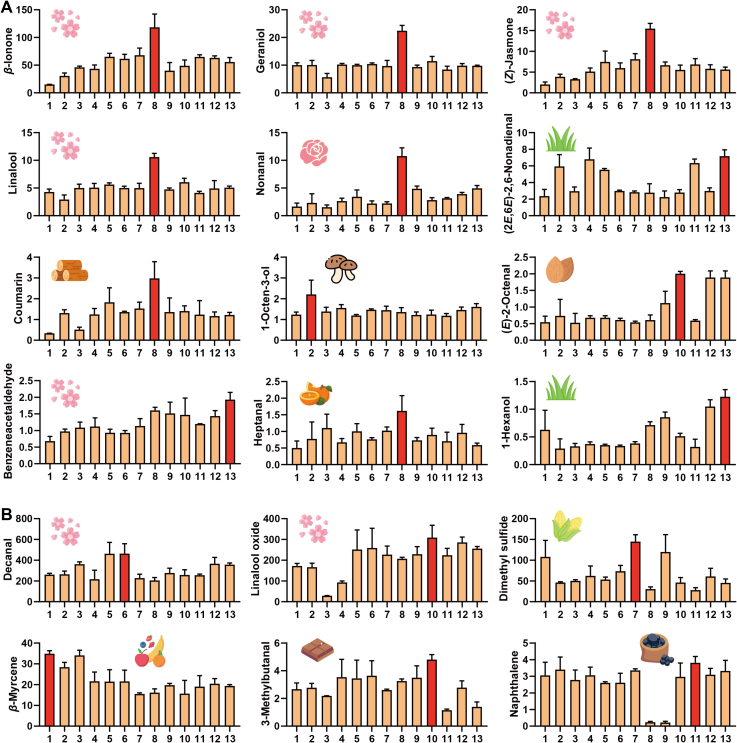
The volatiles identified by the GC-O method can be recognized as key aromas present in tea (Sasaki, Ochiai, Yamazaki, & Sasamoto, 2023). In this study, we consider volatiles that can be detected by sniffing as key candidate volatiles for TH green tea. Sensory panelists used specialized terminology to characterize the volatiles in the sniffed samples, such as floral, herbal, and fruity. Fig. 3A shows the aroma intensities (AIs) detected by GC-O. Briefly, 27 volatiles were identified by olfactory senses, with Als ranging from 0.69 to 2.81. Among them, benzeneacetaldehyde had the highest Als (2.81), which was described as a floral and sweet aroma (Zhang et al., 2023). Additionally, the volatiles with high AIs included linalool (Als = 2.75), cis-linalool oxide (pyranoid) (Als = 2.56), β-ionone (Als = 2.42), (Z)-jasmone (Als = 2.27), and geraniol (Als = 2.25). These volatiles were described as having a floral aroma, as mentioned in TH green tea (Liu et al., 2023; Zhou et al., 2022). In particular, linalool usually forms cis-linalool oxide (pyranoid) through a terpene synthase enzyme-catalyzed reaction. These enzymes are responsible for the synthesis of volatile monoterpenes in plants (Zeng, Watanabe, & Yang, 2018). Although cis-linalool oxide (pyranoid) is an oxidation product of linalool belonging to the same group of aroma odorants with floral attributes, the difference in their thresholds in water is large. The former has an odor threshold of 3000 μg/L, while the latter has an odor threshold of 6 μg/L. Geraniol is a hydrolysate of glycosides, which are enzymatically hydrolyzed from damaged tea tissues during the tea manufacturing process to release aromatic compounds. It is a major component of many essential oils of flowers and has been described as floral (Ho et al., 2015). The distributions and densities of all volatiles with AIs > 1 are plotted in Fig. 3B and Fig. 3C, respectively. Sample 8 has the highest Als for all types of aroma attributes, especially the floral aroma. For the volatiles with floral aroma attributes, Als were also higher in samples 5 and 6, but their contents were not prominent. Whether these volatiles contribute to the formation of floral aroma in TH green tea needs to be further confirmed by OAV.
Fig. 3.
