Abstract
The objective of this paper is to conduct a systematic thematic review of adverse events, safety, and toxicity of traditional ayahuasca plant preparations and its main psychoactive alkaloids (dimethyltryptamine [DMT], harmine, harmaline, and tetrahydroharmine), including discussing clinical considerations (within clinical trials or approved settings). A systematic literature search of preclinical, clinical, epidemiological, and pharmacovigilance data (as well as pertinent reviews and case studies) was conducted for articles using the electronic databases of PubMed and Web of Science (to 6 July 2023) and PsycINFO, ClinicalTrials.gov, and Embase (to 21 September 2022) and included articles in English in peer-reviewed journals. Additionally, reference lists were searched. Due to the breadth of the area covered, we presented the relevant data in a thematic format. Our searches revealed 78 relevant articles. Data showed that ayahuasca or DMT is generally safe; however, some adverse human events have been reported. Animal models using higher doses of ayahuasca have shown abortifacient and teratogenic effects. Isolated harmala alkaloid studies have also revealed evidence of potential toxicity at higher doses, which may increase with co-administration with certain medications. Harmaline revealed the most issues in preclinical models. Nevertheless, animal models involving higher-dose synthetic isolates may not necessarily be able to be extrapolated to human use of therapeutic doses of plant-based extracts. Serious adverse effects are rarely reported within healthy populations, indicating an acceptable safety profile for the traditional use of ayahuasca and DMT in controlled settings. Further randomized, controlled trials with judicious blinding, larger samples, and longer duration are needed.
Keywords: adverse events, safety, toxicology, psychedelic, hallucinogens, dimethyltryptamine, ayahuasca
Introduction
Ayahuasca has been used in the Amazon basin for at least hundreds of years within religious contexts. 1 A psychoactive brew prepared from this plant usually contains DMT (N,N-dimethyltryptamine) in combination with harmala alkaloids. 2 In the 1930s, its use spread to urbanized areas, and it was incorporated into various Brazilian syncretic religions (a blending of two or more religious systems). These churches have now spread internationally and can be found in Australia, North America, and Europe.1,3–5 The word ‘ayahuasca’ is from the Quechua language meaning ‘vine of the souls’ 6 and is used by Indigenous groups in South America to refer to a medicinal botanical boiled water decoction typically made from the Banisteriopsis caapi (ayahuasca) vine and the leaves of Psychotria viridis (chacruna) or Diplopterys cabrerana (chaliponga).2,7
Ayahuasca has dramatically increased in popularity since the turn of the millennium, with increasing numbers of tourists visiting the Amazonas in search of its therapeutic, spiritual, or personal development effects. 8 Use of the ayahuasca preparations outside the Amazon in neo-shamanic ceremonies has also increased in popularity across the globe, with alternative botanical sources of harmala alkaloids and DMT sometimes being used.4,5,9 Vast amounts of anecdotal evidence now exist describing the healing effects of the brew, leading researchers to question the therapeutic potential of DMT-harmaloid preparations. 10
Ayahuasca formulations usually contain the classical psychedelic DMT as well as several beta-carboline (harmala) alkaloids that prevent enzymatic degradation of DMT in the gut, as well as having their own psychoactive and therapeutic profiles.2,6 Both DMT and the beta-carbolines found in ayahuasca have been linked to physiological and psychological markers of improved mental health.11–15 DMT is a potent psychedelic known to produce radical shifts in consciousness, often characterized by profound visionary and emotional experiences.16,17 Pharmacodynamic properties of DMT involve the modulation of serotonergic and dopaminergic signaling via serotonin-1A, -2A, and -2C receptor (5-HT1A, 5-HT2A, and 5-HT2C) agonism, dopamine D2-receptor agonism, and sigma-1 agonism, with the psychedelic effects likely mediated via the 5-HT1A and 5-HT2A receptors. 18 Sigma-1 receptors are suggested to play a key role and be a potential target for addiction treatment, as well as being involved in depression and anxiety, healing of traumatic memories, synaptic plasticity, and cell regeneration.19–24
A further set of potential pathways associated with ayahuasca relates to the harmala alkaloids, containing harmine, harmaline, and tetrahydroharmine. These compounds are monoamine oxidase A (MAO-A) enzyme inhibitors, which serve to make DMT orally active, also increasing serotonin and norepinephrine levels, and have known antidepressant effects. 2 Tetrahydroharmine is a weak SSRI (selective serotonin reuptake inhibitor) itself, while harmine has been associated with increased levels of brain-derived neurotrophic factor, the proliferation of human neural progenitor cells, as well as anti-depressive and anti-inflammatory effects.25-31 The psychotropic properties of harmala alkaloids (which belong to the group of β-carboline derivatives) have been a matter of discussion since the early investigations of psychedelic drugs. 32 β-Carbolines have a diverse range of biological activities including the interaction with benzodiazepine and 5-hydroxy serotonin receptors, demonstrating sedative, anxiolytic, hypnotic, and anticonvulsant activities. 33
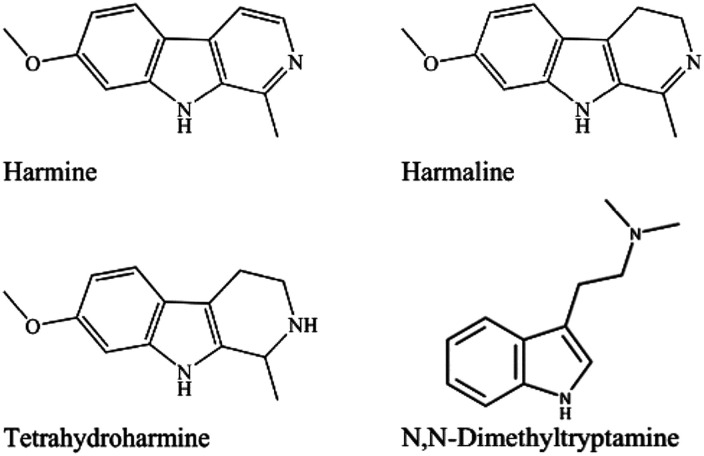
The purpose of this paper is to identify and detail the toxicological profile from preclinical studies and to review the data concerning adverse events (AEs) revealed in both epidemiological and clinical studies of ayahuasca and isolated DMT and harmala alkaloids (harmine, tetrahydroharmine, and harmaline) (Figure 1). While other papers have reviewed ayahuasca and DMT for their use in various specific indications (e.g., exploring the underlying potential anti-depressive or anxiolytic effects, or covering the current human clinical study evidence in the area), no comprehensive integrated paper exists to date that critically appraises potential toxicity and adverse effects while providing clinical observations based on these safety considerations.
Figure 1.
Chemical structure of harmine (CAS no. 442-51-3), harmaline (CAS no. 304-21-2), tetrahydroharmine (CAS no. 17019-01-1), and N,N-dimethyltryptamine (CAS no. 37637-29-9).
Methods
Clinical trials, observational studies, epidemiological data, original research reports, reviews, qualitative studies, systematic and meta-reviews, case reports, and pharmacovigilance databases were sought for potential inclusion to provide a thorough review of the field. In order to include as many relevant sources as possible, there were no exclusions based on the types of animal models (testing ayahuasca or isolated psychoactive alkaloids) used in the studies. No data on the harmala alkaloid tetrahydroharmine were identified. Due to reviewing a series of data from many types of sources, it was deemed that a Preferred Reporting Items for Systematic Reviews and Meta-Analyses 34 checklist and flowchart review was not appropriate (PRISMA is more appropriate for assessing standard structured systematic reviews or meta-analyses which focus on one data type, e.g., clinical trials).
