Abstract
Background
The efficacy and safety of cefiderocol in ICU patients with difficult-to-treat resistance (DTR) non-fermenting Gram-negative bacteria (Nf-GNB) are not as well-established. Consequently, we conducted a cohort study to compare Cefiderocol with the Best Available Therapy (BAT) in ICU patients.
Methods
We included adult patients from 9 different ICUs, including a burn ICU unit, from 2019 to 2023 treated with Cefiderocol for DTR Nf-GNB isolated from the blood or lungs. We matched each patient at a 1:2 ratio based on the same DTR Nf-GBN isolated pathogen, and when possible, within the same type of ICU (burn unit or not). The primary endpoint of the study was the clinical cure at 15 days, with secondary endpoints including clinical cure at 30 days, relapse, and in-ICU mortality. For each outcome, adjusted odds ratios were estimated using bidirectional stepwise regression in a final model, which included 13 preselected confounders.
Results
We included 27 patients with cefiderocol, matched with 54 patients receiving the BAT. Four patients were not exactly matched on the type of ICU unit. Characteristics were comparable between groups, mostly male with a Charlson Comorbidity Index of 3 [1–5], and 28% had immunosuppression. Cefiderocol patients were most likely to have higher number of antibiotic lines. The main DTR Nf-GNB identified was Pseudomonas aeruginosa (81.5%), followed by Acinetobater baumanii (14.8%) and Stenotrophomonas maltophilia (3.7%). Pneumonia was the identified infection in 21 (78.8%) patients in the Cefiderocol group and in 51 (94.4%) patients in the BAT group (p = 0.054). Clinical cure at 15 and 30-day and the in-ICU mortality was comparable between groups, however relapse was higher in the cefiderocol group (8-29.6% vs. 4-7.4%;aOR 10.06[1.96;51.53])
Conclusion
Cefiderocol did not show an improvement in clinical cure or mortality rates compared to BAT in the treatment of DTR Nf-GNB, but it was associated with a higher relapse rate.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01308-z.
Keywords: Cefiderocol, ICU, Relapse, Clinical cure
Key points
What was known:
•The use of cefiderocol remains debated and under-evaluated in patients admitted to the ICU.
What this article tells us:
•In a cohort of 27 patient with cefiderocol treatment, compared with 54 patients treated with best available therapy, no difference was observed compared regarding the 15 and 30-day clinical cure, and in ICU mortality.
•However, a higher risk of relapse was observed in the cefiderocol group: 29% vs. 7%.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01308-z.
Introduction
Cefiderocol is a new siderophore cephalosporin approved by the FDA in 2019 [1, 2]. With a broad spectrum of activity against carbapenemase-producing Gram-negative bacteria, cefiderocol is the first siderophore antibiotic to reach late-stage development and FDA approval, showing good patient tolerance [3].
Non-fermenting Gram-negative bacteria (Nf-GNB), particularly Pseudomonas aeruginosa, are a significant burden in intensive care units. Moreover, these pathogens are at high risk of developing resistance, potentially becoming difficult-to-treat resistant (DTR) organisms, especially in the ICU setting. These bacteria are associated with increased mortality, longer ICU stays, and display a wide disparity in prevalence across Europe. In 2022, the rates of Drug-Resistant (DTR) invasive isolates of Pseudomonas spp. and Carbapenem-resistant Acinetobacter baumannii (CRAB) reached up to 13% and 36%, respectively, as highlighted by the European Centre for Disease Prevention and Control (ECDC) [4].
The current knowledge about cefiderocol is primarily based on randomized controlled trials that compared to carbapenems, which therefore do not specifically address DTR Nf-GNB, or Best Available Therapy (BAT). For instance, in the CREDIBLE-CR trial, cefiderocol was compared to BAT (mainly colistin-based) in 152 patients with DTR GNB infections. This trial demonstrated a higher rate of microbiological eradication and a lower risk of relapse in the cefiderocol arm, although it did not prove a higher rate of clinical success at the time of outcome assessment. Surprisingly, mortality was higher in the cefiderocol arm at both 14 and 28 days, and notably, the study population was not limited to patients admitted to intensive care units. The APEKS-NP trial, focusing on nosocomial pneumonia, involved 292 participants and demonstrated the non-inferiority of cefiderocol in terms of clinical cure, microbiological eradication, and mortality compared to meropenem. However, none of these trials were restricted to ICU patients, and only the CREDIBLE-CR trial focused on carbapenem-resistant pathogen. Some studies suggest that cefiderocol could be a therapeutic alternative to the current treatments available for managing infections caused by multi-drug resistant (MDR) bacteria in the ICU care setting [5, 6]. Therefore, we conducted a retrospective cohort study to evaluate the efficacy and safety of cefiderocol compared to Best Available Therapy in the ICU setting for treating difficult-to-treat Nf-GNB.
