Abstract
CAR-T cell therapies have consolidated their position over the last decade as an effective alternative to conventional chemotherapies for the treatment of a number of hematological malignancies. With an exponential increase in the number of commercial therapies and hundreds of phase 1 trials exploring CAR-T cell efficacy in different settings (including autoimmunity and solid tumors), demand for manufacturing capabilities in recent years has considerably increased. In this review, we explore the current landscape of CAR-T cell manufacturing and discuss some of the challenges limiting production capacity worldwide. We describe the latest technical developments in GMP production platform design to facilitate the delivery of a range of increasingly complex CAR-T cell products, and the challenges associated with translation of new scientific developments into clinical products for patients. We explore all aspects of the manufacturing process, namely early development, manufacturing technology, quality control, and the requirements for industrial scaling. Finally, we discuss the challenges faced as a small academic team, responsible for the delivery of a high number of innovative products to patients. We describe our experience in the setup of an effective bench-to-clinic pipeline, with a streamlined workflow, for implementation of a diverse portfolio of phase 1 trials.
Keywords: chimeric antigen receptors, immunotherapy, manufacturing, automation, quality control
Graphical abstract
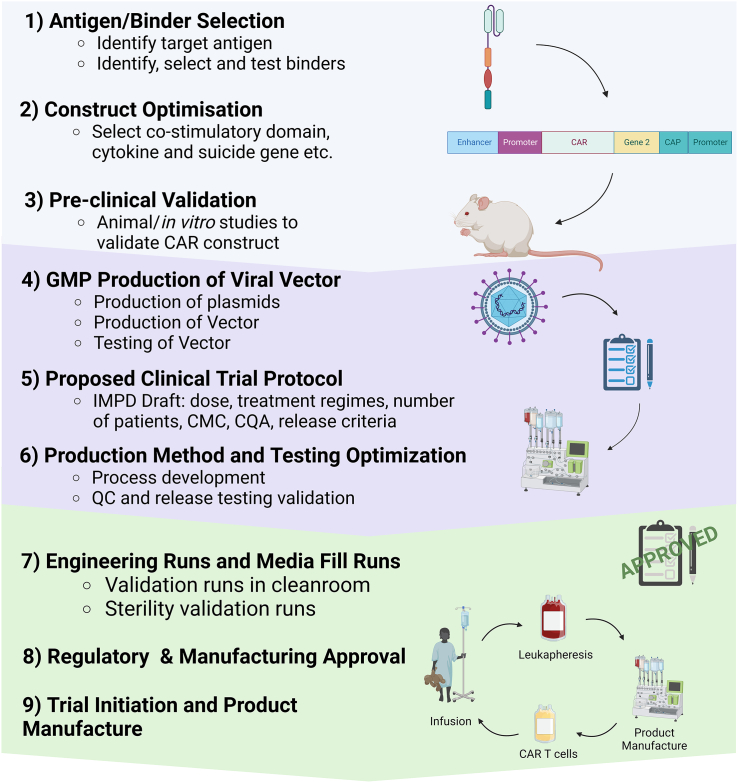
Dias and colleagues assess in this review the current CAR-T cell manufacturing landscape, considering challenges that will need to be addressed to facilitate translation of the next generation of these advanced therapies into clinic from an academic perspective.
Introduction
Chimeric antigen receptor (CAR) T cell therapy has emerged as a ground-breaking immunotherapeutic approach, with remarkable efficacy especially in refractory hematologic malignancies. Data indicate that CAR-T cell manufacturing processes can impact clinical outcomes1,2 and manufacturing science has evolved substantially over the past decade.
As the demand for CAR-T cell therapies grows, with expansion of applicability toward new indications and to attend a yet unmet need in developing countries,3 it becomes essential to implement good manufacturing practice (GMP) strategies that are flexible and robust, with streamlined workflows and optimized production efficiency. In parallel, development of new and more complex products is associated with a heavy demand for assay development and quality control strategies to ensure batch-to-batch consistency and safety.
The enormous innovation in CAR-T cell research and development far surpasses current capacity for clinical manufacture worldwide. Production requires the use of specialized cleanroom facilities and, more importantly, skilled workforce. Gap analysis by work groups both in the UK and the US highlight the shortage of qualified manufacturing staff as one of the main challenges to address in the Cell and Gene Therapy field.4,5 Importantly, a recent review of CAR-T manufacturing processes estimates that over 200 labor hours are required per batch/lot, inclusive of manufacturing, quality assurance (QA), quality control (QC), logistics and supply chain management. This represents 71% of the total costs associated with batch production, with as much as 48% of costs deriving from the manufacturing labor alone.6
This comprehensive review of the CAR-T cell manufacturing landscape seeks to provide critical insights into advancements that have shaped the field, with a focus on novel technical and methodological developments that have the potential to increase product quality and patient access.
Manufacturing
Despite the rapid advance of technology, the general workflow for CAR-T cell manufacture has remained consistent over the years, even from the first clinical trials.7 Briefly, autologous peripheral blood mononuclear cells (PBMCs) are procured via unstimulated leukapheresis, are enriched for T cells using magnetic beads or density-based methods, are then activated and transduced in vitro with a lentiviral or retroviral vector encoding the CAR cassette, and expanded until therapeutic CAR-T cell doses are achieved. This general protocol has been employed with minor modifications over the last decade for hundreds of clinical trials, as well as for commercial production.
Although methods vary between laboratories, technical and methodological developments in CAR-T cell manufacturing have in general focused on four main aspects: (1) a shift from manual methods to closed semi-automated systems (Figure 1), (2) improvement of product phenotype, (3) development of rapid, no-expansion protocols, and (4) the emerging use of genome-editing tools.
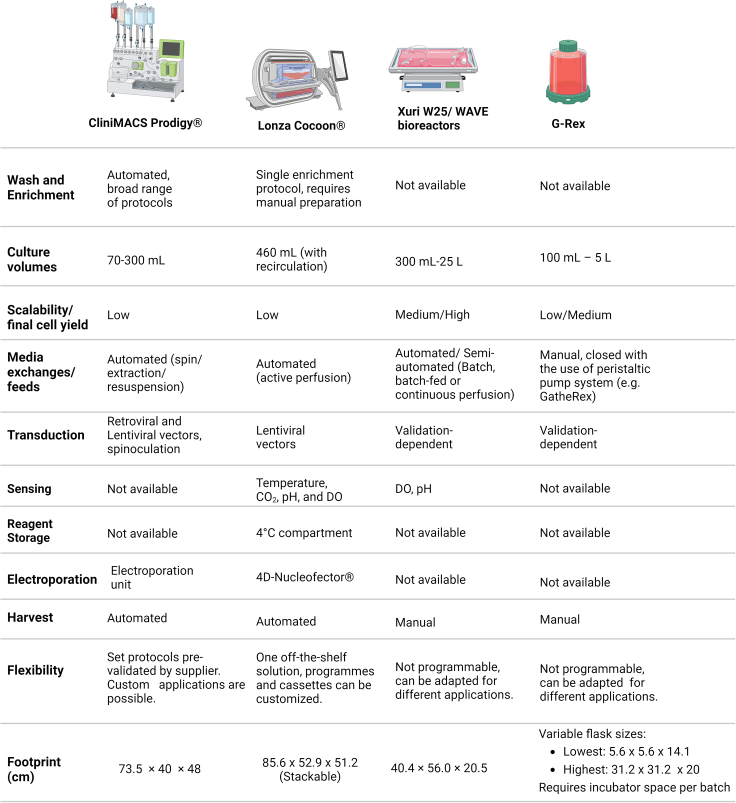
Figure 1.
Overview of the different CAR-T production platforms available in the market
These devices vary widely on level of automation, capacity, and integration with additional processing modules. The footprint has been evaluated taking into account requirements for additional pieces of equipment. Note that volumes and numbers for the CliniMACS Prodigy consider the standard application, but a large-scale protocol has recently been released by the supplier and validated.134
Automation
Starting materials and enrichment
Current CAR-T manufacturing protocols can use a wide range of processing strategies to achieve starting materials with the desired characteristics. Most manufacturing protocols use autologous PBMCs, procured via unstimulated leukapheresis using standard outpatient protocols. This usually permits collection of high T cell yields toward targets set by manufacturers (often in excess of 1 billion T cells), even in patients with low peripheral blood lymphocyte counts, but manufacturing failures still occur due to poor T cell quality and yields.8
More recently, improved T cell activation and expansion technologies have permitted a significant reduction in starting T cell number requirements such that a 25- to 100-fold expansion can often be achieved with newer protocols. In our hands, activation of 25 million T cells is sufficient to consistently achieve therapeutic CAR-T cell doses within a 7-day protocol. This opens a new realm of possibilities, as manufacturing could potentially be possible from a single regular blood draw.9
Bulk PBMCs
Using bulk PBMCs from leukapheresis as starting material for CAR-T cell production is cost effective and simple, but platelet and red blood cell (RBC) contamination can compromise in-process testing and are often associated with aggregation, poor T cell expansion, and low viability.10,11,12 Several processing devices can perform density-based cell separation, cell washing and concentration, and platelets or RBC removal (Table 1).13 A detailed review of the currently available wash-and-concentrate devices is beyond the scope of this review.14
Table 1.
Cell processing devices that can be used in preparation of starting material for CAR-T cell production
| Device | Capabilities/protocols |
|---|---|
| COBE 2991 |
|
| |
| |
| |
| |
| |
| |
| |
| |
| Sepax C-Pro |
|
| |
| |
| |
| |
| Rotea |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| LOVO |
|
| |
| |
|
Purified T cells
Monocyte and macrophage content in the leukapheresis starting material can compromise CAR-T cell manufacture through inhibition of T cell expansion15 and, in patients with high levels of circulating disease, CAR transduction of B cell acute lymphoblastic leukemia (B-ALL) blasts has been associated with leukemia relapse due to epitope masking.16 These risks can be mitigated through a T cell enrichment step, frequently via immunomagnetic cell separation. In our experience, despite an increase in costs, this has resulted in important improvements in product consistency and has reduced manufacturing failure rates.
Lonza has recently incorporated a magnetic selection module into the automated, closed-system Cocoon platform. This allows the selective enrichment and activation of T cells using Dynabeads CD3/CD28.17
Miltenyi’s CliniMACS Cell Separation technology was introduced three decades ago18 and the CliniMACS Plus device is now a well-established system for automated and current GMP (cGMP) immunomagnetic cell enrichment or depletion for hematological progenitor cells grafts19,20 and other applications.21,22,23 This cell selection technology is incorporated into the CliniMACS Prodigy device, an all-in-one solution comprising fully automated cell enrichment and CAR-T cell manufacture including transduction and T cell expansion.
T cell subsets with preferred phenotypes
Selective enrichment of preferred phenotypes has many potential advantages for the development of CAR-T cell products with optimized efficacy and potency. For example, CD19 targeting CAR-T products with defined CD4:CD8 ratios have been shown to display superior antitumor reactivity and in vivo efficacy,24,25 but the manufacture of two separate products for mixing at a given ratio significantly increases costs.
