Abstract
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory process in the airways that results in airflow obstruction. It is mainly linked to cigarette smoke exposure. Th17 cells have a role in the pathogenesis of COPD by secreting pro-inflammatory cytokines, which cause hyperinflammation and progression of the disease.
This study aimed to assess the potential therapeutic effects of nanocurcumin on the Th17 cell frequency and its responses in moderate and severe COPD patients. This study included 20 patients with severe COPD hospitalized in an intensive care unit (ICU) and 20 patients with moderate COPD. Th17 cell frequency, Th17-related factors gene expression (RAR-related orphan receptor t (RORγt), IL-17, IL-21, IL-23, and granulocyte-macrophage colony-stimulating factor), and serum levels of Th17-related cytokines were assessed before and after treatment in both placebo and nanocurcumin-treated groups using flow cytometry, real-time PCR, and ELISA, respectively.
According to our findings, in moderate and severe nanocurcumin-treated COPD patients, there was a substantial reduction in the frequency of Th17 cells, mRNA expression, and cytokines secretion level of Th17-related factors compared to the placebo group. Furthermore, after treatment, the metrics mentioned above were considerably lower in the nanocurcumin-treated group compared to before treatment.
Nanocurcumin has been shown to decrease the number of Th17 cells and their related inflammatory cytokines in moderate and severe COPD patients. As a result, it might be used as an immune-modulatory agent to alleviate the patient's inflammatory state.
Keywords: COPD, Th17, Nanocurcumin, Inflammation, Immunomodulation
1. Introduction
COPD is an airway chronic inflammatory illness characterized by prolonged airflow obstruction and is induced mainly by exposure to harmful substances or gases [1]. Despite developing several drugs to treat COPD, the disease remains one of the major contributors to morbidity and mortality worldwide. There are several challenges in this regard, such as drug delivery to the lungs, inflammation and oxidative stress, multi-targeted approach, and side effects of conventional therapies [[2], [3], [4]]. According to the World Health Organization (WHO), it will be the third most significant cause of death worldwide by 2030. The most common clinical manifestations of COPD are chronic inflammation in the lung, a modification in the structure of the airway resulting in its restriction, and the loss of the alveolar wall, which leads to emphysema.
T helper type 17 (Th17) exerts an important function in COPD-related lung inflammation, mainly through stimulating proinflammatory cytokines, matrix metalloproteinases (MMPs), and chemotactic factors. Th17 is a CD4+ T helper cell that can produce inflammatory factors, including interleukin (IL)-17, IL-21, IL-22, etc. Thus, Th17 and its related cytokines are thought to be therapeutic targets in COPD pathogenesis [5]. Also, it has been reported that increased production of IL-17A, IL-22, and IL-23 (which is required for polarization of Th17) in the bronchial mucosa of patients with COPD suggests that these factors may be essential in the activation of endothelial and T cells, which is seen in COPD patients. This, in turn, may contribute to eliciting neutrophilia and tissue remodeling in the bronchi of patients [6]. In fact, neutrophil migration to the inflammation area may be facilitated mainly through Th17 cells-related cytokines since it has been revealed that IL-17A, IL-17F, and IL-22 have a role in the stimulation of the production of CXCL5, CXCL8, G-CSF, and GM-CSF via airway epithelial cells, which, in turn, facilitate the recruitment, proliferation, and differentiation of these cells [7].
Bronchodilators and corticosteroids are still used extensively in COPD treatment. However, several side effects in administering these regimens have been reported, like diabetes, hypertension, adrenal suppression, eye disorders, and increased susceptibility to infections [8]. Therefore, more effective and safer treatment strategies are needed. On the other hand, because inflammation in COPD is generally resistant to corticosteroid therapy, alternative pharmacological anti-inflammatory agents are required to treat COPD patients more effectively [9]. Various factors are involved in the inflammation resistance to corticosteroid therapy. For example, oxidative stress can interfere with the anti-inflammatory actions of corticosteroids by modifying signaling pathways and reducing the effectiveness of glucocorticoid receptors [10]. In addition to inflammation, oxidative stress is another characteristic of respiratory disorders like COPD; thus, controlling them may aid in preventing disease development.
Curcumin may be an effective treatment method for managing COPD due to its dual actions as an antioxidant and an anti-inflammatory drug [11]. Also, one of the characteristics of COPD patients that must be addressed is glucocorticoid resistance. In this regard, several studies revealed that curcumin operates at the post-translational level through increasing the activity and expression of HDAC2, a vital component of the corticosteroid anti-inflammatory function, thereby correcting steroid resistance. Thus, curcumin may reverse steroid resistance, which is frequent in COPD patients [12]. Currently, SinaCurcumin®, a nanocurcumin product, was created as a nano-range formulation of curcumin employing nanotechnology to enhance the distribution and bioavailability of curcumin and its pharmacological and biological effects [13]. So, in the current study, we measure the modulatory and anti-inflammatory effects of nanocurcumin (SinaCurcumin®) on Th17 cell frequency and its function in moderate and severe COPD patients.
2. Materials and methods
2.1. Study design and patients
The present clinical trial used a randomized, placebo-controlled design to examine nanocurcumin's therapeutic efficiency vs. placebo. A double-blinded technique was used to eliminate bias and psychological effects. We classified patients into moderate and severe categories based on their hospitalization status. Specifically, moderate patients received outpatient care at the clinic, while severe patients required hospitalization. The study comprised 20 patients with moderate COPD and 20 patients with severe COPD hospitalized in the Tabriz University of Medical Sciences' Imam Reza Hospital. Each participant was allocated to a treatment group equally and randomly. Patients with moderate and severe COPD were divided randomly into either the nanocurcumin (n = 10) or placebo (n = 10) groups. In addition, 20 healthy-matched controls were included in evaluating immunological markers at baseline versus the patients. Also, patients and the control group were excluded if they had a background of autoimmune diseases, allergic disorders, leukemia or lymphoma, chronic infections, such as hepatitis B or C, human immunodeficiency viruses, brucellosis, or underlying conditions, according to the exclusion criteria. Written informed consent was acquired from the patient to contribute to the research.
The ethical approval was taken from the Tabriz University of Medical Sciences Research Ethics Committee (IR.TBZMED.REC.1400.767). Moreover, the trial was registered in the Iranian Registry of Clinical Trials (IRCTID: IRCT20200324046851N1). Table 1 represents the demographic information of the patients.
Table 1.
