Abstract
Background and Aims
Introgressive hybridization poses a challenge to taxonomic and phylogenetic understanding of taxa, particularly when there are high numbers of co-occurring, intercrossable species. The genus Quercus exemplifies this situation. Oaks are highly diverse in sympatry and cross freely, creating syngameons of interfertile species. Although a well-resolved, dated phylogeny is available for the American oak clade, evolutionary relationships within many of the more recently derived clades remain to be defined, particularly for the young and exceptionally diverse Mexican white oak clade. Here, we adopted an approach bridging micro- and macroevolutionary scales to resolve evolutionary relationships in a rapidly diversifying clade endemic to Mexico.
Methods
Ecological data and sequences of 155 low-copy nuclear genes were used to identify distinct lineages within the Quercus laeta complex. Concatenated and coalescent approaches were used to assess the phylogenetic placement of these lineages relative to the Mexican white oak clade. Phylogenetic network methods were applied to evaluate the timing and genomic significance of recent or historical introgression among lineages.
Key Results
The Q. laeta complex comprises six well-supported lineages, each restricted geographically and with mostly divergent climatic niches. Species trees corroborated that the different lineages are more closely related to other species of Mexican white oaks than to each other, suggesting that this complex is polyphyletic. Phylogenetic networks estimated events of ancient introgression that involved the ancestors of three present-day Q. laeta lineages.
Conclusions
The Q. laeta complex is a morphologically and ecologically related group of species rather than a clade. Currently, oak phylogenetics is at a turning point, at which it is necessary to integrate phylogenetics and ecology in broad regional samples to figure out species boundaries. Our study illuminates one of the more complicated of the Mexican white oak groups and lays groundwork for further taxonomic study.
Keywords: Coalescence, introgression, syngameon, phylogenetic network, Quercus subsection Leucomexicana, target enrichment
INTRODUCTION
Phylogenetic relationships are often represented graphically by bifurcating trees, which suggest a split of population-level lineages that diverged and remained independent (Linder et al., 2004; Rancilhac et al., 2021). However, hybridization has played an important role in diversification across diverse lineages of the tree of life (Abbott et al., 2013; Mallet et al., 2016; Solís-Lemus and Ané, 2016; Gernandt et al., 2018; Kleinkopf et al., 2019; Taylor and Larson, 2019; Crowl et al., 2020; Rancilhac et al., 2021). Phylogenetic trees can be misleading about evolutionary history if the processes of hybridization (gene flow between lineages) and incomplete lineage sorting (gene divergences that do not track population divergence history) are not modelled explicitly. Recent work has made phylogenetic inference tractable in such cases by reconstructing evolutionary relationships as networks, i.e. phylogenetic trees with interconnected branches, where interconnections represent gene flow in the context of an otherwise divergent process (Maddison and Knowles, 2006; Wen et al., 2008; Solís-Lemus and Ané, 2016; McVay et al., 2017a; Crowl et al., 2020; Rancilhac et al., 2021).
The genus Quercus (the oaks) is renowned for the ease with which species exchange genes within taxonomic sections, almost irrespective of the time elapsed since their divergence from a common ancestor within that section (Palmer, 1948; Hardin, 1975; Belahbib et al., 2001; Eaton et al., 2015; Sullivan et al., 2016; Li et al., 2021). Partly because of this, early attempts to reconstruct the oak phylogeny based on chloroplast DNA and a few nuclear loci faced limited success (Manos et al., 1999; Mayol and Roselló, 2001; Bellarosa et al., 2005; Simeone et al., 2013; Hubert et al., 2014). More recently, analyses based on restriction site-associated DNA sequencing (RAD-seq) data have been useful in reconstructing the relationships and patterns of diversification of the main oak clades (Hipp et al., 2014, 2018, 2020; Cavender-Bares et al., 2015). These studies have demonstrated the need to use a large number of loci across the entire genome to assess the evolutionary relationships of closely related species accurately (Hipp et al., 2014, 2018, 2020; Crowl et al., 2020) and have pointed to introgression events that have yet to be explored across the tree using dense sampling of individuals analysed using phylogenomic methods. Although these studies have provided a solid, well-resolved and dated phylogeny for the monophyletic American oak clade, the evolutionary relationships within some groups remain to be defined, particularly for Mexican species (Valencia-A, 2004; Hipp et al., 2018, 2020).
RAD-seq phylogenetic studies have enabled testing oak introgression hypotheses (e.g. Eaton et al., 2015; McVay et al., 2017a, b; Kim et al., 2018; Ortego et al., 2018), but phylogenetic networks are still underexplored in the genus. In contrast to RAD-seq, which recovers relatively short loci (Ree and Hipp, 2015), targeted enrichment methods can provide a large number of longer low-copy nuclear loci capable of resolving conflicting phylogenetic relationships of non-model organisms, making the inference of reticulate phylogenetic histories against the background of incomplete lineage sorting more straightforward (Weitemier et al., 2014; Folk et al., 2017; Gernandt et al., 2018; Vatanparast et al., 2018; Villaverde et al., 2018; Dodsworth et al., 2019; Loiseau et al., 2019; Hale et al., 2020; Breinholt et al., 2021; Ma et al., 2021). These methods use small RNA or DNA oligonucleotides (called ‘baits’ or ‘probes’) to enrich next-generation sequencing libraries for selected target loci, such that the DNA for target loci is sequenced preferentially, whereas much of the non-target DNA is discarded (Weitemier et al., 2014; Breinholt et al., 2021). Target enrichment has been shown to be cost-effective, efficient in high-throughput sequencing contexts and useful with historical specimens, such as herbarium samples (Gardner et al., 2016; Villaverde et al., 2018; Kleinkopf et al., 2019; Hale et al., 2020; Breinholt et al., 2021).
Understanding the processes shaping biodiversity goes beyond reconstructing phylogenetic networks. Understanding the niche axes along which species have diversified is essential to understanding how they remain distinct from one another, especially when reproductive isolation is incomplete (Van Valen, 1976). Ecological niche models (ENMs) have proved to be complementary to phylogenomic approaches in addressing questions such as whether recently diverged taxa that show limited genetic differences are nonetheless ecologically distinct, supporting the idea that they are separately evolving lineages (Rissler and Apodaca, 2007; Su et al., 2015; Nunes and Pearson, 2017; Lin et al., 2021), or to examine the roles that ecological differences have played in divergence and speciation (Glor and Warren, 2010; Wooten and Gibbs, 2012; Blair et al., 2013; Gutiérrez-Ortega et al., 2020; Calixto-Rojas et al., 2021; Lin et al., 2021). Although most niche models are constructed at the species level, in cases where niche evolution is rapid or local adaptation is suspected, creating separate niche models for lineages within species can demonstrate which genetically divergent groups are also ecologically divergent (Smith et al., 2019; Gutiérrez-Ortega et al., 2020). If these lineages occupy different regions of environmental space, the finding could provide additional support that the lineages identified are diversifying to become discrete species, if not now then in the future (Goudarzi et al., 2019).
In this study, we adopted an approach bridging micro- and macroevolutionary scales to resolve evolutionary relationships in Quercus subsect. Leucomexicana, a rapidly diversifying clade endemic to Mexico, Central America and the southwestern USA (Hipp et al., 2018; Manos and Hipp, 2021). We focus on the taxonomically problematic Quercus laeta complex, which is endemic to Mexico and distributed in different latitudes, environments and elevations (Fig. 1A). The morphological variation, mainly in the shape of the leaf, that characterizes the Q. laeta complex across its geographical distribution has been challenging to sort for taxonomists, who have considered it one of the most polymorphic Mexican oak groups (Fig. 1B–G; Valencia-A, 2004), and as many as six taxa (Quercus bipedalis Trel., Quercus centralis Trel., Quercus obscura Trel., Quercus pallescens Trel., Quercus prinopsis Trel. and Quercus transmontana Trel.) have variously been teased out of or synonymized under Q. laeta (McVaugh, 1974; Romero et al., 2002). In previous taxonomic work, the complex has been characterized based mainly on micromorphological characters, such as papillae and glands on the leaf undersides and the conformation of trichomes on the upper surfaces of the leaves (Liebmann, 1854; Trelease, 1924). More recently, population-level studies, based on microsatellite and morphometric data, have suggested the existence of at least two different entities within Q. laeta that might constitute separate species (Morales-Saldaña et al., 2022). Disentangling the evolutionary history of this complex is clearly essential to clear out its taxonomy and resolve its role in the ecology of Mexican woodlands and savannas.
Fig. 1.
Geographical distribution and morphology of the Quercus laeta complex. (A) Red points indicate localities where Q. laeta has been reported. Representative phenotypes from each lineage identified here: (B) centralis TMVBc lineage; (C) centralis TMVBe lineage; (D) laeta SMS lineage; (E) laeta SMOc lineage; (F) prinopsis lineage; and (G) transmontana lineage.
Here, we performed a broad geographical sampling of Q. laeta complex populations and combined ecological niche comparisons with phylogenetic inference based on target enrichment sequencing data to test whether the Q. laeta complex represents one widespread polymorphic species or, in contrast, there are multiple unrelated cryptic species that converged on a similar phenotype. Likewise, phylogenetic networks allowed us to evaluate whether historical introgression is a primary, secondary or negligible driver of the evolutionary history of the Q. laeta complex.
MATERIALS AND METHODS
Bait design
Baits were designed using the MarkerMiner v.1.0 pipeline (Chamala et al., 2015). Initially, gene and transcriptome data were retrieved from various Quercus references from which to identify conserved single-copy loci. Coding sequences from the Quercus robur (Plomion et al., 2018) and Quercus lobata (Sork et al., 2016) draft genome assemblies, in addition to above-ground tissue transcriptomes for Q. robur (Lesur et al., 2015), Quercus alba (WO454_v2, Hardwood Genomics Project, https://doi.org/10.25504/FAIRsharing.srgkaf), and Q. rubra (RO454_v2, Hardwood Genomics Project, https://doi.org/10.25504/FAIRsharing.srgkaf) were selected based on their relative completeness and quality. Coding sequences retrieved from genome assemblies were treated as transcriptomes within the MarkerMiner wrapper program by masking introns using BedTools (Quinlan and Hall, 2010). The Q. robur coding sequence set was also used as a pseudo-proteome reference for the program by translating masked coding sequences using TransDecoder (Haas, 2016). Single-copy genes were flagged for MarkerMiner using metadata available in the Q. robur annotations. After running MarkerMiner on all provided reference sets, single-copy loci were selected for bait design based on their presence in multiple reference sets and on their length. In addition, Quercus functional genes related to several environmental conditions were added from other studies: waterlogging and budbreak genes from a common garden differential expression experiment using Q. robur and Quercus petraea (Ueno et al., 2013; Lesur et al., 2015); drought tolerance genes from a Q. lobata functional gene meta-analysis (Oney-Birol et al., 2018); and ICE1 and HOS1 from the Q. robur gene annotation (Meireles et al., 2017). In total, 465 genes were used for 2× tiled bait design at Arbor Biosciences, comprising 403 single-copy nuclear loci for phylogenetic inference and 62 functional genes.