CAR-T manufacturing from purified central memory T cell (TCM) or stem-like memory T cell (TSCM) populations also has the potential to enhance antitumor responses.26 Pre-enrichment of CD62L+ cells has been included in some protocols and can be easily achieved with the CliniMACS system.27 However, selection of complex phenotypes would require multiple selection stages, which can become prohibitively expensive and impractical. Turtle et al.25 achieved efficient selection of CD8+ TCM cells using a sequential two-step enrichment including a CD4+/CD14+/CD45RA+ depletion step, followed by CD62L+ positive selection.
Fluorescence-activated cell sorting (FACS) allows multiparameter sorting with much higher purity and can be used when requirements are too complex for adequate results to be achieved with available magnetic-based separation technologies.28 However, most of the currently available sorting systems are not fit for large-scale clinical product manufacture as they are low-throughput devices, with non-sterile fluidics systems and not GMP compliant. Closed-system devices are being developed by multiple companies and should fill in this market gap in the upcoming years. The MACSQuant Tyto allows ten-parameter cell sorting with the use of closed-system cartridges. Unlike with droplet sorters, cells are not subjected to high pressure, decompression, and charges, maintaining sample viability. This technology has been increasingly employed, especially in the field of CAR Tregs.29,30 Other recently released FACS-based cGMP sorting technologies include the Sony GMP-ready CGX10 Cell Isolation System. This device uses a proprietary microfluidic chip and a built-in temperature control cabinet, enabling fully closed multiparametric, fluorescence-based cell sorting.31
Activation
Polyclonal T cell activation is generally required for effective transduction with lentiviral and retroviral vectors and is frequently achieved using CD3 and/or CD28 agonist antibodies. In soluble form these antibodies can only support short-lived T cell activation, but when immobilized they can induce signaling similar to that observed in antigen presentation, resulting in sustained TCR-mediated activation and productive T cell responses.32 Immobilized CD3/CD28 antibodies are available in GMP grade in multiple forms.
CTS Dynabeads (CD3/CD28 magnetic beads) provide T cell isolation and activation within a single reagent. However, one limitation of this approach is the requirement for removal of the magnetic beads at the end of the manufacturing process, which is labor-intensive if done manually, and can lead to considerable cell losses.33 The integrated magnetic separation feature of the Cocoon platform allows automated bead removal, but this is not compatible with the Miltenyi MACS technology, which limits availability of GMP-grade magnetic labeling reagents.
On the other hand, the MACS GMP T cell TransAct activation reagent is a biodegradable polymeric nanomatrix, and does not require removal prior to end of process product formulation. The supplier confirms that the reagent offers no safety concerns by day 7 of a typical manufacture process.
Expamers are polymeric, soluble protein complexes, formed by anti-CD3/CD28 Fab fragments linked to a recombinant Streptactin backbone and designed to activate human primary T cells without the use of solid support while still providing the required contact surface areas. In principle, this soluble reagent could be compatible with any manufacturing platform, and termination of the activation signal can be instantly triggered with addition of non-toxic D-biotin.34 The ability to fine-tune activating signals may have an important role in the determination of T cell fate and may be an important tool for manufacture of products with more favorable phenotypes,35,36,37 although this remains to be evaluated in a clinical setting.
The ImmunoCult Human CD3/CD28 T cell Activator (STEMCELL Technologies) consists of soluble CD3 and CD28 antibody complexes and is available as a GMP compliant reagent for use as an ancillary material in clinical manufacture. This reagent has been demonstrated to be efficient in promoting T cell expansion in the presence of IL-2.38,39 Research efforts are currently focused on novel alternative activation approaches (including engineered viral vectors) and non-activation protocols. LentiSTIM combines activation and transduction steps using viral particles with envelope anti-CD3 and anti-CD28 membrane-bound mitogens, incorporated into their viral envelope and derived from the membrane of producer cell lines.40 The single activation/transduction step may be beneficial to reduce costs and manufacturing times, but its use in clinical-grade manufacturing remains to be evaluated. Alternative activation methods, such as co-stimulation via the CD27 axis has been shown to maintain memory phenotype and improve therapeutic activity of CAR-T cells41 and, more recently, optimized protocols designed to preserve stemness of T cells have explored removal of the activation step42 altogether. These approaches have not yet been tested in GMP manufacturing practice.
Expansion
A diverse range of platforms are available for expansion of suspension cells, varying in terms of capacity, automation level, flexibility of protocols, costs, and integration with external devices for monitoring and/or further processing (Figure 1).
Cell culture bags are available from a range of suppliers as a closed-system alternative to traditional culture flasks. They are inexpensive consumables, but protocols are fully manual, requiring multiple feeds and media exchanges depending on cultivation length. Taking into account the requirements for staff and cleanroom space, two major bottlenecks in current cell and gene therapy manufacturing, cell culture in bags can be impractical in many centers. For this reason, many of the initial CAR-T manufacturing protocols included an initial cultivation in bags, transduction in retronectin-coated bags, but then transfer into a bioreactor for expansion.7,43 Although the multiple bioreactors and automated devices available in the market can have different footprints and requirements in terms of cleanroom space and operation, they can typically be used in lower-grade environments due to closed system operation (e.g., grade D environment in EU GMP as opposed to a grade A environment with a grade B background, as required for open handling steps), they allow parallelization of the production process, and automation of washes and feeds releases staff for execution of other critical processes, therefore allowing an increased manufacturing capacity within a limited cleanroom space and reduced workforce, as is the case in many academic centers.
As an alternative to culture bags and traditional flasks, the G-Rex flasks have a unique gas-permeable membrane and an optimized volume/area ratio, which allows cells to grow completely undisturbed for several days, with no need for feeds, media exchanges, or mechanical motion. The flasks are available with a wide variety of working volumes (100 mL, 500 mL, 1 L, 5 L) and a recommended seeding concentration of 500,000 cells per cm2. They can be used in association with a peristaltic pump, which allows volume reduction and cell harvest within a closed system. However, although the use of the G-Rex culture system can decrease the requirement for manual handling, they still require the use of CO2 incubators and they can only be used for the expansion stage, with seeding, washing, and harvest having to be performed manually, and no integration for the remaining manufacturing steps. Even so, due to its flexibility, adaptability, and cost-effectiveness, the G-Rex system is widely employed for a variety of cell therapy applications.
WAVE bioreactors, and now the Cytiva’s Xuri Cell Expansion System, are cell bag bioreactors, with capacity of up to 25 L. Single-use bags are kept in a heated rocking base, designed to inflate and shake for optimal gas dispersion, and media can be added within a functionally closed system in continuous perfusion or fed-batch mode. Process parameters such as rocking speed, dissolved oxygen, pH, and perfusion rate can be continuously monitored and controlled. These systems are well-established as effective platforms for expansion of CAR-T products and can accommodate a broad range of cell numbers.44 However, the lack of integration with other devices for enrichment, cell wash, and transduction hinder their application.
The Lonza Cocoon is a stand-alone cell-processing device, with a range of capabilities including cell selection, activation, transduction/transfection, expansion, and harvest in an automated and functionally closed system. Protocols can be flexibly developed to be fully integrated using customizable cassettes, tailored to the specific needs of each product. The integrated magnet for cell enrichment is compatible with the use of Dynabeads, but labeling needs to be performed manually or with the use of other devices. Due to media recirculation and continuous monitoring of CO2, dissolved oxygen, and pH levels, cell expansion can be performed with no need for counting and re-seeding, but culture volumes and cell yield are limited. Optimal seeding numbers are reported between 50 and 100 × 106 cells. The Cocoon platform also has the advantage of integration with the Lonza 4D-Nucleofector, a device that has been extensively validated for manufacture of cell and gene therapy products,45,46,47 therefore allowing the easy implementation of non-viral gene delivery protocols.
The CliniMACS Prodigy is another all-in-one alternative that provides automation of all steps of the CAR-T manufacturing process. This device uses the well-established MACS technology for cell enrichment, allowing fully automated cell washing, labeling, and magnetic separation within a closed system. The system comes with pre-programed and validated application-specific processes, including a T cell transduction protocol that allows flexibility in terms of starting material enrichment, definition of transduction and cultivation timelines and volumes, as well as pattern of mechanical agitation. Cells are grown within the device’s cultivation unit from a starting number of up to 200 × 106 in the standard version, in a maximum volume of 300 mL and at a set temperature and CO2%. Furthermore, the device can be readily employed for applications requiring the use of genome-editing tools and non-viral transgene delivery thanks to the availability of an electroporation module that can be integrated for closed-system processing.
However, sensing and monitoring capabilities are limited, and media exchanges must be programmed by the operator according to cell expansion profile.
Formulation and cryopreservation
Automation for final formulation and cryopreservation is one of the main unmet technical needs in the cell and gene therapy field. Although aseptic filling devices are common in the pharmaceutical industry, cell product requirements are highly variable in terms of final composition, volumes, cell numbers, and types of containers, therefore a one-size-fits-all solution may be difficult to achieve. Furthermore, cellular products are especially delicate and require careful handling. Critically, extended exposure to cryoprotectants containing DMSO is toxic to the cells, therefore products must be kept cold and quickly cryopreserved after final dilution.48 For these reasons, the final formulation and cryopreservation of CAR-T product is still performed manually in most centers.
Terumo’s FINIA Fill and Finish System is a closed, automated system that formulates and aliquots cell suspensions to prepare for cryopreservation. Cells and cryoprotectant solution are mixed in a cooled bag and automatically dispensed into up to three dosages and one QC sample in cryobags with volumes set by the user. Cryobags are sealed and ready for cryopreservation in approximately 10 minutes, but the system cannot dispense low-volume doses or work with vials, which remains an unmet need.
Another fluid-handling platform for closed system bioprocessing is Sexton’s Signata CT-5TM. This device uses a peristaltic pump for mixing, rinsing, and filling of cryobags or closed-system cryogenic vials, such as CellSeal vials. The Signata CT-5TM has a smaller footprint than the FINIA, but the process is not fully automated.
Recently launched, the ScaleReady’s Cue system uses syringe pumps, a valving cassette, and a spinning membrane separation technology, to enable high accuracy concentration, formulation, and aliquoting in a functionally closed system. One advantage of this system is the accurate handling of low volumes, supporting final product volumes as low as 2 mL.
Finally, Miltenyi recently released a formulation unit for use with the CliniMACS Prodigy. This attachment allows the cells harvested at the end of a typical T cell transduction process to be resuspended at the correct concentration in the final formulation medium prior to addition of cryoprotectant and subsequent dispensing of defined volumes into cryobags. However, the flexibility of the application is limited, and the device is only suitable for filling of higher volumes in bags, while low volumes and vials would still need to be manually filled.
Future of automation and considerations for phase 1 trials
Given the rapidly growing number of CAR-T products advancing into commercialization and the limited availability of cleanroom space and trained personnel, automation solutions are still required for higher throughput and scaling out to meet the demand of over 100,000 CAR-T batches over the next 10 years for the European market alone.49 Inspiration can be found in other well-established industries, such as the automotive, food, and classical pharmaceutical industries, for which fully automated manufacturing plants are the norm.50
Beyond manufacturing systems, consideration must also be given to the quality control aspects. Batch release testing minimally evaluates quantity (live cell counts), identity (CAR expression), purity (e.g., presence of endotoxin or residual activation beads), and safety (microbiological assessment). Samples are manually processed, and results may take up to 2 weeks, such as in the case of culture-based methods for sterility assessment.
