Summary
In the early days of anaesthesia, the fasting period for liquids was kept short. By the mid-20th century ‘nil by mouth after midnight’ had become routine as the principles of the management of ‘full stomach’ emergencies were extended to include elective healthy patients. Back then, no distinction was made between the withholding of liquids and solids. Towards the end of the last century, recommendations of professional anaesthesiology bodies began to reduce the fasting time of clear liquids to 2 h. This reduction in fasting time was based on the understanding that gastric emptying of clear liquids is rapid, exponential, and proportional to the current filling state of the stomach. Furthermore, there was no evidence of a link between drinking clear liquids and the risk of aspiration. Indeed, most instances of aspiration are caused by failure to identify aspiration risk factors and adjust the anaesthetic technique accordingly. In contrast, long periods of liquid withdrawal cause discomfort and may also lead to serious postoperative complications. Despite this, more than two decades after the introduction of the 2 h limit, patients still fast for a median of up to 12 h before anaesthesia, mainly because of organisational issues. Therefore, some hospitals have decided to allow patients to drink clear liquids within 2 h of induction of anaesthesia. Well-designed clinical trials should investigate whether these concepts are safe in patients scheduled for anaesthesia or procedural sedation, focusing on both aspiration risk and complications of prolonged fasting.
Keywords: 2 h fasting, clear fluid, clear liquid, drink until call, liberal fasting, preoperative fasting, Sip Til Send, unrestricted drinking
The Helsinki Declaration on Patient Safety in Anaesthesiology states: ‘Patients have a right to expect to be safe and protected from harm during their medical care and anaesthesiology has a key role to play improving patient safety perioperatively’.1 Accordingly, the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline ‘Clinical Nutrition in Surgery’ recommends avoidance of long periods of preoperative fasting2 and ‘Choosing Wisely in paediatric anaesthesia’ demands shorter real fasting times.3 This is nothing new. Even in the early days of anaesthesia, it was highlighted that preoperative fasting for several hours would exacerbate existing states of exhaustion. Nevertheless, in the decades that followed, ‘nil by mouth after midnight’ became the norm, even for healthy elective patients with no risk factors for aspiration.
How it all began
When anaesthesia was in its infancy in the mid-19th century, preoperative fasting was recommended to minimise the discomfort of nausea and vomiting.4 In the case of 15-yr-old Hannah Greener, who died under ether anaesthesia in 1848, it remained unclear whether an overdose of ether or aspiration of orally administered brandy led to death.5 The first proven fatal aspiration was published in 1862. As early as 1853, a soldier in Burma vomited during surgery for a bullet wound in the leg and died shortly afterwards. The autopsy showed that the trachea was full of vomitus.4
The British surgeon Sir Joseph Lister published simple, practical guidelines for fasting in 1883, and was the first to distinguish between solids and clear liquids: ‘While it is desirable that there should be no solid matter in the stomach when chloroform is administered, it will be found very salutary to give a cup of tea or beef-tea about two hours before’.4 As ‘several hours of preoperative fasting aggravate existing states of exhaustion’, tea with red wine or cognac up to 45 min before induction of anaesthesia was recommended at the beginning of the 20th century, especially for alcoholics.6
Mendelson's ‘Nil by mouth’
In 1946, the obstetrician Mendelson retrospectively described 66 cases of aspiration of gastric contents during anaesthesia in 44 016 pregnant women, an incidence of 0.15%. Two patients died, both from complete airway obstruction by aspirated solid food. There were no deaths among the 40 patients who aspirated liquid stomach contents; they developed asthma-like symptoms that soon subsided. Mendelson showed that hydrochloric acid or non-neutralised liquid human vomit instilled into the lungs of anaesthetised rabbits caused immediate cyanosis, respiratory distress, and changes on chest radiographs similar to those seen in patients with liquid aspiration.7 Mendelson recommended ‘withholding oral nutrition during delivery to prevent aspiration’ and made no distinction between clear liquids and solids. In the following decades, the fear of Mendelson's syndrome led to the concept of ‘nil by mouth after midnight’. Unfortunately, this applied not only to high-risk patients, but also to healthy patients without risk factors undergoing elective surgery.4
Roberts' threshold for the risk of aspiration
The ‘nil by mouth after midnight’ concept was reinforced when 25 ml in the stomach, present in half of all healthy fasting patients, was adopted as a surrogate marker for a high risk of aspiration.4 Citing his own unpublished data, Roberts stated in 1974: ‘Our preliminary work in the rhesus monkey suggests that 0.4 ml kg−1 body weight is the maximum acid aspirate which does not produce significant changes in the lungs’.8 In 1980, Roberts described that the gastric acid applied directly to the left lung via a tracheostomy tube in this study had a pH of 1.26.9 The number of animals used remained unclear. Roberts went on to write in 1974 that as 0.4 ml kg−1 body weight ‘is equivalent to about 25 ml in the adult human female, we have arbitrarily defined the patient at risk as that patient with at least 25 ml of gastric juice with a pH below 2.5 in the stomach’.8 In the following decades, this threshold was referred to as the ‘critical gastric volume in relation to the risk of aspiration’.
However, fasting elective patients very often exceed the threshold of 0.4 ml kg−1 for gastric residual volume.10,11 Recent studies have arbitrarily set the threshold for distinguishing between an empty and full stomach at 1.5 ml kg−1,11,12 which corresponds to the 95th to 97th percentile of gastric volume in healthy fasting adults. However, the threshold gastric volume that could lead to an increased risk of regurgitation is probably much higher, exceeding 8 ml kg−1 in animal studies.13 To the best of our knowledge, there are no corresponding studies in humans.12
Learning points
-
•
Historically, fasting times have been arbitrarily set with the aim of preventing aspiration.
-
•
For decades, no differentiation has been made between clear liquids and solids (nothing by mouth after midnight).
Gastric emptying
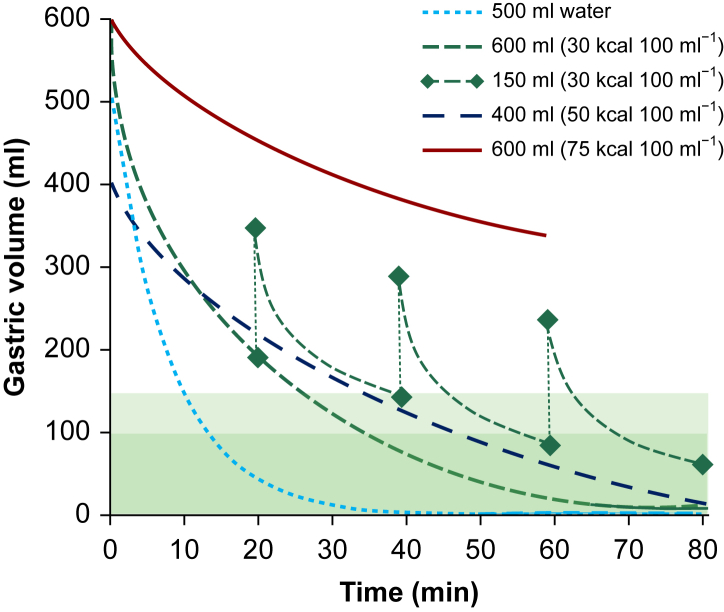
Gastric emptying of clear liquids is mainly affected by the volume of fluid in the stomach and the energy density of the liquid (Fig. 1). Gastric emptying exponentially increases as fluid volume in the stomach increases and is also proportional to the rate of filling.14,15,19, 20, 21, 22 Drinking clear liquids therefore speeds up the emptying of the stomach.15,23
Fig 1.
Gastric emptying after drinking water and clear carbohydrate liquids with three different calorie contents. The green dashed lines between the diamonds represent repeated fluid intake of 150 ml. The range of normal gastric residual volumes is indicated in green (according to data from11,14, 15, 16, 17, 18).
Secondly, the higher the energy density of the liquid, the slower the gastric emptying.16,21,23, 24, 25 Receptors in the small intestine regulate gastric emptying to about 200 kcal per hour to prevent the intestine from receiving more nutrients than it can absorb.14,17 Therefore, liquid gastric emptying depends mainly on the total caloric content.18,26 There were no significant differences in liquid gastric emptying after drinking equal amounts of either orange juice or milk, as long as both had the same number of calories.18
For high-calorie non-clear liquids, calorie and nutrient content affect gastric emptying times.25 The stomach empties carbohydrates faster than proteins, and fat stays in the stomach the longest.27 Analysis of the area under the curve of the gastric content volume–time profile, which may be a more sensitive measure of gastric emptying kinetics, shows that the area under the curve is greater for a high-fat drink than for an isocaloric high-carbohydrate drink.25
In comparison to energy density, osmolality has less of an effect on gastric emptying. Replacing glucose monomers with polymers may increase the rate of gastric emptying, but this osmolality effect is more pronounced at high carbohydrate concentrations.17
Importantly, the gastric emptying rate of liquids shows a high inter-subject variability of about 30%, while the intra-subject variability is <10%.14 The high variability of gastric emptying after liquid intake is also explained by the fact that the stomach emptying is episodic. Consequently, measuring gastric volumes will result in different data depending on the time points just before or just after episodic emptying.
