Abstract
Purpose
We developed and piloted a mobile health app to deliver cognitive behavioral therapy for pain (pain-CBT), remote symptom monitoring, and pharmacologic support for patients with pain from advanced cancer.
Methods
Using an iterative process of patient review and feedback, we developed the STAMP + CBT app. The app delivers brief daily lessons from pain-CBT and pain psychoeducation, adapted for advanced cancer. Daily surveys assess physical symptoms, psychological symptoms, opioid utilization and relief. Just-in-time adaptive interventions generate tailored psychoeducation in response. We then conducted a single-arm pilot feasibility study at two cancer centers. Patients with advanced cancer and chronic pain used the app for 2 or 4 weeks, rated its acceptability and provided feedback in semi-structured interviews. Feasibility and acceptability were defined as ≥ 70% of participants completing ≥ 50% of daily surveys, and ≥ 80% of acceptability items rated ≥ 4/5.
Results
Fifteen participants (female = 9; mean age = 50.3) tested the app. We exceeded our feasibility and accessibility benchmarks: 73% of patients completed ≥ 50% of daily surveys; 87% of acceptability items were rated ≥ 4/5. Participants valued the app’s brevity, clarity, and salience, and found education on stress and pain to be most helpful. The app helped participants learn pain management strategies and decrease maladaptive thoughts. However, participants disliked the notification structure (single prompt with one snooze), which led to missed content.
Conclusion
The STAMP + CBT app was an acceptable and feasible method to deliver psychological/behavioral treatment with pharmacologic support for cancer pain. The app is being refined and will be tested in a larger randomized pilot study. TRN: NCT05403801 (05/06/2022).
Keywords: mHealth, Cognitive behavioral therapy, Opioids, Just-in-time adaptive intervention, Oncology, Pain management
Introduction
Over two-thirds of patients with advanced cancer experience pain [1], which can be devastating to patients’ quality of life [2, 3]. Opioids and other analgesics are the prevailing treatment approach for cancer pain [4]. However, up to 60% of patients experience inadequate relief [5, 6]. A potential reason that pharmacotherapy alone fails to adequately address pain may be the involvement of modulatory psychological factors such as pain catastrophizing, self-efficacy, mood, and disturbed sleep [7–9]. Psychosocial factors also play a critical role in self-management, affecting in-the-moment choices about taking opioids and communicating symptoms to care teams [2, 10–12]. New treatment approaches are needed to address the psycho-behavioral underpinnings of cancer pain, while integrating with pharmacotherapy [13].
Cognitive behavioral therapy for pain (pain-CBT) is the most widely utilized psycho-behavioral treatment for chronic pain, with meta-analyses demonstrating small-to-moderate improvements in pain severity and functioning [14–22]. Pain-CBT differs from traditional CBT because it targets pain-specific psychological processes through pain psychoeducation, challenges maladaptive pain cognitions (e.g., pain catastrophizing) using cognitive restructuring, and teaches coping skills to reduce muscle tension and improve physical function [23–25].
Pain-CBT requires adaptation for advanced cancer populations. Traditional pain-CBT explicitly focuses on minimizing opioid use, whereas cancer patients often need support to achieve proper and sometimes proactive opioid use [13, 26]. Traditional pain-CBT also involves months of lengthy therapy sessions, which may not be feasible given patients’ burden of illness and demanding treatment schedules. A few investigators have delivered slightly abbreviated versions of pain-CBT using telehealth [27–29]. These programs appear feasible among cancer patients undergoing curative treatment and survivors [27–33], yet may still be too time-intensive for advanced cancer populations. To our knowledge, there are no pain-CBT interventions tailored specifically for advanced cancer patients and none that integrate support for pharmacologic aspects of pain management [16].
Mobile health technology (mHealth) is a promising strategy to tailor, deliver, and ultimately disseminate pain-CBT for advanced cancer pain [34–36]. Currently, approximately 85% of Americans use smartphones [37]. Medical systems have also been increasingly turning to remote technology since the COVID-19 pandemic. MHealth interventions may overcome the practical barriers that limit access to traditional behavioral therapies (e.g., travel time, insurance coverage, time spent in session) and allow for delivery of tailored intervention content, including advice and feedback during critical moments [38].
To improve cancer pain outcomes, we sought to adapt and integrate pain-CBT into an existing mHealth intervention app designed to support advanced cancer patients managing chronic pain using opioids (STAMP). We describe a stakeholder-driven, iterative refinement process applied to develop content and app technology for this STAMP + CBT intervention—an mHealth app that integrates pharmacologic and psycho-behavioral treatment. Here, we describe our development process and results of the pre-pilot study.
Methods
Development of pain-CBT content for mHealth delivery
Following the ORBIT model for developing behavioral treatments for chronic diseases [39] and the adapted STROBE checklist for ecological momentary assessment (EMA) studies [40], our goal was to develop an mHealth application for patients with advanced cancer pain that integrates pain-CBT with medical analgesic support (Fig. 1). We adapted a parent intervention, smartphone technology to alleviate malignant pain (STAMP) [41], which includes a patient-facing smartphone app that delivers tailored cancer pain education (e.g., opioid support) and symptom self-management advice in response to daily pain surveys. STAMP + CBT draws from the psychoeducation, surveys, and symptom-support algorithms from STAMP, while incorporating new content adapted from pain-CBT. We adapted traditional modules from pain-CBT manuals [23, 42] to be delivered as 5–10-min daily lessons. The STAMP + CBT app was developed in five iterative stages (see Fig. 1).
Fig. 1.
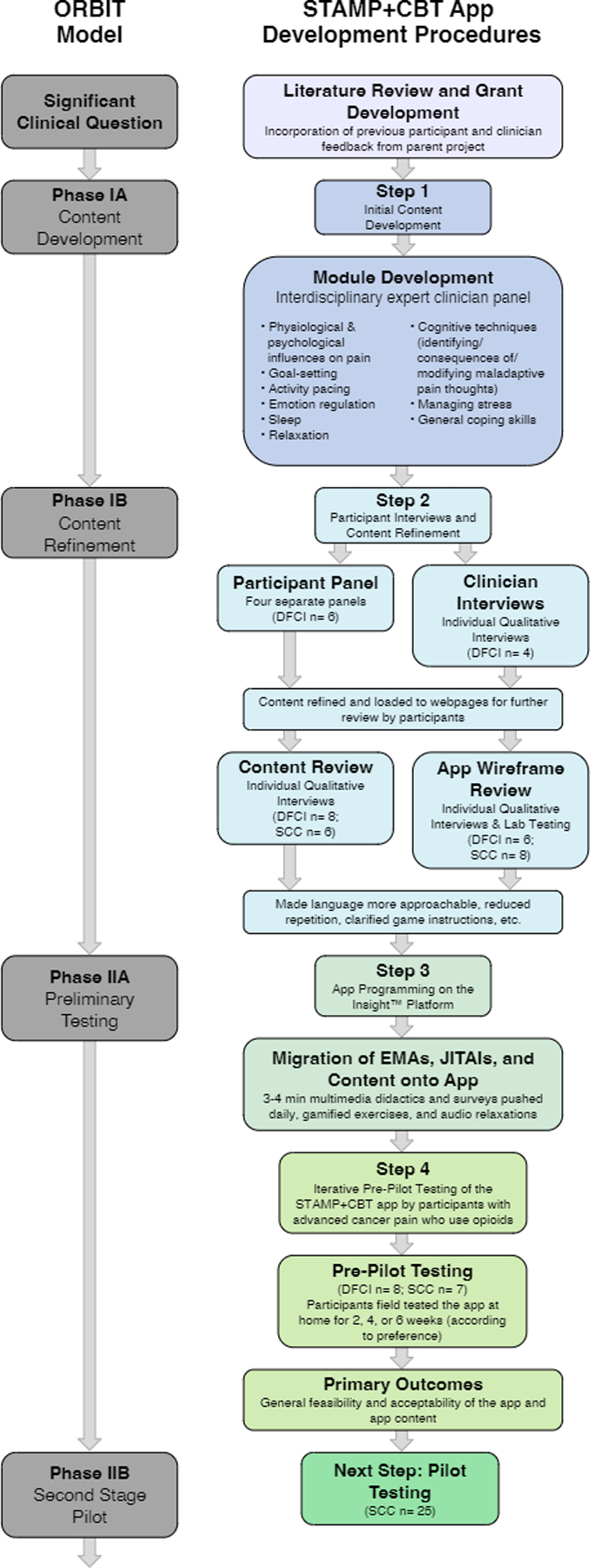
Diagram depicting app development procedures following the ORBIT Model for intervention development
Setting and participants
Two panels (clinician and patient stakeholders) met throughout phases of the project to review draft materials and provide edits and guidance. The interdisciplinary clinician expert panel comprised of oncologists, pain psychologists, and palliative care clinicians. Between 2021 and 2022, participants for all study activities were recruited from palliative care clinics at two NCI-designated cancer centers: the Dana-Farber Cancer Institute (DFCI) in Boston, MA and the Stephenson Cancer Center (SCC) in Oklahoma City, OK. Adults with advanced solid tumor or hematologic malignancies managed with palliative intent were eligible if they had chronic pain related to cancer or cancer treatment, had an average pain ≥ 4/10 [43], were using opioids, and owned a smartphone. Patients were excluded if they had significant cognitive impairment, opioid use disorder, acute pain from recent surgery, or were on hospice. The protocol was approved by the DFCI and SCC IRBs. Participants provided informed consent to participate.
