Abstract
Background
Older humans taking high concentrations of vitamin D3 supplementation for a prolonged time may be at risk of vitamin D toxicity. It is unclear how dietary super-doses (10,000 times greater than the requirement) can affect vitamin D3 status in aged animals. Aged laying hens could be a model to compare vitamin D3 supplementation effects with women in peri- or postmenopausal stages of life.
Objectives
We investigated the dietary super-dose impacts of cholecalciferol (vitamin D3) on vitamin D3 status in aged laying hens in production.
Methods
Forty-eight 68-wk-old Hy-Line Brown laying hens were individually housed in cages with 8 hens per dietary treatment for 11 wk. Hens were randomly assigned to 1 of 6 treatment groups of dietary vitamin D3 supplementation and consumed ad libitum. Supplementation concentrations were 400, 800, 7400, 14,000, 20,000, and 36,000 IU D3/kg of feed. At the end of the study, all hens were sacrificed, and tissue samples and feces were collected. Plasma and egg yolk vitamin D3 metabolites, calcium and phosphorus composition of eggshells, ileal digesta, and feces were measured. Duodenal, ileal, liver, and kidney gene expression levels were also measured.
Results
We observed that increasing dietary vitamin D3 increased plasma vitamin D3 and egg yolk vitamin D3 (P < 0.0001 for both sites). We also observed an increase in plasma 24,25-dihydroxycholecalciferol as dietary vitamin D3 concentrations increased (P < 0.0001). The plasma 25-hydroxycholecalciferol:24,25-dihydroxycholecalciferol ratio exhibited an asymptotic relationship starting at the 14,000 IU/kg D3 treatment.
Conclusions
Dietary super-doses of vitamin D3 led to greater plasma and egg yolk vitamin D3 concentrations, which shows that aged laying hens can deposit excess vitamin D3 in egg yolk. We suggest future research should explore how 24-hydroxylation mechanisms are affected by vitamin D3 supplementation. Further understanding of 24-hydroxylation can help ascertain ways to reduce the risk of vitamin D toxicity.
Keywords: aged laying hen; chicken; dietary vitamin D3; 25-hydroxycholecalciferol; 24,25-dihydroxycholecalciferol; egg; egg yolk vitamin D3; plasma vitamin D3; super-dose
Introduction
Older humans taking extremely high concentrations of vitamin D3 supplementation for a prolonged time may be at risk of vitamin D toxicity [1,2]. People tend to take vitamin D3 supplements to increase or maintain vitamin D3 concentrations [3]. Also, older women take vitamin D3 supplements to manage the hormonal effects of menopause on bone resorption [[4], [5], [6]]. Although vitamin D toxicity is uncommon, vitamin D3 supplements and overfortified foods are the only known means of reaching intoxication levels [1,7]. Vitamin D3 supplementation can be administered through multiple means with the common routes being oral dose supplementation or dietary supplementation.
An important consideration is whether extremely high supplementation of vitamin D3 concentrations over an extended period of time would cause vitamin D toxicity. Laying hens, chickens that have high egg-laying production, have been used to explore questions involving vitamin D3 supplementation [[8], [11]]. Signs of vitamin D toxicity in egg-laying chickens are reduction of food consumption, lower egg production, and reduced growth in younger chickens [10,12]. Laying hens fed a diet containing 68,348 IU of D3/kg of feed over a 48-wk period had reduced body weight and egg production, which is suggestive of vitamin D toxicity [10]. Considering older humans are likely to take vitamin D3 supplements, further exploration of dietary vitamin D3 supplementation effects in older hens is necessary. Characterizing how high concentrations of dietary vitamin D3 affect vitamin D metabolism in older hens may help to better understand how overfortified foods can potentially affect vitamin D metabolism in older animals and humans.
Dietary vitamin D3 supplementation is important for laying hens in production because their bone health is physiologically taxed from egg production [13,14]. Laying hens in commercial farms are fed diets with supplemental vitamin D3 beyond the National Research Council requirements [15,16]. This ensures the hens can lay eggs and maintain adequate calcium (Ca) absorption for eggshell formation and, importantly, bone mineralization [11]. There are a few studies that investigated how very high concentrations of dietary vitamin D3 supplementation affected laying hen production and the metabolic implications pertaining to vitamin D3 status [9,10,17]. Altogether, the aforementioned studies illustrate dietary vitamin D3 supplementation results in vitamin D3-enriched eggs which may be a way to improve vitamin D3 intake for humans. Further understanding of how very high concentrations of dietary vitamin D3 supplementation affect circulating vitamin D3 metabolite concentrations in aged laying hens is relevant to the poultry producers interested in extending the production life of laying hens. Also, understanding the impacts of very high dietary vitamin D3 supplementation in aged laying hens has implications with older women and their vitamin D3 intake from food fortification.
Our study examined dietary vitamin D3 super-dose effects on plasma and egg yolk vitamin D3 metabolites and relative gene expression of vitamin D-related genes in aged laying hens in production. We define “super-dose” as treatment doses >10,000 times greater than requirement. We fed hens diets containing 400, 800, 7400, 14,000, 20,000, and 36,000 IU D3/kg of feed to ascertain vitamin D3 supplementation impacts. Hens consuming diets with vitamin D3 >7400 IU D3/kg were expected to have increased plasma 24,25-dihydroxycholecalciferol [24,25-(OH)2-D3] because 24,25-(OH)2-D3 is an inactive form of vitamin D3 and would suggest that the hens reached vitamin D3 saturation [[18], [19], [20]]. Hens consuming super-doses of vitamin D3 should also lay eggs with increased vitamin D3 content because they would deposit excess vitamin D3 into the egg yolk [9].