Articles were initially identified using the electronic databases PubMed, PsycINFO, Web of Science, and EBSCO, up to 21 September 2022, and only included articles in English and published in peer-reviewed journals. The initial searches were augmented by additional searches of the PubMed and Web of Science databases conducted on 6 July 2023 for any references from 22 September 2022 to 6 July 2023. Furthermore, reference lists and personal databases were searched for additional references. The main database search was split into four systematic search streams: animal models, human clinical trials, epidemiological data, and pharmacovigilance data. The data were displayed in the same order with ayahuasca and DMT/harmalas divided into sections. The term ‘significant’ was used for a P value of <.05. All studies were screened by two independent reviewers (EW and TK) with discrepancies resolved by a third reviewer (JS). Names of authors, year of publication, study type, intervention, and outcome measures were recorded for all included articles. We also drew from reviews by Dos Santos and colleagues27,35 to capture many human studies and case reports.
Search terms used for all databases can be found in the Supplemental information.
Results
Our initial searches revealed 3797 articles (PubMed, n = 1002; EMBASE, n = 240; PsycINFO, n = 565; Web of Science, n = 1967; ClinicalTrials.gov, n = 23). After removal of duplicates, the remaining 2853 titles and abstracts were screened independently by the first two authors (EW and TK) to determine whether they were relevant to our inquiry. After screening the full texts of 255 articles, 78 were chosen for inclusion in this paper. Updated searches on 6 July 2023 revealed 323 additional articles (PubMed, n = 149; Web of Science, n = 174). Seven additional articles were identified for full-text review, and one was chosen for inclusion in this paper. Articles were excluded where titles and/or abstracts did not mention ayahuasca or where adverse effects were not identified through the full-text review. For ease of reading, we segment the information into the following sections: animal model data, human clinical studies, epidemiological data, and pharmacovigilance data. Regarding the plant preparations studied, there were differences in plant sources used and the standardization of DMT and harmalas in some studies. Some studies used standardized isolated synthetic DMT or harmalas, which, where possible, was expressed as g, mg, or mg/kg. Some studies used injection format while others used oral administration. The average ritual/ceremonial oral dose of ayahuasca is 100–150 mL for a 70 kg person (1.0 mg/kg DMT, 1.70 mg/kg harmine, .11 mg/kg harmaline, and 1.36 mg/kg tetrahydroharmine). For reference, the human equivalent of ayahuasca in rat studies is roughly .302 mg/kg body weight (b/w) DMT, while harmalas are 3.34 mg/kg bw harmine and .261 mg/kg bw harmaline.36,37
Animal Model Data
Ayahuasca (B. caapi and P. viridis)
Regarding data that concerns plant-based DMT and harmalas, Pic-Taylor and colleagues 36 administered a single dose of ayahuasca by oral gavage at 30 (9 mg/kg bw DMT) to 50 (15 mg/kg bw DMT) times the regular ritual dose to female Wistar rats. The animals were observed for the next 14 days to determine the acute oral toxicity of ayahuasca. The authors were unable to determine the lethal dose of the extract, but it was deemed to be higher than 50 times the standard dose (standard dose being roughly .302 mg/kg bw DMT, 3.34 mg/kg bw harmine, and .261 mg/kg bw harmaline). The ayahuasca-treated rats showed a general decrease in locomotion when compared to controls in the open field test, and an increase in swimming in the forced swim test when compared to controls. Furthermore, ayahuasca-treated rats displayed higher c-fos expression in areas of the brain associated with serotonergic neurotransmission (dorsal raphe nuclei, amygdala, and hippocampus). It should be noted that although this study led to some neurodegeneration, no permanent damage was found at the end of the 14-day study. A subsequent study treated rats with 0.5X, 1X, and 2X the standard ceremonial dose of ayahuasca. 38 At these doses, ayahuasca caused no evidence of clinical, macroscopic, or hematological toxicity.
Melo Junior and colleagues 39 investigated the mutagenetic, genotoxic, and cytotoxic effects of ayahuasca after a single oral dose by gavage in rats. Doses of 1X, 5X, and 15X, the usual ritualistic dose (.3, 1.5, and 4.5 mg/kg bw DMT), were administered. Ayahuasca was found to have low genotoxicity, with an increased frequency of micronuclei only at the highest exposure level. At 5X dose, it showed a no-observed-adverse-effect level (1.5 mg/kg bw DMT). Cytotoxic effect was measured by the percentage of polychromatic erythrocytes related to the total cells. No cytotoxic effects were observed in this study, and renal, hepatic, and pancreatic function was not significantly affected. 39 Kummrow and colleagues 40 analyzed two ayahuasca samples; findings indicate they were mutagenic in the Salmonella/microsome assay using strains TA98 and TA100 with and without S9, with similar potencies. The authors did not find mutagenic properties in the P. viridis plant, only the B. caapi, concluding that mutagenic activity in the Ames test was most likely due to harmaline. Interestingly, religious groups that work with ayahuasca, such as the Santo Diame, purposefully brew ayahuasca for longer, reducing the levels of harmaline and increasing that of tetrahydroharmine, as tetrahydroharmine is a reduction product of harmaline. Clinical trials investigating the use of ayahuasca have/are also focusing attention on brews with a higher tetrahydroharmine-to-harmaline ratio.
Oliveira and colleagues 41 administered ayahuasca to pregnant Wistar rats by gavage. Three different doses were used: one was representative of the doses typically used in humans (1 mg/kg bw DMT), one 5X, and the other 10X reflective of a typical human dosage level. Doses were administered throughout the gestational period (6–20 days) at approximately the same time every day. The highest of the doses administered resulted in maternal toxicity, leading to decreased weight gain and reduced food intake. Furthermore, embryo-fetal developmental toxicity was dose-dependent. Treatment group doses 5X and 10X experienced significant increases in the dilation of the third ventricle of the brain and renal pelvis, asymmetrically shaped sternebra and cervical rib, decreased fetal body weight, increased incidence of visceral malformation, and skeletal variation such as incomplete ossification of the hyoid bone. Only the 10X dose showed incomplete ossification of the nasal bone and a significant increase of dilated brain lateral ventricle. This study demonstrated that the exposure to ayahuasca can produce dose-dependent maternal toxicity and teratogenic effects in Wistar rats.
Motta and colleagues 37 investigated the maternal and developmental toxicity of ayahuasca in Wistar rats. The rats were treated orally with a single daily dose of ayahuasca on gestation days 6–20 at doses corresponding to one (1X) to eight-fold (8X) the average dose taken by a human adult in a religious ritual (1 mg/kg bw DMT), and the pregnancy outcome was evaluated on GD21. Rats treated with 4X and 8X doses died during the treatment period (44% and 52%), and those that survived showed kidney injury. Rats surviving the 8X dose showed neuronal loss in the hippocampal regions and raphe nuclei, and those from the 2X dose neuronal loss in CA1. Delayed intrauterine growth, induced embryo deaths, and an increased occurrence of fetal anomalies were observed with the 8X dose. At non-lethal doses (1X and 2X), ayahuasca enhanced embryolethality and the incidence of fetal soft tissue and skeletal anomalies. This study demonstrates that ayahuasca may be developmentally toxic and suggests that its use by pregnant women may pose risks to the conceptus.
Oliveira and colleagues 42 subsequently investigated the physical, reflexological, and neurobehavioral postnatal development of Wistar rat offspring exposed to ayahuasca during pregnancy. Alkaloid concentrations based on information from religious groups were: N,N-dimethyltryptamine: .42 mg/mL; harmine: 1.37 mg/mL; harmaline: .62 mg/mL; tetrahydroharmine: .35 mg/mL. The final volume administered to animals was standardized to 1.0 mL/100 g, diluting ayahuasca in potable water. There were no incidences of toxicity in offspring, nor abnormalities in physical or reflexological parameters, as well as no significant changes in maternal weight gain, food or water ingestion, or weight of offspring between the control and experimental groups. Furthermore, offspring showed no abnormalities of central monoamine activity systems and displayed lower levels of general anxiety and social motivation.