Step 1: Initial content development
In Step 1 (ORBIT Phase-1a), we defined the modules and initial app content. The PI (pain psychologist) reviewed pain-CBT manuals and selected primary aspects of the pain-CBT intervention. Working with various digital media consultants, content was drafted to incorporate common challenges specific to advanced cancer, shortened for mHealth delivery, written to a 7th grade reading-level, and presented through a combination of 2D-animated videos, interactive 2D behavioral games [44], audio-recordings, and texts with visuals. Content was then uploaded to an external web-based site (Wix.com), allowing flexible formatting and user-centered design, including color-coded headers, accordion graphical control elements (i.e., click-to-expand features), and paired visuals. Daily surveys-ecological momentary assessments (EMAs) and just-in-time adaptive intervention (JITAI) algorithms and responses were developed using similar procedures.
Step 2: Participant interviews and content refinement
In Step 2 (ORBIT Phase-1b), we refined content based on feedback from both panels and qualitative participant interviews. Following a semi-structured interview guide (Online Resource 1), participants were asked to describe how mood and other psychological factors influenced their pain experience. Thereafter, the interviewer asked the participants to review mock-ups of several wireframes depicting either (a) app educational content or (b) EMA/JITAI components, using a combination of cognitive interviewing techniques to ensure the content was understandable and probes to assess its acceptability, usefulness, and suggested improvements. Results informed content refinement. Participants’ examples of psychological pain factors were used to augment the psychoeducational content, such as highlighting examples of catastrophic pain thoughts within the content and behavioral games [44].
Step 3: Application build
Following ORBIT Phase-2a, we programmed the app on the Insight™ platform—a web-based technology platform that allows researchers to build, disseminate, and test HIPAA-compliant mobile health interventions (Fig. 2) [45]. Users’ app activity is synced with their unique profiles on the Insight™ platform, allowing study staff to monitor when participants receive and respond to notifications, time spent interacting with surveys, and rates of survey and content completion.
Fig. 2.
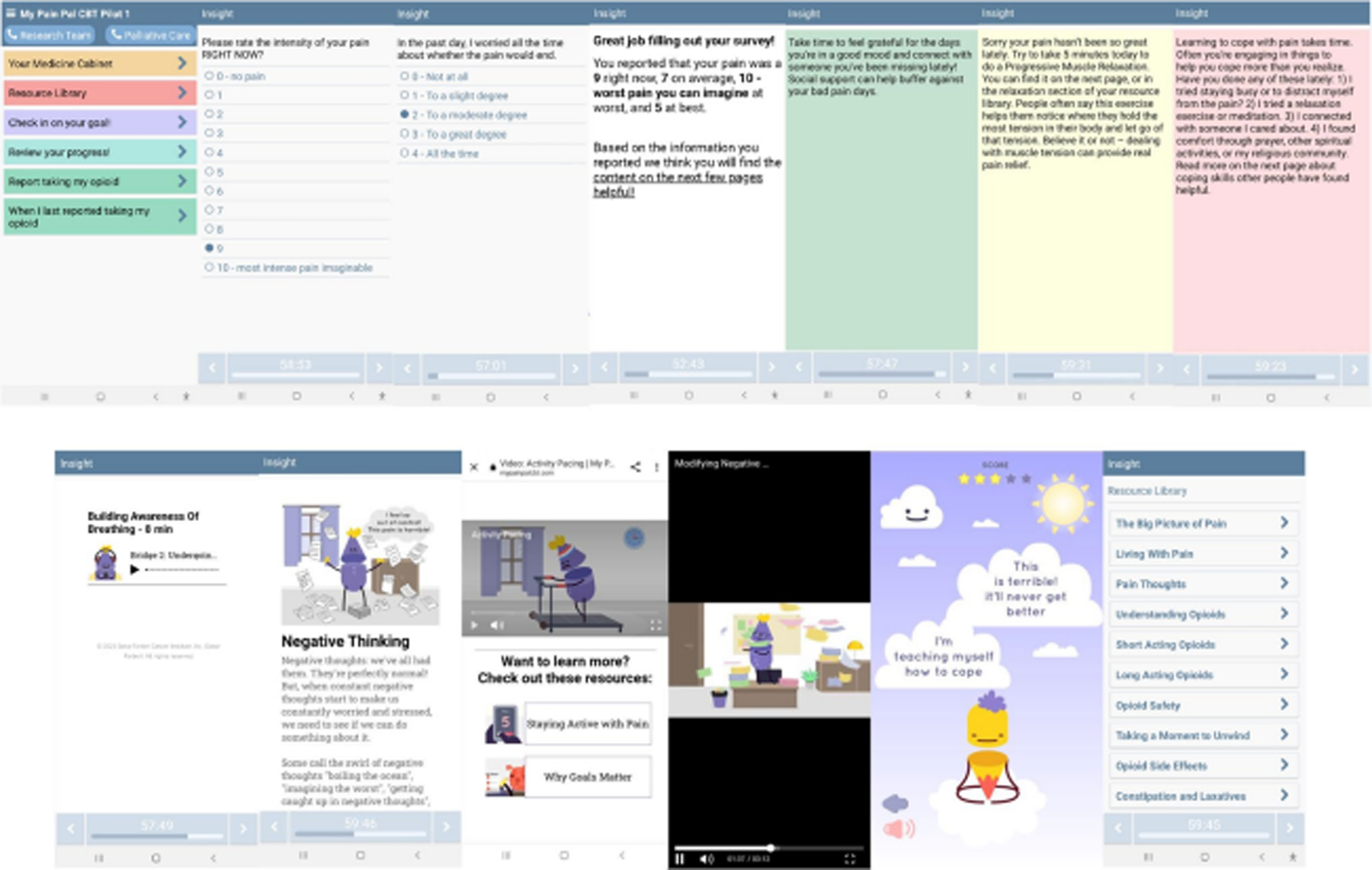
Screenshots of STAMP + CBT app intervention. From top left: app home screen, example pain survey EMA item, example negative thoughts survey EMA item, end of survey summary screen, and stoplight background colors for JITAI feedback and message examples (green, yellow, and red); second line—content examples: breathing relaxation, text with visuals, two 2D video screens, one of the 2D behavioral games, and the homepage for the resource library
Step 4: Single-arm pre-pilot testing of the app
Extending ORBIT Phase-2a, we recruited participants to test the app at home in a single-arm pre-pilot study with a primary goal of assessing feasibility and acceptability of the app. We also sought to identify any technical bugs, gauge the optimal length of the program, and obtain qualitative feedback. Following consent, participants were loaned an Android phone, if needed. Then, study staff inputted their prescribed opioids into the Insight™ dashboard and conducted a 30-min app tutorial. During the tutorial, participants entered a pain-management goal into the app, following SMART principles to ensure that it was sustainable, measurable, attainable, realistic, and time-based [46]. Participants were asked to use the app daily for 2 weeks, after which they could opt to extend their app-use for up to two additional 2-week blocks. Upon study completion, participants were compensated $50, completed a brief survey, and participated in an optional semi-structured debriefing interview.
