Abstract
Juvenile onset recurrent respiratory papillomatosis is a lifelong benign squamous lesion associated with HPV infection, particularly HPV6 and HPV11 genotypes. These lesions are rare, but can lead to laryngeal obturations, which can cause disabling dyspnea, or transform into squamous cell carcinoma. The aim here is to provide an epidemiological, biological and clinical overview of this pathology, particularly in children, in order to understand the issues at stake in terms of research and the development of medical and therapeutic management tools.
Keywords: HPV, Papillomavirus, Papillomatosis, Airway, Children, Head and neck, JRRP
Highlights
-
•
JORRP is a rare and chronic pediatric disease induced by HPV and characterized by the growth of benign tumors in the respiratory tract.
-
•
HPV 6 and 11 are the main genotypes found in JORRP.
-
•
Researchers are actively investigating genetic, immunological, and virological aspects of JORRP to identify novel treatment approaches.
7. Introduction
7.1. Definitions
Respiratory recurrent papillomatosis (RRP) is characterized by the recurrent appearance of multiple contiguous polypoid squamous tumors in the respiratory tree, associated with HPV infection [1]. These papillomas are predominantly distributed at the junctions between respiratory epithelium and squamous epithelium throughout the respiratory tract, from nasal cavities to bronchi. According to a meta-analysis involving 400 patients, the primary site affected is the larynx (97.9 %), followed by the trachea (10.9 %) and the soft palate (10.3 %), with patients potentially presenting lesions at multiple locations [2]. Two age peaks for the onset of RRP are observed: the first corresponds to the juvenile form (JRRP) in children under 5 years old, and the second occurs in patients aged 20–40 years, corresponding to the adult form [1]. RRP is linked to HPV infection, mainly genotypes 6 and 11, classified as low oncogenic risk [3].
The clinical manifestations depend on the extent and progression of the lesions and may include dysphonia, cough, stridor, progressive chronic dyspnea, and even acute dyspnea. In children, the characteristic clinical profile of RRP is the triad of dysphonia, stridor, and dyspnea [4]. In adults, dysphonia is the most common presenting sign. A chronic dyspnea is a permanent or semi-permanent limitation of physical activity but also can also be a long-lasting respiratory impairment at rest due to a respiratory condition. In the present context, the condition is the partial but chronic obstruction by papillomas. A dysphonia is a modified, abnormal voice.
Due to the small diameter of the respiratory airways, symptoms tend to be more severe in children, potentially impacting the prognosis. The clinical course of the disease varies from a mild presentation with occasional spontaneous remission to an aggressive and chronic condition. According to Derkay et al., an aggressive form is defined by more than 4 surgical interventions per year [5]. Dysphonia in adults and dyspnea in children are the most decisive indications for treatment.
RRP is challenging to treat due to its viral nature, recurrent course, and its ability to spread along both the upper and lower respiratory pathways, as well as within the pharyngeal mucosa. For the remainder of this article, we will mainly be discussing juvenile RRP, or JRRP.
7.2. Epidemiology
JRRP is a rare disease; however, it represents the most common benign laryngeal tumor pathology in children. In Canada, through the establishment of a national retrospective database, it was calculated that the incidence between 1994 and 2007 was 0.24 cases per year per 100,000 children aged 14 and under, with a prevalence of 1.11 per 100,000 children. The median age at diagnosis was 4.4 years with a sex ratio close to 1:1 [6]. These figures are similar to a study from 1996 on a population of JRRP patients in Seattle and Atlanta [7]. In Europe, a study covering 50 % of the Danish population between 1969 and 1984 found an incidence of 3.6 cases per year per 100,000 inhabitants under the age of 20 [8].
These epidemiological data already did and will continue to evolve with the introduction of HPV vaccine, especially in countries where HPV vaccination is recommended systematically. In 2018, an Australian study showed a decrease in the incidence of JRRP in children under 14 after the implementation of the national HPV vaccination program in 2007 [9]. More recently, Meites et al. described a decline of the incidence of JRRP in the United States since 2006, when HPV vaccination of females through age 26 years has been recommended. Incidence of JRRP per 100,000 births using national data declined from 2.0 cases in 2004–2005 to 0.5 cases in 2012–2013 (IRR = 0.2, 95 % CI = 0.1-0.4); incidence using state-level data declined from 2.9 cases in 2004–2005 to 0.7 cases in 2012–2013 (IRR = 0.2, 95 % CI = 0.1-0.4) [10].
From a socio-economic perspective, the incidence and prevalence of JRRP in the United States for the year 2006 were described using two databases from private insurances (The Optum Clinformatics®) and state Medicaid public insurances (Truven Marketscan Medicaid). Interestingly, the incidence and prevalence were higher in the population covered by state insurances than in the population covered by private insurances (respective incidence of 1.03 per 100,000 people and 0.51 per 100,000 people, respective prevalence of 2.93 per 100,000 and 1.45 per 100,000) [11]. This raised the question of whether populations with a lower socio-economic status could have been more at risk of developing the disease. To our knowledge however, it was the only report establishing such a link between JRRP and socio-economic status. All other epidemiologic studies with regards social characteristics did not highlight a link with incidence.
7.3. Aetiology and infection mechanism
JRRP is caused by an HPV infection. The prevalence of the HPV genotypes varies between studies, but comparable results are found across large series.
-
-
In the United States, in a cohort of 162 children, 79.6 % of JRRP cases were associated with HPV 6, 15.4 % with HPV 11, 0.6 % with HPV 16 and 1.2 % with a co-infection (or multiple infection) of 2 HPV (1 with HPV 6 and 44, and 1 with HPV 6 and 54) [12].
