Abstract
Rationale
Nicotine cessation is associated with increased consumption of highly palatable foods and body weight gain in most smokers. Concerns about body weight gain are a major barrier to maintaining long-term smoking abstinence, and current treatments for nicotine use disorder (NUD) delay, but do not prevent, body weight gain during abstinence. Glucagon-like peptide-1 receptor (GLP-1R) agonists reduce food intake and are FDA-approved for treating obesity. However, the effects of GLP-1R agonist monotherapy on nicotine seeking and withdrawal-induced hyperphagia are unknown.
Objectives
We screened the efficacy of the long-lasting GLP-1R agonist liraglutide to reduce nicotine-mediated behaviors including voluntary nicotine taking, as well as nicotine seeking and hyperphagia during withdrawal.
Methods
Male and female rats self-administered intravenous nicotine (0.03 mg/kg/inf) for ~21 days. Daily liraglutide administration (25 μg/kg, i.p.) started on the last self-administration day and continued throughout the extinction and reinstatement phases of the experiment. Once nicotine taking was extinguished, the reinstatement of nicotine-seeking behavior was assessed after an acute priming injection of nicotine (0.2 mg/kg, s.c.) and re-exposure to conditioned light cues. Using a novel model of nicotine withdrawal-induced hyperphagia, intake of a high fat diet (HFD) was measured during home cage abstinence in male and female rats with a history of nicotine self-administration.
Results
Liraglutide attenuated nicotine self-administration and reinstatement in male and female rats. Repeated liraglutide attenuated withdrawal-induced hyperphagia and body weight gain in male and female rats at a dose that was not associated with malaise-like effects.
Conclusions
These findings support further studies investigating the translational potential of GLP-1R agonists to treat NUD.
Keywords: Nicotine, glucagon-like peptide-1, body weight, relapse, food intake, withdrawal, relapse, tobacco, obesity
Introduction
Cigarette smoking is the leading cause of preventable disease, disability, and death in the United States (CDC 2020). While smoking cessation at any age significantly increases life expectancy (Taylor et al. 2002), fewer than 1 in 10 smokers who attempt to quit smoking are successful (Babb et al. 2017). Smoking cessation increases consumption of highly palatable foods, and 80–90% of smokers who quit gain weight (Bush et al. 2016; Chao et al. 2019; Perkins et al. 1990; Spring et al. 2003; Stamford et al. 1986; Williamson et al. 1991). Specifically, most smokers who quit gain an average of 5–15 pounds in the first few months of abstinence, while some smokers (13–14%) gain more than 20 pounds (Bush et al. 2016). Concerns about weight gain are a major barrier to achieving long-term smoking abstinence for both men and women (Audrain-McGovern and Benowitz 2011; Klesges et al. 1988; Meyers et al. 1997). Treatments for nicotine use disorder (NUD) like nicotine replacement therapy (NRT), varenicline, and bupropion decrease relapse to nicotine use. However, their long-term efficacy is modest, and these treatments delay, but do not prevent, body weight gain during smoking abstinence (Audrain-McGovern and Benowitz 2011; Bush et al. 2016; Farley et al. 2012; Mills et al. 2012). Therefore, it is critical to identify novel pharmacotherapies that promote sustained smoking abstinence while minimizing post-cessation increases in caloric intake and subsequent body weight gain (Audrain-McGovern and Benowitz 2011; Bush et al. 2016).
Glucagon-like peptide-1 receptor (GLP-1R) agonists reduce food intake in laboratory animals and humans and are FDA-approved for weight loss (Knudsen 2010; Vilsbøll et al. 2007; Wilding et al. 2021). Systemic administration of GLP-1R agonists also decreases the rewarding and reinforcing effects of licit and illicit drugs (Douton et al. 2022; Douton et al. 2020; Egecioglu et al. 2013; Evans et al. 2022; Hernandez and Schmidt 2019; Jerlhag 2019; Merkel et al. 2021; Zhang et al. 2020; Zhang et al. 2021). With regard to nicotine, GLP-1R agonists attenuate nicotine self-administration, nicotine-induced conditioned place preference (CPP), and nicotine-evoked dopamine release in the nucleus accumbens of rodents, suggesting that these medications could be re-purposed for treating NUD (Egecioglu et al. 2013; Tuesta et al. 2017). Consistent with these results, a recent clinical study showed that concurrent administration of a GLP-1R agonist and NRT increased abstinence rates and reduced body weight gain versus NRT alone in human smokers (Yammine et al. 2021). While these findings support GLP-1R agonists as adjunct treatments for NUD, no studies to our knowledge have investigated the efficacy of GLP-1R agonist monotherapy alone to reduce the reinstatement of nicotine-seeking behavior, an animal model of relapse, and prevent nicotine withdrawal-induced hyperphagia and body weight gain (Schmidt et al. 2018).
The first goal of this study was to determine the efficacy of the long-acting GLP-1R agonist liraglutide to attenuate nicotine taking and seeking in male and female rats. To investigate the effects of a GLP-1R agonist on withdrawal phenotypes, we established a novel rodent model of withdrawal-induced hyperphagia and body weight gain following twice daily nicotine self-administration. The second goal of this study was to determine if repeated daily administration of liraglutide during abstinence prevented withdrawal-induced hyperphagia and body weight gain in male and female rats. Our results indicate that GLP-1R agonist monotherapy alone is sufficient to reduce nicotine taking and seeking, as well as nicotine withdrawal-induced hyperphagia and body weight gain in rats.
Materials and methods
Drugs
(−)Nicotine hydrogen tartrate salt (Sigma Aldrich, St. Louis, MO, USA) was dissolved in sterile 0.9% saline (pH was adjusted to 7.4±0.5 with sodium hydroxide). Nicotine doses are reported as freebase concentrations and are based on our previously published nicotine self-administration studies in rats (Ashare et al. 2017; Hopkins et al. 2012; Kimmey et al. 2012). Liraglutide (Abcam, Boston, MA, USA) was dissolved in Tris-buffered 0.9% saline with Tween 80 (pH=8.0). The dose of liraglutide administered and time course of treatment were based on our previous rat studies (Hayes et al. 2011; Liberini et al. 2019).
Animals and housing
Male (n=63) and female (n=58) Sprague-Dawley rats (Rattus norvegicus) weighing 225–250 g were obtained from Taconic Laboratories (Germantown NY, USA). Rats were housed individually on a 12 h/12 h reverse light-dark cycle with water available ad libitum. All rats were mildly food restricted (~25 g chow daily) to ~90–95% of their free-feeding body weight following recovery from surgery. Mild food restriction was used to facilitate acquisition and maintenance of nicotine self-administration similar to previously published studies from our laboratory and others (Ashare et al. 2017; Corrigall and Coen 1989; Fowler and Kenny 2011; Hopkins et al. 2012; Kimmey et al. 2014). All behavioral tests were conducted during the dark phase. All experimental protocols were consistent with the guidelines issued by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Catheter surgery
Drug-naïve rats were handled daily and allowed one week to acclimate to their home cages upon arrival. Prior to surgery, rats were anesthetized with 80 mg/kg ketamine (Midwest Veterinary Supply, Valley Forge, PA, USA) and 12 mg/kg xylazine (Sigma Aldrich, St. Louis, MO, USA). An indwelling silastic catheter (SAI Infusion Technologies, Lake Villa, IL, USA) was inserted into the right external jugular vein, sutured securely in place, and connected to a mesh back-mount that was implanted subcutaneously above the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.2 ml of a solution of the antibiotic Timentin (0.93 mg/ml, Spectrum Chemical, New Brunswick, NJ, USA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH, USA). When not in use, catheters were sealed with plastic obturators. Rats were allowed a minimum of seven days to recover from surgery before behavioral testing commenced.
Nicotine self-administration
Nicotine self-administration was performed as described previously (Ashare et al. 2017; Hopkins et al. 2012; Kimmey et al. 2014; Lee et al. 2014; Maurer et al. 2017; Maurer et al. 2022). Briefly, drug-naïve rats were placed in operant conditioning chambers (Med-Associates Inc., East Fair-field, VT, USA) and allowed to lever press for intravenous infusions of nicotine (0.03 mg/kg nicotine/59 μl saline, infused over 5 s) on a fixed-ratio 1 (FR1) schedule of reinforcement. Each nicotine infusion was paired with a contingent 10 s light cue that was illuminated directly above the active lever (i.e., drug-paired lever). Each infusion was followed by a 20 s timeout period, during which the chamber light was turned off and active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded, and used as a measure of nonspecific behavioral activity. All self-administration sessions were two hours in duration.
Experiment 1: Determine the effects of liraglutide on nicotine taking and seeking in male and female rats
Male (n=18) and female (n=24) rats acquired nicotine self-administration as described above. Nicotine self-administration sessions were 2-h in duration and were conducted once daily five days per week (Mon-Fri). Once a rat achieved >15 infusions of nicotine during three consecutive self-administration sessions on the FR1 schedule, the rat was switched subsequently to a fixed-ratio 3 (FR3) schedule of reinforcement. Rats self-administered nicotine for a total of 19–22 days (Fig. 1A). Rats were then randomly divided into two groups that self-administered equivalent nicotine infusions, and a between-subjects design was used to screen the efficacy of liraglutide to reduce nicotine consumption. Rats were pretreated with vehicle or liraglutide (25 μg/kg, i.p.) 10 min prior to the beginning of their last self-administration test session.
