Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) correlates with poor outcomes, necessitating the identification of prognostic factors from an inflammation-nutritional perspective in locally advanced ICC patients after R0 resection.
Methods
We retrospectively reviewed the medical records of 159 locally advanced ICC patients from Sun Yat-sen University Cancer Center. Univariate and multivariate Cox regression analysis, as well as competing risk analysis, were conducted to explore prognostic variables for locally advanced ICC following surgery. To validate the robustness of our findings, we performed propensity score matching (PSM) analyses to evaluate survival differences based on inflammation-nutritional indexes.
Results
Considering non-cancer-specific death as competing risk factors, both systemic immune-inflammation index (SII, HR: 1.934) and prognostic nutrition index (PNI, HR: 0.604) emerged as significant prognostic variables for locally advanced ICC after R0 resection (P < 0.05). After PSM, the survival benefit between the low and high PNI sets remained clear (median survival time: 15.7 months vs 35.1 months, P = 0.002). Although the 5-year overall survival (OS) rate of the low SII group was higher than that of the high SII group, the difference was not statistically significant (17.5% VS 27.4%, P = 0.112). Other influencing factors included tumor number, tumor diameter, preoperative carcinoembryonic antigen (CEA)and carbohydrate antigen 19–9 (CA19-9) levels, and postoperative adjuvant therapy.
Conclusion
Individual inflammatory and nutritional status significantly impact the prognosis of locally advanced ICC undergoing R0 hapectomy. Oncologists should consider incorporating inflammation-nutritional conditions into the decision-making process for this subset of advanced ICC.
Keywords: locally advanced intrahepatic cholangiocarcinoma, inflammation, nutrition, competing risk analysis, propensity matching analysis
Introduction
Ranked as the second most common hepatic malignancy, intrahepatic cholangiocarcinoma (ICC) accounts for approximately 20% of all liver tumors.1 It is known for its aggressive pathological feature, originating from the intrahepatic biliary epithelium.2 Currently, radical liver resection (LR) is the golden method to treat ICC.3 Unfortunately, despite advances in multidisciplinary treatment, the poor outcome upsets most suffering patients because of the unresectably late stage at diagnosis.4 Most of them receive only palliative chemotherapy after being diagnosed, which makes the median survival time less than two years.1
Currently, there is no universally accepted definition for locally advanced ICC. Lunsford defined locally late-stage ICC as a single lesion or multifocal tumor larger than 2 cm, without invasion of extrahepatic large vessels or lymph nodes.5 Yi et al limited locally advanced ICC to stage N1 or T3/T4, regardless of any N stage, without evidence of distant metastasis.6 Similar to Yi, Moustafa considers stages III and IVa of the AJCC-7th TNM version or stage III of the AJCC-8th TNM version as locally advanced ICC.7 Besides, the standard treatment for locally advanced ICC remains controversial, and some guidelines have attempted to expand the LR indications to a subset of advanced ICC. The National Comprehensive Cancer Network guidelines recommend LR for early stage ICC without metastasis lesions after fully considering the extension and location of the lesions.8 Moustafa reported that patients with locally late-stage ICC who underwent LR had a higher overall survival rate compared with chemotherapy before and after matching.7 However, few studies were performed to figure out the independent factors affecting the prognosis of this subset after R0 resection.
It is generally believed that systemic inflammation can accelerate neoplastic progression.9 Chronic inflammation can stimulate tumorigenesis and accelerate tumor metastasis in multiple ways, such as by creating an immunosuppressive microenvironment. It has been reported that cholelithiasis-induced chronic inflammation and sclerosing cholangitis-induced intrahepatic inflammation can promote the occurrence of cholangiocarcinoma.10 Accurate assessment of systemic inflammation levels in patients with malignancies may help to improve poor outcomes, especially in hepato-biliary tumors. In various solid tumors, a link between the binding of inflammatory markers to different inflammatory cells in patients’ peripheral blood and individual prognosis has been demonstrated.11–13 Recently, the impact of individual nutritional status on malignant progression has attracted increasing attention.14 Nutrition level not only limits tumor development but also confines anti-cancer treatment strategies. Malnourished people often have weakened immune systems, which reduces disease resistance and limits medical options.15,16 Therefore, a comprehensive assessment of the inflammation-nutritional status for those patients may help in making precise medical decisions.
Given the above context, we aim to figure out prognostic factors that influence the prognosis of locally advanced ICC from an inflammation-nutritional viewpoint based on the competing risk and propensity-matching methods.
Methods and Materials
Patient Selection
In this study, we retrospectively analyzed 159 consecutive patients diagnosed with locally advanced ICC who underwent R0 LR at Sun Yat-sen University Cancer Center from December 2004 to December 2018. We regarded R0 LR as a negative margin resection. Due to the lack of a standard definition for locally advanced ICC, we defined the locally advanced ICC subset as stage III of the AJCC-8th edition according to Yi and Moustafa et al6,7 The selection criteria for included cases were as follows: (a) adult individuals, with Eastern Cooperative Oncology Performance Status Score 0 to 1 and preoperative status Child-Pugh A or B, (b) exactly pathological evidence of ICC with R0 resection but no indication of extrahepatic metastasis, (c) no perioperative death or postoperative death within 60 days of hospitalization. We excluded those patients with a history of other malignant diseases, palliative or emergent operation for cancer, or incomplete clinico-pathological records from further analysis. This retrospective study complied with the Helsinki Principles and was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center (ID:B2022-492-01). The need for informed consent was waived due to the nature of the retrospective study, and we conducted a necessarily anonymized process for all included patient data.
Medical Variables
The extracted data included baseline variables (age, gender, and liver cirrhosis), tumor-related characteristics [tumor diameter (≤1cm or >1cm), tumor number (single or multiple), pathological grade (well to moderate or poor to undifferentiated), and microvascular invasion (MVI, absence or presence), and regional lymph node metastasis (negative or positive)], and surgical factors [resection scope (minor and major) and resection margin (0 < x ≤ 1cm and >1cm)]. Major LR was confined as the resection of more than 3 couinaud segments. Besides, Preoperative and postoperative carcino-embryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19-9) levels were extracted. Moreover, we assessed the clinical relationships between several preoperative inflammatory and nutritional indexes [systemic immune-inflammation index (SII), aggregate systemic inflammation index (AISI), systemic inflammation response index (SIRI), gamma-glutamyl-transpeptidase to platelet ratio (GPR), prognostic nutrition index (PNI), and advanced lung cancer index (ALI)] and long-term outcome of locally advanced ICC after R0 resection. The definitions of the above inflammation-nutritional combined indexes have been previously described (Supplementary Table 1).17–22 All the selected patients were followed up regularly after discharge. The follow-up date was censored on June 30th, 2023. Overall Survival (OS) refers to the interval between the date of the LR to the death date.