-
-
In Australia, in a cohort of 47 children, the distribution was 55 % for HPV 11, 43 % for HPV 6, and 2 % for co-infection (or multiple infection) with HPV 6 and 11 [13].
-
-
In Bulgaria, in a cohort of 23 children, the distribution was 61.9 % for HPV 11, 23.8 % for HPV 6, and 14 % for co-infection (or multiple infection) with HPV 6 and 11 [14].
There have also been other reports in the literature of some cases of infection with high oncogenic risk HPVs such as HPV 16, 31, 33, 35, and 39 [15,16].
The transmission of the virus to children is still poorly understood; whereas in adults, the primary factor for HPV transmission is sexual activity. Since HPV types 6 and 11 are also implicated in anogenital warts [17], it has been quickly suspected that the infection is transmitted from mother to child during childbirth. Indeed, it has been demonstrated that the risk of developing JRRP in a child born to a mother with genital condylomas is 231.4 times higher than in a child born to a mother without lesions [18]. However, mother-to-child transmission during delivery is likely not the sole mechanism explaining HPV acquisition. An American study involving 333 mothers and their children examined cervical swabs and oral rinses from the mothers immediately before delivery, and oral and genital swabs from their newborns at least 24 h after delivery. Viral DNA was present in 30 % of all maternal samples, with 27 % in cervical swabs and 3 % in oral rinse fluids; only 1.5 % of newborn samples showed viral DNA (0.6 % in genital swabs and 0.9 % in oral swabs). Among the three mother/child pairs with a positive HPV DNA sample in both the mother and the newborn, there was a discordance in HPV types between the mother and child in two pairs. It is also noteworthy that two HPV-positive newborns were born to HPV-negative mothers [19]. Therefore, it can be assumed that there is also oral transmission of HPV through the newborn's environment. Data from a Spanish study involving 143 mother-child pairs supported this hypothesis. Cervical swabs were performed on mothers during a prenatal visit (median of 31 weeks of gestation) and at a postpartum visit, 6 weeks after delivery. In children oral and anogenital swabs were performed immediately after delivery and at 6 weeks, 3, 6, 12, and 24 months after delivery. At the prenatal visit, 46 % of women had a positive HPV sample, and 21 % on the postpartum testing. In children, 6 % had a positive HPV sample immediately after delivery, and 12.7 % at 6 weeks after delivery. Overall, 16.9 % of children born to HPV-negative mothers were HPV-positive [20]. Furthermore, in the same study, HPV 6 and 11 represented 23 % of the HPV types found in children, whereas these genotypes were found in only 15 % of mothers. These relatively high rates of HPV infections in newborns and the prevalence figures for HPV 6 and 11 in pregnant women (2.45 % and 1.76 %, respectively, according to a meta-analysis including a total of 13,640 pregnant women [21]) contrast with the low prevalence of JRRP. Thus, HPV infection alone is not sufficient to explain the onset of the disease. In the literature, various abnormalities of the innate and adaptive immune systems in combating HPV infection have been identified, which could explain the susceptibility of certain patients to develop JRRP. At the genetic level, one study highlighted the under-representation of two genes encoding the NK cell KIR receptor (3DS1 and 2DS1) in patients with more severe JRRP [22]. KIR receptors activate NK cells against their target cells, resulting in the release of cytotoxic molecules and the secretion of IFNγ, which is necessary for a proper anti-tumor immune response. The innate immune response of patients would therefore be unable to control HPV infection at an early stage.
It also has been shown that JRRP patients have a local Th2-oriented adaptive immune response [23,24], which prevents them from activating the cytotoxic CD8+ T lymphocytes needed to destroy virus-infected cells.
Finally, HPV may evade the immune system by decreasing class I MHC expression of viral antigens in infected cells. Indeed, Vambutas et al. found a decrease in class I MHC and TAP-1 protein expression in JRRP tumoral cells. TAP-1 enables antigens to be transported from the cytosol to the endoplasmic reticulum, where they bind to class I MHC. The authors also demonstrate a significant association between disease severity and decreased TAP-1 protein expression [25].
7.4. Natural history
The clinical course of JRRP is variable and mostly unpredictable. Some patients experience a rapidly progressive disease, requiring frequent endoscopic excisions under general anesthesia to maintain airway patency, and in some cases, emergency tracheotomy may be necessary. Conversely, other patients exhibit a slow-evolving disease with occasional spontaneous remission [26]. In a 2003 American series involving 603 patients, lesion regression was observed in 20 % of cases [27]. The follow-up strategy for these patients is thus a significant challenge because there are currently no reliable markers for disease progression.
Pulmonary dissemination of the disease is sometimes associated, leading to severe respiratory failure. In a large Chinese cohort, 9 % of children had bronchial and pulmonary involvement 7 years after the onset of JRRP, all of them underwent tracheostomy and they had a higher mortality rate (OR = 94.909) compared with children without bronchial and pulmonary involvement [28]. Additionally, very rare cases of transformation of squamous papillomas in the context of JRRP into in situ and/or invasive squamous cell carcinoma have been described, mostly in the lungs and more rarely in the larynx [[29], [30], [31]]. In large North American cohorts, carcinomatous transformation is overall observed in about 1 % of patients with JRRP [6,31]. Several studies highlight an association between pulmonary involvement in JRRP and carcinomatous transformation [31,32]. In a meta-analysis of 1666 children, 16 % of patients with JRPP and a pulmonary involvement developed bronchopulmonary squamous cell carcinoma [31]. HPV 11 infection is also suspected to be associated with a higher risk of carcinomatous transformation compared to HPV 6 infection. Two studies report exclusive HPV11 infection in patients with malignant transformation [30,33]. Finally, a study shown a similar role for E6/E7 of HPV11 in the maintenance of episomes as HPV high-risk E6 and E7 proteins [34]. Little data is available on the integration of HPV into tumor cells of JRRP, and although HPV DNA is generally episomal rather than fused or integrated with host DNA, this information remains unclear for these lesions.
