Abstract
Bovine herpesvirus 1 (BHV-1), like other members of the Alphaherpesvirinae subfamily, establishes latent infection in sensory neurons. Reactivation from latency can occur after natural or corticosteroid-induced stress culminating in recurrent disease and/or virus transmission to uninfected animals. Our previous results concluded that CD4+ T cells in the tonsil and other adjacent lymph nodes are infected and undergo apoptosis during acute infection (M. T. Winkler, A. Doster, and C. Jones, J. Virol. 73:8657–8668, 1999). To test whether BHV-1 persisted in lymphoreticular tissue, we analyzed tonsils of latently infected calves for the presence of viral DNA and gene expression. BHV-1 DNA was consistently detected in the tonsils of latently infected calves. Detection of the latency-related transcript (LRT) in tonsils of latently infected calves required nested reverse transcription-PCR (RT-PCR) suggesting that only a few cells contained viral DNA or that LRT is not an abundant transcript. bICP0 (immediate-early and early transcripts), ribonucleotide reductase (early transcript), and glycoprotein C (late transcript) were not detected by RT-PCR in latently infected calves. When reactivation was initiated by dexamethasone, bICP0 and ribonucleotide reductase transcripts were detected. Following dexamethasone treatment, viral nucleic acid was detected simultaneously in trigeminal ganglionic neurons and lymphoid follicles of tonsil. LRT was detected at 6 and 24 h after dexamethasone treatment but not at 48 h. Dexamethasone-induced reactivation led to apoptosis that was localized to tonsillar lymphoid follicles. Taken together, these findings suggest that the tonsil is a site for persistence or latency from which virus can be reactivated by dexamethasone. We further hypothesize that the shedding of virus from the tonsil during reactivation plays a role in virus transmission.
Bovine herpesvirus 1 (BHV-1) is an important viral pathogen of cattle that can cause severe respiratory disease, conjunctivitis, abortions, vulvovaginitis, balanopostitis, and systemic infection in neonate calves. Secondary bacterial infections resulting in bronchopneumonia and death are common (reviewed in reference 38). BHV-1 belongs to the Alphaherpesvirinae subfamily and shares a number of biological properties with herpes simplex virus 1 (HSV-1) and HSV-2 (13, 33). After initial replication in the mucosa of the upper respiratory tract and eye, latency is established in sensory neurons of trigeminal ganglia (TG). The virus can persist in a latent state for the lifetime of the infected host but can also periodically reactivate (reviewed in references 13 and 33). Successful long-term BHV-1 latency requires at least four distinct events: (i) establishment, (ii) maintenance of the latent state, (iii) reactivation, and (iv) shedding of infectious virus. During productive infection of tissue culture cells, BHV-1 gene expression proceeds in a well-characterized cascade. Immediate-early (IE) gene products are initially expressed, and they subsequently transactivate early (E) and late (L) gene expression (reviewed in reference 13). In contrast to what is found for productive infection of permissive cells, the latency-related transcript (LRT) is the only abundant transcript detected in latently infected neurons (24, 25). LRT is antisense to the IE bICP0 gene. During reactivation, virus is translocated back to the initial site of infection, from which it can spread to other susceptible hosts. The process of viral reactivation occurs after exogenous administration of corticosteroids or elevated levels of natural corticosteroids as a consequence of stress (21, 31, 32). The synthetic corticosteroid dexamethasone (DEX) efficiently reactivates BHV-1 in latently infected rabbits and calves (19, 23, 32).
BHV-1 can infect and replicate in the tonsil during acute infection of cattle (28, 37). T lymphocytes in the tonsil or adjacent lymph nodes are infected and undergo apoptosis (37). Respiratory infections caused by many viruses, including herpesviruses, frequently target the tonsil as an important site for initial replication. For example, the tonsillar epithelium and lymphocytes play a central role in primary Epstein-Barr virus infection and perhaps latency (1). Canine herpesvirus 1 DNA has been detected in tonsils of latently infected dogs (6), and during reactivation virus was recovered from tonsil tissues (20). HSV-1 has been detected in bone marrow of humans (8) and in other nonneural sites in small-animal models (17, 29). Pseudorabies virus (PRV) DNA has also been detected in tonsils (2, 3, 16, 35, 36) of latently infected swine. Nearly 80% of latently infected swine contain detectable levels of the PRV latency-associated transcript in tonsils (9). Although TG is the major site for α-herpesvirus latency following infection, several studies demonstrate that persistence or latency can occur in nonneural sites.
In this study, we demonstrate that BHV-1 DNA was consistently detected in tonsil during latency. LRT, but not bICP0 (IE and E transcripts) and ribonucleotide reductase (RR; E transcript), was detected in tonsils of latently infected calves. Following DEX injection, RR and bICP0 RNA was readily detected. LRT was detected at 6 and 24 h after DEX treatment but not at 48 h. DEX treatment of latently infected calves increased apoptosis in their tonsils. Taken together, the data from this study suggested that the tonsil is a site for BHV-1 latency or persistency and that this virus can be reactivated in vivo.
MATERIALS AND METHODS
Virus and cells.
MDBK cells (American Type Culture Collection, Manassas, Va.) were maintained in Earle's modified Eagle's media (EMEM) with 10% fetal calf serum. The Cooper strain of BHV-1, supplied by the National Veterinary Services Laboratory, Animal and Plant Health Inspection Services, Ames, Iowa, was propagated in MDBK cells with a multiplicity of infection of 0.05. When cytopathic effect (CPE) was evident, virus was harvested, titrated in MDBK cells, aliquoted, and stored at −70°C.
Animals.
Holstein calves (5 to 6 months old) that were seronegative for BHV-1 were used for these studies. Calves were inoculated in the right and left conjunctival sacs and intranasally, 1 ml per site, with 107 50% tissue culture infective doses of BHV-1 Cooper strain/ml. Experiments using animals were done in accordance with the American Association of Laboratory Animal Care guidelines. Calves were housed under strict isolation containment and given antibiotics before and after BHV-1 infection to prevent secondary bacterial infection. The calves can be broken down into four groups: 1, latently infected (≥60 days postinfection (dpi); 2, latently infected and treated with DEX for various times to initiate reactivation; 3, mock infected; and 4, mock infected and treated with DEX for 6, 24, or 48 h.
Nasal and ocular secretions from calves in groups 1 and 2 at days 1, 2, 5, 7, 9, 12, and 30 after DEX-induced reactivation were collected. Swabs were also obtained from mock-infected (groups 3 and 4) and latently infected calves (group 1). Swabs were placed into 2 ml of EMEM containing 0.05 mg of gentamicin/ml immediately after collection, and infectious virus was detected by inoculation with MDBK cells. A sample was considered negative when CPE was not observed after three passages. Each passage was maintained for three consecutive days (37°C in a 5% CO2 incubator). Nasal and ocular swabs were also obtained from calves for DNA extraction for PCR. Alkaline extraction of DNA was performed as described previously (27).
DEX treatment and tissue collection.