Statistical Analysis
All statistical analyses were processed and analyzed by IBM SPSS Statistics 25.0 and R 4.1.3. We take a p-value less than 0.05 in a two-tailed test as statistical significance. Continuous factors are exhibited as “mean±SD”, while categorical factors are expressed as frequency and percentage. We took the median value as the cut-off value to divide the continuous variables into low-value and high-value cohorts. The chi-square or Fisher’s exact tests were taken to assess any difference between groups. Cox proportional hazards analysis was employed to evaluate the prognosis of locally advanced ICC after R0 LR. Kaplan–Meier survival analysis was explored using the Log rank test to examine prognosis difference between groups. Considering traditional Cox analysis overlooking the influence of competing risk events, we performed Cox regression analysis and competing risk analysis. It is reported that competing risk analysis could contribute to accurately evaluating prognosis without overestimating or underestimating the impact of certain variables.23–25 For competing risk analysis, we first took the Fine and Gray analysis to evaluate the sub-hazard ratio of prognosis for locally advanced ICC after R0 LR, considering no cancer-specific death as a competing event.24 Significant variables in univariate analysis were used to perform multivariate competing risk analysis to figure out the independent prognostic factors.25 To minimize selection bias in each group, we conducted a propensity-matching analysis. We first calculated the propensity scores between groups, using logistic regression analysis, and then took the nearest-neighbor matching way without replacement to match the cohort at a 1:1 ratio. The caliper width of 0.2 was set as the standard deviation of the propensity score logit. Variables employed in the matching process were those imbalanced factors between groups.
Results
General Characteristics
Finally, we included 159 patients with locally advanced ICC after R0 resection in this study. Their demographic and clinicopathological data are exhibited in Table 1. Among them, 62.3% were male, and 46.5% had liver cirrhosis. The average tumor diameter was 6.6 cm, and most were of poorly differentiated to undifferentiated grade. About 51.6% of patients accepted major LR, 58.5% got surgical margin >1cm, and 40.9% acquired adjuvant postoperative therapy.
Table 1.
Basic Clinicopathological Characteristics in Locally Advanced Intrahepatic Cholangiocarcinoma
| Variables | Total (n=159, %) | Variables | Total (n=159, %) |
|---|---|---|---|
| Age, (years) | 55.3±10.6 | Surgical Margin | |
| Gender | >1cm | 93 (58.5) | |
| Female | 60 (37.7) | ≤ 1cm | 66 (41.5) |
| Male | 99 (62.3) | RLNM | |
| Liver Cirrhosis | Negative | 120 (75.5) | |
| No | 23 (14.5) | Positive | 39 (24.5) |
| Yes | 74 (46.5) | Adjuvant postoperative therapy | |
| Unknown | 62 (39) | No | 94 (59.1) |
| Tumor Diameter, (cm) | 6.6 ± 2.8 | Yes | 65 (40.9) |
| Tumor number | CEA, (U/mL) | 15.7 ± 39.2 | |
| Single | 103 (64.8) | CA19-9, (U/mL) | 927.2 ± 2835.4 |
| Multiple | 56 (35.2) | pCEA, (U/mL) | 3.9 ± 6.8 |
| Grade | pCA19-9, (U/mL) | 87.8 ± 321.2 | |
| Well/Moderate | 51 (32.1) | SII | 650.5 ± 458.8 |
| Poor/Undifferentiated | 108 (67.9) | AISI | 364.9 ± 410.3 |
| MVI | SIRI | 11 ± 9.5 | |
| Absent | 116 (73) | GPR | 0.6± 0.8 |
| Present | 43 (27) | PNI | 52.9 ± 10.1 |
| Resection Scope | ALI | 50.9 ± 80.2 | |
| Minor | 77 (48.4) | ||
| Major | 82 (51.6) |
Abbreviations: MVI, microvascular invasion; RLNM, regional lymph node metastasis; CEA, carcino-embryonic antigen; CA19-9, carbohydrate antigen 19-9; pCEA, postoperative CEA; pCA19-9, postoperative CA19-9; SII, systemic immune- inflammation index; AISI, aggregate systemic inflammation index; SIRI, systemic inflammation response index; GPR, gamma-glutamyl-transpeptidase to platelet ratio; PNI, prognostic nutrition index; ALI, advanced lung cancer index.
Prognostic Survival Analyses for Locally Advanced ICC After R0 LR
The median survival time of the selected sets was 23.97 months (2.8 to 182.13 months). The 1-, 3-, and 5-year OS rates of the whole set were 70.7%, 36.6%, and 25.7%, respectively. Multivariate Cox regression analysis results showed that tumor diameter (HR: 1.87, 95% CI: 1.215–2.878, P = 0.004), tumor number (HR: 2.331, 95% CI: 1.592–3.413, P < 0.001), surgical margin (HR: 1.504, 95% CI: 1.025–2.207, P = 0.037), adjuvant postoperative therapy (HR: 0.532, 95% CI: 0.361–0.784, P = 0.001), CEA (HR: 1.72, 95% CI: 1.161–2.548, P = 0.007), CA19-9 (HR: 1.996, 95% CI: 1.345–2.962, P = 0.001), SII (HR: 1.99, 95% CI: 1.327–2.986, P = 0.001), GPR (HR: 1.485, 95% CI: 1.011–2.181, P = 0.044), and PNI (HR: 0.448, 95% CI: 0.306–0.658, P < 0.001) were significantly prognostic variables for locally advanced ICC after R0 resection (P < 0.05, Table 2). However, taking non-cancer-specific death into consideration, only tumor diameter (HR: 1.76, 95% CI: 1.066–2.906, P = 0.027), tumor number (HR: 1.83, 95% CI: 1.204–2.782, P = 0.005), adjuvant postoperative therapy (HR: 0.585, 95% CI: 0.388–0.884, P = 0.011), CEA (HR: 1.603, 95% CI: 1.029–2.498, P = 0.037), CA19-9 (HR: 2.097, 95% CI: 1.379–3.191, P = 0.001), SII (HR: 1.934, 95% CI: 1.079–3.465, P = 0.027), and PNI (HR: 0.604, 95% CI: 0.392–0.929, P = 0.022) were preserved after multivariate competing risk regression analysis (Table 2). The results of univariate Fine and Gray analysis are exhibited in Supplementary Figure 1. The survival curves of selected variables are shown in Supplementary Figure 2.
