Abstract
Garcinia celebica L. syn. Garcinia hombroniana Pierre belongs to the family Clusiaceae, is indigenous to Southeast Asian countries. This review aims to provide updated, comprehensive and categorized information on the phytoconstituents and pharmacological effects of this species. The data collection mainly involved searches through databases named Scopus, Google Scholar, Pubmed and Springer Link. Approximately 100 phytochemicals were recorded in this review, with various classes of compounds such as triterpenoids, flavonoids, benzophenones, xanthones, depsidones and sterols identified. The most abundant compounds isolated belong to two chemical classes: triterpenoids and xanthones. Their extracts and pure compounds have been reported for their antibacterial, antiparasitic, hepatoprotective, antioxidant, antidiabetic, antituberculosis, antiplatelet aggregation, anti-neuraminidase and cholinesterase inhibitory activities. This review will provide a comprehensive understanding between the phytochemical components and its medicinal uses that may serve as a valuable resource for future drug development.
Keywords: Garcinia celebica, Garcinia hombroniana, Phytochemicals, Pharmacological effects, Bioactive compounds
Highlights
-
•
The phytochemical constituents of G. celebica are reviewed.
-
•
The pharmacological aspect of extracts and pure compounds is comprehensively discussed.
-
•
The relationship between medicinal uses, chemistry and pharmacology is also described.
1. Introduction
Garcinia celebica L. (synonym Garcinia hombroniana Pierre), known as Seashore Mangosteen in English, and manggis hutan in Malay is an important medicinal plant of South East Asia and belongs to the family Clusiaceae. G. celebica is an accepted species according to the “The World Flora Online” [1]. Initially, the scientific name established was G. hombroniana as Pierre grouped G. cornea and G. celebica under the same category based on their morphological and geographical distribution. However, it was later found that G. hombroniana, G. cornea and G. celebica were referring to the same species based on their morphological evidence from the literature and herbarium specimens. Hence, it was decided that the valid taxonomic name used was G. celebica as it was published earlier than G. cornea and G. hombroniana [2,3]. Nevertheless, until now the name G. hombroniana was still popularly used by some researchers. Other names for the species include seashore mangosteen, Puli mangosteen, Beraus and Waaa.
The word Garcinia was named in honor of the French naturalist Laurent Garcin during the 18th century by Linnaeus for his contributions in the botanical field [4]. Garcinia, taxonomically classified within the Guttiferae or Clusiaceae family, large genus includes 400 species distributed primarily in Africa, tropical Asia and Polynesia. It is a rich sources of medicinal compounds for instance hydroxycitric acid, procyanidines, garcinol, α-mangostin, cambogin, and gambogic acid making this genus a rich repository of useful compounds [[5], [6], [7], [8]].
Many species of the genus Garcinia have long since been used to treat several ailments. Studies have shown the pericarp and seed extracts from G. brasiliensis Mart. Demonstrated excellent antioxidant, anti-inflammatory, leishmanicidal, and antiprotozoal properties [[9], [10], [11]]. The most famous Garcinia species is G. mangostana L, in which xanthones isolated from this species were reported to exhibit anticancer activity [12]. The phytochemicals present in G. celebica possess substantial pharmacological effects that have been empirically used in traditional medicine for various purposes [13,14]. The decoction of the root of G. celebica was utilized for preventing infections after childbirth in folk medicines, while the root and leaf parts were used as ailments for relieving itchiness and treating skin diseases [[15], [16], [17], [18]].
Based on our extensive search regarding phytoconstituents and pharmacological effects of the genus Garcinia, there has been no review thus far that has reviewed its composition and properties despite the availability of several reports on G. celebica. There is great potential in further venturing into G. celebica aimed at providing direction toward improving its prospect to be developed into modern medicines. In this comprehensive review, all findings from previous studies to date focusing G. celebica will be compiled. This review aims to link the studies that cover the medicinal uses, phytochemical constituents and pharmacological effects of G. celebica to achieve ethnopharmacological relevance.