Materials and methods
Study setting
This retrospective, multicentric study was conducted across nine different university intensive care units, including a burn unit. The study adhered to the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments. The study received approval from the Institutional Review Board (CSE-HCL – IRB 00013204-22_547).
Eligibility criteria
We included adult patients (18 years or older) admitted to the ICU between January 1, 2019, and June 1, 2023, who were treated with Cefiderocol for a difficult-to-treat (DTR) infection caused by non-fermenting Gram-negative bacteria (Nf-GBN) with an identified infection site in the blood (bloodstream infection) or the lungs (pneumonia). Patients with infections caused by more than one DTR Nf-GBN or with a duration of treatment of less than 48 h were excluded from the study.
The chosen definition of DTR Nf-GNB was established for Pseudomonas aeruginosa and Acinetobacter baumanii as resistance to all fluoroquinolones and all β-lactam categories (except for Cefiderocol, Ceftazidime avibactam, Ceftolozane/tazobactam), including carbapenems. For Stenotrophomonas maltophilia, resistance to Trimethoprim/sulfamethoxazole was also a requirement [7].
Afterward, a matching process was carried out for every germ, at a 1:2 ratio, pairing them with another ICU patient exhibiting identical microorganism, DTR profile, and ideally, being situated in the same type of ICU (either a standard ICU or a burn unit ICU).
Data sources
The data about the Nf-GBN were obtained from the microbiological laboratory database (Clinisys GLIMS ®, Glasgow, Scotland), and the electronic health record (IntelliSpace Critical Care and Anesthesia (ICCA) ®, Philips, Amsterdam, Netherlands). The electronic health record then provided the following variables: Socio-demographic data and baseline characteristics: Age, sex, Charlson Comorbidity Index, organ transplantation, type of ICU admission (Medical, Surgical, or Burn), Immunosuppression (Immunosuppression (based on a patient getting an immuosuppressive treatment (chemotherapy, radiation, long term or recent high dosesteroids) or having an immuosuppressive condition (e.g. leukemia, lymphoma, multiple myeloma, AIDS), or with a HLA-DR dose < 8000 antibodies bound per cell [8, 9]), number of antibiotic lines before the infection (antibiotic line defined as the number of antibiotic/ association of antibiotic previously used during the intensive care hospitalization), and organ failure on the day of the infection, defined as follows:
Hemodynamic failure: requirement of vasoactive amines during the last 24 h of the infection.
Renal: Continuous Renal Replacement Therapy or Acute Kidney Injury KDIGO III.
Respiratory: Requirement of mechanical ventilation.
Neurologic: GCS < 9 without sedative drugs.
Characteristics of the infection: Days from admission to infection, localization, associated pathogen isolated, antibiotic used. The administration of the cefiderocol was performed intermittently at 2 g per 8 h, and the dosage was adapted to the renal clearance.
Adverse events attributable to cefiderocol or BAT: diarrhea, candidiasis, skin rash, cytolysis, Clostridioides difficile infection.
Follow-up of the infection: clinical cure at 15-day and 30-day (defined by the absence of antimicrobial treatment at 15 days for the same infection site, and the absence of clinical or biological signs of infection), relapse (defined as another infection after clinical cure caused by the same DTR Nf-GNB), duration of mechanical ventilation, delay from infection to ICU discharge, length of ICU stay, and in-ICU mortality.
Outcome
Our primary endpoint was the clinical cure 15 days after the infection. The secondary endpoints included clinical cure at 30 days, relapse, and in-ICU mortality.
Statistical methods
Descriptive statistics were expressed by the median and interquartile range [IQR] for quantitative variables and by the number and percentage (%) for qualitative variables. Differences between groups were estimated using the Wilcoxon rank-sum test for quantitative variables, and the Chi-squared test for qualitative variables or Fisher’s exact test when applicable.
To estimate the impact of the use of cefiderocol on each of the outcomes, a full model was built with 15 confounder selected a priori : sex, age, SAPS II, immunosuppression, transplantation, Charlson comorbidity index, type of ICU admission (medical, surgical, or burn), presence of hemodynamic, renal, respiratory and neurologic organ failure at the time of infection, pathogen of the infection, previous use of new β-lactam/β-lactamase inhibitor (Ceftazidime avibactam or Ceftolozane/tazobactam), presence of a polymicrobial infection and delay between ICU admission and occurrence of the infection. Then, a bidirectional Stepwise Algorithm was used to select the final model using the best Akaike information criterion. The treatment group was forced into the model. The final impact of the cefiderocol treatment was expressed using adjusted Odds Ratio associated with their 95% confidence interval [95%CI]. As a sensitivity analysis, the Relapse was also studied using competing risk regression, with death or discharge of ICU treated as competing event. The same confounder and variable selection process was performed. The statistical threshold was set at 0.05. Analyses were performed using the R software v3.4.3 [10].
