Abstract
Background
Local anaesthetics are widely used for their analgesic and anaesthetic properties in the perioperative setting, including surgical procedures to excise malignant tumours. Simultaneously, chemotherapeutic agents remain a cornerstone of cancer treatment, targeting rapidly dividing cancer cells to inhibit tumour growth. The potential interactions between these two drug classes have drawn increasing attention and there are oncological surgical contexts where their combined use could be considered. This review examines existing evidence regarding the interactions between local anaesthetics and chemotherapeutic agents, including biological mechanisms and clinical implications.
Methods
A systematic search of electronic databases was performed as per Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. Selection criteria were designed to capture in vitro, in vivo, and clinical studies assessing interactions between local anaesthetics and a wide variety of chemotherapeutic agents. Screening and data extraction were performed independently by two reviewers. The data were synthesised using a narrative approach because of the anticipated heterogeneity of included studies.
Results
Initial searches yielded 1225 relevant articles for screening, of which 43 met the inclusion criteria. The interactions between local anaesthetics and chemotherapeutic agents were diverse and multifaceted. In vitro studies frequently demonstrated altered cytotoxicity profiles when these agents were combined, with variations depending on the specific drug combination and cancer cell type. Mechanistically, some interactions were attributed to modifications in efflux pump activity, tumour suppressor gene expression, or alterations in cellular signalling pathways associated with tumour promotion. A large majority of in vitro studies report potentially beneficial effects of local anaesthetics in terms of enhancing the antineoplastic activity of chemotherapeutic agents. In animal models, the combined administration of local anaesthetics and chemotherapeutic agents showed largely beneficial effects on tumour growth, metastasis, and overall survival. Notably, no clinical study examining the possible interactions of local anaesthetics and chemotherapy on cancer outcomes has been reported.
Conclusions
Reported preclinical interactions between local anaesthetics and chemotherapeutic agents are complex and encompass a spectrum of effects which are largely, although not uniformly, additive or synergistic. The clinical implications of these interactions remain unclear because of the lack of prospective trials. Nonetheless, the modulation of chemotherapy effects by local anaesthetics warrants further clinical investigation in the context of cancer surgery where they could be used together.
Clinical trial registration
Open Science Framework (OSF, project link: https://osf.io/r2u4z)
Keywords: anaesthesia, cancer, cancer recurrence, chemotherapy, local anaesthetics
Cancer remains a formidable challenge to global health, representing a leading cause of morbidity and mortality worldwide with almost 10 million cancer-related deaths in 2020.1 Oncology is a rapidly evolving field, with significant progress made recently not only with the development of novel therapies, but also with deepening of our understanding of cancer biology and metastasis.2 Despite these medical advances, surgery remains the primary curative strategy for solid organ cancers. An area of growing speculation is the potential interaction between local anaesthetics, which are commonly used in perioperative settings for anaesthesia and pain management, and chemotherapeutic agents.
Cancer progression is a highly complex, multifactorial process involving primary tumour growth, release of circulating tumour cells, and the subsequent development of distant metastases.3 Metastasis is not only a consequence of the intrinsic characteristics of cancer cells but is also influenced by the host microenvironment and systemic factors.4 Research suggests that invasive surgical procedures may have an impact on tumour growth and the likelihood of metastasis.5 This raises questions about how the patient's perioperative journey, including choice of drugs administered perioperatively, may influence cancer progression.6 Surgical tissue trauma initiates multiple physiological signalling pathways resulting in a complex interplay of adrenergic, inflammatory, and immune responses which are intended to cause wound healing but, inadvertently, are also implicated in stimulating the growth of residual cancer cells. Significant preclinical experimental research has revealed how anaesthetic and analgesic techniques may modulate these pathways and possibly alter the risk of cancer progressing or recurring after operation.7 For instance, the use of volatile anaesthetic agents leads to higher postoperative serum pro-inflammatory cytokine concentrations.8
Some retrospective clinical studies have detected an association between anaesthetic type and risk of postoperative cancer recurrence, although this association has not uniformly been found.9,10 Although few prospective randomised controlled trials (RCTs) have been completed to date, almost all published RCTs have not confirmed a definite link between technique or agent used and long-term cancer outcomes. For example, there is no high-quality evidence that propofol is superior to volatile agents or that epidural use conveys an advantage in terms of overall survival or cancer recurrence.11 The exception is a large RCT involving women undergoing breast cancer surgery of curative intent. Almost 1600 women were randomly allocated to an active arm (received peritumoral infiltration of the local anaesthetic lidocaine 0.5% up to 4.5 mg kg−1 body weight, 10 min before surgical excision), compared with a control group, who did not receive local anaesthetic infiltration. In the local anaesthetic and control groups, 5-yr disease-free survival rates were 87% and 83% (hazard ratio [HR] 0.74; 95% confidence interval [CI] 0.58–0.95; P=0.017) and 5-yr overall survival rates were 90% and 86%, respectively (HR 0.71; 95% CI 0.53–0.94; P=0.019). No toxic effects of lidocaine were observed. Although it has been the subject of some criticism (e.g. for lacking a placebo injection in the control group), this trial is the first to report a positive difference after a single perioperative intervention on long-term oncological outcomes.12
Lidocaine and other local anaesthetics have been examined extensively in preclinical studies with substantial laboratory evidence that they can inhibit cancer biology both in vitro and in vivo.13 The mechanisms underlying this effect appear multifactorial with evidence that local anaesthetics have direct inhibitory effects on cancer cells via numerous cellular signalling pathways: they may damage mitochondria directly, they may help to preserve immune cell function, and may inhibit both angiogenesis and inflammation.14,15 These potentially beneficial physiological effects were also detected in clinical translational studies: for instance, i.v. lidocaine infusion has an anti-inflammatory effect and reduces postoperative serum pro-inflammatory cytokine concentrations.16
Surgical procedures involving concurrent administration of local anaesthetics and chemotherapeutic agents might at first glance appear limited, but are in fact quite frequent and likely to be steadily increasing. Intraoperative chemotherapy use was previously largely confined to hyperthermic intraperitoneal chemotherapy (HIPEC) using mitomycin C, oxaliplatin, and others, during cytoreductive surgery (CRS) for ovarian malignancies with peritoneal carcinomatosis and pseudomyxoma peritonei, but subsequently this technique was expanded to treat advanced colorectal and gastric malignancies.17 In 2018 at least 3800 patients underwent CRS/HIPEC worldwide, although this estimate was based on a limited survey of 19 countries so may have underestimated the true figure.18 In some HIPEC cases termed ‘bidirectional HIPEC’, intraoperative i.v. chemotherapy (e.g. i.v. cisplatin) is co-administered with intraperitoneal chemotherapy.19 Heated intracavitary chemotherapy combined with CRS is also used in the thoracic cavity for the treatment of malignant pleural mesothelioma.20 Infusion of chemotherapeutic agents into the portal vein or hepatic artery using catheters placed during surgery to excise primary colorectal tumours has been studied, with mixed results being reported.21,22 Very recently, some centres have examined whether intraoperative i.v. chemotherapy during colorectal cancer excisional surgery may improve cancer outcomes, with promising initial results.23 Intraoperative intravesical chemotherapy has been used during nephroureterectomy to reduce bladder urothelial carcinoma recurrence.24 Given the trend in management patterns towards a more interventional approach and a shift in attitudes towards viewing advanced cancers as a chronic illness where multiple, aggressive resections are considered appropriate, it may be expected that procedures involving intraoperative or very early postoperative chemotherapy will increase in frequency.25,26
Looking ahead then, it appears likely that procedures where local anaesthetics and chemotherapeutic agents are co-administered will become more common. Local anaesthetic either given systemically or absorbed into the circulation (from epidural, regional block, infiltration, etc.) may interact with chemotherapeutic drugs similarly given either systemically or absorbed via the peritoneum, pleura or bladder. Local anaesthetics could potentially act to synergistically enhance the anticancer effects of intraoperative chemotherapy, in which case short-term (hours-to-days) exposure to local anaesthetic may potentially improve the patient's long-term survival or cancer recurrence risk. Or local anaesthetic may in fact inhibit chemotherapy effects and should be avoided to reduce the risk of adverse outcomes. Understanding the potential impact of local anaesthetics on cancer recurrence and metastasis is therefore of paramount importance and will allow for optimal perioperative care of the cancer patient to achieve the best long-term outcomes. To the best of our knowledge, this review is the first to evaluate existing literature regarding the interactions between local anaesthetics and chemotherapy.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines.27 The PRISMA checklist can be found in the Supplementary Materials. After initial exploratory scoping searches it was evident that no human clinical studies would be eligible for inclusion and the large majority of included studies would be in vitro in nature with a smaller number of in vivo studies. Therefore, this review was ineligible for registration in the PROSPERO international prospective register of systematic reviews; instead, the review methodology was predefined and prospectively registered on the Open Science Framework (OSF, project link: https://osf.io/r2u4z), a similar registry including a wider range of review types. The following databases were searched for relevant published literature: PubMed, Scopus, Web of Science and Embase. The search strategy used for each database is available on the OSF registration page. The database searches were performed on 2 August 2023. If any relevant references were found in study bibliographies, then these were included also. In order to be included in the review the returned studies had to meet the following inclusion criteria: (i) preclinical or clinical studies examining cancer; (ii) studies where local anaesthetic agents are combined with a chemotherapy drug (any agent, with common agents specifically identified, see OSF page); (iii) studies where the effect of each drug on its own is compared with both used in combination; (iv) in vitro studies which examine outcomes including cell viability, proliferation, migration, protein/gene expression, etc., or clinical outcomes (survival, recurrence, metastasis, tumour sizes, etc.) for any in vivo or clinical studies. The exclusion criteria were: (i) studies not published in English; (ii) studies that do not involve combination use of a local anaesthetic and a chemotherapeutic agent (e.g. both are studied but not used in combination). The Covidence online systematic review platform (Veritas Health Innovation, Melbourne, Australia) was used to manage references, identify duplicates, screen studies, and aid with the data extraction process. Studies were screened for eligibility by two independent reviewers (AA, TPW). Studies meeting the inclusion criteria based on their title or abstract, or where their eligibility was uncertain, proceeded to full-text assessment. Each full text was assessed in duplicate by the same reviewers to determine their eligibility for inclusion. Where there was disagreement between the two reviewers as to eligibility, a third reviewer (DJB) made a final decision on inclusion. For eligible references, data were extracted including author, year published, study type (in vivo or in vitro), cancer type, local anaesthetic(s) examined, local anaesthetic concentration used, chemotherapeutic agent(s) examined, interaction observed and mechanism of action studied (if any). As there were no clinical studies to include, no risk of bias or quality assessment was performed as there are no established methods for performing these assessments for in vitro and in vivo laboratory studies. Given this limitation, and the anticipated high degree of heterogeneity of the included studies, a narrative synthesis of the extracted data and subsequent findings was performed.