Taxon sampling
A dataset of 56 samples grouped into two subsets was used (Supplementary Data Table S1). The first (subset 1) corresponded to 36 samples collected for this study from 15 populations of the Q. laeta complex throughout its range in Mexico. In each population, up to five randomly selected trees were sampled, separated from each other by a minimum of 50 m. Also, in those populations where other oaks from the Leucomexicana clade occurred, one or two individuals per species were sampled for the phylogenetic and reticulation analysis. Subset 2 consisted of 20 samples comprising 10 species from the Leucomexicana clade that occurred in sympatry with the complex, plus previously collected samples of three additional species that have been identified as being closely related to the Q. laeta complex in former phylogenomic studies (Hipp et al., 2018, 2020). Voucher specimens were deposited at the Herbarium of the Facultad de Ciencias of the Universidad Nacional Autónoma de México (FCME). Geographical information and voucher details for the 56 samples are shown in the Supplementary Data (Table S1).
DNA extraction, library preparation and sequencing
Genomic DNA was extracted from fresh leaves using the cetyltrimethyl ammonium bromide protocol, with an additional phenol–chloroform cleaning step (Lefort and Douglas, 1999). We checked the DNA concentration of each extraction with a Qubit fluorometer 4.0 (Thermo Fisher Scientific, Waltham, MA, USA) using a high-sensitivity kit and with a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Isolated genomic DNA was sonicated to obtain fragments of size >550 bp using a Covaris E220 Focused-ultrasonicator (Wohurn, MA, USA) with Covaris microtubes.
Library preparation was done with the KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA, USA) following the manufacturer’s protocol. Library concentrations were quantified using a Qubit fluorometer 4.0 (Thermo Fisher Scientific) with a high-sensitivity kit. Solution-based hybridization and enrichment was carried out through the MYBaits target enrichment system (Arbor Biosciences, Ann Arbor, MI, USA) following the standard MYBaits v.5.02 protocol (https://arborbiosci.com/mybaits-manual/). Libraries were pooled according to phylogenetic proximity, following previous phylogenomic studies (Hipp et al., 2018). We used the above-described custom baits designed for oaks that target 465 putatively single-copy genes. The target-enriched libraries were sequenced on an Illumina MiSeq at The Field Museum of Natural History (Chicago, IL, USA) using MiSeq reagent kit v.3 (600 cycles, 2 × 300 bp paired-end reads).
Gene assembly, alignment and filtering
We used FastQC v.0.11.8 (Andrews, 2010) to assess the quality of Illumina raw reads. Multiplexed reads were separated using their barcode sequences and combined into paired fastq files (R1 and R2) for each sample individually. We then used Trimmomatic v.0.36 (Bolger et al., 2014) to remove adapter sequences and low‐quality reads with a 4 bp sliding window, a quality threshold of Q30 and a minimum sequence length of 30. Consensus target sequences were assembled using HybPiper v.1.2 (Johnson et al., 2016), which defaults to ignoring heterozygotic positions, generating a single consensus sequence per individual, with potentially heterozygous bases called as the nucleotide with the highest read frequency. HybPiper used BWA v.0.7.1 (Li and Durbin, 2009) to align reads to the reference nuclear gene sequences and SAMtools v.0.1.19 (Li et al., 2009) to sort the reads into separate directories for each gene. Subsequently, reads mapped for each locus were assembled de novo into contigs, with the best k-mer automatically detected by SPAdes v.3.10.1 (Bankevich et al., 2012).
The resulting gene files were imported to Geneious v.8.1.9 and aligned individually with MAFFT v.7.3 (Katoh et al., 2002; Katoh and Standley, 2013). Following Gernandt et al. (2018) and Villaverde et al. (2018), after a visual inspection, we proposed the following ad hoc criteria to identify and exclude alignments with poor quality: (1) alignment length of <500 bp; (2) <20 % of sites identical; (3) pairwise identity of <90 %; and (4) genes detected as paralogues. The percentage of identical sites and pairwise identity statistics were calculated in the Geneious alignment view (Kearse et al., 2012); paralogues were detected using HybPiper v.1.2 (Johnson et al., 2016). After data clean-up, a total of 155 genes were retained for analysis (additional details are in the Results).
Inference of phylogenetic relationships and genetic distinctiveness within the Q. laeta complex
Initially, we inferred phylogenetic relationships at the population level within the Q. laeta complex to identify hypothetical lineages. For this, we evaluated phylogenetic relationships by maximum likelihood (ML), including only samples belonging to the Q. laeta complex (subset 1; see Supplementary Data Table S1). For ML analysis, all 155 loci retained after data cleaning and filtering were concatenated into a super-matrix, and a phylogenetic tree was inferred in RAxML v.8.2.9 (Stamatakis, 2014), using the GTRCAT implementation of the general time-reversible model that affords substantial savings in computational time for large phylogenetic datasets, with branch support assessed using 1000 fast bootstraps. Additionally, to assess the genetic distinctiveness and cohesion of groups within the Q. laeta complex, non-metric multidimensional scaling (NMDS) on a pairwise genetic distance matrix was used to cluster samples. The distance matrix for NMDS was calculated under the GTRGAMMA model in RAxML v.8.2.9 (Stamatakis, 2014), then converted from long to wide format for use in the monoMDS function of the R package vegan v.2.5-2 (Oksanen et al., 2018). NMDS ordination was conducted using default parameters with a maximum number of random starts in search of a stable solution of 1000 (maxit = 1000) from K = 1 to K = 5.
Coalescent species delimitation
To evaluate species limits within the Q. laeta complex, we used the Bayesian phylogenetic and phylogeography (BPP) program v.4.3.8 (Flouri et al., 2018), running analysis type ‘A11’, which estimates a species tree while simultaneously running the species delimitation algorithm (Yang, 2015). We separated individuals into hypothetical species based on lineages obtained from the previous concatenated analysis; thus, the BPP analysis contained six potential species (see Results; Fig. 2). Following the approach of Leaché and Fujita (2010) and Fujita et al. (2012), we used two combinations of priors that represent different effective population sizes and ages for the root in the species tree. These priors are the species divergence times (τ) and the population sizes (θ), with the gamma distribution specified as G. The first combination assumes a larger effective population size θ ~ G (1, 10) and deep divergence τ ~ G (1, 10), whereas the second prior combination assumes a smaller effective population size θ ~ G (2, 2000 and a shallow divergence time τ ~ G (2, 2000). For each scenario, we performed two independent runs to check that our results were consistent, running 200 000 Markov chain Monte Carlo generations, sampled every five generations and using 20 000 burn-in generations.
Fig. 2.
Tree based on maximum likelihood (155 loci and 302 321 bp) with the GTRCAT model and 1000 bootstraps. Bootstrap support values of >50 % are indicated above the branches. Colours and labels to the right of the tree indicate the different lineages proposed.
Inference of phylogenetic relationships among the Q. laeta lineages and other species from the Leucomexicana subsection
Once different lineages were identified within the Q. laeta complex, we tested whether Q. laeta lineages form a clade relative to the broader set of Mexican white oaks, using a sample defined by a previous phylogenetic study (Hipp et al., 2020). For this purpose, we evaluated phylogenetic relationships using both an ML analysis of a concatenated matrix of all genes and estimation of the species tree using the multispecies coalescent (MSC) based on gene tree phylogenies inferred individually. For analysis of the concatenated data matrix, we conducted ML analysis in RAxML v.8.2.9 (Stamatakis, 2014), using the GTRCAT approximation with 1000 fast bootstraps to evaluate branch support. To test whether the Q. laeta complex forms a clade, we compared the ML tree with a tree optimized under the constraint that the Q. laeta complex forms a clade. We performed the comparison using the Kishino–Hasegawa paired-sites test (Kishino and Hasegawa, 1989), which tests whether two selected phylogenetic trees differ significantly in likelihood for the same molecular dataset. The test was implemented in IQ-TREE v.2.2.6 under the GTR+I+G model, with significance testing using 1000 RELL bootstraps.
Because concatenation approaches can produce erroneous tree topologies in the presence of incomplete lineage sorting (Liang et al., 2015; Edwards et al., 2016), potentially producing high support for the incorrect tree (Bapteste et al., 2008; Warnow, 2015), we used two coalescent-based species tree methods that accommodate incomplete lineage sorting, namely ASTRAL-III v.5.7.8 (Zhang et al., 2018) and SVDQuartets (Chifman and Kubatko, 2014) as implemented in PAUP* v.4.0a167 (Swofford, 2002). Both ASTRAL-III and SVDQuartets allow analysis of multiple individuals per species, while estimating the relationship amongst species, not individuals. We assigned individuals based on: (1) the identity of their corresponding taxonomic species; and (2) the results of the concatenated phylogenetic analyses. For the ASTRAL-III analysis, a single gene tree was reconstructed for each locus by ML in RAxML using the GTRCAT model with 1000 fast bootstraps. The 155 gene trees reconstructed were used as input for the species tree reconstruction in ASTRAL-III v.5.7.3 (Zhang et al., 2018). Finally, for each branch in the ASTRAL species tree, we recovered the local posterior probability (LPP) support (Sayyari and Mirarab, 2016). For the SVDQuartets analysis, PAUP v.4.0a169 (Swofford, 2002) was applied to the concatenated matrix, with 100 bootstrap replicates for branch support and all possible quartets evaluated. Both the ML and MSC trees were displayed graphically in FigTree v.1.4.0 (Rambaut, 2012).