Mock-infected calves and latently infected calves, 60 dpi, were given a single dose of water-soluble DEX (catalogue no. D2915; Sigma Chemical Co., St. Louis, Mo.) by the intravenous route (0.4 mg/kg of body weight). TG and pharyngeal tonsil were obtained from mock-infected, mock-infected and DEX-treated (6, 24, and 48 h), latently infected (60 to 70 dpi), and latently infected and DEX-treated (6, 24, and 48 h) calves. Tissue samples were subjected to nucleic acid extractions and processed by routine histological methods or processed for electron microscopy.
Preparation of CD4+ and CD8+ lymphocytes from peripheral blood mononuclear cells.
Peripheral blood lymphocytes (PBL) from mock-infected calves and calves latently infected with BHV-1 at 1, 2, 5, 7, 9, 12, and 30 days after DEX treatment were prepared by density gradient centrifugation on Histopaque 1083 (Sigma Chemical Co.) as previously described (37).
Flow cytometry.
Indirect immunofluorescence analysis was performed according to standard techniques. PBL were incubated with primary monoclonal antibodies, mouse immunoglobulin G1 (IgG1) anti-bovine CD4 (MCA834; Serotec, Oxford, United Kingdom), mouse IgG2a anti-bovine CD8 (MCA837), and mouse IgG2a anti-bovine WC1 (MCA838) for 1 h at 4°C as described before (37).
In situ hybridization.
Double-stranded DNA probes specific for BHV-1 were synthesized and labeled by PCR with digoxigenin-dUTP (Boehringer Mannheim Corp., Indianapolis, Ind.). PCR conditions using ICP4, RR, and gC upstream and downstream primers have been previously described and are listed below (12, 30). The coding sequence for the BHV-1 gC glycoprotein was contained in plasmid pKS92-2 and was obtained from S. I. Chowdhury (Kansas State University). In situ hybridization was performed as described before in RNase-free conditions (37) using 0.1 ng of each probe/ml added to the prehybridization mixture.
Immunohistochemistry.
Tissues sections, deparaffinized and rehydrated in a graded ethanol series, as described above, were treated with 0.05% protease (protease XIV; Sigma) in Tris buffer (0.05 M Tris, pH 7.6) for 7 min at room temperature. Nonspecific binding was blocked by incubation with 5% normal swine serum (Sigma) for 45 min at room temperature. The monoclonal antibody directed against the BHV-1 gD glycoprotein (MM113) was obtained from S. Srikumaran (University of Nebraska, Lincoln). Immunohistochemistry was performed as previously described (34, 37).
Nucleic acid extractions.
DNA and RNA extraction from bovine tissues (mock-infected and BHV-1-infected calves) was performed as described previously (30, 37).
DNase I treatment, RT, and PCRs.
DNase I treatment, reverse transcription (RT), and PCR were done as described before using primers specific for BHV-1 genes (30). The IE gene tested was the bICP0 gene, and the primers were +TTCTCTGGGCTCGGGGCTGC and −AGAGGTCGAACAAACACCCGCGGT. The internal-hybridization primer for the bICP0 gene was CCGCAAGGGCGGCGCGCTAGC.
The E gene that was tested was the RR gene, and the primers were +GACGCCTGCTCGCTGCTATCC and −GCCTGTGTAGTTGGTGCTGCGGC. The internal-hybridization primer for the RR gene was TTTCCTTTGGCCCTGATGACTGCCGAG.
The L gene tested was the gC gene, and the primers were +GAGCAAAGCCCCGCCGAAGGA and −TACGAACAGCAGCACGGGCGG. The internal-hybridization primer for the gC gene was GAACCTGCCCACGCGCTGAAAC.
The nested primer pairs used to amplify a 276-bp external segment of LRT (nucleotides [nt] 1672 to 1693 and 1947 to 1924) were +CGCTCCCCTTCGTCCCTCCTCA and −AGAGGTCGACAAACACCCGCGGT. The internal primer pair for an 81-bp segment (nt 1755 to 1775 and 1835 to 1815) was +TTCTCTGGGCTCGGGGCTGC and −GACGAGACCCCCGATTGCCG (12). Nested PCR was performed with 5 μl of the reaction products from the first amplification using procedures previously described for RT-PCR. Amplified products were electrophoresed in a 2% agarose gel. The oligonucleotide that was used as a hybridization probe to detect the amplified LRT product spans nt 1781 to 1800 (5′-GCGCTTGCGCCGCGGGGGCT-3′). This oligonucleotide was labeled by incubation with [γ-32P]ATP and polynucleotide kinase. All oligonucleotides are listed in the 5′-to-3′ direction.
Southern blotting.
PCR products were electrophoresed in 2% agarose gels. The DNA in the gel was denatured, neutralized, and transferred to nylon membranes. Radioactive probes were prepared by incubating the respective oligonucleotides with [γ-32P]ATP and polynucleotide kinase. Probes, hybridization, and washing conditions were as previously described (30).
In situ detection of apoptosis.
Tissue sections, 4 to 5 μm thick, on Superfrost Plus slides were prepared as described previously (37). Apoptosis in tissue was examined by using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (Boehringer-Mannheim Corp.). Slides were counterstained with methyl green (Vector Laboratories), and a cover slip was added with Permount (Fisher).
Electron microscopy.
Lymphoid tissues from mock-infected and latently infected calves (48 h after DEX treatment) were cut into 1-mm3 cubes and fixed in 2% buffered glutaraldehyde. Samples were postfixed in 1% osmium tetroxide in phosphate buffer and were stained in-block with uranyl acetate. After dehydration, samples were embedded in Epon araldite, cut to thin sections (70 nm thick), stained with lead citrate and uranyl acetate, and then examined with a Phillips 210 microscope.
Statistical analyses.
Differences in CD4+, CD8+, and γ/δ lymphocyte percentages were analyzed by independent t test using a two-tailed P value, and a P value <0.05 was the criterion for statistical significance.
RESULTS
DEX-induced BHV-1 reactivation.
DEX induces reactivation of latent BHV-1 in cattle when multiple doses are administered by the intramuscular or intravenous route (15, 31, 32). A single intravenous dose will synchronously induce reactivation of latent BHV-1 in rabbits (23). We tested whether a single dose of DEX (0.4 mg/kg) administered intravenously induced reactivation in latently infected calves. All five latently infected calves had specific serum neutralizing antibody titers (1:32 to 1:64) against BHV-1. Virus was not recovered from nasal or ocular swabs and viral sequences were not amplified from swabs prior to DEX injection (Table 1). Thus, by standard criteria that are clinically used to define latency, these calves were latently infected. Reactivated virus was recovered from ocular and nasal swabs in 100% of the animals at 5 days posttreatment (Table 1). PCR results from swabs confirmed the virus isolation results. The BHV-1 genome was amplified at 5 and 7 days postreactivation in 100% of the swab specimens (Table 1). PCR-positive results were obtained at 9 days posttreatment for some samples and at 12 and 30 days posttreatment in nasal swabs from one calf (Table 1). Thus, a single dose of DEX was sufficient to consistently induce reactivation in latently infected calves.
