Abstract
Coenzyme Q10 (CoQ10) is an important cofactor and antioxidant for numerous cellular processes, and its deficiency has been linked to human disorders including mitochondrial disease, heart failure, Parkinson’s disease, and hypertension. Unfortunately, treatment with exogenous CoQ10 is often ineffective, likely due to its extreme hydrophobicity and high molecular weight. Here, we show that less hydrophobic CoQ species with shorter isoprenoid tails can serve as viable substitutes for CoQ10 in human cells. We demonstrate that CoQ4 can perform multiple functions of CoQ10 in CoQ-deficient cells at markedly lower treatment concentrations, motivating further investigation of CoQ4 as a supplement for CoQ10 deficiencies. In addition, we describe the synthesis and evaluation of an initial set of compounds designed to target CoQ4 selectively to mitochondria using triphenylphosphonium. Our results indicate that select versions of these compounds can successfully be delivered to mitochondria in a cell model and be cleaved to produce CoQ4, laying the groundwork for further development.
Keywords: coenzyme Q10 (CoQ10), ubiquinone, mitochondrial respiratory chain complex, mitochondrial therapeutics, membrane lipid, bioenergetics, antioxidant, ferroptosis, pyrimidine biosynthesis
Coenzyme Q (CoQ) is a ubiquitous redox-active lipid with critical functions throughout the cell. It is composed of a polyisoprenoid tail that anchors it in lipid bilayers and a benzoquinone head group that can accept and donate electrons. CoQ is required for shuttling electrons from complexes I and II to complex III in the electron transport chain (ETC) and participates in myriad mitochondrial and extramitochondrial processes including pyrimidine biosynthesis, ferroptosis suppression, fatty acid oxidation, sulfide detoxification, and membrane antioxidation (1). These diverse and important functions are reflected in the wide spectrum of clinical diseases that arise from deficiency of CoQ10 (the predominant CoQ species found in humans). Genetic defects in CoQ10 biosynthesis cause phenotypes varying from nephropathy and myopathy to fatal multiorgan disease (2). In addition, secondary deficits in CoQ10 levels have been observed in aging (3) and in numerous common conditions such as neurodegenerative diseases (4), cardiomyopathy (5), and primary mitochondrial diseases (6).
CoQ10 supplementation has emerged as an attractive therapeutic candidate for these disorders. CoQ10 treatment has been the subject of numerous clinical trials and is among the most common supplements taken in the Western world (5). Despite widespread interest and use, CoQ10 treatment is frequently ineffective (7, 8, 9). Poor bioavailability and cell delivery due to the large size and extreme hydrophobicity of CoQ10 are often cited as reasons for treatment failure (1, 8, 10). Transportation of the lipophilic CoQ10 across the gut epithelium and through the aqueous environment of the body is a major barrier to effective supplementation, with only 2% of enterally administered CoQ reaching the bloodstream (11) and an even smaller fraction reaching mitochondria and other target membranes in the cell (12, 13). Thus, a less hydrophobic CoQ analog or an analog better directed to mitochondria could more efficiently reach target membranes and represent attractive alternatives for CoQ10 supplementation.
The hydrophobicity of CoQ is primarily determined by its long polyisoprenoid tail (Table 1), which plays a critical role in anchoring CoQ within the core of the lipid bilayer to facilitate its interactions with membrane-embedded proteins. The specific number of isoprene units in the tail (denoted by a subscript) varies between organisms. For example, humans, Saccharomyces cerevisiae, and Escherichia coli have ten, six, and eight isoprene units in the tail of their dominant CoQ form, respectively. Although the reason for this variation remains poorly understood, it suggests that reducing the hydrophobicity of the CoQ tail may not compromise its function in humans.
Table 1.
Octanol-water partition coefficients (P) of CoQ species with increasing isoprenoid tail lengths as calculated using Advanced Chemistry Development (ACD) Software
| Coenzyme Q species | Octanol-water partition coefficient (LogP) |
|---|---|
| Coenzyme Q0 | 0.12 |
| Coenzyme Q1 | 2.61 |
| Coenzyme Q2 | 4.65 |
| Coenzyme Q3 | 6.68 |
| Coenzyme Q4 | 8.72 |
| Coenzyme Q5 | 10.75 |
| Coenzyme Q6 | 12.79 |
| Coenzyme Q7 | 14.82 |
| Coenzyme Q8 | 16.86 |
| Coenzyme Q9 | 18.89 |
| Coenzyme Q10 | 20.93 |
CoQ analogs with tail modifications have been investigated as CoQ10 substitutes. For instance, idebenone, a CoQ mimetic approved for treatment of Leber hereditary optic neuropathy (14), consists of the benzoquinone head group of CoQ with a fully saturated 10-carbon acyl chain capped by a hydroxyl group in place of the isoprenoid tail. The dramatic decrease in hydrophobicity and structural differences likely prevent its direct substitution for CoQ10 in the ETC; rather, its cellular effects occur through entirely separate mechanisms (15, 16, 17). Moreover, studies involving CoQ species with shortened isoprenoid tail lengths have been met with inconsistent outcomes (18). These short-chain quinones can act as cellular antioxidants and donate cytoplasmic electrons to complex III in the context of complex I impairment (16, 18); however, their ability to directly substitute for CoQ10 has not been fully established. In yeast, CoQ2 can rescue CoQ-deficient respiratory growth (19); in Drosophila, CoQ4 can rescue impaired neural growth from loss of CoQ (20). In human systems, CoQ6 was unable to increase CoQ-dependent oxidative phosphorylation (OxPhos) activities in HL-60 cells (21), and CoQ2 did not increase ATP levels in CoQ-deficient fibroblasts (17). Conversely, a separate study showed that CoQ4 restored ATP levels and CoQ-dependent OxPhos in CoQ-deficient patient fibroblasts (22). In addition to potential benefits, shorter chain quinones have also been associated with adverse effects in cells, such as the induction of reactive oxygen species (ROS) and apoptosis (21, 23, 24, 25).
Mitochondria-targeting CoQ species have also been developed as potential CoQ10 substitutes. Most notably, the molecule MitoQ is composed of the CoQ head group linked to triphenylphosphonium (TPP), a mitochondriotropic moiety. While MitoQ rapidly accumulates in mitochondria and exhibits potent antioxidant behavior, the attached TPP prevents the head group from properly interacting with ETC complexes (26), and thus it is unable to substitute for CoQ10 in supporting OxPhos. Here, toward establishing a more effective CoQ therapeutic, we examined a series of CoQ tail lengths across multiple assays to clarify the minimum hydrophobicity required for CoQ to function in human cells and developed a series of CoQ analogs with reversible TPP linkages to explore their ability to enhance the functional delivery of CoQ.
