Abstract
In this two-center prospective cohort study of children on ECMO, we assessed a panel of plasma brain injury biomarkers using exploratory factor analysis (EFA) to evaluate their interplay and association with outcomes. Biomarker concentrations were measured daily for the first 3 days of ECMO support in 95 participants. Unfavorable composite outcome was defined as in-hospital mortality or discharge Pediatric Cerebral Performance Category > 2 with decline ≥ 1 point from baseline. EFA grouped 11 biomarkers into three factors. Factor 1 comprised markers of cellular brain injury (NSE, BDNF, GFAP, S100β, MCP1, VILIP-1, neurogranin); Factor 2 comprised markers related to vascular processes (vWF, PDGFRβ, NPTX1); and Factor 3 comprised the BDNF/MMP-9 cellular pathway. Multivariable logistic models demonstrated that higher Factor 1 and 2 scores were associated with higher odds of unfavorable outcome (adjusted OR 2.88 [1.61, 5.66] and 1.89 [1.12, 3.43], respectively). Conversely, higher Factor 3 scores were associated with lower odds of unfavorable outcome (adjusted OR 0.54 [0.31, 0.88]), which is biologically plausible given the role of BDNF in neuroplasticity. Application of EFA on plasma brain injury biomarkers in children on ECMO yielded grouping of biomarkers into three factors that were significantly associated with unfavorable outcome, suggesting future potential as prognostic instruments.
Keywords: Extracorporeal membrane oxygenation, Child, Brain injury, Biomarkers, Neurologic outcome
Subject terms: Biomarkers, Brain injuries, Paediatrics, Therapeutics
Introduction
Extracorporeal membrane oxygenation (ECMO) is a technique that provides support in neonatal and pediatric patients with severe refractory cardiopulmonary failure or cardiac arrest. It is a potentially lifesaving intervention, and technological advancements over the last decades have made utilization increasingly prevalent in critical care medicine. The Extracorporeal Life Support Organization (ELSO) registry most recently reported that during the five-year period between 2018 and 2022, there were a total of 12,196 pediatric and 7575 neonatal ECMO cases at 557 centers. In these populations, the indications for ECMO spanned 31–51% for respiratory support, 38–47% for cardiac support, and 11–22% for extracorporeal cardiopulmonary resuscitation (ECPR)1. The overall rate of survival to hospital discharge was 59–61%, which encompassed 69–70% for respiratory indications, 52–60% for cardiac indications, and 40–47% for ECPR indications1.
Acute neurologic injury is one of the major complications of ECMO, occurring in 13–36% of children on ECMO support2–4. Acute neurologic injury during ECMO includes intracranial hemorrhage, thromboembolic stroke, hypoxic-ischemic injury, and seizures, and is associated with increased mortality2 and long-term neurologic disability among survivors5,6. While morbidity and mortality on ECMO are certainly not limited to neurologic etiologies, neurologic injury is one of the main drivers of outcome in the neonatal and pediatric ECMO populations3.
The pathophysiology of neurologic injury in critically ill patients requiring ECMO support is complex and not yet well understood. Several neuromonitoring methods are used for early diagnosis of neurologic injury, therapy guidance, avoidance of secondary insults, and neuroprognostication3,4. However, it is likely that only multimodal monitoring can capture said complexity. Plasma brain injury biomarkers have been proposed as additions to multimodal monitoring and have been previously shown to be associated with neurologic injury in critically ill children, including those on ECMO support. These include biomarkers of primary structural damage but also of secondary cascade of injury and repair in the brain7, such as markers of neuronal injury: neuron-specific enolase (NSE)8,9, of neuroregeneration: brain-derived neurotrophic factor (BDNF)9, of astrocytic injury: glial fibrillary acidic protein (GFAP)9,10 and S100β8,9,11, and of neuroinflammation: monocyte chemoattractant protein 1 (MCP1)9.
Additionally, biomarkers that are relevant in neonatal or adult brain injury and thus may have pediatric implications include: platelet-derived growth factor receptor-beta (PDGFRβ)12, visinin-like protein 1 (VILIP-1)13,14, matrix metalloproteinase 9 (MMP-9)15–19, neurogranin (NRGN)20,21, creatine kinase B type (CK-BB)11, and von Willebrand factor (vWF)22–24. Lastly, biomarkers that have been identified in vitro or in animal models of brain injury but not yet established clinically, include neuronal pentraxin 1 (NPTX1)25–28 and peroxiredoxin-6 (PRDX6)29,30. Understanding the role of these biomarkers that represent multiple neuronal cell subtypes and neurologic injury pathways during neonatal and pediatric ECMO can potentially lead to improvements in neuromonitoring, identification of potential therapeutic targets, reduction in ECMO-related morbidity and mortality, and neuroprognostication among survivors.
In this study, we aimed to assess if use of exploratory factor analysis (EFA) on an expanded panel of brain injury biomarkers previously evaluated in various types of brain injury could lead to better understanding of the interplay of these biomarkers and their association with unfavorable outcomes and abnormal neuroimaging in critically ill children on ECMO support.