Fig. 1.
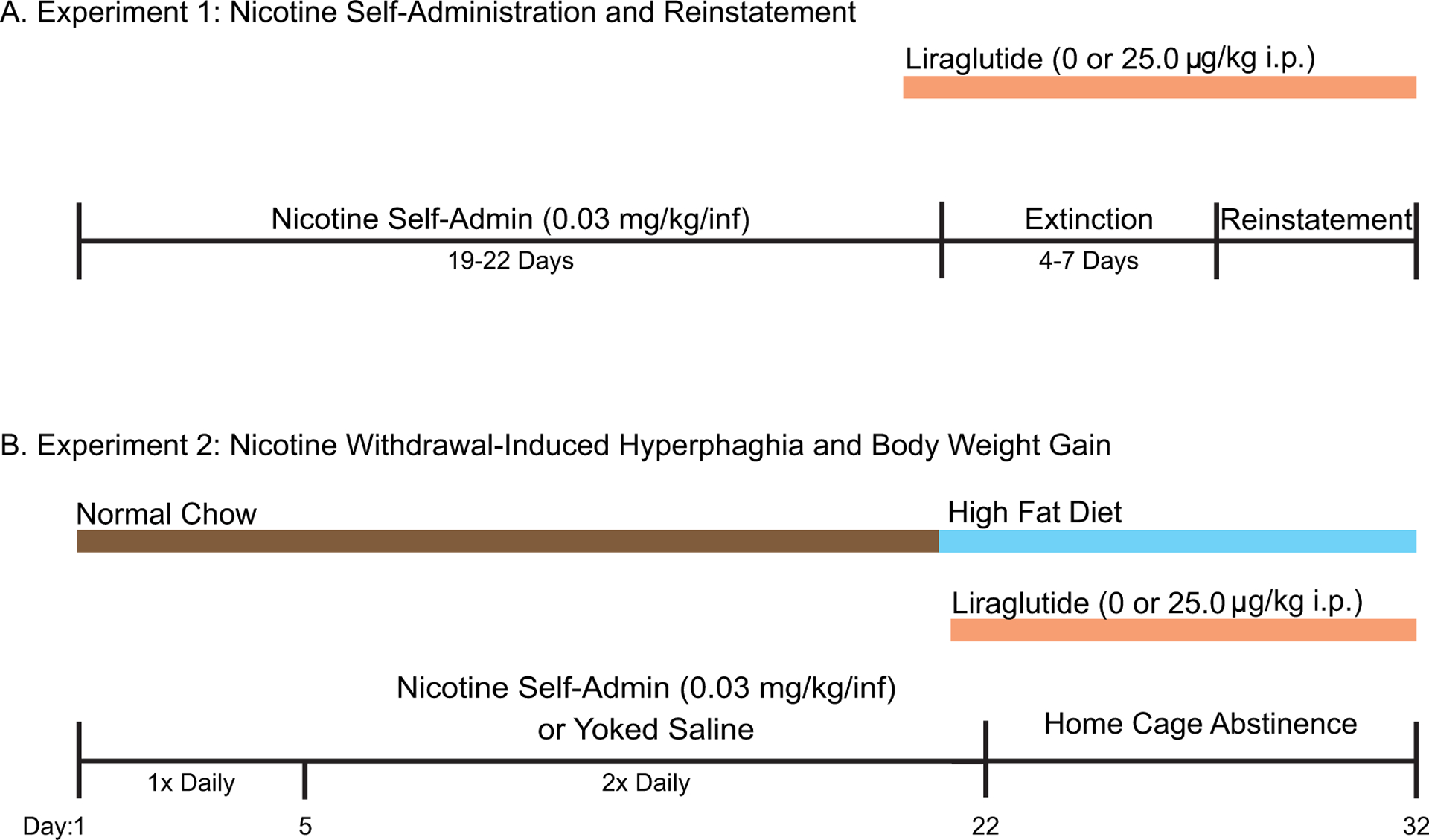
Experimental Timeline. (A) Experiment 1: Rats self-administered nicotine in 2-hour sessions for 19–22 days. Nicotine-taking behavior was then extinguished by replacing the nicotine solution with a saline solution. Reinstatement of nicotine-seeking behavior was then assessed. Rats were pretreated daily with vehicle or liraglutide prior to their last nicotine self-administration session and subsequent extinction and reinstatement sessions. (B) Experiment 2: Rats self-administered nicotine or received yoked saline injections for 22 days during two daily 2-h operant sessions. Rats were pretreated with vehicle or liraglutide prior to the last nicotine self-administration session. Treatments continued daily throughout the subsequent withdrawal period. Rats experienced 10 consecutive days of withdrawal in their home cages.
Rats continued to receive daily treatments of vehicle or liraglutide (25 μg/kg, i.p.) as they proceeded through the extinction and reinstatement phases of the experiment (Fig. 1A). After the effects of acute liraglutide were tested on nicotine self-administration, drug taking was extinguished by replacing the nicotine solution with saline and turning off the drug-paired cue light. Daily extinction sessions continued until responding on the active lever was <25% of the total active lever responses completed on the second to last day of nicotine self-administration (i.e., the day prior to investigating the acute effects of liraglutide pretreatment on nicotine self-administration). It took 4–7 days for all rats to meet this criterion. Once nicotine taking was extinguished, rats entered the reinstatement phase of the experiment. The ability of an acute priming injection of nicotine (0.2 mg/ kg, s.c.) and re-exposure to the cue light to reinstate drug-seeking behavior was assessed. The priming dose of nicotine was based on our previously published reinstatement studies in rats (Kimmey et al. 2014; Lee et al. 2014; Maurer et al. 2017). During reinstatement test sessions, every 3rd lever press resulted in an infusion of saline and illumination of the cue light previously paired with nicotine taking during the self-administration phase.
Experiment 2: Determine the effects of repeated liraglutide on nicotine withdrawal-induced hyperphagia and body weight gain in male and female rats
Separate groups of drug-naïve male (n=45) and female (n=34) rats were individually housed in custom-made hanging wire-bottom cages throughout Experiment 2 in order to measure daily food and water intake. Rats acquired nicotine self-administration on a FR1 schedule of reinforcement as described above. Each rat that was allowed to respond for contingent infusions of nicotine was paired with a yoked rat that received infusions of saline. While lever pressing for the yoked saline rats had no scheduled consequences, these rats received the same number and temporal pattern of infusions as self-administered by their paired nicotine-experimental rat (Maurer et al. 2022). This yoked-pair design allowed us to measure intake of food, water, and kaolin as well as body weight in rats undergoing identical experimental conditions but without exposure to nicotine. Importantly, this design allowed for direct comparisons between the effects of liraglutide in nicotine-experienced rats versus yoked saline controls to identify potential interactions between liraglutide and nicotine experience on food intake, pica, and body weight gain. Nicotine self-administration sessions were conducted once daily for five days, and then twice daily for 17 additional days for a total of 22 continuous days of self-administration (Fig. 1B). Beginning on self-administration day 15, all rats received an additional 5 g of chow each day until they were feeding ad libitum. Previous studies showed that nicotine-exposed male rats with access to normal chow did not develop hyperphagia during abstinence (Grunberg et al. 1984). Consistent with these findings, we found that male rats with access to normal chow did not develop hyperphagia during abstinence following once daily nicotine self-administration sessions in our pilot studies (Figure S1). Importantly, human smokers demonstrate increased preference for palatable foods (Perkins et al. 1990; Spring et al. 2003), and nicotine withdrawal increases caloric intake specifically via increased consumption of high fat foods (Anker et al. 2021). These results suggest that the availability of palatable food may be necessary for hyperphagia to develop during nicotine withdrawal in rats. Therefore, beginning on self-administration day 20, rats were given ad libitum access to a high fat diet (HFD; Rodent Diet D12492; Research Diets, New Brunswick, New Jersey), which continued through the completion of the experiment (Fig. 1B). Both nicotine-experimental and yoked saline rats were divided randomly into vehicle or liraglutide treatment groups with equivalent body weights and nicotine exposure (i.e., saline/vehicle, saline/liraglutide, nicotine/vehicle, and nicotine/liraglutide). Starting on the final day of nicotine self-administration, rats were pretreated with vehicle or liraglutide (25 μg/kg, i.p.) 10 min prior to the beginning of the operant test session. Rats then continued to receive daily systemic infusions of vehicle or liraglutide for 10 consecutive withdrawal days in their home cages. Food and water intake were measured daily. Our model used twice daily (i.e., two daily two-hour operant sessions separated by 3 hours) nicotine self-administration, in contrast to previous studies where nicotine was delivered via subcutaneous minipumps, experimenter-delivered infusions, or non-contingent nicotine infusions (Bellinger et al. 2003; Bellinger et al. 2010; Bishop et al. 2002; Levin et al. 1987; Miyata et al. 2001). Given that smokers smoke compulsively throughout the day to maintain plasma nicotine levels, our model of hyperphagia during nicotine withdrawal more accurately parallels human smokers and adds an important translational component to the current literature (Benowitz 2009; Lerman et al. 2007; Shiffman et al. 2014).