2. Methodology
Information on the ethnobotanical use of Garcinia celebica was retrieved from Google Scholar, Pubmed, Scopus and Springer Link electronic databases from 2000 to 2024. Specific keywords used for the collection of data were “Garcinia celebica L.”,“Garcinia hombroniana”, “phytoconstituents”, “phytochemical constituents”, “pharmacological effects”, “antioxidant”, “cytotoxic”, “anticancer”, “platelet aggregation inhibitory activity”, “antibacterial”, “antimicrobial”, “antiviral”, “antiparasitic”, “antifungal”, “antituberculosis”, “anti-inflammatory”, “antidiabetic”, “hepatoprotective.” The inclusion criteria for the articles are as follows: the full text of relevant original research articles written in English or Malay reporting the pharmacological activities of G. celebica or its related compounds that contribute to bioactivities; cellular and/or animal and toxicological studies were included in this review. A total of 41 relevant studies were included in this review.
3. Phytochemical constituents
Phytochemical studies have been extensively carried out on different parts of G. celebica resulting in the identification of various classes of secondary metabolites including benzophenones, triterpenoids, xanthones, flavonoids and bisflavonoids. In addition to these classes, sesquiterpenoids, sesquiterpenes, depisidones, sterols, benzoic acid derivatives, coumaric acids, fatty acids and ionone-derived glycosides also enrich the diversity of the phytochemistry of G. celebica [13,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. To date, as many as 100 compounds have been identified in this review according to our search. A comprehensive list of the compounds from G. celebica and their chemical structures are presented in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10 and Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, respectively.
Table 1.
Benzophenones isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 1 | (2,4-dihydroxy-6-methoxyphenyl)(3,5-dihydroxyphenyl)methanone monohydrate | Bark | Ethyl actate | [29] |
| 2 | 2,3′,4,5′-tetrahydroxy-6-methoxybenzophenone | Root | Acetone | [17] |
| Bark | Ethyl acetate | [17] | ||
| 3 | Garcihombrianone | Root | Acetone | [18] |
| 4 | 2,3′,4,4′-tetrahydroxy-6-methoxybenzophenone | Bark | Ethyl acetate | [17] |
| 5 | 2,3′,4,6-tetrahydroxybenzophenone | Bark | Ethyl acetate | [17] |
| 6 | 3,5,3′,5′-tetrahydroxy-4-methoxybenzophenone | Twig | Ethyl acetate | [30] |
| 7 | (−)‐Cycloxanthochymol | Root bark | Ethyl acetate | [31] |
| 8 | Isoxanthochymol | Root bark | Ethyl acetate | [31] |
| 9 | Xanthochymol | Root bark | Ethyl acetate | [31] |
Table 2.
Triterpenoids isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 10 | (22Z,24E)-3β-hydroxycycloart-14,22,24-trien-26-oic acid | Bark | Dichloromethane | [32] |
| 11 | Garcihombronane G | Bark | Dichloromethane | [32] |
| Leaf | Methanol | [33] | ||
| 12 | Garcihombronane J | Bark | Dichloromethane | [32] |
| Leaf | Methanol | [33] | ||
| 13 | 3β-acetoxy-9α-hydroxy-17,14-friedolanostan-14,24-dien-26-oic acid | Bark | Dichloromethane | [32] |
| 14 | Garcihombronane F/(22Z, 24E)-3β,9α-dihydroxy-17,14-friedolanostan-14,22,24-trien-26-oic acid | Bark | Dichloromethane | [32] |
| Leaf | Methanol | [33] | ||
| Twig | Methanol | [14] | ||
| Twig | Ethyl acetate | [30] | ||
| 15 | 3β, 23α-dihydroxy-17,14-friedolanostan-8,14,24-trien-26-oic acid | Twig | Ethyl acetate | [32] |
| 16 | (E)-3β,9α-dihydroxylanosta-24-en-26-oic acid | Bark | Ethyl acetate | [34] |
| 17 | 3,23-dioxo-9,16-lanostadien-26-oic acid | Bark | Ethyl acetate | [34] |
| 18 | (24E)-3-oxo-17,14-friedolanosta-8,14,24-trien-26-oic acid | Bark | Ethyl acetate | [34] |