The results of GC-O for tea samples. (A) The ion spectrum of tea samples detected by GC–MS and the corresponding aroma scores evaluated by GC-O; (B) The volatiles with aroma scores over 1 and corresponding aroma descriptions in tea samples; (C) Density maps of the aroma scores evaluated by GC-O of tea samples.
3.4. Contribution of aroma-active volatiles by OAV
The volatiles with a relative odor activity value (ROAV) > 0.5 were selected as candidate contributing volatiles. Then, the chemical standards of these volatiles were employed for absolute qualification (Tables S4, S5). Among them, geraniol (42.28–168.25 μg/L), dimethyl sulfide (9.11–43.44 μg/L) and β-myrcene (18.59–41.87 μg/L) showed high contents in these 13 batches of tea samples. The contribution of geraniol to green tea is well recognized by its floral aroma attribute. Geraniol synthase acts as a precursor of geraniol. The appropriate fixation and temperature of first-drying in this study may have accelerated the breakdown of geraniol synthase (Ho et al., 2015; Yin, Kong, et al., 2022). Interestingly, dimethyl sulfide was the lowest in tea sample 8 compared to the other samples, which was formed by the thermal decomposition of S-methyl methionine during brewing or the thermal decomposition of precursors during drying (Ho et al., 2015). Dimethyl sulfide has a pleasant odor at low content (0.3 μg/L in water). Its odor is often described as similar to that of cooked corn (Zhai et al., 2022). The highest content of β-myrcene (41.87 ± 1.74 μg/L) was found in tea sample 1, with odor attributes described as woody, tropical fruit and herbal. Specific aromas may vary depending on the type of tea, variety and preparation method, which may have contributed to the low aroma of the tea broth in sample 1 (Chen et al., 2022).
The aroma profile is a complex system that results from the interaction of several factors and is not solely determined by the concentration of volatiles. Generally, volatiles with OAVs >1 are considered significant contributors to the aroma profile (Hao et al., 2023; Wang, Ma, et al., 2020). The OAV results are shown in Fig. 4, with a total of 18 volatiles having an OAV > 1. The average OAVs of all samples of decanal, linalool oxide, dimethyl sulfide and β-ionone were generally higher than those of the other volatiles, which were 305.54, 208.15, 66.85, and 55.50, respectively (Fig. 4). Among them, decanal had the highest OAV (305.54), and its contribution to green tea has been confirmed (Zhang et al., 2020). Linalool and β-ionone are both derived from carotenoids. Therefore, a higher drying temperature accelerated enzymatic decomposition, which may have contributed to its higher OAV (Ho et al., 2015).
Fig. 4.
The volatiles with OAV > 1 in 13 groups of tea samples. (A) Volatiles detected by HS-SPME; (B) Volatiles detected by SBSE.
The OAV of each volatile differed in 13 tea samples (Fig. 5). Floral-like volatiles, including β-ionone, geraniol, (Z)-jasmone, linalool, nonanal, and benzeneacetaldehyde, had higher contents in sample 8 than in the other samples, which was in agreement with a previous report (Zhou et al., 2022). The enrichment of these volatiles may be an important reason why sample 8 shows a high aroma score and floral aroma. Accordingly, we finally identified decanal, linalool oxide, β-ionone, geraniol, (Z)-jasmone, linalool, nonanal, and benzeneacetaldehyde as the 8 floral-like volatiles that contribute significantly to the floral aroma of TH green tea. This is consistent with previously reported results (Zhou et al., 2022).
Fig. 5.
The volatiles with OAV > 1 in 13 groups of tea samples and the corresponding aroma descriptions. (A) Volatiles detected by HS-SPME; (B) Volatiles detected by SBSE.