Clear liquids quickly leave the stomach. There is a functional tunnel along the small curvature of the stomach which allows liquids to pass directly into the duodenum within 10 min, bypassing the greater part of the stomach.28 When volunteers drink 500 ml of water, more than half of the water has left the stomach after 10 min, and the stomach is empty after 20 min.18 If a subject drinks 600 ml of a carbohydrate drink (30 g L−1 glucose), half of the liquid will have left the stomach after 15 min and the stomach will be empty after 30 min. If subjects drink 600 ml of the carbohydrate drink first and then continue drinking 150 ml every 20 min, they will have ingested a total volume of 900 ml in less than an hour, consumed the energy of a small breakfast, and still have an empty stomach.14,15 The gastric emptying time after drinking tea or coffee with milk (up to about one-fifth of the total volume) is comparable to that of water or pure tea.29, 30, 31, 32 Trauma, such as fractures of the radius or hip, does not delay gastric emptying of clear liquids,33,34 nor does obesity35 or diabetes mellitus.36 Gastric emptying is independent of age37 and is not affected by carbonated liquids.38,39
In addition to oral liquid intake, 2.5 L of saliva and gastric juice pass through the stomach each day.40 Their secretion can increase up to 600 ml h−1 in the cephalic secretion phase (triggered, for example, by thoughts of food). Therefore, fasting does not guarantee an empty stomach.
Gastric emptying of solid food
Differentiating between gastric emptying of solids and liquids is very important. In addition to the digestion and breakdown of food, the stomach also has a reservoir function.19 This function does not apply to water and clear liquids with low energy densities, which pass through the stomach quickly, proportionally to the rate of filling.
According to European guidelines, breastfeeding should be encouraged up to 3 h before anaesthesia, milk and light solid foods up to 4 h and solid foods up to 6 h before induction of anaesthesia for children.41 In adults, solid food should be prohibited for 6 h before elective surgery.32 But American guidelines explicitly point out that both the amount and type of foods ingested have to be considered when determining an appropriate fasting period for solids. A longer fasting time (e.g. 8 h or more) may be needed after fried foods, fatty foods, or meat and for patients with coexisting diseases or conditions that can affect gastric emptying (see below).42
Learning points
-
•
Clear liquids leave the stomach very quickly, with a half-life of 10–15 min for clear liquids that have no calories or low calorie content.
-
•
The amount and type of solid food consumed must be considered when determining a suitable fasting period.
Risk factors for pulmonary aspiration
Recent studies have defined the normal gastric fluid volume as up to 1.5 ml kg−1.11 But 1.5 ml kg−1 gastric fluid volume represents the upper end of normal baseline gastric secretions. Therefore, with this definition up to 6–13% of patients who have formally fasted will have an increased gastric fluid volume.11,43, 44, 45, 46 Cho and colleagues47 found a fasting gastric volume >1.5 ml kg−1 in 31% of gynaecological patients and Zhou and colleagues48 in 48% of patients with diabetes. However, the incidence of perioperative aspiration is only 0.02–0.04% in retrospective studies49,50 and 0.02–0.07% in elective adults and children in prospective studies.51, 52, 53 Therefore, gastric volume does not appear to be a suitable surrogate marker for the risk of pulmonary aspiration and, in particular, for the risk of pneumonia.
Sonographic evidence of fluid in the stomach is not associated with a higher risk of aspiration. Drinking clear liquids fills the stomach but also increases the compliance of the gastric fundus while intragastric pressure is stable until the gastric volume is 1 L or less.19,24 But if the pressure in the stomach increases, the tone of the lower oesophageal sphincter also increases, so that the barrier pressure between the stomach and oesophagus is maintained.54,55
Pre-existing conditions
Comorbidities are not independent risk factors for pulmonary aspiration.50 Laryngeal incompetence, for example in neuromuscular diseases, leads to dysphagia. In this case, the risk of aspiration is increased only during the swallowing process.
Liquids pass through the oesophagus within 1 s when the body is in an upright position. Even in severe swallowing disorders such as achalasia, gastric emptying is not affected. Reflux disease is a disorder of motility and function of the oesophagus or cardia that does not affect gastric emptying.
Conditions that may be associated with delayed gastric emptying are often cited as reasons for the need for a 2-h liquid fast. But it is often the case that no distinction is made between the gastric emptying of solids and liquids. This distinction is crucial, and is important to understand. Aspiration of clear liquids is rarely of clinical significance, whereas aspiration of solids often leads to serious complications.56 However, gastric emptying is usually delayed only for solids. Gastric ultrasound in patients with risk factors for gastroparesis revealed the presence of solid food in the stomach in 12.5% of fasted patients.57
In patients with gastroparesis, gastric emptying of clear liquids is usually unaffected, and sometimes even accelerated. One example is diabetic gastroparesis, which primarily affects solids.58, 59, 60 Gastric emptying after carbohydrate drinks is even faster in patients with type 2 diabetes than in those without diabetes.61 Similarly, vagotomy results in rapid fluid and delayed solid emptying.58 Some drugs, such as semaglutide, delay gastric emptying for solids.62 Perioperative semaglutide use was associated with increased residual gastric volume, but in 85.2% of 33 patients, solid content was observed.62 Only four patients met the study criteria for an increased gastric fluid volume of >0.8 ml kg−1, but this is a normal fluid level.
Aspiration pneumonia
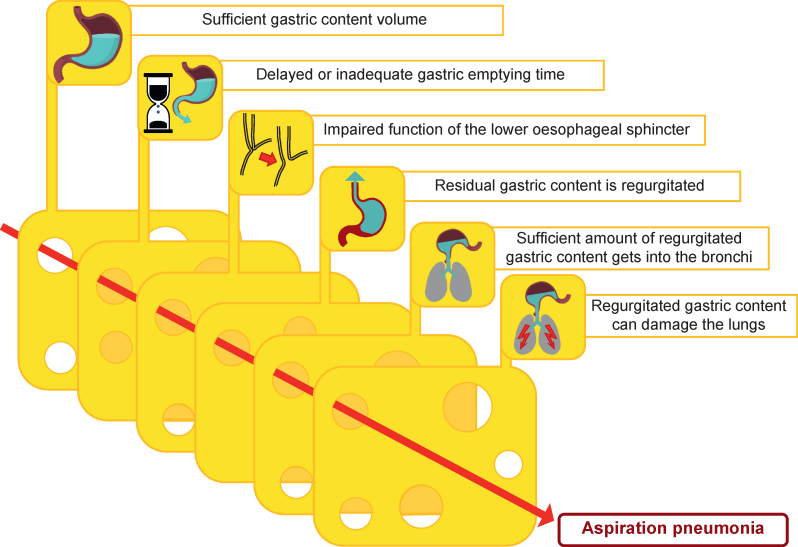
Based on the aforementioned findings, pulmonary aspiration in elective patients is the result of the coincidence of several factors (Fig. 2):
-
o
A sufficient volume of gastric content must be present.
-
o
Delayed or inadequate gastric emptying time.
-
o
The function of the lower oesophageal sphincter must be impaired or unable to withstand the applied pressure (e.g. reflex reactions of the patient during tracheal intubation under insufficiently deep anaesthesia).
-
o
The residual gastric content must be regurgitated.
-
o
Sufficient regurgitated gastric content must reach the bronchi.
-
o
The regurgitated gastric content must be harmful to the lungs.
Fig 2.
Factors that must coincide for patients to develop aspiration pneumonia.
The presence or occurrence of individual factors does not lead to aspiration pneumonia.
Prevention of aspiration
Most aspiration events are as a result of failure to recognise the risk factors for aspiration (see Table 1) and to adjust the anaesthetic technique accordingly.63,64 Therefore, good preoperative patient assessment and staff training with adaptation of anaesthetic techniques can prevent aspiration. Particular attention should be paid to the indication and correct performance of a rapid sequence induction. In an online survey, 1921 members of the European Society of Anaesthesiology from 56 countries were asked about their clinical practice of rapid sequence induction.65 The majority (61.7%) of the respondents preoxygenated patients with O2 100% for 3 min and 65.9% administered opioids during rapid sequence induction. In all patients where rapid sequence induction was indicated, the Sellick manoeuvre was used by 38.5% and was never used by 37.4% of the respondents. The remaining respondents only performed the Sellick manoeuvre in certain patient groups. First-line medications for haemodynamically stable adult patients were propofol (90.6%) and suxamethonium (56.0%). Manual ventilation (inspiratory pressure <12 cm H2O) was used by 35.5% of the respondents.