Methods
Animal husbandry
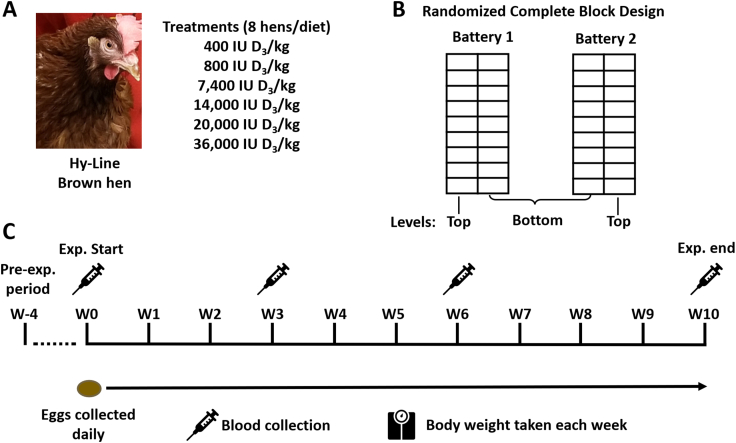
The hens used in our study were from North Carolina State University’s maintained poultry flock. Forty-eight 68-wk-old Hy-Line Brown laying hens were housed at North Carolina State University, Raleigh, NC, and fed experimental diets for 11 wk (Figure 1). Hens were individually housed in cages between 2 2-level (top level and bottom level) battery cages with 8 hens per treatment. Each hen was individually fed via their own trough feeder with side barriers to reduce cross-feeding between hens and randomly assigned to a treatment group. The experimental design was a randomized complete block design with 6 levels of dietary vitamin D3 supplementation blocked by cage level. Vitamin D3 supplementation concentrations were formulated to be 250, 500, 1500, 15,000, 30,000, and 60,000 IU D3/kg of feed, but the analyzed vitamin D3 concentrations in the feed were 400, 800, 7400, 14,000, 20,000, and 36,000 IU D3/kg of feed (Table 1 and Supplementary Table 1). The source of vitamin D3 used in the study was the crystalline vitamin D3 from Alfa Aesar. We refer to the 6 different analyzed vitamin D3 concentrations as the named treatment groups for our study. In our study, the 400 and 800 IU/kg vitamin D3 treatments were formulated to meet the National Research Council [16] requirements for laying hens. The 7400, 14,000, 20,000, and 36,000 IU/kg D3 treatments were dietary super-dose treatments for D3 intake. Prior to the start of the experiment, all hens were fed the same diet (400 IU D3/kg of feed) for 1 mo as a washout period. Hens were fed the diet and water ad libitum. North Carolina State University’s Institutional Animal Care and Use Committee approved all methods for this study, protocol ID number: 18-093-A.
FIGURE 1.
Study design. (A) Forty-eight 68-wk Hy-Line Brown hens were used in the study and were randomly assorted into 1 of 6 treatment groups and fed the diet with the corresponding dietary vitamin D3 supplementation concentration. (B) Hens were individually housed in cages of a 2-leveled battery cage in a randomly assigned complete block design (n = 8/diet). (C) The experimental timeline in which hens were fed the same basal diet (400 IU D3/kg) for 4 wk as a washout period (W-4). Hens were started on the experimental diets at week 0 (W0), eggs were collected daily, and weekly body weight was taken until the end of the study. On weeks 0, 3, 6, and 10, blood was collected from the brachial (wing) vein from all hens to measure ionized blood calcium using an i-STAT blood analyzer. Blood was centrifuged down, and plasma was collected to measure vitamin D3 metabolite concentrations. The study ended on week 10, and all hens were sacrificed, and sample tissues were collected.
TABLE 1.
Ingredient composition of the experimental basal diet
| Ingredient name | % |
|---|---|
| Corn | 63.40 |
| Soybean meal, 46% crude protein | 20.60 |
| Calcium carbonate | 9.20 |
| Poultry fat | 2.33 |
| Dicalcium phosphate1 | 1.95 |
| L-lysine-hydrochloride, 78.8% | 1.01 |
| Sodium bicarbonate | 0.57 |
| Vitamin premix2 | 0.25 |
| Mineral premix3 | 0.20 |
| Choline chloride, 60% choline | 0.20 |
| DL-Methionine, 99% | 0.14 |
| Salt | 0.10 |
| Selenium premix4 | 0.05 |
Dicalcium phosphate contains 19.79% calcium, 17.91% phosphorus, and 17.73% available phosphorus.
Provided as milligrams per kilogram of diet: 125 mg ethoxyquin; 25 mg niacinamide;10 mg calcium pantothenate; 6.7 mg DL-α-tocopherol; 3.6 mg riboflavin; 3 mg pyridoxine hydrochloride; 1.8 mg thiamine hydrochloride; 0.55 mg folic acid; 0.55 mg menadione sodium bisulfite; 0.516 mg retinol acetate; 0.15 mg biotin; 0.01 mg cyanocobalamin.
Trace minerals provided per kg of premix: 60 g manganese sulfate, 60 g zinc sulfate, 40 g iron sulfate, 5 g copper sulfate, 1.25 g calcium iodate.
Selenium premix provided selenium at 0.3 mg/kg of diet.
Sample Collection
Egg collection started 24 h after the hens were started on the experimental diets. Eggs were collected every morning and stored at 7°C for egg quality analyses. Shell strength and elasticity were measured using methods described by Redhead et al. [21], and shell thickness was also measured using calipers. Egg quality measurements were done by selecting 2 eggs at random per week for each replicate. Starting on Mondays, the first egg laid for the week by each hen was selected for egg yolk collection. Eggs were cracked open in a dim-lighted room to reduce the photodegradative impacts of light on vitamin D3 in the yolk. The egg yolk was separated from albumen and placed in a small plastic container wrapped in aluminum foil and stored at 4°C for a year until they were freeze-dried using a freeze-dryer (FreeZone 6 Liter Benchtop Freeze Dry System; Labconco). On weeks 0, 3, 6, and 10, blood was collected from the brachial wing vein from all hens to measure ionized blood Ca using an i-STAT blood analyzer (Abaxis) using CG8+ cartridges (Abaxis). The remaining blood was centrifuged down to collect plasma, which was stored at –80°C. All hens were sacrificed by cervical dislocation, and tissue samples were collected from 43 hens (minimum of 7 hens per treatment) due to time constraints. The duodenum, ileum, liver, and kidney were collected and stored in RNAlater at –20°C until RNA extractions were performed. The feces and ileal digesta were collected immediately after the hens were sacrificed, along with the humerus and tibia bones. The ileal digesta, feces, humerus, and tibia were collected for measuring Ca and phosphorus (P) composition.
