Abstract
Adhesion receptors expressed on the surfaces of tumor-activated endothelial cells provide an advantageous locus for targeting gene therapy vectors to angiogenic tissues and/or tumor vasculature. In this study, we engineered a series of Asn-Gly-Arg (NGR)-containing congeners of the presumptive cell binding motif contained within the ninth type III repeat of fibronectin and displayed these tumor vasculature targeting motifs (TVTMs) within the context of Moloney murine leukemia envelope “escort” proteins. Comparative studies of envelope incorporation into viral particles and evaluation of the cell binding properties of the targeted vectors revealed critical structural features, thus identifying a subset of optimal TVTMs. Utilizing a modified ELISA to evaluate viral binding to target cells, we observed a significant down-regulation of TVTM-virion binding to human endothelial cells following sustained (48-h) exposure to VEGF. Normalized for equivalent titers (106 CFU/ml), as assayed on NIH 3T3 cells, vectors displaying TVTM escort proteins significantly enhanced the transduction efficiency from 12.2 to 37.4% in human KSY-1 endothelial cell cultures (P < 0.001) and from 0.4 to 4.1% in human umbilical vein endothelial cell (HUVEC) cultures (P < 0.001). In summary, these studies utilized an engineering approach to identify a subset of TVTMs that are stably incorporated as envelope “escort” proteins into retroviral vectors and that, by functioning to improve the binding efficiency and transduction of both HUVEC and KSY1 endothelial cells, may have therapeutic potential for targeting gene delivery to the tumor-associated vasculature.
Neoplastic cells within solid tumors develop an intimate and complex relationship with nonneoplastic tissues, including vascular endothelial cells, stromal cells, and extracellular matrix (16). These histologic features of solid tumors, which make up more than 90% of all human cancers, taken together with the emerging mechanisms of angiogenesis that accompany tumor growth and metastasis (16, 27), have promoted the concept of targeting to tumor vasculature as a compelling therapeutic strategy (11, 18). Accordingly, antiangiogenic gene therapy directed against microvascular endothelial cells that have been recruited into the tumor beds has been developed (19) and employed (23, 58) with considerable success (7).
Vascular endothelial growth factor (VEGF), a selective endothelial cell mitogen (13, 32, 59) and mediator of vascular permeability (53, 57), is an important factor in driving the growth, metastasis, and angiogenesis of solid tumors (12, 50). Within the tumor microenvironment, there is a reported up-regulation of both VEGF and its cognate receptor(s) on tumor vascular endothelium (8). Both oncogenic transformation and hypoxic conditions that are found in most solid tumors act synergistically to modulate VEGF expression (20, 40). Moreover, recent studies of VEGF expression in tumor stromal cells versus tumor cells have also focused on the importance of stromal fibroblasts as a source of VEGF and hence as a contributor to tumor angiogenesis (22). Additionally, both fibroblast growth factor type 2 exposure (46) and hypoxia (9, 61) serve to up-regulate the expression of the VEGF receptor(s) on endothelial cells. Thus, the VEGF-receptor complex is a highly specific marker of tumor endothelium (15, 33) and can be used for the targeting and/or imaging of tumor vasculature (8).
In addition to expression of angiogenic growth factors, endothelial cells within the activated microvasculature of solid tumors express αv integrins, which are virtually absent from the cells of established blood vessels (10, 21, 26). Indeed, fundamental roles for inducible integrin receptors and extracellular matrix (ECM) proteins in angiogenesis are well established (57). The integrin αvβ3, for example, is expressed preferentially on vascular cells during the proliferative and invasive phases of angiogenesis and serves as a multifunctional adhesion receptor that binds to a number of ECM proteins which typically constitute a provisional matrix (including collagen, fibronectin, vitronectin, and fibrinogen) and which are reported to promote cell proliferation (1, 38). Normally undetectable in quiescent blood vessels, αvβ3 becomes highly expressed upon stimulation by angiogenic growth factors or tumor cell supernatant (10). Conversely, blockade of αvβ3 function by specific antibodies or peptide antagonists results in unscheduled apoptosis of proliferating endothelial cells (10), suggesting that the αvβ3 receptor system provides a critical survival signal that facilitates vascular cell proliferation (57). Endothelial cell apoptosis appears to be mediated by p53 and accompanied by induction of the cell cycle inhibitor p21WAF1/CIP1 (57). Thus, the interaction between the inducible αvβ3 adhesion receptor and the provisional ECM directly regulates growth arrest signals and promotes endothelial cell survival (57).
Growth factor and/or adhesion receptors that are selectively expressed on the surface of activated endothelial cells provide an advantageous locus for targeting drugs and gene therapy vectors to angiogenic tissues. Molecular screens for high-affinity targeting motifs have been developed using designated panels of monoclonal antibodies (31) and random phage display peptide libraries (45). Remarkably, many of the peptide ligands isolated by random phage display technology, either (i) by panning for peptides that bind the integrin αvβ1 (34, 35) or αvβ3 (29) in vitro or (ii) by isolating peptides with homing specificity for tumor blood vessels in vivo (5), exhibit sequences that correspond to identifiable ligand-receptor contact points within the primary structure of fibronectin. Specifically, Asn-Gly-Arg (NGR)-containing sequences identified in vitro and in vivo bear striking similarities to the 9th type III repeat of fibronectin, while Arg-Gly-Asp (RGD) sequences correspond closely to the 10th type III repeat of fibronectin (5, 34, 35). From this point, we will use the term “tumor vasculature targeting motif” (TVTM) to concisely describe the tumor-homing and accumulation phenomenology of fibronectin-derived (NGR) congeners originally characterized by phage display technology (5). NGR motifs exhibit a greater homing ratio (tumor/control organs) than do comparable RGD motifs, and the two motifs have differential affinities for defined integrins and peptide competition kinetics, suggesting that NGR and RGD peptides bind to different cellular receptors (5). In the present study, we constructed a number of NGR-bearing peptide congeners displayed within the context of Moloney murine leukemia virus (MoMLV) envelope escort proteins. Escort proteins are defined as noninfectious Env proteins that accompany the infectious wild-type (WT) Env to provide a gain-in-function phenotype (i.e., targeting) to the composite vector. Escort proteins consist of ligands or peptides that replace the deleted receptor binding domains of the modified ecotropic Env construct, CEEC, which bears an amphotropic (CAE) instead of the ecotropic (CEE+) hinge region. The performance of these chimeric vectors was evaluated in terms of in vitro targeting and transduction of activated endothelial cells.
