Abstract
Purpose
Although up to 20% of people with type 2 diabetes (DM) have normal BMI (< 25 kg/m2), it remains unclear whether there is a difference in the development of cardiac dysfunction between those with normal and higher BMI. Furthermore, little is known about the relationship of visceral fat with BMI or fitness in asymptomatic patients with DM.
Methods
We prospectively enrolled asymptomatic patients with DM and divided into two groups: BMI ≥ 25kg/m2 (overweight/obese group) versus < 25kg/m2(normal-weight group). Resting echocardiogram followed by exercise stress echocardiogram and exercise gas exchange analysis (in a subgroup) was performed. Cardiac function was evaluated using left ventricular longitudinal strain (LVLS), E/e’, and relative wall thickness (RWT). In addition, epicardial fat thickness (EFT) was measured to estimate visceral fat.
Results
Normal-weight patients with DM had more EFT compared with overweight/obese patients (0.66 ± 0.17 cm vs. 0.59 ± 0.22 cm, p < 0.05), despite the overlap between the groups. There was no significant difference in the prevalence of LV remodeling (p = 0.49), impaired LVLS (p = 0.22), or increased E/e’ (p = 0.26), and these were consistently observed when matched for race. The majority of patients (63%) achieved ≥ 85% of percent peak-predicted VO2. At peak, there was no significant difference in peak VO2 normalized by eLBM (36.4 ± 7.7 vs. 37.8 ± 7.1 ml/kg eLBM/min, p = 0.43) while VO2 normalized by weight (23.6 ± 6.5 vs. 29.6 ± 6.7 ml/kg/min, p < 0.001) and VO2 ratio (5.7 ± 1.7 vs. 7.3 ± 2.4 METs, p = 0.001) were significantly lower in patients with obese/overweight group. There was no significant difference between patients with higher and lower EFT.
Conclusions
Patients with DM and normal BMI have excess epicardial fat compared to those with overweight/obese. Epicardial fat was not directly linked to prevalence of subclinical dysfunction.
Keywords: Diabetic cardiomyopathy, Epicardial fat, Early stage heart failure, Exercise capacity
Introduction
The prevalence of type 2 diabetes in our society is increasing and it has been associated with a high burden of morbidity and mortality caused by accelerated cardiovascular disease [1]. Clinical studies have demonstrated a strong relationship between visceral fat content and metabolic diseases, such as type 2 diabetes mellitus (DM) and liver steatosis. Epicardial fat is a unique adipose tissue located between the myocardium and the visceral layer of pericardium. This tissue is characterized by highly active fatty acid metabolism and highly expressed thermogenic genes. Observational cross-sectional studies repeatedly show a correlation between increased epicardial fat, coronary artery disease severity and incident cardiovascular events or mortality [2]. This could be in part mediated by proinflammatory and proatherogenic cytokines [3, 4] associated with epicardial fat. Although computed Tomography, is the reference standard to assess epicardial fat [5], echocardiography has been recently used as an easy alternative and cost-effective way to assess the burden of epicardial fat [6].
While the majority of patients with type 2 diabetes are overweight/obese, normal weight diabetes, defined by a body mass index (BMI) < 25 kg/m2, is recognized as a different phenotype, more common in Asian populations and older adults. Compared to overweight/obese subgroups, patients with normal weight at diagnosis of type 2 diabetes may potentially have higher mortality than those who are overweight or obese [7]. However, it remains unclear whether these patients are more susceptible to the development subclinical (stage B) heart failure [8]. Furthermore, BMI does not indicate visceral adiposity and the association between BMI and visceral fat has not been well explored in asymptomatic patients with DM.
We therefore first sought to determine whether patients with normal weight diabetes have increased epicardial adiposity when compared to controls. We further sought to assess the difference between those who are obese and normal weight in patients with DM. We secondly sought to determine the prevalence of subclinical heart failure and its relationship with exercise capacity in asymptomatic patients with DM. We also evaluated whether there was a difference in the impact on cardiac function according to the BMI as well as its association with epicardial fat.
Methods
Study population
We prospectively recruited patients with type-2 DM participating in exercise intervention clinical trials (NCT02448498 and NCT02061579) from Stanford Diabetes Research Center who agreed to participate in cardiac phenotype sub-study, where resting echocardiographic assessment followed by exercise stress echocardiograms were performed to rule out significant coronary artery disease. Those participants were asymptomatic and willing to perform exercise intervention without previous diagnosis of heart failure. Patients were then divided into two groups according to BMI ≥ 25 kg/m2(overweight group) and < 25 kg/m2(normal-weight group). Patients were excluded if a diagnosis of chronic kidney disease, neuropathy, diagnosed liver disease, or active malignancy were present. In addition, 100 age- and sex-matched controls were randomly selected from the Stanford Healthy Aging Cardiovascular Institute database for the purpose of comparison between specific echocardiographic parameters. The study was approved by the Stanford Institutional Review Board and all participants gave written informed consent.
Echocardiography
Echocardiography was performed using commercially available echocardiographic systems (EPIQ 7 C; Philips Medical Imaging, Eindhoven, the Netherlands), according to the American Society of Echocardiography guideline recommendations [9]. Image analyses were performed on Xcelera workstation by trained cardiologists from the Biomarker and Imaging Core Laboratory at Stanford Cardiovascular Institute (Y.K. and T.N.). Relative wall thickness (RWT) was calculated as (2*inferolateral wall thickness) / (LV internal dimension). Left ventricular (LV) mass was obtained using area-length method. LVEF was calculated using biplane Simpson’s method. LV longitudinal strain was measured using Lagrangian strain by manual tracing from the apical views, computing the myocardial length in end-diastole and end-systole in the following formula: as described before [10]. LV global longitudinal strain (GLS) represents the average values of longitudinal strain from the apical 4-, 3-, and 2-chamber views. Transmitral pulsed-wave Doppler and tissue Doppler imaging were acquired from apical 4-chamber view to obtain early (E) and late (A) diastolic flow velocity as well as early diastolic (e’) velocity of the mitral annulus at septal and lateral. E/e’ ratio was obtained by the average of septal and lateral sites. Left atrial (LA) volumes were obtained using biplane area-length method and LA strain was measured using Lagrangian strain by manual tracing from apical 4- and 2-chamber as previously described [11]. LA reservoir strain was calculated with QRS onset as where represents the maximum length and represents the minimum length (QRS).