Ca and P content of various sites
Eggshells from weeks 0, 3, 4, 6, and 9 were washed with warm water to help with removing the shell membrane by hand and dried for 48 h at room temperature. Dried eggshells were preweighed and further dried at 68°C for 72 h using a dry oven (Blue M) and weighed again. Eggshells were crushed into fine powder and subjected to acid digestion to measure the Ca and P composition of eggshells. Feces and ileal digesta were also subjected to the same steps as eggshells. Dried samples were weighed and then placed in a muffle furnace at 500°C overnight to ash samples. The ashed samples were processed by North Carolina State University’s Environmental and Agricultural Testing Service laboratory. Ashed samples were dehydrated in 2 mL of distilled water and 4 mL of 6 N hydrochloric acid. The resulting sample was mixed and heated to warm to the touch. The heated solution was poured into a volumetric flask, and deionized water was added to have a working solution of 50 mL. The flask was inverted 12 times to mix, and the resulting solution was filtered using #40 filter paper into 15 mL centrifuge tubes for analysis. Ca and P were measured by inductively coupled plasma optical emission spectrometry.
The humerus and tibia were wrapped in petroleum ether-moistened cheesecloth and placed in a desiccator for 72 h to extract fat and moisture from the bones. Fat-extracted bones were preweighed and dried for 24 h at 100°C to evaporate petroleum ether residues. Fat- and moisture-free bones were weighed and ashed using the same methods as eggshell, feces, and ileal digesta for Ca and P composition and measured by inductively coupled plasma optical emission spectrometry.
RNA extraction and qPCR
Total RNA was extracted from the duodenum, ileum, liver, and kidney using Qiagen’s RNeasy Mini Kit. The extracted RNA was diluted and normalized to ∼200 ng/μL for the liver and 60 ng/μL for the duodenum, ileum, and kidney. The tissues’ RNA was reverse transcribed to cDNA using Applied Biosystems’ High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific) and their recommended steps to make a 20 μL working solution. The cycling procedure for reverse transcription started at 25°C for 10 min, 37°C for 120 min, 85°C for 5 min, then held at 5°C indefinitely until storage or use.
Genes amplified for qPCR were vitamin D receptor (VDR), 1α-hydroxylase (CYP27C1), 24-hydroxylase (CYP24A1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene (Table 2). qPCR was conducted using PowerUP SYBR Green Master Mix (Life Technologies) following the manufacturer’s protocol and using Applied Biosystems StepOnePlus Real-Time PCR System. The cycling procedure started at 95°C for 10 min, then 40 cycles at 95°C for 15 s for denaturing and 15 s at 60°C for annealing. All samples were analyzed in triplicates.
TABLE 2.
Primer sequences for qPCR
| Gene | Orientation | Primer sequence (5’ – 3’) | Size (bp) | Accession # |
|---|---|---|---|---|
| VDR | Forward | TGCCTCCAGTCTGGCATCTC | 297 | NM_205098.1 |
| Reverse | GGTGATTTTGCAGTCCCCGT | |||
| CYP27C1 | Forward | ATGATTGGCGTCCCCTTCAG | 177 | XM_422077.41 |
| Reverse | TCCACGCTTTCACTCACACA | |||
| CYP24A1 | Forward | AAACCCTGGAAAGCCTATCG | 133 | NM_204979.12 |
| Reverse | CCAGTTTCACCACCTCCTTG | |||
| GAPDH | Forward | TGTTGTTGACCTGACCTGCC | 291 | NM_204305.1 |
| Reverse | CTGGCTCACTCCTTGGATGC |
CYP24A1, 24-hydroxylase; CYP27C1, 1α-hydroxylase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; qPCR, quantitative polymerase chain reaction; VDR, vitamin D receptor; NCBI, National Center for Biotechnology Information.
The NCBI record for CYP27C1 was removed due to standard genome annotation processing. However, in our study, the CYP27C1 primers were made and used in 2019 when the record was available.
The NCBI record for CYP24A1 was removed because of insufficient support for the transcript and protein. In our study, the CYP24A1 primers were made and used in 2019 when the record was available.
Vitamin D3 metabolites
Plasma from the week 10 timepoint from hens from the 400, 800, 14,000, and 36,000 IU D3/kg groups were respectively pooled (n = 4/treatment) and sent to Heartland Assays for measuring vitamin D3, 25-hydroxycholecalciferol (25-OH-D3), and 24,25-(OH)2-D3, inactive form of vitamin D3, using LC-MS/MS. Freeze-dried egg yolk from week 10 timepoint from the same hens were pooled (15 g/sample) like plasma, but only samples from 400, 14,000, and 36,000 IU D3/kg groups (n = 4/treatment) were analyzed for vitamin D3 and 25-OH-D3 from Heartland Assays.
Statistical analysis
We conducted statistical analyses using general linear models using SAS 9.4 for all statistical tests, and the Tukey-Kramer test was used for multiple comparisons of differences between dietary treatments. We utilized repeated measures to account for the temporal effects of weekly body weight, feed intake, egg production, eggshell quality, and ionized blood Ca. Dietary vitamin D3 concentration was the independent variable for Ca and P composition, plasma and egg yolk vitamin D3 metabolite concentrations, and gene expression data. Plasma vitamin D3 was below the detection limit (<0.5 ng/mL) for the 400 and 800 IU D3/kg treatments, so those samples were set to 0.4 as an arbitrary value for statistics to account for model building. All vitamin D3 metabolite data exhibited heteroscedasticity and were transformed using the natural logarithm function. Transformed data exhibited linear and homoscedastic relationships and were used for statistics. We did not observe a cage-level effect in any analysis, so the blocking variable was omitted from all statistical tests. All mRNA relative expressions were normalized using 2-CΔΔT with GAPDH as the housekeeping gene. Statistical significance was established at P < 0.05.
Results
Hens’ production performance was not influenced by dietary vitamin D3
To determine if dietary super-doses of vitamin D3 affected the hens’ production value, we measured the hens’ weekly body weights and egg production. Dietary super-doses of vitamin D3 did not affect the body weight of these laying hens (P = 0.08; Supplementary Table 2), but there was an interaction between dietary vitamin D3 concentration over time on feed intake (P < 0.0001). However, there was constant feed wastage throughout the study so this effect could be inflated. A dietary trend was observed for egg production for the entire study duration (P = 0.07; Supplementary Table 3). Eggshell strength and eggshell thickness were not affected by dietary vitamin D3 concentrations (P = 0.19, P = 0.72, respectively; Supplementary Table 3). There was a trending interaction between dietary vitamin D3 concentrations over time in which there was a decrease in eggshell elasticity (P = 0.07).
