Abstract
Although in vitro experiments have demonstrated the potential of flavonoid compounds in regulating blood pressure, there is still a lack of evidence from large population studies. We conducted a cross‐sectional study using the National Health and Nutrition Examination Survey to investigate the relationship between flavonoid intake levels (natural log transformation) and hypertension events. A total of 15 752 participants aged over 20 years were included, and a weighted multivariable logistic regression analysis was performed to explore the relationship between total flavonoids, five sub types intake, and hypertension events. Smooth curve fitting was used to explore potential nonlinear relationships. Higher total flavonoids intake was associated with a lower risk of hypertension than the lowest group. The adjusted odds ratios (95% CIs) were 0.79 (0.70–0.88) for total flavonoids intake. Elevated total flavonoids intake levels were significantly and linearly associated with a lower risk of hypertension. For each unit increase in the total flavonoids intake level, the adjusted ORs for risk of hypertension decrease by 5% (OR 0.95; 95% CI, 0.92–0.98). In addition, in restricted cubic spline regression, we found that flavan‐3‐ols, anthocyanidins, and flavonols intake were linearly and negatively related to prevalence of hypertension. Flavones intake showed nonlinear associations with prevalence of hypertension with inflection points of ‐1.90. Within a certain range, a negative correlation exists between flavonoids intake and hypertension events. This finding provides insights into dietary modifications in the prevention of hypertension.
Keywords: cross‐sectional study, flavonoids intake, hypertension, NHANES, U.S. adults
1. INTRODUCTION
Hypertension represents a significant global public health challenge with increasing prevalence. 1 Previous surveys have reported that 85.7 million adults in the United States suffer from hypertension. 2 Hypertension, caused by vascular sclerosis and endothelial dysfunction, burdens cardiovascular and cerebrovascular systems heavily, leading to severe cardiovascular and cerebrovascular diseases. 3 , 4 Hypertension is influenced by both genetic and environmental factors, 5 and controlling diet and lifestyle can reduce its incidence.
Flavonoid compounds are present in fruits and vegetables and belong to a class of polyphenolic compounds. 6 They may regulate vascular function through eNOS, provide antioxidant function, and alleviate endothelial dysfunction. 7 Some in vitro experiments also support the antihypertensive activity of flavonoid compounds. 6 Consumption of a healthy plant‐based diet may also reduce the incidence of hypertension. Although flavonoid intake has the potential to control hypertension, observational studies have reported inconsistent associations between flavonoid intake and hypertension. 8 , 9 It could be attributed to population heterogeneity, inadequate intake, and interactions between flavonoid intake and intestinal flora. Therefore, we investigated the relationship between flavonoid intake and hypertension risk in American adults. This study aims to provide insights for the prevention of hypertension.
2. MATERIALS AND METHODS
2.1. Data source and study population
The National Health and Nutrition Examination Study (NHANES) is a thorough study that uses a nationally representative sample to assess the American population's physical health and nutritional consumption. We used NHANES data from 2007−2008, 2009−2010, and 2017−2018, which contained information on the consumption of flavonoids, in our investigation. Participants who were under 20 years of age (n = 12 218), pregnant (n = 171), without hypertension or missing flavonoid intake data (n = 1799) were excluded. Finally, in the analysis, there were 15 752 individuals (Figure 1).
FIGURE 1.
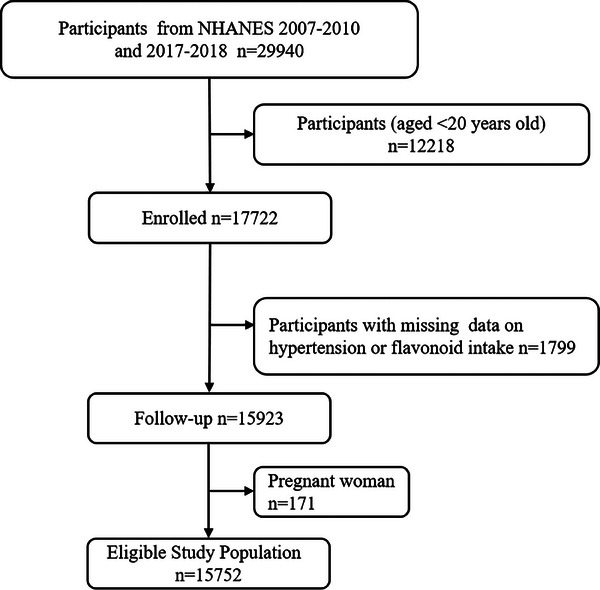
Flowchart for the research.
2.2. Dietary intakes
Two 24‐h‐long interviews on eating habits were conducted as part of the NHANES, during which participants provided information about the amount of food and beverages they had consumed the day before. Based on the Food and Nutrient Database for Dietary Studies (FNDDS), individual nutrients and flavonoid intake were calculated. If persons finished both food recall interviews, the mean intake of nutrients was calculated. We obtained data on the intake of 29 individual flavonoids by six types (Table S1). Data sources and calculation can be found in the FNDDS flavonoid database (https://www.ars.usda.gov/northeast‐area/beltsville‐md‐bhnrc/beltsville‐human‐nutrition‐research‐center/food‐surveys‐research‐group/docs/fndds‐flavonoid‐database/).