To upscale manufacturing capacity, two main strategies can be employed: (1) the use of parallel all-in-one systems, working simultaneously or (2) modular integration of diverse platforms, each performing specific sub-processes of the CAR-T manufacturing protocol. While the first approach may be more easily available based on current technology, the latter certainly optimizes the use of space and resources and is the most commonly adopted approach in modern assembly lines in different industries. However, full automation will require better integration between different devices, as well as implementation of new real-time data monitoring and processing systems. Fully automated, smart manufacturing plants, using real-time data analysis, with digital communication of all devices and integration of concepts of machine learning to further minimize the need for human intervention are a reality within the Pharma 4.0 concept and have the potential to make CAR-T cell therapies more accessible. All these technical innovations must be adaptable to account for the biological advancements in CAR immunotherapy and they must be in line with the requirements of regulatory agencies.
Setting up fully automated smart plants is only feasible if processes are demonstrated capable and well defined, and proven to be suitable under a broad range of conditions. This is possible with an extensive characterization of products and processes in the development phase, with the assistance of a quality by design (QbB) approach.51 In the case of early phase development and manufacturing for phase 1 trials, it is common that critical quality attributes are still being defined. Therefore, processes must be flexible enough to be fine-tuned as data become available and models are improved. Furthermore, early-stage clinical trials typically recruit a small number of patients. Therefore, for phase 1 trials the focus is usually evaluating a number of different products, but a small number of batches for each, which makes flexibility a fundamental requirement of any platforms used. In the academic setting, process development for phase 1 trials should focus on evaluation of technologies and on the optimization of manufacturing processes to reliably generate the best CAR T products. Given that these are mostly first-in-human studies, where cutting edge developments are evaluated, the use of research-grade reagents and platforms are generally deemed acceptable by regulatory authorities, where no GMP alternatives exist (e.g., EU GMP guidelines in EudraLex, volume 4, part IV, 7.13).
However, because changes in the manufacturing processes can be cumbersome when moving to commercialization, processes must be developed taking into account scaling-out for later stages. This may require early discussions and collaboration with suppliers, and automation has the advantage of facilitating technology transfer between different manufacturing sites.
Improvement of product phenotype
It is clear that CAR-T persistence is critical to ensure sustained remission. Determinants of response and resistance to CAR-T cell therapy have been sought by multiple groups and, although highly dependent on engineered construct, such as the choice of co-stimulatory domains,52 sustained responses for the same product have been demonstrated to be associated with specific phenotypes, such as the frequency of early memory subsets, with an oxidative phosphorylation (OXPHOS) directed metabolism and low expression of exhaustion and senescence markers.53,54 Importantly, CAR-T cell manufacturing strategies can have a significant impact on final product phenotype and should be evaluated with care.
The differential capacity of CD4 and CD8 CAR-T cells to proliferate and persist has been demonstrated in vivo24 and their respective roles in initial and long-term responses may differ. These two subsets appear to exert synergistic activities with respect to expansion and antitumor activity24 and the benefits of CAR-T products with a fixed CD4:CD8 ratio have been evaluated.55 In this regard, manufacturing protocols may have a critical role in determining efficacy by influencing predominance of each subset in the final drug product. Turtle et al. have demonstrated that the frequency of CD4 and CD8 T cells is substantially different between patients and healthy donors, with often an inverted CD4:CD8 ratio in heavily pre-treated patients. Beyond selective enrichment in the starting material, the frequency of each subset can also be influenced by activation method and culture conditions. For example, anti-CD3/CD28 beads, the activation method of choice for most manufacturers, are known to favor CD4 expansion over CD8.24,56,57 On the other hand, the use of soluble CD3-targeting activation methods can have the opposite effect.56 This is likely associated with the differential activation threshold of each subset, as duration of stimulation for optimal T cell expansion has been demonstrated to differ between CD4 and CD8 T cells.58 While CD4 expansion is favored upon sustained antigen exposure, CD8 T cells are able to proliferate and differentiate into effector T cells in response to transient antigen presentation, but rapidly become exhausted, or activation-induced nonresponsive.59
As manufacturing data accumulate, the development of models to predict final phenotype based on starting material characteristics and culture conditions have emerged as potential solutions to some of the manufacturing hurdles associated with the variability of autologous patient material.60 This can be an alternative to the expensive approaches used today to obtain a fixed ratio of CAR-T cell products by optimization of manufacturing protocols in a patient-specific manner to determine the activation method and culture conditions best suited for the starting CD4:CD8 ratio, considering variables such as differentiation stage and response to antigen exposure and cytokines.
In our experience, T cell activation using TransAct CD3/CD28 beads for 4 days, followed by cultivation in a combination of IL-7 and IL-15 can favor expansion of CD4+ cells, with final products often having a skewed CD4:CD8 ratio.61 However, even if under-represented in the infused product, CD8+ cells tend to expand quickly in vivo in response to antigen stimulation and typically represent the majority of CAR+ cells detected in the patient’s blood at early follow-up,62 with a lower CD4/CD8 ratio in the blood at days 6–41 post infusion being associated with improved responses.63 This dynamic feature complicates the assessment of the individual contribution of each T cell subset in the final products to clinical responses.
It is also increasingly clear that therapy efficacy is linked to the differentiation stage of the cells infused, with naive (TN), TCM, and TSCM lymphocytes related to a better long-term response due to their ability to proliferate and persist longer.64 Therefore, strategies to develop products enriched in these compartments and to uncouple T cell expansion from differentiation are constantly being sought. Beyond introduction of shorter or no-expansion protocols, which are discussed in more details below, TN and/or TSCM can be enriched for CAR-T cell manufacture via multi-stage magnetic selection24 or FACS.26,65
Activation method, strength, and duration of the stimulus seem to be critical in determining final product phenotype. Anti-CD3/CD28 bead-treated T cells typically assume a TEM phenotype by day 14, but transient stimulation with antigen-presenting cells has been shown to provide superior expansion of CD8 TSCM, while improving cytokine secretion, anti-tumor effects, and in vivo persistence.58 Furthermore, earlier wash and removal of activation beads has been reported beneficial for T cell expansion in automated manufacturing protocols.66
Composition of the cultivation medium, presence of serum or sera substitutes, and exogenous cytokines have a determinant role in final product phenotype but are complex to evaluate. Small adjustments to media formulations, such as an increased potassium content, have been described to preserve T cells in a stem-like state where they retain the capacity to expand.67 On the other hand, while IL-2 supplementation has been common practice to support cell expansion, it can drive differentiation into TEM and favor proliferation of Tregs, which can hinder therapy efficacy.68 New combinations of cytokines including IL-7, IL-15, IL-18, and IL-21 are becoming increasingly common.1
Overall, improving product phenotype for sustained responses will depend on a profound understanding of T cell biology, signaling, and immunological memory. Understanding the mechanisms associated with the transformation of quiescent naive cells into fast-proliferating effectors, and then again into quiescent memory cells, provides key information on what are the determining features of a good product and, therefore, which cellular processes can be rewired for better phenotype. We know that T cell activation is coupled to a shift in cell metabolism from basal OXPHOS into a highly metabolically active state using glycolysis to support the energy requirements for rapid expansion.69 Conversely T cell stimulation in the presence of glycolysis inhibitors can enhance the generation of memory cells and improve antitumor activity.70 On the other hand, differentiation into memory T cells requires entry into a primed state, where cells remain quiescent, but can rapidly respond to a previously encountered antigen. This is associated with a shift toward fatty acid oxidation and OXPHOS and a higher mitochondrial reserve and increased levels of cardiolipin.69 The impact of different metabolites of cultivation medium and tumor microenvironment on epigenetic signatures and determination of T cell fate has been reviewed by Akbari et al.71
Induction of a less-differentiated phenotype can also be induced with the use of pharmacological inhibitors of critical T cell signaling pathways such as PI3K/AKT/mTOR to uncouple T cell proliferation from differentiation and exhaustion.72,73,74,75 Similar results have been demonstrated with the use of ibrutinib to block interleukin-2-inducible T cell kinase/Bruton’s tyrosine kinase signaling during autologous manufacture for chronic lymphocytic leukemia patients.76 Similarly, addition of dasatinib during manufacture, a clinically available tyrosine kinase inhibitor that inhibits essential proximal CAR signaling kinases, has been shown to prevent exhaustion of CAR-T cells and has been implemented in phase 1 trials.77,78 Despite the advantages of these tools, it is important to mention that inclusion of additional components in the cultivation medium can be a logistical and regulatory burden, especially if the product is taken beyond phase 1. The regulatory status of these drugs, which need to be manufactured under GMP compliance and qualified as suitable for their use as ancillary materials, must be taken into account. A close interaction with suppliers from the earliest stages of development is essential to ensure that the correct grade material will be available for large-scale production. Furthermore, residual levels should be carefully evaluated to minimize risk to patients.
In vivo, repeated exposure to antigen and tonic signaling are associated with terminal differentiation and exhaustion. This has been demonstrated to be determined by CAR design, especially the choice of co-stimulatory domains.78,79 For example, whereas CD28-co-stimulated CARs are often described to have better effector function, persistence, and long-term responses are poor when compared with 4-1BB-co-stimulated CARs, which tend to exhibit a memory-like phenotype.79,80 This is associated with strong activation mediated by CD28ζ, which drives rapid T cell differentiation and high effector function preceding T cell contraction.81 Other contributing factors include CAR expression levels, promoter strength, hinge, and transmembrane domains and scFv characteristics. For example, we have demonstrated that a lower-affinity CD19 binder leads to enhanced expansion and persistence of CAR-T cells post-infusion,43,61 which is associated with maintenance of a TSCM phenotype.82,83 Changes in manufacturing protocols can also be implemented to prevent loss of function due to excessive tonic signaling, which is often associated with clustering of receptors in the plasma membrane.80 Optimal CAR expression levels can be achieved with choice of the correct expression platform84 and fine-tuning of multiplicity of infection used for viral vectors. Clustering of receptors can also be prevented by regulating the ionic strength of the cultivation medium, with a high-salt treatment significantly reducing the tonic signaling index.85
Furthermore, regardless of product phenotype at infusion, the harsh immunosuppressive and anaerobic tumor microenvironment can lead to T cell exhaustion and senescence,86 a concern particularly in the case of solid tumors. Engineering strategies are being evaluated now by many teams and can include PD-1 knockout using Crispr-Cas9,87 the use of a dominant negative TGF-β receptor to block TGF-β signaling88 co-expression or secretion of cytokines, such as IL-7 or IL-15,89,90,91 use of constitutively active receptors92,93 or hypoxia-activated CARs with the use of an HIF-1a subdomain.94 Although some of these approaches rely on the use of multicistronic vectors and therefore change little of the manufacturing requirements, quality control becomes increasingly complex and potency characteristics challenging to evaluate. With the use of genome-editing tools, features like efficacy of gene editing, genotoxicity, tumorigenicity, and immunogenicity are critical quality attributes that need to be evaluated during development and/or at batch release.95
Rapid manufacturing protocols
The applicability of CAR-T cell therapy is still limited by manufacturing capacity worldwide, and strategies to decease vein-to-vein time are highly desirable. Beyond decreasing patient wait times, rapid manufacturing protocols also have the potential advantage of limiting the impacts of ex vivo expansion on product quality.