Results
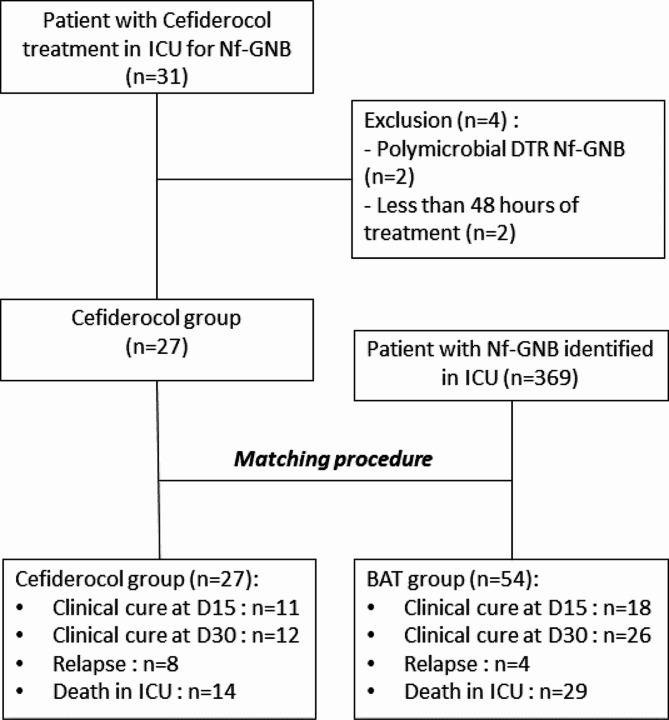
Out of the 31 patients treated with cefiderocol for DTR Nf-GNB, 2 patients with more than one DTR Nf-GBN and 2 patients with less than 48 h of treatment were excluded, leaving a final total of 27 patients in the cefiderocol group (Fig. 1). We applied an exact matching procedure based on the identical pathogen, the same DTR profile, and if possible, the same type of ICU for 27 patients, resulting in a comparison group of 54 patients receiving the Best Available Therapy (BAT). Only 4 matched patients could not be precisely matched based on the ICU profile; as a result, 4 patients in the cefiderocol group from the burn unit were matched with 4 patients in a conventional ICU unit.
Fig. 1.
Flow chart
Nf-GNB : Non fermenting Gram-Negative Bacteria; BAT : Best available treatment
Description of the population (table 1)
Table 1.
Patient characteristics
| Variable | Cefiderocol group n = 27 |
BAT group n = 54 |
p value |
|---|---|---|---|
| Sexe, Male | 20 (74.1%) | 35 (64.8%) | 0.556 |
| Age, year | 58 [42–65] | 60 [45–67] | 0.968 |
| Charlson Comorbidity Index | 4 [1–5] | 3 [1–5] | 0.781 |
| Immunosupression | 11 (40.7%) | 12 (22.2%) | 0.139 |
| Organ transplantation | 5 (18.5%) | 10 (18.5%) | 1.000 |
| Lung | 1 | 8 | |
| Heart | 1 | 2 | |
| Liver | 1 | 0 | |
| Liver-Kidney | 1 | 0 | |
| Bone Marrow | 1 | 0 | |
| Type of ICU admission | 0.341 | ||
| Medical | 13 (48.1%) | 17 (31.5%) | |
| Surgical | 4 (14.8%) | 11 (20.4%) | |
| Burn | 10 (37.0%) | 17 (31.5%) | 0.222 |
| Previous antibiotic line | 0.010 | ||
| 0 | 0 (0.0%) | 0 (0.0%) | |
| 1 | 2 (7.4%) | 15 (27.8%) | |
| 2 | 10 (37.0%) | 26 (48.1%) | |
| ≥ 3 | 15 (55.6%) | 13 (24.1%) | |
| Previous use of new β-lactam/β-lactamase inhibitor | 10 (37.0%) | 6 (11.1%) | 0.014 |
| SAPS II score | 55 [42–66] | 47 [43–66] | 0.217 |
| Delay from admission to infection, days | 29 [16–54] | 25 [10–59] | 0.346 |
| Organ failure at the occurrence of infection | |||
| Hemodynamic failure | 21 (77.8%) | 43 (79.6%) | 1.000 |
| Renal Failure | 13 (48.1%) | 28 (51.9%) | 0.937 |
| Respiratory failure | 25 (92.6%) | 53 (98.1%) | 0.256 |
| Neurologic failure | 9 (33.3%) | 22 (40.7%) | 0.686 |
The patients included were predominantly male (55–67.9%), with an average age of 58 [44–67] years old and were mostly admitted for medical reasons, followed by burn and surgical admission. They had a Charlson Comorbidity Index of 3 [1–5] and 28.4% (23 patients) presented with immunosuppression. No statistical differences were observed between the groups. During their ICU stay, 25 (92.6%) patients in the Cefiderocol group and 53 (98.1%) in the BAT group required mechanical ventilation (p = 0.597).