These results should, however, be interpreted with caution, as the high frequency of administration and high doses are not representative of average human clinical (.75 mg/kg DMT) or ceremonial (.9 mg/kg DMT) consumption. Moreover, pregnant women usually drink ayahuasca less frequently during this period, rather than increasing consumption. 43 Research into ayahuasca consumption in pregnant women is relatively underexplored, potentially due to the unlikelihood of encountering this in real-life settings due to its potent nature and ethical concerns. Studies suggest that adolescents exposed to it in utero and throughout childhood do not present with a greater degree of neuropsychological, psychological, or psychiatric abnormalities.44–46
Another reproductive toxicity study administered ayahuasca to male Wistar rats every other day for 70 days. 27 1X, 2X, 4X, and 8X the regular dose (.302 mg/kg bw DMT, 3.34 mg/kg bw harmine, .261 mg/kg bw harmaline) was used. Rats receiving either a 4X or 8X dose showed a significant decrease in body weight and food consumption. There was an increase in the relative size of the brain and stomach of rats who had received 8X the regular dose of ayahuasca. Those in the 4X group showed an increase in serum testosterone levels as well as a decrease in spermatic reserves and transit time in the epididymis, with no significant changes in any other reproductive endpoints. Santos et al 27 conclude the NOAEL is at 2X (potential reproductive toxicity was observed at 4X) and consider the ‘non-monotonic’ response as potential reproductive toxicity related to endocrine disruption, all related to increased testosterone and resultant decrease in spermatic reserves and transit time.
Syrian Rue (Peganum harmala)
Peganum harmala is a plant that has been used in traditional medicine throughout the Middle Eastern and Asiatic region since the first century A.D. and remains in use for therapeutic purposes. Harmine is the primary active constituent of the plant seeds. Wang and colleagues 47 investigated the subchronic toxicity and concomitant toxicokinetics of total alkaloid extracts from seeds of P. harmala after oral administration for 4 weeks in rats. The subchronic toxicity and concomitant toxicokinetics of the harmala extract were evaluated after 28-day oral administration in rats at daily dose levels of 15 mg/kg, 45 mg/kg, and 150 mg/kg. The signs of toxicity and mortality were monitored and recorded daily, while the body weight and average food consumption were measured weekly.
A study by Lamchouri and colleagues 48 evaluated the use and manipulation of therapeutic doses of an aqueous extract of P. harmala. Wistar rats were orally dosed acutely, and the LD50 obtained was 2.70+/−.05 g/kg. In chronic studies, aqueous extract of P. harmala administered orally six times a week at doses of 1 g/kg, 1.35 g/kg, and 2 g/kg during a 3-month period was also found to increase liver transaminases. Changes in blood glucose and creatinine were not significant. No significant gross changes were found at necropsy. Histologic study showed liver degeneration and spongiform changes in the central nervous system (CNS) in rats treated with 2 g/kg dose but not at the lower dose of 1 g/kg. With respect to the human dose, it is noted that commonly only 2 g to 4 g of P. harmala seeds are prescribed (equating to about .05 g/kg), thus far below the levels tested in animal models showing any adverse effects.
Following initial repeated exposure to a high dose (150 mg/kg/day) of the harmala extract, excitotoxic reaction (e.g., tremor) was observed but tolerated on the fourth day after multiple dosing. Significant alterations in lipid metabolism and blood glucose in liver were observed; however, these biomarkers recovered after 4 weeks of drug withdrawal and were not considered adverse effects. There were no significant gender differences in most indexes of subchronic toxicity observed throughout the study period, except food consumption and total body weight. In summary, the results revealed that the NOAEL from P. harmala extract in rats in this study was 45 mg/kg/day.
Isolated DMT
Animal studies suggest a fatal dosage of oral DMT would be 20 times that of the standard ritualistic ayahuasca practice. The oral LD50 of DMT in mice was reported as 47 mg/kg intraperitoneally and 32 mg/kg intravenously. 49
A study conducted by Borbély and colleagues 50 investigated the neurogenic and anti-neuroinflammatory effects of DMT in a mouse model of Alzheimer’s disease. DMT was intraperitoneally injected at a concentration of 1 mg/kg–1 into male C57BL/6 wild-type mice (n = 80), treated with either amyloid-β peptide 1–42-oligomers (polymer whose molecules consist of relatively few repeating units) or vehicle (phosphate-buffered saline [PBS]) as a control. Results showed that DMT significantly reduced the number of neuronal stem cells and neuronal density. DMT bound moderately to sigma-1 receptor (S1R), with a high affinity for 5-HT receptors, and negatively influenced neurogenesis. Moreover, DMT significantly reduced the Aβ1–42-induced hyperreactive astrogliosis. However, this did not have a significant effect on microglial activation. Conflicting findings regarding the relationship between DMT and neuroinflammation have been published in the literature, which may be explained by the use of different protocols (injection, dose, and survival time). Further experiments are required to elucidate the accuracy of the adverse effects of DMT on neurogenesis and neuroinflammation.
Gillin and colleagues 51 injected DMT intraperitoneally at 3 mg/kg to five male cats twice a day for 7–15 days. DMT produced consistent changes in coordination, posture, pupil dilation, propensity for vomiting, staring, and apparent hallucinations. No long-lasting effects were noted once administration was discontinued after the final day of the study. In terms of EEG changes, autonomic effects, and apparent hallucinatory or staring behavior, the results indicate that tolerance to DMT does not develop.
Gillin’s study noted in proof 51 to a study conducted by Cole and Piper reporting that no tolerance developed in squirrel monkeys to the disruptive effects of DMT injected intramuscularly at .0 Μmoles/kg, 5.4 Μmoles/kg, and 10.8 Μmoles/kg doses on appetitively conditioned behavior when DMT was administered once daily for 36–38 days.
Isolated Harmala Alkaloids
Studies reported mixed results regarding toxicological analyses of ayahuasca alkaloids harmine and harmaline during pregnancy. Harmaline was given in a single daily dose of .4 mg/day subcutaneously to three groups of mice on days 1–6, 4–7, and 6–11 of gestational pregnancy, and results indicated slight to no deleterious effects on early pregnancy. 52 The experiment was repeated on mice during later stages (days 11–16) of pregnancy with a higher dose of .8 mg/day and concluded that harmaline had no adverse effects on pregnancy. In contrast, Kamel and colleagues 53 discovered multiple abortifacient effects in white pregnant rats after subcutaneously injecting a 2:1 mixture of harmine and harmaline (.1055 g/kg bw) for five consecutive days. Significant tremors, characterized by high-frequency and amplitude movements in the head and neck region, were observed following a single intraperitoneal injection of harmaline at higher doses (50 mg/kg in rats; 100 mg/kg in mice). 54 In contrast, lower doses of harmaline (5 mg/kg in rats; 10 mg/kg in mice) resulted in intermittent tremors with lesser amplitudes. The onset of tremors occurred rapidly within a span of 5 minutes after harmaline injection. Notably, there was an evident relationship between the dose administered and the severity of the observed tremors within a 7-day interval. Both high and low doses of harmaline produced no neurotoxic effects in mice. Immunohistochemical studies revealed significant degeneration of Purkinje cells that was associated with activated microgliosis in the cerebellar cortex, following the administration of harmaline in rats.54–56
Human Clinical Studies
Ayahuasca
A number of studies into ayahuasca/DMT usage in humans have been documented in the literature, highlighting similar effects across testing conditions with minimum adverse effects. DMT dose ranges are commonly between 30 mg and 120 mg orally, with lower doses needed if using via inhalation or intravenous administration. A systematic review of 28 human ayahuasca studies reported that neither acute nor long-term use was associated with increased psychopathology or cognitive deficits. 35 Ayahuasca was well tolerated and produced positive psychological and behavioral changes in two separate observational studies with both only reporting vomiting as a mild adverse effect. Only individuals with current or prior psychiatric symptomatology, family history of psychosis, 57 or those who used DMT concurrently with other drugs (e.g., cannabis and other hallucinogens) were found to experience psychotic symptoms or disorders in a systematic review describing eight case studies. 27 According to Gable, 49 there were between 13 and 24 instances of unspecified psychotic incidents out of approximately 25,000 ayahuasca sessions in the UDV (União do Vegetal) context over a span of 5 years, indicating a rate below .1%. 35
Yeniocak and colleagues 58 documented a rare case of ayahuasca ingestion that led to a 59-year-old man being brought to the emergency department after experiencing hallucinations, agitated behavior, aggression, nausea, and vomiting. Hypertension and mydriasis were determined. Symptomatic treatment was administered, and the patient was discharged following 24-hr clinical observation.