Ad libitum food and water intake
On each day of withdrawal, food spillage was collected on papers placed beneath the hanging wire cages. Cumulative chow intake was measured as the difference in weight of the food hoppers between two time points to the nearest 0.1 g minus the weight of spilled crumbs. Feeding measurements, body weight, and water intake were measured daily, 24-h post infusion.
Pica/kaolin intake
Pica is a model of malaise-like behavior in which rats consume a non-nutritive substance, such as kaolin clay, in response to an emetic agent (Mitchell et al. 1976). To measure pica in Experiment 2, rats were habituated to ad libitum kaolin clay in their hanging wire cages for four days before withdrawal, similar to our previously published pica studies in nicotine-experienced rats (Ashare et al. 2017; Hopkins et al. 2012; Kimmey et al. 2014). Kaolin intake was measured as the difference in weight of the kaolin hopper between two time points to the nearest 0.1 g minus the weight of spilled crumbs. Intake was measured 24-h post liraglutide injection during each day of withdrawal.
Statistics
For all self-administration and reinstatement experiments, total active and inactive lever responses were analyzed with two-way analyses of variance (ANOVAs) with drug treatment and lever as factors. Total number of infusions were analyzed with one-way ANOVAs. Percent of control body weight was calculated by normalizing each rat’s body weight to the average body weight of the yoked saline/vehicle treated controls on the same day (body weight/average saline/vehicle body weight × 100). Daily 24-h food intake and percent of control body weight data were analyzed with two-way ANOVAs. Water and kaolin intake were analyzed with repeated measures (RM) two-way ANOVAs. Two-way ANOVAs were used to analyze cumulative food intake data. Saline vs. nicotine experience and drug treatment were factors in these analyses. All data are presented as mean ± SEM. To ensure robust and reliable results, the behavioral effects of liraglutide were tested in at least two cohorts of rats per experiment. Experimenters were blinded to treatment assignments during the testing phase of each experiment.
Results
Acute liraglutide administration attenuates voluntary nicotine taking in male and female rats
The efficacy of liraglutide was tested in male and female rats self-administering nicotine on a FR3 schedule of reinforcement on their final day of self-administration. Total lever responses in male rats pretreated with vehicle (n=9) or 25 μg/kg liraglutide (n=9) were analyzed with a two-way ANOVA, which revealed significant main effects of lever [F(1, 32)=57.74, p<0.0001] and treatment [F(1, 32)=13.74, p<0.001]. Subsequent pairwise analyses indicated that total active lever responses were significantly decreased in liraglutide-treated male rats compared to vehicle-treated controls (Bonferroni, p<0.01) (Fig. 2A). Consistent with these findings, liraglutide-treated male rats self-administered significantly less nicotine compared to control rats [t(16)=2.43, p<0.05] (Fig. 2B). Total lever responses in female rats pretreated with vehicle (n=11) or 25 μg/kg liraglutide (n=13) were analyzed with a two-way ANOVA, which revealed significant main effects of lever [F(1, 44)=62.06, p<0.0001] and treatment [F(1, 44)=4.30, p<0.05]. Subsequent pairwise analyses revealed that total active lever responses were significantly decreased in liraglutide-treated female rats compared to vehicle-treated controls (Bonferroni, p<0.05) (Fig. 2C). Female rats pretreated with liraglutide also self-administered significantly less nicotine infusions than control rats [t(22)=2.22, p<0.05] (Fig. 2D). These studies indicate that GLP-1R agonist monotherapy is sufficient to attenuate nicotine self-administration in male and female rats.
Fig. 2.
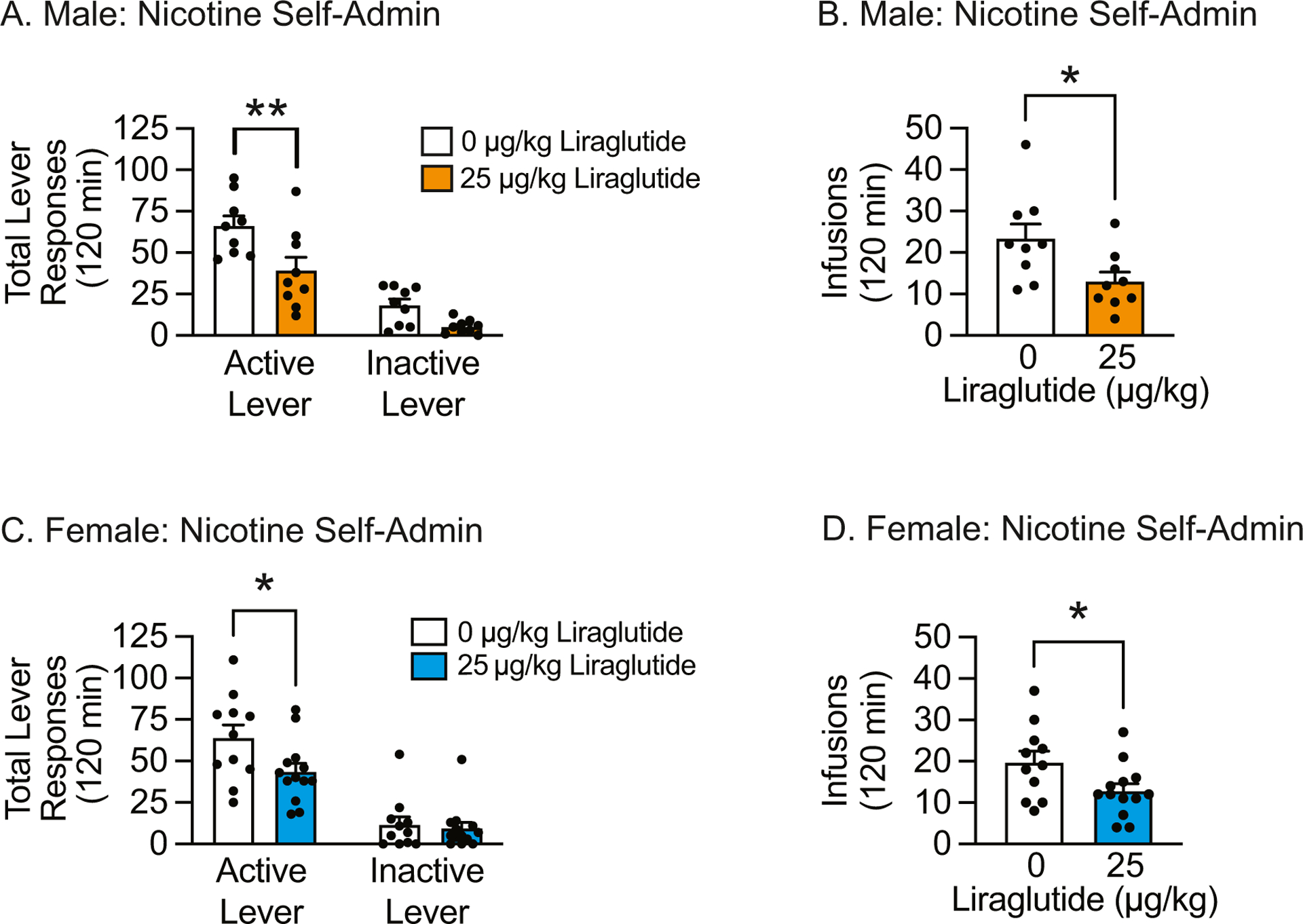
Acute liraglutide administration attenuated nicotine self-administration in male and female rats. Total active lever responses (A & C) and nicotine infusions (B & D) were significantly reduced in both male and female rats treated with 25 μg/kg liraglutide versus vehicle-treated controls on their final day of nicotine self-administration. (males: n=9/treatment; females: n=11 vehicle, n=13 liraglutide; * p<0.05,** p<0.01, Bonferroni or un-paired t-test).
Repeated liraglutide administration during abstinence attenuates nicotine seeking in male and female rats
To determine if repeated administration of liraglutide is sufficient to attenuate the reinstatement of nicotine-seeking behavior, male and female rats were pretreated daily with systemic vehicle or liraglutide before extinction and reinstatement test sessions. Repeated liraglutide administration significantly decreased the number of days required to reach extinction criteria in male [t(13)=2.82, p<0.05] (Fig. 3A) and female [t(17)=2.73, p<0.05] (Fig. 3C) rats. Total lever responses during subsequent reinstatement test sessions were analyzed with separate two-way ANOVAs, which revealed significant main effects of lever [F(1, 24)=60.86, p<0.0001] and treatment [F(1, 24)=5.31, p<0.05] in male rats (Fig. 3B) and a significant lever × treatment interaction [F(1, 36)=6.03, p<0.05] in female rats (Fig. 3D). Subsequent pairwise analyses indicated that total active lever responses were significantly decreased in male (n=7) and female (n=12) rats pretreated with liraglutide compared to vehicle-treated controls (n=8 male rats & n=7 female rats) (Bonferroni, p<0.05). Taken together, these results demonstrate that repeated liraglutide administration during abstinence is sufficient to attenuate nicotine reinstatement in male and female rats.