Clinical baseline characteristics of patients with COPD, patients with AECOPD and healthy controls.
| Item | Healthy controls (n = 20) | Moderate COPD (n = 20) | Severe COPD (n = 20) | P* Value | P** Value | P*** Value |
|---|---|---|---|---|---|---|
| Age, years | 62.6 ± 4.3 | 63.4 ± 6.3 | 64.3 ± 8.5 | 0.756 | 0.456 | 0.644 |
| Gender (Male/Female) | 13/7 | 15/5 | 16/4 | |||
| BMI (kg/m2), mean ± SEM | 23.2 ± 1.36 | 22.2 ± 1.35 | 23.1 ± 1.7 | 0.669 | 0.755 | 0.690 |
| History of smoke, n (%) | 5 (25) | 15 (75) | 16 (80) | |||
| Smoking pack-years | 7.54 | 34.75 | 51.45 | <0.001 | <0.001 | <0.001 |
| FEV1/FVC (mean ± SEM) | 84.5 ± 2.5 | 61.3 ± 2.92 | 45.9 ± 4.16 | <0.001 | <0.001 | 0.008 |
| FEV1% (% predicted), mean ± SD | 95.15 ± 4.5 | 47.25 ± 8.55 | 48.2 ± 7.9 | <0.001 | <0.001 | 0.570 |
| Leukocytes ( × 109/L) | 6.72 (4.4; 6.45) | 7.5 (6.2; 11.3) | 9.25 (7.5; 13.32) | <0.001 | <0.001 | <0.001 |
| Lymphocytes ( × 109/L) | 2.3 (1.3; 3.4) | 2.5 (1.5; 3.5) | 4.5 (1.8; 6.2) | 0.009 | <0.001 | <0.001 |
| Granulocytes ( × 109/L) | 3.1 (1.9; 4.7) | 4.15 (1.98; 6.32) | 6.35 (1.85; 9.2) | 0.003 | <0.001 | <0.001 |
Comparison between 2 groups was determined by t-test. P < 0.05 was considered significant. COPD = chronic obstructive pulmonary disease, BMI = body mass index, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity. FEV1, FEV1 predicted, and FEV1/FVC were determined post bronchodilator. ∗Comparison between healthy controls and moderate COPD patients. ∗∗Comparison between healthy controls and severe COPD patients. ∗∗∗Comparison between moderate COPD patients and severe COPD.
2.2. Intervention procedure
Patients in the nanocurcumin group got 80 mg SinaCurcumin® (Exir Nano) capsules twice daily for 21 days. To increase curcumin oral bioavailability, patients were given nanocurcumin oral capsules. A placebo pill was also given to the placebo group twice daily (every 12 h) for 21 days. Curcumin nanomicelles are licenced curcumin medicines (SinaCurcumin®) for oral administration. SinaCurcumin® soft gels have included a nano micelle containing 80 mg curcumin. The curcumin encapsulation percentage in nano micelle format is nearly 100 %, while the size of the nano micelles is approximately 10 nm.
2.3. Blood collection, isolation of PBMCs, and cell culture
Using standard venipuncture techniques and heparinized syringes, 8 ml of fresh blood samples were collected from COPD patients and control subjects to isolate the peripheral blood mononuclear cells (PBMCs) in Imam Reza Hospital of Tabriz. Patients' PBMCs were isolated from the blood via a density-gradient method using the standard Ficoll (lymphosep) 1.077 g/mL (Biosera, UK) and next centrifuged 25 min at 450g and rinsed twice with PBS (phosphate buffer saline) (Sigma-Aldrich, Schnelldorf, Germany). Then, cells (approximately 5 × 106) were cultured in a medium containing 10 % fetal bovine serum (FBS), 200 mM l-glutamine, 100 U/mL penicillin, and 10 ng/mL phosphomolybdic acid (eBioscience, San Diego, CA, USA). Then, the isolated cells were placed in a 5 % CO2 incubator (48 h at 37 °C). Flow cytometry was performed to count the cells, and real-time polymerase chain reaction (RT-PCR) was employed to examine gene expression in the cells.
2.4. Flow cytometry
Flow cytometry was performed using monoclonal antibodies against surface and intracellular markers to determine the number of Th17 cells in COPD patients and control. Isolated PBMCs were incubated in a 5 % CO2 humidified incubator (37 °C for 5 h) with 1.7 g/ml monensin, 25 ng/ml PMA, and 1 g/ml ionomycin (all from eBioscience). PBMCs were then incubated at 4 °C with allophycocyanin-conjugated anti-CD4 for 15 min, following phycoerythrin (PE)-conjugated anti-IL-17A staining, to quantify Th17 cells (eBioscience). FITC mouse immunoglobulin G1 and PE, κ-isotype control were used as isotype controls. The cell count was evaluated using a FACgmSCalibur Flowcytometer and FlowJo software (Becton Dickinson).
2.5. Evaluation by real-time PCR
Real-time PCR was utilized to determine the expression patterns of Th17-related factors such as RORγt, IL-17, IL-21, IL-23, and GM-CSF in cultured PBMCs from COPD patients by the SYBR Green technique and the corresponding primers. Therefore, the RNX-PLUS Solution extracted total RNA from COPD patients' separated cells (SinaClon, Tehran, Iran). The cDNA was then produced utilizing random hexamer primers and Revert Aid™ reverse transcriptase (Thermo Fisher Scientific). The mRNA expression of the genes mentioned above was assessed via real-time PCR: DNA denaturation was conducted (10 s at 95 °C), repeated 40 cycles. Then, the annealing step was 10 s at 60 °C, followed by a 20 s extension step at 72 °C. Electrophoresis was performed using 2 % agarose gel and Biosystems (Seqlab, Gottingen, Germany) to demonstrate the amplification of genes and DNA sequencing. Also, the curves were plotted using the six standards, which were prepared from serial dilutions (10-fold) derived from concentrated gene specimens. The 2−ΔΔCT formula, mainly known as the comparative Ct approach, was performed to determine the data. Eventually, target gene expression was evaluated with β-actin (as a housekeeping gene). Table 2 lists the primer sequences in detail.
Table 2.
Primer sequence.
| Gene | Primer | Sequence |
|---|---|---|
| RORγt | Forward Reverse |
ACTCAAAGCAGGAGCAATGGAA AGTGGGAGAAGTCAAAGATGGA |
| IL-17 | Forward Reverse |
CATAACCGGAATACCAATACCAAT GGATATCTCTCAGGGTCCTCATT |
| IL-21 | Forward Reverse |
ACATCCCTAGGGCTCTGTGATG GAGCAGCAGCAGCAGGAGCAAGGGG |
| IL-23 | Forward Reverse |
GGACAACAGTCAGTTCTGCTT CACAGGGCTATCAGGGAGC |
| GM-CSF | Forward Reverse |
GGAGCATGTGAATGCCATCCAG CTGGAGGTCAAACATTTCTGAGAT |
| β-Actin | Forward Reverse |
AGAGCTACGAGCTGCCTGAC AGCACTGTGTTGGCGTACAG |
RORγt, retinoic acid-related orphan receptor γt; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
2.6. Measurements of cytokine levels by ELISA
The concentrations of cytokines IL-17, IL-21, IL-23, and GM-CSF secreted by Th17 cells were quantified in serum samples from COPD patients and controls using an enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource). In brief, the ELISA steps were completed in this sequence: 100 μl of coating antibody were used for coating the 96-well assay plates, then incubated at 4 °C overnight and rinsed with Tween-20-containing PBS (0.05 %). After that, plates were incubated at room temperature (for 2 h) on a shaker with a blocking buffer comprising 2 % milk in PBS, followed by a PBS wash. After that, 100 μl of standards and experimental group samples were put into a plate and incubated for an hour. 100 μl of biotinylated antibody was added to the plate and incubated for 1 h to improve detection sensitivity. The avidin-biotin-peroxidase complex was then added and incubated for 30 min. After, the plates were incubated for 30 min with 100 μl of tetramethylbenzidine substrate, and eventually, the reaction was terminated. The absorbance of the samples and standards were read using a Medgenix ELISA reader at 450 nm (BP-800; Biohit).