Assessing incongruence among gene trees and species trees
Bootstrap support can be high even when a low number of genes support a clade (Pease et al., 2018; Minh et al., 2020). Therefore, we examined gene and site concordance factors (gCF and sCF, respectively) to quantify topological conflict around each branch of the ML concatenated tree in IQ‐TREE‐2 v.2.1.2 (Minh et al., 2020). For every branch of the concatenated tree, the gCF and sCF represent the percentage of decisive gene trees and the percentage of alignment sites, respectively, containing that branch (Minh et al., 2020). In addition, the ASTRAL quartet score and the normalized quartet score were computed to summarize the proportion of induced quartet trees (from individual single-locus gene trees) in the ASTRAL species tree. Also, we recovered the quartet support (Sayyari and Mirarab, 2016), which measures the conflict of genes around each branch of the ASTRAL species tree (Zhang et al., 2018)
Inferring ancient introgression
We used the maximum pseudolikelihood method Species Networks applying Quartets (SNaQ) (Solís‐Lemus and Ané, 2016) to estimate a phylogenetic network among the Mexican white oak species sampled, allowing for both hybridization and incomplete lineage sorting. To reduce the computational burden of estimating a large network (>30 taxa) and account for sensitivity of SNaQ to taxon sampling, we analysed four subsets of data that reflected a range of possible reticulation events. We focused our analysis on samples that showed conflicting phylogenetic signals in the concatenated analysis and in the species trees. Networks were inferred with the SnaQ method implemented in the Julia v.1.7.2 (Bezanson et al., 2017) package PhyloNetworks v.0.11.0 (Solís‐Lemus and Ané, 2016; Solís‐Lemus et al., 2017), based on concordance factors from 155-gene-tree sets for each of the four taxon samples. We used RAxML v.8 (Stamatakis, 2014) to obtain gene trees with bootstrap support for each gene (with the GTRCAT model and 100 bootstrap replicates) and used these trees to estimate quartet concordance factors (CFs) with PhyloNetworks (function readTrees2CF). These CFs were then used to reconstruct phylogenetic networks under incomplete lineage sorting. Using the species tree obtained with ASTRAL-III as a starting tree, we then estimated the best phylogenetic network with a varying number of hybridization events (h) allowed (0 ≤ h ≤ 3). To ensure convergence, we performed ten independent runs under each value of h. Pseudolikelihood scores for each value of h were plotted in R v.3.4.4 (R Development Core Team, 2018), and the best network model was selected by examining at what value of h the pseudolikelihood score plateaus, as recommended by Solís-Lemus et al. (2017).
Niche differentiation among lineages within the Q. laeta complex
Localities for the ENMs for each of the identified lineages in the Q. laeta complex were based on the populations included in this study (Supplementary Data Table S1), combined with records obtained from the careful revision conducted by the authors of the specimens present in the collections of the Herbarium of Facultad de Ciencias, UNAM (FCME) and the National Herbarium of Mexico (MEXU). We did not consider records from online databases, because close visual inspection of specimens was necessary to differentiate among the lineages identified here. Given that the spatial correlation of the localities might cause model overfitting (Dormann et al., 2007, 2013), specimen records were thinned using the spThin package in R (Aiello-Lammens et al., 2015), which randomly selects points of presence with ≥5 km distance among locations using 100 replicas. In the end, we obtained 56 presence data points partitioned into the centralis TMVBc (Trans-Mexican Volcanic Belt central; 7), centralis TMVBe (Trans-Mexican Volcanic Belt eastern; 7), laeta SMS (Sierra Madre del Sur; 6), laeta SMOc (Sierra Madre Occidental; 10), prinopsis (17) and transmontana (9) lineages. Although this sampling is low, several studies have revealed that as few as five localities can produce biologically meaningful models (Pearson et al., 2007; Shcheglovitova and Anderson, 2013; Galante et al., 2018).
The accessibility area ‘M’ was defined based on the shapefile of ecoregions from the World Wildlife Fund (Olson et al., 2001), selecting those ecoregions where at least one point of occurrence was found. We used the 19 bioclimatic variables obtained from the WorldClim database at 30 arc-second spatial resolution (~1 km2; Fick and Hijmans, 2017; available at https://worldclim.org/data/worldclim21.html). For selection of the bioclimatic variables, we explored three datasets based on different criteria: (set 1) removal of one variable from each pair of variables for which Pearson product–moment correlations were high (r ≥ 0.8); (set 2) identification of variables that contribute most strongly to models using the jackknife in Maxent, followed by removal of one variable from each highly correlated pair of variables (r > 0.80); and (set 3) inclusion of only variables with variance inflation factor values less than ten (following Brauner and Shacham, 1998; Guisan et al., 2002).
Model calibration consisted of evaluating candidate models created with 13 distinct regularization multipliers (from 0.1 to 1.3, at intervals of 0.1), different combinations of three feature classes (linear; linear and quadratic; linear, quadratic and hinge) and the three sets of environmental variables (set 1, set 2 and set 3) with a distinct number of variables each. Best parameter settings were selected considering statistical significance (partial Receiver Operating Characteristic, ROC; Peterson et al., 2008), predictive power (omission rates E = 5 %; Anderson et al., 2003) and complexity level (Akaike information criterion corrected for small sample size; Warren et al., 2010), in that order (Cobos et al., 2019). Final models were generated with ten bootstrap replicates, using Maxent v.3.4.1 (Phillips et al., 2006) and the results of model calibration obtained from application of the kuenm R package (Cobos et al., 2019).
To test the degree of climate niche differentiation among lineages, we used the niche similarity test implemented in ENMTools (Warren et al., 2008, 2010). This test uses randomization to compute a null distribution and estimate whether the ENMs from different lineages are like each other more than expected by chance, based on environmental differences in the environment in which they occur. The test was run using the ‘background.test’ function in R package ENMTools (Warren et al., 2010) with 100 replicates, sampling a total of 30 000 random points, and was analysed with a two-tailed test (Warren et al., 2008). Niche overlap among lineages was measured via Schoener’s D and a modified Hellinger’s I metric (Warren et al., 2008).
RESULTS
Targeted enrichment
A total of 465 genes from 465 targets were assembled successfully for at least one sample. From the 465 genes assembled, four genes (0.86 %) showed an alignment length of <500 bp, 16 genes (3.44 %) were identified as paralogues, 93 genes (20 %) showed pairwise identity of <90 %, and 197 genes (42.36 %) showed <20 % of sites identical, meaning that 310 loci were removed. The remaining 155 genes were carried forward for subsequent analysis. Detailed information about these 155 genes is shown in the Supplementary Data (Table S2). Individual gene alignments ranged in length from 606 to 4689 bp, and the aligned length of the concatenated 56‐taxon and 155-loci super-matrix was 302 321 bp, with 8078 parsimony-informative sites and 23.02 % of missing data.
Inference of phylogenetic relationships, genetic distinctiveness and coalescent species delimitation within the Q. laeta complex
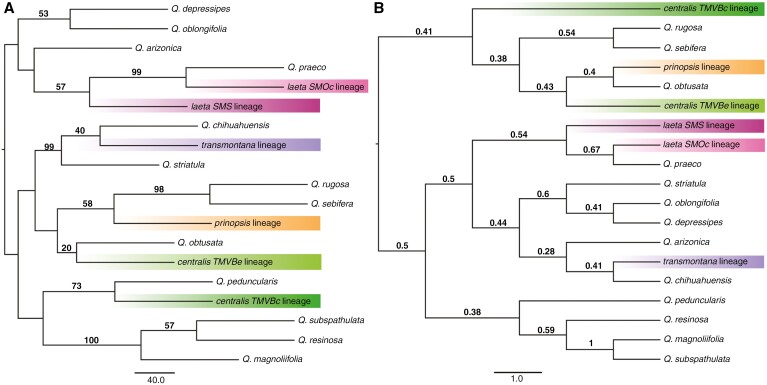
The ML analysis recovered up to six well-supported lineages. Lineage designations were based on the synonyms of Q. laeta and on the geographical regions where these lineages were sampled. Accordingly, these six lineages were called centralis TMVBc, centralis TMVBe, laeta SMS, laeta SMOc, prinopsis and transmontana (Fig. 2). In the tree, bootstrap support (BS) was high (BS > 90) for both the backbone and the relationships among the six major lineages (Fig. 2). Both the transmontana lineage (BS = 96) and the prinopsis lineage (BS = 96) were recovered as monophyletic groups. Support for the centralis TMVBc and centralis TMVBe lineages was also high (BS = 100 and 99, respectively). The laeta SMS lineage (BS = 91) comprised the population from the Sierra El Cuale in the west of Jalisco, in the Sierra Madre del Sur physiographic province, and the laeta SMOc lineage (BS = 99) corresponded to the populations distributed in the sierras of Nayarit and Durango, in the Sierra Madre Occidental physiographic province (Fig. 2).
The NMDS genetic ordination (Fig. 3B) showed that the stress values decrease rapidly from K = 1 to 2 to 3 (stress = 0.254, 0.162 and 0.118, respectively), showing decreases of <0.028 per additional dimension after that, indicating little added information for more than three dimensions. These three NMDS axes (Fig. 3B, as two two-dimensional plots) showed a clear separation of the transmontana lineage from centralis, laeta and prinopsis lineages, although with weaker separation among the other groups (Fig. 3B).
Fig. 3.
(A) Results from Bayesian phylogenetic and phylogeography analysis. Support values reported above each branch are based on the algorithm setting for two different priors corresponding to large population size and deep divergence and to small population size and a shallow divergence time. (B) Ordination of Quercus laeta individuals sequenced, based on genetic data. Ordination is based on a three-dimensional non-metric multidimensional scaling (NMDS) analysis of the pairwise genetic distance estimated using the GTRCAT nucleotide substitution model in RAxML.
In the BPP analyses, we found that the different prior combinations for θ and τ had a low influence on model selection, because in all runs under both demographic scenarios BPP delimitation suggested the existence of six species within the Q. laeta complex, with a posterior probability of one (Fig. 3A).
Phylogenetic relationships among lineages of the Q. laeta complex and other Leucomexicana species
The trees recovered by both ML of the concatenated matrix and species tree methods under the MSC suggest that the Q. laeta complex is polyphyletic (Figs 4 and 5). Furthermore, all methods support Quercus sebifera as sister to Q. rugosa and a clade containing Quercus magnoliifolia, Quercus resinosa and Quercus subspathulata with high bootstrap support.