Fig. 3.
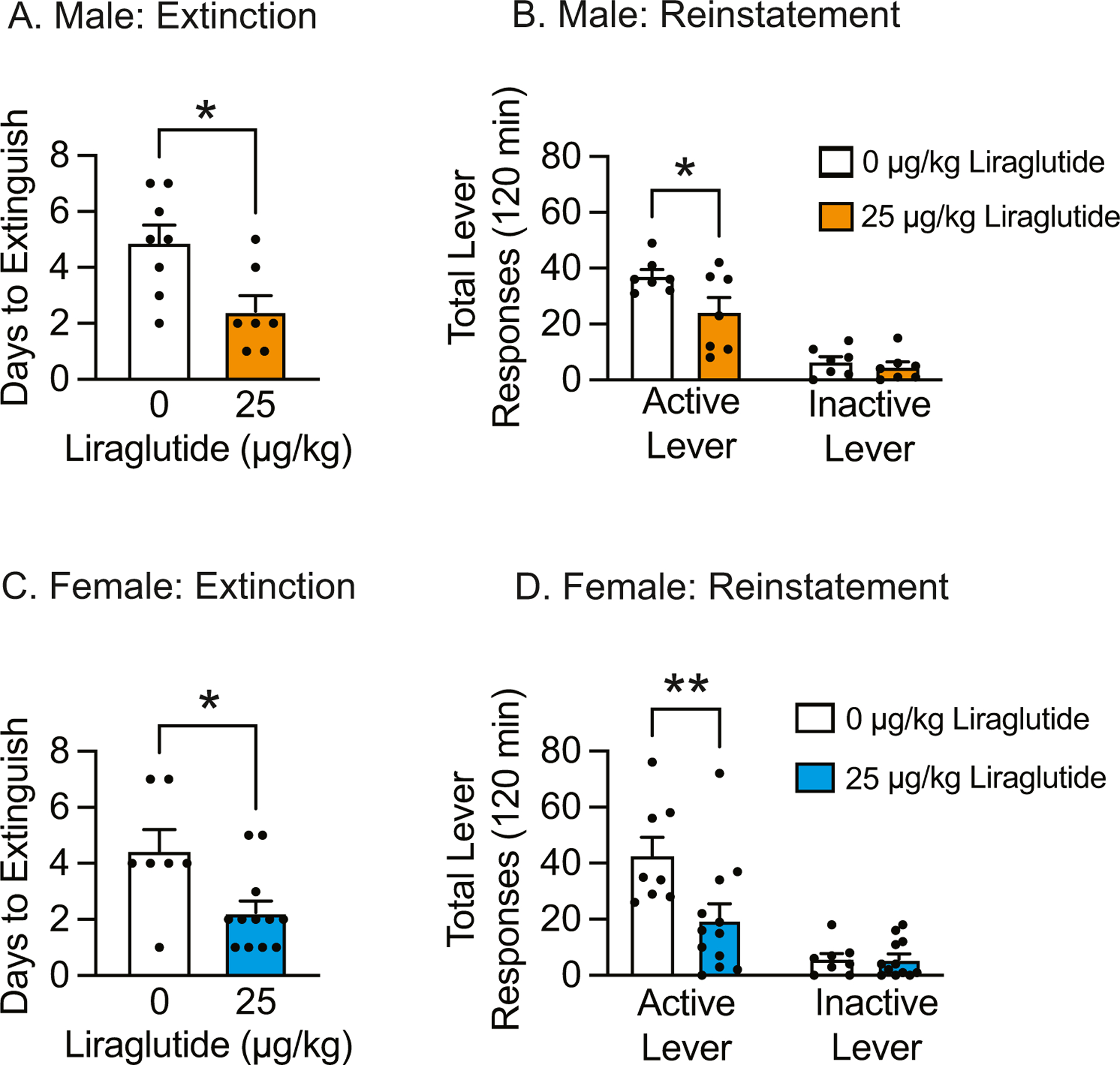
Repeated liraglutide administration during abstinence facilitated extinction of drug taking and attenuated subsequent nicotine-seeking behavior. (A) Repeated liraglutide administration decreased total days required to extinguish nicotine taking in male rats (n=7 vehicle, n=8 liraglutide; *p<0.05, un-paired t-test). (B) Repeated liraglutide administration significantly attenuated active lever responding during reinstatement tests in male rats (*p<0.05, Bonferroni). (C) Repeated liraglutide administration decreased total days required to extinguish nicotine taking in female rats (n=12 vehicle, n=7 liraglutide; *p<0.05, un-paired t-test). (D) epeated liraglutide administration significantly attenuated active lever responding during reinstatement tests in female rats (**p<0.01, Bonferroni).
Repeated liraglutide administration prevents nicotine withdrawal-induced hyperphagia and body weight gain in male rats
To determine if liraglutide reduces nicotine withdrawal-induced hyperphagia, male and female rats were pretreated with vehicle or 25 μg/kg liraglutide starting on the last day of nicotine self-administration and throughout the subsequent 10-day abstinence period. Male nicotine-experienced and yoked saline control rats were randomly divided into four treatment groups: yoked saline/vehicle (n=8), yoked saline/liraglutide (n=14), nicotine/vehicle (n=10), and nicotine/liraglutide (n=13). On the first day of treatment, acute liraglutide reduced nicotine self-administration (Figure S2A & 2B), consistent with our findings in Fig. 2. Acute liraglutide pretreatment also decreased 24-h HFD intake (Figure S2C) and water intake (Figure S2E), but did not affect body weight or kaolin intake in male rats (Figure S2D & 2F).
Daily food intake data during withdrawal were analyzed with two-way ANOVAs, which revealed significant nicotine × liraglutide interactions on withdrawal days 9 [F(1, 41)=5.10, p<0.05] and 10 [F(1, 41)=4.48, p<0.05] (Fig. 4A). Subsequent pairwise analyses revealed a significant increase in HFD intake in the nicotine/vehicle group compared to the saline/vehicle controls on withdrawal day 10, a significant decrease in HFD intake in nicotine/liraglutide rats compared to nicotine/vehicle rats on withdrawal days 1 and 5–10, and a significant decrease in HFD intake in saline/liraglutide rats compared to saline/vehicle rats on withdrawal days 3 and 7 (Tukey’s, p<0.05). Cumulative HFD intake over the 10-day withdrawal period was analyzed with a two-way ANOVA, which revealed significant main effects of liraglutide [F(1, 41)=36.53, p<0.0001] and nicotine [F(1, 41)=4.93, p<0.05] (Fig. 4B). There was no significant liraglutide × nicotine interaction [F(1, 41)=3.06, p=0.088]. Subsequent pairwise analyses indicated that liraglutide administration significantly reduced consumption of HFD in both the saline- and nicotine-experienced rats (Tukey’s, p<0.05). While not statistically significant, there was a trend towards increased hyperphagia over the 10-day withdrawal period in the nicotine-experienced rats versus the saline controls (Tukey’s, p=0.06). Body weight data were analyzed with a RM two-way ANOVA, which revealed a significant time × treatment interaction [F(18, 261)=3.17, p<0.0001] (Fig. 4C). Separate pairwise analyses indicated that body weight was significantly reduced in nicotine-experienced rats treated with liraglutide compared to nicotine-experienced rats treated with vehicle on withdrawal days 4–10 (Tukey’s, p<0.05). No effects of liraglutide were found on daily water intake (Fig. 4D) or kaolin consumption (Fig. 4E) during withdrawal. These results demonstrate that repeated liraglutide is sufficient to attenuate nicotine withdrawal-induced hyperphagia and body weight gain in male rats.
Fig. 4.