Before the administration of the antibiotic, none of the patients were antibiotic naive. The Cefiderocol group was more likely to have a higher number of previous antibiotic (15 (55.6%) patients with ≥ 3 lines of antibiotic treatment) compared to the BAT group (13 (24.1%) patients with ≥ 3 lines of antibiotic). Details of the previous antimicrobial therapies used in the ICU can be found in Supplementary Table 1. Notably, the cefiderocol group had a higher rate of previous use of previous use of new β-lactam/β-lactamase inhibitor (10 (37.0%) vs. 6 (11.1%)). Organ failure at the time of infection was comparable, primarily involving respiratory and hemodynamic failure.
Characteristics of the infection (table 2)
Table 2.
Characteristic of the infection
| Variable | Cefiderocol group n = 27 |
BAT group n = 54 |
p value |
|---|---|---|---|
| Pathogen identified | |||
| Pseudomonas aeruginosa | 22 (81.5%) | 44 (81.5%) | |
| Acinetobacter baumannii | 4 (14.8%) | 8 (14.8%) | |
| Stenotrophomonas maltophilia | 1 (3.7%) | 2 (3.7%) | |
| Localization of the infection | 0.054 | ||
| Pneumonia | 21 (77.8%) | 51 (94.4%) | |
| Bloodstream infection | 6 (22.2%) | 3 (5.6%) | |
| Polymicrobial infection | 22 (81.5%) | 36 (66.7%) | 0.257 |
| Number of associated pathogen | 0.147 | ||
| 1 | 13 (59.1%) | 27 (75.0%) | |
| 2 | 5 (22.7%) | 8 (22.2%) | |
| ≥3 | 4 (18.2%) | 1 (2.8%) | |
| Antibiotic therapy | |||
| Cefiderocol | 27 (100%) | 0 (0%) | |
| Ceftazidime avibactam | 0 (0%) | 28 (51.9%) | |
| Ceftolozane/tazobactam | 0 (0.0%) | 8 (14.8%) | |
| Carbapenem | 0 (0.0%) | 2 (3.7%) | |
| Imipenem/cilastatin/relebactam | 0 (0.0%) | 2 (3.7%) | |
| Aztreonam | 0 (0.0%) | 2 (3.7%) | |
| Trimethoprim/sulfamethoxazole | 0 (0.0%) | 1 (1.9%) | |
| Fluoroquinolone | 0 (0.0%) | 6 (11.1%) | |
| Colistin | |||
| Intravenous | 1 (3.7%) | 14 (25.9%) | |
| Inhaled | 6 (22.2%) | 16 (29.6%) | |
| Duration of antimicrobial therapy | 13 [8–15] | 14 [12–15] | 0.241 |
The predominant DTR Nf-GNB identified was Pseudomonas aeruginosa (81.5%), followed by Acinetobacter baumannii (14.8%) and Stenotrophomonas maltophilia (3.7%). Pneumonia was the identified infection in 21 (78.8%) patients in the Cefiderocol group and in 51 (94.4%) patients in the BAT group (p = 0.054). An additional microorganism was present in 22 (81.5%) patients in the Cefiderocol group and 36 (66.7%) patients in the BAT group (p = 0.257). Further details regarding the associated microorganisms can be found in Supplementary Table 2. The duration of antibiotic was 13 [8–16] days in the Cefiderocol group and 14 [12–15] days in the BAT group (p = 0.241). In the Cefiderocol group, 7 patients also received Colistin treatment, predominantly inhaled Colistin. The Cefiderocol was adapted to the renal clearance in 20 (74.1%) of the patient. In the BAT group, the most commonly used antibiotic was Ceftazidime-avibactam (28 patients, 51.9%), followed by Colistin and Ceftolozane/tazobactam. In the Cefiderocol group, we noticed a higher associated rate of resistance to new β-lactam (Supplementary Table 3).
Outcome of the patients (table 3)
Table 3.