Fig. 4.
Concatenated maximum likelihood tree inferred in RAxML, with concatenated matrix (155 loci and 302 321 bp). Bootstrap support values of >50 % are indicated above the branches.
Fig. 5.
Phylogenetic hypotheses of relationships among the different lineages of the Quercus laeta complex and other species of the Leucomexicana clade. (A) Species tree obtained by SVDQuartets. Bootstrap support values are indicated above the branches. (B) Species tree obtained by ASTRAL-III. Local posterior probability values expressed as percentages are shown above branches.
The ML analysis of the concatenated matrix under the GTRCAT substitution model yielded a topology where Quercus chihuahuensis and one sample of Quercus peduncularis (pedun_Coy1) were placed in a clade with transmontana individuals, and this clade was sister to Quercus oblongifolia. In turn, the other sample of Q. peduncularis (pedun_Coy2) was sister to a clade containing Q. resinosa, Q. magnoliifolia and Q. subspathulata (BS = 78) (Fig. 4). The samples of laeta SMS were supported as monophyletic (BS = 99) and more closely related to the clade of Quercusdepressipes–Quercus arizonica (BS = 61) than to laeta SMOc individuals, with one of these (laeta_1165) being placed with Quercus praeco (BS = 68). In addition, prinopsis samples formed a clade (BS = 71), except for two samples that were sister to a clade containing Quercus obtusata and one sample of centralis TMVBe (centr_TET4) (BS = 49). Finally, centralis TMVBc individuals were recovered as monophyletic (BS = 98) and placed sister to one sample of centralis TMVBe (centr_TUL7) (BS = 67) (Fig. 4). The Kishino–Hasegawa test rejected monophyly of the Q. laeta complex (log-likelihood difference = 980.31, P < 0.001). This strongly suggested that our target enrichment dataset is sufficiently conclusive to evaluate monophyly of the group, and thus, alongside other analyses presented here, supports segregation of Q. laeta into two or more distinct species (Supplementary Data Table S3).
Gene and site concordance factors calculated for the ML concatenated tree were generally low, indicating poor concordance among gene trees despite relatively high bootstrap values in shallow nodes of the tree. Gene concordance factors ranged from 0 to 18.39 % across the dataset (Supplementary Data Fig. S1), indicating that few gene trees contain the branches present in the ML tree. In contrast, site concordance factors ranged from 29.57 to 69.57 %, with sCF values close to the neutral value (~33 % = no informative decisive sites; Minh et al., 2020) mainly in the deeper nodes, pointing to a lack of a clear signal at this level (Supplementary Data Fig. S2). A comparison between bootstrap, gCF and sCF values showed that low bootstrap values coincided with the lowest gCF and sCF values, but also revealed that high bootstrap values occurred with low gCF and sCF values, illustrating that bootstrap values do not entirely capture the variation in the underlying data (Supplementary Data Fig. S2).
In the SVDQuartets tree (Fig. 5A), individuals of transmontana formed a clade sister to Q. chihuahuensis (BS = 40), while Q. peduncularis was sister to a centralis TMVBc clade (BS = 73). Furthermore, a laeta SMOc clade was strongly supported as sister to Q. praeco (BS = 99) and, in turn, this clade was sister to laeta SMS (BS = 57). As found in the ML analysis of the concatenated matrix, centralis TMVBe was sister to Q. obtusata, although with low support (BS = 20), and prinopsis was recovered as sister to the Q. sebifera–Q. rugosa clade (BS = 58).
The ASTRAL species tree also showed polyphyly of the Q. laeta complex and recovered relationships consistent, in part, with the results from the SVDQuartets tree (Fig. 5B). The transmontana clade was sister to Q. chihuahuensis (LPP = 0.41), and this clade, in turn, was sister to Q. arizonica (LPP = 0.29). The laeta SMOc clade was recovered as sister to Q. praeco (LPP = 0.67) and, in turn, this clade was sister to laeta SMS (LPP = 0.54). In contrast to the SVDQuartets tree, prinopsis was placed as sister to Q. obtusata (LPP = 0.40) and this clade was sister to centralis TMVBe (LPP = 0.43). As in the concatenated analysis, Q. peduncularis was more closely related to a clade containing Q. magnoliifolia, Q. resinosa and Q. subspathulata than to the centralis TMVBc lineage.
The normalized quartet score from ASTRAL, which measures gene tree discordance (Singh et al., 2022), was 0.38 (7 955 760 unnormalized), suggesting a high level of incomplete lineage sorting, although this quartet score would also be affected by gene flow. Furthermore, the quartet support computed for all nodes in the species tree showed branch-specific quartet scores ranging from 32 to 100 %, where higher quartet scores indicate that a large proportion of the gene trees share the same topology as the inferred species tree, whereas values close to 33 % point to high levels of discordance (Supplementary Data Fig. S3).
Evidence of reticulation in the Q. laeta complex
Phylogenetic networks estimated to ascertain whether introgression occurred over the evolutionary history of Q. laeta lineages pinpointed introgression events in each of the four datasets (Supplementary Data Table S4; Fig. S4). Based on the slope heuristic, SNaQ analysis favoured networks with a single reticulation edge (h1) for all datasets. In all datasets, the phylogenetic networks recovered the Q. laeta complex as polyphyletic (Fig. 6). The four datasets recovered different reticulate relationships through several Leucomexicana lineages. Two analyses showed Q. transmontana arising from a lineage that is either of hybrid origin with Q. laeta as one parent (Fig. 6A) or derived from the ancestor of the lineage that gave rise to Q. laeta, with introgression from a distant relative (Fig. 6B). The other two networks (Fig. 6C, D) pointed to introgression histories for Q. striatula or Q. rugosa. Numerous species (Q. chihuahuensis, Q. oblongifolia, Q. peduncularis, Q. rugosa, laeta SMOc and transmontana lineages) were implicated in multiple networks (Fig. 6A–D).
Fig. 6.
Phylogenetic networks as inferred by Species Networks applying Quartets (SNaQ) from four different datasets. γ = inheritance probabilities. Bootstrap support values are indicated above branches.
Environmental differentiation among lineages
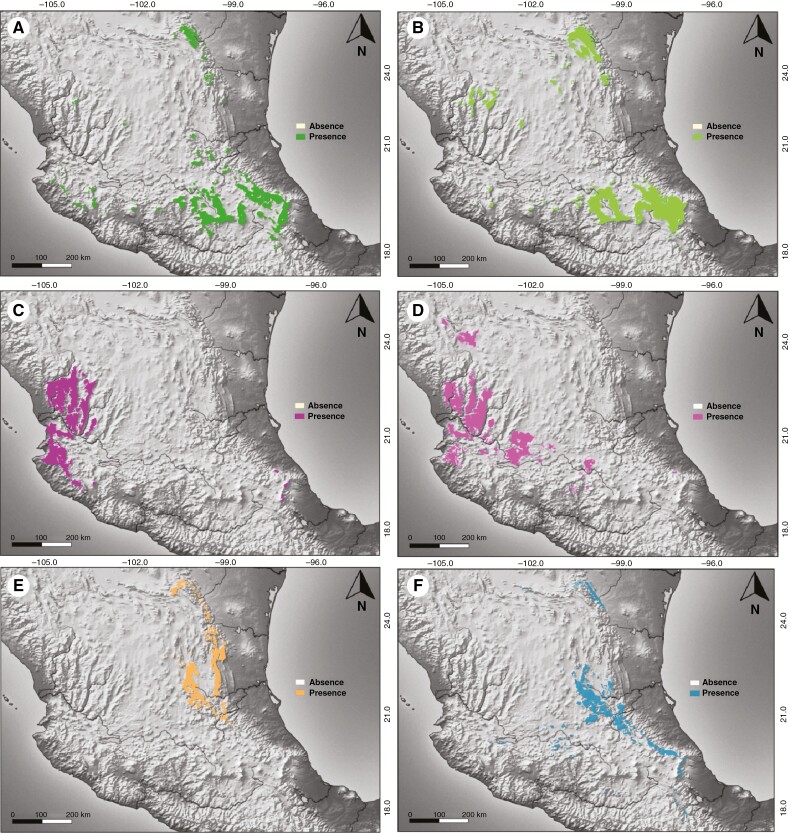
A total of 234 candidate models were generated and tested for each lineage (1404 in total), of which 37 (2.63 % of all models tested) passed the three selection criteria. The evaluation of multiple models identified different sets of environmental variables and parameters to produce the best model for each lineage (Supplementary Data Table S5). The ENMs for the different lineages showed a high predictive power based on AUC values (centralis TMVBc AUC = 0.93; centralis TMVBe AUC = 0.91; laeta SMOc AUC = 0.97; laeta SMS AUC = 0.95; prinopsis AUC = 0.97; transmontana AUC = 0.96). Overall, the ENMs suggested that the different lineages were allopatric, although certain lineages showed parapatric patterns (Table 1). The ENM showed that the highest environmental suitability both for centralis TMVBc and centralis TMVBe occurred in the temperate forests of the centre and eastern region of the Trans-Mexican Volcanic Belt, while laeta SMS and laeta SMOc were restricted to the temperate forests of western Mexico (Jalisco, Nayarit and Durango). In contrast, transmontana and prinopsis had more xeric affinities. The highest environmental suitability for the transmontana lineage was on the mountain areas of the central and southern region of the Sierra Madre Oriental (SMOr) and in isolated mountain ranges of the Mexican Plateau, while prinopsis was restricted to the centre and northern region of the SMOr. Also, the ENMs showed potential contact zones among prinopsis and transmontana in the central region of the SMOr, between centralis TMVBc and centralis TMVBe in the Trans-Mexican Volcanic Belt and between laeta SMS and laeta SMOc in western Mexico (Fig. 7).
Table 1.
