Abstract
Background
Adenovirus is a known cause of hepatitis in immunocompromised children, but not in immunocompetent children. In April, 2022, following multiple reports of hepatitis of unknown aetiology and adenovirus viraemia in immunocompetent children in the USA and UK, the US Centers for Disease Control and Prevention (CDC) and jurisdictional health departments initiated national surveillance of paediatric acute hepatitis of unknown aetiology. We aimed to describe the clinical and epidemiological characteristics of children identified with hepatitis of unknown aetiology between Oct 1, 2021, and Sept 30, 2022, in the USA and to compare characteristics of those who tested positive for adenovirus with those who tested negative.
Methods:
In this national surveillance investigation in the USA, children were identified for investigation if they were younger than 10 years with elevated liver transaminases (>500 U/L) who had an unknown cause for their hepatitis and onset on or after Oct 1, 2021. We reviewed medical chart abstractions, which included data on demographics, underlying health conditions, signs and symptoms of illness, laboratory results, vaccination history, radiological and liver pathology findings, diagnoses and treatment received, and outcomes. Caregiver interviews were done to obtain information on symptoms and health-care utilisation for the hepatitis illness, medical history, illness in close contacts or at school or daycare, diet, travel, and other potential exposures. Blood, stool, respiratory, and tissue specimens were evaluated according to clinician discretion and available specimens were submitted to CDC for additional laboratory testing or pathology evaluation.
Findings:
Surveillance identified 377 patients from 45 US jurisdictions with hepatitis of unknown aetiology with onset from Oct 1, 2021, to Sept 30, 2022. The median age of patients was 2·8 years (IQR 1·2–5·0) and 192 (51%) were male, 184 (49%) were female, and one patient had sex unknown. Only 22 (6%) patients had a notable predisposing underlying condition. 347 patients (92%) were admitted to hospital, 21 (6%) subsequently received a liver transplant, and nine (2%) died. Among the 318 patients without notable underlying conditions, 275 were tested for adenovirus. Of these 116 (42%) had at least one positive specimen, and species F type 41 was the most frequent type identified (19 [73%] of 26 typed specimens were HAdV-41). Proportions of patients who had acute liver failure, received a liver transplant, and died were similar between those who tested positive for adenovirus compared with those who tested negative. Adenovirus species F was detected by polymerase chain reaction in nine pathology liver evaluations, but not by immunohistochemistry in seven of the nine with adequate liver tissue available. Interviews with caregivers yielded no common exposures.
Interpretation:
Adenovirus, alone or in combination with other factors, might play a potential role in acute hepatitis among immunocompetent children identified in this investigation, but the pathophysiologic mechanism of liver injury is unclear. To inform both prevention and intervention measures, more research is warranted to determine if and how adenovirus might contribute to hepatitis risk and the potential roles of other pathogens and host factors.
Funding:
None
Introduction
Severe acute hepatitis is a rare condition in young children that can result in acute liver failure, need for liver transplantation, and in rare instances, death.1,2 There are multiple known causes of paediatric acute hepatitis, including infectious (eg, hepatitis viruses A–E), immunological, metabolic, vascular, and toxin-related or drug-related causes.3 However, it is not unusual for the causes of hepatitis in children to remain unknown, with some estimates suggesting that a third of children with acute liver failure have no identified cause.4,5 Because paediatric acute hepatitis is rare, advancing our understanding of the causes has historically been challenging.
In late 2021 and early 2022, a cluster of previously healthy children with hepatitis of unknown aetiology, a high proportion of whom tested positive for adenovirus, was identified in the USA.6 Concurrently, additional clusters in the UK7 drew international attention because adenovirus, a known cause of hepatitis in immunocompromised children, is not known to cause hepatitis in previously healthy children.8,9 On April 21, 2022, the US Centers for Disease Control and Prevention (CDC) issued a health advisory requesting that clinicians report patients younger than 10 years with hepatitis of unknown aetiology to public health authorities.10 Similar investigations were also initiated internationally.11
By early July, 2022, 35 countries reported 1010 probable cases of severe acute hepatitis of unknown aetiology in children aged 16 years or younger.12 Although adenovirus, particularly human adenovirus type 41 (HAdV-41), was the most detected pathogen among patients under investigation globally (ranging from 9% to 66%),12,13 whether it caused these illnesses remains unknown. Other potential contributing factors, such as coinfections or host factors, could also have had a causal role. More data are needed to further describe the clinical and epidemiological characteristics of paediatric acute hepatitis of unknown aetiology, particularly among previously healthy children with no known predisposing factors for hepatitis.14
The main objective of this study was to describe the clinical and epidemiological characteristics of children identified with hepatitis of unknown aetiology between Oct 1, 2021, and Sept 30, 2022, in the USA to provide a thorough overview of the clinical course and potential exposures. We also aimed to compare characteristics of those who tested positive for adenovirus compared with those who tested negative.
Methods
Study design and participants
Children were reported and investigated as part of this national surveillance investigation if they were younger than age 10 years with acute hepatitis of unknown aetiology and had hepatitis onset on or after Oct 1, 2021, in the USA. This investigation was reviewed by CDC and was conducted in accordance with applicable federal law and CDC policy 45 C.F.R. part 46.102(l)(2). Because this project was determined to be public health surveillance and not human subject research, neither consent nor ethical approval were required.
Acute hepatitis was defined as elevated (>500 U/L) aspartate aminotransferase (AST) or alanine amino transferase (ALT). Treating clinicians determined whether the acute hepatitis was of unknown cause on the basis of diagnostic investigations. Patients with a confirmed cause determined by a clinician (eg, hepatitis A–E, drug-induced liver injury, or definitive autoimmune hepatitis) were not included in this investigation. Patients were reported by clinical providers to health departments and subsequently to CDC or identified by health departments using electronic queries of syndromic surveillance or electronic health records, and were confirmed by clinical providers. This analysis included patients identified both retrospectively and prospectively.
Procedures
Investigations included medical chart abstractions, caregiver interviews, laboratory testing, and pathological evaluation of liver tissue specimens. Medical chart abstractions were completed by clinicians and jurisdictional health departments and included data on demographics, underlying health conditions, signs and symptoms of illness, laboratory results, history of SARS-CoV-2 testing, vaccination history, radiological and liver pathology findings, diagnoses and treatment received, and outcomes. Interviews were conducted by jurisdictional health departments with a parent or guardian to obtain information on symptoms and health-care utilisation for the hepatitis illness, medical history, illness in close contacts or at school or daycare, diet, travel, and other potential exposures. Interviews were only conducted for caregivers of children with hepatitis onset before Aug 15, 2022. For patients with onset on Aug 15, 2022, or later, interview data were not collected because of a drastic decline in case counts and preliminary data analysis suggested no common exposures.
Clinical diagnostic investigations for infectious and non-infectious causes of hepatitis in patients were based on clinician discretion and was not standardised (ie, testing varied based on the individual patient and clinical judgement). In accordance with the CDC-issued health advisory, adenovirus nucleic acid amplification testing (eg, PCR) of blood, respiratory, or stool samples from patients was requested at the discretion of the treating clinician and conducted at a diagnostic or reference laboratory.
Residual adenovirus positive specimens were typed at select reference laboratories or the CDC laboratory using Sanger sequencing of the six hypervariable regions of the hexon gene. A phylogenetic tree was constructed to compare sequences of the hexon hypervariable region of HAdV-41 detected in these specimens to sequences from representative HAdV-41 sequences. Formalin-fixed, paraffin-embedded (FFPE) liver biopsy, explant, or autopsy tissue specimens underwent routine evaluation at the clinical institutions and data from pathology reports were abstracted using a standardised form. Any available residual FFPE tissue specimens were submitted to CDC for further pathological evaluation and infectious disease testing.
Current SARS-CoV-2 infection was defined as a positive real-time RT-PCR or antigen test within 14 days of hepatitis onset, while history of SARS-CoV-2 infection was defined according to serology, medical record documentation of a positive real-time RT-PCR or antigen test more than 14 days before hepatitis onset, or parental report of previously confirmed COVID-19. For applicable cases, acute infections were defined on the basis of real-time RT-PCR or serology indicators of acute infection (eg, immunoglobulin M result).
Two CDC paediatricians (HLK and PAG) independently reviewed the medical conditions noted in the chart abstraction to assess whether patients’ underlying medical conditions might have contributed to the development of hepatitis. All patients were categorised as follows: (1) no underlying medical conditions or underlying medical conditions that were unlikely to contribute to hepatitis; (2) underlying medical conditions that, in the right clinical circumstance, could possibly contribute to hepatitis; and (3) notable or specific underlying conditions that probably directly caused or contributed to hepatitis (hereafter described as patients with notable underlying medical conditions). Immunocompromising conditions, such as previous liver transplant and immunosuppressive therapy, were categorised as notable conditions that probably contribute to hepatitis. All other conditions were reviewed individually to determine the appropriate category (appendix p 2). Discordant categorisations were resolved after discussion and consensus between the two paediatricians. Patients in categories 1 and 2 are hereafter described as immunocompetent patients without notable underlying conditions.