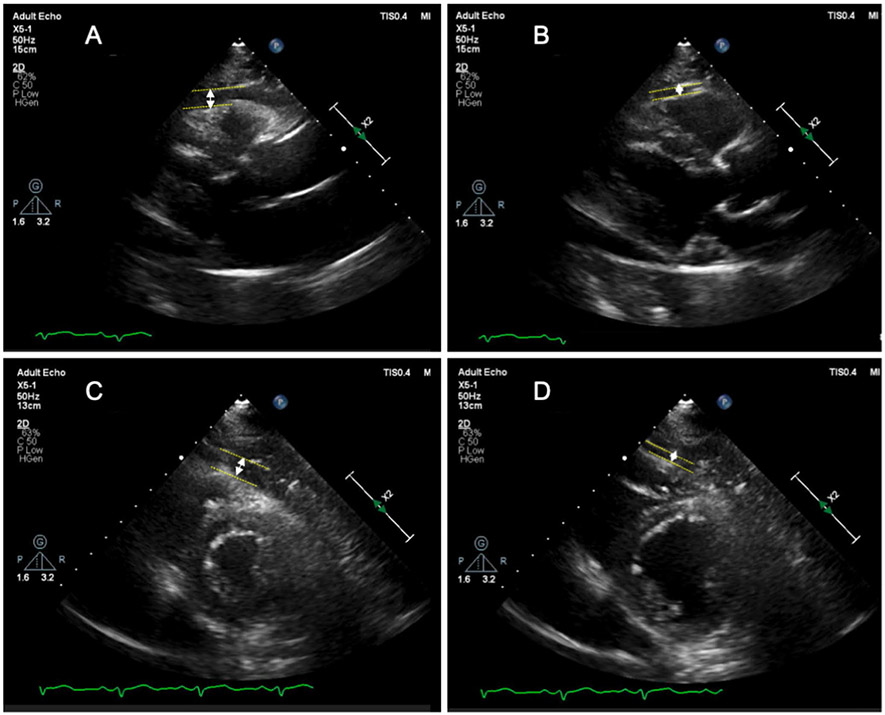
Epicardial and pericardial fat thickness were measured according to the previously published methods in end-systole [12] and end-diastole [13, 14]. Epicardial fat thickness was identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium. Pericardial fat thickness was identified as the hypoechoic space anterior to the epicardial fat and parietal pericardium and it does not significantly change size during the cardiac cycle [15]. They were measured perpendicularly, on the free wall of the right ventricle from both parasternal long-axis and short-axis views at mid ventricle (Fig. 1).
Fig. 1.
Epicardial fat thickness measurement by echocardiography. The epicardial fat thickness was measured on parasternal long axis (A and B) and parasternal short axis view (C and D) at end-systole and end-diastole. EAT thickness was defined as distance from outer side of myocardium to pericardium and measured perpendicularly in front of the right ventricular free wall
Subclinical HF was evaluated by LV morphology and systolic and diastolic function. Abnormal morphology was considered present if patients exhibited LV concentric remodeling (RWT > 0.42) or LV hypertrophy (LV mass index > 95 g/m2 for women and LV mass index > 115 g/m2 for men). Systolic dysfunction was defined as LVGLS using an absolute value < 16%, based on previous literature [16]. Diastolic function was categorized according to the progression of diastolic dysfunction: normal (0.75 < E/A < 1.5 and E/e’ < 10); mild defined as impaired relaxation without evidence of increased filling pressures (E/A ≤ 0.75 and E/e’ < 10); moderate defined as impaired relaxation associated with moderate elevation of filling pressures or pseudo-normal filling (0.75 < E/A < 1.5 and E/e’≥ 10), and severe defined as advanced reduction in compliance or reversible or fixed restrictive filling (E/A > 1.5 and E/e’ ≥ 10) as previously described and validated [17, 18] and diastolic dysfunction was defined by the presence of elevation of filling pressure (i.e.. E/e’ ratio ≥ 10).
All patients performed exercise echocardiography using a standard symptom-limited ramp protocol. Exercise protocols were individualized according to the predetermined exercise capacity of the participant being tested. This was determined by the participant’s answers to the Veterans Specific Activity Questionnaire [19]. Regional wall motion was compared before and after exercise and patients with ischemia were identified by inducible wall motion abnormalities.
Among those enrolled, cardiopulmonary exercise (CPX) tests were completed during exercise echocardiography on a treadmill with an integrated metabolic cart (Quark CPET, CosMed USA Inc., Concord, CA, USA), using breath-by-breath data capture and analysis [19] in participants who agreed. Peak VO2 was calculated as the highest average VO2 over 30s during the last phase of exercise [20]. Peak predicted VO2 was obtained using the FRIEND equation [21] and percent peak-predicted VO2 (ppVO2) was calculated as 100 x [measured peak VO2 / peak-predicted VO2]. To assess the peak exercise capacity, peak VO2 as well as VO2 ratio calculated as peak VO2 divided by standing VO2 (METs) were used since peak values vary depending on the individuals.
Statistical analysis
Variables are presented as counts and percentages or mean and standard deviation. Categorical variables were compared using Pearson’s chi square test or Fisher’s exact test, as appropriate. Comparison between groups was performed using Student or Welch t-test or Mann-Whitney U test, as appropriate if two groups and one-way ANOVA or Kruskal-Wallis test was used if three or more groups were compared, and post-hoc analysis was performed with Turkey-HSD multiple comparison tests or Games-Howell, as appropriate. Receiver-operator characteristic (ROC) curves analysis was performed to determine the sensitivity and specificity of epicardial fat thickness to differentiate subjects with DM from age and sex-matched controls. Multivariable logistic regression analysis was performed to detect the independent correlations with the presence of each cardiac characteristic (i.e., cardiac morphology change, systolic and diastolic dysfunction) or the number of cardiac abnormalities present using the covariates age, sex, BMI, systolic blood pressure, heart rate and HbA1c. P values < 0.05 were considered statistically significant. Analyses were performed using SPSS version 21 (SPSS Inc, Chicago, Illinois, USA).