Results of background tests for pairwise comparisons of Quercus laeta lineages based on 100 replicates.
| Pairwise comparison of lineages | Range overlap | Schoener’s D |
P-value background test |
Inference |
|---|---|---|---|---|
| centralis TMVBc–centralis TMVBe | Parapatric | 0.60 | 0.029 | Overlapping |
| centralis TMVBc–laeta SMS | Allopatric | 0.61 | 0.019 | Overlapping |
| centralis TMVBc–laeta SMOc | Allopatric | 0.38 | 0.009 | Divergent |
| centralis TMVBc–prinopsis | Allopatric | 0.27 | 0.009 | Divergent |
| centralis TMVBc–transmontana | Allopatric | 0.55 | 0.009 | Overlapping |
| centralis TMVBe–laeta SMS | Allopatric | 0.40 | 0.009 | Divergent |
| centralis TMVBe–laeta SMOc | Allopatric | 0.26 | 0.009 | Divergent |
| centralis TMVBe–prinopsis | Allopatric | 0.24 | 0.009 | Divergent |
| centralis TMVBe–transmontana | Allopatric | 0.49 | 0.009 | Divergent |
| laeta SMS–laeta SMOc | Parapatric | 0.65 | 0.019 | Overlapping |
| laeta SMS–prinopsis | Allopatric | 0.14 | 0.009 | Divergent |
| laeta SMS–transmontana | Allopatric | 0.36 | 0.009 | Divergent |
| laeta SMOc–prinopsis | Allopatric | 0.09 | 0.009 | Divergent |
| laeta SMOc–transmontana | Allopatric | 0.22 | 0.009 | Divergent |
| prinopsis–transmontana | Parapatric | 0.48 | 0.009 | Divergent |
Abbreviations: SMOc, Sierra Madre Occidental; SMS, Sierra Madre del Sur; TMVBc, Trans-Mexican Volcanic Belt central; TMVBe, Trans-Mexican Volcanic Belt eastern.
Fig. 7.
Reconstruction of the ecological niche models (ENMs) for the six major lineages detected in the Quercus laeta complex: (A) binary distribution for centralis TMVBc lineage; (B) binary distribution for centralis TMVBe lineage; (C) binary distribution for laeta SMS lineage; (D) binary distribution for laeta SMOc lineage; (E) binary distribution for prinopsis lineage; and (F) binary distribution for transmontana lineage.
According to the jackknife test, different combinations of variables explained environmental suitability for the different lineages (Supplementary Data Table S5). The results of the background test showed that Schoener’s D values ranged from 0.09 to 0.65 depending on the pair of lineages compared, where the laeta SMOc–prinopsis comparison had the lowest similarity value of all pairwise comparisons, whereas the laeta SMS–laeta SMOc comparison had the greatest similarity value (Table 1). All observed values of D were significantly lower than the null distributions, hence the null hypothesis could be rejected in all cases (Table 1; Supplementary Data Fig. S5).
DISCUSSION
Species are the basis of biodiversity research, underlying our inferences in evolution, conservation and biogeography. Studying speciation in rapidly diversifying lineages that are also subject to introgression is particularly tricky, but also important for our understanding in the biodiversity field; therefore, merging ecology with genomics will be essential to figuring out these groups (Carneiro et al., 2023; Jia et al., 2023).
Our study combines multilocus data based on target enrichment sequencing with the evaluation of ecological niche differentiation to identify evolutionary lineages at multiple levels (populations, species and deeper clades) in a plant group characterized by introgressive hybridization. Three points are worth highlighting based on the results. First, our data suggest the existence of up to six lineages within the Q. laeta complex, each geographically and ecologically restricted. This concordance between ecological and genetic differentiation is the hallmark of species boundaries in the oak syngameon, because reproductive isolation takes tens of millions of years to evolve (Van Valen, 1976; Kremer and Hipp, 2020). Second, our study demonstrates that the Q. laeta complex is polyphyletic. Third, the low support for relationships within the Mexican white oaks is attributable, at least in part, to introgressive hybridization. Although our analyses did not partition tree support between processes of incomplete lineage sorting and historical or recent introgression, they demonstrate that introgression is playing a role. The difficulty of recognizing species and deeper lineages in the Mexican white oaks is probably tied up with the very same processes, such as introgressive hybridization, that have most probably contributed to their diversification.
Relationships among lineages in the Q. laeta complex and other Leucomexicana species: evolutionary and ecological implications
Recent phylogenomic analyses have greatly contributed to our understanding of the relationships among American oaks (Hipp et al., 2018, 2020). Several of these analyses have investigated the potential effects of lineage sorting and introgression (Eaton et al., 2015; McVay et al., 2017a, b; Ortego et al., 2018; Crowl et al., 2020; Zhou et al., 2022) and point to the need for region-by-region phylogenetic network studies. The Mexican oaks still present numerous ecological and taxonomic questions, and we expect that introgression will affect phylogenetic resolution disproportionately in the relatively young and highly diverse clades of Mexican oaks (Hipp et al., 2020). Within the subsection Leucomexicana, Q. laeta is a clear example of the degree of conflict among gene trees within a young oak clade.
Previous studies have recovered inconsistent relationships among species of the Q. laeta complex and close relatives of the subsection Leucomexicana. One of the first phylogenetic studies to include Q. laeta (Manos et al., 1999) reported Q. rugosa as sister to Q. laeta, a result also reported by Hubert et al. (2014) although with a lower support (BS < 50). Both studies had limited taxonomic sampling from Mexico. Later studies with more samples reported Q. arizonica as sister to Q. laeta (Hipp et al., 2018) and suggested that the Q. laeta complex was polyphyletic, with a close relationship to Q. chihuahuensis (Hipp et al., 2020). Considering our results together with the previous phylogenetic relationships of Quercus species proposed by Hipp et al. (2020), the lineages detected here are clearly scattered in different clades across Leucomexicana, supporting the polyphyly of the Q. laeta complex, suggesting that the Q. laeta lineages recognized here are more deeply divergent than previously suspected and supporting the hypothesis that these lineages correspond to up to six different species. In addition, although the objective of our study was not to present an exhaustive phylogeny of the subsection Leucomexicana, and although we found widespread gene tree discordance in certain clades, our results also provide advances in the understanding of the relationships among American oaks. The Q. subspathulata–Q. resinosa–Q. magnoliifolia and Q. rugosa–Q. sebifera clades, in addition to the close relationship between the laeta SMOc lineage and Q. praeco, are stable across the different analyses.
The addition of numerous samples of the Q. laeta complex, however, introduces phylogenetic complexity. One of our most interesting results was the detection of four lineages that showed a clear geographical pattern with independent phylogenetic affinities. The laeta SMS and laeta SMOc lineages, located in western Mexico, are suggested in the MSC trees to have a previously unsuspected close relationship with Q. praeco. However, the ML concatenated analysis also suggested phylogenetic affinities with Q. arizonica–Q. depressipes (BS = 61), although concordance factors demonstrate discordance at the gene and nucleotide levels. The other geographically structured lineages correspond to centralis TMVBc and centralis TMVBe, for which recovered phylogenetic relationships were ambiguous and poorly supported. This low support in conjunction with low gCF and sCF values suggests that there is not enough information at this level to resolve phylogenetic affinities for these lineages. Nevertheless, all analyses placed centralis TMVBc and centralis TMVBe in separate clades, where they showed closer phylogenetic affinities to other species of the Leucomexicana subsection than to each other, supporting that they are polyphyletic, a result also shown in the molecular ordination.
Our results based on different phylogenetic networks are sensitive to taxon sampling (cf. Karimi et al., 2020), yet in combination they suggest events of ancient introgression that involved the ancestors of three present-day Q. laeta lineages (laeta, prinopsis and transmontana), but none among centralis lineages. In this context, the discordant phylogenetic relationships proposed among Q. chihuahuensis, Q. oblongifolia, Q. peduncularis and the transmontana lineage could be explained by ancient introgression events, which, together with rapid diversification of the Mexican white oaks (Hipp et al., 2018, 2020), probably has given these lineages too little time to accumulate informative genomic polymorphisms.
Modes of speciation in Mexican oaks
Little is known about the relative importance of sympatric and allopatric speciation in oaks (Lazic et al., 2021), and the Mexican diversification might be compatible with either scenario. High rates of lineage diversification in the Mexican oaks are associated with high rates of evolution along moisture gradients (Hipp et al., 2018). What is not clear is whether this is driven by ecological diversification in sympatry or by increased opportunities for allopatric speciation.
Our work supports the phylogenetic analyses associating rates of lineage diversification with rates of ecological diversification in the Mexican oaks, because climatic niche divergence is common among the lineages of the Q. laeta complex. More importantly, our work lends support to the importance of allopatric speciation in the genus: in the ENM projection, 12 of 15 comparisons suggest allopatric patterns, with lineages occupying geographically and ecologically distinct habitats, with the exception of the centralis TMVBc–centralis TMVBe and laeta SMS–laeta SMOc lineages, which showed parapatric patterns with a significant degree of niche similarity. In the case of the prinopsis–transmontana lineages, a parapatric pattern was also observed, with a potential contact zone in the central region of the SMOr, although evidence of genetic admixture between these two lineages was not observed. Our results thus suggest that mountain barriers might have played key roles in speciation and diversification through the effects of topographic complexity on ecological stratification, environmental heterogeneity and limitation of gene flow (Rodríguez-Correa et al., 2015; Hipp et al., 2018; Barret et al., 2019).
Although we are approaching good answers to the questions such as ‘How many oaks are there?’ and ‘Why do oaks remain distinct in the face of gene flow?’, we are still far from having an answer to the question, ‘How do oak species come into existence?’ (Abadie et al., 2012; Bodénès et al., 2016; Kremer and Hipp, 2020). Our work points to the importance of combining phylogenomics and ecology to address this question.
Delimitation of major lineages: the need for a taxonomic reappraisal
This work supports that oak species diversity in Mexico is underestimated, despite recent taxonomic descriptions and reappraisal (Valencia et al., 2016; González-Villarreal, 2018; McCauley et al., 2019; McCauley and Oyama, 2020; Valencia and Coombes, 2020; Morales-Saldaña et al., 2022). Quercus transmontana, delimited using genetic and morphometric data by Morales-Saldaña et al. (2022), was recovered in this study as a monophyletic group and strongly supported by the BPP method with a posterior probability of one. Also, the species exhibits strong ecological niche differentiation from the rest of the lineages. Together, these lines of evidence suggest that Q. transmontana is as distinct as any other closely related Mexican white oak species by phylogenetic, morphological, genetic and ecological criteria. On the contrary, Q. laeta was also recognized by genetic and morphometric data (Morales-Saldaña et al., 2022), but in the present study it was split into up to five lineages, each one supported by the ML and BPP analysis. The background test showed that among these lineages, prinopsis, distributed along dry oak forests in the centre and northern region of the SMOr, is the most ecologically divergent, which, in conjunction with results from both ML and BPP analyses, suggests that it also merits recognition as an independent species (Q. prinopsis).
