Graphical abstract
Keywords: Manganese, Hyperactivity, Autism, Neurodevelopment, Neurotoxicity
Highlights
-
•
Mn exposure seems to be associated with hyperactivity and inattention scores in ADHD.
-
•
Mn-induced hyperactivity is associated with dopaminergic dysfunction.
-
•
Epidemiological data on the association between Mn exposure and ASD are scarce.
-
•
Neuronal oxidative stress and neuroinflammation mediate the role of Mn in ADHD/ASD.
-
•
Mn-induced neuronal damage and altered neurogenesis may contribute to ASD/ADHD.
Abstract
The objective of the present narrative review was to synthesize existing clinical and epidemiological findings linking manganese (Mn) exposure biomarkers to autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD), and to discuss key pathophysiological mechanisms of neurodevelopmental disorders that may be affected by this metal. Existing epidemiological data demonstrated both direct and inverse association between Mn body burden and ASD, or lack of any relationship. In contrast, the majority of studies revealed significantly higher Mn levels in subjects with ADHD, as well as direct relationship between Mn body burden with hyperactivity and inattention scores in children, although several studies reported contradictory results. Existing laboratory studies demonstrated that impaired attention and hyperactivity in animals following Mn exposure was associated with dopaminergic dysfunction and neuroinflammation. Despite lack of direct evidence on Mn-induced neurobiological alterations in patients with ASD and ADHD, a plethora of studies demonstrated that neurotoxic effects of Mn overexposure may interfere with key mechanisms of pathogenesis inherent to these neurodevelopmental disorders. Specifically, Mn overload was shown to impair not only dopaminergic neurotransmission, but also affect metabolism of glutamine/glutamate, GABA, serotonin, noradrenaline, thus affecting neuronal signaling. In turn, neurotoxic effects of Mn may be associated with its ability to induce oxidative stress, apoptosis, and neuroinflammation, and/or impair neurogenesis. Nonetheless, additional detailed studies are required to evaluate the association between environmental Mn exposure and/or Mn body burden and neurodevelopmental disorders at a wide range of concentrations to estimate the potential dose-dependent effects, as well as environmental and genetic factors affecting this association.
1. Introduction
Manganese (Mn) is an essential metal involved in numerous biological processes due to the functioning of Mn-containing metalloenzymes like arginase, Mn-superoxide dismutase, and glutamine synthetase (Avila et al., 2013). The existing data demonstrate a significant role of Mn in redox homeostasis, energy metabolism, regulation of immune response and neurodevelopment (Erikson and Aschner, 2019). Mn deficiency has been associated with multiple disturbances, including reproductive dysfunction (Boyer et al., 1984), impaired carbohydrate metabolism (Baly et al., 1984), as well as altered brain functioning (Takeda, 2003). Specifically, mutations in SLC39A8, a metal transporter responsible for cellular Mn uptake, associated with Mn deficiency result in cerebral atrophy with subsequent developmental delay, dystonia, and seizures (Riley et al., 2017). Nutritional deficiency of Mn is rather rare due to high content of Mn in the foodstuffs (Martins et al., 2020b). Furthermore, laboratory in vitro and in vivo studies demonstrated that Mn overload also possesses significant adverse effects on brain functioning due to its neurotoxic effects including induction of neuronal oxidative stress and apoptosis, neuroinflammation, and dysregulation of neurotransmitter metabolism to name a few (Tinkov et al., 2021c). Correspondingly, Mn overexposure was shown to contribute significantly to neurodegeneration in Parkinson’s disease and Alzheimer’s disease (Martins et al., 2019), as well as Huntington’s disease and amyotrophic lateral sclerosis (Martins et al., 2020a). Several studies demonstrated that developmental Mn overexposure may affect neurodevelopment resulting in behavioral deficits and reduced cognitive performance (Liu et al., 2020). Moreover, Mn overexposure may be associated with increased prevalence of neurodevelopmental disorders (NDD), including ASD (Blazewicz et al., 2022, Skogheim et al., 2021) and ADHD (Shih et al., 2018). However, other epidemiological studies yielded contradictory results demonstrating the lack of difference or even lower Mn levels in children with these NDDs (Qin et al., 2018, Hawari et al., 2020). Therefore, the objective of the present narrative review was a to synthesize the existing clinical and epidemiological findings linking Mn exposure biomarkers to ASD and ADHD, and to discuss key mechanisms of neurodevelopmental disorders pathogenesis that may be affected by exposure to this metal.
2. Epidemiological findings on Mn and ASD
Autism spectrum disorder (ASD) is a major public health concern, being observed in one child of 45 (Zablotsky et al., 2015). However, the etiology and its underlying pathophysiology remains incomplete. Factors such as genetics, environment, and epigenetic changes are frequently implicated, and ASD is considered a multifactorial disorder. In this regard, anomalies in essential elements, such as Mn, are environmental factors presumed to play a role on the pathophysiology of ASD (Baj et al., 2021). Therefore, epidemiological studies have focused on the association between Mn levels and ASD.
Recently, two hundred and twenty-seven children (107 with ASD and 120 controls) aged 3 to 14 years were recruited in Morocco and essential elements (copper, iron, manganese, selenium, and zinc) levels were evaluated in hair. The results showed a significant decrease in levels of essential elements in children with ASD when compared with control children. The authors reported that gender was a significant predictor of hair levels of Mn where boys had significantly lower Mn levels (26 %) than girls (Ouisselsat et al., 2023). Likewise, hair samples obtained from children diagnosed with ASD showed reduced levels of essential elements, including Mn, in comparison to children in the control group. Moreover, a significantly elevated prevalence of social withdrawal, sleep and eating disturbances, as well as speech and language disorders were observed in individuals with ASD compared to the control group (Al-Ayadhi, 2005). Zhao et al. (2023) demonstrated a significant inverse correlation between whole blood Mn and total CNBS score as well as domains associated with language skills in children with ASD, suggesting that Mn induced adverse effects on cognitive ability (Zhao et al., 2023).
Mn concentration was evaluated in blood plasma in children with ASD and in unaffected children in China. Some factors associated with Mn exposure and bioaccumulation such as body mass index (BMI), passive smoking, and seafood consumption, were investigated. The results showed lower Mn levels in children with ASD (13.5 μg/L) than the unaffected children (21.4 μg/L). However, no significant difference was found in Mn concentrations in blood of children with ASD and unaffected children in relation to the assessed factors (Qin et al., 2018). Corroborating these findings, another Chinese Han population study reported no significant difference between ASD cases and controls for the concentration of Mn in serum (Ma et al., 2022). At the same time, our previous study involving 70 children with ASD and 70 healthy children from Moscow (Russia) revealed significantly higher serum Mn levels only in boys with ASD when compared to the respective control group (2.4 vs 2 ng/ml), whereas no significant group difference was noted in girls (Skalny et al., 2017). Moreover, the results of the Norwegian Mother, Father and Child Cohort Study demonstrated a positive association between the fourth quartile of maternal whole blood Mn levels and increased odds of ASD in children (Skogheim et al., 2021). Examination of 129 Polish adolescents with ASD and 86 neurotypical examinees revealed more than a threefold higher urinary Mn levels in ASD (0.043 ± 0.026 µg/d) cases in comparison to controls (0.012 ± 0.002 µg/d) (Błażewicz et al., 2022).
Similarly, a study with Jamaican children with ASD and without ASD found no significant association between Mn exposure and ASD in univariable General Linear Models (GLM) analysis and in a multivariable GLM adjusting for paternal age, parental education, place of child’s birth, consumption of root vegetables, cabbage, saltwater fish, and cakes/buns (Rahbar et al., 2014). On the other hand, in Jamaican children, Rahbar et al., identified a significant interaction between GSTP1 gene and Mn blood concentrations. Their findings suggested that among children with the Ile/Ile genotype for GSTP1, those with Mn levels ≥ 12 µg/L had approximately four times higher odds of ASD than those with Mn levels < 12 µg/L considered normal (p = 0.03). Furthermore, the study indicated an interaction between Mn and GST gene, suggesting that ASD status may modify the association between GST genes and blood Mn concentration (Rahbar et al., 2015). In this respect, a study on the association of ASD in Pakistani children with Mn and genotype frequencies of three GST genes (GSTP1, GSTM1, GSTT1) found no association with the essential metal concentration and three GST genes (Rahbar et al., 2021). GST is a family of phase II drug metabolizing enzymes involved in detoxification of a variety of xenobiotics. GST inhibition increases susceptibility to neurotoxic agents, contributing to a variety of neurological disorders (Dasari et al., 2018) including ASD (Mandic-Maravic et al., 2021). Vescovi et al. (1989) demonstrated that Mn exposure inhibited brain GST in a dose-dependent manner, indicative of the potential role of GST inhibition as a mediator of Mn-induced neurotoxicity (Vescovi et al., 1989).
A study using primary teeth as biomarkers was carried out to evaluate and compare the concentration of Mn in ASD children and a control group in India. The results showed that Mn concentrations were three times higher in teeth of ASD children group compared to children in the control group, although the number of examinees per group was only six (Kaur et al., 2021).
As mentioned before, environmental factors are associate with ASD development. In conflict zones, such as in war, children may be exposed to several environmental pollutions and food shortages, which can adversely impact their nutrition, leading to essential metal deficiencies. Interestingly, during the Syrian crisis, Hawari et al., (2020) examined whole blood Mn levels in 31 ASD children and 30 healthy children that were born or grown during the Syrian crisis. it was reported that the incidence of ASD, ADHD were associated with an increase in the whole blood lead and a decrease in Mn or both (Hawari et al., 2020). The association between lead and ASD was recently reviewed (Tizabi et al., 2023). The authors suggested that the conditions stemming from the Syrian crisis may also influence the nutrition of those children. Besides, genetic variations, such as polymorphisms in Mn transporter genes SLC30A10 and SLC39A8, can impact Mn homeostasis, directly influencing the levels of this metal in the bloodstream (Wahlberg et al., 2018).
The concentration of Mn in hair samples of children with ASD was studied to address alterations in trace element and mineral status in children with NDDs. Mn levels were significantly reduced in children with ASD when compared with neurotypical controls (Skalny et al., 2020b). Absence of Mn overexposure in the studied children may explain a connection between low Mn levels and adverse neurodevelopment (Skalny et al., 2020b). Likewise, Fiori et al., observed decreased levels of Mn in patients with ASD, as well as an inverse correlation between hair Mn levels and cognitive functions (Fiore et al., 2020). However, contradictory results can be found in the literature regarding association of Mn levels and ASD. A meta-analysis conducted by Saghazadeh and Rezaei found that the mean difference in blood and hair Mn concentrations between ASD and control individuals was not significant (Saghazadeh and Rezaei, 2017). Other studies have also reported no significant differences between Mn blood and hair levels in ASD and control groups (Geier et al., 2012, Skalny et al., 2017).
The brain is exquisitely vulnerable to neurotoxic effects of xenobiotics during childhood (Martins et al., 2021, Oulhote et al., 2014), and exposures to environmental factors in the early stages of life, particularly during critical windows (CWs) of development, have the potential to influence health throughout the entire lifespan. Indeed, several studies demonstrated that Mn exposure interferes with neurodevelopment. Based on evaluation of dentine Mn content and magnetic resonance imaging at age of age 16–23 years, it was reported that Mn exposure affected intrinsic functional connectivity in dorsal striatum, occipital and frontal lobes, and middle frontal gyrus (upon prenatal exposure), putamen and cerebellum (upon perinatal exposure), and putamen, occipital, frontal, and temporal lobes (upon early postnatal exposure), circuitries implicated in cognition and motor functions (Rechtman et al., 2023).
Growing evidence suggests that maternal exposure to air pollution in the perinatal period may increase the risk of ASD in children. In this respect, a stronger association was observed in boys compared with girls for pollutants (including Mn), suggesting a sex-specific interaction (Roberts et al., 2013). Moreover, evidence suggests that metals could exert gender-specific influences on social behavior by affecting dopamine function (Curtis et al., 2010). Additionally, children living in communities near a ferro-manganese alloy plant had diminished IQ and neuropsychological performance in tests evaluating executive functions such as response inhibition, strategic visual formation, and verbal working memory (Carvalho et al., 2014). Hence, environmental exposure to Mn in early-life may alter cognitive function in children (Lucchini et al., 2017).
A Swedish study employing biomarkers derived from tooth matrices that assess the absorption of various elements with high temporal precision in early development, and a well-characterized set of twins as subjects, reported distinctions between individuals with ASD and those without ASD during specific prenatal and postnatal intervals. The authors showed that Mn levels were consistently lower in cases both pre- and postnatally, and this deficiency was more significant 4 months after birth in ASD group, suggesting that systemic elemental dysregulation during specific developmental windows may play an important role in ASD etiology (Arora et al., 2017).
Approximately one-third of children diagnosed with ASD belong to the neurodevelopmental regression (NDR) subtype. Children in this category exhibit typical developmental progress during infancy but experience a decline in social and language skills, typically occurring between the first and second years of life (Brister et al., 2022, Maenner et al., 2020). A possible explanation for NDR is related to mitochondrial dysfunction, mainly in children with ASD (Delhey et al., 2017, Shoffner et al., 2010). Elevated concentration of Mn during prenatal exposure was linked to diminished mitochondrial respiration and glycolysis in individuals with ASD with a history of NDR, suggesting that baseline Mn level could be a crucial factor linked to the modulating impact of Mn on both the severity of ASD and its physiological aspects (Frye et al., 2020).
Although several studies have evaluated the potential impact of early-life exposure to Mn on cognitive functions and its association with ASD and NDR, the mechanism and pathways that are involved are not completely understood. and additional studies are necessary to elucidate the involvement of Mn in these neurological alterations.
3. Epidemiological and laboratory findings on Mn and ADHD
ADHD is a neurodevelopmental disorder characterized by behavioral disturbances including inattention, impulsivity, and hyperactivity. Despite a high prevalence of ADHD in the population, the potential causes of ADHD are still debatable (Nigg et al., 2020). It is proposed that, along with biological and genetic factors, exposure to environmental toxins may also significantly contribute to the development of ADHD (Aghaei et al., 2019).
Several previous studies demonstrated an association between environmental Mn exposure and ADHD. Specifically, a nationwide study in Denmark demonstrated showed that exposure to high levels of Mn in drinking water (>100 ug/l) is was associated with 51 % and 20 % increase in risk of ADHD-inattentive subtype in females and males, respectively, whereas no relationship between Mn exposure and ADHD combined subtype was revealed (Schullehner et al., 2020). Soil Mn levels were also found to be associated with inattention and ADHD scores in girls and to a lesser extent with hyperactivity scores in boys, while SLC30A10 rs12064812 and SLC39A8 rs13107325 significantly modified this association by increasing the risk of adverse behavioral outcome in girls exposed to Mn (Broberg et al., 2019). A previous case report revealed that a significant elevation of Mn concentration in drinking water Mn concentration was associated with high whole blood, urinary, and hair Mn levels, along with inattention, poor verbal and visual memory in a 10-year old boy (Woolf et al., 2002), demonstrating that impaired attention and memory may be considered a symptom of Mn overload.
Epidemiological studies also addressed the association between systemic Mn levels in the human body and ADHD status. A study in the United Arab Emirates demonstrated that each 1 ppb increase in blood Mn levels was associated with a nearly 80 % increase in the odds of ADHD in school-aged children (Yousef et al., 2011). In addition, children with higher Mn exposure as evidenced by detectable nail Mn levels were characterized by reduced sustained attention as compared to their counterparts with less Mn exposure (nail Mn < LOD) (Sears et al., 2021). Higher serum Mn concentrations in children with ADHD, especially inattentive type, were significantly decreased by treatment with methylphenidate (MPH), a drug commonly used for ADHD management, (Farias et al., 2010), indirectly supporting the association between ADHD severity and circulating Mn levels. Correspondingly, the results of a preliminary meta-analysis revealed a significant association between an increase in blood and hair Mn levels and ADHD in children, while after exclusion of studies on hair metal levels the association was only nearly significant (Shih et al., 2018).