Outcomes of the patients
| Variable | Cefiderocol group n = 27 |
BAT group n = 54 |
p value |
|---|---|---|---|
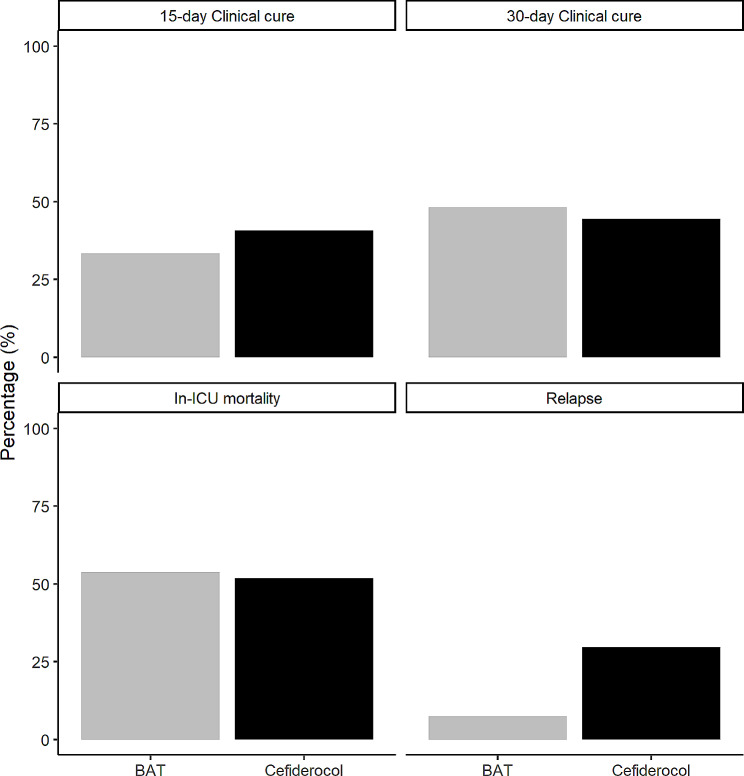
| Clinical cure at 15-day | 11 (40.7%) | 18 (33.3%) | 0.682 |
| Clinical cure at 30-day | 12 (44.4%) | 26 (48.1%) | 0.937 |
| Relapse | 8 (29.6%) | 4 (7.4%) | 0.017 |
| Delay infection-relapse, days | 43 [35–46] | 22 [15–31] | 0.106 |
| Duration of mechanical ventilation, days | 93 [46–122] | 59 [30–75] | 0.050 |
| Delay from infection to ICU discharge, days | 47 [16–77] | 29 [14–44] | 0.090 |
| Length of ICU stay, days | 95 [47–134] | 65 [36–97] | 0.087 |
| In ICU mortality | 14 (51.9%) | 29 (53.7%) | 1.000 |
The clinical cure at 15 days was similar between groups, with 40.7% (11 patients) in the cefiderocol group and 33.3% (18 patients) in the BAT group achieving cure (p = 0.682). However, the rate of relapse was higher in the cefiderocol group, with 8 (29.6%) patients experiencing relapse compared to 4 (7.4%) patients in the BAT group (p = 0.017) Figure 2. No emergence of resistance of cefiderocol was noted in the 8 relapses of the cefiderocol group, and cefiderocol was used to treat this relapse in 7 of the 8 relapses. In-ICU mortality, duration of mechanical ventilation, and time from infection to ICU discharge were comparable between the groups. The incidence of adverse events was similar between the cefiderocol and BAT groups, as documented in Supplementary Table 4.
Fig. 2.
Description of the clinical cure, relapse and clinical cure
These comparisons across different endpoints were confirmed after adjusting for main confounding variables, particularly regarding the higher risk of relapse, with an adjusted odds ratio (aOR) of 10.06 [1.96;51.53], p = 0.005 (Table 4; Supplementary Table 5). These results were confirmed in the sensitivity analysis (sub-Hazard Ratio 8.38 [1.91;36.67]; Supplementary Table 6).
Table 4.
Impact of the Cefiderocol on the different endpoints
| Variable | Adjusted Odds Ratio | p value |
|---|---|---|
| Clinical cure at 15-day | 1.65 [0.54;5.08] | 0.382 |
| Clinical cure at 30-day | 0.75 [0.26;2.19] | 0.597 |
| Relapse | 10.06 [1.96;51.53] | 0.005 |
| In ICU mortality | 1.11 [0.37;3.35] | 0.855 |
Discussion
The use of cefiderocol in the ICU is currently under-evaluated in the intensive care setting. The findings of this present study suggest that the use of cefiderocol, compared to the BAT, does not differ significantly in clinical cure or in-ICU mortality, but it does suggest a higher rate of infection relapse.