3.5. Recombination of floral-like aroma active volatiles
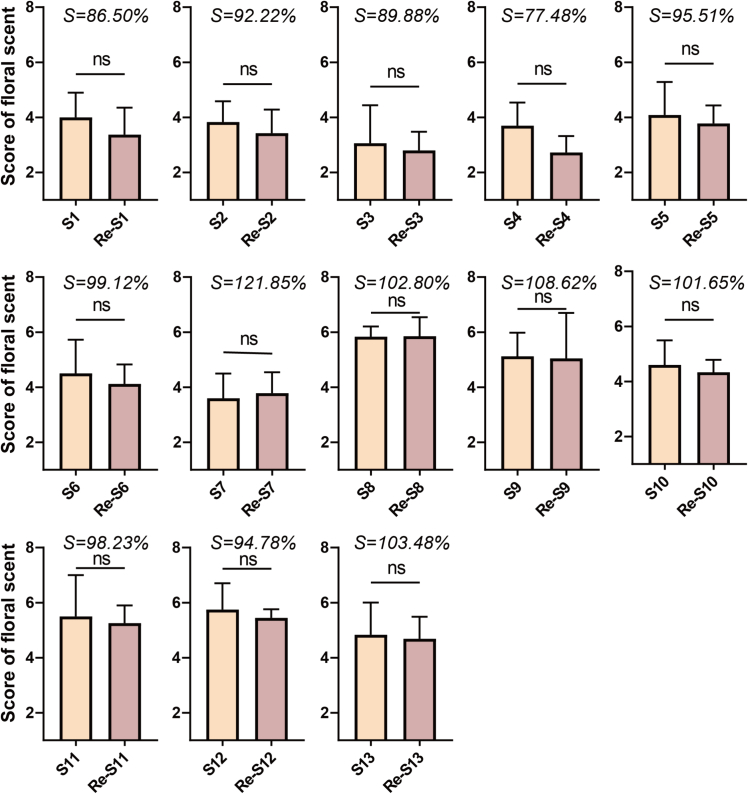
Aroma recombination provides a more precise identification of the contribution of individual volatiles to the aroma characterization of tea (He et al., 2023; Wei et al., 2024; Zhang et al., 2023). The 8 volatiles were selected as contributing volatiles and thus added in this study. The similarity between tea broth and reconstituted solution under 13 groups of different optimized conditions was revealed (Fig. 6). Samples 8, 11, and 12 belong to floral green tea. Therefore, they achieved high floral aroma scores of 5.96, 5.28, and 5.45 by QDA. The reasons for this may be that the aroma profile depends, first, on the type and concentration of volatiles present in the substance, and their interaction affects the perception of aroma (Guo et al., 2021). The concentration of floral-like volatiles was generally higher in floral green tea samples than in other samples. Floral-like volatiles may interact with each other during volatilization to form new flavor combinations. Such interactions may enhance the aroma perception of floral fragrances (Li, Han, Mei, Wang, & Han, 2024). Overall, the overall characterization of the floral aroma was highly reproduced, with the similarity of the samples being above 75% (Fig. 6). The ANOVA showed that there was no significant difference (p < 0.05) between the floral scores of the original tea samples. In summary, the aroma recombination confirmed that decanal (floral, sweet), linalool oxide (floral, sweet), β-ionone (floral, violet), geraniol (floral), (Z)-jasmone (floral), linalool (floral, sweet), nonanal (rose, orange), and benzeneacetaldehyde (floral) were floral-like aroma active volatiles in TH green tea.
Fig. 6.
The results of recombination tests for the floral aroma scores of tea samples by using the 8 floral volatiles.