Results
Short-chain CoQ analogs support OxPhos
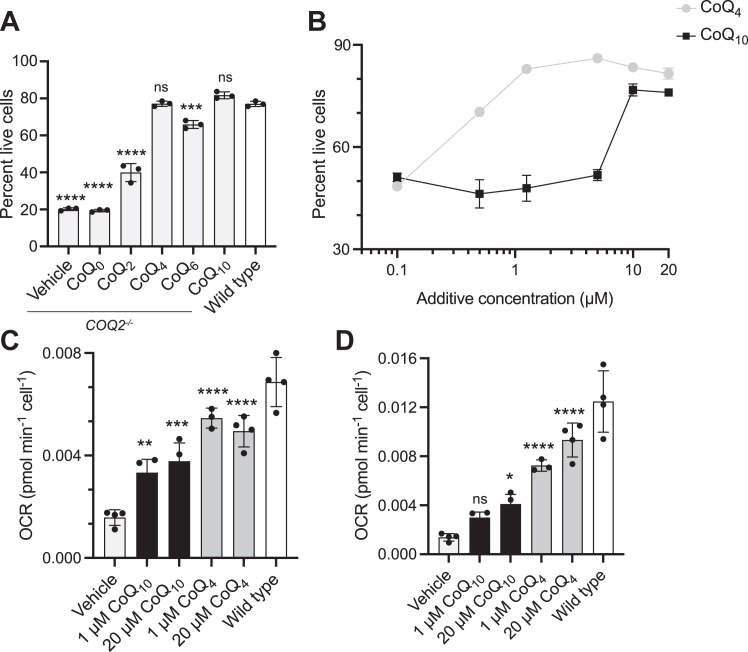
Although decreasing the tail length of CoQ may improve its bioavailability, its tail must still be sufficiently lipophilic to partition CoQ into the inner leaflet of the mitochondrial inner membrane to interact with ETC complexes. To find this balance between aqueous solubility and functionality, we sought to identify the minimum isoprenoid tail length required for CoQ to support OxPhos. Compromised OxPhos function causes cell death in media containing galactose as the sole carbon source (27). Accordingly, HepG2 COQ2−/− cells, which lack a key CoQ10 biosynthetic enzyme and are CoQ10 deficient, had increased rates of cell death in galactose media, which were reduced with 20 μM CoQ10 supplementation (Fig. 1A). Surprisingly, CoQ4 supplementation at this concentration was equally effective at preventing cell death. CoQ2 supplementation initially maintained viability in galactose (Fig. S1A), but extended treatment caused cell death, likely due to inherent toxicity of the compound (24, 25) (Fig. S1B). CoQ4 showed no evidence of cellular toxicity or increased ROS production at concentrations up to 100 μM (Fig. S2).
Figure 1.
Short-chain CoQ analogs support OxPhos.A, percent live wild-type or COQ2−/− HepG2 cells after 72 h of incubation in galactose media with 20 μM indicated CoQ additive. Cells were pretreated with CoQ additives for 48 h to ensure sufficient uptake. Live cells defined as not staining for AAD-7 or annexin-V. n = three independent technical replicates, and error bars indicate standard deviation. Significance calculated using a one-way ANOVA. B, survival of COQ2−/− HepG2 cells in galactose as in (A) over a titration of CoQ4 and CoQ10 concentrations. n = two independent technical replicates, and error bars indicate standard deviation. C and D, oxygen consumption rates (OCR) indicating (C) basal respiration and (D) maximal respiration after 3 μM FCCP treatment in COQ2−/− and wild-type HepG2 cells after 24 h incubation with CoQ additives. n = four independent technical replicates, and error bars indicate standard deviation. Significance calculated using a one-way ANOVA. ns, not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. For exact p values, see Table S1. CoQ, coenzyme Q; OxPhos, oxidative phosphorylation.
We repeated this analysis across a range of concentrations using CoQ4 and CoQ10 and found that, comparatively, 10-fold lower concentrations of the former were needed to rescue cell viability (Fig. 1B). Importantly, we confirmed that CoQ4 supplementation also increased basal and maximal respiration in CoQ10-deficient cells to a greater extent than CoQ10 supplementation (Fig. 1, C and D). CoQ4 supplementation additionally rescued mitochondrial polarization, ATP production, and oxygen consumption rate (OCR) in CoQ10-deficient cells grown in galactose media to a similar or greater extent than CoQ10 supplementation (Fig. S3). Overall, these results indicate that a tail length of four isoprene units is sufficient to support proper OxPhos function in human cells. Interestingly, while 1 μM of CoQ10 was not sufficient to rescue viability in galactose, it was able to increase basal OCR to a similar level as 20 μM CoQ10, suggesting that other functions of CoQ might contribute to viability in galactose media.
CoQ4 can support ferroptosis defense and pyrimidine biosynthesis
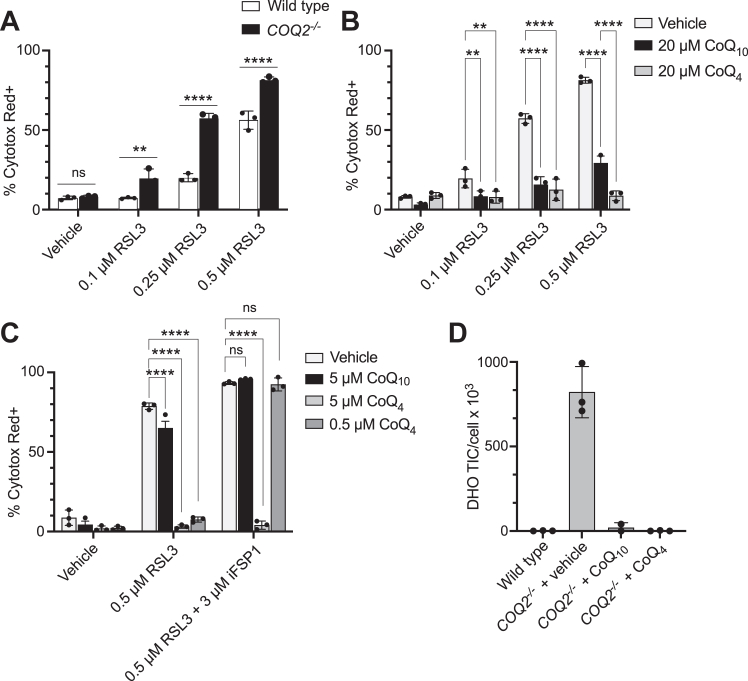
Next, we examined whether CoQ4 could participate in CoQ10 roles beyond the ETC, such as ferroptosis defense and de novo pyrimidine biosynthesis. Reduced CoQ10 (CoQ10H2) at the plasma membrane helps prevent ferroptosis through radical trapping and suppression of lipid peroxidation and is regenerated by the oxidoreductase FSP1 for continued ferroptosis suppression (28, 29). This pathway works in parallel with another major ferroptosis defense mechanism mediated by GPX4, a hydroperoxidase that neutralizes lipid peroxides using glutathione. Thus, the HepG2 COQ2−/− cells, which lack CoQ10 at the plasma membrane, were more sensitive to the GPX4 inhibitor RSL3 (Fig. 2A). This RSL3 sensitivity was decreased by CoQ10 supplementation and completely ablated by CoQ4 (Fig. 2B). Inhibition of FSP1 by its inhibitor iFSP1 blocked the ability of both CoQ10 and CoQ4 (at low concentrations) to prevent ferroptosis (Fig. 2C). Higher CoQ4 supplementation concentrations caused resistance to FSP1 inhibition, likely because there were sufficient amounts of reduced CoQ4 at the plasma membrane to preclude the need for FSP1-mediated regeneration.
Figure 2.
CoQ4can participate in ferroptosis suppression and pyrimidine biosynthesis.A, cell death of wild-type and COQ2−/− HepG2 cells after 24 h of treatment with GPX4-inhibitor RSL3. Cell death determined by high levels of Cytotox Red labeling. n = three independent technical replicates, and error bars indicate standard deviation. Significance calculated using a two-way ANOVA. B, cell death of COQ2−/− HepG2 cells after 24 h of treatment with RSL3 following 24 h of preincubation with 20 μM CoQ4 or CoQ10. n = three independent technical replicates, error bars indicate standard deviation. Significance is calculated using a two-way ANOVA. C, cell death of COQ2−/− HepG2 cells after 24 h of treatment with RSL3 or iFSP1 following 24 h of preincubation with indicated concentrations of CoQ4 or CoQ10. n = three independent technical replicates, and error bars indicate standard deviation. Significance calculated using a two-way ANOVA. D, total ion counts of dihydroorotate normalized to cell count in HAP1 wild-type and COQ2−/− cells after 24 h of treatment with 20 μM CoQ4 or CoQ10. n = three independent technical replicates, and error bars indicate standard deviation. ns, not significant, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. For exact p values see Table S1. CoQ, coenzyme Q; DHO,dihydroorotate.