Results
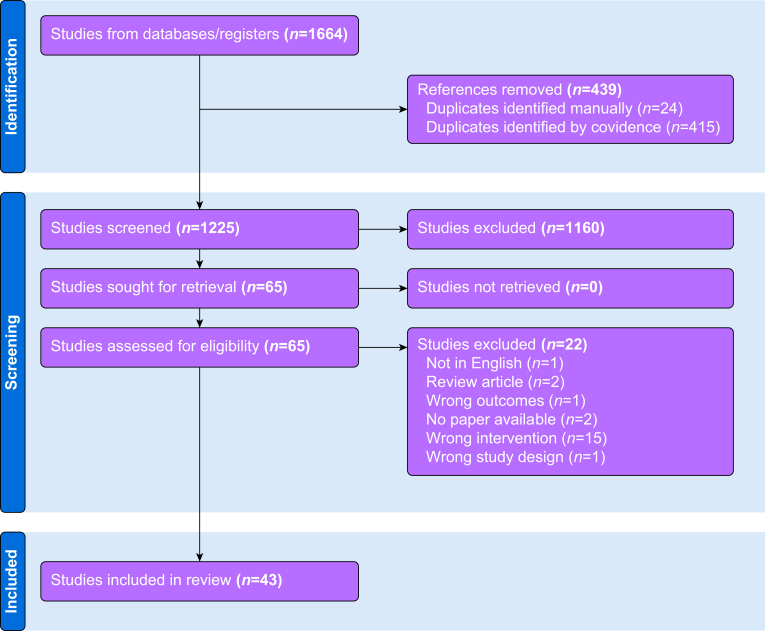
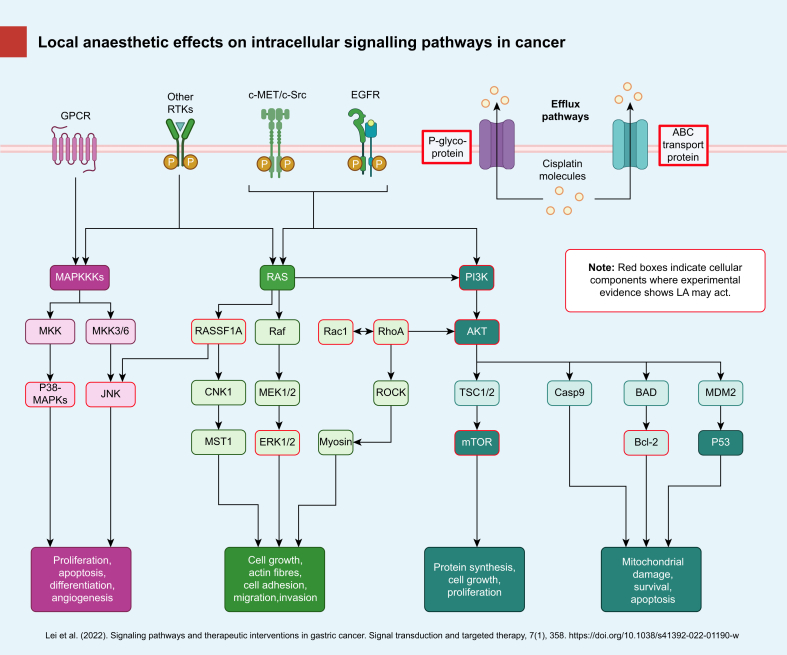
Figure 1 shows the PRISMA flow diagram for the review process. Initial searches retrieved 1664 references; 439 duplicates were identified and removed leaving 1225 references to be screened by title and abstract. Screening resulted in 65 references that were assessed as full-text articles. After full-text analysis, 43 references were eligible for data extraction. Summary extracted data are presented in Table 1 (studies involving lidocaine and other amino amide local anaesthetics) and Table 2 (studies involving the amino ester procaine). In vitro experiments were described in 30 studies, five were in vivo animal model experiments and eight involved both in vitro and in vivo approaches. No clinical study matching the inclusion criteria was identified. Ten different local anaesthetics were examined in preclinical studies: lidocaine, bupivacaine, levobupivacaine, ropivacaine, dibucaine, mepivacaine, procaine, tetracaine, benzocaine, and butacaine. Lidocaine was the most frequently studied local anaesthetic with 27 published articles: 18 involving lidocaine alone and nine studied lidocaine along with other local anaesthetics. Procaine was studied in 17 reports: eight with procaine alone, five where procaine was used as a complex with cisplatin (known as DPR) and four where procaine was examined along with other local anaesthetics. Twenty-three chemotherapeutic agents were analysed across the 42 studies; the most commonly studied were cisplatin (18 studies) and 5-fluorouracil (eight). A hypothetical biochemical mechanism of action was examined in 27 studies, such as deoxyribonucleic acid (DNA) demethylation, P-glycoprotein expression, etc. A total of 16 different cancer types were examined between the 43 included studies, some of which examined more than one type. The most commonly studied were breast cancer (11) and leukaemia (11). The large majority of included studies (38/43, 88.4%) reported potentially beneficial anticancer effects of combined local anaesthetic/chemotherapeutic agent use compared with sole use of either agent. Typical beneficial effects seen in vitro were enhanced cytotoxicity or reduced resistance to the chemotherapeutic agent, and typical in vivo effects seen were improved survival or reduced tumour size. Three studies did not detect beneficial effects attributable to combination local anaesthetic/chemotherapeutic agent treatment in vitro or in vivo,28, 29, 30 and two reported potentially harmful effects.31,32 Potential cellular mechanisms of action responsible for the experimental effects observed were investigated by 26 studies, with numerous candidate mechanisms identified ranging from drug efflux protein effects, DNA demethylation, inhibition or promotion of intracellular signalling pathways controlling apoptosis, etc. These putative mechanisms of action are shown schematically in Fig 2.
Fig 1.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Table 1.
Summary extracted data from studies examining amino amide local anaesthetics which meet systematic review selection criteria. 5-FU, 5-fluorouracil; 8-OHdG, 8-hydroxy-2' -deoxyguanosine; ABCG2, ATP-binding cassette G2; AKT, protein kinase B; Bax, BCL2-associated X; Bcl-2, B-cell lymphoma 2; c-Met, c-mesenchymal-epithelial transition factor; c-Src, Proto-oncogene tyrosine-protein kinase Src; DDP, diamminedichloroplatinum; EMT, epithelial-to-mesenchymal transition; ERK1/2, extracellular signal-related kinase 1/2; GSK3B, Glycogen synthase kinase-3 beta; IL-6, interleukin-6; mTOR, mammalian target of rapamycin; miR, micro RNA; MLC, myosin light chain; MMP, matrix-metalloproteinase; MRP, multidrug resistance-associated protein; NLC-DTX, nanostructured lipid carrier-docetaxel; PI3K, phosphoinositide 3-kinase; Rac1, Ras-related C3 botulinum toxin substrate 1; RARβ2, retinoic acid receptor beta 2; RASSF1A, Ras association domain-containing protein 1; ROCK, Rho-associated, coiled-coil containing protein kinase 1; ROS, reactive oxygen species; SCC, squamous cell carcinoma; SIRT, sirtuin 1; SOX4, SRY-related HMG-box 4; TGF, transforming growth factor.