Early cell therapy manufacturing protocols, such as those developed for tumor-infiltrating lymphocyte therapy (TILs) in the 1980s, required extended in vitro expansion with high doses of IL-2, aiming to achieve therapeutic doses of hundreds of billions of cells.96 While effective doses for CAR-T cell therapies have been established to be 1,000-fold lower than for TILs, at least for hematological malignancies,97 early CAR-T manufacturing protocols still involved between 9 and 14 days of expansion,7 leading to progressive T cell differentiation in culture. To investigate whether this period could be shortened, Ghassemi et al.98 demonstrated that products harvested from culture between days 3 and 5 exhibited less differentiation and enhanced effector function in vitro when compared with the standard 9-day protocol, highlighting the potential importance of curtailing the in vitro manufacturing period.
The feasibility of a next-day manufacturing protocol, where T cells are enriched and activated with CD3/CD28 Dynabeads, transduced after 24 h with a CD19 CAR-encoding lentiviral vector, and frozen the next day with no expansion step, has been evaluated in a phase 1 trial (NCT03825718). These products exhibited better ex vivo expansion upon stimulation with CD19-expressing cells and a less-differentiated and less-exhausted phenotype.99 Furthermore, when treated with low doses, ranging from 104 to 105 cells/kg, 23 out of 25 adult and pediatric B-ALL patients achieved minimal residual disease-negative remission on day 28 after infusion. However, because most patients underwent allogeneic hematopoietic stem cell transplantation within 3 months post CAR-T cell therapy, evaluation of long-term responses was not possible.
On the commercial side, Novartis announced T-Charge as their next-generation CAR-T manufacturing platform. Here, T cells are enriched, activated, and transduced on the same day with a lentiviral vector encoding the same CD19-targeting CAR construct used for tisagenlecleucel, and then harvested, washed, and formulated after 2 days in culture. When tested clinically at doses 25 times lower than tisagenlecleucel (which is manufactured in a typical 10-day process), this platform provided products with maintained T cell stemness and with promising overall safety and excellent CAR-T cell expansion.100
An alternative approach for rapid CAR-T manufacture described recently involves the optimization of lentiviral transduction of non-activated T cells, aiming to further reduce the irreversible differentiation of T cells triggered by T cell receptor activation with CD3/CD28 antibodies.42 Cells manufactured with this protocol were demonstrated to be potent in vitro and in vivo, but transduction efficiency is still suboptimal when compared with the standard activated products.
The use of no-expansion protocols is currently limited by costs of viral vector required for transduction of a large cell number. However, since data so far indicate that non-expanded cells may have a superior phenotype, it is likely that effective doses can potentially be significantly lower.
Lastly, prior to widespread adoption of rapid manufacturing methods, the effective removal of residual activation beads and free viral particles, as well as other impurities in the final formulated product, needs to be carefully evaluated. Extensive washes are likely to be required and a rigorous evaluation of the risks associated with residual bound beads is needed to ensure product quality and patient safety.
Genome-editing technologies
Transcription activator-like effector nucleases and clustered regularly interspaced short palindromic repeats-Cas9 (CRISPR-Cas9) now offer powerful genome-editing tools to create allogenic, off-the-shelf CAR-T products,101,102 with locus-specific insertion of the CAR transgene103 and the potential for multiplexed editing to manipulate critical T cell signaling pathways.104 The role of CRISPR-Cas9 in the development of next-generation CAR-T therapies has been reviewed in detail by Dimitri et al.105
Targeted delivery of GMP-grade editing components (in the form of proteins, DNA, and/or RNA) to T cells is a critical step and is most frequently achieved using electroporation. This is efficient and versatile, but can lead to significant cell death and may not be suitable for all cell types.106 Pulse format, duration and intensity, as well as cargo concentration and ratios need to be carefully optimized at the pre-clinical stage. Closed-system, GMP-compliant electroporation devices are now available in the market. The Lonza 4D-Nucleofector has a large-scale unit with a single-use weldable tubing-set that can be connected to the Cocoon system. Miltenyi has also released the CliniMACS Electroporator, which can be connected to the CliniMACS Prodigy for automated transfection. Lastly, MaxCyte has also released the ExPERT GMP Processing Assemblies, which allow large-scale flow electroporation in a closed system.
Lipid nanoparticles (LNPs) have been demonstrated to be an effective, and likely gentler, delivery method for ex vivo gene editing,107 with in vivo clinical efficacy demonstrated over the past 2 years with COVID-19 mRNA vaccines.108 These LNPs can be generated and loaded with mRNA cargo using a variety of chemical methods,108 but microfluidic mixing has the advantage of allowing precise control of particle size, high reproducibility, and high encapsulation efficiency.109 Furthermore, this method is scalable and can be automated for clinical production. The NanoAssemblr platforms (Precision Nanosystems) can formulate mRNA-loaded LNPs with a variety of sizes and compositions, with a choice of devices suitable for formulation volumes from 25 μL to >10 L.
However, while genome-editing platforms offer solutions augmenting CAR-T cell therapies, they are not without drawbacks. Multiple disputes on intellectual property ownership are centered around the CRISPR-Cas9 technology, and the use for human applications may require complex licensing agreements.110 Furthermore, from a manufacturing perspective, gene editing is a complex procedure that requires highly skilled operators and adds significant costs to CAR-T cell products that are already expensive, which may prove a bottleneck for any products to progress beyond phase 1.
Gene editing is associated with genotoxicity, linked to double-stranded breaks, insertion and deletions (or INDELS),111 bystander and off-target edits,112 and chromosomal aberrations.113,114,115 For this reason, advanced, and sometimes costly, quality testing procedures need to be implemented to ensure the safety of gene edited CAR-T cell products. During product development, the genome-editing components and protocols must be optimized and verified not only to ensure functionality of the edited cells and the intended downstream biological modifications but also to minimize off-target effects.116,117 Assessments of off-target editing frequency, interchromosomal and intrachromosomal rearrangements, and residual editing components are also required. This typically includes a combination of genome-wide analyses (e.g., in silico or cellular-based assays) and verification of sites identified using methods with adequate sensitivity to detect low-frequency events.118 Release testing also includes evaluation of on-target editing efficiency.
Recently, base-editing technology has emerged as a potentially safer alternative to traditional CRISPR-Cas9. In brief, instead of the double-strand breaks promoted by Cas9, this system uses a catalytically disabled nuclease fused to a deaminase enzyme that is capable of mediating highly precise base conversions, which can be directed to precise positions to create premature stop codons or disrupt splice sites.119
Lastly, although GMP manufacture of RNA can be prohibitively expensive, significant progress has been made over the last few years with the use of circular RNA (circRNA). Compared with linear mRNA, circRNA would appear to be easier to manufacture and may potentially be used in much lower concentrations. In one exemplar preclinical CAR-T manufacturing study, base editing was used for increased PD-1 knockout efficiency.47
Quality control and release testing
As CAR-T cell products increase in complexity, development of adequate quality control assays becomes a critical challenge. It is essential that enough pre-clinical data are available to allow determination of the main critical quality attributes of each product and the use of testing methods that are fit for purpose from the offset, so that appropriate product specification can be defined. The regulatory requirements for release testing depend on the phase of clinical development. For phase 1 trials, regulatory bodies accept that assays may still require optimization; however, minimum requirements in terms of safety, identity, purity, and potency must be met. Typical quality control requirements for CAR-T cell products are described in Table 2.
Table 2.
Examples of CAR-T cell quality control requirements
| Parameter | Tests |
|---|---|
| Quantity |
|
| Viability |
|
| Safety |
|
| |
| |
| |
| Identity |
|
| Purity |
|
| |
| |
| Potency |
|
|
Often, product testing represents the biggest bottleneck for delivery of autologous therapies. Culture-based sterility assessment methods typically take 10–15 days to produce results, limiting the turnaround times even if rapid manufacturing methods are employed. More importantly, a limited number of licensed testing sites are available in Europe to carry out more complex assays, such as detection of replication-competent lentiviruses and retroviruses, or high sensitivity detection of off-target effects following the use of genome-editing tools, and backlogs can cause significant delays to product delivery. Therefore, it is essential that manufacturing advancements are accompanied by the implementation of automated and streamlined testing methods, developed in accordance with the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceuticals for Human Use guidelines and demonstrated to be robust and appropriate for the specific stage of development. The latest innovations applicable to the testing of CAR-T cell products are discussed below. Many of these new technologies are being developed by the biotechnology industry, and their evaluation in the academic setting in the context of early-stage clinical trial offers many advantages. While this model of collaboration allows generation of datasets that can be used for validation and demonstration of suitability for the intended application, it also allows an extended characterization and a better understanding of the products through the use of innovative methods.
Safety
Safety assessment minimally comprises assays to ensure that the final product is free from microbial contamination, mycoplasma, and impurities, such as endotoxin.
Sterility assessment remains a major obstacle to rapid release of CAR-T cell products. The introduction of colorimetric and fluorescence-based CO2 measurements of metabolic activity (e.g., BacT/Alert 3D and BD BACTEC systems) or adenosine triphosphate detection by bioluminescence (Rapid Milliflex Detection System) has allowed for faster evaluation of microbiological contamination. However, a minimum of 7 days incubation at 35°C–37°C is still recommended for an accurate assessment.
Rapid sterility testing methods have recently become available, such as the Microsart ATMP Sterile Release kit (Sartorius). This is a PCR-based assay that uses TaqMan probes for detection of a broad range of Gram-positive and Gram-negative bacterial and fungal contaminants (results are obtained within 3 h; therefore implementation of this assay would allow for rapid release of ATMP products). DNA-based methods are already widely employed for detection of mycoplasma specimen in CAR-T cell products. However, because these methods require extended sample handling for DNA extraction and preparation of the qPCR assay, risks of cross-contamination must be considered carefully and mitigated accordingly. Sample handling in a sterile, DNA-free environment is essential, with the use of isolators recommended by the manufacturer.
Safety evaluation of cell therapy products has gained a whole new dimension with the introduction of genome-editing tools. Off-target effects need to be carefully evaluated at the development stage to ensure guides designed are optimal. This can be done with the use of prediction tools, but also experimentally with off-target profiling methods for whole-genome analysis, including GUIDE-seq, DIGENOME-seq, CIRCLE-seq, or SITE-seq. The variety of methodologies and their advantages have been reviewed previously.120 Assessing breaks or deletions at these potential off-target sites, as well as the desired editing event, requires the use of whole-genome sequencing of the edited products and amplification of the candidate off-target sites, followed by deep sequencing to provide adequate sensitivity for low frequency events.118 In case of methods that induce double-strand breaks, such as CRISPR-Cas9, the risk of large deletions or insertions, inversions of gene fragments, and chromosomal translocations is also a concern and should be evaluated.121
Identity and quantity
The main purpose of identity assessment is to define the cellular composition of the final products, particularly the expression of the CAR. Detection of the CAR can usually be performed by flow cytometry, and it can either detect the CAR itself or surrogate marker genes. Direct detection of the CAR molecule is performed using CAR-specific reagents: these can either be an antibody specifically raised against the binder, antibodies against the stalk region (such the IgG portion), or the antigen-Fc detection proteins. Absolute quantification can be obtained with the use of counting beads or with the use of a dual platform approach, where absolute cell numbers are obtained with the use of automated hematological counters. As flow-based detection assays are mostly CAR specific, the method needs to be optimized and validated for each cell product, ideally demonstrating specificity, precision, accuracy, linearity, sensitivity, and range.122 Traditional flow cytometry methods can be variable, and results are often operator dependent, which is often an issue in the GMP setting. It is critical that the use of an optimized method is allied with standardized instructions for gating and adequate training. Although the custom nature of these detection assays represents a challenge for the setup of multicenter proficiency programs, some of these concerns can likely be addressed with the help of automated analysis tools, currently under development, which use a variety of unsupervised and supervised machine learning algorithms to automatically identify populations of interest.123 Furthermore, automated sample preparation workstations are now available for integration with a variety of devices, such as the BD FACSDuet for the BD FACS Lyric, the Biomek i5 for the Beckman Coulter Cytoflex, and the Sysmex PS-10. Lastly, cartridge-based technologies are also in implementation for product phenotyping, as is the case of the Accellix platform.