Ionized blood Ca is affected by dietary vitamin D3 concentrations
We also examined if ionized blood Ca in hens was influenced by dietary vitamin D3 concentrations throughout the study. There was no temporal effect on ionized blood Ca (P = 0.65). With week 0 considered as a covariate, there was a dietary effect on ionized blood Ca (P = 0.002, Supplementary Table 4). It is not known why there is no clear trend in ionized blood Ca relative to the dietary treatment, but one possibility could be related to when the hen laid an egg prior to the blood collection, which may influence circulating Ca concentrations.
Fecal Ca is affected by dietary vitamin D3 concentrations, but not ileal digesta or eggshell Ca or P
We had the eggshell, ileal digesta, and fecal Ca and P measured to determine if dietary vitamin D3 fed to our hens would reduce the excretion or loss of Ca and P. There was no dietary effect on eggshell Ca and P (P = 0.64 for both) and ileal digesta Ca or P (P = 0.74 and 0.09, respectively). There was a dietary effect with fecal Ca with hens fed 14,000 IU/kg D3 diet had 10.6% ± 0.8% Ca by weight in their feces, whereas all other treatments were 8.0–9.0%, with the exception of 36,000 IU/kg D3 fed hens which had 7.36% ± 0.43% fecal Ca (P = 0.03, Supplementary Table 5). There was no dietary effect on fecal P (P = 0.76).
Humerus is more Ca and P dense than tibia
We assessed if dietary vitamin D3 would improve bone Ca and P in hens. However, no dietary effects of vitamin D3 supplementation were observed on bone Ca or P (P = 0.79 and 0.63, respectively). Humerus bones have a higher percentage by weight, Ca and P, than tibia bones (P = 0.020 and 0.015, respectively; Supplementary Figure 1).
Dietary super-dosage concentrations of vitamin D3 increased plasma and egg yolk vitamin D3 metabolites
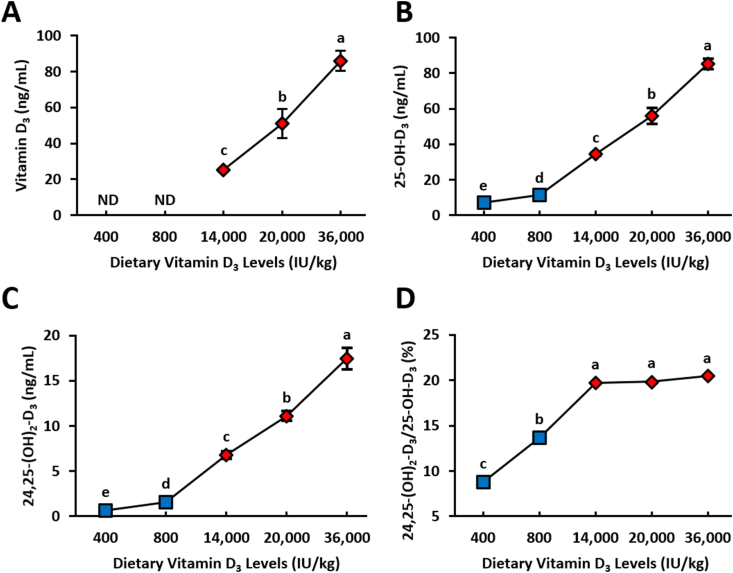
We had plasma and egg yolk vitamin D3 metabolites measured by LC-MS/MS to determine if dietary vitamin D3 affected plasma and egg yolk concentrations. There was a significant increase in plasma concentration of vitamin D3, 25-OH-D3, and 24,25-(OH)2-D3 of hens fed dietary super-dose concentrations of vitamin D3 (D3: P = 0.0002; 25-OH-D3: P < 0.0001; 24,25-OH-D3: P < 0.0001; Figure 2A–C). Although plasma vitamin D3 concentration was below the limit of detection for the 400 and 800 IU D3/kg treatments, the plasma vitamin D3 concentration had a strong positive correlation with 25-OH-D3 and 24,25-(OH)2-D3 concentrations (r = 0.95, P < 0.0001; r = 0.92, P < 0.0001; respectively, data not shown). Plasma vitamin D3 and 25-OH-D3 had similar concentration values when dietary vitamin D3 increased, with both metabolites having ∼85 ng/mL at 36,000 IU D3/kg treatment. Although plasma 24,25-(OH)2-D3 concentration was lower than vitamin D3 and 25-OH-D3, 24,25-(OH)2-D3 was affected by dietary treatment, and 24,25-(OH)2-D3 also exhibited a similar rate of increase relative to dietary treatment like vitamin D3 and 25-OH-D3. The percentage ratio of 24,25-(OH)2-D3 to 25-OH-D3 increased as dietary vitamin D3 concentrations increased and reached an asymptote at the super-dose levels (P < 0.0001; Figure 2D). The 24,25-(OH)2-D3: 25-OH-D3 ratio percentage ranged from 8.7% to 20.5%, with all super-dose-fed hens having a ratio of ∼20%.
FIGURE 2.
Vitamin D3 metabolite plasma concentrations of 78-wk Hy-Line Brown laying hens fed different concentrations of dietary vitamin D3. (A) Cholecalciferol [vitamin D3; 400 and 800 IU treatment concentrations were below limit of detection and were not determined (ND)] (B) 25-hydroxycholecalciferol (25-OH-D3) (C) 24,25-dihydroxycholecalciferol [24,25-(OH)2-D3] (D) ratio of 24,25-(OH)2-D3/25-OH-D3 presented as a percentage. Blue squares denote standard NRC range vitamin D3 concentrations (400 and 800 IU D3/kg) in diet (n = 4), and red diamonds denote super-dose concentrations of vitamin D3 (14,000, 20,000, and 36,000 IU D3/kg) in diet (n = 4). Samples were reported as means ± SEM. Samples with common letters were not significantly different from each other (general linear models, P < 0.0001). NRC, National Research Council; SEM, standard error of the mean.