2.7. Statistical analysis
The statistical analysis was done utilizing SPSS PC Statistics Software (version 25). The Shapiro–Wilk test was used to ensure that the data distribution was normal. To examine the statistical differences in immunologic parameters between the healthy control group, the COPD patient group, and between pre-treatment and post-treatment patient groups, the unpaired Student's t-test and the paired Student's t-test were used. Also, one-way ANOVA test was used for multiple comparisons. The scale data were reported using mean ± standard deviation, and a p < 0.05 was considered statistically significant. GraphPad Prism was performed to create the graphs (version 7.00).
3. Results
3.1. Th17 cells frequency in moderate and severe COPD patients and controls
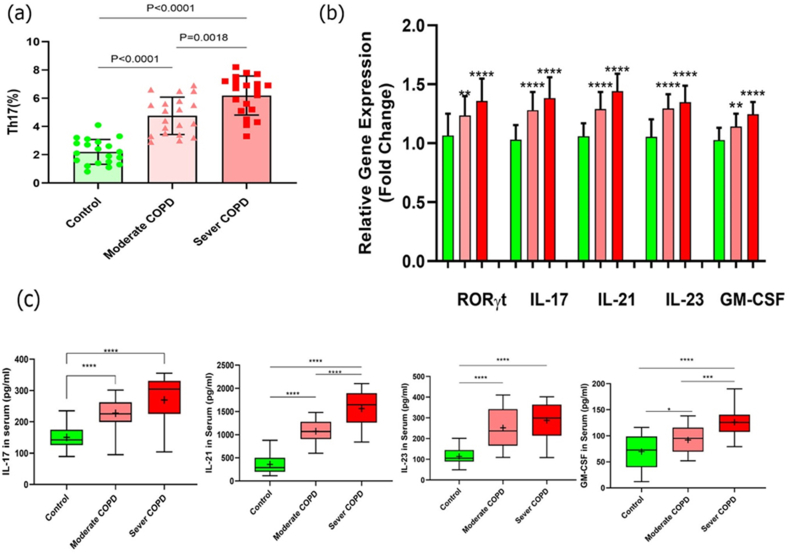
Flow cytometry evaluated the circulating Th17 cell frequency in PBMCs from moderate and severe COPD patients and healthy subjects. As a result, our findings revealed that the frequency of Th17 cells in COPD patients was substantially higher in both moderate and severe patients when compared to control groups (P < 0.0001 and P < 0.0001, respectively). Furthermore, severe patients had a substantially higher frequency of Th17 cells than moderate patients (P = 0.0018) (Fig. 1a and Table 3).
Fig. 1.
Increase of Th17 cell frequency and its relative factors in moderate and severe COPD compared to controls. (a) Th17 cell frequency was significantly greater in patients with moderate (p < 0.0001) and severe (p < 0.0001) COPD compared to controls. Also, the number of Th17 cells was higher in the severe stage when compared to the moderate stage (p = 0.0018). (b) Gene expression levels of Th17-related markers (RORγt, IL-17, IL-21, IL-23, and GM-CSF) were exhibited to be significantly higher in both moderate (P = 0.0044, P < 0.0001, P < 0.0001, p < 00.0001, and p = 00.0018 respectively) and severe (P < 0.0001, P < 0.0001, P < 0.0001, P < 0.0001, and P < 0.0001 respectively) COPD patients when compared to healthy controls. (c) Cytokine secretion levels were substantially elevated in the moderate patient group compared to healthy groups, as well as in the severe COPD compared to control groups, as follows: IL-17 (P < 0.0001, and P < 0.0001), IL-21 (P < 0.0001, and P < 0.0001), IL-23 (P < 0.0001, and P < 0.0001), and GM-CSF (P = 0.0242, and P < 0.0001) respectively. Furthermore, serum secretion levels of IL-21 and GM-CSF cytokine were remarkably increased in the severe patient group compared to the moderate group (P < 0.0001, and P = 0.0003); however, serum concentrations of IL-17 and IL-23 were not notably different in the severe stage in comparison to the moderate stage patients (P < 0.0515, and P = 0.2333). Moderate group, n = 20; severe group, n = 20; and control group, n = 20. The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; Th17, T-helper 17; IL, interleukin; RORγt, RAR-related orphan receptor γt; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Table 3.
Frequency and gene expression levels of Th17 respective parameters in COPD patients and healthy controls.
| Moderate COPD | Controls | P value | Severe COPD | Controls | P value | |
|---|---|---|---|---|---|---|
| Th17 mean ± SD (%) | 4.755 ± 1.326 | 2.200 ± 0.8784 | <0.0001 | 6.190 ± 1.383 | 2.200 ± 0.8784 | <0.0001 |
| RORγt | 1.234 ± 0.1645 | 1.067 ± 0.1843 | 0.0044 | 1.358 ± 0.04254 | 1.067 ± 0.1843 | <0.0001 |
| IL-17 | 1.281 ± 0.1526 | 1.031 ± 0.1230 | <0.0001 | 1.382 ± 0.1770 | 1.031 ± 0.1230 | <0.0001 |
| IL-21 | 1.292 ± 0.1427 | 1.060 ± 0.1090 | <0.0001 | 1.441 ± 0.1492 | 1.060 ± 0.1090 | <0.0001 |
| IL-23 | 1.294 ± 0.1231 | 1.054 ± 0.1498 | <0.0001 | 1.348 ± 0.1388 | 1.054 ± 0.1498 | <0.0001 |
| GM-CSF | 1.141 ± 0.1095 | 1.028 ± 0.1029 | 0.0018 | 1.246 ± 0.1038 | 1.028 ± 0.1029 | <0.0001 |
Note: The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. Th17, T‐helper 17; COPD, Chronic obstructive pulmonary disease; RORγt, RAR‐related orphan receptorγt; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin.
3.2. Th17-related transcription factor and cytokine mRNA expression levels in moderate and severe COPD patients in comparison to control subjects
The gene expression levels of RORγt transcription factor and cytokines such as IL-17, IL-21, IL-23, and GM-CSF were assessed in moderate and severe COPD patients and also control groups employing qRT-PCR to examine responses of Th17 cells and their potential function in inflammation of COPD patients. Our data revealed that all of the factors mentioned above had considerably higher expression levels in both moderate and severe patient groups than in the control group. RORγt, IL-17, IL-21, IL-23, and GM-CSF mRNA expression levels in the moderate patient group vs. control groups and the severe patient group vs. control groups are shown in Fig. 1b and Table 3.