In contrast, the status of the centralis TMVBc, centralis TMVBe, laeta SMS and laeta SMOc lineages is more ambiguous. Considering ML and BPP results, each one of these lineages could be considered as a different species. However, these genetic differentiation patterns could be a consequence of strong geographical structure and not necessarily of speciation processes (Freudenstein et al., 2017; Sukumaran and Knowles, 2017). Also, our ecological niche analysis showed a significant degree of niche similarity between centralis TMVBc and centralis TMVBe, which occur in the temperate forests of the centre and eastern region of the Trans-Mexican Volcanic Belt with annual mean temperatures of 11–16 °C and annual precipitation of ~760–1000 mm. In turn, laeta SMS and laeta SMOc are restricted to the temperate forests of western Mexico with annual mean temperatures of 16–20 °C and annual precipitation of 800–1400 mm. Considering that there is no evidence of divergence at the morphometric level (Morales-Saldaña et al., 2022) and that differentiation at the micromorphological level has not been evaluated, we suggest that these patterns represent initial stages of the speciation processes between centralis TMVBc–centralis TMVBe and between laeta SMS–laeta SMOc. Therefore, we propose centralis TMVBc and centralis TMVBe as a single species (Q. centralis) and that laeta SMS and laeta SMOc should be maintained under the single name (Q. laeta) for the time being.
Although the putative species name designations presented in this paper are based on the names proposed by Trelease (1924), older names exist that might have nomenclatural priority. Nomenclatural research accompanied by a quantitative study of the variation of micromorphological leaf traits is advisable for the lineages proposed here before they can be redescribed formally.
Conclusion
Currently, oak phylogenetics is at a turning point, at which it is necessary to integrate phylogenetics and ecology in broad regional samples to figure out species boundaries. The present study highlights the importance of taking approaches that bridge the micro- and macroevolutionary scales to resolve evolutionary relationships and species boundaries in young, potentially reticulate species complexes. Using target enrichment and ecological niche modelling, we recognized up to six evolutionarily independent lineages, greatly increasing the recognized species diversity of the Q. laeta complex. Also, inferred species trees corroborated that the different lineages comprising the Q. laeta complex are more closely related to other species than to each another. Finally, the existence of significant ecological niche differentiation among the Q. laeta complex lineages suggests that ecological differentiation has been a part of evolutionary divergence among these closely related white oaks, either driving speciation or resulting from it. Our work points out complications in one of the thorniest of the Mexican white oak groups and it lays the groundwork for further taxonomic study.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: gene concordance factor (gCF) and site concordance factor (sCF) calculated for maximum likelihood concatenated tree. Figure S2: (A) Relationship between site concordance factor (sCF) and gene concordance factor (gCF). (B) Relationship between gCF and bootstrap support. (C) Relationship between sCF and bootstrap support. Figure S3: species tree inferred from ASTRAL analyses based on 155 loci. Figure S4: negative log pseudolikelihood profile for each number of hybridization events for each dataset inferred using SNaQ. Figure S5: climatic niche differentiation between the major lineages identified in the Quercus laeta complex. Table S1: list of samples used in this study for Q. laeta complex and for species from the Leucomexicana clade that have been closely related to the Q. laeta complex with GPS coordinates of localities. Individual label includes abbreviation for species, site abbreviation, and individual number. Table S2: gene list used in this study. Table S3: results for constrained tree tests. Table S4: the negative log pseudolikelihood for phylogenetic network estimation. Table S5: set of WorldClim variables used for the construction of the ecological niche modelling for Quercus laeta lineages.
ACKNOWLEDGEMENTS
We thank Oscar De Luna, Gonzalo Contreras, Ricardo Gaytán and Maribel Arenas for their help with field collection. We thank the Field Museum of Natural History for allowing the use of sequencing equipment and for training and support. We thank the Laboratorio Nacional de Análisis y Síntesis Ecológica (LANASE‐UNAM) for providing computational resources for data analysis.
Contributor Information
Saddan Morales-Saldaña, Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México (UNAM), Antigua Carretera a Pátzcuaro No. 8701, Col. Ex-Hacienda de San José de la Huerta, Morelia, 58190, Michoacán, México.
Andrew L Hipp, The Morton Arboretum, Lisle, IL 60532-1293, USA; The Field Museum, Chicago, IL 60605, USA.
Susana Valencia-Ávalos, Herbario de la Facultad de Ciencias, Departamento de Biología Comparada, Universidad Nacional Autónoma de México (UNAM), 04510, Ciudad de México, México.
Marlene Hahn, The Morton Arboretum, Lisle, IL 60532-1293, USA.
M Socorro González-Elizondo, CIIDIR Unidad Durango, Instituto Politécnico Nacional, Durango, México.
David S Gernandt, Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México (UNAM), 04510, Ciudad de México, México.
Kasey K Pham, Department of Biology, University of Florida, Gainesville, FL 32611, USA.
Ken Oyama, Escuela Nacional de Estudios Superiores Unidad Morelia, Universidad Nacional Autónoma de México (UNAM), Antigua Carretera a Pátzcuaro No. 8701, Col. Ex‐Hacienda de San José de la Huerta, Morelia, 58190, Michoacán, México.
Antonio González-Rodríguez, Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México (UNAM), Antigua Carretera a Pátzcuaro No. 8701, Col. Ex-Hacienda de San José de la Huerta, Morelia, 58190, Michoacán, México.
FUNDING
The authors thank funding provided by the National Geographic Society grant NGS-73961R-20 and The Morton Arboretum. This study constitutes a partial fulfilment of the Graduate Program in Biological Sciences of the National Autonomous University of Mexico (UNAM) for SM-S. Saddan Morales-Saldaña thanks Posgrado en Ciencias Biológicas–UNAM and Consejo Nacional de Ciencia y Tecnología (CONACYT) for the PhD scholarship and financial support to develop graduate studies at UNAM (scholarship/CVU: 483720/624511).
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
DATA AVAILABILITY
The data underlying this article will be shared on request to the corresponding author.
LITERATURE CITED
- Abadie P, Roussel G, Dencausse B, et al. 2012. Strength, diversity and plasticity of postmating reproductive barriers between two hybridizing oak species (Quercus robur L. and Quercus petraea (Matt) Liebl.). Journal of Evolutionary Biology 25: 157–173. doi: 10.1111/j.1420-9101.2011.02414.x [DOI] [PubMed] [Google Scholar]
- Abbott R, Albach D, Ansell S, et al. 2013. Hybridization and speciation. Journal of Evolutionary Biology 26: 229–246. [DOI] [PubMed] [Google Scholar]
- Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP.. 2015. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38: 541–545. [Google Scholar]
- Anderson RP, Lew D, Peterson AT.. 2003. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecological Modelling 162: 211–232. [Google Scholar]
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (3 December 2018, date last accessed).
- Bankevich A, Nurk S, Antipov D, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19: 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapteste E, Susko E, Leigh J, Ruiz-Trillo I, Bucknam J, Doolittle WF.. 2008. Alternative methods for concatenation of core genes indicate a lack of resolution in deep nodes of the prokaryotic phylogeny. Molecular Biology and Evolution 25: 83–91. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Sinn BT, King LT, et al. 2019. Phylogenomics, biogeography and evolution in the American genus Brahea (Arecaceae). Botanical Journal of the Linnean Society 190: 242–259. [Google Scholar]
- Belahbib N, Pemonge MH, Ouassou A, Sbay H, Kremer A, Petit RJ.. 2001. Frequent cytoplasmic exchanges between oak species that are not closely related: Quercus suber and Q. ilex in Morocco. Molecular Ecology 10: 2003–2012. [DOI] [PubMed] [Google Scholar]
- Bellarosa R, Simeone MC, Papini A, Schirone B.. 2005. Utility of ITS sequence data for phylogenetic reconstruction of Italian Quercus spp. Molecular Phylogenetics and Evolution 34: 355–370. [DOI] [PubMed] [Google Scholar]
- Bezanson J, Edelman A, Karpinski S, Shah VB.. 2017. Julia: a fresh approach to numerical computing. SIAM Review 59: 65–98. [Google Scholar]
- Blair ME, Sterling EJ, Dusch M, Raxworthy CJ, Pearson RG.. 2013. Ecological divergence and speciation between lemur (Eulemur) sister species in Madagascar. Journal of Evolutionary Biology 26: 1790–1801. [DOI] [PubMed] [Google Scholar]
- Bodénès C, Chancerel E, Ehrenmann F, Kremer A, Plomion C.. 2016. High-density linkage mapping and distribution of segregation distortion regions in the oak genome. DNA Research 23: 115–124. doi: 10.1093/dnares/dsw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B.. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner N, Shacham M.. 1998. Role of range and precision of the independent variable in regression of data. AIChE Journal 44: 603–611. [Google Scholar]
- Breinholt JW, Carey SB, Tiley GP, et al. 2021. A target enrichment probe set for resolving the flagellate land plant tree of life. Applications in Plant Sciences 9: e11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto‐Rojas M, Lira‐Noriega A, Rubio‐Godoy M, Pérez‐Ponce de León G, Pinacho‐Pinacho CD.. 2021. Phylogenetic relationships and ecological niche conservatism in killifish (Profundulidae) in Mesoamerica. Journal of Fish Biology 99: 396–410. [DOI] [PubMed] [Google Scholar]
- Carneiro Muniz A, Santiago de Oliviera Buzatti R, Pires de Lemos-Filho J, et al. 2023. Genomic signatures of ecological divergence between savanna and forest populations of a Neotropical tree. Annals of Botany 132: 523–540. doi: 10.1093/aob/mcad120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender‐Bares J, González‐Rodríguez A, Eaton DA, Hipp AA, Beulke A, Manos PS.. 2015. Phylogeny and biogeography of the American live oaks (Quercus subsection Virentes): a genomic and population genetics approach. Molecular Ecology 24: 3668–3687. [DOI] [PubMed] [Google Scholar]
- Chamala S, García N, Godden GT, et al. 2015. MarkerMiner 1.0: a new application for phylogenetic marker development using angiosperm transcriptomes. Applications in Plant Sciences 3: 1400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifman J, Kubatko L.. 2014. Quartet inference from SNP data under the coalescent model. Bioinformatics 30: 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos ME, Peterson AT, Barve N, Osorio-Olvera L.. 2019. kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ 7: e6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl AA, Manos PS, McVay JD, Lemmon AR, Lemmon EM, Hipp AL.. 2020. Uncovering the genomic signature of ancient introgression between white oak lineages (Quercus). The New Phytologist 226: 1158–1170. [DOI] [PubMed] [Google Scholar]
- Dodsworth S, Pokorny L, Johnson MG, et al. 2019. Hyb-Seq for flowering plant systematics. Trends in Plant Science 24: 887–891. [DOI] [PubMed] [Google Scholar]
- Dormann CF, McPherson J, Araújo M, et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30: 609–628. [Google Scholar]
- Dormann CF, Elith J, Bacher S, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. [Google Scholar]