Table 1.
| Full stomach | Recent eating |
| Ileus (paralytic, non-paralytic) | |
| Pregnancy | |
| Delayed gastric emptying (traumatised patients, pyloric spasm) | |
| Gastric hypersecretion (pain, stress) | |
| Advanced chronic disease resulting in gastroparesis (diabetes mellitus/chronic kidney disease/neuromuscular disorders) | |
| Oesophageal sphincter | Severe reflux disease |
| Oesophageal disorders: Zenker's diverticulum, strictures | |
| Previous oesophageal-gastric surgery | |
| Morbid obesity | |
| Laryngeal reflexes | Inadequate depth of anaesthesia |
| Traumatic brain injury, cerebral infarction | |
| Neuromuscular disorders | |
| Others | Emergency surgery |
| Difficult airway | |
| Inadequate use of first generation supraglottic airway devices |
An international survey of 10 003 anaesthetists from 141 countries demonstrated that preferences for positioning (head-up or head-down), nasogastric tube use, and cricoid force application during rapid sequence intubation vary substantially, but were routinely performed for a hypothetical patient with intestinal obstruction.66 When anaesthetists were asked to identify the most important learning point from their experience with aspiration, their response was to address gastric decompression before anaesthesia. This includes placing a nasogastric tube if not already present, applying suction through it, administering a small amount of saline to unblock a potentially obstructed tube, and changing the patient position on the operating table to facilitate gastric emptying. For more details on rapid sequence induction, see the review by Collins and O'Sullivan.71
However, there is a lack of evidence from randomised controlled trials for many of the interventions that constitute rapid sequence induction.65,72 In particular, cricoid pressure is controversial, but a large, controlled, randomised trial from France found no advantage in terms of pneumonia and mortality in the cricoid pressure group, but a significantly higher rate of more difficult laryngoscopy.73 Contrary to Sellick's assumption,74 more recent sonographic studies have shown that the oesophagus is displaced laterally rather than dorsally to the cervical spine when cricoid pressure is applied.75 However, prophylactic strategies to prevent aspiration also carry potential risks of side-effects (e.g. rapid sequence induction with unexpected difficult intubation); therefore a critical appraisal of the risk factors is needed.76
There is no evidence of a link between drinking clear liquids and the risk of aspiration in elective patients.12 Of course, emergency patients with an indication for rapid sequence induction must remain fasting from the time of diagnosis, even for clear liquids. In cases of doubt, gastric ultrasound can be used as a ‘point-of-care’ procedure when considering whether to place a gastric tube before induction of anaesthesia and perform a controlled ‘rapid sequence induction’. A scientific evaluation of this topic is expected in the recommendations of new guidelines on perioperative fasting. The usefulness of ultrasound in assessing gastric contents and the associated risk of aspiration is one of the main topics of this upcoming guideline.77 Where possible, performing surgery with regional anaesthesia may be an alternative.
Learning points
-
•
Most cases of aspirations are associated with failure to recognise risk factors for aspiration and failure to adjust the anaesthetic technique accordingly.
-
•
Gastroparesis mainly delays the gastric emptying of solids, but not liquids.
Prolonged liquid fasting
International guidelines have recommended 2-h clear liquid fasting before induction of anaesthesia in adults.32,42,67, 78, 79 In clinical practice, however, adult patients fast for a median of 9–12 h.11,80, 81, 82, 83, 84 Prolonged liquid deprivation not only causes discomfort such as thirst, anxiety, fatigue, and postoperative nausea,80,82,85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 but can also lead to serious postoperative complications. The duration of preoperative liquid fasting is a modifiable precipitating factor of delirium in the post-anaesthesia recovery room (odds ratio 2.69) and on the ward (odds ratio 10.57).101 Preoperative dehydration quadruples the number of postoperative complications after hip fracture, especially neurological, cardiovascular, renal, or respiratory problems.102 Dehydrated tumour nephrectomy patients have an increased risk of postoperative renal failure.103 Preoperative oral rehydration solution supplementation significantly reduced post-spinal transient ischaemic electrocardiographic changes in older patients.104
In adults, prolonged fasting resulted in perioperative hyperglycaemia, insulin resistance, increased interleukin-6 levels, urinary ketone excretion, impaired cardiac output, and psychosomatic status, and decreased muscle strength compared with drinking carbohydrate drinks 2 h before induction of anaesthesia.91,93,95,98,105, 106, 107, 108, 109, 110, 111, 112, 113, 114 Perioperative hyperglycaemia is associated with an increased risk of infection, reoperation, and mortality in patients undergoing visceral surgery.115 Patients who drink coffee regularly have an increased risk of postoperative caffeine withdrawal headache.116
During long periods of fasting, children also experience thirst, hunger, anxiety, vomiting, and pain.117, 118, 119 Prolonged preoperative fasting may be a risk factor for postoperative emergence delirium in children.120,121 Optimised preoperative fasting management reduces fasting time, decreases ketone body concentration, and helps to stabilise mean arterial blood pressure during induction of anaesthesia in children.122,123
Up to 13% of patients deliberately ignore fasting recommendations for eating or drinking in order to avoid fasting for several hours.124, 125, 126, 127, 128 Some patients would lie about how long they had fasted if they were told that their operation would be delayed or cancelled because they had eaten or drunk something.125,127,128 Approximately 2–3% of patients do not adhere to the fasting rules for solids, putting themselves at serious risk. Although pulmonary aspiration of clear liquids is rarely of clinical significance, aspiration of solid food often leads to serious complications.56
Reasons for prolonged liquid fasting
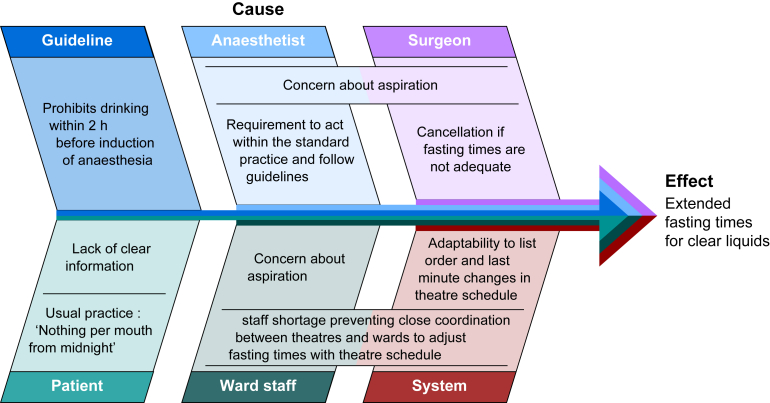
The main reasons for prolonged liquid fasting are summarised in the Ishikawa diagram in Figure 3. Systemic reasons focussed on problems in the organisational implementation of guidelines. Human reasons are based on concerns about aspiration if the 2 h limit is not met. To better understand the latter, knowledge of the risk factors for aspiration in elective patients and knowledge of gastric emptying—pathophysiology and clinical studies—are required (see above).
Fig 3.
Ishikawa diagram summarising the main reasons for prolonged liquid fasting.
Quality improvement measures to implement the 2 h liquid fasting have not yet resulted in liquid withdrawal times in the range recommended by guidelines.129, 130, 131, 132, 133 In a survey of Indian anaesthetists, only 20% reported following the ASA guideline.134 In an international survey, only about half of the anaesthetists recommended that patients continue to drink clear liquids up to 2 h before surgery.135 The main reasons given for these discrepancies were uncertainty of scheduling of the surgical procedures and failure to implement the guideline.134,135 In an operating theatre the exact time to start anaesthesia is often unpredictable and affected by short-term re-scheduling.56 Up to 12–16% of operations have been cancelled on the day that they were scheduled, mainly because other operations took longer than planned, emergency cases overwhelmed theatre capacity, or operations were cancelled because of limited personal or material resources or patient reasons.136,137 In fact, neither patients nor hospital staff know exactly what time intake of clear liquid should be stopped.56 Close communication between the operating theatre and ward would be necessary but is often limited because of the workload, staff shortages, and surgeons' concerns that the anaesthesia department may refuse to anaesthetise the patient if the liquid fasting time was too short.56,134
Giving i.v. fluids does not seem to be a solution. Oral liquid withdrawal in patients who receive i.v. fluids while waiting for surgery is almost twice as long as in patients who do not receive i.v. fluid replacement (12.5 vs 6.5 h), and they experience significantly more thirst and thirst-related distress.138 However, infusions should be given if they are needed because of pre-existing conditions or other medical reasons.
Learning points
-
•
Prolonged liquid fasting reduces patient comfort and may lead to serious postoperative complications.
-
•
The main reasons for the failure to implement the recommendation of 2 h are the variable timing of surgical procedures and the (historical) fear of aspiration.
And today
Current guidelines recommend 2 h of liquid fasting,32,42,78,79 which translates into median fasting times of up to 12 h in clinical practice.80,83,129 Because many anaesthetists refuse to induce anaesthesia in patients who have liquid fasting for <2 h, mostly for medico-legal reasons, significant reductions in prolonged fasting times have not yet been achieved. The lack of implementation of guidelines puts patients at risk in several ways. Reduced wellbeing increases the perioperative stress response, dehydration increases the incidence of severe postoperative complications, and lack of energy intake leads to insulin resistance and, thus, muscle breakdown. Patients put themselves at risk by deliberately breaking the fasting recommendations for solids.
Strict adherence to the recommendation of at least 2 h of liquid withdrawal is therefore incompatible with the recommendation that patients should not fast for >2 h because of the risk of postoperative complications. Fulfilling both requirements of the guidelines—at least 2 h, but not >2 h—appears to be practically impossible, especially for hospitals providing emergency care. To decide which of the two guideline requirements is considered more important, anaesthetists are increasingly carrying out a risk assessment between these two options:
-
-
adherence to the 2-h limit, which in practice usually results in excessively prolonged liquid fasting times or
-
-
allowing shorter liquid fasting times even shorter than 2 h to reduce perioperative complications.
The current adult guidelines only consider studies that investigated liquid fasting times between 2 and 4 h vs >4 h, or conclude that drinking up to 2 h before induction of anaesthesia has no effect on, or even reduces, gastric residual volume.32,42 There is neither evidence nor any theoretical pathophysiological explanation for harm after clear liquid fasting for <2 h. Therefore, a recent international multidisciplinary consensus statement recommends that all patients with no or low risk of aspiration should receive unrestricted clear liquids before procedural sedation.139
This is why more and more anaesthetists consider concepts such as ‘Sip Til Send’, ‘drink until called’ or ‘unrestricted drinking before surgery’.42,52,83,85,140, 141, 142
Learning points
-
•
To date, there is no system that makes it possible to comply with both recommendations of the guidelines—at least 2 h, but not much more than 2 h.
-
•
As a result, an increasing number of hospitals are weighing up the pros and cons of prolonged liquid fasting or of going below the recommendation of 2 h.