Materials and methods
Study population
This prospective observational cohort study enrolled neonatal and pediatric patients on ECMO support at two academic, quaternary care, urban, pediatric intensive care units between July 2010 and June 2015. Characteristics of the cohort have been previously described5. Informed consent was obtained from all parents within 24 h of ECMO initiation. A total of 99 children were enrolled in the parent study. Of these, 95 subjects had longitudinal blood samples obtained daily during the first 3 days of the ECMO course. Platelet-poor plasma aliquots that had not undergone any freeze–thaw cycles and had been stored at -80 °C in a freezer with continuous temperature monitoring were used for the current study. We collected data from neuroimaging reports of serial head ultrasounds obtained daily during the ECMO course in infants with open anterior fontanel as part of routine clinical protocols at the two participating institutions, and of brain computed tomography (CT) and/or brain magnetic resonance imaging (MRI) studies that were obtained based on clinical indications at the discretion of the clinical team, during the ECMO course and up to six weeks after decannulation. Clinical imaging reports noting abnormal findings were then classified as postasphyxial injury, arterial ischemic stroke, and intracranial hemorrhage, noting that imaging studies frequently detected more than one type of injury. Types of neuroimaging findings and time to first abnormal neuroimaging are presented in Supplemental Table 1. Pediatric Cerebral Performance Category (PCPC) scores at baseline and hospital discharge were ascertained from the medical records locally and in real-time by trained data abstractors who were blinded to biomarker results31,32. This study was approved by the Johns Hopkins Medicine and the Children’s National Institutional Review Boards at the two participating centers and performed in accordance with relevant guidelines and regulations.
Measurement of brain injury biomarkers
Thirteen brain injury biomarkers were measured using chemiluminescent immunoassays (Meso Scale Discovery, Gaithersburg, MD). Of the 13 biomarkers, 11 were selected for the present study based on detectability in ≥ 50% of samples: brain-derived neurotrophic factor (BDNF), glial fibrillary acidic protein (GFAP), monocyte chemoattractant protein 1 (MCP1), matrix metalloproteinase 9 (MMP-9), neurogranin (NRGN), neuronal pentraxin 1 (NPTX1), neuron-specific enolase (NSE), platelet-derived growth factor beta (PDGFRβ), S100β, visinin-like protein 1 (VILIP-1), and von Willebrand factor (vWF). Markers not included in the analysis were peroxiredoxin-6 (PRDX6), which had more than half of samples above the upper limit of quantification, and creatine kinase B type (CKBB), for which the quantity of plasma available was insufficient to assay in all participants.
Descriptive analysis of biomarkers
Of all available samples from a given participant, the highest value, or peak, of any given biomarker measured daily for the first 3 days of ECMO support was used for EFA. Biomarkers had right-skewed distributions and were therefore natural log-transformed. Seventy-two of 295 biomarker observations lay above their upper limit of quantification (ULOQ), all related to 5 (GFAP, MCP1, MMP-9, S100β, VILIP-1) of the 11 biomarkers included in the analysis. To utilize EFA, which requires numerical values for all data, we imputed values for these 72 observations using truncated log-normal distributions for each biomarker. Specifically, we fit a normal distribution to the observed data, extrapolated the curve above the ULOQ, and drew values randomly from the distribution above the ULOQ.
EFA methods
We used EFA to detect combinations of biomarkers that indicated unique processes agnostic to covariates and outcomes of interest. EFA identifies groups of markers that are highly correlated and thus likely to reflect the same process. Log-transformed biomarker values (observed and imputed) were standardized to create distributions with a mean of 0 and a standard deviation of 1. Principal components analysis was used to produce proportion criteria and create a scree plot used to reduce the brain injury biomarkers to an optimal number of factors that retained the most amount of total variance in the original variables. The factors were derived using generalized weighted least squares methods and oblique rotation on the peak measurements to transform the factors and obtain factor loadings, which represent the strength and direction of the relationship between the biomarkers and the underlying (latent) relationships. For factor interpretation, only biomarkers with factor loadings ≥ 0.3 were considered; however, the final factor scores were based on all variables. The standardized factor scores for each participant were derived using the tenBerge regression scoring method, which preserves the correlation between factors for an oblique solution33.
The factors were then included as dependent variables in linear regression models to evaluate associations with clinical characteristics including participant age, sex, and ECMO indication. Next, the factors were included as independent variables in multivariable logistic regression models. The primary outcome for the first set of models was a composite unfavorable outcome, defined as in-hospital mortality or decline in neurofunctional status defined as discharge PCPC > 2 with decline ≥ 1 from baseline PCPC5. The secondary outcome for the second set of logistic models was new abnormal neuroimaging during the ECMO course or within 6 weeks of ECMO decannulation, evaluated among the subset of patients with available neuroimaging. These logistic regression models included each factor separately and summarized the association between each factor and unfavorable outcome, unadjusted and adjusted for age, sex, and ECMO indication. All analyses were performed using R 3.6.0 (R Core Team, Vienna, Austria).
Results
Demographic and clinical characteristics of the parent cohort (n = 99) have been previously described5. Summary characteristics of the 95 participants included in this analysis are presented in Table 1.
Table 1.
Demographic and clinical characteristics of children supported on ECMOa.