TABLE 1.
PCR analysis from swabs of BHV-1-infected calves during latency and after DEX-induced reactivation
| Calf | Swab | Procedure | Presence of virus at latencya or indicated no. of dprb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latency | 1 | 2 | 5 | 7 | 9 | 12 | 30 | |||
| 34 | Nasal | Virus isolationc | − | − | − | + | + | − | − | − |
| PCRd | − | − | − | + | + | + | + | + | ||
| Ocular | Virus isolation | − | − | − | + | + | − | − | − | |
| PCR | − | − | − | + | + | + | − | − | ||
| 35 | Nasal | Virus isolation | − | − | − | + | − | − | − | − |
| PCR | − | − | − | + | + | − | − | − | ||
| Ocular | Virus isolation | − | − | − | + | − | − | − | − | |
| PCR | − | − | − | + | + | + | − | − | ||
| 36 | Nasal | Virus isolation | − | − | − | + | + | − | − | − |
| Ocular | Virus isolation | − | − | − | + | + | − | − | − | |
| 20 | Nasal | Virus isolation | − | − | − | + | − | − | − | − |
| Ocular | Virus isolation | − | − | − | + | − | − | − | − | |
| 29 | Nasal | Virus isolation | − | − | − | + | + | − | − | − |
| Ocular | Virus isolation | − | − | − | + | − | − | − | − | |
BHV-1-seronegative calves were inoculated with 107 50% tissue culture infective doses of BHV-1 per ml. Latently infected calves (60 dpi) were treated with 0.4 mg/kg of DEX intravenously. At the indicated times, swabs were collected and subjected to virus isolation and PCR.
dpr, days postreactivation.
Virus isolation was performed by inoculating MDBK cells. A sample was considered negative when characteristic CPE was not observed after three passages. Each passage was maintained for three consecutive days at 37°C in humidified 5% CO2 incubator.
DNA was isolated from swabs by alkaline extraction. PCR was performed using gC gene primers as described in Materials and Methods.
Detection of viral nucleic acid in tonsils of latently infected calves and after DEX-induced reactivation.
To determine if BHV-1 persists and can be reactivated in tonsillar tissue, six latently infected calves (60 dpi) and six mock-infected calves were injected with a single intravenous dose of DEX (0.4 mg/kg). A previous finding demonstrated that BHV-1 infects CD4+ cells in the tonsillar epithelia during acute infection of cattle (37), suggesting that this could be a site for persistence or latency. Mock-infected or latently infected calves were euthanized 6, 24, and 48 h after DEX treatment (two calves for each time), and TG and tonsils were collected. Two other latently infected calves were not treated with DEX. Consistent with the results in Table 1, infectious virus was not detected in nasal and ocular swabs from any latently infected calf prior to DEX treatment. Following enzymatic digestion of tonsils with collagenase and cocultivation with bovine kidney cells (MDBK) on collagen-treated plates, infectious BHV-1 was isolated from latently infected calves 48 h after DEX treatment.
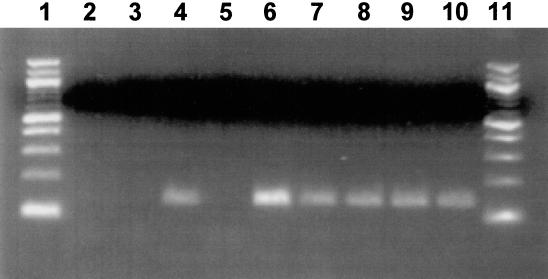
PCR was performed with RR gene-specific primers to determine if viral DNA was present in tonsils of latently infected calves. The expected product of 150 bp in tonsils from latently infected calves (Fig. 1, lane 7) and from latently infected calves that were treated with DEX for 6, 24, and 48 h was amplified (Fig. 1, lanes 8 to 10, respectively). No PCR product from the no-template control (lane 2), from mock-infected MDBK cells (lane 3), and from tonsillar tissue of a mock-infected calf (lane 5) was amplified. The two positive controls, MDBK cells infected for 18 h with BHV-1 (lane 4) and a tonsil from a BHV-1-infected calf at 7 dpi (lane 6), contained the 150-bp PCR product. Seven out of eight latently infected calves were positive for BHV-1 DNA by PCR, demonstrating that BHV-1 DNA was consistently detected in the tonsils of latently infected calves.
FIG. 1.
Detection of BHV-1 DNA in tonsils. Tonsils were collected from euthanized calves. DNA extraction and PCR conditions are described in Materials and Methods. Lanes: 1 and 11, molecular weight marker (100-bp DNA ladder); 2, no-template control; 3, mock-infected MDBK cells; 4, MDBK cells infected with BHV-1 for 18 h; 5, tonsil from a mock-infected calf; 6, tonsil from a BHV-1-infected calf (7 dpi); 7, tonsil from a latently infected calf (60 dpi); 8 to 10, tonsil from latently infected calves at 6, 24, and 48 h after DEX treatment, respectively. One microgram of DNA was used for each PCR except for the positive controls. The results are representative of three different infected calves at each time point.
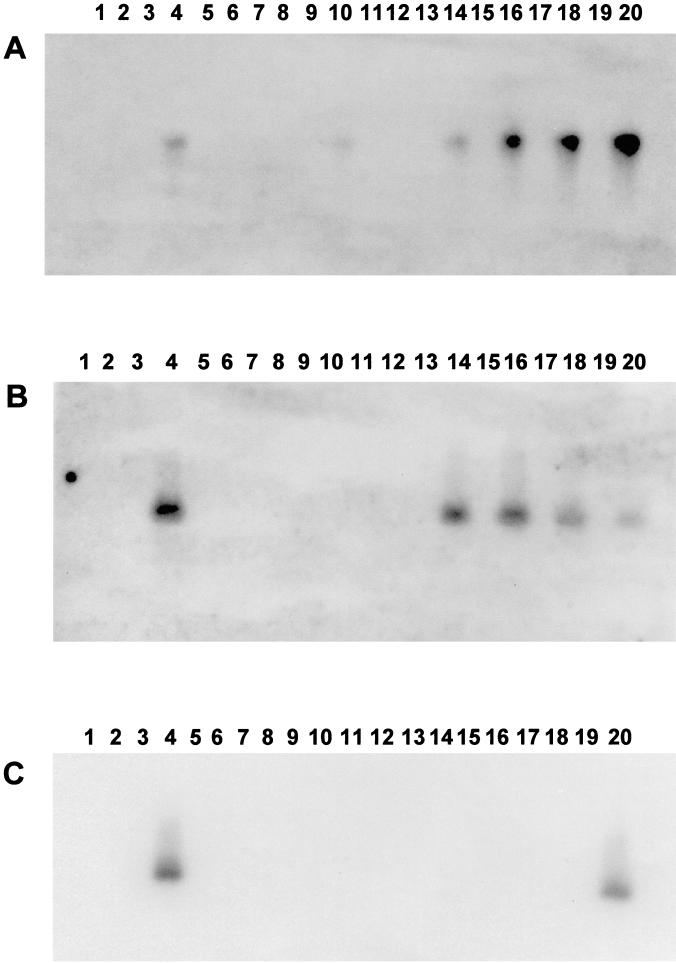
Viral gene expression in tonsillar tissue of latently infected calves was compared to that in tonsillar tissue of calves that were treated with DEX to initiate reactivation. RT-PCR was performed with primers that detect IE (bICP0), E (RR), and L (gC) transcripts. Viral gene expression in three calves was examined at each time point after DEX-induced reactivation, and Fig. 2 shows representative data for two calves at each time point. bICP0 cDNA in the tonsil of one calf at 6 h was amplified (Fig. 2A, lane 10), as was that in all three calves at 24 (Fig. 2A, lanes 14 and 16) and 48 h (Fig. 2A, lanes 18 and 20) after treatment. RR transcripts were detected in the tonsil after DEX treatment in two out of three calves at 24 h (Fig. 2B, lanes 14 and 16) and in three out of three calves at 48 h (Fig. 2B, lanes 18 and 20). gC RNA was detected in only one calf at 48 h after DEX treatment (Fig. 2C, lane 20). bICP0, RR, and gC transcripts were not detected in mock-infected (Fig. 2, lanes 2) or latently infected calves (Fig. 2, lanes 6 and 8). As expected, all three viral transcripts were detected in tonsil prepared from a calf infected with BHV-1 for 7 days (Fig. 2, lanes 4). When reverse transcriptase was omitted from RT reaction mixtures, amplified products were not detected in any positive sample (Fig. 2). Thus, bICP0 and RR expression were consistently detected in the tonsil at 24 and 48 h after DEX treatment but not in latently infected calves.