MATERIALS AND METHODS
Molecular cloning of MoMLV-based escort proteins displaying TVTMs.
TVTM inserts with cohesive ends were cloned into the CEE+ (ecotropic)-delta hinge envelope (Env) construct (62), designated CEEC, which was modified from CEE+ by substitution of an amphotropic proline-rich hinge region containing three unique restriction sites (AvrII, PstI, and StuI) and an NgoMI restriction site (62). The MoMLV-based Env construct was cut with BstEII and AvrII, and the linearized env plasmid was verified by restriction analysis on agarose gels and purified by the Gene Clean method (Bio 101, Vista, Calif.) prior to ligation with the respective TVTM insert and T4 DNA ligase (New England Biolabs, Beverly, Mass.) for either 3 h at room temperature (RT) or overnight at 4°C. In the resulting escort constructs, a TVTM peptide flanked by glycine linkers replaced the entire receptor binding region of the MoMLV ecotropic Env surface (SU) protein, between the BstEII site at the amino terminus and the AvrII site located proximal to the transmembrane (TM) domain. After ligation, the various constructs of plasmid DNA were transformed into XL1 Blue strain of Escherichia coli and grown on Luria-Bertani agar plates under ampicillin selection. Plasmid DNA was extracted from selected transformed clones using QIAprep Miniprep Kits (Qiagen, Valencia, Calif.). Each construct was confirmed by enzyme digestion and analysis of the respective inserts followed by direct DNA sequence analysis using the T7 Sequenase sequencing kit (Amersham Life Science, Inc., Cleveland, Ohio).
Generation of retroviral vector stocks.
Retroviral vectors bearing WT Env and/or TVTM-bearing escort protein constructs were assembled using a three- or four-plasmid transient transfection system (56) in which the WT amphotropic or ecotropic Env protein was coexpressed. The packaging components gag-pol, the WT env, the chimeric env, and a retroviral vector bearing a nucleus-targeted β galactosidase expression construct expressed from cytomegalovirus promoters were placed on separate plasmids, each containing the simian virus 40 origin of replication. A 10-μg portion of each plasmid (pcgp, either pCAE, pCEE+ or pCEEC, pESCORT, and pcnBg) was cotransfected by the calcium phosphate method into 293T cells, which express SV40 large T antigen. The producer cells were subsequently treated with 10 mM sodium butyrate for 8 to 12 h to facilitate virion production, and retroviral supernatants were harvested at 48 h after transfection.
Viral processing and incorporation of chimeric Env proteins into retroviral vectors.
The level of expression of the nascent WT Env proteins gp70 and/or the chimeric Env escort proteins in 293T cell lysates was evaluated by Western analysis, using a rat monoclonal 83A25 antibody against the C terminus of the SU domain of gp70, as previously described (64). To evaluate Env incorporation into virions, viral particles were purified from soluble proteins and cell debris on a 20% sucrose gradient (in phosphate-buffered saline PBS) and the virion-associated proteins were subjected to Western analysis using anti-gp70 and anti-p30 antibodies (64).
Determination of viral titers.
The infectious titers of test retroviral supernatants were standardized and quantified based on the expression of a nucleus-targeted β galactosidase reporter gene (55), as determined by light microscopy. Briefly, 2.5 × 104 NIH 3T3 cells in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (D10 medium) were plated in each well of six-well plates 1 day prior to transduction. The medium was replaced with 1-ml volumes of serial dilutions of the respective retroviral supernatant with 8 μg of Polybrene per ml for 2 h, after which 1 ml of fresh D10 medium was added to the cultures, which were maintained overnight at 37°C under 5% CO2. The respective cultures were then stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemical stain 48 h after transduction to detect the presence of nucleus-targeted β-galactosidase in transduced cells (cells with blue-green nuclei). Viral titers were expressed as the number of β-galactosidase-positive CFU per milliliter of vector supernatant.
Viral binding to human endothelial cells.
KSY1 Kaposi's human sarcoma cells (CRL-11448) and human umbilical vein epithelial cells (HUVEC) (CC-2517) were obtained from the American Type Cell Culture Collection (Bethesda, Md.) and Clonetics (San Diego, Calif.), respectively. For quantification of viral binding, 106 KSY1 cells or HUVEC were suspended in D10 medium in a microcentrifuge tube, and were centrifuged for 15 s, after which time 1 ml of test vector supernatant was added (viral titers were normalized at 106 CFU/ml). The mixture was incubated with gentle shaking at RT for 30 min. The cells were washed twice with D10 medium and then resuspended in 300 μl in the presence of a rat monoclonal 83A25 antibody directed against the C terminus of the gp70 MoMLV Env protein (17) and incubated at RT for 1 h. The cells were again washed twice with D10 medium and then incubated in 500 μl of 1:2,500 horseradish peroxidase-conjugated goat anti-rat immunoglobulin G (Zymed Laboratories Inc.) at RT for 30 min. After being washed, the cells were incubated in 500 μl of 1:1,000 rat peroxidase anti-peroxidase antibody (Sternberger Monoclonals, Inc.) at RT for 30 min. After being washed again, the cells were resuspended in 100 μl of TMB single solution (Zymed Laboratories Inc.), and transferred to a 96-well enzyme-linked immunosorbent assay (ELISA) plate, where the intensity of the color reaction (blue) was measured by determining the optical density at 650 nm (OD650) nm on a Rainbow Spectra ELISA reader (TECAN US, Inc.).
Transduction of human endothelial cells.