Results
Among 202 patients with DM enrolled, 5 patients were excluded; one due to severely reduced ventricular function at rest, one due to exercise induced sustained ventricular tachycardia without hemodynamic compromise which resolved during early recovery, two due to exercise induced wall motion abnormalities and one due to inability to assess images. Therefore, 197 patients were included in the final analysis. Patients were then divided into two groups; 133 patients in the overweight/obese group and 64 patients in the normal-weight group. Overall, patients with DM presented higher BMI and body surface area compared with controls (Table 1). Among patients with DM, per study design, BMI and body surface area were significantly lower in the normal-weight group compared with the overweight/obese group; however, there were no significant differences in other clinical characteristics except for the racial and ethnicity categories (Table 1).
Table 1.
Clinical characteristics
| Controls (N = 100) |
Entire Diabetes (N = 197) |
Over-weight/ obese (N = 133) |
Normal- weight (N = 64) |
|
|---|---|---|---|---|
| Age (years) | 58.6 ± 11.2 | 59.5 ± 10.6 | 59.5 ± 11.5 | 59.6 ± 9.0 |
| Male, n (%) | 57 (57) | 115 (58) | 73 (54) | 40 (63) |
| Body surface area (m2) | 1.87 ± 0.20 | 1.97 ± 0.29* | 2.08 ± 0.28 | 1.75 ± 0.15# |
| Body mass index (kg/m2) | 24.8 ± 3.3 | 29.8 ± 7.3* | 32.9 ± 6.6 | 23.2 ± 1.6# |
| HbA1c | 7.6 ± 1.0 | 7.5 ± 1.1 | ||
| White | 88 (88) | 60 (30) | 52 (39) | 8 (13) |
| Asian | 11 (11) | 98 (50) | 48 (36) | 50 (78) |
| African American/Black | 0 | 15 (8) | 11 (8) | 4 (6) |
| Other | 1 (1) | 24 (12) | 22 (17) | 2 (3) |
| Heart rate (bpm) | 60 ± 10 | 73 ± 11* | 73 ± 11 | 73 ± 11 |
| Systolic blood pressure (mmHg) | 120 ± 13 | 135 ± 16* | 136 ± 16 | 133 ± 16 |
| Diastolic blood pressure (mmHg) | 74 ± 9 | 81 ± 11* | 81 ± 10 | 80 ± 12 |
| Hypertension | 8 (8) | 133 (68) | 93 (70) | 40 (62) |
| Echocardiographic parameters | ||||
| Epicardial fat in systole (cm) | 0.30 ± 0.10 | 0.61 ± 0.21* | 0.59 ± 0.22 | 0.66 ± 0.17# |
| indexed to height (cm/m) | 0.18 ± 0.06 | 0.37 ± 0.13 | 0.35 ± 0.14 | 0.40 ± 0.11# |
| Epicardial fat in diastole (cm) | 0.11 ± 0.07 | 0.38 ± 0.17* | 0.34 ± 0.17 | 0.46 ± 0.14# |
| indexed to height (cm/m) | 0.06 ± 0.04 | 0.23 ± 0.06* | 0.20 ± 0.10 | 0.27 ± 0.09# |
| Pericardial fat in systole (cm) | 0.36 ± 0.16 | 0.50 ± 0.25* | 0.51 ± 0.24 | 0.47 ± 0.25 |
| Pericardial fat in diastole (cm) | 0.33 ± 0.15 | 0.46 ± 0.23* | 0.45 ± 0.22 | 0.46 ± 0.26 |
| Cardiac function parameters | ||||
| Interventricular septum (cm) | 0.67 ± 0.16 | 0.90 ± 0.19* | 0.90 ± 0.18 | 0.89 ± 0.20 |
| Inferolateral wall thickness (cm) | 0.76 ± 0.14 | 0.89 ± 0.14* | 0.90 ± 0.14 | 0.86 ± 0.14 |
| LV internal diameter (cm) | 4.9 ± 0.9 | 4.6 ± 0.6* | 4.7 ± 0.6 | 4.4 ± 0.5 |
| Relative wall thickness | 0.30 ± 0.06 | 0.39 ± 0.08* | 0.39 ± 0.07 | 0.40 ± 0.10 |
| LV mass index (g/m2) | 60.5 ± 16.4 | 57.7 ± 14.0 | 59.1 ± 13.9 | 53.7 ± 12.8 |
| LVEF (%) | 62.0 ± 10.0 | 65.1 ± 5.4* | 65.3 ± 5.8 | 64.7 ± 4.4 |
| LVGLS (%)-absolute value | 19.1 ± 2.1 | 16.6 ± 1.8* | 16.6 ± 1.9 | 16.8 ± 1.4 |
| E/A | 1.06 ± 0.35 | 0.90 ± 0.35* | 0.91 ± 0.35 | 0.90 ± 0.35 |
| Average e’ (cm/s) | 8.6 ± 2.1 | 6.8 ± 2.1* | 6.9 ± 1.9 | 7.0 ± 1.8 |
| Average E/e’ | 7.9 ± 2.2 | 10.6 ± 3.1* | 10.8 ± 3.2 | 10.1 ± 2.6 |
| LA volume index (ml/m2) | 25.1 ± 6.8 | 27.2 ± 5.8* | 26.9 ± 6.2 | 27.8 ± 5.0 |