Statistical analysis
Demographic characteristics and clinical outcomes were summarised for patients and stratified by underlying condition categories. Detailed clinical and exposure descriptions were restricted to immunocompetent patients without notable underlying conditions. The reason for excluding children with notable underlying conditions was, first, that the relationship between hepatitis and the various factors explored in the analyses might be confounded by underlying conditions and, second, adenovirus (a focus of this investigation) is a rare but recognised cause of hepatitis in immunocompromised children. Categorical variables were described using proportions and compared between strata using Fisher’s exact test or Yates’ continuity adjusted χ2 test and continuous variables were described using medians and IRQs and compared using Wilcoxon rank sum test. Select demographics, clinical features, and exposures were stratified by adenovirus viraemia (positive vs negative in any blood specimen) and any adenovirus test results (positive vs negative in any specimen). The expected proportion of Hispanic or Latino patients was calculated using the state-based proportions of Hispanic or Latino people younger than 10 years from the population estimates from the US 2020 bridged-race vintage postcensal file15 and weighting by the number of patients reported by state. The proportion of Hispanic or Latino patients was compared with the expected proportion of Hispanic or Latino patients using χ2 test. Deaths were defined as any death related to the hepatitis episode, regardless of the time between hepatitis onset and death. Missing data were excluded from analyses. REDCap electronic data tools16,17 hosted at CDC were used for data management and SAS (version 9.4) was used for statistical analyses.
Role of the funding source
There was no funding provided for this investigation.
Results
A total of 377 children younger than 10 years with hepatitis of unknown aetiology with onset from Oct 1, 2021, to Sept 30, 2022, were reported to CDC from 45 US jurisdictions. The number of children presenting with hepatitis of unknown aetiology peaked during the week of May 1, 2022, near the time that the CDC health advisory was issued on April 21, 2022, with a subsequent steady decline in reported cases (figure 1). The median age among patients was 2·8 years (IQR 1·2–5·0; table 1). 192 (51%) patients were male and 184 (49%) were female (one patient had sex unknown). There were 347 (92%) patients who required hospital admission, 21 (6%) who received a liver transplant, and nine (2%) who died. Among 352 patients with known race and ethnicity, 153 (43%) were Hispanic or Latino, 130 (37%) were non-Hispanic White, and 38 (11%) were non-Hispanic Black. The proportion of reported Hispanic or Latino patients was significantly higher than the expected proportion of Hispanic or Latino patients based on census estimates (43% vs 24%; p<0·0001). 259 (69%) of 377 patients had no underlying conditions or had underlying conditions that were unlikely to contribute to hepatitis, while 59 (16%) had underlying conditions that possibly contributed to their hepatitis, and 22 (6%) patients had notable underlying medical conditions that probably contributed to their hepatitis; 37 (10%) had unknown underlying conditions. Among 332 patients with completed chart abstraction, 279 (84%) patients reported at least one sign or symptom of gastrointestinal illness, including vomiting (211 [64%] of 332) and diarrhoea (160 [48%] of 332) during their illness or before hepatitis onset.
Figure 1.
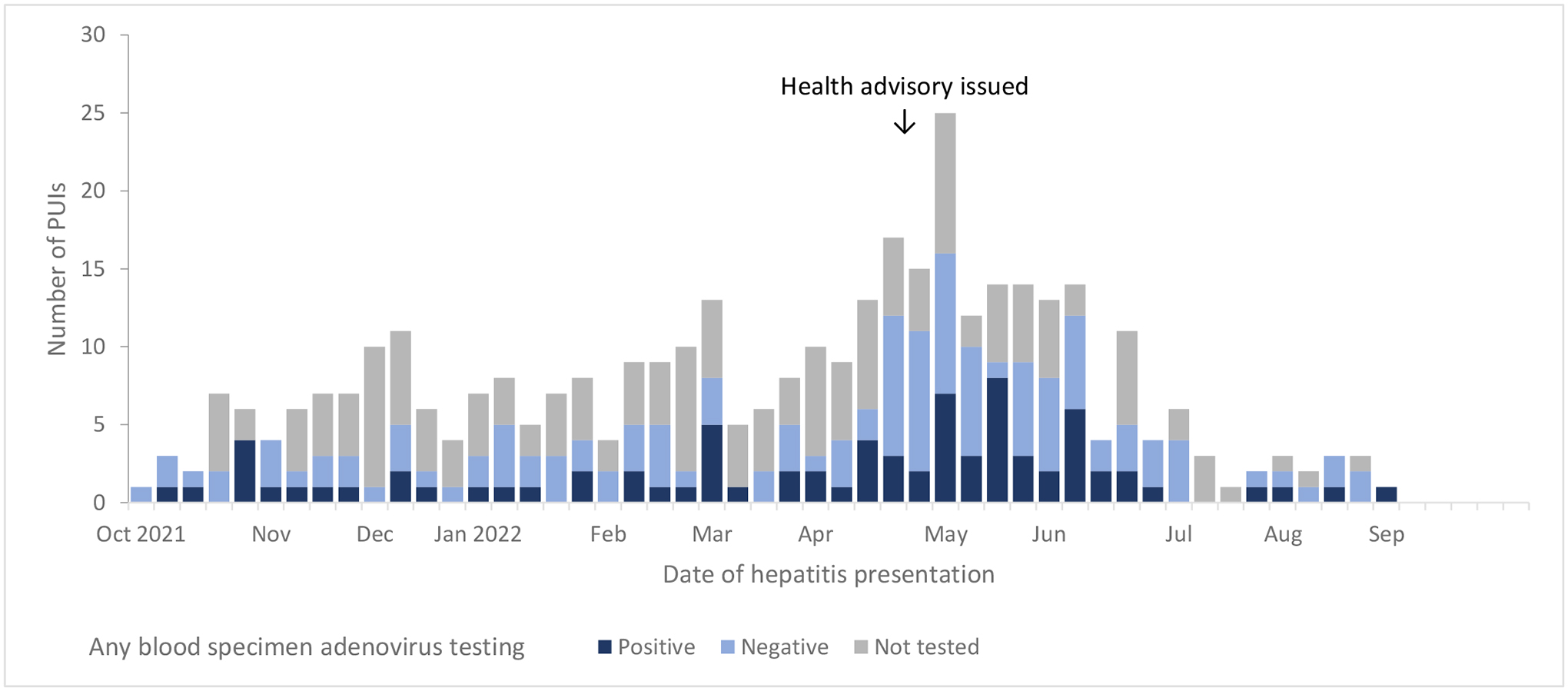
Epidemiologic curve of reported children with acute hepatitis of unknown etiology in the United States from October 1, 2021 – September 30, 2022, stratified by adenovirus testing results among any blood specimen type (N=377). The trend in cases should be interpreted in the context of potential reporting bias, given the cluster in identified children around the time the health advisory was issued.
Table 1.
Demographic characteristics and patient outcomes among all US patients with hepatitis of unknown etiology from October 1, 2021 – September 30, 2022, stratified by underlying condition category (N=377).