Measures and outcomes
Participants completed a modified version of the Acceptability E-scale, rating the app on a 5-point Likert scale on the following five domains: understandability, enjoyability, ease of use, time required to use it, and overall satisfaction [47]. Acceptability was pre-defined as ≥ 80% of all acceptability items rated as ≥ 4/5. Feasibility of app use was pre-defined as ≥ 70% of participants completing ≥ 50% of the daily surveys.
Qualitative interviews and analysis
Study staff (DRA, KA, SD) conducted semi-structured interviews over Zoom assessing participants’ overall experience using the app, what prompted them to use it, what they liked and did not like, features used or not, how helpful they found it, what they learned, and impacts on their pain self-management (Online Resource 1). Interviews were audio-recorded and transcribed verbatim, and analyzed using framework analysis [48]. We derived a codebook focused on participants’ pain coping experiences, as well as themes regarding the understandability, relevance, and perceived acceptability and usability of the intervention. Framework analysis was used to summarize feedback and outline recommendations.
Results
Overview of the STAMP + CBT App
The STAMP + CBT app retained the core components of the parent app including its virtual “medicine cabinet” which hosted participants’ opioid regimens, education related to pharmacologic aspects of pain self-management, and daily surveys assessing pain, medication use, and side-effects. STAMP + CBT further integrated daily psychological assessments, content, and algorithms specific to pain-CBT.
The app included two types of patient-centered interactions: pushed and patient-initiated. For pushed content, one daily notification sent multi-media psychoeducation while another prompted completion of daily symptom surveys (EMAs). Notifications rang aloud and vibrated for 30 seconds and paused for 30 seconds, repeating five times. Participants could snooze notifications once for 45 minutes but could not access the prompts between notifications. All content, including resources about pain, analgesics, and pain-CBT, was patient-initiated and was accessible at any time from the home screen (see Fig. 3).
Fig. 3.
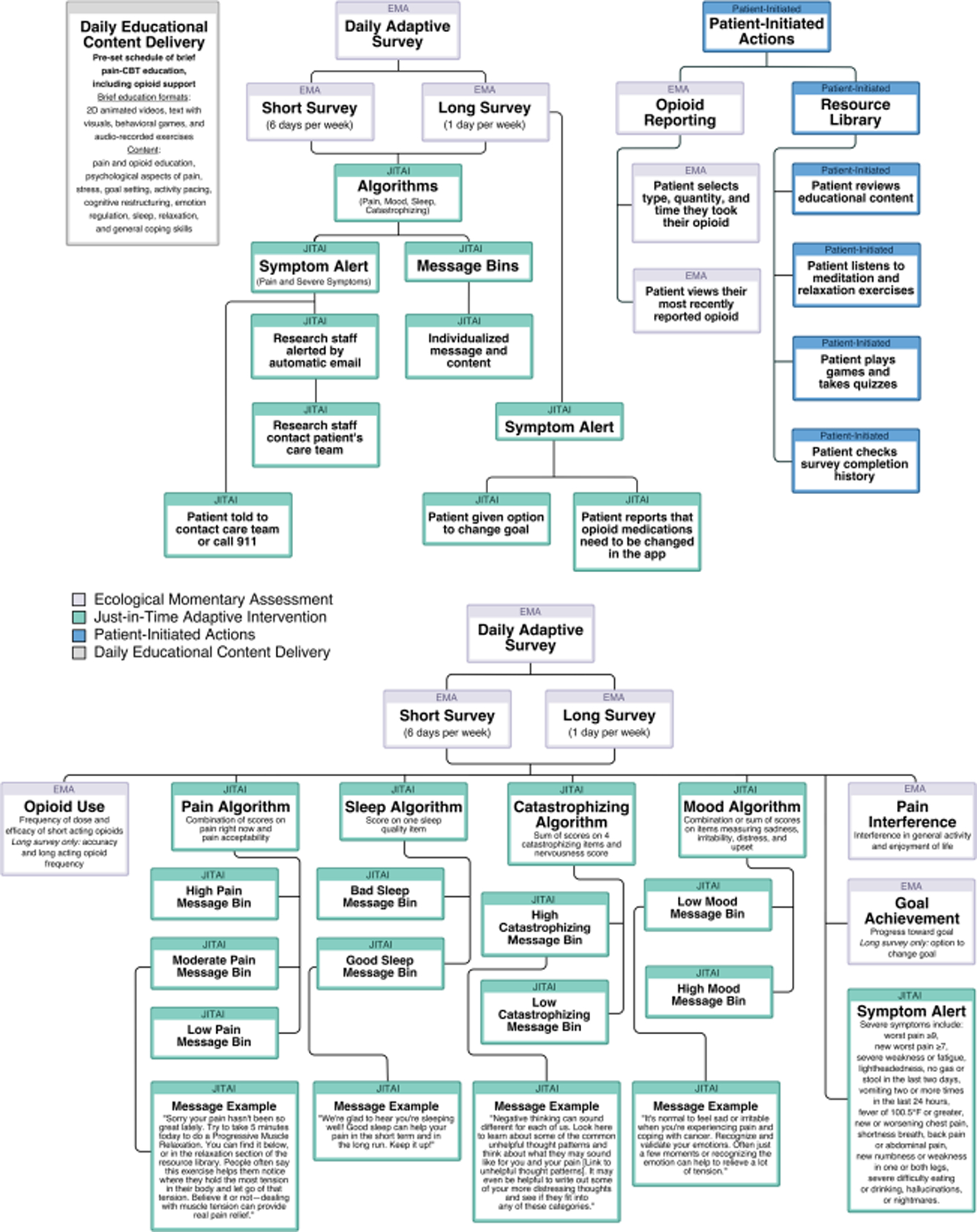
Flowchart summary of STAMP + CBT app features and functionalities, including pusheda and patient-initiated content. EMAb and JITAIc survey components, and example “message bins”d. aIf patients did not respond to or dismissed a push notification, they were not able to interact with that content. Content was delivered on a pre-determined schedule to mirror content delivery in pain-CBT modules, beginning with pain- and opioid-specific medical advice, biopsychosocial aspects of pain, stress and pain, goals and activity pacing, cognitive restructuring, emotional processing, and relaxation exercises. bUpon completion of EMAs, patients were shown a summary of their responses (see Fig. 2). On following screens, they could review individualized messages and advice, which were paired with links to pain-CBT content. cWhen patients reported severe symptoms, the app prompted them to contact their palliative care provider using the clinic phone number on the home screen or to call 911. The same algorithm also pushed an automated email that listed their severe symptoms to the study team. The study team then shared this report with the patient’s care team through secure email to encourage them to reach out accordingly. Patients were made aware that study staff monitored the app only during business hours. dEach “message bin” consisted of customized messages and paired educational content delivered on a rotating basis with a green, yellow, or red background corresponding to mild, moderate, or severe symptoms
“Pushed” ecological momentary assessments
STAMP + CBT uses two EMAs: a daily survey pushed six times per week and a slightly longer survey pushed weekly, both of which include validated measures with minor modifications to assess pain severity and interference (Brief Pain Inventory) [43], mood (five items from the Positive And Negative Affect Schedule) [49], pain catastrophizing (four items from the Pain Catastrophizing Scale) [50], and sleep quality [51]. Additional items assess other severe symptoms, opioid analgesic use and relief, pain acceptability, and progress toward self-identified goals. The weekly survey included several additional items pertaining to analgesic utilization and side effects.