8. Diagnosis
8.1. Clinical features
As discussed above, JRRP can present clinically with a wide variety of respiratory symptoms, from simple dysphonia to severe obstructive respiratory distress. Depending on the severity of the disease, these symptoms may have progressed over many months, or much more rapidly in a matter of weeks or even days. Stridor, obstructive inspiratory dyspnea and signs of hypoxia and hypercapnia can be seen in the most severe forms, requiring emergency treatment. In cases of chronic obstruction, obstructive sleep apnea syndrome (OSAS), delayed growth and weight loss are possible [35].
8.2. Endoscopic examination and pulmonary assessment
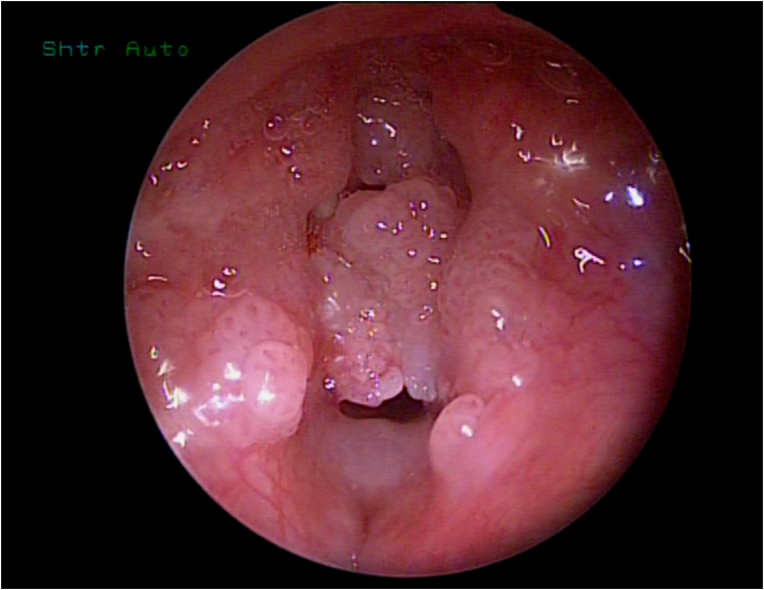
Diagnosis is strongly suspected on fiberoptic examination, which can be performed at any age and in almost any clinical situation, showing papillomas of typical appearance: raspberry-like, sessile or pedunculated, preferentially affecting the larynx and frequently obstructive (see Fig. 1).
Fig. 1.
Larynx, endoscopic view. The lumen is almost completely obstructed by papillomas. Vocal folds are not identifiable.
Confirmation of the diagnosis of JRRP is made by direct laryngoscopy under general anesthesia with anatomopathological and virological sampling. Laryngotracheal endoscopy using optics is the most reliable method for diagnosing JRRP, as it enables direct visualization of lesions, evaluation of their extent, and collection of biopsy samples for anatomopathological diagnosis and viral genotyping. This endoscopy is also useful for therapeutic planning. Analysis of the lesions ensures a definitive diagnosis [4].
The procedure allows lesion mapping with gradation of severity, enabling intra- and inter-individual comparisons via the Derkay-Wiatrak score; biopsies for pathological confirmation of diagnosis and viral typing; and surgical treatment [36].
The severity of PRR can be assessed using the Derkay - Coltrera grading system, which ranks the extent of papillomatosis at defined subsites in the aerodigestive tract.
This scoring system was developed to enable monitoring of a child's disease progression, accurate communication between surgeons and appropriate management [5].
A chest CT scan is recommended at the time of initial diagnosis. If this CT scan is normal and if endoscopic controls show no progression of the disease towards the bronchi, a control CT-scan is recommended every 5 years [35].
8.3. Pathology
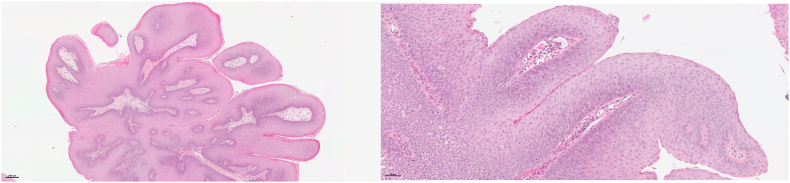
The microscopic lesion of JRRP is the squamous papilloma. It is a benign tumor with a papillary architecture, composed of a branching network of fibro-vascular axes lined by squamous epithelium (Fig. 2). It is often associated with signs of HPV infection: cells exhibit koilocytic features (clear perinuclear halo, enlarged hyperchromatic and irregular nucleus, binucleation). Hyperplasia of parabasal cells in the lower half of the epithelium can also be observed. Mitoses along the basal layer may be present but do not have a pejorative character. Surface keratinization, typically in the form of parakeratosis, may occur. Lastly, rarely, lesions of squamous epithelial dysplasia could be observed [1].
Fig. 2.