In addition to evidence linking ADHD diagnosis to higher Mn body burden, several studies demonstrated that systemic Mn levels in the human body is positively associated with various ADHD severity and behavioral disorders scores. Specifically, blood Mn levels were significantly associated with Child Behavior Checklist (CBCL) total problem score, as well as specific problems including anxiety/depression, social problems, delinquent behavior, aggressive behavior, internalizing problems, and externalizing problems in children with ADHD (Hong et al., 2014). Polymorphisms in Mn transporters including SLC30A10 rs1776029 (AA) and rs12064812 (TT), and SLC39A8 rs13107325 (CC), leading to increased blood Mn levels, were also associated with higher scores of ADHD-related behavior assessed by Conners' subscales ADHD-index and DSM-IV total in comparison to carriers of other alleles (Wahlberg et al., 2018). Hair Mn content was shown to be associated with total CBCL scores, total externalizing behavior and inattention scores in girls, but not in boys from communities located near a ferro-manganese alloy plant (Menezes-Filho et al., 2014). Furthermore, the level of Mn in hair samples was associated with Revised Conners’ Rating Scale for parents (CPRS-R) T-scores on oppositional and hyperactivity subscales in children exposed to Mn through tap water (Bouchard et al., 2007). Despite the lack of significant group difference in hair Mn levels between ADHD cases and controls, subjects with abnormal Mn levels were characterized by higher prevalence of ADHD. Moreover, hair Mn concentration directly correlated with total Korean ADHD Rating Scale (K-ARS) values (Shin et al., 2015).
Finally, Mn level in tooth enamel, a marker of prenatal Mn exposure, was associated with parent and teacher ratings of externalizing and attention problems in children (Ericson et al., 2007). Despite a significant association between prenatal and postnatal dentine Mn content and adverse behavioral outcome including hyperactivity problems both in boys and girls, higher prenatal and postnatal deciduous tooth dentine Mn levels were also associated with better memory, cognitive and motor function in boys (Mora et al., 2015).
Nonetheless, several studies not only failed to reveal the association between Mn overexposure and ADHD risk or severity, but also reported contradictory results. Specifically, no significant association between umbilical cord blood Mn levels and ADHD in children was observed (Ode et al., 2015). In our previous study, we also did not observe significant alterations in serum Mn levels in children with ADHD aged 4–9 years old (Skalny et al., 2020a).
In contrast, as mentioned earlier in Syrian children born or grown during the Syrian crisis with ADHD, blood Mn levels were nearly 10 % lower than in the neurotypical controls (Hawari et al., 2020). Our previous findings also demonstrated a significant reduction in hair Mn levels in children with ADHD (Skalny et al., 2020b).
The results from the Italian Public Health Impact of Metals Exposure Study demonstrated that prenatal and early postnatal (0–1.5 years old) Mn exposure evaluated by deciduous tooth Mn content was associated with lower teacher and parent −reported inattention scores, suggesting a potential protective effect of Mn against ADHD (Broberg et al., 2019). Generally, these findings corroborate the results of the “Norwegian Mother, Father and Child Cohort Study,” which revealed a slight U-shaped association between maternal blood Mn levels at week 17 of gestation and the odds of ADHD, suggesting that either deficiency or overload of Mn during development may lead to ADHD (Skogheim et al., 2021).
In vivo laboratory studies demonstrated that Mn exposure can induce a variety of neurobehavioral deficits, including hyperactivity, altered attention, and impulsivity, all characteristics of ADHD. Specifically, early postnatal Mn exposure (25 or 50 mg Mn/kg/day orally) was shown to induce persistent attentional dysfunction in adult rats, while learning and impulse control were not affected (Beaudin et al., 2017a). Mn-induced attention deficits were also evident in non-human primates following intravenous injection of 15–20 mg/kg/week MnSO4 monohydrate (Schneider et al., 2015). In addition, maternal and early postnatal Mn exposure (2–4 mg/mL Mn in drinking water) increased locomotor activity (McDaniel et al., 2022), although this effect was evident only in females (Phattanarudee et al., 2009). At the same time, hyperactivity induced by Mn-containing drinking water (1.0 mg/ml MnCl2·4H2O) was evident at 5–7 weeks of exposure, but not at longer periods (Nachtman et al., 1986). Interestingly, Mn-induced increased motor activity was also replaced by activity reduction at 8 months of exposure through drinking water (5.0 mg/ml) (Bonilla, 1984).
Other laboratory studies demonstrated that behavioral alterations resembling those in ADHD are associated with impaired dopaminergic signaling and to a lesser extent other neurotransmitters. Specifically, preweaning Mn exposure (25–50 mg/kg/day orally) was shown to induce hyperactivity, as well as memory and learning alterations associated with reduced D1 receptor and DAT protein levels in dorsal striatum and nucleus accumbens along with increased D2 receptor protein expression in prefrontal cortex (Kern et al., 2010). An increase in behavioral reactivity in an open field following early postnatal Mn exposure (25–50 mg/kg/day orally) was associated with a reduction in evoked norepinephrine outflow, decrease in TH, DAT, NET, and D1 receptor protein levels in prefrontal cortex, in parallel with up-regulation of D2R protein levels and astrocyte reactivity assessed by GFAP (Conley et al., 2020). Another study demonstrated that preweaning Mn exposure (50 mg/kg/day orally) resulted in attention deficits associated with down-regulation of Th, dat, and Dnmt3a (DNA methyltransferase 3a) gene expression in prefrontal cortex. Hypermethylation of these genes can result in inflammation, alteration in cell development, and neuronal systems via mTOR signaling changes in the prefrontal cortex (Santiago et al., 2023). Dietary exposure of young mice to Mn (2400 ppm Mn in chow) significantly increased extracellular dopamine clearance, thus reducing the duration of dopamine in the synapse This was associated with locomotor hyperactivity and significant reductions in striatal DA, 3-MT, as well as 5-HT and 5-HIIA levels. However, these effects were significantly affected by YAC128 genotype (Wilcox et al., 2022). A significant increase in hyperactivity in 100 mg/kg Mn-exposed rats (via gavage) treated with amphetamine, an indirect agonist of dopamine, norepinephrine and serotonin, or with MK-801, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, indicates involvement of other transmitters in Mn-induced hyperactivity (Amos-Kroohs et al., 2015).
It has also been demonstrated that increased spontaneous locomotion in MnCl2 (200 mg/kg intragastrical) and methylcyclopentadienyl manganese tricarbonyl (1–4 mg/kg i.g.)-treated rats was associated with structural alterations in substantia nigra, reduction of TH-positive neurons and microglia activation (Zhu et al., 2022).
The association between chronic postnatal Mn exposure (50 mg Mn/kg/day orally) and ADHD is indirectly supported by the observation that MPH could prevent Mn-induced decrease in striatal DA levels (Beaudin et al., 2015). Inhibition of Mn-induced attentional deficits by MPH was independent of D1, D2, or α2A dopamine receptor signaling. However, D2R and D1R signaling were responsible for Mn-induced sensorimotor deficits and protective effects of MPH, respectively (Beaudin et al., 2023). In turn, MPH treatment did not improve Mn-induced selective attention alterations while improving impulse control (Beaudin et al., 2017b).
An earlier study by Pappas et al. (1997) demonstrated that perinatal exposure to 10 mg/ml Mn in drinking water induced hyperactivity in rats in association with cerebral cortex thickening, although no significant alterations in TH activity, dopamine, and GFAP levels were observed (Pappas et al., 1997), suggesting involvement of other potential mechanisms in addition to dopamine. Specifically, administration of Mn with drinking water (0.4 g/l) for 6 weeks significantly increased locomotor activity in mice while decreasing grip strength and swimming or climbing time. These effects were associated with an increase in striatal 5-HIAA and nigral GFAP levels, whereas no significant alteration in nigral and striatal TH was observed, suggesting a role for nigral and striatal astrocytes in Mn-induced neurobehavioral deficits (Krishna et al., 2014). It is also notable that co-exposure to Mn (50 mg/kg/day) and MPTP induced hyperactive behavior, while the latter was not observed in astrocyte-specific knockout (KO) mice lacking I kappa B kinase 2, an upstream regulator of NF-κB pathway. Collectively, these data suggest a role for microglia and astrocytes in Mn/MPTP-induced hyperactivity (Hammond et al., 2020).
In contrast, several studies demonstrated a significant decrease in locomotor activity following oral 15 and 59 mg/kg b.w Mn exposure via gavage (Vezer et al., 2007). Severe Mn overload in brain resulting from ZIP14 knockout was associated with reduced locomotor activity in comparison to WT mice (Aydemir et al., 2017). In agreement, a number of studies demonstrated that the effect of intraperitoneal Mn exposure (5, 10 and 20 mg/kg) on locomotor activity appears to be dose-specific, when only high doses of Mn possess inhibitory effect on activity (Cordova et al., 2013). Therefore, similar to ASD, additional carefully designed studies are required to clarify the association between Mn levels and ADHD.
4. Considerations regarding inconsistencies in epidemiological studies
Taken together, existing epidemiological data demonstrated significant contradictions on the associations between Mn body burden in children and neurodevelopmental disorders (Table 1). Several studies demonstrated that blood Mn levels in ASD cases may be most increased or unchanged, while a single study reported a decline in circulating Mn levels. In contrast, analysis of hair revealed a significant decrease in Mn content in children with ASD. Single studies using urine and toenails as biological samples detected higher Mn levels in ASD patients compared with controls. In contrast, data on Mn levels in ADHD patients were more uniform. The majority of studies revealed increased hair, blood, and teeth Mn content in children with ADHD, with only single study demonstrating opposite results. While considering only the studies with clearly estimated source of Mn exposure (proximity to ferromanganese alloy plants, coal ash storage sites, agricultural areas of frequent maneb/mancozeb use), Mn body burden in subjects with ADHD significantly exceeded the levels in controls. Taken together, these findings provide stronger evidence for the association of Mn exposure with ADHD rather than ASD.
Table 1.
A summary of the main findings of research examining the relationship between Mn and its correlation with Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD).
| Country of origin | Number of participants (age) | Design | Potential exposure source | Exposure biomarkers |
Main outcomes | Reference |
|---|---|---|---|---|---|---|
| Morocco | 107 ASD and 120 controls, (aged 3 to 14 years) | Case- Control study | Not specified | Hair | Boys had significantly lower Mn levels (26 %) than girls; ASD and gender were significant predictors of Mn levels | Ouisselsat et al., 2023 |
| Saudi Arabia | 65 ASD 80 control |
Case- Control study | Not specified | Hair | Children diagnosed with ASD revealed reduced levels of essential elements, including Mn, in comparison to children in the control group | Al-Ayadhi, 2005 |
| China | 30 ASD 30 Control |
Case- Control study | Not specified | Blood and urine |
Correlation between whole blood Mn levels and symptoms | Zhao et. 2023 |
| China | 34 ASD 38 Control |
Case- Control study | Not specified | Blood | No significant difference was found in Mn concentrations | Qin et al., 2018 |
| China (Han population) |
92 ASD 91 Control |
Case- Control study | Not specified | Serum | No significant difference | Ma et al., 2022 |
| Russia | 70 ASD 70 Control |
Case- Control study | Not specified | Serum | Significantly higher serum Mn levels only in boys with ASD, no significant in girls. | Skalny et al., 2017 |
| Norway | 397ASD 705 ADHD 1034 control |
Cohort Study | Not specified | Blood | Positive association between the fourth quartile of maternal whole blood Mn levels and increased odds of ASD in children | Skogheim et al., 2021 |
| Poland | 129 ASD 86 Control |
Case- Control study | Not specified | Urine | Threefold higher urinary Mn levels in ASD cases in comparison to controls | Błażewicz et al 2022 |
| Jamaica | 109 ASD 109 Control |
Case- Control study | Diet | Blood | No significant association between Mn levels and ASD | Rahbar et al., 2014 |
| Jamaica | 100 ASD 100 Control |
Case- Control study | Not specified | Blood | Interaction between GSTP1 gene and Mn blood concentration | Rahbar et al., 2015 |
| Pakistan | 30 ASD 30 Control |
Case- Control study | Not specified | Blood | No association between Mn concentration and GSTP1 gene | Rahbar et al., 2021 |
| India | 6 ASD 6 Control |
Case- Control study | Not specified | Teeth | Mn concentrations were three times higher in teeth of ASD children group as compared to the children in the control group | Kaur et al., 2021 |
| Syria | 31 ASD 30 Control |
Case- Control study | Military-related pollution | Blood | Blood Mn levels were lower in the group of ASD when compared with control group | Hawari et al., 2020 |
| Russia | 52 ASD 53 Control |
Case- Control study | Not specified | Hair | Mn levels was significantly reduced in children with ASD when compared with controls | Skalny et al., 2020b |
| Italy | 34 male 14 female |
Cross-sectional study | Not specified | Hair | Mn decreased in patients with ASD compared with age-related reference values | Fiore et al., 2020 |
| USA | 15 male 3 female |
Cross-sectional study | Not specified | Hair | No correlation between Mn levels and ASD | Geier et al., 2012 |
| Denmark | 643,401 children born 1992–2007 for clinical diagnoses of ADHD | Cohort Study | Drinking water | Mn in drinking water |
High levels of Mn in drinking are associated with 51 % and 20 % increase in risk of ADHD-inattentive subtype in females and males | Schullehner et al., 2020 |
| Italy | 645 children (aged 11–14 years) | Cross-sectional study | Soil | Mn in soil and blood |
Mn levels were associated with inattention and ADHD scores in girls | Broberg et al., 2019 |
| USA | 1 child (10-year old) |
Case Report | Drinking water | Blood, urine and hair |
Mn was associated with high whole blood, urinary, and hair Mn levels, along with inattention, poor verbal and visual memory | Woolf et al., 2002 |
| United Arab Emirates | 18 ADHD 74 control |
Case- Control study | Not specified | Blood | Blood Mn levels was associated with a nearly 80 % increase in the odds of ADHD in school-aged children | Yousef et al., 2011 |
| USA | 255 children |
Cross-sectional study | Coal ash storage sites | Nail | Children with detectable nail Mn levels had reduced sustained attention | Sears et al., 2021 |
| Brazil | 74 ADHS 67 control |
Case- Control study | Not specified | Serum | Higher serum Mn concentrations in children with ADHD | Farias et al., 2010 |
| Korea | 890 children (aged 8–11 years) | Cross-sectional study | Not specified | Blood | Blood Mn levels were significantly associated with Child Behavior Checklist (CBCL) total problem score | Hong et al., 2014 |
| Italy | 686 children (ages 11–14) | Cross-sectional study | Ferromanganese alloy plants | Blood | Polymorphisms in Mn transporters are associated to increased blood Mn levels were also associated with higher scores of ADHD-related behavior | Wahlberg et al., 2018 |
| Brazil | 34 boys and 36 girls (aged 7–12 years) |
Cross-sectional study | Ferromanganese alloy plants | Hair | Hair Mn content was associated with total CBCL scores in girls, but not in boys | Menezes- Filho et al. 2014 |
| Canada | 24 boys and 22 girls (6–15 years) | Cross-sectional study | Tap water | Hair | Mn in hair samples was associated with Revised Conners’ Rating Scale for parents (CPRS-R) T-scores | Bouchard et al., 2007 |
| Korea | 40 ADHD 43 Control |
Case- Control study | Not specified | Hair | Hair Mn concentration directly correlated with total Korean ADHD Rating Scale (K-ARS) values | Shin et al., 2015 |
| USA | 27 children | Cross-sectional study | Not specified | Tooth | Mn exposure, was associated with parent and teacher ratings of externalizing and attention problem | Ericson et al., 2007 |
| USA | 248 children (aged 7, 9, and/or 10.5 years) |
Cohort study | Area of maneb/mancozeb use | Tooth | Dentine Mn content and adverse behavioral outcome including hyperactivity problems | Mora et al., 2015 |
| Sweden | 166 ADHD 166 Control |
Case- Control study | Not specified | Umbilical cord serum |
No significant association between umbilical cord blood Mn levels and ADHD in children | Ode et al.,2015 |
| Syria | 29 ADHD 30 Control |
Case- Control study | Not specified | Blood | Children born with ADHD had blood Mn levels nearly 10 % lower than in the neurotypical controls | Hawari et al., 2020 |
| Russia | 68 ADHD 68 Control |
Case- Control study | Not specified | Serum | No significant alterations in serum Mn levels in children with ADHD | Skalny et al., 2020a |
| Russia | 52 ADHD 53 Control |
Case- Control study | Not specified | Hair | Significant reduction in hair Mn levels in children with ADHD | Skalny et al., 2020b |
| Italy | 645 children (aged 11–14 years) | Cross-sectional study | Not specified | Tooth | Deciduous tooth Mn content was associated with lower teacher and parent −reported inattention scores | Broberg et. al 2019 |
| Norway | 705 ADHD 1034 controls |
Cohort Study | Not specified | Blood | Association between maternal blood Mn levels at week 17 of gestation and the odds of ADHD | Skogheim et al.,2021 |
Conflicting findings may be due to differences in design and existence of confounding factors. For example, small size of groups, possible exposure to toxic metals and other xenobiotics, nutritional status, and different matrices employed to analyze Mn levels (e.g., hair, blood, serum, teeth).