CoQ also plays a key role in the pyrimidine de novo biosynthetic pathway as a cofactor for dihydroorotate dehydrogenase (DHODH); thus, HAP1 COQ2−/− cells accumulated dihydroorotate (DHO), the substrate of DHODH (Fig. 2D). Supplementation of both CoQ4 and CoQ10 restored DHO to wildtype levels (Fig. 2D), suggesting that DHODH can use CoQ4 to synthesize orotate. Altogether, these results indicate that CoQ4 can fulfill roles of CoQ10 outside of the ETC.
CoQ4 can ameliorate loss of cell viability due to statin treatment
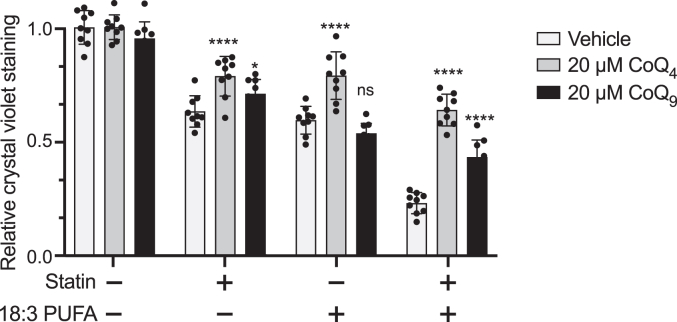
Statin treatment can reduce levels of CoQ10 in the body (8), as the mevalonate pathway synthesizes the precursors for the isoprenoid tail of CoQ. This decrease in CoQ10 is hypothesized to contribute to myopathy associated with statin use in patients (30). Accordingly, CoQ10 treatment can decrease statin-induced toxicity (31) and mitochondrial dysfunction (32) in cellular and rodent models. To test the ability of CoQ4 to alleviate statin-induced toxicity, we measured cell viability of C2C12 cells treated with simvastatin in the presence or absence of the polyunsaturated fatty acid linolenic acid (C18:3) (used to further sensitize the cells to statin-induced loss of cellular CoQ). In each case, CoQ4 restored viability equally or more effectively than CoQ9 (the predominant form of CoQ in rodent cells) (Fig. 3). These results suggest a potential therapeutic application for CoQ4 in alleviating statin-related dysfunction in clinical settings.
Figure 3.
CoQ4can ameliorate statin-induced loss of cell viability. Cell viability of C2C12 myocytes after 48 h of treatment with 50 μM simvastatin, 50 μM linolenic acid (C18:3), or a combination. Cells were pretreated with 20 μM CoQ4 or CoQ9 for 24 h prior to addition of statin and polyunsaturated fatty acids to ensure adequate uptake. Cell viability was assessed with crystal violet, with normalization to vehicle-treated cells with no CoQ pretreatment. n = nine independent technical replicates, error bars indicate standard deviation. Significance calculated using a two-way ANOVA. ns, not significant, ∗∗∗∗p < 0.0001. For exact p values, see Table S1. CoQ, coenzyme Q.
CoQ4 can be preferentially enriched in mitochondria
While CoQ4 is much less hydrophobic than CoQ10, it still contains 20 aliphatic carbons in its tail and retains considerable lipophilicity (Table 1). Therefore, there will likely still be significant barriers to its bioavailability and cellular delivery. We hypothesized that a reversible linkage to TPP, a mitochondria-targeting group, could increase the delivery of functional CoQ4. TPP is a well-studied lipophilic cation known to selectively transport cargo into mitochondria, driving concentrations of cargo in the mitochondrial matrix 100- to 1000-fold higher than extracellular concentrations. Moreover, TPP-conjugated compounds often bypass traditional transport mechanisms, instead directly passing through membranes to reach the mitochondrial matrix (33). Exogenous CoQ taken up by the cell primarily becomes trapped in lysosomes during transport (12, 13); thus, bypassing the cellular transport mechanisms with TPP could increase the amount of CoQ reaching target membranes.
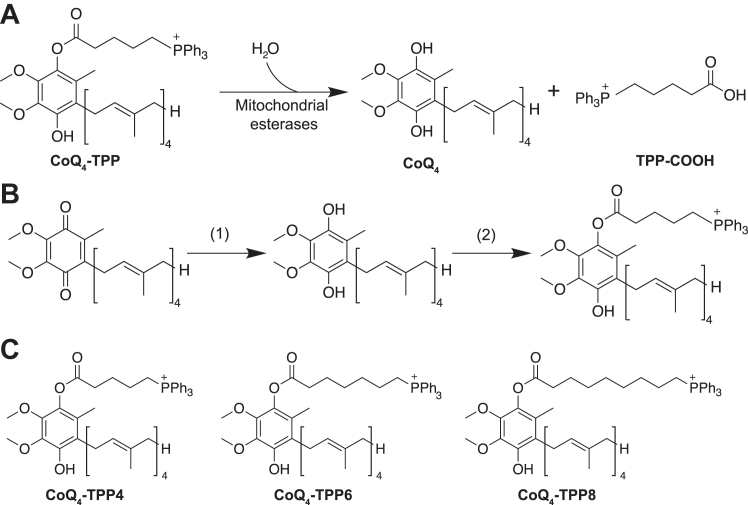
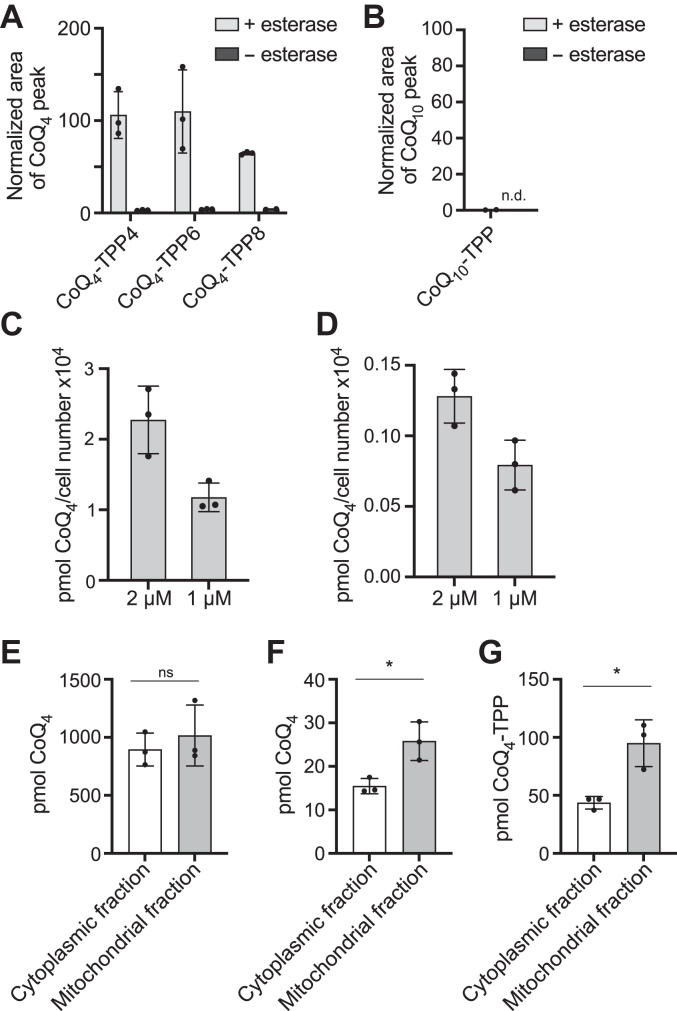
Studies with MitoQ have demonstrated that the irreversible attachment of TPP to CoQ prevents proper interactions with complex I and complex III, likely due to steric hindrance and improper membrane portioning. Therefore, we attached the TPP moiety to the head group of CoQ through an ester linkage (Fig. 4A). Esters are often labile in cells and can be hydrolyzed by resident nonspecific esterases to remove TPP-containing moieties (34, 35). We synthesized three CoQ4-TPP compounds with varying acyl linker chain lengths (Fig. 4B). Porcine liver esterase, a representative esterase, hydrolyzed all three compounds to a large extent in vitro (Fig. 5A), while a similar CoQ10-TPP compound was unable to be hydrolyzed (Fig. 5B). Incubating cells with CoQ4-TPP resulted in the dose-dependent production of free CoQ4 (Fig. 5D), although the resulting levels were 10-fold lower than levels from treatment with exogenous unmodified CoQ4 (Fig. 5C). Importantly, while unmodified CoQ4 treatment resulted in comparable amounts of CoQ4 in the cytoplasmic and mitochondrial fractions of the cell, CoQ4-TPP increased relative CoQ4 levels in the mitochondrial fraction compared to the cytoplasmic fraction (Figs. 5, E–G and S4), despite having diminished cellular uptake compared to CoQ4. Unfortunately, further investigations of CoQ4-TPP were limited by the toxicity of the compound (Fig. S5). While optimization of the delivery system to reduce toxicity and improve hydrolysis is required, these data provide proof-of-concept that CoQ4 can be reversibly linked to a mitochondria-targeting group and preferentially delivered to mitochondria, providing a platform for further study into CoQ targeting strategies.