Microscopic image of JRRP. A: Papillary architecture of squamous lesion (H&E stain, 5× magnification). B: The squamous epithelium shows hyperplasia of basal cells (1) and a layer of surface parakeratosis (2). The papillary axis is richly vascularized (3) (H&E stain, 20× magnification).
8.4. Complementary examinations
As mentioned above, additional tests should include at least viral typing and a chest CT scan. Depending on the case, bronchial fibroscopy and pulmonary function tests may also be indicated.
At each endoscopy, biopsies should be performed to check for the appearance of dysplasia.
8.5. Prognostic factors
Many studies have attempted to identify severity risk factors to predict the progression of the disease in patients. A literature review including 32 articles representing 2200 patients with JRRP concluded that the primary risk factors for severity are early age at diagnosis and infection with HPV 11 [37].
Regarding the HPV genotype, caution is advised in interpreting the results. In studies finding a correlation between clinical severity (excluding any carcinomatous transformation) and HPV 11 infection, there is sometimes an earlier age at diagnosis among patients infected with HPV 11 [13,[38], [39], [40]], potentially constituting a confounding factor.
Several studies have investigated the utility of immunohistochemical markers for assessing severity. Ahn et al. examined the density of cells expressing CD8, CD4, FoxP3, PD-1, or PD-L1 in biopsies from 39 patients with papillomas. Only the density of CD8+ cells was inversely correlated with the severity of the disease (p = 0.01) [41]. Another study involving papillomas from 12 patients found a trend between a higher number of cells marked by the anti-p53 antibody and increased disease activity; however, this association was not statistically significant (p = 0.1) [42]. A recent study on a cohort of 48 patients, with automated analysis of immunohistochemical staining using anti-p53 and anti-p63 antibodies, does not support the usefulness of these antibodies for stratifying the risk of disease aggressiveness [43]. Finally, a high transcription of mRNA for E6 and E7 proteins of HPV 6 and 11 appears to be correlated with a more aggressive disease [44].
9. Management
There is currently no curative treatment for JRRP. Surgical excision of papillomas remains the principal resource, primarily to ensure airway patency and, when possible, improve the voice. Several surgical options are currently available: laryngoscopy using cold instruments, laser (notably CO2) and coblation. A tracheostomy, although not recommended, is sometimes necessary [45].
9.1. Endoscopic management
Endoscopic procedures are the gold standard in the treatment of JRRP and are performed under general anesthesia. Papillomas are removed using, depending on local conditions and the surgeon's preference, cold steel instruments, a microdebrider, laser, or a radiofrequency/coblation device. A microdebrider is a wand with fast-rotating blades and selective suction, enabling debridement of affected tissue. When papillomas are simultaneously present in the larynx and proximal trachea, its use is an effective solution for their removal.
In some case of mild laryngeal lesions in adults, an office-based procedure using local anesthesia and flexible scopes is sometimes possible [46].
Depending of the severity of the disease, the need for endoscopic procedures is highly variable, ranging from one every few years to an emergency desobstruction every month or less.
Furthermore, the recurrence of excision surgeries and the extent of lesions can lead to morbidity, ranging from simple adhesions to laryngotracheal stenosis and result in vocal impairment or respiratory discomfort.
9.2. Medical treatment
Numerous medical treatments have been described to treat JRRP, most of them without any statistically acceptable proof, but only two are currently used on a regular basis, their efficacy being fairly well established.
9.2.1. Cidofovir
Cidofovir is a cytosine nucleotide analog that blocks replication of DNA viruses by inhibiting viral DNA polymerase [47].
Cidofovir is mainly used as an intralesional injection, during endoscopy, and reserved for severe forms of the disease. Cidofovir appears to be effective in increasing the interval between procedures, thereby reducing the number of surgeries required.
An international multicenter retrospective study of 635 JRRP patients conducted by Tjon et al. showed that intralesional cidofovir is one of the mainstays of complementary treatment for JRRP, and does not cause toxicity [47,48].
Earlier works have suggested that cidofovir might be associated with an increased risk of malignant transformation. This has never been proven, to stay cautious, its injection is contraindicated in cases of high-grade dysplasia. Regular biopsies are therefore necessary during disease monitoring, particularly when cidofovir treatment is being considered or has already been carried out [49,50].
9.2.2. Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting human vascular endothelial growth factor A (VEGF-A). Bevacizumab has no effect on viral DNA or apoptosis mechanisms, but has a direct antiangiogenic action that affects tumor growth and is responsible for the therapeutic effect. It reduces lesion size and improves airway permeability, but does not eradicate the viral infection.
Bevacizumab is a recent adjuvant treatment option for the most aggressive cases of JRRP, or when lungs are involved. Although no randomized clinical trial (RCT) has been conducted to date, published data are promising, with few or no significant sub-lesional side effects, but with systemic effects during IV infusions (headache, bleeding, thrombosis, impact on ovarian function, etc.). Administered intravenously, it has demonstrated benefit in patients with JRRP poorly controlled by surgery and other adjuvants, and has shown promising results in patients with lung disease, resulting in improvement or stabilization of disease progression. Best et al. have shown, following a case series study, that systemic bevacizumab appears to be promising in the most resistant and aggressive JRRP. The case study by Zur et al. concerning the use of intravenous bevacizumab also points in this direction [[51], [52], [53], [54]].
Intralesional injections of bevacizumab have also been studied and seem promising. They however, like the cidofovir, require an endoscopy under general anesthesia to be delivered [55].