Among randomized controlled trials conducted to evaluate the efficacy of cefiderocol, three are noteworthy. The first was a comparison of cefiderocol versus imipenem-cilastatin for treating complicated urinary tract infections caused by Gram-negative uropathogens. This phase 2 trial included 452 patients, primarily with infections caused by Enterobacterales, and observed a higher clinical success rate in the cefiderocol arm [2]. The CREDIBLE-CR trial randomized 152 patients to receive either cefiderocol (101 patients) or the BAT (51 patients) [11]. These patients had DTR-GNB documented pneumonia, bloodstream infection, or sepsis, in various settings (ICU or not). The trial reported similar clinical success rates at the end of treatment in both groups (66% versus 58%) but noted a higher mortality rate in the cefiderocol arm at 14, 28 days, and at the end of the study. Lastly, the APEKS-NP trial included 145 participants in the cefiderocol arm and 152 in the meropenem arm, all suffering from pneumonia with Multi Drug Resistant GNB, most of which were Enterobacterales [12]. This trial showed comparable 14-day mortality rates and clinical cures. However, none of these trials specifically focused on DTR Nf-GNB or on the unique conditions of the ICU-care setting.
Therefore, the retrospective cohort study by Russo et al. specifically focused on Carbapenem-Resistant Acinetobacter baumannii in COVID-19 associated Ventilator-Associated Pneumonia (VAP) [13]. The study, which involved 73 patients with VAP, compared Cefiderocol-containing regimens with Colistin-containing regimens. The results showed that Cefiderocol was associated with better survival (HR 0.44 [0.22;0.66]). Despite the well-known impact on mortality of COVID-associated Ventilator-Associated Pneumonia (VAP), and its high incidence which could potentially explain the significant impact of treatment on mortality rates, the patients on the Colistin regimen had a surprisingly high 30-day mortality rate (98.1%) and only 19 patients in this study were treated with Cefiderocol [14, 15].
These results are inconsistent with other studies, such as the cohort from Mazzitelli, study involving 111 patients with Carbapenem-Resistant Acinetobacter baumannii infections [16]. This study showed a higher, yet not statistically significant, mortality rate in the cefiderocol group compared to the colistin group (51% vs. 37%, p = 0.130). These results were confirmed in several study and meta-analysis, which reported better outcomes in the specific setting of Carbapenem-Resistant Acinetobacter baumannii infections with cefiderocol [17–20].
In another instance, Wicky et al. reported on 16 ICU patients treated with cefiderocol for DTR Nf-GNB. In this descriptive study, they observed a clinical cure rate of 68.7% and an ICU mortality rate of 31.3%. Notably, persistent colonization was found in 81.3% of the patients, and a relapse was documented in 56.3% of them. The duration of antibiotic therapy was short, averaging 8 [7-13.5] days, and 31.3% of the patients received a combination of antibiotics, mainly with colistin [21].
A possible reason for the high relapse rate could be that more patients in the cefiderocol group had used new β-lactam/β-lactamase inhibitor before, which might indicate that cefiderocol was given as a rescue therapy in 37% of the patients (compared to 11% in the BAT group). Even though many patients in the cefiderocol group had not used new β-lactam/β-lactamase inhibitor before, and that this prior use was not finally associated with this outcome, this remains a potential explanation for the high relapse rate. Another observation is the use of cefiderocol in a context of higher resistance.
There are also specific concerns about the emergence of resistance during treatment with cefiderocol. As described by some authors, doubts exist regarding its efficacy against non-fermenting GNB, with several resistance mechanisms identified for cefiderocol [24, 25]. Moreover, difficulties for cefiderocol laboratory testing were also reported by EUCAST, choosing disc diffusion method for resistance screening as performed in our study [26]. Additionally, questions remain about whether to use cefiderocol as monotherapy or in combination with other antibiotics, but currently, there is no clinical data available to support either practice [27].
We observed a notably high percentage of polymicrobial infections : the successive exposure to antimicrobial agents across multiple infection episodes favors the emergence of highly resistant strains and the coexistence of multiple pathogens within the host – 70% in our cohort. Furthermore, the prolonged ICU stays preceding these infections further exacerbate the likelihood of polymicrobial involvement, and the ICU-acquired immunosuppression.
Despite the known adverse events attributable to cefiderocol, we confirmed that it remains safe for use in ICU care, likely because the antibiotics administered in the BAT group had more severe adverse events. Indeed, the toxicity of cefiderocol has been extensively assessed in randomized controlled trials and preclinical studies [28, 29]. It has the advantage of probably presenting no clinically significant drug-drug interaction (DDI) [30].
However, this study is subject to several notable limitations that must be acknowledged. Firstly, the relatively low number of patients included in the study could potentially limit the generalizability of the findings. Furthermore, there was a lack of matching based on the site of infection. This oversight could result in variations in the treatment outcomes, as the efficacy of cefiderocol may differ depending on the infection site, a factor not accounted for in our study design. Additionally, the retrospective nature of the study introduces inherent biases, as it relies on pre-existing data and lacks the stringent controls of prospective research: for example, the ‘clinical cure’ was not assessed in a blinded manner, not standardized – based on the medical note, physiological and biological parameters at the time of the evaluation - and is therefore not a objective endpoint such as mortality. Finally, on the matched DTR Nf-GNB, we observed a higher proportion of resistance, especially to other new β-lactam. This constatation, associated to the higher number of previous antimicrobial line might explain the higher rate of relapse rate in the cefiderocol group. Therefore, these results need should be confirmed through larger real-life data cohorts. Moreover, specific randomized controlled trials are necessary to validate our observations and ensure that the conclusions drawn are robust and applicable to broader clinical practices.