In a double-blind placebo-controlled clinical trial by Riba and colleagues, 17 eighteen volunteers with prior experience in the use of psychedelics received single oral doses of encapsulated freeze-dried ayahuasca (.60 mg and .85 mg of DMT/kg of body weight) and placebo. Ayahuasca produced significant subjective effects, peaking between 1.5 and 2 h, involving perceptual modifications and increases in ratings of positive mood and activation. Diastolic blood pressure showed a significant increase at the high dose (9 mmHg at 75 min), whereas systolic blood pressure and heart rate were moderately and non-significantly increased.
In respect to ayahuasca data pertaining to specialized populations, data are limited on the effects of ayahuasca consumption in those who are pregnant and breastfeeding, as well as the impact this may have on children in the long term.59,60 Anecdotal evidence does, however, suggest that ayahuasca consumption is not associated with negative effects when consumed during pregnancy or breastfeeding. 60 No specific data on elderly or pediatric cohorts was revealed.
Although there is evidence indicating that the safety profile of ayahuasca is acceptable, some cases have emerged wherein consumption has been linked to psychosis. 27 Various syncretic churches such as UDV have reported these incidents. However, drawing causation poses a challenge due to variables like concurrent substance use, pre-existing conditions, and temporality, among others. 49 This estimate corresponds to rates observed in the population over time. In contrast, AEs related to other drugs, such as cannabis, are reported to result in two-to-three times elevated risk for developing psychotic disorders. 61
Isolated DMT
A case study by Paterson and colleagues 62 described a 42-year-old man with no significant past psychiatric history who was admitted to the emergency department for potential substance-induced psychosis attributable to repeated use of DMT. The symptoms included disinhibited behavior, elevated effect, disorganized thought processes, and delusions. The patient disclosed recent and repeated use of DMT as well as long-term cannabis use. It is possible that a combination of these drugs may have caused the psychosis. Following 3 weeks of treatment, the patient’s symptoms resolved successfully, and the patient was discharged.
D’Souza and colleagues 63 conducted a clinical trial to explore the safety, tolerability, and efficacy of intravenous DMT (.1 mg/kg to .3 mg/kg) in 7 volunteers with treatment-resistant major depressive disorder. The most common AEs reported were transient anxiety prior to administration and drug onset (n = 6), transient headache (n = 2) during onset and after resolution of drug effects, and transient hypertension (n = 2) before dose and during onset of effects. While some AEs were confirmed to be related to DMT effects, others appeared unrelated. The only serious AE occurred in a female participant who had significant asymptomatic bradycardia and hypotension while receiving .3 mg/kg. However, prompt intervention in the form of placing her into a Trendelenburg position coupled with intravenous saline infusion prevented any complications or sequelae from arising. Finally, no participants reported clinically relevant psychotic symptoms throughout their participation in this study. 63
Isolated Harmala Alkaloids
Case studies involving Syrian Rue have revealed that perceived intoxication can occur, and this is due to the MAOI (monoamine oxidase inhibitors) modulatory effects from the β-carbolines (in principle from harmaline).
Frison and colleagues 64 described a case study of alkaloid intoxication following ingestion of a self-made preparation of P. harmala seeds purchased online. An 18-year-old male was admitted to the emergency department of a regional hospital. The patient presented psychomotor agitation, visual hallucinations, diffuse tremors, ataxia, and vomiting. The specific dose is unknown; however, it is estimated to be high. The symptoms observed in the patient can be explained by the concomitant presence of a high dose of β-carbolines, possibly interacting with specific receptors and mediating hallucinogenesis. The patient rapidly and fully recovered and was discharged the next day.
Epidemiological Studies
A large observational survey of 10,836 consumers of ayahuasca from more than 50 countries demonstrated that adverse mental health effects such as emotional-cognitive effects (42.0%) and altered perception effects (38.3%) in the weeks or months following consumption were commonly reported by 55.9% of all respondents and 51.2% of respondents that had only used ayahuasca on a single occasion. 65 Generally, these were mild and transient and considered to be part of a positive process of psychological growth or integration of the psychedelic experience. Approximately 12% of those reporting psychological distress sought professional support to cope with their feelings/experiences. Acute physical adverse effects such as vomiting and nausea were also commonly reported (69.9%), with 2.3% reported requiring subsequent medical attention. Potentially more serious adverse events less commonly reported were breathing difficulties (7.3%), chest pains (4.7%), fainting (4.2%), and fits/seizures (1.4%).
Other analysis of a subset of individuals from the same dataset with diagnosed anxiety (n = 1125) or depression (n = 1571) identified 2.7% and 4.5% of individuals with these conditions, respectively, considered their symptoms to have worsened as a result of their ayahuasca consumption.66,67 As with the larger sample, post consumption adverse mental health effects were common but usually mild and transient; however, a slightly higher proportion (13.9%) reported seeking professional support to help deal with such effects. Two other papers from this study examining broader mental health and psychological well-being effects, and impacts on drug and alcohol use, have reported the number of mental health adverse effects reported to be negatively associated with longer-term mental health status, as well as an increased likelihood of recent drug use for individuals with a prior drug use disorder diagnosis.66,68
A cross-sectional study was conducted on ayahuasca users from several ‘União do Vegetal’ religious institutions in Brazil. 69 A total of 614 participants answered an online questionnaire to evaluate the safety of ritual ayahuasca in terms of adverse effects and risk factors. The most frequently reported adverse effects were transient gastrointestinal effects (nausea and vomiting). However, they were mostly mild and seemed to be well-tolerated by users. Fifty participants who self-reported a psychiatric diagnosis (depression and anxiety were the most prevalent) experienced adverse effects more frequently. The use of psychiatric medication was reported by 31 participants, but no indication of increased adverse effects due to drug-drug interactions was found. Many participants reported persistent physical (42%) or psychological (21%) adverse effects following the ayahuasca ceremony, although further details on the nature of these effects and their duration were not reported. Moreover, out of 614 participants, only 1.47% necessitated medical aid due to persistent negative reactions. However, the majority of these adverse reactions were short-lived and did not pose a significant threat to healthy individuals.
A review of the ritualistic use of DMT and harmala alkaloid decoctions identified a safety margin (20 mg) comparable to codeine, mescaline, or methadone, but with minimal risk of dependence or psychological disturbance. 49 A range of studies have also observed reductions in symptoms of ‘panic-like disorders’ and reduced suicidality and depression following DMT administration.35,70 Furthermore, studies indicate that both short- and long-term use of DMT/ayahuasca has limited safety concerns when consumed by healthy individuals. 59
Pharmacovigilance Data
A search of VigiAccess (the WHO pharmacovigilance database) revealed 61 records of potential side effects reported since 2005 for ayahuasca, DMT, peganum harmala, or harmala alkaloids as referenced in Table 1.
Table 1.
VigiAccess (the WHO pharmacovigilance database) results of ayahuasca, DMT, Peganum harmala, harmala alkaloids, and Psychotria viridis side effects (ADRs = adverse drug reactions).
| VigiAccess | Total Reports | Most Reported Potential Side Effect | Highest Geographical Distribution | Years Reported |
|---|---|---|---|---|
| Ayahuasca(Banisteriopsis caapi) | 2 | General disorders and administration site conditions (25%, 2 ADRs) | Europe (50%) and Americas (50%) | 2009 and 2011 |
| Dimethyltryptamine | 12 | Psychiatric disorders (20%, 6 ADRs) | Europe (67%) | 2015–2022 |
| Peganum harmala (wild rue) | 47 | Gastrointestinal disorders (25%, 21 ADRs) | Africa (89%) | 2005–2022 |
| Harmala alkaloids (harmine, harmaline, tetrahydroharmine) | 0 | — | — | — |
| Psychotria viridis | 0 | — | — | — |
Considerations to Reduce AEs and Improve Therapeutic Effects
Generally speaking, when DMT-harmala-based preparations are administered within a hospital-based research setting, the quality of the altered state of consciousness experienced by patients can be influenced by several key factors. These include dose and purity of the preparation, method of preparation, consumption setting, user intention, and expectations, as well as any pre-existing mental or physical health conditions that may exist. Additionally, past experiences with psychoactive substances and medication usage should also be taken into account when considering successful treatment outcomes. The proper consideration and management of these factors are essential to minimize adverse events during therapy sessions (see Table 2).