The efficacy of bevacizumab and cidofovir have been compared, even their mechanisms of action and administration modalities are very different. Both seem to have a similar effectiveness on JRRP, suggesting that a combined approach may be a future interesting option [56].
9.2.3. Others
Another avenue of research concerns inhibitors of an apoptosis protein expressed on the surface of T lymphocytes: PD-1 (Programmed cell Death protein 1). The association of this molecule with its ligand PDL-1, expressed by tumor cells, is thought to help the virus escape from the host immune system, thereby promoting papilloma growth. The study by Ahn et al. shows a significant presence of these two molecules in papillomas. Targeting the PD-1 pathway therefore represents a promising strategy for the treatment of JRRP. A non-randomized Phase II trial for the treatment of adults with severe tracheal, bronchial, pulmonary or laryngeal PRR with the anti-PD-1 monoclonal antibody pembrolizumab is currently underway [41].
Numerous drugs have been tested and abandoned, due to lack of sufficient proof of efficacy: indole-3-carbinol (I3C), dietary supplementation with cruciferous vegetable derivatives (cabbage, broccoli), interferon alpha, ribavarin, acyclovir, propranolol, cimetedin, heat shock protein 65, celecoxib and other Cox-2 inhibitors, retinoids,- vitamin A metabolites and analogues, local injections of mumps vaccine …
9.3. Role of vaccination
9.3.1. In the management of the disease
Several studies support the use of the HPV vaccine as an adjuvant treatment for JRRP. As these HPV vaccine are used for prophylaxis, the mechanism underlying the potential therapeutic efficacy on JRRP is unknown. One hypothesis is that vaccination may prevent disease recurrence by avoiding reinfection after surgical removal [57].
In the study by Yui et al., in 2018, comparison of pre- and post-vaccination periods showed a significant increase in mean times between surgical procedures (3.5 months vs. 12.8 months), as well as a significant reduction in the number of surgical procedures performed per year and post-vaccination (2.7 vs. 0.81) [58]. After vaccination, 5 patients showed no sign of papilloma recurrence for more than 12 months The meta-analysis by Rosenberg et al. also showed a reduction in the number of surgical procedures in vaccinated patients, as did the 10 patients in the study by Papaioannou et al. [59,60]. Isolated case reports of children with PRR have also been published in which the vaccination seemed to induce an increase in the time between surgical procedures or complete remission.
Thus, despite the low level of evidence (retrospective studies with small samples of patients, case reports, possible biases) concerning a possible therapeutic effect of the anti-HPV vaccine on JRRP, and after explaining to patients the current low level of evidence for this vaccination on the evolution of JRRP, it would appear justified to offer this vaccine as an adjuvant treatment to previously unvaccinated patients, in compliance with its MA (from age 9). Some teams offer it, after explaining to parents, outside of the MA before age 9, in cases of particularly aggressive disease in young children [61].
Finally, preliminary studies on the efficiency of DNA vaccines against RRP have been conducted in adults and showed promising results [62].
9.3.2. Vaccination for prevention
The most promising result in recent years has been the publication of a report by an Australian team following the implementation of a systematic HPV vaccination program in adolescents. The quadrivalent vaccine was introduced in 2007, first in girls, then in boys. Vaccination coverage (at least 2 doses) has reached over 80 % in girls and over 75 % in boys. A decline in the annual incidence of JRRP was observed, from 0.16 to 0.02 per 100,000 children between 2012 and 2016, with even between 2016 and 2022 two years without any new cases on Australian territory. Of the 15 Australian JRRP cases reported between 2012 and 2016, none of the children's mothers had been vaccinated. This study was the first demonstration that the HPV vaccine plays a role in the prevention and eradication of JRRP. Comparable results are hoped for in other countries that extended vaccination a few years ago [9].
10. Social and economic burden of the disease
Due to the recurring nature of the disease, involving repeated endoscopies and expensive treatments, the costs of patient care are substantial. In the United States, to treat a patient throughout the duration of their illness, the cost ranges from $60,000 to $470,000 [63]. It is estimated that, each year, the overall cost amounts to $150 million [64].
11. Conclusion
JRRP lesions are exceptional and may be associated with major complications such as pernicious recurrence or cancerization. Despite numerous studies, it is to date difficult to determine which factors induce unfavorable outcomes, apart from clinical criteria. Endoscopic procedure is the actual reference treatment, and can be combined with medical therapies. Preventive vaccination of mothers-to-be is the best hope that these lesions never appear in their very young children. Therapeutic vaccination trials are also underway.
12. Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-to-profit sectors.
CRediT authorship contribution statement
Charles Lepine: Writing – original draft, Formal analysis, Data curation. Nicolas Leboulanger: Writing – original draft, Data curation, Conceptualization. Cécile Badoual: Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nicolas LEBOULANGER and Cécile BADOUAL have been invited by MSD France the November 10, 2023 to their annual French meeting on HPV-related diseases.
Acknowledgments
The authors thank Dr Peter STERN for the invitation to publish in Tumor Virus Research and Pr François SIMON for his kind reviewing of this manuscript.
Data availability
No data was used for the research described in the article.