| 19 | (22Z,24E)-9α-hydroxy-3-oxo-17,13-friedolanosta-12,22,24-trien-26-oic acid | Bark | Ethyl acetate | [34] |
| 20 | (22Z,24E)-3-oxo-17,14-friedolanosta-8,14,22,24-tetraen-26-oic acid | Bark | Ethyl acetate | [34] |
| 21 | (22Z,24E)-9α-hydroxy-3-oxo-13α,30-cyclo-17,13-friedolanosta-22,24-dien-26-oic acid | Bark | Ethyl acetate | [34] |
| 22 | Mangiferolic acid | Bark | Ethyl acetate | [34] |
| Bark | Dichloromethane and ethyl acetate | [35] | ||
| 23 | (22Z,24E)-9α-hydroxy-3-oxo-17,14-friedolanosta-14,22,24-trien-26-oic acid | Bark | Ethyl acetate | [34] |
| 24 | (24E)-3β-acetoxy-9α-hydroxy-17,14-friedolanosta-14,24-dien-26-oic acid | Bark | Ethyl acetate | [34] |
| 25 | (22Z,24E)-3β-acetoxy-9α-hydroxy-17,14-friedolanosta-14,22,24-trien-26-oic acid | Bark | Ethyl acetate | [34] |
| 26 | Garcihombronane B/(24E)-3α,9,23-trihydroxy-17,14-friedolanostan-14,24-dien-26-oate | Pericarp | Dichloromethane | [36] |
| Bark | Dichloromethane | [17] | ||
| Leaf | n-Hexane | [37] | ||
| Leaf | Methanol | [33] | ||
| Twig | Methanol | [14] | ||
| Twig | Ethyl acetate | [30] | ||
| Pericarp | Dichloromethane | [38] | ||
| 27 | Garcihombronane C | Pericarp | Dichloromthane | [36] |
| Leaf | Methanol | [33] | ||
| Pericarp | Dichloromethane | [38] | ||
| 28 | Friedelin/Friedeline | Leaf | n-Hexane | [37] |
| Leaf | Ethyl acetate | [39] | ||
| Leaf | Chloroform | [40] | ||
| Twigs | Ethyl acetate | [30] | ||
| 29 | Methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat | Leaf | n-Hexane | [37] |
| Leaf | Ethanol | [41] | ||
| 30 | Garcihombronane D/3β-hydroxy-23-oxo-9,16-lanostadien-26-oic acid | Leaf | Ethyl acetate | [39] |
| Leaf | Chloroform | [40] | ||
| Bark | Dichloromethane | [17] | ||
| Leaf | Methanol | [33] | ||
| Twig | Methanol | [14] | ||
| Twig | n-Hexane | [30] | ||
| Pericarp | Dichloromethane | [38] | ||
| 31 | 2β-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid | Bark | Dichloromethane | [42] |
| 32 | Taraxerol | Bark | Dichloromethane | [42] |
| Leaf | Chloroform | [42] | ||
| 33 | Taraxerone | Bark | Dichloromethane | [42] |
| 34 | Betulin | Bark | Dichloromethane | [42] |
| Bark | Dichloromethane and ethyl acetate | [35] | ||
| Leaf | Chloroform | [40] | ||
| 35 | Betulinic acid | Bark | Dichloromethane | [42] |
| Bark | Dichloromethane and ethyl acetate | [35] | ||
| Leaf | Chloroform | [40] | ||
| 36 | Garcihombronane N/18(13 → 17)-abeo-3β -acetoxy-9α,13β-lanost-24E-en-26-oic acid |
Bark | Dichloromethane | [17] |
| 37 | Friedelan-3-one | Bark | Dichloromethane | [17] |
| 38 | Lupeol | Bark | Dichloromethane | [17] |
| Twig | n-Hexane | [30] | ||
| Bark | Dichloromethane and ethyl acetate | [35] | ||
| Leaf | Chloroform | [40] | ||
| 39 | Garcihombronane H | Leaf | Methanol | [33] |
| 40 | Garcihombronane I | Leaf | Methanol | [33] |
| 41 | Garcihombronane E/3α-Hydroxy-23-oxo-9,16-lanostadien-26-oic acid | Leaf | Methanol | [33] |
| Twig | Ethyl acetate | [30] | ||
| Pericarp | Dichloromethane | [38] | ||
| 42 | Methyl (25R)-3β-hydroxy-23-oxo-9,15-lanostadien-26-oate | Twig | Ethyl acetate | [31] |
| 43 | Garcihombronane K/(24E)-3β-hydroxy-9α-hydroxy-17,14-friedolanosta-14,24-dien-26-oic acid | Twig | Methanol | [30] |
| 44 | Garcihombronane L/Methyl (24E)-3α-9α-23α-trihydroxy-15-oxo-17,14-friedolanosta-8(14),24-dien-26-oate | Twig | Methanol | [14] |
| 45 | Glutin-5-en-3β-ol | Twig | n-Hexane | [30] |
| 46 | (24E)-3α-hydroxy-17,14-friedolanostan-8,14,24-trien-26-oic acid | Pericarp | Dichloromethane | [38] |
| 47 | Lupeol acetate | Bark | Dichloromethane and ethyl acetate | [35] |
| 48 | 3β-acetoxy-lup-12,20(29)-diene | Bark | Dichloromethane and ethyl acetate | [35] |
| 49 | Ursolic acid | Bark | Dichloromethane and ethyl acetate | [35] |
Table 3.