| LA reservoir strain (%) | 41.2 ± 17.6 | 29.3 ± 9.4* | 27.9 ± 8.9 | 32.2 ± 9.7# |
p < 0.05 vs. control
p < 0.05 vs. over-weight population
Epicardial fat
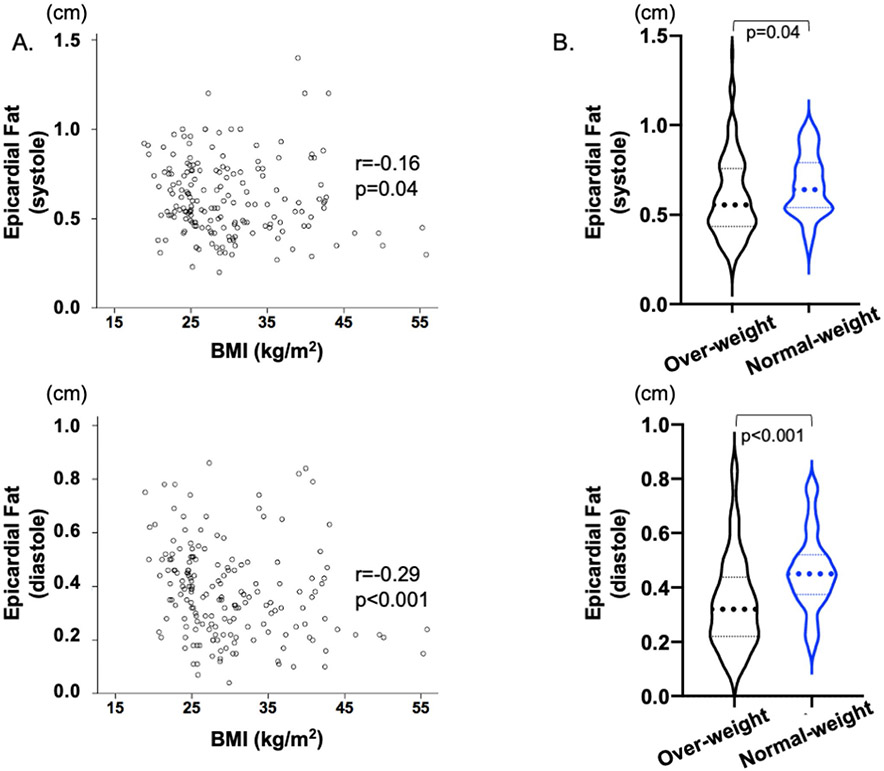
Patients with DM had significantly higher epicardial as well as pericardial fat compared with controls (Table 1), and this was observed even among those with normal weight (Table 2). ROC comparison analysis found that epicardial fat differentiated subjects with DM from controls better than pericardial fat (systole: best cut-off 0.41 cm with sensitivity 85% and specificity 86% (AUC 0.923, p < 0.001), and diastole: best cut-off 0.17 cm with sensitivity 91% and specificity 84% (AUC 0.947, p < 0.001)) and that there was no difference between epicardial fat at systole and diastole (p = 0.12). Furthermore, normal weight patients with DM had statistically more epicardial fat thickness compared with overweight/obese patients, having weak inverse association with each other (Fig. 2A and B), although there was overlap between the two groups.
Table 2.
Epicardial fat thickness between subjects with diabetes and sex matched control in Normal Weight
| Control (N = 55) |
Diabetes (N = 62) |
P value | |
|---|---|---|---|
| Age (years) | 60 ± 12 | 60 ± 9 | 0.95 |
| Male, n (%) | 27 (50) | 39 (63) | 0.14 |
| Epicardial fat in systole (cm) | 0.29 ± 0.10 | 0.67 ± 0.17 | < 0.001 |
| indexed to height (cm/m) | 0.17 ± 0.06 | 0.40 ± 0.11 | < 0.001 |
| Epicardial fat in diastole (cm) | 0.10 ± 0.07 | 0.46 ± 0.14 | < 0.001 |
| indexed to height (cm/m) | 0.06 ± 0.04 | 0.27 ± 0.08 | < 0.001 |
| Pericardial fat in systole (cm) | 0.33 ± 0.15 | 0.46 ± 0.25 | 0.001 |
| Pericardial fat in diastole (cm) | 0.31 ± 0.14 | 0.45 ± 0.26 | 0.001 |
Fig. 2.
Epicardial fat and BMI in the Diabetes group. (A) There was a weak inverse association between epicardial fat and BMI. (B) Overweight/obese patients had less epicardial fat compared with normal weight patients
Cardiac phenotype: cardiac morphology, LV systolic and diastolic function
As shown in Tables 1, patients with DM had higher relative wall thickness (RWT), impaired LVGLS as well as higher E/e’ ratio. LA volume was slightly larger but within normal range in patients with DM, while LA reservoir strain was significantly reduced compared to controls (29.3 ± 9.4% for patients with DM vs. 41.2 ± 17.6% for controls, p < 0.001). There were no significant differences in abnormal cardiac morphology or LV systolic or diastolic function between the overweight/obese and normal weight groups except for E/e’ which was significantly higher in overweight/obese group than the normal-weight group (p = 0.005).
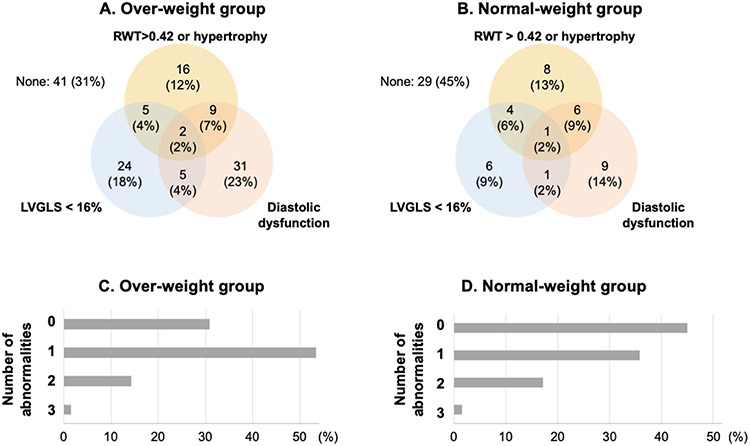
Figure 3 A and B show the Venn diagrams demonstrating overlap between patients with three cardiac features in overweight/obese group (A) and normal-weight (B) patients with DM. The prevalence of diastolic dysfunction was the highest in overweight/obese group (35%), while the prevalence of LV hypertrophy was the highest in normal weight group (30%) followed by diastolic dysfunction (27%). The majority of the patients had at least one cardiac abnormality (Fig. 3C and D) and there was no significant difference between the two groups (Table 3). In terms of LA function, LAVI was comparable between the two groups; however, LA reservoir strain was more reduced in the overweight/obese group compared to the normal-weight group (27.9 ± 8.9% vs. 32.2 ± 9.7%, p = 0.003).
Fig. 3.