- Eaton DA, Hipp AL, González‐Rodríguez A, Cavender‐Bares J.. 2015. Historical introgression among the American live oaks and the comparative nature of tests for introgression. Evolution 69: 2587–2601. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Xi Z, Janke A, et al. 2016. Implementing and testing the multispecies coalescent model: a valuable paradigm for phylogenomics. Molecular Phylogenetics and Evolution 94: 447–462. [DOI] [PubMed] [Google Scholar]
- Fick SE, Hijmans RJ.. 2017. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Flouri T, Jiao X, Rannala B, Yang Z.. 2018. Species tree inference with BPP using genomic sequences and the multispecies coalescent. Molecular biology and evolution 35: 2585–2593. doi: 10.1093/molbev/msy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk RA, Mandel JR, Freudenstein JV.. 2017. Ancestral gene flow and parallel organellar genome capture result in extreme phylogenomic discord in a lineage of angiosperms. Systematic Biology 66: 320–337. [DOI] [PubMed] [Google Scholar]
- Freudenstein JV, Broe MB, Folk RA, Sinn BT.. 2017. Biodiversity and the species concept—lineages are not enough. Systematic Biology 66: 644–656. [DOI] [PubMed] [Google Scholar]
- Fujita MK, Leaché AD, Burbrink FT, McGuire JA, Moritz C.. 2012. Coalescent-based species delimitation in an integrative taxonomy. Trends in Ecology & Evolution 27: 480–488. [DOI] [PubMed] [Google Scholar]
- Galante PJ, Alade B, Muscarella R, Jansa SA, Goodman SM, Anderson RP.. 2018. The challenge of modeling niches and distributions for data-poor species: a comprehensive approach to model complexity. Ecography 41: 726–736. [Google Scholar]
- Gardner EM, Johnson MG, Ragone D, Wickett NJ, Zerega NJ.. 2016. Low‐coverage, whole‐genome sequencing of Artocarpus camansi (Moraceae) for phylogenetic marker development and gene discovery. Applications in Plant Sciences 4: 1600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernandt DS, Aguirre-Dugua X, Vázquez‐Lobo A, et al. 2018. Multi‐locus phylogenetics, lineage sorting, and reticulation in Pinus subsection Australes. American Journal of Botany 105: 711–725. [DOI] [PubMed] [Google Scholar]
- Glor RE, Warren D.. 2010. Testing ecological explanations for biogeographic boundaries. Evolution; International Journal of Organic Evolution 65: 673–683. [DOI] [PubMed] [Google Scholar]
- González-Villarreal LM. 2018. Dos nuevas especies de encinos (Quercus: Fagaceae), adicionales para la Flora de Jalisco y Áreas Colindantes, en el Occidente de México. Ibugana 9: 47–71. [Google Scholar]
- Goudarzi F, Hemami MR, Rancilhac L, et al. 2019. Geographic separation and genetic differentiation of populations are not coupled with niche differentiation in threatened Kaiser’s spotted newt (Neurergus kaiseri). Scientific Reports 9: 6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Edwards TC, Hastie T.. 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecological Modelling 157: 89–100. [Google Scholar]
- Gutiérrez‐Ortega JS, Salinas‐Rodríguez MM, Ito T, et al. 2020. Niche conservatism promotes speciation in cycads: the case of Dioon merolae (Zamiaceae) in Mexico. New Phytologist 227: 1872–1884. [DOI] [PubMed] [Google Scholar]
- Haas BJ. 2016. TransDecoder v3.0.1. https://github.com/TransDecoder/TransDecoder (15 March 2024, date last accessed).
- Hale H, Gardner EM, Viruel J, Pokorny L, Johnson MG.. 2020. Strategies for reducing per‐sample costs in target capture sequencing for phylogenomics and population genomics in plants. Applications in Plant Sciences 8: e11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW. 1975. Hybridization and introgression in Quercus alba. Journal of the Arnold Arboretum 56: 336–363. [Google Scholar]
- Hipp AL, Eaton DA, Cavender-Bares J, Fitzek E, Nipper R, Manos PS.. 2014. A framework phylogeny of the American oak clade based on sequenced RAD data. PLoS One 9: e93975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipp AL, Manos PS, González‐Rodríguez A, et al. 2018. Sympatric parallel diversification of major oak clades in the Americas and the origins of Mexican species diversity. New Phytologist 217: 439–452. [DOI] [PubMed] [Google Scholar]
- Hipp AL, Manos PS, Hahn M, et al. 2020. Genomic landscape of the global oak phylogeny. The New Phytologist 226: 1198–1212. [DOI] [PubMed] [Google Scholar]
- Hubert F, Grimm GW, Jousselin E, Berry V, Franc A, Kremer A.. 2014. Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity 12: 405–423. [Google Scholar]
- Jia Y, Liu ML, López-Pujol J, et al. 2023. The hybridization origin of the Chinese endemic herb genus Notopterygium (Apiaceae): evidence from population genomics and ecological niche analysis. Molecular Phylogenetics and Evolution 182: 107736. [DOI] [PubMed] [Google Scholar]
- Johnson MG, Gardner EM, Liu Y, et al. 2016. HybPiper: extracting coding sequence and introns for phylogenetics from high-throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: 1600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi N, Grover CE, Gallagher JP, Wendel JF, Ané C, Baum DA.. 2020. Reticulate evolution helps explain apparent homoplasy in floral biology and pollination in baobabs (Adansonia; Bombacoideae; Malvaceae). Systematic Biology 69: 462–478. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BY, Wei X, Fitz‐Gibbon S, et al. 2018. RADseq data reveal ancient, but not pervasive, introgression between Californian tree and scrub oak species (Quercus sect. Quercus: Fagaceae). Molecular Ecology 27: 4556–4571. [DOI] [PubMed] [Google Scholar]
- Kishino H, Hasegawa M.. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. Journal of Molecular Evolution 29: 170–179. [DOI] [PubMed] [Google Scholar]
- Kleinkopf JA, Roberts WR, Wagner WL, Roalson EH.. 2019. Diversification of Hawaiian Cyrtandra (Gesneriaceae) under the influence of incomplete lineage sorting and hybridization. JSE: Journal of Systematics and Evolution 57: 561–578. [Google Scholar]
- Kremer A, Hipp AL.. 2020. Oaks: an evolutionary success story. The New Phytologist 226: 987–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic D, Hipp AL, Carlson JE, Gailing O.. 2021. Use of genomic resources to assess adaptive divergence and introgression in oaks. Forests 12: 690. [Google Scholar]
- Leaché AD, Fujita MK.. 2010. Bayesian species delimitation in West African forest geckos (Hemidactylus fasciatus). Proceedings of the Royal Society B: Biological Sciences 277: 3071–3077. doi: 10.1098/rspb.2010.0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort F, Douglas GC.. 1999. An efficient micro-method of DNA isolation from mature leaves of four hardwood tree species Acer, Fraxinus, Prunus and Quercus. Annals of Forest Science 56: 259–263. [Google Scholar]
- Lesur I, Le Provost G, Bento P, et al. 2015. The oak gene expression atlas: insights into Fagaceae genome evolution and the discovery of genes regulated during bud dormancy release. BMC Genomics 16: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R.. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al.; 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wei G, El-Kassaby YA, Fang Y.. 2021. Hybridization and introgression in sympatric and allopatric populations of four oak species. BMC Plant Biology 21: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zhenxiang X, Charles CD.. 2015. Coalescent Methods Are Robust to the Simultaneous Effects of Long Branches and Incomplete Lineage Sorting. Molecular Biology and Evolution 32: 791–805. doi: 10.1093/molbev/msu331 [DOI] [PubMed] [Google Scholar]
- Liebmann FM. 1854. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og Dets Medlemmers Arbeider. Copenhagen: Bianco Lunos Bogtrykkeri. [Google Scholar]
- Lin HY, Gu KJ, Li WH, Zhao YP.. 2021. Integrating coalescent‐based species delimitation with ecological niche modeling delimited two species within the Stewartia sinensis complex (Theaceae). Journal of Systematics and Evolution 60: 1037–1048. [Google Scholar]
- Linder CR, Moret BME, Nakhleh L, Warnow T.. 2004. Network (reticulate) evolution: biology, models, and algorithms. In: The Pacific Symposium on Biocomputing. The Big Island, Hawaii: Proceedings of the PSB04, 1–41. [Google Scholar]
- Loiseau O, Olivares I, Paris M, et al. 2019. Targeted capture of hundreds of nuclear genes unravels phylogenetic relationships of the diverse Neotropical palm tribe Geonomateae. Frontiers in Plant Science 10: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZY, Nie ZL, Ren C, Liu XQ, Zimmer EA, Wen J.. 2021. Phylogenomic relationships and character evolution of the grape family (Vitaceae). Molecular Phylogenetics and Evolution 154: 106948. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Knowles LL.. 2006. Inferring phylogeny despite incomplete lineage sorting. Systematic Biology 55: 21–30. [DOI] [PubMed] [Google Scholar]
- Mallet J, Besansky N, Hahn MW.. 2016. How reticulated are species? Bioessays 38: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos PS, Hipp AL.. 2021. An updated infrageneric classification of the North American oaks (Quercus subgenus Quercus): review of the contribution of phylogenomic data to biogeography and species diversity. Forests 12: 786. [Google Scholar]
- Manos PS, Doyle JJ, Nixon KC.. 1999. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). Molecular Phylogenetics and Evolution 12: 333–349. [DOI] [PubMed] [Google Scholar]
- Mayol M, Rosselló JA.. 2001. Why nuclear ribosomal DNA spacers (ITS) tell different stories in Quercus. Molecular Phylogenetics and Evolution 19: 167–176. [DOI] [PubMed] [Google Scholar]
- McCauley RA, Oyama K.. 2020. A re-evaluation of taxonomy in Quercus section Lobatae subsection Racemiflorae (Fagaceae), resurrection of the name Q. pennivenia and description of a new taxon, Q. huicholensis. Phytotaxa 471: 247–257. [Google Scholar]
- McCauley RA, Cortés-Palomec AC, Oyama K.. 2019. Species diversification in a lineage of Mexican red oak (Quercus section Lobatae subsection Racemiflorae)—the interplay between distance, habitat, and hybridization. Tree Genetics & Genomes 15: 27. [Google Scholar]
- McVaugh R. 1974. Fagaceae. In: McVaugh R, ed. Flora Novo‐galiciana, Vol. 12. United States: Contributions from the University of Michigan herbarium, 50–30. [Google Scholar]
- McVay JD, Hauser D, Hipp AL, Manos PS.. 2017b. Phylogenomics reveals a complex evolutionary history of lobed-leaf white oaks in western North America. Genome 60: 733–742. [DOI] [PubMed] [Google Scholar]
- McVay JD, Hipp AL, Manos PS.. 2017a. A genetic legacy of introgression confounds phylogeny and biogeography in oaks. Proceedings of the Royal Society B: Biological Sciences 284: 20170300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles JE, Beulke A, Borkowski DS, Romero-Severson J, Cavender-Bares J.. 2017. Balancing selection maintains diversity in a cold tolerance gene in broadly distributed live oaks. Genome 60: 762–769. [DOI] [PubMed] [Google Scholar]
- Minh BQ, Hahn MW, Lanfear R.. 2020. New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37: 2727–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Saldaña S, Valencia‐Ávalos S, Oyama K, Sánchez ET, Hipp AL, González‐Rodríguez A.. 2022. Even more oak species in Mexico? Genetic structure and morphological differentiation support the presence of at least two specific entities within Quercus laeta. JSE: Journal of Systematics and Evolution 60: 1124–1139. [Google Scholar]
- Nunes LA, Pearson RG.. 2017. A null biogeographical test for assessing ecological niche evolution. Journal of Biogeography 44: 1331–1343. [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2018. vegan: community ecology package. R package version 2.5-2. https://CRAN.Rproject.org/package=vegan (27 September 2022, date last accessed).