Sesquiterpenoid and sesquiterpene from G. celebica.
Table 4.
Depsidone and xanthones isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 54 | Garcinisidone H | Bark | Ethyl acetate | [34] |
| 55 | Macluraxanthone | Bark | Ethyl acetate | [34] |
| 56 | Toxyloxanthone B | Bark | Ethyl acetate | [34] |
| Twig | Methanol | [14] | ||
| 57 | Nigrolineaxanthone A | Bark | Ethyl acetate | [34] |
| 58 | Nigrolineaxanthone E | Bark | Ethyl acetate | [34] |
| 59 | 6-deoxyjacareubin | Bark | Ethyl acetate | [34] |
| 60 | 6-deoxyisojacareubin | Bark | Ethyl acetate | [34] |
| 61 | Morusignin A | Bark | Ethyl acetate | [34] |
| 62 | Isocudraniaxanthone B | Bark | Ethyl acetate | [35] |
| 63 | Garcihomxanthone | Root | Acetone | [18] |
| 64 | Garceduxanthone | Root | n-Hexane | [18] |
| 65 | Cheffouxanthone | Root | n-Hexane | [18] |
| Twig | Methanol | [14] | ||
| 66 | Norathyriol/1,3,6,7-tetrahydroxyxanthone | Root | Acetone | [18] |
| Bark | Ethyl acetate | [17] | ||
| Twig | Methanol | [14] | ||
| 67 | Garcihombronone A/1,5,8-trihydroxy-furano [2,3-c]xanthone | Twig | Methanol | [14] |
| 68 | Garcihombronone B/4-(3,7-dimethyl-6-hydroxy-2,7-octadienyl)-1,3,5,8-tetrahydroxyxanthone | Twig | Methanol | [14] |
| 69 | Garcihombronone C/1,3,8-trihydroxy-4,6-dimethoxyxanthone | Twig | Methanol | [14] |
| 70 | Garcihombronone D/1,3,7-trihydroxy-4,6-dimethoxyxanthone | Twig | Methanol | [14] |
| 71 | Bangangxanthone A | Twig | Methanol | [14] |
| 72 | Gentisein/1,3,7-trihydroxyxanthone | Twig | Methanol | [14] |
| 73 | 1,3,6,7-tetrahydroxy-8-prenylxanthone | Twig | Methanol | [14] |
| 74 | 1,7-dihydroxyxanthone | Twig | n-Hexane | [30] |
| 75 | 1,3,6-trihydroxy-7-methoxy-2,8-(3-methyl-2-butenyl)xanthone | Bark | Dichloromethane and ethyl acetate | [35] |
Table 5.