2.3. Definition of hypertension
After 5 min of rest, trained physicians took the patient's blood pressure three times at 30 s apart (and occasionally four times) using a consistent methodology. An individual was classified as suffering from hypertension if they fulfilled any or all of the following descriptions: 1) average systolic blood pressure (SBP) ≥140 mmHg, 2) average diastolic blood pressure (DBP) ≥ 90 mmHg, 3) currently taking antihypertensive medication, and 4) self‐reported of hypertension by questionnaire.
2.4. Covariate definitions
·The study incorporated potential factors that may confuse. Socioeconomic considerations included age, sex (male, female), race/ethnicity (non‐Hispanic whites, non‐Hispanic blacks, and others), education level (below high school, high school, above high school), smoking status (never smoker, former smoker, current smoker), drinking status (nondrinker, low‐to‐moderate drinker, heavy drinker), family income poverty ratio (≤ 1.0, 1−3, > 3), leisure‐time physical activity (inactive, insufficiently active, active), energy intake (continuous), BMI (continuous), and health‐related illnesses, including diabetes (yes, no, borderline), cardiovascular disease (yes, no), and cancer (yes, no). Laboratory tests included glycohemoglobin (continuous) and estimated glomerular filtration rate (eGFR) (continuous). Less than 100 cigarettes must have been smoked in a lifetime to qualify as a nonsmoker. Former smokers have never smoked again after smoking more than 100 cigarettes, whereas current smokers continuously smoke. In terms of drinking status, men with < 2 drinks/day and women < 1 drink/day were defined as low to moderate drinkers, while men with ≥ 2 drinks/day and women with ≥ 1 drink/day were defined as heavy drinkers. Diet modification in the Renal Disease formula was used to compute the estimated eGFR. 10 According to inactive, inadequately active, and active categories, leisure‐time physical activity was calculated using moderate and vigorous‐intensity physical activity. 11 We have conducted all research following the NHANES guidelines, and publicly available details are available at https://www.cdc.gov/nchs/nhanes/.
2.5. Statistical analysis
The complex survey design factors, including sample weights, stratification, and clustering, were considered in the NHANES analysis to allow nationally representative estimates. 12 , 13 We used “WTDRD1, SDMVPSU, SDMVSTRA” to conduct the weighted analyses using the R package ‘‘survey. 13 Baseline characteristics were analyzed by a weighted linear regression model for continuous variables and a weighted Chi‐square test for categorical variables. Mean (CI%) was used for continuous variables and percentage (CI%) for categorical variables. CI% was derived from adjusted Wald tests for differences in weighted categories. 14 Although isoflavones are included in total flavonoids, they were not analyzed separately as the intake in the United States is too low and is not expected to produce biological effects. 15 , 16 In addition, considering the skewed flavonoids intake distribution, the data were transformed into natural logarithms and expressed in quartiles. Two models of weighted multivariate logistic regression analyses were used to examine the relationship between the consumption of total flavonoids, five sub types and the risk of hypertension. Model 1 included race/ethnicity as non‐Hispanic white, non‐Hispanic black, or other, age as a continuous variable, sex as male or female, and gender. If the covariate changed the resulting estimate by more than 10% or had a regression coefficient p < .1, it was considered a potential confounder in Model 2. To prevent multicollinearity, variables with a variance inflation factor (VIF) greater than five were excluded from the models. Model 2 included further adjustments for education level (less than high school, high school or college, or more), alcohol consumption status (none, light, or heavy), smoking status (never, former, or current), poverty‐to‐income ratio (≤ 1, 1−3, or > 3), energy intake (continuous), and further adjustments for leisure‐time physical activity (inactive, insufficiently active, or active), eGFR (continuous), BMI (continuous), glycated hemoglobin (continuous), diabetes (yes, no, or borderline), cardiovascular disease (yes, no), and cancer (yes, no). Additionally, restricted cubic splines were used with three knots (10th, 50th, and 90th percentiles) to examine dose‐response associations. We then calculated OR for flavonoids and outcomes by running a log‐likelihood ratio test for the non‐linearity of the smooth curve fit, contrasting the segmented regression model to the single‐linear (non‐segmented) model while accounting for relevant confounders.
Likewise, except for the variables, the adjustment technique was the same as in Model 2. Analyses were divided into groups according to the following factors: sex, age (≤ 60 years or > 60 years), race/ethnicity (non‐Hispanic white, non‐Hispanic black, or others), cancer (yes, no), cardiovascular disease (yes, no), BMI (< 30.00 or ≥ 30.00), glycohemoglobin (< 6.5% or ≥6.5%), and calorie consumption (continuous) to explore interactions, and finally, employed multiple estimates and chained equation techniques based on five replications in the R MI process to account for the missing data to prevent a deterioration in the effectiveness and bias of the statistical analyses owing to the direct exclusion of missing values. Weighted multivariate logistic regression analysis was performed on the five newly created data sets, and the outcomes were merged. We performed sensitivity analyses to assess the influence of multiple Imputation on the results. The link between total flavonoids intake after transformed into natural logarithms and the risk of hypertension was first investigated using data before multiple imputations (n = 12 628). Finally, we also used generalized linear regression to analyze the association among HbA1c, FBG, triglycerides, cholesterol, LDL‐C, HDL‐C, hs‐CRP, and total flavonoids intake. R 4.2.1 (http://www.R‐project.org) were used for all analyses. A two‐sided p value of less than .05 was deemed statistically significant.