| Overall (n = 95) | Favorable (n = 43) | Unfavorableb (n = 52) | |
|---|---|---|---|
| Demographic characteristics | |||
| Male | 52 (55) | 21 (49) | 31 (6) |
| Age group | |||
| Neonate, < 1 mo | 44 (46) | 24 (56) | 20 (38) |
| Infant, 1 mo-< 1 y | 24 (25) | 7 (16) | 17 (33) |
| Child, 1-< 12 y | 19 (20) | 7 (16) | 12 (23) |
| Adolescent, ≥ 12 y | 8 (8) | 5 (12) | 3 (6) |
| Weight, kg | 4.0 [3.1, 11.9] | 4.0 [3.2, 11.9] | 4.0 [3.0, 11.7] |
| ECMO characteristics | |||
| Primary indication for ECMO | |||
| Respiratory | 31 (33) | 22 (51) | 9 (17) |
| Non-respiratory | 64 (67) | 21 (49) | 43 (83) |
| ECMO mode: venoarterial | 88 (93) | 38 (88) | 50 (96) |
| ECMO duration, days | 4.9 [3.2, 10.2] | 4.6 [3.5, 7.5] | 5.4 [3.2, 12.5] |
| Neuroimaging (n = 84) | |||
| Neuroimaging during or within 6 weeks post-ECMO decannulation | |||
| Daily HUS during or post-ECMO | 34 (40) | 11 (31) | 23 (48) |
| Daily HUS during ECMO, brain CT during or post-ECMO, and/or brain MRI post-ECMO | 32 (38) | 18 (50) | 14 (29) |
| At least one brain CT during or post-ECMO and/or brain MRI post-ECMO, without HUS | 18 (21) | 7 (19) | 11 (23) |
| Outcomes | |||
| Composite unfavorable outcomeb | 52 (55) | NA | 52 (100) |
| In-hospital mortality | 42 (44) | NA | 42 (81) |
| Abnormal neuroimaging (n = 84) | 42 (50) | 15 (42) | 27 (56) |
ECMO, extracorporeal membrane oxygenation, PCPC, Pediatric Cerebral Performance Category, HUS, head ultrasound, CT, computed tomography, MRI, magnetic resonance imaging.
aContinuous variables are presented as medians [P25, P75] and categorical variables as counts (frequencies).
bComposite unfavorable outcome: in-hospital mortality or decline in neurofunctional status defined as discharge PCPC > 2 with decline ≥ 1 from baseline PCPC among survivors.
Supplemental Fig. 1 displays the distribution of daily plasma levels of each biomarker during the first 3 days of ECMO support. The distributions of peak plasma biomarker levels are summarized in Supplemental Table 2, which also includes counts and frequencies of biomarker levels above the ULOQ which occurred in 5 biomarkers (GFAP, MCP1, MMP-9, S100β, and VILIP-1). These counts above the ULOQ correspond to the 72 observations that were imputed using the log-normal models shown in Supplemental Fig. 2.
Correlations among the standardized log-transformed peak plasma biomarker levels are shown in Table 2, with moderately strong relationship (correlation coefficient ≥ 0.30) noted for: GFAP and S100β, MCP1, VILIP-1, NSE, BDNF, NRGN; S100β and MCP1, VILIP-1, NSE; MCP1 and VILIP-1, NSE; VILIP-1 and NSE; NSE and NRGN; BDNF and MMP-9; vWF and PDGFRβ, NPTX1; and PDGFRβ and NPTX1. We also observed a moderately strong inverse correlation between VILIP-1 and MMP-9. Factor loading and variance explained results from the EFA are shown in Table 3. Three brain injury factors were identified, accounting for 44% of the total variance in the biomarker data. Factor 1 was characterized by the biomarkers GFAP, S100β, MCP1, VILIP-1, NSE, BDNF, and NRGN; factor 2 by NPTX1, vWF, and PDGFRβ; and factor 3 by BDNF and MMP-9.
Table 2.
Pearson correlations among the standardized log-transformed peak plasma biomarker levels (n = 95).
| Marker | Pearson correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GFAP | S100β | MCP1 | VILIP-1 | NSE | BDNF | NRGN | vWF | PDGFRβ | NPTX1 | MMP-9 | |
| GFAP | 1.00 | 0.50 a,b | 0.41 a,b | 0.42 a,b | 0.47 a,b | 0.32 a,c | 0.30 a,c | 0.20c | 0.15 | 0.16 | − 0.08 |
| S100β | – | 1.00 | 0.47 a,b | 0.44 a,b | 0.33 a,c | 0.18 | 0.10 | 0.26c | 0.20 | 0.17 | − 0.14 |
| MCP1 | – | – | 1.00 | 0.40 a,b | 0.33 a,c | 0.10 | 0.24c | 0.12 | 0.14 | 0.17 | − 0.23c |
| VILIP-1 | – | – | – | 1.00 | 0.41 a,b | 0.12 | 0.20c | 0.18 | 0.12 | 0.27c | − 0.32 a,c |
| NSE | – | – | – | – | 1.00 | 0.24c | 0.33 a,c | 0.29c | 0.13 | 0.29c | − 0.26c |
| BDNF | – | – | – | – | 1.00 | 0.24c | 0.04 | 0.05 | 0.09 | 0.35 a,b | |
| NRGN | – | – | – | – | – | – | 1.00 | 0.18 | 0.29c | 0.23c | 0.13 |
| vWF | – | – | – | – | – | – | – | 1.00 | 0.30 a,c | 0.46 a,b | 0.00 |
| PDGFRβ | – | – | – | – | – | – | – | – | 1.00 | 0.36 a,b | − 0.05 |
| NPTX1 | – | – | – | – | – | – | – | – | – | 1.00 | − 0.02 |
| MMP-9 | – | – | – | – | – | – | – | – | – | – | 1.00 |
BDNF, brain-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; MCP1, monocyte chemoattractant protein 1; MMP-9, matrix metalloproteinase 9; NRGN, neurogranin, NPTX1, neuronal pentraxin 1; NSE, neuron-specific enolase; PDGFRβ, platelet derived growth factor receptor beta; VILIP-1, visinin-like protein 1, vWF, von Willebrand factor.
aCorrelation coefficient ≥ 0.30 (moderately strong relationship).
bp < 0.001.
c0.001 ≤ p < 0.05.
Table 3.