FIG. 2.
Viral gene expression in the tonsil after DEX treatment. RNA extraction, RT, and PCR were performed as described in Materials and Methods. cDNAs were PCR amplified with genes for bICP0 (A), RR (B), and gC (C) and the respective hybridization primers used to detect specific amplification products as described in Materials and Methods. Lanes: 1 and 2, tonsil from a mock-infected calf without and with reverse transcriptase, respectively; 3 and 4, tonsil from a BHV-1-infected calf (7 dpi) without and with reverse transcriptase, respectively; 5 and 6, tonsil from a latently infected calf (60 dpi) without and with reverse transcriptase, respectively; 7 and 8, tonsil from a latently infected calf (60 dpi) without and with reverse transcriptase, respectively; 9 and 10, tonsil from a latently infected calf at 6 h after DEX treatment without and with reverse transcriptase, respectively; 11 and 12, tonsil from a latently infected calf at 6 h after DEX treatment without and with reverse transcriptase, respectively; 13 and 14, tonsil from a latently infected calf at 24 h after DEX treatment without and with reverse transcriptase, respectively; 15 and 16, tonsil from a latently infected calf at 24 h after DEX treatment without and with reverse transcriptase, respectively; 17 and 18, tonsil from a latently infected calf at 48 h after DEX treatment without and with reverse transcriptase, respectively; 19 and 20, tonsil from a latently infected calf at 48 h after DEX treatment without and with reverse transcriptase, respectively.
Localization of viral nucleic acid in tonsil and TG by in situ hybridization.
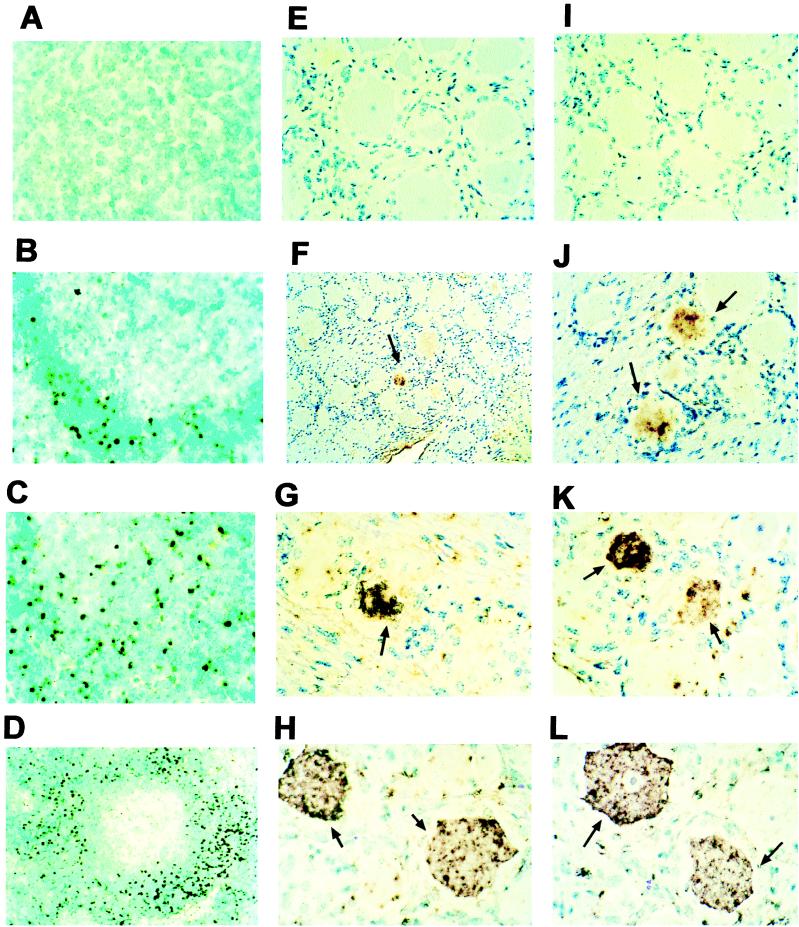
Digoxigenin-labeled ICP4, RR, and gC gene probes were used to localize viral nucleic acid in tonsillar tissues. Double-stranded DNA probes were prepared to detect viral mRNA and DNA and thus enhance the sensitivity of in situ hybridization. BHV-1 nucleic acid was not detected in tonsils from mock-infected calves at 6, 24, and 48 h after DEX treatment (data not shown). In general, the in situ hybridization signal of latently infected calves was similar to that of mock-infected calves (Fig. 3A). In a few sections, low levels of viral nucleic acid were detected in lymphoid follicles of latently infected calves. BHV-1 nucleic acid was consistently detected in lymphoid follicles at 6, 24, and 48 h after DEX treatment (Fig. 3B to D, respectively). The number and intensity of positive cells increased from 6 to 48 h after DEX injection, which is consistent with viral reactivation.
FIG. 3.
In situ hybridization of sections from tonsils and TG of infected calves. The ICP4, RR, and gC probes as well as in situ hybridization conditions are described in Materials and Methods. Shown are tonsil sections from a latently infected calf (A) and from latently infected calves at 6 (B), 24 (C), and 48 h (D) after DEX treatment; TG from a mock-infected calf (E); TG from a latently infected calf (60 dpi) (I); TG from latently infected calves at 6 h after DEX treatment (F and J); TG from latently infected calves at 24 h after DEX treatment (G and K); and TG from latently infected calves at 48 h after DEX treatment (H and L). The tissue sections were counterstained with methyl green. Arrows, viral nucleic acid-positive neurons.