| 1st author and year | Study type | Cancer type (cell line) | Local anaesthetic(s) studied | Local anaesthetic concentration | Chemotherapy agent(s) studied | Interaction observed (e.g. no effect, synergistic effect, additive effect, etc) | Mechanism(s) studied (if any) |
|---|---|---|---|---|---|---|---|
| Li 201451 | In vitro | Breast (MCF-7, MDA-MB-231) | Lidocaine | 10 μM–1 mM | Cisplatin | Lidocaine enhanced the cytotoxic effects of cisplatin | Upregulation of RARβ2 and RASSF1A (promoters of tumour suppressor genes) |
| Xing 201746 | Both | Hepatocellular (HepG2) | Lidocaine | 100 μM–10 mM in vitro, 30 mg kg−1 twice weekly in vivo | Cisplatin | Lidocaine–cisplatin treatment was more cytotoxic in vitro and suppressed tumour growth more effective in vivo than either agent used alone | Bcl-2, Bax, and cleaved caspase-3 activation, activation of ERK1/2, p-38 cascade |
| Yang 201949 | In vitro | Lung cancer (A549/DDP) | Lidocaine | 1–100 μM | Cisplatin | Lidocaine reduced cisplatin resistance | miR-21 expression |
| Liu 202247 | In vitro | Skin squamous cell carcinoma (A431) | Lidocaine | 0–10 mM | Cisplatin | Lidocaine reduces cisplatin resistance | miR-30c/SIRT1 pathway activation |
| Freeman 201844 | In vivo | Breast (4T1 murine) | Lidocaine | 1.5 mg kg−1 bolus then 2 mg kg−1 h−1 infusion | Cisplatin | Enhanced effect in terms of reduced pulmonary metastases, no effect on liver metastases | No significant difference in serum IL-6 noted |
| Zhang 202048 | In vitro | Gastric (MGC-803, MGC-803/DDP) | Lidocaine | 25 μM–200 μM | Cisplatin | Lidocaine reduced cisplatin resistance | Inhibition of miR-10b, AKT/mTOR and β-catenin pathway repression |
| Gao 201845 | Both | Breast (MDA-MB-231, MCF-7) | Lidocaine | In vitro 12 mg kg−1 (murine model) | Cisplatin | Nanogel loaded lidocaine has a synergistic anticancer effect with co-loaded cisplatin both in vitro and in vivo | N/A |
| Lazo 198552 | In vitro | Leukaemia (L1210 murine) | Lidocaine | 0–10 mM | Cisplatin, bleomycin, mitomycin C, etoposide | Lidocaine potentiates bleomycin, cisplatin and etoposide cytotoxicity | N/A |
| Zeng 202143 | In vitro | Gastric (MKN45) | Lidocaine | 10 mM | Cisplatin, 5-FU | Lidocaine enhanced sensitivity of cells to chemotherapeutic agents | Phosphorylation levels of c-Met and c-Src were reduced by lidocaine treatment |
| Zhang 201940 | In vitro | Choriocarcinoma (JEG-3, JAR) | Lidocaine | 10–1000 μM | 5-FU | Lidocaine potentiated 5-FU cytotoxicity | ATP-binding cassette (ABC) transport protein expression—expression of ABCG2, P-glycoprotein, MRP1, MRP2, PI3K/AKT pathway inhibition |
| Wang 201741 | In vitro | Melanoma (SK-MEL-2) | Lidocaine | 10–1000 μM | 5-FU | Lidocaine enhances sensitivity of melanoma cells to 5-FU | Lidocaine induced expression of miR-493 and downregulated expression of SOX4 perhaps by inactivation of PI3K/AKT and TGF-TGF-β pathways |
| Polekova 199258 | In vitro | Leukaemia (L1210, murine) | Lidocaine | 0–2 mM | Vincristine | Lidocaine reversed cancer cell resistance to vincristine | P-glycoprotein and MDR1 (multidrug resistance) gene expression |
| Kim 201929 | In vitro | Oral SCC (KBV20C, MDR cells) | Lidocaine | 5 μM | Vincristine | Lidocaine had no additional effect on cell viability when combined with vincristine | Inhibition of the P-glycoprotein cell efflux protein |
| Wall 201931 | In vivo | Breast (4T1 murine) | Lidocaine | 1.5 mg kg−1 bolus + 2 mg kg−1 h−1 infusion | Bosutinib | Bosutinib reversed the antimetastatic effect of lidocaine, lidocaine reduced MMP-2 expression | Src, MMP-2/9 inhibition |
| Wall 202130 | In vitro | Breast (4T1 murine) | Lidocaine | 5 μM–3 mM | Bosutinib | No effect of combination therapy at therapeutic concentrations | N/A |
| Han 202262 | Both | Breast cancer (MDA-MB231 and 453) | Lidocaine | 0–3 mM | Palbociclib | Palbociclib effects enhanced by local anaesthetic (in vivo/in vitro) | Inhibition of PI3K/AKT/GSK3B and EMT signalling |
| Yang 201855 | Both | Bladder (BIU-87) | Lidocaine | 1.25–5 mg ml−1in vitro, 2.5–5 mg ml−1in vivo per week | Mitomycin C, pirarubicin | In vitro lidocaine enhances cytotoxicity of both chemotherapeutic agents, in vivo lidocaine/MMC prolonged survival and reduced mean bladder wet weight compared with solo therapy | N/A |
| De Moura 202161 | Both | Melanoma (B16–F10 murine, SK-MEL-103) | Lidocaine | 30 μM–10 mM | Docetaxel (DTX) | Addition of lidocaine to NLC-DTX and HGel-NLC-DTX systems increased their cytotoxicity in vitro; addition of lidocaine decreased tumour growth in vivo | Nanostructured lipid carriers (NLC) combined with the antineoplastic docetaxel, formed a hybrid gel (NLC-in-hydrogel) for topical application |
| Zheng 202056 | In vitro | Melanoma (A375, A431) | Lidocaine, ropivacaine, bupivacaine | 250 μM–2 mM | Dacarbazine, vemurafenib | Ropivacaine and lidocaine (but not bupivacaine) enhanced the antimigratory, antiproliferative and pro-apoptotic effects of vemurafenib and dacarbazine | Ropivacaine and lidocaine decreased RhoA, Rac1, and Ras activity; bupivacaine did not affect RhoA, Rac1, and Ras activity |
| Brummelhuis 202159 | In vitro | Ovarian (OVCAR3, OVCAR5, T47D, KURAMOCHI, JHOS4) | Lidocaine, bupivacaine, benzocaine, procaine | Lidocaine (0.2–125 mM), bupivacaine (6 μM–3.75 mM), benzocaine (8 μM–5 mM), procaine (0.02–12.5 mM) | Carboplatin, paclitaxel | Additive effect of local anaesthetics to chemotherapeutic agent effect | Voltage-gated sodium channel (VGSC) inhibition |
| Lirk 201457 | In vitro | Breast (BT-20, MCF-7) | Lidocaine, bupivacaine, ropivacaine | 10–309.2 μM | Decitabine (DAC) | No effect of local anaesthetic/DAC combination on cell viability, lidocaine and ropivacaine cause DNA demethylation—this effect is additive (but not supra-additive) when lidocaine is combined with DAC | DNA demethylation |
| Dorr 199028 | In vitro | Leukaemia (L-1210, RL-1210) | Lidocaine, procaine | 250–350 μM | Mitomycin C | Local anaesthetics did not reverse resistance to mitomycin-C | P-glycoprotein expression |
| Mizuno 198253 | In vitro | Breast (FM3A murine) | Lidocaine, procaine, dibucaine, butacaine, tetracaine | 0.2 mM–12 mM | Bleomycin | All local anaesthetics enhanced bleomycin cytotoxicity | N/A |
| Mizuno 198254 | In vitro | Breast (FM3A, HeLa) | Lidocaine, procaine, dibucaine, butacaine, tetracaine | 0–10 mM | Peplomycin | All local anaesthetics enhanced the cytotoxicity of peplomycin, this was enhanced further by hyperthermia | N/A |
| Chen 202050 | In vitro | Hepatoma (HepG2, BEL-7402) | Lidocaine, ropivacaine, bupivacaine | 0.05, 0.5, 5 mM | Cisplatin | Chemotherapeutic effect enhanced by local anaesthetics | Unregulated RASSF1A expression |
| Zhu 202042 | In vitro | Oesophageal (OE19, SK-GT-4) | Lidocaine, ropivacaine, bupivacaine, mepivacaine | 10–100 μM | 5-FU, paclitaxel | Local anaesthetics augmented the effects of chemotherapeutic agent drugs in inhibiting growth and inducing apoptosis | Mitochondrial dysfunction and oxidative damage (decreased oxygen consumption rate, increased intercellular ROS and 8-OHdG levels), decreased Rac1 activity, no effect on RhoA |
| Meireles 201860 | In vitro | Prostate (PC3) | Lidocaine, ropivacaine, levobupivacaine | Lidocaine (426.7–853.4 nM), ropivacaine (36.4–273.3 nM), levobupivacaine (43.3–173.4 nM) | Docetaxel | Local anaesthetics enhanced chemo-induced inhibition of cell proliferation | N/A |
| Zheng 201866 | In vitro | Leukaemia (CD34, K562, LAMA84) | Ropivacaine | 100–1000 μM | Dasatinib, imatinib | Local anaesthetic/chemotherapeutic agent combination causes greater growth inhibition and apoptosis induction than either agent used alone | PI3K/Akt/mTOR pathway inhibition, increased caspase-3 activation |
| Gong 201865 | In vitro | Breast (MDA-MB-468, SkBr) | Ropivacaine | 0.1–1 mM | 5-FU | Enhanced effects of 5-FU on inhibiting cell growth, survival, and colony formation | Inhibition of mitochondrial respiration (inhibition of phosphorylation of Akt, mTOR, rS6, and EBP1) |
| Dan 201867 | In vitro | Gastric cancer (SNU1, AGS) | Bupivacaine | 10 μM–5 mM | 5-FU | Inhibitory chemotherapeutic effects augmented by bupivacaine | Inhibition of RhoA/ROCK/MLC |
Table 2.