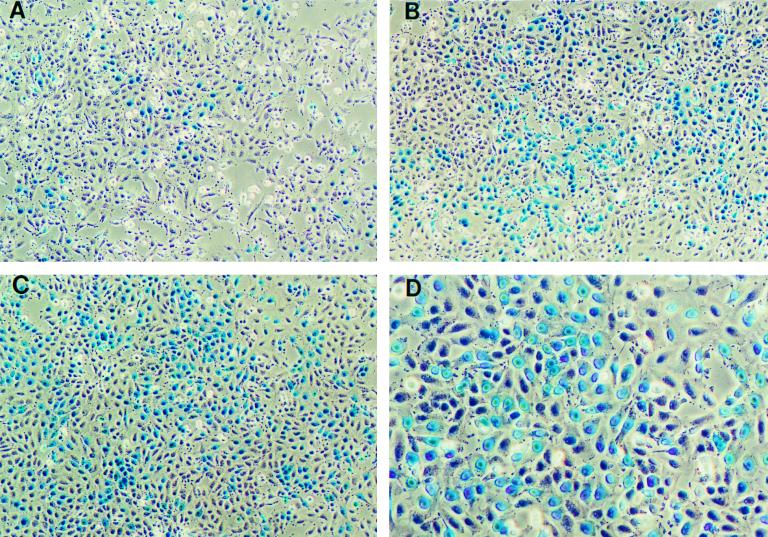
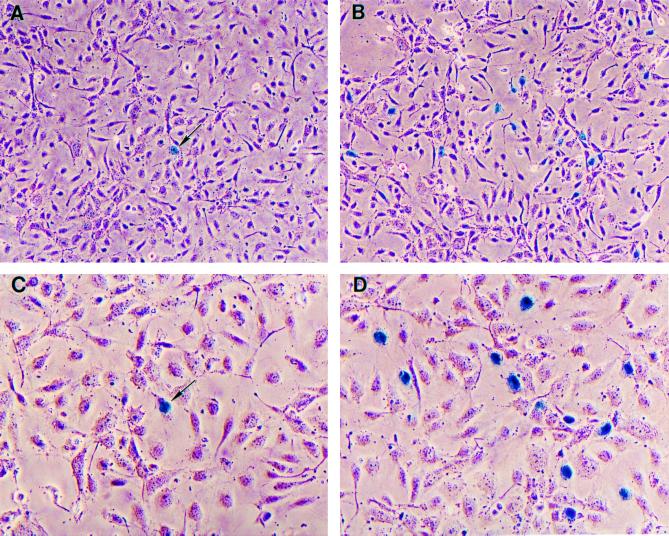
KSY1 cells or HUVEC were cultured on 1% gelatin-coated dishes containing RPMI 1640 supplemented with either KSY1 medium (2% fetal calf serum, 1% sodium pyruvate, 1% essential amino acids, 1% nonessential amino acids, 1 mM glutamine, 1% penicillin-streptomycin) or HUVEC medium (Iscove's modified Dulbecco's/Ham F-12 medium supplemented with 15% fetal calf serum, 1% penicillin-streptomycin, 45 μg of heparin per ml, and 10 μg of endothelial cell growth supplement per ml. For transduction experiments, 0.5 × 105 to 1.0 × 105 KSY1 cells or HUVEC in 3 ml of KSY1 medium or HUVEC medium, respectively, were plated into each gelatin-coated well in six-well plates and allowed to attach overnight at 37°C. The following morning, the medium was replaced with 1 ml of fresh KSY1 or HUVEC medium. The cultures were transduced with 1 ml of each test vector supernatant normalized for equivalent viral titers in the presence of Polybrene (8 μg/ml) at 37°C for 30 min. Thereafter, 2.5 ml of fresh medium was added to the cultures, which were then incubated overnight at 37°C. The medium was replaced with fresh medium, and the cultures were further incubated at 37°C for another 48 h. The cells were then stained with X-Gal stain to visualize the presence of nucleus-targeted β galactosidase activity under light microscopy. To quantify the resulting transduction efficiency, five low-power (×10) fields of each test group were photographed (∼1,500 cells per field) for KSY1 cells, and 10 low-power fields were photographed (∼500 cells per field) for HUVEC. Transduction efficiency was expressed as a percentage by dividing the number of positive cells (cells with blue-staining nuclei) by the total number of cells per low-power field (magnification, ×100).
RESULTS
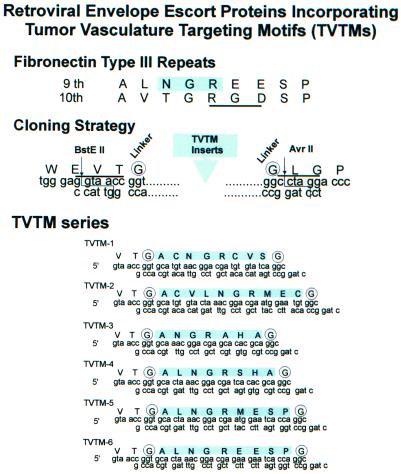
Six NGR-bearing TVTMs were selected for comparative evaluation (Fig. 1). Two presumably cyclic peptides (TVTM1 and TVTM2) and one linear peptide (TVTM3) previously demonstrated selectivity for tumor vasculature in vivo when expressed on the surface of filamentous phage (5). The remaining three TVTMs (TVTM4, TVTM5, and TVTM6) represent novel designs. TVTM4 introduces specific modifications of TVTM3 including a hydrophobic residue (Leu) N-terminal and a polar residue (Ser) C-terminal to the core NGR motif. TVTM5 and TVTM6 constitute congeners of NGR motifs designed to examine the influence of adjacent C-terminal residues (Glu-Glu-Ser-Pro) present in the ninth fibronectin type III repeat (37). Glycine residues were included as linkers flanking each of the TVTMs in an effort to add flexibility to the secondary structures and facilitate folding of the chimeric retroviral envelope proteins. Each of the six TVTMs was encoded into cDNA sequences, prepared as double-stranded oligodeoxynucleotides, and cloned into a modified CEEC vector (see Materials and Methods) in which a unique cloning site (specifically AvrII) has been added proximal to the proline-rich hinge region of the gp70 surface protein. The respective targeted ecotropic Env, termed escort proteins, specifically designed to accompany the WT Env, were prepared by ligation of the TVTM inserts into the BstEII and AvrII cloning sites, thus replacing the entire receptor binding domain of the ecotropic Env protein with the respective TVTM/linker construct. Notably, the primary structure of the specified cloning sites within the CEE+ vector roughly approximates the primary structures flanking the core cell binding motifs (NGR and RGD) of the 9th and 10th type III repeats found within fibronectin (Fig. 1).