Mn levels in blood serum/plasma and red blood cells appeared to be sensitive markers of Mn exposure (Zheng et al., 2011), correlating with ambient Mn levels (Hoet et al., 2012). However, the most recent systematic analysis demonstrate that blood Mn cannot be considered a useful biomarker of non-occupational Mn exposure (Shilnikova et al., 2022). In addition, homeostatic control of circulating Mn levels may also limit the utility of blood as a sample for assessment of environmental Mn exposure (Chojnacka et al., 2010).
Although urinary Mn may reflect Mn exposure to certain extent, one should consider that up to 95 % of Mn is excreted via bile with feces (Aschner et al., 2007), and urinary Mn level cannot be a sensitive biomarker (Hoet and Roels, 2014).
The second most frequently used sample for investigation of the relationship between Mn exposure and neurodevelopmental disorders is hair. Although it has been demonstrated that hair may be used as a biomarker of environmental Mn exposure in children (Eastman et al., 2013), including Mn intake with drinking water (Ntihabose et al., 2018), certain limitations including the impact of hair dyeing, pigmentation, and lack of systematic data on its correlation with other biomarkers, thus stating that data on hair Mn accumulation should be treated with caution (Shilnikova et al., 2022). Moreover, Skröder et al. (2017) demosntrated that hair Mn did not correlate significantly with red blood cell, urinary, or water metal content in children (Skroder et al., 2017). In contrast, the results of a systematic review and meta-analysis demonstrate that hair is the most reliable biomarker of Mn exposure in children (Liu et al., 2020).
Similarly to hair, toenails were used as a substance for assessment of long-term trace element exposure (Gutierrez-Gonzalez et al., 2019). It is proposed that toenails may be a better biomarker of Mn exposure (Shilnikova et al., 2022), being more sensitive than urinary or blood levels (Laohaudomchok et al., 2011).
Taken together, the existing data demonstrate that all biomarkers have some limitations and the outcome of the studies should be critically evaluated. In turn, further studies assessing multiple biomarkers of Mn exposure in parallel with environmental Mn levels in children with ADHD and ASD are warranted to investigate the potential contribution of Mn exposure at different environmental levels to neurodevelopmental disorders.
5. Mn toxicity and the particular mechanisms of neurodevelopmental disorders
Given that Mn promotes developmental neurotoxicity, various studies have explored the molecular mechanisms of such toxicity in relation to ASD and ADHD. These studies have focused on impaired neurogenesis including neuronal damage, oxidative stress, mitochondrial dysfunction, neuroinflammation, and neurotransmitter alterations (da Silva et al., 2023, Ijomone et al., 2020), which are discussed in detail below.
5.1. Neurogenesis and neuronal damage
Both ASD and ADHD have been shown to be associated with impaired neurogenesis following exposure to various environmental stressors (Kaushik and Zarbalis, 2016, Nunez-Jaramillo et al., 2021). Despite being essential for neuronal health at physiological levels (Horning et al., 2015) high doses of Mn exert significant neurotoxic effects resulting in a decrease in neuronal differentiation and viability. Specifically, Mn overexposure was shown to reduce neurogenesis (Adamson et al., 2018) at least partially due to down-regulation of BDNF signaling (Kikuchihara et al., 2015). Such exposure also resulted in cytoskeletal alterations and impaired neurite structure (Parsons-White and Spitzer, 2018). These effects may be due to Mn-induced alterations in astrocytes’ ability to promote neurogenesis, mediated through redox-dependent mechanisms (Giordano et al., 2009). Finally, undifferentiated neurons were shown to be more sensitive to Mn neurotoxicity (Hernandez et al., 2011), thus supporting the epidemiological and laboratory evidence of adverse neurodevelopmental effects of Mn exposure.
In addition to altered neurogenesis, developmental exposure to Mn significantly increased hyperactivity and aggressive behavior due to induction of apoptosis in developing granule cells and altered neuronal migration (Wang et al., 2012). It has been demonstrated that Mn exposure induces neuronal apoptosis by down-regulation of Bcl-2 expression and increasing proapoptotic p53, Bax, caspase-3 (Nkpaa et al., 2019) and p53 expression (Wan et al., 2014). Mn-induced apoptosis was also shown to be associated with mitofusin 2 down-regulation (Liu et al., 2017b). In addition to apoptosis, Mn exposure was also shown to be associated with neuronal ferroptosis (Zhang et al., 2023a) through repression of amyloid precursor protein and H-Ferritin translation, resulting in increased accumulation of catalytically active neurotoxic iron (Venkataramani et al., 2018).
5.2. Oxidative stress
Mn is crucial for mitochondrial function, as it plays a key role in the mitochondrial-specific SOD enzyme (Zhang et al., 2023b). However, neurons and astrocyte mitochondria are especially susceptible to excessive Mn, which can result in mitochondrial dysfunction through processes such as neuroinflammation and impaired mitochondrial repair (Martins et al., 2023, Morcillo et al., 2021, Smith et al., 2017). Oxidative stress may serve as a crucial connection between mitochondrial dysfunction and ASD. Indeed, reactive oxygen species (ROS) generated from environmental toxicants with pro-oxidant properties may lead to mitochondrial dysfunction (Rossignol and Frye, 2012). In this regard, lymphoblastoid cell lines derived from patients with ASD were more susceptible to oxidative stress and had significant physiological abnormalities in mitochondrial function (Rose et al., 2014). In addition, a positive correlation between mitochondria dysfunction and ASD was also reported (Shoffner et al., 2010).
As observed in ASD, ADHD is also associated with mitochondrial dysfunction. Specifically, a cellular model of ADHD was characterized by a significant reduction in mitochondrial complex V activity and mitochondrial respiration, along with reduction of mitochondrial membrane potential and oxidative stress (Verma et al., 2016) Moreover, in spontaneously hypertensive rats (SHR) used as an in vivo model of ADHD, increased ROS generation was revealed in cortex, striatum, and hippocampus, along with a decrease in prefrontal cortex and hippocampal GPX activity (Leffa et al., 2017). The results of certain clinical studies also demonstrated increased levels of systemic oxidative stress biomarkers (Verlaet et al., 2019). However, other studies have reported lower levels of malondialdehyde and 8-hydroxy-2′-deoxyguanosine (8-OHDG) in ADHD patients (Bulut et al., 2013). Despite these contradictions, an earlier meta-analysis demonstrated a significant association between ADHD and systemic oxidative stress, but not antioxidant status (Joseph et al., 2015).
Oxidative stress, depletion of cellular antioxidant defenses, and formation of toxic metabolites are also potential underlying mechanisms in early-life Mn exposure (Martinez-Finley et al., 2013). Peres et al. reported that low-level exposure to Mn during a critical neurodevelopmental period leads to decreased levels of non-protein thiols and alterations in antioxidant defense system that affects motor coordination and cognitive function in rats (Peres et al., 2015). Although the role of Mn overload in development of oxidative stress was discussed in a number of excellent reviews (Farina et al., 2013), it is noteworthy that Mn promotes mitochondrial H2O2 production in SH-SY5Y cells both in physiologic and toxicologic range (Fernandes et al., 2017). Mn was shown to increase H2O2 production by complex II and Kreb’s cycle with its subsequent emission by mitochondrial permeability transition (Bonke et al., 2016). In addition, Mn interaction with SIRT1 may also lead to alterations in redox homeostasis and mitochondrial dysfunction (Tinkov et al., 2021b). Therefore, Mn effects on mitochondrial redox signaling and oxidative stress (Smith et al., 2017) may, at least in part, be responsible for its role in pathobiology of ASD and ADHD.
5.3. Neuroinflammation
Several studies have proposed that neuroinflammation may be a pivotal factor in the pathological progression of both ASD (Al-Bishri, 2023, Frankovich et al., 2023) and ADHD (Dunn et al., 2019). In a genetic mouse model of ASD, elevated levels of interferon gamma (IFN-γ) and monocyte chemoattractant protein 1 (MCP-1) indicative of neuroinflammation were noted in the hippocampus. Furthermore, increased microglial activity, also reflective of neuroinflammation, was observed in the hippocampus and prefrontal cortex of such mice (Duarte-Campos et al., 2023). The results of a systematic review demonstrated that ADHD is associated with increased systemic levels of proinflammatory cytokines, as well as cytokine gene polymorphisms (Anand et al., 2017). Iba1-immunopositive microglia and TNF-α protein levels are detected in animal models of this disorder (Fang et al., 2023). Given that Mn promotes inflammatory effects in the brain, it is possible to speculate that high levels of Mn contribute to neuroinflammation associated with ASD and ADHD.
Mn also induced neuroinflammation in different brain regions (Sarkar, 2021), including thalamus (Deng et al., 2023) and striatum (Pajarillo et al., 2022). This is due to increases in NLRP3, CASP1, IL-1β, IL-18 (Fang et al., 2021), COX-2 expression and p38 MAPK signaling (Li et al., 2018). Interestingly, treatment with Mn chelator, sodium p-aminosalicylic acid, ameliorated these effects. In addition to direct NLRP3 inflammasome activation, Mn exposure increases exosome-mediated transfer of inflammasome adaptor protein ASC which further promoted NLRP3 activation (Sarkar et al., 2019).
Proinflammatory effect of Mn in brain was shown to be mediated by NF-κB signaling. Specifically, Mn was shown to facilitate ROS production and increase IκB kinase (IKK-β) phosphorylation resulting in nuclear NF-κB p65 translocation and subsequent proinflammatory signaling (Rizor et al., 2021), including inflammasome activation (Zhao et al., 2019). Correspondingly, treatment with sodium p-aminosalicylic acid significantly down-regulated NF-κB mRNA expression leading to reduced TNF-α and IL-1β production by microglia (Li et al., 2021). Microglial activation following Mn exposure was shown to be at least partially mediated by down-regulation of SIRT1 expression resulting NF-κB phosphorylation and STAT3 acetylation (Cong et al., 2021). In addition, the role of SIRT1 down-regulation in Mn-induced neuroinflammation was shown to be associated with inhibition of FOXO3/LC3B-mediated autophagy in microglia cells (Yan et al., 2023).
Mn-induced up-regulation of LRRK2 expression in microglia was shown to be responsible for microglia activation and subsequent neuroinflammation (Chen et al., 2018). Specifically, LRRK2 expression was shown to be essential for up-regulation of TNF-α and IL-1β expression, and NLRP3 inflammasome activation in BV-2 microglia cells (Pajarillo et al., 2023). The role of LRRK2 in Mn-induced neuroinflammation and apoptosis in microglia and macrophages was shown to be at least partially mediated by p38 MAPK activation (Kim et al., 2019).
Generally, glial activation serves as a robust marker of neuroinflammation and is a prominent pathological feature observed in individuals exposed to Mn (Martinez-Hernandez et al., 2023). The activation of glial cells is also significant in enhancing Mn neurotoxicity by triggering the release of ROS and inflammatory mediators, such as proinflammatory cytokines (Harischandra et al., 2019). Mn triggered proinflammatory events in astrocytes by impairing mitochondrial bioenergetics, which led not only to the release of proinflammatory cytokines, but also intensified the inflammatory response caused by aggregated α-synuclein (Sarkar et al., 2018). While all these studies strongly support the neuroinflammatory effects of Mn, further studies are required to delineate the contribution of Mn to neuroinflammation in the context of ASD and ADHD.
5.4. Neurotransmitter metabolism
Recently, a study using a metabolomic approach demonstrated that children with ASD had alteration in metabolic pathways linked to neurotransmitters (Brister et al., 2022). In fact, ASD has been associated with a perturbation of the dopaminergic system (Paval, 2017), such as decreased release of dopamine in the prefrontal cortex and reduced neural response in the nucleus accumbens (Ernst et al., 1997, Scott-Van Zeeland et al., 2010). Interestingly, Mn has frequently been linked to dopaminergic dysfunction, leading to structural, functional, and neurochemical changes in the dopaminergic system. Thus, it is considered an environmental risk factor for ASD (Ijomone et al., 2020). A number of other neurotransmitters, including GABA, glutamate, serotonin (Marotta et al., 2020), and norepinephrine (Beversdorf, 2020) were shown to be involved in ASD pathogenesis. Dysregulation of neurotransmitter metabolism, namely alteration of dopamine and norepinephrine signaling, was also shown to be involved in pathogenesis of ADHD (Mehta et al., 2019). However, a number of studies demonstrated that altered glutamatergic (Elia et al., 2020), serotoninergic (van Rooij et al., 2015), and GABAergic (Edden et al., 2012) neurotransmission may also be involved in ADHD development. Despite limited epidemiological and laboratory evidence on the role of Mn in etiology of ASD or ADHD, established effects of Mn on neurotransmitters implicated in these neurological disorders provide a potential mechanism for its involvement in their etiology (Soares et al., 2020).
Dopamine. Given the role of DA signaling in ADHD and ASD development, the latter may be at least partially mediated by Mn-induced perturbations in dopaminergic homeostasis. Specifically, in a model of SLC30A10-KO-induced neuronal Mn overload a significant reduction in dopamine release was revealed (Taylor et al., 2023). Similar findings were obtained for Slc39a14 (Rodichkin et al., 2021). It has also been demonstrated that Mn significantly reduced neuronal DA uptake through DAT internalization in HEK cells (Roth et al., 2013). Similar findings were obtained in vivo, where exposure to Mn in early postnatal period resulted in reduced DAT expression and dopamine uptake in striatum and nucleus accumbens, and decreased striatal DA efflux (McDougall et al., 2008). A study in Cynomolgus macaques also demonstrated that Mn exposure significantly affected DA release through presynaptic mechanisms (Guilarte et al., 2008). Mn-induced dopaminergic toxicity was shown to be at least partially mediated by striatal axon attractant netrin-1 expression involved in regulation of DA transmission (Wen et al., 2022).
Effects of Mn on dopaminergic transmission were primarily due to its interaction with TH, the rate limiting enzyme in DA synthesis. Early life exposure to Mn was shown to increase TH protein levels and phosphorylation at Ser 40, 31, and 19 at PND14, while at later periods (PND70) a significant decrease in TH protein level along with increased TH phosphorylation at Ser40 and Ser19 was observed (Peres et al., 2016). It has been demonstrated that a significant decrease in dopamine levels and TH and D1 dopamine receptor expression in SNpc following Mn exposure in rats occurred through inhibition of autophagy (Zhang et al., 2013). Inhibition of TH activity following Mn exposure may be at least partially mediated by down-regulation of c-RET transcription and protein ubiquitination (Kumasaka et al., 2017). Chronic Mn exposure was also shown to inhibit TH through up-regulation of PKCδ and PP2A activity (Zhang et al., 2011). Mn-induced damage in dopaminergic SH-SY5Y was shown to be mediated by BNIP3-dependent mitophagy (Huang et al., 2021). In addition to neuronal death, Mn exposure was shown to induce cytoskeletal dysfunction in dopaminergic neurons (Stanwood et al., 2009).