Factor loadings showing the strength of relationship between factors and individual biomarkers (n = 95).
| Biomarker | Factor Loadings for Brain Injury Biomarkers | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| GFAP | 0.76 | − 0.02 | 0.08 |
| S100β | 0.61 | 0.05 | − 0.09 |
| MCP1 | 0.58 | 0.01 | − 0.16 |
| VILIP-1 | 0.57 | 0.09 | − 0.24 |
| NSE | 0.54 | 0.18 | − 0.08 |
| BDNF | 0.45 | − 0.06 | 0.53 |
| NRGN | 0.30 | 0.27 | 0.24 |
| vWF | 0.02 | 0.63 | 0.01 |
| PDGFRβ | 0.01 | 0.53 | 0.02 |
| NPTX1 | − 0.01 | 0.73 | 0.01 |
| MMP-9 | − 0.14 | 0.03 | 0.83 |
| Variance explained, % | 21 | 13 | 10 |
| Cumulative variance | 21 | 34 | 44 |
BDNF, brain-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; MCP1, monocyte chemoattractant protein 1; MMP-9, matrix metalloproteinase 9; NRGN, neurogranin, NPTX1, neuronal pentraxin 1; NSE, neuron-specific enolase; PDGFRβ, platelet-derived growth factor receptor beta; VILIP-1, visinin-like protein 1, vWF, von Willebrand factor.
Bold indicates salient factors using criterion of factor loadings ≥ 0.3.
To evaluate the relationship between brain injury biomarker factor scores and exposures (age, sex, and ECMO indication), we performed univariable linear regression analyses where each factor score was treated as the dependent variable while the exposures were the independent variables. Factor 1 score was associated with ECMO indication: participants with non-respiratory ECMO indications had mean factor 1 scores + 0.78 higher (p < 0.001) than participants with respiratory ECMO indications. Factor 2 and 3 scores were not significantly associated with any of the exposures (Table 4).
Table 4.
Unadjusted associations of the three brain injury biomarker factors with the exposures of interest (n = 95).
| Exposure | Brain injury biomarker factorsa | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Est (95% CI) | p | Est (95% CI) | p | Est (95% CI) | p | |
| Sex: Male vs Female (ref) |
0.31 (− 0.09, 0.72) |
0.131 |
0.04 (− 0.37, 0.45) |
0.849 |
− 0.08 (− 0.49, 0.33) |
0.708 |
| Age: Neonate vs Non-neonate (ref) |
− 0.23 (− 0.63, 0.18) |
0.276 |
0.29 (− 0.12, 0.69) |
0.166 |
− 0.32 (− 0.73, 0.08) |
0.119 |
| ECMO indication: Non-respiratory vs Respiratory (ref) |
0.78 (0.37, 1.18) |
< 0.001 |
0.34 (− 0.09, 0.77) |
0.118 |
− 0.19 (− 0.62, 0.25) |
0.390 |
ECMO, extracorporeal membrane oxygenation; CI, confidence interval.
aBrain injury biomarker factors:
Factor 1: GFAP, S100β, MCP1, VILIP-1, NSE, BDNF, NRGN.
Factor 2: NPTX1, vWF, PDGFRβ.
Factor 3: BDNF, MMP-9.
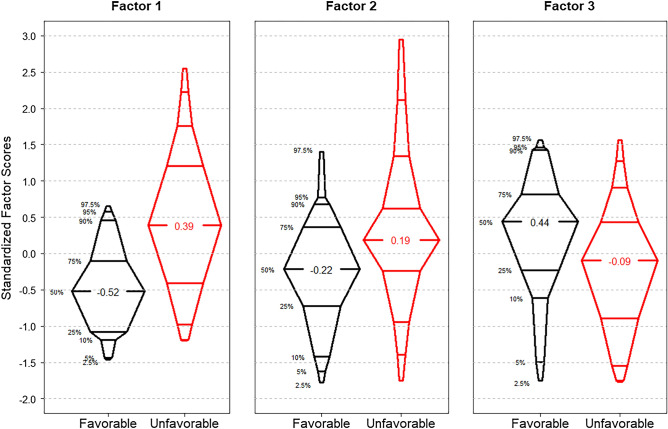
The distributions of all three brain injury biomarker factor scores by unfavorable versus favorable outcome are graphically displayed in Fig. 1. These distributions indicate that an unfavorable outcome had higher median factor 1 and 2 scores compared to a favorable outcome (+ 0.39 versus -0.52, p < 0.001, and + 0.19 versus -0.22, p = 0.033, respectively). Conversely, lower median factor 3 scores were seen in unfavorable outcomes compared to favorable outcomes (-0.09 versus + 0.44, p = 0.008).
Figure 1.
Distributions of the three brain injury biomarker factors by outcome (n = 95). The black plots show those with favorable outcome (n = 43), and the red plots show those with unfavorable outcome (n = 52). Wilcoxon rank sum comparisons between factor scores for favorable versus unfavorable outcomes rendered: p < 0.001 for Factor 1, p = 0.033 for Factor 2, and p = 0.008 for Factor 3.
Table 5 presents results from multivariable logistic regression analyses where the composite unfavorable outcome was the dependent variable, and each brain injury biomarker factor score was the exposure of interest in separate models. Brain injury factors 1 and 2 were associated with higher odds of unfavorable outcome (adjusted OR 2.88, 95% CI 1.61–5.66, and adjusted OR 1.89, 95% CI 1.12–3.43, respectively), while brain injury factor 3 was associated with lower odds of unfavorable outcome (adjusted OR 0.54, 95% CI 0.31–0.88). The results of the full multivariable logistic regression models are presented in Supplemental Table 3.
Table 5.