Purity
Process-related impurities include residual components of the cultivation media and buffers, selection or activation beads, viral vectors, enzymes, and DNA and RNA used for genome editing. Typically, these impurities are removed with the inclusion of extensive washing steps during the manufacture and formulation procedures. However, the presence of residual bound beads could be a concern, especially in the case of rapid manufacturing protocols. Although elements such as guide RNA and Cas9 proteins are expected to be degraded in vivo, new testing methods will be needed to assess their residual levels in products involving genome editing. For CAR-T cell products, especially autologous ones, the relevance of product-related impurities may be difficult to assess. For example, although residual blasts could have a clear impact on product quality and patient safety,16 the significance of residual untransduced T cells or NK cells is debatable. However, even if not forming part of the product specification for release, a broad characterization of the cellular content of each CAR-T batch is recommended and can be reviewed retrospectively in light of clinical responses.
Potency
For cell therapy products, potency assays are typically challenging to develop, as the determinants of therapy efficacy are complex and not fully understood. It is recommended that these assays are defined based on the expected mechanism of action, and, in the case of CAR-T cells, this generally includes assessment of cytotoxicity or cytokine secretion upon co-culture with cells expressing the targeted antigen. However, traditional methods, such as 51Cr release assays or evaluation of IFN-γ secretion by ELISA are complicated, labor intensive, and lack standardization.
Real-time cell analysis platforms, such as the impedance-based xCELLigence or the fluorescence-based Incucyte, can offer advantages over endpoint assays by providing information on killing kinetics, which is critical for identification of subtle differences among CAR-T products.124 Furthermore, microfluidics platforms are currently being developed and could represent an important step toward full automation for cytotoxicity analysis, allowing evaluation of multiple targets or even 3D tumor models in a single chip.125,126
Cytotoxicity data is often associated with additional assays, such as extended phenotyping for exhaustion and memory markers, or cytokine secretion profile, to provide a more comprehensive characterization of CAR-T products. High-throughput single-cell analysis platforms are useful for a systematic evaluation of the features of engineered CAR T cells and provide invaluable information on how they can be improved. Examples are RNA-seq platforms for single-cell transcriptome profiling127,128 and the IsoPlexis single-cell secretomics platform.38,74,129 As clinical data emerge, the variety of factors influencing in vivo efficacy are being explored by multiple groups and will provide additional insights on the critical quality attributes that determine product potency. This will likely inform the requirements of release-testing assays and help define potency criteria for CAR-T batches.
Set up of phase 1 trials and challenges for academic teams
As a general rule, the path for CAR-T cell therapy development starts with academic discoveries, later spun out into start-ups or licensed to pharmaceutical companies. The importance of academic-industry collaborations in the delivery of the CAR-T products licensed to date is clear, but academic centers remain the primary players in the development of new modalities of these therapies.130 The academic/hospital setting has the advantage of a close proximity between researchers and patients, favoring translational research, and many of these centers already have licensed GMP facilities, conveniently located for production of autologous therapies.
With regard to the regulatory framework, a phase-appropriate approach is generally adopted by most agencies, including the EMA and FDA, when assessing the requirements for manufacture of ATMPs, including CAR-T cell therapies for use in clinical trials. For early-stage development, safety is a primary focus, but it is generally understood that other quality attributes may not be fully characterized. Encouraging innovation requires that regulatory requirements are adjusted to match the reality of academic centers and, in line with this, the EMA recently set up a pilot aiming to support academic and non-profit organizations on the translation of basic research developments into medicines for unmet needs. The program offers enhanced regulatory support, and participants benefit from available regulatory flexibilities and development support measures, such as fee reductions and waivers and details can be found in the EMA website at https://www.ema.europa.eu/en/news/ema-pilot-offers-enhancedsupport-academic-and-non-profit-developers-advanced-therapy-medicinal-products.
In our experience, an effective bench-to-bedside workflow (Figure 2) benefits from a close collaboration between research teams, manufacturers, and regulators from the earliest stages of development. Implementation of a robust manufacturing platform that can deliver consistent results, but is also flexible to adapt to new requirements and keep up with pre-clinical innovation, is also essential. In this setting, all-in-one automated devices have clear advantages, providing an easy-to-adapt starting point for new protocols, minimizing requirements with cleanroom, specialized workforce, and training. Academic manufacture needs to be dynamic, meeting the increased demand for new phase 1 trials, which typically recruit a limited number of patients, but require the delivery of highly innovative products, and often also be capable of scaling up manufacture for phase 2. A focus on product development, based on a QbD approach, is key and must take into account future requirements for later phases and scaling-out.
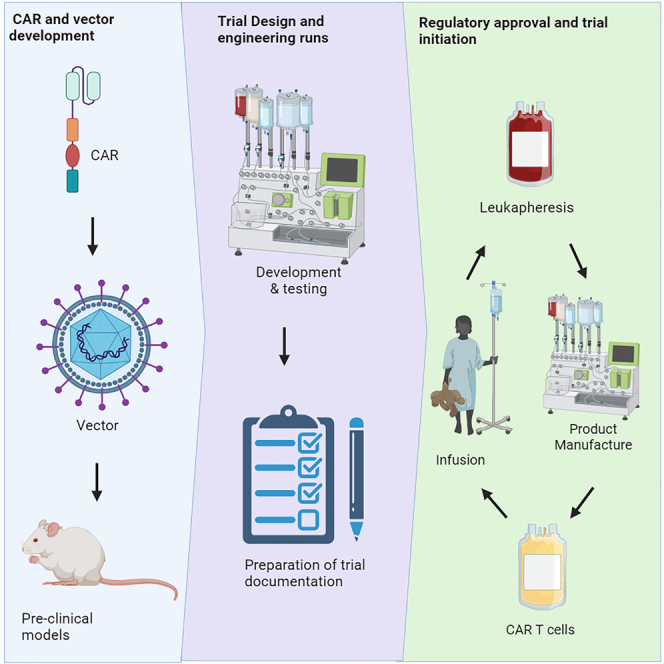
Figure 2.
Bench-to-bedside workflow used in the UCL program for translation and delivery of new phase 1 CAR-T cell studies
New target antigens and specific binders are identified in the research lab and CAR constructs optimized for the desired application (e.g., co-stimulatory domains, cytokine secretion, suicide or marker genes, etc.). The chosen CARs are validated in vitro and with the use of animal models and, if applicable, genome-editing strategies are also validated. A viral vector batch is then manufactured under GMP compliance. At this stage, it is expected that a proposed trial protocol is available, indicating the treatment regimens and required doses, as this will help defining the critical quality attributes of the drug product. Based on this information, the proposed manufacturing protocol is adjusted, a testing plan is devised, and testing methods optimized, before scale-up engineering runs are completed to show that products can be manufactured with the desired characteristics. All data on literature review and trial rationale, ethical considerations, risk-benefit analysis, pre-clinical validation data, detailed manufacturing methods and compliance, drug product characteristics, and proposed protocol are submitted for regulatory review by the MHRA in the UK. Once a CTA is granted, internal protocols are followed to complete the required validations and obtain the relevant approvals. The team is then trained on the pre-approved manufacturing and testing methods and products are manufacture in accordance with issued SOPs. Each batch manufactured undergoes detailed review by the quality team and the Qualified Person (QP) prior to release and results are constantly monitored to continuously improve processes. CAR, chimeric antigen receptors; CMC, chemistry, manufacturing, and control; CQA, critical quality attributes; QC, quality control; cGMP, current good manufacturing practice; MHRA, Medicines and Healthcare Products Regulatory Agency; CTA, clinical trial authorization.
High costs and recruitment of trained manufacturing personnel are the main challenges currently faced by academic CAR-T manufacturers. Skills shortage is a critical problem in the Cell and Gene Therapy field as a whole but this is exacerbated in academic teams due to the high demand and competition from industry. On-the-job learning through an effective training program and collaboration with the University’s teaching activities to provide practical experience and internships for students enrolled in life science degrees can help narrow this gap, but the establishment of streamlined, standardized, and effective protocols, relying on automation, and with minimal hands-on requirements is essential. Overall, adoption of new manufacturing technologies and development of simple manufacturing processes will be critical in increasing availability, quality, and applicability of CAR-T cell therapies.
The setup of the academic CAR T program at University College London (UCL) illustrates many of the challenges and solutions discussed above for the delivery of a phase 1 clinical trial. A manufacturing platform based on the use of the CliniMACS Prodigy was implemented within the Center for Cell, Gene and Tissue Therapeutics (CCGTT), at the Royal Free Hospital, in London, and has been adapted for CAR-T cell production for 4 completed and 5 on-going phase 1 studies, with 5 more currently in implementation. These trials have recruitment cohorts of 12–40 patients and funding sources are varied, ranging from government bodies, charities, and non-profit organizations, as well as partnerships with the biotechnology industry. With a focus on the improvement of manufacturing processes and development of new clinical products, and benefiting from the close links with hospital sites and research scientists, delivery of over 100 CAR-T product batches with diverse requisites was facilitated by the use of a standardized protocol that is used as a starting point, but is also adaptable for a diverse range of requirements, such as autologous or allogeneic use, different cultivation conditions and different kinds of viral vectors (lentiviral/retroviral and concentrated/unconcentrated). Because the manufacturing science team consists of a small group of six to ten scientists, responsible for all stages of process development, assay development, preparation of product dossiers for regulatory submission, clinical manufacturing, QC testing, quality assurance, and logistics of all CAR-T batches and working in a multiuser facility where space is limited and disputed, automation and reduction of hands-on time are essential, as is the support of a core CCGTT team for facility management, quality management, and regulatory guidance. The clinical development pipeline adopted by the UCL program is described in Figure 2.
Conclusions
The CAR-T cell manufacturing landscape is rapidly evolving. Automation currently emerges as a cornerstone, streamlining processes and enhancing scalability while mitigating the challenges posed by skills shortage and lack of consistency. Efforts to improve clinical outcomes rely on the robust characterization of CAR-T products, and will include not only engineering advancements but also improved manufacturing strategies to overcome terminal differentiation. Rapid manufacturing protocols, epitomized by next-day methodologies, not only tackle capacity limitations but are also expected to have an important impact on product efficacy. However, quality control remains a bottleneck to be addressed for further reduction of vein-to-vein times. Furthermore, the use of genome-editing technologies, notably CRISPR-Cas9, reshapes the therapeutic landscape, offering unparalleled precision in CAR-T cell engineering, but are associated with challenges such as genotoxicity and extensive requirements in safety evaluation and quality control implementation.