FIG. 1.
Schematic diagram of the TVTM series of retroviral escort proteins. TVTMs were designed to target retroviral vectors to the tumor vasculature. TVTM1, TVTM2, and TVTM3 bind selectively to up-regulated integrins in in vitro panning assays and accumulate selectively in the tumor vasculature in vivo. TVTM4, TVTM5, and TVTM6 represent novel designs.
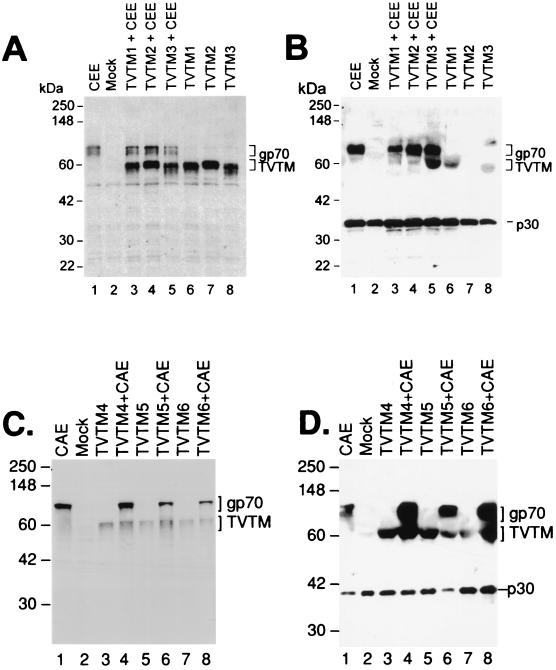
Upon transient transfection, all six TVTM Env proteins were expressed in human 293T retroviral vector producer cells, each exhibiting an apparent molecular mass of ∼60 kDa. As seen in Fig. 2A and C, the expression of the six Env escort proteins was not impaired by coexpression of WT Env proteins, which confer vector tropism and infectivity (i.e., ecotropic [CEE] or amphotropic [CAE]). Each of the TVTM escort proteins could be detected in purified viral particles; however, notable differences in incorporation efficiencies were observed (Fig. 2B and D). Five out of the six TVTM proteins were stably incorporated into viral particles in the absence of the WT CEE or CAE Env, the exception being TVTM2 and TVTM3, which was consistently lower in terms of incorporation efficiency. For TVTM3 and TVTM6, coexpression of a WT envelope facilitates the incorporation of the modified escort protein, presumably due to structural complementation of the tertiary structures (3, 63). Remarkably, only the linear peptide sequences (TVTM3 to TVTM6) were incorporated stoichiometrically with the WT Env, which is indicative of proper assembly, processing, and stable incorporation into viral particles. Examination of corresponding viral titers on NIH 3T3 cells, which range from nil (for TVTM Env alone) to 1.6 × 108 for the TVTM2 + WT CEE vector (Table 1), confirm that the observed fusion and infectivity are provided solely by the coexpression and fusogenic properties of the WT (CEE or CAE) envelope partner.
FIG. 2.
Modified envelope protein expression and incorporation into retroviral vectors displaying TVTMs. (A and C) Comparative Env protein expression levels of WT CEE, WT CAE, TVTMs alone, WT CEE plus TVTMs, and WT CAE plus TVTMs in 293T cell lysates. (B and D) Comparative levels of virion incorporation of retroviral vectors bearing WT CEE, WT CAE, TVTMs alone, WT CEE plus TVTMs, and WT CAE plus TVTMs. Western analysis of gp70 and gag proteins was conducted using a rat monoclonal antibody, 83A25, against the C terminus of the gp70 Env and a polyclonal antibody against p30.
TABLE 1.
Viral titers of retroviral vectors displaying TVTMs
| Vector | Titer on NIH 3T3 cells (CFU/ml)a | Vector | Titer on NIH 3T3 cells (CFU/ml)a |
|---|---|---|---|
| CEE | 1.7 × 108 | CAE | 5.6 × 106 |
| CEE + TVTM1 | 1.7 × 107 | CAE + TVTM2 | 0.6 × 106 |
| CEE + TVTM2 | 1.6 × 108 | CAE + TVTM2 | 1.5 × 106 |
| CEE + TVTM3 | 5.8 × 107 | CAE + TVTM3 | 0.8 × 106 |
| CEE + TVTM4 | 1.9 × 107 | CAE + TVTM4 | 0.6 × 106 |
| CEE + TVTM5 | 2.5 × 107 | CAE + TVTM5 | 2.2 × 106 |
| CEE + TVTM6 | 1.9 × 107 | CAE + TVTM6 | 2.5 × 106 |
Results are expressed as arithmetic means of duplicate determinations.
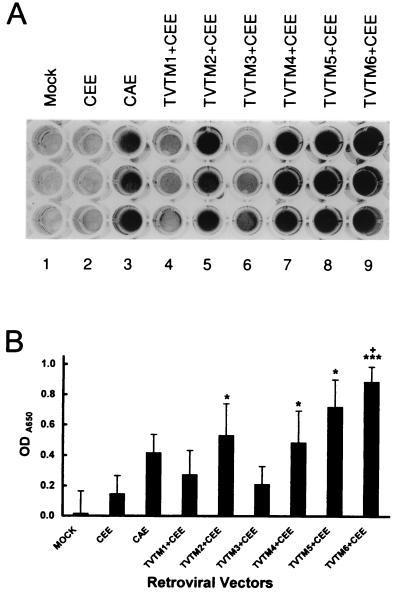
To examine the binding of TVTM-bearing retroviral vectors to activated endothelial cells in vitro, we used KSY1 cells, which exhibit a constitutive (autocrine) expression of both VEGF and VEGF receptors (39). The test vectors were coexpressed with ecotropic CEE (rodent-specific) envelope partners, which do not recognize or infect human cells. Figure 3 demonstrates high-affinity binding of TVTM2-, TVTM4-, and TVTM-6-bearing viral particles to KSY1 cells compared to that of vectors bearing WT CEE Env (P < 0.05), which was equal to or greater than that of the CAE (amphotropic) envelope-bearing (positive control) vectors (P < 0.05 for TVTM6 plus CEE). In contrast, the cell binding affinities of TVTM1- and TVTM3-bearing vectors were noted to be substantially lower than those of TVTM2-, TVTM4-, TVTM5- or TVTM-6-bearing vectors. Minimal binding was observed with the vector bearing only WT CEE (ecotropic) Env.