Just-in-time adaptive intervention components
Several JITAIs were used to tailor messages and content in response to EMAs (see Fig. 3), with algorithms categorizing symptom responses by severity with a “message bin” of customized messages and paired educational content for varying levels of pain, mood, pain catastrophizing, and sleep disturbance.
“Pushed” daily content delivery
Each day, participants received a brief lesson or exercise drawn from pain-CBT in the form of text with visuals, 2D-animated videos, audio-recorded relaxation exercises, or behavioral games. In addition to pharmacologic education, content followed the traditional schedule of pain-CBT training modules [psychological and physiological influences on pain, goal-setting, activity pacing, cognitive techniques (i.e., identifying and modifying maladaptive pain thoughts), emotion regulation, sleep, relaxation, managing stress, and coping skills] while highlighting challenges commonly encountered by advanced cancer populations.
Patient-initiated content
Patient-initiated aspects of the app included a resource library containing links to all educational content, separate sections for participants to report and review their most recent opioid use, a medication library that housed a list of participants’ active opioid prescriptions, and tabs to review their goal and completed assignments.
Phase 1: Refinements driven by qualitative feedback on app wireframes and content
In total, 28 participants (14 from DFCI and 14 from SCC) provided feedback on app wireframes of educational content and EMA/JITAI components (Fig. 1; Table 1). Online resource 2 describes participant feedback and actions taken to refine content. The majority of feedback focused on shortening text, adding advanced cancer-specific examples, and removing jargon.
Table 1.
Participant characteristics
| Variable |
N (%) or M (SD) |
|||
|---|---|---|---|---|
| Content and wireframe review |
Iterative pre-pilot |
|||
| DFCI (N = 14) | SCC (N = 14) | SCC (N = 7) | DFCI (N = 8) | |
| Female | 8 (57%) | 7 (50%) | 4 (57%) | 5 (63%) |
| Age (years) | 54.3 (9.7) | 51.6 (12.4) | 46.0 (8.9) | 52.0 (9.6) |
| Race/ethnicity | ||||
| American Indian/Alaska Native | 1 (7%) | |||
| Black/African American | 1 (7%) | 1 (13%) | ||
| White | 14 (100%) | 12 (86%) | 7 (100%) | 7 (88%) |
| Hispanic | 2 (29%) | |||
| Current/historical opioid use | ||||
| Short-acting | 13 (93%) | 14 (100%) | 7 (100%) | 8 (100%) |
| Long-acting | 8 (57%) | 8 (57%) | 4 (57%) | 6 (75%) |
| Cancer type* | ||||
| Head and neck | 3 (21%) | 2 (14%) | 2 (29%) | 1 (13%) |
| Breast | 2 (14%) | 1 (7%) | 2 (25%) | |
| Upper gastrointestinal | 2 (25%) | |||
| Colorectal | 3 (21%) | 2 (14%) | 1 (14%) | |
| Lung | 3 (21%) | 1 (7%) | ||
| Hemopoietic | 2 (14%) | 1 (14%) | 1 (13%) | |
| Kidney | 2 (14%) | 1 (7%) | ||
| Prostate | 1 (7%) | 1 (13%) | ||
| Neuroendocrine | 1 (7%) | |||
| Pancreatic | 1 (7%) | 1 (14%) | ||
| Cervical | 1 (7%) | 1 (14%) | ||
| Ovarian | 1 (7%) | 1 (14%) | ||
| Polycythemia vera | 1 (7%) | |||
| Other | 1 (7%) | 1 (7%) | 1 (13%) | |
| Received a study phone | 5 (71%) | 4 (50%) | ||
| Rural address | 0 | 3 (21%) | 2 (29%) | 0 |
Rurality was identified using zip code analysis of RUCA codes [58]. Some participants were diagnosed with multiple primary cancer types. Samples were recruited from the Stephenson Cancer Center (SCC) in Oklahoma City, OK and the Dana-Farber Cancer Institute (DFCI) in Boston, MA
Phase 2: Pre-pilot testing of STAMP + CBT
A total of 15 patients participated in the pre-pilot study. Most were white (n = 14, 93%) and female (n = 9, 60%). They had a variety of cancer types (Table 1) and 60% (n = 9) used a study phone (Table 1). One participant tested the app for the minimum of 2 weeks, whereas 14 opted to extend testing to 4 weeks.
App utilization, feasibility, and acceptability
Participants who tested the app for 4 weeks (n = 14; 2 weeks n = 1) completed an average of 53% of the daily surveys (median = 61%, 17/28 days) with 73% (n = 11) completing ≥ 50% of their daily EMA surveys, and 27% (n = 4) completing ≥ 75%, surpassing our pre-defined feasibility benchmark. Participants reviewed a median of 32% of pushed-educational content with 43% (n = 6) reviewing ≥ 50%. They used the opioid reporting feature a median of 6.0 times (range 0–123 times) and accessed the resource library a median of 1.0 times (range 0–17 times). Very few participants used the tab presenting content completion, the list of current opioids, and educational content links within JITAI messages. Because of app bugs such as blocked notifications, connection problems, crashing surveys, and difficulties syncing notification schedules, four participants needed to recreate/restart their accounts.
Surpassing our acceptability benchmark, 87% of all acceptability items were rated as ≥ 4/5 (see Table 2). The cohort mean acceptability rating across all items was 4.34 (sd = 0.67, range 1–5), with individual item ratings being highest for understandability (median = 5/5), ease of use (median = 5/5), and satisfaction (median = 4.5/5), and slightly lower ratings for enjoyability (median = 4/5) and time to complete the app (median = 4/5).
Table 2.
Field user acceptability testing (N = 14)
| Median | Mean (SD) | |
|---|---|---|
| How easy was the My Pain Pal app for you to use? | 5 | 4.57 (0.51) |
| How understandable were the questions in the My Pain Pal app? | 5 | 4.93 (0.27) |
| How much did you enjoy using the My Pain Pal app? | 4 | 3.93 (0.73) |
| Was the amount of time it took to complete the My Pain Pal Daily questionnaire acceptable? | 4 | 3.86 (1.29) |
| How would you rate your overall satisfaction with the My Pain Pal app? | 4.5 | 4.43 (0.65) |
Measures: Five items from the Acceptability E-Scale [47] were included. Each item is rated on a 5-point Likert scale, where 5 represents the most favorable response, 3 represents a neutral response, and 1 represents the most negative response
Qualitative feedback from phase 2 pre-pilot of STAMP + CBT
Most participants (73%, n = 11) completed debriefing interviews. Most feedback was positive and fell into the following categories: (1) The role of this mHealth intervention within their experience of advanced cancer and pain management and (2) Feedback about the intervention and its usability (see Table 3 for representative quotes).