3. RESULTS
3.1. Characteristics of participants
There were 15 752 participants in all, and 7408 of them had hypertension, making up 47% of the population. The weighted study population with hypertension exhibited a higher prevalence among the elderly population, males, non‐Hispanic Whites, and non‐Hispanic Blacks, as well as individuals with lower educational attainment and moderate family income. Moreover, this patient cohort had a notable inclination towards lower energy intake and reduced physical activity levels. And diabetes, cancer, and heart disease are more frequently present alongside them. Additionally, they displayed higher BMI, glycohemoglobin and lower eGFR levels (Table 1).
TABLE 1.
Baseline characteristics of people, weighted.
| Characteristics | Total | Non‐hypertension | Hypertension | p |
|---|---|---|---|---|
| Patients, no. | 15 752 | 8344 | 7408 | |
| Age, years | 47.63 (47.04,48.23) | 41.14 (40.51,41.76) | 57.02 (56.45,57.60) | <.001 |
| Sex (%) | <.001 | |||
| Male | 48.84 (47.97,49.70) | 47.44 (46.28,48.61) | 50.85 (49.54,52.17) | |
| Female | 51.16 (50.30,52.03) | 52.56 (51.39,53.72) | 49.15 (47.83,50.46) | |
| Race/ethnicity (%) | <.001 | |||
| Non‐Hispanic White | 67.41 (63.60,71.00) | 66.09 (62.37,69.63) | 69.31 (65.10,73.22) | |
| Non‐Hispanic Black | 11.25 (9.61,13.13) | 9.53 (8.16,11.09) | 13.75 (11.51,16.35) | |
| Others | 21.34 (18.39,24.61) | 24.38 (21.18,27.88) | 16.94 (14.18,20.11) | |
| Education level (%) | <.001 | |||
| Below high school | 14.77 (13.44,16.22) | 14.77 (13.44,16.22) | 18.54 (16.93,20.26) | |
| High school | 23.89 (21.90,25.99) | 23.89 (21.90,25.99) | 27.50 (25.91,29.16) | |
| Above high school | 61.34 (58.59,64.02) | 61.34 (58.59,64.02) | 53.96 (51.57,56.33) | |
| Smoking status (%) | <.001 | |||
| Never smoker | 55.23 (53.38,57.08) | 58.09 (55.59,60.56) | 51.10 (49.39,52.82) | |
| Former smoker | 24.91 (23.78,26.07) | 20.27 (18.79,21.83) | 31.61 (30.31,32.95) | |
| Current smoker | 19.86 (18.53,21.25) | 21.64 (19.76,23.64) | 17.28 (15.95,18.71) | |
| Drinking status (%) | <.001 | |||
| Nondrinker | 19.89 (18.66,21.17) | 16.53 (15.24,17.91) | 24.64 (22.88,26.49) | |
| Low‐to‐moderate drinker | 70.33 (69.20,71.44) | 73.74 (72.44,75.00) | 65.50 (63.81,67.15) | |
| Heavy drinker | 9.78 (8.89,10.76) | 9.73 (8.74,10.82) | 9.86 (8.67,11.19) | |
| Family income‐poverty ratio (%) | .008 | |||
| ≤ 1.0 | 13.65 (12.47,14.93) | 14.14 (12.88,15.50) | 12.94 (11.47,14.56) | |
| 1‐3 | 35.98 (33.83,38.18) | 34.52 (32.17,36.95) | 38.11 (35.47,40.82) | |
| > 3 | 50.37 (47.88,52.86) | 51.34 (48.44,54.24) | 48.95 (46.24,51.67) | |
| Leisure‐time physical activity (%) | <.001 | |||
| Inactive | 47.32 (45.10,49.55) | 41.18 (39.09,43.29) | 56.20 (53.47,58.90) | |
| Insufficiently active | 17.66 (16.63,18.74) | 17.90 (16.61,19.26) | 17.32 (15.98,18.75) | |
| Active | 35.02 (33.07,37.01) | 40.92 (38.85,43.03) | 26.48 (24.27,28.81) | |
| Energy intake (kcal) | 1988.00 (1474.00,2668.00) | 2027.00 (1526.00,2710.00) | 1921.00 (1409.00,2608.00) | <.001 |
| <1500 kcal/day | 26.27 (25.14,27.43) | 23.75 (22.48,25.07) | 29.90 (28.31,31.55) | |
| ≥1500 kcal/day | 73.73 (72.57,74.86) | 76.25 (74.93,77.52) | 70.10 (68.45,71.69) | |
| Cardiovascular disease (%) | 8.76 (8.03,9.56) | 3.28 (2.78,3.86) | 16.69 (15.31,18.16) | <.001 |
| Cancer (%) | 10.14 (9.38,10.96) | 6.71 (5.92,7.61) | 15.11 (14.00,16.28) | <.001 |
| Diabetes (%) | <.001 | |||
| Yes | 9.66 (8.94,10.42) | 3.73 (3.23,4.30) | 18.23 (16.87,19.68) | |
| No | 88.34 (87.61,89.03) | 94.93 (94.20,95.58) | 78.80 (77.53,80.02) | |
| Borderline | 2.00 (1.76,2.28) | 1.34 (1.04,1.73) | 2.97 (2.49,3.53) | |
| BMI kg/m2 | 29.08 (28.84,29.31) | 27.71 (27.46,27.96) | 31.06 (30.76,31.35) | <.001 |
| eGFR (mg/min/1.73 m2) | 94.23 (93.30,95.16) | 100.66 (99.64,101.68) | 84.88 (84.01,85.76) | <.001 |
| Glycohemoglobin (%) | 5.64 (5.62,5.66) | 5.45 (5.43,5.47) | 5.91 (5.87,5.94) | <.001 |
| Total flavonoids intake (mg/day) | 235.67 (220.19,251.16) | 233.78 (217.20,250.36) | 238.42 (218.48,258.35) | .631 |
For continuous variables: weighted mean (95% CI), and for categorical variables: weighted percentage (95% CI).