Multivariable model for association of the three brain injury biomarker factors with unfavorable outcome at hospital dischargea (n = 95).
| Brain injury biomarker factorsc | Unadjusted | Adjustedb | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| 1 | 3.33 (1.94, 6.32) | < 0.001 | 2.88 (1.61, 5.66) | 0.001 |
| 2 | 1.75 (1.12, 2.89) | 0.020 | 1.89 (1.12, 3.43) | 0.025 |
| 3 | 0.59 (0.36, 0.91) | 0.021 | 0.54 (0.31, 0.88) | 0.020 |
aUnfavorable outcome at hospital discharge is defined as in-hospital mortality or discharge Pediatric Cerebral Performance Category (PCPC) > 2 with decline ≥ 1 point from baseline PCPC.
bAdjusted for age, sex, and ECMO indication.
cBrain injury biomarker factors:
Factor 1: GFAP, S100β, MCP1, VILIP-1, NSE, BDNF, NRGN.
Factor 2: NPTX1, vWF, PDGFRβ.
Factor 3: BDNF, MMP-9.
Among the subset of 84 participants who had neuroimaging studies completed during ECMO or within 6 weeks after ECMO decannulation, factor 1 was associated with higher odds of abnormal neuroimaging (adjusted OR 2.38, 95% CI 1.38–4.45). Factors 2 and 3 were not significantly associated with abnormal neuroimaging. The results of these multivariable logistic regression models are presented in Supplemental Table 4. Of note, abnormal neuroimaging was not statistically associated with the composite primary outcome, unadjusted (OR 1.80, 95% CI 0.76–4.37) or when adjusting for age, sex, and ECMO indication (adjusted OR 1.45, 95% CI 0.56–3.80).
Discussion
In this two-center prospective observational study of 95 neonatal and pediatric patients on ECMO, we found that 11 circulating biomarkers could be grouped via EFA as a data reduction technique. Specifically, biomarker levels clustered together in three distinct brain injury factors that are biologically plausible based on what is currently known about originating cells and function of each of the biomarkers studied. More importantly, the results indicate that these factors were associated with unfavorable outcome at hospital discharge and new abnormal neuroimaging during or within 6 weeks after ECMO decannulation.
Brain injury factor 1 grouped the following biomarkers: GFAP, S100β, MCP1, NSE, BDNF, VILIP-1, and NRGN. This group encompasses biomarkers of cellular brain injury: neuronal injury, astrocytic injury, and neuroinflammation. To first address markers of neuronal injury, NSE is a glycolytic enzyme localized mostly in neuronal cytoplasm. It has been previously associated with unfavorable outcomes after pediatric cardiac arrest34. BDNF is a member of the neurotrophins family of growth factors and mediates neuronal growth, differentiation, regeneration, and survival. It has been implicated in providing neuroplasticity and neuroprotection through reduction in secondary brain injury. BDNF has been associated with pediatric traumatic brain injury (TBI)35, favorable outcomes in neonatal hypoxic-ischemic encephalopathy (HIE)36,37, and decreased functional impairment in pediatric neurocritical illness38. Next, to address markers of astrocytic injury, GFAP is a cytoskeletal filament protein found in mature astrocytes, and its expression is increased during reactive astrogliosis after neurologic injury39. It has been shown to predict neurologic injury and outcomes in pediatric patients on cardiopulmonary bypass40,41, in pediatric patients with sickle cell disease42 and severe TBI43, and in HIE37,44,45. S100β is a calcium-binding protein localized predominately in the cytoplasm of astrocytes and involved in neuronal growth and survival12,48 and has been associated with unfavorable outcome after pediatric cardiac arrest34 and in neonatal HIE46. Finally, to address neuroinflammation, MCP1 is a small cytokine in the CC chemokine family that plays a key role in recruiting immune factors and cells to sites of inflammation. In the central nervous system, it is found in neurons, astrocytes, microglia, and infiltrating macrophages and thus involved in acute neuroinflammation, neuronal injury and death. MCP1 has been shown to be elevated after neonatal HIE47. These five biomarkers (GFAP, S100β, MCP1, NSE, BDNF) have all been previously studied in pediatric ECMO. GFAP, S100β, NSE and MCP1 have been associated with neurologic injury and unfavorable outcomes in pediatric ECMO9,48. NSE has also been associated with abnormal neuroimaging in these studies9. BDNF’s neuroprotective factors have also been previously investigated in pediatric ECMO, but no studies have found associations with survival, outcomes, or neuroimaging9. Together, these five biomarkers represent three major categories of neurologic injury pathophysiology—neuronal injury, astrocytic injury, and neuroinflammation—and thus it is fitting for them to be agnostically grouped into one factor.
Two less studied biomarkers, VILIP-1 and NRGN, were also grouped into brain injury factor 1. VILIP-1 is a neuronal calcium sensor protein that has been studied in adult neurodegenerative diseases49, stroke14, and TBI13. The only clinical study in pediatrics found that serum and cerebrospinal fluid levels of VILIP-1 were associated with acute encephalopathy with biphasic seizures and late reduced diffusion50. NRGN is a brain-specific postsynaptic calmodulin-binding protein involved in the protein kinase C signaling pathway. Elevated levels have been observed in children with sickle cell disease and stroke51 and associated with worse neonatal HIE grades and developmental outcomes37. The addition of these two biomarkers with more well-established markers of brain injury in one grouped factor suggests that they may warrant further investigation in pediatric brain injury.