Finally, the role of Mn in dopaminergic dysfunction may be mediated by its ability to induce apoptosis in dopaminergic neurons (Ding et al., 2020, Pajarillo et al., 2020). Dopaminergic neuron damage following Mn exposure was shown to be mediated by microglia activation with subsequent up-regulation of iNOS, TNF-α, and IL-1β gene and protein expression. The key role of microglia was confirmed by the finding that inhibition of its activation ameliorated neuronal damage (Zhao et al., 2009). Thus, a variety of molecular mechanisms may mediate Mn-induced toxicity in dopaminergic neurons.
Glutamate/glutamine. In addition to dysregulation of dopamine signaling in brain, both ASD and ADHD were shown to be at least partially mediated by glutamatergic/GABAergic dysfunction (Purkayastha et al., 2015), where both are also affected by Mn. Specifically, alteration in neurotransmitter release from glutamatergic neurons was shown to precede toxic effects of Mn (Moberly et al., 2012). Correspondingly, Mn exposure was associated with increased extracellular glutamate levels in AF5 rat neural-derived cell line (Crooks et al., 2007). It has been demonstrated that Mn exposure inhibits glutamate uptake through down-regulation of GLT-1 and GLAST transporters (Mutkus et al., 2005). Mn also affects astrocytic GLAST mRNA expression. Thus, it was reported that Mn led to differential regulation of GLAST, taurine transporter (MT), and metallothionein in cultured rat astrocytes. Specifically, exposure to 500 μM MnCl2 decreased mRNA levels of GLAST and MT, but the same concentration of Mn increased tau-T mRNA levels. Furthermore, aspartate uptake was significantly attenuated in Mn exposed astrocytes, whereas, taurine uptake remained unchanged (Erikson and Aschner, 2002).
Mn was also shown to inhibit Gln transporters including SNAT3, SNAT2, LAT2, and ASCT2 in primary rat astrocytes (Sidoryk-Wegrzynowicz et al., 2009). Such inhibition was shown to involve Mn-induced PKCδ signaling (Sidoryk-Wegrzynowicz et al., 2011) with subsequent SNAT3 ubiquitination and protein degradation (Sidoryk-Wegrzynowicz et al., 2010). In addition, Mn-induced inhibition of excitatory amino acid transporter (EAAT) 1 expression was shown to be mediated by increased binding of YY1 to the EAAT1 promoter (Karki et al., 2015).
Mn also inhibited glutamine synthetase activity both in vitro (Deng et al., 2012) and in vivo (Burton et al., 2009). Prevention of Mn-induced alterations in GS, phosphate-activated glutaminase, GLAST, and GLT1 by PAS-Na treatment also supports the role of Mn in dysregulation of glutamatergic neurotransmission (Li et al., 2020).
Mn exposure down-regulated striatal NMDA receptor subunits NR1, NR2B (Xu et al., 2010), and NR2A (Xu et al., 2009). In addition, Mn impaired striatal interaction between DR1 and NMDAR leading to memory and learning deficits (Song et al., 2016). Thus, Mn can interact with glutamatergic transmission at several molecular sites and levels.
GABA. Mn exposure was shown to increase striatal and to a lesser extent cortical GABA level (Gwiazda et al., 2002). An increase in GABA levels in thalamus and adjacent basal ganglia was also observed in Mn-exposed smelters (Dydak et al., 2011). Indeed, it has been suggested that Mn-induced locomotor dysfunction might be associated with partial activation of substantia nigra GABAergic neurons rather than alterations in dopaminergic system (Yang et al., 2011). An increase in extracellular GABA levels in striatum following Mn treatment was mediated by GAT inhibition (Fordahl et al., 2010), although this effect was region-specific (Anderson et al., 2008). Mn exposure also reduced GAD expression in basal ganglia (Stanwood et al., 2009). Finally, Mn toxicity was also shown to disrupt GABAAR mRNA and protein expression (Ou et al., 2012). Thus, it may be concluded that Mn affects various molecular stages of GABA in region-specific manner.
Serotonin (5-HT). Rats intraperitoneally exposed to Mn had reduced striatal 5-HT content (Wu et al., 2018), in agreement with the results of the earlier study by Kimura et al. (1978) (Kimura et al., 1978). Inhalation of Mn increased hippocampal but not striatal 5-HT content, while this increase was abolished by curcumin treatment (Schmitz et al., 2014). Chronic manganism was associated with reduction in 5-HT content in frontal cortex and hippocampus (Huang et al., 2015). In addition to a decrease in brain 5-HT levels, Mn exposure was shown to reduce 5-HIAA/5-HT ratio in prefrontal cortex and striatum while increasing this ratio in hippocampus (Blecharz-Klin et al., 2012). A significant decrease in 5-HT, but not 5-HIAA, was also observed in frontal cortex of Mn-exposed rats (Bouabid et al., 2014). A decrease in neuronal 5-HT levels is more reflective of exposure to Mn(III) rather than Mn(II) (Reaney and Smith, 2005). Serotonin transporter expression was also found to be down-regulated by Mn exposure in blue mussel Mytilus edulis (Fraser et al., 2018).
In contrast, several studies demonstrated a significant increase in brain 5-HT levels following Mn exposure (Vorhees et al., 2014). In this regard, Mn effect appeared to be related to its chemical formula, where MnO2 increased striatal 5-HT levels, whereas MnCl2 led to a decrease in 5-HT levels in this area (Nielsen et al., 2017).
Norepinephrine (NE). An earlier study demonstrated Mn-induced increase in NE turnover in rat brain stem and hypothalamus (Autissier et al., 1982). Mn exposure promotes catecholamine oxidation with the formation of adrenochrome that significantly reduces cell viability through repression of gene expression (Ueda et al., 2020). Mn exposure was shown to reduce extracellular NE levels in caudate putamen, while decreasing NE uptake in the locus coeruleus. In addition, Mn treatment down-regulated NE transporter and α2 adrenergic receptor mRNA and protein expression (Anderson et al., 2009). Correspondingly, reduction of locomotor activity in adult SD rats following Mn exposure was associated with reduced NE content in frontal cortex (Bouabid et al., 2014). It is noteworthy that noradrenergic system has been implicated in both ASD and ADHD. Thus, cognitive processes, such as arousal, working memory, and response inhibition, all of which are typically affected in ADHD are regulated at least to some extent by NE. Moreover, the norepinephrine transporter (NET) is an important target for frequently prescribed medication in ADHD (Vanicek et al., 2014; Ulke et al. 2019). In regard to ASD, which is frequently associated with anxiety and hyperarousal, a recent review updates the role of NE in this disorder and suggests potential targeting of this neurotransmitter. This is due to the fact that a number of functional indices of the sympathetic/parasympathetic balance are altered in individuals with ASD, and that neuropsychopharmacological effects of α2 agonists and β-adrenergic antagonists make agents targeting these receptors of particular interest (Beversdorf, 2020).
5.5. Epigenetics
Epigenetic mechanisms were shown to be involved in the development of neurodevelopmental disorders, including ADHD (Cecil and Nigg, 2022) and ASD (Loke et al., 2015). Indeed, laboratory studies demonstrated have corroborated the ability of Mn to induce epigenetic changes in the brain. Specifically, promotor hypermethylation and down-regulation of Mid1, Atp1a3, Nr2f1, and Pvalb transcription were all shown to be involved in Mn-induced alterations in neurogenesis (Wang et al., 2013). Prenatal Mn exposure also led to altered DNA methylation in the placenta, affecting neurodevelopment (Maccani et al., 2015).
Mn had a significant impact on histone modifications modulating neuronal gene expression. Specifically, Mn reduced H3K27 acetylation at Mn-SOD promoter resulting in inhibition of its expression and neuronal oxidative stress (Yang et al., 2022). Moreover, Mn exposure significant increased H3K9, H3K14, H3K18, H3K56 and H3K79 acetylation, while down-regulating H3K27, H3K36, H4K91, H4K79, H4K31, H2BK16 and H2BK20 in rat striatum, at least partially contributing to antioxidant enzyme dysregulation and oxidative stress (Ao et al., 2024). In addition, modulation of H3K18 acetylation was shown to mediate inhibition of glutathione transferase omega 1 (GSTO1) gene expression, contributing to hippocampal oxidative stress (Chen et al., 2024b). Mn-induced alterations in histone acetylation were shown to be dependent on up-regulation of HDAC3 and HDAC4 expression, as well as down-regulation of HAT in PC12 cells and SHSY5Y cells (Guo et al., 2018).
Finally, several neurotoxic effects of Mn were shown to be mediated by modulation of micro RNA expression. Specifically, down-regulation of miR-125b-2-3p, miR-138-5p, and miR-155 was shown to mediate Mn-induced ferroptosis (Chen et al., 2024a), autophagic dysregulation (Ma et al., 2019), and neuroinflammation (Grogg et al., 2016) in neuronal cells. These studies establish the propensity of Mn to interfere with a host of epigenetic mechanisms known to be involved in pathogenesis of neurodevelopmental disorders.
5.6. Other potential mechanisms
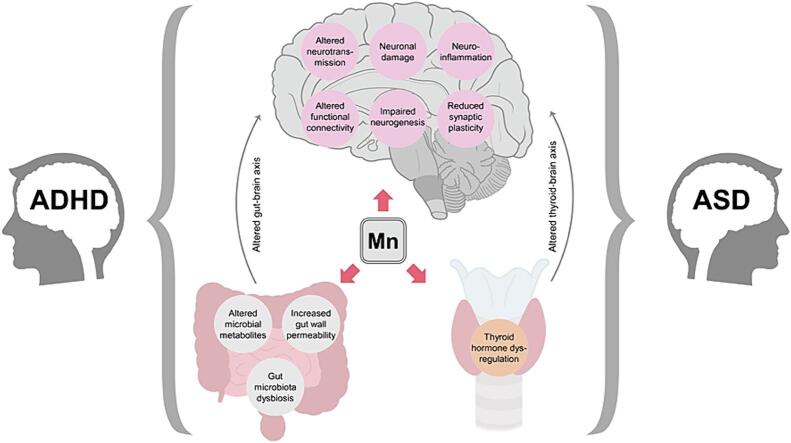
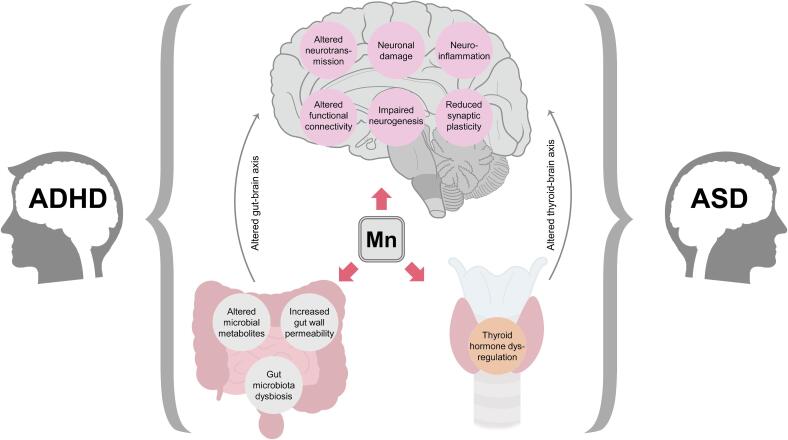
In addition to oxidative stress, impaired neurogenesis, neuronal damage, neurotransmitter dysregulation, and neuroinflammation, pathogenesis of neurodevelopmental disorders may be significantly impacted by dysregulation of thyroid hormone signaling, which may at least partially mediate the effect of endocrine-disrupting chemicals in altered neurodevelopment (Salazar et al., 2021) (Fig. 1). Mn ions can disrupt the binding, transport, and activity of thyroid hormones at the tissue level both directly and indirectly. One potential mechanism by which Mn directly impairs thyroid hormones involves the dysfunction of deiodinase enzymes responsible for the activation and deactivation of thyroid hormones, crucial in metabolic, signaling, and regulatory processes related to the formation and conversion of thyroid hormones (Ou et al., 2019). Another mechanism involves the binding of Mn ions to dopaminergic receptors. Dopamine suppresses TSH secretion, influencing the thyroid hormone levels, and damage to dopaminergic receptors may lead to non-developmental deficits (Soldin and Aschner, 2007). Moreover, thyroid hormone significantly contributes to neural development, and early-life hypothyroidism may result in impairments of cognition, learning, attention, and intellectual capabilities (Bernal, 2000, Obsekov et al., 2023). A study using Slc30a10 single knockout mice which have Mn accumulation found that they develop hypothyroidism due to Mn-induced inhibition of thyroxine production (Liu et al., 2017a). In agreement, a case-control study involving adolescents, the presence of thyroid dysfunction and exposure to Mn were linked to an elevated risk of ASD and an augmentation in the severity of ASD symptoms in comparison to non-ASD controls (Blazewicz et al., 2022). Despite the lack of direct evidence supporting the role of Mn in thyroid hormone dysregulation in ADHD patients, the results of a recent study demonstrate that maternal hypothyroidism is associated with increased hazard of ADHD in the offspring (Rotem et al., 2022).
Fig. 1.
Proposed mechanisms linking Mn overexposure with ASD and ADHD. Being a neurotoxic metal, Mn overload was shown to induce neuronal damage, neuroinflammation, impaired neurogenesis, dysregulation of neurotransmitter metabolism with subsequent alterations in neuronal signal transduction, as well as reduced synaptic plasticity and altered functional connectivity, all known to be involved in pathogenesis of neurodevelopmental disorders. In addition to its direct adverse effects on neurons, it is proposed that Mn-induced dysregulation of gut microbiota and thyroid hormone production may result in alterations of gut-brain and thyroid-brain axes which have also been implicated in the pathogenesis of both ASD and ADHD.
Gut microbiota (GM) may have a significant impact on brain functioning through the gut-brain axis, and thus play a key role in development of neuropsychiatric diseases (Ghaisas et al., 2016); Tizabi et al. 2023). Indeed, children with ASD were characterized by altered GM (Iglesias-Vazquez et al., 2020) as well as microbial metabolites (Kang et al., 2018), supporting the role of gut microbiota in ASD pathogenesis (Li et al., 2017) Tizabi et al. 2023).
Similarly, significant alterations of GM composition were revealed in ADHD (Wang et al., 2022). These GM alterations may contribute to the ADHD pathology through modulation of dopaminergic neurotransmission, neuroinflammation, and altered short-chain fatty acid levels (Shirvani-Rad et al., 2022). Although direct evidence on the impact of Mn on GM in ASD and ADHD is still lacking, previous findings demonstrate that modulation of GM may mediate adverse effects of Mn on neurotransmitter metabolism and neuroinflammation (Tinkov et al., 2021a). Studies demonstrated that Mn-induced changes of gut microbiota composition may correlate significantly with behavioral alterations in mice (Zhu et al., 2020). In turn, healthy fecal microbiota transplantation was shown to improve neurotoxic effects of Mn overexposure (Liu et al., 2022, Wang et al., 2020). Taken together, these findings provide indirect evidence for the potential interplay between Mn toxicity and gut microbiota in pathogenesis of neurodevelopmental disorders. Nonetheless, further detailed studies are necessary.