Academic teams play a crucial role in driving innovation and are essential for translating fundamental CAR-T research into clinical applications. Regulatory frameworks must adapt to the specific requirements of early-stage clinical trials, ensuring safety while creating an environment that supports innovation. As the field progresses, the alignment of technological advancements in manufacturing and quality control, associated with regulatory flexibility, can offer solutions to many of the current bottlenecks, accelerating the development and increasing availability of CAR-T cell products to a growing range of indications.
Acknowledgments
We acknowledge the hard work of the UCL CAR-T manufacturing team on the development of manufacturing methods and delivery of trial products: Ms Louisa Green, Ms Mhairi Vaughan, Ms Evie Lewin, Ms Eleanor Wooderson, Ms Lais de Castro, Ms Silvia Rondinelli, and Ms Jiahe Zhang. We also thank the CCGTT team for their support and expert advice: Dr Owen Bain, Ms Maryam Sekhavat, and Ms Fiona O’Brien. The UCL CAR-T cell program receives research funding from the ECMC, NIHR (II-C3-0714-20005), MRC (MR/S037144/1), CRUK (CTRQQR-2021\100004), Wellcome Trust (200145/Z/15/Z), Autolus Therapeutics, The Jon Moulton Charity Trust, and Abbie’s Army.
Author contributions
J.D., J.G., G.A., and C.R. wrote, reviewed, and approved the manuscript.
Declaration of interests
C.R., honoraria Novartis, Kite/Gilead, Miltenyi, BMS/Cengene, Autolus, Amgen, BD Biosciences.
References
- 1.Watanabe N., Mo F., McKenna M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.876339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaartinen T., Luostarinen A., Maliniemi P., Keto J., Arvas M., Belt H., Koponen J., Mäkinen P.I., Loskog A., Mustjoki S., et al. Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy. 2017;19:689–702. doi: 10.1016/j.jcyt.2017.03.067. [DOI] [PubMed] [Google Scholar]
- 3.Odstrcil M.S., Lee C.J., Sobieski C., Weisdorf D., Couriel D. Access to CAR T-cell therapy: Focus on diversity, equity and inclusion. Blood Rev. 2024;63 doi: 10.1016/j.blre.2023.101136. [DOI] [PubMed] [Google Scholar]
- 4.Fekete N., Walker R., Pike N. 2023. Workforce Report: Gap Analysis for the Cell and Gene Therapy Sector.https://alliancerm.org/wp-content/uploads/2023/03/ARM-Workforce-Gap-Analysis.pdf Alliance for Regenerative Medicine. [Google Scholar]
- 5.Griesenbach U., Rafiq Q., Kirby J., Ferraiuolo L., Przysowa J., Miller A., Roberts C., Salman S., Sebastian S., Mountcastle S., et al. 2023. Addressing the Skills Gap in Cell & Gene Therapies.https://genetherapyhubs.uk/application/files/7716/8805/1659/IHfGT_Skills_Gaps_Doc_June_2023.pdf Innovation Hubs for Gene Therapies. [Google Scholar]
- 6.Spink K., Steinsapir A. The long road to affordability: a cost of goods analysis for an autologous CAR-T process. Cell Gene Ther. Insights. 2018;4:1105–1116. doi: 10.18609/cgti.2018.108. [DOI] [Google Scholar]
- 7.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly M.A., Malhi A., Cheok K.P.L., Ings S., Balsa C., Keane H., Jalowiec K., Neill L., Peggs K.S., Roddie C. A novel predictive algorithm to personalize autologous T-cell harvest for chimeric antigen receptor T-cell manufacture. Cytotherapy. 2023;25:323–329. doi: 10.1016/j.jcyt.2022.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Palen K., Zurko J., Johnson B.D., Hari P., Shah N.N. Manufacturing chimeric antigen receptor T cells from cryopreserved peripheral blood cells: time for a collect-and-freeze model? Cytotherapy. 2021;23:985–990. doi: 10.1016/j.jcyt.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Ino K., Ageitos A.G., Singh R.K., Talmadge J.E. Activation-induced T cell apoptosis by monocytes from stem cell products. Int. Immunopharmacol. 2001;1:1307–1319. doi: 10.1016/S1567-5769(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 11.McFarland D.C., Zhang C., Thomas H.C., Tl R. Confounding effects of platelets on flow cytometric analysis and cell-sorting experiments using blood-derived cells. Cytometry A. 2006;69:86–94. doi: 10.1002/cyto.a.20207. [DOI] [PubMed] [Google Scholar]
- 12.Stroncek D.F., Ren J., Lee D.W., Tran M., Frodigh S.E., Sabatino M., Khuu H., Merchant M.S., Mackall C.L. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. doi: 10.1016/j.jcyt.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu M., Lezzar D.L., Vörös E., Shevkoplyas S.S. Traditional and emerging technologies for washing and volume reducing blood products. J. Blood Med. 2019;10:37–46. doi: 10.2147/JBM.S166316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li A., Kusuma G.D., Driscoll D., Smith N., Wall D.M., Levine B.L., James D., Lim R. Advances in automated cell washing and concentration. Cytotherapy. 2021;23:774–786. doi: 10.1016/j.jcyt.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Borquez-Ojeda O., Stefanski J., Du F., Qu J., Chaudhari J., Thummar K., Zhu M., Shen L.B., Hall M., et al. Depletion of high-content CD14+ cells from apheresis products is critical for successful transduction and expansion of CAR T cells during large-scale cGMP manufacturing. Mol. Ther. Methods Clin. Dev. 2021;22:377–387. doi: 10.1016/j.omtm.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruella M., Xu J., Barrett D.M., Fraietta J.A., Reich T.J., Ambrose D.E., Klichinsky M., Shestova O., Patel P.R., Kulikovskaya I., et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perret R., Olsen K. Magnetic selection for consistent cellular starting material in autologous cell therapy manufacture. Cell Gene Ther. Insights. 2022;8:97–112. doi: 10.18609/cgti.2022.027. [DOI] [Google Scholar]
- 18.Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 19.Haastrup E., Ifversen M.R.S., Heilmann C., Fischer-Nielsen A. Depletion of αβ+ T and B Cells Using the CliniMACS Prodigy: Results of 10 Graft-Processing Procedures from Haploidentical Donors. Transfus. Med. Hemother. 2019;46:446–449. doi: 10.1159/000497074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keever-Taylor C.A., Devine S.M., Soiffer R.J., Mendizabal A., Carter S., Pasquini M.C., Hari P.N., Stein A., Lazarus H.M., Linker C., et al. Characteristics of CliniMACS® System CD34-Enriched T Cell-Depleted Grafts in a Multicenter Trial for Acute Myeloid Leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Protocol 0303. Biol. Blood Marrow Transplant. 2012;18:690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudousquié C., Boand V., Lingre E., Dutoit L., Balint K., Danilo M., Harari A., Gannon P.O., Kandalaft L.E. Development and Optimization of a GMP-Compliant Manufacturing Process for a Personalized Tumor Lysate Dendritic Cell Vaccine. Vaccines. 2020;8:25. doi: 10.3390/vaccines8010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priesner C., Esser R., Tischer S., Marburger M., Aleksandrova K., Maecker-Kolhoff B., Heuft H.-G., Goudeva L., Blasczyk R., Arseniev L., et al. Comparative Analysis of Clinical-Scale IFN-γ-Positive T-Cell Enrichment Using Partially and Fully Integrated Platforms. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skorska A., Müller P., Gaebel R., Große J., Lemcke H., Lux C.A., Bastian M., Hausburg F., Zarniko N., Bubritzki S., et al. GMP-conformant on-site manufacturing of a CD133+ stem cell product for cardiovascular regeneration. Stem Cell Res. Ther. 2017;8:33. doi: 10.1186/s13287-016-0467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J., Riddell S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turtle C.J., Hanafi L.-A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M., et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arcangeli S., Bove C., Mezzanotte C., Camisa B., Falcone L., Manfredi F., Bezzecchi E., El Khoury R., Norata R., Sanvito F., et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J. Clin. Invest. 2022;132 doi: 10.1172/JCI150807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priesner C., Aleksandrova K., Esser R., Mockel-Tenbrinck N., Leise J., Drechsel K., Marburger M., Quaiser A., Goudeva L., Arseniev L., et al. Automated Enrichment, Transduction, and Expansion of Clinical-Scale CD62L + T Cells for Manufacturing of Gene Therapy Medicinal Products. Hum. Gene Ther. 2016;27:860–869. doi: 10.1089/hum.2016.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fritsche E., Volk H.-D., Reinke P., Abou-El-Enein M. Toward an Optimized Process for Clinical Manufacturing of CAR-Treg Cell Therapy. Trends Biotechnol. 2020;38:1099–1112. doi: 10.1016/j.tibtech.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Bluestone J.A., Buckner J.H., Fitch M., Gitelman S.E., Gupta S., Hellerstein M.K., Herold K.C., Lares A., Lee M.R., Li K., et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putnam A.L., Safinia N., Medvec A., Laszkowska M., Wray M., Mintz M.A., Trotta E., Szot G.L., Liu W., Lares A., et al. Clinical Grade Manufacturing of Human Alloantigen-Reactive Regulatory T Cells for Use in Transplantation. Am. J. Transplant. 2013;13:3010–3020. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A. Enabling GMP-ready cell sorting. Cell Gene Ther. Insights. 2023;8:1339–1344. doi: 10.18609/cgti.2022.194. [DOI] [Google Scholar]
- 32.Arndt B., Poltorak M., Kowtharapu B.S., Reichardt P., Philipsen L., Lindquist J.A., Schraven B., Simeoni L. Analysis of TCR activation kinetics in primary human T cells upon focal or soluble stimulation. J. Immunol. Methods. 2013;387:276–283. doi: 10.1016/j.jim.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Keskar V., Sood A., Loghin E., Kovacs E., Duthie R.S., Liu S., Park J.H., Chadwick C., Smith R., Brown M., et al. Novel DNA-based T-Cell Activator Promotes Rapid T-Cell Activation and Expansion. J. Immunother. 2020;43:231–235. doi: 10.1097/CJI.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poltorak M.P., Graef P., Tschulik C., Wagner M., Cletiu V., Dreher S., Borjan B., Fraessle S.P., Effenberger M., Turk M., et al. Expamers: a new technology to control T cell activation. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-74595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehlhop-Williams E.R., Bevan M.J. Memory CD8+ T cells exhibit increased antigen threshold requirements for recall proliferation. J. Exp. Med. 2014;211:345–356. doi: 10.1084/jem.20131271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Stipdonk M.J., Lemmens E.E., Schoenberger S.P. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 37.Wang X., Simeoni L., Lindquist J.A., Saez-Rodriguez J., Ambach A., Gilles E.D., Kliche S., Schraven B. Dynamics of Proximal Signaling Events after TCR/CD8-Mediated Induction of Proliferation or Apoptosis in Mature CD8+ T Cells. J. Immunol. 2008;180:6703–6712. doi: 10.4049/jimmunol.180.10.6703. [DOI] [PubMed] [Google Scholar]