Comparison of subclinical cardiac dysfunction between overweight/obese and normal weight groups. The panels A and B represent the Venn diagrams demonstrating the overlap between patients with abnormal values of morphology change (RWT > 0.42 or LV hypertrophy), LV systolic (LVGLS < 16%) and diastolic dysfunction in Over- (A) and Normal-weight (B) group. The panels C and D show the ratio of patients according to the number of cardiac abnormalities in over/obese (C) and normal-weight (D) groups
Table 3.
Comparison of cardiac phenotypes between Overweight/obese and Normal weight groups
| Overweight/obese | Normal weight |
P value |
|
|---|---|---|---|
| Entire population | N = 133 | N = 64 | |
| RWT > 0.42, LV hypertrophy, n (%) | 32 (24) | 19 (30) | 0.49 |
| GLS < 16%, n (%) | 36 (27) | 12 (19) | 0.22 |
| Diastolic dysfunction, n (%) | 47 (35) | 17 (27) | 0.26 |
| Asian population | N = 47 | N = 51 | |
| RWT > 0.42, LV hypertrophy, n (%) | 10 (21) | 15 (29) | 0.49 |
| GLS < 16%, n (%) | 10 (21) | 9 (18) | 0.80 |
| Diastolic dysfunction, n (%) | 13 (28) | 12 (26) | 0.65 |
B. Independent associates with cardiac dysfunction
Exercise stress ECG test and exercise capacity
No patients with DM presented chest pain during the exercise test. Fifteen patients (8%) had ST segment depression (defined as ST segment ≥ 1 mm depression) on exercise ECG, and five (3%) had multiple isolated premature ventricular contractions.
Of 197 patients, 98 participants underwent CPX testing (Table 4). There was no significant difference in peak VO2 normalized by eLBM (overweight/obese vs. normal weight: 36.4 ± 7.7 vs. 37.8 ± 7.1 ml/kg eLBM/min, p = 0.43) while VO2 normalized by weight (23.6 ± 6.5 vs. 29.6 ± 6.7 ml/kg/min, p < 0.001) and VO2 ratio (5.7 ± 1.7 vs. 7.3 ± 2.4 METs, p = 0.001) were significantly lower in patients with obese/overweight group. On the contrary, percent peak-predicted VO2 was higher in obese/overweight group.
Table 4.
Cardiopulmonary exercise parameters based on BMI
| Over weight/ obese (N = 72) |
Normal weight (N = 26) |
P value | |
|---|---|---|---|
| Age (years) | 60.0 ± 11.9 | 57.5 ± 8.0 | 0.27 |
| Male sex, n (%) | 37 (51) | 18 (69) | 0.17 |
| Rest | |||
| Heart rate (bpm) | 79 ± 12 | 77 ± 11 | 0.46 |
| Systolic blood pressure (mmHg) | 137 ± 16 | 133 ± 15 | 0.24 |
| VO2 (ml/min) | 420 ± 121 | 323 ± 60 | < 0.001 |
| VO2 (ml/kg/min) | 4.6 ± 1.0 | 4.9 ± 1.1 | 0.13 |
| Peak | |||
| Heart rate (bpm) | 153 ± 19 | 159 ± 18 | 0.12 |
| Systolic blood pressure (mmHg) | 199 ± 23 | 185 ± 19 | 0.008 |
| Hypertensive response, n (%) | 31 (43) | 4 (15) | 0.02 |
| VO2 (mL/min) | 2352 ± 795 | 2306 ± 710 | 0.23 |
| VO2 (ml/kg/min) | 23.6 ± 6.5 | 29.6 ± 6.7 | < 0.001 |
| VO2 ratio peak/rest (METs) | 5.7 ± 1.7 | 7.3 ± 2.4 | 0.001 |
| ppVO2 (%) | 117 ± 48.7 | 89.1 ± 17.1 | < 0.001 |
| RER | 1.09 ± 0.09 | 1.16 ± 0.09 | 0.001 |
| VE/VCO2 slope | 30.4 ± 4.3 | 30.4 ± 4.1 | 0.97 |
When compared CPX parameters between patients with higher EFT and lower EFT, there was no significant difference observed in resting VO2 normalized by weight (overweight/obese vs. normal weight: 4.7 ± 1.1 vs. 4.6 ± 1.0 ml/kg/min, p = 0.82), normalized by lean mass (7.0 ± 1.7 vs. 7.0 ± 1.4 ml/kg eLBM/min, p = 0.90) as well as peak VO2 normalized by weight (24.6 ± 6.2 vs. 24.8 ± 6.6 ml/kg/min, p = 0.86), normalized by lean mass (36.0 ± 7.5 vs. 37.2 ± 7.1 ml/kg eLBM/min, p = 0.47), peak VO2 ratio (6.0 ± 1.7 vs. 6.0 ± 1.7 METs, p = 0.98).
Comparison of cardiac phenotypes in asian population
Since there was a racial difference between the overweight/obese and normal-weight groups, we also compared the echocardiographic parameters between those groups in Asian patients; 48 overweight/obese patients vs. 50 normal weight patients. Overweight/obese patients presented higher E/e’ than normal weight patients (Table 5). However, similar to the comparison in the entire population, using the thresholds to assess cardiac abnormalities, there was no significant difference between the two groups (Table 3). Furthermore, there were no significant differences in the number of cardiac abnormalities between the two groups (p = 0.94), however, LA reservoir strain was lower in overweight/obese group compared with normal weight group (27.6 ± 8.8% vs. 32.4 ± 9.9%, p = 0.01).
Table 5.