Summary extracted data from preclinical studies examining the amino ester local anaesthetic procaine which meet systematic review selection criteria. 5-FU, 5-fluorouracil; DDP, diamminedichloroplatinum; DPR, cis-diamminechloro-[2-(diethylamino)ethyl 4-amino-benzoate, N4]-chlorideplatinum(II); PAX9, Paired box gene 9.
| 1st author and year | Study type | Cancer type (cell line) | Local anaesthetic(s) studied | Local anaesthetic concentration | Chemotherapy agent(s) studied | Interaction observed (e.g. no effect, synergistic effect, additive effect, etc) | Mechanism(s) studied (if any) |
|---|---|---|---|---|---|---|---|
| Bhol 202272 | Both | Oral squamous cell carcinoma (OSCC) (CAL33, FaDu) | Procaine | 0.1–1 mM | In vitro—cisplatin, 5-FU, doxorubicin, docetaxel; in vivo—cisplatin | Procaine enhanced the in vitro chemotherapeutic sensitivity of all drugs tested when used in combination; in vivo, combination procaine/cisplatin reduced tumour volume compared with single agent | Procaine suppresses DNA methyltransferase and increases the expression of PAX9, a transcription factor downregulated in OSCC |
| Esposito 199076 | Both | Leukaemia (P388 murine, CCRF-CEM, K-562) | Procaine | 1–100 mM in vitro, 20–100 mg kg−1in vivo | Cisplatin | No effect on cytotoxicity when procaine added to cisplatin in vitro, procaine increased tolerated dose of cisplatin in mice with reduction in nephrotoxicity | N/A |
| Esposito 199677 | In vivo | Leukaemia (P388 murine) | Procaine | 40 mg kg−1 | Cisplatin | In vivo section examined cisplatin vs cisplatin + procaine and cisplatin + procainamide; combined local anaesthetic agent/chemotherapeutic agent improved survival vs cisplatin alone | N/A |
| Viale 200178 | In vivo | Leukaemia (P388 murine) | Procaine | 40 mg kg−1 | Cisplatin | Procaine–cisplatin increased survival and cure rate compared with cisplatin treatment alone | N/A |
| Viale 199479 | In vitro | Leukaemia (P388 murine) | Procaine (as DPR with cisplatin) | 0–33.2 μM DPR | Cisplatin | Similar activity of DPR and cisplatin observed | N/A |
| Viale 199683 | Both | Leukaemia (P388, L1210—murine) | Procaine (as DPR with cisplatin) | 0.025–1.04 μM DPR in vitro; 0.5–10 mg kg−1 DPR in vivo | Cisplatin | In vitro: synergistic effects of combined DDP/DPR on cell survival compared to either agent alone. In vivo: DPR/DDP combo synergistically reduced tumour size and increased tumour free mice | N/A |
| Mariggio 200481 | In vitro | Multiple types including neuroblastoma, leukaemia, lung cancer | Procaine (as DPR with cisplatin) | Varied, ranges from 0.07 plus or minus 0.02 μM to 48.3 plus or minus 14.9 μM DPR | Cisplatin | Procaine–cisplatin complex (as DPR) causes greater inhibition of cell viability and enhances apoptosis in some cancer cell lines compared with cisplatin alone | DNA fragmentation, DNA inter-strand cross-linking, p53 upregulation by DPR |
| Viale 200380 | In vitro | Leukaemia (HL60, K562, Molt-4) | Procaine (as DPR with cisplatin) | 0.01–100 μM | Cisplatin, carboplatin | Procaine–cisplatin (as DPR) inhibits colony formation more than cisplatin or carboplatin alone | N/A |
| Viale 199882 | In vitro | Ovarian (M5076—murine, A2780) | Procaine (as DPR with cisplatin) | DPR 0.13–4.15 μM M5076, 0.065–1.04 μM A2780 | Cisplatin, 5-FU, doxorubicin, mitomycin-c, Taxol | Synergistic or additive effects observed for most DPR–other agent combinations in terms of cytotoxicity | N/A |
| Ali 201873 | In vitro | Breast cancer (MCF-7) | Procaine | 5–50 μM | Doxorubicin | Procaine enhanced the cytotoxic and apoptotic effect of doxorubicin | DNA binding by procaine |
| Sabit 201632 | In vitro | Colon (HCT116) | Procaine | 3–5 μM | Carboplatin, erlotinib, vorinostat, Na phenylbutyrate | Procaine and low-dose carboplatin effectively reduced DNA methylation, but higher doses of some drugs increased cell proliferation and methylation | DNA fragmentation |
| Godal 196974 | In vivo | Sarcoma | Procaine | 4 mg kg−1 day−1 | Nitrogen mustard (HN3) | Procaine co-treatment increased survival | Vasodilation induced by procaine |
| Chlebowski 198275 | In vitro | Melanoma (SHG) | Procaine | 0.64 mg ml−1 | Doxorubicin | Combination treatment caused greater cytotoxic effects than single use of either agent | N/A |
Fig 2.
Simplified schematic of selected intracellular signalling pathways in cancer and points at which experimental evidence suggests local anaesthetic agents may act. The basic elements of pathways involving PI3K/AKT/mTOR, MAPK, c-Met, p53, drug efflux mechanisms and the overall cell functions affected are shown. ABC, ATP-binding cassette transporters; AKT, protein kinase B; BAD, Bcl-xl/Bcl-2-associated death promoter; Bcl-2, B-cell lymphoma 2; c-Met, c-mesenchymal-epithelial transition factor; Casp9, cysteinyl aspartate specific proteinase 9; c-Src, Proto-oncogene tyrosine-protein kinase Src; CNK1, connector enhancer of KSR; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-related kinase 1/2; GPCRs, G-protein-coupled receptors; JNK, jun amino-terminal kinase; LA, local anaesthetic; MAPKKKs, mitogen-activated protein kinase kinase kinases; MDM2, murine double minute 2; MEK, mitogen-activated protein kinase kinase; MKK, mitogen-activated protein kinase kinase; MST1, mammalian sterile 20 kinase 1; mTOR, mammalian target of rapamycin; p38-MAPKs, p38 group of mitogen-activated protein kinases; p53, tumor protein 53; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; Rac1, Ras-related C3 botulinum toxin substrate 1; Raf, rapidly accelerated fibrosarcoma; RAS, rat sarcoma; RASSF1A, Ras association domain-containing protein 1; RhoA, Ras homolog family member A; ROCK, Rho-associated, coiled-coil containing protein kinase 1; RTKs, receptor tyrosine kinases; TSC1/2, tuberous sclerosis complex 1/2. Figure created using Biorender.com.
Discussion
Local anaesthetic toxicity and experimental concentrations
When evaluating experimental use of local anaesthetics, care must be taken to consider the concentrations being examined. Local anaesthetic systemic toxicity is an obvious concern with any local anaesthetic and clinicians must ensure that doses do not exceed known safe maximum limits e.g. bupivacaine 2 mg kg−1 given as a peripheral nerve block should maintain peak plasma concentration below the toxic concentration for bupivacaine of 1–2 μg ml−1.33 Although systemic absorption of submaximal doses of local anaesthetic from the epidural space, peripheral nerve block or tissue infiltration will result in low circulating plasma concentrations below the toxic threshold, the local tissue concentrations of local anaesthetic at the site of injection are much higher and may be temporarily as high as the molar concentration of the undiluted injectate (e.g. bupivacaine 0.5% has a molarity of 15.4 mM, the molarity of lidocaine 2% is 85.3 mM). Many of the in vitro studies included in this review used molar local anaesthetic concentrations that would be toxic if they reflected plasma concentrations in a human, which would limit the applicability of their results when considering i.v. lidocaine or systemic absorption from neuraxial or regional techniques. However, such concentrations may be safely achievable locally in tissue if local anaesthetic is administered as peritumoral infiltration (as performed with lidocaine in the recently reported breast cancer trial by Badwe and colleagues12) or by direct application to a surface (e.g. peritoneum). The 13 in vivo local anaesthetic/chemotherapeutic agent experiments included in the review are particularly useful then, because not only are animal models more translatable to human physiology, but in vivo studies are also largely restricted to using non-toxic doses mirroring what could safely be used in clinical practice.