Table 3.
Themes and sample quotes
|
Role of STAMP + CBT in the cancer pain experience | |
|---|---|
| Theme | Theme description and sample quotes |
| Convenience of tools and resources |
Participants valued convenient access to tools and resources, especially symptom tracking, and factored app convenience into their assessment of usability “I just assumed the app would be a constant companion…I think of this as part of your healthcare provider team.” (50-year-old female, breast cancer, patient stakeholder panel) “I think it’d be helpful to have it [pain reporting] there on the phone so you can track it.” (71-year-old male, prostate cancer) |
| Mood and pain |
Participants noted a bidirectional relationship between their mood and pain “When I’m really having a bad day, it’s like man, why is this hurting so bad? And then I kinda started putting them two together, just from reading and understanding what y’all were saying, and it helped…. I put them two together and it’s like, wow, it really does; it really does you know, affect how you feel.” (57-year-old male, head and neck cancer) “It’s just getting emotional about anything… That’ll make my pain feel worse than it is.” (38-year-old female, neuroendocrine cancer) “It made me think more about how I was feeling, you know, not just physically but emotionally as well” (47-year-old male, upper gastrointestinal cancer) |
| Timing of intervention |
Participants felt that the app would be beneficial to people who were recently diagnosed with cancer “I hope you guys get this up and running, you know, as soon as possible… I wish I could have had it back then. But, you know, for future patients and all that, I think it’s a great idea.” (53-year-old male, head and neck cancer) “I mean, when I first got diagnosed what six years ago, it really would have been helpful as far as trying to keep track of how many pills I’ve took for, for you know, my pain… And I could see where it would have helped. It would have helped the doctors and me.” (57-year-old male with head and neck cancer) |
| Psychological and behavioral coping skill development |
Interacting with the app prompted the development of psychological and behavioral coping skills, such as modifying negative thoughts, working towards goals, and practicing relaxation “I’m managing my pain well now. I’ve learned you know, like stay on top of the pain, don’t get behind your pain” (46-year-old-female, desmoid tumors). “It’s been hard, but I’m learning to just be, you know, more relaxed and not get upset about things ‘cause it makes you feel worse. [Laughs]… because, you know, – I think ‘cause it just makes it easier on myself, and maybe easier to deal with the people that I deal with on my – on a daily basis.” (51-year-old female, multiple myeloma) “I think it, for me…, it helped. I mean, to put the two things together. … I put the coping part over here and the, the pain, or the pills over here. I never, I haven’t put them together yet. But I’ve learned with, with the pain, what’s left over after I take the pain pill, I’ve learned how to deal with the coping part of it over here. I mean, what, compartmentalizing it, you know? I guess that’s how I kind of deal with some things.” (57-year-old male with head and neck cancer) “Is a lot of, um, self-talk and even just having, like, the examples was super helpful because again, you can just stay those, you know if you’re having trouble figuring out what to say, or how to get thoughts, you know, to be a little bit more positive. Having those examples was huge. Um, that was big. …” (38 year-old-female with breast cancer) |
| Sleep, stress, and pain |
Participants noticed a connection between their stress, sleep quality, and pain “…when you’re pumping out the bad hormones through stress… it’s just compounding your pain you’re having or your misery you’re having.” (62-year-old male, head and neck cancer) “If I’m not sleeping well, I have a harder time coping with my pain… So, if my pain is bad, it makes it harder for me to sleep which makes it harder for me to cope with the pain.” (50-year-old Female, breast cancer) |
| Opioids are not enough |
Though opioids were helpful, participants felt that opioids worked best in conjunction with other forms of pain management “…it [the app] just helps you with your thinking and what you want to do and it just helps ease a little bit more than what the meds can do. Even if it’s just thinking different, it helps.” (52-year-old female with pancreatic cancer) “And I mean, there’s only so much you can do for physical pain, without being just completely zonked out with whatever it is, oxys or Percocets or [sniffles] –your body’s gonna react to it however – however it does. But, you know, at least the mental part, you can do some kinda Zen-like things. Maybe breathing or meditation, or whatever. Or, maybe you get out and walk or – or, you know, have some kind of physical thing to just kinda get out of your head.” (53-year-old male, head and neck cancer) “It’s a combination of that [stress management techniques] with the medications. We’ve tried it without, and then we’re in agreement that the combination of two of those things together seem to really be the right recipe for me.” (49-year-old female, thyroid cancer) |
| Prior pain management education |
Participants received varying levels of pain management education from healthcare providers “Did they? Um, I’m sure we talked about it [coping skills to manage pain]. Um, so a lot of it was such a blur. I was just, I mean, they’re really good doctors. I don’t recall being real in-depth about it, though.” (53-year-old male, head and neck cancer) “I believe so [have been provided pain management education]. It’s dos – proper dosing is mostly what I have.” (68-year-old male, kidney cancer) |
| Iterations and developments required to refine the intervention | |
| Themes | Sample quotes |
| Content delivery |
In general, participants felt that app content was understandable, tonally appropriate, and engaging. Participants appreciated the availability of multiple forms of content (i.e., articles, images, videos, games) “One thing I do like this app… is that” it’s “really informal, friendly language, which is I think what people want these days. It’s very clear and relaxed in some of its wording, which is nice.” (59-year-old male, cecal cancer) “Well, like I said, a lot of people don’t – they’re gonna be turned off by the reading stuff… maybe they would be more apt to, uh, enjoy playing a game.” (54-year-old male, head and neck cancer) “…It made it fun for, for you to do. Like the game part and the reading part, you know. …You broke it up a little bit to where this wasn’t a one monotonous, you know, ‘here I am doing this every day, and the same thing every day.’” (57-year-old-male with head and neck cancer) |
| Visuals and aesthetics |
Many participants enjoyed the design of app features, graphics, and videos “I enjoyed the look of the video. I appreciate that it was kind of cartoony and fun and then a little bit silly…. Anything that can get somebody to smile when you’re in this much pain is good.” (38-year-old female, neuroendocrine cancer) “Like I said earlier, I feel like there might be some older people that are a little turned off by the animation, but, for me, it – like I said, it’s kinda just simple and relatable Information that is definitely pertaining to my particular experience.” (37-year-old female, prostate cancer) “If you’re looking for something to read, and you’re having a problem, then …yeah, and it’s got the little pictures on there that kinda break it up.” (77-year-old female, lung cancer) |
| EMAs/JITAIs |
While some participants felt that daily EMAs prompted reflection, others found them repetitive. Many participants noticed that the end of survey messages (JITAIs) corresponded to their survey answers “Those [EMAs], again, go into what I was saying earlier about how I liked those because …it made me more reflective in thinking about what I was actually going through. So, I thought those were all great.” (47-year-old man, upper gastrointestinal cancer) “I think it’s very well balanced, it asks about how much you are stressed and nervous…it also asks about positive things. I like that there is balance there.” (37-year-old female, desmoid fibromatosis) “I don’t think I’d want to answer these questions every day. People might find it repetitive if they’re getting this every day and the answers don’t change day to day.” (59-year-old male, cecal cancer) |
| Smartphone use |
Most participants were comfortable with smartphones and used them regularly “But if it was on the app, I love it. I just love everything in one spot. Because everything I do is on my phone, as you know. So, I definitely would. It’d just be so readily available, and I think it would just be more in my face, too.” (49-year-old female, thyroid cancer) “I mean, I been three days without picking up my phone.” (58-year-old female, lung cancer) |
| Relevance |
Participants felt the app was relevant to their experience of advanced cancer and chronic cancer-related pain “But I noticed so much of what I do is in those. And the way I think, it’s in there. And that’s good to know that I’m not alone in that other people do the same thing I do” (51-year-old female, cervical cancer) “I’m finding that things like that can be so preachy and so overwhelming to someone who is in the middle of it. And I did not detect that tone at all…. That’s a very difficult thing to do… Because if someone’s in pain, it’s easier for them to be angry when they’re told to stop and look at their thoughts. And I didn’t feel any of that defensiveness.” (67-year-old female with pancreatic cancer, participant stakeholder panel) |
| Usability |
By and large, participants felt the app was easy to use and could see themselves using it. Some felt that their pain would preclude them from using the app “I think that it would be helpful. I mean, five or ten minutes a day is literally like, laying in bed waking up in the morning, you know? You can kind of start your day on a positive note. And, you know, start organized.” (37-year-old female, pancreatic cancer) “I have my phone out all the time, so if it’s short then I’d do it daily at the end of the day.” (54-year-old female, colorectal cancer and leukemia) “But I always was in pain and, so you know, when you’re in pain you don’t want to sit there and learn anything you just wanna curl— curl up and get better.” (64-year-old female, breast cancer) |
Role of STAMP + CBT in the cancer pain experience
Relevance
Many participants expressed that app content was directly relevant to their experiences coping with pain, medical uncertainty, and alteration of their relationships. In contrast, a few participants noted a lack of relevance because their pain had abated or they had ceased taking opioids since enrolling. Many participants felt that the app was a convenient tool to track their pain and opioid use, and improved their pain self-management by helping them to create better medication routines.