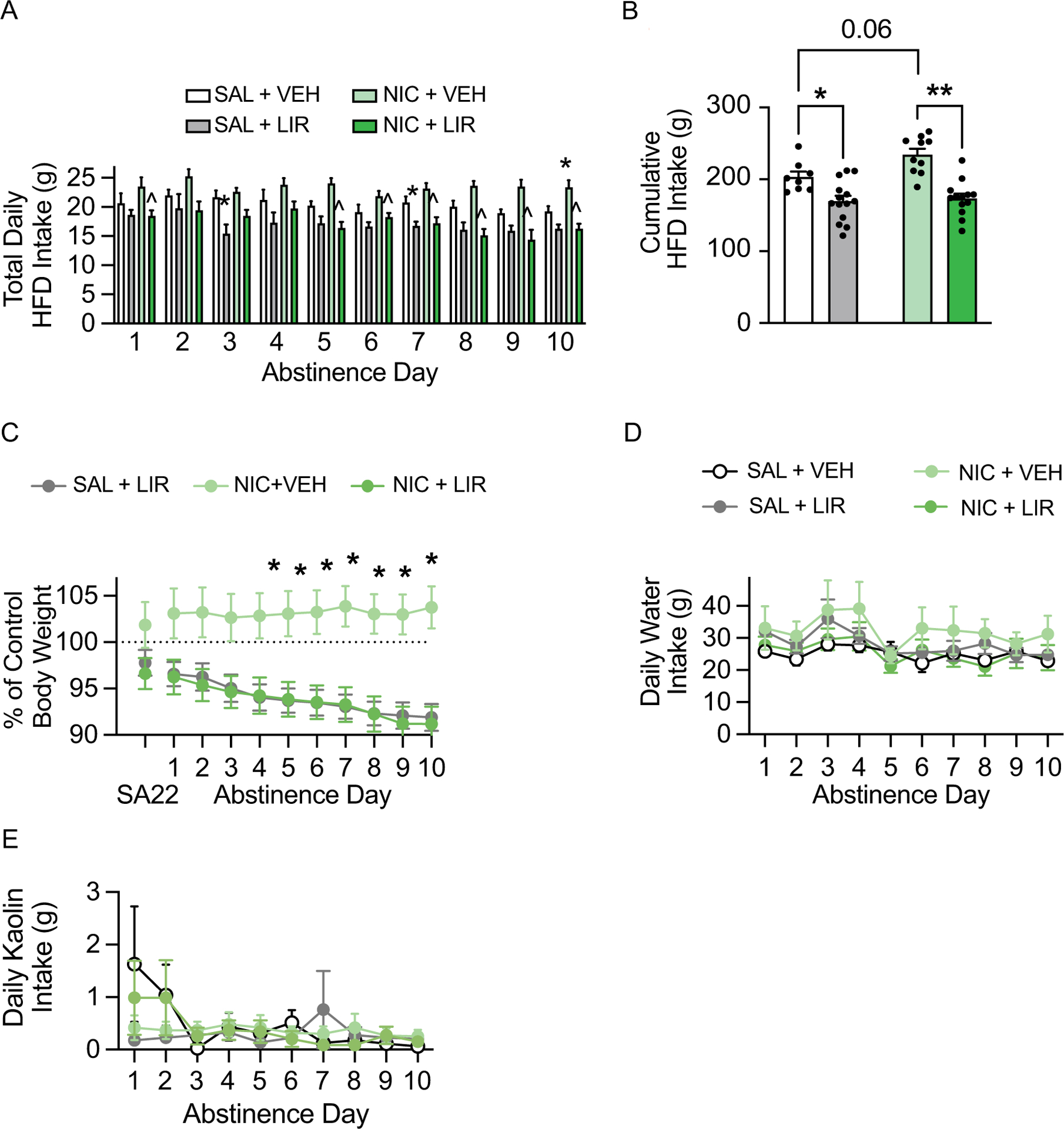
Repeated liraglutide administration prevented nicotine withdrawal-induced hyperphagia and body weight gain in male rats. (A) Repeated liraglutide administration attenuated daily HFD intake during nicotine abstinence (n= 8 saline/vehicle, 14 saline/liraglutide, 10 nicotine/vehicle, 13 nicotine/liraglutide; *p<0.05 vs saline/vehicle, ^p<0.05 vs nicotine/vehicle, Tukey’s). (B) Repeated liraglutide administration decreased cumulative HFD intake during nicotine abstinence in both saline- and nicotine-experienced male rats (*p<0.05, **p<0.0001, Tukey’s). (C) Repeated liraglutide administration prevented body weight gain during withdrawal in nicotine-experienced male rats (*p<0.05, Tukey’s). There were no effects of repeated liraglutide on daily water intake (D) and kaolin intake (E) during nicotine withdrawal in male rats.
Repeated liraglutide administration prevents nicotine withdrawal-induced hyperphagia and body weight gain in female rats
Female nicotine-experienced and yoked saline control rats were randomly divided into four treatment groups: yoked saline/vehicle (n=9), yoked saline/liraglutide (n=6), nicotine/vehicle (n=8), and nicotine/liraglutide (n=11). On the first day of treatment, acute liraglutide attenuated nicotine self-administration in female rats (Figure S3A & 3B), consistent with our findings in Fig. 2. Acute liraglutide pretreatment also decreased 24-h HFD intake (Figure S3C), but did not affect body weight, water consumption, or kaolin intake in female rats (Figure S3D–F).
Daily HFD intake data during withdrawal were analyzed with two-way ANOVAs, which revealed significant main effects of nicotine on daily HFD intake on withdrawal days 2 [F(1, 33)=17.31, p<0.001], 4 [F(1, 33)=12.16, p<0.01], 7 [F(1, 33)=5.62 p<0.05], and 8 [F(1, 33)=10.21, p<0.01], and significant main effects of liraglutide on withdrawal days 1 [F(1, 33)=16.21, p<0.001], 2 [F(1, 33)=17.31, p<0.001], 3 [F(1, 33)=12.61, p<0.01], 4 [F(1, 33)=15.21, p<0.0001], 5 [F(1, 33)=9.35, p<0.01], 6 [F(1, 33)=6.00, p<0.05], 7 [F(1, 33)=4.97, p<0.05], 8 [F(1, 33)=13.44, p<0.001], 9 [F(1, 33)=17.21, p<0.001], and 10 [F (1, 33)=26.30, p<0.0001] (Fig. 5A). Subsequent pairwise analyses revealed a significant increase in HFD intake in the nicotine/vehicle group compared to the saline/vehicle controls on withdrawal days 2, 4, and 8 (Tukey’s, p<0.05), and a significant decrease in HFD intake in nicotine/liraglutide rats compared to nicotine/vehicle rats on withdrawal days 1–6 and 8–10 (Tukey’s, p<0.01). Cumulative HFD intake over the 10-day withdrawal period was analyzed with a two-way ANOVA, which revealed significant main effects of liraglutide [F(1, 33)=29.42, p<0.0001] and nicotine [F(1, 33)=8.59, p<0.01], as well as a significant liraglutide × nicotine interaction [F(1,33)=4.39, p<0.05] (Fig. 5B). Subsequent pairwise analyses indicated that nicotine taking significantly increased HFD intake in vehicle-treated rats (Tukey’s, p<0.01), and liraglutide administration significantly reduced HFD intake in nicotine-experienced rats (Tukey’s, p<0.0001). Body weight data were analyzed with a RM two-way ANOVA, which revealed a significant time × treatment interaction [F (18, 225) = 3.67, p<0.0001] (Fig. 5C). Body weight was significantly reduced in nicotine-experienced rats treated with liraglutide compared to nicotine-experienced rats treated with vehicle on withdrawal days 6–10 (Tukey’s, p<0.05). There were no effects of liraglutide on water intake (Fig. 5D) and kaolin consumption (Fig. 5E) during withdrawal. These results demonstrate that repeated liraglutide is sufficient to attenuate nicotine withdrawal-induced hyperphagia and body weight gain in female rats.
Fig. 5.
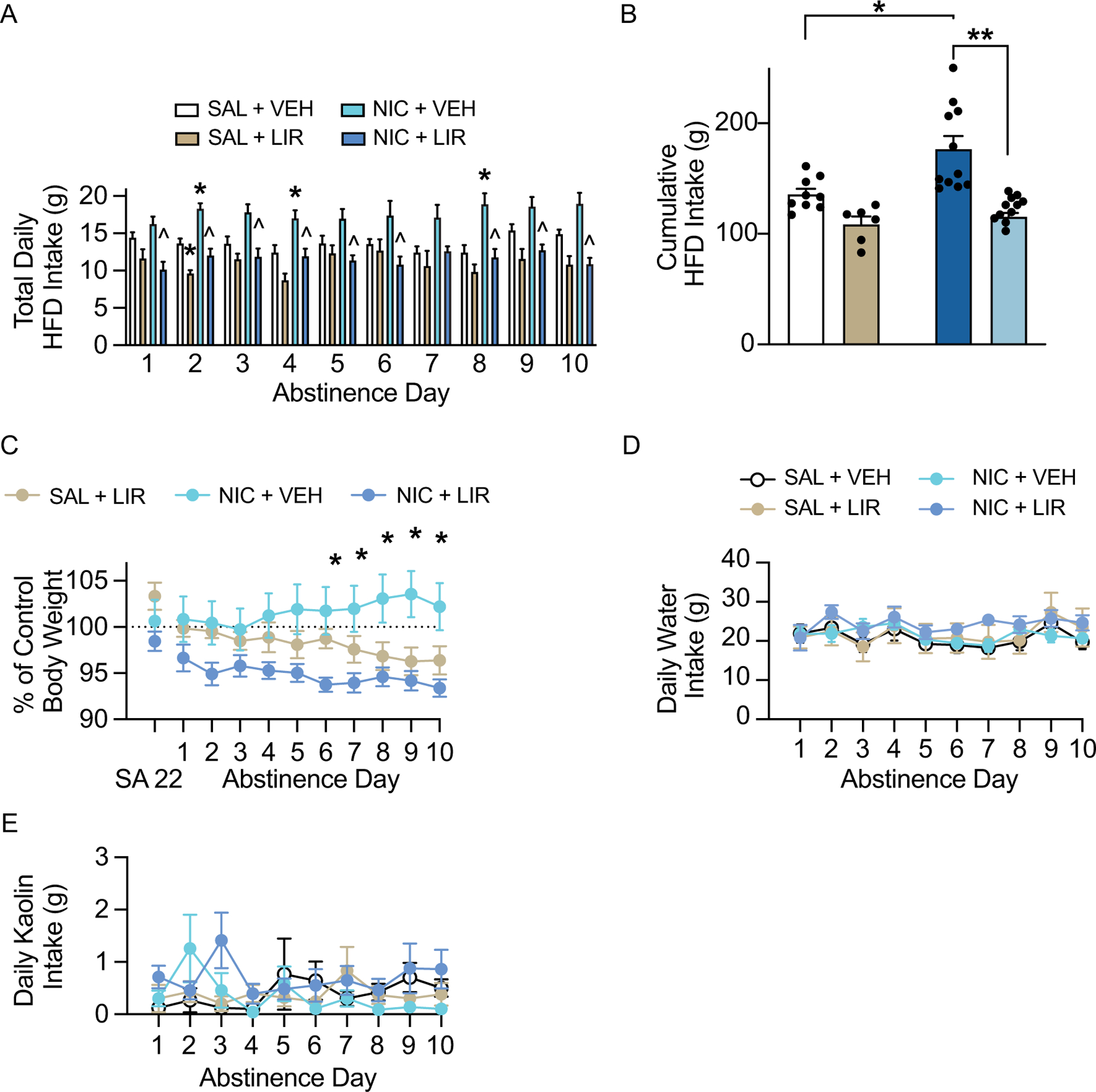
Repeated liraglutide administration prevented nicotine withdrawal-induced hyperphagia and body weight gain in female rats. (A) Repeated liraglutide administration attenuated daily HFD intake during nicotine abstinence (n=9 saline/vehicle, 6 saline/liraglutide, 8 nicotine/vehicle, 11 nicotine/liraglutide: *p<0.05 vs saline/ vehicle, ^p<0.05 vs nicotine/vehicle, Tukey’s). (B) Repeated liraglutide administration decreased cumulative HFD intake during nicotine abstinence in nicotine-experienced female rats (*p<0.01, **p<0.0001, Tukey’s). (C) Repeated liraglutide administration prevented body weight gain during withdrawal in nicotine-experienced female rats (*p<0.05, Tukey’s). There were no effects of repeated liraglutide on daily water intake (D) and kaolin intake (E) during nicotine withdrawal in female rats.