Amino amide local anaesthetics—lidocaine
Lidocaine is the prototype amino amide local anaesthetic and is in widespread use worldwide, appearing on the World Health Organisation's List of Essential Medicines (alongside bupivacaine).34 With a short half-life and relatively low toxicity, lidocaine is the only amino amide local anaesthetic which can safely be administered as an i.v. infusion and toxicity is not typically encountered below plasma concentrations of 5 μg ml−1 (approximately 22μM).14 I.V. lidocaine (typically given as a bolus of 1–2 mg kg−1 followed by an infusion of 1–2 mg kg−1 h−1) has been advocated as a perioperative analgesic.35 Only one large RCT has examined perioperative i.v. lidocaine and cancer outcomes, with no effect on overall survival or disease-free survival detected in patients undergoing pancreatectomy for pancreatic cancer, despite a significant survival benefit being detected in a prior large retrospective study.36,37 However, pancreatic cancer is notoriously aggressive, with an almost uniformly dismal prognosis, so how generalisable this result is to other cancers is unclear—other prospective trials examining perioperative i.v. lidocaine are underway or planned.38,39 Otherwise, lidocaine may be administered via the epidural route, used in regional nerve blocks or given as local infiltration (where its use has previously been associated with improved cancer outcomes after surgery for early breast cancer).12
Lidocaine and 5-fluorouracil
Four in vitro studies examined lidocaine and 5-fluorouracil used in combination and all demonstrated beneficial antineoplastic effects associated with combination treatment. Zhang and colleagues40 studied combination effects on choriocarcinoma cells and found that lidocaine in low concentrations on its own had no effect on cell viability or apoptosis, but at the same concentrations it potentiated 5-fluorouracil cytotoxicity. Mechanistically, this was associated with reduced expression of the ABC efflux protein and inhibition of the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, a vital cellular signalling pathway that contributes to cell cycle regulation, and increased expression of which is associated with cancer progression and chemotherapy resistance. Inhibition of the PI3K/AKT/mTOR pathway was also found by another group studying lidocaine and 5-fluorouracil effects on melanoma cells. This effect may be mediated by increased expression of the microRNA (miRNA) miR-493, inactivating PI3K/AKT and transforming growth factor- ß (TGF-ß) leading to downregulated expression of SOX4, a transcription factor involved in cell differentiation and proliferation which is often aberrantly expressed in malignancies.41 One study examining four amino amide local anaesthetics (lidocaine, ropivacaine, mepivacaine, bupivacaine) found that all substantially augmented the inhibitory effects of 5-fluorouracil in oesophageal cancer cells; this effect may be mediated through inhibition of mitochondrial respiration and Ras-related C3 botulinum toxin substrate 1 (Rac1), a member of the Ras family of GTPases which act as molecular switches to control cytoskeletal rearrangement and dysfunction of which is implicated in oncogenesis.42 Lidocaine also enhanced the in vitro effect of 5-fluorouracil in a study examining gastric cancer cells, an effect that may be associated with lidocaine-related inhibition of the c-mesenchymal-epithelial transition factor (c-Met)/Proto-oncogene tyrosine-protein kinase Src (c-Src) pathways leading to decreased B-cell lymphoma 2 (Bcl-2) levels and increased levels of Bax and cleaved caspase-3, resulting in apoptosis.43
Lidocaine and cisplatin
Ten studies examined the interaction between lidocaine and cisplatin on cancer biology: seven were in vitro studies, one solely in vivo, and two combined both approaches. All 10 studies reported some form of beneficial antineoplastic effect when combination therapy was used compared with either drug used alone. Freeman and colleagues44 randomly allocated mice with breast cancer to receive either cisplatin, lidocaine, or both, or neither (control) while undergoing resection of the primary tumour under sevoflurane anaesthesia. The pulmonary metastatic burden was measured 14 days after operation; cisplatin reduced lung metastases compared with control, and the addition of lidocaine to cisplatin enhanced this effect. Gao and colleagues45 developed a nanogel to deliver cisplatin directly at the site of breast cancer and found that the introduction of lidocaine to the gel not only increased cisplatin-induced apoptosis both in vitro and in a mouse model of breast cancer, but also reduced metastasis in the animal model. Additionally, another group developed a murine model of hepatocellular carcinoma and examined lidocaine and cisplatin treatment in vitro and in vivo. Again, combination lidocaine–cisplatin treatment was significantly more cytotoxic in vitro and suppressed tumour growth more effectively in vivo than either agent used alone.46 These findings were associated with an increased ratio of Bax/Bcl-2 and cleaved caspase-3 activation and activation of the extracellular signal-related kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) signalling pathways, suggesting that lidocaine induces apoptosis through these mechanisms.
The seven in vitro studies involved a variety of cancer types. The addition of lidocaine potentiated the effects of cisplatin in each of these experiments. A range of putative cellular mechanisms which may explain this interaction were examined. Lidocaine-induced alteration of miRNA expression was examined as a potential mechanism. The miRNAs are small, non-coding RNAs which influence gene expression by binding to target messenger RNA (mRNA) and inhibiting translation. Dysregulated miRNA expression by cancer cells can instigate phenotypical changes in cells within the tumour microenvironment, promoting tumorigenesis and chemotherapy resistance by targeting oncogenes. Three studies found that individual miRNA expression was altered by lidocaine leading to an effect on oncogenic cellular signalling pathways influencing cancer progression—miR30c and sirtuin 1 (SIRT1) in cutaneous squamous cell carcinoma, miR-10b and AKT/mTOR and β-catenin in gastric cancer, and miR-21 and phosphatase and tensin homolog (PTEN)/PI3K/AKT and programmed cell death 4 (PDCD4)/jun amino-terminal kinase (JNK) in lung cancer.47, 48, 49 Two other studies identified upregulated expression of the tumour suppressor gene Ras association domain family 1A (RASSF1A) as a potential mechanism for the observed effect of lidocaine potentiating the cytotoxicity of cisplatin on breast cancer and hepatocellular carcinoma cells in vitro.50,51
Lidocaine and other chemotherapeutic agents
Fourteen studies examined the effects of lidocaine and other (non-5-fluorouracil, non-cisplatin) chemotherapeutic agents (one solely in vivo, 10 in vitro, and three with both in vivo and in vitro experiments). These involved a wide range of chemotherapeutic agents including cytotoxic antibiotics (mitomycin-C,28 bleomycin,52,53 peplomycin54), anthracyclines (pirarubicin55), other alkylating agents (dacarbazine56), antimetabolites (decitabine57), vinca alkaloids (vincristine29,58), taxanes (paclitaxel,59 docetaxel60,61), cyclin-dependent kinase inhibitors (palbociclib62), and Src/BCR-ABL inhibitors (bosutinib30,31). A wide range of cancers were studied but breast cancer was the most common (6/14). Nine studies found that lidocaine potentiated the antineoplastic effects of the chemotherapeutic agent when tested in vitro. Of note, four studies did not detect any alteration of chemotherapeutic agent effects by the tested local anaesthetic during combination treatment. Three studies examined P-glycoprotein expression, activity, or both as a potential mechanism by which lidocaine may interact with chemotherapeutic agents.28,29,58 P-glycoprotein facilitates drug efflux across the cell membrane and out of the cancer cell, thus providing a means by which drug resistance can develop and which provides a site where local anaesthetics can act to reduce P-glycoprotein activity and enhance chemotherapeutic agent efficacy.63
One in vivo study examining whether lidocaine may act via inhibition of the Src oncogene studied the combination of i.v. lidocaine and bosutinib (a BCR-ABL, Src tyrosine kinase inhibitor) on metastatic outcomes after excision of primary tumours in a mouse model of breast cancer.31 While bosutinib alone had no effect on postoperative pulmonary metastases, lidocaine on its own reduced metastases, and unusually, the combination of bosutinib/lidocaine actually reversed the beneficial antimetastatic effects seen with lidocaine alone. A number of explanations were postulated for this phenomenon, including competitive binding at receptor sites. In contrast to the results of this study, three other experiments found that lidocaine significantly enhanced the effects of the chemotherapeutic agent being studied when given as combined treatment in murine cancer models: docetaxel in melanoma, mitomycin-C in bladder cancer, and palbociclib in breast cancer.55,61,62 The beneficial effects attributed to combination local anaesthetic/chemotherapeutic agent treatment in these animal models included reduced tumour growth61,62 and increased survival.55
Other amino amide local anaesthetics
Eleven of the included studies examined non-lidocaine amide local anaesthetics, with individual studies often examining multiple local anaesthetics. The most frequently examined were ropivacaine (7/11) and bupivacaine (6/11), two agents used in everyday clinical practice. All 11 were in vitro experiments. Again, a wide range of cancers and chemotherapeutic agents were studied, the most frequent being breast cancer (4/11) and 5-fluorouracil (3/11), respectively. The 11 studies reported almost uniformly beneficial anticancer in vitro effects when local anaesthetic was added to chemotherapeutic agent with the inhibitory effects of the chemotherapeutic agent being augmented by those of the local anaesthetic during combination use. The influence of local anaesthetic/chemotherapeutic agent on the Ras and Ras homolog family member A (RhoA)/Rac1 signalling pathways were assessed by three studies. Ras is an oncogene coding for a GTPase which stimulates cellular proliferation and survival through, amongst others, the PI3K/AKT/mTOR pathway, and is frequently found in mutated form in cancer and was examined by several included studies.64, 65, 66 RhoA and Rac1 are also GTPases and control actin fibre dynamics, cellular adhesion, and cell migration. One study found that in melanoma cells, lidocaine and ropivacaine, but not bupivacaine, decreased Ras, RhoA, and Rac1 expression, and augmented the antimigratory, antiproliferative and pro-apoptotic effects of the chemotherapeutic agents vemurafenib and dacarbazine when used in combination.56 Despite the lack of effect of bupivacaine on these GTPases observed in this melanoma study, another study found that bupivacaine did have inhibitory effects on RhoA and Rac1 in gastric cancer cells; this finding was associated with inhibition of migration and was additive when combined with 5-fluorouracil.67 A further study examining lidocaine, bupivacaine, ropivacaine, and mepivacaine found that all tested local anaesthetics significantly enhanced the antiproliferative and pro-apoptotic effects of both 5-fluorouracil and paclitaxel in oesophageal cancer cells, and although RhoA was unaffected, Rac1 activity was significantly depressed by ropivacaine and bupivacaine.42 Bupivacaine appeared to be the only local anaesthetic tested that inhibited cell migration at clinically relevant concentrations (10 μM).