6. Conclusions
Taken together, existing epidemiological data on the association between Mn exposure biomarkers and ASD and ADHD are rather contradictory. Despite findings on increased Mn body burden in patients with ASD, a growing body of evidence purports an inverse association between systemic Mn levels and ASD. In contrast, most findings attest to higher systemic Mn levels in ADHD patients, as well as a significant association between Mn biomarkers and severity of behavioral deficits in ADHD. However, in several studies, a significant decrease in Mn body burden in ADHD was observed. The results of clinical and epidemiological studies corroborate laboratory data, consistent with behavioral patterns characteristic of ADHD (hyperactivity, attention deficits, impulsivity) in Mn-exposed animals. These studies also highlight potential mechanisms linking Mn exposure to ADHD-specific behavioral deficits, including alterations in dopaminergic neurotransmission and to a lesser extent dysregulation of other neurotransmitters, as well as glial activation and neuroinflammation. Despite the lack of direct evidence, existing data demonstrate that Mn overexposure can perturb mechanisms inherent to neurodevelopmental disorders, including altered neurogenesis and neuronal damage, mitochondrial dysfunction and oxidative stress, neuroinflammation, alterations in dopaminergic, glutamatergic, serotoninergic, GABAergic, and adrenergic neurotransmission, as well as thyroid hormone and gut-brain axis. In contrast to laboratory data demonstrating the potential influence of Mn toxicity on pathways associated with ASD and ADHD, contradictions in the epidemiological studies’ outcomes likely reflect differences in environmental levels of Mn in various cohorts, as well as genetic heterogeneity of the examined subjects, as clearly demonstrated for polymorphisms of genes involved in detoxification. Moreover, several studies revealed a slight but significant U-shaped association between Mn exposure and ADHD risk, that may be indicative of the potential role of both Mn deficiency and excess in neurodevelopmental disorders. Therefore, further detailed studies are required to evaluate the association between environmental Mn exposure and/or Mn body burden and neurodevelopmental disorders in a wide range of concentrations to estimate the potential dose-dependent effects, as well as environmental and genetic factors affecting this association. Taken together, one should consider the potential risks of adverse neurodevelopment upon overexposure to Mn, especially in view of the convincing laboratory data on Mn neurotoxicity.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This paper was supported by the Peoples' Friendship University of Russia (RUDN University) Strategic Academic Leadership Program (award no. 202717-0-000 «Development of a scientifically based methodology for the ecological adaptation of foreign students to the new environmental conditions»). ABB and MA were supported in part by a grant from the National Institute of Environmental Health Sciences (NIEHS) R01ES10563.
Data availability
No data was used for the research described in the article.
References
- Adamson S.X., Shen X., Jiang W., Lai V., Wang X., Shannahan J.H., Cannon J.R., Chen J., Zheng W. Subchronic Manganese Exposure Impairs Neurogenesis in the Adult Rat Hippocampus. Toxicol. Sci. 2018;163:592–608. doi: 10.1093/toxsci/kfy062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaei M., Janjani H., Yousefian F., Jamal A., Yunesian M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ. Res. 2019;173:135–156. doi: 10.1016/j.envres.2019.03.030. [DOI] [PubMed] [Google Scholar]
- Al-Ayadhi L.Y. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosciences (Riyadh) 2005;10:213–218. [PubMed] [Google Scholar]
- Al-Bishri W.M. Glucose transporter 1 deficiency, AMP-activated protein kinase activation and immune dysregulation in autism spectrum disorder: Novel biomarker sources for clinical diagnosis. Saudi J Biol Sci. 2023;30 doi: 10.1016/j.sjbs.2023.103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs R.M., Bloor C.P., Qureshi M.A., Vorhees C.V., Williams M.T. Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, sucrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluramine, amphetamine, and MK-801. Toxicol. Rep. 2015;2:1046–1056. doi: 10.1016/j.toxrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand D., Colpo G.D., Zeni G., Zeni C.P., Teixeira A.L. Attention-deficit/hyperactivity disorder and inflammation: what does current knowledge tell us? A systematic review. Front Psychiatry. 2017;8:228. doi: 10.3389/fpsyt.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.G., Fordahl S.C., Cooney P.T., Weaver T.L., Colyer C.L., Erikson K.M. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29:1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.G., Fordahl S.C., Cooney P.T., Weaver T.L., Colyer C.L., Erikson K.M. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res. 2009;1281:1–14. doi: 10.1016/j.brainres.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao C., Tang S., Yang Y., Liu Y., Zhao H., Ban J., Li J. Identification of histone acetylation modification sites in the striatum of subchronically manganese-exposed rats. Epigenomics. 2024;16:5–21. doi: 10.2217/epi-2023-0364. [DOI] [PubMed] [Google Scholar]
- Arora M., Reichenberg A., Willfors C., Austin C., Gennings C., Berggren S., Lichtenstein P., Anckarsater H., Tammimies K., Bolte S. Fetal and postnatal metal dysregulation in autism. Nat. Commun. 2017;8:15493. doi: 10.1038/ncomms15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M., Guilarte T.R., Schneider J.S., Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autissier N., Rochette L., Dumas P., Beley A., Loireau A., Bralet J. Dopamine and norepinephrine turnover in various regions of the rat brain after chronic manganese chloride administration. Toxicology. 1982;24:175–182. doi: 10.1016/0300-483x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- Avila D.S., Puntel R.L., Aschner M. Manganese in health and disease. Met. Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir T.B., Kim M.H., Kim J., Colon-Perez L.M., Banan G., Mareci T.H., Febo M., Cousins R.J. Metal Transporter Zip14 (Slc39a14) Deletion in Mice Increases Manganese Deposition and Produces Neurotoxic Signatures and Diminished Motor Activity. J. Neurosci. 2017;37:5996–6006. doi: 10.1523/JNEUROSCI.0285-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj J., Flieger W., Flieger M., Forma A., Sitarz E., Skorzynska-Dziduszko K., Grochowski C., Maciejewski R., Karakula-Juchnowicz H. Autism spectrum disorder: Trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci. Biobehav. Rev. 2021;129:117–132. doi: 10.1016/j.neubiorev.2021.07.029. [DOI] [PubMed] [Google Scholar]
- Baly D.L., Curry D.L., Keen C.L., Hurley L.S. Effect of manganese deficiency on insulin secretion and carbohydrate homeostasis in rats. J. Nutr. 1984;114:1438–1446. doi: 10.1093/jn/114.8.1438. [DOI] [PubMed] [Google Scholar]
- Beaudin, S.A., Howard, S., Santiago, N., Strupp, B.J. and Smith, D.R. 2023. Methylphenidate alleviates cognitive dysfunction from early Mn exposure: Role of catecholaminergic receptors. bioRxiv. [DOI] [PubMed]
- Beaudin S.A., Strupp B.J., Lasley S.M., Fornal C.A., Mandal S., Smith D.R. Oral methylphenidate alleviates the fine motor dysfunction caused by chronic postnatal manganese exposure in adult rats. Toxicol. Sci. 2015;144:318–327. doi: 10.1093/toxsci/kfv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin S.A., Strupp B.J., Strawderman M., Smith D.R. Early Postnatal Manganese Exposure Causes Lasting Impairment of Selective and Focused Attention and Arousal Regulation in Adult Rats. Environ. Health Perspect. 2017;125:230–237. doi: 10.1289/EHP258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin S.A., Strupp B.J., Uribe W., Ysais L., Strawderman M., Smith D.R. Methylphenidate alleviates manganese-induced impulsivity but not distractibility. Neurotoxicol. Teratol. 2017;61:17–28. doi: 10.1016/j.ntt.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal, J. 2000. Thyroid Hormones in Brain Development and Function. In: K.R. Feingold, B. Anawalt, M.R. Blackman, A. Boyce, G. Chrousos, E. Corpas, W.W. de Herder, K. Dhatariya, K. Dungan, J. Hofland, S. Kalra, G. Kaltsas, N. Kapoor, C. Koch, P. Kopp, M. Korbonits, C.S. Kovacs, W. Kuohung, B. Laferrere, M. Levy, E.A. McGee, R. McLachlan, M. New, J. Purnell, R. Sahay, A.S. Shah, F. Singer, M.A. Sperling, C.A. Stratakis, D.L. Trence and D.P. Wilson (Eds), Endotext, South Dartmouth (MA).
- Beversdorf D.Q. The Role of the Noradrenergic System in Autism Spectrum Disorders. Implications for Treatment. Semin Pediatr Neurol. 2020;35 doi: 10.1016/j.spen.2020.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazewicz A., Grywalska E., Macek P., Mertowska P., Mertowski S., Wojnicka J., Durante N., Makarewicz A. Research into the Association of Cadmium and Manganese Excretion with Thyroid Function and Behavioral Areas in Adolescents with Autism Spectrum Disorders. J. Clin. Med. 2022:11. doi: 10.3390/jcm11030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecharz-Klin K., Piechal A., Joniec-Maciejak I., Pyrzanowska J., Widy-Tyszkiewicz E. Effect of intranasal manganese administration on neurotransmission and spatial learning in rats. Toxicol. Appl. Pharmacol. 2012;265:1–9. doi: 10.1016/j.taap.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Bonilla E. Chronic manganese intake induces changes in the motor activity of rats. Exp. Neurol. 1984;84:696–700. doi: 10.1016/0014-4886(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Bonke E., Siebels I., Zwicker K., Drose S. Manganese ions enhance mitochondrial H(2)O(2) emission from Krebs cycle oxidoreductases by inducing permeability transition. Free Radic. Biol. Med. 2016;99:43–53. doi: 10.1016/j.freeradbiomed.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Bouabid S., Delaville C., De Deurwaerdere P., Lakhdar-Ghazal N., Benazzouz A. Manganese-induced atypical parkinsonism is associated with altered Basal Ganglia activity and changes in tissue levels of monoamines in the rat. PLoS One. 2014;9:e98952. doi: 10.1371/journal.pone.0098952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M., Laforest F., Vandelac L., Bellinger D., Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brister D., Rose S., Delhey L., Tippett M., Jin Y., Gu H., Frye R.E. Metabolomic Signatures of Autism Spectrum Disorder. J Pers Med. 2022;12 doi: 10.3390/jpm12101727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg K., Taj T., Guazzetti S., Peli M., Cagna G., Pineda D., Placidi D., Wright R.O., Smith D.R., Lucchini R.G., Wahlberg K. Manganese transporter genetics and sex modify the association between environmental manganese exposure and neurobehavioral outcomes in children. Environ. Int. 2019;130 doi: 10.1016/j.envint.2019.104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut M., Selek S., Bez Y., Cemal Kaya M., Gunes M., Karababa F., Celik H., Savas H.A. Lipid peroxidation markers in adult attention deficit hyperactivity disorder: new findings for oxidative stress. Psychiatry Res. 2013;209:638–642. doi: 10.1016/j.psychres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Burton N.C., Schneider J.S., Syversen T., Guilarte T.R. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol. Sci. 2009;111:131–139. doi: 10.1093/toxsci/kfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C.F., Menezes-Filho J.A., de Matos V.P., Bessa J.R., Coelho-Santos J., Viana G.F., Argollo N., Abreu N. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2014;45:301–308. doi: 10.1016/j.neuro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Cecil C.A.M., Nigg J.T. Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential. Mol. Diagn. Ther. 2022;26:581–606. doi: 10.1007/s40291-022-00609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Ao C., Liu Y., Yang Y., Liu Y., Ming Q., Li C., Zhao H., Ban J., Li J. Manganese induces oxidative damage in the hippocampus by regulating the expression of oxidative stress-related genes via modulation of H3K18 acetylation. Environ. Toxicol. 2024;39:2240–2253. doi: 10.1002/tox.24102. [DOI] [PubMed] [Google Scholar]
- Chen J., Su P., Luo W., Chen J. Role of LRRK2 in manganese-induced neuroinflammation and microglial autophagy. Biochem. Biophys. Res. Commun. 2018;498:171–177. doi: 10.1016/j.bbrc.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Chen H., Wu J., Zhu X., Ma Y., Li Z., Lu L., Aschner M., Su P., Luo W. Manganese-induced miR-125b-2-3p promotes anxiety-like behavior via TFR1-mediated ferroptosis. Environ. Pollut. 2024;344 doi: 10.1016/j.envpol.2023.123255. [DOI] [PubMed] [Google Scholar]
- Chojnacka K., Zielinska A., Gorecka H., Dobrzanski Z., Gorecki H. Reference values for hair minerals of Polish students. Environ. Toxicol. Pharmacol. 2010;29:314–319. doi: 10.1016/j.etap.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Cong L., Lei M.Y., Liu Z.Q., Liu Z.F., Ma Z., Liu K., Li J., Deng Y., Liu W., Xu B. Resveratrol attenuates manganese-induced oxidative stress and neuroinflammation through SIRT1 signaling in mice. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112283. [DOI] [PubMed] [Google Scholar]
- Conley T.E., Beaudin S.A., Lasley S.M., Fornal C.A., Hartman J., Uribe W., Khan T., Strupp B.J., Smith D.R. Early postnatal manganese exposure causes arousal dysregulation and lasting hypofunctioning of the prefrontal cortex catecholaminergic systems. J. Neurochem. 2020;153:631–649. doi: 10.1111/jnc.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova F.M., Aguiar A.S., Jr., Peres T.V., Lopes M.W., Goncalves F.M., Pedro D.Z., Lopes S.C., Pilati C., Prediger R.D., Farina M., Erikson K.M., Aschner M., Leal R.B. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch. Toxicol. 2013;87:1231–1244. doi: 10.1007/s00204-013-1017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks D.R., Welch N., Smith D.R. Low-level manganese exposure alters glutamate metabolism in GABAergic AF5 cells. Neurotoxicology. 2007;28:548–554. doi: 10.1016/j.neuro.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J.T., Hood A.N., Chen Y., Cobb G.P., Wallace D.R. Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: an animal model of autism. Behav. Brain Res. 2010;213:42–49. doi: 10.1016/j.bbr.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva B.S., Grevet E.H., Silva L.C.F., Ramos J.K.N., Rovaris D.L., Bau C.H.D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discov Ment Health. 2023;3:2. doi: 10.1007/s44192-022-00030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhey, L.M., Nur Kilinc, E., Yin, L., Slattery, J.C., Tippett, M.L., Rose, S., Bennuri, S.C., Kahler, S.G., Damle, S., Legido, A., Goldenthal, M.J. and Frye, R.E. 2017. The Effect of Mitochondrial Supplements on Mitochondrial Activity in Children with Autism Spectrum Disorder. J Clin Med 6. [DOI] [PMC free article] [PubMed]
- Deng Y., Xu Z., Xu B., Xu D., Tian Y., Feng W. The protective effects of riluzole on manganese-induced disruption of glutamate transporters and glutamine synthetase in the cultured astrocytes. Biol. Trace Elem. Res. 2012;148:242–249. doi: 10.1007/s12011-012-9365-1. [DOI] [PubMed] [Google Scholar]
- Deng Y., Peng D., Yang C., Zhao L., Li J., Lu L., Zhu X., Li S., Aschner M., Jiang Y. Preventive treatment with sodium para-aminosalicylic acid inhibits manganese-induced apoptosis and inflammation via the MAPK pathway in rat thalamus. Drug Chem. Toxicol. 2023;46:59–68. doi: 10.1080/01480545.2021.2008127. [DOI] [PubMed] [Google Scholar]