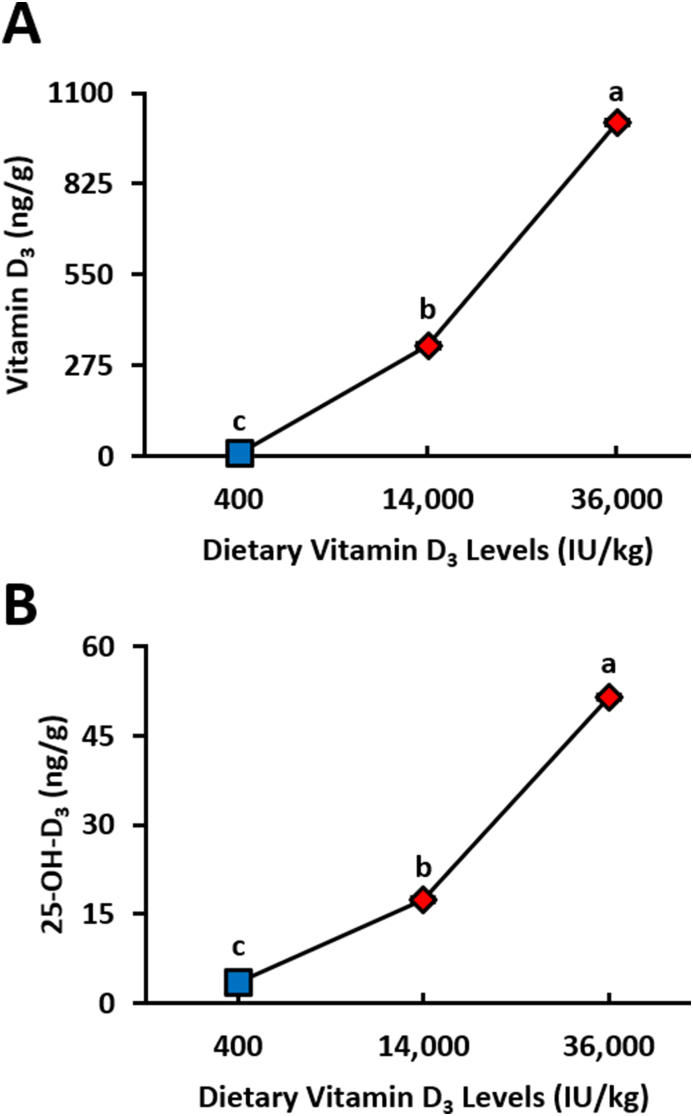
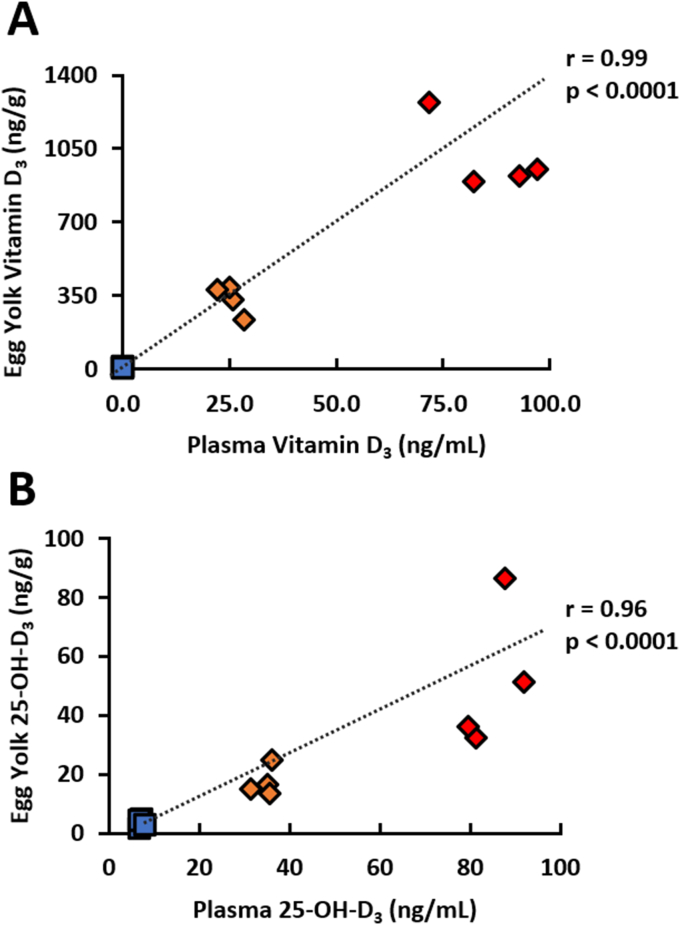
Egg yolk vitamin D3 increased drastically as hens’ dietary vitamin D3 intake increased (P < 0.0001; Figure 3A and B). Egg yolk 25-OH-D3 was also significantly increased in concentration as hens’ dietary vitamin D3 increased (P < 0.0001), but the rate of increase was much lower compared with egg yolk vitamin D3. Egg yolk vitamin D3 was strongly and positively correlated with plasma vitamin D3 (r = 0.99, P < 0.0001, Figure 4A). Egg yolk 25-OH-D3 also had a strong positive correlation with plasma 25-OH-D3 (r = 0.96, P < 0.0001, Figure 4B).
FIGURE 3.
Egg yolk vitamin D3 metabolite concentrations from 78-wk Hy-Line Brown laying hens fed different concentrations of dietary vitamin D3. (A) Cholecalciferol (vitamin D3) (B) 25-hydroxycholecalciferol (25-OH-D3). Blue squares denote standard NRC range vitamin D3 concentrations (400 IU D3/kg) in diet (n = 4), and red diamonds denote super-dose concentrations of vitamin D3 (14,000 and 36,000 IU D3/kg) in diet (n = 4). Samples were reported as means ± SEM; however, error bar values were narrow and overlapped by the marker for each sample. Samples with common letters were not significantly different from each other (general linear models, P < 0.0001). NRC, National Research Council; SEM, standard error of the mean.
FIGURE 4.