Figure 4.
Schematic of CoQ4-TPP hydrolysis and synthetic scheme.A, schematic of CoQ4-TPP hydrolysis by mitochondrial esterases to produce CoQ4 and a carboxy-TPP side product (TPP-COOH). B, chemical synthesis of CoQ4-TPP. Reaction conditions: (1) NaBH4 in methanol/isopropanol (2) 4-carboxybutyl triphenylphosphonium bromide, N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide, 4-dimethylaminopyridine in dichloromethane. C, structures of CoQ4-TPP compounds synthesized for this study. CoQ, coenzyme Q; TPP, triphenylphosphonium.
Figure 5.
CoQ4-TPP can be hydrolyzed to produce CoQ4.A and B, CoQ production from CoQ-TPP species after 24 h of incubation with porcine liver esterase, normalized to equivalent CoQ4 (A) or CoQ10 (B) area indicating 100% turnover. CoQ levels measured by HPLC-ECD. n = three independent technical replicates, and error bars indicate standard deviation. C and D, levels of CoQ4 after 24 h of incubation of wild-type HepG2 cells with unmodified CoQ4 (C) or CoQ4-TPP (D) measured by HPLC-ECD after cellular lipid extraction. n = three independent technical replicates, and error bars indicate standard deviation. E and F, CoQ4 levels in different subcellular compartments after 3 h of incubation of HepG2 cells with 10 μM CoQ4 (E) or CoQ4-TPP4 (F). G, CoQ4-TPP levels in different subcellular compartments after 3 h of incubation of HepG2 cells with 10 μM CoQ4-TPP4. n = three independent technical replicates, and error bars indicate standard deviation. Significance calculated with a two-tailed, unpaired t test. ns, not significant, ∗p < 0.05. For exact p values see Table S1. CoQ, coenzyme Q; TPP, triphenylphosphonium.
Discussion
Stemming from its myriad critical cellular roles, CoQ10 deficiency leads to a variety of human diseases. CoQ10 supplementation is often recommended for numerous conditions including primary mitochondrial diseases, heart failure, Parkinson’s disease, and hypertension (5, 36). CoQ10 is widely used as a nutritional supplement, with a global market size estimated to be nearly 600 million USD per year (8). Unfortunately, while CoQ10 supplementation has clear benefits in isolated cases (5, 37, 38), evidence supporting its clinical efficacy is often weak, and treatment failure is common, likely because the extreme hydrophobicity and large size of CoQ10 limits its uptake and distribution to target membranes. Here, we further demonstrate that the less hydrophobic CoQ4 can fulfill multiple functions of CoQ10 in a cellular model, positing that it could be a viable therapeutic alternative for CoQ supplementation, and show that CoQ4 can be preferentially targeted to mitochondria through linkage to TPP.
Surprisingly, we found that CoQ4 could rescue CoQ-deficiency phenotypes in cells at much lower treatment concentrations than CoQ10. This likely reflects increased CoQ4 delivery to target membranes rather than enhanced CoQ4 functionality. Indeed, in a reconstituted system, complex I displayed decreased catalytic efficacy with shorter CoQ species including CoQ4 (39). CoQ4 must reach multiple cellular membranes to fulfill CoQ’s various cellular roles. Its movement throughout the cell could be mediated by the endomembrane system, as has been shown for CoQ10 (40), or could occur through a separate mechanism. Given that CoQ4 has been shown to exchange between phospholipid bilayers of two vesicle populations while CoQ10 cannot (19), it is possible that CoQ4 does not require the same level of active transport between membranes as CoQ10 but instead can move through the cell without relying on dedicated protein machinery.
Assessing the bioavailability and cellular delivery of CoQ4 in an animal model will provide further insight into its potential as a CoQ10 substitute. While CoQ4 is much less hydrophobic than CoQ10, it still possesses a highly hydrophobic 20-carbon tail and could experience similar bioavailability barriers as CoQ10. Idebenone, a CoQ analog with a fully saturated 10-carbon tail, is much less lipophilic than CoQ10 yet is still poorly water soluble, which presents many challenges to its use (41, 42, 43). Thus, additional strategies to boost CoQ4 delivery will likely be required to develop an effective therapeutic.
Toward further improving CoQ delivery, we trialed reversibly attaching TPP to the CoQ head group, as TPP can drive cargo molecules to high concentrations intracellularly and specifically in mitochondria. Encouragingly, we observed release of free CoQ4 that was enriched in mitochondria. Unexpectedly, there was a substantial amount of CoQ4 and CoQ4-TPP in the cytoplasmic fraction as well. We suspect that this discrepancy from the usual profound enrichment of TPP compounds in mitochondria is due to the known disruption of the inner mitochondrial membrane potential caused by hydrophobic TPP molecules (44, 45, 46), which would decrease the electrochemical gradient driving CoQ4-TPP into mitochondria and likely also contribute to the observed toxicity. A less disruptive mitochondria targeting group, such as the novel TPP-CF3 derivatives (45), could potentially target CoQ4 more effectively. Alternative targeting groups could additionally alter the lipophilicity of the molecule and could result in improved cellular uptake compared to the CoQ4-TPP. Additionally, we surmise that the toxicity of our compound arises from the unhydrolyzed CoQ4-TPP, as joint treatment with unmodified CoQ4 and TPP-COOH resulted in high intracellular levels without evident toxicity. Therefore, more labile linker constructs would likely decrease the toxicity of the compound while also leading to higher levels of CoQ4 enrichment. Overall, our work encourages further exploration of less hydrophobic CoQ analogs in therapeutic regimens and provides proof-of-concept that CoQ species can be reversibly linked to a targeting moiety to enhance delivery to mitochondria.