Comparison among Race matched Asian population
| Overweight/ obese (N = 48) |
Normal weight (N = 50) |
P value | |
|---|---|---|---|
| Age (years) | 58.1 ± 12.3 | 58.5 ± 9.0 | 0.88 |
| Male, n (%) | 26 (55) | 33 (66) | 0.31 |
| BSA (m2) | 1.92 ± 0.27 | 1.74 ± 0.15 | < 0.001 |
| BMI (kg/m2) | 30.0 ± 5.5 | 23.1 ± 1.6 | < 0.001 |
| Race | 0.14 | ||
| Asian Indian, n (%) | 26 (54) | 25 (50) | |
| Chinese, n (%) | 15 (31) | 21 (42) | |
| Others, n (%) | 7 (15) | 4 (8) | |
| Hypertension, n (%) | 34 (72) | 41 (80) | 0.48 |
| HbA1c (%) | 7.3 (6.9-8.0) | 7.2 (6.7–7.8) | 0.29 |
| Echocardiographic parameters | |||
| Interventricular septum (cm) | 0.89 ± 0.23 | 0.88 ± 0.18 | 0.77 |
| Inferolateral wall thickness (cm) | 0.88 ± 0.15 | 0.85 ± 0.14 | 0.25 |
| LV internal diameter (cm) | 4.6 ± 0.6 | 4.4 ± 0.5 | 0.02 |
| Relative wall thickness | 0.38 ± 0.07 | 0.39 ± 0.08 | 0.56 |
| LV mass index (g/m2) | 54.0 ± 13.0 | 54.2 ± 13.9 | 0.94 |
| LVEF (%) | 65.9 ± 7.5 | 65.0 ± 4.2 | 0.47 |
| LVGLS (%) | −16.6 ± 2.2 | −17.0 ± 1.3 | 0.23 |
| Average e’ (cm/s) | 6.4 ± 1.7 | 7.2 ± 1.9 | 0.04 |
| Average E/e’ | 11.6 ± 3.9 | 9.6 ± 2.7 | 0.005 |
| Stroke volume index (ml/m2) | 32.1 ± 8.4 | 34.1 ± 9.6 | 0.28 |
| Maximal LA volume index (ml/m2) | 25.5 ± 5.4 | 27.9 ± 4.9 | 0.02 |
| LA reservoir strain (%) | 27.6 ± 8.8 | 32.4 ± 9.9 | 0.01 |
| Epicardial fat in systole (cm) | 0.57 ± 0.21 | 0.66 ± 0.18 | 0.04 |
| Epicardial fat in diastole (cm) | 0.33 ± 0.15 | 0.45 ± 0.14 | < 0.001 |
| Pericardial fat in systole (cm) | 0.52 ± 0.22 | 0.47 ± 0.24 | 0.34 |
| Pericardial fat in diastole (cm) | 0.44 ± 0.20 | 0.47 ± 0.26 | 0.59 |
Multivariable analysis found that higher BMI was independently associated with concentric remodeling/hypertrophy and diastolic dysfunction, resulting in the positive relationship with the number of cardiac abnormalities (Table 6). However, lower BMI is associated with higher epicardial fat in patients with asymptomatic DM. HbA1c was not retained in the model as an independent associate of cardiac dysfunction.
Table 6.
Independent associates with cardiac dysfunction
| Clinical parameters (odds ratio [95%CI] by 1SD increase, p value) |
|
|---|---|
| Concentric remodeling or left ventricular hypertrophy | Age (1.68 [1.11–2.49], p = 0.008) BMI (1.43 [1.00-2.01], p = 0.04) |
| Impaired LV longitudinal strain | Age (2.26 [1.52–3.65], p < 0.001) Heart rate (1.76 [1.19–2.61], p = 0.004) |
| Diastolic dysfunction | BMI (1.44 [1.04-2.00], p = 0.02) Heart rate (1.50 [1.06–2.14], p = 0.03) |
| The number of cardiac abnormalities | Age (1.77 [1.30–2.41], p < 0.001) BMI (1.73 [1.29–2.32], p < 0.001) |
| Epicardial fat thickness in systole | None |
| Epicardial fat thickness in diastole | Age (1.52 [1.00-2.05], p = 0.04) BMI (0.64 [0.42–0.93], p = 0.02) |
Covariates include age, sex, BMI, the presence of hypertension or dyslipidemia, HbA1c, heart rate, systolic blood pressure
Discussion
The main finding of our study is that patients with DM have significantly higher epicardial adiposity compared to subjects without abnormal glycemic control. In addition, compared to patients with overweight/obese DM, patients with normal weight DM had a significant increase in epicardial fat layer. Normal weight DM was also commonly associated with stage B heart failure although their exercise capacity and LA strain were more preserved than patients with higher BMI. These finding was consistent among Asian participants.
Epicardial fat tissue (EFT) is a visceral adipose tissue that surrounds the myocardium and pericardium. Previous studies have suggested that the measurements of the tissue are a substitute for visceral fat [3, 12] and observational cross-sectional studies showed a correlation between increased epicardial fat and the risk of incident cardiovascular events or mortality [2]. Our study found that patients with DM had higher epicardial fat compared to those without DM regardless of BMI, and normal-weight patients with DM had slightly higher epicardial fat compared to those with overweight/obese although there was an overlap. Biochemically, epicardial and pericardial fat are different. Epicardial fat is metabolically active and source of several adipokines, potential interactions through paracrine or vasocrine mechanisms between epicardial fat and myocardium are strongly suggested [3, 22], whereas the role pericardial fat as source of adipokines is still partially unknown. These studies support our finding that epicardial fat is more sensitive to differentiate patients with DM from controls and further studies are warranted and of interest to elucidate the mechanism of how this difference between the two groups appears using metabolic biomarker profile.
Controversial point of the EFT measurement by echocardiography is which time in the cardiac cycle is the most suitable for measuring; at systole or diastole. Some recommend the measurement during systole to prevent possible deformation by epicardial fat compression during diastole [12] and others in diastole to coincide with other imaging modalities such as CT and MRI [13, 14]. Similar results were obtained in terms of the correlation with BMI or cardiac function in our study, however, larger studies are needed to confirm this finding.
Obesity is regarded as an independent risk factor for heart failure and myocardial dysfunction [23] and various epidemiologic studies have provided evidence for a link between obesity and heart failure [24-27]. For example the Framingham Heart Study demonstrated that each BMI increment by 1 unit is associated with a 5% increased heart failure risk in men and 7% in women after adjusting for other cardiovascular risk factors, and the risk of heart failure was found to be increased across the entire spectrum of BMI [27] and the Multi-Ethnic Study of Atherosclerosis (MESA) found BMI as a risk factor in addition to DM [28]. In our study, we observed a high prevalence of subclinical heart failure in patients with DM, irrespective of BMI refocusing the attention to adiposity. It is also important to mention that BMI may not accurately reflect visceral adiposity especially in patients of South-Asian or Asian race. In the MESA, pericardial adipose tissue was associated with increased risk of all-cause cardiovascular disease and atherosclerosis burden [29] and excess visceral adipose tissue as a predictor for the development of cardiovascular risk factors over time, independently of total body fat mass or subcutaneous adipose tissue levels [30]. Evidence also indicates that the distribution of excess adiposity is an important determinant of cardiovascular risk; visceral and ectopic adiposity confer a much higher risk than subcutaneous adiposity [31]. It appears that the distribution of fat, rather than BMI itself, is more directly associated with cardiovascular risk. Future studies could refocus on body composition rather than BMI as a significant proportion of Asian individuals can have dysfunction without increased BMI.