- 38.Gatla H., Uth N., Levinson Y., Navaei A., Sargent A., Ramaswamy S., Friedrich Ben-Nun I. Enabling Allogeneic T Cell-Based Therapies: Scalable Stirred-Tank Bioreactor Mediated Manufacturing. Front. Med. Technol. 2022;4 doi: 10.3389/fmedt.2022.850565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Auw N., Serfling R., Kitte R., Hilger N., Zhang C., Gebhardt C., Duenkel A., Franz P., Koehl U., Fricke S., Tretbar U.S. Comparison of two lab-scale protocols for enhanced mRNA-based CAR-T cell generation and functionality. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-45197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labbé R.P., Vessillier S., Rafiq Q.A. Lentiviral Vectors for T Cell Engineering: Clinical Applications, Bioprocessing and Future Perspectives. Viruses. 2021;13:1528. doi: 10.3390/v13081528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaeger-Ruckstuhl C.A., Lo Y., Fulton E., Waltner O.G., Shabaneh T.B., Simon S., Muthuraman P.V., Correnti C.E., Newsom O.J., Engstrom I.A., et al. Signaling via a CD27-TRAF2-SHP-1 axis during naive T cell activation promotes memory-associated gene regulatory networks. Immunity. 2024;57:287–302.e12. doi: 10.1016/j.immuni.2024.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghassemi S., Durgin J.S., Nunez-Cruz S., Patel J., Leferovich J., Pinzone M., Shen F., Cummins K.D., Plesa G., Cantu V.A., et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 2022;6:118–128. doi: 10.1038/s41551-021-00842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghorashian S., Kramer A.M., Onuoha S., Wright G., Bartram J., Richardson R., Albon S.J., Casanovas-Company J., Castro F., Popova B., et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 44.Smith T.A. CAR-T Cell Expansion in a Xuri Cell Expansion System W25. Methods Mol. Biol. 2020;2086:151–163. doi: 10.1007/978-1-0716-0146-4_11. [DOI] [PubMed] [Google Scholar]
- 45.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S., et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgiadis C., Rasaiyaah J., Gkazi S.A., Preece R., Etuk A., Christi A., Qasim W. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia. 2021;35:3466–3481. doi: 10.1038/s41375-021-01282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodruff R., Parekh F., Lamb K., Mekkaoui L., Allen C., Smetanova K., Huang J., Williams A., Toledo G.S., Lilova K., et al. Large-scale manufacturing of base-edited chimeric antigen receptor T cells. Mol. Ther. Methods Clin. Dev. 2023;31 doi: 10.1016/j.omtm.2023.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fry L.J., Querol S., Gomez S.G., McArdle S., Rees R., Madrigal J.A. Assessing the toxic effects of DMSO on cord blood to determine exposure time limits and the optimum concentration for cryopreservation. Vox Sang. 2015;109:181–190. doi: 10.1111/vox.12267. [DOI] [PubMed] [Google Scholar]
- 49.Thielen F. Erasmus University Rotterdam; 2020. Current Challenges in Health Technology Assessment: Assessing Costs and Cost-Effectiveness of Novel Treatments in Haemato-Oncology. [Google Scholar]
- 50.Blache U., Weiss R., Boldt A., Kapinsky M., Blaudszun A.-R., Quaiser A., Pohl A., Miloud T., Burgaud M., Vucinic V., et al. Advanced Flow Cytometry Assays for Immune Monitoring of CAR-T Cell Applications. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.658314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu L.X., Amidon G., Khan M.A., Hoag S.W., Polli J., Raju G.K., Woodcock J. Understanding pharmaceutical quality by design. AAPS J. 2014;16:771–783. doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guedan S., Posey A.D., Jr., Shaw C., Wing A., Da T., Patel P.R., McGettigan S.E., Casado-Medrano V., Kawalekar O.U., Uribe-Herranz M., et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O’Connor R.S., Hwang W.-T., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanjireddy P.M., Olejniczak S.H., Buxbaum N.P. Targeting of chimeric antigen receptor T cell metabolism to improve therapeutic outcomes. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turtle C.J., Hay K.A., Hanafi L.-A., Li D., Cherian S., Chen X., Wood B., Lozanski A., Byrd J.C., Heimfeld S., et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor–Modified T Cells After Failure of Ibrutinib. J. Clin. Oncol. 2017;35:3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Kurlander R.J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: Differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl. Med. 2010;8:104. doi: 10.1186/1479-5876-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maus M.V., Thomas A.K., Leonard D.G.B., Allman D., Addya K., Schlienger K., Riley J.L., June C.H. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 58.Kagoya Y., Nakatsugawa M., Ochi T., Cen Y., Guo T., Anczurowski M., Saso K., Butler M.O., Hirano N. Transient stimulation expands superior antitumor T cells for adoptive therapy. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tham E.L., Mescher M.F. The Poststimulation Program of CD4 Versus CD8 T Cells (Death Versus Activation-Induced Nonresponsiveness)1. J. Immunol. 2002;169:1822–1828. doi: 10.4049/jimmunol.169.4.1822. [DOI] [PubMed] [Google Scholar]
- 60.Zhang D.K.Y., Adu-Berchie K., Iyer S., Liu Y., Cieri N., Brockman J.M., Neuberg D., Wu C.J., Mooney D.J. Enhancing CAR-T cell functionality in a patient-specific manner. Nat. Commun. 2023;14:506. doi: 10.1038/s41467-023-36126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roddie C., Dias J., O’Reilly M.A., Abbasian M., Cadinanos-Garai A., Vispute K., Bosshard-Carter L., Mitsikakou M., Mehra V., Roddy H., et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2021;39:3352–3363. doi: 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talleur A.C., Qudeimat A., Métais J.Y., Langfitt D., Mamcarz E., Crawford J.C., Huang S., Cheng C., Hurley C., Madden R., et al. Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv. 2022;6:5737–5749. doi: 10.1182/bloodadvances.2021006293.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Tong C., Lu Y., Wu Z., Guo Y., Liu Y., Wei J., Wang C., Yang Q., Han W. Characteristics of premanufacture CD8+ T cells determine CAR-T efficacy in patients with diffuse large B-cell lymphoma. Signal Transduct. Target. Ther. 2023;8:409. doi: 10.1038/s41392-023-01659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westin J.R., Oluwole O.O., Kersten M.J., Miklos D.B., Perales M.-A., Ghobadi A., Rapoport A.P., Sureda A., Jacobson C.A., Farooq U., et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023;389:148–157. doi: 10.1056/NEJMoa2301665. [DOI] [PubMed] [Google Scholar]
- 65.Joedicke J.J., Großkinsky U., Gerlach K., Künkele A., Höpken U.E., Rehm A. Accelerating clinical-scale production of BCMA CAR T cells with defined maturation stages. Mol. Ther. Methods Clin. Dev. 2022;24:181–198. doi: 10.1016/j.omtm.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spiegel J.Y., Patel S., Muffly L., Hossain N.M., Oak J., Baird J.H., Frank M.J., Shiraz P., Sahaf B., Craig J., et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vodnala S.K., Eil R., Kishton R.J., Sukumar M., Yamamoto T.N., Ha N.-H., Lee P.-H., Shin M., Patel S.J., Yu Z., et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019;363 doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmadzadeh M., Rosenberg S.A. IL-2 administration increases CD4+CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corrado M., Pearce E.L. Targeting memory T cell metabolism to improve immunity. J. Clin. Invest. 2022;132 doi: 10.1172/JCI148546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D., et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akbari B., Hosseini Z., Shahabinejad P., Ghassemi S., Mirzaei H.R., O’Connor R.S. Metabolic and epigenetic orchestration of (CAR) T cell fate and function. Cancer Lett. 2022;550 doi: 10.1016/j.canlet.2022.215948. [DOI] [PubMed] [Google Scholar]
- 72.Funk C.R., Wang S., Chen K.Z., Waller A., Sharma A., Edgar C.L., Gupta V.A., Chandrakasan S., Zoine J.T., Fedanov A., et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139:523–537. doi: 10.1182/blood.2021011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klebanoff C.A., Crompton J.G., Leonardi A.J., Yamamoto T.N., Chandran S.S., Eil R.L., Sukumar M., Vodnala S.K., Hu J., Ji Y., et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2 doi: 10.1172/jci.insight.95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehra V., Agliardi G., Dias Alves Pinto J., Shafat M.S., Garai A.C., Green L., Hotblack A., Arce Vargas F., Peggs K.S., van der Waart A.B., et al. AKT inhibition generates potent polyfunctional clinical grade AUTO1 CAR T-cells, enhancing function and survival. J. Immunother. Cancer. 2023;11 doi: 10.1136/jitc-2023-007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q., Ding J., Sun S., Liu H., Lu M., Wei X., Gao X., Zhang X., Fu Q., Zheng J. Akt inhibition at the initial stage of CAR-T preparation enhances the CAR-positive expression rate, memory phenotype and in vivo efficacy. Am. J. Cancer Res. 2019;9:2379–2396. [PMC free article] [PubMed] [Google Scholar]
- 76.Fan F., Yoo H.J., Stock S., Wang L., Liu Y., Schubert M.-L., Wang S., Neuber B., Hückelhoven-Krauss A., Gern U., et al. Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients. Int. J. Cancer. 2021;148:419–428. doi: 10.1002/ijc.33212. [DOI] [PubMed] [Google Scholar]
- 77.Majzner R.G., Ramakrishna S., Yeom K.W., Patel S., Chinnasamy H., Schultz L.M., Richards R.M., Jiang L., Barsan V., Mancusi R., et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber E.W., Lynn R.C., Sotillo E., Lattin J., Xu P., Mackall C.L. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3:711–717. doi: 10.1182/bloodadvances.2018028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cappell K.M., Kochenderfer J.N. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 2021;18:715–727. doi: 10.1038/s41571-021-00530-z. [DOI] [PubMed] [Google Scholar]
- 80.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feucht J., Sadelain M. Function and evolution of the prototypic CD28ζ and 4-1BBζ chimeric antigen receptors. Immunooncol. Technol. 2020;8:2–11. doi: 10.1016/j.iotech.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biasco L., Izotova N., Rivat C., Ghorashian S., Richardson R., Guvenel A., Hough R., Wynn R., Popova B., Lopes A., et al. Clonal expansion of T memory stem cells determines early anti-leukemic responses and long-term CAR T cell persistence in patients. Nat. Cancer. 2021;2:629–642. doi: 10.1038/s43018-021-00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michelozzi I.M., Gomez-Castaneda E., Pohle R.V.C., Cardoso Rodriguez F., Sufi J., Puigdevall Costa P., Subramaniyam M., Kirtsios E., Eddaoudi A., Wu S.W., et al. Activation priming and cytokine polyfunctionality modulate the enhanced functionality of low-affinity CD19 CAR T cells. Blood Adv. 2023;7:1725–1738. doi: 10.1182/bloodadvances.2022008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gomes-Silva D., Mukherjee M., Srinivasan M., Krenciute G., Dakhova O., Zheng Y., Cabral J.M.S., Rooney C.M., Orange J.S., Brenner M.K., Mamonkin M. Tonic 4-1BB Costimulation in Chimeric Antigen Receptors Impedes T Cell Survival and Is Vector-Dependent. Cell Rep. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen J., Qiu S., Li W., Wang K., Zhang Y., Yang H., Liu B., Li G., Li L., Chen M., et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 2023;33:341–354. doi: 10.1038/s41422-023-00789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin M.-Z., Jin W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., Li N., Feng K., Chen M., Zhang Y., Liu Y., Yang Q., Nie J., Tang N., Zhang X., et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell. Mol. Immunol. 