Discussion
The present findings indicate that GLP-1R monotherapy alone is sufficient to reduce nicotine-mediated behaviors in rats. Acute and repeated administration of the GLP-1R agonist liraglutide attenuated nicotine self-administration and reinstatement, respectively, in male and female rats. Repeated administration of liraglutide during abstinence also prevented nicotine withdrawal-induced hyperphagia and body weight gain in male and female rats. At the dose tested, liraglutide did not elicit pica in nicotine-experienced rats, indicating that the suppressive effects of liraglutide on nicotine-mediated behaviors and withdrawal-induced hyperphagia are likely not due to adverse malaise-like effects. Taken together, these findings support further studies investigating the clinical potential of GLP-1R agonists as novel pharmacotherapies for treating nicotine use disorder (NUD).
Recent preclinical studies showed that systemic administration of a dipeptidyl peptidase-4 (DPPIV) inhibitor – a drug that blocks the breakdown of endogenous GLP-1 – and a short-acting GLP-1R agonist decreased the rewarding effects of nicotine in rodents (Egecioglu et al. 2013; Tuesta et al. 2017). Consistent with these effects, chemogenetic activation of GLP-1-producing neurons in the nucleus tractus solitarius, the primary source of central GLP-1, attenuated nicotine taking whereas knockdown or pharmacological inhibition of GLP-1Rs increased nicotine consumption in male mice (Tuesta et al. 2017). Together, these studies indicate that activation of central GLP-1Rs is sufficient to reduce nicotine-mediated behaviors. Our study extends these findings by showing that the GLP-1R agonist liraglutide attenuated nicotine self-administration in both male and female rats. This study is the first, to our knowledge, to demonstrate the efficacy of a GLP-1R agonist in decreasing voluntary nicotine taking in female rats. The neural mechanisms underlying the suppressive effects of GLP-1R agonists on nicotine taking are likely to be complex and incorporate multiple neural circuits involved in reward and aversion. While our study does not investigate these potential mechanisms, previous studies showed that activation of presynaptic GLP-1Rs on medial habenula terminals in the interpeduncular nucleus promoted avoidance-like behaviors that served to titrate nicotine intake and decrease nicotine consumption (Tuesta et al. 2017). GLP-1Rs are expressed throughout the brain, including the mesolimbic dopamine system (Merchenthaler et al. 1999). Given that GLP-1R agonists decrease nicotine-evoked dopamine release in the nucleus accumbens (NAc) (Egecioglu et al. 2013), it is possible and likely that activating GLP-1Rs in the NAc and ventral tegmental area (VTA) may also attenuate nicotine taking and seeking via effects on striatal dopamine signaling. This hypothesis is supported by studies showing that activation of GLP-1Rs in the mesoaccumbens pathway is sufficient to reduce drug taking and seeking as well as drug-evoked phasic dopamine cell firing (Fortin and Roitman 2017; Hernandez et al. 2018; Hernandez et al. 2019; Schmidt et al. 2016). Understanding the central mechanisms underlying the suppressive effects of GLP-1R agonists on nicotine taking may lead to novel GLP-1R-based approaches to treating NUD and optimizing the health benefits of smoking cessation.
We also discovered that activating GLP-1Rs during abstinence decreased the number of days required to extinguish nicotine taking and attenuated subsequent nicotine-seeking behavior in male and female rats. These results are consistent with previous studies showing that systemic administration of the GLP-1R agonist exendin-4 facilitated extinction of cocaine-induced CPP and decreased subsequent cocaine priming-induced reinstatement of CPP in mice (Zhu et al. 2021). Together, these findings suggest that the combination of GLP-1R agonist treatment and daily extinction training may facilitate extinction learning-related plasticity. Emerging evidence indicates that GLP-1R activation increases glutamate signaling and AMPA receptor trafficking in drug-naïve rats (Liu et al. 2017; Mietlicki-Baase et al. 2013; Ohtake et al. 2014; Park et al. 2018). Since enhanced glutamatergic signaling including upregulation of AMPA receptors facilitates extinction and decreases drug seeking (Chesworth and Corbit 2017; Sutton et al. 2003), these findings suggest that GLP-1R agonists may enhance glutamate-mediated synaptic plasticity and facilitate extinction learning to reduce nicotine seeking during abstinence. Repeated liraglutide effectively reduced drug seeking elicited by both a priming injection of nicotine and re-exposure to conditioned light cues that were previously paired with nicotine infusions during the self-administration phase of the experiment. Given that different neural circuits and mechanisms regulate drug-versus cue-primed reinstatement of drug seeking (Schmidt et al. 2018), it will be important to investigate the effects of GLP-1R agonists on these systems to more fully realize the translational potential of these pharmacotherapies for NUD.
The majority of smokers who quit gain weight (Bush et al. 2016; Chao et al. 2019; Stamford et al. 1986), and concerns about body weight gain are a major barrier to achieving long-term smoking abstinence (Bush et al. 2016). Smoking cessation-induced body weight gain is mediated, in part, by hyperphagia and increased consumption of highly palatable foods (Anker et al. 2021; Epstein et al. 2004; Perkins et al. 1990; Spring et al. 2003). Previous preclinical studies have modeled increased food intake during nicotine abstinence. However, these studies are limited in their face validity in that nicotine was delivered via subcutaneous minipumps, experimenter-delivered infusions, or non-contingent nicotine infusions (Bellinger et al. 2003; Bellinger et al. 2010; Bishop et al. 2002; Levin et al. 1987; Miyata et al. 2001). Here, we established a novel rodent model of withdrawal-induced hyperphagia and body weight gain following twice daily nicotine self-administration. We found that withdrawal following voluntary nicotine taking increased consumption of a high fat diet (HFD) in male and female rats. Interestingly, food intake was significantly increased over more withdrawal days in female versus male rats. This is consistent with previous studies demonstrating increased hyperphagia and rate of body weight gain in female, but not male rats, with access to bland food during withdrawal following nicotine exposure via osmotic minipump (Grunberg et al. 1984; Grunberg et al. 1986). These results suggest that females may be more susceptible to nicotine withdrawal-induced hyperphagia regardless of food palatability, a hypothesis also supported by clinical studies showing that women gain more weight than men during smoking cessation attempts (Kasteridis and Yen 2012; Williamson et al. 1991). Parallel studies using a standard chow diet did not produce withdrawal-induced hyperphagia following nicotine self-administration in male rats (Figure S1). These results are consistent with a previous nicotine osmotic minipump study that showed no hyperphagia during abstinence in male rats with access to standard chow (Grunberg et al. 1984). Overall, these results are consistent with human epidemiological data indicating that nicotine withdrawal increased motivation for/ consumption of highly palatable foods (Anker et al. 2021; Epstein et al. 2004; Perkins et al. 1990; Spring et al. 2003), demonstrating that this phenotype is driven, in part, by consumption of a Western diet. In contrast to the present study, nicotine abstinence increased intake of standard chow in food-restricted female mice (Mannucci et al. 2005). Together with our findings, this suggests that nicotine may have complex reinforcement-enhancing effects on food that are unmasked under conditions of food scarcity or high palatability (Caggiula et al. 2001; Donny et al. 2011). The neural mechanisms underlying the effects of Ex-4 on withdrawal-induced hyperphagia are unknown. However, previous studies have demonstrated that nicotine affects feeding behavior via complex interactions with cholinergic receptors (Jo et al. 2002). Furthermore, nicotine withdrawal has been shown to alter dopamine signaling dynamics (Epping-Jordan et al. 1998; Jo et al. 2002; Zhang et al. 2012). Given that GLP-1Rs have been shown to play a role in cholinergic signaling and GLP-1R agonism suppresses phasic dopamine responses to a food-predictive cue (Konanur et al. 2020; Tuesta et al. 2017), future studies should investigate the impact of nicotine withdrawal and subsequent GLP-R agonist treatment on measures of cholinergic and dopaminergic activity.