References
- 1.El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J., editors. WHO Classification of Head and Neck Tumours. fourth ed. International Agency for Research on Cancer; Lyon: 2017. [Google Scholar]
- 2.Kashima H., Mounts P., Leventhal B., Hruban R.H. Sites of predilection in recurrent respiratory papillomatosis. Ann. Otol. Rhinol. Laryngol. 1993;102:580–583. doi: 10.1177/000348949310200802. [DOI] [PubMed] [Google Scholar]
- 3.Donne A.J., Hampson L., Homer J.J., Hampson I.N. The role of HPV type in recurrent respiratory papillomatosis. Int. J. Pediatr. Otorhinolaryngol. 2010;74:7–14. doi: 10.1016/j.ijporl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Fortes H.R., von Ranke F.M., Escuissato D.L., Araujo Neto C.A., Zanetti G., Hochhegger B., et al. Recurrent respiratory papillomatosis: a state-of-the-art review. Respir. Med. 2017;126:116–121. doi: 10.1016/j.rmed.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Derkay C.S., Malis D.J., Zalzal G., Wiatrak B.J., Kashima H.K., Coltrera M.D. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope. 1998;108:935–937. doi: 10.1097/00005537-199806000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Campisi P., Hawkes M., Simpson K., Canadian Juvenile Onset Recurrent Respiratory Papillomatosis Working Group The epidemiology of juvenile onset recurrent respiratory papillomatosis derived from a population level national database. Laryngoscope. 2010;120:1233–1245. doi: 10.1002/lary.20901. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong L.R., Preston E.J., Reichert M., Phillips D.L., Nisenbaum R., Todd N.W., et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis Off Publ Infect Dis Soc Am. 2000;31:107–109. doi: 10.1086/313914. [DOI] [PubMed] [Google Scholar]
- 8.Lindeberg H., Elbrønd O. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965-1984. Clin. Otolaryngol. Allied Sci. 1990;15:125–131. doi: 10.1111/j.1365-2273.1990.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 9.Novakovic D., Cheng A.T.L., Zurynski Y., Booy R., Walker P.J., Berkowitz R., et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J. Infect. Dis. 2018;217:208–212. doi: 10.1093/infdis/jix498. [DOI] [PubMed] [Google Scholar]
- 10.Meites E., Stone L., Amiling R., Singh V., Unger E.R., Derkay C.S., et al. Significant declines in juvenile-onset recurrent respiratory papillomatosis following human papillomavirus (HPV) vaccine introduction in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73:885–890. doi: 10.1093/cid/ciab171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsico M., Mehta V., Chastek B., Liaw K.-L., Derkay C. Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex. Transm. Dis. 2014;41:300–305. doi: 10.1097/OLQ.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 12.Amiling R., Meites E., Querec T.D., Stone L., Singh V., Unger E.R., et al. Juvenile-onset recurrent respiratory papillomatosis in the United States, epidemiology and HPV types-2015-2020. J Pediatr Infect Dis Soc. 2021;10:774–781. doi: 10.1093/jpids/piab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbott M., Cossart Y.E., Kan A., Konopka M., Chan R., Rose B.R. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J. Clin. Microbiol. 1997;35:3098–3103. doi: 10.1128/jcm.35.12.3098-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draganov P., Todorov S., Todorov I., Karchev T., Kalvatchev Z. Identification of HPV DNA in patients with juvenile-onset recurrent respiratory papillomatosis using SYBR Green real-time PCR. Int. J. Pediatr. Otorhinolaryngol. 2006;70:469–473. doi: 10.1016/j.ijporl.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Dickens P., Srivastava G., Loke S.L., Larkin S. Human papillomavirus 6, 11, and 16 in laryngeal papillomas. J. Pathol. 1991;165:243–246. doi: 10.1002/path.1711650308. [DOI] [PubMed] [Google Scholar]
- 16.Peñaloza-Plascencia M., Montoya-Fuentes H., Flores-Martínez S.E., Fierro-Velasco F.J., Peñaloza-González J.M., Sánchez-Corona J. Molecular identification of 7 human papillomavirus types in recurrent respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 2000;126:1119–1123. doi: 10.1001/archotol.126.9.1119. [DOI] [PubMed] [Google Scholar]
- 17.Jamshidi M., Shekari M., Nejatizadeh A.A., Malekzadeh K., Baghershiroodi M., Davudian P., et al. The impact of human papillomavirus (HPV) types 6, 11 in women with genital warts. Arch. Gynecol. Obstet. 2012;286:1261–1267. doi: 10.1007/s00404-012-2416-1. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg M.J., Thorsen P., Lindeberg H., Grant L.A., Shah K.V. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet. Gynecol. 2003;101:645–652. doi: 10.1016/s0029-7844(02)03081-8. [DOI] [PubMed] [Google Scholar]