Experimental procedures
General cell culture
HepG2 lines (ATCC) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Thermo) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (R&D Systems, S11550) and 1x penicillin-streptomycin (Pen/Strep; Thermo, 15140122) at 37 ˚C and 5% CO2. The Genome Engineering and iPSC Center at Washington University in St Louis generated the HepG2 COQ2−/− cell lines. HAP1 lines (Horizon Discovery) were cultured in Iscove’s Modified Dulbecco’s Medium supplemented with 10% heat-inactivated FBS and 1x penicillin–streptomycin at 37 ˚C and 5% CO2. C2C12 lines (ATCC) were cultured in DMEM (Thermo) supplemented with 10% heat-inactivated FBS and 1x penicillin–streptomycin at 37 ⁰C and 5% CO2. Lines were regularly tested for mycoplasma contamination. Cell counts were obtained using a Muse Cell Analyzer (Luminex) and the Muse Count and Viability Assay (Luminex).
Galactose rescue
Wildtype and COQ2−/− HepG2 cells were plated at 75,000 cells/well in 6-well plates. After allowing cells to adhere overnight, medium was exchanged to DMEM containing either vehicle (isopropanol), CoQ0 (Sigma, D9150), CoQ2 (Sigma, C8081), CoQ4 (Sigma, C2470), CoQ6 (Avanti, 900150O), or CoQ10 (Sigma, C9538) in isopropanol. After 48 h of incubation, cells were washed with Dulbecco’s phosphate buffered saline (dPBS), and medium was replaced with glucose-free DMEM containing 10 mM of galactose, 1 mM pyruvate, and 50 μg/ml uridine along with CoQ additives or vehicle. After 72 h, cell apoptosis was assessed by the Muse Annexin-V and Dead Cell Kit (Luminex). For shorter CoQ2 supplementation, cells were incubated with 20 μM CoQ4 for 24 h prior to galactose media replacement, and apoptosis was assessed after 48 h in galactose.
Seahorse measurements
An Agilent Seahorse XFe96 Analyzer was used to determine OCRs. HepG2 wildtype and COQ2−/− cells were plated at 30K cells per well in XF96 microplates (Agilent, 102416-100) with media containing CoQ species. The next day, cell media were exchanged for XF assay medium supplemented with 25 mM glucose, 1 mM pyruvate, 4 mM glutamine (Agilent, 03680-100), and CoQ species (Sigma). Oxygen consumption rate was measured at baseline and after injections of 2 μM oligomycin, 3 μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and 0.5 μM rotenone/antimycin A (Agilent, 103015-100). For galactose OCR measurements, HepG2 wildtype and COQ2−/− cells were preincubated with either vehicle, 20 μM CoQ4 (Sigma, C2470), or 20 μM CoQ10 (Sigma, C9538) in isopropanol for 72 h, then plated at 20K cells/well with CoQ species and allowed to adhere overnight. Cells were washed with dPBS, and medium was replaced with glucose-free DMEM containing 10 mM of galactose, 1 mM pyruvate, and 50 μg/ml uridine. The next day, cell media were exchanged for XF assay medium supplemented with 25 mM glucose, 1 mM pyruvate, and 4 mM glutamine (Agilent, 03680-100), and baseline oxygen consumption rates were measured. For all measurements, rates were normalized to cell count per well obtained immediately prior to XF assay medium exchange, obtained by Sartorius IncuCyte S3 live-cells analysis system.
Ferroptosis sensitivity
Wildtype and COQ2−/− HepG2 cells were plated at 25,000 cells/well in a 24-well plate and allowed to adhere overnight. Medium was exchanged to DMEM with 1 mM pyruvate and 50 μg/ml uridine along with either vehicle (isopropanol) or CoQ additives (Sigma) in isopropanol. After 48 h of incubation, cells were washed with dPBS. Media were replaced with DMEM with 1 mM pyruvate, 50 μg/ml uridine, and IncuCyte Cytotox Red Reagent (Fisher, NC1015259); along with RSL3 (Sigma, SML2234), iFSP1 (Fisher, NC1755669), or vehicle (DMSO). Cell death was quantified after 24 h using a Sartorius IncuCyte S3 live-cells analysis system.
Dihydroorotate and ATP measurements
For DHO measurements, HAP1 wildtype and COQ2−/− cells were plated at 500,000 cells/well in a 6-well plate and allowed to adhere overnight. Media were exchanged to DMEM containing either vehicle, 20 μM CoQ4 (Sigma, C2470) or 20 μM CoQ10 (Sigma, C9538) in isopropanol. After 24 h of incubation, cells were washed three times with cold dPBS, then incubated at −80 ˚C with cold LC-MS grade 80:20 methanol/water (v/v) for 15 min. Cells collected and centrifuged at 16,000g for 5 min. Supernatant was collected and dried under nitrogen flow. For ATP measurements, HepG2 wildtype and COQ2−/− cells were preincubated with either vehicle, 20 μM CoQ4 (Sigma, C2470) or 20 μM CoQ10 (Sigma, C9538) in isopropanol for 72 h, then plated at 500,000 cells/well in a 6-well plate with CoQ species and allowed to adhere overnight. Cells were washed with dPBS, and medium was replaced with glucose-free DMEM containing 10 mM of galactose, 1 mM pyruvate, and 50 μg/ml uridine. After 24 h, metabolites were extracted as above. To measure the metabolite species, samples were resuspended in LC-MS grade water. Samples were analyzed using a Thermo Q-Exactive mass spectrometer coupled to a Vanquish Horizon UHPLC. Analytes were separated on a 100 × 2.1 mm, 1.7 μM Acquity UPLC BEH C18 Column (Waters), with a 0.2 ml min−1 flow rate and with a gradient of solvent A (97:3 H2O/methanol, 10 mM TBA, 9 mM acetate, pH 8.2) and solvent B (100% methanol). The gradient is as follows: 0 min, 5% B; 2.5 min, 5% B; 17 min, 95% B; 21 min, 95% B; and 21.5 min, 5% B. Data were collected in full-scan negative mode. Setting for the ion source was as follows: 10 aux gas flow rate, 35 sheath gas flow rate, 2 sweep gas flow rate, 3.2 kV spray voltage, 320 °C capillary temperature, and 300 °C heater temperature. The metabolites reported here were identified based on exact m/z and retention times determined with chemical standards.
Mitochondrial membrane potential analysis
HepG2 wildtype and COQ2−/− cells were plated at 4,000 cells/well in a 24-well plate and allowed to adhere overnight. Media were exchanged to glucose-free DMEM containing 10 mM of galactose, 1 mM pyruvate, and 50 μg/ml uridine with either vehicle, 5 μM CoQ4 (Sigma, C2470), or 5 μM CoQ10 (Sigma, C9538) in isopropanol. After 48 h of incubation, cells were washed with dPBS and incubated for 5 min with vehicle or 6.66 μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), then 100 nM TMRM was added. After 30 min, red fluorescence and cell number were determined using a Sartorius IncuCyte S3 live-cells analysis system.
Statin toxicity assay
C2C12 cells were plated at 10,000 cells/well in a 96-well plate and allowed to adhere overnight. The next day, medium was replaced with fresh DMEM containing either vehicle (isopropanol) or 20 μM CoQ additive (Sigma) in isopropanol. After 24 h, cells were washed with dPBS, and media were replaced with DMEM containing 10 mM galactose with varying concentrations of simvastatin (Sigma, S6196) in isopropanol and/or linolenic acid (Sigma, L2376) in isopropanol. After 48 h, media were removed, and cells were washed three times with dPBS. Crystal violet staining solution (0.5% crystal violet in 25% methanol) was added to the cells and allowed to incubate for 20 min with gentle rocking. After removing the staining solution, cells were washed three times with dPBS, and remaining crystal violet was dissolved with 100% methanol. Absorbance was read at 540 nm in a microplate reader (Cytation3).