What approaches have individual centres implemented to shorten liquid fasting times?
In paediatric anaesthesia, liberal fasting regimens are much more common than in adult anaesthesia, where there are only a few published approaches to the implementation of shorter fasting times.
For children
For children, the recommendation for clear liquid fasting was reduced to 1 h in 2022.41 The extent to which liberalisation of the preoperative liquid fasting time to the new recommendation of 1 h41 will be translated into clinical reality remains to be seen. In a quality management project involving around 16 000 children, the introduction of the 1 h limit only reduced the median liquid fasting time from 9 to 6 h.143 Implementation of the 1-h fasting instruction reduced the median effective fasting time for clear fluids to 2.6 h in a prospective, observational, multi-institutional cohort study of 22 766 paediatric anaesthetics.144 In the NIKs study, clinics with a ‘drink until call’ regimen had the shortest liquid fasting times of 1.8 h (n=4188) compared with clinics with a 6/4/1 regimen (2.5 h, n=7163) or a 6/4/2 regimen (3.7 h, n=742.)145 The Uppsala working group, which has been practising ‘no limitations on clear liquid intake until called to theatre’ in children for >20 yr, reported a median fasting time of 1 h.49,146 Allowing children to drink clear liquids until premedication significantly reduces the actual fasting time from 3.9 h to 48 min.147 In none of the studies was a shorter fasting time an independent risk factor for aspiration.49,144,145,147
For adults
To find out what approaches individual centres have taken to reduce fasting times in adults, we conducted a literature search (PubMed) to identify relevant articles. The search terms were: fasting and anaesthesia, language English and date limit was set from year 2020 onwards. We identified 425 articles to assess for relevance. Of these, 148 articles were relevant to preoperative liquid fasting and 14 reported approaches to reduce liquid fasting times. From the secondary literature, we added another study published in 2018.
Intensive training to implement the 2 h limit
Intensive training can reduce liquid fasting time to some extent. In two recent studies, liquid fasting time before surgery was significantly reduced by education/training interventions. However, the fasting times achieved were still significantly longer than those recommended by the guidelines, at >5 h.129,130 The introduction of a fasting guideline reminder via a mobile phone SMS by Zia and colleagues131 in addition to a written hospital policy reduced median liquid fasting times by 3 h, but only 13.6% of patients fasted appropriately. Komatsu and colleagues132 reported a reduction in liquid fasting time from 243 to 180 min through multidisciplinary interventions in a perioperative management centre. However, only 8% of patients consumed >200 ml of clear liquid at baseline and at the end of the study.
Different approaches to implement ‘Sip Til Send’
In 2021, Sands and colleagues141 submitted freedom of information requests to all acute National Health Service England Hospital Trusts. The results revealed that 21 out of 100 Trusts now have preoperative intake guidelines that allow water after the 2-h cut-off recommended by current national guidance and 15 Trusts allow water to be sipped up to the point of sending for the patient for theatre. None of these Trusts are reporting increased rates of adverse events in the current literature or safety publications.141
In some centres, only drinking water is allowed. In the UK, Kannan140 started a ‘THINK DRINK’ campaign in which, on the day of arrival, surgical patients get a ‘welcome drink’ of water on admission and are allowed unlimited sips of water until being called. Patients showed a reduction in the mean fasting time for liquids to 2 h. Daly and colleagues100 allowed one glass of water (160 ml) per hour for women undergoing elective Caesarean delivery under spinal anaesthesia. Liquid fasting time was reduced from almost 8 h to 55 min with significant reductions in thirst, nausea, discomfort, light-headedness, and anxiety. However, in a study by Bouvet and colleagues148 allowing only limited water consumption does not ensure high patient satisfaction, at least during delivery. The authors concluded that fresh clear liquids, unrestricted amounts of liquids and sweet liquids could improve patient comfort.
In one British study and one Dutch study, all clear liquids were allowed; however, the amount that could be consumed was limited. The ‘Sip Til Send’ policy implemented by Checketts,142 allowing one 170 ml glass of clear liquid per hour until patients are sent for their procedure, reduced liquid fasting times from 6 h to 17 min. They did not observe an increase in reported adverse events in >12 000 patients through ongoing governance monitoring. The Dutch study by Marsman and colleagues52 reduced the median fasting time to 74 min by allowing clear liquids to be consumed until arrival in the operating theatre, with a maximum of 1 glass per hour and an additional glass with premedication (see below for more details).
In two studies, patients were allowed to drink all clear liquids without restrictions on the amount. In the UK, McCracken and Montgomery85 allowed >4700 day case patients unrestricted clear oral fluids before operation until transfer to the theatre. They demonstrated a significant reduction in nausea without adverse events of pulmonary aspiration of gastric contents requiring hospital admission. A German quality improvement study used fasting cards to implement unrestricted drinking of clear liquids until called to the theatre. Using this approach, they reduced the median liquid fasting time from 12 to 2.1 h.83 Patients who were allowed unrestricted drinking before surgery drank wisely and according to their needs.149 Neither sonographically nor gastroscopically did the ‘Sip Til Send’ regimen result in a clinically relevant increase in residual gastric volume.150,151
Learning points
-
•
In children, liberal liquid fasting regimens even below the recommended 1 h are much more common than in adults.
-
•
In adults, quality improvement measures have not yet resulted in liquid fasting times in the range of the recommendation of 2 h.
-
•
When drinking was allowed within 2 h before induction of anaesthesia, there was a significant reduction in fasting times for liquids.
Perspectives
However, no adequately powered studies have demonstrated the safety of short liquid fasting times in patients, particularly with regard to the risk of aspiration. The authors of the updated American guideline have calculated that, with a baseline incidence of 1.1/10 000 cases of aspiration in elective patients, 214 000 participants per study arm would be required in a two-arm study to demonstrate a two-fold increase (power, 80%; α, 0.05).79 So far, no study has met this requirement for the number of cases. However, the data from Marsman and colleagues52 are a first step.
In 2023, Marsman and colleagues52 achieved a median liquid fasting time of 74 min in 16 815 patients with a liberal liquid fasting policy (for details see above), with no significant increase in the incidence of regurgitation or aspiration compared with 59 636 control patients who followed standard fasting rules. Four patients of the liberal fasting group aspirated; three of them developed an aspiration pneumonia. One of these patients had a liquid fasting time of 2.09 h, one aspirated food, and in one patient an ileus was missed. Patients with regurgitation (n=146) had a mean liquid fasting duration of 2.52 h compared with patients without regurgitation (n=76 305) with a liquid fasting duration of 2.36 h.
What is coming next?
-
•
With the new upcoming guideline ‘Perioperative Fasting in Adults’, a systematic literature review from 2010 onwards on the impact of preoperative fasting on perioperative outcomes is expected, together with a revision of the recommendations.77
-
•
Eurofast, a large multicentre study, will monitor the incidence of pulmonary aspiration in 180 000 paediatric patients in relation to liquid fasting time. The study is expected to be completed by the end of June 2024.152
-
•
A multicentre randomised controlled study on ‘Preoperative Fasting Versus Not Fasting in Critically Ill Patients’.153
-
•
An ongoing systematic review on ‘Abbreviation of preoperative fasting in surgical patients’ is analysing articles published in the last 10 yr and is expected to be completed by the end of February 2024.154
Learning points
-
•
Adequately powered studies demonstrating the safety of liberal liquid fasting regimes in patients, particularly regarding the risk of aspiration, are lacking.
Conclusion
Attempts to reduce fasting times through quality improvement measures have failed so far. Concepts such as ‘Sip Til Send’ have achieved significant reductions in fasting times and improvements in patient well-being. However, these concepts need to be further investigated in well-designed, large clinical trials to assess patient safety, focusing on both the risk of aspiration and the complications of prolonged fasting.
Authors’ contributions
Conception and interpretation of this work: all authors
Drafting of the manuscript: AR
Critical revisions of the manuscript: PM, EN
Approved the final version of the manuscript: all authors
Accountable for all aspects of the work: all authors
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to thank Susan Hinkson, Ib Jammer, and Alexander Nagrebetsky for proofreading the manuscript.