Association between plasma and egg yolk vitamin D3 metabolite concentrations from 78-wk Hy-Line Brown laying hens fed different concentrations of dietary vitamin D3. (A) Cholecalciferol (vitamin D3) (B) 25-hydroxycholecalciferol (25-OH-D3). Blue squares denote the standard NRC range vitamin D3 concentration (400 IU D3/kg, n = 4) in diet; orange (14,000 IU D3/kg, n = 4) and red diamonds (36,000 IU D3/kg, n = 4) denote super-dose concentrations of vitamin D3 in diet (Pearson correlation). NRC, National Research Council.
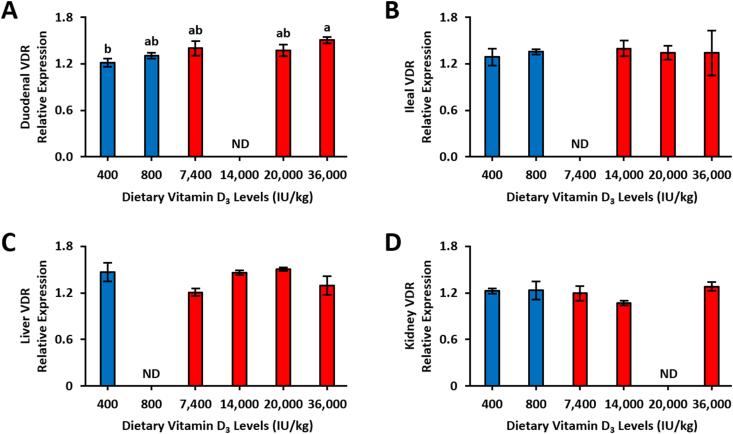
Dietary super-doses of vitamin D3 intake affected VDR expression and kidney CYP24A1 expression
Considering VDR is a ligand-activated transcription factor responsible for exerting vitamin D’s physiologic effects [22], we measured VDR expression in multiple tissues to determine if dietary vitamin D3 concentrations would affect VDR expression. Hens fed higher concentrations of dietary vitamin D3 had upregulated duodenal VDR expression (P = 0.036; Figure 5A). There was no dietary effect on VDR expression from the ileum, liver, and kidney (P = 0.96, 0.17, 0.32, respectively; Figure 5B–D). We also examined if vitamin D3 super-dosages would affect the gene expression of vitamin D hydroxylase enzymes in the kidney. Unexpectedly, kidney 24-OHase expression was lower in hens-fed diets with super-dose concentrations of vitamin D3 (P = 0.0006, Supplementary Figure 2A). No differences were observed with kidney 1α-OHase expression (P = 0.81, Supplementary Figure 2B).
FIGURE 5.
Relative gene expression of vitamin D receptor (VDR) in the duodenum, ileum, liver, and kidney of 78-wk Hy-Line Brown laying hens fed different concentrations of dietary vitamin D3. (A) Duodenal VDR (n = 2–6) (B) ileal VDR (n = 2–5) (C) liver VDR (n = 2–4) (D) kidney VDR (n = 2–4). Tissues were analyzed using qPCR normalized against glyceraldehyde phosphate dehydrogenase (housekeeping gene) expression. Blue bars denote standard NRC range vitamin D3 concentrations in the diet, and red bars denote super-dose concentrations of vitamin D3 in the diet. All samples were analyzed in triplicates and reported as means ± SEM. ND = not detected or below limit of detection. Bars with common letters were not significantly different from each other (general linear models, P < 0.05). NRC, National Research Council; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean.
Discussion
Our results suggest that dietary super-doses of vitamin D3 greatly increased plasma and egg yolk D3 concentrations. Increased plasma vitamin D3 indicates these hens absorbed vitamin D3 from their diets. Although the inactive vitamin D3 metabolite, 24,25-(OH)2-D3, had a lower measured value than vitamin D3 and 25-OH-D3, its slope and rate of increase had the same rate of increase. Increasing plasma 24,25-(OH)2-D3 concentrations highlights that these hens were likely trying to reduce their circulating vitamin D3 concentrations (Figure 6). In addition, egg yolk vitamin D3 drastically increased, whereas yolk 25-OH-D3 had a smaller rate of increase. Altogether, there is a strong association between dietary vitamin D3 concentrations and plasma and egg yolk vitamin D3 metabolite concentrations.
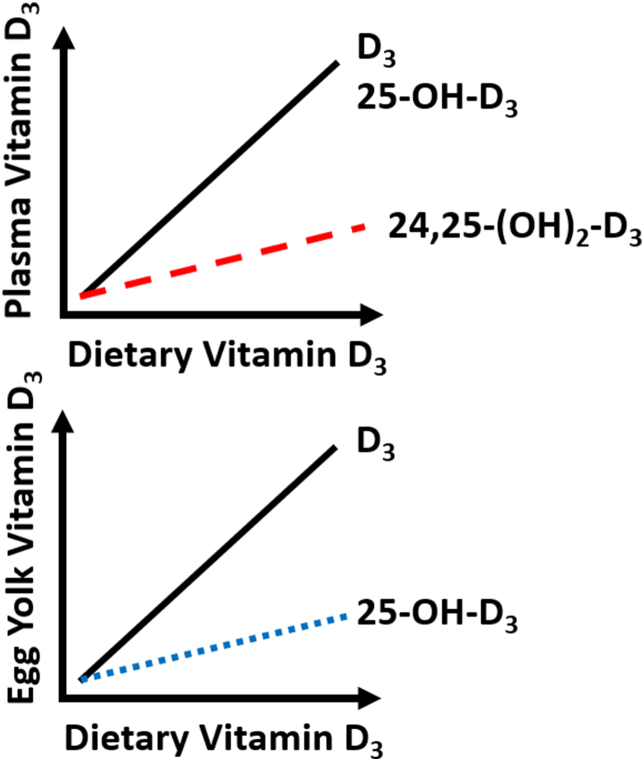
FIGURE 6.
Dietary super-doses of vitamin D3 fed to aged laying hens cause drastic increases in plasma and egg yolk vitamin D3 metabolites. Egg yolk vitamin D3 concentrations were strongly correlated to the dietary concentrations of vitamin D3 fed to the hens. Egg yolk 25-hydroxycholecalciferol (25-OH-D3) concentrations were also dependent on dietary vitamin D3; however, 25-OH-D3 increased at a lower rate. For plasma vitamin D3 metabolites, vitamin D3 and 25-OH-D3 concentrations increased relative to dietary vitamin D3 concentrations fed to the hens. Plasma 24,25-dihydroxycholecalciferol [24,25-(OH)2-D3] concentrations were also dependent on dietary vitamin D3, but the rate of increase was lower.