| Stratified by whether the patient had underlying conditions that could contribute to their hepatitis*,† | ||||
|---|---|---|---|---|
| Overall | ||||
| Characteristic | No. (%) | No | Possibly | Likely |
| All patients | N=377 | N=259 | N=59 | N=22 |
| Age, years, median (IQR) | 2.8 (1.2–5.0) | 2.9 (1.5–5.1) | 3.1 (1.2–5) | 1.7 (0.5–3.0) |
| Sex | ||||
| Male | 192 (51%) | 134 (52%) | 28 (47%) | 11 (50%) |
| Female | 184 (49%) | 125 (48%) | 31 (53%) | 11 (50%) |
| Unknown | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Race and ethnicity ‡ | ||||
| Hispanic or Latino | 153 (41%) | 110 (43%) | 24 (41%) | 9 (41%) |
| White, non-Hispanic | 130 (35%) | 90 (35%) | 20 (34%) | 7 (32%) |
| Black, non-Hispanic | 38 (10%) | 25 (10%) | 7 (12%) | 4 (18%) |
| Asian, non-Hispanic | 11 (3%) | 10 (4%) | 0 (0%) | 0 (0%) |
| Multiple, non-Hispanic | 10 (3%) | 5 (2%) | 4 (7%) | 0 (0%) |
| American Indian/Alaskan, non-Hispanic | 6 (2%) | 4 (2%) | 2 (3%) | 0 (0%) |
| Hawaiian/Other Pacific Islander, non-Hispanic | 4 (1%) | 3 (1 %) | 0 (0%) | 1 (5%) |
| Unknown/missing | 25 (7%) | 12 (5%) | 2 (3%) | 1 (5%) |
| Outcome | ||||
| Hospitalized | 347 (92%) | 244 (94%) | 50 (85%) | 21 (95%) |
| Length of stay (days) | 4 (2–9) | 4 (2–8.5) | 3.5 (2–9) | 3 (2–18) |
| Received liver transplant | 21 (6%) | 15 (6%) | 3 (5%) | 0 (0%) |
| Died | 9 (2%) | 5 (2%) | 2 (3%) | 2 (9%) |
| Measure of severity of acute hepatitis § | ||||
| Patients with completed chart abstraction§ | 332 (88%) | 250 (97%) | 57 (97%) | 21 (95%) |
| AST, median (IQR) | 1761 (717–3577) | 2076 (806–3763) | 1268 (645–2931) | 890 (552–2037) |
| ALT, median (IQR) | 1662 (667–2994) | 1846 (784–3279) | 1010 (565–2134) | 906 (626–1567) |
| INR, median (IQR) | 1.3 (1.1–1.9) | 1.3 (1.1–2.0) | 1.3 (1.0–1.6) | 1.2 (1.1–1.5) |
| Hepatomegaly | 113 (34%) | 89 (36%) | 17 (30%) | 7 (33%) |
| Splenomegaly | 36 (11%) | 28 (11%) | 4 (7%) | 4 (19%) |
| Ascites | 38 (11%) | 28 (11%) | 6 (11%) | 4 (19%) |
| Acute hepatitis with acute liver failure# | 84 (25%) | 68 (27%) | 14 (25%) | 2 (10%) |
| ALF based on coding** | 63 (19%) | 51 (20%) | 9 (16%) | 2 (10%) |
| Hepatic encephalopathy# | 39 (12%) | 30 (12%) | 9 (16%) | 0 (0%) |
| Hepatic lymphohistiocytosis# | 4 (1%) | 1 (<1%) | 2 (4%) | 1 (5%) |
| Signs and symptoms during illness or prior to hepatitis onset § | ||||
| Any respiratory | 165 (50%) | 127 (51%) | 28 (49%) | 8 (38%) |
| Cough/rhinorrhea | 137 (41%) | 104 (42%) | 24 (42%) | 7 (33%) |
| Congestion | 67 (20%) | 48 (19%) | 12 (21%) | 7 (33%) |
| Any gastrointestinal | 279 (84%) | 217 (87%) | 46 (81%) | 14 (67%) |
| Vomiting | 211 (64%) | 166 (66%) | 34 (60%) | 10 (48%) |
| Diarrhea | 160 (48%) | 130 (52%) | 21 (37%) | 9 (43%) |
| Abdominal pain | 143 (43%) | 114 (46%) | 23 (40%) | 6 (29%) |
| Any systemic | 296 (89%) | 227 (91%) | 51 (89%) | 15 (71%) |
| Fatigue | 205 (62%) | 163 (65%) | 32 (56%) | 10 (48%) |
| Decreased appetite | 204 (61%) | 162 (65%) | 33 (58%) | 8 (38%) |
| Fever | 165 (50%) | 121 (48%) | 30 (53%) | 12 (57%) |
| Hepatitis signs and symptoms | 215 (65%) | 180 (72%) | 28 (49%) | 7 (33%) |
| Jaundice | 190 (57%) | 161 (64%) | 24 (42%) | 5 (24%) |
| Dark-colored urine | 87 (26%) | 75 (30%) | 11 (19%) | 1 (5%) |
| Days from gastrointestinal, respiratory, or systemic sign/symptom onset to presentation for hepatitis illness | ||||
| Gastrointestinal to hepatitis presentation, days, median (IQR) | 3 (1–7) | 3 (1–7) | 3 (1–6) | 1 (1–4) |
| Respiratory to hepatitis presentation, days, median (IQR) | 4 (2–9) | 4 (2–10) | 3 (2–8) | 4 (2–14) |
| Systemic signs/symptoms to hepatitis presentation, days, median (IQR) | 3 (1–6) | 3 (1–6) | 2 (1–5) | 1 (0–2) |
| Gastrointestinal, respiratory, or systemic signs/symptoms to hepatitis presentation, days, median (IQR) | 4 (2–8) | 4 (2–8) | 4 (2–7) | 2 (0–10) |
Abbreviations: ALF = acute liver failure; ALT= alanine aminotransferase; AST= aspartate transaminase; IQR= interquartile range;
Underlying condition strata: “No”: Those with no underlying medical conditions or underlying medical conditions unlikely to contribute to hepatitis, “Possibly”: Those with some underlying medical conditions that in the right circumstances, possibly could contribute and/or result in worsening hepatitis, “Likely”: patients with significant or specific comorbid medical conditions that likely could directly cause and/or contribute to hepatitis.
Four patients with underlying conditions marked unknown and 33 without complete medical record abstraction could not be classified into one of the underlying condition strata.
Race and ethnicity data were obtained from parental report, if available, and if parental report was not available then race and ethnicity were obtained from the medical chart abstraction.
The denominators for all measures of severity of acute hepatitis and for signs and symptoms during illness or prior to hepatitis onset only included patients with completed chart abstractions.
Acute liver failure, hepatic encephalopathy, and hepatic lymphohistiocytosis were defined based on documentation written from the chart abstraction indicating these outcomes occurred.
Acute liver failure based on coding was defined as INR >1.5 AND hepatic encephalopathy or INR >2 (without hepatic encephalopathy)
Patients with notable underlying medical conditions were younger (median age 1·7 years [IQR 0·5–3·0] vs 2·9 years [1·5–5·1]), had lower AST and ALT concentrations, and reported fewer gastrointestinal, systemic, and hepatitis signs and symptoms compared with patients with no underlying conditions. The proportions of patients who were admitted to hospital, received a liver transplant, or died were not significantly different by underlying condition group. The median number of days from onset of gastrointestinal, respiratory, or systemic signs or symptoms to presentation for hepatitis illness was shorter, although not statistically significant, among patients with notable underlying conditions than in patients with no underlying conditions (median 2·0 days [0·0–10·0] vs 4·0 [2·0–8·0]). All further analyses were restricted to the 318 patients without notable underlying medical conditions.
Adenovirus testing was conducted in 275 (86%) of 318 patients without notable underlying medical conditions with 116 (42%) of the 275 positive in at least one specimen type (table 2; figure 2; appendix p 3). Among 275 patients without notable underlying medical conditions who had any adenovirus testing performed, 35 (13%) had only blood specimens tested, 59 (21%) had only respiratory specimens tested, and three (1%) had only stool tested, while the remaining 178 (65%) had a combination of different specimen types tested (appendix p 5). 15 patients had concurrent whole blood and plasma tested, among whom 14 (93%) had concordance of adenovirus results between the two specimen types (appendix p 6). Among patients without notable underlying conditions, 19 (73%) of 26 typed specimens were HAdV-41 and 17 (94%) of 18 whole blood specimens were HAdV-41 (appendix p 7). Phylogenetic analysis of HAdV-41 sequences from the 19 specimens suggests differences in the HAdV-41 hexon sequences associated with these cases (appendix p 8). The median (IQR) adenovirus viral load was slightly higher among eight patients with acute liver failure and available viral load data than in 25 patients without acute liver failure (15 854 copies per mL [1782–57 376] vs 5132 copies per mL [500–11 060]).
Table 2.