Exercise capacity is a strong predictor of cardiovascular events and survival in patients with DM [32, 33]. In our study, a majority of participants had preserved exercise capacity despite a high prevalence of subclinical cardiac dysfunction. This may be because LV filling is compensated with greater atrial compliance and pump function which do not impair exercise capacity. Further studies are warranted to determine the extent to which more detailed parameters such as cardiac function at peak exercise predicts reduced exercise capacity. While a majority of patients maintained exercise capacity, a smaller proportion of overweight/obese participants had abnormal exercise capacity as measured by peak VO2 normalized to resting values (METs) and exercise tolerance was lower in overweight/obese participants compared to normal weight participants. Several studies have recommended to use peak VO2 normalized by lean mass for comparison of cardiopulmonary fitness across different categories of body mass [34-37]. Upon normalized by lean mass, there was no significant difference in exercise capacity between the two groups.
In contrast to prior studies that highlighted an association between higher epicardial fat thickness in type 2 diabetes and impaired cardiopulmonary performance [38] or that reported an inverse correlation between epicardial adipose tissue and peak VO2 [39], our study did not reveal any significant relationship between EFT and peak VO2. Our study included a larger number of normal weight participants, primarily of Asian descent. It is noteworthy that Asians typically exhibit a tendency to accumulate a greater amount of visceral fat in comparison to subcutaneous fat compared to African, Hispanic, and Caucasian populations. This inclination towards higher visceral fat accumulation in Asians is largely attributed to inherent genetic distinctions [40]. Consequently, our study noted higher EFT in the normal weight group compared to the overweight/obese group. This disparity can be attributed to the distinct adipose deposition patterns among different ethnicities and serves as one of the explanations for the discordance between our findings and those of previous studies.
Another important finding is that LA reservoir strain was more reduced in overweight/obese patients with DM compared to normal-weight. There is mounting evidence that LA dysfunction contributes to exercise capacity [41, 42]. For example, Kusunose et al. demonstrated a strong association between reduced LA strain and impaired exercise capacity in unselected individuals referred for exercise stress echocardiogram [43]. Impaired LA function can impose greater hemodynamic stress on the pulmonary vasculature and has been shown to be a robust correlate of elevated pulmonary vascular resistance and peak volume of oxygen, above and beyond traditional markers of diastolic function including the E/e’ ratio and LA volume [44]. Additionally, chronic pulmonary venous congestion in the setting of abnormal LA mechanics results in altered pulmonary artery compliance, which hinders oxygen delivery and gas exchange [45]. As the interplay between atrial phasic function and ventricular mechanics is essential for modulation of cardiovascular performance, it is not surprising that exercise capacity was lower in overweight/obese patients with DM who presented worse LA reservoir strain and higher E/e’ compared to those with normal-weight.
There are several limitations in our study. First, the patients were selected for participation in a rehabilitation program and thus they may have been more fit and motivated. Despite this fact, there was a relatively high prevalence of early stage HF in the study population. Second, we did not analyze the relationship with dyslipidemia which frequently coexisted in patients with diabetes. Further analysis will be needed to assess the relationship with cardiac maladaptation with complete lipid profiles. Third, we assessed echocardiographic parameters at rest and not at peak or post exercise. Since cardiac dysfunction in patients with DM often manifests itself during exercise, further evaluation of diastolic and systolic function during exercise may be helpful for more sensitive diagnosis. In addition, BNP and NT-BNP were not routinely obtained.
Conclusion
Patients with normal weight DM have a significant increased epicardial adiposity as well as a high prevalence of stage B heart failure comparable to overweight/obese DM.
Acknowledgements
This work was supported by the Stanford Diabetes Research Center P30DK116074, the National Institutes of Health under award number R18DK09639405 and R01DK081371 as well as the grand from Philips Royal Precision Medicine Initiative.
Footnotes
Conflict of interest The authors declare that they have no potential conflict of interest with respect to the research.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Chan JCN, Gregg EW, Sargent J, Horton R (2016) Reducing global diabetes burden by implementing solutions and identifying gaps: a Lancet Commission [Internet]. The Lancet. p. 1494–5. 10.1016/s0140-6736(16)30165-9 [DOI] [PubMed] [Google Scholar]
- 2.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS et al. (2008) Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample [Internet]. Circulation. p. 605–13. 10.1161/circulationaha.107.743062 [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Corradi D, Sharma AM (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2:536–543 [DOI] [PubMed] [Google Scholar]
- 4.Baker AR, da Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS et al. (2006) Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorter PM, van Lindert ASR, de Vos AM, Meijs MFL, van der Graaf Y, Doevendans PA et al. (2008) Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease [Internet]. Atherosclerosis. p. 896–903. 10.1016/j.atherosclerosis.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Willens HJ (2009) Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 22:1311–1319 quiz 1417–8 [DOI] [PubMed] [Google Scholar]
- 7.Carnethon MR, De Chavez PJD, Biggs ML, Lewis CE, Pankow JS, Bertoni AG et al. (2012) Association of Weight Status With Mortality in Adults With Incident Diabetes [Internet]. JAMA. 10.1001/jama.2012.9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun J-J, Colvin MM et al. (2022) 2022 American College of Cardiology/American Heart Association/Heart failure society of America Guideline for the management of Heart failure: executive Summary. J Card Fail 28:810–830 [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al. (2015) Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging [Internet]. Journal of the American Society of Echocardiography. p. 1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y Ariyama M, Kobayashi Y, Giraldeau G, Fleischman D, Kozelj M et al. (2016) Comparison of left ventricular manual versus automated derived longitudinal strain: implications for clinical practice and research [Internet]. The International Journal of Cardiovascular Imaging. p. 429–37. 10.1007/s10554-015-0804-x [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Moneghetti KJ, Boralkar K, Amsallem M, Tuzovic M, Liang D et al. (2017) Challenging the complementarity of different metrics of left atrial function: insight from a cardiomyopathy-based study [Internet]. European Heart Journal - Cardiovascular Imaging. p. 1153–62. 10.1093/ehjci/jew121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U et al. (2003) Epicardial Fat from Echocardiography: A New Method for Visceral Adipose Tissue Prediction [Internet]. Obesity Research. p. 304–10. 10.1038/oby.2003.45 [DOI] [PubMed] [Google Scholar]