FIG. 3.
KSY1 cell binding affinities of retroviral vectors bearing TVTM escort proteins coexpressed with the ecotropic (CEE) envelope protein. The comparative binding affinities of vectors bearing WT Env (CEE* or CAE+) versus vectors displaying TVTMs to KSY-1 cells are shown as varying degrees of darkened ELISA wells (A), which are then expressed as OD650 readings on a Rainbow Spectra ELISA reader (B). The levels of significance of the differences between TVTM2, TVTM4, TVTM5, and TVTM6 plus CEE versus CEE alone (wild-type Env) are indicated by asterisks. ∗, P < 0.05; ∗∗∗, P < 0.001. The levels of significance of the difference between TVTM plus CEE and CAE is indicated by the plus sign. +, P < 0.05.
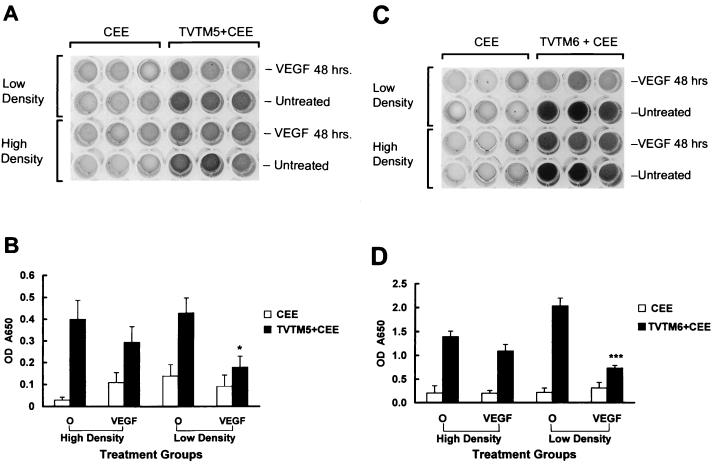
Next, we examined the cell binding properties of TVTM-bearing retroviral vectors on HUVEC before and after pretreatment with VEGF. As in the previous binding studies, the WT CEE Env was coexpressed with the TVTM escort proteins instead of the WT CAE Env to prevent vector fusion and entry, which would confound the interpretation of the results. A marked increase in cell binding was provided by coexpression of the selected TVTM5 escort protein compared to the WT CEE Env alone (P < 0.01). However, the observed increase in cell binding was significantly reduced by exposure of the HUVEC to VEGF for 48 h (P < 0.01). Additional studies were designed to examine the influence of cell density, as well as VEGF pretreatment, on TVTM receptor expression on human endothelial cells. The results of these studies (Fig. 4A and B) revealed a significant down-regulation of TVTM receptor expression on KSY1 cells exposed to VEGF when cultured under low-density conditions (P < 0.05) and to a lesser degree on cells when cultured at high densities. Similar results, obtained with HUVEC (Fig. 4C and D), demonstrate that TVTM receptor expression and/or binding to vectors displaying TVTM6 was highest in cells grown under low-density conditions and was again reduced by pretreatment with VEGF (P < 0.001).
FIG. 4.
Density-dependent VEGF-induced down-regulation of TVTM receptors in KSY1 cells. The comparative binding affinities of vectors bearing WT Env versus vectors displaying TVTMs to low- or high-density KSY1 cells following treatment with VEGF are shown as varying degrees of darkened ELISA wells (A), which are then expressed as OD650 readings on a Rainbow Spectra ELISA reader (B). The comparative binding affinities of vectors bearing WT env versus vectors displaying TVTMs to low- or high-density HUVE cells following treatment with VEGF are shown as varying degrees of darkened ELISA wells (C), which are then expressed as OD650 readings on a Rainbow Spectra ELISA reader (D). ∗, P < 0.05 compared to no VEGF; ∗∗∗, P < 0.001 compared to no VEGF.
As in the cell binding studies described above, test vector supernatants were prepared for cellular transduction studies, with the exception that an amphotropic Env partner (WT CAE) was used to enable the transduction of human cells. The resultant vectors were then normalized for equivalency of titer (106 CFU/ml), based on the transduction of NIH 3T3 cells (Table 1). The results of a representative study of KSY1 cell transduction are shown in Fig. 5. Under these comparative conditions, vectors displaying TVTM escort proteins significantly enhanced the transduction efficiency from approximately 12.2 ± 1.4% (mean ± standard error of the mean) for the WT CAE vector alone to 37.4 ± 1.7% for the WT CAE plus TVTM5 vector (P < 0.001) and 31.0 ± 2.5% for the WT CAE plus TVTM6 vector (P < 0.001). Further, a 10-fold increase in transduction efficiency was observed in HUVEC (from 0.4% for the WT CAE vector alone to 4.2% for the WT CAE plus TVTM6 vector) (P < 0.001) (Fig. 6, Table 2). These results demonstrate cell-specific targeting and transduction by enhancement of viral binding to an unidentified yet dynamic endothelial cell receptor for TVTMs.
FIG. 5.
Enhanced transduction of KSY1 cells by TVTM-bearing retroviral vectors. (A) Representative KSY1 cells transduced with a retroviral vector bearing WT envelope. (B) Representative KSY1 cells transduced with a retroviral vector displaying TVTM5. (C and D) Representative KSY1 cells transduced with a retroviral vector displaying TVTM6. Positive cells expressing nucleus-targeted β-galactosidase activity are shown as cells with blue-staining nuclei. Magnifications, ×100 (A to C) and ×400 (D).
FIG. 6.
Enhanced transduction of HUVEC by TVTM-bearing retroviral vectors. (A and C) Representative HUVEC transduced with a retroviral vector bearing WT envelope. (B and D) Representative HUVEC transduced with a retroviral vector displaying TVTM6. Positive cells expressing nucleus-targeted β-galactosidase activity are shown as cells with blue-staining nuclei. Magnifications, ×100 (A and B) and ×400 (C and D).