3.3. Assessing the serum concentration of Th17 cells cytokines in moderate and severe COPD patients compared to controls
The amounts of IL-17, IL-21, IL-23, and GM-CSF cytokines were measured in serum specimens of patients and control groups utilizing ELISA. Our results demonstrated that serum levels of all four cytokines were significantly higher in the moderate COPD group compared to control groups (P < 0.0001, P < 0 0.0001, P < 0.0001, P = 0.0242, respectively) and in the severe COPD group compared to control ones (P < 0.0001, P < 0.0001, P < 0.0001, P < 0.0001, respectively). Furthermore, there was a substantial elevation in the serum levels of IL-21 and GM-CSF (P < 0.0001 and P = 0.0003) cytokine in the severe COPD patient group compared to the moderate patient group; however, there was no significant alteration in the serum levels of IL-17 and IL-23 (P = 0.0515 and P = 0.2333) in the severe stage patients compared to the moderate stage patients (Fig. 1c and Table 4).
Table 4.
The serum concentration of Th17-related cytokines in moderate and severe COPD groups and control.
| Moderate COPD | Controls | P value | Severe COPD | Controls | P value | Severe COPD | Moderate COPD | P value | |
|---|---|---|---|---|---|---|---|---|---|
| IL-17 (pg/ml) | 227.4 ± 49.16 | 150.4 ± 37.60 | <0.0001 | 269.9 ± 80.76 | 150.4 ± 37.60 | <0.0001 | 269.9 ± 80.76 | 227.4 ± 49.16 | 0.0515 |
| IL-21 (pg/ml) | 1074 ± 239.7 | 359.5 ± 215.4 | <0.0001 | 1564 ± 402.3 | 359.5 ± 215.4 | <0.0001 | 1564 ± 402.3 | 1074 ± 239.7 | <0.0001 |
| IL-23 (pg/ml) | 252.0 ± 96.33 | 113.6 ± 41.07 | <0.0001 | 282.6 ± 89.45 | 113.6 ± 41.07 | <0.0001 | 282.6 ± 89.45 | 252.0 ± 96.33 | 0.2333 |
| GM-CSF (pg/ml) | 92.15 ± 25.87 | 69.60 ± 34.27 | 0.0242 | 126.2 ± 28.26 | 69.60 ± 34.27 | <0.0001 | 126.2 ± 28.26 | 92.15 ± 25.87 | 0.0003 |
Note: The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. Abbreviations: Th17, T‐helper 17; COPD, Chronic obstructive pulmonary disease; RORγt, RAR‐related orphan receptorγt; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin.
3.4. Gene expression levels of Th17 cell parameters in moderate COPD after treatment with nanocurcumin compared to the placebo group
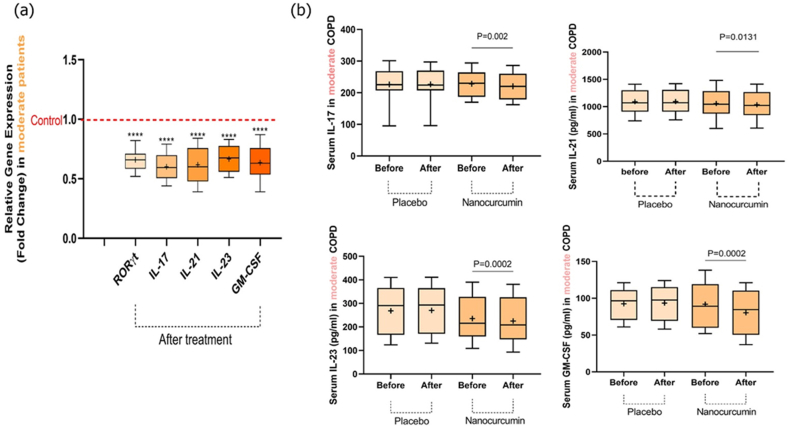
The mRNA expression levels of Th17-related factors, RORγt, IL-17, IL-21, IL-23, and GM-CSF, were assessed before and after treatment in moderate COPD patients using qRT-PCR. In the nanocurcumin-treated group, we discovered substantial alterations in gene expression levels of the factors mentioned earlier than in the placebo group. According to this, a significant decrease was detected in the expression levels of RORγt (P < 0.0001), IL-17 (P < 0.0001), IL-21 (P < 0.0001), IL-23 (P < 0.0001), and GM-CSF (P < 0.0001) after treatment with nanocurcumin compared to placebo groups (Fig. 2a and Table 5).
Fig. 2.
Expression levels of Th17-related transcription factor and its cytokines, as well as cytokine secretion levels in placebo and nanocurcumin-treated groups in moderate COPD patients. (a) We detected a substantial decline in the mRNA expression levels of the Th17-related marker in the nanocurcumin-treated group compared to pretreatment (RORγt (P < 0.0001), IL-17 (P < 0.0001), IL-21 (P < 0.0001), IL-23 (P < 0.0001), and GM-CSF (P < 0.0001)). (b) Serum concentration levels of IL-17 (P = 0.002), IL-21 (P = 0.0131), IL-23 (P = 0.0002), and GM-CSF (P = 0.0002) were significantly lower in the nanocurcumin-treated group after treatment in comparison before treatment. While, in the placebo-treated group, there was no remarkable alteration in cytokine serum levels before and after treatment (P = 0.5505, P = 0.1233, P = 0.0777, and P = 0.2729, respectively). Moderate group, n = 20; nanocurcumin-treated group, n = 10; placebo-treated group, n = 10. The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; Th17, T-helper 17; mRNA, messenger RNA; IL, interleukin; RORγt, RAR-related orphan receptor γt; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Table 5.
Expression levels of Th17-related markers in placebo and nanocurcumin groups after treatment.
| Moderate COPD | Severe COPD | |||||
|---|---|---|---|---|---|---|
| Nanocurcumin | Placebo | P value | Nanocurcumin | Placebo | value | |
| RORγt | 0.6590 ± 0.08850 | 1.004 ± 0.1193 | <0.0001 | 0.6070 ± 0.09031 | 0.9640 ± 0.09070 | <0.0001 |
| IL-17 | 0.6000 ± 0.1098 | 1.004 ± 0.1193 | <0.0001 | 0.8630 ± 0.04945 | 0.9640 ± 0.09070 | 0.0063 |
| IL-21 | 0.6180 ± 0.1555 | 1.004 ± 0.1193 | <0.0001 | 0.6930 ± 0.1268 | 0.9640 ± 0.09070 | <0.0001 |
| IL-23 | 0.6670 ± 0.1110 | 1.004 ± 0.1193 | <0.0001 | 0.7360 ± 0.09501 | 0.9640 ± 0.09070 | <0.0001 |
| GM-CSF | 0.6350 ± 0.1449 | 1.004 ± 0.1193 | <0.0001 | 0.8570 ± 0.07514 | 0.9640 ± 0.09070 | 0.0101 |
Note: The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; RORγt, RAR‐related orphan receptorγt; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin.
3.5. Th17 cells cytokine concentration in serum samples of moderate COPD
ELISA was performed to measure the serum concentration of Th17 cell cytokines in moderate COPD in both placebo and nanocurcumin-treated patients. As expected, our data showed that there was a remarkable reduction in serum concentration of IL-17 (P = 0.002), IL-21 (P = 0.0131), IL-23 (P = 0.0002), and GM-CSF (P = 0.0002) in the group treated with nanocurcumin compared to pretreatment condition. In contrast, after treatment in the placebo group, there was no remarkable alteration compared to before treatment in the levels of cytokines mentioned above (P = 0.5505, P = 0.1233, P = 0.0777, and P = 0.2729, respectively) (Fig. 2b and Table 6).