In our study and in other studies of hyperphagia during nicotine abstinence, nicotine-experienced rats gained weight throughout the withdrawal period (Bellinger et al. 2003; Bellinger et al. 2010; Levin et al. 1987; Miyata et al. 2001). However, these withdrawal periods are relatively short (i.e., 7–14 days) and the nicotine-experienced rats did not weigh more than yoked saline control rats at the end of abstinence. In contrast, former smokers weigh more than never smokers (Klesges et al. 1989), and their greatest weight gain occurs six months after quitting smoking (Klesges et al. 1997). Therefore, one limitation of these preclinical studies is their relatively short duration of withdrawal, which may not be sufficient to reveal weight gain above controls. Tolerance did not develop to the effects of repeated liraglutide on hyperphagia and body weight gain in our model. While these promising findings suggest that liraglutide may maintain its efficacy in attenuating nicotine withdrawal-induced hyperphagia and body weight gain over prolonged periods of time, more comprehensive studies incorporating different doses of liraglutide, and longer withdrawal periods are required to determine the long-term effects of GLP-1R agonists. Interestingly, our results suggest that the effects of liraglutide on body weight may incubate throughout withdrawal and be sex-dependent (i.e., greater in females than males). These potential incubation effects should be studied in future studies that incorporate longer withdrawal periods. The most notable adverse effect of GLP-1R agonists is nausea or malaise, which can limit patient compliance (Bettge et al. 2017; Vilsbøll et al. 2012). We identified a behaviorally selective dose of liraglutide that did not produce malaise-like effects (i.e., pica) in nicotine-experienced rats. These results indicate that liraglutide is well-tolerated at a relatively low dose that prevents withdrawal-induced hyperphagia and body weight gain in nicotine-experienced rats. Since diverse patient populations smoke, it will also be important to screen the effects of GLP-1R agonists in rodent models of obesity and type II diabetes to enhance the translational potential of these findings.
Our preclinical findings are consistent with a recent clinical study that showed that administration of the GLP-1R agonist exenatide in combination with NRT improved smoking abstinence rates, reduced withdrawal symptoms and craving, and mitigated post-cessation weight gain compared to NRT alone (Yammine et al. 2021). Smokers treated with exenatide had a 19.5% higher rate of abstinence and achieved a 5.6 lb body weight loss after six weeks of treatment compared to placebo-treated controls. Importantly, those treated with exenatide and NRT reported adverse effects that were mild in severity and did not result in treatment discontinuation. Adverse effects did not occur at a rate significantly different than the control group, and there were no reports of nausea, hypoglycemia, or vomiting. Coupled with our findings that GLP-1R monotherapy alone did not produce malaise-like effects in rats, this research suggests that liraglutide alone may be well-tolerated in treatment-seeking smokers. While the mechanisms underlying reduced body weight gain in former smokers treated with exenatide and NRT are not clear, our preclinical studies demonstrate that GLP-1R monotherapy prevented withdrawal-induced hyperphagia in nicotine-experienced rats given access to a HFD. In addition to investigating the efficacy of GLP-1R monotherapy in human smokers, it will also be important to determine how GLP-1R activation during nicotine withdrawal affects macronutrient preference and food intake.
Conclusion
In summary, our studies showed that GLP-1R agonist mono-therapy is sufficient to attenuate voluntary nicotine taking and seeking, and nicotine withdrawal-induced hyperphagia and body weight gain in male and female rats. These findings support and expand an emerging literature that suggests that GLP-1R agonists may be efficacious treatments for NUD. Future studies should compare the efficacy and side effect profiles of liraglutide monotherapy versus a combinatorial therapy with NRT to determine the most efficacious and safe treatment. The efficacy of next-generation GLP-1R agonists with improved pharmacokinetics (e.g., semaglutide) and dual agonists of GLP-1Rs and other neuropeptide receptor systems including glucose-dependent insulinotropic polypeptide (GIP) and neuropeptide type 2 receptors (Y2Rs) should also be assessed (Hayes et al. 2021; Merkel et al. 2021). These results support further clinical trials of liraglutide and other GLP-1R agonists for preventing nicotine withdrawal-induced hyperphagia and body weight gain. Findings from this study raise the intriguing possibility that GLP-1R monotherapy alone could reduce nicotine withdrawal-induced hyperphagia and body weight gain and thereby remove one of the biggest obstacles toward achieving long-term abstinence in human smokers.
Supplementary Material
Acknowledgements
The authors would like to thank Sana Zeb, Kael Ragnini, and Victoria Chinaka for their technical contributions to this project.
Funding
This work was supported by the following grants from the National Institutes of Health (NIH): R01 DA037897 and R21 DA045792 (H.D.S.), R01 DK105155 (H.D.S. and M.R.H.), R01 CA206058 (J.A.-M.), and training grant T32 DA028874 and fellowship F31 DA058451 (R.J.H.). R.L.A. was supported in part by an investigator-initiated grant from Novo Nordisk.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00213-023-06376-w.
Disclosure The authors declare no other competing financial interests.
References
- Anker JJ, Nakajima M, Raatz S, Allen S, al’ Absi M (2021) Tobacco withdrawal increases junk food intake: The role of the endogenous opioid system. Drug Alcohol Depend 225:108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD (2017) Repeated administration of an acetylcho-linesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry 7:e1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz NL (2011) Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther 90:164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, Jamal A (2017) Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 65:1457–1464 [DOI] [PubMed] [Google Scholar]
- Bellinger L, Cepeda-Benito A, Wellman PJ (2003) Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol Biochem Behav 74:495–504 [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Wellman PJ, Harris RB, Kelso EW, Kramer PR (2010) The effects of chronic nicotine on meal patterns, food intake, metabolism and body weight of male rats. Pharmacol Biochem Behav 95:92–99 [DOI] [PubMed] [Google Scholar]
- Benowitz NL (2009) Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49:57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA (2017) Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes Metab 19:336–347 [DOI] [PubMed] [Google Scholar]
- Bishop C, Parker G, Coscina D (2002) Nicotine and its withdrawal alter feeding induced by paraventricular hypothalamic injections of neuropeptide Y in Sprague-Dawley rats. Psychopharmacology 162:265–272 [DOI] [PubMed] [Google Scholar]
- Bush T, Lovejoy JC, Deprey M, Carpenter KM (2016) The effect of tobacco cessation on weight gain, obesity and diabetes risk. Obesity (Silver Spring, Md) 24:1834–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530 [DOI] [PubMed] [Google Scholar]
- CDC (2020) Smoking Cessation: A Report of the Surgeon General. CDC Center for Disease Control and Prevention [Google Scholar]
- Chao AM, Wadden TA, Ashare RL, Loughead J, Schmidt HD (2019) Tobacco Smoking, Eating Behaviors, and Body Weight: A Review. Curr Addict Rep 6:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth R, Corbit LH (2017) Recent developments in the behavioural and pharmacological enhancement of extinction of drug seeking. Addic Biol 22:3–43 [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99:473–478 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF (2011) The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol Behav 104:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douton JE, Acharya NK, Stoltzfus B, Sun D, Grigson PS, Nyland JE (2022) Acute glucagon-like peptide-1 receptor agonist liraglutide prevents cue-, stress-, and drug-induced heroin-seeking in rats. Behav Pharmacol 33:364–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douton JE, Augusto C, Stoltzfus B, Carkaci-Salli N, Vrana KE, Grigson PS (2020) Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behav Pharmacol 32(4):265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013) The Glucagon-Like Peptide 1 Analogue Exendin-4 Attenuates the Nicotine-Induced Locomotor Stimulation, Accumbal Dopamine Release, Conditioned Place Preference as well as the Expression of Locomotor Sensitization in Mice. PLoS ONE 8:e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79 [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW Jr, Jaroni JL, Saad FG, Crystal-Mansour S, Lerman C (2004) Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav 81:511–517 [DOI] [PubMed] [Google Scholar]
- Evans B, Stoltzfus B, Acharya N, Nyland JE, Arnold AC, Freet CS, Bunce SC, Grigson PS (2022) Dose titration with the glucagon-like peptide-1 agonist, liraglutide, reduces cue- and drug-induced heroin seeking in high drug-taking rats. Brain Res Bull 189:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley AC, Hajek P, Lycett D, Aveyard P (2012) Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 1:Cd006219. [DOI] [PubMed] [Google Scholar]