TABLE 2.
Transduction efficiency of retroviral vectors displaying TVTMs
| Vector | Transduction efficiencya (%) in:
|
|
|---|---|---|
| KSY-1 cells (n = 5) | HUVEC (n = 10) | |
| CAE | 12.2 ± 1.4 | 0.4 ± 0.1 |
| CAE + TVTM5 | 37.4 ± 1.7c | NDb |
| CAE + TVTM6 | 31.0 ± 2.5c | 4.2 ± 0.5c |
Results are expressed as arithmetic mean ± standard error of the mean.
ND, not determined.
P < 0.01 compared to CAE (vector bearing WT envelope).
DISCUSSION
Fibronectin, a ubiquitous adhesive glycoprotein found in relatively high concentrations in plasma as well as ECM, functions to mediate cell-ECM interactions during development, wound healing, and hemostasis. Soluble fibronectin is generally a dimer composed of two nonidentical (alternatively spliced) subunits linked covalently by a pair of disulfide bonds, while the insoluble matrix form of fibronectin is arrayed as oligomers and fibrils (6). The primary structure of the fibronectin molecule is mosaic, consisting of a series of structurally distinct domains that interact with either ECM components or cell membrane receptors and are linked by flexible peptide segments (6, 37). Of particular interest is the central cell binding domain that is recognized by the integrin receptors of adherent cells (41, 54). Among the active sites that have been identified within these domains (30) are the RGD motif of the 10th type III repeat (48, 51) and the NGR motif of the 9th type III repeat (29, 34, 35). While the binding of αvβ1 and αvβ3 integrins to RGD-bearing proteins has been definitively shown (29, 34–36), the cellular receptor(s) selective for the NGR cell binding motif remains to be identified (5). Extensive deletion studies of fibronectin have demonstrated the importance of the RGD cell binding domain within the 10th type III repeat (43). However, additional domains contribute to the overall binding affinities, and a synergistic interaction specifically with adjacent type III repeats has been reported (4, 42). Filamentous phage displaying NGR motifs exhibit dissimilar integrin binding affinities and displacement kinetics from those of phage displaying RGD motifs, indicating that the cellular receptor(s) for NGR motifs is not identical to that of RGD (5, 29). Moreover, the tumor homing ratio of the NGR-bearing phage in terms of adherence to tumor-associated vasculature versus normal vasculature in vivo is severalfold greater than that of the RGD-bearing phage (5), thus confirming that the RGD and NGR cell binding motifs are indeed functionally distinct.
In this study, we examined the performance of a series of NGR-bearing peptide congeners, termed TVTMs, as a prelude to deploying retroviral vectors in pursuit of tumor vasculature and/or metastatic cancer. The TVTMs were displayed within the context of MoMLV Env escort proteins, defined as noninfectious Env proteins that accompany the infectious WT Env to provide a gain-in-function phenotype to the composite vector. In contrast to the cyclic configurations found to be advantageous when similar motifs were displayed within the context of either soluble peptides (24, 47, 49) or the surface proteins of filamentous phage (29, 34, 35, 44), we found that the cyclic NGR congeners (TVTM1 and TVTM2) were readily expressed in human producer cells (Fig. 2A) but either were poorly incorporated into virions (TVTM2) (Fig. 2B) or failed to exhibit the expected high-affinity binding properties (TVTM1) (Fig. 3). For example, the cyclic TVTM2, which exhibits sequences flanking the core NGR motif that are very similar to those of TVTM5, was poorly incorporated into viral particles (Fig. 2B) but bound relatively well to target cells (Fig. 3). In contrast, the linear TVTMs were more readily incorporated into viral particles (Fig. 2B and D), and, unlike free peptides or filamentous phage, the endothelial cell binding properties of chimeric vectors bearing linear TVTM designs were superior (Fig. 3). Although general interference of the additional cysteine residues that generate the cyclic motifs with protein folding, disulfide bond formation, and secretion of the modified envelope proteins cannot be ruled out, it appears that the secondary and tertiary structures of the MoMLV envelope proteins may be more constrained than those of the filamentous phage or free peptides. In any event, since incorporation of retroviral envelope proteins into viral particles is an important feature of retroviral vector production, the linear peptide motifs are considered to be more favorable for this purpose.
The results of this study demonstrate that both addition and substitution of amino acid residues flanking the core NGR motif have profound effects on target cell binding. The cell binding affinity of TVTM4 is significantly greater than that of TVTM3, drawing attention first to the addition of a Ser residue immediately C-terminal to the NGR motif and second to the addition of a Leu residue immediately N-terminal to this motif. Interestingly, the C-terminal Ser residue is not present on the 9th type III repeat (NGR) of fibronectin yet is evident from random alterations of the 10th type III repeat (29, 35) and is conserved in a broad spectrum of proteins that have sequences similar to the RGD cell attachment-promoting sequence of fibronectin (48). The N-terminal Leu residue flanking the NGR motif of TVTM4, as well as TVTM5 and TVTM6, is found in the 9th type III repeat of native fibronectin (Fig. 1) and again in screens of random phage display libraries (35). TVTM5 and TVTM6 exhibit stable incorporation into retroviral particles and comparatively greater cell binding characteristics. These constructs represent linear peptides in which the flanking residues closely approximate the 9th type III repeat of fibronectin, including an N-terminal Ala-Leu leader sequence and one (TVTM5) or two (TVTM6) negatively charged Glu residues C-terminal to the core NGR motif, followed by a Ser-Pro sequence, constituting what appears to be a type I beta turn (60) present in both the 9th and 10th type III repeats of native fibronectin.