Conclusion
In conclusion, our study provides important insights into the efficacy and safety of cefiderocol in the treatment of DTR Nf-GNB in ICU settings. We observed that while cefiderocol is comparable to the best available therapy (BAT) in terms of clinical cure and in-ICU mortality, it may be associated with a higher rate of infection relapse.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Dr Mathias GERNEZ for his help in data collection.
Author contributions
CHV, AF wrote the original draft of the manuscript, and participated in conceptualization formal analysis, Methodology, supervision and validation. AK, OD, JPR participated in the data curation, conceptualization and reviewing and editing the manuscript substantially. BB, JPR JLF FA LA OD JCR ACL participated in reviewing and editing the manuscript substantially.
Data availability
Upon reasonable request.
Declarations
Ethics approval and consent to participate
The study adhered to the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments. The study received approval from the Institutional Review Board (CSE-HCL – IRB 00013204-22_547).
Consent for publication
Not applicable.
Financial disclosures
None.
Conflict of interest
Arnaud Friggeri participated as a member of the Data Safety Monitoring Board or Advisory Board, receiving payments or honoraria for lectures, presentations, as well as for attending meetings and/or travel, on behalf of Shionogi & Company.
Footnotes
The original online version of this article was revised: The supplementary material 2 has been removed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/24/2024
A Correction to this paper has been published: 10.1186/s13613-024-01317-y
References
- 1.Naseer S, Weinstein EA, Rubin DB, Suvarna K, Wei X, Higgins K, et al. US Food and Drug Administration (FDA): benefit-risk considerations for Cefiderocol (Fetroja®) Clin Infect Dis off Publ Infect Dis Soc Am. 2021;72:e1103–11. doi: 10.1093/cid/ciaa1799. [DOI] [PubMed] [Google Scholar]
- 2.Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18:1319–28. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 3.Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, Safety, and tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-negative Bacteria, in healthy subjects. Antimicrob Agents Chemother 2018;62. [DOI] [PMC free article] [PubMed]
- 4.EARS. Antimicrobial resistance in the EU/EEA (EARS-Net): Annual Epidemiological Report for 2019. 11/11/2019 ed.
- 5.Gijón D, García-Castillo J, Fernández-López MC, Bou G, Siller M, Calvo-Montes J, et al. In vitro activity of cefiderocol and other newly approved antimicrobials against multi-drug resistant gram-negative pathogens recovered in intensive care units in Spain and Portugal. Rev Esp Quimioter Publicacion Soc Esp Quimioter. 2024;37:69–77. doi: 10.37201/req/098.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbier F, Hraiech S, Kernéis S, Veluppillai N, Pajot O, Poissy J, et al. Rationale and evidence for the use of new beta-lactam/beta-lactamase inhibitor combinations and cefiderocol in critically ill patients. Ann Intensive Care. 2023;13:65. doi: 10.1186/s13613-023-01153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2022 Guidance on the treatment of extended-spectrum β-lactamase Producing enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat Resistance (DTR-P. aeruginosa) Clin Infect Dis off Publ Infect Dis Soc Am. 2022 doi: 10.1093/cid/ciac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Vacheron C-H, Lepape A, Venet F, Monneret G, Gueyffier F, Boutitie F, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients presenting sepsis-induced immunosuppression: the GRID randomized controlled trial. J Crit Care. 2023;78:154330. doi: 10.1016/j.jcrc.2023.154330. [DOI] [PubMed] [Google Scholar]
- 10.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing,Vienna, Austria. URLhttps://www.R-project.org/. 2017.