ROS measurements and cell growth assay
Cells were plated at 40,000 cells/well in a 24-well plate and allowed to adhere overnight. Cell media were exchanged to DMEM containing 5 μM dichlorodihydrofluorescein diacetate (Abcam, ab113851) and incubated for 30 min at 37 °C. Cells were washed twice with dPBS, and medium was replaced with DMEM containing the CoQ additives (Sigma), menadione (Sigma, M5625), or vehicle (isopropanol). Using a Sartorius IncuCyte S3 live-cells analysis system, green fluorescence was determined after 24 hours of incubation to obtain ROS measurements, and cell number was obtained after 48 hours of incubation to calculate fold change.
Synthesis of CoQ-TPP compounds
To synthesize CoQ4-TPP compounds, CoQ4 (100 mg, 2.26 μmol) was added to isopropanol/methanol (10 ml). CoQ4 for synthesis is obtained from WuXi Chemicals. NaBH4 (Sigma, 480886) was added to CoQ until color change from orange to colorless was observed, indicating reduction. Reaction quenched with 1M hydrochloric acid (20 ml), diluted with dichloromethane (15 ml), and washed with 1M hydrochloric acid (50 ml) then brine (50 ml). Organic layer was sealed under argon. 4-dimethylaminopyridine (25 mg, 2.0 μmol; Sigma, 107700), N-(3-dimethylaminopropyl)-N’ethylcarbodiimide hydrochloride (45 mg, 2.0 μmol; Sigma, 03450), and 4-carboxybutyl- (Sigma, 157945); 6-carboxyhexyl (WuXi Chemicals) or 8-carboxyoctyl (Toronto Research Chemicals, C178905) triphenylphosphonium bromide (100 mg, 2.0 μmol) were dissolved in dichloromethane and added dropwise to reduced CoQ. Reaction was stirred at room temperature for 72 h, and product formation was confirmed by TLC. Solvent was removed under reduced pressure, and column chromatography on silica gel with 2:1 ethyl acetate:methanol gave products as yellow gel. Remaining silica gel was filtered with acetonitrile elution. The yield calculated at 5 to 10% for monoacylated products.
CoQ4-TPP4: 1H NMR 7.87 (t, 6H), 7.77 (t, 3H), 7.68 (t, 6H), 5.09 (m, 4H), 4.00 (m, 2H), 3.87 (m, 2H), 3.67 (s, 2H), 3.65 (s, 2H), 3.32 (d, 1H), 3.09 (d, 1H), 2.11 (d, 3H), 2.05 (d, 5H, 1.97 (d, 5H), 1.68 (s, 9H), 1.60 (m, 12H), 1.23 (s, 6H), 0.89 (m, 3H). 13C NMR 134.89, 133.85, 130.49, 124.42, 118.92, 118.24, 96.15, 60.83, 39.75, 32.94, 29.72, 26.79, 25.72, 25.42, 21.88, 20.39, 17.72, 16.03, 12.12. HRMS (ESI-MS, m/z) calculated 801.46, found 801.465.
CoQ4-TPP6: 1H NMR 7.89 (t, 6H), 7.78 (t, 3H), 7.70 (t, 6H), 5.09 (m, 4H), 3.97 (m, 2H), 3.88 (m, 2H), 3.76 (s, 2H), 3.33 (d, 1H), 3.14 (d, 1H), 2.56 (M, 2H), 2.12 (s, 2H), 2.05 (d, 5H, 1.97 (d, 6H), 1.76 (s, 15H), 1.68 (m, 9H), 1.60 (s, 9H), 1.46 (m, 3H), 0.93 (m, 2H). 13C NMR 172.11, 144.87, 137.64, 134.89, 133.75, 130.4, 124.43, 118.99, 118.3, 96.15, 60.98, 39.75, 33.72, 29.81, 28.53, 26.80, 25.73, 24.5, 22.45, 17.72, 16.02, 12.12. HRMS (ESI-MS, m/z) calculated 829.50, found 829.495.
CoQ4-TPP8: 1H NMR 7.86 (t, 6H), 7.79 (t, 3H), 7.71 (t, 6H), 5.10 (m, 4H), 4.0 (m, 2H), 3.9 (m, 3H), 3.79 (m, 2H), 3.7 (m, 1H), 3.34 (d, 1H), 3.2 (d, 1H), 2.55 (m, 1H), 2.48 (m, 1H), 2.05 (m, 6H), 2.0 (m, 6H), 1.81 (m, 18H), 1.68 (s, 6H), 1.60 (m, 12H), 1.26 (m, 9H), 0.89 (m, 2H). 13C NMR 135.99, 134.87, 133.74, 130.50, 130.40, 124.42, 119.02, 118.34, 96.14, 39.75, 29.73, 28.83, 27.36, 26.79, 25.73, 24.99, 24.15, 22.72, 17.72, 16.05. HRMS (ESI-MS, m/z) calculated 857.53, found 857.526.
High performance liquid chromatography–electrochemical detection measurement of CoQ levels
Samples were injected into HPLC (Ultimate 3000, Thermo Scientific) with an electrochemical detector (ECD-3000RS). The first electrode (6020RS) was set to +600 mV and placed before the column (Thermo Scientific, Betasil C18, 100 × 2.1 mm, 3 μM particle) to oxidize all quinones. The second electrode (6011RS) was set to −600 mV to reduce all quinones exiting the column, and the third electrode was set at +600 mV to measure redox active species. Peaks were quantified with Chromeleon 7.2.10 software. To measure CoQ4 levels, extracted lipids were resuspended in methanol, and the mobile phase was 95% methanol 5% 1M ammonium acetate pH 4.4 in water. To measure CoQ10 levels, extracted lipids were resuspended in isopropanol, and the mobile phase was 78% methanol, 20% isopropanol, and 2% 1M ammonium acetate pH 4.4 in water.
Representative esterase hydrolysis
CoQ-TPP compounds were incubated at 200 μM at 37 °C for 24 h in KCl buffer (120 mM KCl, 10 mM Hepes, 1 mM EGTA, pH = 7.2) containing either 1 mg/ml porcine liver esterase (Sigma, E2884) or a buffer control along with CoQ2 (Sigma, C8081) as an internal standard. To extract CoQ4, cold LC-MS grade methanol was added, and sample was incubated at −80 °C for 15 min. Sample was vortexed for 5 min at 4 °C in a disruptor genie set to max (3000 rpm) and centrifuged at 16,000g for 5 min. Supernatant was collected and dried under nitrogen flow, and CoQ species were measured by high performance liquid chromatography–electrochemical detection (HPLC-ECD). To extract CoQ10, 400 μl petroleum ether was added to reaction. Samples were vortexed for 10 min at 4 °C in a disruptor genie set to max (3000 rpm), then centrifuged at 1000g for 3 min. Petroleum ether layer was collected, and 400 μl fresh petroleum ether added to reaction mixture. Samples were vortexed for 3 min at 4 °C in a disruptor genie set to max (3000 rpm), then centrifuged at 1000g for 3 min. Petroleum ether layer was collected and combined with prior petroleum ether, dried under nitrogen flow, and CoQ species measured by HPLC-ECD.