To compare viral nucleic acid accumulation in tonsil during reactivation to that in TG, in situ hybridization was performed with TG samples at 6, 24, and 48 h after DEX-induced reactivation. BHV-1 nucleic acid was not detected in mock- and latently infected TG (Fig. 3E and I, respectively). A positive in situ hybridization signal was detected in TG neurons at 6 (F and J), 24 (G and K) and 48 h (H and L) after DEX treatment. The intensity of the positive signal and the number of positive neurons increased from 6 to 24 h after DEX treatment. The intensity and number of in situ hybridization-positive neurons were roughly equivalent after 24 and 48 h of DEX treatment. By RT-PCR, bICP0 expression was also detected in latently infected calves after DEX treatment (M. T. Winkler and C. Jones, unpublished data). Taken together, the experiments shown in Fig. 2 and 3 demonstrated that DEX treatment of latently infected calves led to increased levels of viral nucleic acids (RNA and DNA) in TG and tonsil.
LRT is expressed in tonsils during latency.
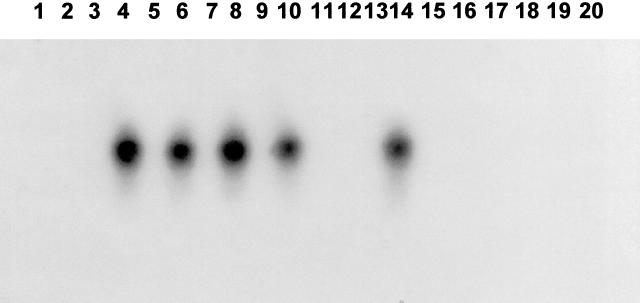
During latent infection of neurons, BHV-1 gene expression is restricted to LRT (reviewed in reference 13). To test whether LRT was expressed in tonsils of latently infected calves, total RNA was prepared from tonsils of latently infected calves and DEX-treated calves and LRT expression was assessed by nested RT-PCR. An 80-bp fragment was detected in three out of three tonsils from latently infected calves (Fig. 4, lanes 6 and 8), two out of three calves 6 h after DEX treatment (lane 10 and 12), and two out of three calves 24 h after DEX treatment (lanes 14 and 16). No product was amplified in two out of two tonsils 48 h after DEX treatment (lanes 18 and 20). When reverse transcriptase was omitted from RT reaction mixtures, amplified products were not detected in the positive samples. As expected, the LRT-specific band was not detected in mock-infected calves (lane 2). LRT was detected in total RNA from TG of a latently infected calf after the first PCR (lane 4). For the nested PCR, the TG sample was diluted 1:100 and 1 μl was added to the PCR mixture. LRT was not detected in tonsils unless nested PCR was performed (data not shown). Except for latently infected calves that were treated with DEX for 48 h, LRT was consistently detected in tonsils of latently infected calves.
FIG. 4.
Detection of LRT in tonsil of latently infected calves. Nested PCR was performed to detect LRT. RT-PCR, PCR conditions, and Southern blot analysis were as described in Materials and Methods. Lanes 1 and 2, mock-infected calves; lanes 3 and 4, TG of a latently infected calf (60 dpi); lanes 5 to 8, tonsils of latently infected animals; lanes 9 to 12, two different latently infected calves that were treated with DEX for 6 h; lanes 12 to 16, two different latently infected calves that were treated with DEX for 24 h. Lanes 17 to 20, two different latently infected calves that were treated with DEX for 48 h. The odd-numbered lanes were the results of nested PCR without reverse transcriptase, and the even-numbered lanes are the results of PCR with reverse transcriptase.
DEX-induced reactivation induces apoptosis in the tonsil.
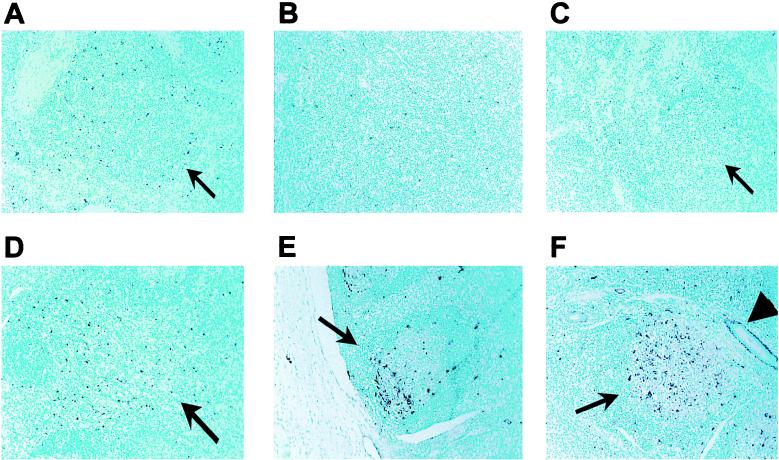
To test whether BHV-1 reactivation led to apoptosis in tonsils, TUNEL assays were performed with tissue sections. A previous study demonstrated that BHV-1 induces apoptosis in lymphoid tissues during acute infection (37). Since DEX can induce apoptosis in several different cell types, it was reasonable to hypothesize that DEX-induced reactivation leads to apoptosis in tonsils. Tonsillar tissue contained many TUNEL-positive cells at 24 and 48 h after DEX treatment of latently infected calves (Fig. 5E and F). TUNEL-positive cells were predominantly localized to the corticomedullary junction in germinal centers of lymphoid follicles (Fig. 5E). At 48 h after treatment, TUNEL-positive cells were detected in the entire lymphoid follicle (Fig. 5F). Furthermore, TUNEL-positive cells were detected in tonsillar crypts 48 h after DEX treatment when these crypts were adjacent to follicles. Only a few TUNEL-positive cells were observed in mock-infected calves treated with DEX for 6, 24, or 48 h (Fig. 5A to C, respectively). Latently infected calves treated with DEX for 6 h also contained low levels of TUNEL-positive cells (Fig. 5D). Prior to DEX treatment, tonsil from latently infected calves contained little or no TUNEL-positive cells (M. T. Winkler and C. Jones, unpublished data). Taken together, these data suggested that viral reactivation and DEX were necessary for extensive apoptosis in tonsil.
FIG. 5.
TUNEL analysis of tonsils from latently infected calves after DEX treatment. Thin sections were prepared, and TUNEL assays were performed. (A to C) Tonsils from mock-infected calves at 6, 24, and 48 h after DEX treatment, respectively. (D to F) Tonsils from calves latently infected with BHV-1 (60 dpi) at 6, 24, and 48 h after DEX treatment, respectively. Tissue was counterstained with methyl green. Dark purple, TUNEL-positive cells; arrows, positions of lymphoid follicles; arrowhead (F), tonsillar crypt that is bound by the epithelium.