Building psychological and behavioral skills
Many participants were unaware that psychological factors influenced pain (“[I] had no clue that the two even connected,” 51-year-old female, cervical cancer), and credited the app with helping them understand these factors (“I was reading this and, I started putting the two together… your emotions really do affect how you’re hurting,” 57-year-old male, head and neck cancer). Several participants also articulated how the app gave them insights into pain catastrophizing. For example, one participant stated “[I got into] quite a rabbit hole worrying about that stuff [pain and cancer], and that’s super helpful that the app could kind of delve into that,” (54-year-old male, head and neck cancer). Multiple participants described learning to modify maladaptive thinking patterns: “I was a person that was like it’s just never gonna end, …the app really helped me refocus to thinking, you know, it will get better, we can do this…it just changed the way I thought about it.” (52-year-old female, pancreatic cancer).
A few participants reported that the app helped them acquire new pain coping skills, such as activity pacing, which they used to achieve their pain management goals. The goals participants inputted varied from improving medication use (e.g., staying consistent with medications) to engaging in meaningful activities (e.g., play with dogs, spend time with family), to improving other aspects of health (e.g., getting better sleep, walking more, gaining weight). Participants were generally open to incorporating psychological/behavioral coping skills into their pain management routines, and several wished that they would have learned these principles earlier. A 54-year-old male with head and neck cancer stated “[I could] see where that [the app] would have really helped me, if I had had access to it earlier.”
Feedback on content, delivery, and usability
Content delivery and style
Most participants liked and even looked forward to receiving the “pushed” daily educational content, appreciating its importance, clarity, brevity, and use of visuals. One participant stated, “But for me, it’s simple, … people that are going through treatment… they would understand needing the simplicity” (37-year-old female, pancreatic cancer). In contrast, fewer participants engaged with educational content “pushed” in response to EMAs and two participants felt it was not a helpful mode of delivery, saying “if I’m entering pain meds, it’s because I’m in pain…So, I’m not sitting reading…” (70-year-old male, upper gastrointestinal cancer). Participants generally under-utilized content in the resource library and explained that they forgot it was available or was difficult to locate.
Participants were satisfied with the visual design of the app. While most appreciated the animated characters used to depict abstract concepts, a few felt that they were somewhat “childish.” A few participants particularly enjoyed the behavioral games, with one noting ‘[they] kinda made you think… about what you’d actually been reading” (57-year-old male, head and neck cancer). Several found the audio-recorded relaxations helpful and used them to cope with sleeplessness or stress.
EMAs and JITAIs
Many participants liked being asked about psychological factors in addition to physical symptoms within the EMAs, but some thought the questions were repetitive to receive daily. Many were frustrated that the EMAs expired an hour after they were pushed and missed surveys for this reason. The tailored messages (JITAI) delivered at the end of surveys were considered appropriate and generally helpful, with one participant stating “with the way I answered questions, it seemed that it [the app] would kind of, remember things about that and, steer me in certain ways… I thought it was helpful” (54-year-old male, head and neck cancer). However, participants did not want to review the educational content linked to these messages and perceived this to be burdensome.
Adaptations resulting from pre-pilot
In response to qualitative feedback and app-use data, features that were rarely used or considered unhelpful were removed, including the list of current opioids and content links within JITAI messages. Daily EMAs and “pushed” educational content were made accessible on the app home screen (instead of being solely available through push notifications) and the 1-hour expiration was eliminated. The platform was not capable of letting participants set their own app notification times; however, a comment feature where participants could communicate requests to the study team was added. Finally, based on participant choice of testing duration, the next iteration of the intervention will run for 4 weeks.
Discussion
Here, we describe the development and refinement of STAMP + CBT, which to our knowledge, is the first app-based intervention to deliver pain-CBT and pharmacologic support for patients with cancer pain. This intervention has many innovative features, including its use of mHealth to deliver components of pain-CBT, pharmacologically-oriented cancer pain psycho-education, and its use of EMAs and algorithms (i.e., JITAI’s) to personalize symptom and psychological support, giving real-time feedback and advice in response to participants’ pain, mood, stress, and sleep symptomatology. In this initial pilot, the app was deemed feasible and acceptable. Moreover, many participants described improving their pain coping skills as a direct result of using the app.
While the goal of this mHealth intervention is not to replace in-person psycho-behavioral pain management treatment, it may succeed in making pain-CBT more accessible to patients and integrating with their oncology care. Many of the current interventions either provide static pain education or focus on electronic symptom-based monitoring, most of which are unlikely to promote behavior change to help patients gain proven behavioral skills [30, 52]. STAMP + CBT meets an important unmet need for patients with cancer, who have very limited access to pain-CBT [27–33, 53] due to a lack of trained psychologists, siloed behavioral care, and stigma related to seeking mental health care [54].
Although this pilot study was small, the results are encouraging. We exceeded our acceptability and feasibility benchmarks with 73% of participants completing more than half of their daily EMAs, despite the fact that surveys expired an hour after push notifications. While participants completed relatively less of their daily educational content, it is unclear how much pain-CBT content is required to be effective; even single-session pain-CBT interventions have demonstrated meaningful improvements in pain outcomes [55–57]. Our qualitative debriefing interviews suggest that they engaged with the content in a meaningful way and implemented several core principles of pain-CBT in their pain self-management. While we cannot ascertain which app components were most impactful, interviews suggested that participants benefitted from varied engagement opportunities, including written and video education, behavioral games (i.e., teaching cognitive restructuring), and daily surveys that facilitated reflection. Participants noted that the daily surveys helped them make connections between psychological factors and their pain—a factor participants appreciated since many reported that pain management education, especially pain psychology education, was mostly absent in their cancer treatment. In contrast, participants did not want to engage with longer education at the time of symptom reporting and requested greater variety in their daily surveys. We have modified the app in response and are testing this updated version in a larger pilot.