Table 6.
The serum concentration of Th17-related cytokines in moderate and severe COPD after treatment with nanocurcumin.
| Moderate COPD patients |
||||||
|---|---|---|---|---|---|---|
| Placebo |
Nanocurcumin |
|||||
| Before | After | P value | Before | After | P value | |
| IL-17 (pg/ml) | 226.1 ± 56.54 | 226.6 ± 55.89 | 0.5505 | 228.6 ± 43.62 | 220.4 ± 45.42 | 0.0020 |
| IL-21(pg/ml) | 1090 ± 220.4 | 1094 ± 219.5 | 0.1233 | 1057 ± 268.4 | 1036 ± 256.6 | 0.0131 |
| IL-23 (pg/ml) | 268.4 ± 103.1 | 270.2 ± 101.0 | 0.0777 | 235.6 ± 91.49 | 225.4 ± 94.01 | 0.0002 |
| GM-CSF (pg/ml) |
92.40 ± 21.16 |
93.40 ± 23.19 |
0.2729 |
91.90 ± 31.07 |
80.30 ± 30.73 |
0.0002 |
| Severe COPD patients | ||||||
| Placebo | Nanocurcumin | |||||
| Before |
After |
P value |
Before |
After |
P value |
|
| IL-17 (pg/ml) | 268.0 ± 73.79 | 268.7 ± 74.88 | 0.4874 | 271.7 ± 91.19 | 258.1 ± 93.09 | 0.0003 |
| IL-21(pg/ml) | 1554 ± 318.7 | 1555 ± 316.8 | 0.7577 | 1574 ± 489.8 | 1550 ± 476.7 | 0.0080 |
| IL-23 (pg/ml) | 312.7 ± 63.26 | 315.5 ± 64.69 | 0.0593 | 262.5 ± 107.2 | 237.8 ± 98.15 | 0.0004 |
| GM-CSF (pg/ml) | 118.7 ± 26.62 | 121.4 ± 26.51 | 0.0543 | 133.6 ± 29.23 | 119.1 ± 20.57 | 0.0043 |
Note: The results were reported as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL, interleukin.
3.6. Expression levels of Th17 cell factors in severe COPD after treatment with nanocurcumin compared to the placebo group
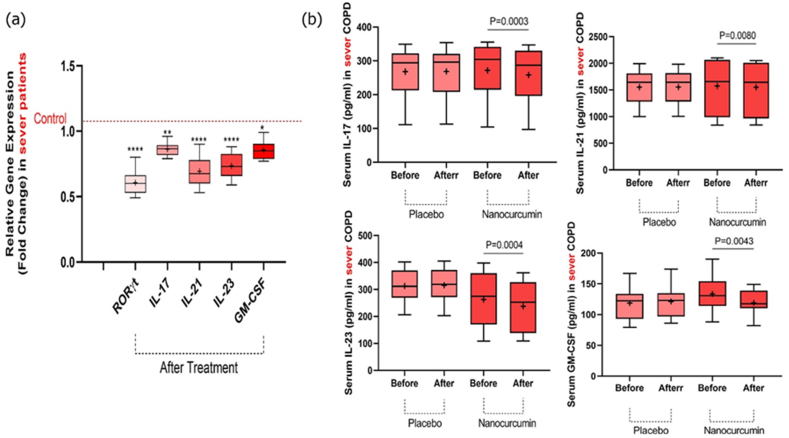
mRNA expression levels of RORγt, IL-17, IL-21, IL-23, and GM-CSF were evaluated by qRT-PCR in severe COPD patients before and after the intervention in both nanocurcumin and placebo-treated groups. We revealed that the expression levels of RORγt (P < 0.0001), IL-17 (P = 0.0063), IL-21 (P < 0.0001), IL-23 (P < 0.0001), and GM-CSF (P = 0.0101) factors were significantly lower in the group treated with nanocurcumin after treatment compared to the placebo group (Fig 3a and Table 5).
3.7. Th17 cells cytokine concentration in serum samples of severe COPD
The serum concentration of Th17 cell-related cytokines was evaluated in the severe stage of COPD patients using the ELISA in both the placebo and nanocurcumin-treated groups. Our results demonstrated that the serum concentration of IL-17 (P = 0.0003), IL-21 (P = 0.0080), IL-23 (p = 0.0004), and GM-CSF (P = 0.0043) cytokines were significantly lower in the group treated with nanocurcumin after treatment in comparison to before treatment. However, in the placebo-treated group, there was no remarkable variation either before or after treatment (P = 0.4874, P = 0.7577, P = 0.0593, and P = 0.0543, respectively) (Fig. 3b and Table 6).
Fig. 3.
mRNA expression levels of Th17 transcription factor and its relevant cytokines, as well as serum concentration of cytokines in placebo and nanocurcumin-treated groups in the severe COPD patients. (a) The nanocurcumin-treated group exhibited a substantial decrease in the expression levels of RORγt (P < 0.0001), IL-17 (P = 0.0063), IL-21 (P < 0.0001), IL-23 (P < 0.0001), and GM-CSF (P = 0.0101) factors in comparison with the control (before treatment). (b) Cytokine serum levels of IL-17 (P = 0.0003), IL-21 (P = 0.0080), IL-23 P = 0.0004), and GM-CSF (P = 0.0043) were substantially lower in the nanocurcumin-treated group after treatment compared to pretreatment. While in the placebo-treated group, there was no remarkable alteration in cytokine serum concentration before and after treatment (P = 0.4874, P = 0.7577, P = 0.0593, and P = 0.0543, respectively). Severe group, n = 20; nanocurcumin-treated group, n = 10; placebo group, n = 10. The results were described as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; Th17, T-helper 17; IL, interleukin; mRNA, messenger RNA; GM-CSF, granulocyte-macrophage colony-stimulating factor; RORγt, RAR-related orphan receptor γt.
3.8. Th17 lymphocyte frequency in the circulation of moderate and severe COPD patients
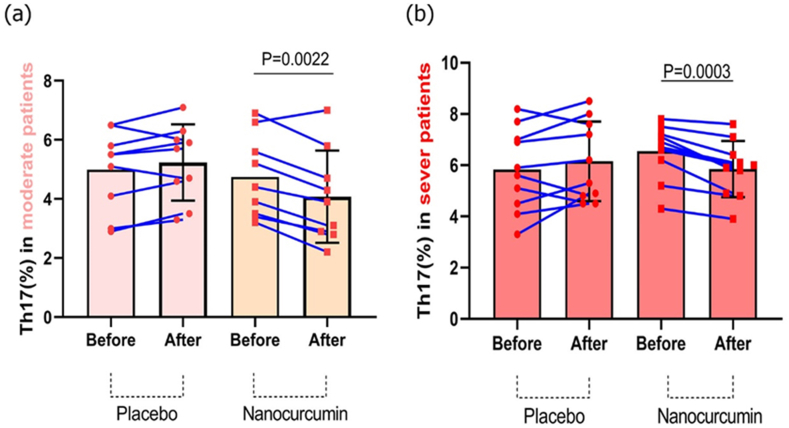
Flow cytometry was performed to examine the Th17 lymphocyte frequency in both moderate and severe COPD patients in the placebo and nanocurcumin-treated groups. Based on our results, in the moderate stage, the nanocurcumin-treated group had a substantially lower number of Th17 cells after treatment compared to before treatment (P = 0.0022). However, the placebo-treated group exhibited no significant alterations before and after treatment (Fig. 4a). Similarly, in the severe COPD stage, the Th17 cell frequency was considerably reduced in the group treated with nanocurcumin post-treatment compared to pre-treatment (P = 0.0003). Still, no considerable decrease was found in the placebo group before and after treatment (Fig. 4b). Finally, our results demonstrated that in both moderate and severe patients, Th17 cell count was significantly decreased after treatment with nanocurcumin compared to placebo groups.