Handling editor: Phil Hopkins
References
- 1.Mellin-Olsen J., Staender S., Whitaker D.K., Smith A.F. The Helsinki Declaration on patient safety in anaesthesiology. Eur J Anaesthesiol. 2010;27:592–597. doi: 10.1097/EJA.0b013e32833b1adf. [DOI] [PubMed] [Google Scholar]
- 2.Weimann A., Braga M., Carli F., et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 2021;40:4745–4761. doi: 10.1016/j.clnu.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Becke K., Eich C., Höhne C., et al. Choosing wisely in pediatric anesthesia: an interpretation from the German scientific working group of paediatric anaesthesia (WAKKA) Pediatr Anesth. 2018;28:588–596. doi: 10.1111/pan.13383. [DOI] [PubMed] [Google Scholar]
- 4.Maltby J.R. Fasting from midnight – the history behind the dogma. Best Pract Res Clin Anaesthesiol. 2006;20:363–378. doi: 10.1016/j.bpa.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Knight P.R., Bacon D.R. An unexplained death. Anesthesiology. 2002;96:1250–1253. doi: 10.1097/00000542-200205000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Goerig M., Schulte am Esch J. In: 50 jahre dtsch ges für anästhesiol intensivmed. Schüttler J., editor. Springer; Berlin, Heidelberg: 2003. Die Anästhesie in der ersten Hälfte des 20. Jahrhunderts; pp. 27–65.http://link.springer.com/10.1007/978-3-642-18198-6_2 Available from: [Google Scholar]
- 7.Mendelson C.L. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 8.Roberts R.B., Shirley M.A. Reducing the risk of acid aspiration during Cesarean section. Anesth Analg. 1974;53:859–868. doi: 10.1213/00000539-197453060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R.B., Shirley M.A. Antacid therapy in obstetrics. Anesthesiology. 1980;53:83. doi: 10.1097/00000542-198007000-00025. 83. [DOI] [PubMed] [Google Scholar]
- 10.Søreide E., Holst-Larsen H., Reite K., Mikkelsen H., Søreide J.A., Steen P.A. Effects of giving water 20-450 ml with oral diazepam premedication 1-2 h before operation. Br J Anaesth. 1993;71:503–506. doi: 10.1093/bja/71.4.503. [DOI] [PubMed] [Google Scholar]
- 11.Van de Putte P., Vernieuwe L., Jerjir A., Verschueren L., Tacken M., Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118:363–371. doi: 10.1093/bja/aew435. [DOI] [PubMed] [Google Scholar]
- 12.Van de Putte P., Perlas A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anaesthesia. 2018;73:274–279. doi: 10.1111/anae.14164. [DOI] [PubMed] [Google Scholar]
- 13.Plourde G., Hardy J.-F. Aspiration pneumonia: assessing the risk of regurgitation in the cat. Can Anaesth Soc J. 1986;33:345–348. doi: 10.1007/BF03010748. [DOI] [PubMed] [Google Scholar]
- 14.Leiper J.B. Fate of ingested fluids: factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. 2015;73:57–72. doi: 10.1093/nutrit/nuv032. [DOI] [PubMed] [Google Scholar]
- 15.Rehrer N., Brouns F., Beckers E., ten Hoor F., Saris W.H. Gastric emptying with repeated drinking during running and bicycling. Int J Sports Med. 1990;11:238–243. doi: 10.1055/s-2007-1024799. [DOI] [PubMed] [Google Scholar]
- 16.Nygren J., Thorell A., Jacobsson H., et al. Preoperative gastric emptying effects of anxiety and oral carbohydrate administration. Ann Surg. 1995;222:728–734. doi: 10.1097/00000658-199512000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vist G.E., Maughan R.J. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol. 1995;486:523–531. doi: 10.1113/jphysiol.1995.sp020831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okabe T., Terashima H., Sakamoto A. Determinants of liquid gastric emptying: comparisons between milk and isocalorically adjusted clear fluids. Br J Anaesth. 2015;114:77–82. doi: 10.1093/bja/aeu338. [DOI] [PubMed] [Google Scholar]
- 19.Jolliffe D.M. Practical gastric physiology. Contin Educ Anaesth Crit Care Pain. 2009;9:173–177. [Google Scholar]
- 20.Hunt J.N., Spurrell W.R. The pattern of emptying of the human stomach. J Physiol. 1951;113:157–168. doi: 10.1113/jphysiol.1951.sp004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones K.L., O’Donovan D., Russo A., et al. Effects of drink volume and glucose load on gastric emptying and postprandial blood pressure in healthy older subjects. Am J Physiol Gastrointest Liver Physiol. 2005;289:G240–G248. doi: 10.1152/ajpgi.00030.2005. [DOI] [PubMed] [Google Scholar]
- 22.Mudie D.M., Murray K., Hoad C.L., et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 ml dose of water in the fasted state. Mol Pharm. 2014;11:3039–3047. doi: 10.1021/mp500210c. [DOI] [PubMed] [Google Scholar]
- 23.Costill D.L., Saltin B. Factors limiting gastric emptying during rest and exercise. J Appl Physiol. 1974;37:679–683. doi: 10.1152/jappl.1974.37.5.679. [DOI] [PubMed] [Google Scholar]
- 24.Goyal R.K., Guo Y., Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil. 2019;31 doi: 10.1111/nmo.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali M., Uslu A., Bodin L., Andersson H., Modiri A.-R., Frykholm P. Effects of caloric and nutrient content of oral fluids on gastric emptying in volunteers: a randomised crossover study. Br J Anaesth. 2024;132:260–266. doi: 10.1016/j.bja.2023.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Du T., Hill L., Ding L., et al. Gastric emptying for liquids of different compositions in children. Br J Anaesth. 2017;119:948–955. doi: 10.1093/bja/aex340. [DOI] [PubMed] [Google Scholar]
- 27.Betts J.G., Desaix P., Johnson E., et al. OpenStax College, Rice University; Houston, Texas: 2013. Anatomy & physiology. [Google Scholar]
- 28.Pal A., Brasseur J.G., Abrahamsson B. A stomach road or “Magenstrasse” for gastric emptying. J Biomech. 2007;40:1202–1210. doi: 10.1016/j.jbiomech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Irwin R., Gyawali I., Kennedy B., Garry N., Milne S., Tan T. An ultrasound assessment of gastric emptying following tea with milk in pregnancy: a randomised controlled trial. Eur J Anaesthesiol. 2020;37:303–308. doi: 10.1097/EJA.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 30.Hillyard S., Cowman S., Ramasundaram R., Seed P.T., O’Sullivan G. Does adding milk to tea delay gastric emptying? Br J Anaesth. 2014;112:66–71. doi: 10.1093/bja/aet261. [DOI] [PubMed] [Google Scholar]
- 31.Larsen B., Larsen L.P., Sivesgaard K., Juul S. Black or white coffee before anaesthesia?: a randomised crossover trial. Eur J Anaesthesiol. 2016;33:457–462. doi: 10.1097/EJA.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 32.Smith I., Kranke P., Murat I., et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–569. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 33.Hellström P.M., Samuelsson B., Al-Ani A.N., Hedström M. Normal gastric emptying time of a carbohydrate-rich drink in elderly patients with acute hip fracture: a pilot study. BMC Anesthesiol. 2017;17:23. doi: 10.1186/s12871-016-0299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steedman D.J., Payne M.R., McClure J.H., Prescott L.F. Gastric emptying following Colles’ fracture. Arch Emerg Med. 1991;8:165–168. doi: 10.1136/emj.8.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiraishi T., Kurosaki D., Nakamura M., et al. Gastric fluid volume change after oral rehydration solution intake in morbidly obese and normal controls: a magnetic resonance imaging-based analysis. Anesth Analg. 2017;124:1174–1178. doi: 10.1213/ANE.0000000000001886. [DOI] [PubMed] [Google Scholar]
- 36.Frank J.W., Saslow S.B., Camilleri M., Thomforde G.M., Dinneen S., Rizza R.A. Mechanism of accelerated gastric emptying of liquids and hyperglycemia in patients with type II diabetes mellitus. Gastroenterology. 1995;109:755–765. doi: 10.1016/0016-5085(95)90382-8. [DOI] [PubMed] [Google Scholar]
- 37.Bonner J.J., Vajjah P., Abduljalil K., et al. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm Drug Dispos. 2015;36:245–257. doi: 10.1002/bdd.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachwieja J.J., Costill D.L., Beard G.C., Robergs R.A., Pascoe D.D., Anderson D.E. The effects of a carbonated carbohydrate drink on gastric emptying, gastrointestinal distress, and exercise performance. Int J Sport Nutr. 1992;2:239–250. doi: 10.1123/ijsn.2.3.239. [DOI] [PubMed] [Google Scholar]
- 39.Pouderoux P., Friedman N., Shirazi P., Ringelstein J.G., Keshavarzian A. Effect of carbonated water on gastric emptying and intragastric meal distribution. Dig Dis Sci. 1997;42:34–39. doi: 10.1023/a:1018820718313. [DOI] [PubMed] [Google Scholar]
- 40.Hall J.E., Guyton A.C. 12th Edn. Saunders Elsevier; USA: 2011. Textbook of medical physiology. [Google Scholar]
- 41.Frykholm P., Disma N., Andersson H., et al. Pre-operative fasting in children: a guideline from the European society of anaesthesiology and intensive care. Eur J Anaesthesiol. 2022;39:4–25. doi: 10.1097/EJA.0000000000001599. [DOI] [PubMed] [Google Scholar]
- 42.American Society of Anesthesiologists Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 2017;126:376–393. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 43.Chang J.-E., Kim H., Won D., et al. Ultrasound assessment of gastric content in fasted patients before elective laparoscopic cholecystectomy: a prospective observational single-cohort study. Can J Anaesth. 2020;67:810–816. doi: 10.1007/s12630-020-01668-7. [DOI] [PubMed] [Google Scholar]