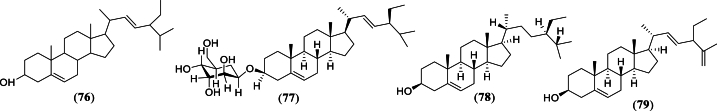
Sterols isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 76 | Stigmasterol | Pericarp | n-Hexane | [36] |
| Bark | Dichloromethane | [17] | ||
| Twig | n-Hexane | [30] | ||
| 77 | Stigmasterol glucoside | Bark | Dichloromethane | [17] |
| Leaf | Chloroform | [40] | ||
| 78 | β-sitosterol | Bark | Dichloromethane and ethyl acetate | [35] |
| 79 | 22-dehydroclerosterol | Bark | Dichloromethane and ethyl acetate | [35] |
Table 6.
Flavonoids isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 80 | Catechin | Leaf | Ethyl acetate | [37] |
| Leaf | Ethanol | [44] | ||
| 81 | 3,3′,4′,5,7-pentahydroxyflavone | Bark | Ethyl acetate | [17] |
| 82 | 3,3′,5,5′,7-pentahydroxyflavanone | Bark | Ethyl acetate | [17] |
| 83 | 3,3′,4′,5,5′,7-hexahydroxyflavone | Bark | Ethyl acetate | [17] |
| 84 | 4′,5,7-trihydroxyflavanone-7-rutinoside | Bark | Ethyl acetate | [17] |
| 85 | Vitexin | Leaf | Methanol | [33] |
| 86 | Isovitexin | Leaf | Methanol | [33] |
Table 7.
Bisflavonoids isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 87 | (2R, 3S) volkensiflavone-7-O-rhamnopyranoside | Bark | Ethyl acetate | [45] |
| 88 | Volkensiflavone | Bark | Ethyl acetate | [45] |
| Twig | Methanol | [45] | ||
| 89 | 4″-O-methyl-volkensiflavone | Bark | Ethyl acetate | [45] |
| 90 | Volkensiflavone-7-O-glucopyranoside | Bark | Ethyl acetate | [45] |
| 91 | Morelloflavone | Bark | Ethyl acetate | [45] |
| 92 | 3″-O-methyl-morelloflavone | Bark | Ethyl acetate | [45] |
| 93 | Morelloflavone-7-O-glucopyranoside | Bark | Ethyl acetate | [45] |
Table 8.
Benzoic acid derivatives and coumaric acid isolated from G. celebica.
| Structure number | Compound | Plant part | Extract | References |
|---|---|---|---|---|
| 94 | 4-hydroxybenzoic acid/p-Hydroxybenzoic acid | Twig | Methanol | [14] |
| Bark | Dichloromethane and ethyl acetate | [35] | ||
| Leaf | Chloroform | [40] | ||
| 95 | Protocatechuic acid methyl ester | Twig | Methanol | [14] |
| 96 | p-hydroxycinnamate | Bark | Dichloromethane and ethyl acetate | [35] |
Table 9.
Ionone-derived glycosides isolated from G. celebica.
Table 10.
Fatty acids isolated from G. celebica.
Fig. 1.
Benzophenones from G. celebica.
Fig. 2.
Triterpenoids from G. celebica.
Fig. 3.
Sesquiterpenoid and sesquiterpenes found in G. celebica.
Fig. 4.
Depsidones and xanthones from G. celebica.
Fig. 5.
Sterols from G. celebica.
Fig. 6.
Flavonoids from G. celebica.
Fig. 7.
Bisflavonoids from G. celebica.
Fig. 8.

Benzoic acids derivatives and coumaric acid isolated from G. celebica.
Fig. 9.
Ionone-derived glycosides isolated from G. celebica.
Fig. 10.
Fatty acids from G. celebica.
3.1. Benzophenones
Based on literature reports, nine benzophenones from different parts of G. celebica were found in this species. These compounds are listed in Table 1 and their structures are depicted in Fig. 1. Among those, 2,3′,4,5′-tetrahydroxy-6-methoxybenzophenone (2) and garcihombrianone (3) were first reported from this species while the three known polyprenylated benzophenones—(−)-cycloxanthochymol (7), isoxanthochymol (8), and xanthochymol (9) were isolated for the first time in this species [18,45,31].