2021;18:2188–2198. doi: 10.1038/s41423-021-00749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kloss C.C., Lee J., Zhang A., Chen F., Melenhorst J.J., Lacey S.F., Maus M.V., Fraietta J.A., Zhao Y., June C.H. Dominant-Negative TGF-β Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol. Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y., Sun C., Landoni E., Metelitsa L., Dotti G., Savoldo B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clin. Cancer Res. 2019;25:2915–2924. doi: 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 90.Golumba-Nagy V., Kuehle J., Hombach A.A., Abken H. CD28-ζ CAR T Cells Resist TGF-β Repression through IL-2 Signaling, Which Can Be Mimicked by an Engineered IL-7 Autocrine Loop. Mol. Ther. 2018;26:2218–2230. doi: 10.1016/j.ymthe.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurton L.V., Singh H., Najjar A.M., Switzer K.C., Mi T., Maiti S., Olivares S., Rabinovich B., Huls H., Forget M.-A., et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hunter M.R., Prosser M.E., Mahadev V., Wang X., Aguilar B., Brown C.E., Forman S.J., Jensen M.C. Chimeric γc cytokine receptors confer cytokine independent engraftment of human T lymphocytes. Mol. Immunol. 2013;56:1–11. doi: 10.1016/j.molimm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 93.Zhao Z., Li Y., Liu W., Li X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/4795171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Juillerat A., Marechal A., Filhol J.M., Valogne Y., Valton J., Duclert A., Duchateau P., Poirot L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 2017;7 doi: 10.1038/srep39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shim G., Kim D., Park G.T., Jin H., Suh S.-K., Oh Y.-K. Therapeutic gene editing: delivery and regulatory perspectives. Acta Pharmacol. Sin. 2017;38:738–753. doi: 10.1038/aps.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hopewell E.L., Cox C., Pilon-Thomas S., Kelley L.L. Tumor-infiltrating lymphocytes: Streamlining a complex manufacturing process. Cytotherapy. 2019;21:307–314. doi: 10.1016/j.jcyt.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rotte A., Frigault M.J., Ansari A., Gliner B., Heery C., Shah B. Dose–response correlation for CAR-T cells: a systematic review of clinical studies. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2022-005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghassemi S., Nunez-Cruz S., O’Connor R.S., Fraietta J.A., Patel P.R., Scholler J., Barrett D.M., Lundh S.M., Davis M.M., Bedoya F., et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol. Res. 2018;6:1100–1109. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., He J., Zhang X., Li J., Wang Z., Zhang Y., Qiu L., Wu Q., Sun Z., Ye X., et al. Next-day manufacture of a novel anti-CD19 CAR-T therapy for B-cell acute lymphoblastic leukemia: first-in-human clinical study. Blood Cancer J. 2022;12:104. doi: 10.1038/s41408-022-00694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dickinson M.J., Barba P., Jäger U., Shah N.N., Blaise D., Briones J., Shune L., Boissel N., Bondanza A., Mariconti L., et al. A Novel Autologous CAR-T Therapy, YTB323, with Preserved T-cell Stemness Shows Enhanced CAR T-cell Efficacy in Preclinical and Early Clinical Development. Cancer Discov. 2023;13:1982–1997. doi: 10.1158/2159-8290.CD-22-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benjamin R., Jain N., Maus M.V., Boissel N., Graham C., Jozwik A., Yallop D., Konopleva M., Frigault M.J., Teshima T., et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial. Lancet. Haematol. 2022;9:e833–e843. doi: 10.1016/S2352-3026(22)00245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K., et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 103.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J.C., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Y., Zu C., Zhang M., Wei G., Li W., Fu S., Hong R., Zhou L., Wu W., Cui J., et al. Safety and efficacy of CRISPR-based non-viral PD1 locus specifically integrated anti-CD19 CAR-T cells in patients with relapsed or refractory Non-Hodgkin’s lymphoma: a first-in-human phase I study. eClinicalMedicine. 2023;60 doi: 10.1016/j.eclinm.2023.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dimitri A., Herbst F., Fraietta J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer. 2022;21:78. doi: 10.1186/s12943-022-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yip B.H. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules. 2020;10 doi: 10.3390/biom10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vavassori V., Ferrari S., Beretta S., Asperti C., Albano L., Annoni A., Gaddoni C., Varesi A., Soldi M., Cuomo A., et al. Lipid nanoparticles allow efficient and harmless ex vivo gene editing of human hematopoietic cells. Blood. 2023;142:812–826. doi: 10.1182/blood.2022019333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung H.N., Lee S.-Y., Lee S., Youn H., Im H.-J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics. 2022;12:7509–7531. doi: 10.7150/thno.77259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maeki M., Uno S., Niwa A., Okada Y., Tokeshi M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release. 2022;344:80–96. doi: 10.1016/j.jconrel.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nature Editorial Licensing for profit and for good. Nat. Biotechnol. 2022;40:439. doi: 10.1038/s41587-022-01296-0. [DOI] [PubMed] [Google Scholar]
- 111.Kosicki M., Allen F., Steward F., Tomberg K., Pan Y., Bradley A. Cas9-induced large deletions and small indels are controlled in a convergent fashion. Nat. Commun. 2022;13:3422. doi: 10.1038/s41467-022-30480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papathanasiou S., Markoulaki S., Blaine L.J., Leibowitz M.L., Zhang C.-Z., Jaenisch R., Pellman D. Whole chromosome loss and genomic instability in mouse embryos after CRISPR-Cas9 genome editing. Nat. Commun. 2021;12:5855. doi: 10.1038/s41467-021-26097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rayner E., Durin M.-A., Thomas R., Moralli D., O’Cathail S.M., Tomlinson I., Green C.M., Lewis A. CRISPR-Cas9 Causes Chromosomal Instability and Rearrangements in Cancer Cell Lines, Detectable by Cytogenetic Methods. CRISPR J. 2019;2:406–416. doi: 10.1089/crispr.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boroviak K., Fu B., Yang F., Doe B., Bradley A. Revealing hidden complexities of genomic rearrangements generated with Cas9. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-12740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chapman J.E., Gillum D., Kiani S. Approaches to Reduce CRISPR Off-Target Effects for Safer Genome Editing. Appl. Biosaf. 2017;22:7–13. doi: 10.1177/1535676017694148. [DOI] [Google Scholar]
- 117.Naeem M., Alkhnbashi O.S. Current Bioinformatics Tools to Optimize CRISPR/Cas9 Experiments to Reduce Off-Target Effects. Int. J. Mol. Sci. 2023;24:6261. doi: 10.3390/ijms24076261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo C., Ma X., Gao F., Guo Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1143157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rees H.A., Liu D.R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodríguez T.C., Dadafarin S., Pratt H.E., Liu P., Amrani N., Zhu L.J. In: Progress in Molecular Biology and Translational Science. Singh V., editor. Academic Press; 2021. Chapter Three - Genome-wide detection and analysis of CRISPR-Cas off-targets; pp. 31–43. [DOI] [PubMed] [Google Scholar]
- 121.Hunt J.M.T., Samson C.A., Rand A.d., Sheppard H.M. Unintended CRISPR-Cas9 editing outcomes: a review of the detection and prevalence of structural variants generated by gene-editing in human cells. Hum. Genet. 2023;142:705–720. doi: 10.1007/s00439-023-02561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selliah N., Eck S., Green C., Oldaker T., Stewart J., Vitaliti A., Litwin V. Flow Cytometry Method Validation Protocols. Curr. Protoc. Cytom. 2019;87 doi: 10.1002/cpcy.53. [DOI] [PubMed] [Google Scholar]
- 123.Cheung M., Campbell J.J., Thomas R.J., Braybrook J., Petzing J. Assessment of Automated Flow Cytometry Data Analysis Tools within Cell and Gene Therapy Manufacturing. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lisby A.N., Carlson R.D., Baybutt T.R., Weindorfer M., Snook A.E. In: Methods in Cell Biology. Spada S., Galluzzi L., editors. Academic Press; 2022. Chapter 5 - Evaluation of CAR-T cell cytotoxicity: Real-time impedance-based analysis; pp. 81–98. [DOI] [PubMed] [Google Scholar]
- 125.Paterson K., Paterson S., Mulholland T., Coffelt S.B., Zagnoni M. Assessment of CAR-T Cell-Mediated Cytotoxicity in 3D Microfluidic Cancer Co-Culture Models for Combination Therapy. IEEE Open J. Eng. Med. Biol. 2022;3:86–95. doi: 10.1109/OJEMB.2022.3178302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong K.U., Shi J., Li P., Wang H., Jia Y., Deng C., Jiang L., Wong A.H.-H. Assessment of chimeric antigen receptor T cytotoxicity by droplet microfluidics in vitro. Antib. Ther. 2022;5:85–99. doi: 10.1093/abt/tbac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sheih A., Voillet V., Hanafi L.-A., DeBerg H.A., Yajima M., Hawkins R., Gersuk V., Riddell S.R., Maloney D.G., Wohlfahrt M.E., et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat. Commun. 2020;11:219. doi: 10.1038/s41467-019-13880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X., Peticone C., Kotsopoulou E., Göttgens B., Calero-Nieto F.J. Single-cell transcriptome analysis of CAR T-cell products reveals subpopulations, stimulation, and exhaustion signatures. OncoImmunology. 2021;10 doi: 10.1080/2162402X.2020.1866287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim M.Y., Jayasinghe R., Devenport J.M., Ritchey J.K., Rettig M.P., O’Neal J., Staser K.W., Kennerly K.M., Carter A.J., Gao F., et al. A long-acting interleukin-7, rhIL-7-hyFc, enhances CAR T cell expansion, persistence, and anti-tumor activity. Nat. Commun. 2022;13:3296. doi: 10.1038/s41467-022-30860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang V., Gauthier M., Decot V., Reppel L., Bensoussan D. Systematic Review on CAR-T Cell Clinical Trials Up to 2022: Academic Center Input. Cancers. 2023;15 doi: 10.3390/cancers15041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vormittag P., Gunn R., Ghorashian S., Veraitch F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 132.Fesnak A., Lin C., Siegel D.L., Maus M.V. CAR-T Cell Therapies From the Transfusion Medicine Perspective. Transfus. Med. Rev. 2016;30:139–145. doi: 10.1016/j.tmrv.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chiche-Lapierre C.E., Tramalloni D., Chaput N., Lapierre V. Comparative Analysis of Sepax S-100, COBE 2991, and Manual DMSO Removal Techniques From Cryopreserved Hematopoietic Stem Cell Apheresis Product. Cytotherapy. 2016;18:S47. doi: 10.1016/j.jcyt.2016.03.112. [DOI] [Google Scholar]
- 134.Francis N., Braun M., Neagle S., Peiffer S., Bohn A., Rosenthal A., Olbrich T., Lollies S., Ilsmann K., Hauck C., et al. Development of an automated manufacturing process for large-scale production of autologous T cell therapies. Mol. Ther. Methods Clin. Dev. 2023;31 doi: 10.1016/j.omtm.2023.101114. [DOI] [PMC free article] [PubMed] [Google Scholar]