Amino ester local anaesthetic agents
Seventeen studies examined the effects of combined treatment with procaine and a chemotherapeutic agent (11 were in vitro studies, three in vivo, and three combined both in vitro and in vivo elements). Procaine (often known by its trade name Novocain) is one of the oldest synthetic local anaesthetics, and although its systemic toxicity is relatively low, it has a high pKa and a low lipid solubility conferring a slow onset time and short duration of action limiting its clinical utility. Historically, i.v. procaine has been used as a sole agent to provide general anaesthesia, as when given in sufficient doses (1.25–2.5 g over 10 min) it will induce reduced consciousness without respiratory depression or cardiovascular collapse. Unsurprisingly, the overall patient experience was frequently unpleasant.68 Although still used for dental anaesthesia, the clinical use of procaine has largely been supplanted by newer amide local anaesthetics which are less allergenic and have more favourable onset and offset profiles. However, a closely related derivative, chloroprocaine, has found a useful niche as a short-acting agent for spinal anaesthesia in day case surgery.
Procaine has activity outside of voltage-gated sodium channel blockade with antagonistic effects at acetylcholine receptors, N-methyl-d-aspartate (NMDA) receptors, the serotonin receptor-ion channel complex, amongst others.69 One potentially beneficial antineoplastic effect of procaine is its recognised ability to demethylate DNA.70 DNA methylation is an epigenetic modification involved in regulation of gene expression and when occurring abnormally may result in dysregulated gene expression leading to malignant transformation. DNA hypermethylation resulting in the silencing of tumour suppressor genes is a hallmark of many cancers, and DNA demethylating agents such as 5-aza-2′-deoxycytidine (decitabine) can be effective chemotherapeutic drugs.71 Recent evidence has shown that in oral squamous cell carcinoma, procaine inhibits DNA methyltransferase, thereby preventing DNA methylation and promoting the expression of the tumour suppressor gene PAX9, eventually leading to inhibition of cell growth and stimulation of apoptosis in vitro and in vivo.72 The effect of procaine on DNA methylation was also identified when used in conjunction with carboplatin in colon cancer cells, leading to inhibition of cancer cell proliferation, although interestingly this effect was abolished at higher doses.32 Procaine may also affect DNA by binding directly to certain regions within the double helix, a finding associated with enhanced doxorubicin cytotoxicity when used in combination in breast cancer cells.73 The ability of procaine to act as a DNA demethylating agent or otherwise interact with DNA opens a potential door to its use in cancer therapeutics, particularly if additive or synergistic effects with a chemotherapeutic agent can be achieved clinically.
Historically the beneficial antineoplastic effects of procaine were recognised from as early as the 1960s and developed further over the subsequent decades. In 1969 it was observed that procaine enhanced survival in a rat model of sarcoma when combined with nitrogen mustard (an early chemotherapeutic agent) compared with nitrogen mustard given alone, whereas procaine alone had no beneficial effect on survival. This effect was attributed to vasodilatation induced by procaine improving perfusion of the sarcoma by the chemotherapeutic agent.74 In the early 1980s, researchers found that procaine enhanced the cytotoxicity of bleomycin (and its derivative peplomycin) to breast cancer cells and doxorubicin to melanoma cells in vitro.53,54,75 Beginning in the early 1990s, Esposito and colleagues76,77 and Viale and colleagues78 began experiments examining procaine used along with cisplatin in a murine model of leukaemia, finding that procaine increased the tolerated dose of cisplatin, reduced nephrotoxicity, and improved survival. The same group subsequently developed a novel single molecule combination of procaine and cisplatin known as DPR.79,80 This was tested across a range of cancer types in both in vitro and in vivo settings and found to have greater antineoplastic effects compared with cisplatin use alone.81, 82, 83 Despite these promising findings, DPR has not found its way into mainstream clinical use as a chemotherapy agent. Esters other than procaine (e.g. benzocaine, butacaine, tetracaine) have rarely been studied in combination with chemotherapeutic agents, but have been associated with beneficial additive effects in the few in vitro studies that have been performed.54,84
Limitations
As with any review, the accuracy of the conclusions we can infer from the evidence is limited by the number, nature, and quality of the included studies themselves. Laboratory studies, unlike clinical trials, lack a recognised framework with which to systematically assess bias, such as the Cochrane Risk of Bias 2 tool (RoB2). Similarly, there are no consensus-based tools with which to examine the quality of the evidence provided by in vitro and in vivo experiments in a systematic manner as there are for clinical studies (i.e. the GRADE system, Grading of Recommendations, Assessment, Development and Evaluation). The studies included in this review are heterogenous in terms of the local anaesthetics, chemotherapeutic agents, drug concentrations, cancer types, animal models, and outcomes studied. This renders formal meta-analysis inappropriate, and therefore interpretation and synthesis of the results requires a less structured and defined narrative approach, providing a more qualitative rather than quantitative overview of the published evidence. Publication bias favouring studies with positive results may be present also, and it may be that other laboratory studies with negative results were performed but were not published (or were rejected for publication).
Conclusions
Despite a relatively small evidence base of 43 published preclinical studies, a large majority (almost 90%) of these demonstrated beneficial interactions between local anaesthetics and chemotherapeutic agents in terms of effects on cancer biology across a broad spectrum of cancers. Reassuringly, many of the beneficial in vitro effects reported were also detected in animal models, with no evidence of systemic toxicity reported when using conventional doses. Positive results with a good safety profile in animal models present a promising signal that similar results may potentially be seen with translation to human clinical studies. However, no such study has been performed to date. Although the local anaesthetic concentrations used in many of the in vitro experiments often exceeded safe plasma concentrations and therefore would not translate to use of i.v. local anaesthetic, these concentrations could be achieved clinically through either local infiltration, topical or intra-cavity application. Perhaps the most obvious cohort in whom interactions between local anaesthetic and chemotherapeutic agent therapy could be studied are those patients undergoing HIPEC where the chemotherapy given into the peritoneal space could possibly interact with, for example, lidocaine given i.v., bupivacaine given via the epidural space and absorbed systemically, or ropivacaine given directly into the peritoneal cavity. As the use of intraoperative chemotherapy becomes more common and broadens in scope, the opportunities for combined local anaesthetic/chemotherapeutic agent use are likely to increase. Whether such combined use may benefit patients in terms of improved clinical cancer outcomes will remain unknown until the completion of suitably powered randomised clinical studies.