3.2. Triterpenoids
An abundance of studies demonstrated the presence of triterpenoids 10–49 (Fig. 2) from various parts of G. celebica (Table 2). Compounds 10–14 were isolated from dichloromethane bark and methanol leaf extracts [33,32], while compounds 15 and 16 were found in ethyl acetate twig and bark extracts [30,34]. The number of compounds recorded from this class was the highest among other classes in this review. Garcihombronane B or (24E)-3α,9,23-trihydroxy-17,14-friedolanostan-14,24-dien-26-oate (26) is the most predominant triterpenoid found in the pericarp, bark, twig and leaf of this plant [14,17,33,[30], [34], [35], [36], [38]]. Compounds 28 and 29 were found in n-hexane leaf extract [37]. 2β-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid (31), taraxerol (32), taraxerone (33), betulin (34), betulinic acid (35), garcihombronane N (36), friedelan-3-one (37) and lupeol (38) were found in dichloromethane bark extract [17,35,42,40]. Furthermore, compound 42 was isolated from ethyl acetate twig extract while compound 45 was isolated from n-hexane twig extract [29]. Lupeol acetate (47), 3β-acetoxy-lup-12,20(29)-diene (48) and ursolic acid (49) were isolated from dichloromethane and ethyl acetate bark extract [35].
3.3. Sesquiterpenoids and sesquiterpenes
Leucodin (50) was isolated from the dichloromethane and ethyl acetate bark extracts of G. celebica [35]. Specifically, the major components found in essential oil from the leaf of G. celebica were α-copaene (61.25 %) (51), germacrene D (6.72 %) (52) and β-caryophyllene (5.85 %) (53). This was the first report that reported the chemical composition of sesquiterpenes rich essential oil from G. celebica (Fig. 3 and Table 3) [43].
3.4. Depsidones and xanthones
Garcinisidone H (54) was isolated from the ethyl acetate bark extract of G. celebica [34]. Xanthone 55–75 were discovered to be present in G. celebica in many studies [14,17,18,30,34,35]. Furthermore, garcihomxanthone (63) was isolated from the acetone extract of the root of G. celebica (Fig. 4 and Table 4) [18].
3.5. Sterols
A total of four sterols (compounds 76–79) were reported found in G. celebica from several studies (Fig. 5 and Table 5) [17,30,35,36,40].
3.6. Flavonoids
Flavonoids 80–86 were present in the leaf and bark of G. celebica (Fig. 6 and Table 6) [17,33,37,44]. Catechin (80) was obtained after isolation from ethyl acetate and ethanol leaf extracts [37,44]. Vitexin (85) and isovitexin (86) on the other hand, were isolated from methanolic leaf extract [33].
3.7. Bisflavonoids
Investigation of the bisflavonoids found in G. celebica was pioneered by Jamila et al. (2014) as they reported the isolation of compounds 87–93 from the bark of ethyl acetate and methanolic twig extract (Fig. 6 and Table 6) [45]. (2R, 3S) volkensiflavone-7-O-rhamnopyranoside (87), was firstly discovered from this plant while compounds 88–93 known as 3 → 8 rotameric bisflavonoids.
3.8. Benzoic acid derivatives and coumaric acids
Two benzoic acid derivatives, 4-hydroxybenzoic acid or p-hydroxybenzoic acid (94) and protocatechuic acid methyl ester (95) were found in the twig and bark of G. celebica [35,40]. Meanwhile, p-hydroxycinnamate (96) was found in the dichloromethane and ethyl acetate of the bark extract (Fig. 8 and Table 8) [35].
3.9. Ionone-derived glycosides
Compounds 97 and 98, namely, blumenol C 9-O-β-d-apiofuranosyl-(1 → 6)-β-d-glucopyranoside and vomifoliol 9-O-β-d-apiofuranosyl-(1 → 6)-β-d-glucopyranoside were isolated from the methanolic leaf extract of G. celebica (Fig. 9 and Table 9) [33].
3.10. Fatty acids
Stearic acid (99) was reported to be found in dichloromethane and ethyl acetate extracts while palmitic acid (100) in chloroform leaf extract of G. celebica (Fig. 10 and Table 10) [35,40].
4. Pharmacological effects of Garcinia celebica
This review extensively screened all the studies to gather relevant information regarding its pharmacological effects. The pharmacological activities of the extracts and isolated compounds from G. celebica were all compiled and summarized in Table 11 and Table 12. However, the effects were not extensively studied.