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51: 933–938. [Google Scholar]
- Oney-Birol S, Fitz-Gibbon S, Chen JM, Gugger PF, Sork VL.. 2018. Assessment of shared alleles in drought-associated candidate genes among southern California white oak species (Quercus sect. Quercus). BMC Genetics 19: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Gugger PF, Sork VL.. 2018. Genomic data reveal cryptic lineage diversification and introgression in Californian golden cup oaks (section Protobalanus). The New Phytologist 218: 804–818. [DOI] [PubMed] [Google Scholar]
- Palmer EJ. 1948. Hybrid oaks of North America. Journal of the Arnold Arboretum 29: 1–48. [Google Scholar]
- Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT.. 2007. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography 34: 102–117. [Google Scholar]
- Pease JB, Brown JW, Walker JF, Hinchliff CE, Smith SA.. 2018. Quartet sampling distinguishes lack of support from conflicting support in the green plant tree of life. American Journal of Botany 105: 385–403. [DOI] [PubMed] [Google Scholar]
- Peterson AT, Papeş M, Soberón J.. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling 213: 63–72. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE.. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- Plomion C, Aury JM, Amselem J, et al. 2018. Oak genome reveals facets of long lifespan. Nature Plants 4: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM.. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.R-project.org/ (27 September 2022, date last accessed). [Google Scholar]
- Rambaut A. 2012. FigTree. Tree figure drawing tool version 1.4.0. http://tree.bio.ed.ac.uk/software/figtree/ (14 December 2022, date last accessed).
- Rancilhac L, Irisarri I, Angelini C, et al. 2021. Phylotranscriptomic evidence for pervasive ancient hybridization among Old World salamanders. Molecular Phylogenetics and Evolution 155: 106967. [DOI] [PubMed] [Google Scholar]
- Ree RH, Hipp AL.. 2015. Inferring phylogenetic history from restriction site associated DNA (RADseq). In: Hoerandl E, Appelhaus M, eds. Next generation sequencing in plant systematics. Koenigstein: Koeltz Scientific Books, 181–204. [Google Scholar]
- Rissler LJ, Apodaca JJ.. 2007. Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Systematic Biology 56: 924–942. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Correa H, Oyama K, MacGregor-Fors I, González-Rodríguez A.. 2015. How are oaks distributed in the Neotropics? A perspective from species turnover, areas of endemism, and climatic niches. International Journal of Plant Sciences 176: 222–231. [Google Scholar]
- Romero SR, Carlos E, Zenteno R, de Lourdes M, Enríquez A.. 2002. El género Quercus (Fagaceae) en el estado de México. Annals of Missouri Botanical Garden 89: 551–593. [Google Scholar]
- Sayyari E, Mirarab S.. 2016. Fast coalescent-based computation of local branch support from quartet frequencies. Molecular Biology and Evolution 33: 1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheglovitova M, Anderson RP.. 2013. Estimating optimal complexity for ecological niche models: a jackknife approach for species with small sample sizes. Ecological Modelling 269: 9–17. [Google Scholar]
- Simeone MC, Piredda R, Papini A, Vessella F, Schirone B.. 2013. Application of plastid and nuclear markers to DNA barcoding of Euro-Mediterranean oaks (Quercus, Fagaceae): problems, prospects and phylogenetic implications. Botanical Journal of the Linnean Society 172: 478–499. [Google Scholar]
- Singh P, Irisarri I, Torres‐Dowdall J, et al. 2022. Phylogenomics of trophically diverse cichlids disentangles processes driving adaptive radiation and repeated trophic transitions. Ecology and Evolution 12: e9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Godsoe W, Rodríguez-Sánchez F, Wang HH, Warren D.. 2019. Niche estimation above and below the species level. Trends in Ecology & Evolution 34: 260–273. doi: 10.1016/j.tree.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Solís-Lemus C, Ané C.. 2016. Inferring phylogenetic networks with maximum pseudolikelihood under incomplete lineage sorting. PLoS Genetics 12: e1005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solís-Lemus C, Bastide P, Ané C.. 2017. PhyloNetworks: a package for phylogenetic networks. Molecular Biology and Evolution 34: 3292–3298. [DOI] [PubMed] [Google Scholar]
- Sork VL, Fitz-Gibbon ST, Puiu D, et al. 2016. First draft assembly and annotation of the genome of a California endemic oak Quercus lobata Née (Fagaceae). G3 (Bethesda) 6: 3485–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wu G, Li L, Liu J.. 2015. Species delimitation in plants using the Qinghai–Tibet Plateau endemic Orinus (Poaceae: Tridentinae) as an example. Annals of Botany 116: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran J, Knowles LL.. 2017. Multispecies coalescent delimits structure, not species. Proceedings of the National Academy of Sciences of the United States of America 114: 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AR, Owusu SA, Weber JA, Hipp AL, Gailing O.. 2016. Hybridization and divergence in multi-species oak (Quercus) communities. Botanical Journal of the Linnean Society 181: 99–114. [Google Scholar]
- Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10 win32. Sunderland: Sinauer Associates. [Google Scholar]
- Taylor SA, Larson L.. 2019. Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nature Ecology & Evolution 3: 170–177. [DOI] [PubMed] [Google Scholar]
- Trelease W. 1924. The American Oaks. Memoirs of the National Academy of Sciences 20: 1–255. [Google Scholar]
- Ueno S, Klopp C, Leplé JC, et al. 2013. Transcriptional profiling of bud dormancy induction and release in oak by next-generation sequencing. BMC Genomics 14: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-A S. 2004. Diversidad del género Quercus (Fagaceae) en México. Botanical Sciences 75: 33–53. [Google Scholar]
- Valencia S, Coombes AJ.. 2020. Nomenclatural revision and lectotypification of five species of Mexican oaks: Quercus (Fagaceae). Phytotaxa 428: 81–92. [Google Scholar]
- Valencia S, Rosales JLS, Arellano OJS.. 2016. A new species of Quercus, section Lobatae (Fagaceae) from the Sierra Madre Oriental, Mexico. Phytotaxa 269: 120–126. [Google Scholar]
- Van Valen L. 1976. Ecological species, multispecies, and oaks. Taxon 25: 233–239. [Google Scholar]
- Vatanparast M, Powell A, Doyle JJ, Egan AN.. 2018. Targeting legume loci: a comparison of three methods for target enrichment bait design in Leguminosae phylogenomics. Applications in Plant Sciences 6: e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaverde T, Pokorny L, Olsson S, et al. 2018. Bridging the micro‐ and macroevolutionary levels in phylogenomics: Hyb‐Seq solves relationships from populations to species and above. New Phytologist 220: 636–650. [DOI] [PubMed] [Google Scholar]
- Warnow T. 2015. Concatenation analyses in the presence of incomplete lineage sorting. PLoS Currents 7. doi: 10.1371/currents.tol.8d41ac0f13d1abedf4c4a59f5d17b1f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M.. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62: 2868–2883. [DOI] [PubMed] [Google Scholar]
- Warren DL, Glor RE, Turelli M.. 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33: 607–611. [Google Scholar]
- Weitemier K, Straub SC, Cronn RC, et al. 2014. Hyb‐Seq: combining target enrichment and genome skimming for plant phylogenomics. Applications in Plant Sciences 2: 1400042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Yu Y, Zhu J, Nakhleh L.. 2008. Inferring phylogenetic networks using PhyloNet. Systematic Biology 67: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten JA, Gibbs HL.. 2012. Niche divergence and lineage diversification among closely related Sistrurus rattlesnakes. Journal of Evolutionary Biology 25: 317–328. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2015. The BPP program for species tree estimation and species delimitation. Current Zoology 61: 854–865. doi: 10.1093/czoolo/61.5.854 [DOI] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S.. 2018. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BF, Yuan S, Crowl AA, et al. 2022. Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the Northern Hemisphere. Nature Communications 13: 1320. doi: 10.1038/s41467-022-28917-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on request to the corresponding author.