Fig. 4.
The Th17 lymphocytes frequency in placebo and nanocurcumin-treated groups in moderate and severe COPD patients. (a) There was a significant decline in the frequency of Th17 cells after treatment in the nanocurcumin-treated group compared to pre-treatment condition (P = 0.0022); but, before and after treatment in the placebo group, there was no considerable alteration in number of Th17 lymphocytes. (b) In severe COPD, the Th17 lymphocytes frequency was found to be considerably lower in the group treated with nanocurcumin compared to before treatment condition (P = 0.0003); however, before and after treatment in the placebo group, no significant alteration in the count of Th17 lymphocytes was observed.
Moderate group, n = 20; severe group, n = 20; placebo group, n = 10; and nanocurcumin group, n = 10. The results were described as a mean ± SD. P < 0.05 was considered statistically significant. COPD, Chronic obstructive pulmonary disease; Th17, T-helper 17.
4. Discussion
COPD impacts approximately 10 percent of persons over the age of 40 worldwide, and it is a primary cause of mortality in the United States [14]. Inflammation is mainly caused by smoking in COPD patients [15]. However, in smokers with COPD, inflammation remains long even after cessation of smoking, and unfortunately, the capacity of the lung tends to worsen [15], presumably because the colonization of bacteria in smoke-damaged airways maintains inflammation and airway injury [16,17]. As a result, in addition to withdrawing smoking, further approaches that slow the advancement of severe COPD are very appealing.
Th17 was recently demonstrated to mediate neutrophilic and macrophage inflammation, implying that Th17 cells may play an essential role in severe, steroid-resistance COPD [18]. The function of Th17 and IL-17 in COPD pathogenesis remains mostly hypothetical. However, the function of IL-17 in increasing the secretion of cytokine and the involvement of macrophages and neutrophils in facilitating COPD pathogenesis have sparked interest in a possible association [19]. Elevated IL-17 production boosts neutrophil recruitment throughout the disease's onset and development, resulting in chronic inflammation and emphysema [5]. IL-17 cytokine in sputum was detected at low levels in patients with COPD [20]. Also, it recently exhibited that COPD subjects had an elevated number of IL-17-positive cells in the submucosa and IL-22 and IL-23-staining cells in the bronchial epithelium [6].
The Food and Drug Administration (FDA) approved several drugs for the treatment of COPD, including long-acting beta-2 agonists (LABAs) indacaterol, formoterol, salmeterol, and arformoterol; long-acting muscarinic antagonist (LAMA) tiotropium (theophylline); LABA/inhaled corticosteroid (ICS) combinations formoterol/budesonide and salmeterol/fluticasone [21,22]. Both LAMAs and LABAs have been revealed to have anti-inflammatory activities in vitro and in animals, but the therapeutic importance of these findings has yet to be determined. In addition, clinical investigations have linked ICS usage to a higher risk of side effects, including candidiasis, hoarseness, and pneumonia [23].
Furthermore, numerous anti-inflammatory drugs, such as anti-cytokine and anti-chemokine medications, are being developed for COPD treatment. In COPD patients, reduced dyspnea was observed in a pilot study with a monoclonal antibody against IL-8 (ABX-IL8), implying anti-inflammatory properties [24].
Because the TNF-α cytokine has a key role in the inflammatory process, a TNF-α targeting drug (such as Etanercept) was considered a promising treatment option for COPD [25]. Nevertheless, infliximab, the anti-TNF-α antibody, did not enhance the life quality or exacerbations of patients with COPD and exhibited a trend for increased pneumonia and cancer cases [22]. Therefore, further research is required to discover or design therapeutic medicines that target particular inflammatory targets. These findings prompted us to examine the anti-inflammatory and therapeutic potential of SinaCurcumin®, a new compound of curcumin, in patients with COPD.
Curcumin is a plant-derived compound with immunomodulatory, anti-inflammatory, antitumor, anti-angiogenic, anti-invasive, and antioxidant activities in various diseases [26]. The rapid metabolism of curcumin, poor solubility in water, negligible oral bioavailability, and systemic clearance have all been recognized as critical therapeutic problems [26]. To address these problems, nano-curcumin as biodegradable particles, also employed in our study, were utilized to increase curcumin's stability, solubility, half-life, and free radical scavenging capacity [27]. Also, nanocurcumin has substantially greater bioavailability upon oral administration than primary curcumin. Following oral intake, SinaCurcumin® soft gels release and transit across the small intestine in approximately 15 min. These micromicelles may break down in an undisturbed water layer after passing through the small intestine [13].
Based on both the beneficial anti-inflammatory and immune-modulatory activity of nanocurcumin and the importance of Th17 cells in inflammation, in the present study, we assessed the effects of nanocurcumin on the count of Th17 lymphocytes, the gene expression levels of related markers (RORγt, IL-17, IL-21, IL-23, and GM-CSF), and serum concentration of cytokine in both moderate and severe COPD patients. According to our findings, nanocurcumin reduced the amount of Th17 lymphocytes in moderate and severe COPD patients compared to those who received a placebo. Furthermore, we discovered that in the group treated with nanocurcumin of both moderate and severe COPD, both expression levels and serum concentration of Th17-related inflammatory factors were significantly lower after treatment than before treatment. In contrast, the placebo group exhibited no significant variations post-treatment compared to the pre-treatment condition.
As far as we know, an elevated number of Th17 lymphocytes and their related factors, a reduced number of regulatory T (Treg) cells, the disruption of the Th17/Treg cells balance, as well as hyperinflammation all play an important role in the pathogenesis of several immunological diseases, including SARS-CoV2 [28], MS [29], ankylosing spondylitis (AS) [30,31], Behcet's disease [32], Recurrent implantation failure [33,34], recurrent pregnancy loss [35,36], and recurrent miscarriage [37]. In the course of COPD, the lack of balance between pro-inflammatory and anti-inflammatory immune cell responses regulated by Th17 and Treg has been demonstrated to play a critical function in the pathogenesis of COPD [38].
According to our findings, the number of Th17 cells and related parameters rises in the PBMCs of moderate and severe COPD. This is consistent with the results of Xiang-Nan Li [39]. Also, Tiyaki Ito et al. revealed that the COPD mice model showed an elevated proinflammatory response (including high expression of the TNF-α, NF-κB, CD4, CD8, CD20, IL-6, and IL-17 parameters) with a simultaneously diminished anti-inflammatory response such as TGF-β, IL-10, and FOXP3 markers) in comparison with the control mice [40].