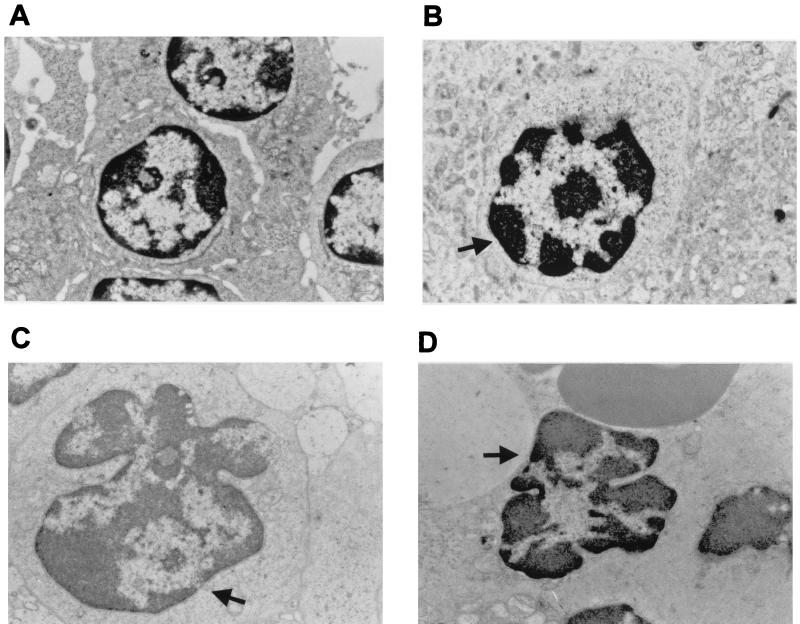
Electron microscopy was performed with tonsil from latently infected calves at 48 h after treatment. Cells located in the lymphoid follicles contained condensed chromatin and apoptotic bodies shaped like kidney beans or half-moons (Fig. 6B to D), which are morphological hallmarks of apoptosis. The size and morphology of the apoptotic cells suggested that they were lymphocytes. In sharp contrast, lymphocytes within a tonsillar follicle of mock-infected calves did not contain condensed chromatin or apoptotic bodies. Thus, the data in Fig. 5 and 6 demonstrate that apoptosis occurs in the tonsils of latently infected calves treated with DEX.
FIG. 6.
Electron microscopy of tonsils from mock-infected calves and calves latently infected with BHV-1 at 48 h after DEX treatment. Calves were euthanized, and tonsils were collected, fixed in 2% buffered glutaraldehyde, and embedded in Epon araldite. Thin sections were stained with lead citrate and uranyl acetate and then examined with a Phillips 210 microscope. (A) Tonsil from a mock-infected calf. Magnification, ×13,300. (B to D) Tonsils from calves latently infected with BHV-1 at 48 h after DEX treatment. Magnification, ×19,000. Arrows, prominent apoptotic bodies and condensed chromatin in apoptotic lymphocytes.
A transient decrease in T cells in blood during acute infection of cattle was observed (37). In large part, this was due to increased apoptosis. Thus, it was of interest to test whether DEX-induced reactivation led to decreased levels of CD4+, CD8+, and γ/δ T-lymphocyte populations in blood. After DEX treatment, CD4+ and CD8+ lymphocyte populations in PBL were not significantly different from those in latently infected calves (P > 0.05) (Table 2). A selective depletion of the T-lymphocyte subpopulation that expresses the γ/δ form of the T-cell receptor was observed at 1 and 2 days after DEX treatment (P < 0.05). The levels of γ/δ T cells remained lower than the normal levels even at 30 days posttreatment. A reduction in circulating T lymphocytes following DEX treatment has been observed in γ/δ but not in α/β T cells (7, 21). In contrast to results for acute infection, we did not see a transient decrease in T cells (CD4+ and CD8+) following DEX treatment of latently infected calves. The significance of the selective depletion of γ/δ T cells after DEX treatment for viral reactivation was not clear.
TABLE 2.
Comparison of CD4+ and CD8+ T lymphocytes during latency and after DEX-induced reactivation
| T cells | Calf | Mock-infected | % of total T lymphocytes in mock-infected calves or in calves at latencya or at indicated no. of dprc
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latency | 1 | 2 | 5 | 7 | 9 | 12 | 30 | |||
| CD4 | 34 | 22 | 22.3 | 26.1 | 24.5 | 27.7 | 28.7 | 24.9 | 27.1 | 21.5 |
| 35 | 18.5 | 18.9 | 23.6 | 27.2 | 23.3 | 21.8 | 27.6 | 28.8 | 27.8 | |
| CD8 | 34 | 14.2 | 20.5 | 13.8 | 14.7 | 19 | 16.6 | 26.4 | 27.1 | 33 |
| 35 | 13.8 | 13.3 | 22 | 12.8 | 12.6 | 8.7 | 15.8 | 14.3 | 21.1 | |
| γ/δ | 34 | 23.4 | 13.3b | 2.0b | 4.6b | 8.9b | 5.9b | 6.3b | 7.5b | 8.3b |
| 35 | 20.6 | 19.3 | 3.2b | 6.5b | 16.2 | 14.6 | 14.3 | 13.3 | 15.6 | |
BHV-1-seronegative calves were inoculated with 107 50% tissue culture infective doses of BHV-1 per ml. Latently infected calves (60 dpi) were treated with 0.4 mg/kg of DEX intravenously. At the indicated times, CD4+, CD8+, and γ/δ T lymphocytes were prepared from PBL after density gradient centrifugation on Histopaque 1083. Mononuclear cells were incubated with monoclonal antibodies against bovine CD4, CD8, or WC1. A secondary antibody conjugated to fluorescein isothiocyanate was then incubated with the samples, and flow cytometry was performed.
Significant difference (P < 0.05; independent t test) compared with mock-infected calves.
dpr, days postreactivation.
DISCUSSION
In this study, we demonstrated that a single intravenous injection of DEX led to reactivation of latent BHV-1 in calves, which is consistent with the rabbit model for BHV-1 (24). We further demonstrated that viral DNA persists in the tonsil and can be reactivated. Following DEX treatment, extensive apoptosis occurred in the tonsils of latently infected calves but not uninfected calves. The ability of BHV-1 to persist and reactivate in tonsil may be important for virus transmission.
BHV-1 DNA was not detected in TG of latently infected calves by in situ hybridization, which is consistent with other studies (4, 15, 23, 26). Thus, it was expected that in situ hybridization would not detect viral DNA in tonsils of latently infected calves. However, BHV-1 DNA was consistently detected in tonsils by PCR at 60 dpi. Because in situ hybridization localized viral nucleic acid in germinal centers of tonsil during reactivation (Fig. 3), it is hypothesized that viral DNA is present in lymphoid cells. This hypothesis is supported by the recent finding that BHV-1 DNA is detected in peripheral blood cells for long periods of time after infection (11). Additional studies will be necessary to determine what cell type BHV-1 DNA is harbored in and whether the DNA is circular, as in latently infected neurons. A nonneuronal site of latency has been proposed for tonsil following infection of swine with PRV. PRV DNA was consistently detected in some studies (5, 9, 35, 36) but not others (3, 18). Nonneural sites for latency or persistence have also been described for HSV-1 (8, 17, 29) and may be important for ocular disease (17). Thus, nonneural sites of latency or persistence may be a normal outcome of acute infection by certain α-herpesviruses.