Table 2.
DMT-harmala-based variables that may reduce adverse effects in hospital-based research.
| Preparation/Screening | Dosage | Set and Setting | Integration |
|---|---|---|---|
| To ensure the desired outcomes during the therapy sessions, proper preparation is vital. Patients need to be fully informed about the risks and benefits of treatment, including potential side effects. 16 | Dosing should be carefully considered, ideally by both the participant and the administrator. | An important factor that influences the outcome of therapy is the mindset or ‘set’ of patients. Patients should approach this with an open mindset, being willing to explore their inner selves and confront any negative emotions or traumas that may arise during sessions. | After the therapy session, it is important to offer adequate integration support to patients. 66 |
| Appropriate doses may differ depending on the participant. | This can include follow-up therapy sessions, group meetings, or individual check-ins to help the patient process and integrate their experiences. | ||
| The patient can be aided through the use of preparatory sessions prior to treatment, where patients can learn relaxation techniques and receive guidance on managing challenging experiences. | Numerous factors should be considered, such as the weight of the participant, previous psychedelic experience, and the intention of the participant. 67 | The setting in which DMT-harmala-based therapy is administered should be carefully chosen to ensure comfort and safety for patients. 67 | This support can help patients better understand and integrate the insights gained from their therapy sessions into their daily lives, potentially leading to long-term benefits for their mental health. |
| Additionally, patients should undergo a thorough psychiatric evaluation before being approved for therapy to screen for any underlying mental health or physical conditions that may exacerbate the risks of treatment. 71 | A series of controlled clinical trials described ayahuasca dosages as: low (.5 mg/DMT/kg), medium (.75 mg/DMT/kg), and high (1 mg–1.4 mg//DMT/kg). a 65 | The environment should be welcoming with comfortable seating and space for patients to lie down if necessary. It should also be free from distractions and provide privacy for patients to explore their inner experiences. Participants should be accompanied by a therapist with whom they feel safe. |
aThe data above are only presented as examples.
When referring to DMT-harmala formulations in hospital-based research for therapeutic context, one approach for clinics to minimize AE typically employs four types of sessions: screening, preparation, dosing (one to three sessions with moderate to high doses of a psychedelic), and integration sessions. During the preparation sessions, the therapist or co-therapist team encourages the patient to explore their life history and to help the patient understand their symptoms and intentions, with an emphasis on the potential for emotional and psychological growth. 72 They also discuss what to expect during the psychedelic session and work on developing a therapeutic alliance.
During experimental sessions, DMT-harmala-based preparations are administered in a reclining chair or bed in a comfortable room that is decorated and warm, so that the subject does not feel intimidated in the way they might in a hospital or medical office setting. 16 Following ingestion, the participant is encouraged to focus their attention inward and is often offered the option of listening to music and wearing eye shades. During the session, the therapists maintain a non-threatening, neutral therapeutic stance and listen empathically. The effects of the brew and the participants’ thought content drive the experience. The therapist’s goal is to facilitate a sense of safety, trust, and openness. 73 After the experimental sessions, during the integration sessions, the therapists work with the patient to achieve meaningful long-term change through the identification of insights or by interpreting thoughts or ideas that arose during the psychedelic session.
This specific approach draws inspiration from the key factors known to mitigate potential adverse effects based on knowledge acquired from traditional use. 67 The ethical frameworks and cultural assumptions shaping DMT-harmala-based therapy are not thoroughly examined, leaving practitioners to navigate universal concepts of mental health and ethics alongside traditional ontologies of healing, care, and safety. 66 Striking a balance between these conflicting approaches should occur to prevent potential ethical dilemmas among healers, mental health practitioners, and retreat centers that deal with ayahuasca usage. 5
Drug Interactions
Regarding general drug interactions, there is a potential risk associated with the use of MAOIs in conjunction with tyramine. 74 The MAOIs present in ayahuasca prevent the breakdown of tyramine and monoamine neurotransmitters. 75 Dangerously high levels of tyramine may occur when MAOIs are introduced to the body exogenically in combination with the aforementioned drugs, potentially resulting in hypertensive crisis. 75 Furthermore, there is a theoretical concern for ayahuasca to be associated with serotonin syndrome in extremely rare cases when used in combination with psychiatric drugs that target 5HT1 and 2a receptors. 76 The condition is thought to be potentially life-threatening when these receptors are over-stimulated due to excessively high levels of serotonin. Symptoms usually include confusion, vomiting, nausea, and euphoria, which overlap with the expected subjective effects of the brew, followed by convulsions, tremors, loss of consciousness, and potentially death. 76 However, no clear/confirmed cases of 5HT syndrome related to ayahuasca consumption have been found in the scientific literature.
Application of Rescue Medications and Supportive Care
When encountering acute distress resulting from the use of psychedelic substances in clinical settings, it is advisable to prioritize supportive care and utilize approaches that were introduced to patients during preparatory psychotherapy sessions. However, in certain cases, individuals may be administered a ‘rescue’ medication.
If the participant is severely agitated or experiencing any other severe psychological distress at the completion of the 8-hour dose session, the following interventional process may be considered. Firstly, if a participant is agitated or at risk of any self-harm or suicidal behavior at the end of the dose session, the trial therapists should remain with the participant for at least two more hours. During this time, the trial therapists can consider implementing emotion regulation interventions, facilitate anxiety management and grounding techniques (learned in preparatory psychotherapy sessions), and will assist the participant to find coherence and perspective in their experiences. If the participant becomes distressed at the completion of one of the post-integrative therapy sessions, at least one trial therapist should remain with the participant for at least two additional hours until such time that the therapist deems the participant safe enough to return home. If a participant remains severely anxious, agitated or in danger of self-harm or suicide, or is otherwise deemed to be psychologically unstable at the completion of a two-hour stabilization period, the psychiatrist/medical practitioner may decide between the following potential options:
1. The psychiatrist or medical professional may prescribe a benzodiazepine and/or antipsychotic as a ‘rescue medication’. This medication will be recorded as appropriate. Therapists will NOT prescribe a selective serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), or monoamine oxidase inhibitor (MAOI) in this context. Residual symptoms will be addressed during the follow-up psychotherapy visits with the therapists.
2. Hospitalization: In the highly unlikely event that a participant should become psychotic, arrangements will be made to stabilize them. In the first instance, the psychiatrist or medical team member within the trial team may administer appropriate medications available on-site to manage acute psychotic symptoms. If necessary, the participant may be transferred to the Emergency Department for triage or admitted directly to the mental health inpatient unit for monitoring of psychiatric state.
It should, however, be noted that such interventions have been warned against. Grof and Grof 73 describe Spiritual Emergency as an expanded way of being, involving enhanced emotional and psychosomatic health, greater freedom of personal choices, and deeper connection with other people, nature, and the cosmos. They explain that this is a change to values, attitudes, life purpose, and identity due to spiritual experience, and that such experiences are commonplace with mystics and shamans. 73 Lukoff and colleagues 72 describe various criteria to determine spiritual emergency. These criteria include good pre-episodic function, acute onset of symptoms during a 3-month period (or less), stressful precipitants to the psychotic episode, and a positive exploratory attitude to the experience. Lukoff and colleagues 72 advise against medicalization and hospitalization, which he warns may interrupt the process and leave the patient without symptomatic resolution, instead emphasizing the importance of intense psychotherapy. As with most clinical trials investigating psychedelics to date, Lukoff and colleagues 72 and Grof and Grof 73 emphasize the importance of avoiding giving psychedelics to participants with risk factors for psychosis. Regardless, it is recognized that medical patient care is vital and that, in some cases, administration of appropriate pharmacotherapies and hospitalization may be required.