The second brain injury factor grouped: vWF, PDGFRβ, and NPTX1. Interestingly, two of the three markers are involved in vascular processes (vWF and PDGFRβ). vWF is a glycoprotein integrally involved in hemostasis through platelet and collagen adhesion and the intrinsic coagulation cascade. It has been previously studied as a marker of endothelial activation and injury in adults with TBI with mixed results22–24. PDGFRβ is a growth factor receptor that is released with pericyte damage and thus has been used as an indicator of microvascular injury and blood–brain barrier disruption in adult TBI, stroke, spinal cord injury, multiple sclerosis, and glioblastoma12. NPTX1 is a neuronal pentraxin protein preferentially secreted at excitatory synapses and has a role in regulating mitochondria-driven neuronal death in hypoxic-ischemic animal models25–28. None of these three markers have been previously studied in pediatric neurologic injury or critical illness. However, given the association with unfavorable outcomes in this study, they may warrant further investigation into vascular-related pathophysiology in pediatric brain injury.
Brain injury factor 3 grouped MMP-9 and BDNF. This is fitting as BDNF has been shown to upregulate MMP-9, a proteinase involved in extracellular matrix degradation, in terms of expression and enzymatic activity52. In turn, MMP-9 helps convert pro-BDNF to its activated form. MMP-9 has been previously associated in adult ischemic stroke severity, lesion volume, and hemorrhagic conversion15–18. It has not been as well studied in pediatrics, though elevated levels have been found in neonatal encephalopathy19 and in neuroinflammatory conditions53. This factor’s association with lower levels of unfavorable neurologic outcomes in neonatal and pediatric ECMO is supported by the previously discussed neuroprotective properties of BDNF. Interestingly, this suggested neuroprotective property of MMP-9 stands in contrast to previously demonstrated pathogenic properties of other proteases, such as calpain, in many neuropathologies including neonatal encephalopathy54–56. Additionally, the agnostic grouping of these two biomarkers involved in the same signaling pathway supports that EFA did in fact group combinations that indicate a unique pathophysiologic process.
In summary, EFA agnostically grouped 11 biomarkers into three factors that have compelling shared properties and appear to cluster together, with factor 1 represented by markers of cellular injury, factor 2 represented by markers of microvascular injury, and factor 3 represented by the BDNF and MMP-9 cellular pathway. This study provides insight into a diverse set of biomarkers that have had varying degrees of prior investigation in neonatal and pediatric ECMO and critical illness. For GFAP, S100β, and NSE, we support previously published associations with outcomes in pediatric ECMO. While BDNF and its neuroprotective role have been previously demonstrated, this is the first study to support the role in neonatal and pediatric ECMO. Additionally, we newly describe associations of VILIP-1, NRGN, vWF, PDGFRβ, and MMP-9, which have only been previously studied in infant, pediatric, or adult brain injury and not in neonatal and pediatric ECMO. We also newly describe clinical associations of NPTX1, which has only been previously studied in in vitro and animal models of brain injury. The agnostic grouping suggests that further investigations into clinical or biochemical associations of biomarkers within each factor may be of interest.
This study had several limitations. First, in our study population prior to ECMO, 20% had a pre-existing neurologic diagnosis, 40% had cardiac arrest, and 21% of children with available neuroimaging had acute abnormal findings5. Given this high prevalence of pre-ECMO neurologic conditions, it is difficult to specifically attribute these biomarker patterns to neurologic injury in critical illness versus neurologic injury on ECMO. Additionally, in this study, neuroimaging was obtained based on clinical indications and protocols, with head ultrasounds obtained daily for infants and brain CTs obtained when the clinical team had concerns for an acute neurological event. Given that neuroimaging cannot be consistently captured in a standardized manner in this patient population, the exact timing of an acute neurological event cannot be ascertained, with imaging lagging the actual time of onset of the event. Also, for this reason, abnormal neuroimaging is generally deemed not informative as a primary outcome; it was thus treated as a secondary outcome in our study. Furthermore, in the neonatal and pediatric ECMO population, it has been shown that abnormal neuroimaging findings during ECMO have limited associations with long-term neurodevelopmental outcomes in survivors3. Lastly, the definition of unfavorable outcome based on PCPC lacks granularity, however ongoing and future studies of blood-based biomarkers will continue to evaluate long-term outcomes as they relate to specific patterns of injury during critical illness with ECMO support.
Conclusions
In this two-center prospective observational study of neonatal and pediatric ECMO, we found that 11 circulating biomarkers could be grouped via EFA to suggest three brain injury factors. The first brain injury factor grouped markers of cellular brain injury. Higher levels were associated with unfavorable survival and neurofunctional outcomes as well as with new abnormal neuroimaging. The second brain injury factor grouped markers related to vascular processes. Higher levels were also associated with unfavorable outcome. Lastly, the third brain injury factor grouped the BDNF and MMP-9 cellular pathway. This pathway was associated with neuroprotective properties, with lower levels associated with unfavorable outcome.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the contributing centers as well as the patients described herein and the medical staff who made their complex medical care possible.
Author contributions
M.M.B., D.K.N., and C.C. contributed with conceptualization and methodology. M.M.B., J.R., and D.K.N. contributed with data curation and formal analysis. V.H. contributed with writing—original draft. J.R., D.K.N., J.M.S., A.D.E., N.P., D.R., J.J., C.C., G.B.S., J.N.W., and M.M.B. contributed with writing—review & editing. A.D.E., N.P., D.R., J.J., G.B.S., and J.N.W. also contributed with methodology. M.M.B. contributed with funding acquisition.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R21HD096389, MMB) and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (K23NS076674, MMB).
Data availability
The data used in this study are not publicly available because the study sites retain data ownership, and the data contain information that can compromise the privacy of research participants. MMB can be contacted to request data from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61388-6.