Table 2.
The Results of Prognosis Analyses for Overall Survival in Locally Advanced Intrahepatic Cholangiocarcinoma
| Variables | Cox regression Analysis | Competing Risk Regression Analysis | |||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Fine–Gray analysis P value | Multivariate Analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||||
| ≤ 65 | Ref. | – | – | – | 0.044 | – | – |
| > 65 | 1.446 (0.928–2.253) | 0.103 | – | – | – | – | |
| Gender | |||||||
| Female | Ref. | – | – | – | 0.299 | – | – |
| Male | 1.193 (0.824–1.726) | 0.351 | – | – | – | – | |
| Liver Cirrhosis | |||||||
| No | Ref. | – | – | – | 0.263 | – | – |
| Yes | 1.31 (0.74–2.32) | 0.354 | – | – | – | – | |
| Unknown | 1.611 (0.904–2.871) | 0.105 | – | – | – | – | |
| Tumor Diameter | |||||||
| ≤ 5cm | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| > 5cm | 2.523 (1.703–3.738) | < 0.001 | 1.87 (1.215–2.878) | 0.004 | 1.76 (1.066–2.906) | 0.027 | |
| Tumor number | |||||||
| Single | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| Multiple | 1.969 (1.372–2.826) | < 0.001 | 2.331 (1.592–3.413) | < 0.001 | 1.83 (1.204–2.782) | 0.005 | |
| Grade | |||||||
| Well/Moderate | Ref. | – | – | – | 0.119 | – | – |
| Poor/Undifferentiated | 1.366 (0.932–2.003) | 0.11 | – | – | – | – | |
| MVI | |||||||
| Absence | Ref. | – | – | – | 0.177 | – | – |
| Presence | 1.412 (0.952–2.096) | 0.807 | – | – | – | – | |
| Resection Scope | |||||||
| Minor | Ref. | – | – | – | < 0.001 | – | – |
| Major | 2.131 (1.473–3.082) | < 0.001 | – | – | – | – | |
| Surgical Margin(cm) | |||||||
| (1,+∞) | Ref. | – | Ref. | 0.037 | 0.01 | – | – |
| (0,1) | 1.65 (1.151–2.364) | 0.006 | 1.504 (1.025–2.207) | – | – | ||
| RLNM | |||||||
| Negative | Ref. | – | – | – | < 0.001 | – | – |
| Positive | 2.172 (1.456–3.239) | < 0.001 | – | – | – | – | |
| Adjuvant postoperative therapy | |||||||
| No | Ref. | – | Ref. | – | 0.041 | Ref. | – |
| Yes | 0.618 (0.426–0.898) | 0.012 | 0.532 (0.361–0.784) | 0.001 | 0.585 (0.388–0.884) | 0.011 | |
| CEA | |||||||
| Low | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| High | 2.129 (1.486–3.05) | < 0.001 | 1.72 (1.161–2.548) | 0.007 | 1.603 (1.029–2.498) | 0.037 | |
| CA19–9 | |||||||
| Low | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| High | 2.416 (1.674–3.485) | < 0.001 | 1.996 (1.345–2.962) | 0.001 | 2.097 (1.379–3.191) | 0.001 | |
| pCEA | |||||||
| Low | Ref. | – | – | – | 0.037 | – | – |
| High | 1.649 (1.061–2.561) | 0.026 | – | – | – | – | |
| Unknown | 1.567 (0.991–2.478) | 0.055 | – | – | – | – | |
| pCA19–9 | |||||||
| Low | Ref. | – | – | – | 0.001 | – | – |
| High | 2.272 (1.489–3.467) | < 0.001 | – | – | – | – | |
| Unknown | 1.749 (1.086–2.816) | 0.021 | – | – | – | – | |
| SII | |||||||
| Low | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| High | 2.543 (1.755–3.684) | < 0.001 | 1.99 (1.327–2.986) | 0.001 | 1.934 (1.079–3.465) | 0.027 | |
| AISI | |||||||
| Low | Ref. | – | – | – | < 0.001 | – | – |
| High | 2.004 (1.398–2.871) | < 0.001 | – | – | – | – | |
| SIRI | |||||||
| Low | Ref. | – | – | – | 0.49 | – | – |
| High | 0.927 (0.65–1.322) | 0.675 | – | – | – | – | |
| GPR | |||||||
| Low | Ref. | – | Ref. | – | 0.003 | – | – |
| High | 2.035 (1.414–2.929) | < 0.001 | 1.485 (1.011–2.181) | 0.044 | – | – | |
| PNI | |||||||
| Low | Ref. | – | Ref. | – | < 0.001 | Ref. | – |
| High | 0.349 (0.24–0.506) | < 0.001 | 0.448 (0.306–0.658) | < 0.001 | 0.604 (0.392–0.929) | 0.022 | |
| ALI | |||||||
| Low | Ref. | – | – | – | < 0.001 | – | – |
| High | 0.437 (0.303–0.63) | < 0.001 | – | – | – | – | |
Abbreviations: MVI, microvascular invasion; RLNM, regional lymph node metastasis; CEA, carcino-embryonic antigen; CA19-9, carbohydrate antigen 19–9; pCEA, postoperative CEA; pCA19-9, postoperative CA19-9; SII, systemic immune- inflammation index; AISI, aggregate systemici nflammation index; SIRI, systemic inflammation response index; GPR, gamma-glutamyl- transpeptidase to platelet ratio; PNI, prognostic nutrition index; ALI, advanced lung cancer index.
PSM Analyses for SII and PNI Variables
Considering that SII and PNI are significantly associated with outcomes for locally advanced ICC following surgery in multivariate Cox regression analysis and competing risk analysis, we performed PSM analysis to compare their prognostic difference.