Th17 cells secrete IL-17, the proinflammatory cytokine, which can cause epithelial cells, bronchial fibroblasts, and smooth muscle cells to become activated. These cells are then encouraged to produce cytokines such as IL-6, IL-8, and G-CSF, which enhance the local proliferation of neutrophil granulocytes in the airway and worsen inflammatory responses in the airway and lungs [41]. Overall, elevated expression of Th17-related cytokines such as IL-17A, IL-17F, IL-21, IL-22, IL-23, and GM-CSF in COPD patients might imply that these factors have a role in the stimulation of T cell and endothelial cells and in turn, inducing chronic inflammation which observed in COPD patients [6].
Curcumin's prospective advantages in COPD have been proven in several studies. According to Moghaddam et al., curcumin prevented the migration of neutrophils to the lungs in a mouse model of a K-ras-driven lung tumor in which Haemophilus influenzae generated COPD-like airway inflammation [42]. Yuan et al. reported that curcumin suppressed the IkappaB-alpha protein degradation and the cyclooxygenase-2 expression, decreasing airway inflammation in a mouse model of COPD stimulated by LPS (lipopolysaccharide) and cigarette smoke [43]. In a clinical trial including subjects with moderate COPD, Funamoto et al. found that those who took highly absorbable curcumin had considerably lower oxidized LDL – alpha-1-antitrypsin LDL – than those who took a placebo [44].
In a similar research, Tahmasebi et al. evaluated the nanocurcumin on Th17 lymphocyte frequency and its immunomodulatory properties in mild and severe COVID-19 patients. They found that in both mild and severe COVID-19 patients, nanocurcumin decreased the number of Th17 lymphocytes and also reduced their related markers, such as RORγt, IL-17, IL-21, IL-23, and GM-CSF [45]. Also, in line with this study, Zhang et al. revealed that curcumin remarkably reduced the number of macrophages and neutrophils in bronchoalveolar lavage fluid (BALF) from COPD rats. They also discovered that curcumin administration significantly lowered IL-6, IL-8, and TNF-α levels in the serum and BALF of COPD rats [46].
Furthermore, Valizadeh et al. revealed that nanocurcumin had an anti-inflammatory impact in patients with COVID-19 by regulating inflammatory factors such as IL-1β, IL-6, TNF-, and IL-18. According to their results, nanocurcumin treatment significantly reduced IL-6 mRNA expression and serum secretion levels, as also the concentration of IL-1β in supernatant and serum samples of patients with COVID-19, compared to the pre-treatment condition, which could result in improvement of clinical manifestation and recovery of the patient [27]. In another study, Dolati et al. evaluated the effects of nanocurcumin on the number of Th17 lymphocytes and its related inflammatory factors (RORγt, IL-17A, and IL-23) in MS patients compared to healthy controls. Their finding revealed that the number of Th17 cells, the expression levels of RORγt and IL-17A parameters, and the serum concentration of the IL-17A cytokine all decreased significantly. Also, nanocurcumin did not impact IL-23 cytokine expression and secretion levels [29]. Another supportive research found that nanocurcumin has a therapeutic modulatory effect on Th17-related factors in ankylosing spondylitis patients [31]. Curcumin has been shown to suppress the cytokine storm caused by the excess production of proinflammatory factors by inhibiting key signaling pathways such as NF-κB and mitogen-activated protein kinase [47,48]. In a study by Possebon et al., the therapeutic effects of four medicinal herbs (Arctium lappa, Plantago major, Mikania glomerata Spreng, and Equisetum arvense) with anti-inflammatory properties were examined in the COPD model. Their results indicated the synergistic effects of these herbal medicines can be used as a potential therapeutic strategy [49]. However, more research is needed to understand the advantages of curcumin over other drugs. Also, to better understand the anti-inflammatory effects of nanocurcumin, it is better to evaluate other immune cells in future studies, such as Th1/Th2 or Th1/Th17 axis. Also, evaluating other important cytokines, such as IL-6 and TGF-β, may be helpful in understanding the therapeutic potential of curcumin.
5. Conclusions
Our findings suggest nanocurcumin's usefulness as a potent immunomodulatory drug that might be beneficial in mitigating Th17 lymphocyte responses, alleviating inflammation, and accelerating COPD patients' rehabilitation. It is noteworthy that more research is needed to validate our results regarding nanocurcumin's beneficial anti-inflammatory and immunomodulatory impacts in COPD and to understand better the molecular processes altered by nanocurcumin therapy.
Funding
This study was supported by the NIMAD Institute of Iran (Grant No:4000622)..
Data availability
Data will be made available on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declare of competing interests
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Amirhossein Mardi: Writing – review & editing, Writing – original draft, Investigation. Samaneh Abdolmohammadi-Vahid: Formal analysis, Data curation. Sarvin Alizadeh Sadeghi: Formal analysis, Data curation. Sajad Jafarzadeh: Formal analysis, Data curation. Sanaz Abbaspour-Aghdam: Visualization, Validation. Ali Hazrati: Visualization, Validation. Haleh Mikaeili: Project administration, Methodology. Hamed Valizadeh: Project administration, Methodology. Armin Sadeghi: Formal analysis, Data curation. Majid Ahmadi: Writing – review & editing, Supervision, Conceptualization. Mehdi Nadiri: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Majid Ahmadi reports financial support was provided by National Institute for Medical Research Development. Majid Ahmadi reports a relationship with Tabriz University of Medical Sciences that includes: employment. Majid Ahmadi has patent pending to NA. NA If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Majid Ahmadi, Email: ahmadi.m@tbzmed.ac.ir.
Mehdi Nadiri, Email: mehdi.nadiri@yahoo.com.