References
- 1.Extracorporeal Life Support Organization. ECLS Registry Report, 1–41 (2023).
- 2.Barrett CS, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr. Crit. Care. Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 3.Felling RJ, et al. Neuromonitoring during ECMO support in children. Neurocrit Care. 2023;39:701–713. doi: 10.1007/s12028-023-01675-8. [DOI] [PubMed] [Google Scholar]
- 4.Pandiyan P, et al. Clinical guidelines for routine neuromonitoring in neonatal and pediatric patients supported on extracorporeal membrane oxygenation. ASAIO J. 2023;69:895–900. doi: 10.1097/MAT.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 5.Bembea MM, et al. Neurologic outcomes in a two-center cohort of neonatal and pediatric patients supported on extracorporeal membrane oxygenation. ASAIO J. 2020;66:79–88. doi: 10.1097/MAT.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle K, et al. Neurologic outcomes after extracorporeal membrane oxygenation: A systematic review. Pediatr. Crit. Care. Med. 2018;19:760–766. doi: 10.1097/PCC.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendes Arent A, de Souza LF, Walz R, Dafre AL. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed. Res. Int. 2014;2014:723060. doi: 10.1155/2014/723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Au AK, et al. Brain-specific serum biomarkers predict neurological morbidity in diagnostically diverse pediatric intensive care unit patients. Neurocrit Care. 2018;28:26–34. doi: 10.1007/s12028-017-0414-7. [DOI] [PubMed] [Google Scholar]
- 9.Bembea MM, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit. Care Med. 2015;43:2202–2211. doi: 10.1097/CCM.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 10.Fink EL, et al. Association of blood-based brain injury biomarker concentrations with outcomes after pediatric cardiac arrest. JAMA Netw. Open. 2022;5:e2230518. doi: 10.1001/jamanetworkopen.2022.30518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagdyman N, Kömen W, Ko HK, Müller C, Obladen M. Early biochemical indicators of hypoxic-ischemic encephalopathy after birth asphyxia. Pediatr. Res. 2001;49:502–506. doi: 10.1203/00006450-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Dias DO, et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021;12:5501–5505. doi: 10.1038/s41467-021-25585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahim P, et al. Serum visinin-like protein-1 in concussed professional ice hockey players. Brain Inj. 2015;29:872–876. doi: 10.3109/02699052.2015.1018324. [DOI] [PubMed] [Google Scholar]
- 14.Stejskal D, Sporova L, Svestak M, Karpisek M. Determination of serum visinin like protein-1 and its potential for the diagnosis of brain injury due to the stroke: A pilot study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2011;155:263–268. doi: 10.5507/bp.2011.049. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: A systematic review. J. Stroke Cerebrovasc Dis. 2011;20:47–54. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos M, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. doi: 10.1161/01.STR.0000046764.57344.31. [DOI] [PubMed] [Google Scholar]
- 17.Demir R, et al. Relationship between plasma metalloproteinase-9 levels and volume and severity of infarct in patients with acute ischemic stroke. Acta Neurol. Belg. 2012;112:351–356. doi: 10.1007/s13760-012-0067-4. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich NH, Dehmel T, Wittsack HJ, Kieseier BC, Seitz RJ. Peripheral blood levels of matrix metalloproteinase-9 predict lesion volume in acute stroke. Neurol. Sci. 2013;34:379–382. doi: 10.1007/s10072-012-0999-8. [DOI] [PubMed] [Google Scholar]
- 19.Bednarek N, et al. Increased MMP-9 and TIMP-1 in mouse neonatal brain and plasma and in human neonatal plasma after hypoxia-ischemia: A potential marker of neonatal encephalopathy. Pediatr. Res. 2012;71:63–70. doi: 10.1038/pr.2011.3. [DOI] [PubMed] [Google Scholar]
- 20.De Vos A, et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017;17:170–178. doi: 10.1186/s12883-017-0945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Korley FK, Dai M, Everett AD. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem. 2015;48:843–848. doi: 10.1016/j.clinbiochem.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Oliveira CO, et al. Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J. Neurotrauma. 2007;24:1331–1338. doi: 10.1089/neu.2006.0159. [DOI] [PubMed] [Google Scholar]
- 23.Genét GF, et al. Trauma-induced coagulopathy: Standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J. Neurotrauma. 2013;30:301–306. doi: 10.1089/neu.2012.2612. [DOI] [PubMed] [Google Scholar]
- 24.Yokota H, et al. Cerebral endothelial injury in severe head injury: The significance of measurements of serum thrombomodulin and the von Willebrand factor. J. Neurotrauma. 2002;19:1007–1015. doi: 10.1089/089771502760341929. [DOI] [PubMed] [Google Scholar]
- 25.Al Rahim M, Thatipamula S, Hossain MA. Critical role of neuronal pentraxin 1 in mitochondria-mediated hypoxic-ischemic neuronal injury. Neurobiol. Dis. 2013;50:59–68. doi: 10.1016/j.nbd.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell JC, Kishimoto K, O'Driscoll C, Hossain MA. Neuronal pentraxin 1 induction in hypoxic-ischemic neuronal death is regulated via a glycogen synthase kinase-3α/β dependent mechanism. Cell. Signal. 2011;23:673–682. doi: 10.1016/j.cellsig.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thatipamula S, Al Rahim M, Zhang J, Hossain MA. Genetic deletion of neuronal pentraxin 1 expression prevents brain injury in a neonatal mouse model of cerebral hypoxia-ischemia. Neurobiol. Dis. 2015;75:15–30. doi: 10.1016/j.nbd.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thatipamula S, Hossain MA. Critical role of extracellularly secreted neuronal pentraxin 1 in ischemic neuronal death. BMC Neurosci. 2014;15:133–143. doi: 10.1186/s12868-014-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Bonilla L, Iadecola C. Peroxiredoxin sets the brain on fire after stroke. Nat. Med. 2012;18:858–859. doi: 10.1038/nm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shichita T, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012;18:911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- 31.Fiser DH. Assessing the outcome of pediatric intensive care. J. Pediatr. 1992;121:68–74. doi: 10.1016/S0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 32.Fiser DH, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit. Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro A, Ten Berge JM. Statistical inference of minimum rank factor analysis. Psychometrika. 2002;67:79–94. doi: 10.1007/BF02294710. [DOI] [Google Scholar]