- 19.Smith E.M., Parker M.A., Rubenstein L.M., Haugen T.H., Hamsikova E., Turek L.P. Evidence for vertical transmission of HPV from mothers to infants. Infect. Dis. Obstet. Gynecol. 2010 doi: 10.1155/2010/326369. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellsagué X., Drudis T., Cañadas M.P., Goncé A., Ros R., Pérez J.M., et al. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect. Dis. 2009;9:74. doi: 10.1186/1471-2334-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P., Xu L., Sun Y., Wang Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol. Infect. 2014;142:1567–1578. doi: 10.1017/S0950268814000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonagura V.R., Du Z., Ashouri E., Luo L., Hatam L.J., DeVoti J.A., et al. Activating killer cell immunoglobulin-like receptors 3DS1 and 2DS1 protect against developing the severe form of recurrent respiratory papillomatosis. Hum. Immunol. 2010;71:212–219. doi: 10.1016/j.humimm.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonagura V.R., Hatam L., DeVoti J., Zeng F., Steinberg B.M. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol Orlando Fla. 1999;93:302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 24.Bonagura V.R., Hatam L.J., Rosenthal D.W., de Voti J.A., Lam F., Steinberg B.M., et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS Acta Pathol Microbiol Immunol Scand. 2010;118:455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vambutas A., Bonagura V.R., Steinberg B.M. Altered expression of TAP-1 and major histocompatibility complex class I in laryngeal papillomatosis: correlation of TAP-1 with disease. Clin. Diagn. Lab. Immunol. 2000;7:79–85. doi: 10.1128/cdli.7.1.79-85.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasca R.A., Clarke R.W. Recurrent respiratory papillomatosis. Arch. Dis. Child. 2006;91:689–691. doi: 10.1136/adc.2005.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves W.C., Ruparelia S.S., Swanson K.I., Derkay C.S., Marcus A., Unger E.R. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 2003;129:976–982. doi: 10.1001/archotol.129.9.976. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q., Li Y., Ma L., Xiao Y., Wang H., Ding Y., et al. Long-term outcomes of juvenile onset recurrent respiratory papillomatosis with pulmonary involvement. Laryngoscope. 2021;131:EE2277–E2283. doi: 10.1002/lary.29376. [DOI] [PubMed] [Google Scholar]
- 29.Go C., Schwartz M.R., Donovan D.T. Molecular transformation of recurrent respiratory papillomatosis: viral typing and P53 overexpression. Ann. Otol. Rhinol. Laryngol. 2003;112:298–302. doi: 10.1177/000348940311200402. [DOI] [PubMed] [Google Scholar]
- 30.Reidy PM, Dedo HH, Rabah R, Field JB, Mathog RH, Gregoire L, et al. Integration of Human Papillomavirus Type 11 in Recurrent Respiratory Papilloma-Associated Cancer. Laryngoscope n.d.;114:1906–1909. 10.1097/01.mlg.0000147918.81733.49. [DOI] [PubMed]
- 31.Gélinas J.-F., Manoukian J., Côté A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: a systematic review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2008;72:433–452. doi: 10.1016/j.ijporl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Karatayli-Ozgursoy S., Bishop J.A., Hillel A., Akst L., Best S.R.A. Risk factors for dysplasia in recurrent respiratory papillomatosis in an adult and pediatric population. Ann. Otol. Rhinol. Laryngol. 2016;125:235–241. doi: 10.1177/0003489415608196. [DOI] [PubMed] [Google Scholar]
- 33.Gerein V., Rastorguev E., Gerein J., Draf W., Schirren J. Incidence, age at onset, and potential reasons of malignant transformation in recurrent respiratory papillomatosis patients: 20 years experience. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2005;132:392–394. doi: 10.1016/j.otohns.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Oh S.T., Longworth M.S., Laimins L.A. Roles of the E6 and E7 proteins in the life cycle of low-risk human papillomavirus type 11. J. Virol. 2004;78:2620–2626. doi: 10.1128/jvi.78.5.2620-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor C., Balakrishnan K., Bottero S., Boudewyns A., Campisi P., Carter J., et al. International Pediatric Otolaryngology Group (IPOG): juvenile-onset recurrent respiratory papillomatosis consensus recommendations. Int. J. Pediatr. Otorhinolaryngol. 2020;128 doi: 10.1016/j.ijporl.2019.109697. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox L.J., Hull B.P., Baldassari C.M., Derkay C.S. Diagnosis and management of recurrent respiratory papillomatosis. Pediatr. Infect. Dis. J. 2014;33:1283–1284. doi: 10.1097/INF.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 37.Niyibizi J., Rodier C., Wassef M., Trottier H. Risk factors for the development and severity of juvenile-onset recurrent respiratory papillomatosis: a systematic review. Int. J. Pediatr. Otorhinolaryngol. 2014;78:186–197. doi: 10.1016/j.ijporl.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Wiatrak B.J., Wiatrak D.W., Broker T.R., Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114:1–23. doi: 10.1097/01.mlg.000148224.83491.0f. [DOI] [PubMed] [Google Scholar]
- 39.Buchinsky F.J., Donfack J., Derkay C.S., Choi S.S., Conley S.F., Myer C.M., et al. Age of child, more than HPV type, is associated with clinical course in recurrent respiratory papillomatosis. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabah R., Lancaster W.D., Thomas R., Gregoire L. Human papillomavirus-11-associated recurrent respiratory papillomatosis is more aggressive than human papillomavirus-6-associated disease. Pediatr. Dev. Pathol. 2001;4:68–72. doi: 10.1007/s100240010105. [DOI] [PubMed] [Google Scholar]
- 41.Ahn J., Bishop J.A., Roden R.B.S., Allen C.T., Best S.R.A. The PD-1 and PD-L1 pathway in recurrent respiratory papillomatosis. Laryngoscope. 2018;128:E27–E32. doi: 10.1002/lary.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perdana R.F., Herawati S., Yusuf M., Kristyono I., Nugroho P.S. Correlation between P53 and KI67 with aggressiveness factor in recurrent respiratory papillomatosis. Sys. Rev. Pharm. 2020;11:6. [Google Scholar]