The identification of the C-NGR-C motif (within the CNGRCVSGCAGRC phage) as the active site responsible for the greatest homing ratio (tumor/control organ) ascertained from random phage display libraries (5) is remarkable, considering the structure and function of the fibronectin cell binding domain (35, 41). Conceptually, it is plausible that nonmalignant but activated endothelial cells, under the influence of the tumor microenvironment, express an adhesion molecule and/or receptor complex that is not highly expressed on quiescent endothelial cells and that this receptor recognizes a specific motif of fibronectin (i.e., NGR) that has been conserved and canalized by natural selection into a high-affinity interaction. If, in fact, the functional selectivity lies in the expression of the undefined “TVTM receptor” and not in the exquisite sequence selectivity of the binding peptide (which is inherent within the primary structure of the ubiquitous fibronectin molecule), it might well be advantageous to use fibronectin-like sequences per se, as in TVTM5 and TVTM6. Indeed, TVTM4, TVTM5, and TVTM6 are the most favorable in terms of retroviral vector production, stability of membrane proteins in retroviral particles, and binding interactions of the resulting TVTM-bearing virions to proliferating endothelial cells and transformed KSY1 cells. With regard to the presumptive TVTM receptor, it is pertinent to note that pharmacological regulation of the cell binding properties of TVTM-bearing vectors was also observed in these studies (Fig. 4), since the TVTM binding properties of both normal and KSY1 endothelial cells in culture were highest in cells grown under low-density conditions and were reduced significantly by prolonged (48-h) pretreatment with VEGF. The observed down-regulation of TVTM binding to target cells by VEGF, as well as increased cell density, suggests that other growth-regulatory molecules may be implicated in the physiological up-regulation of the putative TVTM cellular receptor.
In terms of retroviral vector targeting and prospective gene therapy, the development of a targeted injectable vector which exhibits suitable affinities, selectivity, and stability for application in vivo remains a principal objective (2). Although a number of creative systems for targeting retroviral vectors have been designed (14), the most successful approach to date involves the insertion of a ligand that recognizes an ECM component into a portion of the MoMLV surface (SU) protein to concentrate the vector on the ECM in the vicinity of target cells (25). Similarly, colocalization of retroviral particles and target cells on specific fibronectin fragments increases the transduction of cultured cells in vitro (28). Utilizing minimal or optimal peptide sequences, determined by random phage display technology to home specifically to tumor blood vessels, targeted anticancer drug-peptide conjugates were developed that exhibited enhanced efficacy and reduced toxicity when injected into the circulation of nude mice bearing human breast carcinoma xenografts (5). The present study expands our understanding of these TVTMs and their cognate receptors and extends the potential utility of a defined subset of TVTMs to include targeted retroviral vectors.
In summary, we used an engineering approach to examine the performance of a designed series of NGR-bearing peptide congeners, including a series of linear peptides that approximate the 9th type III repeat in fibronectin, and have determined that three novel designs (TVTM4, TVTM5, and TVTM6), presented in the context of MoMLV Env escort proteins, including strategic linkers and cloning sites, are most suitable in terms of protein expression, retroviral vector production, and cell binding affinities. These optimized TVTM-bearing Env escort proteins were further demonstrated to function as targeting elements which served to increase the retroviral cell binding affinity and transduction efficiency in human endothelial cells, illustrating a potential utility for improving gene delivery in therapeutic angiogenesis and/or antiangiogenesis-anticancer strategies.
ACKNOWLEDGMENTS
We are grateful to Carlan Wendler, Heather C. Gordon, and Michelle D. Whitley for technical assistance.
This study was supported in part by grants from the American Heart Association Great Western States awarded to F.L.H. (1157-GI1) and E.M.G. (1156-GI1) and in part by Genetic Therapy Inc./Novartis Pharmaceuticals, Gaithersburg, Md.
REFERENCES
- 1.Adams J C, Watt F M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 2.Anderson W F. Gene therapy. Sci Am. 1995;273:124–128. [PubMed] [Google Scholar]
- 3.Anderson W F. Human gene therapy. Nature. 1998;392:25–33. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 4.Aota S, Nagai T, Yamada K M. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266:15938–15943. [PubMed] [Google Scholar]
- 5.Arap W, Pasquilani R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 6.Ayad S, Boot-Handford R P, Humphries M J, Kadler K E, Shuttleworth C A. The extracellular matrix facts book, section II. 2nd ed. San Diego, Calif: Academic Press, Harcourt Brace & Co., Publishers; 1998. The extracellular proteins; pp. 149–152. [Google Scholar]
- 7.Bergers G, Javaherian K, Lo K M, Folkman J, Hanahan D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science. 1999;248:808–812. doi: 10.1126/science.284.5415.808. [DOI] [PubMed] [Google Scholar]
- 8.Brekken R A, Huang X, King S W, Thorpe P E. Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res. 1998;58:1952–1959. [PubMed] [Google Scholar]
- 9.Brogi, E., G. Schatteman, T. Wu, E. A. Kim, L. Varticovski, B. Keyt, J. M. Isner. Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J. Clin. Investig. 97:469–476. [DOI] [PMC free article] [PubMed]
- 10.Brooks P C, Montgomery A M P, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 11.Burrows F J, Thorpe P E. Vascular targeting: a new approach to the therapy of solid tumors. Pharmacol Ther. 1994;64:155–174. doi: 10.1016/0163-7258(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 12.Claffey K P, Brown L F, del Aguila L F, Tognazzi K, Yeo K T, Manseau E J, Dvorak H F. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 13.Connolly D T, Heuvelman D M, Nelson R, Olander J V, Eppley B L, Delfino J J, Siegel N R, Leimgruber R M, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Investig. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosset F L, Morling F J, Takeuchi U, Weiss R A, Collins M K, Russell S J. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–22. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvorak H F, Nagy J A, Dvorak A M. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells. 1991;3:77–85. [PubMed] [Google Scholar]