Despite the promising results, the study has several limitations. First, this small pilot did not examine efficacy and participants may not be representative of general cancer populations. Our next phase of testing will include more patients, track recruitment rates and reasons for refusal, and assess our anticipated primary and secondary outcomes (pain interference and pain intensity, catastrophizing, depression, anxiety, and quality of life). Third, although mobile health has the potential to scale behavioral pain treatments, older and historically disadvantaged populations experience greater barriers to using digital technologies. Moreover, some patients may prefer or require in-person treatment [34]. Even still, this study indicates that for patients who report some comfort with technology, app-based delivery of pain-CBT with opioid support is feasible and acceptable.
Supplementary Material
Acknowledgements
The Insight™ mHealth platform was developed by the University of Oklahoma Health Sciences Center and funded by the P30 and Tobacco Settlement Endowment Trust grants.
Funding
This work was funded by the National Palliative Care Research Center (PI Azizoddin), the National Institutes of Health-National Cancer Institute (NIH-NCI R21CA263838, PI Azizoddin; NIH-NCI K08CA266937, PI Azizoddin), and Tobacco Settlement Endowment Trust (TSET-R23-02).
Footnotes
Declarations
Ethics approval and consent to participate The study protocol was approved by Dana-Farber Cancer Institute (No: 20–453 exempted 12/07/2020; No: 21–719 approved 5/23/2022, TRN: NCT05403801 registered 05/06/2022) and Stephenson Cancer Center (No: 13725 approved 12/13/2021) Institutional Review Boards. Participants provided informed consent to participate and study activities conformed to the 1964 Helsinki Declaration and following amendments.
Competing interests MSB is the primary inventor of the Insight™ mHealth Platform, which was used to develop the app described in this manuscript. MSB receives royalties related to the Insight™ Platform, but he did not receive royalties in this case. The remaining authors have no relevant financial or non-financial interests to disclose.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00520-024-08307-7.
References
- 1.Van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA(2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag 51(6):1070–90.e9. 10.1016/j.jpainsymman.2015.12.340 [DOI] [PubMed] [Google Scholar]
- 2.Kwon JH (2014) Overcoming barriers in cancer pain management. J Clin Oncol 32(16):1727–1733. 10.1200/JCO.2013.52.4827 [DOI] [PubMed] [Google Scholar]
- 3.O’Mahony S, Goulet J, Kornblith A, Abbatiello G, Clarke B, Kless-Siegel S, Breitbart W, Payne R (2005) Desire for hastened death, cancer pain and depression: report of a longitudinal observational study. J Pain Symptom Manag 29(5):446–457. 10.1016/j.jpainsymman.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ (1994) Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 330(9). 10.1056/NEJM199403033300902 [DOI] [PubMed] [Google Scholar]
- 5.Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19(12):1985–91. 10.1093/annonc/mdn419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deandrea S, Corli O, Consonni D, Villani W, Greco MT, Apolone G (2014) Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manag 47(1):55–76. 10.1016/j.jpainsymman.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 7.Porter LS, Keefe FJ (2011) Psychosocial issues in cancer pain. Curr Pain Headache Rep 15(4):263–270. 10.1007/s11916-011-0190-6 [DOI] [PubMed] [Google Scholar]
- 8.Keefe FJ, Abernethy AP, Campbell LC (2005) Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol 56:601–30. 10.1146/annurev.psych.56.091103.070302 [DOI] [PubMed] [Google Scholar]
- 9.Zaza C, Baine N (2002) Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manag 24(5):526–542 [DOI] [PubMed] [Google Scholar]
- 10.Gunnarsdottir S, Donovan HS, Serlin RC, Voge C, Ward S (2002) Patient-related barriers to pain management: the Barriers Questionnaire II (BQ-II). Pain 99(3):385–396. 10.1016/S0304-3959(02)00243-9 [DOI] [PubMed] [Google Scholar]
- 11.Mah K, Tran KT, Gauthier LR, Rodin G, Zimmermann C, Warr D, Librach SL, Moore M, Shepherd FA, Gagliese L (2018) Do correlates of pain-related stoicism and cautiousness differ in younger and older people with advanced cancer? J Pain 19(3). 10.1016/j.jpain.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Potter VT, Wiseman CE, Dunn SM, Boyle FM (2003) Patient barriers to optimal cancer pain control. Psychooncology 12(1):153–160. 10.1002/pon.627 [DOI] [PubMed] [Google Scholar]
- 13.Wright EM, El-Jawahri A, Temel JS, Carr A, Safren SA, Park ER, Pirl WF, Bruera E, Traeger L (2019) Patient patterns and perspectives on using opioid regimens for chronic cancer pain. J Pain Symptom Manag 57(6):1062–1079. 10.1016/j.jpainsymman.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturgeon JA (2014) Psychological therapies for the management of chronic pain. Psychol Res Behav Manag 7:115–124. 10.2147/PRBM.S44762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannsen M, Farver I, Beck N, Zachariae R (2013) The efficacy of psychosocial intervention for pain in breast cancer patients and survivors: a systematic review and meta-analysis. Breast Cancer Res Treat 138(3):675–690. 10.1007/s10549-013-2503-4 [DOI] [PubMed] [Google Scholar]
- 16.Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ (2014) Psychological and behavioral approaches to cancer pain management. J Clin Oncol 32(16):1703–11. 10.1200/JCO.2013.54.4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorin S, Krebs P, Badr H, Janke EA, Jim HS, Spring B, Mohr DC, Berendsen MA, Jacobsen PB (2012) Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol 30(5):539–547. 10.1200/jco.2011.37.0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehde DM, Dillworth TM, Turner JA (2014) Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 69(2):153–166. 10.1037/a0035747 [DOI] [PubMed] [Google Scholar]
- 19.Veehof MM, Oskam M-J, Schreurs KMG, Bohlmeijer ET (2011) Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. PAIN® 152(3):533–42. 10.1016/j.pain.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG (2016) Acceptance- and mindfulness- based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther 45(1):5–31. 10.1080/16506073.2015.1098724 [DOI] [PubMed] [Google Scholar]
- 21.Knoerl R, Smith EML, Weisberg J (2015) Chronic pain and cognitive behavioral therapy: an integrative review. West J Nurs Res 38(5):596–628. 10.1177/019394591561586 [DOI] [PubMed] [Google Scholar]
- 22.Williams ACdC, Eccleston C, Morley S (2012) Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 11. 10.1002/14651858.CD007407.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorn B (2017) Cognitive therapy for chronic pain: a step-by-step guide. Guilford Publications [Google Scholar]
- 24.Murphy JL, McKellar JD, Raffa SD, Clark ME, Kerns RD, Karlin BE (2017) Cognitive behavioral therapy for chronic pain among veterans: therapist manual. Mass Gen Hosp Handb Behav Med 93–114. 10.1007/978-3-319-29294-6_5 [DOI] [Google Scholar]
- 25.Ciarrochi JV, Bailey A (2008) A CBT Practitioner’s guide to ACT: how to bridge the gap between cognitive behavioral therapy & acceptance and commitment therapy. New Harbinger Publications [Google Scholar]
- 26.Bulls HW, Hoogland AI, Craig D, Paice J, Chang YD, Oberoi-Jassal R, Rajasekhara S, Haas M, Bobonis M, Gonzalez BD, Portman D, Jim HSL (2019) Cancer and opioids: patient experiences with stigma (COPES)-a pilot study. J Pain Symptom Manag 57(4):816–819. 10.1016/j.jpainsymman.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somers TJ, Kelleher SA, Westbrook KW, Kimmick GG, Shelby RA, Abernethy AP, Keefe FJ (2016) A small randomized controlled pilot trial comparing mobile and traditional pain coping skills training protocols for cancer patients with pain. Pain Res Treat 2016:2473629. 10.1155/2016/2473629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somers TJ, Abernethy AP, Edmond SN, Kelleher SA, Wren AA, Samsa GP, Keefe FJ (2015) A pilot study of a mobile health pain coping skills training protocol for patients with persistent cancer pain. J Pain Symptom Manag 50(4):553–558. 10.1016/j.jpainsymman.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somers TJ, Kelleher SA, Dorfman CS, Shelby RA, Fisher HM, Rowe Nichols K, Sullivan KM, Chao NJ, Samsa GP, Abernethy AP, Keefe FJ (2018) An mHealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth Uhealth. 6(3):e66. 10.2196/mhealth.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva EH, Lawler S, Langbecker D (2019) The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: a systematic review. J Cancer Survivorship: Res Pract 13(1):97–107. 10.1007/s11764-018-0730-8 [DOI] [PubMed] [Google Scholar]