Although this study demonstrated that LRT was present in tonsils of latently infected calves, nested PCR was required for detection (Fig. 4). LRT is readily detected in TG of latently infected calves without the use of nested PCR (10, 12, 25, 30). This suggested that LRT is not an abundant transcript in tonsils of latently infected calves or that only a minor population of cells was persistently or latently infected. It is also possible that other viral transcripts, but not LRT, are abundantly expressed in tonsils of latently infected calves. Forty-eight hours after DEX treatment, LRT was not detected in tonsil (Fig. 5). This is consistent with a previous study that concluded that the number of LRT-positive trigeminal ganglionic neurons decreased when latently infected rabbits were injected with DEX (23). DEX also represses the LRT promoter activity in transiently transfected bovine cells (14). Thus, repression of LRT by DEX in tonsils and TG correlates with reactivation. Since LRT is alternatively spliced in TG (10, 12), it will be interesting to determine if there are novel species expressed in tonsil and if other regions of the viral genome are expressed. Detecting LRT in tonsils of latently infected calves, but not bICP0 and RR, does not necessarily mean these cells were latently infected because productively infected cells express LRT (reviewed in reference 13). Furthermore, nested RT-PCR was not used to detect bICP0 and RR, and thus the assay may not have detected low levels of these transcripts if they were expressed in only a minor population of cells.
Following DEX treatment, viral nucleic acid in neurons and tonsils was readily detectable by in situ hybridization. As soon as 6 h after DEX treatment, a few viral nucleic acid-containing neurons were detected. A similar effect was observed in tonsils of latently infected calves. At 24 and 48 h after DEX treatment, the numbers of virus-positive cells in TG and tonsils were much higher. In latently infected rabbits, in situ hybridization detected extensive viral transcription 15 to 18 h after DEX treatment (23). The number of hybridizing neurons in reactivating TG appeared to be higher than that in tonsils. Virus-positive neurons were found in clusters distributed throughout TG, but hybridizing cells in the tonsil did not appear to be abundant. However, we could not detect viral transcription by in situ hybridization or RT-PCR in TG before it was detected in tonsils during reactivation. Taken together, the data from this study suggested that DEX induced viral transcription and reactivation of latent or persistent genomes in tonsil. It is also possible that virus reactivation in TG contributed to virus infection and gene expression in the tonsil at late times after reactivation.
This study demonstrated that long-term persistence (chronic infection) or latency occurred in lymphoid follicles. BHV-1 DNA is also present in PBL of latently infected calves (11; M. T. Winkler and C. Jones, unpublished results), demonstrating that viral DNA is retained in lymphoid cells long after acute infection. Although we cannot completely rule out a chronic or persistent infection, we favor the possibility that latency occurs in tonsillar lymphoid cells and probably blood. This hypothesis is supported by several findings. First, viral nucleic acids were not detected in tonsils by in situ hybridization until after DEX-induced reactivation. Since viral nucleic acid was detected in the tonsil and TG at 6 h after DEX treatment, it is unlikely that reactivation occurred in TG and then spread to the tonsil. Second, infectious virus was only detected in tonsils of calves that were treated with DEX (M. T. Winkler and C. Jones, unpublished results), making it unlikely that virus is being shed during latency. Recent studies have demonstrated that spontaneous reactivation leads to higher levels of HSV-1-neutralizing antibodies in rabbits (22) and BHV-1-neutralizing antibodies in cattle after DEX-induced reactivation (15). The animals that were infected in this study seroconverted, but neutralizing antibody levels were stable during latency, suggesting that spontaneous shedding was not occurring. Calves infected with BHV-1 and housed under strict isolation, as described in this study, are not prone to spontaneous reactivation unless treated with DEX. Although lymphoid cells and sensory neurons have very different phenotypes, they are highly differentiated cells that may lack factors necessary for productive infection but that have the potential to establish latency. The lack of permissive transcription factors or the presence of cellular factors that repress viral transcription is believed to be a significant factor during establishment of latency (reviewed in reference 13). Developing a better understanding of virus-host interactions in lymphoid cells will help us understand the mechanism of viral pathogenesis, immunosuppression, and virus transmission.
ACKNOWLEDGMENTS
This research was supported by grants from the USDA (9702394 and 9802064) and the Center for Biotechnology, UN-L.
We thank F. Osorio and C. Wood for critically reviewing the manuscript. We acknowledge K. Arumuganathan (Center for Biotechnology, University of Nebraska, Lincoln) for assistance with flow cytometry and S. I. Chowdhury (Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University) for the gC plasmid. We are grateful to T. Bargar for assistance with electron microscopy, S. Srikumaran for the monoclonal antibody directed against gD, and T. Holt for technical assistance. We thank J.-H. Sur (Plum Island Animal Disease Center, African Swine Fever Virus Research Group) for the ISH protocol. We also thank B. Clowser, M. Klintworth, J. Wilkinson, and T. Green for assistance with cattle experiments. Finally, we thank R. Olmscheid and V. Johns for assistance with histological preparations.