Pathogens detected among US patients with hepatitis of unknown etiology without significant underlying conditions from October 1, 2021 – September 30, 2022 (N=318).
| Characteristic | No. (%) |
|---|---|
| Patients | N=318* |
| Adenovirus positivity, no. positive/total no. tested (%) | |
| Any specimen type | 116/275 (42%) |
| Whole blood | 53/119 (45%) |
| Plasma | 22/85 (26%) |
| Serum | 8/17 (47%) |
| Any blood | 71/189 (38%) |
| Stool | 28/101 (28%) |
| Respiratory | 52/222 (23%) |
| Liver | 9/61 (15%) |
| SARS-CoV-2 | |
| Acute SARS-CoV-2 infection, no. positive/total no. tested (%) | 24/290 (8%) |
| History of SARS-CoV-2 infection† | 54/308 (18%) |
| Serology | 14/29 (48%) |
| Date of past infection, median (IQR) | Dec 21, 2021 (Aug 21, 2021–Jan 13, 2022) |
| Days since last infection, median (IQR) | 144 (92–230) |
| Gastrointestinal (GI) pathogens ‡ | 28/99 (28%) |
| Escherichia coli (EAEC, EPEC, ETEC, O157, EIEC) | 11/81 (14%) |
| Norovirus GI/GII | 4/84 (5%) |
| Rotavirus | 7/84 (8%) |
| Sapovirus | 6/81 (7%) |
| Salmonella § | 2/81 (3%) |
| Other GI pathogen# | 8/97 (8%) |
| Respiratory pathogens ‡ | 97/259 (37%) |
| Metapneumovirus | 13/235 (6%) |
| Rhinovirus/Enterovirus** | 73/252 (29%) |
| Human Coronavirus†† | 12/235 (5%) |
| Parainfluenza (1–4) | 9/235 (4%) |
| Influenza A‡‡ | 4/235 (2%) |
| RSV | 5/235 (2%) |
| Other respiratory pathogen§§ | 9/241 (4%) |
| Other viral testing | 45/212 (21%) |
| Acute EBV## | 18/87 (21%) |
| Acute CMV*** | 20/163 (12%) |
| HHV-6††† | 10/43 (23%) |
| Varicella-zoster virus††† | 1/13 (8%) |
| HSV-1††† | 1/54 (2%) |
| Hepatitis A – E‡‡‡ | 0/262 (0%) |
| Parvovirus B19 | 0/29 (0%) |
| Pathogen Count | |
| Median (range) | 1 (0, 12) |
| No Pathogen Detected | 100/304 (33%) |
| 1 pathogen | 116/304 (38%) |
| 2 pathogens | 45/304 (15%) |
| ≥3 pathogens | 43/304 (14%) |
Abbreviations: CMV=cytomegalovirus; EAEC= Enteroaggregative Escherichia coli (E. coli); EBV=Epstein-Barr Virus; EPEC= Enteropathogenic E. Coli; ETEC= Enterotoxigenic E. coli; EIEC= Enteroinvasive Escherichia coli; IQR= interquartile range; RSV=respiratory syncytial virus; HHV=human herpes virus.
This includes patients with no underlying medical conditions or underlying medical conditions unlikely to contribute to hepatitis (N=259), and patients with some underlying medical conditions that in the right circumstances, possibly could contribute and/or result in worsening hepatitis (N=59).
Based on 1) serology, 2) documentation of a positive SARS-CoV-2 PCR or antigen test more than 14 days prior to hepatitis onset, or 3) parental report of history of COVID-19 illness more than 14 days prior to hepatitis onset with a confirmed test result.
Most respiratory and GI pathogens tested as part of a panel but panel targets vary and panels used by clinical lab/facility also vary, thus the denominator of negative test results may be over-estimated.
Two patients positive by stool culture; 1 for s. enteriditis, 1 for unspecified salmonella spp
Eight patients positive for Campylobacter (2), C.difficile (3), Plesiomonas shigelloides (1), Vibrio nonsp. (1), Y.enterocolitica (1), Giardia (1), astrovirus (3)
Combines testing for rhinovirus/enterovirus via multiplex respiratory panel as well as testing via enterovirus specific NAAT (unknown specimen types).
Includes testing for the common HCVs (e.g. 229E, HKU1, HL63, OC43), not SARS-CoV-2
All influenza A serotype specific results combined (i.e., A/H3, A/H1N1, etc.)
Nine patients positive for parapertussis (1), pertussis (2), chlamydia pnuemoniae (1), mycoplasma (1), acinetobacter (1), klebsiella (1), p.aeruginosa (1), s.maltophilia (2), and unspecified respiratory pathogens (6)
Acute EBV defined as positive EBV viral capsid antigen (VCA) immunoglobulin M (IgM) or early antigen (EA) immunoglobulin G (IgG) test result, or diagnosis of primary EBV in the medical chart.
Acute CMV based on clinical diagnosis, verified by PCR IgM result.
HHV-6, Varicella-zoster virus, and HSV-1 defined as positive PCR or IgM; IgG antibody results were not included.
One patient with Hepatitis E IgM positive but IgG negative on initial and repeat testing, thus clinician expressed concern of false positive Hepatitis E results. Hepatitis virus testing using either antibody or RNA/DNA diagnostic tests were abstracted from the medical chart.
Figure 2.
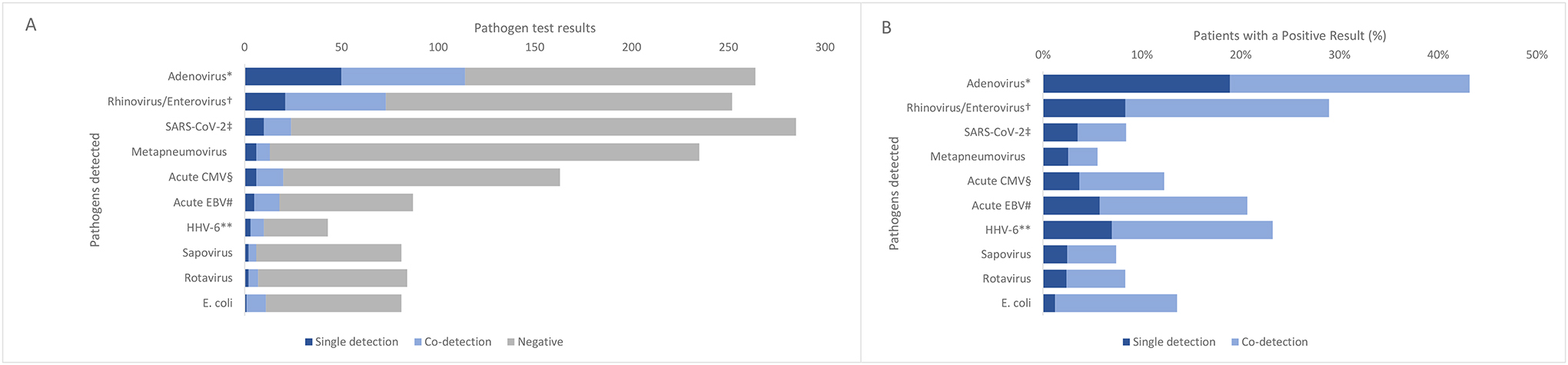
Absolute numbers (A) of single detection, co-detection, and negative test results for pathogens tested for and proportions (B) of patients with a positive single detection or co-detection result, among patients without significant underlying conditions (N=318).
Abbreviations: EBV=Epstein-Barr Virus; HHV=human herpes virus.
* Among any specimen type
† Combines testing for rhinovirus/enterovirus via multiplex panel as well as testing via enterovirus specific NAAT
‡ Current SARS-CoV-2 infection defined as a positive PCR or antigen test within 14 days of hepatitis onset.
§ Acute CMV based on clinical diagnosis, verified by PCR IgM result.
# Acute EBV defined as positive EBV viral capsid antigen (VCA) immunoglobulin M (IgM) or early antigen (EA) immunoglobulin G (IgG) test result, or diagnosis of primary EBV in the medical chart.
** HHV-6 defined as positive PCR or IgM; IgG antibody results were not included.
Adenovirus was the most prevalent pathogen detected (table 2, figure 2). 290 (91%) of 318 patients were tested for acute SARS-CoV-2, of whom 24 (8%) had a current SARS-CoV-2 infection. Of 304 patients tested for at least one pathogen, 88 (29%) tested positive for multiple concurrent pathogens. Only 88 (27%) of the 318 patients were age-eligible18,19 to receive the COVID-19 vaccine and nine (3%) had received at least one dose before illness onset.
Among liver biopsy specimens from 79 patients without notable underlying conditions who underwent routine evaluation at the clinical institutions, 53 (67%) had findings of acute or active hepatitis, and none had viral or intranuclear inclusions or smudge cells (appendix p 9). There were findings potentially consistent with autoimmune hepatitis in eight (10%) patients, fibrosis in 18 (23%) patients, and portal inflammation in 31 (39%) patients. FFPE liver tissue specimens from 25 patients without notable underlying conditions were submitted to CDC. Adenovirus species F was detected in liver tissue from nine patients by conventional PCR20 and confirmed by sequencing, eight of which also had detection of adenovirus from one or more other specimens. Of these nine patients, seven had adequate liver tissue that was available for performance of immunohistochemistry and adenovirus was not detected by immunohistochemistry in any of these specimens. Liver tissue samples from patients without notable underlying conditions examined at CDC demonstrated varying degrees of active hepatitis; changes were non-specific and not typical for adenovirus hepatitis, with no viral inclusions observed. Electron microscopy was performed on liver tissue specimens from six of these patients as previously reported, and no viral particles were observed.14,20
Among 314 patients without notable underlying conditions with hepatitis onset before Aug 15, 2022, 192 (61%) had a caregiver interviewed. No common exposures related to travel, family structure, diet, water source, or toxins were identified (appendix p 10). Acetaminophen use (known to cause liver injury at high doses) in the 2 months before hepatitis onset was the most frequently reported medication used (18 [16%] of 112; appendix p 10). However, 161 patients had medical record documentation of acetaminophen drug level testing and only one patient was identified with potential acetaminophen toxicity. 83 (44%) of 187 patients attended a daycare or school during the month preceding the hepatitis illness. Among patients born prior to 2021, the median number of months of in-person child-care was zero (IQR 0–4) in 2020 compared to 5 months (4–9) in 2021. 85 (48%) of 176 caregivers reported that the patient had another acute illness, primarily respiratory or gastrointestinal, in the 2 months before hepatitis onset. 92 (63%) of 146 caregivers sought medical care with the patient’s primary care provider for their hepatitis illness.