Although a laying hen’s physiologic status affects egg quality [23], high concentrations of dietary vitamin D3 have been shown to reduce egg quality but not affect egg production [24]. Mattila et al. [9] and Wen et al. [10] reported that laying hens fed diets with greater concentrations of dietary vitamin D3 throughout their production cycle increased egg yolk vitamin D3 content. The egg production and egg yolk vitamin D3 data in our study were similar to the 2 aforementioned studies, even though the hens in our study were older. Signs of vitamin D toxicity in laying hens are reduction in egg production and food consumption [12]. However, the hens in our study did not show any drastic differences with either of those which suggests they were in no danger of vitamin D toxicity. A novel finding we observed was that vitamin D3 was deposited more readily into the yolk compared with 25-OH-D3. One possibility is that excess circulating 25-OH-D3 was transferred to the egg yolk as a way to lower circulating 25-OH-D3 concentrations. Vitamin D3 is readily converted to 25-OH-D3 in the liver by the 25-hydroxylase, whereas CYP27C1 and CYP24A1 are tightly controlled by parathyroid hormone and fibroblast growth factor 23 [25,26]. Supplementing laying hen diets with 25-OH-D3 increased egg yolk 25-OH-D3 and reduced egg yolk vitamin D3 [27]. It seems likely that the vitamin D3 metabolite composition in egg yolk is influenced by whatever dietary vitamin D3 isoform is fed to the laying hens.
The biologic significance of 24,25-(OH)2-D3 is to reduce 25-OH-D3’s plasma concentration [28]. A recent study involving laying hens showed how plasma 24,25-(OH)2-D3 did not change over time after an egg was laid [29]. In our study, the hens’ plasma 24,25-(OH)2-D3 increased relative to dietary vitamin D3 concentrations. However, unlike plasma D3 and 25-OH-D3, the rate of increase with plasma 24,25-(OH)2-D3 was miniscule. The rate of increase for 24,25-(OH)2-D3 relative to D3 and 25-OH-D3 at super-dosage level off at 20%, highlighting a possible asymptotic relationship. The asymptote suggests that 24-hydroxylation activity hit its maximal limit. It is not clear if VDR expression is associated with plasma 24,25-(OH)2-D3 concentrations or 24-hydroxylation activity because there was little difference in VDR expression across multiple tissues in this study.
Our study has several strengths that highlight its impact on advancing nutritional knowledge. A significant strength of our study is plasma and egg yolk vitamin D3 metabolite concentration ranges across treatment groups. This illustrates the experimental design captured a broad range of dietary vitamin D3 supplementation concentration effects on plasma vitamin D3 metabolites that future research studies can focus on a specific range to build off our findings. Our study provides novel observations of laying hen plasma 25-OH-D3 concentrations relative to dietary vitamin D3 supplementation that can be valuable for the poultry industry to consider with vitamin D3 status. The plasma 24,25-(OH)2-D3 data are the most exciting finding of our study. Further understanding of the asymptotic relationship of the super-dose concentrations with plasma 24,25-(OH)2-D3 concentrations can open new knowledge about24,25-(OH)2-D3’s value as a biomarker for vitamin D metabolism. One possibility of 24,25-(OH)2-D3’s use as a biomarker is determining a circulating level range that can be used as an early warning sign to suggest a person or animal is starting to approach vitamin D toxicity.
There were a few limitations with this study that were realized when data were collected. We should have investigated the kidney histopathology of these hens because soft-tissue calcification or renal kidney failure could result from the hens reaching vitamin D toxicity [30,31]. However, Mattila et al. [9] did not observe any pathologic issues in kidneys from 67-wk-old hens fed 15,000 IU D3/kg of feed. The smaller sample sizes and missing treatment groups from the qPCR results were because some tissue samples would not yield RNA for cDNA synthesis, even after multiple extraction attempts. All tissue samples, except for plasma, were temporarily stored in a 7°C cold room until freezer space was available, which could have caused a reduction in RNA quality. It is important to note that the CYP27C1 gene may not encode CYP27C1 [32] (Table 2 footnote). We also stored the egg yolk in a refrigerator for about a year before the yolk was freeze-dried; however, our findings are similar to Wen et al. [10] to hint toward minimal vitamin D3 degradation (Table 3). This could indicate how stable vitamin D3 is when it is stored in cold, dark conditions.
TABLE 3.
Comparison of egg yolk vitamin D3 from eggs laid by hens fed different dietary concentrations of vitamin D3 in this study compared with Wen et al. 2019 study [10]
Our study indicates that feeding super-doses of dietary vitamin D3 to aged laying hens increases their plasma and egg yolk vitamin D3. Importantly, there is a possible metabolic limit of 24-hydroxylation to remove excess circulating vitamin D3. Investigating 24-hydroxylation mechanisms will be important to understanding vitamin D3 supplementation impacts in geriatric animals for improving bone health and vitamin D metabolism in older humans.
Author contributions
The authors’ responsibilities were as follows – MFW, KAL: designed research; MFW, DDH, NCT, KAL: conducted research; MFW, KAL: analyzed data; MFW, KAL: wrote manuscript; MFW, KAL: prepared experimental diets for study; MFW, PMP: prepared samples for ashing; MFW, DDH: prepared and shipped plasma and egg yolk samples to Heartland Assays; MFW, KAL: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This research was supported by the United States Poultry and Egg Association (KAL) and the United States Poultry and Egg Association had no involvement or restrictions regarding publication.
Data availability
Data described in the current study will be made available from the corresponding author upon request.