Discussion
The present systematic thematic review presented evidence from data associating ayahuasca or DMT/harmala alkaloid intake with adverse events or toxicity and formulated clinical observations based on the results.
In summary, animal models tolerated ayahuasca well at low to moderate doses. Our findings in this regard are supported by another recent systematic review that focused solely on animal studies. 77 Adverse events only arose when pregnant mice were given multiple high doses of ayahuasca. Additionally, Santos et al 78 determined the NOAEL for male rats to be 2X the standard human dosage, with potential reproductive toxicity occurring at 4X. The researchers suggest that this non-monotonic response may be associated with endocrine disruption, specifically linked to an increase in testosterone levels and a subsequent decrease in both spermatic reserves and transit time.
Within human clinical studies, healthy populations reported vomiting as the only mild adverse effect of ayahuasca. Epidemiological studies show that adverse events reported for ayahuasca were generally minor and transient, though these were severe and ongoing for a small proportion of users, and professional mental health support was sometimes required. Physical health adverse events were similarly mild and transient; however, there a small proportion of people reported potentially serious AEs and subsequent medical attention because of these. Severe adverse events requiring hospitalization or subsequent professional/medical assistance for users should temper their future use of the compounds. Clinical trials of DMT/ayahuasca reported common AEs such as vomiting and nausea. Other less frequent effects were transient anxiety, transient headache, hypertension, breathing difficulties, chest pains, fainting, and fits/seizures. Asymptomatic bradycardia was observed in one rare case.
Concerning general drug interactions, there is a theoretical risk associated with the use of ayahuasca-related beta-carbolines (MAOIs) in conjunction with tyramine. Ayahuasca has been associated with extremely rare case studies of serotonin syndrome when used in combination with psychiatric drugs, but no confirmed cases have been found. Syrian Rue case studies have revealed that perceived intoxication can occur due to the MAOI modulatory effects from the β-carbolines (in principle from harmaline). Anecdotal evidence suggests that ayahuasca consumption in humans is not associated with negative effects when consumed during pregnancy or breastfeeding. No specific data on elderly or pediatric cohorts were revealed.
The adverse effects described in the present systematic review are associated with several contributing factors, and not only ayahuasca or DMT intake. Many cases involve individuals with a personal or family history of psychosis, non-psychotic bipolar disorder, or concomitant use of other drugs. Therefore, these individuals have a different profile from those who participate in controlled studies under medical direction where a full mental health screening is performed, and the use of other contraindicated drugs is not allowed. Previous reviews of the adverse effects of psychedelics in non-controlled/recreational settings demonstrated that in reports from the field, it is very difficult to tease apart pre-existing psychopathology, drug/alcohol abuse, family history, and other important features such as proper preparation, guidance, and integration of drug effects. Thus, it is difficult to establish a causal relationship between psychedelic use and most of these cases. These data suggest that the performance of a psychiatric and drug use history before ayahuasca or DMT administration in controlled settings may reduce the occurrence of psychotic experiences. Thereby, it is important to screen out individuals with a personal or family history of schizophrenia or schizophreniform disorders, psychotic depression or mania, or ongoing manic or psychotic symptomatology.
Limitations are acknowledged in our review, including the fact that the human studies were generally small (including case studies) and varied in research design, making comparisons difficult. Future studies are needed to establish consistency in the results – they should describe timing and severity of adverse effects more extensively. Some studies included patients with prior psychedelic experience, which may bias results and limit generalizability. Full transparency about AEs is a responsibility of clinicians, particularly in a nascent field fueled by the enthusiasm of pioneering researchers. Understanding the full spectrum of unpleasant, potentially harmful, and transformative treatment-related events is crucial to inform future therapists who may otherwise be suboptimally prepared to handle challenging and potentially destabilizing patient experiences, particularly in larger groups of patients with more complex and potentially comorbid conditions. Several points of strength and novelty, however, exist in this manuscript, including the broad coverage of ayahuasca and its active constituents and the various sources of data assessed.
In conclusion, the scientific literature suggests that acute ayahuasca administration to healthy volunteers has an acceptable safety profile with a narrow safety margin and that long-term ayahuasca consumption is generally not associated with cognitive or psychiatric problems but should be avoided by those with certain pre-existing mental conditions and pregnant women. Further research is now advised to determine more precise and focused psychological and medical support frameworks to minimize the potential of adverse effects.
Supplemental Material
Supplemental Material for Ayahuasca and Dimethyltryptamine Adverse Events and Toxicity Analysis: A Systematic Thematic Review by Eleanor White, Tom Kennedy, Simon Ruffell, Daniel Perkins, and Jerome Sarris in the International Journal of Toxicology.
Author Contributions: White, E. contributed to conception and design, contributed to acquisition, analysis, and interpretation, drafted the manuscript, and critically revised the manuscript; Kennedy, T. contributed to acquisition, drafted the manuscript, and critically revised the manuscript; Sarris, J. contributed to conception and design, contributed to acquisition and analysis, drafted the manuscript, and critically revised the manuscript; Ruffell, S. contributed to conception, contributed to acquisition, and critically revised the manuscript; Perkins, D. contributed to conception, contributed to acquisition, and critically revised the manuscript. All authors agree to be accountable for all aspects of work, ensuring integrity and accuracy.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosures: JS and DP are directors of a not-for-profit medicinal psychedelics research institute named Psychae institute, which has received funding from the biotechnology sector, and has a connected commerical arm which raises funding for researcher. JS and DP hold equity in the attached commerical entity. SR is the director of a non-for-profit research organisation called Onaya.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Eleanor White https://orcid.org/0000-0002-6498-0242
References
- 1.Shanon B. The Antipodes of the Mind: Charting the Phenomenology of the Ayahuasca Experience. Oxford: Oxford University Press; 2002. [Google Scholar]
- 2.Ruffell S, Netzband N, Bird C, Young AH, Juruena MF. The pharmacological interaction of compounds in ayahuasca: a systematic review. Br J Psychiatr. 2020;42(6):646-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowell JT, Adams PC. The routes of a plant: ayahuasca and the global networks of Santo Daime. Soc Cult Geogr. 2017;18(2):137-157. doi: 10.1080/14649365.2016.1161818 [DOI] [Google Scholar]
- 4.Trichter S. Ayahuasca beyond the Amazon: the benefits and risks of a spreading tradition. J Transpers Psychol. 2010;42(2):131-148 [Google Scholar]
- 5.Tupper KW. Ayahuasca healing beyond the Amazon: the globalization of a traditional indigenous entheogenic practice. Global Network. 2009;9(1):117-136. doi: 10.1111/j.1471-0374.2009.00245.x [DOI] [Google Scholar]