A higher proportion of patients who tested positive for adenovirus were Hispanic or Latino compared with those who tested negative (table 3). Compared with patients who tested negative, more patients who tested positive for adenovirus reported diarrhoea and jaundice (table 3). Among patients who tested positive for adenovirus, 46 (63%) of 73 had a caregiver report of another acute illness, primarily respiratory or gastrointestinal, in the 2 months before hepatitis onset and 27 (42%) of 65 had reports of acute illness, primarily respiratory or gastrointestinal, in household members or other close contacts in the 2 months before hepatitis onset. Among patients who tested negative for adenovirus, fewer had a reported acute illness in the 2 months before hepatitis onset (30 [35%] of 85; p=0.0008) and 39 (47%) of 83 had a reported acute illness in a household member or other close contact in the 2 months before hepatitis onset. The proportion of severe outcomes, including hospital admission, liver transplant or death, and markers of hepatitis severity, including acute liver failure and hepatic encephalopathy, were similar among patients who tested positive for adenovirus and those who tested negative. Findings were similar when stratified by adenovirus viraemia. The proportion of severe outcomes and markers of hepatitis severity were similar between patients with detection of HAdV-41 and patients with non-HAdV-41 hexon sequence detected, although the small sample size limits robust comparisons between sequence types (appendix p 7). The higher proportion of transplant recipients among patients who had HAdV-41 (four [22%] of 18) and non-HAdV-41 (one [14%] of seven) sequences detected than among the overall population (21 [6%] of 377) is probably biased by higher proportions of specimens available for sequencing among patients who received a liver transplant.
Table 3.
Select demographics, clinical features, and risk factors from the interview, among patients without underlying conditions and with adenovirus test results (N=275) stratified by adenovirus test results among any specimen and adenovirus test results among blood specimens.
| Stratified by any adenovirus positive specimen | p-value | Stratified by adenovirus viremia | p-value | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Yes | No | Yes | No | |||
| Patients | N=116 | N=159 | N=71 | N=118 | |||
| Age, yrs, median (IQR) | 2.6 (1.3, 4.2) | 3 (1.4, 5.6) | 0.20 | 2.8 (1.3, 4.5) | 2.9 (1.2, 5.3) | 0.80 | |
| Race and ethnicity | 0.35* | 0.20* | |||||
| Hispanic or Latino | 61 (53%) | 62 (39%) | 0.063† | 42 (59%) | 47 (40%) | 0.022† | |
| White, non-Hispanic | 33 (28%) | 58 (36%) | 17 (24%) | 48 (41%) | |||
| Black, non-Hispanic | 12 (10%) | 15 (9%) | 7 (10%) | 11 (9%) | |||
| Asian, non-Hispanic | 3 (3%) | 6 (4%) | 1 (1%) | 3 (3%) | |||
| Multiple race, non-Hispanic | 1 (1%) | 5 (3%) | 1 (1%) | 2 (2%) | |||
| American Indian or Alaska Native, non-Hispanic | 3 (3%) | 3 (2%) | 1 (1%) | 2 (2%) | |||
| Native Hawaiian or other Pacific Islander, non-Hispanic | 2 (2%) | 1 (1%) | 1 (1%) | 1 (1%) | |||
| Unknown or missing | 1 (1%) | 9 (6%) | 1 (1%) | 4 (3%) | |||
| Outcome | |||||||
| Hospitalized | 113 (97%) | 150 (94%) | 0.25 | 69 (97%) | 114 (97%) | 1.0 | |
| Received liver transplant | 7 (6%) | 11 (7%) | 1.0 | 7 (10%) | 9 (8%) | 0.79 | |
| Died | 1 (1%) | 5 (3%) | 0.41 | 1 (1%) | 3 (3%) | 0.65 | |
| Measure of severity of acute hepatitis § | |||||||
| Acute hepatitis with acute liver failure | 29/114 (25%) | 46/150 (31%) | 0.41 | 22/70 (31%) | 43/112 (38%) | 0.34 | |
| ALF based on coding# | 21/114 (18%) | 32/150 (21%) | 0.64 | 15/70 (21%) | 34/112 (30%) | 0.17 | |
| Hepatic encephalopathy | 16/114 (14%) | 22/150 (15%) | 1.0 | 13/70 (19%) | 24/112 (21%) | 0.71 | |
| AST, median (IQR) | 2173 (1010–3803) | 1628 (642–2866) | 0.0050 | 2524 (1442–4126) | 1871 (946–3239) | 0.016 | |
| ALT, median (IQR) | 2889 (1041–4361) | 1627 (745–3203) | 0.014 | 3234 (1715–4932) | 2263 (1078–4156) | 0.018 | |
| Signs and symptoms during illness | |||||||
| Diarrhea | 66 (57%) | 68 (43%) | 0.028 | 46 (65%) | 53 (45%) | 0.011 | |
| Fever | 56 (48%) | 80 (50%) | 0.81 | 36 (51%) | 56 (47%) | 0.65 | |
| Jaundice | 82 (71%) | 89 (56%) | 0.017 | 61 (86%) | 67 (57%) | <0.0001 | |
| Other exposures or risk factors | |||||||
| Parental report of other illnesses in the child 2 months prior to hepatitis illness onset that required treatment or care | 46/73 (63%) | 30/85 (35%) | 0.0008 | 34/52 (65%) | 26/66 (39%) | 0.0055 | |
| Parental report of any new illnesses or infections in household members or other close contacts in the 2 months prior to hepatitis illness onset | 27/65 (42%) | 39/83 (47%) | 0.62 | 20/45 (44%) | 29/65 (45%) | 1.0 | |
| Household size, median (range) | 5 (2–13) | 4 (2–9) | 0.91 | 5 (2–13) | 4 (2–8) | 0.10 | |
| Number of months of in-person child-care or daycare attendance in 2020, median (IQR) ** | 0 (0–4) | 0.5 (0–4) | 0.96 | 0 (0–4) | 0 (0–3) | 0.51 | |
| Number of months of in-person child-care or daycare attendance in 2021, median (IQR) †† | 5 (3–9) | 5 (4–9) | 0.50 | 5 (3.5–9) | 5 (4–9) | 0.95 | |
Abbreviations: ALF = acute liver failure; ALT= alanine aminotransferase; AST= aspartate transaminase; IQR= interquartile range;
Fisher’s exact test p-value comparing the difference in distribution of all race and ethnicity groups between patients who tested positive and negative for adenovirus.
Yates’ continuity adjusted chi-squared test p-value comparing the proportion of patients who were Hispanic or Latino vs non-Hispanic or Latino between patients who tested positive and negative for adenovirus.
indicates cell sizes too small for appropriate chi-square estimation
Acute liver failure, hepatic encephalopathy, and hepatic lymphohistiocytosis were defined based on documentation written from the chart abstraction indicating these outcomes occurred.
Acute liver failure based on coding was defined as INR >1.5 AND hepatic encephalopathy or INR >2 (without hepatic encephalopathy)
Denominator includes 48 patients who were born prior to 2020.
Denominator includes 65 patients who were born prior to 2021.
Discussion
We describe detailed clinical and epidemiological data from children with acute hepatitis of unknown aetiology in the USA, who were reported to CDC between Oct 1, 2021, and Sept 30, 2022. Overall, findings were consistent with preliminary evaluations of the US interim data published in June, 2022.14 Most children were previously healthy, with only 6% of patients having a notable predisposing underlying condition. Adenovirus was the most commonly detected pathogen among those tested, although it remains unclear if adenovirus was the causative agent for hepatitis in these children. Children who tested positive for adenovirus were similar to those who tested negative for adenovirus for most demographic characteristics and markers of hepatitis severity. Diagnostic testing for pathogens varied, but co-detection of pathogens was documented in almost a third of patients. No other common exposures were identified. Future case–control, pathophysiological, and immunological evaluations are warranted to elucidate the role of adenovirus, alone or in conjunction with other co-factors, in the development of paediatric acute hepatitis.