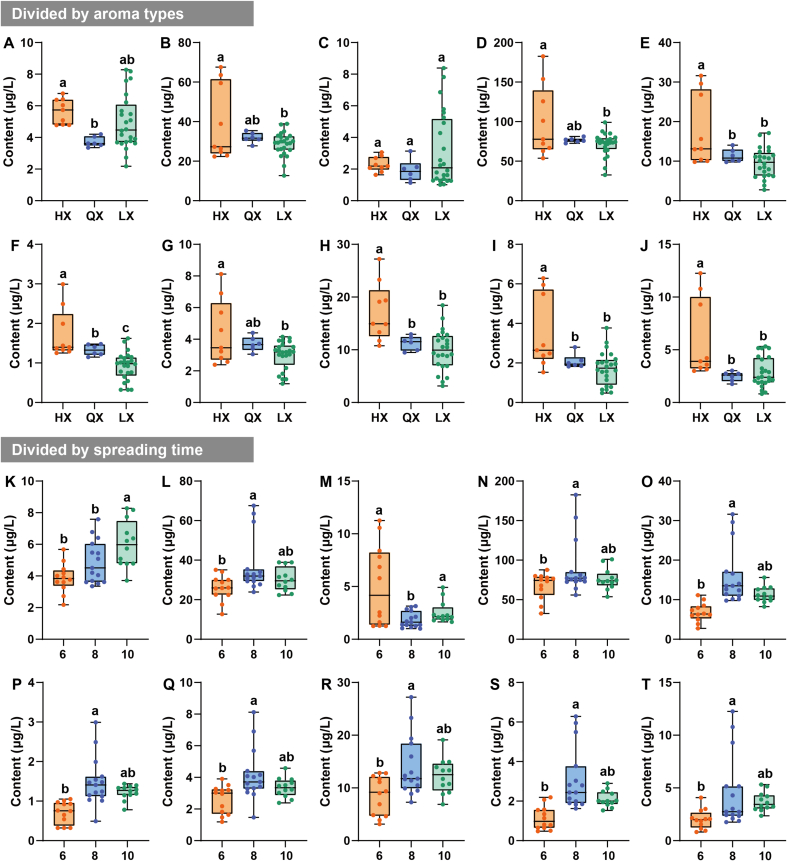
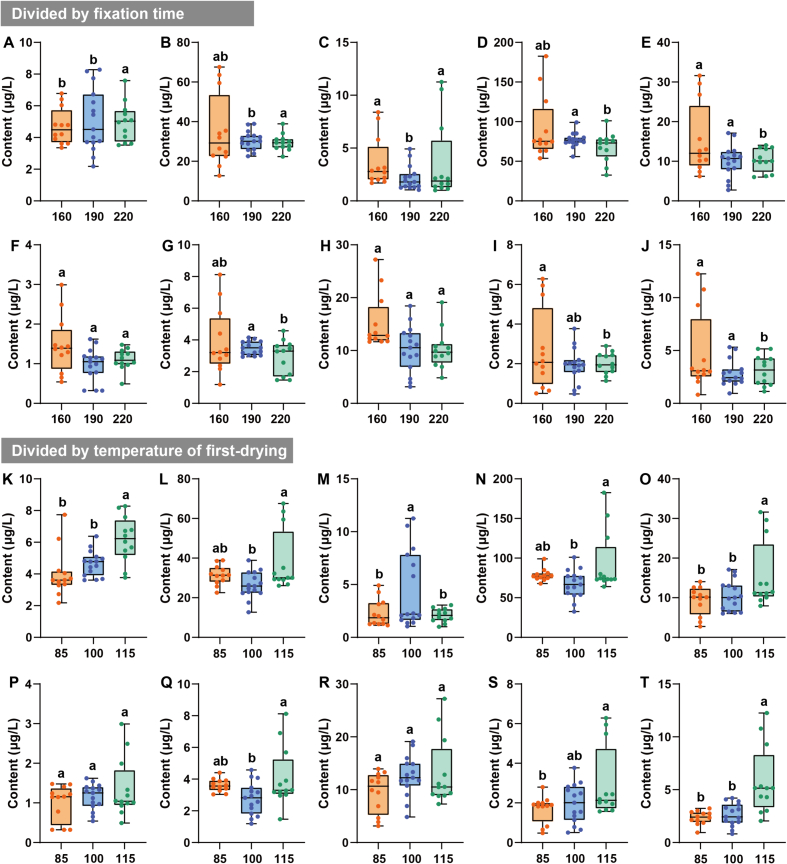
3.6. Enrichment patterns of floral-like aroma-active volatiles during processing
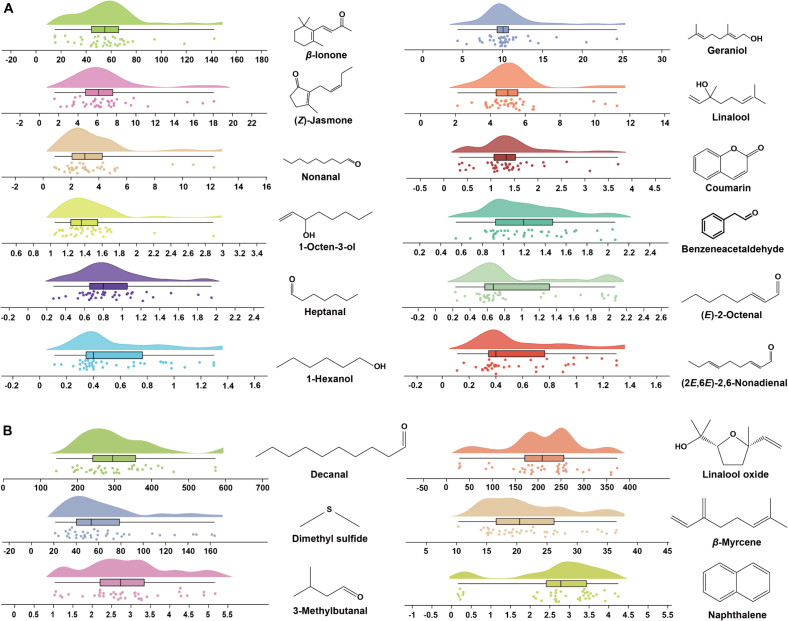
The aroma types of green teas can be classified as flowery (Huaxiang in Chinese, HX), chestnut-like (Lixiang in Chinese, LX), fresh (Qingxiang in Chinese, QX), and others. For the different aroma types, the content of floral-like volatiles was generally higher in HX than in both QX and LX (Fig. 7). For spreading time, the contents of floral-like volatiles were higher at 8–10 h than at 6 h. This further indicates that longer spreading time is more likely to yield floral-like volatiles in subsequent processing (Qiao et al., 2023; Xie et al., 2023). Spreading is a dehydration stress process in plants, which may be the result of reaching the saturation point of dehydration stress where no more water can be lost under certain conditions, thus shifting to the next process (Gu et al., 2021). Spreading step likewise influence the type, content and aroma profile of volatiles. For example, β-ionone is the key floral volatile identified in this study. The spreading step may cause photo-oxidation of β-carotene under UV irradiation and cleavage reaction of the β-ionone double bond on cyclohexene to produce ring-like aroma volatiles (Ho et al., 2015). Furthermore, linalool, geraniol, linalool oxide, and coumarin have likewise been shown to be accessible by hydrolyzing their glycosidic precursors during the spreading step (Ho et al., 2015). Therefore, considering the convenience of processing and cost control, a spreading time of 8 h is more favorable for accurate processing (Qiao et al., 2023). A reasonable drying temperature can retain the natural aroma and colour of green tea and control enzyme activity and oxidation reactions (Wang, Ouyang, et al., 2022). Overall, the fixation time at 160 s had an enhancing effect on the enrichment of seemingly floral aroma volatiles (Fig. 8). The contents of cis-linalool oxide (pyranoid) (3.63 ± 2.35 μg/L) and nonanal (4.92 ± 3.72 μg/L) were high, but the OAVs were <1. Therefore, they do not contribute significantly to the perceived attributes of floral in TH green tea (Hao et al., 2023). It is noteworthy that linalool and geraniol were more enriched at 85 °C. Benzeneacetaldehyde, (Z)-jasmone, β-ionone, and nonanal were enriched at 115 °C, which may be related to the temperature at which the chemical bonds of the precursors of different volatiles were broken, and all of them had OAVs over 1 (Qiu et al., 2023). The different drying temperatures mainly affected the aroma types of the final green tea product. The 10 floral-like aroma compounds were mostly alcohols, aldehydes, ketones and heterocyclic compounds. They are produced through biochemical actions such as thermal degradation of precursors, oxidation, and hydrolysis, and their synthesis requires catalysis at a certain temperature. Therefore, the formation of these floral-like volatiles is accelerated to varying degrees (Ho et al., 2015; Wang, Qu, et al., 2022; Yin, Kong, et al., 2022). Temperatures of 100–115 °C would be beneficial for the enrichment of floral-like volatiles.
Fig. 7.
Box plots of the volatiles with floral aroma varied in tea samples with different aroma types and spreading time. (A) Benzeneacetaldehyde; (B) Linalool; (C) cis-Linalool oxide (pyranoid); (D) Geraniol; (E) (Z)-Jasmone; (F) β-lonone; (G) (Z)-Geraniol; (H) Indole; (I) Methyl jasmonate; (J) Nonanal; (K) Benzeneacetaldehyde; (L) Linalool; (M) cis-Linalool oxide (pyranoid); (N) Geraniol; (O) (Z)-Jasmone; (P) β-lonone; (Q) (Z)-Geraniol; (R) Indole; (S) Methyl jasmonate; (T) Nonanal.