- 44.Alcarraz P., Servente L., Kuster F., et al. Preoperative fasting for the infusion of “yerba mate”: a randomized clinical trial with ultrasound evaluation of gastric contents. Braz J Anesthesiol. 2022;72:757–761. doi: 10.1016/j.bjane.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaitra T.S., Palta S., Saroa R., Jindal S., Jain A. Assessment of residual gastric volume using point-of-care ultrasonography in adult patients who underwent elective surgery. Ultrasound J. 2023;15:7. doi: 10.1186/s13089-023-00307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasanin A., Abdelmottaleb A., Elhadi H., Arafa A.S., Mostafa M. Evaluation of gastric residual volume using ultrasound in fasting patients with uncomplicated appendicitis scheduled for appendectomy. Anaesth Crit Care Pain Med. 2021;40 doi: 10.1016/j.accpm.2021.100869. [DOI] [PubMed] [Google Scholar]
- 47.Cho E.-A., Huh J., Lee S.H., et al. Gastric ultrasound assessing gastric emptying of preoperative carbohydrate drinks: a randomized controlled noninferiority study. Anesth Analg. 2021;133:690–697. doi: 10.1213/ANE.0000000000005411. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L., Yang Y., Yang L., et al. Point-of-care ultrasound defines gastric content in elective surgical patients with type 2 diabetes mellitus: a prospective cohort study. BMC Anesthesiol. 2019;19:179. doi: 10.1186/s12871-019-0848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson H., Zarén B., Frykholm P. Low incidence of pulmonary aspiration in children allowed intake of clear fluids until called to the operating suite. Paediatr Anaesth. 2015;25:770–777. doi: 10.1111/pan.12667. [DOI] [PubMed] [Google Scholar]
- 50.Warner M.A., Warner M.E., Weber J.G. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Habre W., Disma N., Virag K., et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. 2017;5:412–425. doi: 10.1016/S2213-2600(17)30116-9. [DOI] [PubMed] [Google Scholar]
- 52.Marsman M., Kappen T.H., Vernooij L.M., van der Hout E.C., van Waes J.A., van Klei W.A. Association of a liberal fasting policy of clear fluids before surgery with fasting duration and patient well-being and safety. JAMA Surg. 2023;158:254–263. doi: 10.1001/jamasurg.2022.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker R.W.M. Pulmonary aspiration in pediatric anesthetic practice in the UK: a prospective survey of specialist pediatric centers over a one-year period. Anderson B., editor. Pediatr Anesth. 2013;23:702–711. doi: 10.1111/pan.12207. [DOI] [PubMed] [Google Scholar]
- 54.Jones M.J., Mitchell R.W., Hindocha N. Effect of increased intra-abdominal pressure during laparoscopy on the lower esophageal sphincter. Anesth Analg. 1989;68:63–65. [PubMed] [Google Scholar]
- 55.Zacchi P., Mearin F., Humbert P., Formiguera X., Malagelada J.-R. Effect of obesity on gastroesophageal resistance to flow in man. Dig Dis Sci. 1991;36:1473–1480. doi: 10.1007/BF01296818. [DOI] [PubMed] [Google Scholar]
- 56.Morrison C.E., Ritchie-McLean S., Jha A., Mythen M. Two hours too long: time to review fasting guidelines for clear fluids. Br J Anaesth. 2020;124:363–366. doi: 10.1016/j.bja.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Schwisow S., Falyar C., Silva S., Muckler V.C. A protocol implementation to determine aspiration risk in patients with multiple risk factors for gastroparesis. J Perioper Pract. 2022;32:172–177. doi: 10.1177/1750458921996925. [DOI] [PubMed] [Google Scholar]
- 58.Tack J. In: Textbook of clinical gastroenterology and hepatology. 2nd Edn. Hawkey C.J., Bosch J., Richter J.E., Garcia-Tsao G., Chan F.K.L., editors. Oxford: Wiley-Blackwell; 2012. Gastric motility disorders. [Google Scholar]
- 59.Xiao M.Z.X., Englesakis M., Perlas A. Gastric content and perioperative pulmonary aspiration in patients with diabetes mellitus: a scoping review. Br J Anaesth. 2021;127:224–235. doi: 10.1016/j.bja.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Rousset J., Coppere Z., Vallee A., et al. Ultrasound assessment of the gastric content among diabetic and non-diabetic patients before elective surgery: a prospective multicenter study. Minerva Anestesiol. 2022;88:23–31. doi: 10.23736/S0375-9393.21.15603-2. [DOI] [PubMed] [Google Scholar]
- 61.Oberoi A., Giezenaar C., Rigda R.S., et al. Effects of co-ingesting glucose and whey protein on blood glucose, plasma insulin and glucagon concentrations, and gastric emptying, in older men with and without type 2 diabetes. Diabetes Obes Metab. 2023;25:1321–1330. doi: 10.1111/dom.14983. [DOI] [PubMed] [Google Scholar]
- 62.Queiroz Silveira S., da Silva L.M., de Campos Vieira Abib A., et al. Relationship between perioperative semaglutide use and residual gastric content: a retrospective analysis of patients undergoing elective upper endoscopy. J Clin Anesth. 2023;87 doi: 10.1016/j.jclinane.2023.111091. [DOI] [PubMed] [Google Scholar]
- 63.Cook T.M., Woodall N., Frerk C. Major complications of airway management in the UK: results of the fourth national audit project of the royal college of anaesthetists and the difficult airway society. Part 1: anaesthesia. Br J Anaesth. 2011;106:617–631. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 64.Kluger M.T., Culwick M.D., Moore M.R., Merry A.F. Aspiration during anaesthesia in the first 4000 incidents reported to webAIRS. Anaesth Intensive Care. 2019;47:442–451. doi: 10.1177/0310057X19854456. [DOI] [PubMed] [Google Scholar]
- 65.Klucka J., Kosinova M., Zacharowski K., et al. Rapid sequence induction: an international survey. Eur J Anaesthesiol. 2020;37:435–442. doi: 10.1097/EJA.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zdravkovic M., Berger-Estilita J., Sorbello M., Hagberg C.A. An international survey about rapid sequence intubation of 10,003 anaesthetists and 16 airway experts. Anaesthesia. 2020;75:313–322. doi: 10.1111/anae.14867. [DOI] [PubMed] [Google Scholar]
- 67.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896–905. doi: 10.1097/00000542-199903000-00034. [DOI] [PubMed] [Google Scholar]
- 68.Asai T. Editorial II: Who is at increased risk of pulmonary aspiration? Br J Anaesth. 2004;93:497–500. doi: 10.1093/bja/aeh234. [DOI] [PubMed] [Google Scholar]
- 69.Janda M., Scheeren T.W.L., Nöldge-Schomburg G.F.E. Management of pulmonary aspiration. Best Pract Res Clin Anaesthesiol. 2006;20:409–427. doi: 10.1016/j.bpa.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Robinson M., Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Contin Educ Anaesth Crit Care Pain. 2014;14:171–175. [Google Scholar]
- 71.Collins J., O’Sullivan E.P. Rapid sequence induction and intubation. BJA Educ. 2022;22:484–490. doi: 10.1016/j.bjae.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neilipovitz D.T., Crosby E.T. No evidence for decreased incidence of aspiration after rapid sequence induction. Can J Anesth. 2007;54:748–764. doi: 10.1007/BF03026872. [DOI] [PubMed] [Google Scholar]
- 73.Birenbaum A., Hajage D., Roche S., et al. Effect of cricoid pressure compared with a sham procedure in the rapid sequence induction of anesthesia: the IRIS randomized clinical trial. JAMA Surg. 2019;154:9. doi: 10.1001/jamasurg.2018.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sellick B.A. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;278:404–406. doi: 10.1016/s0140-6736(61)92485-0. [DOI] [PubMed] [Google Scholar]
- 75.Tsung J.W., Fenster D., Kessler D.O., Novik J. Dynamic anatomic relationship of the esophagus and trachea on sonography: implications for endotracheal tube confirmation in children. J Ultrasound Med. 2012;31:1365–1370. doi: 10.7863/jum.2012.31.9.1365. [DOI] [PubMed] [Google Scholar]
- 76.Apfel C.C., Roewer N. Ways to prevent and treat pulmonary aspiration of gastric contents. Curr Opin Anaesthesiol. 2005;18:157–162. doi: 10.1097/01.aco.0000162834.33474.e0. [DOI] [PubMed] [Google Scholar]
- 77.Bilotta F., Nagrebetsky A., Rüggeberg A., et al. Ongoing guideline: Guideline on perioperative Fasting in Adult. https://www.esaic.org/uploads/2019/03/template-for-website-adult-fasting-complete.pdf Available from:
- 78.Feldheiser A., Aziz O., Baldini G., et al. Enhanced Recovery after Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi G.P., Abdelmalak B.B., Weigel W.A., et al. 2023 American society of anesthesiologists practice guidelines for preoperative fasting: carbohydrate-containing clear liquids with or without protein, chewing gum, and pediatric fasting duration—a modular update of the 2017 American society of anesthesiologists practice guidelines for preoperative fasting. Anesthesiology. 2023;138:132–151. doi: 10.1097/ALN.0000000000004381. [DOI] [PubMed] [Google Scholar]
- 80.Seyhan Ak E., Türkmen A., Sinmaz T., Biçer Ö.S. Evaluation of thirst in the early postoperative period in patients undergoing orthopedic surgery. J Perianesth Nurs. 2023;3:448–453. doi: 10.1016/j.jopan.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Falconer R., Skouras C., Carter T., Greenway L., Paisley A.M. Preoperative fasting: current practice and areas for improvement. Updates Surg. 2014;66:31–39. doi: 10.1007/s13304-013-0242-z. [DOI] [PubMed] [Google Scholar]
- 82.Tosun B., Yava A., Açıkel C. Evaluating the effects of preoperative fasting and fluid limitation: the effects of preoperative fasting. Int J Nurs Pract. 2015;21:156–165. doi: 10.1111/ijn.12239. [DOI] [PubMed] [Google Scholar]