- 6.McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10(2):195-223. [DOI] [PubMed] [Google Scholar]
- 7.Wolff TJ, Ruffell S, Netzband N, Passie T. A phenomenology of subjectively relevant experiences induced by ayahuasca in Upper Amazon vegetalismo tourism. Journal of Psychedelic Studies. 2019;3(3):295-307. doi: 10.1556/2054.2019.007 [DOI] [Google Scholar]
- 8.Kavenská V, Simonová H. Ayahuasca tourism: participants in shamanic rituals and their personality styles, motivation, benefits and risks. J Psychoact Drugs. 2015;47(5):351-359. doi: 10.1080/02791072.2015.1094590 [DOI] [PubMed] [Google Scholar]
- 9.Gearin AK. Whatever you want to believe’: kaleidoscopic individualism and ayahuasca healing in Australia. Aust J Anthropol. 2015;26(3):442-455. doi: 10.1111/taja.12143 [DOI] [Google Scholar]
- 10.Winkelman M. Drug tourism or spiritual healing? Ayahuasca seekers in Amazonia. J Psychoact Drugs. 2005;37(2):209-218. doi: 10.1080/02791072.2005.10399803 [DOI] [PubMed] [Google Scholar]
- 11.Aaghaz S, Sharma K, Jain R, Kamal A. β-Carbolines as potential anticancer agents. Eur J Med Chem. 2021;216:113321. [DOI] [PubMed] [Google Scholar]
- 12.Barceloux DG. Ayahuasca, harmala alkaloids, and dimethyltryptamines. Medical Toxicology of Drug Abuse. Hoboken, NJ: John Wiley and Sons, Inc; 2012. [Google Scholar]
- 13.Djamshidian A, Poewe W, Högl B. Impact of impulse control disorders on sleep-wake regulation in Parkinson’s disease. Parkinson’s Dis. 2015;2015:970862. doi: 10.1155/2015/970862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moura DJ, Richter MF, Boeira JM, Pêgas Henriques JA, Saffi J. Antioxidant properties of beta-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis. 2007;22(4):293-302. doi: 10.1093/mutage/gem016 [DOI] [PubMed] [Google Scholar]
- 15.Samoylenko V, Rahman MM, Tekwani BL, et al. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J Ethnopharmacol. 2010;127(2):357-367. doi: 10.1016/j.jep.2009.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos RG, Grasa E, Valle M, et al. Pharmacology of ayahuasca administered in two repeated doses. Psychopharmacology (Berl. 2012;219(4):1039-1053. doi: 10.1007/s00213-011-2434-x [DOI] [PubMed] [Google Scholar]
- 17.Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Therapeut. 2003;306(1):73-83. doi: 10.1124/jpet.103.049882 [DOI] [PubMed] [Google Scholar]
- 18.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323(5916):934-937. doi: 10.1126/science.1166127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frecska E, Szabo A, Winkelman MJ, Luna LE, McKenna DJ. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J Neural Transm. 2013;120(9):1295-1303. doi: 10.1007/s00702-013-1024-y [DOI] [PubMed] [Google Scholar]
- 20.Inserra A. Hypothesis: the psychedelic ayahuasca heals traumatic memories via a sigma 1 receptor-mediated epigenetic-mnemonic process. Front Pharmacol. 2018;9(330):330. doi: 10.3389/fphar.2018.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr Pharmaceut Des. 2012;18(7):902-919. doi: 10.2174/138161212799436601 [DOI] [PubMed] [Google Scholar]
- 22.Sabino V, Hicks C, Cottone P. Sigma receptors and substance use disorders. Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Berlin: Springer; 2017:177-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambo DO, Lebowitz JJ, Khoshbouei H. The sigma-1 receptor as a regulator of dopamine neurotransmission: a potential therapeutic target for methamphetamine addiction. Pharmacol Ther. 2018;186:152-167. doi: 10.1016/j.pharmthera.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng TY, Hung DT, Su TP, Tsai SYA. Loss of sigma-1 receptor chaperone promotes astrocytosis and enhances the Nrf2 antioxidant defense. Oxid Med Cell Longev. 2017;2017:4582135. doi: 10.1155/2017/4582135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Br J Psychiatr. 2013;35(Suppl 2):112-120. doi: 10.1590/1516-4446-2013-1098 [DOI] [PubMed] [Google Scholar]
- 26.Dakic V, Maciel RM, Drummond H, Nascimento JM, Trindade P, Rehen SK. Harmine stimulates proliferation of human neural progenitors. PeerJ. 2016;4:2727. doi: 10.7717/peerj.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dos Santos RG, Bouso JC, Hallak JE. Ayahuasca, dimethyltryptamine, and psychosis: a systematic review of human studies. Ther Adv Psychopharmacol. 2017;7(4):141-157. doi: 10.1177/2045125316689030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dos Santos RG, Hallak JEC. Effects of the natural β-carboline alkaloid harmine, a main constituent of ayahuasca, in memory and in the Hippocampus: a systematic literature review of preclinical studies. J Psychoact Drugs. 2016;49:1-10. Published online. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Wu J, Gong Y, et al. Harmine produces antidepressant-like effects via restoration of astrocytic functions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;79:258-267. doi: 10.1016/j.pnpbp.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Li M, Tan S, Wang C, Fan S, Huang C. Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochem Biophys Res Commun. 2017;489(3):332-338. doi: 10.1016/j.bbrc.2017.05.126 [DOI] [PubMed] [Google Scholar]
- 31.Morales-García JA, Fuente Revenga M, Alonso-Gil S, et al. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci Rep. 2017;7(1):1-13. doi: 10.1038/s41598-017-05407-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naranjo C. Psychotropic properties of the harmala alkaloids. In: Efron D, Holmstedt B, Kline N, eds. Ethnopharmacologic Search for Psychoactive Drugs: Proceedings of a Symposium Held in San Francisco: National Institute of Mental Health Public Health Service, U.S. Dept of Health Education and Welfare; 1967. [Google Scholar]
- 33.Cao R, Peng W, Wang Z, Xu A. Beta-Carboline alkaloids: biochemical and pharmacological functions. Curr Med Chem. 2007;14(4):479-500. doi: 10.2174/092986707779940998 [DOI] [PubMed] [Google Scholar]
- 34.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372(4):141-143. doi: 10.1097/wnf.0000000000000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dos Santos RG, Balthazar FM, Bouso JC, Hallak JE. The current state of research on ayahuasca: a systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J Psychopharmacol. 2016;30(12):1230-1247. doi: 10.1177/0269881116652578 [DOI] [PubMed] [Google Scholar]
- 36.Pic-Taylor A, da Motta LG, de Morais JA, et al. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav Process. 2015;118:102-110. doi: 10.1016/j.beproc.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 37.da Motta LG, de Morais JA, Tavares ACAM, et al. Maternal and developmental toxicity of the hallucinogenic plant-based beverage ayahuasca in rats. Reprod Toxicol. 2018;77:143-153. doi: 10.1016/j.reprotox.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 38.Colaço CS, Alves SS, Nolli LM, et al. Toxicity of ayahuasca after 28 days daily exposure and effects on monoamines and brain-derived neurotrophic factor (BDNF) in brain of Wistar rats. Metab Brain Dis. 2020;35(5):739-751. doi: 10.1007/s11011-020-00547-w [DOI] [PubMed] [Google Scholar]
- 39.Melo Junior W, Souza Filho J, Grisolia C, Caldas E, Pic-Taylor A. Genotoxic evaluations in Wistar rats of the hallucinogenic plant extract ayahuasca. Int J Phytomed. 2016;8:249-2225. [Google Scholar]
- 40.Kummrow F, Maselli BS, Lanaro R, Costa JL, Umbuzeiro GA, Linardi A. Mutagenicity of ayahuasca and their constituents to the salmonella/microsome assay. Environ Mol Mutagen. 2019;60(3):269-276. doi: 10.1002/em.22263 [DOI] [PubMed] [Google Scholar]
- 41.Oliveira CDR, Moreira CQ, de Sá LRM, Spinosa HS, Yonamine M. Maternal and developmental toxicity of ayahuasca in Wistar rats. Birth Defects Res B Dev Reprod Toxicol. 2010;89(3):207-212. doi: 10.1002/bdrb.20244 [DOI] [PubMed] [Google Scholar]
- 42.Oliveira CDRd, Moreira CQ, Spinosa Hd S, Yonamine M. Neurobehavioral, reflexological and physical development of Wistar rat offspring exposed to ayahuasca during pregnancy and lactation. Rev bras farmacogn. 2011;21(6):1065-1076. [Google Scholar]
- 43.Labate BC. Consumption of ayahuasca by children and pregnant women: medical controversies and religious perspectives. J Psychoact Drugs. 2011;43(1):27-35. doi: 10.1080/02791072.2011.566498 [DOI] [PubMed] [Google Scholar]
- 44.Da Silveira DX, Grob CS, de Rios MD, et al. Ayahuasca in adolescence: a preliminary psychiatric assessment. J Psychoact Drugs. 2005;37(2):129-133. doi: 10.1080/02791072.2005.10399792 [DOI] [PubMed] [Google Scholar]
- 45.de Rios MD, Grob CS, Lopez E, da Silviera DX, Alonso LK, Doering-Silveira E. Ayahuasca in adolescence: qualitative results. J Psychoact Drugs. 2005;37(2):135-139. doi: 10.1080/02791072.2005.10399793 [DOI] [PubMed] [Google Scholar]
- 46.Doering-Silveira E, Lopez E, Grob CS, et al. Ayahuasca in adolescence: a neuropsychological assessment. J Psychoact Drugs. 2005;37(2):123-128. doi: 10.1080/02791072.2005.10399791 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Wang H, Zhang L, et al. Subchronic toxicity and concomitant toxicokinetics of long-term oral administration of total alkaloid extracts from seeds of Peganum harmala Linn: a 28-day study in rats. J Ethnopharmacol. 2019;238:111866. doi: 10.1016/j.jep.2019.111866 [DOI] [PubMed] [Google Scholar]
- 48.Lamchouri F, Settaf A, Cherrah Y, et al. Experimental toxicity of Peganum harmala seeds. Ann Pharm Fr. 2002;60(2):123-129 [PubMed] [Google Scholar]
- 49.Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34. doi: 10.1111/j.1360-0443.2006.01652.x [DOI] [PubMed] [Google Scholar]
- 50.Borbély E, Varga V, Szögi T, et al. Impact of two neuronal sigma-1 receptor modulators, PRE084 and DMT, on neurogenesis and neuroinflammation in an aβ1–42-injected, wild-type mouse model of AD. Int J Mol Sci. 2022;23(5):2514. doi: 10.3390/ijms23052514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillin JC, Cannon E, Magyar R, Schwartz M, Wyatt RJ. Failure of N,N-dimethyltryptamine to evoke tolerance in cats. Biol Psychiatr. 1973;7(3):213-220. [PubMed] [Google Scholar]
- 52.Poulson E, Robson JM. The effect of amine oxidase inhibitors on pregnancy. J Endocrinol. 1963;27(2):147-155. doi: 10.1677/joe.0.0270147 [DOI] [PubMed] [Google Scholar]
- 53.Kamel SH, Ibrahim TM, Hamza SM. Effect of harmine and harmaline hydrochloride on pregnancy in white rats. Zentralblatt für Veterinarmed A. 1971;18(3):230-233. doi: 10.1111/j.1439-0442.1971.tb00573.x [DOI] [PubMed] [Google Scholar]
- 54.Miwa H, Kubo T, Suzuki A, Kihira T, Kondo T. A species-specific difference in the effects of harmaline on the rodent olivocerebellar system. Brain Res. 2006;1068(1):94-101. doi: 10.1016/j.brainres.2005.11.036 [DOI] [PubMed] [Google Scholar]
- 55.O’Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55(2):303-310. doi: 10.1016/0306-4522(93)90500-f [DOI] [PubMed] [Google Scholar]
- 56.O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells: a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17(22):8828-8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szmulewicz AG, Valerio MP, Smith JM. Switch to mania after ayahuasca consumption in a man with bipolar disorder: a case report. Int J Bipolar Disord. 2015;3(1):4. doi: 10.1186/s40345-014-0020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeniocak S, Kalkan A, Aguş T, DemiRel A, Akkoç İ, KatiPoglu B. A rare case: ayahuasca tea intoxication. Journal of Emergency Medicine Case Reports. 2019;10:75. [Google Scholar]
- 59.Dos Santos RG. A critical evaluation of reports associating ayahuasca with life-threatening adverse reactions. J Psychoact Drugs. 2013;45(2):179-188. doi: 10.1080/02791072.2013.785846 [DOI] [PubMed] [Google Scholar]
- 60.Labate B, Jungaberle H. The Internationalization of Ayahuasca. Münster: LIT Verlag Münster; 2011. Published online. [Google Scholar]
- 61.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184(2):110-117. doi: 10.1192/bjp.184.2.110 [DOI] [PubMed] [Google Scholar]
- 62.Paterson NE, Darby WC, Sandhu PSN. N,N-Dimethyltryptamine-Induced psychosis. Clin Neuropharmacol. 2015;38(4):141-143. doi: 10.1097/wnf.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 63.D’Souza DC, Syed SA, Flynn LT, Safi-Aghdam H, Cozzi NV, Ranganathan M. Exploratory study of the dose-related safety, tolerability, and efficacy of dimethyltryptamine (DMT) in healthy volunteers and major depressive disorder. Opharmacology. 2022;47(10):1854-1862. doi: 10.1038/s41386-022-01344-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frison G, Favretto D, Zancanaro F, Fazzin G, Ferrara SD. A case of beta-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci Int. 2008;179(2):37-43. doi: 10.1016/j.forsciint.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 65.Bouso JC, Andión Ó, Sarris JJ. et al. Adverse effects of ayahuasca: results from the global ayahuasca survey. PLOS Glob Public Health. 2022;2(11):0000438. doi: 10.1371/journal.pgph.0000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perkins D, Schubert V, Simonová H, et al. Influence of context and setting on the mental health and wellbeing outcomes of ayahuasca drinkers: results of a large international survey. Front Pharmacol. 2021;12(469):623979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarris J, Perkins D, Cribb L, et al. Ayahuasca use and reported effects on depression and anxiety symptoms: an international cross-sectional study of 11,912 consumers. Journal of Affective Disorders Reports. 2021;4:100098. doi: 10.1016/j.jadr.2021.100098 [DOI] [Google Scholar]
- 68.Perkins D, Opaleye E, Simonova H, et al. Associations between ayahuasca consumption in naturalistic settings and current alcohol and drug use: results of a large international cross-sectional survey. Drug and Alcohol Review. 2022;41:265. Published online. [DOI] [PubMed] [Google Scholar]
- 69.Durante Í, Dos Santos RG, Bouso JC, Hallak JE. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Br J Psychiatr. 2021;43(4):362-369. doi: 10.1590/1516-4446-2020-0913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murphy-Beiner A, Soar K. Ayahuasca’s ‘afterglow’: improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacology. 2020;237(4):1161-1169. doi: 10.1007/s00213-019-05445-3 [DOI] [PubMed] [Google Scholar]
- 71.Sanches RF, de Lima Osório F, Dos Santos RG, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol. 2016;36(1):77-81. doi: 10.1097/JCP.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 72.Lukoff D, Lu FG, Turner R. Diagnosis: a transpersonal clinical approach to religious and spiritual problems. In: Scotton BW, Chinen AB, Battista JR, eds. Textbook of Transpersonal Psychiatry and Psychology. New York, NY: Basic Books; 1996:231-249. [Google Scholar]
- 73.Grof C, Grof S. Spiritual emergency: the understanding and treatment of transpersonal crises. International Journal of Transpersonal Studies. 2017;36(2):30-43. [Google Scholar]
- 74.Callaway JC, Airaksinen MM, McKenna DJ, Brito GS, Grob CS. Platelet serotonin uptake sites increased in drinkers of ayahuasca. Psychopharmacology. 1994;116(3):385-387. doi: 10.1007/BF02245347 [DOI] [PubMed] [Google Scholar]
- 75.Dalgarno P. Buying Ayahuasca and other entheogens online: a word of caution. Addiction Res Theor. 2008;16(1):1-4. [Google Scholar]
- 76.Callaway JC, Grob CS. Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for severe adverse interactions. J Psychoact Drugs. 1998;30(4):367-369. doi: 10.1080/02791072.1998.10399712 [DOI] [PubMed] [Google Scholar]
- 77.Daldegan-Bueno D, Simionato NM, Favaro VM, Maia LO. The current state of ayahuasca research in animal models: a systematic review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2023;125:110738. doi: 10.1016/j.pnpbp.2023.110738 [DOI] [PubMed] [Google Scholar]
- 78.Santos AdFA, Vieira ALS, Pic-Taylor A, Caldas ED. Reproductive effects of the psychoactive beverage ayahuasca in male Wistar rats after chronic exposure. Revista Brasileira de Farmacognosia. 2017;27:353-360. doi: 10.1016/j.bjp.2017.01.006 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Ayahuasca and Dimethyltryptamine Adverse Events and Toxicity Analysis: A Systematic Thematic Review by Eleanor White, Tom Kennedy, Simon Ruffell, Daniel Perkins, and Jerome Sarris in the International Journal of Toxicology.