Fig. 8.
Box plots of the volatiles with floral aroma varied in tea samples with different fixation time and first-drying temperature. (A) Benzeneacetaldehyde; (B) Linalool; (C) cis-Linalool oxide (pyranoid); (D) Geraniol; (E) (Z)-Jasmone; (F) β-lonone; (G) (Z)-Geraniol; (H) Indole; (I) Methyl jasmonate; (J) Nonanal; (K) Benzeneacetaldehyde; (L) Linalool; (M) cis-Linalool oxide (pyranoid); (N) Geraniol; (O) (Z)-Jasmone; (P) β-lonone; (Q) (Z)-Geraniol; (R) Indole; (S) Methyl jasmonate; (T) Nonanal.
4. Conclusion
In summary, the optimal processing conditions for flowery-like Taiping Houkui green teas were obtained by RSM with the spreading time of 8.97 h, fixation time of 162.3 s, and temperature of first-drying of 103.32 s. GC–MS, GC-O, and aroma recombination confirmed the importance of flowery volatiles, including decanal, linalool oxide, β-lonone, geraniol, (Z)-jasmone, linalool, nonanal, and benzeneacetaldehyde. Furthermore, the accumulations of these volatiles were promoted with the extending spreading duration (8–10 h), reducing fixation duration (160–190 s), and increasing drying temperature (100–115 °C). In the future, there is still a need to further explore the importance of other aspects for the quality formation of flowery-like green teas, such as tea plant varieties and cultivation measures. Overall, this study provides new perspectives for the precise enhancement of floral odorants for green tea, thus benefiting targeted quality control in the industrial production of green tea.
CRediT authorship contribution statement
Yujie Wang: Writing – review & editing, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis. Nanfeng Liu: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Tianzi Yu: Writing – review & editing, Formal analysis. Jing Gao: Data curation. Yulin Fan: Validation, Supervision. Wenya Wang: Validation, Supervision. Junhan Wang: Validation, Supervision. Yida Wu: Validation, Supervision. Jixin Zhang: Validation, Supervision. Jingming Ning: Resources, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support of the National Key Research and Development Program of China (2021YFD1601102), the China Postdoctoral Science Foundation (2022M720192), the Agricultural and Material Technology and Equipment Project for Specialists of Science and Technology of Anhui Province (2022296906020011), the Natural Science Research Project of Anhui Higher Education Institution (KJ2021A0144).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101427.
Contributor Information
Yujie Wang, Email: wangyj@ahau.edu.cn.
Jingming Ning, Email: ningjm1998009@163.com.
Appendix A. Supplementary data
Supplementary material: Supplementary data 1
Data availability
Data will be made available on request.
References
- Béliveau R., Gingras D. Green tea: Prevention and treatment of cancer by nutraceuticals. The Lancet. 2004;364(9439):1021–1022. doi: 10.1016/S0140-6736(04)17076-1. [DOI] [PubMed] [Google Scholar]
- Chen J., Yang Y., Deng Y., Liu Z., Shen S., Zhu J.…Jiang Y. Characterization of the key differential volatile components in different grades of Dianhong congou tea infusions by the combination of sensory evaluation, comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry, and odor activity value. LWT - Food Science and Technology. 2022;165 doi: 10.1016/j.lwt.2022.113755. [DOI] [Google Scholar]
- Chen Q., Zhu Y., Dai W., Lv H., Mu B., Li P., Tan J., Ni D., Lin Z. Aroma formation and dynamic changes during white tea processing. Food Chemistry. 2019;274(15):915–924. doi: 10.1016/j.foodchem.2018.09.072. [DOI] [PubMed] [Google Scholar]