- 31.Dorfman CS, Kelleher SA, Winger JG, Shelby RA, Thorn BE, Sutton LM, Keefe FJ, Gandhi V, Manohar P, Somers TJ (2019) Development and pilot testing of an mHealth behavioral cancer pain protocol for medically underserved communities. J Psychosoc Oncol 37(3):335–349. 10.1080/07347332.2018.1479327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelleher SA, Fisher HM, Hyland K, Miller SN, Amaden G, Diachina A, Pittman AS, Winger JG, Sung A, Berchuck S, Samsa G, Somers TJ (2022) Hybrid-delivered cognitive behavioral symptom management and activity coaching intervention for patients undergoing hematopoietic stem cell transplant: findings from intervention development and a pilot randomized trial. J Psychosoc Oncol.Online ahead of print 1–19. 10.1080/07347332.2022.2152519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelleher SA, Fisher HM, Winger JG, Somers TJ, Uronis HE, Wright AN, Keefe FJ (2021) Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support Care Cancer 29:5361–5369. 10.1007/s00520-021-06126-8 [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Bruin Md, Burton CD, Bond CM, Clausen MG, Murchie P (2018) What are the current challenges of managing cancer pain and could digital technologies help? BMJ Support Palliat Care 8(2):204–212. 10.1136/bmjspcare-2016-001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allsop MJ, Taylor S, Bennett MI, Bewick BM (2019) Understanding patient requirements for technology systems that support pain management in palliative care services: a qualitative study. Health Informatics J 25(3):1105–1115. 10.1177/1460458217740724 [DOI] [PubMed] [Google Scholar]
- 36.Goodman W, Bagnall A-M, Ashley L, Azizoddin D, Muehlensiepen F, Blum D, Bennett MI, Allsop M (2022) The extent of engagement with telehealth approaches by patients with advanced cancer: systematic review. JMIR Cancer 8(1):e33355. 10.2196/33355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pew Research Center. Mobile Fact Sheet 2021. Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/ [Google Scholar]
- 38.Castelnuovo G, Zoppis I, Santoro E, Ceccarini M, Pietrabissa G, Manzoni GM, Corti S, Borrello M, Giusti EM, Cattivelli R, Melesi A, Mauri G, Molinari E, Sicurello F (2015) Managing chronic pathologies with a stepped mHealth-based approach in clinical psychology and medicine. Front Psychol 6(407). 10.3389/fpsyg.2015.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, Laraia B, Olster DH, Perna FM, Peterson JC, Epel E, Boyington JE, Charlson ME (2015) From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol 34(10):971–982. 10.1037/hea0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y, Skelton K, Dunton G, Bruening M (2016) A systematic review of methods and procedures used in ecological momentary assessments of diet and physical activity research in youth: an adapted STROBE checklist for reporting EMA studies (CRE-MAS). J Med Internet Res 18(6):e151. 10.2196/jmir.4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azizoddin DR, Adam R, Kessler D, Wright AA, Kematick B, Sullivan C, Zhang H, Hassett MJ, Cooley ME, Ehrlich O, Enzinger AC (2021) Leveraging mobile health technology and research methodology to optimize patient education and self-management support for advanced cancer pain. Support Care Cancer 29:5741–5751. 10.1007/s00520-021-06146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otis J (2007) Managing chronic pain: a cognitivebehavioral therapy approach. Oxford University Press [Google Scholar]
- 43.Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, Shaw M, Li Y, Hay J, Cleeland CS, Scher HI, Breitbart WS, Basch E (2011) Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manag 41(3):558–565. 10.1016/j.jpainsymman.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azizoddin DR, Thomas TH (2022) Game changer: is palliative care ready for games? JCO Clin Cancer Inform 6:e2200003. 10.1200/CCI.22.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.TSET Health Promotion Research Center. Mobile Health Technology 2023. [cited 2023]. Available from: https://healthpromotionresearch.org/MHealth [Google Scholar]
- 46.Hooker SA, Punjabi A, Justesen K, Boyle L, Sherman MD (2018) Using these brief interventions, you can help your patients make healthy behavior changes. Fam Pract Manag 25(2):31–36 [PubMed] [Google Scholar]
- 47.Tariman JD, Berry DL, Halpenny B, Wolpin S, Schepp K (2011) Validation and testing of the Acceptability E-scale for web-based patient-reported outcomes in cancer care. Appl Nurs Res 24(1):53–8. 10.1016/j.apnr.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritchie J, Lewis J, Nicholls CM, Ormston R (2014) Qualitative research practice: a guide for social science students and researchers, 2nd edn. SAGE Publications [Google Scholar]
- 49.Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54(6):1063–1070. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 50.Sullivan MJL, Bishop SR, Pivik J (1995) The pain catastrophizing scale: development and validation. Psychol Assess 7(4):524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 51.Stone AA, Broderick JE, Junghaenel DU, Schneider S, Schwartz JE (2016) PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. J Clin Epidemiol 74:194–206. 10.1016/j.jclinepi.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 52.Zheng C, Chen X, Weng L, Guo L, Xu H, Lin M, Xue Y, Lin X, Yang A, Yu L, Xue Z, Yang J (2020) Benefits of mobile apps for cancer pain management: systematic review. JMIR Mhealth Uhealth 8(1):e17055. 10.2196/17055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dempster PG, Bewick BM, Jones R, Bennett MI (2012) Management of cancer pain in the community: perceptions of current UK information technology systems and implications for future development. J Health Inform 18(4):284–93. 10.1177/1460458212445341 [DOI] [PubMed] [Google Scholar]
- 54.Darnall BD (2021) Psychological treatment for chronic pain: improving access and integration. Pyschological Sci Public Interes 22(2):45–51. 10.1177/15291006211033612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darnall BD, Roy A, Chen AL, Ziadni MS, Keane RT, You DS, Slater K, Poupore-King H, Mackey I, Kao MC, Cook KF, Lorig K, Zhang D, Hong J, Tian L, Mackey SC (2021) Comparison of a single-session pain management skills intervention with a single-session health education intervention and 8 sessions of cognitive behavioral therapy in adults with chronic low back pain: a randomized clinical trial. Jama Netw Open 4(8):e2113401. 10.1001/jamanetworkopen.2021.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziadni MS, Gonzalez-Castro L, Anderson S, Krishnamurthy P, Darnall BD (2021) Efficacy of a single-session “empowered relief” zoom-delivered group intervention for chronic pain: randomized controlled trial conducted during the COVID-19 pandemic. J Med Internet Res 23(9):e29672. 10.2196/29672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dochat C, Wooldridge JS, Herbert MS, Lee MW, Afari N (2021) Single-session acceptance and commitment therapy (ACT) interventions for patients with chronic health conditions: a systematic review and meta-analysis. J Contextual Behav Sci 20:52–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Service ER. Rural-Urban Commuting Area Codes: U.S. Department of Agriculture; [updated Monday, August 17, 2020]. Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