Hispanic or Latino children were overrepresented when compared with the 2020 US census. The reason for this overrepresentation is unclear; it could be related to an unidentified exposure, socioeconomic health disparities that might have increased the risk for infection or decreased access to primary care among Hispanic or Latino populations, or host factors that predispose some children to more severe hepatitis than others. One small study among a White ethnicity population in the UK found that genetic markers known as human leukocyte antigen (HLA) alleles could be associated with increased susceptibility to hepatitis.21 More research is needed to elucidate the relationship between HLA alleles and paediatric hepatitis of unknown aetiology, especially in diverse populations.
Adenovirus was the most commonly detected pathogen among patients in this investigation, consistent with findings globally.12 HAdV-41, which typically causes gastrointestinal symptoms, remains of particular interest. HAdV-41 was the most frequent genotype detected, consistent with genotyping done in the UK.22 Although most adenovirus-positive specimens were not available for genotyping in our investigation, the high proportion of diarrhoea among patients who tested positive for adenovirus is further suggestive of enteric HAdV-41. These HAdV-41 strains cluster closely to known HAdV-41 hexon sequences in phylogenetic analysis, suggesting that these infections were not caused by a new adenovirus strain.20 Children who tested positive for adenovirus were similar to those who tested negative for adenovirus for most characteristics and did not have more severe illness than did those who tested negative for adenovirus.
The high prevalence of adenovirus and HAdV-41 in this study alone does not implicate this pathogen as a causative agent. However, a case–control investigation in the UK found a strong association between adenovirus and paediatric hepatitis of unknown aetiology, and there has been ecological evidence of a correlation between adenovirus and hepatitis.22,23 Taken together, these findings suggest some role for this pathogen; however, potential mechanisms of action remain poorly understood. There was detection of adenovirus by conventional PCR in the liver, but pathological evaluations of liver specimens among immunocompetent patients without notable underlying conditions in this investigation generally did not show classic features of adenovirus hepatitis seen in immunocompromised individuals.20,24 Detection of adenovirus by conventional PCR in the liver does not differentiate between liver cell infection versus viraemia from blood in the liver. The lack of direct cytopathic effect of HAdV-41 on the liver in the subset of patients with liver pathology evaluations suggests that if HAdV-41 plays a role in acute hepatitis in immunocompetent children, it might be indirectly involved in liver injury. There is growing evidence of the role of co-factors, including HLA alleles and adeno-associated virus 2 (AAV2), in the association between adenovirus and acute hepatitis of unknown aetiology.21,25,26 As previously reported, a subset of 14 patients from this US investigation underwent more advanced metagenomic testing, of whom 13 (93%) had AAV2 detected, a significantly higher proportion of AAV2 detection than the control group.25 These findings have been corroborated by two independent UK studies.21,26
At least one pathogen was detected in two thirds of previously healthy patients and co-infections were identified in almost a third of patients. The significance of these detections and their contribution to hepatitis are difficult to interpret. Several detected pathogens (eg, rhinoviruses and enteroviruses) are common among children, especially since COVID-19 social distancing and masking restrictions were no longer routinely recommended in many settings in late 2021 and 2022, leading to increases in pathogen detections to equal to or higher than pre-pandemic levels.27 Other pathogens (eg, cytomegalovirus, Epstein-Barr Virus, or human herpesvirus 6) are known potential contributors to liver disfunction, but it is unclear if these infections contributed to hepatitis in patients in whom these pathogens were detected.3,28 Current or previous infection with SARS-CoV-2, part of an autoimmune and superantigen-mediated immune-cell activation hypothesis early in this investigation,29,30 was not common among US patients and was not significantly associated with hepatitis in UK investigations.22
A caregiver interview was completed for most patients. No common exposures or risk factors were identified among children with acute hepatitis of unknown aetiology included in this report. COVID-19 vaccination is unlikely to be related to these hepatitis illnesses, given the low percentage of children who had received the vaccine and that only a quarter of children were age-eligible to receive the COVID-19 vaccine by the time of their illness. Findings from caregiver interviews show that children had opportunities for exposures to infectious pathogens in the 2 months before illness onset, following periods of reduced potential exposures during the COVID-19 pandemic. Acetaminophen toxicity was not a common finding, although acetaminophen use was frequently reported by caregivers. Another finding from these interviews was the high proportion of caregivers who sought care for their child’s illness from their primary care providers. Although not unexpected given the vital role of paediatricians, it warrants a reminder for general paediatric clinicians to remain aware of acute hepatitis of unknown aetiology and provide education to caregivers about the potential escalation to severe illness and the need for prompt clinical care if symptoms worsen.
Although this investigation was initiated on the basis of concerns of an outbreak or unexplained increase in paediatric acute hepatitis of unknown aetiology, and a potential association to adenovirus, it was not possible to directly compare the 377 children with acute hepatitis of unknown aetiology identified through this newly initiated surveillance to cases from previous years because historical data are scarce. A previous evaluation of trends in hepatitis-associated emergency department visits and hospital admissions, liver transplants, and adenovirus stool testing results among children in the USA from 2017 to 2022 did not suggest an increase in paediatric hepatitis of unspecified cause or detection of adenovirus types 40 or 41 in children above pre-COVID-19 pandemic baseline levels.23 The UK did see an increase in hepatitis and adenovirus-positive stool specimen test results among children aged 1–4 years when comparing 2022 with previous years, although similar trend analyses were inconclusive in other countries.22,31,32 Evidence of adenovirus detection in banked liver specimens from UK immunocompetent children with acute liver failure in 2017–19 suggest that the potential association between adenovirus and acute hepatitis might have existed previously and been unrecognised until this more recent 2021–22 investigation. As of Aug 30, 2023, the USA continues to receive reports on only a small number of children with hepatitis of unknown aetiology since Oct 1, 2022,33 and other countries have not reported new cases since July, 2022.12,22 This decrease in cases might support one hypothesis that the peak in cases in the USA and globally in April–May of 2022 might have partially been related to changes in exposure patterns to adenovirus or other co-factors such as AAV2 following a lifting of COVID-19-related non-pharmaceutical interventions in many settings. Additionally, increased reporting in April–May of 2022 and the subsequent decline cases reported over the following months might have been due to variability in clinician engagement and reporting patterns.
This investigation was subject to several limitations. The method for identification of patients varied over time and across jurisdictions. Patients with hepatitis onset before April, 2022, were identified retrospectively and some jurisdictions used syndromic surveillance34 to identify patients, in addition to clinician reporting, or had more engaged clinicians reporting from specialty transplant or liver and digestive centres. Although all patients were identified with acute hepatitis based on the treating clinician’s diagnostic investigations, patients presenting due to an underlying chronic liver disease might have been included in this investigation. Clinicians might also have reported patients with some evidence of potential alternative cause, for which evidence was suggestive but not sufficient to confirm these as definitive causes. There might have been underreporting of less severe illnesses, limiting generalisability of our findings. Tests done to detect pathogens were influenced by the health advisory, clinical presentation, and clinician judgment, thus not all patients received diagnostic tests for the same spectrum of pathogens. Finally, the findings reported here are limited by the lack of a comparison group. A case–control evaluation in the USA is underway to prospectively identify matched controls to evaluate unresolved questions related to adenovirus, SARS-CoV-2, AAV2, and host factors such as HLA type.
In summary, the cause of hepatitis in these children remains unknown. The high prevalence of adenovirus detection aligns with other studies suggesting a potential role of adenovirus, particularly type HAdV-41; however, the mechanism of action is unclear. To inform both prevention and intervention measures, more research is warranted to determine if and how adenovirus might contribute to hepatitis risk and the potential roles of other pathogens and host factors such as AAV2 and HLA. Further investigations should include diverse populations, particularly those of Hispanic or Latino ethnicity, given the overrepresentation of this population seen in our investigation.
Supplementary Material
Research in context.
Evidence before this study
Paediatric acute hepatitis can commonly have no known cause. In-depth investigations into paediatric acute hepatitis of unknown aetiology began in 2022 when clusters of children with hepatitis of unknown aetiology and adenovirus viraemia were identified in multiple countries. We searched PubMed for original research articles published on paediatric hepatitis of unknown aetiology between Oct 1, 2021, and May 17, 2023, using the terms (hepatitis) AND (unknown cause OR unknown etiology OR adeno OR adenovirus) AND (pediatric OR children) in the title or abstract with no language restrictions. The search returned 90 publications, of which 17 were unrelated to paediatric acute hepatitis of unknown aetiology, 35 were editorials or commentaries, 12 were reviews, and 26 were relevant studies. As of July, 2022, a global report summarising recent investigations reported on 1010 children identified from 35 countries, that showed adenovirus as the most commonly detected pathogen (range 9–66%). Several studies reported on small numbers of children (eg, case reports, case series, or single-centre retrospective reviews). Comprehensive clinical and epidemiological data from large-scale investigations were limited to an interim analysis of patients under investigation in the USA and four technical briefings from the UK’s investigation. Three studies identified other potential contributing factors in paediatric hepatitis of unknown aetiology, including adeno-associated virus 2 and human leukocyte antigen alleles.