REFERENCES
- 1.Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications of the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- 2.Balasch M, Pujols J, Segales J, Plana-Duran J, Pumarola M. Study of the persistence of Aujeszky's disease (pseudorabies) virus in peripheral blood mononuclear cells and tissues of experimentally infected pigs. Vet Microbiol. 1998;62:171–183. doi: 10.1016/s0378-1135(98)00208-9. [DOI] [PubMed] [Google Scholar]
- 3.Brockmeier S L, Lager K M, Mengeling W L. Comparison of in vivo reactivation, in vitro reactivation and polymerase chain reaction for detection of latent pseudorabies virus infection in swine. J Vet Diagn Investig. 1993;5:505–509. doi: 10.1177/104063879300500401. [DOI] [PubMed] [Google Scholar]
- 4.Brown T M, Osorio F A, Rock D L. Detection of latent pseudorabies virus in swine using in situ hybridization. Vet Microbiol. 1990;24:273–280. doi: 10.1016/0378-1135(90)90177-w. [DOI] [PubMed] [Google Scholar]
- 5.Brown T T, Shin K O, Fuller F J. Detection of pseudorabies viral DNA in tonsillar epithelial cells of latently infected pigs. Am J Vet Res. 1995;56:587–594. [PubMed] [Google Scholar]
- 6.Burr P D, Campbell M E, Nicolson L, Onions D E. Detection of canine herpesvirus 1 in a wide range of tissues using the polymerase chain reaction. Vet Microbiol. 1996;53:227–237. doi: 10.1016/s0378-1135(96)01227-8. [DOI] [PubMed] [Google Scholar]
- 7.Burton J L, Kehrli M E., Jr Effects of dexamethasone on bovine circulating T lymphocyte populations. J Leukoc Biol. 1996;59:90–99. doi: 10.1002/jlb.59.1.90. [DOI] [PubMed] [Google Scholar]
- 8.Cantin E, Chen J, Gaidulis L, Valo Z, McLaughlin-Taylor E. Detection of herpes simplex virus DNA sequences in human blood and bone marrow cells. J Med Virol. 1994;42:279–286. doi: 10.1002/jmv.1890420315. [DOI] [PubMed] [Google Scholar]
- 9.Cheung A K. Investigation of pseudorabies virus DNA and RNA in trigeminal ganglia and tonsil tissues of latently infected swine. Am J Vet Res. 1995;56:45–50. [PubMed] [Google Scholar]
- 10.Devireddy L R, Jones C. Alternative splicing of the latency-related transcript of bovine herpesvirus type 1 yields RNAs containing unique open reading frames. J Virol. 1998;72:7294–7301. doi: 10.1128/jvi.72.9.7294-7301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs M, Hubert P, Detterer J, Rzia H-J. Detection of bovine herpesvirus type 1 in blood from naturally infected cattle by using a sensitive PCR that discriminates between wild-type virus and virus lacking glycoprotein E. J Clin Microbiol. 1999;37:2498–2507. doi: 10.1128/jcm.37.8.2498-2507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain A, Schang L, Jones C. Identification of gene products encoded by the latency-related gene of bovine herpesvirus type 1. J Virol. 1995;69:5345–5352. doi: 10.1128/jvi.69.9.5345-5352.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones C. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv Virus Res. 1998;51:81–133. doi: 10.1016/s0065-3527(08)60784-8. [DOI] [PubMed] [Google Scholar]
- 14.Jones C, Delhon G, Bratanich A, Kutish G, Rock D. Analysis of the transcriptional promoter which regulates the latency-related transcript of bovine herpesvirus 1. J Virol. 1990;64:1164–1170. doi: 10.1128/jvi.64.3.1164-1170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, C., T. J. Newby, T. Holt, A. Doster, M. Stone, J. Ciacci-Zanella, C. J. Webster, and M. W. Jackwood. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine, in press. [DOI] [PubMed]
- 16.Lokensgard J R, Thawley D G, Molitor T W. Pseudorabies virus latency: restricted transcription. Arch Virol. 1990;110:129–136. doi: 10.1007/BF01310709. [DOI] [PubMed] [Google Scholar]
- 17.Maggs D J, Chang E, Naisse M P, Mitchell W J. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J Virol. 1998;72:9166–9172. doi: 10.1128/jvi.72.11.9166-9172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mengeling W L, Lager K M, Voltz D M, Brockmeier S L. Effect of various vaccination procedures on shedding, latency, and reactivation of attenuated and virulent pseudorabies virus in swine. Am J Vet Res. 1992;53:2164–2173. [PubMed] [Google Scholar]
- 19.Narita M, Inui S, Nanba K, Shimizu Y. Recrudescence of infectious bovine rhinotracheitis virus and associated neural changes in calves treated with dexamethasone. Am J Vet Res. 1981;42:1192–1197. [PubMed] [Google Scholar]
- 20.Okuda Y, Ishida K, Hashimoto A, Yamaguchi T, Fukushi H, Hirai K, Carmichael L E. Virus reactivation in bitches with a medical history of herpesvirus infection. Am J Vet Res. 1993;54:551–554. [PubMed] [Google Scholar]
- 21.Oldham G, Howard C J. Suppression of bovine lymphocyte responses to mitogens following in vivo and in vitro treatment with dexamethasone. Vet Immunol Immunopathol. 1992;30:161–177. doi: 10.1016/0165-2427(92)90136-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perng G C, Slanina S M, Yuhkt A, Ghiasi H, Nesburn A B, Wechsler S L. Herpes simplex virus type 1 serum neutralizing antibody titers increase during latency in rabbits latently infected with latency-associated transcript (LAT)-positive but not LAT-negative viruses. J Virol. 1999;73:9669–9672. doi: 10.1128/jvi.73.11.9669-9672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock D, Lokensgard J, Lewis T, Kutish G. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J Virol. 1992;66:2484–2490. doi: 10.1128/jvi.66.4.2484-2490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rock D L. Latent infection with bovine herpesvirus type 1. Semin Virol. 1993;5:233–240. [Google Scholar]
- 25.Rock D L, Beam S L, Mayfield J E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987;61:3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rock D L, Reed D E. Persistent infection with bovine herpesvirus type 1: rabbit model. Infect Immun. 1982;35:371–373. doi: 10.1128/iai.35.1.371-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudbeck L, Dissing J. Rapid, simple alkaline extraction of human genomic DNA from whole blood, buccal epithelial cells, semen and forensic stains for PCR. BioTechniques. 1998;25:588–592. doi: 10.2144/98254bm09. [DOI] [PubMed] [Google Scholar]
- 28.Schuh J C L, Bielefeldt Ohmann H, Babiuk L A, Doige C E. Bovine herpesvirus-1-induced pharyngeal tonsil lesions in neonatal and weaning calves. J Comp Pathol. 1992;106:243–253. doi: 10.1016/0021-9975(92)90053-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scriba M. Extraneural localization of herpes simplex virus in latently infected guinea pigs. Nature. 1977;267:529–531. doi: 10.1038/267529a0. [DOI] [PubMed] [Google Scholar]
- 30.Shang L M, Jones C. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J Virol. 1997;71:6786–6795. doi: 10.1128/jvi.71.9.6786-6795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheffy B E, Rodman S. Activation of latent infectious bovine rhinotracheitis infection. J Am Vet Med Assoc. 1973;163:850–851. [Google Scholar]
- 32.Sheffy B E, Davies D H. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc Soc Exp Biol Med. 1972;140:974–976. doi: 10.3181/00379727-140-36592. [DOI] [PubMed] [Google Scholar]
- 33.Spear A. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 34.Stroop W G, Rock D L, Fraser N W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Investig. 1984;51:27–38. [PubMed] [Google Scholar]
- 35.Wheeler J G, Osorio F A. Investigation of sites of pseudorabies virus latency, using polymerase chain reaction. Am J Vet Res. 1991;52:1799–1803. [PubMed] [Google Scholar]
- 36.White A K, Ciacci-Zanella J, Galeota J, Ele S, Osorio F A. Comparison of the abilities of serologic tests to detect pseudorabies-infected pigs during the latent phase of infection. Am J Vet Res. 1996;57:608–611. [PubMed] [Google Scholar]
- 37.Winkler M T C, Doster A, Jones C. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. J Virol. 1999;73:8657–8668. doi: 10.1128/jvi.73.10.8657-8668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyler R, Engels M, Schwyzer M. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1) In: Witman G, editor. Herpesviruses diseases of cattle, horses, and pigs, developments in veterinary medicine. Boston, Mass: Kluver Academic Publishers; 1989. p. 172. [Google Scholar]