- 83.Rüggeberg A., Nickel E.A. Unrestricted drinking before surgery: an iterative quality improvement study. Anaesthesia. 2022;77:1386–1394. doi: 10.1111/anae.15855. [DOI] [PubMed] [Google Scholar]
- 84.Abdullah Al Maqbali M. Preoperative fasting for elective surgery in a regional hospital in Oman. Br J Nurs. 2016;25:798–802. doi: 10.12968/bjon.2016.25.14.798. [DOI] [PubMed] [Google Scholar]
- 85.McCracken G.C., Montgomery J. Postoperative nausea and vomiting after unrestricted clear fluids before day surgery: a retrospective analysis. Eur J Anaesthesiol. 2018;35:337–342. doi: 10.1097/EJA.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 86.Walker E.M.K., Bell M., Cook T.M., Grocott M.P.W., Moonesinghe S.R. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth. 2016;117:758–766. doi: 10.1093/bja/aew381. [DOI] [PubMed] [Google Scholar]
- 87.Guerrier G., Bernabei F., Giannaccare G., et al. The StarvAnx study-comparison between the effects of non-fasting vs. fasting strategy on surgical outcomes, anxiety and pain in patients undergoing cataract surgery under topical anesthesia: a randomized, crossover, controlled trial. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.916225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang Y., Yan X., Liao Y. The effect of shortening the preoperative fasting period on patient comfort and gastrointestinal function after elective laparoscopic surgery. Am J Transl Res. 2021;13:13067–13075. [PMC free article] [PubMed] [Google Scholar]
- 89.Hausel J., Nygren J., Lagerkranser M., et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344–1350. doi: 10.1097/00000539-200111000-00063. [DOI] [PubMed] [Google Scholar]
- 90.Li J., Wang Y., Xiao Y., et al. Effect of different preoperative fasting time on safety and postoperative complications of painless gastrointestinal endoscopy for polyps in patients. Am J Transl Res. 2021;13:8471–8479. [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan Y., Shi G., Chen H., et al. Effects of preoperative oral enzyme-hydrolyzed rice flour solution on gastric emptying and insulin resistance in patients undergoing laparoscopic cholecystectomy: a prospective randomized controlled trial. BMC Anesthesiol. 2023;23:52. doi: 10.1186/s12871-023-02012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saeed F., Liu T.-Y., Chung W., Rich H. NPO at midnight: reassessing unnecessary pre-endoscopy fasting. R Med J. 2013;2021(104):35–38. [PubMed] [Google Scholar]
- 93.Kaška M., Grosmanová T., Havel E., et al. The impact and safety of preoperative oral or intravenous carbohydrate administration versus fasting in colorectal surgery – a randomized controlled trial. Wien Klin Wochenschr. 2010;122:23–30. doi: 10.1007/s00508-009-1291-7. [DOI] [PubMed] [Google Scholar]
- 94.Breuer-P J., von Dossow V., von Heymann C., et al. Preoperative oral carbohydrate administration to ASA iii-iv patients undergoing elective cardiac surgery. Anesth Analg. 2006;103:1099–1108. doi: 10.1213/01.ane.0000237415.18715.1d. [DOI] [PubMed] [Google Scholar]
- 95.Rizvanović N., Nesek Adam V., Čaušević S., Dervišević S., Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. 2019;34:1551–1561. doi: 10.1007/s00384-019-03349-4. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Zhu Z., Li H., et al. Effects of preoperative oral carbohydrates on patients undergoing ESD surgery under general anesthesia: a randomized control study. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Su D., Sun Y., Hu Z., Wei Z., Jia J. Influence of different preoperative fasting times on women and neonates in cesarean section: a retrospective analysis. BMC Pregnancy Childbirth. 2019;19:104. doi: 10.1186/s12884-019-2254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z.G., Wang Q., Wang W.J., Qin H.L. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg. 2010;97:317–327. doi: 10.1002/bjs.6963. [DOI] [PubMed] [Google Scholar]
- 99.Yildiz H., Gunal S.E., Yilmaz G., Yucel S. Oral carbohydrate supplementation reduces preoperative discomfort in laparoscopic cholecystectomy. J Invest Surg. 2013;26:89–95. doi: 10.3109/08941939.2012.699998. [DOI] [PubMed] [Google Scholar]
- 100.Daly S., Mohamed O., Loughrey J., Kearsley R., Drew T. ‘Sip ‘til Send’: a prospective study of the effect of a liberal fluid fasting policy on patient reported and haemodynamic variables at elective caesarean delivery. Int J Obstet Anesth. 2024;57 doi: 10.1016/j.ijoa.2023.103956. [DOI] [PubMed] [Google Scholar]
- 101.Radtke F.M., Franck M., MacGuill M., et al. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur J Anaesthesiol. 2010;27:411–416. doi: 10.1097/EJA.0b013e3283335cee. [DOI] [PubMed] [Google Scholar]
- 102.Ylinenvaara S.I., Elisson O., Berg K., Zdolsek J.H., Krook H., Hahn R.G. Preoperative urine-specific gravity and the incidence of complications after hip fracture surgery: a prospective, observational study. Eur J Anaesthesiol. 2014;31:85–90. doi: 10.1097/01.EJA.0000435057.72303.0e. [DOI] [PubMed] [Google Scholar]
- 103.Ellis R.J., Del Vecchio S.J., Kalma B., et al. Association between preoperative hydration status and acute kidney injury in patients managed surgically for kidney tumours. Int Urol Nephrol. 2018;50:1211–1217. doi: 10.1007/s11255-018-1901-2. [DOI] [PubMed] [Google Scholar]
- 104.Mj H., Jain G., Gupta P., Kalia R.B., Talawar P. Role of preoperative oral rehydration solution on myocardial ischaemia during orthopaedic surgery under spinal anaesthesia: a prospective randomised study. Turk J Anaesthesiol Reanim. 2023;51:388–394. doi: 10.4274/TJAR.2023.231206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuill K.A., Richardson R.A., Davidson H.I.M., Garden O.J., Parks R.W. The administration of an oral carbohydrate-containing fluid prior to major elective upper-gastrointestinal surgery preserves skeletal muscle mass postoperatively—a randomised clinical trial. Clin Nutr. 2005;24:32–37. doi: 10.1016/j.clnu.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Lidder P., Thomas S., Fleming S., Hosie K., Shaw S., Lewis S. A randomized placebo controlled trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Dis. 2013;15:737–745. doi: 10.1111/codi.12130. [DOI] [PubMed] [Google Scholar]
- 107.Gava M.G., Castro-Barcellos H.M., Caporossi C., Aguilar-Nascimento J.E. Enhanced muscle strength with carbohydrate supplement two hours before open cholecystectomy: a randomized, double-blind study. Rev Col Bras Cir. 2016;43:54–59. doi: 10.1590/0100-69912016001011. [DOI] [PubMed] [Google Scholar]
- 108.Bellwood H., Rozdarz K.M., Riordan J. Incidence of urinary ketosis and the effect of carbohydrate drink supplementation during fasting for elective caesarean section: audit. J Perioper Pract. 2022;32:280–285. doi: 10.1177/17504589211009099. [DOI] [PubMed] [Google Scholar]
- 109.Faria M.S.M., de Aguilar-Nascimento J.E., Pimenta O.S., Alvarenga L.C., Dock-Nascimento D.B., Slhessarenko N. Preoperative fasting of 2 hours minimizes insulin resistance and organic response to trauma after video-cholecystectomy: a randomized, controlled, clinical trial. World J Surg. 2009;33:1158. doi: 10.1007/s00268-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 110.Nygren J., Soop M., Thorell A., Sree Nair K., Ljungqvist O. Preoperative oral carbohydrates and postoperative insulin resistance. Clin Nutr. 1999;18:117–120. doi: 10.1054/clnu.1998.0019. [DOI] [PubMed] [Google Scholar]
- 111.Okabayashi T., Nishimori I., Yamashita K., et al. Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids. 2010;38:901–907. doi: 10.1007/s00726-009-0297-9. [DOI] [PubMed] [Google Scholar]
- 112.Gümüs K., Pirhan Y., Aydın G., Keloglan S., Tasova V., Kahveci M. The effect of preoperative oral intake of liquid carbohydrate on postoperative stress parameters in patients undergoing laparoscopic cholecystectomy: an experimental study. J Perianesth Nurs. 2021;36:526–531. doi: 10.1016/j.jopan.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Lee B., Soh S., Shim J.-K., Kim H.Y., Lee H., Kwak Y.-L. Evaluation of preoperative oral carbohydrate administration on insulin resistance in off-pump coronary artery bypass patients: a randomised trial. Eur J Anaesthesiol. 2017;34:740–747. doi: 10.1097/EJA.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 114.Yilmaz N., Cekmen N., Bilgin F., Erten E., Ozhan M.Ö., Coşar A. Preoperative carbohydrate nutrition reduces postoperative nausea and vomiting compared to preoperative fasting. J Res Med Sci. 2013;18:827–832. [PMC free article] [PubMed] [Google Scholar]
- 115.Kwon S., Thompson R., Dellinger P., Yanez D., Farrohki E., Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257:8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fennelly M., Galletly D.C., Purdie G.I. Is caffeine withdrawal the mechanism of postoperative headache? Anesth Analg. 1991;72:449–453. doi: 10.1213/00000539-199104000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Al-Robeye A.M., Barnard A.N., Bew S. Thirsty work: exploring children’s experiences of preoperative fasting. Pediatr Anesth. 2020;30:43–49. doi: 10.1111/pan.13759. [DOI] [PubMed] [Google Scholar]