- 13.Nelson AJ, Worthley MI, Psaltis PJ, Carbone A, Dundon BK, Duncan RF et al. (2009) Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel R, Alharthi M, Jiamsripong P, Cha S, Mookadam F (2010) Epicardial fat and its association with cardiovascular risk: A cross-sectional observational study [Internet]. Heart Views. p. 103. 10.4103/1995-705x.76801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobellis G (2020) Epicardial adipose tissue: from cell to Clinic. Springer [Google Scholar]
- 16.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH (2013) Normal Ranges of Left Ventricular Strain: A Meta-Analysis [Internet]. Journal of the American Society of Echocardiography. p. 185–91. 10.1016/j.echo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 17.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ (2003) Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289:194–202 [DOI] [PubMed] [Google Scholar]
- 18.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM et al. (2000) Clinical utility of Doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous doppler-catheterization study. Circulation 102:1788–1794 [DOI] [PubMed] [Google Scholar]
- 19.Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M et al. (1991) Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol 17:1334–1342 [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF et al. (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122:191–225 [DOI] [PubMed] [Google Scholar]
- 21.Kokkinos P, Kaminsky LA, Arena R, Zhang J, Myers J (2017) New Generalized equation for Predicting maximal oxygen uptake (from the Fitness Registry and the importance of Exercise National Database). Am J Cardiol 120:688–692 [DOI] [PubMed] [Google Scholar]
- 22.Sacks HS, Fain JN (2007) Human epicardial adipose tissue: A review [Internet]. American Heart Journal. p. 907–17. 10.1016/j.ahj.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 23.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX et al. (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the obesity Committee of the Council on Nutrition, Physical Activity, and metabolism. Circulation 113:898–918 [DOI] [PubMed] [Google Scholar]
- 24.Hubert HB, Feinleib M, McNamara PM, Castelli WP(1983)Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67:968–977 [DOI] [PubMed] [Google Scholar]
- 25.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK (2001) Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 161:996–1002 [DOI] [PubMed] [Google Scholar]
- 26.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJV (2006) Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew–Paisley study) [Internet]. European Heart Journal. p. 96–106. 10.1093/eurheartj/ehi506 [DOI] [PubMed] [Google Scholar]
- 27.Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG et al. (2002) Obesity and the Risk of Heart Failure [Internet]. New England Journal of Medicine. p. 305–13. 10.1056/nejmoa020245 [DOI] [PubMed] [Google Scholar]
- 28.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E et al. (2008) Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (multi-ethnic study of atherosclerosis) study. J Am Coll Cardiol 51:1775–1783 [DOI] [PubMed] [Google Scholar]
- 29.Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE et al. (2017) Pericardial, but not hepatic, Fat by CT is Associated with CV Outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 10:1016–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS (2015) Association Between Visceral and Subcutaneous Adipose Depots and Incident Cardiovascular Disease Risk Factors [Internet]. Circulation. p. 1639–47. 10.1161/circulationaha.114.015000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okura T, Nakata Y, Yamabuki K, Tanaka K (2004) Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol 24:923–929 [DOI] [PubMed] [Google Scholar]
- 32.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL et al. (2004) Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 27:83–88 [DOI] [PubMed] [Google Scholar]
- 33.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN (2000) Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132:605–611 [DOI] [PubMed] [Google Scholar]
- 34.Savonen K, Krachler B, Hassinen M et al. (2012) The current standard measure of cardiorespiratory fitness introduces confounding by body mass: the DR’s EXTRA study. Int J Obes 36:1135–1140 [DOI] [PubMed] [Google Scholar]
- 35.Goran M, Fields DA, Hunter GR et al. (2000) Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord 24:841–848 [DOI] [PubMed] [Google Scholar]
- 36.Krachler B, Savonen K, Komulainen P et al. (2015) Cardiopulmonary fitness is a function of lean mass, not total body weight: the DR’s EXTRA study. Eur J Prev Cardiol 22:1171–1179 [DOI] [PubMed] [Google Scholar]
- 37.Osman AF, Mehra MR, Lavie CJ et al. (2000) The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol 36:2126–2131 [DOI] [PubMed] [Google Scholar]
- 38.Nesti L, Pugliese NR, Chiriacò M et al. (2023) Epicardial adipose tissue thickness is associated with reduced peak oxygen consumption and systolic reserve in patients with type 2 diabetes and normal heart function. Diabetes Obes Metab 25:177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pugliese NR, Paneni F, Mazzola M et al. (2021) Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail 23:1858–1871 [DOI] [PubMed] [Google Scholar]
- 40.Williams R, Periasamy M (2020) Genetic and environmental factors contributing to visceral adiposity in asian populations. Endocrinol Metab (Seoul) 35:681–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donal E, Raud-Raynier P, De Place C, Gervais R, Rosier A, Roulaud M et al. (2008) Resting echocardiographic assessments of left atrial function and filling pressure interest in the understanding of exercise capacity in patients with chronic congestive heart failure. J Am Soc Echocardiogr 21:703–710 [DOI] [PubMed] [Google Scholar]
- 42.von Roeder M, Rommel K-P, Kowallick JT, Blazek S, Besler C, Fengler K et al. (2017) Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging [Internet]. ;10. 10.1161/CIRCIMAGING.116.005467 [DOI] [PubMed] [Google Scholar]
- 43.Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH (2012) Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 98:1311–1317 [DOI] [PubMed] [Google Scholar]
- 44.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A et al. (2016) Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ Cardiovasc Imaging [Internet]. ;9. 10.1161/CIRCIMAGING.115.003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naeije R, Chesler N (2012) Pulmonary Circulation at Exercise [Internet]. Comprehensive Physiology. p. 711–41. 10.1002/cphy.c100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.