- Ding H., Wang F., Su L., Zhao L., Hu B., Zheng W., Yao S., Li Y. Involvement of MEK5/ERK5 signaling pathway in manganese-induced cell injury in dopaminergic MN9D cells. J. Trace Elem. Med Biol. 2020;61 doi: 10.1016/j.jtemb.2020.126546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Campos J.F., Vazquez-Moreno C.N., Martinez-Marcial M., Chavarria A., Ramirez-Carreto R.J., Velasco Velazquez M.A., De La Fuente-Granada M., Gonzalez-Arenas A. Changes in neuroinflammatory markers and microglial density in the hippocampus and prefrontal cortex of the C58/J mouse model of autism. Eur. J. Neurosci. 2023 doi: 10.1111/ejn.16204. [DOI] [PubMed] [Google Scholar]
- Dunn G.A., Nigg J.T., Sullivan E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019;182:22–34. doi: 10.1016/j.pbb.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydak U., Jiang Y.M., Long L.L., Zhu H., Chen J., Li W.M., Edden R.A., Hu S., Fu X., Long Z., Mo X.A., Meier D., Harezlak J., Aschner M., Murdoch J.B., Zheng W. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman R.R., Jursa T.P., Benedetti C., Lucchini R.G., Smith D.R. Hair as a biomarker of environmental manganese exposure. Environ. Sci. Tech. 2013;47:1629–1637. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A., Crocetti D., Zhu H., Gilbert D.L., Mostofsky S.H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J.E., Crinella F.M., Clarke-Stewart K.A., Allhusen V.D., Chan T., Robertson R.T. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007;29:181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Erikson K., Aschner M. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology. 2002;23:595–602. doi: 10.1016/s0161-813x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Erikson K.M., Aschner M. Manganese: Its Role in Disease and Health. Met Ions. Life Sci. 2019;19 doi: 10.1515/9783110527872-016. [DOI] [PubMed] [Google Scholar]
- Ernst M., Zametkin A.J., Matochik J.A., Pascualvaca D., Cohen R.M. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350:638. doi: 10.1016/s0140-6736(05)63326-0. [DOI] [PubMed] [Google Scholar]
- Fang Z., Shen G., Amin N., Lou C., Wang C., Fang M. Effects of Neuroinflammation and Autophagy on the Structure of the Blood-Brain Barrier in ADHD Model. Neuroscience. 2023;530:17–25. doi: 10.1016/j.neuroscience.2023.08.025. [DOI] [PubMed] [Google Scholar]
- Farias A.C., Cunha A., Benko C.R., McCracken J.T., Costa M.T., Farias L.G., Cordeiro M.L. Manganese in children with attention-deficit/hyperactivity disorder: relationship with methylphenidate exposure. J. Child Adolesc. Psychopharmacol. 2010;20:113–118. doi: 10.1089/cap.2009.0073. [DOI] [PubMed] [Google Scholar]
- Farina M., Avila D.S., da Rocha J.B., Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem. Int. 2013;62:575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Hao L., Bijli K.M., Chandler J.D., Orr M., Hu X., Jones D.P., Go Y.M. From the Cover: Manganese Stimulates Mitochondrial H2O2 Production in SH-SY5Y Human Neuroblastoma Cells Over Physiologic as well as Toxicologic Range. Toxicol. Sci. 2017;155:213–223. doi: 10.1093/toxsci/kfw196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M., Barone R., Copat C., Grasso A., Cristaldi A., Rizzo R., Ferrante M. Metal and essential element levels in hair and association with autism severity. J. Trace Elem. Med Biol. 2020;57 doi: 10.1016/j.jtemb.2019.126409. [DOI] [PubMed] [Google Scholar]
- Fordahl S.C., Anderson J.G., Cooney P.T., Weaver T.L., Colyer C.L., Erikson K.M. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. Neurotoxicology. 2010;31:639–646. doi: 10.1016/j.neuro.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankovich J., Nanda H., Mellins E.D., Jyonouchi H., Boles R.G., Walker S.J., Gaitanis J., Frye R.E. Synchrony 2022: The Role of Neuroinflammation in Behavioral Exacerbations in Autism Spectrum Disorder. J Pers Med. 2023;13 doi: 10.3390/jpm13071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M., Fortier M., Foucher D., Roumier P.H., Brousseau P., Fournier M., Surette C., Vaillancourt C. Exposure to low environmental concentrations of manganese, lead, and cadmium alters the serotonin system of blue mussels. Environ. Toxicol. Chem. 2018;37:192–200. doi: 10.1002/etc.3942. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Cakir J., Rose S., Delhey L., Bennuri S.C., Tippett M., Palmer R.F., Austin C., Curtin P., Arora M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry. 2020;10:223. doi: 10.1038/s41398-020-00905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier D.A., Kern J.K., King P.G., Sykes L.K., Geier M.R. Hair toxic metal concentrations and autism spectrum disorder severity in young children. Int. J. Environ. Res. Public Health. 2012;9:4486–4497. doi: 10.3390/ijerph9124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaisas S., Maher J., Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., Pizzurro D., VanDeMark K., Guizzetti M., Costa L.G. Manganese inhibits the ability of astrocytes to promote neuronal differentiation. Toxicol. Appl. Pharmacol. 2009;240:226–235. doi: 10.1016/j.taap.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Grogg M.W., Braydich-Stolle L.K., Maurer-Gardner E.I., Hill N.T., Sakaram S., Kadakia M.P., Hussain S.M. Modulation of miRNA-155 alters manganese nanoparticle-induced inflammatory response. Toxicol Res (camb) 2016;5:1733–1743. doi: 10.1039/c6tx00208k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T.R., Burton N.C., McGlothan J.L., Verina T., Zhou Y., Alexander M., Pham L., Griswold M., Wong D.F., Syversen T., Schneider J.S. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J. Neurochem. 2008;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Zhang Z., Wang Q., Zhang J., Wang L., Zhang Q., Li H., Wu S. Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage. Neurotoxicology. 2018;65:255–263. doi: 10.1016/j.neuro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Gonzalez E., Garcia-Esquinas E., de Larrea-Baz N.F., Salcedo-Bellido I., Navas-Acien A., Lope V., Gomez-Ariza J.L., Pastor R., Pollan M., Perez-Gomez B. Toenails as biomarker of exposure to essential trace metals: A review. Environ. Res. 2019;179 doi: 10.1016/j.envres.2019.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda R.H., Lee D., Sheridan J., Smith D.R. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Hammond S.L., Bantle C.M., Popichak K.A., Wright K.A., Thompson D., Forero C., Kirkley K.S., Damale P.U., Chong E.K.P., Tjalkens R.B. NF-kappaB Signaling in Astrocytes Modulates Brain Inflammation and Neuronal Injury Following Sequential Exposure to Manganese and MPTP During Development and Aging. Toxicol. Sci. 2020;177:506–520. doi: 10.1093/toxsci/kfaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra D.S., Ghaisas S., Zenitsky G., Jin H., Kanthasamy A., Anantharam V., Kanthasamy A.G. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019;13:654. doi: 10.3389/fnins.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawari I., Eskandar M.B., Alzeer S. The Role of Lead, Manganese, and Zinc in Autism Spectrum Disorders (ASDs) and Attention-Deficient Hyperactivity Disorder (ADHD): a Case-Control Study on Syrian Children Affected by the Syrian Crisis. Biol. Trace Elem. Res. 2020;197:107–114. doi: 10.1007/s12011-020-02146-3. [DOI] [PubMed] [Google Scholar]
- Hernandez R.B., Farina M., Esposito B.P., Souza-Pinto N.C., Barbosa F., Jr., Sunol C. Mechanisms of manganese-induced neurotoxicity in primary neuronal cultures: the role of manganese speciation and cell type. Toxicol. Sci. 2011;124:414–423. doi: 10.1093/toxsci/kfr234. [DOI] [PubMed] [Google Scholar]
- Hoet P. and Roels H. A., in Manganese in Health and Disease, ed. L. Costa and M. Aschner, The Royal Society of Chemistry, 2014, ch. 14, pp. 355-401.
- Hoet P., Vanmarcke E., Geens T., Deumer G., Haufroid V., Roels H.A. Manganese in plasma: a promising biomarker of exposure to Mn in welders. A Pilot Study. Toxicol Lett. 2012;213:69–74. doi: 10.1016/j.toxlet.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Hong S.B., Kim J.W., Choi B.S., Hong Y.C., Park E.J., Shin M.S., Kim B.N., Yoo H.J., Cho I.H., Bhang S.Y., Cho S.C. Blood manganese levels in relation to comorbid behavioral and emotional problems in children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2014;220:418–425. doi: 10.1016/j.psychres.2014.05.049. [DOI] [PubMed] [Google Scholar]
- Horning K.J., Caito S.W., Tipps K.G., Bowman A.B., Aschner M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Wen Q., Huang J., Luo M., Xiao Y., Mo R., Wang J. Manganese (II) chloride leads to dopaminergic neurotoxicity by promoting mitophagy through BNIP3-mediated oxidative stress in SH-SY5Y cells. Cell. Mol. Biol. Lett. 2021;26:23. doi: 10.1186/s11658-021-00267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Vazquez L., Van Ginkel Riba G., Arija V., Canals J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients. 2020;12 doi: 10.3390/nu12030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijomone O.M., Olung N.F., Akingbade G.T., Okoh C.O.A., Aschner M. Environmental influence on neurodevelopmental disorders: Potential association of heavy metal exposure and autism. J. Trace Elem. Med Biol. 2020;62 doi: 10.1016/j.jtemb.2020.126638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph N., Zhang-James Y., Perl A., Faraone S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015;19:915–924. doi: 10.1177/1087054713510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.W., Ilhan Z.E., Isern N.G., Hoyt D.W., Howsmon D.P., Shaffer M., Lozupone C.A., Hahn J., Adams J.B., Krajmalnik-Brown R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018;49:121–131. doi: 10.1016/j.anaerobe.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Karki P., Kim C., Smith K., Son D.S., Aschner M., Lee E. Transcriptional Regulation of the Astrocytic Excitatory Amino Acid Transporter 1 (EAAT1) via NF-kappaB and Yin Yang 1 (YY1) J. Biol. Chem. 2015;290:23725–23737. doi: 10.1074/jbc.M115.649327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Suneja B., Jodhka S., Kaur J., Singh A., Singh S.R. Metallic insignia in primary teeth: A biomarker for Autism Spectrum Disorders. J. Indian Soc. Pedod. Prev. Dent. 2021;39:61–66. doi: 10.4103/jisppd.jisppd_485_20. [DOI] [PubMed] [Google Scholar]
- Kaushik G., Zarbalis K.S. Prenatal Neurogenesis in Autism Spectrum Disorders. Front. Chem. 2016;4:12. doi: 10.3389/fchem.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C.H., Stanwood G.D., Smith D.R. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchihara Y., Abe H., Tanaka T., Kato M., Wang L., Ikarashi Y., Yoshida T., Shibutani M. Relationship between brain accumulation of manganese and aberration of hippocampal adult neurogenesis after oral exposure to manganese chloride in mice. Toxicology. 2015;331:24–34. doi: 10.1016/j.tox.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Kim J., Pajarillo E., Rizor A., Son D.S., Lee J., Aschner M., Lee E. LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia. PLoS One. 2019;14:e0210248. doi: 10.1371/journal.pone.0210248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Yagi N., Itokawa Y. Effect of subacute manganese feeding on serotonin metabolism in the rat. J. Environ. Pathol. Toxicol. 1978;2:455–461. [PubMed] [Google Scholar]
- Krishna S., Dodd C.A., Hekmatyar S.K., Filipov N.M. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch. Toxicol. 2014;88:47–64. doi: 10.1007/s00204-013-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumasaka M.Y., Yajima I., Ohgami N., Ninomiya H., Iida M., Li X., Oshino R., Tanihata H., Yoshinaga M., Kato M. Manganese-Mediated Decrease in Levels of c-RET and Tyrosine Hydroxylase Expression In Vitro. Neurotox. Res. 2017;32:661–670. doi: 10.1007/s12640-017-9783-0. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W., Lin X., Herrick R.F., Fang S.C., Cavallari J.M., Christiani D.C., Weisskopf M.G. Toenail, blood, and urine as biomarkers of manganese exposure. J. Occup. Environ. Med. 2011;53:506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffa D.T., Bellaver B., de Oliveira C., de Macedo I.C., de Freitas J.S., Grevet E.H., Caumo W., Rohde L.A., Quincozes-Santos A., Torres I.L.S. Increased Oxidative Parameters and Decreased Cytokine Levels in an Animal Model of Attention-Deficit/Hyperactivity Disorder. Neurochem. Res. 2017;42:3084–3092. doi: 10.1007/s11064-017-2341-6. [DOI] [PubMed] [Google Scholar]
- Li J., Deng Y., Peng D., Zhao L., Fang Y., Zhu X., Li S., Aschner M., Ou S., Jiang Y. Sodium P-aminosalicylic Acid Attenuates Manganese-Induced Neuroinflammation in BV2 Microglia by Modulating NF-kappaB Pathway. Biol. Trace Elem. Res. 2021;199:4688–4699. doi: 10.1007/s12011-021-02581-w. [DOI] [PubMed] [Google Scholar]
- Li Q., Han Y., Dy A.B.C., Hagerman R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017;11:120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.J., Qin W.X., Peng D.J., Yuan Z.X., He S.N., Luo Y.N., Aschner M., Jiang Y.M., Liang D.Y., Xie B.Y., Xu F. Sodium P-aminosalicylic acid inhibits sub-chronic manganese-induced neuroinflammation in rats by modulating MAPK and COX-2. Neurotoxicology. 2018;64:219–229. doi: 10.1016/j.neuro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Li Z.C., Wang F., Li S.J., Zhao L., Li J.Y., Deng Y., Zhu X.J., Zhang Y.W., Peng D.J., Jiang Y.M. Sodium Para-aminosalicylic Acid Reverses Changes of Glutamate Turnover in Manganese-Exposed Rats. Biol. Trace Elem. Res. 2020;197:544–554. doi: 10.1007/s12011-019-02001-0. [DOI] [PubMed] [Google Scholar]
- Liu C., Hutchens S., Jursa T., Shawlot W., Polishchuk E.V., Polishchuk R.S., Dray B.K., Gore A.C., Aschner M., Smith D.R., Mukhopadhyay S. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J. Biol. Chem. 2017;292:16605–16615. doi: 10.1074/jbc.M117.804989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Xin Y., Li Q., Shang Y., Ping Z., Min J., Cahill C.M., Rogers J.T., Wang F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: a systematic review and meta-analysis. Environ. Health. 2020;19:104. doi: 10.1186/s12940-020-00659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yang J., Lu C., Jiang S., Nie X., Han J., Yin L., Jiang J. Downregulation of Mfn2 participates in manganese-induced neuronal apoptosis in rat striatum and PC12 cells. Neurochem. Int. 2017;108:40–51. doi: 10.1016/j.neuint.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang X., Ta X., Luo M., Chang X., Wang H. Fecal microbiome transplantation attenuates manganese-induced neurotoxicity through regulation of the apelin signaling pathway by inhibition of autophagy in mouse brain. Ecotoxicol. Environ. Saf. 2022;242 doi: 10.1016/j.ecoenv.2022.113925. [DOI] [PubMed] [Google Scholar]
- Loke Y.J., Hannan A.J., Craig J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015;6:107. doi: 10.3389/fneur.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R., Placidi D., Cagna G., Fedrighi C., Oppini M., Peli M., Zoni S. Manganese and Developmental Neurotoxicity. Adv Neurobiol. 2017;18:13–34. doi: 10.1007/978-3-319-60189-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., Zhang, Y., Ji, H., Chen, L., Chen, T., Guo, C., Zhang, S., Jia, J. and Niu, P. 2019. Overexpression of miR-138-5p suppresses MnCl(2) -induced autophagy by targeting SIRT1 in SH-SY5Y cells. Environ Toxicol 34, 539-547. [DOI] [PubMed]