- Fortin SM, Roitman MF (2017) Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav 176:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ (2011) Intravenous nicotine self-administration and cue-induced reinstatement in mice: Effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Morse DE (1984) Effects of nicotine on body weight and food consumption in rats. Psychopharmacology (Berl) 83:93–98 [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Winders SE (1986) Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl) 90:101–105 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Borner T, De Jonghe BC (2021) The Role of GIP in the Regulation of GLP-1 Satiety and Nausea. Diabetes 70:1956–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ (2011) Comparative Effects of the Long-Acting GLP-1 Receptor Ligands, Liraglutide and Exendin-4, on Food Intake and Body Weight Suppression in Rats. Obesity 19:1342–1349 [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD (2018) Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD (2019) Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addict Biol 24:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Schmidt HD (2019) Central GLP-1 receptors: Novel molecular targets for cocaine use disorder Physiology and Behavior. Elsevier Inc., pp 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD (2012) Galantamine, an Acetylcholinesterase Inhibitor and Positive Allosteric Modulator of Nicotinic Acetylcholine Receptors, Attenuates Nicotine Taking and Seeking in Rats. Neuropsychopharmacology 37:2310–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E (2019) Gut-brain axis and addictive disorders: A review with focus on alcohol and drugs of abuse Pharmacology and Therapeutics. Elsevier Inc., pp 1–14 [DOI] [PubMed] [Google Scholar]
- Jo Y-H, Talmage DA, Role LW (2002) Nicotinic receptor-mediated effects on appetite and food intake. Journal of Neurobiology 53:618–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasteridis P, Yen ST (2012) Smoking cessation and body weight: evidence from the Behavioral Risk Factor Surveillance Survey. Health Serv Res 47:1580–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD (2012) Done-pezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addic Biol 19(4):539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey BA, Rupprecht LE, Hayes MR, Schmidt HD (2014) Done-pezil, an acetylcholinesterase inhibitor, attenuates nicotine self-administration and reinstatement of nicotine seeking in rats. Addic Biol 19:539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA (1988) Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol 7:575–589 [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME (1989) Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull 106:204–230 [DOI] [PubMed] [Google Scholar]
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, Ray JW, Shadish WR (1997) How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. J Consult Clin Psychol 65:286–291 [DOI] [PubMed] [Google Scholar]
- Knudsen LB (2010) Liraglutide: the therapeutic promise from animal models. Int J Clin Pract 64:4–11 [DOI] [PubMed] [Google Scholar]
- Konanur VR, Hsu TM, Kanoski SE, Hayes MR, Roitman MF (2020) Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiol Behav 215:112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Arreola AC, Kimmey BA, Schmidt HD (2014) Administration of the nicotinic acetylcholine receptor agonists ABT-089 and ABT-107 attenuates the reinstatement of nicotine-seeking behavior in rats. Behav Brain Res 274:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA (2007) Translational research in medication development for nicotine dependence. Nat Rev Drug Discov 6:746–762 [DOI] [PubMed] [Google Scholar]
- Levin ED, Morgan MM, Galvez C, Ellison GD (1987) Chronic nicotine and withdrawal effects on body weight and food and water consumption in female rats. Physiology & Behavior 39:441–444 [DOI] [PubMed] [Google Scholar]
- Liberini CG, Koch-Laskowski K, Shaulson E, McGrath LE, Lipsky RK, Lhamo R, Ghidewon M, Ling T, Stein LM, Hayes MR (2019) Combined Amylin/GLP-1 pharmacotherapy to promote and sustain long-lasting weight loss. Scie Rep 9:8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, Pang ZP (2017) Enhanced AMPA Receptor Trafficking Mediates the Anorexigenic Effect of Endogenous Glucagon-like Peptide-1 in the Paraventricular Hypothalamus. Neuron 96:897–909.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci C, Catania MA, Adamo EB, Bellomo M, Caputi AP, Calapai G (2005) Long-Term Effects of High Doses of Nicotine on Feeding Behavior and Brain Nitric Oxide Synthase Activity in Female Mice. J Pharmacol Sci 98:232–238 [DOI] [PubMed] [Google Scholar]
- Maurer JJ, Sandager-Nielsen K, Schmidt HD (2017) Attenuation of nicotine taking and seeking in rats by the stoichiometry-selective alpha4beta2 nicotinic acetylcholine receptor positive allosteric modulator NS9283. Psychopharmacology (Berl) 234:475–484 [DOI] [PubMed] [Google Scholar]
- Maurer JJ, Wimmer ME, Turner CA, Herman RJ, Zhang Y, Ragnini K, Ferrante J, Kimmey BA, Crist RC, Christopher Pierce R, Schmidt HD (2022) Paternal nicotine taking elicits heritable sex-specific phenotypes that are mediated by hippocampal Satb2. Mol Psychiatry 27:3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology 403:261–280 [DOI] [PubMed] [Google Scholar]
- Merkel R, Moreno A, Zhang Y, Herman R, Ben Nathan J, Zeb S, Rahematpura S, Stecyk K, Milliken BT, Hayes MR, Doyle RP, Schmidt HD (2021) A novel approach to treating opioid use disorders: Dual agonists of glucagon-like peptide-1 receptors and neuro-peptide Y(2) receptors. Neurosci Biobehav Rev 131:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH (1997) Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol 65:448–452 [DOI] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Christopher Pierce R, Hayes MR (2013) The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305:1367–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO (2012) Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med 44:588–597 [DOI] [PubMed] [Google Scholar]
- Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK (1976) Poison induced pica in rats. Physiol Behav 17:691–697 [DOI] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ (2001) Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav 74:169–176 [DOI] [PubMed] [Google Scholar]
- Ohtake N, Saito M, Eto M, Seki K (2014) Exendin-4 promotes the membrane trafficking of the AMPA receptor GluR1 subunit and ADAM10 in the mouse neocortex. Regul Pept 190–191:1–11 [DOI] [PubMed] [Google Scholar]
- Park SW, Mansur RB, Lee Y, Lee JH, Seo MK, Choi AJ, McIntyre RS, Lee JG (2018) Liraglutide Activates mTORC1 Signaling and AMPA Receptors in Rat Hippocampal Neurons Under Toxic Conditions. Front Neurosci 12:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Sexton JE, Pastor S (1990) Effects of smoking cessation on consumption of alcohol and sweet, high-fat foods. J Subst Abuse Treat 2:287–297 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR (2016) Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41:1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt Heath D, Rupprecht Laura E, Addy Nii A (2018) Neurobiological and Neurophysiological Mechanisms Underlying Nicotine Seeking and Smoking Relapse. Molecular Neuropsychiatry 4:169–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG (2014) Smoking Patterns and Stimulus Control in Intermittent and Daily Smokers. PLoS One 9:e89911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pagoto S, McChargue D, Hedeker D, Werth J (2003) Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav 76:351–360 [DOI] [PubMed] [Google Scholar]
- Stamford BA, Matter S, Fell RD, Papanek P (1986) Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr 43:486–494 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW (2003) Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421:70–75 [DOI] [PubMed] [Google Scholar]
- Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA (2002) Benefits of smoking cessation for longevity. Am J Public Health 92:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu XA, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ (2017) GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nature Neuroscience 20:708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges J-P, Verhoeven R, Bugánová I, Madsbad S (2007) Liraglutide, a Long-Acting Human Glucagon-Like Peptide-1 Analog, Given as Monotherapy Significantly Improves Glycemic Control and Lowers Body Weight Without Risk of Hypoglycemia in Patients With Type 2 Diabetes. Diabetes Care 30:1608–1610 [DOI] [PubMed] [Google Scholar]
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, MTD T, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF, Group SS (2021) Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 384:989. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T (1991) Smoking cessation and severity of weight gain in a national cohort. N Engl J Med 324:739–745 [DOI] [PubMed] [Google Scholar]
- Yammine L, Green CE, Kosten TR, de Dios C, Suchting R, Lane SD, Verrico CD, Schmitz JM (2021) Exenatide Adjunct to Nicotine Patch Facilitates Smoking Cessation and May Reduce Post-Cessation Weight Gain: A Pilot Randomized Controlled Trial. Nicotine Tob Res 23:1682–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA (2012) Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry 71:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, Schmidt HD (2020) Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology 45:451–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rahematpura S, Ragnini KH, Moreno A, Stecyk KS, Kahng MW, Milliken BT, Hayes MR, Doyle RP, Schmidt HD (2021) A novel dual agonist of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors attenuates fentanyl taking and seeking in male rats. Neuropharmacology 192:108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Hong T, Li H, Jiang S, Guo B, Wang L, Ding J, Gao C, Sun Y, Sun T, Wang F, Wang Y, Wan D (2021) Glucagon-Like Peptide-1 Agonist Exendin-4 Facilitates the Extinction of Cocaine-Induced Condition Place Preference. Front Syst Neurosci 15:711750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