3.2. Association between total flavonoids, five sub types, and hypertension
After natural log transformation for total flavonoids and five sub types, total flavonoids, flavan‐3‐ols, and anthocyanidins were negatively associated with prevalence of hypertension. In Model 2, we found that compared to the lowest quartile, quartile 4 of total flavonoids intake (OR = 0.78, 95% CI: 0.70–0.88), flavan‐3‐ols intake (OR = 0.83, 95% CI: 0.74–0.93), anthocyanidins intake (OR = 0.83, 95% CI: 0.71–0.97) showed significant negative associations with the prevalence of hypertension (Tables 2 and 3).
TABLE 2.
Odds ratios (95% CIs) for hypertension according to quartiles of total flavonoid intake (natural log transformation), weighted.
| Exposure | Non‐adjusted model | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Total flavonoid intake (natural log transformation) | ||||||
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.95 (0.83,1.18) | .47 | 0.93 (0.63,1.37) | .02 | 0.98 (0.84,1.15) | .83 |
| Q3 | 0.91 (0.79,1.04) | .20 | 0.71 (0.61,0.82) | <.01 | 0.87 (0.75,1.02) | .11 |
| Q4 | 0.87 (0.78,0.96) | .01 | 0.65 (0.58,0.72) | <.01 | 0.78 (0.70,0.88) | <.01 |
| P for trend | <0.01 | <0.01 | <0.01 | |||
Model 1: Adjusted for age (continuous), sex (male or female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, or others).
Model 2: Adjusted for age (continuous), sex (male or female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, or others), drinking status (none, mild, or heavy), smoking status (never, former, or current), education level (below high school, high school, or college or above), poverty income ratio (≤ 1, 1−3, or > 3), energy intake (continuous), physical activity in leisure time (inactive, insufficiently active, or active), eGFR (continuous), BMI (continuous), glycated hemoglobin (continuous), diabetes (yes, no, or borderline), cardiovascular disease (yes, no), and cancer (yes, no).
TABLE 3.
Multiple logistic regression associations of five sub types of flavonoid intake (natural log transformation) with hypertension in adults, weighted.
| Flavonoid intake | Quartiles of flavonoids (natural log transformation) | p for trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Flavan‐3‐ols | |||||
| Non‐adjusted model | Reference | 0.88 (0.78, 0.99) | 0.81 (0.70, 0.95) | 0.91 (0.82, 1.01) | .21 |
| Model 1 | Reference | 0.81 (0.70, 0.94) | 0.69 (0.58, 0.81) | 0.73 (0.65, 0.83) | <.01 |
| Model 2 | Reference | 0.92 (0.80, 1.05) | 0.82 (0.69, 0.96) | 0.83 (0.74, 0.93) | .01 |
| Anthocyanidins | |||||
| Non‐adjusted model | Reference | 1.06 (0.88, 1.27) | 0.96 (0.85, 1.10) | 0.93 (0.83, 1.05) | .11 |
| Model 1 | Reference | 0.96 (0.77, 1.20) | 0.83 (0.71, 0.96) | 0.71 (0.61, 0.82) | <.01 |
| Model 2 | Reference | 0.98 (0.79, 1.22) | 0.88 (0.75, 1.04) | 0.83 (0.71, 0.97) | <.01 |
| Flavones | |||||
| Non‐adjusted model | Reference | 0.94 (0.85, 1.03) | 0.91 (0.82, 0.99) | 0.86 (0.78, 0.94) | <.01 |
| Model 1 | Reference | 0.83 (0.75, 0.93) | 0.83 (0.75, 0.93) | 0.80 (0.72, 0.90) | <.01 |
| Model 2 | Reference | 0.88 (0.78, 0.99) | 0.91 (0.81, 1.02) | 0.90 (0.80, 1.01) | .13 |
| Flavanones | |||||
| Non‐adjusted model | Reference | 0.95 (0.84, 1.06) | 1.03 (0.92, 1.16) | 1.08 (0.96, 1.20) | .09 |
| Model 1 | Reference | 0.88 (0.77, 1.00) | 0.87 (0.76, 0.99) | 0.85 (0.74, 0.97) | .02 |
| Model 2 | Reference | 0.91 (0.79, 1.05) | 1.02 (0.88, 1.17) | 0.96 (0.84, 1.11) | 1.00 |
| Flavonols | |||||
| Non‐adjusted model | Reference | 0.87 (0.80, 0.95) | 0.80 (0.73, 0.88) | 0.80 (0.73, 0.88) | <.01 |
| Model 1 | Reference | 0.86 (0.77, 0.96) | 0.81 (0.73, 0.90) | 0.83 (0.75, 0.92) | <.01 |
| Model 2 | Reference | 0.92 (0.83, 1.03) | 0.91 (0.81, 1.01) | 0.92 (0.82, 1.03) | .16 |
Model 1: Adjusted for age (continuous), sex (male or female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, or others).