- Ma J., Wu J., Li H., Wang J., Han J., Zhang R. Association Between Essential Metal Elements and the Risk of Autism in Chinese Han Population. Biol. Trace Elem. Res. 2022;200:505–515. doi: 10.1007/s12011-021-02690-6. [DOI] [PubMed] [Google Scholar]
- Maccani J.Z., Koestler D.C., Houseman E.A., Armstrong D.A., Marsit C.J., Kelsey K.T. DNA methylation changes in the placenta are associated with fetal manganese exposure. Reprod. Toxicol. 2015;57:43–49. doi: 10.1016/j.reprotox.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner M.J., Shaw K.A., Baio J., EdS, Washington A., Patrick M., DiRienzo M., Christensen D.L., Wiggins L.D., Pettygrove S., Andrews J.G., Lopez M., Hudson A., Baroud T., Schwenk Y., White T., Rosenberg C.R., Lee L.C., Harrington R.A., Huston M., Hewitt A., Esler A., Hall-Lande J., Poynter J.N., Hallas-Muchow L., Constantino J.N., Fitzgerald R.T., Zahorodny W., Shenouda J., Daniels J.L., Warren Z., Vehorn A., Salinas A., Durkin M.S., Dietz P.M. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta R., Risoleo M.C., Messina G., Parisi L., Carotenuto M., Vetri L., Roccella M. The Neurochemistry of Autism. Brain Sci. 2020;10 doi: 10.3390/brainsci10030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Finley E.J., Gavin C.E., Aschner M., Gunter T.E. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic. Biol. Med. 2013;62:65–75. doi: 10.1016/j.freeradbiomed.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez M.I., Acosta-Saavedra L.C., Hernandez-Kelly L.C., Loaeza-Loaeza J., Ortega A. Microglial Activation in Metal Neurotoxicity: Impact in Neurodegenerative Diseases. Biomed Res. Int. 2023;2023:7389508. doi: 10.1155/2023/7389508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.C., Jr., Morcillo P., Ijomone O.M., Venkataramani V., Harrison F.E., Lee E., Bowman A.B., Aschner M. New Insights on the Role of Manganese in Alzheimer's Disease and Parkinson's Disease. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16193546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.C., Jr., Gubert P., Villas Boas G.R., Meirelles Paes M., Santamaria A., Lee E., Tinkov A.A., Bowman A.B., Aschner M. Manganese-induced neurodegenerative diseases and possible therapeutic approaches. Expert Rev. Neurother. 2020;20:1109–1121. doi: 10.1080/14737175.2020.1807330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.C., Krum B.N., Queiros L., Tinkov A.A., Skalny A.V., Bowman A.B., Aschner M. Manganese in the diet: bioaccessibility, adequate intake, and neurotoxicological effects. J. Agric. Food Chem. 2020;68:12893–12903. doi: 10.1021/acs.jafc.0c00641. [DOI] [PubMed] [Google Scholar]
- Martins A.C., Jr., Ruella Oliveira S., Barbosa F., Jr., Tinkov A.A., Santamaria A., Lee E., Bowman A.B., Aschner M. Evaluating the risk of manganese-induced neurotoxicity of parenteral nutrition: review of the current literature. Expert Opin. Drug Metab. Toxicol. 2021;17:581–593. doi: 10.1080/17425255.2021.1894123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.C., Virgolini M.B., Avila D.S., Scharf P., Li J., Tinkov A.A., Skalny A.V., Bowman A.B., Rocha J.B.T., Aschner M. Mitochondria in the spotlight: C. elegans as a model organism to evaluate xenobiotic-induced dysfunction. Cells. 2023;12 doi: 10.3390/cells12172124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel K.L., Beasley T.E., Oshiro W.M., Huffstickler M., Moser V.C., Herr D.W. Impacts of a perinatal exposure to manganese coupled with maternal stress in rats: Tests of untrained behaviors. Neurotoxicol. Teratol. 2022;91 doi: 10.1016/j.ntt.2022.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall S.A., Reichel C.M., Farley C.M., Flesher M.M., Der-Ghazarian T., Cortez A.M., Wacan J.J., Martinez C.E., Varela F.A., Butt A.E., Crawford C.A. Postnatal manganese exposure alters dopamine transporter function in adult rats: Potential impact on nonassociative and associative processes. Neuroscience. 2008;154:848–860. doi: 10.1016/j.neuroscience.2008.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho J.A., de Carvalho-Vivas C.F., Viana G.F., Ferreira J.R., Nunes L.S., Mergler D., Abreu N. Elevated manganese exposure and school-aged children's behavior: a gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Moberly A.H., Czarnecki L.A., Pottackal J., Rubinstein T., Turkel D.J., Kass M.D., McGann J.P. Intranasal exposure to manganese disrupts neurotransmitter release from glutamatergic synapses in the central nervous system in vivo. Neurotoxicology. 2012;33:996–1004. doi: 10.1016/j.neuro.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A.M., Arora M., Harley K.G., Kogut K., Parra K., Hernandez-Bonilla D., Gunier R.B., Bradman A., Smith D.R., Eskenazi B. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ. Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo P., Cordero H., Ijomone O.M., Ayodele A., Bornhorst J., Gunther L., Macaluso F.P., Bowman A.B., Aschner M. Defective Mitochondrial Dynamics Underlie Manganese-Induced Neurotoxicity. Mol. Neurobiol. 2021;58:3270–3289. doi: 10.1007/s12035-021-02341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutkus L., Aschner J.L., Fitsanakis V., Aschner M. The in vitro uptake of glutamate in GLAST and GLT-1 transfected mutant CHO-K1 cells is inhibited by manganese. Biol. Trace Elem. Res. 2005;107:221–230. doi: 10.1385/BTER:107:3:221. [DOI] [PubMed] [Google Scholar]
- Nachtman J.P., Tubben R.E., Commissaris R.L. Behavioral effects of chronic manganese administration in rats: locomotor activity studies. Neurobehav. Toxicol. Teratol. 1986;8:711–715. [PubMed] [Google Scholar]
- Nielsen B.S., Larsen E.H., Ladefoged O., Lam H.R. Subchronic, Low-Level Intraperitoneal Injections of Manganese (IV) Oxide and Manganese (II) Chloride Affect Rat Brain Neurochemistry. Int. J. Toxicol. 2017;36:239–251. doi: 10.1177/1091581817704378. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Sibley M.H., Thapar A., Karalunas S.L. Development of ADHD: Etiology, Heterogeneity, and Early Life Course. Annu Rev Dev Psychol. 2020;2:559–583. doi: 10.1146/annurev-devpsych-060320-093413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkpaa K.W., Awogbindin I.O., Amadi B.A., Abolaji A.O., Adedara I.A., Wegwu M.O., Farombi E.O. Ethanol Exacerbates Manganese-Induced Neurobehavioral Deficits, Striatal Oxidative Stress, and Apoptosis Via Regulation of p53, Caspase-3, and Bax/Bcl-2 Ratio-Dependent Pathway. Biol. Trace Elem. Res. 2019;191:135–148. doi: 10.1007/s12011-018-1587-4. [DOI] [PubMed] [Google Scholar]
- Ntihabose R., Surette C., Foucher D., Clarisse O., Bouchard M.F. Assessment of saliva, hair and toenails as biomarkers of low level exposure to manganese from drinking water in children. Neurotoxicology. 2018;64:126–133. doi: 10.1016/j.neuro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Nunez-Jaramillo L., Herrera-Solis A., Herrera-Morales W.V. ADHD: reviewing the causes and evaluating solutions. J. Pers. Med. 2021:11. doi: 10.3390/jpm11030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obsekov V., Ghassabian A., Mukhopadhyay S., Trasande L. Manganese and thyroid function in the national health and nutrition examination survey, 2011–2012. Environ. Res. 2023;222 doi: 10.1016/j.envres.2023.115371. [DOI] [PubMed] [Google Scholar]
- Ode A., Rylander L., Gustafsson P., Lundh T., Kallen K., Olofsson P., Ivarsson S.A., Rignell-Hydbom A. Manganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhood. Environ. Res. 2015;137:373–381. doi: 10.1016/j.envres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Ou C.Y., He Y.H., Sun Y., Yang L., Shi W.X., Li S.J. Effects of Sub-Acute Manganese Exposure on Thyroid Hormone and Glutamine (Gln)/Glutamate (Glu)-gamma- Aminobutyric Acid (GABA) Cycle in Serum of Rats. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16122157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouisselsat M., Maidoumi S., Elmaouaki A., Lekouch N., Pineau A., Sedki A. Hair trace elements and mineral content in moroccan children with autism spectrum disorder: a case-control study. Biol. Trace Elem. Res. 2023;201:2701–2710. doi: 10.1007/s12011-022-03365-6. [DOI] [PubMed] [Google Scholar]
- Oulhote Y., Mergler D., Barbeau B., Bellinger D.C., Bouffard T., Brodeur M.E., Saint-Amour D., Legrand M., Sauve S., Bouchard M.F. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect. 2014;122:1343–1350. doi: 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E., Rizor A., Son D.S., Aschner M., Lee E. The transcription factor REST up-regulates tyrosine hydroxylase and antiapoptotic genes and protects dopaminergic neurons against manganese toxicity. J. Biol. Chem. 2020;295:3040–3054. doi: 10.1074/jbc.RA119.011446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E., Demayo M., Digman A., Nyarko-Danquah I., Son D.S., Aschner M., Lee E. Deletion of RE1-silencing transcription factor in striatal astrocytes exacerbates manganese-induced neurotoxicity in mice. Glia. 2022;70:1886–1901. doi: 10.1002/glia.24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E., Kim S., Digman A., Dutton M., Son D.S., Aschner M., Lee E. The role of microglial LRRK2 kinase in manganese-induced inflammatory neurotoxicity via NLRP3 inflammasome and RAB10-mediated autophagy dysfunction. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas B.A., Zhang D., Davidson C.M., Crowder T., Park G.A., Fortin T. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol. Teratol. 1997;19:17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Parsons-White A.B., Spitzer N. Environmentally relevant manganese overexposure alters neural cell morphology and differentiation in vitro. Toxicol. In Vitro. 2018;50:22–28. doi: 10.1016/j.tiv.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Paval D. A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017;39:355–360. doi: 10.1159/000478725. [DOI] [PubMed] [Google Scholar]
- Peres T.V., Eyng H., Lopes S.C., Colle D., Goncalves F.M., Venske D.K., Lopes M.W., Ben J., Bornhorst J., Schwerdtle T., Aschner M., Farina M., Prediger R.D., Leal R.B. Developmental exposure to manganese induces lasting motor and cognitive impairment in rats. Neurotoxicology. 2015;50:28–37. doi: 10.1016/j.neuro.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Peres T.V., Ong L.K., Costa A.P., Eyng H., Venske D.K., Colle D., Goncalves F.M., Lopes M.W., Farina M., Aschner M., Dickson P.W., Dunkley P.R., Leal R.B. Tyrosine hydroxylase regulation in adult rat striatum following short-term neonatal exposure to manganese. Metallomics. 2016;8:597–604. doi: 10.1039/c5mt00265f. [DOI] [PubMed] [Google Scholar]
- Purkayastha P., Malapati A., Yogeeswari P., Sriram D. A Review on GABA/Glutamate Pathway for Therapeutic Intervention of ASD and ADHD. Curr. Med. Chem. 2015;22:1850–1859. doi: 10.2174/0929867322666150209152712. [DOI] [PubMed] [Google Scholar]
- Qin Y.Y., Jian B., Wu C., Jiang C.Z., Kang Y., Zhou J.X., Yang F., Liang Y. A comparison of blood metal levels in autism spectrum disorder and unaffected children in Shenzhen of China and factors involved in bioaccumulation of metals. Environ. Sci. Pollut. Res. Int. 2018;25:17950–17956. doi: 10.1007/s11356-018-1957-7. [DOI] [PubMed] [Google Scholar]
- Rahbar M.H., Samms-Vaughan M., Dickerson A.S., Loveland K.A., Ardjomand-Hessabi M., Bressler J., Shakespeare-Pellington S., Grove M.L., Pearson D.A., Boerwinkle E. Blood manganese concentrations in Jamaican children with and without autism spectrum disorders. Environ. Health. 2014;13:69. doi: 10.1186/1476-069X-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar M.H., Samms-Vaughan M., Ma J., Bressler J., Dickerson A.S., Hessabi M., Loveland K.A., Grove M.L., Shakespeare-Pellington S., Beecher C., McLaughlin W., Boerwinkle E. Synergic effect of GSTP1 and blood manganese concentrations in Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2015;18:73–82. doi: 10.1016/j.rasd.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar M.H., Ibrahim S.H., Azam S.I., Hessabi M., Karim F., Kim S., Zhang J., Gulzar Ali N., Loveland K.A. Concentrations of lead, mercury, arsenic, cadmium, manganese, and aluminum in the blood of pakistani children with and without autism spectrum disorder and their associated factors. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18168625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney S.H., Smith D.R. Manganese oxidation state mediates toxicity in PC12 cells. Toxicol. Appl. Pharmacol. 2005;205:271–281. doi: 10.1016/j.taap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Rechtman E., Navarro E., de Water E., Tang C.Y., Curtin P., Papazaharias D.M., Ambrosi C., Mascaro L., Cagna G., Gasparotti R., Invernizzi A., Reichenberg A., Austin C., Arora M., Smith D.R., Lucchini R.G., Wright R.O., Placidi D., Horton M.K. Early-Life Critical Windows of Susceptibility to Manganese Exposure and Sex-Specific Changes in Brain Connectivity in Late Adolescence. Biol Psychiatry Glob Open Sci. 2023;3:460–469. doi: 10.1016/j.bpsgos.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L.G., Cowley M.J., Gayevskiy V., Roscioli T., Thorburn D.R., Prelog K., Bahlo M., Sue C.M., Balasubramaniam S., Christodoulou J. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J. Inherit. Metab. Dis. 2017;40:261–269. doi: 10.1007/s10545-016-0010-6. [DOI] [PubMed] [Google Scholar]
- Rizor A., Pajarillo E., Nyarko-Danquah I., Digman A., Mooneyham L., Son D.S., Aschner M., Lee E. Manganese-induced reactive oxygen species activate IkappaB kinase to upregulate YY1 and impair glutamate transporter EAAT2 function in human astrocytes in vitro. Neurotoxicology. 2021;86:94–103. doi: 10.1016/j.neuro.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.L., Lyall K., Hart J.E., Laden F., Just A.C., Bobb J.F., Koenen K.C., Ascherio A., Weisskopf M.G. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses' Health Study II participants. Environ. Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodichkin A.N., Edler M.K., McGlothan J.L., Guilarte T.R. Behavioral and neurochemical studies of inherited manganese-induced dystonia-parkinsonism in Slc39a14-knockout mice. Neurobiol. Dis. 2021;158 doi: 10.1016/j.nbd.2021.105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S., Frye R.E., Slattery J., Wynne R., Tippett M., Pavliv O., Melnyk S., James S.J. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One. 2014;9:e85436. doi: 10.1371/journal.pone.0085436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D.A., Frye R.E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem R.S., Chodick G., Davidovitch M., Bellavia A., Weisskopf M.G. Maternal Thyroid Anomalies and Attention-Deficit Hyperactivity Disorder in Progeny. Am. J. Epidemiol. 2022;191:430–440. doi: 10.1093/aje/kwab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J.A., Li Z., Sridhar S., Khoshbouei H. The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology. 2013;35:121–128. doi: 10.1016/j.neuro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Rezaei N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;79:340–368. doi: 10.1016/j.pnpbp.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Salazar P., Villaseca P., Cisternas P., Inestrosa N.C. Neurodevelopmental impact of the offspring by thyroid hormone system-disrupting environmental chemicals during pregnancy. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111345. [DOI] [PubMed] [Google Scholar]
- Santiago, N.A., He, B., Howard, S.L., Beaudin, S., Strupp, B.J. and Smith, D.R. 2023. Developmental Manganese Exposure Causes Lasting Attention Deficits Accompanied by Dysregulation of mTOR Signaling and Catecholaminergic Gene Expression in Brain Prefrontal Cortex. bioRxiv.