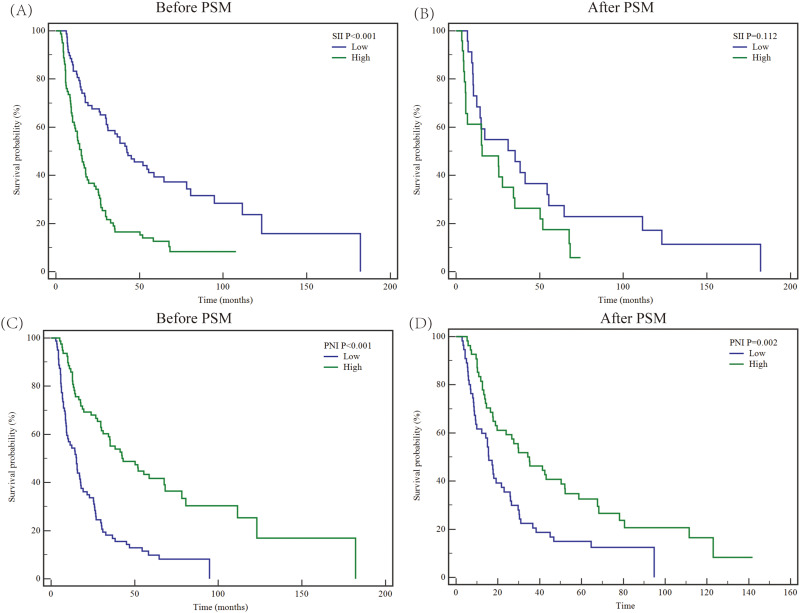
The baseline characteristics between the High SII value group (n = 80) and low SII value group (n = 79) exhibited significant differences (Table 3). Besides, according to the Kaplan-Meier survival analyses, the 1-, 3-, and 5-year OS rates in the high SII value group were 58.3%, 16.5%, and 12.5%, respectively, which were lower than that of in low SII value group (1-, 3-, 5-year OS rates: 83.3%, 57.2%, and 39.4%, P < 0.05, Figure 1A). After the PSM process, 48 patients were matched (High SII value group = 24; low SII value group = 24). No significant difference was found in baseline characteristics between groups (all P > 0.05, Table 3). The 5-year OS rate in the high SII group was lower than that of the low SII group though without statistical significance (17.5% VS 27.4%, P = 0.112, Figure 1B).
Table 3.
Baseline Characteristics Before and After Propensity Score Matching for SII
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Low SII (n=79) | High SII (n=80) | P value | Low SII (n=24) | High SII (n=24) | P value | |
| Age (years) | ||||||
| ≤ 65 | 66 (83.5) | 65 (81.3) | 0.836 | 20 (83.3) | 20 (83.3) | 1 |
| > 65 | 13 (16.5) | 15 (18.7) | 4 (16.7) | 4 (16.7) | ||
| Gender | ||||||
| Male | 46 (58.2) | 53 (66.3) | 0.329 | 17 (70.8) | 14 (58.3) | 0.547 |
| Female | 33 (41.8) | 27 (33.7) | 7 (29.2) | 10 (41.7) | ||
| Liver Cirrhosis | ||||||
| No | 12 (15.2) | 11 (13.8) | 0.459 | 2 (8.3) | 5 (20.8) | 0.445 |
| Yes | 40 (50.6) | 34 (42.5) | 7 (29.2) | 7 (29.2) | ||
| Unknown | 27 (34.2) | 35 (43.7) | 15 (62.5) | 12 (50) | ||
| Tumor Diameter | ||||||
| ≤ 5cm | 45 (57) | 18 (22.5) | < 0.001 | 8 (33.3) | 6 (25) | 0.752 |
| > 5cm | 34 (43) | 62 (77.5) | 16 (66.7) | 18 (75) | ||
| Tumor number | ||||||
| Single | 57 (72.2) | 46 (57.5) | 0.068 | 18 (75) | 15 (62.5) | 0.534 |
| Multiple | 22 (27.8) | 34 (42.5) | 6 (25) | 9 (37.5) | ||
| Grade | ||||||
| Well/Moderate | 28 (35.4) | 23 (28.7) | 0.399 | 9 (37.5) | 10 (41.7) | 1 |
| Poor/Undifferentiated | 51 (64.6) | 57 (71.3) | 15 (62.5) | 14 (58.3) | ||
| MVI | ||||||
| Absence | 62 (78.5) | 54 (67.5) | 0.153 | 21 (87.5) | 18 (75) | 0.461 |
| Presence | 17 (21.5) | 26 (32.5) | 3 (12.5) | 6 (25) | ||
| Resection Scope | ||||||
| Minor | 50 (63.3) | 27 (33.7) | < 0.001 | 12 (50) | 12 (50) | 1 |
| Major | 29 (36.7) | 53 (66.3) | 12 (50) | 12 (50) | ||
| Surgical Margin | ||||||
| ≤ 1cm | 32 (40.5) | 34 (42.5) | 0.872 | 13 (54.2) | 12 (50) | 1 |
| > 1cm | 47 (59.5) | 46 (57.5) | 11 (45.8) | 12 (50) | ||
| RLNM | ||||||
| Negative | 68 (86.1) | 52 (65) | 0.003 | 17 (70.8) | 18 (75) | 1 |
| Positive | 11 (13.9) | 28 (35) | 7 (29.2) | 6 (25) | ||
| Adjuvant postoperative therapy | ||||||
| No | 41 (51.9) | 53 (66.3) | 0.077 | 10 (41.7) | 17 (70.8) | 0.08 |
| Yes | 38 (48.1) | 27 (33.7) | 14 (58.3) | 7 (29.2) | ||
| CEA | ||||||
| Low | 42 (53.2) | 39 (48.8) | 0.635 | 9 (37.5) | 11 (45.8) | 0.77 |
| High | 37 (46.8) | 41 (51.2) | 15 (62.5) | 13 (54.2) | ||
| CA19-9 | ||||||
| Low | 47 (59.5) | 33 (41.2) | 0.027 | 9 (37.5) | 8 (33.3) | 1 |
| High | 32 (40.5) | 47 (58.8) | 15 (62.5) | 16 (66.7) | ||
| pCEA | ||||||
| Low | 52 (65.8) | 45 (56.3) | 0.348 | 14 (58.3) | 12 (50) | 0.54 |
| High | 15 (19) | 16 (20) | 3 (12.5) | 6 (25) | ||
| Unknown | 12 (15.2) | 19 (23.7) | 7 (29.2) | 6 (25) | ||
| pCA19-9 | ||||||
| Low | 53 (67.1) | 41 (51.2) | 0.125 | 13 (54.2) | 13 (54.2) | 0.91 |
| High | 14 (17.7) | 22 (27.5) | 4 (16.7) | 5 (20.8) | ||
| Unknown | 12 (15.2) | 17 (21.3) | 7 (29.2) | 6 (25) | ||
| AISI | ||||||
| Low | 66 (83.5) | 14 (17.5) | < 0.001 | 13 (54.2) | 13 (54.2) | 1 |
| High | 13 (16.5) | 66 (82.5) | 11 (45.8) | 11 (45.8) | ||
| SIRI | ||||||
| Low | 48 (60.8) | 33 (41.2) | 0.017 | 16 (66.7) | 9 (37.5) | 0.082 |
| High | 31 (39.2) | 47 (58.8) | 8 (33.3) | 15 (62.5) | ||
| GPR | ||||||
| Low | 44 (55.7) | 36 (45) | 0.206 | 10 (41.7) | 9 (37.5) | 1 |
| High | 35 (44.3) | 44 (55) | 14 (58.3) | 15 (62.5) | ||
| ALI | ||||||
| Low | 15 (19) | 64 (80) | < 0.001 | 10 (41.7) | 11 (45.8) | 1 |
| High | 64 (81) | 16 (20) | 14 (58.3) | 13 (54.2) | ||
| PNI | ||||||
| Low | 30 (38) | 50 (62.5) | 0.003 | 11 (45.8) | 14 (58.3) | 0.564 |
| High | 49 (62) | 30 (37.5) | 13 (54.2) | 10 (41.7) | ||
Abbreviations: SII, systemic immune-inflammation index; RLNM, regional lymph node metastasis; MVI, microvascular invasion; CEA, carcino-embryonic antigen; CA19-9, carbohydrate antigen 19–9; pCEA, postoperative CEA; pCA19-9, postoperative CA19-9; AISI, aggregate systemic inflammation index; SIRI, systemic inflammation response index; GPR, gamma-glutamyl-transpeptidase to platelet ratio; PNI, prognostic nutrition index; ALI, advanced lung cancer index.
Figure 1.
Kaplan-Meier survival analyses to estimate the prognosis of locally advanced intrahepatic cholangiocarcinoma after R0 resection stratified by SII and PNI variables before and after PSM analyses. Plots of Kaplan–Meier survival curves for SII before (A) and after (B) PSM. Plots of Kaplan–Meier survival curves for PNI before (C) and after (D) PSM. SII, systemic immune-inflammation index; PNI, prognostic nutrition index; PSM, propensity matching analysis.
Similarly, the medical data between the High PNI value group (n = 79) and the low PNI value group (n = 80) showed statistical differences (Table 4). The 5-year OS rate in the High PNI value group was 41.6%, which is higher than the low PNI value group (9.9%, P < 0.001, Figure 1C). After the PSM process, 110 patients were matched (High PNI value group = 55; low PNI value group = 55). The baseline data between groups were similar (Table 4). However, the median survival time in the high PNI group was significantly longer than in the low PNI group (35.1 months VS 15.7 months, P = 0.002, Figure 1D).
Table 4.
Baseline Characteristics Before and After Propensity Score Matching for PNI
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Low PNI (n=80) | High PNI (n=79) | P value | Low PNI (n=55) | High PNI (n=55) | P value | |
| Age (years) | ||||||
| ≤ 65 | 64 (80) | 67 (84.8) | 0.533 | 45 (81.8) | 47 (85.5) | 0.797 |
| > 65 | 16 (20) | 12 (15.2) | 10 (18.2) | 8 (14.5) | ||
| Gender | ||||||
| Male | 50 (62.5) | 49 (62) | 1 | 34 (61.8) | 37 (67.3) | 0.69 |
| Female | 30 (37.5) | 30 (38) | 21 (38.2) | 18 (32.7) | ||
| Liver Cirrhosis | ||||||
| No | 9 (11.3) | 14 (17.7) | 0.457 | 6 (10.9) | 9 (16.3) | 0.545 |
| Yes | 40 (50) | 34 (43) | 26 (47.3) | 21 (38.2) | ||
| Unknown | 31 (38.7) | 31 (39.3) | 23 (41.8) | 25 (45.5) | ||
| Tumor Diameter | ||||||
| ≤ 5cm | 25 (31.3) | 38 (48.1) | 0.036 | 21 (38.2) | 24 (43.6) | 0.698 |
| > 5cm | 55 (68.7) | 41 (51.9) | 34 (61.8) | 31 (56.4) | ||
| Tumor number | ||||||
| Single | 51 (63.7) | 52 (65.8) | 0.868 | 32 (58.2) | 36 (65.5) | 0.556 |
| Multiple | 29 (36.3) | 27 (34.2) | 23 (41.8) | 19 (34.5) | ||
| Grade | ||||||
| Well/Moderate | 22 (27.5) | 29 (36.7) | 0.237 | 17 (30.9) | 18 (32.7) | 1 |
| Poor/Undifferentiated | 58 (72.5) | 50 (63.3) | 38 (69.1) | 37 (67.3) | ||
| MVI | ||||||
| Absence | 53 (66.3) | 63 (79.7) | 0.074 | 39 (70.9) | 42 (76.4) | 0.666 |
| Presence | 27 (33.7) | 16 (20.3) | 16 (29.1) | 13 (23.6) | ||
| Resection Scope | ||||||
| Minor | 32 (40) | 45 (57) | 0.039 | 26 (47.3) | 29 (52.7) | 0.703 |
| Major | 48 (60) | 34 (43) | 29 (52.7) | 26 (47.3) | ||
| Surgical Margin | ||||||
| ≤ 1cm | 37 (46.3) | 29 (36.7) | 0.261 | 26 (47.3) | 20 (36.4) | 0.334 |
| > 1cm | 43 (53.7) | 50 (63.3) | 29 (52.7) | 35 (63.6) | ||
| RLNM | ||||||
| Negative | 55 (68.8) | 65 (82.3) | 0.065 | 38 (69.1) | 44 (80) | 0.274 |
| Positive | 25 (31.2) | 14 (17.7) | 17 (30.9) | 11 (20) | ||
| Adjuvant postoperative therapy | ||||||
| No | 51 (63.7) | 43 (54.4) | 0.261 | 34 (61.8) | 30 (54.5) | 0.562 |
| Yes | 29 (36.3) | 36 (45.6) | 21 (38.2) | 25 (45.5) | ||
| CEA | ||||||
| Low | 31 (38.8) | 50 (63.3) | 0.003 | 22 (40) | 33 (60) | 0.056 |
| High | 49 (61.2) | 29 (36.7) | 33 (60) | 22 (40) | ||
| CA19-9 | ||||||
| Low | 31 (38.8) | 49 (62) | 0.004 | 28 (50.9) | 30 (54.5) | 0.849 |
| High | 49 (61.2) | 30 (38) | 27 (49.1) | 25 (45.5) | ||
| pCEA | ||||||
| Low | 50 (62.4) | 47 (59.4) | 0.927 | 34 (61.9) | 31 (56.4) | 0.613 |
| High | 15 (18.8) | 16 (20.3) | 8 (14.5) | 12 (21.8) | ||
| Unknown | 15 (18.8) | 16 (20.3) | 13 (23.6) | 12 (21.8) | ||
| pCA19-9 | ||||||
| Low | 40 (50) | 54 (68.4) | 0.01 | 30 (54.5) | 35 (63.6) | 0.613 |
| High | 26 (32.5) | 10 (12.7) | 12 (21.8) | 9 (16.4) | ||
| Unknown | 14 (17.5) | 15 (19) | 13 (23.6) | 11 (20) | ||
| SII | ||||||
| Low | 30 (37.5) | 49 (62) | 0.002 | 25 (45.5) | 29 (52.7) | 0.567 |
| High | 50 (62.5) | 30 (38) | 30 (54.5) | 26 (47.3) | ||
| AISI | ||||||
| Low | 33 (41.3) | 47 (59.5) | 0.027 | 25 (45.5) | 29 (52.7) | 0.567 |
| High | 47 (58.7) | 32 (40.5) | 30 (54.5) | 26 (47.3) | ||
| SIRI | ||||||
| Low | 45 (56.3) | 36 (45.6) | 0.206 | 36 (65.5) | 24 (43.6) | 0.035 |
| High | 35 (43.7) | 43 (54.4) | 19 (34.5) | 31 (56.4) | ||
| GPR | ||||||
| Low | 31 (38.8) | 49 (62) | 0.004 | 26 (47.3) | 26 (47.3) | 1 |
| High | 49 (61.2) | 30 (38) | 29 (52.7) | 29 (52.7) | ||
| ALI | ||||||
| Low | 53 (66.3) | 30 (32.9) | < 0.001 | 28 (50.9) | 24 (43.6) | 0.567 |
| High | 27 (33.7) | 76 (67.1) | 27 (49.1) | 31 (56.4) | ||
Abbreviations: PNI, prognostic nutrition index; RLNM, regional lymph node metastasis; MVI, microvascular invasion; CEA, carcino-embryonic antigen; CA19-9, carbohydrate antigen 19–9; pCEA, postoperative CEA; pCA19-9, postoperative CA19-9; SII, systemic immune-inflammation index; AISI, aggregate systemic inflammation index; SIRI, systemic inflammation response index; GPR, gamma-glutamyl-transpeptidase to platelet ratio; ALI, advanced lung cancer index.
Discussion
In this study, our team investigated the long-term outcome of locally advanced ICC after R0 resection. Traditional Cox proportional hazards analysis often ignores the impact of competing risk events, which may lower the accuracy of conclusions, and overestimate or underestimate the significance of certain variables.23 To reduce the potential bias from competing risk events, we conducted Cox regression combined with competing risk analysis. After these two-step processes, we found that two tumor-related variables (tumor diameter and tumor number), adjuvant postoperative therapy, two tumor-associated laboratory variables (CEA and CA19-9), and two inflammation-nutritional indexes (SII and PNI) remained significantly different (P < 0.05). To further verify the clinical value of inflammation-nutritional variables on the prognosis of locally advanced ICC after hepatectomy, we conducted PSM analysis between SII and PNI subgroups (low-value group vs high-value group). Before PSM, survival differences were found in the subsets of SII and PNI with imbalanced distribution in baseline data. After PSM, mortal differences were only detected in subgroup analysis of the PNI variable (P < 0.05). Notely, the survival benefit was superior in the low SII value group even though no statistical significance was found (15.5 months vs 35.23 months).
Systemic inflammatory condition exerts a great role in tumorigenesis and malignant progression. Chronic inflammation can re-shape the tumor immune microenvironment into an immunological suppression status through several mechanisms such as recruitment of regulatory T cells, Type 2 macrophage polarization, CD8+ T cell exhaustion, and secretion of inflammatory cytokine.26–30 Reversely, tumors themselves could trigger a long-term proinflammatory and inflammatory response that contributes to several deleterious impacts during the process of malignant progression like cachexia.31 These positive and bidirectional causalities between systemic inflammation and cancer gain increasing attention from clinicians and scientists. In addition, individual nutrition condition has the potential to impact the immune landscape, which in turn, may contribute to exacerbating inflammation and remodeling tumor ecology.32 The malignant proliferation of tumor cells could accelerate the exhaustion of systemic nutrition to induce cachexy that disturbs the anti-cancer treatment and shortens the survival time. To better make medical decisions for cancer patients, oncologists are going to realize the importance of serum inflammation-nutrition-based scores. Numerous articles have reported that such combined indexes could reflect systemic inflammatory and nutritional status and predict prognoses.13,15,17,18 PNI, known as an inflammation-nutritional evaluation score, was proposed by Buzby and then modified by Onodera.33,34 It was calculated by albumin and lymphocytes that has the proved ability to assess the outcomes of numerous malignancies.35 Based on the results of the final multivariate competing risk analysis, patients with lower PNI scores tend to have poorer long-term outcomes (5-year OS rate less than 10%, P < 0.05). Strikingly, after balancing the unbalanced variables between subgroups by PSM process, the high PNI value set still had a longer median survival time (35.1 months). These findings were similar to Sun et al.35 Besides, the results of the SII index showed inversely prognostic evidence by competing risk analysis. Although without statistical significance after the PSM process, the median survival time in the high SII score group, which means a higher inflammation level, was shorter than that of the low score set. Therefore, considering the clinical association between these inflammation-nutritional indexes and the prognosis of locally advanced ICC after surgery, we strongly recommend their clinical application.
Except for inflammatory and nutritional factors, we found that multifocal tumor and tumor diameter were statistically prognostic variables for locally advanced ICC with LR after removing the potential influence of competing risk events. As known, multifocal lesions can be regarded as stage II according to the 8th AJCC-TNM system. However, recently, Lamarca et al conducted a large cohort study and found that ICC patients with multiple tumors suffered poorer prognoses than other early stage.36 Therefore, they suggested that ICC patients with multifocal lesions should be regarded as M1 stage. Results from Spolverato and our findings reflected their points to some extent.37 The 8th AJCC-TNM staging system does not excessively subdivide tumor diameter, which classifies tumors larger than 5 cm as T1b. Strikingly, we surprisingly found that a larger tumor tends to have a poor outcome after resection (5-year OS rate: 12.5%, P < 0.001). Technically, large ICCs usually meet the difficulties of requiring complex liver resection, major vascular invasion, and insufficient remnant liver volume.37–39 Combined with our findings, surgeons should make comprehensive and sufficient therapeutic strategies for locally advanced ICC patients. Furthermore, the surgical resection margin did not exhibit survival benefit after excluding competing risk events. Whether a wide surgical margin could contribute to a better prognosis remains controversial.38 A retrospective multicenter study reported that the prognostic value of surgical margin depended on the context of lymph node metastasis. For patients with positive lymph nodes, a wide surgical margin did not accompany a prolonged survival. Inversely, the survival benefits in individuals without lymph node invasion were positive with the width of surgical margin.40 Regrettably, limited by the limited number of included cases, we did not conduct a subgroup analysis for surgical resection to figure out the potential association between them. Patients included in our study did not accept routine lymph node dissection without any radiological evidence of lymph node metastasis. Therefore, we did not explore the survival influence of the number of lymphectomy. Importantly, according to the results of multivariate analysis, regional lymph node metastasis did not exhibit survival difference clearly in locally advanced ICC patients with R0 resection after multivariate processes. Recently, Moustafa et al performed another PSM analysis targeting locally advanced ICC, utilizing the Surveillance, Epidemiology, and End Results database to evaluate the prognostic difference between LR and chemotherapy.7 Similar to our findings, they reported that lymph node metastases did not show a clear survival difference after the PSM process. Following these, another retrospective study reported a marginal prognostic difference (P = 0.07) between IIIa (negative lymph node invasion) and IIIb (positive lymph node invasion).41 However, most of them did not take the impact of competing risk events on survival evaluation into consideration, and ignored the role of inflammation-nutritional variables and other laboratory tests.
The BILCAP 3 phase trial failed to achieve the intended primary endpoint of OS that the survival benefit was not statistically significant after adjustments; however, nowadays, ICC patients are usually supplemented with adjuvant chemotherapy based on capecitabine.42,43 Similarly, a meta-analysis by Mavrou revealed that adjuvant treatments did not exhibit a prolonged survival benefit after primary LR.44 Reversely, other multicenter or retrospective studies reported that ICC patients with high-risk factors such as advanced tumor stage could benefit from postoperative adjuvant treatments.45–47 The above points were echoed by our multivariate analyses, which showed that postoperative adjuvant treatments could accompany an improved outcome. Owing to a lack of standard regimens for postoperative adjuvant treatment, in our study, adjuvant regimens vary among times and attending doctors, and include gemcitabine, cisplatin, capecitabine, S-1, radiotherapy, immunotherapy, and targeted drugs. Due to the limited number of included patients, we did not compare the survival difference among different adjuvant strategies. Further trials will need to explore the impact of postoperative adjuvant regimens for locally advanced ICC following surgery.
Several limitations existed in this study. Firstly, this study was conducted at a single institution and included a limited number of locally advanced ICC individuals after R0 LR. Secondly, limited by the trait of retrospective study, the findings we concluded need further large-scale multicenter prospective studies to verify accuracy. Thirdly, we did not explore those patients who were diagnosed with locally advanced ICC without surgery or R1 resection. Whether the associations we found could be applied to these subsets should be taken seriously. Finally, we did not explore the prognostic value of neoadjuvant systemic therapy in this study, which may bring locally advanced ICC patients with survival benefits. Further large-scale studies should be conducted to elucidate the potential associations undertaking comprehensive prognostic factors.
Conclusions
In summary, we figured out that one’s inflammation-nutritional condition could impact the long-term outcome of locally advanced ICC after R0 resection by competing risk regression analysis.
Data Sharing Statement
Data are requestable from the corresponding author upon reasonable request.
Ethical Approval Statement
This retrospective study was approved by the local Ethics Committee (ID: B2022-492-01). The need for informed consent was waived due to the nature of the retrospective study, and we conducted a necessarily anonymized process for all included patient data.