Authors’ contributions
Writing, review, and approval of the final version of the manuscript: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2024.100284.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehlen P., Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 4.Raskov H., Orhan A., Salanti A., Gögenur I. Premetastatic niches, exosomes and circulating tumor cells: early mechanisms of tumor dissemination and the relation to surgery. Int J Cancer. 2020;146:3244–3255. doi: 10.1002/ijc.32820. [DOI] [PubMed] [Google Scholar]
- 5.Alieva M., van Rheenen J., Broekman M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin Exp Metastasis. 2018;35:319–331. doi: 10.1007/s10585-018-9896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 7.Duff S., Connolly C., Buggy D.J. Adrenergic, inflammatory, and immune function in the setting of oncological surgery: their effects on cancer progression and the role of the anesthetic technique in their modulation. Int Anesthesiol Clin. 2016;54:48–57. doi: 10.1097/AIA.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 8.Yoo Y.C., Shim J.K., Song Y., Yang S.Y., Kwak Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86:414–422. doi: 10.1038/ki.2013.532. [DOI] [PubMed] [Google Scholar]
- 9.Wigmore T.J., Mohammed K., Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 10.Biki B., Mascha E., Moriarty D.C., Fitzpatrick J.M., Sessler D.I., Buggy D.J. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 11.Rahmani L.S., Abdelaatti A., Wall T.P., Buggy D.J. Anaesthesia and cancer recurrence: the influence of perioperative anaesthetic technique on cancer recurrence after surgery. Curr Opin Anaesthesiol. 2023;36:361–368. doi: 10.1097/ACO.0000000000001261. [DOI] [PubMed] [Google Scholar]
- 12.Badwe R.A., Parmar V., Nair N., et al. Effect of peritumoral infiltration of local anesthetic before surgery on survival in early breast cancer. J Clin Oncol. 2023;41:3318–3328. doi: 10.1200/JCO.22.01966. [DOI] [PubMed] [Google Scholar]
- 13.Chamaraux-Tran T.N., Piegeler T. The amide local anesthetic lidocaine in cancer surgery-potential antimetastatic effects and preservation of immune cell function? A narrative review. Front Med (Lausanne) 2017;4:235. doi: 10.3389/fmed.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall T.P., Buggy D.J. Perioperative intravenous lidocaine and metastatic cancer recurrence - a narrative review. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.688896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson M.E., Uhl C.B., Spittler K.H., Wang H., Gores G.J. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology. 2004;101:1184–1194. doi: 10.1097/00000542-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Castro I., Carvalho P., Vale N., Monjardino T., Mourão J. Systemic anti-inflammatory effects of intravenous lidocaine in surgical patients: a systematic review and meta-analysis. J Clin Med. 2023;12:3772. doi: 10.3390/jcm12113772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugarbaker P.H. A narrative review of what can HIPEC do. Eur J Surg Oncol. 2023;49 doi: 10.1016/j.ejso.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Bushati M., Rovers K.P., Sommariva A., et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI) Eur J Surg Oncol. 2018;44:1942–1948. doi: 10.1016/j.ejso.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Glockzin G., Schlitt H.J., Piso P. Bidirectional intraperitoneal chemotherapy with oxaliplatin in the context of CRS and HIPEC in patients with peritoneal carcinomatosis. J Clin Oncol. 2011;29(4_suppl):568. [Google Scholar]
- 20.Bongiolatti S., Mazzoni F., Salimbene O., et al. Induction chemotherapy followed by pleurectomy decortication and hyperthermic intraoperative chemotherapy (HITHOC) for early-stage epitheliod malignant pleural mesothelioma-a prospective report. J Clin Med. 2021;10:5542. doi: 10.3390/jcm10235542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fielding L.P., Hittinger R., Grace R.H., Fry J.S. Randomised controlled trial of adjuvant chemotherapy by portal-vein perfusion after curative resection for colorectal adenocarcinoma. Lancet. 1992;340:502–506. doi: 10.1016/0140-6736(92)91708-g. [DOI] [PubMed] [Google Scholar]
- 22.Laffer U., Metzger U., Aeberhard P., et al. Adjuvant perioperative portal vein or peripheral intravenous chemotherapy for potentially curative colorectal cancer: long-term results of a randomized controlled trial. Int J Colorectal Dis. 2008;23:1233–1241. doi: 10.1007/s00384-008-0543-8. [DOI] [PubMed] [Google Scholar]
- 23.Hu X., Zheng Z., Han J., et al. Effect of intra-operative chemotherapy with 5-fluorouracil and leucovorin on the survival of patients with colorectal cancer after radical surgery: a retrospective cohort study. Chin Med J (Engl) 2023;136:830–839. doi: 10.1097/CM9.0000000000002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriarty M.A., Uhlman M.A., Bing M.T., et al. Evaluating the safety of intraoperative instillation of intravesical chemotherapy at the time of nephroureterectomy. BMC Urol. 2015;15:45. doi: 10.1186/s12894-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuwirth M.G., Alexander H.R., Karakousis G.C. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2015;7:18–28. doi: 10.3978/j.issn.2078-6891.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers B.D., Felder S., Veerapong J., et al. Repeat cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is not associated with prohibitive complications: results of a multiinstitutional retrospective study. Ann Surg Oncol. 2020;27:4883–4891. doi: 10.1245/s10434-020-08482-x. [DOI] [PubMed] [Google Scholar]
- 27.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorr R.T., Liddil J.D. Modulation of mitomycin C-induced multidrug resistance in vitro. Cancer Chemother Pharmacol. 1991;27:290–294. doi: 10.1007/BF00685114. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.Y., Kim K.S., Kim I.S., Yoon S. Histamine receptor antagonists, loratadine and azelastine, sensitize P-gp-overexpressing antimitotic drug-resistant KBV20C cells through different molecular mechanisms. Anticancer Res. 2019;39:3767–3775. doi: 10.21873/anticanres.13525. [DOI] [PubMed] [Google Scholar]
- 30.Wall T.P., Crowley P.D., Buggy D.J. The effect of lidocaine and bosutinib on 4T1 murine breast cancer cell behaviour in vitro. Anticancer Res. 2021;41:2835–2840. doi: 10.21873/anticanres.15064. [DOI] [PubMed] [Google Scholar]
- 31.Wall T.P., Crowley P.D., Sherwin A., Foley A.G., Buggy D.J. Effects of lidocaine and Src inhibition on metastasis in a murine model of breast cancer surgery. Cancers (Basel) 2019;11:1414. doi: 10.3390/cancers11101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabit H., Samy M.B., Said O.A., El-Zawahri M.M. Procaine induces epigenetic changes in HCT116 colon cancer cells. Genet Res Int. 2016;2016 doi: 10.1155/2016/8348450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longhorn R.K., Cross M. Bupivacaine toxicity. J Cardiothorac Vasc Anesth. 2000;14:759–760. doi: 10.1053/jcan.2000.18671. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organisation . 2023. Model list of essential Medicines.https://iris.who.int/bitstream/handle/10665/371090/WHO-MHP-HPS-EML-2023.02-eng.pdf?sequence=1 [Google Scholar]
- 35.Yang W., Yan S., Yu F., Jiang C. Appropriate duration of perioperative intravenous administration of lidocaine to provide satisfactory analgesia for adult patients undergoing colorectal surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2023;136:494–506. doi: 10.1213/ANE.0000000000006347. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Yang L., Zhu X., et al. Association between intraoperative intravenous lidocaine infusion and survival in patients undergoing pancreatectomy for pancreatic cancer: a retrospective study. Br J Anaesth. 2020;125:141–148. doi: 10.1016/j.bja.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H., Qu M., Guo K., et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br J Anaesth. 2022;129:244–253. doi: 10.1016/j.bja.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Dubowitz J.A., Cata J.P., De Silva A.P., et al. Volatile anaesthesia and peri-operative outcomes related to cancer: a feasibility and pilot study for a large randomised control trial. Anaesthesia. 2021;76:1198–1206. doi: 10.1111/anae.15354. [DOI] [PubMed] [Google Scholar]
- 39.West R., Soo C.P., Murphy J., Vizcaychipi M.P., Ma D. A protocol for a pilot study to assess the feasibility of a randomised clinical trial of perioperative intravenous lidocaine on colorectal cancer outcome after surgery (FLICOR trial) BJA Open. © 2023 The Author(s) 2023;6 doi: 10.1016/j.bjao.2023.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., Pang W., Liu H., Wang J. Lidocine potentiates the cytotoxicity of 5-fluorouracil to choriocarcinoma cells by downregulating ABC transport proteins expression. J Cell Biochem. 2019;120:16533–16542. doi: 10.1002/jcb.28913. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Xie J., Liu W., Zhang R., Huang S., Xing Y. Lidocaine sensitizes the cytotoxicity of 5-fluorouacil in melanoma cells via upregulation of microRNA-493. Pharmazie. 2017;72:663–669. doi: 10.1691/ph.2017.7616. [DOI] [PubMed] [Google Scholar]
- 42.Zhu G., Zhang L., Dan J., Zhu Q. Differential effects and mechanisms of local anesthetics on esophageal carcinoma cell migration, growth, survival and chemosensitivity. BMC Anesthesiol. 2020;20:126. doi: 10.1186/s12871-020-01039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng W., Xing Z.T., Tan M.Y., Wu Y.W., Zhang C.Y. Lidocaine suppresses the malignant behavior of gastric cancer cells via the c-Met/c-Src pathway. Exp Ther Med. 2021;21:1–7. doi: 10.3892/etm.2021.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman J., Crowley P.D., Foley A.G., et al. Effect of perioperative lidocaine and cisplatin on metastasis in a murine model of breast cancer surgery. Anticancer Res. 2018;38:5599–5606. doi: 10.21873/anticanres.12894. [DOI] [PubMed] [Google Scholar]
- 45.Gao X., Yang H., Wu M., et al. Targeting delivery of lidocaine and cisplatin by nanogel enhances chemotherapy and alleviates metastasis. ACS Appl Mater Interfaces. 2018;10:25228–25240. doi: 10.1021/acsami.8b09376. [DOI] [PubMed] [Google Scholar]
- 46.Xing W., Chen D.T., Pan J.H., et al. Lidocaine induces apoptosis and suppresses tumor growth in human hepatocellular carcinoma cells in vitro and in a xenograft model in vivo. Anesthesiology. 2017;126:868–881. doi: 10.1097/ALN.0000000000001528. [DOI] [PubMed] [Google Scholar]
- 47.Liu T., Jiang F., Yu L.-Y., Wu Y.-Y. Lidocaine represses proliferation and cisplatin resistance in cutaneous squamous cell carcinoma via miR-30c/SIRT1 regulation. Bioengineered. 2022;13:6359–6370. doi: 10.1080/21655979.2022.2031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., Gu G., Li X., Zhang C. Lidocaine alleviates cisplatin resistance and inhibits migration of MGC-803/DDP cells through decreasing miR-10b. Cell Cycle. 2020;19:2530–2537. doi: 10.1080/15384101.2020.1809914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Q., Zhang Z., Xu H., Ma C. Lidocaine alleviates cytotoxicity-resistance in lung cancer A549/DDP cells via down-regulation of miR-21. Mol Cell Biochem. 2019;456:63–72. doi: 10.1007/s11010-018-3490-x. [DOI] [PubMed] [Google Scholar]
- 50.Chen D., Yan Y., Xie J., et al. Amide-type local anesthetics may suppress tumor cell proliferation and sensitize human hepatocellular carcinoma cells to cisplatin via upregulation of RASSF1A expression and demethylation. J Cancer. 2020;11:7312. doi: 10.7150/jca.46630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K., Yang J., Han X. Lidocaine sensitizes the cytotoxicity of cisplatin in breast cancer cells via up-regulation of RARβ2 and RASSF1A demethylation. Int J Mol Sci. 2014;15:23519–23536. doi: 10.3390/ijms151223519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazo J.S., Braun I.D., Meandzija B., Kennedy K.A., Pham E.T., Smaldone L.F. Lidocaine potentiation of bleomycin A2 cytotoxicity and DNA strand breakage in L1210 and human A-253 cells. Cancer Res. 1985;45:2103–2109. [PubMed] [Google Scholar]
- 53.Mizuno S., Ishida A. Selective enhancement of bleomycin cytotoxicity by local anesthetics. Biochem Biophys Res Commun. 1982;105:425–431. doi: 10.1016/0006-291x(82)91451-6. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno S., Ishida A. Selective enhancement of the cytotoxicity of the bleomycin derivative, peplomycin, by local anesthetics alone and combined with hyperthermia. Cancer Res. 1982;42:4726–4729. [PubMed] [Google Scholar]
- 55.Yang X., Zhao L., Li M., et al. Lidocaine enhances the effects of chemotherapeutic drugs against bladder cancer. Sci Rep. 2018;8:1–7. doi: 10.1038/s41598-017-19026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Q., Peng X., Zhang Y. Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of Ras and RhoA signaling independent of sodium channel blockade. BMC Anesthesiol. 2020;20:43. doi: 10.1186/s12871-020-00957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lirk P., Hollmann M., Fleischer M., Weber N., Fiegl H. Lidocaine and ropivacaine, but not bupivacaine, demethylate deoxyribonucleic acid in breast cancer cells in vitro. Br J Anaesth. 2014;113(suppl_1):i32–i38. doi: 10.1093/bja/aeu201. [DOI] [PubMed] [Google Scholar]
- 58.Polekova L., Barancik M., Mrazova T., et al. Adaptation of mouse leukemia cells L1210 to vincristine. Evidence for expression of P-glycoprotein. Neoplasma. 1992;39:73–77. [PubMed] [Google Scholar]
- 59.Brummelhuis I.S., Fiascone S.J., Hasselblatt K.T., Frendl G., Elias K.M. Voltage-gated sodium channels as potential biomarkers and therapeutic targets for epithelial ovarian cancer. Cancers (Basel) 2021;13:5437. doi: 10.3390/cancers13215437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meireles I., Marques I., Abrantes A., et al. Effect of pain management drugs in metastatic prostate cancer: in vitro studies. ESMO Open. 2018;3:A205. [Google Scholar]
- 61.de Moura L.D., Ribeiro L.N., de Carvalho F.V., et al. Docetaxel and lidocaine co-loaded (NLC-in-hydrogel) hybrid system designed for the treatment of melanoma. Pharmaceutics. 2021;13:1552. doi: 10.3390/pharmaceutics13101552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han B.S., Jung K.H., Lee J.E., et al. Lidocaine enhances the efficacy of palbociclib in triple-negative breast cancer. Am J Cancer Res. 2022;12:3083–3098. [PMC free article] [PubMed] [Google Scholar]
- 63.Tian Y., Lei Y., Wang Y., Lai J., Wang J., Xia F. Mechanism of multidrug resistance to chemotherapy mediated by P-glycoprotein. Int J Oncol. 2023;63:119. doi: 10.3892/ijo.2023.5567. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soriano O., Alcón-Pérez M., Vicente-Manzanares M., Castellano E. The crossroads between RAS and RHO signaling pathways in cellular transformation, motility and contraction. Genes (Basel) 2021;12:819. doi: 10.3390/genes12060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong X., Dan J., Li F., Wang L. Suppression of mitochondrial respiration with local anesthetic ropivacaine targets breast cancer cells. J Thorac Dis. 2018;10:2804–2812. doi: 10.21037/jtd.2018.05.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Q., Peng X., Yu H. Local anesthetic drug inhibits growth and survival in chronic myeloid leukemia through suppressing PI3K/Akt/mTOR. Am J Med Sci. 2018;355:266–273. doi: 10.1016/j.amjms.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Dan J., Gong X., Li D., Zhu G., Wang L., Li F. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomed Pharmacother. 2018;103:823–828. doi: 10.1016/j.biopha.2018.04.106. [DOI] [PubMed] [Google Scholar]
- 68.Wright G.V. Intravenous procaine hydrochloride. Anaesthesia. Oct 1950;5(4):201–207. doi: 10.1111/j.1365-2044.1950.tb12684.x. [DOI] [PubMed] [Google Scholar]
- 69.Benson B.E., Carson R.E., Kiesewetter D.O., et al. A potential cholinergic mechanism of procaine's limbic activation. Neuropsychopharmacology. 2004;29:1239–1250. doi: 10.1038/sj.npp.1300404. [DOI] [PubMed] [Google Scholar]
- 70.Villar-Garea A., Fraga M.F., Espada J., Esteller M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res. 2003;63:4984–4989. [PubMed] [Google Scholar]
- 71.Gradinaru D., Ungurianu A., Margina D., Moreno-Villanueva M., Bürkle A. Procaine–the controversial geroprotector candidate: new insights regarding its molecular and cellular effects. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/3617042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhol C.S., Mishra S.R., Patil S., et al. PAX9 reactivation by inhibiting DNA methyltransferase triggers antitumor effect in oral squamous cell carcinoma. Biochim Biophys Acta Mol Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2022.166428. [DOI] [PubMed] [Google Scholar]
- 73.Ali M.S., Farah M.A., Al-Lohedan H.A., Al-Anazi K.M. Comprehensive exploration of the anticancer activities of procaine and its binding with calf thymus DNA: a multi spectroscopic and molecular modelling study. RSC Adv. 2018;8:9083–9093. doi: 10.1039/c7ra13647a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Godál A., Medzihradský J., Knotek J., Ujházy V. Attempt to increase the effect of regional chemotherapy in experimental tumours by procaine. Neoplasma. 1969;16:389–392. [PubMed] [Google Scholar]
- 75.Chlebowski R.T., Block J.B., Cundiff D., Dietrich M.F. Doxorubicin cytotoxicity enhanced by local anesthetics in a human. Cancer Treat Rep. 1982;66:121–125. [PubMed] [Google Scholar]
- 76.Esposito M., Fulco R.A., Collecchi P., et al. Improved therapeutic index of cisplatin by procaine hydrochloride. J Natl Cancer Inst. 1990;82:677–684. doi: 10.1093/jnci/82.8.677. [DOI] [PubMed] [Google Scholar]
- 77.Esposito M., Viale M., Vannozzi M.O., et al. Effect of the antiarrhythmic drug procainamide on the toxicity and antitumor activity of cis-diamminedichloroplatinum(II) Toxicol Appl Pharmacol. 1996;140:370–377. doi: 10.1006/taap.1996.0233. [DOI] [PubMed] [Google Scholar]
- 78.Viale M., Vánnozzi M.O., Mandys V., Esposito M. Time-dependent influence of procaine hydrochloride on cisplatin antitumor activity in P388 tumor bearing mice. Anticancer Res. 2001;21:485–487. [PubMed] [Google Scholar]
- 79.Viale M., Melioli G., Pasquetti W., Meta M., Cafaggi S., Esposito N. Modifications of cell-cycle induced by dpr, a new platinum-triamine complex containing procaine. Int J Oncol. 1994;4:1047–1051. doi: 10.3892/ijo.4.5.1047. [DOI] [PubMed] [Google Scholar]
- 80.Viale M., Minetti S., Ottone M., Lerza R., Parodi B., Pannacciulli I. Preclinical in vitro evaluation of hematotoxicity of the cisplatin-procaine complex DPR. Anticancer Drugs. 2003;14:163–166. doi: 10.1097/00001813-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Mariggiò M.A., Cafaggi S., Ottone M., et al. Inhibition of cell growth, induction of apoptosis and mechanism of action of the novel platinum compound cis-diaminechloro-[2-(diethylamino) ethyl 4-amino-benzoate, N(4)]-chloride platinum (II) monohydrochloride monohydrate. Invest New Drugs. 2004;22:3–16. doi: 10.1023/b:drug.0000006170.38419.c9. [DOI] [PubMed] [Google Scholar]
- 82.Viale M., Pastrone I., Pellecchia C., Vannozzi M.O., Cafaggi S., Esposito M. Combination of cisplatin-procaine complex DPR with anticancer drugs increases cytotoxicity against ovarian cancer cell lines. Anticancer Drugs. 1998;9:457–463. doi: 10.1097/00001813-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 83.Viale M., Vannozzi M.O., Merlo F., Cafaggi S., Parodi B., Esposito M. Cisplatin combined with the new cisplatin-procaine complex DPR: in vitro and in vivo studies. Eur J Cancer. 1996;32A:2327–2333. doi: 10.1016/s0959-8049(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 84.Mizuno S., Ishida A., Uehara Y., Nanjo T. Modulation of the cytotoxicity of the antitumor antibiotic peplomycin by membrane-interacting drugs and by increased levels of calcium ions. Gan. 1983;74:767–776. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.