- Feng Z., Li Y., Li M., Wang Y., Zhang L., Wan X., Yang X. Tea aroma formation from six model manufacturing processes. Food Chemistry. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- Gamero A., Wesselink W., de Jong C. Comparison of the sensitivity of different aroma extraction techniques in combination with gas chromatography–mass spectrometry to detect minor aroma compounds in wine. Journal of Chromatography A. 2013;1272:1–7. doi: 10.1016/j.chroma.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Gu D., Yang J., Wu S., Liao Y., Zeng L., Yang Z. Epigenetic regulation of the Phytohormone abscisic acid accumulation under dehydration stress during postharvest processing of tea (Camellia sinensis) Journal of Agricultural and Food Chemistry. 2021;69(3):1039–1048. doi: 10.1021/acs.jafc.0c07220. [DOI] [PubMed] [Google Scholar]
- Guo X., Schwab W., Ho C.T., Song C., Wan X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chemistry. 2021;376 doi: 10.1016/j.foodchem.2021.131933. [DOI] [PubMed] [Google Scholar]
- Habib A., Landa E.N., Holbrook K.L., Walker W.S., Lee W.Y. Rapid, efficient, and green analytical technique for determination of fluorotelomer alcohol in water by stir bar sorptive extraction. Chemosphere. 2023;338 doi: 10.1016/j.chemosphere.2023.139439. [DOI] [PubMed] [Google Scholar]
- Hao Z., Feng J., Chen Q., Lin H., Zhou X., Zhuang J., Wang J., Tan Y., Sun Z., Wang Y., Yu B. Comparative volatiles profiling in milk-flavored white tea and traditional white tea Shoumei via HS-SPME-GC-TOFMS and OAV analyses. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Zhou J., Li Y., Zhang D., Ntezimana B., Zhu J., Wang X., Xu W., Wen X., Chen Y., Yu Z., Wang Y., Ni D. The aroma characteristics of oolong tea are jointly determined by processing mode and tea cultivars. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.-T., Zheng X., Li S. Tea aroma formation. Food Science and Human Wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Huang W., Lu G., Deng W.-W., Ning J. Effects of different withering methods on the taste of Keemun black tea. LWT - Food Science and Technology. 2022;166 doi: 10.1016/j.lwt.2022.113791. [DOI] [Google Scholar]
- Lancioni C., Castells C., Candal R., Tascon M. Headspace solid-phase microextraction: Fundamentals and recent advances. Advances in Sample Preparation. 2022;3 doi: 10.1016/j.sampre.2022.100035. [DOI] [Google Scholar]
- Li J., Han S., Mei X., Wang M., Han B. Changes in profiles of volatile compounds and prediction of the storage year of organic green tea during the long-term storage. Food Chemistry. 2024;437 doi: 10.1016/j.foodchem.2023.137831. [DOI] [PubMed] [Google Scholar]
- Liu N., Shen S., Huang L., Deng G., Wei Y., Ning J., Wang Y. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Research International. 2023;169 doi: 10.1016/j.foodres.2023.112845. [DOI] [PubMed] [Google Scholar]
- Nogueira J.M.F. Novel sorption-based methodologies for static microextraction analysis: A review on SBSE and related techniques. Analytica Chimica Acta. 2012;757:1–10. doi: 10.1016/j.aca.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Qiao D., Zhu J., Mi X., Xie H., Shu M., Chen M., Li R., Liu S., Wei C. Effects of withering time of fresh leaves on the formation of flavor quality of Taiping Houkui tea. LWT - Food Science and Technology. 2023;182 doi: 10.1016/j.lwt.2023.114833. [DOI] [Google Scholar]
- Qiu D., Duan R., Wang Y., He Y., Li C., Shen X., Li Y. Effects of different drying temperatures on the profile and sources of flavor in semi-dried golden pompano (Trachinotus ovatus) Food Chemistry. 2023;401 doi: 10.1016/j.foodchem.2022.134112. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Ochiai N., Yamazaki Y., Sasamoto K. Solvent-assisted stir bar sorptive extraction and gas chromatography–mass spectrometry with simultaneous olfactometry for the characterization of aroma compounds in Japanese Yamahai-brewed sake. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134640. [DOI] [PubMed] [Google Scholar]
- Wang B., Qu F., Wang P., Zhao L., Wang Z., Han Y., Zhang X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT - Food Science and Technology. 2022;161 doi: 10.1016/j.lwt.2022.113394. [DOI] [Google Scholar]