Extraction of CoQ species
Wildtype HepG2 cells wells were seeded at 3,000,000 cells/well in a 6-well plate and allowed to adhere overnight. Medium was exchanged to DMEM containing either vehicle (isopropanol), CoQ4 (Sigma, C2470), or CoQ4-TPP. After 24 h, cells were trypsinized and pelleted (600g, 5 min). Pellet was washed with dPBS) + 10% isopropanol followed by dBPS + 30% isopropanol. Pellet was flash frozen in 500 μl cold methanol with 2 μM of CoQ6 (Avanti, 900150O) as an internal standard. Cells were lysed by vortexing for 10 min at 4 °C in a disruptor genie set to max (3000 rpm) speed. Sample was spun 5 min 16,000g, and the supernatant was collected. Five hundred microliter of cold methanol was added, and sample was vortexed for 3 min and then spun as above. Supernatant was again collected and combined with supernatant from previous step and dried under nitrogen gas.
Mitochondria subfractionation and CoQ extraction
HepG2 cells were seeded in 15 cm plates and allowed to adhere overnight. Media were exchanged for DMEM with 10 μM either CoQ4-TPP or CoQ4 (Sigma, C2470). After 3 h, mitochondrial fractions were isolated from cells through differential centrifugation as described previously (Frezza, 2007). Briefly, cells and media were collected and washed with dPBS then dPBS + 10% isopropanol. Cells were resuspended in isolation buffer (10 mM Tris-Mops, pH 7.4, 1 mM EGTA/Tris, 200 mM sucrose) and homogenized with 50 strokes of a glass-Teflon potter. Whole cell and nuclei were pelleted (10 min × 600g, 4 °C), supernatant collected and centrifuged to produce mitochondrial fraction (10 min × 7000g, 4 °C). Supernatant of hard spin collected as cytoplasmic fraction. Protein content in fractions was quantified using Pierce BCA Protein Assay Kit. (Thermo, 23225). Fifty microgram protein from each fraction saved for later analysis by Western blot.
To extract CoQ4, the mitochondrial fraction was resuspended in an equivalent amount of isolation buffer to cytoplasmic fraction. Four hundred microliters of chloroform with CoQ6 (Avanti, 900150O) as an internal standard was added, and the sample was vortexed for 10 min at 4 °C in a disruptor genie set to max (3000 rpm) speed. Chloroform was collected, and process is repeated. Lipids were dried and measured with HPLC-ECD.
To assess fraction purity, the 50 μg protein collected was methanol precipitated. Sample was solubilized in radioimmunoprecipitation assay buffer, and 1 ml methanol was added. Sample was pelleted (20 min, 16,000g), and supernatant was discarded. 1 ml 90:10 methanol:water (v/v) was added, sample was pelleted (20 min, 16,000g), and supernatant was discarded. Precipitated protein was resuspended in sample buffer (Invitrogen, NP0007) to a final concentration of 1 mg/ml. Ten microgram of protein from each sample was separated on a NuPAGE 10% Bis-Tris gel (Thermo, NP0303BOX) with a protein standard (Licor, 928-60000). Resolved proteins were then transferred to PVDF membrane (Fisher, IPFL00010), probed with primary antibodies (Abcam, 154856; Cell Signaling, 9644S) followed by HRP-conjugated secondary antibody (Cell Signaling Technologies, 7074S, 7076S) and ECL substrate (Thermo, 34579, 34094). Blots were imaged and analyzed using Azure5 Imaging System (version 1.9.0.0406).
CoQ-TPP toxicity cell counts
Wildtype HepG2 cells seeded at 150,000 cells/well in 6-well plate and allowed to adhere overnight. Media were exchanged for DMEM containing CoQ-TPP additives and incubated for 24 h. Cells were washed with dPBS, collected, and counted using a Muse Cell Analyzer and the Muse Count and Viability Assay (Luminex).
Data availability
All data contained within the manuscript.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank members of the Pagliarini and Fan labs for helpful discussions throughout this project. We thank P. Cobra for assistance with NMR data acquisition.
Author contributions
L. H. S. and D. J. P. conceptualization; L. H. S., S. R., A. Y. S., J. F., and D. J. P. methodology; L. H. S., S. R., and A. Y. S. formal analysis; L. H. S., S. R., and A. Y. S. investigation; L. H. S. and D. J. P. writing–original draft; S. R., A. Y. S., and J. F. writing–review and editing; J. F. and D. J. P. supervision; D. J. P. resources; D. J. P. visualization; D. J. P. project administration; D. J. P. funding acquisition.
Funding and additional information
This work was supported by National Institutes of Health (NIH) awards R35GM131795 (D. J. P.), T32GM008505 (L. H. S), T32GM140935 (L. H. S. and A. Y. S.), T32AG000213 (A. Y. S.), and funds from the BJC Investigator Program (D. J. P.). This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grant P41GM136463, P41GM103399 (NIGMS). Equipment was purchased with funds from the University of Wisconsin-Madison, the NIH P41GM136463, P41GM103399, S10RR02781, S10RR08438, S10RR023438, S10RR025062, and S10RR029220), the NSF (DMB-8415048, OIA-9977486, and BIR-9214394), and the USDA. This study made use of the Washington University in St Louis Genome Engineering and iPSC Center for cell line generation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Ursula Jakob
Supporting information
References
- 1.Guerra R.M., Pagliarini D.J. Coenzyme Q biochemistry and biosynthesis. Trends Biochem. Sci. 2023;48:463–476. doi: 10.1016/j.tibs.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desbats M.A., Lunardi G., Doimo M., Trevisson E., Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ10) deficiency. J. Inherit. Metab. Dis. 2015;38:145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 3.Kalén A., Appelkvist E.-L., Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 4.Jiménez-Jiménez F.J., Alonso-Navarro H., García-Martín E., Agúndez J.A.G. Coenzyme Q10 and Parkinsonian Syndromes: a systematic review. J. Pers. Med. 2022;12:975. doi: 10.3390/jpm12060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayer A., Macdonald P., Stocker R. CoQ10 function and role in heart failure and Ischemic heart disease. Annu. Rev. Nutr. 2015;35:1–39. doi: 10.1146/annurev-nutr-071714-034258. [DOI] [PubMed] [Google Scholar]
- 6.Navas P., Cascajo M.V., Alcázar-Fabra M., Hernández-Camacho J.D., Sánchez-Cuesta A., Rodríguez A.B.C., et al. Secondary CoQ10 deficiency, bioenergetics unbalance in disease and aging. Biofactors. 2021;47:551–569. doi: 10.1002/biof.1733. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy C., Köller Y., Surkova E. Effect of Coenzyme Q10 on statin-associated myalgia and adherence to statin therapy: a systematic review and meta-analysis. Atherosclerosis. 2020;299:1–8. doi: 10.1016/j.atherosclerosis.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Hekimi S. The efficacy of coenzyme Q10 treatment in alleviating the symptoms of primary coenzyme Q10 deficiency: a systematic review. J. Cell. Mol. Med. 2022;26:4635–4644. doi: 10.1111/jcmm.17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z.-G., Sun M.-X., Zhang W.-L., Wang W.-W., Jin Y.-M., Xie C.-L. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol. Sci. 2017;38:215–224. doi: 10.1007/s10072-016-2757-9. [DOI] [PubMed] [Google Scholar]
- 10.López-Lluch G., del Pozo-Cruz J., Sánchez-Cuesta A., Cortés-Rodríguez A.B., Navas P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition. 2019;57:133–140. doi: 10.1016/j.nut.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Aberg F., Appelkvist E.-L., Dallner G., Ernster L. Uptake of dietary coenzyme Q supplement is limited in Rats. J. Nutr. 1994;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- 12.Bentinger M., Dallner G., Chojnacki T., Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic. Bio. Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- 13.Padilla-López S., Jiménez-Hidalgo M., Martín-Montalvo A., Clarke C.F., Navas P., Santos-Ocaña C. Genetic evidence for the requirement of the endocytic pathway in the uptake of coenzyme Q6 in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2009;1788:1238–1248. doi: 10.1016/j.bbamem.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carelli V., Morgia C.L., Valentino M.L., Rizzo G., Carbonelli M., Negri A.M.D., et al. Idebenone treatment in Leber’s hereditary optic neuropathy. Brain. 2011;134:e188. doi: 10.1093/brain/awr180. [DOI] [PubMed] [Google Scholar]