- 16.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 17.Evans L H, Morrison F G, Malik J, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoprotein of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidler I J. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Antiangiogenic gene therapy. Proc Natl Acad Sci USA. 1998;95:9064–9066. doi: 10.1073/pnas.95.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe J A, Jiang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedlander M, Brooks P C, Shaffer R W, Kincaid C M, Varner J A, Cheresh D A. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 22.Fukumura D, Xavier R, Sugiura T, Chen Y, Park E, Lu N, Selig M, Neilsen G, Taksir T, Jain R K, Seed B. Tumor with VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 23.Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Angiostatin gene transfer: inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with mitosis arrest. Proc Natl Acad Sci USA. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurrath M, Muller G, Kessler H, Aumailley M, Timpl R. Conformation activity studies of rationally designed potent anti-adhesive RGD peptides. Eur J Biochem. 1992;210:911–921. doi: 10.1111/j.1432-1033.1992.tb17495.x. [DOI] [PubMed] [Google Scholar]
- 25.Hall F L, Gordon E M, Wu L, Zhu N L, Skotzko M J, Starnes V A, Anderson W F. Targeting retroviral vectors to vascular lesions by genetic engineering of the MoMuLV gp70 envelope protein. Hum Gene Ther. 1997;8:2183–2192. doi: 10.1089/hum.1997.8.18-2183. [DOI] [PubMed] [Google Scholar]
- 26.Hammes H P, Brownlee M, Jonczyk A, Sutter A, Preissner K T. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 28.Hanenberg H, Xiao X L, Dilloo D, Hashino K, Kato I, Williams D A. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 29.Healy J M, Murayama O, Maeda T, Yoshino K, Sekiguchi K, Kikuchi M. Peptide ligands for integrin αvβ3 selected from random phage display libraries. Biochemistry. 1995;34:3948–3955. doi: 10.1021/bi00012a012. [DOI] [PubMed] [Google Scholar]
- 30.Humphries M J, Komoriya A, Akiyama S K, Olden K, Yamada K M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- 31.Johnson R C, Zhu D, Augustin-Voss H G, Pauli B V. Lung endothelial dipeptidyl peptidase IV is an adhesion molecule for lung-metastatic rat breast and prostate carcinoma cells. J Cell Biol. 1993;121:1423–1432. doi: 10.1083/jcb.121.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 33.Ke-Lin Q H, Nagy J A, Eckelhoefer I A, Masse E M, Dvorak A M, Dvorak H F. Vascular targeting of solid and ascites tumours with antibodies to vascular endothelial growth factor. Eur J Cancer. 1996;32A:2467–2473. doi: 10.1016/s0959-8049(96)00391-7. [DOI] [PubMed] [Google Scholar]
- 34.Koivunen E, Gay D A, Ruoslahti E. Selection of peptides to the α5β1 integrin from phage display library. J Biol Chem. 1993;268:20205–20210. [PubMed] [Google Scholar]
- 35.Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J Cell Biol. 1994;124:373–379. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Bio/Technology. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 37.Kornblihtt A R, Umezawa K, Vibe-Pederson K, Barelle F E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985;4:1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C Q, Bissell M J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 39.Masood R, Cai J, Zheng T, Smith D L, Naidu Y, Gill P S. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:979–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazure N M, Chen E Y, Yeh P, Laderoute K R, Giaccia A J. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436–3440. [PubMed] [Google Scholar]
- 41.Mohri H. Fibronectin and integrins interactions. J Investig Med. 1996;44:429–438. [PubMed] [Google Scholar]
- 42.Nagai T, Yamakawa N, Aota S, Yamada S S, Akiyama S K, Olden K, Yamada K M. Monoclonal antibody characterization of two distinct sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991;114:1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obara M, Kang M S, Yamada K M. Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional biological assays for the purified cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988;53:649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- 44.O'Neil K T, Hoess R H, Jackson S A, Ramachandran N S, Mousa S A, DeGrado W F. Identification of novel peptide antagonists for GPIIb/IIa from a conformationally constrained phage peptide library. Proteins. 1992;14:509–515. doi: 10.1002/prot.340140411. [DOI] [PubMed] [Google Scholar]
- 45.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 46.Pepper M S, Mandriota S J. Regulation of vascular endothelial growth factor receptor-2 (Flk-1) expression in vascular endothelial cells. Exp Cell Res. 1998;241:414–425. doi: 10.1006/excr.1998.4072. [DOI] [PubMed] [Google Scholar]
- 47.Pfaff M, Tangemann K, Muller B, Gurrath M, Muller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by αIIβ3, αVβ3, and α5β1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- 48.Pierschbacher M D, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 49.Piersbacher M D, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xa on binding specificity in cell adhesion. J Biol Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- 50.Plate K H, Breier G, Weich H A, Mennel H D, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 51.Ruoslahti E, Pierschbacher M D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:5985–5988. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 52.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 53.Senger D R, Perruzzi C A, Feder J, Dvorak H F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 54.Skorstengaard K, Jensen M S, Sahl O, Petersen T E, Magnusson S. Purification of a complete primary structure of the heparin-, cell-, and DNA-binding domain of bovine plasma fibronectin. Eur J Biochem. 1986;161:441–453. doi: 10.1111/j.1432-1033.1986.tb10464.x. [DOI] [PubMed] [Google Scholar]
- 55.Skotzko M, Wu L, Anderson W F, Gordon E M, Hall F L. Retroviral Vector-mediated gene transfer of antisense cyclin G1 (CYCG1) inhibits proliferation of human osteogenic sarcoma cells. Cancer Res. 1995;55:5493–5498. [PubMed] [Google Scholar]
- 56.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Gaetano R, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stromblad S, Cheresh D A. Cell adhesion and angiogenesis. Trends Cell Biol. 1996;6:462–466. doi: 10.1016/0962-8924(96)84942-7. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka T, Cao Y, Folkman J, Fine H A. Viral vector-targeted antiagniogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res. 1998;58:3362–3369. [PubMed] [Google Scholar]
- 59.Thomas K A. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996;271:602–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- 60.Vulliet P R, Hall F L, Mitchell J P, Hardie D G. A novel growth factor-sensitive protein kinase in pheochromocytoma. Proc West Pharmacol Soc. 1988;31:255–258. [PubMed] [Google Scholar]
- 61.Waltenberger J, Mayr U, Pentz S, Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation. 1996;94:1647–1654. doi: 10.1161/01.cir.94.7.1647. [DOI] [PubMed] [Google Scholar]
- 62.Wu B W, Cannon P M, Gordon E M, Hall F L, Anderson W F. Characterization of the proline-rich region of murine leukemia virus envelope protein. J Virol. 1998;72:5383–5391. doi: 10.1128/jvi.72.7.5383-5391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Y, Lee S, Anderson W F. Functional interactions between monomers of the retroviral envelope protein complex. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu N L, Cannon P M, Chen D, Anderson W F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J Virol. 1998;72:1632–1639. doi: 10.1128/jvi.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]