- 11.Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21:226–40. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 12.Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21:213–25. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 13.Russo A, Bruni A, Gullì S, Borrazzo C, Quirino A, Lionello R, et al. Efficacy of cefiderocol- vs colistin-containing regimen for treatment of bacteraemic ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19. Int J Antimicrob Agents. 2023;62:106825. doi: 10.1016/j.ijantimicag.2023.106825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vacheron C-H, Lepape A, Savey A, Machut A, Timsit JF, Comparot S, et al. Attributable mortality of ventilator-associated Pneumonia among patients with COVID-19. Am J Respir Crit Care Med. 2022;206:161–9. doi: 10.1164/rccm.202202-0357OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vacheron C-H, Lepape A, Savey A, Machut A, Timsit JF, Vanhems P, et al. Increased incidence of Ventilator-Acquired Pneumonia in Coronavirus Disease 2019 patients: a Multicentric Cohort Study. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzitelli M, Gregori D, Sasset L, Trevenzoli M, Scaglione V, Lo Menzo S, et al. Cefiderocol-based versus colistin-based regimens for severe carbapenem-resistant Acinetobacter baumannii infections: a propensity Score-Weighted, Retrospective Cohort Study during the first two years of the COVID-19 pandemic. Microorganisms. 2023;11:984. doi: 10.3390/microorganisms11040984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rando E, Cutuli SL, Sangiorgi F, Tanzarella ES, Giovannenze F, De Angelis G, et al. Cefiderocol-containing regimens for the treatment of carbapenem-resistant A. Baumannii ventilator-associated pneumonia: a propensity-weighted cohort study. JAC-Antimicrob Resist. 2023;5:dlad085. doi: 10.1093/jacamr/dlad085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falcone M, Tiseo G, Leonildi A, Della Sala L, Vecchione A, Barnini S, et al. Cefiderocol- compared to colistin-based regimens for the treatment of severe infections caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2022;66:e0214221. doi: 10.1128/aac.02142-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascale R, Pasquini Z, Bartoletti M, Caiazzo L, Fornaro G, Bussini L, et al. Cefiderocol treatment for carbapenem-resistant Acinetobacter baumannii infection in the ICU during the COVID-19 pandemic: a multicentre cohort study. JAC-Antimicrob Resist. 2021;3:dlab174. doi: 10.1093/jacamr/dlab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onorato L, de Luca I, Monari C, Coppola N. Cefiderocol either in monotherapy or combination versus best available therapy in the treatment of carbapenem-resistant Acinetobacter baumannii infections: a systematic review and meta-analysis. J Infect 2024;0. [DOI] [PubMed]
- 21.Wicky P-H, Poiraud J, Alves M, Patrier J, d’Humières C, Lê M, et al. Cefiderocol Treatment for Severe Infections due to difficult-to-treat-resistant non-fermentative gram-negative Bacilli in ICU patients: a Case Series and Narrative Literature Review. Antibiot Basel Switz. 2023;12:991. doi: 10.3390/antibiotics12060991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc. 2015;16:147–53. doi: 10.1177/1751143714564816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsube T, Saisho Y, Shimada J, Furuie H. Intrapulmonary pharmacokinetics of cefiderocol, a novel siderophore cephalosporin, in healthy adult subjects. J Antimicrob Chemother. 2019;74:1971–4. doi: 10.1093/jac/dkz123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhu J, Chen L, Du H. Cefiderocol: clinical application and emergence of resistance. Drug Resist Updat. 2024;72:101034. doi: 10.1016/j.drup.2023.101034. [DOI] [PubMed] [Google Scholar]
- 25.Smoke SM, Brophy A, Reveron S, Iovleva A, Kline EG, Marano M, et al. Evolution and transmission of Cefiderocol-Resistant Acinetobacter baumannii during an outbreak in the burn Intensive Care Unit. Clin Infect Dis off Publ Infect Dis Soc Am. 2023;76:e1261–5. doi: 10.1093/cid/ciac647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.eucast. Cefiderocol susceptibility testing. at < https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=493&cHash=22779384b74c8cf2c55aa3f7fd69d173.
- 27.Corcione S, De Benedetto I, Pinna SM, Vita D, Lupia T, Montrucchio G, et al. Cefiderocol use in Gram negative infections with limited therapeutic options: is combination therapy the key? J Infect Public Health. 2022;15:975–9. doi: 10.1016/j.jiph.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga Y, Sonoyama T, Casanova L, Nagata TD, Echols R, De Gregorio F, et al. 1292. Safety Profile of the Novel Siderophore Cephalosporin Cefiderocol in Randomized Phase 2 and phase 3 Clinical studies of Serious Gram-negative infections. Open Forum Infect Dis. 2020;7:S661–2. doi: 10.1093/ofid/ofaa439.1475. [DOI] [Google Scholar]
- 29.Timsit J-F, Paul M, Shields RK, Echols R, Baba T, Yamano Y, et al. Cefiderocol for the treatment of infections due to Metallo-Beta-Lactamase-Producing pathogens in the CREDIBLE-CR and APEKS-NP phase 3 Randomized studies. Clin Infect Dis off Publ Infect Dis Soc Am. 2022 doi: 10.1093/cid/ciac078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsube T, Miyazaki S, Narukawa Y, Hernandez-Illas M, Wajima T. Drug-drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol. 2018;74:931–8. doi: 10.1007/s00228-018-2458-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request.