References
- 1.Angelis N., et al. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014;6(Suppl 1):S167. doi: 10.3978/j.issn.2072-1439.2014.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cazzola M., et al. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Expet Opin. Drug Deliv. 2020;17(5):635–646. doi: 10.1080/17425247.2020.1739021. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves P.B., Romeiro N.C. Multi-target natural products as alternatives against oxidative stress in chronic obstructive pulmonary disease (COPD) Eur. J. Med. Chem. 2019;163:911–931. doi: 10.1016/j.ejmech.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 4.van Haarst A., McGarvey L., Paglialunga S. Review of drug development guidance to treat chronic obstructive pulmonary disease: US and EU perspectives. Clin. Pharmacol. Ther. 2019;106(6):1222–1235. doi: 10.1002/cpt.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Rouzic O., et al. Th17 cytokines: novel potential therapeutic targets for COPD pathogenesis and exacerbations. Eur. Respir. J. 2017;50(4) doi: 10.1183/13993003.02434-2016. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano A., et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin. Exp. Immunol. 2009;157(2):316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aujla S.J., Dubin P.J., Kolls J.K. Interleukin-17 in pulmonary host defense. Exp. Lung Res. 2007;33(10):507–518. doi: 10.1080/01902140701756604. [DOI] [PubMed] [Google Scholar]
- 8.Miravitlles M., et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev. 2021;30(160) doi: 10.1183/16000617.0075-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matera M.G., Cazzola M., Page C. Prospects for COPD treatment. Curr. Opin. Pharmacol. 2021;56:74–84. doi: 10.1016/j.coph.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Ingawale D.K., Mandlik S.K., Patel S.S. An emphasis on molecular mechanisms of anti-inflammatory effects and glucocorticoid resistance. J. Compl. Integr. Med. 2015;12(1):1–13. doi: 10.1515/jcim-2014-0051. [DOI] [PubMed] [Google Scholar]
- 11.Abbaspour-Aghdam S., et al. Immunomodulatory role of Nanocurcumin in COVID-19 patients with dropped natural killer cells frequency and function. Eur. J. Pharmacol. 2022;933 doi: 10.1016/j.ejphar.2022.175267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meja K.K., et al. Curcumin restores corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2. Am. J. Respir. Cell Mol. Biol. 2008;39(3):312–323. doi: 10.1165/rcmb.2008-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatamipour M., et al. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iranian journal of basic medical sciences. 2019;22(3):282. doi: 10.22038/ijbms.2019.32873.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbert R., et al. Global burden of COPD: systematic review and meta-analysis. Eur. Respir. J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro S.D. End-stage chronic obstructive pulmonary disease: the cigarette is burned out but inflammation rages on. Am. J. Respir. Crit. Care Med. 2001;164(3):339–340. doi: 10.1164/ajrccm.164.3.2105072c. [DOI] [PubMed] [Google Scholar]
- 16.Rennard S.I. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest. 1998;113(4):235S–241S. doi: 10.1378/chest.113.4_supplement.235s. [DOI] [PubMed] [Google Scholar]
- 17.Saetta M., et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163(6):1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 18.Alcorn J.F., Crowe C.R., Kolls J.K. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 19.Curtis J.L., Freeman C.M., Hogg J.C. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc. Am. Thorac. Soc. 2007;4(7):512–521. doi: 10.1513/pats.200701-002FM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barczyk A., Pierzchala W., Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 21.Singh D., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 22.Rennard S.I., et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175(9):926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S., et al. Inflammation in COPD: implications for management. Am. J. Med. 2012;125(12):1162–1170. doi: 10.1016/j.amjmed.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Mahler D.A., et al. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126(3):926–934. doi: 10.1378/chest.126.3.926. [DOI] [PubMed] [Google Scholar]
- 25.Matera M.G., Calzetta L., Cazzola M. TNF-α inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm. Pharmacol. Therapeut. 2010;23(2):121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Catanzaro M., et al. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules. 2018;23(11):2778. doi: 10.3390/molecules23112778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valizadeh H., et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharm. 2020;89 doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeghi A., et al. Th17 and Treg cells function in SARS‐CoV2 patients compared with healthy controls. J. Cell. Physiol. 2021;236(4):2829–2839. doi: 10.1002/jcp.30047. [DOI] [PubMed] [Google Scholar]
- 29.Dolati S., et al. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int. Immunopharm. 2018;61:74–81. doi: 10.1016/j.intimp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadi M., et al. The effects of nanocurcumin on Treg cell responses and treatment of ankylosing spondylitis patients: a randomized, double‐blind, placebo‐controlled clinical trial. J. Cell. Biochem. 2020;121(1):103–110. doi: 10.1002/jcb.28901. [DOI] [PubMed] [Google Scholar]
- 31.Hajialilo M., et al. Nanocurcumin: a novel strategy in treating ankylosing spondylitis by modulating Th17 cells frequency and function. J. Cell. Biochem. 2019;120(7):12027–12038. doi: 10.1002/jcb.28488. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi M., et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet's disease. J. Cell. Physiol. 2019;234(4):3985–3994. doi: 10.1002/jcp.27207. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadi M., et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst. Biol. Reprod. Med. 2017;63(6):350–359. doi: 10.1080/19396368.2017.1390007. [DOI] [PubMed] [Google Scholar]
- 34.Ahmadi M., et al. Sirolimus as a new drug to treat RIF patients with elevated Th17/Treg ratio: a double-blind, phase II randomized clinical trial. Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105730. [DOI] [PubMed] [Google Scholar]
- 35.Abdolmohammadi Vahid S., et al. Altered T‐cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. J. Cell. Physiol. 2019;234(4):4924–4933. doi: 10.1002/jcp.27290. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadi M., et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: non randomized, open-label clinical trial. Immunol. Lett. 2017;192:12–19. doi: 10.1016/j.imlet.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Jafarzadeh S., et al. Intravenous immunoglobulin G treatment increases live birth rate in women with recurrent miscarriage and modulates regulatory and exhausted regulatory T cells frequency and function. J. Cell. Biochem. 2019;120(4):5424–5434. doi: 10.1002/jcb.27821. [DOI] [PubMed] [Google Scholar]
- 38.Wang H., et al. Imbalance of peripheral blood T h17 and T reg responses in patients with chronic obstructive pulmonary disease. The clinical respiratory journal. 2015;9(3):330–341. doi: 10.1111/crj.12147. [DOI] [PubMed] [Google Scholar]
- 39.Li X.-N., Pan X., Qiu D. Imbalances of Th17 and Treg cells and their respective cytokines in COPD patients by disease stage. Int. J. Clin. Exp. Med. 2014;7(12):5324. [PMC free article] [PubMed] [Google Scholar]
- 40.Ito J.T., et al. Th17/Treg imbalance in COPD progression: a temporal analysis using a CS-induced model. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0209351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oboki K., et al. Th17 and allergy. Allergol. Int. 2008;57(2):121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 42.Moghaddam S., et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30(11):1949–1956. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J., et al. Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-κB signaling and COX-2 in cigarette smoke-induced COPD mice. Inflammation. 2018;41(5):1804–1814. doi: 10.1007/s10753-018-0823-6. [DOI] [PubMed] [Google Scholar]
- 44.Funamoto M., et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:2029. doi: 10.2147/COPD.S104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tahmasebi S., et al. Immunomodulatory effects of Nanocurcumin on Th17 cell responses in mild and severe COVID‐19 patients. J. Cell. Physiol. 2021;236(7):5325–5338. doi: 10.1002/jcp.30233. [DOI] [PubMed] [Google Scholar]
- 46.Zhang M., et al. Curcumin ameliorates alveolar epithelial injury in a rat model of chronic obstructive pulmonary disease. Life Sci. 2016;164:1–8. doi: 10.1016/j.lfs.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Li Q., et al. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARγ-NF-κB signaling pathway. Food Funct. 2019;10(12):7983–7994. doi: 10.1039/c9fo02159k. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan P.S., et al. Intranasal curcumin regulates chronic asthma in mice by modulating NF-ĸB activation and MAPK signaling. Phytomedicine. 2018;51:29–38. doi: 10.1016/j.phymed.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Possebon L., et al. Anti-inflammatory actions of herbal medicines in a model of chronic obstructive pulmonary disease induced by cigarette smoke. Biomed. Pharmacother. 2018;99:591–597. doi: 10.1016/j.biopha.2018.01.106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.