Added value of this study
To our knowledge, no comprehensive surveillance for paediatric acute hepatitis of unknown aetiology has been done in the USA before this investigation. This study provides updated and detailed information on children with acute hepatitis of unknown aetiology in the USA, encompassing a year of data from Oct 1, 2021, to Sept 30, 2022, obtained through extensive medical chart abstractions, detailed interviews, and additional laboratory and pathological evaluations of available residual specimens. This is the first study to broadly describe clinical and epidemiological findings in US children with hepatitis of unknown aetiology, including frequency in which they tested positive for adenovirus. It also is the first US study to compare children with hepatitis of unknown aetiology who tested positive for adenovirus with those who tested negative for adenovirus.
Implications of all the available evidence
These data support the growing evidence that adenovirus is a frequently detected pathogen among previously healthy children with hepatitis of unknown aetiology, although there is little evidence to indicate direct viral cytopathic effect on the liver. These data suggest that children with hepatitis of unknown aetiology who tested positive for adenovirus did not have more severe illness than those who tested negative for adenovirus. Further research is needed to confirm the role of adenovirus and of other potential contributing factors, such as coinfections and genetic host factors, and to elucidate the exact pathophysiological mechanism of liver injury.
Acknowledgments:
There was no funding for this investigation. We would like to acknowledge the contributions of the following individuals. Tyler Chavers, Jasmine D. Ruffin, Sadia Sleweon, Melisa M. Shah, Jana M. Ritter, Shelby Chastain-Potts, Ji In Park, Elizabeth Lee, Negar Rassaei, Hannah Bullock, CDC; Amanda Mandi, Robert Ramaekers, Iowa Department of Health and Human Services; Judy Ditchen, Charleston Area Medical Center; Clancey Collins, Liam Hicks, Xandy Peterson Pompa, Priscilla Lauro, Mike Reh, Arizona Department of Health Services; Sam Burt, Stacy Holzbauer, Sarah Lim, Ruth Lynfield, Anna Strain, Minnesota Department of Health; Sharon Watkins, Allison Longenberger, Lauren Orkis, Pennsylvania Department of Health; Michele McClenagha, Bryon Backenson, Donna Gowie, New York State Department of Health; Sylvianette Luna, Mónica M. Allende Quirós, Carmen J. Rodríguez Caquías, Puerto Rico Department of Health; Wes Stubblefield, Burnestine P. Taylor, Evelyn F. Geeter, Ali Martin, Veronica Quintero, Kelly Haywood, Lori Lloyd, Alabama Department of Public Health; Wesley G. Willeford, Jefferson County Department of Health; Sandra Henley, Mobile County Health Department; Judy Chen, Tingting Gu-templin, Page Keating, Ramona Lall, Joel Ackelsberg, New York City Department of Health and Mental Hygiene; Kathleen Winter, Dimple Patel, Kentucky Department for Public Health; Matt McHugh, Maeve Marsh, Ann & Robert H. Lurie Children’s Hospital of Chicago; Seth Eckel, Jim Collins, Michigan Department of Health and Human Services; Samuel Dominguez, Kevin Messacar, Amy Feldman, Children’s Hospital Colorado; Laura Taylor, New Jersey Department of Health; Joanne Sitaras, Cleveland Clinic;, Cleveland Clinic; Cincinnati Children’s Hospital; Nationwide Children’s Hospital; Chandni Patel, Virginia Department of Health; Victoria Pyne, Delaware Department of Health and Social Service; Viral Hepatitis Program, Delaware Department of Health and Social Service Division of Public Health Office of Infectious Disease Epidemiology; Michele D. Honeycutt, Arkansas Children’s Hospital; Bobby L. Boyanton Jr, Arkansas Children’s Hospital and University of Arkansas for Medical Sciences; Jessica Snowden, University of Arkansas for Medical Sciences; Amanda Jara, Tracy Kavanaugh, Jessica Pavlick, Georgia Department of Public Health; Kristina J. Herndon, Children’s Healthcare of Atlanta; Kimberly D. Dillon, Idaho Division of Public Health; Victoria O’Dell, Central District Health; Jeffrey Weigel, Panhandle Health District; Simon Ogbamikael, Erik Rist, Jonathan Plitnick, Meghan Fuschino, Jennifer Laplante, Wadsworth Center, NYSDOH; April Hatada, Carol A. Glaser, California Department of Public Health; Sara Chronister, Charla A. DeBolt, Sofia Husain, Esther Lam, Kali Turner, James S. Miller, Mellisa Roskosky, Washington State Department of Health; Jennifer Nybo, Tacoma-Pierce County Health Department; Hilary Armstrong, Eileen Benoliel, Shauna Clark, Jennifer Lenahan, Christopher White, Public Health – Seattle and King County; Susan Babcock, Snohomish Health District; Matthew Collins, Jesse Knibbs, Mary-Elizabeth Steppig, Lauren Milroy, Jena Rasor, Layne Mounsey, Connor Martin, Indiana Department of Health; Catherine M. Brown, Lawrence C. Madoff, Dylan B. Tierney, Steve Fleming, Rosa Hernandez, Sandra C. Smole, Mary DeMartino, Marisa Nazareno, Glen R. Gallagher, Massachusetts Department of Public Health.
Hepatitis of Unknown Etiology Group:
Rachel M Burke PhD, Eleanor Burnett MPH, Xiaoyan Lu MS, Melissa M Coughlin PhD, Bettina Bankamp PhD, and Everardo M Vega PhD (Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC); Brooke Leitgeb MS, Lindsey B C Estetter MS, Luciana Silva-Flannery PhD, and Roosecelis B Martines MD (Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC); Debra A Wadford PhD, Christina Morales PhD, and Chao-Yang Pan MPH (California Department of Public Health); Michael Popowich BS and Daryl M Lamson BS (Laboratory of Viral Diseases, Wadsworth Center, New York Department of Health); Kirsten St. George PhD (Laboratory of Viral Diseases, Wadsworth Center, New York Department of Health; Department of Biomedical Science, University of Albany, State University of New York); Elizabeth Cebelinski BS and Anna Panek BS (Minnesota Department of Health); L Amanda Ingram MPH (Alabama Department of Public Health); Stephanie Ayers-Millsap MPH (Jefferson County Department of Health); Theresa Dulski MD (CDC Epidemic Intelligence Service, Arkansas Department of Health); Tameika Reed BS (Arkansas Children’s Hospital); Lydia Sietsema MHS (Arkansas Children’s Northwest); Adrian Savella MPH (Arizona Department of Health Services); Mary P Derby PhD (Pima County Health Department); James Matthews MPH (Maricopa County Department of Public Health); Kentaro F Abe MPH, Kyle R Rizzo MPH, and Lauren J Stockman MPH, (California Department of Public Health, Division of Communicable Disease Control); Bonnie L Dao MPH (Los Angeles County Department of Public Health); Paul Gacek MPH, Quyen Phan MPH, and Christina Langer MPH (Connecticut Department of Public Health); Alexis Burakoff MD and Diana M Tapay MD (Colorado Department of Public Health and Environment); Michael Kacka (CDC Epidemic Intelligence Service, Colorado Department of Public Health and Environment); Nikki Kupferman MS (Delaware Department of Health and Social Service, Division of Public Health: Office of Infectious Diseases); Ashley Gent MPH and Dalton Dessi MPH, (Florida Department of Health); Melissa Tobin-D’Angelo MD and Ami Gandhi MPH (Georgia Department of Public Health); Kris K Carter DVM (CDC Career Epidemiology Field Officer, Idaho Division of Public Health); Matthew T Leslie PhD and Lori Saathoff-Huber MPH (Illinois Department of Public Health); Nicole Stone MPH (Indiana Department of Health); Bethany Hodge MD and Amanda Odegard BSN (Kentucky Department for Public Health); Julia Hand MSPH (Louisiana Department of Health); Juliana Jacoboski MPH and Christine Nguyen MPH (Massachusetts Department of Public Health); Chloe Manchester MSc and Emer Smith MPH (Maine Center for Disease Control and Prevention); Macey Ladisky MPH and Geoff Brousseau MPH (Michigan Department of Health and Human Services); Genny Grilli MPH (Minnesota Department of Health); Mari Freitas MPH (CDC/CSTE Applied Epidemiology Fellow, Minnesota Department of Health); Alexandra Berkley MPH, (Missouri Department of Health and Senior Services); Michael Do MD (Commonwealth Healthcare Corporation, Saipan, Northern Mariana Islands); Jennifer Hanson RN and Carla Boutwell RN (Mississippi State Department of Health); Lindsey VanderBusch MPH, Shari Renton MPH, and Rachel Goebel MPH (North Dakota Department of Health and Human Services); Matthew Donahue MD and Derek Julian MPH (Nebraska Department of Health and Human Services); Alice I Sato MD (Children’s Hospital and Medical Center, University of Nebraska Medical Center Department of Pediatrics); Krystle Mallory BSN and Deanna Bridges MPH (New Hampshire Division of Public Health Services, Bureau of Infectious Disease Control); Deepam Thomas MPH (New Jersey Department of Health); Anna M Stadelman PhD, Mika Gehre PhD, and Nora Holzinger MPH (Epidemiology and Response Division, New Mexico Department of Health); Melissa Peek-Bullock MPH and Victoria Sepcic MPH (Nevada Department of Health and Human Services); Nitin M Ghadge MPH, Youjung Byun PhD, and Bridget J Anderson PhD (New York State Department of Health); Dominique Balan MPH and Mike Antwi MD (NYC Department of Health and Mental Hygiene, Bureau of Communicable Disease); Brandi Taylor (Ohio Department of Health); Courtney Dewart PhD (Centers for Disease Control and Prevention and Ohio Department of Health); Ashlyn Wayman MPH and Marie Solberg MPH (Oklahoma State Department of Health); Hannah Lund MPH (CSTE Applied Epidemiology Fellow, Pennsylvania Department of Health); Nottasorn Plipat MD and Jennifer Wallace (Pennsylvania Department of Health); Abby L Berns MPH and Patricia McAuley MSN (Rhode Island Department of Health, Center for Acute Infectious Disease Epidemiology); Iris Cardona Gerena MD and Melissa Marzán Rodríguez DrPH (Puerto Rico Department of Health); Chelsea Campbell PharmD (South Carolina Department of Health and Environmental Control, Division of Acute Disease Epidemiology); Joshua Clayton PhD (South Dakota Department of Health); Jessica Schultz, MPH, (Tennessee Department of Health); Ryan Wallace MPH (Texas Department of State Health Services); Amelia Prebish Salmanson MPH (Utah Department of Health & Human Services); Dawn Saady MS and Tabatha Heaton MPH (Virginia Department of Health); Kimberly Carlson RN and Amanda Dodd MPH, (Washington State Department of Health); Thomas Haupt MS (Wisconsin Department of Health Services); Stephanie D. McLemore RN and Maria C. del Rosario MD (West Virginia Department of Health and Human Resources)
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention nor of the collaborating departments of health.
Declarations of interest
We declare no competing interests.
Data sharing statement
Inquiries regarding access to the data may be submitted to CDC (ncirddvdgast@cdc.gov). Data will be made available upon request in a manner that is compliant with all local and U.S. government laws and regulations and that protects human subjects’ patient confidentiality.
REFERENCES
- 1.Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology 1995; 21: 240–52. [PubMed] [Google Scholar]
- 2.Squires R, Alonso E. Acute liver failure in children. Cambridge: Cambridge University Press, 2014. [Google Scholar]
- 3.Squires JE, McKiernan P, Squires RH. Acute liver failure: an update. Clin Liver Dis 2018; 22: 773–805. [DOI] [PubMed] [Google Scholar]
- 4.Narkewicz MR, Horslen S, Hardison RM, et al. A learning collaborative approach increases specificity of diagnosis of acute liver failure in pediatric patients. Clin Gastroenterol Hepatol 2018; 16: 1801–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squires RH Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr 2006; 148: 652–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JM, Buchfellner M, Britt W, et al. Acute hepatitis and adenovirus infection among children— Alabama, October 2021– February 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 638–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh K, Tayler R, Pollock L, et al. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Euro Surveill 2022; 27: 2200318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hierholzer JC. Adenoviruses in the immunocompromised host. Clin Microbiol Rev 1992; 5: 262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1998; 27: 1194–200. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Recommendations for adenovirus testing and reporting of children with acute hepatitis of unknown etiology. 2022. https://emergency.cdc.gov/han/2022/han00462.asp (accessed Feb 13, 2023).
- 11.WHO. Multi-country— acute, severe hepatitis of unknown origin in children. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 (accessed Feb 13, 2023).
- 12.WHO. Severe acute hepatitis of unknown aetiology in children— multi-country. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400 (accessed Feb 13, 2023).
- 13.UK Health Security Agency. Investigation into acute hepatitis of unknown aetiology in children in England: technical briefing 4. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1094573/acute-hepatitis-technical-briefing-4.pdf (accessed Feb 13, 2023).
- 14.Cates J, Baker JM, Almendares O, et al. Interim Analysis of acute hepatitis of unknown etiology in children aged <10 years—United States, October 2021–June 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 852–58. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. Vintage 2020 bridged-race postcensal population estimates. 2021. https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#vintage2020 (accessed Sept 4, 2023).
- 16.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) —a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. CDC recommends COVID-19 vaccines for young children. 2022. https://www.cdc.gov/media/releases/2022/s0618-children-vaccine.html (accessed Feb 13, 2023).
- 19.Woodworth KR, Moulia D, Collins JP, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years— United States, November 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutierrez Sanchez LH, Shiau H, Baker JM, et al. A case series of children with acute hepatitis and human adenovirus infection. N Engl J Med 2022; 387: 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho A, Orton R, Tayler R, et al. Adeno-associated virus 2 infection in children with non-A-E hepatitis. Nature 2023; 617: 555–63. [DOI] [PubMed] [Google Scholar]
- 22.Mandal S, Simmons R, Ireland G, et al. Paediatric acute hepatitis of unknown aetiology: a national investigation and adenoviraemia case-control study in the UK. Lancet Child Adolesc Health 2023; online Sept 26. 10.1016/S2352-4642(23)00215-8. [DOI] [PubMed] [Google Scholar]
- 23.Kambhampati AK, Burke RM, Dietz S, et al. Trends in acute hepatitis of unspecified etiology and adenovirus stool testing results in children—United States, 2017–2022. MMWR Morb Mortal Wkly Rep 2022; 71: 797–802. [DOI] [PubMed] [Google Scholar]
- 24.Kelgeri C, Couper M, Gupte GL, et al. Clinical spectrum of children with acute hepatitis of unknown cause. N Engl J Med 2022; 387: 611–19. [DOI] [PubMed] [Google Scholar]
- 25.Servellita V, Sotomayor Gonzalez A, Lamson DM, et al. Adeno-associated virus type 2 in US children with acute severe hepatitis. Nature 2023; 617: 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morfopoulou S, Buddle S, Torres Montaguth OE, et al. Genomic investigations of unexplained acute hepatitis in children. Nature 2023; 617: 564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol 2023; 21: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsunoda T, Inui A, Iwasawa K, et al. Acute liver dysfunction not resulting from hepatitis virus in immunocompetent children. Pediatr Int 2017; 59: 551–56. [DOI] [PubMed] [Google Scholar]
- 29.Brodin P, Arditi M. Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens. Lancet Gastroenterol Hepatol 2022; 7: 594–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deep A, Alexander E. Children with acute hepatitis of unknown cause. N Engl J Med 2022; 387: 1907–08. [DOI] [PubMed] [Google Scholar]
- 31.van Beek J, Fraaij P, Giaquinto C, et al. Case numbers of acute hepatitis of unknown aetiology among children in 24 countries up to 18 April 2022 compared to the previous 5 years. Euro Surveill 2022; 27: 2200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Kleine RH, Lexmond WS, Buescher G, et al. Severe acute hepatitis and acute liver failure of unknown origin in children: a questionnaire-based study within 34 paediatric liver centres in 22 European countries and Israel, April 2022. Euro Surveill 2022; 27: 2200369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Children with acute hepatitis of unknow etiology. Investigation updates. 2022. https://www.cdc.gov/ncird/investigation/hepatitis-unknown-cause/updates.html (accessed Aug 30, 2023).
- 34.Henning KJ. What is syndromic surveillance? MMWR Suppl 2004; 53: 5–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Inquiries regarding access to the data may be submitted to CDC (ncirddvdgast@cdc.gov). Data will be made available upon request in a manner that is compliant with all local and U.S. government laws and regulations and that protects human subjects’ patient confidentiality.