- Sarkar S. Mechanism of Gene-Environment Interactions Driving Glial Activation in Parkinson's Diseases. Curr Environ Health Rep. 2021;8:203–211. doi: 10.1007/s40572-021-00320-w. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Malovic E., Harischandra D.S., Ngwa H.A., Ghosh A., Hogan C., Rokad D., Zenitsky G., Jin H., Anantharam V., Kanthasamy A.G., Kanthasamy A. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology. 2018;64:204–218. doi: 10.1016/j.neuro.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Rokad D., Malovic E., Luo J., Harischandra D.S., Jin H., Anantharam V., Huang X., Lewis M., Kanthasamy A., Kanthasamy A.G. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aat9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A.E., de Oliveira P.A., de Souza L.F., da Silva D.G., Danielski S., Santos D.B., de Almeida E.A., Prediger R.D., Fisher A., Farina M., Dafre A.L. Interaction of curcumin with manganese may compromise metal and neurotransmitter homeostasis in the hippocampus of young mice. Biol. Trace Elem. Res. 2014;158:399–409. doi: 10.1007/s12011-014-9951-5. [DOI] [PubMed] [Google Scholar]
- Schneider J.S., Williams C., Ault M., Guilarte T.R. Effects of chronic manganese exposure on attention and working memory in non-human primates. Neurotoxicology. 2015;48:217–222. doi: 10.1016/j.neuro.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schullehner J., Thygesen M., Kristiansen S.M., Hansen B., Pedersen C.B., Dalsgaard S. Exposure to Manganese in Drinking Water during Childhood and Association with Attention-Deficit Hyperactivity Disorder: A Nationwide Cohort Study. Environ. Health Perspect. 2020;128:97004. doi: 10.1289/EHP6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland A.A., Dapretto M., Ghahremani D.G., Poldrack R.A., Bookheimer S.Y. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears L., Myers J.V., Sears C.G., Brock G.N., Zhang C., Zierold K.M. Manganese body burden in children is associated with reduced visual motor and attention skills. Neurotoxicol. Teratol. 2021;88 doi: 10.1016/j.ntt.2021.107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J.H., Zeng B.Y., Lin P.Y., Chen T.Y., Chen Y.W., Wu C.K., Tseng P.T., Wu M.K. Association between peripheral manganese levels and attention-deficit/hyperactivity disorder: a preliminary meta-analysis. Neuropsychiatr. Dis. Treat. 2018;14:1831–1842. doi: 10.2147/NDT.S165378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilnikova N., Karyakina N., Farhat N., Ramoju S., Cline B., Momoli F., Mattison D., Jensen N., Terrell R., Krewski D. Biomarkers of environmental manganese exposure. Crit. Rev. Toxicol. 2022;52:325–343. doi: 10.1080/10408444.2022.2095979. [DOI] [PubMed] [Google Scholar]
- Shin D.W., Kim E.J., Lim S.W., Shin Y.C., Oh K.S., Kim E.J. Association of hair manganese level with symptoms in attention-deficit/hyperactivity disorder. Psychiatry Investig. 2015;12:66–72. doi: 10.4306/pi.2015.12.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvani-Rad S., Ejtahed H.S., Ettehad Marvasti F., Taghavi M., Sharifi F., Arzaghi S.M., Larijani B. The Role of Gut Microbiota-Brain Axis in Pathophysiology of ADHD: A Systematic Review. J. Atten. Disord. 2022;26:1698–1710. doi: 10.1177/10870547211073474. [DOI] [PubMed] [Google Scholar]
- Shoffner J., Hyams L., Langley G.N., Cossette S., Mylacraine L., Dale J., Ollis L., Kuoch S., Bennett K., Aliberti A., Hyland K. Fever plus mitochondrial disease could be risk factors for autistic regression. J. Child Neurol. 2010;25:429–434. doi: 10.1177/0883073809342128. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M., Lee E., Albrecht J., Aschner M. Manganese disrupts astrocyte glutamine transporter expression and function. J. Neurochem. 2009;110:822–830. doi: 10.1111/j.1471-4159.2009.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M., Lee E.S., Ni M., Aschner M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia. 2010;58:1905–1912. doi: 10.1002/glia.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M., Lee E., Mingwei N., Aschner M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia. 2011;59:1732–1743. doi: 10.1002/glia.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny A.V., Simashkova N.V., Skalnaya A.A., Klyushnik T.P., Bjorklund G., Skalnaya M.G., Tinkov A.A. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab. Brain Dis. 2017;32:1675–1684. doi: 10.1007/s11011-017-0056-7. [DOI] [PubMed] [Google Scholar]
- Skalny A.V., Mazaletskaya A.L., Ajsuvakova O.P., Bjorklund G., Skalnaya M.G., Chao J.C., Chernova L.N., Shakieva R.A., Kopylov P.Y., Skalny A.A., Tinkov A.A. Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD) J. Trace Elem. Med Biol. 2020;58 doi: 10.1016/j.jtemb.2019.126445. [DOI] [PubMed] [Google Scholar]
- Skalny A.V., Mazaletskaya A.L., Ajsuvakova O.P., Bjorklund G., Skalnaya M.G., Notova S.V., Chernova L.N., Skalny A.A., Burtseva T.I., Tinkov A.A. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD) J. Trace Elem. Med Biol. 2020;61 doi: 10.1016/j.jtemb.2020.126539. [DOI] [PubMed] [Google Scholar]
- Skogheim T.S., Weyde K.V.F., Engel S.M., Aase H., Suren P., Oie M.G., Biele G., Reichborn-Kjennerud T., Caspersen I.H., Hornig M., Haug L.S., Villanger G.D. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021;152 doi: 10.1016/j.envint.2021.106468. [DOI] [PubMed] [Google Scholar]
- Skroder H., Kippler M., Nermell B., Tofail F., Levi M., Rahman S.M., Raqib R., Vahter M. Major Limitations in Using Element Concentrations in Hair as Biomarkers of Exposure to Toxic and Essential Trace Elements in Children. Environ. Health Perspect. 2017;125 doi: 10.1289/EHP1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Fernandes J., Go Y.M., Jones D.P. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem. Biophys. Res. Commun. 2017;482:388–398. doi: 10.1016/j.bbrc.2016.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares A.T.G., Silva A.C., Tinkov A.A., Khan H., Santamaria A., Skalnaya M.G., Skalny A.V., Tsatsakis A., Bowman A.B., Aschner M., Avila D.S. The impact of manganese on neurotransmitter systems. J. Trace Elem. Med Biol. 2020;61 doi: 10.1016/j.jtemb.2020.126554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin O.P., Aschner M. Effects of manganese on thyroid hormone homeostasis: potential links. Neurotoxicology. 2007;28:951–956. doi: 10.1016/j.neuro.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Deng Y., Yang X., Bai Y., Xu B., Liu W., Zheng W., Wang C., Zhang M., Xu Z. Manganese-Disrupted Interaction of Dopamine D1 and NMDAR in the Striatum to Injury Learning and Memory Ability of Mice. Mol. Neurobiol. 2016;53:6745–6758. doi: 10.1007/s12035-015-9602-7. [DOI] [PubMed] [Google Scholar]
- Stanwood G.D., Leitch D.B., Savchenko V., Wu J., Fitsanakis V.A., Anderson D.J., Stankowski J.N., Aschner M., McLaughlin B. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J. Neurochem. 2009;110:378–389. doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res. Brain Res. Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Taylor C.A., Grant S.M., Jursa T., Melkote A., Fulthorpe R., Aschner M., Smith D.R., Gonzales R.A., Mukhopadhyay S. SLC30A10 manganese transporter in the brain protects against deficits in motor function and dopaminergic neurotransmission under physiological conditions. Metallomics. 2023;15 doi: 10.1093/mtomcs/mfad021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov A.A., Martins A.C., Avila D.S., Gritsenko V.A., Skalny A.V., Santamaria A., Lee E., Bowman A.B., Aschner M. Gut Microbiota as a Potential Player in Mn-Induced Neurotoxicity. Biomolecules. 2021:11. doi: 10.3390/biom11091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov A.A., Nguyen T.T., Santamaria A., Bowman A.B., Buha Djordjevic A., Paoliello M.M.B., Skalny A.V., Aschner M. Sirtuins as molecular targets, mediators, and protective agents in metal-induced toxicity. Arch. Toxicol. 2021;95:2263–2278. doi: 10.1007/s00204-021-03048-6. [DOI] [PubMed] [Google Scholar]
- Tinkov A.A., Paoliello M.M.B., Mazilina A.N., Skalny A.V., Martins A.C., Voskresenskaya O.N., Aaseth J., Santamaria A., Notova S.V., Tsatsakis A., Lee E., Bowman A.B., Aschner M. Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Okamoto Y., Aoki A., Jinno H. Catecholamine oxidation-mediated transcriptional inhibition in Mn neurotoxicity. J. Toxicol. Sci. 2020;45:619–624. doi: 10.2131/jts.45.619. [DOI] [PubMed] [Google Scholar]
- van Rooij D., Hartman C.A., van Donkelaar M.M., Bralten J., von Rhein D., Hakobjan M., Franke B., Heslenfeld D.J., Oosterlaan J., Rommelse N., Buitelaar J.K., Hoekstra P.J. Variation in serotonin neurotransmission genes affects neural activation during response inhibition in adolescents and young adults with ADHD and healthy controls. World J. Biol. Psychiatry. 2015;16:625–634. doi: 10.3109/15622975.2015.1067371. [DOI] [PubMed] [Google Scholar]
- Venkataramani V., Doeppner T.R., Willkommen D., Cahill C.M., Xin Y., Ye G., Liu Y., Southon A., Aron A., Au-Yeung H.Y., Huang X., Lahiri D.K., Wang F., Bush A.I., Wulf G.G., Strobel P., Michalke B., Rogers J.T. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J. Neurochem. 2018;147:831–848. doi: 10.1111/jnc.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaet A.A.J., Breynaert A., Ceulemans B., De Bruyne T., Fransen E., Pieters L., Savelkoul H.F.J., Hermans N. Oxidative stress and immune aberrancies in attention-deficit/hyperactivity disorder (ADHD): a case-control comparison. Eur. Child Adolesc. Psychiatry. 2019;28:719–729. doi: 10.1007/s00787-018-1239-4. [DOI] [PubMed] [Google Scholar]
- Verma P., Singh A., Nthenge-Ngumbau D.N., Rajamma U., Sinha S., Mukhopadhyay K., Mohanakumar K.P. Attention deficit-hyperactivity disorder suffers from mitochondrial dysfunction. BBA Clin. 2016;6:153–158. doi: 10.1016/j.bbacli.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezer T., Kurunczi A., Naray M., Papp A., Nagymajtenyi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am. J. Ind. Med. 2007;50:841–852. doi: 10.1002/ajim.20485. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Graham D.L., Amos-Kroohs R.M., Braun A.A., Grace C.E., Schaefer T.L., Skelton M.R., Erikson K.M., Aschner M., Williams M.T. Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicol. Rep. 2014;1:1046–1061. doi: 10.1016/j.toxrep.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg K.E., Guazzetti S., Pineda D., Larsson S.C., Fedrighi C., Cagna G., Zoni S., Placidi D., Wright R.O., Smith D.R., Lucchini R.G., Broberg K. Polymorphisms in Manganese Transporters SLC30A10 and SLC39A8 Are Associated With Children's Neurodevelopment by Influencing Manganese Homeostasis. Front. Genet. 2018;9:664. doi: 10.3389/fgene.2018.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C., Ma X., Shi S., Zhao J., Nie X., Han J., Xiao J., Wang X., Jiang S., Jiang J. Pivotal roles of p53 transcription-dependent and -independent pathways in manganese-induced mitochondrial dysfunction and neuronal apoptosis. Toxicol. Appl. Pharmacol. 2014;281:294–302. doi: 10.1016/j.taap.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Wang N., Gao X., Zhang Z., Yang L. Composition of the Gut Microbiota in Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Front Endocrinol (lausanne) 2022;13 doi: 10.3389/fendo.2022.838941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ohishi T., Shiraki A., Morita R., Akane H., Ikarashi Y., Mitsumori K., Shibutani M. Developmental exposure to manganese chloride induces sustained aberration of neurogenesis in the hippocampal dentate gyrus of mice. Toxicol. Sci. 2012;127:508–521. doi: 10.1093/toxsci/kfs110. [DOI] [PubMed] [Google Scholar]
- Wang L., Shiraki A., Itahashi M., Akane H., Abe H., Mitsumori K., Shibutani M. Aberration in epigenetic gene regulation in hippocampal neurogenesis by developmental exposure to manganese chloride in mice. Toxicol. Sci. 2013;136:154–165. doi: 10.1093/toxsci/kft183. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang S., Yang F., Xin R., Wang S., Cui D., Sun Y. The gut microbiota confers protection in the CNS against neurodegeneration induced by manganism. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110150. [DOI] [PubMed] [Google Scholar]
- Wen Y., Qi Z., Li J., Zhang L., Wang S., Cui R., Xu B., Liu W., Xu Z., Deng Y. Suppression of axonal attractant netrin-1 injured dopaminergic neuronal and motor function of mice during manganese overexposure. Metallomics. 2022;14 doi: 10.1093/mtomcs/mfac019. [DOI] [PubMed] [Google Scholar]
- Wilcox J.M., Consoli D.C., Paffenroth K.C., Spitznagel B.D., Calipari E.S., Bowman A.B., Harrison F.E. Manganese-induced hyperactivity and dopaminergic dysfunction depend on age, sex and YAC128 genotype. Pharmacol. Biochem. Behav. 2022;213 doi: 10.1016/j.pbb.2022.173337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf A., Wright R., Amarasiriwardena C., Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Xu Z.F., Deng Y. Effect of manganese exposure on intracellular Ca2+ homeostasis and expression of NMDA receptor subunits in primary cultured neurons. Neurotoxicology. 2009;30:941–949. doi: 10.1016/j.neuro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Xu B., Xu Z.F., Deng Y. Manganese exposure alters the expression of N-methyl-D-aspartate receptor subunit mRNAs and proteins in rat striatum. J. Biochem. Mol. Toxicol. 2010;24:1–9. doi: 10.1002/jbt.20306. [DOI] [PubMed] [Google Scholar]
- Yan D., Yang Y., Lang J., Wang X., Huang Y., Meng J., Wu J., Zeng X., Li H., Ma H., Gao L. SIRT1/FOXO3-mediated autophagy signaling involved in manganese-induced neuroinflammation in microglia. Ecotoxicol. Environ. Saf. 2023;256 doi: 10.1016/j.ecoenv.2023.114872. [DOI] [PubMed] [Google Scholar]
- Yang Y., An J., Wang Y., Luo W., Wang W., Mei X., Wu S., Chen J. Intrastriatal manganese chloride exposure causes acute locomotor impairment as well as partial activation of substantia nigra GABAergic neurons. Environ. Toxicol. Pharmacol. 2011;31:171–178. doi: 10.1016/j.etap.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Yang Y., Liu Y., Zhang A.L., Tang S.F., Ming Q., Ao C.Y., Liu Y., Li C.Z., Yu C., Zhao H., Chen L., Li J. Curcumin protects against manganese-induced neurotoxicity in rat by regulating oxidative stress-related gene expression via H3K27 acetylation. Ecotoxicol. Environ. Saf. 2022;236 doi: 10.1016/j.ecoenv.2022.113469. [DOI] [PubMed] [Google Scholar]
- Yousef S., Adem A., Zoubeidi T., Kosanovic M., Mabrouk A.A., Eapen V. Attention deficit hyperactivity disorder and environmental toxic metal exposure in the United Arab Emirates. J. Trop. Pediatr. 2011;57:457–460. doi: 10.1093/tropej/fmq121. [DOI] [PubMed] [Google Scholar]
- Zablotsky B., Black L.I., Maenner M.J., Schieve L.A., Blumberg S.J. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. Natl Health Stat Report. 2015;87:1–20. [PubMed] [Google Scholar]
- Zhang J., Cao R., Cai T., Aschner M., Zhao F., Yao T., Chen Y., Cao Z., Luo W., Chen J. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox. Res. 2013;24:478–490. doi: 10.1007/s12640-013-9392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Kanthasamy A., Anantharam V., Kanthasamy A. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol. Appl. Pharmacol. 2011;254:65–71. doi: 10.1016/j.taap.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wu L., Zhang J., Wang X., Yang X., Xin Y., Chen L., Li J., Niu P. Multi-omics analysis reveals Mn exposure affects ferroptosis pathway in zebrafish brain. Ecotoxicol. Environ. Saf. 2023;253 doi: 10.1016/j.ecoenv.2023.114616. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhang J., Wu L., Chen L., Niu P., Li J. Glutamine supplementation reverses manganese neurotoxicity by eliciting the mitochondrial unfolded protein response. iScience. 2023;26 doi: 10.1016/j.isci.2023.107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Cai T., Liu M., Zheng G., Luo W., Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol. Sci. 2009;107:156–164. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
- Zhao G., Liu S.J., Gan X.Y., Li J.R., Wu X.X., Liu S.Y., Jin Y.S., Zhang K.R., Wu H.M. Analysis of Whole Blood and Urine Trace Elements in Children with Autism Spectrum Disorders and Autistic Behaviors. Biol. Trace Elem. Res. 2023;201:627–635. doi: 10.1007/s12011-022-03197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Fu S.X., Dydak U., Cowan D.M. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32:1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.F., Lu L.L., Fang Y.Y., Wu J., Huang Z.Y., Zheng X.W., Song H.X., Aschner M., Song C., Jiang Y.M. Methylcyclopentadienyl manganese tricarbonyl alter behavior and cause ultrastructural changes in the substantia nigra of rats: comparison with inorganic manganese chloride. Neurochem. Res. 2022;47:2198–2210. doi: 10.1007/s11064-022-03606-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.