- Wang B., Yu M., Tang Y., Wang Y., Xia T., Song H. Characterization of odor-active compounds in Dahongpao Wuyi rock tea (Camellia sinensis) by sensory-directed flavor analysis. Journal of Food Composition and Analysis. 2023;123 doi: 10.1016/j.jfca.2023.105612. [DOI] [Google Scholar]
- Wang H., Hua J., Jiang Y., Yang Y., Wang J., Yuan H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109479. [DOI] [PubMed] [Google Scholar]
- Wang H., Ouyang W., Yu Y., Wang J., Yuan H., Hua J., Jiang Y. Analysis of non-volatile and volatile metabolites reveals the influence of second-drying heat transfer methods on green tea quality. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.-Q., Ma W.-J., Shi J., Zhu Y., Lin Z., Lv H.-P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Research International. 2020;130 doi: 10.1016/j.foodres.2019.108908. [DOI] [PubMed] [Google Scholar]
- Wei Y., Zhang J., Li T., Zhao M., Song Z., Wang Y., Ning J. GC–MS, GC–O, and sensomics analysis reveals the key odorants underlying the improvement of yellow tea aroma after optimized yellowing. Food Chemistry. 2024;431 doi: 10.1016/j.foodchem.2023.137139. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang L., Deng Y., Yuan H., Zhu J., Jiang Y., Yang Y. Characterization of the key odorants in floral aroma green tea based on GC-E-nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chemistry. 2023;427 doi: 10.1016/j.foodchem.2023.136641. [DOI] [PubMed] [Google Scholar]
- Yang Y., Chen J., Jiang Y., Qian M., Deng Y., Xie J., Li J., Wang J., Dong C., Yuan H. Aroma dynamic characteristics during the drying process of green tea by gas phase electronic nose and gas chromatography-ion mobility spectrometry. LWT - Food Science and Technology. 2022;154(15) doi: 10.1016/j.lwt.2021.112691. [DOI] [Google Scholar]
- Yin P., Kong Y.-S., Liu P.-P., Wang J.-J., Zhu Y., Wang G.-M., Sun M.-F., Chen Y., Guo G.-Y., Liu Z.-H. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends in Food Science & Technology. 2022;129:221–232. doi: 10.1016/j.tifs.2022.09.013. [DOI] [Google Scholar]
- Yin X., Huang J.A., Huang J., Wu W., Tong T., Liu S.…Zhang S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT - Food Science and Technology. 2022;164 doi: 10.1016/j.lwt.2022.113656. [DOI] [Google Scholar]
- Zeng L., Watanabe N., Yang Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Critical Reviews in Food Science and Nutrition. 2018;59(14):2321–2334. doi: 10.1080/10408398.2018.1506907. [DOI] [PubMed] [Google Scholar]
- Zhai X., Wang J., Wang H., Xue M., Yao X., Li M., Yu J., Zhang L., Wan X. Formation of dimethyl sulfide from the decomposition of S-methylmethionine in tea (Camellia sinensis) during manufacturing process and infusion brewing. Food Research International. 2022;162 doi: 10.1016/j.foodres.2022.112106. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xia D., Li T., Wei Y., Feng W., Xiong Z.…Ning J. Effects of different over-fired drying methods on the aroma of Lu’an Guapian tea. Food Research International. 2023;173 doi: 10.1016/j.foodres.2023.113224. [DOI] [PubMed] [Google Scholar]
- Zhang T., Fang K., Ni H., Li T., Li L.J., Li Q.B., Chen F. Aroma enhancement of instant green tea infusion using β-glucosidase and β-xylosidase. Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2020.126287. [DOI] [PubMed] [Google Scholar]
- Zhou H., Liu Y., Yang J., Wang H., Ding Y., Lei P. Comprehensive profiling of volatile components in Taiping Houkui green tea. LWT - Food Science and Technology. 2022;163 doi: 10.1016/j.lwt.2022.113523. [DOI] [Google Scholar]
- Zhu Y., Lv H.-P., Shao C.-Y., Kang S., Zhang Y., Guo L., Dai W.-D., Tan J.-F., Peng Q.-H., Lin Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Research International. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Supplementary data 1
Data Availability Statement
Data will be made available on request.