Model 2: Adjusted for age (continuous), sex (male or female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, or others), drinking status (none, mild, or heavy), smoking status (never, former, or current), education level (below high school, high school, or college or above), poverty income ratio (≤ 1, 1−3, or > 3), energy intake (continuous), physical activity in leisure time (inactive, insufficiently active, or active), eGFR (continuous), BMI (continuous), glycated hemoglobin (continuous), diabetes (yes,no, or borderline), cardiovascular disease (yes, no), and cancer (yes, no).
3.3. Restricted cubic spline analysis of the association between total flavonoids, five subtypes intake, and prevalence of hypertension
The dose‐response relationship between total flavonoids, five subtypes intake (natural log transformation), and the adjusted odds ratio for hypertension was depicted using restricted cubic splines. A linearity association between total flavonoids intake and hypertension was observed (Figure 2). Additionally, a combination of logistic proportional odds models with a two‐segmented logistic proportional odds model was not find the non‐linear relationship between total flavonoids intake levels and the hypertension mentioned above rates (p for log‐likelihood ratio > .05). For each unit increase in the total flavonoids intake (natural log transformation), the adjusted ORs for risk of hypertension decrease by 5% (OR 0.95; 95% CI, 0.92–0.98) (Table 4). At the same time, restricted cubic splines showed the flavan‐3‐ols, anthocyanidins, and flavonols intake were linearly and negatively associated with prevalence of hypertension (flavan‐3‐ols intake: p for nonlinearity = .909, Figure 3A; anthocyanidins intake: p for nonlinearity = .371, Figure 3B; flavonols intake: p for nonlinearity = .053, Figure 3E). In addition, flavones intake (p for nonlinearity = .023) was nonlinearly and negatively correlated with prevalence of hypertension with inflection points of −1.90 (Figure 3C and Table 5).
FIGURE 2.
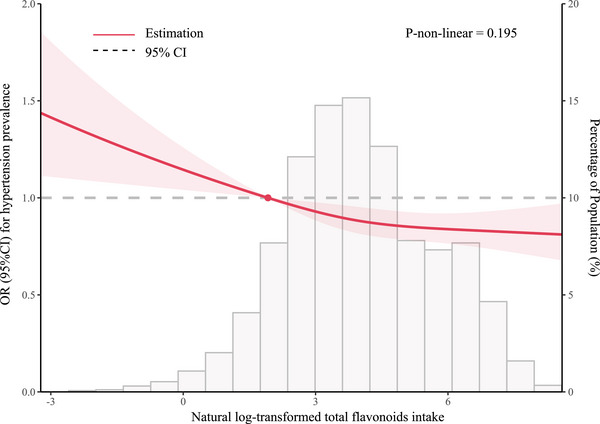
Restricted cubic spline analyses of the association of flavonoid intake (natural log transformation) with hypertension. Heavy central lines represent the estimated adjusted hazard ratios. The 95% confidence interval is represented by the red band. The adjustment strategy is the same as the Model 2.
TABLE 4.
Nonlinearity addressing of total flavonoid intake (natural log transformation) and risk of hypertension.
| Risk of hypertension | |
|---|---|
| Outcome: | OR, 95% CI, p |
| Fitting model by standard linear regression | 0.95 (0.92–0.98) < .01 |
| Fitting model by two‐piecewise linear regression | |
| Inflection point | 2.92 |
| < Inflection point | 0.98 (0.92–1.05) .54 |
| ≥ Inflection point | 0.92 (0.88–0.97) < .01 |
| p for the log‐likelihood ratio test | .25 |
The adjustment strategy is the same as the Model 2.
FIGURE 3.
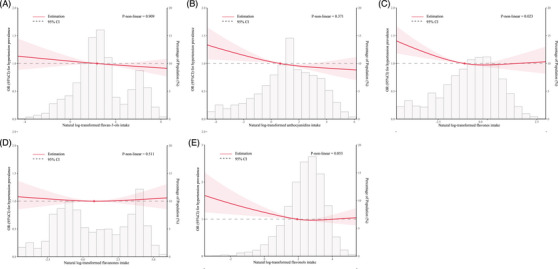
Restricted cubic spline analyses of the association of five sub types (A: flavan‐3‐ols, B: anthocyanidins, C: flavones, D: flavanones, E: flavonols) of flavonoid intake (natural log transformation) with hypertension. Heavy central lines represent the estimated adjusted hazard ratios. The 95% confidence interval is represented by the red band. The adjustment strategy is the same as the Model 2.
TABLE 5.
Nonlinearity addressing of flavones intake (natural log transformation) and risk of hypertension.
| Risk of hypertension | |
|---|---|
| Outcome: | OR, 95% CI, p |
| Fitting model by standard linear regression | 0.96 (0.94–0.99) < .01 |
| Fitting model by two‐piecewise linear regression | |
| Inflection point | −1.9 |
| < Inflection point | 0.86 (0.80–0.93) < .01 |
| ≥ Inflection point | 1.02 (0.97–1.06) .44 |
| p for the log‐likelihood ratio test | <.01 |
The adjustment strategy is the same as the Model 2.