- 118.Engelhardt T., Wilson G., Horne L., Weiss M., Schmitz A. Are you hungry? Are you thirsty? – fasting times in elective outpatient pediatric patients: pediatric fasting guidelines. Pediatr Anesth. 2011;21:964–968. doi: 10.1111/j.1460-9592.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y., Tai J., Nan Y. Effect of fasting time before anesthesia on postoperative complications in children undergoing adenotonsillectomy. Ear Nose Throat J. 2022 doi: 10.1177/01455613221078344. Advance Access published on Feb 18. [DOI] [PubMed] [Google Scholar]
- 120.Khanna P., Saini K., Sinha R., Nisa N., Kumar S., Maitra S. Correlation between duration of preoperative fasting and emergence delirium in pediatric patients undergoing ophthalmic examination under anesthesia: a prospective observational study. Pediatr Anesth. 2018;28:547–551. doi: 10.1111/pan.13381. [DOI] [PubMed] [Google Scholar]
- 121.Balkaya A.N., Yılmaz C., Baytar Ç., et al. Relationship between fasting times and emergence delirium in children undergoing magnetic resonance imaging under sedation. Medicina (Kaunas) 2022;58:1861. doi: 10.3390/medicina58121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dennhardt N., Beck C., Huber D., et al. Optimized preoperative fasting times decrease ketone body concentration and stabilize mean arterial blood pressure during induction of anesthesia in children younger than 36 months: a prospective observational cohort study. Pediatr Anesth. 2016;26:838–843. doi: 10.1111/pan.12943. [DOI] [PubMed] [Google Scholar]
- 123.Simpao A.F., Wu L., Nelson O., et al. Preoperative fluid fasting times and postinduction low blood pressure in children. Anesthesiology. 2020;133:523–533. doi: 10.1097/ALN.0000000000003343. [DOI] [PubMed] [Google Scholar]
- 124.Cantellow S., Lightfoot J., Bould H., Beringer R. Parents’ understanding of and compliance with fasting instruction for pediatric day case surgery: parents’ adherence to fasting instruction. Pediatr Anesth. 2012;22:897–900. doi: 10.1111/j.1460-9592.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- 125.Lim H., Lee H., Ti L. An audit of preoperative fasting compliance at a major tertiary referral hospital in Singapore. Singapore Med J. 2014;55:18–23. doi: 10.11622/smedj.2014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Laffey J.G., Carroll M., Donnelly N., Boylan J.F. Instructions for ambulatory surgery — patient comprehension and compliance. Ir J Med Sci. 1998;167:160–163. doi: 10.1007/BF02937929. [DOI] [PubMed] [Google Scholar]
- 127.Walker H., Thorn C., Omundsen M. Patients’ understanding of pre-operative fasting. Anaesth Intensive Care. 2006;34:358–361. doi: 10.1177/0310057X0603400317. [DOI] [PubMed] [Google Scholar]
- 128.Singla K., Bala I., Jain D., Bharti N., Samujh R. Parents’ perception and factors affecting compliance with preoperative fasting instructions in children undergoing day care surgery: a prospective observational study. Indian J Anaesth. 2020;64:210. doi: 10.4103/ija.IJA_794_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Witt L., Lehmann B., Sümpelmann R., Dennhardt N., Beck C.E. Quality-improvement project to reduce actual fasting times for fluids and solids before induction of anaesthesia. BMC Anesthesiol. 2021;21:254. doi: 10.1186/s12871-021-01468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Noort H.H.J., Lamers C.R., Vermeulen H., Huisman-de Waal G., Witteman B.J.M. Patient education regarding fasting recommendations to shorten fasting times in patients undergoing esophagogastroduodenoscopy: a controlled pilot study. Gastroenterol Nurs. 2022;45:342–353. doi: 10.1097/SGA.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zia F., Cosic L., Wong A., et al. Effects of a short message service (SMS) by cellular phone to improve compliance with fasting guidelines in patients undergoing elective surgery: a retrospective observational study. BMC Health Serv Res. 2021;21:27. doi: 10.1186/s12913-020-06039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Komatsu S., Yamashita C., Yatabe T., Kuriyama N., Nakamura T., Nishida O. Effect of multidisciplinary interventions in perioperative management center on duration of preoperative fasting: a single-center before-and-after study. Fujita Med J. 2022;8:108–113. doi: 10.20407/fmj.2021-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davies A., Pang W.S., Fowler T., Dewi F., Wright T. Preoperative fasting in the department of plastic surgery. BMJ Open Qual. 2018;7 doi: 10.1136/bmjoq-2017-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Panjiar P., Kochhar A., Vajifdar H., Bhat K. A prospective survey on knowledge, attitude and current practices of pre-operative fasting amongst anaesthesiologists: a nationwide survey. Indian J Anaesth. 2019;63:350. doi: 10.4103/ija.IJA_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Merchant R.N., Chima N., Ljungqvist O., Kok J.N.J. Preoperative fasting practices across three anesthesia societies: survey of practitioners. JMIR Perioper Med. 2020;3 doi: 10.2196/15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seim A.R., Fagerhaug T., Ryen S.M., et al. Causes of cancellations on the day of surgery at two major university hospitals. Surg Innov. 2009;16:173–180. doi: 10.1177/1553350609335035. [DOI] [PubMed] [Google Scholar]
- 137.Schofield W.N., Rubin G.L., Piza M., et al. Cancellation of operations on the day of intended surgery at a major Australian referral hospital. Med J Aust. 2005;182:612–615. doi: 10.5694/j.1326-5377.2005.tb06846.x. [DOI] [PubMed] [Google Scholar]
- 138.Carey S., Waller J., Wang L.Y., Ferrie S. Qualifying thirst distress in the acute hospital setting – validation of a patient-reported outcome measure. J Perioper Nurs. 2021;34:e38–e44. [Google Scholar]
- 139.Green S.M., Leroy P.L., Roback M.G., et al. An international multidisciplinary consensus statement on fasting before procedural sedation in adults and children. Anaesthesia. 2020;75:374–385. doi: 10.1111/anae.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kannan S. Will one hour less make any difference? Eur J Anaesthesiol. 2020;37:52. doi: 10.1097/EJA.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 141.Sands R., Wiltshire R., Isherwood P. Preoperative fasting guidelines in national Health service England Trusts: a thirst for progress. Br J Anaesth. 2022;129:e100–e102. doi: 10.1016/j.bja.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 142.Checketts M.R. Fluid fasting before surgery: the ultimate example of medical sophistry? Anaesthesia. 2023;78:147–149. doi: 10.1111/anae.15925. [DOI] [PubMed] [Google Scholar]
- 143.Isserman R., Elliott E., Subramanyam R., et al. Quality improvement project to reduce pediatric clear liquid fasting times prior to anesthesia. Pediatr Anaesth. 2019;29:698–704. doi: 10.1111/pan.13661. [DOI] [PubMed] [Google Scholar]
- 144.Schmitz A., Kuhn F., Hofmann J., et al. Incidence of adverse respiratory events after adjustment of clear fluid fasting recommendations to 1 h: a prospective, observational, multi-institutional cohort study. Br J Anaesth. 2024;132:66–75. doi: 10.1016/j.bja.2023.10.009. [DOI] [PubMed] [Google Scholar]
- 145.Beck C.E., Rudolph D., Mahn C., et al. Impact of clear fluid fasting on pulmonary aspiration in children undergoing general anesthesia: results of the German prospective multicenter observational (NiKs) study. Pediatr Anaesth. 2020;30:892–899. doi: 10.1111/pan.13948. [DOI] [PubMed] [Google Scholar]
- 146.Andersson H., Hellström P.M., Frykholm P. Introducing the 6-4-0 fasting regimen and the incidence of prolonged preoperative fasting in children. Pediatr Anaesth. 2018;28:46–52. doi: 10.1111/pan.13282. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt A.R., Buehler K.P., Both C., et al. Liberal fluid fasting: impact on gastric pH and residual volume in healthy children undergoing general anaesthesia for elective surgery. Br J Anaesth. 2018;121:647–655. doi: 10.1016/j.bja.2018.02.065. [DOI] [PubMed] [Google Scholar]
- 148.Bouvet L., Garrigue J., Desgranges F.-P., Piana F., Lamblin G., Chassard D. Women’s view on fasting during labor in a tertiary care obstetric unit. A prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;253:25–30. doi: 10.1016/j.ejogrb.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 149.Rüggeberg A., Nickel E.A. Unrestricted drinking before surgery: a structured patient interview. Anaesthesia. 2023;78:911–913. doi: 10.1111/anae.15997. [DOI] [PubMed] [Google Scholar]
- 150.Harnett C., Connors J., Kelly S., Tan T., Howle R. Evaluation of the ‘Sip Til Send’ regimen before elective caesarean delivery using bedside gastric ultrasound: a paired cohort pragmatic study. Eur J Anaesthesiol. 2024;41:129–135. doi: 10.1097/EJA.0000000000001926. [DOI] [PubMed] [Google Scholar]
- 151.Marsman M., Pouw N., Moons L.M.G., van Klei W.A., Kappen T.H. Gastric fluid volume in adults after implementation of a liberal fasting policy: a prospective cohort study. Br J Anaesth. 2021;127:e85–e87. doi: 10.1016/j.bja.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 152.Frykholm P., Modiri A. Ongoing Clinical Trial NCT05519969. https://ichgcp.net/clinical-trials-registry/NCT05519969 Available from:
- 153.Nagrebetsky A., Vidal Melo M. Ongoing Clinical Trial: Preoperative Fasting Versus Not Fasting in Critically Ill Patients. https://www.pcori.org/research-results/2023/preoperative-fasting-versus-not-fasting-critically-ill-patients Available from:
- 154.Smith J., Mistrinel M., de Santana Lemos C. Ongoing Systematic Review. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=432494 Available from: