Abstract
Background and Aims
The Glu504Lys polymorphism in the aldehyde dehydrogenase 2 (ALDH2) gene is closely associated with myocardial ischaemia/reperfusion injury (I/RI). The effects of ALDH2 on neutrophil extracellular trap (NET) formation (i.e. NETosis) during I/RI remain unknown. This study aimed to investigate the role of ALDH2 in NETosis in the pathogenesis of myocardial I/RI.
Methods
The mouse model of myocardial I/RI was constructed on wild-type, ALDH2 knockout, peptidylarginine deiminase 4 (Pad4) knockout, and ALDH2/PAD4 double knockout mice. Overall, 308 ST-elevation myocardial infarction patients after primary percutaneous coronary intervention were enrolled in the study.
Results
Enhanced NETosis was observed in human neutrophils carrying the ALDH2 genetic mutation and ischaemic myocardium of ALDH2 knockout mice compared with controls. PAD4 knockout or treatment with NETosis-targeting drugs (GSK484, DNase1) substantially attenuated the extent of myocardial damage, particularly in ALDH2 knockout. Mechanistically, ALDH2 deficiency increased damage-associated molecular pattern release and susceptibility to NET-induced damage during myocardial I/RI. ALDH2 deficiency induced NOX2-dependent NETosis via upregulating the endoplasmic reticulum stress/microsomal glutathione S-transferase 2/leukotriene C4 (LTC4) pathway. The Food and Drug Administration-approved LTC4 receptor antagonist pranlukast ameliorated I/RI by inhibiting NETosis in both wild-type and ALDH2 knockout mice. Serum myeloperoxidase–DNA complex and LTC4 levels exhibited the predictive effect on adverse left ventricular remodelling at 6 months after primary percutaneous coronary intervention in ST-elevation myocardial infarction patients.
Conclusions
ALDH2 deficiency exacerbates myocardial I/RI by promoting NETosis via the endoplasmic reticulum stress/microsomal glutathione S-transferase 2/LTC4/NOX2 pathway. This study hints at the role of NETosis in the pathogenesis of myocardial I/RI, and pranlukast might be a potential therapeutic option for attenuating I/RI, particularly in individuals with the ALDH2 mutation.
Keywords: Aldehyde dehydrogenase 2, Myocardial ischaemia/reperfusion injury, NETosis, Microsomal glutathione S-transferase 2, Leukotriene C4 receptor antagonist
Structured Graphical Abstract
Structured Graphical Abstract.
The ALDH2 deletion increases myocardial DAMP release during myocardial I/RI. Undergoing the stimulation of DAMPs, ALDH2 deletion induces NOX2-dependent NET formation by upregulating the ER stress/Mgst2/LTC4 pathway, resulting in aggravated myocardial I/RI. ALDH2, aldehyde dehydrogenase 2; DAMP, damage-associated molecular pattern; I/RI, ischaemia/reperfusion injury; NOX2, NADPH oxidase 2; NET, neutrophil extracellular trap; ER, endoplasmic reticulum; Mgst2, microsomal glutathione S-transferase 2; LTC4, leukotriene C4; ROS, reactive oxygen species.
See the editorial comment for this article ‘Repurposing of an antiasthmatic drug may reduce NETosis and myocardial ischaemia/reperfusion injury’, by M. Amponsah-Offeh, https://doi.org/10.1093/eurheartj/ehae201.
Translational Perspective.
The Glu504Lys (rs671) polymorphism causes significantly reduced ALDH2 activity. It exists in ∼8% of the world population. This study shows high serum MPO–DNA complex and LTC4 levels are associated with ALDH2 mutation and predictive indicators of adverse left ventricular remodelling in STEMI patients after PCI. ALDH2 knockout mice exhibit increased neutrophil recruitment and NETosis, and the extent of myocardial damage during myocardial I/RI is substantially attenuated by NETosis abolish or LTC4 receptor antagonist pranlukast. These findings hint at the therapeutic value of targeting NETosis as a novel clinical option for myocardial I/RI, particularly in individuals with the ALDH2 mutation.
Introduction
Timely reperfusion by percutaneous coronary intervention (PCI) is one of the most effective treatment options to rescue the moribund myocardium after acute myocardial infarction (AMI).1 Despite early reperfusion, ∼7% of patients die, and 22% of survivors might develop chronic heart failure 1 year after AMI.2 Myocardial ischaemia/reperfusion injury (I/RI) is a widely recognized phenome that can occur after reperfusion3 and contribute to up to 50% of the final infarct size.4
Previous studies have shown that the immune response plays a central role during I/RI, and the early immune response is mainly mediated by innate immune cells, including macrophages and neutrophils.5,6 Ischaemia/reperfusion injury could stimulate the release of nuclear damage-associated molecular patterns (DAMPs), such as high mobility group box 1 (HMGB1) and extracellular histones by damaged cardiomyocytes (CMs),7–9 followed by cascade favouring the recruitment and activation of immune cells.9 Restriction of the neutrophil infiltration enhanced myocardial salvage.10 Neutrophil extracellular traps (NETs), a novel aspect of neutrophil biology, have recently been reported to increase myocardial I/RI.11–13 In response to DAMPs, a subset of neutrophils could release chromatin structures decorated with histones, cytoplasmic, and granular proteins.14 This process is referred to as NET formation (NETosis). NETosis could increase intracellular calcium and activate peptidylarginine deiminase 4 (PAD4), which converts specific positively charged arginine residues to neutral citrulline residues. This often occurs on histones, resulting in citrullinated histones, such as citrullinated histone H3 (citH3).15 Accumulating evidence has demonstrated that rapid release of cytotoxic NETs may cause severe myocardial injury11,12 and vascellum fungi.16,17
Aldehyde dehydrogenase 2 (ALDH2) is a key alcohol metabolism enzyme. Approximately 30%–50% of the East Asian population (∼8% of the world) carry the ALDH2 rs671 (Glu504Lys) polymorphism.18 The ALDH2 rs671 G > A polymorphism is associated with pathogenesis of various cardiovascular diseases, including myocardial I/RI.19–22 Our previous studies demonstrated the protective effect of ALDH2 in various cardiovascular models, including I/RI23–26; however, it remains unclear whether ALDH2 is involved in NETosis during I/RI. Myocardial I/RI models were thus established on the wild-type (WT), ALDH2 knockout, PAD4 knockout, and ALDH2/PAD4 double knockout mice to unveil the role of ALDH2 in NETosis during myocardial I/RI and possible underlying mechanisms. A cohort of ST-elevation myocardial infarction (STEMI) patients undergoing primary PCI (pPCI) was analysed to evaluate the predictive value of myeloperoxidase (MPO)–DNA complex and leukotriene C4 (LTC4) for adverse left ventricular remodelling (LVR).
Methods
Detailed experimental materials and methods are available in the Supplementary data online, Supplementary Methods.
Results
The human ALDH2 rs671 polymorphism predicts NETosis vulnerability
Fifteen healthy volunteers with different ALDH2 rs671 genotypes (GG, GA, and AA; n = 5/each genotype) were enrolled to investigate the association between genotype and NETosis (Figure 1A). NETosis in all of the neutrophils (GG, GA, and AA) were assessed at 2 h after phorbol 12-myristate 13-acetate (PMA, 100 nM) or vehicle stimulation. The ALDH2 rs671 mutant genotypes (GA and AA) promoted neutrophil NETosis (Figure 1B) and death (Figure 1C and D). The citH3 levels, commonly used to evaluate NETosis, were significantly higher in the GA and AA genotype neutrophils than in GG genotype neutrophils (Figure 1E). These results suggest that human neutrophils carrying ALDH2 rs671 mutant genotypes (GA and AA) are more prone to NETosis than those carrying the ALDH2 rs671 WT genotype (GG).
Figure 1.
The human ALDH2 rs671 (Glu504Lys) polymorphism predicts NETosis vulnerability. (A) Human peripheral neutrophils were isolated from healthy volunteers. ALDH2 rs671 genotypes (GG, GA, and AA, n = 5 of each genotype) were treated by PMA. (B) The MPO–DNA complex levels were assessed in the human neutrophils treated by PMA or vehicle for 2 h (n = 5 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Immunofluorescence staining of the neutrophils treated by PMA for 2 h with staining for CD66b and nuclei. (C) Quantification (n = 5 of each group, one-way ANOVA using Tukey’s multiple comparisons test) and (D) representative immunofluorescence images of the human neutrophils treated by PMA for 2 h with staining for CD66b (green) and nuclei (blue). Scale bar, 20 μm. (E) Quantification (n = 5 of each group, one-way ANOVA using Tukey’s test) and representative bands of citH3 protein measured by Western blot assay. The data were presented as the means ± SD of five independent biological experiments. ALDH2, aldehyde dehydrogenase 2; NETosis, neutrophil extracellular trap (NET) formation; PMA, phorbol 12-myristate 13-acetate; citH3, citrullinated histone H3
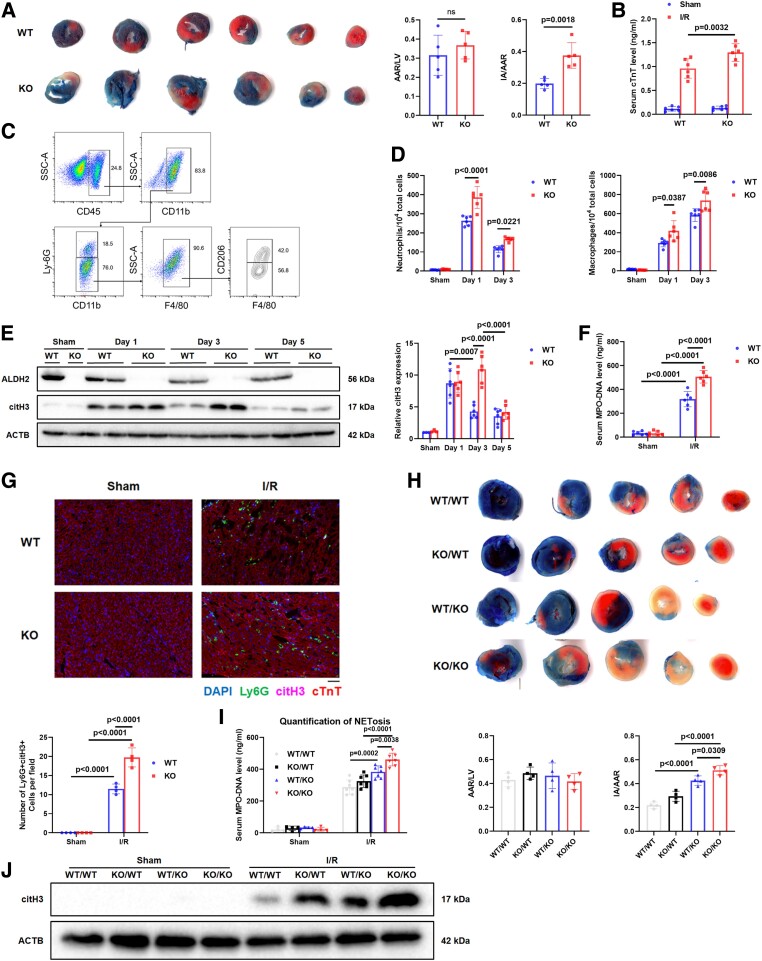
Increased NETosis during myocardial ischaemia/reperfusion injury in ALDH2-KO mice
Infarct area (IA)/area at risk (AAR) ratio (Figure 2A) and serum cardiac troponin T (cTnT) levels (Figure 2B) were higher in ALDH2-KO mice than WT mice at Day 3 post-I/RI (45 min of ischaemia followed by reperfusion). The influx of neutrophils and macrophages in the peripheral blood and hearts of the mice at Days 1 and 3 post-I/RI were then quantified by flow cytometry. The gating strategy for neutrophils (CD45+Ly6G+), pro-inflammatory M1 macrophages (CD45+F4/80+CD206−), and anti-inflammatory M2 macrophages (CD45+F4/80+CD206+) is presented in Figure 2C. Flow cytometry data demonstrated significantly increased myocardial neutrophil and macrophage infiltration in the ALDH2-KO mice at Days 1 and 3 post-I/RI compared with WT mice (Figure 2D). The M1 and M2 macrophage polarization was similar between the two groups (see Supplementary data online, Figure S1A–D). In terms of neutrophil-mediated NETosis, the citH3 levels in the AAR tissues of the ALDH2-KO mice were higher than those of the WT mice at Days 1, 3, and 5 post-I/RI (Figure 2E). Consistently, serum levels of MPO-DNA complex were significantly higher in the ALDH2-KO mice than in WT mice at Day 3 post-I/RI (Figure 2F). Immunofluorescence analysis also showed a higher percentage of infiltratory Ly6G+ neutrophils containing citH3 in the AAR tissues of ALDH2-KO mice than those of WT mice at Day 3 post-I/RI (Figure 2G).
Figure 2.
Increased NETosis during myocardial I/RI in ALDH2-KO mice. (A–G) Myocardial I/R surgery (45 min of ischaemia followed by reperfusion) was performed in wild-type mice and ALDH2-KO (KO) mice. (A) Representative images and quantification of cross-sections stained with TTC and Evans blue to determine the extent of I/RI at 3 days post-I/R (n = 5 per group, unpaired two-tailed Student's t-test). (B) The serum cTnT levels were measured by ELISA assay (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (C) Gating strategy for flow cytometric analysis of leucocyte population isolated from the hearts of WT and KO subjected to sham operation or I/R surgery at 1 and 3 days post-I/R. (D) Flow cytometry–based quantification of neutrophils and macrophages (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (E) Representative bands and quantification of citH3 protein from the hearts of WT and KO subjected to sham operation or I/R surgery at 1, 3, and 5 days post-I/R. (sham n = 3 of each group; I/R Days 1, 3, and 5, n = 6 of each group, two-way ANOVA using Sidak’s multiple comparisons test). (F) The serum MPO–DNA complex levels were assessed in WT and KO mice 3 days post-I/R (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (G) Representative immunofluorescence images and quantification of WT and KO of hearts at 3 days post-I/R with staining for Ly6G, cTnT, citH3, and nuclei (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 50 μm. (H–J) Myocardial I/R surgery (45 min of ischaemia followed by reperfusion) was performed in WT/WT, KO/WT, WT/KO, and KO/KO mice (sham group: per n = 4; I/R group: per n = 10). (H) Representative images and quantification of cross-sections stained with TTC and Evans blue to determine the extent of I/RI at 3 days (n = 4 of each group, one-way ANOVA using Tukey’s multiple comparisons test). (I) The serum MPO–DNA complex levels were measured by ELISA assay (sham n = 4 of each group; I/R n = 7 of each group, two-way ANOVA using Sidak’s multiple comparisons test). (J) Quantification of citH3 protein measured by Western blot assay. The data were presented as the means ± SD of at least four independent biological experiments. ALDH2, aldehyde dehydrogenase 2; NETosis, neutrophil extracellular trap (NET) formation; I/RI, ischaemia/reperfusion injury; TTC, triphenyl tetrazolium chloride; cTnT, cardiac troponin T; WT, wild type; KO, knockout; citH3, citrullinated histone H3
To further investigate whether ALDH2 deficiency in bone marrow (BM) or heart played an important role in NET formation and myocardial I/RI, allogeneic BM transplantation was performed in mice according to the following scheme: WT recipient/WT BM donor (WT/WT), WT recipient/ALDH2-KO BM donor (WT/KO), ALDH2-KO recipient/WT BM donor (KO/WT), and ALDH2-KO recipient/ALDH2-KO BM donor (KO/KO). The genotype of the WT recipients was ALDH2+CD45.1+CD45.2−, whereas the genotypes of the WT and KO BM donors were ALDH2+CD45.1−CD45.2+ and ALDH2−CD45.1−CD45.2+, respectively. In addition, the genotypes of the ALDH2-KO recipients, WT, and ALDH2-KO BM donors were ALDH2−CD45.1−CD45.2+, ALDH2+CD45.1+CD45.2−, and ALDH2−CD45.1−CD45.2+, respectively (see Supplementary data online, Figure S1E). Peripheral blood cells stained with anti-CD45.1 and anti-CD45.2 were identified by flow cytometry (see Supplementary data online, Figure S1F) while ALDH2 expression was assessed by Western blot (see Supplementary data online, Figure S1G). The four groups underwent sham or myocardial I/R operation for 3 days. The triphenyl tetrazolium chloride (TTC) and Evans blue staining showed a greater extent of myocardial I/RI in ALDH2-KO BM group than in WT BM group (Figure 2H). Neutrophils and macrophages from ALDH2-KO BM exhibited stronger recruitment of neutrophil and macrophage than those from WT BM (see Supplementary data online, Figure S1H and I). Similarly, the mice with ALDH2-KO BM showed enhanced NETosis than WT BM at Day 3 post-I/RI, evidenced by significantly elevated serum MPO–DNA complex levels (Figure 2I) and citH3 protein expression (Figure 2J). The ALDH2 deletion in hearts also exhibited an increase of myocardial injury and NETosis compared with WT hearts (Figure 2G–J; Supplementary data online, Figure S1H and I). These findings suggest that NETosis are involved in ALDH2 deficiency-mediated exacerbation of myocardial I/RI.
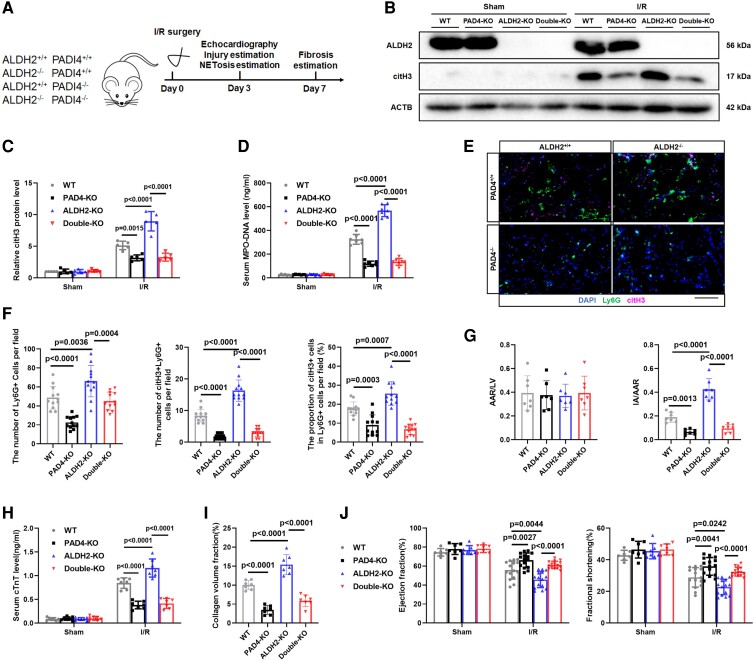
NETosis plays a core role in the ALDH2 deficiency-mediated exacerbation of myocardial ischaemia/reperfusion injury
Next, we investigated whether the exacerbated myocardial I/RI observed in response to ALDH2 deficiency was due to increased NETosis. PAD4 plays an essential role in chromatin decondensation and NETosis.27 Deficiency of PAD4 reduces NETosis formation and myocardial I/R injury in mice.11 Global PAD4- and ALDH2/PAD4 double-KO (double-KO) mice were generated (Figure 3A). There was no difference on myocardial morphology and cardiac function assessed by haematoxylin-eosin (HE) staining and echocardiography between WT, ALDH2-KO, PAD4-KO, and double-KO mice (see Supplementary data online, Figure S1J and K). Reduced citH3 levels were observed in the AAR tissues of the PAD4-KO mice and the double-KO mice at Day 3 post-I/RI compared with those of WT mice, indicating that PAD4-KO significantly reversed the elevated citH3 levels caused by ALDH2 deficiency (Figure 3B and C). The citH3 levels were similar between the PAD4-KO and double-KO mice (Figure 3C). Serum MPO–DNA complex levels showed a trend similar to that of citH3 levels in the AAR tissues (Figure 3D). Next, immunofluorescence was applied to quantify the number of infiltrated Ly6G+ and Ly6G+citH3+ double-positive neutrophils in the myocardial AAR tissues at Day 3 post-I/RI (Figure 3E). Proportion of these cells was used as an indicator of NETosis. ALDH2 deficiency promoted NETosis, which was reversed in the PAD4-KO and double-KO mice compared with both ALDH2-KO and WT mice. NETosis levels were similar between the PAD4-KO and double-KO mice (Figure 3E and F). These results suggest that PAD4 deficiency significantly inhibits NETosis in response to myocardial I/RI as well as the ALDH2 deficiency-mediated enhancement of NETosis.
Figure 3.
NETosis plays a core role in the ALDH2 deficiency-mediated exacerbation of myocardial I/RI. Myocardial I/R surgery (45 min of ischaemia followed by reperfusion) was performed in wild-type (WT) mice and global PAD4 knockout mice (PAD4-KO) and global ALDH2/PAD4 double knockout mice (double-KO). (A) Experimental strategy of myocardial I/R model. (B) Representative bands and (C) quantification of citH3 protein from WT, PAD4-KO, ALDH2-KO, and double-KO of sham hearts and hearts 3 days post-I/R (n = 5 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (D) The serum MPO–DNA complex levels were assessed in WT, PAD4-KO, ALDH2-KO, and double-KO mice at 3 days post-I/R (n = 7 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (E) Representative immunofluorescence images and (F) quantification of Ly6G+ cell and citH3+Ly6G+ cell in WT, PAD4-KO, ALDH2-KO, and double-KO of hearts at 3 days post-I/R with staining for Ly6G, cTnT, citH3, and nuclei (n = 12 of each group, one-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 100 μm. (G) Quantification of cross-sections stained with TTC and Evans blue (n = 7 of each group, one-way ANOVA using Tukey’s multiple comparisons test). (H) The serum cTnT levels were measured by ELISA assay (n = 7 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (I) The degree of fibrosis was assessed by Masson's trichrome staining (n = 7 of each group, one-way ANOVA using Tukey’s multiple comparisons test). (J) Echocardiography was performed to assess EF and FS of the left ventricle in WT, PAD4-KO, ALDH2-KO, and double-KO groups subjected to sham operation or I/R surgery at 3 days post-I/R (n = 7 of each sham group and n = 15 of each I/R group, two-way ANOVA using Sidak’s multiple comparisons test). The data were presented as the means ± SD of at least five independent biological experiments. NETosis, neutrophil extracellular trap (NET) formation; ALDH2, aldehyde dehydrogenase 2; I/RI, ischaemia/reperfusion injury; PAD4, peptidylarginine deiminase 4; Ly6G, lymphocyte antigen 6 complex locus G6D; citH3, citrullinated histone H3; cTnT, cardiac troponin T; TTC, triphenyl tetrazolium chloride; EF, ejection fraction; FS, fractional shortening
The effects of NETosis abolition on the extent of I/RI were then investigated. Higher IA/AAR ratio (Figure 3G; Supplementary data online, Figure S2A) and serum cTnT levels (Figure 3H) of ALDH2-KO mice hinted greater extent of I/RI compared with WT mice. NETosis abolition substantially reduced the extent of myocardial damage in both ALDH2-KO and WT mice. The extent of myocardial damage was similar between the PAD4-KO and double-KO mice. Masson's trichrome staining demonstrated similar trends in cardiac collagen content in these mice, thus indicating that PAD4 deficiency ameliorated cardiac fibrosis and myocardial damage after I/RI (Figure 3I; Supplementary data online, Figure S2B). Cardiac function was evaluated by echocardiography. There were no differences in cardiac function among the WT, ALDH2-KO, PAD4-KO, and double-KO mice after sham surgery. After I/R surgery, PAD4-KO mice exhibited preserved left ventricular ejection fraction (LVEF) and fractional shortening (FS) compared with those of WT mice. Similar results were also observed between the double-KO and ALDH2-KO mice. Cardiac function was similar between the PAD4-KO and double-KO mice (Figure 3J; Supplementary data online, Figure S2C). These results suggest that blocking NETosis via PAD4 knockout lessens the ALDH2 deficiency-mediated enhanced I/RI in terms of infarct size, cardiac fibrosis, and cardiac dysfunction.
NETosis-targeting drugs ameliorate myocardial ischaemia/reperfusion injury
To assess the therapeutic potential of NETosis inhibitors for attenuating myocardial I/RI, GSK484 hydrochloride (GSK484, 10 mg/kg),28 a selective and reversible PAD4 inhibitor, or DNase1 (5 mg/kg), which has been shown to inhibit NET function, was applied to mice on the day of surgery alone or continued daily for another 2 days post-I/R (see Supplementary data online, Figure S3A). The infarct size and NETosis reductions were more significant post GSK484 and DNase I injection daily for 3 days after I/R surgery than single injection on the day of surgery (see Supplementary data online, Figure S2D–G). Significantly lower citH3 levels in the AAR tissues were evidenced from GSK484- or DNase1-treated WT mice than in those from vehicle-treated mice at Day 3 post-I/R. A similar therapeutic effect was observed in the ALDH2-KO mice (see Supplementary data online, Figure S3B and C). Serum MPO–DNA complex levels were also significantly decreased in the GSK484- and DNase1-treated WT and ALDH2-KO mice (see Supplementary data online, Figure S3D). In WT mice, GSK484 or DNase1 reduced the IA at Day 3 post-I/R. GSK484 and DNase1 also significantly decreased IA/AAR ratio of ALDH2-KO mice at Day 3 post-I/R (see Supplementary data online, Figure S3E). Enzyme-linked immunosorbent assay (ELISA) revealed that serum cTnT level changes were consistent with the myocardial IA changes of these six groups (see Supplementary data online, Figure S3F). Cardiac fibrosis was assessed at Day 7 post-I/R. Masson's trichrome staining revealed higher collagen content in ALDH2-KO mice compared with WT mice (see Supplementary data online, Figure S3G), which was significantly reduced by GSK484 or DNase1 treatment in both WT and ALDH2-KO mice (see Supplementary data online, Figure S3G). Echocardiography was performed on the WT and ALDH2-KO mice of the sham, I/RI + vehicle, I/RI + GSK484, and I/RI + DNase1 groups at Day 3 post-I/RI. NETosis inhibition by GSK484 or DNase1 significantly improved cardiac function in both WT and ALDH2-KO mice at Day 3 post-I/R (see Supplementary data online, Figure S3H).
Myocardial ischaemia/reperfusion injury leads to the increased release of DAMPs in ALDH2-KO mice
Compared with WT CMs, ALDH2-KO CMs exhibited increased cell death (Figure 4A) and released more DAMPs, including HMGB1 and nucleosomes, after hypoxia/reoxygenation (H/R; Figure 4B and C). Hypoxia/reoxygenation also increased the expression of necroptotic proteins [phosphorylated receptor-interacting protein 3 (p-RIP3), phosphorylated mixed lineage kinase domain-like pseudokinase (p-MLKL)] in WT CMs. This effect was exacerbated in ALDH2-KO CMs (Figure 4D). Next, neutrophils were treated with the supernatants from these CMs, and supernatant from H/R-treated ALDH2-KO CMs induced more NETosis than that of the WT CMs on neutrophils, as shown by higher Ly6G+citH3+ cells and overall citH3 levels (Figure 4E and F).
Figure 4.
Myocardial I/RI leads to the increased release of DAMPs in ALDH2-KO mice. (A) Propidium iodide (PI) was added to detect the loss of plasma membrane integrity, and fluorescent images were obtained by confocal microscopy. Quantification of PI+cTnT+ cells elevated died cardiomyocytes (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 100μm. The cellular supernatant (B) HMGB1 and (C) nucleosomes were measured by ELISA assay (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (D) Representative bands of necroptosis protein p-RIP3 and p-MLKL in WT and KO cardiomyocytes treated by control or H/R. These cell supernatants were used to treat neutrophils. (E) Representative immunofluorescence images and quantification of Ly6G+citH3+ double-positive cells with staining for Ly6G, citH3, and nuclei (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 50 μm. (F) Representative bands of citH3 protein. (G) Quantification of HMGB1, p-RIP3, and p-MLKL proteins measured by Western blot assay. The serum (H) nucleosome and (I) HMGB1 levels were measured by ELISA assay (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). The data were presented as the means ± SD of four independent biological experiments. ALDH2, aldehyde dehydrogenase 2; I/RI, ischaemia/reperfusion injury; DAMPs, damage-associated molecular patterns; cTnT, cardiac troponin T; HMGB1, high mobility group box 1; p-RIP3, phosphorylated receptor-interacting protein 3; p-MLKL, phosphorylated mixed lineage kinase domain-like pseudokinase; H/R, hypoxia/reoxygenation; Ly6G, lymphocyte antigen 6 complex locus G6D; citH3, citrullinated histone H3
To evaluate the effect of ALDH2 on DAMP release after myocardial I/RI, allogeneic BM transplantation was performed in mice according to the following scheme: WT recipient/WT BM donor (WT/WT) and ALDH2-KO recipient/WT BM donor (KO/WT). Western blot showed higher necroptotic protein and HMGB1 levels in KO/WT mice than in WT/WT mice (Figure 4G). Serum HMGB1 and nucleosome levels significantly increased at Day 3 post-I/RI in the KO/WT mice compared with WT/WT mice (Figure 4H and I).
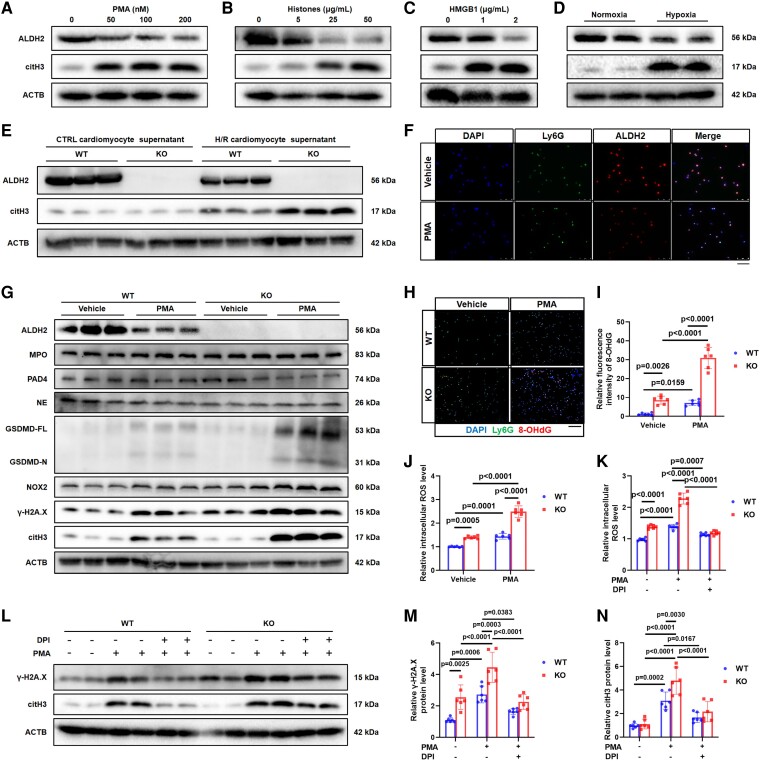
ALDH2 deficiency promotes NOX2-dependent NETosis in neutrophils
PMA, hypoxia, and DAMPs have been shown to induce NETosis. Accordingly, stimulation with these factors resulted in ALDH2 downregulation (Figure 5A–D). Then, WT and ALDH2-KO neutrophils were treated with DAMPs from control and H/R-treated CM supernatants. Western blot revealed that the H/R-treated CM supernatant decreased ALDH2 expression in WT neutrophils. More ALDH2-KO neutrophils underwent NETosis than WT neutrophils (Figure 5E). Immunofluorescence analysis also showed decreased ALDH2 levels after PMA-induced NETosis (Figure 5F). These data suggest that ALDH2 deficiency promotes NETosis in response to various stimuli.
Figure 5.
ALDH2 deficiency promotes NOX2-dependent NETosis in neutrophils. (A–D) ALDH2 and citH3 protein levels were obtained from WT neutrophils stimulated with PMA (0, 50, 100, and 200 nM), histones (0, 5, 25, and 50 μg/mL), HMGB1 (0, 1, and 2 μg/mL), and hypoxia for 4 h. (E) The WT cardiomyocytes were treated by control or H/R, and the supernatant was used to treat WT and KO neutrophils. Representative bands of citH3 protein. (F) Representative immunofluorescence images of neutrophils with staining with Ly6G, ALDH2, and nuclei. Scale bar, 50 μm. WT and KO neutrophils were treated by PMA (100 nM). (G) NETosis relevant proteins MPO, NE, NOX2, PAD4, GSDMD-FL/-N, γ-H2A.X, and citH3 were assessed in neutrophils. (H) Representative immunofluorescence images and (I) quantification of 8-OHdG in neutrophils with staining for Ly6G, 8-OHdG, and nuclei (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 50 μm. (J) Fluorescence intensity of ROS in neutrophils. WT and KO neutrophils were treated by DPI (10 μM) and PMA (100 nM; n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (K) Fluorescence intensity of ROS in neutrophils (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (L) Representative bands and (M and N) quantification of citH3 and γ-H2A.X proteins (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). The data were presented as the means ± SD of at least six independent biological experiments. ALDH2, aldehyde dehydrogenase 2; NETosis, neutrophil extracellular trap (NET) formation; NOX2, NADPH oxidases 2; citH3, citrullinated histone H3; PMA, phorbol 12-myristate 13-acetate; HMGB1, high mobility group box 1; H/R, hypoxia/reoxygenation; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; Ly6G, lymphocyte antigen 6 complex locus G6D; MPO, myeloperoxidase; NE, neutrophil elastase; PAD4, peptidylarginine deiminase 4; GSDMD, gasdermin D; γ-H2A.X, gamma-histone 2A family member X; DPI, diphenyleneiodonium chloride; ROS, reactive oxygen species
The caspase-11 (CASP11)/gasdermin D (GSDMD), NOX2/reactive oxygen species (ROS), MPO/neutrophil elastase (NE), and PAD4/citH3 pathways have been demonstrated as the main mechanisms of NETosis. To avoid overwhelming neutrophil NETosis at 4 h after stimulation, which might unmake the protein expression of various pathway proteins, the activation of these pathways was evaluated in WT and KO neutrophils after 2-h PMA stimulation, and the most pronounced increases were observed in NOX2 and gamma-histone 2A family member X (γ-H2A.X) expression (Figure 5G). Enhanced oxidative DNA damage [8-hydroxy-2′-deoxyguanosine (8-OHdG)] and ROS accumulation were observed in the ALDH2-KO group compared with the WT group (Figure 5H–J), an effect mediated by NOX2.29 Although NOX2 signal activation was observed in untreated ALDH2-KO neutrophils compared with WT neutrophils, citH3 levels did not increase, indicating that NOX2 activation was not key pathway to induce NETosis.30 Next, NOX2 inhibitor diphenyleneiodonium chloride (DPI) was used to treat PMA-induced NETosis in WT and ALDH2-KO neutrophils. Diphenyleneiodonium chloride significantly reduced ROS production (Figure 5K) as well as citH3 and γ-H2A.X levels (Figure 5L–N) in both WT and ALDH2-KO neutrophils. Furthermore, DPI effectively blocked the increased NETosis caused by ALDH2 deficiency (Figure 5K–N). These results indicate that ALDH2 deficiency-enhanced NETosis is predominantly mediated by ROS accumulation and oxidative DNA damage through activating NOX2. Next, the different NET components isolated from WT and ALDH2-KO neutrophils were identified after 4-h PMA stimulation in case of overwhelming NETosis. Western blot revealed that MPO, NE, and citH3 protein levels did not differ between WT and ALDH2-KO NETs (see Supplementary data online, Figure S4A). Additional analysis revealed that the enzymatic activities of MPO and NE were also similar between WT and KO NETs (see Supplementary data online, Figure S4B and C). Altogether, these results indicate that ALDH2 deficiency promotes NETosis without altering NET composition.
ALDH2 protects cardiomyocytes, cardiac fibroblasts, and endothelial cells from neutrophil extracellular trap–induced damage
Previous studies have reported that NET components directly damage living cells.17 The impact of ALDH2 on NET-induced damage in cardiac cells was explored. Cardiomyocytes, cardiac fibroblast (CFs), and endothelial cells (ECs) were treated for 24 h with equal amounts (500 ng/mL) of NET contents isolated from either WT or ALDH2-KO neutrophils. The highest levels of necroptotic (p-RIP3 and p-MLKL) and apoptotic [cleaved caspase-3 (CASP3) and BCL2-asssociated X (BAX)] proteins were observed in ALDH2-KO CMs treated with the ALDH2-KO NET contents, followed by WT CMs treated with the ALDH2-KO NET contents and ALDH2-KO CMs treated with the WT NET contents. WT CMs treated with WT NET contents showed the lowest levels of apoptosis and necroptosis (see Supplementary data online, Figure S5A). Likewise, detection of HMGB1 levels by real-time quantitative polymerase chain reaction (RT-qPCR) and extracellular HMGB1 levels by ELISA showed the highest HMGB1 levels were found in ALDH2-KO CMs treated with ALDH2-KO NET contents and the lowest HMGB1 levels in WT CMs treated with WT NET contents (see Supplementary data online, Figure S5B and C). Fibroblasts have been shown to activate inflammatory responses and promote fibrosis after myocardial damage.31 Fibrotic collagen type I alpha 1 (Col1α1) and alpha-smooth muscle actin (α-SMA) protein levels (see Supplementary data online, Figure S5D) and pro-inflammatory cytokine Tnf-α, Il-1β, and Il-6 mRNA levels were also the highest in ALDH2-KO CMs treated with ALDH2-KO NET contents, followed by WT CMs treated with ALDH2-KO NET contents and ALDH2-KO CMs treated with WT NET contents, and the lowest in WT CMs treated with WT NET contents (see Supplementary data online, Figure S5E–G). Finally, death levels in cardiac microvascular endothelial cells (CMECs) in response to NET-induced damage were evaluated. Western blot and annexin V/propidium iodide assay revealed the highest levels of death levels (including apoptosis and necroptosis) in ALDH2-KO CMECs treated with ALDH2-KO NET contents, followed by WT CMECs treated with KO NET contents and KO CMECs treated with WT NET contents (see Supplementary data online, Figure S5H–J). WT CMECs treated with WT NET contents exhibited the lowest of death levels.
The therapeutic potential of ALDH2 overexpression on myocardial I/R injury was explored via tail vein injection of AAV2-Control or AAV2-ALDH2 (see Supplementary data online, Figure S6A). ALDH2 overexpression attenuated the formation of NETs (see Supplementary data online, Figure S6B), cardiac fibrosis (see Supplementary data online, Figure S6C), and infarct size (see Supplementary data online, Figure S6D and E) and improved the cardiac angiogenesis (see Supplementary data online, Figure S6F) and function (see Supplementary data online, Figure S6G and H).
ALDH2 deficiency-induced NOX2-dependent NETosis is mediated by the ER stress/Mgst2/LTC4 pathway
Proteomic analysis identified several differentially expressed proteins in WT neutrophils vs. ALDH2-KO neutrophils after 2-h PMA stimulation. These proteins were involved in oxidative and ER stress, glutathione metabolism, and NETosis (see Supplementary data online, Figure S7A and B). Proteomic analysis further showed that proteins associated with NETosis were activated (see Supplementary data online, Figure S7C). A heatmap of differential proteins is shown in Figure 6A, from which Mgst2 was selected as the differential downstream gene (Figure 6B).
Figure 6.
ALDH2 deficiency-induced NOX2-dependent NETosis is mediated by the ER stress/ microsomal glutathione S-transferase 2 (Mgst2)/LTC4 pathway. Proteomic analysis of WT and KO neutrophils stimulated by PMA for 2 h. (A) The heatmap showing the most significant enrichment proteins. (B) The volcano plot showing the most significant enrichment proteins. (C) ER stress/Mgst2/LTC4/NOX2 pathway is activated in ALDH2-deficienct neutrophils. (D) Representative bands of proteins Mgst2, NOX2, γ-H2A.X, and citH3 in WT and KO neutrophils treated by vehicle or PMA. (E) Representative immunofluorescence images and (F) quantification of LTC4 in neutrophils with staining for Ly6G, LTC4, and nuclei (n = 5 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 50 μm. WT and KO neutrophils were treated by pranlukast (10 μM) and PMA (100 nM). (G) Representative bands and (H–J) quantification of proteins NOX2, γ-H2A.X, and citH3 (n = 4 of each group, two-way ANOVA using Tukey’s multiple comparisons test). The data were presented as the means ± SD of at least four independent biological experiments. ALDH2, aldehyde dehydrogenase 2; NOX2, NADPH oxidases 2; NETosis, neutrophil extracellular trap (NET) formation; ER, endoplasmic reticulum; Mgst2, microsomal glutathione S-transferase 2; LTC4, leukotriene C4; PMA, phorbol 12-myristate 13-acetate; γ-H2A.X, gamma-histone 2A family member X; citH3, citrullinated histone H3; Ly6G, lymphocyte antigen 6 complex locus G6D
ALDH2 is important for the removal of endogenous aldehydes, such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA),32 leading to alleviation of endoplasmic reticulum (ER) stress,33 which is an important process in NETosis.34 Accumulated lipid peroxidation (MDA and 4-HNE) and ER stress activation [C/EBP homologous protein (CHOP) and Glucose-Regulated Protein 78 (GRP78)] during NETosis were found in ALDH2-KO neutrophils compared with WT neutrophils (see Supplementary data online, Figure S7D). Eliminating lipid peroxidation by N-acetylcysteine (NAC) or inhibiting ER stress by 4-PBA reduced GRP78, NOX2, and citH3 expression (see Supplementary data online, Figure S8A and B). These results showed that ALDH2 deficiency promoted ER stress through lipid peroxide accumulation, thereby exacerbating NOX2-dependent NETosis (Figure 6C).
LTC4 synthase Mgst2 has been reported to play a key role in the intracellular signalling of ER stress, leading to NOX4-induced ROS accumulation, oxidative DNA damage, and cell death,35 which was consistent with GO and KEGG enrichment analyses. Therefore, Mgst2 may be a key trigger for the endogenous signalling of NOX2-dependent NETosis (Figure 6C). Mgst2 protein levels were upregulated in PMA-treated WT neutrophils for 2 h, whereas these levels were further upregulated in ALDH2-KO neutrophils (Figure 6D). LTC4 level changes were consistent with that of Mgst2 (Figure 6E and F). The PMA-induced upregulation of Mgst2 was inhibited by 4-PBA, indicating ER stress-dependent Mgst2 regulation (see Supplementary data online, Figure S8C). Next, Mgst2 expression was inhibited by si-RNA (si-Mgst2) in HL-60 cells to investigate its role in NOX2-dependent NETosis. Mgst2 inhibition led to reduced expression of NOX2, γ-H2A.X, and citH3 after 2-h PMA stimulation (see Supplementary data online, Figure S8D). LTC4 receptor antagonist pranlukast was applied to explore whether ALDH2 mediates NETosis via Mgst2/LTC4 signalling. The results showed that pranlukast inhibited NOX2, γ-H2A.X, and citH3 expression in PMA-treated WT neutrophils. Pranlukast also prevented the ALDH2 deficiency-induced exacerbation of NETosis, indicating the importance of Mgst2/LTC4 signalling in NOX2-dependent NETosis (Figure 6G–J). These findings suggest pranlukast, a commonly used clinical drug, might be a potential therapy option to inhibit NETosis, particularly for individuals with ALDH2 deficiency.
LTC4 receptor antagonist pranlukast ameliorates myocardial ischaemia/reperfusion injury in ALDH2-KO mice via NETosis inhibition
Pranlukast (.5 mg/kg, once daily) was intraperitoneally administered to mice for 3 days after myocardial I/R surgery36 (Figure 7A). The infarct size and NETosis reduction were more significant post pranlukast injection daily for 3 days after I/R surgery than single injection on the day of surgery (see Supplementary data online, Figure S2D–G). It significantly reduced citH3 expression in the AAR tissues of both WT and ALDH2-KO mice compared with those of vehicle-treated mice at Day 3 post-I/R (Figure 7B). Serum MPO–DNA complex and LTC4 levels showed similar trends to that of myocardial citH3 expression (Figure 7C and D). The infiltratory Ly6G+ and Ly6G+citH3+ double-positive neutrophils were detected in the myocardial AAR tissues of the mice by immunofluorescence, and the proportion of these cells was used as an indicator of NETosis (Figure 7E and F). Higher NETosis was observed in the ALDH2-KO mice at Day 3 post-I/R compared with WT mice. Pranlukast effectively reduced NETosis in both WT and ALDH2-KO mice after I/R, and no significant differences were observed between these two groups at 3 days post-I/RI (Figure 7E and F).
Figure 7.
LTC4 receptor antagonist pranlukast ameliorates myocardial I/RI in ALDH2-KO mice via NETosis inhibition. Myocardial I/R surgery (45 min of ischaemia followed by reperfusion) was performed in wild-type (WT) mice and ALDH2-KO mice; sham operation mice as control. The mice were given vehicle or pranlukast treatment for 3 days after surgery. (A) Experimental strategy of myocardial I/R model. (B) Representative bands of proteins ALDH2 and citH3 in the infarct area of hearts from I/R mice at 3 days post-I/R. (C) The serum MPO–DNA complex levels and (D) the serum LTC4 levels were assessed by ELISA assay (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (E) Representative immunofluorescence images and (F) quantification of Ly6G+cells and Ly6G+citH3+ double-positive cells with staining for Ly6G, citH3, and nuclei (n = 12 of each group, two-way ANOVA using Tukey’s multiple comparisons test). Scale bar, 50 μm. (G) Serum cTnT levels were assessed by ELISA assay (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (H) Representative images and quantification of cross-sections stained with TTC and Evans blue to determine the extent of I/RI at 3 days post-I/R (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (I) The degree of fibrosis was assessed by Masson's trichrome staining (n = 6 of each group, two-way ANOVA using Tukey’s multiple comparisons test). (J) Echocardiography was performed to assess EF and FS of the left ventricle in WT + vehicle, WT + pranlukast, KO + vehicle, and KO + pranlukast groups subjected to sham operation or I/R surgery at 3 days post-I/R (n = 7 of each sham group and n = 15 of each I/R group, two-way ANOVA using Sidak’s multiple comparisons test). The data were presented as the means ± SD of at least six independent biological experiments. LTC4, leukotriene C4; ALDH2, aldehyde dehydrogenase 2; NETosis, neutrophil extracellular trap (NET) formation; I/RI, ischaemia/reperfusion injury; citH3, citrullinated histone H3; MPO, myeloperoxidase; Ly6G, lymphocyte antigen 6 complex locus G6D; cTnT, cardiac troponin T; TTC, triphenyl tetrazolium chloride; EF, ejection fraction; FS, fractional shortening
Next, the extent of post-I/R myocardial damage was evaluated in the mice after pranlukast treatment. The ALDH2-KO mice exhibited higher serum cTnT level and IA/AAR ratio after I/RI compared with WT mice (Figure 7G and H). The difference disappeared after pranlukast treatment between the two groups. ALDH2-KO mice also exhibited a higher overall collagen volume fraction than WT mice, representing a greater extent of fibrosis in these mice. Again, pranlukast treatment reduced fibrosis in both WT and ALDH2-KO mice 7 days after I/R (Figure 7I; Supplementary data online, Figure S9A). Cardiac function was evaluated by echocardiography on Day 3 post-I/R. The cardiac function of ALDH2-KO mice was worse than WT mice at Day 3 post-I/R; pranlukast improved the cardiac function in both ALDH2-KO and WT mice (Figure 7J; Supplementary data online, Figure S9B and C).
To verify the NETosis-dependent protective effect of pranlukast, PAD4-KO mice and ALDH2/PAD4 double-KO mice were generated and intraperitoneally administered with pranlukast for 3 days after myocardial I/R surgery. Serum MPO–DNA complex and cTnT levels were similar between vehicle and pranlukast treatment in the PAD4-KO mice and ALDH2/PAD4 double-KO mice (see Supplementary data online, Figure S9D and E). The pharmacological therapeutic effect of pranlukast on the IA/AAR ratio and cardiac function was not found in the PAD4-KO mice and ALDH2/PAD4 double-KO mice at 3 days post-I/R (see Supplementary data online, Figure S9F and G).
Additionally, the therapeutic effects of GSK484, DNase I, or pranlukast on female mice after myocardial I/R were explored. In female mice, ALDH2 deficiency also exacerbated NETosis and myocardial I/RI. The infarct size and NETosis were reduced by treatment with GSK484, DNase I, or pranlukast daily for 3 days after I/R surgery in both WT and ALDH2-KO female mice (see Supplementary data online, Figure S10A–C).
These results indicate that pranlukast demonstrates great therapeutic potential for inhibiting NETosis and reducing the extent of I/RI, particularly in individuals with ALDH2 deficiency.
High MPO–DNA complex and LTC4 levels predict adverse left ventricular remodelling in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention
A nested case–control study was conducted within a cohort of 308 consecutive STEMI patients post pPCI between October 2021 and March 2023. Thirty patients were excluded based on exclusion criteria. A total of 278 patients were finally enrolled (Figure 8A). During the 6-month follow-up period, 7 patients died from cardiovascular causes, and 2 patients died from non-cardiovascular causes. Echocardiography data at 6-month post pPCI were not available from 19 patients. The patients who carried ALDH2 rs671 mutant genotypes (GA, AA) showed higher levels of MPO–DNA (Figure 8B) and LTC4 (Figure 8C) than ALDH2 rs671 wild genotype GG. Of the remaining 250 patients, 65 (26.0%) developed adverse LVR [defined as a relative increase in left ventricular end-diastolic volume index (LVEDVi) ≥ 20% within 6 months post pPCI]. Patients who developed LVR were more likely to have higher levels of MPO–DNA and LTC4. Baseline characteristics are described in Table 1. Changes of echocardiography-derived parameters from baseline to 6 months after PCI between LVR group and non-LVR group are described in Table 2. The association between MPO–DNA or LTC4 and LVR was investigated by univariate and multivariate logistic regression models. In Model 1, after adjusting for age and sex, MPO–DNA and LTC4 were correlated with LVR, respectively (odds ratio [OR] 3.94; 95% confidence interval [CI] 2.14–7.55; P < .001; OR 3.24; 95% CI 1.79–6.07; P < .001). In Model 2, after adjusting for age, sex, heart rate (HR), diabetes, hypertension, cardiac troponin I, and total ischaemic time, the association between MPO–DNA or LTC4 and LVR remained significant (OR 3.93; 95% CI 2.09–7.69; P < .001; OR 3.23; 95% CI 1.73–6.22; P < .001; Table 3). Receiver operating characteristic (ROC) analysis showed an area under the curve (AUC) of .653 (95% CI .579–.727) and .668 (95% CI .594–.743) for MPO–DNA and LTC4 in predicting LVR (Figure 8D). MPO–DNA and LTC4 significantly improved the prediction efficacy for LVR on top of traditional risk factor including age, sex, HR, diabetes, hypertension, cTnI, and total ischaemic time. Receiver operating characteristic analysis showed AUC of .634 (95% CI .551–.717), .721 (95% CI .650–.792), and .701 (95% CI .622–.780) for MI risk model, MI risk model + MPO–DNA, and MI risk model + LTC4. Myocardial infarction risk model + MPO–DNA and MI risk model + LTC4 both had higher AUC values than MI risk model, respectively (.634 vs. .721, P = .01; .634 vs. .701, P = .02; Figure 8E and Table 4). Moreover, the addition of MPO–DNA or LTC4 to the conventional model improved the risk reclassification [net reclassification improvement (MPO–DNA: .645, 95% CI .388–.901; LTC4: .561, 95% CI .298–.824, both P < .01) and integrated discrimination improvement (MPO-DNA: .059, 95% CI .027–.090; LTC4: .062, 95% CI .032–.095, both P < .01)] for the LVR incidence (Table 4).
Figure 8.
High MPO–DNA complex and LTC4 levels predict adverse LVR in STEMI patients undergoing pPCI. (A) Flowchart of study participants. (B) The serum MPO–DNA complex levels and (C) the serum LTC4 levels were assessed by ELISA assay. (D) Receiver operating characteristic curve analysis of MPO–DNA and LTC4 for predicting LVR prevalence. (E) Receiver operating characteristic curve analysis of myocardial infarction risk model and its modifications for predicting LVR prevalence. The data were presented as the means ± SD. LVR is defined as a relative increase in LVEDVi ≥20% within 6 months post pPCI. MPO, myeloperoxidase; LTC4, leukotriene C4; STEMI, ST-elevation myocardial infarction; pPCI, primary percutaneous coronary intervention; LVR, left ventricular remodelling; LVEDVi, left ventricular end-diastolic volume index; CMR, cardiac magnetic resonance; IS, infarct size; LVLS, left ventricular longitudinal systolic strain
Table 1.
Baseline variables between left ventricular remodelling group and non-left ventricular remodelling group
| Non-LVR | LVR | P value | |
|---|---|---|---|
| n = 185 | n = 65 | ||
| Demographics | |||
| Age, median [IQR], years | 63 (55–70) | 63 (52–70) | .596 |
| Sex, female, n (%) | 34 (18.4) | 9 (13.9) | .521 |
| Systolic blood pressure, median [IQR], mmHg | 128 (117–139) | 130 (120–134) | .881 |
| Heart rate, median [IQR], b.p.m. | 75 (70–80) | 74 (66–80) | .165 |
| Body mass index, median [IQR], kg/m2 | 24.4 (22.7–26.0) | 24.5 (22.5–26.4) | .858 |
| Clinical history | |||
| Current smoker, n (%) | 99 (53.5) | 36 (55.4) | .908 |
| Diabetes, n (%) | 61 (33.0) | 23 (35.4) | .840 |
| Hypertension, n (%) | 109 (58.9) | 30 (46.2) | .102 |
| Atrial fibrillation, n (%) | 10 (5.4) | 5 (7.7) | .546 |
| Laboratory measurements | |||
| WBC, median [IQR], 109/L | 11.2 (9.6–13.6) | 11.2 (9.1–14.1) | .935 |
| RBC, mean ± SD, 1012/L | 4.67 ± .63 | 4.72 ± .65 | .567 |
| Platelets, median [IQR], 109/L | 237 (203–287) | 232 (207–290) | .965 |
| Total cholesterol, median [IQR], mmol/L | 4.63 (3.96–5.50) | 4.77 (4.20–5.74) | .308 |
| LDL-C, median [IQR], mmol/L | 3.02 (2.48–3.83) | 3.28 (2.70–3.83) | .326 |
| HDL-C, median [IQR], mmol/L | .98 (.84–1.15) | 1.02 (.84–1.24) | .268 |
| cTnI, median [IQR], ng/mL | 143.79 (57.05–361.80) | 194.41 (76.19–441.80) | .158 |
| NT-proBNP, median [IQR], pg/mL | 1184 (531–2510) | 1352 (536–3030) | .872 |
| D-Dimer, median [IQR], mg/L FEU | .40 (.26–.68) | .41 (.27–.83) | .412 |
| MPO–DNA, median [IQR], ng/mL | 39.8 (28.9–54.4) | 49.1 (43.2–64.1) | <.001 |
| LTC4, median [IQR], pg/mL | 428.2 (314.1–563.6) | 530.3 (438.4–661.6) | <.001 |
| Procedure characteristics | |||
| Total ischaemic time, median [IQR], min | 259 (180–318) | 262 (178–379) | .395 |
| Time of door to balloon, median [IQR], min | 90 (73–106) | 89 (68–113) | .900 |
| TIMI flow grade before PCI, n (%) | .412 | ||
| 0 | 123 (66.49) | 49 (75.38) | |
| 1 | 23 (12.43) | 6 (9.23) | |
| 2 | 39 (21.08) | 10 (15.38) | |
| TIMI flow Grade 3 after PCI, n (%) | 159 (86.0) | 50 (76.9) | .135 |
| Number of stents, mean ± SD, n | 1.42 ± .60 | 1.40 ± .70 | .826 |
| Medical therapy during hospitalization | |||
| Antiplatelet agent use, n (%) | 182 (98.4) | 63 (96.9) | .607 |
| Statin use, n (%) | 180 (97.3) | 62 (95.4) | .432 |
| ACEI/ARB use, n (%) | 148 (80.0) | 50 (76.9) | .728 |
| β-Blocker use, n (%) | 164 (88.7) | 61 (93.9) | .336 |
| Diuretic use, n (%) | 111 (60.0) | 45 (69.2) | .241 |
| Genotype | .077 | ||
| GG, n (%) | 96 (51.9) | 31 (47.7) | |
| GA, n (%) | 69 (37.3) | 32 (49.2) | |
| AA, n (%) | 20 (10.8) | 2 (3.1) | |
| Echocardiography measurements | |||
| LVEF, median [IQR], % | 55 (51–58) | 55 (47–59) | .919 |
| IVS, median [IQR], cm | 1.06 (.94–1.24) | 1.09 (1.01–1.24) | .178 |
| LVDd, mean ± SD, cm | 4.85 ± .59 | 4.75 ± .63 | .255 |
| LVPW, median [IQR], cm | .98 (.91–1.07) | .98 (.89–1.06) | .662 |
| LVEDVi, median [IQR], mL/1.73 m2 | 90 (77–110) | 80.0 (72–91) | .002 |
| LVESVi, median [IQR], mL/1.73 m2 | 41 (33–54) | 36 (32–43) | .020 |
| Echocardiography measurements at 6 months post pPCI | |||
| LVEF, median [IQR], % | 56 (51–60) | 55 (46–60) | .291 |
| IVS, median [IQR], cm | 1.03 (.91–1.22) | 1.08 (.94–1.23) | .508 |
| LVDd, median [IQR], cm | 4.74 (4.38–5.14) | 4.96 (4.60–5.30) | .031 |
| LVPW, median [IQR], cm | .95 (.88–1.03) | .97 (.87–1.06) | .671 |
| LVEDVi, median [IQR], mL/1.73 m2 | 88 (76–110) | 129 (111–148) | <.001 |
| LVESVi, median [IQR], mL/1.73 m2 | 39 (32–50) | 62 (44–75) | <.001 |
LVR, left ventricular remodelling; WBC, white blood cell; RBC, red blood cell; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide; TIMI, thrombolysis in myocardial infarction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; LVEF, left ventricular ejection fraction; IVS, interventricular septum; LVDd, left ventricular end-diastolic dimensions; LVPW, left ventricular posterior wall; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index.
Table 2.
Changes of echocardiography-derived parameters from baseline to 6-month post percutaneous coronary intervention between left ventricular remodelling group and non-left ventricular remodelling group
| Non-LVR | LVR | P value | |
|---|---|---|---|
| n = 185 | n = 65 | ||
| LVEF, mean ± SD, % | 2.26 ± 15.08 | 1.00 ± 11.73 | .624 |
| IVS, mean ± SD, % | −3.38 ± 13.51 | −2.82 ± 12.64 | .824 |
| LVDd, mean ± SD, % | 1.35 ± 10.95 | 5.67 ± 11.68 | .052 |
| LVPW, mean ± SD, % | −2.64 ± 14.09 | .72 ± 19.69 | .324 |
| LVEDVi, mean ± SD, % | 1.69 ± 14.61 | 62.76 ± 19.12 | <.001 |
| LVESVi, mean ± SD, % | 2.31 ± 27.19 | 63.07 ± 33.97 | <.001 |
LVR, left ventricular remodelling; LVEF, left ventricular ejection fraction; IVS, interventricular septum; LVDd, left ventricular end-diastolic dimension; LVPW, left ventricular posterior wall; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index.
Table 3.
Association of left ventricular remodelling presence with MPO–DNA and LTC4
| MPO–DNA < 43.16 ng/mL | MPO–DNA ≥ 43.16 ng/mL | P value | LTC4 < 462.07 pg/mL | LTC4 ≥ 462.07 pg/mL | P value | |
|---|---|---|---|---|---|---|
| Unadjusted | 1 (ref.) | 3.96 (2.15–7.57) | <.001 | 1 (ref.) | 3.25 (1.79–6.08) | <.001 |
| Model 1 | 1 (ref.) | 3.94 (2.14–7.55) | <.001 | 1 (ref.) | 3.24 (1.79–6.07) | <.001 |
| Model 2 | 1 (ref.) | 3.93 (2.09–7.69) | <.001 | 1 (ref.) | 3.23 (1.73–6.22) | <.001 |
Model 1: adjusted for age and sex. Model 2: Model 1 + HR + diabetes + hypertension + cTnI+ total ischaemic time.
HR, heart rate; cTnI, cardiac troponin I.
Table 4.
Comparison of the predictive value of MPO–DNA and LTC4 on myocardial infarction risk model for left ventricular remodelling using area under the curve, net reclassification improvement, and integrated discrimination improvement
| AUC | P value | Continuous NRI (95% CI) | P value | IDI (95% CI) | P value | |
|---|---|---|---|---|---|---|
| MI risk model vs. model + MPO–DNA | .634 vs. .721 | .01 | .645 (.388–.901) | <.01 | .059 (.027–.090) | <.01 |
| MI risk model vs. model + LTC4 | .634 vs. .701 | .02 | .561 (.298–.824) | <.01 | .062 (.032–.095) | <.01 |
MI, myocardial infarction; AUC, area under the curve; NRI, net reclassification improvement; IDI, integrated discrimination improvement.
Furthermore, 110 out of the 250 patients in our cohort with 12-month cardiac magnetic resonance (CMR) examination post PCI underwent further analysis. The baseline characteristics and CMR-derived parameters at 12 months after pPCI are described in Supplementary data online, Table S1. Similarly, MPO–DNA and LTC4 concentrations showed moderate correlations with the CMR-measured infarct size and LV global longitudinal peak systolic strain after adjusting for TIMI flow grade before pPCI and infarct-related artery at baseline (infarct size: MPO–DNA: r = .390, P < .001; LTC4: r = .379, P < .001; LV longitudinal strain: MPO–DNA: r = .307, P = .001; LTC4: r = .303, P = .001). Fifteen patients were randomly selected and analysed twice by the same observer after 4 weeks and another observer. The correlation coefficients of intraobserver and interobserver agreement for CMR-measured infarct size were .982 (.850–.994) and .956 (.878–.985), and the correlation coefficients of intraobserver and interobserver agreement for CMR-measured LV global longitudinal peak systolic strain were .925 (.794–.974) and .821 (.523–.940).
Discussion
Mitochondrial enzyme ALDH2 has been shown capable of attenuating myocardial I/RI, predominantly via its protective effects in CMs19,20,23 and macrophage.37,38 However, its role in BM-derived cells is poorly understood. This study provides evidence that NET-mediated myocardial injury might be a novel mechanism contributing to the exacerbation of myocardial I/RI in response to ALDH2 deficiency. Present results indicated that ALDH2 deficiency might enhance I/RI by inducing NOX2-dependent NETosis via the ER stress/Mgst2/LTC4 pathway. Furthermore, serum MPO–DNA complex and LTC4 levels could be used as predictive indicators of adverse LVR after pPCI in STEMI patients. Drugs targeting NETosis including LTC4 receptor antagonists might be potential promising options for attenuating I/RI, particularly in patients with ALDH2 mutation.
The ALDH2 Glu504Lys polymorphism is a common genetic variant in the East Asian population.39 Mounting evidence suggests that ALDH2 exerts cardioprotective effects through various pathways, including ischaemic preconditioning,40,41 autophagy,20 and the renin–angiotensin system (RAS).42 Here, the association between ALDH2 Glu504Lys polymorphism and NETosis is newly defined. In vitro studies demonstrated that neutrophils from healthy volunteers carrying ALDH2 rs671 mutant genotypes (GA, AA) were more prone to NETosis than those carrying the ALDH2 rs671 WT genotype (GG). The ALDH2 knockout mice were thus used to evaluate the role of ALDH2 in NETosis during myocardial I/RI in this study. In vivo, neutrophil recruitment and NETosis were significantly elevated after myocardial I/RI in ALDH2-KO mice and in recipient mice after allogeneic transplantation with ALDH2-KO BM. Targeting NETosis via PAD4 genetic knockout or drugs (GSK484 and DNase 1) reduced myocardial IA and fibrosis and improved cardiac function. This therapeutic effect was more significant in ALDH2-KO mice. Above results thus suggest that ALDH2 plays a cardioprotective role not only in CMs but also in neutrophils by regulating neutrophil recruitment and NETosis during I/RI.
DAMPs have been described as NETosis irritants.28 Previous studies20,43 demonstrated that ALDH2 could attenuate H/R-induced CM injury, but it remains unclear whether ALDH2 deficiency in CMs contributes to increased DAMP release. Both in vitro and in vivo experiments revealed a higher number of dying CMs and greater DAMP release in ALDH2-KO models following I/RI, which may partially explain increased NETosis in ALDH2-KO mice. Neutrophil extracellular trap–induced cytotoxicity has been reported in many studies,17,44–46 but its exact mechanism was not fully clear. This study demonstrated the protective role of ALDH2 against NET-induced cytotoxicity in CMs, CFs, and ECs. Although ALDH2 deficiency did not affect core NET structure (MPO and NE), it exacerbated the cytotoxicity of NETs to various cardiac cells. NOX2 generates large amounts of ROS, which is essential for NOX-dependent NETosis.30 Present results showed that ALDH2 deficiency could promote ROS generation and oxidative DNA damage via NOX2 pathway activation, resulting in increased NETosis. This is the first study to systematically demonstrate the role of ALDH2 in NETosis, involving DAMP release, NET formation, and cytotoxicity of NETs post-I/R.
Myocardial I/RI triggers both ER stress and oxidative stress as part of its mechanism of action.47,48 Dvash et al.35 described the ER stress–activated Mgst2/LTC4 pathway as the major executor of ROS generation and subsequent DNA damage in epithelial cells, fibroblasts, and pre-keratinocytes. Here, the role of the Mgst2/LTC4 pathway in NETosis in ALDH2-KO neutrophils was presented. Endogenous LTC4 signalling triggers NETosis in a NOX2-dependent manner, supplementing the mechanism that depends on ROS generation. LTC4 receptor antagonists have demonstrated beneficial effects on myocardial injury.49,50 Consistently, the beneficial role of LTC4 receptor antagonist pranlukast was confirmed in this myocardial I/RI mouse model, particularly in ALDH2-KO mice. These findings suggest that inhibiting LTC4 activity through the use of LTC4 receptor antagonists holds great clinical significance for patients with MI, particularly for those of the East Asian population carrying the ALDH2 Glu504Lys polymorphism.
Clinical data revealed that serum biomarkers MPO–DNA complex and LTC4 levels were independently associated with adverse LVR and might be used as predictive indicators of adverse LVR in STEMI patients after pPCI. Moreover, the moderate correlations between serum MPO–DNA/LTC4 and CMR parameters infarct size/LV strain hinted the potential role of NETosis in predicting the prognosis of STEMI patients. These findings add to the growing clinical evidence of the detrimental effects of NETosis during myocardial I/RI.
However, this study also had several limitations. First, this study was conducted with small sample size in a single centre. The sample size estimation was not pre-defined, partly due to the lack of previous published studies on the matter. Therefore, post hoc statistical power test was performed, and results were .997 and .980 for MPO–DNA and LTC4, respectively, so group sample sizes of 125 in high-level MPO–DNA or LTC4 and 125 in low-level MPO–DNA or LTC4 could achieve >90% power to detect a difference between the group proportions of .248 for MPO–DNA and .216 for LTC4. Second, LVR parameter was only defined by LVEDVi from echocardiography in this study; although this index has been used in many studies and LVEDVi defined adverse LVR as significantly related to worse prognosis of STEMI patients,51–53 the LVR defined by comprehensive indexed LVR needs to be tested in future studies. Third, LVR as an alternative outcome for I/R injury may have some potential limitations. Future studies are needed to evaluate the association between NETosis and I/R injury with more solid outcome index such as major adverse cardiac events, and much larger patient cohort is needed for that purpose. Fourth, data of 110 out of the 250 patients in our cohort, who also underwent CMR examination at 12 months post PCI, were analysed, and CMR measurements at admission were not available. Although MPO–DNA and LTC4 concentrations showed moderate correlation with the CMR-measured infarct size and LV global longitudinal peak systolic strain after adjusting for TIMI flow grade before pPCI and infarct-related artery at baseline, the LVR could not be defined in these patients. Lastly, due to the relatively short follow-up time, adverse LVR (≥20% relative change in LVEDVi) instead of a major adverse cardiovascular event was set as outcome to evaluate the association between MPO–DNA, LTC4, and IR/I. Future studies with larger patient cohort and long-term follow-up with solid outcome indexes are needed.
In conclusion, the present study demonstrates that ALDH2 deficiency exacerbates myocardial I/RI in both mice and patients, predominantly by promoting NETosis via the ER stress/Mgst2/LTC4/NOX2 pathway. Pharmacotherapeutic targeting of NETosis or the use of LTC4 receptor antagonists might be promising therapeutic approaches for attenuating myocardial I/RI, particularly in individuals with the ALDH2 mutation.
Supplementary Material
Acknowledgements
The authors wish to thank MD Lei Yin from Shengli Clinical Medical College of Fujian Medical University and MD Minzhi Lyu from the Clinical Research Unit of Zhongshan Hospital, Fudan University, for their help and support.
Contributor Information
Kun Yang, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China.
Rifeng Gao, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China; Department of Cardiology, The Fifth People’s Hospital of Shanghai, Fudan University, 128 Ruili Road, Shanghai 200240, China; Department of Cardiac Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, 88 Jiefang Road, Hangzhou 310009, China.
Hanchuan Chen, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China.
Jingjing Hu, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China; Department of Cardiology, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou 310006, China.
Peng Zhang, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China; Department of Cardiology, Minhang Hospital affiliated to Fudan University, 170 Xinsong Road, Shanghai 201100, China.
Xiang Wei, Department of Cardiology, The Fifth People’s Hospital of Shanghai, Fudan University, 128 Ruili Road, Shanghai 200240, China.
Jiaran Shi, Department of Cardiology, Lihuili Hospital Facilitated to Ningbo University, 57 Xingning Road, Ningbo 315040, China.
Yinyin Chen, Department of Radiology, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, China; Department of Medical Imaging, Fudan University, 180 Fenglin Road, Shanghai 200032, China.
Liwei Zhang, Department of Cardiology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, 134 Dongjie Road, Fuzhou 350001, China.
Juntao Chen, Department of Urology, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai 200032, China.
Yang Lyu, Department of Cardiology, The Fifth People’s Hospital of Shanghai, Fudan University, 128 Ruili Road, Shanghai 200240, China.
Zhen Dong, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China.
Wei Wei, Department of Cardiology, Shanghai Chest Hospital, Shanghai Jiao Tong University, 241 West Huaihai Road, Shanghai 200030, China.
Kai Hu, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China.
Yansong Guo, Department of Cardiology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, 134 Dongjie Road, Fuzhou 350001, China; Fujian Provincial Key Laboratory of Cardiovascular Disease, Fujian Provincial Center for Geriatrics, Fujian Provincial Clinical Research Center for Severe Acute Cardiovascular Diseases, 134 Dongjie Road, Fuzhou 350001, China; Fujian Heart Failure Center Alliance, 134 Dongjie Road, Fuzhou 350001, China.
Junbo Ge, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China; Institutes of Biomedical Sciences, Fudan University, 131 Dongan Road, Shanghai 200032, China; Key Laboratory of Viral Heart Diseases, National Health Commission, 180 Fenglin Road, Shanghai 200032, China; Key Laboratory of Viral Heart Diseases, Chinese Academy of Medical Sciences, 180 Fenglin Road, Shanghai 200032, China.
Aijun Sun, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, 180 Fenglin Road, Shanghai 200032, China; Institutes of Biomedical Sciences, Fudan University, 131 Dongan Road, Shanghai 200032, China.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no disclosure of interest for this contribution.
Data Availability
The data underlying this article will be shared upon reasonable request by the corresponding author.
Funding
This study was supported by a grant to A.S. from the Key Project of the National Natural Science Foundation of China (82130010), Basic Research Projects of Shanghai Science and Technology Commission (22JC1400500), and Innovation Program of Shanghai Municipal Education Commission; to Y.G. from the National Natural Science Foundation of China General Program (Grant Numbers 81873495 and 82171569), Heart Failure Center Research Foundation of Fujian Provincial Hospital (supported by the Fujian Provincial Department of Finance), National Key Clinical Specialty Construction Project of China (Cardiovascular Medicine 2021), and Cardiovascular Disease Clinical and Research Platform (National Regional Medical Center); and to P.Z. from Projects of the Minhang District Science Committee of Shanghai (2022MHZ042).
Ethical Approval
All experimental procedures were approved by the Animal Care Ethics Committee of Zhongshan Hospital, Fudan University (No. 2021-034) and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Fujian Provincial Hospital Ethics Committee provided ethical approval for this study, and the protocol was carried out in accordance with the Declaration of Helsinki (K2019-07-011). The Zhongshan Hospital, Fudan University Ethics Committee provided ethical approval for this study, and the protocol was carried out in accordance with the Declaration of Helsinki (B2022-016R).
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022;145:e153–639. 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–31. 10.1056/NEJMoa1505489 [DOI] [PubMed] [Google Scholar]
- 3. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–35. 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 5. Liao Y-H, Xia N, Zhou S-F, Tang T-T, Yan X-X, Lv B-J, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol 2012;59:420–9. 10.1016/j.jacc.2011.10.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang T, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation 2019;139:663–78. 10.1161/CIRCULATIONAHA.118.036044 [DOI] [PubMed] [Google Scholar]
- 7. Shah M, He Z, Rauf A, Beikoghli Kalkhoran S, Heiestad CM, Stenslokken KO, et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc Res 2022;118:1115–25. 10.1093/cvr/cvab139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silvis MJM, Kaffka Genaamd Dengler SE, Odille CA, Mishra M, van der Kaaij NP, Doevendans PA, et al. Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol 2020;11:599511. 10.3389/fimmu.2020.599511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 2011;8:292–300. 10.1038/nrcardio.2011.38 [DOI] [PubMed] [Google Scholar]
- 10. Mauler M, Herr N, Schoenichen C, Witsch T, Marchini T, Hardtner C, et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation 2019;139:918–31. 10.1161/CIRCULATIONAHA.118.033942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014;123:141–8. 10.1182/blood-2013-07-514992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge L, Zhou X, Ji W-J, Lu R-Y, Zhang Y, Zhang Y-D, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol 2015;308:H500–9. 10.1152/ajpheart.00381.2014 [DOI] [PubMed] [Google Scholar]
- 13. Hashemi P, Nouri-Vaskeh M, Alizadeh L, Baghbanzadeh A, Badalzadeh R, Askari E, et al. NETosis in ischemic/reperfusion injuries: an organ-based review. Life Sci 2022;290:120158. 10.1016/j.lfs.2021.120158 [DOI] [PubMed] [Google Scholar]
- 14. Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-works with dire consequences for health. Circ Res 2019;125:470–88. 10.1161/CIRCRESAHA.119.314581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boeltz S, Amini P, Anders HJ, Andrade F, Bilyy R, Chatfield S, et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ 2019;26:395–408. 10.1038/s41418-018-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, et al. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018;123:33–42. 10.1161/CIRCRESAHA.117.312494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019;569:236–40. 10.1038/s41586-019-1167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu F, Sun Y, Shang R, Li M, Cui L, Cui Z, et al. The Glu504Lys polymorphism of aldehyde dehydrogenase 2 contributes to development of coronary artery disease. Tohoku J Exp Med 2014;234:143–50. 10.1620/tjem.234.143 [DOI] [PubMed] [Google Scholar]
- 19. Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 2008;321:1493–5. 10.1126/science.1158554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J 2011;32:1025–38. 10.1093/eurheartj/ehq253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan G, Roy B, Palaniyandi SS. Diabetic aldehyde dehydrogenase 2 mutant (ALDH2*2) mice are more susceptible to cardiac ischemic-reperfusion injury due to 4-hydroxy-2-nonenal induced coronary endothelial cell damage. J Am Heart Assoc 2021;10:e021140. 10.1161/JAHA.121.021140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C-H, Ferreira JCB, Mochly-Rosen D. ALDH2 and cardiovascular disease. Adv Exp Med Biol 2019;1193:53–67. 10.1007/978-981-13-6260-6_3 [DOI] [PubMed] [Google Scholar]
- 23. Li W, Yin L, Sun X, Wu J, Dong Z, Hu K, et al. Alpha-lipoic acid protects against pressure overload-induced heart failure via ALDH2-dependent Nrf1-FUNDC1 signaling. Cell Death Dis 2020;11:599. 10.1038/s41419-020-02805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L-L, Ding Z-W, Yin P-P, Wu J, Hu K, Sun A-J, et al. Hypertrophic preconditioning cardioprotection after myocardial ischaemia/reperfusion injury involves ALDH2-dependent metabolism modulation. Redox Biol 2021;43:101960. 10.1016/j.redox.2021.101960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun X, Gao R, Li W, Zhao Y, Yang H, Chen H, et al. Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact Mater 2021;6:2058–69. 10.1016/j.bioactmat.2020.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun A, Cheng Y, Zhang Y, Zhang Q, Wang S, Tian S, et al. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J Mol Cell Cardiol 2014;71:92–104. 10.1016/j.yjmcc.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 27. Ling S, Xu J-W. NETosis as a pathogenic factor for heart failure. Oxid Med Cell Longev 2021;2021:6687096. 10.1155/2021/6687096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015;62:600–14. 10.1002/hep.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffmann MH, Griffiths HR. The dual role of reactive oxygen species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic Biol Med 2018;125:62–71. 10.1016/j.freeradbiomed.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 30. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A 2015;112:2817–22. 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255–65. 10.1038/nrcardio.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimura M, Yokoyama A, Higuchi S. Aldehyde dehydrogenase-2 as a therapeutic target. Expert Opin Ther Targets 2019;23:955–66. 10.1080/14728222.2019.1690454 [DOI] [PubMed] [Google Scholar]
- 33. Yang M-Y, Wang Y-B, Han B, Yang B, Qiang Y-W, Zhang Y, et al. Activation of aldehyde dehydrogenase 2 slows down the progression of atherosclerosis via attenuation of ER stress and apoptosis in smooth muscle cells. Acta Pharmacol Sin 2018;39:48–58. 10.1038/aps.2017.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, et al. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis 2021;12:606. 10.1038/s41419-021-03896-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dvash E, Har-Tal M, Barak S, Meir O, Rubinstein M. Leukotriene C4 is the major trigger of stress-induced oxidative DNA damage. Nat Commun 2015;6:10112. 10.1038/ncomms10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin J-R, Fang S-C, Tang S-S, Hu M, Long Y, Ghosh A, et al. Hippocampal CysLT1R knockdown or blockade represses LPS-induced depressive behaviors and neuroinflammatory response in mice. Acta Pharmacol Sin 2017;38:477–87. 10.1038/aps.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong S, Li L, Zhang Y-L, Zhang L, Lu J, Guo S, et al. Acetaldehyde dehydrogenase 2 interactions with LDLR and AMPK regulate foam cell formation. J Clin Invest 2019;129:252–67. 10.1172/JCI122064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J, Zhao X, Guo Y, Liu Z, Wei S, Yuan Q, et al. Macrophage ALDH2 (aldehyde dehydrogenase 2) stabilizing Rac2 is required for efferocytosis internalization and reduction of atherosclerosis development. Arterioscler Thromb Vasc Biol 2022;42:700–16. 10.1161/ATVBAHA.121.317204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goodnough CL, Gross ER. Precision medicine considerations for the management of heart disease and stroke in East Asians. Cardiol Plus 2020;5:101–8. 10.4103/cp.cp_17_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Contractor H, Stottrup NB, Cunnington C, Manlhiot C, Diesch J, Ormerod JO, et al. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic Res Cardiol 2013;108:343. 10.1007/s00395-013-0343-3 [DOI] [PubMed] [Google Scholar]
- 41. Ueta CB, Campos JC, Albuquerque RPE, Lima VM, Disatnik M-H, Sanchez AB, et al. Cardioprotection induced by a brief exposure to acetaldehyde: role of aldehyde dehydrogenase 2. Cardiovasc Res 2018;114:1006–15. 10.1093/cvr/cvy070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koda K, Salazar-Rodriguez M, Corti F, Chan NY-K, Estephan R, Silver RB, et al. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 2010;122:771–81. 10.1161/CIRCULATIONAHA.110.952481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang R, Xue M-Y, Liu B-S, Wang W-J, Fan X-H, Zheng B-Y, et al. Aldehyde dehydrogenase 2 preserves mitochondrial morphology and attenuates hypoxia/reoxygenation-induced cardiomyocyte injury. World J Emerg Med 2020;11:246–54. 10.5847/wjem.j.1920-8642.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumar SVR, Kulkarni OP, Mulay SR, Darisipudi MN, Romoli S, Thomasova D, et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J Am Soc Nephrol 2015;26:2399–413. 10.1681/ASN.2014070673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol 2020;36:191–218. 10.1146/annurev-cellbio-020520-111016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsourouktsoglou TD, Warnatsch A, Ioannou M, Hoving D, Wang Q, Papayannopoulos V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep 2020;31:107602. 10.1016/j.celrep.2020.107602 [DOI] [PubMed] [Google Scholar]
- 47. Jin JK, Blackwood EA, Azizi K, Thuerauf DJ, Fahem AG, Hofmann C, et al. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ Res 2017;120:862–75. 10.1161/CIRCRESAHA.116.310266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang F, Pulinilkunnil T, Flibotte S, Nislow C, Vlodavsky I, Hussein B, et al. Heparanase protects the heart against chemical or ischemia/reperfusion injury. J Mol Cell Cardiol 2019;131:29–40. 10.1016/j.yjmcc.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 49. Becher UM, Ghanem A, Tiyerili V, Furst DO, Nickenig G, Mueller CF. Inhibition of leukotriene C4 action reduces oxidative stress and apoptosis in cardiomyocytes and impedes remodeling after myocardial injury. J Mol Cell Cardiol 2011;50:570–7. 10.1016/j.yjmcc.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 50. Ito T, Toki Y, Hieda N, Okumura K, Hashimoto H, Ogawa K, et al. Protective effects of a thromboxane synthetase inhibitor, a thromboxane antagonist, a lipoxygenase inhibitor and a leukotriene C4, D4 antagonist on myocardial injury caused by acute myocardial infarction in the canine heart. Jpn Circ J 1989;53:1115–21. 10.1253/jcj.53.1115 [DOI] [PubMed] [Google Scholar]
- 51. Evans S, Weinheimer CJ, Kovacs A, Williams JW, Randolph GJ, Jiang W, et al. Ischemia reperfusion injury provokes adverse left ventricular remodeling in dysferlin-deficient hearts through a pathway that involves TIRAP dependent signaling. Sci Rep 2020;10:14129. 10.1038/s41598-020-71079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qin Y, Yu Y, Dong H, Bian X, Guo X, Dong S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int J Med Sci 2012;9:413–23. 10.7150/ijms.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi GJ, Takeda M, et al. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia-reperfusion injury in pigs in vivo. Coron Artery Dis 2010;21:304–11. 10.1097/mca.0b013e32833aec62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request by the corresponding author.