3.4. Stratified analysis and sensitivity analysis
Results between total flavonoids intake (natural log transformation) and the risk of hypertension were consistent after stratification of studies by sex (male), age (< 60), race/ethnicity (non‐Hispanic white), cardiovascular disease (no), cancer (no), BMI (< 30.00 or ≥ 30.00), glycohemoglobin (< 6.5%), and energy intake (low and high) (Figure 4). In sensitivity analyses, the characteristics of raw data and data after multiple imputations were similar except family income‐poverty ratio and drinking status (Table S2). Similar results were observed when we excluded participants with missing covariate data to investigate the association among the take of total flavonoids (natural log transformation) and the risk of hypertension (Table S3). Finally, a cross‐sectional investigation indicated that total flavonoids (natural log transformation) intake increment was related to a lower hs‐CRP level in the population without hypertension (adjusted‐β = −0.18; 95% CI: −0.31 to −0.04), but not associated with other metabolic risk factors, including HbA1c, fasting blood glucose, triglycerides, cholesterol, LDL‐C, and HDL‐C (Table S4).
FIGURE 4.
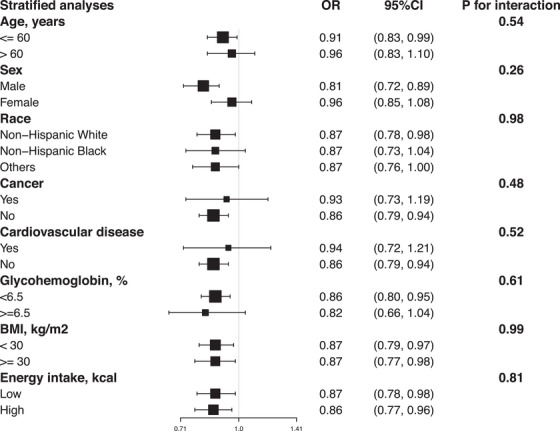
Forest plots of stratified analyses of total flavonoid intake (natural log transformation) and the risk of hypertension. With the exception of the variable itself, the adjustment approach is the same as Model 2.
4. DISCUSSION
In this cross‐sectional research, we demonstrated that higher total flavonoids were significantly and linearly associated with a lower prevalence of hypertension. Significant inverse relationships between flavan‐3‐ols, anthocyanidins, flavonols intake, and prevalence of hypertension were also revealed in the restricted cubic splines. In addition, the inverse relationships between flavones intake and hypertension were nonlinear. These findings may influence clinical practice and help individuals decrease the prevalence of hypertension.
The relationship between flavonoid intake and hypertension may vary among different populations. A cohort study of young Australian women demonstrated a L‐shaped relationship between flavonoid intake and hypertension. 17 Similarly, an 18‐year cohort study in China demonstrated the same results. 18 Although our study is cross‐sectional, it suggests a linear negative correlation. Prospective studies of middle‐aged and older Americans suggest that intake of anthocyanins, flavones, and flavonoid‐3‐ol compounds may help prevent hypertension and that the benefits were greatest in participants younger than 60 years, 19 which is consistent with our study. Although in vitro experiments have shown that flavonoid compounds can protect the cardiovascular system and lower blood pressure through various mechanisms, 20 our stratified analysis revealed that total flavonoids intake does not reduce the risk of hypertension in individuals with other diseases or those over 60. This result may be caused by an imbalance between damage and benefit, with cumulative damage over decades possibly outweighing the ability of flavonoids to affect endothelial function and blood pressure in older adults beneficially. 19 The potential explanation is that the detrimental effects of other diseases on the cardiovascular system outweigh the benefits of flavonoids, and the influence of flavonoids on hypertension is more apparent in healthy individuals. Additionally, these data highlight the importance of dietary intervention strategies for lowering blood pressure before middle age. A cross‐sectional study in the Iranian population also demonstrated a lower prevalence of hypertension with increased flavonoid intake. 21 As is well known, blueberries, orange juice, and some fruits contain large amounts of anthocyanins, which can be easily incorporated into the diet. 22 Several large prospective studies have also demonstrated the strong negative correlation between higher intake of flavonols, anthocyanins, and flavan‐3‐ols on blood pressure. 19 , 23 In addition, previous studies of people who regularly consumed flavonoid‐rich diets, most of whom were women, showed higher benefits. One study attributed this result to natural differences in hormonal protection, 9 but we believe exposure to risk factors also plays a key role. Our stratified analyses, after adjusting for confounding factors such as smoking and alcohol consumption, showed that negative correlation were not stronger in women than in men. Moreover, the heterogeneity of foods and beverages in different countries, due to differences in the food composition tables and dietary assessment methods used, also contributed to possible differences in the results of different studies. Since we used a complete flavonoid database, we estimated flavonoid intake more accurately than in previous flavonoid studies. 24 However, due to differences in geography, species, agriculture, and lifestyle habits of people in different regions, the flavonoid content of foods varies greatly, even in similar populations, and the lack of biomarker labeling of metabolic processes may be a large source of heterogeneity. 25 In addition, misclassification of flavonoid intake is inevitable, and important foods that have previously contained flavonoids may have been missed. 17 Nonetheless, several interventional studies of flavonoid intake have found that the use of cocoa flavan‐3‐ol for a certain period resulted in an average reduction of 3−6 units in systolic and diastolic blood pressure 26 and that the intake of specific flavonoids is associated with vascular mechanisms of action, such as improvement of vascular endothelial function, vasodilatation, and antagonism of calcium ion signaling. 27 These provides evidence for the relationship between flavonoid intake and hypertension.
Nitric oxide (NO) plays a significant role in the pathophysiology of hypertension. Decreased bioavailability of NO, elevated levels of reactive oxygen species (ROS), weakened function of the antioxidant system, and aberrant signaling pathways contribute to the various adverse consequences associated with hypertension. 28 Oxidative stress, the release of pro‐inflammatory factors, endoplasmic reticulum stress, and related signal cascades have been implicated in developing hypertension and its related complications. 29 , 30 Endothelin‐1 (ET‐1), an effective vasoconstrictor involved in regulating cardiovascular diseases, triggers increased ROS generation in blood vessels, reduces the bioavailability of NO, and induces endothelial dysfunction, thereby exacerbating hypertension. 31
Flavonoids exert their effects through antioxidant stress, downregulation of inflammatory cytokine expression in endothelial cells, and influence on endothelial function and vascular homeostasis. 32 They produce local factors such as prostacyclins, nitric oxide, and endothelins within the vessel wall and lumen. 33 Animal experiments have shown that they significantly lower blood pressure, improve aortic vasodilation, and increase NO levels in endothelial cells by inhibiting cAMP‐specific phosphodiesterase and subsequent cascade reactions. 34 , 35 In vitro studies have demonstrated that flavonoid compounds reduce oxidative damage by scavenging free radicals. 36 Additionally, some studies have reported a lower level of established inflammatory biomarkers, such as C‐reactive protein, associated with a higher intake of flavonoids. 37 , 38 This is consistent with our study, which suggests that total flavonoids intakes increment were associated with lower hs‐CRP. Flavonoid compounds possess antioxidant, anti‐inflammatory, and anti‐thrombotic activities. 39 They have therapeutic effects on endothelial dysfunction, myocardial ischemia, cardiomyocyte, and vascular damage. 40 Although it remains unclear whether flavonoid compounds in the human body can enhance the preventive effects against cardiovascular events through mutual interactions and metabolism of intestinal flora, 41 their wide range of pharmacological actions provides compelling evidence for dietary adjustments to prevent hypertension and cardiovascular diseases. 42
Our study has several strengths. To the best of our knowledge, this is the first large cross‐sectional study to investigate the association between flavonoid intake and hypertension risk in the adult population using the NHANES database. Second, we analyzed the relationship between total flavonoid intake, five subtypes, and hypertension while adjusting for more confounding variables. Third, we used a larger and more comprehensive flavonoid database, and compared to previous flavonoid research, we were able to more accurately estimate flavonoid intake, 16 and explored the relationship between flavonoid intake and hypertension in different population groups, which provides insights into the dietary habits of diverse populations and the potential benefits of flavonoid compounds.
However, it is important to recognize the study's shortcomings. First, due to the cross‐sectional nature of this study, we cannot establish causality. Second, the intake of flavonoids was only estimated at the baseline, which might not be an indicator of intake throughout follow‐up. Third, we did not stratify high blood pressure by severity. Finally, individuals often adopt healthier lifestyles and dietary habits after being diagnosed with hypertension, and unknown confounders factors related to different dietary habits among different population groups may introduce biases.
5. CONCLUSIONS
This cross‐sectional study demonstrates a negative correlation between the increased intake of total flavonoids within a certain range and the prevalence of hypertension, particularly among individuals without comorbidities. Further interventional studies are needed to clarify the potential effects of flavonoid intake on hypertension.
AUTHOR CONTRIBUTIONS
Data collection, analysis, and manuscript writing were all assisted by Ben Hu. The analytical procedures were confirmed by Linlin Hou, who also read the text. The text was edited by Yan Wang for key information. Some data was gathered by Jun Feng. The final manuscript was reviewed and approved by all writers.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to the contributors to the NHANES project. No funding was received for this study.
Hu B, Wang Y, Feng J, Hou L. The association between flavonoids intake and hypertension in U.S. adults: A cross‐sectional study from The National Health and Nutrition Examination Survey. J Clin Hypertens. 2024;26:573–583. 10.1111/jch.14807
DATA AVAILABILITY STATEMENT
The datasets were accessible from NHANES 2007−2010 and 2017−2018 (https://www.cdc.gov/nchs/nhanes/index.htm). The corresponding author will provide the datasets upon reasonable request.
REFERENCES
- 1. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982‐1004. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnier M, Damianaki A. Hypertension as cardiovascular risk factor in chronic kidney disease. Circ Res. 2023;132(8):1050‐1063. [DOI] [PubMed] [Google Scholar]
- 4. Saiz LC, Gorricho J, Garjon J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Db Syst Rev. 2022;11(11):CD010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olczak KJ, Taylor‐Bateman V, Nicholls HL, Traylor M, Cabrera CP, Munroe PB. Hypertension genetics past, present and future applications. J Intern Med. 2021;290(6):1130‐1152. [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Xie L, Liu K, et al. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: a review. Pharmacol Res. 2021;174:105919. [DOI] [PubMed] [Google Scholar]
- 7. Das M, Devi KP, Belwal T, et al. Harnessing polyphenol power by targeting eNOS for vascular diseases. Crit Rev Food Sci. 2023;63(14):2093‐2118. [DOI] [PubMed] [Google Scholar]
- 8. Sohrab G, Ebrahimof S, Hosseinpour‐Niazi S, Yuzbashian E, Mirmiran P, Azizi F. Association of dietary intakes of total polyphenol and its subclasses with the risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metab Syndr Relat D. 2018;16(6):274‐281. [DOI] [PubMed] [Google Scholar]
- 9. Grosso G, Stepaniak U, Micek A, et al. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur J Nutr. 2018;57(4):1535‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 11. Zhao G, Li C, Ford ES, et al. Leisure‐time aerobic physical activity, muscle‐strengthening activity and mortality risks among US adults: the NHANES linked mortality study. Brit J Sport Med. 2014;48(3):244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernaez R, Mclean J, Lazo M, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound‐defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol H. 2013;11(9):1183‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson CL, Paulose‐Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2(161):1‐24. [PubMed] [Google Scholar]
- 14. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. New Engl J Med. 2015;373(14):1307‐1317. [DOI] [PubMed] [Google Scholar]
- 15. Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129(3):758S‐767S. [DOI] [PubMed] [Google Scholar]
- 16. Sebastian RS, Wilkinson EC, Goldman JD, et al. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US adults. J Nutr. 2015;145(6):1239‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Do RV, Schoenaker D, Kent K, Weston‐Green K, Charlton K. Association between flavonoid intake and risk of hypertension in two cohorts of Australian women: a longitudinal study. Eur J Nutr. 2021;60(5):2507‐2519. [DOI] [PubMed] [Google Scholar]
- 18. Lin X, Zhao J, Ge S, et al. Dietary polyphenol intake and risk of hypertension: an 18‐y nationwide cohort study in China. Am J Clin Nutr. 2023;118(1):264‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cassidy A, O'Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93(2):338‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maaliki D, Shaito AA, Pintus G, El‐Yazbi A, Eid AH. Flavonoids in hypertension: a brief review of the underlying mechanisms. Curr Opin Pharmacol. 2019;45:57‐65. [DOI] [PubMed] [Google Scholar]
- 21. Sohrab G, Hosseinpour‐Niazi S, Hejazi J, Yuzbashian E, Mirmiran P, Azizi F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int J Food Sci Nutr. 2013;64(6):661‐667. [DOI] [PubMed] [Google Scholar]
- 22. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agr Food Chem. 2006;54(11):4069‐4075. [DOI] [PubMed] [Google Scholar]
- 23. Lajous M, Rossignol E, Fagherazzi G, et al. Flavonoid intake and incident hypertension in women. Am J Clin Nutr. 2016;103(4):1091‐1098. [DOI] [PubMed] [Google Scholar]
- 24. Erdman JJ, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31‐June 1, 2005, Washington, DC. J Nutr. 2007;137(3 Suppl 1):718S‐737S. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta‐analysis of prospective cohort studies. Brit J Nutr. 2014;111(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 26. Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid‐rich foods, and cardiovascular risk: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(1):38‐50. [DOI] [PubMed] [Google Scholar]
- 27. Hedayati N, Bemani NM, Mohammadinejad A, Mohajeri SA. Beneficial effects of celery (Apium graveolens) on metabolic syndrome: a review of the existing evidences. Phytother Res. 2019;33(12):3040‐3053. [DOI] [PubMed] [Google Scholar]
- 28. Puzserova A, Bernatova I. Blood pressure regulation in stress: focus on nitric oxide‐dependent mechanisms. Physiol Res. 2016;65(Suppl 3):S309‐S342. [DOI] [PubMed] [Google Scholar]
- 29. Oyarce MP, Iturriaga R. Contribution of oxidative stress and inflammation to the neurogenic hypertension induced by intermittent hypoxia. Front Physiol. 2018;9:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlisle RE, Werner KE, Yum V, et al. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J Hypertens. 2016;34(8):1556‐1569. [DOI] [PubMed] [Google Scholar]
- 31. Gao Y, Chen T, Raj JU. Endothelial and smooth muscle cell interactions in the pathobiology of pulmonary hypertension. Am J Resp Cell Mol. 2016;54(4):451‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamagata K, Yamori Y. Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J Cardiovasc Pharm. 2020;75(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 33. Li PG, Sun L, Han X, Ling S, Gan WT, Xu JW. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt‐independent and PKA‐dependent mechanism. Pharmacology. 2012;89(3‐4):220‐228. [DOI] [PubMed] [Google Scholar]
- 34. Ko WC, Shih CM, Leu IJ, Chen TT, Chang JP. Mechanisms of relaxant action of luteolin in isolated guinea pig trachea. Planta Med. 2005;71(5):406‐411. [DOI] [PubMed] [Google Scholar]
- 35. Si H, Wyeth RP, Liu D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur J Nutr. 2014;53(1):269‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673‐751. [PubMed] [Google Scholar]
- 37. Tong J, Zeng Y, Xie J, Xiao K, Li M, Cong L. Association between flavonoid and subclasses intake and metabolic associated fatty liver disease in U.S. adults: results from National Health and Nutrition Examination Survey 2017–2018. Front Nutr. 2022;9:1074494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Li D, Shan Y, et al. Luteolin intake is negatively associated with all‐cause and cardiac mortality among patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2023;15(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. [DOI] [PubMed] [Google Scholar]
- 40. Wallace TC. Anthocyanins in cardiovascular disease. Adv Nutr. 2011;2(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24(2):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khan J, Deb PK, Priya S, et al. Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules. 2021;26(13):4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The datasets were accessible from NHANES 2007−2010 and 2017−2018 (https://www.cdc.gov/nchs/nhanes/index.htm). The corresponding author will provide the datasets upon reasonable request.