- 34.Fink EL, et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit. Care Med. 2014;42:664–674. doi: 10.1097/01.ccm.0000435668.53188.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tylicka M, et al. BDNF and IL-8, but not UCHL-1 and IL-11, are markers of brain injury in children caused by mild head trauma. Brain Sci. 2020;10:665. doi: 10.3390/brainsci10100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massaro, A. N. et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr.194, 67–75. e1 (2018). [DOI] [PubMed]
- 37.Dietrick B, et al. Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J. Pediatr. 2020;226:71–79.e5. doi: 10.1016/j.jpeds.2020.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madurski C, et al. Serum biomarkers of regeneration and plasticity are associated with functional outcome in pediatric neurocritical illness: An exploratory study. Neurocrit. Care. 2021;35:457–467. doi: 10.1007/s12028-021-01199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/A:1007677003387. [DOI] [PubMed] [Google Scholar]
- 40.Brunetti MA, et al. Glial fibrillary acidic protein in children with congenital heart disease undergoing cardiopulmonary bypass. Cardiol. Young. 2014;24:623–631. doi: 10.1017/S1047951113000851. [DOI] [PubMed] [Google Scholar]
- 41.Magruder JT, et al. Association of nadir oxygen delivery on cardiopulmonary bypass with serum glial fibrillary acid protein levels in paediatric heart surgery patients. Interact. Cardiovasc. Thorac. Surg. 2016;23:531–537. doi: 10.1093/icvts/ivw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage WJ, et al. Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am. J. Hematol. 2011;86:427–429. doi: 10.1002/ajh.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser DD, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr. Crit. Care. Med. 2011;12:319–324. doi: 10.1097/PCC.0b013e3181e8b32d. [DOI] [PubMed] [Google Scholar]
- 44.Douglas-Escobar MV, et al. UCH-L1 and GFAP serum levels in neonates with hypoxic-ischemic encephalopathy: A single center pilot study. Front. Neurol. 2014;5:273. doi: 10.3389/fneur.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ennen CS, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 2011;205:251.e1–251.e7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaparro-Huerta V, et al. Proinflammatory cytokines, enolase and s-100 as early biochemical indicators of hypoxic-ischemic encephalopathy following perinatal asphyxia in newborns. Pediatr. Neonatol. 2017;58:70–76. doi: 10.1016/j.pedneo.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins DD, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 2012;32:1888–1896. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gazzolo D, Masetti P, Meli M, Grutzfeld D, Michetti F. Elevated S100B protein as an early indicator of intracranial haemorrhage in infants subjected to extracorporeal membrane oxygenation. Acta Paediatr. 2002;91:218–221. doi: 10.1111/j.1651-2227.2002.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 49.Braunewell K, Klein-Szanto AJ. Visinin-like proteins (VSNLs): Interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+ -sensor proteins. Cell Tissue Res. 2009;335:301–316. doi: 10.1007/s00441-008-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasegawa S, et al. Serum and cerebrospinal fluid levels of visinin-like protein-1 in acute encephalopathy with biphasic seizures and late reduced diffusion. Brain Dev. 2014;36:608–612. doi: 10.1016/j.braindev.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Lance EI, et al. Proteomic discovery in sickle cell disease: Elevated neurogranin levels in children with sickle cell disease. Proteomics Clin. Appl. 2021;15:e2100003. doi: 10.1002/prca.202100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuzniewska B, et al. Brain-derived neurotrophic factor induces matrix metalloproteinase 9 expression in neurons via the serum response factor/c-Fos pathway. Mol. Cell. Biol. 2013;33:2149–2162. doi: 10.1128/MCB.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubarski K, Mania A, Małecki P, Mazur-Melewska K, Figlerowicz M. Inflammatory markers combined with metalloproteinase-9, neopterin, and S100B concentrations may indicate the pathogenesis of central nervous system diseases in children. J. Child Neurol. 2022;37:707–716. doi: 10.1177/08830738221106663. [DOI] [PubMed] [Google Scholar]
- 54.Knopp RC, et al. Extending the calpain-cathepsin hypothesis to the neurovasculature: Protection of brain endothelial cells and mice from neurotrauma. ACS Pharmacol. Transl. Sci. 2021;4:372–385. doi: 10.1021/acsptsci.0c00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caba E, Brown QB, Kawasaki B, Bahr BA. Peptidyl alpha-keto amide inhibitor of calpain blocks excitotoxic damage without affecting signal transduction events. J. Neurosci. Res. 2002;67:787–794. doi: 10.1002/jnr.10163. [DOI] [PubMed] [Google Scholar]
- 56.Blomgren K, et al. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: A mechanism of "pathological apoptosis"? J. Biol. Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are not publicly available because the study sites retain data ownership, and the data contain information that can compromise the privacy of research participants. MMB can be contacted to request data from this study.