- 43.Lépine C., Klein P., Voron T., Mandavit M., Berrebi D., Outh-Gauer S., et al. Histological severity risk factors identification in juvenile-onset recurrent respiratory papillomatosis: how immunohistochemistry and AI algorithms can help? Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.596499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lépine C., Voron T., Berrebi D., Mandavit M., Nervo M., Outh-Gauer S., et al. Juvenile-onset recurrent respiratory papillomatosis aggressiveness: in situ study of the level of transcription of HPV E6 and E7. Cancers. 2020;12 doi: 10.3390/cancers12102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derkay C.S., Bluher A.E. Update on recurrent respiratory papillomatosis. Otolaryngol. Clin. 2019;52:669–679. doi: 10.1016/j.otc.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Bar R., Mattei A., Haddad R., Giovanni A. Laryngeal office-based procedures: a safe approach. Am. J. Otolaryngol. 2023;45 doi: 10.1016/j.amjoto.2023.104128. [DOI] [PubMed] [Google Scholar]
- 47.Tran M.N., Galt L., Bashirzadeh F. Recurrent respiratory papillomatosis: the role of cidofovir. Respirol Case Rep. 2018;6 doi: 10.1002/rcr2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjon Pian Gi R.E.A., Ilmarinen T., van den Heuvel E.R., Aaltonen L.M., Andersen J., Brunings J.W., et al. Safety of intralesional cidofovir in patients with recurrent respiratory papillomatosis: an international retrospective study on 635 RRP patients. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2013;270:1679–1687. doi: 10.1007/s00405-013-2358-7. [DOI] [PubMed] [Google Scholar]
- 49.Broekema F.I., Dikkers F.G. Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2008;265:871–879. doi: 10.1007/s00405-008-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inglis A.F. Cidofovir and the black box warning. Ann. Otol. Rhinol. Laryngol. 2005;114:834–835. doi: 10.1177/000348940511401104. [DOI] [PubMed] [Google Scholar]
- 51.Best S.R., Mohr M., Zur K.B. Systemic bevacizumab for recurrent respiratory papillomatosis: a national survey. Laryngoscope. 2017;127:2225–2229. doi: 10.1002/lary.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zur K.B., Fox E. Bevacizumab chemotherapy for management of pulmonary and laryngotracheal papillomatosis in a child. Laryngoscope. 2017;127:1538–1542. doi: 10.1002/lary.26450. [DOI] [PubMed] [Google Scholar]
- 53.So R.J., Hidalgo Lopez J.C., Ballestas S.A., Klein A.M., Steuer C., Shin D.M., et al. Efficacy of systemic bevacizumab for recurrent respiratory papillomatosis with pulmonary involvement. Laryngoscope. 2024;134:577–581. doi: 10.1002/lary.30893. [DOI] [PubMed] [Google Scholar]
- 54.Derkay C.S., Wikner E.E., Pransky S., Best S.R., Zur K., Sidell D.R., et al. Systemic use of bevacizumab for recurrent respiratory papillomatosis: who, what, where, when, and why? Laryngoscope. 2023;133:2–3. doi: 10.1002/lary.30180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guragain R., Gyawali B.R. Intralesional bevacizumab as adjuvant therapy for juvenile onset recurrent respiratory papillomatosis: a systematic review. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. 2023;75:1296–1301. doi: 10.1007/s12070-022-03204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zagzoog F.H., Mogharbel A.M., Alqutub A., Bukhari M., Almohizea M.I. Intralesional cidofovir vs. bevacizumab for recurrent respiratory papillomatosis: a systematic review and indirect meta-analysis. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2023 doi: 10.1007/s00405-023-08279-0. [DOI] [PubMed] [Google Scholar]
- 57.Young D.L., Moore M.M., Halstead L.A. The use of the quadrivalent human papillomavirus vaccine (gardasil) as adjuvant therapy in the treatment of recurrent respiratory papilloma. J Voice Off J Voice Found. 2015;29:223–229. doi: 10.1016/j.jvoice.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Yiu Y., Fayson S., Smith H., Matrka L. Implementation of routine HPV vaccination in the management of recurrent respiratory papillomatosis. Ann. Otol. Rhinol. Laryngol. 2019;128:309–315. doi: 10.1177/0003489418821695. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg T., Philipsen B.B., Mehlum C.S., Dyrvig A.-K., Wehberg S., Chirilǎ M., et al. Therapeutic use of the human papillomavirus vaccine on recurrent respiratory papillomatosis: a systematic review and meta-analysis. J. Infect. Dis. 2019;219:1016–1025. doi: 10.1093/infdis/jiy616. [DOI] [PubMed] [Google Scholar]
- 60.Papaioannou V.-A., Lux A., Voigt-Zimmermann S., Arens C. Treatment outcomes of recurrent respiratory papillomatosis : retrospective analysis of juvenile and adult cases. HNO. 2018;66:7–15. doi: 10.1007/s00106-017-0378-0. [DOI] [PubMed] [Google Scholar]
- 61.Benedict J.J., Derkay C.S. Recurrent respiratory papillomatosis: a 2020 perspective. Laryngoscope Investig Otolaryngol. 2021;6:340–345. doi: 10.1002/lio2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mau T., Amin M.R., Belafsky P.C., Best S.R., Friedman A.D., Klein A.M., et al. Interim results of a Phase 1/2 open-label study of INO-3107 for HPV-6 and/or HPV-11-Associated recurrent respiratory papillomatosis. Laryngoscope. 2023;133:3087–3093. doi: 10.1002/lary.30749. [DOI] [PubMed] [Google Scholar]
- 63.Marsico M., Mehta V., Wentworth C. 2009. Estimating the Disease Burden of Juvenile Onset RRP in the US Using Large Administrative Databases – Preliminary Pilot Results. [Google Scholar]
- 64.Derkay C.S. Task force on recurrent respiratory papillomas. A preliminary report. Arch. Otolaryngol. Head Neck Surg. 1995;121:1386–1391. doi: 10.1001/archotol.1995.01890120044008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.