Acknowledgments
We thank Gavin Conant, Peter Ferket, Matt Koci, and Shannon Madden for their comments and edits to the initial manuscript; Taylor Jones for her help with the RNA extraction, cDNA synthesis, and qPCR experiments; David Dickey for statistical assistance; Jeff Hall and Zach Spivey for their help with taking care of the hens and collecting samples; Liza Lentz of the Environmental and Agricultural Testing Service laboratory, Department of Crop and Soil Sciences, at North Carolina State University who performed the mineral composition experiments and analyses; and John Rathmacher of Heartland Assays for quantifying the plasma and egg yolk vitamin D3.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2024.102156.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jones G. Pharmacokinetics of vitamin D toxicity. Am. J Clin. Nutr. 2008;88(2):582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 2.Marcinowska-Suchowierska E., Kupisz-Urbańska M., Łukaszkiewicz J., Płudowski P., Jones G. Vitamin D toxicity–A clinical perspective. Front Endocrinol. 2018;9:550. doi: 10.3389/fendo.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick M.F. Vitamin D deficiency. N Engl. J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Tang B.M., Eslick G.D., Nowson C., Smith C., Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Stuck A.E., Staehelin H.B., Orav E.J., et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2009;169(6):551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari H.A., Willett W.C., Orav E.J., Lips P., Meunier P.J., Lyons R.A., et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl. J Med. 2012;367(1):40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 7.Klontz K.C., Acheson D.W. Dietary supplement–induced vitamin D intoxication, N Engl. J Med. 2007;357(3):308–309. doi: 10.1056/NEJMc063341. [DOI] [PubMed] [Google Scholar]
- 8.Mattila P., Rokka T., Könkö K., Valaja J., Rossow L., Ryhänen E.L. Effect of cholecalciferol-enriched hen feed on egg quality. J Agric. Food Chem. 2003;51(1):283–287. doi: 10.1021/jf020743z. [DOI] [PubMed] [Google Scholar]
- 9.Mattila P., Valaja J., Rossow L., Venäläinen E., Tupasela T. Effect of vitamin D2-and D3-enriched diets on egg vitamin D content, production, and bird condition during an entire production period. Poult. Sci. 2004;83(3):433–440. doi: 10.1093/ps/83.3.433. [DOI] [PubMed] [Google Scholar]
- 10.Wen J., Livingston K.A., Persia M.E. Effect of high concentrations of dietary vitamin D3 on pullet and laying hen performance, skeleton health, eggshell quality, and yolk vitamin D3 content when fed to W36 laying hens from day of hatch until 68 wk of age. Poult. Sci. 2019;98(12):6713–6720. doi: 10.3382/ps/pez386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari R., White D., House J.D., Kim W.K. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020;99(1):364–373. doi: 10.3382/ps/pez502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soares J.H., Jr., Kaetzel D.M., Allen J.T., Swerdel M.R. Toxicity of a vitamin D steroid to laying hens. Poult. Sci. 1983;62(1):24–29. doi: 10.3382/ps.0620024. [DOI] [PubMed] [Google Scholar]
- 13.Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83(2):193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- 14.DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am. J Clin. Nutr. 2004;80(6):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;79(7):1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- 16.Council N.R. National Academies Press; 1994. Nutrient requirements of poultry. [Google Scholar]
- 17.Mattila P., Lehikoinen K., Kiiskinen T., Piironen V. Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J Agric. Food Chem. 1999;47(10):4089–4092. doi: 10.1021/jf990183c. [DOI] [PubMed] [Google Scholar]
- 18.van Leeuwen J.P.T.M., van den Bemd G.-J.C.M., van Driel M., Buurman C.J., Pols H.A.P. 24,25-dihydroxyvitamin D3 and bone metabolism. Steroids. 2001;66(3–5):375–380. doi: 10.1016/S0039-128X(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y., DeLuca H.F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- 20.Holick M.F., Schnoes H.K., DeLuca H.F., Gray R.W., Boyle I.T., Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- 21.Redhead A.K., Sanders E., Vu T.C., Malheiros R.D., Anderson K.E., Toomer O.T. The effects of high-oleic peanuts as an alternate feed ingredient on performance, ileal digestibility, apparent metabolizable energy, and histology of the small intestine in laying hens. Transl. Anim. Sci. 2021;5(1):txab015. doi: 10.1093/tas/txab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haussler M.R., Haussler C.A., Bartik L., Whitfield G.K., Hsieh J.C., Slater S., et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008;66(10):S98–S112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 23.Travel A., Nys Y., Bain M. Improving the safety and quality of eggs and egg products. Elsevier; 2011. Effect of hen age, moult, laying environment and egg storage on egg quality; pp. 300–329. [Google Scholar]
- 24.Ameenuddin S., Sunde M.L., DeLuca H.F., Cook M.E. Excessive cholecalciferol in a layers diet: decline in some aspects of reproductive performance and increased bone mineralisation of progeny. Br. Poult. Sci. 1986;27(4):671–677. doi: 10.1080/00071668608416926. [DOI] [PubMed] [Google Scholar]
- 25.Shinki T., Jin C.H., Nishimura A., Nagai Y., Ohyama Y., Noshiro M., et al. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha, 25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol. Chem. 1992;267(19):13757–13762. doi: 10.1016/S0021-9258(18)42278-8. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner. Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 27.Duffy S.K., Rajauria G., Clarke L.C., Kelly A.K., Cashman K.D., O’Doherty J.V. The potential of cholecalciferol and 25-hydroxyvitamin D3 enriched diets in laying hens, to improve egg vitamin D content and antioxidant availability. Innov. Food Sci. Emerg. Technol. 2017;44:109–116. doi: 10.1016/j.ifset.2017.07.007. [DOI] [Google Scholar]
- 28.Bikle D. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2000. Vitamin D: production, metabolism, and mechanisms of action. [Internet] [Google Scholar]
- 29.Sinclair-Black M., Garcia-Mejia R.A., Blair L.R., Angel R., Arbe X., Cavero D., et al. Circadian regulation of calcium and phosphorus homeostasis during the oviposition cycle in laying hens. Poult. Sci. 2024;103(2) doi: 10.1016/j.psj.2023.103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner. Res. 2007;22(2):V64–V68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 31.Morrissey R.L., Cohn R.M., Empson R.N., Jr., Greene H.L., Taunton O.D., Ziporin Z.Z. Relative toxicity and metabolic effects of cholecalciferol and 25-hydroxycholecalciferol in chicks. J Nutr. 1977;107(6):1027–1034. doi: 10.1093/jn/107.6.1027. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair-Black M., Garcia R.A., Ellestad L.E. Physiological regulation of calcium and phosphorus utilization in laying hens. Front Physiol. 2023;14 doi: 10.3389/fphys.2023.1112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the current study will be made available from the corresponding author upon request.