- 15.Gueven N., Woolley K., Smith J. Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10. Redox Biol. 2015;4:289–295. doi: 10.1016/j.redox.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erb M., Hoffmann-Enger B., Deppe H., Soeberdt M., Haefeli R.H., Rummey C., et al. Features of idebenone and related short-chain quinones that rescue ATP levels under conditions of impaired mitochondrial complex I. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López L.C., Quinzii C.M., Area E., Naini A., Rahman S., Schuelke M., et al. Treatment of CoQ10 deficient fibroblasts with ubiquinone, CoQ analogs, and Vitamin C: time- and compound-dependent effects. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suárez-Rivero J.M., Pastor-Maldonado C.J., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Munuera-Cabeza M., et al. Coenzyme Q10 analogues: benefits and challenges for therapeutics. Antioxidants. 2021;10:236. doi: 10.3390/antiox10020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James A.M., Cochemé H.M., Murai M., Miyoshi H., Murphy M.P. Complementation of coenzyme Q-deficient yeast by coenzyme Q analogues requires the isoprenoid side chain. FEBS J. 2010;277:2067–2082. doi: 10.1111/j.1742-4658.2010.07622.x. [DOI] [PubMed] [Google Scholar]
- 20.Grant J., Saldanha J.W., Gould A.P. A Drosophila model for primary coenzyme Q deficiency and dietary rescue in the developing nervous system. Dis. Model. Mech. 2010;3:799–806. doi: 10.1242/dmm.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Ayala D.J.M., López-Lluch G., García-Valdés M., Arroyo A., Navas P. Specificity of coenzyme Q10 for a balanced function of respiratory chain and endogenous ubiquinone biosynthesis in human cells. Biochim. Biophys. Acta. 2005;1706:174–183. doi: 10.1016/j.bbabio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Cerqua C., Casarin A., Pierrel F., Fonseca L.V., Viola G., Salviati L., et al. Vitamin K2 cannot substitute Coenzyme Q10 as electron carrier in the mitochondrial respiratory chain of mammalian cells. Sci. Rep. 2019;9:6553. doi: 10.1038/s41598-019-43014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devun F., Walter L., Belliere J., Cottet-Rousselle C., Leverve X., Fontaine E. Ubiquinone analogs: a mitochondrial permeability transition pore-dependent pathway to selective cell death. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esaka Y., Nagahara Y., Hasome Y., Nishio R., Ikekita M. Coenzyme Q2 induced p53-dependent apoptosis. Biochim. Biophys. Acta. 2005;1724:49–58. doi: 10.1016/j.bbagen.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T., Mine Y., Okamoto T. Intracellular reduction of coenzyme Q homologues with a short isoprenoid side chain induces apoptosis of HeLa cells. J. Biochem. 2018;163:329–339. doi: 10.1093/jb/mvy002. [DOI] [PubMed] [Google Scholar]
- 26.James A.M., Sharpley M.S., Manas A.-R.B., Frerman F.E., Hirst J., Smith R.A.J., et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007;282:14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 27.Robinson B.H., Petrova-Benedict R., Buncic J.R., Wallace D.C. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem. Med. Metab. Biol. 1992;48:122–126. doi: 10.1016/0885-4505(92)90056-5. [DOI] [PubMed] [Google Scholar]
- 28.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 29.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschetti A., Dagda R.K., Ryan R.O. Coenzyme Q nanodisks counteract the effect of statins on C2C12 myotubes. Nanomedicine. 2021;37 doi: 10.1016/j.nano.2021.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan R.A., Garcia-Smith R., Bisoffi M., Conn C.A., Trujillo K.A. Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: implications for statin-induced rhabdomyolysis. Eur. J. Pharmacol. 2013;711:1–9. doi: 10.1016/j.ejphar.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 32.El-Ganainy S.O., El-Mallah A., Abdallah D., Khattab M.M., El-Din M.M.M., El-Khatib A.S. Elucidation of the mechanism of atorvastatin-induced myopathy in a rat model. Toxicology. 2016;359:29–38. doi: 10.1016/j.tox.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Murphy M.P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta. 2008;1777:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Prag H.A., Kula-Alwar D., Pala L., Caldwell S.T., Beach T.E., James A.M., et al. Selective delivery of Dicarboxylates to mitochondria by conjugation to a lipophilic cation via a cleavable linker. Mol. Pharm. 2020;17:3526–3540. doi: 10.1021/acs.molpharmaceut.0c00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ripcke J., Zarse K., Ristow M., Birringer M. Small-molecule targeting of the mitochondrial compartment with an Endogenously cleaved reversible Tag. Chembiochem. 2009;10:1689–1696. doi: 10.1002/cbic.200900159. [DOI] [PubMed] [Google Scholar]
- 36.Pastor-Maldonado C.J., Suárez-Rivero J.M., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Munuera-Cabeza M., et al. Coenzyme Q10: novel formulations and medical trends. Int. J. Mol. Sci. 2020;21:8432. doi: 10.3390/ijms21228432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández-Camacho J.D., Bernier M., López-Lluch G., Navas P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinzii C.M., DiMauro S., Hirano M. Human coenzyme Q10 deficiency. Neurochem. Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedor J.G., Jones A.J.Y., Luca A.D., Kaila V.R.I., Hirst J. Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I. Proc. Natl. Acad Sci. U. S. A. 2017;114:12737–12742. doi: 10.1073/pnas.1714074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández-Ayala D.J.M., Brea-Calvo G., López-Lluch G., Navas P. Coenzyme Q distribution in HL-60 human cells depends on the endomembrane system. Biochim. Biophys. Acta. 2005;1713:129–137. doi: 10.1016/j.bbamem.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Venuti V., Crupi V., Fazio B., Majolino D., Acri G., Testagrossa B., et al. Physicochemical Characterization and antioxidant activity evaluation of idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion complex. Biomolecules. 2019;9:531. doi: 10.3390/biom9100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbone C., Pignatello R., Musumeci T., Puglisi G. Chemical and technological delivery systems for idebenone: a review of literature production. Expert Opin. Drug Deliv. 2012;9:1377–1392. doi: 10.1517/17425247.2012.724396. [DOI] [PubMed] [Google Scholar]
- 43.Bodmer M., Vankan P., Dreier M., Kutz K.W., Drewe J. Pharmacokinetics and metabolism of idebenone in healthy male subjects. Eur. J. Clin. Pharmacol. 2009;65:493. doi: 10.1007/s00228-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 44.Gottwald E.M., Duss M., Bugarski M., Haenni D., Schuh C.D., Landau E.M., et al. The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 2018;6:1–9. doi: 10.14814/phy2.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkarni C.A., Fink B.D., Gibbs B.E., Chheda P.R., Wu M., Sivitz W.I., et al. A novel triphenylphosphonium carrier to target mitochondria without Uncoupling oxidative phosphorylation. J. Med. Chem. 2021;64:662–676. doi: 10.1021/acs.jmedchem.0c01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reily C., Mitchell T., Chacko B.K., Benavides G.A., Murphy M.P., Darley-Usmar V.M. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013;1:86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data contained within the manuscript.