Table 11.
Pharmacological effects of crude extract and fraction from G. celebica.
| Pharmacological activity | Plant part | Assay type | References |
|---|---|---|---|
| Antioxidant | Leaf | FRAP | [13] |
| Bark | FRAP | [26] | |
| Bark | DPPH, FRAP, Folin-Ciocalteu and ABTS | [22] | |
| Rootbark | DPPH, ABTS and FRAP | [31] | |
| Leaf | FRS, FRP and FIC | [27] | |
| Pericarp | FRS, FRP and FIC | [27] | |
| Fruit pulp | FRS, FRP and FIC | [27] | |
| Fruit | TBARS | [21] | |
| Bark | DPPH, ABTS and FRAP | [24] | |
| Fruit | DPPH, ABTS and FRAP | [24] | |
| Leaf | Lipoxygenase enzyme inhibition | [13] | |
| Stembark | Lipoxygenase enzyme inhibition | [26] | |
| Cholinesterase inhibitor | Bark | Ellman | [22] |
| Cytotoxic | Stem bark | MTT | [46] |
| Root bark | PrestoBlue | [31] | |
| Bark | MTT | [25] | |
| Leaf | MTT and Annexin | [47] | |
| Leaf | MTT | [23] | |
| Leaf | MTT | [43] | |
| Leaf | MTT | [47] | |
| Stem bark | Brine shrimp test | [19] | |
| Antibacterial | Stem bark and pericarp | Disc diffusion, MIC, MBC | [36] |
| Leaf | Disc diffusion, MIC, MBC | [48] | |
| Bark | Disc diffusion, MIC | [22] | |
| Leaf | Disc diffusion | [20] | |
| Stem bark | Disc diffusion | [19] | |
| Anti-neuraminidase | Leaf | MUNANA | [37] |
| Antiparasitic | Leaf | Antitrypanosomal | [23] |
| Leaf | Antitrypanosomal | [49] | |
| Leaf | Antiplasmodial | [44] | |
| Leaf | Antiplasmodial | [28] | |
| Root bark | Antiplasmodial | [31] | |
| Antiaggregant | Leaf | Platelet aggregation | [21] |
| Twig | Platelet aggregation | [21] | |
| Fruit | Platelet aggregation | [21] | |
| Antituberculosis | Bark | Collins's BACTEC 460 | [24] |
| Antifungal | Leaf | Candida albicans agar dilution method | [49] |
| Antiviral | Stem bark | JFH1 strain of hepatitis C | [46] |
| Hepatoprotective | Bark | ALP, ALT and AST | [25] |
| Antidiabetic | Leaf | α-glucosidase | [50] |
| Fruit | α-glucosidase and α-amylase | [24] |
Abbrevations: ABTS, 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid); ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase DPPH, 2,2-diphenyl-1-picrylhydrazyl; FRAP, ferric ion reducing antioxidant power; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction; MUNANA, 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid; TBARS, thiobarbituric acid reactive substances.
Table 12.
Pharmacological activity of compounds from G. celebica.