Author Contributions
All authors made huge contribution to this study: I) concept and design of the study: Guizhong Huang, Zehui Yao, Pu Xi, Chongyu Zhao, Xiaohui Li, Zexian Chen and Xiaojun Lin; II) execution, acquisition of data, analysis and interpretation: Guizhong Huang, Zehui Yao, Pu Xi, Chongyu Zhao, Xiaohui Li, Zexian Chen and Xiaojun Lin; III) literature search and manuscript preparation: Guizhong Huang, Chongyu Zhao and Pu Xi; IV) manuscript editing and review: Guizhong Huang, Zehui Yao, Pu Xi, Zexian Chen and Xiaojun Lin. V) manuscript revision: Guizhong Huang, Zehui Yao, Pu Xi, Chongyu Zhao, Xiaohui Li, Zexian Chen and Xiaojun Lin. All authors read and approved the final manuscript to be published. All authors agreed on the journal to which the article has been submitted and to be responsible for all aspects of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–232. doi: 10.1016/j.bpg.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma–evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087 [DOI] [PubMed] [Google Scholar]
- 5.Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337–348. doi: 10.1016/S2468-1253(18)30045-1 [DOI] [PubMed] [Google Scholar]
- 6.Yi SW, Kang DR, Kim KS, et al. Efficacy of concurrent chemoradiotherapy with 5-fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2014;73(1):191–198. doi: 10.1007/s00280-013-2340-5 [DOI] [PubMed] [Google Scholar]
- 7.Moustafa M, Fasolo E, Bassi D, et al. The impact of liver resection on survival for locally advanced intrahepatic cholangiocarcinoma tumors: a propensity score analysis. Eur J Surg Oncol. 2020;46(4):632–637. doi: 10.1016/j.ejso.2019.11.502 [DOI] [PubMed] [Google Scholar]
- 8.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 9.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy AG, Makarova-Rusher OV, Greten TF. The case for immune-based approaches in biliary tract carcinoma. Hepatology. 2016;64(5):1785–1791. doi: 10.1002/hep.28635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–3458. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casadei Gardini A, Marisi G, Canale M, et al. Radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of overall survival and recurrence-free survival. Onco Targets Ther. 2018;11:6555–6567. doi: 10.2147/OTT.S170836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi: 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 14.Prado CMM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of Chemotherapy Toxicity and Time to Tumor Progression in metastatic breast Cancer patients receiving Capecitabine Treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242 [DOI] [PubMed] [Google Scholar]
- 15.Read JA, Choy ST, Beale PJ, et al. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer. 2006;55(1):78–85. doi: 10.1207/s15327914nc5501_10 [DOI] [PubMed] [Google Scholar]
- 16.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66(2):460S–463S. doi: 10.1093/ajcn/66.2.460S [DOI] [PubMed] [Google Scholar]
- 17.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 18.Dziedzic EA, Gąsior JS, Tuzimek A, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553. doi: 10.3390/ijms23179553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369–1376. doi: 10.1136/gutjnl-2015-309260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Wang S, Yang J, et al. The predictive value of hematological inflammatory markers for acute kidney injury and mortality in adults with hemophagocytic Lymphohistiocytosis: a retrospective analysis of 585 patients. Int Immunopharmacol. 2023;122:110564. doi: 10.1016/j.intimp.2023.110564 [DOI] [PubMed] [Google Scholar]
- 21.Mullen JL, Buzby GP, Matthews DC, et al. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann Surg. 1980;192(5):604–613. doi: 10.1097/00000658-198019250-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Shahbal H, Parpia S, et al. Trials using composite outcomes neglect the presence of competing risks: a methodological survey of cardiovascular studies. J Clin Epidemiol. 2023;160:1–13. doi: 10.1016/j.jclinepi.2023.05.015 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen PK, Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Stat Med. 2012;31(11–12):1074–1088. doi: 10.1002/sim.4385 [DOI] [PubMed] [Google Scholar]
- 26.Iglesias-Escudero M, Arias-González N, Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Mol Cancer. 2023;22(1):26. doi: 10.1186/s12943-023-01714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41(3):450–465. doi: 10.1016/j.ccell.2023.02.014 [DOI] [PubMed] [Google Scholar]
- 28.Giles JR, Globig AM, Kaech SM, et al. CD8+ T cells in the cancer-immunity cycle. Immunity. 2023;56(10):2231–2253. doi: 10.1016/j.immuni.2023.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baessler A, Vignali DAA. T Cell Exhaustion. Annu Rev Immunol. 2024;42. doi: 10.1146/annurev-immunol-090222-110914 [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Saeed AFUH, Liu Q, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8(1):207. doi: 10.1038/s41392-023-01452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136 [DOI] [PubMed] [Google Scholar]
- 32.Siracusa F, Tintelnot J, Cortesi F, et al. Diet and immune response: how today’s plate shapes tomorrow’s health. Trends Immunol. 2023;2023:8. [DOI] [PubMed] [Google Scholar]
- 33.Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–167. doi: 10.1016/0002-9610(80)90246-9 [DOI] [PubMed] [Google Scholar]
- 34.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 35.Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–1549. doi: 10.1007/s00432-014-1714-3 [DOI] [PubMed] [Google Scholar]
- 36.Lamarca A, Santos-Laso A, Utpatel K, et al. Liver metastases of intrahepatic cholangiocarcinoma: implications for an updated staging system. Hepatology. 2021;73(6):2311–2325. doi: 10.1002/hep.31598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spolverato G, Ejaz A, Kim Y, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2014;18(7):1284–1291. doi: 10.1007/s11605-014-2533-1 [DOI] [PubMed] [Google Scholar]
- 38.Cillo U, Spolverato G, Vitale A, et al. Liver resection for advanced intrahepatic cholangiocarcinoma: a cost-utility analysis. World J Surg. 2015;39:2500–2509. doi: 10.1007/s00268-015-3150-1 [DOI] [PubMed] [Google Scholar]
- 39.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 40.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254(5):824–829. doi: 10.1097/SLA.0b013e318236c21d [DOI] [PubMed] [Google Scholar]
- 41.Kim Y, Moris DP, Zhang XF, et al. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: a surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol. 2017;116(6):643–650. doi: 10.1002/jso.24720 [DOI] [PubMed] [Google Scholar]
- 42.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, Phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 43.Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. doi: 10.1016/j.ctarc.2021.100354 [DOI] [PubMed] [Google Scholar]
- 44.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–574. doi: 10.1001/jamasurg.2013.5137 [DOI] [PubMed] [Google Scholar]
- 45.Miura JT, Johnston FM, Tsai S, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangio- carcinoma. Ann Surg Oncol. 2015;22(11):3716–3723. doi: 10.1245/s10434-015-4501-8 [DOI] [PubMed] [Google Scholar]
- 46.Altman AM, Kizy S, Marmor S, et al. Adjuvant chemotherapy for intrahepatic cholangiocarcinoma: approaching clinical practice consensus? Hepatobiliary Surg Nutr. 2020;9(5):577–586. doi: 10.21037/hbsn.2019.06.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reames BN, Bagante F, Ejaz A, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB. 2017;19(10):901–909. doi: 10.1016/j.hpb.2017.06.008 [DOI] [PubMed] [Google Scholar]