| Compound | Structure number | Chemical class | Pharmacological activity | References |
|---|---|---|---|---|
| 2,3′,4,5′-tetrahydroxy-6-methoxybenzophenone | 2 | Benzophenone | Antioxidant, anticholinesterase and cytotoxic | [17,32] |
| 2,3′,4,4′-tetrahydroxy-6-methoxybenzophenone | 4 | Benzophenone | Antioxidant | [17] |
| 2,3′,4,6-tetrahydroxybenzophenone | 5 | Benzophenone | Antioxidant | [17] |
| 3,5,3′,5′-tetrahydroxy-4-methoxybenzophenone | 6 | Benzophenone | Antioxidant and platelet aggregation | [30] |
| (22Z,24E)-3β-hydroxycycloart-14,22,24-trien-26-oic acid | 10 | Triterpenenoid | Anticholinesterase | [32] |
| Garcihombronane G | 11 | Triterpenoid | Anticholinesterase | [32] |
| Garcihombronane J | 12 | Triterpenoid | Anticholinesterase | [32] |
| 3β-acetoxy-9α-hydroxy-17,14-friedolanostan-14,24-dien-26-oic acid | 13 | Triterpenoid | Anticholinesterase | [32] |
| Garcihombronane F/(22Z, 24E)-3β, 9α-dihydroxy-17,14-friedolanostan-14,22,24-trien-26-oic acid | 14 | Triterpenoid | Anticholinesterase and platelet aggregation | [32,30] |
| 3β, 23α-dihydroxy-17,14-friedolanostan-8,14,24-trien-26-oic acid | 15 | Triterpenoid | Anticholinester ase | [32] |
| (22Z,24E)-9α-hydroxy-3-oxo-17,14-friedolanosta-14,22,24-trien-26-oic acid | 23 | Triterpenoid | Cytotoxic | [34] |
| (24E)-3β-acetoxy-9α-hydroxy-17,14-friedolanosta-14,24-dien-26-oic acid | 24 | Triterpenoid | Cytotoxic | [34] |
| (22Z,24E)-3β-acetoxy-9α-hydroxy-17,14-friedolanosta-14,22,24-trien-26-oic acid | 25 | Triterpenoid | Cytotoxic | [34] |
| Garcihombronane B/(24E)-3α,9,23-trihydroxy-17,14-friedolanostan-14,24-dien-26-oate | 26 | Triterpenoid | Antioxidant, anticholinesterase, antibacterial and antineuraminidase | [32,30,36,37] |
| Garcihombronane C | 27 | Triterpenoid | Antibacterial | [36] |
| Methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat | 29 | Triterpenoid | Cytotoxic and antineuraminidase | [41] |
| Garcihombronane D/3β-hydroxy-23-oxo-9,16-lanostadien-26-oic acid | 30 | Triterpenoid | Anticholinesterase, antiparasitic and platelet aggregation | [32,30,39] |
| 2β-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid | 31 | Triterpenoid | Antioxidant | [42] |
| Taraxerol | 32 | Triterpenoid | Anticholinesterase | [32] |
| Betulin | 34 | Triterpenoid | Anticholinesterase | [32] |
| Betulinic acid | 35 | Triterpenoid | Anticholinesterase | [32] |
| Garcihombronane N/18(13 → 17)-abeo-3β-acetoxy-9α,13β-lanost-24E-en-26-oic acid | 36 | Triterpenoid | Cytotoxic | [17] |
| Ursolic acid | 49 | Triterpenoid | Antibacterial | [35] |
| Leucodin | 50 | Sesquiterpenoid | Antibacterial | [35] |
| Macluraxanthone | 55 | Xanthone | Cytotoxic | [34] |
| Nigrolineaxanthone E | 57 | Xanthone | Cytotoxic | [34] |
| Cheffouxanthone | 65 | Xanthone | Antibacterial | [14] |
| Norathyriol/1,3,6,7-tetrahydroxyxanthone | 66 | Xanthone | Antioxidant | [17] |
| Bangangxanthone A | 71 | Xanthone | Antibacterial | [14] |
| 1,7-dihydroxyxanthone | 74 | Xanthone | Antioxidant and platelet aggregation | [30] |
| 1,3,6-trihydroxy-7-methoxy-2,8-(3-methyl-2-butenyl)xanthone | 75 | Xanthone | Antioxidant and Antibacterial | [35] |
| Catechin | 80 | Flavonoid | Antineuraminidase and antiparasitic | [37,44] |
| Stigmasterol | 76 | Sterol | Antibacterial | [36] |
| 3,3′,4′,5,7-pentahydroxyflavone | 81 | Flavonoid | Antioxidant | [17] |
| 3,3′,5,5′,7-pentahydroxyflavanone | 82 | Flavonoid | Antioxidant | [17] |
| 3,3′,4′,5,5′,7-hexahydroxyflavone | 83 | Flavonoid | Antioxidant | [17] |
| 4′,5,7-trihydroxyflavanone-7-rutinoside | 84 | Flavonoid | Antioxidant | [17] |
| (2R, 3S) volkensiflavone-7-O-rhamnopyranoside | 87 | Bisflavonoid | Antioxidant | [45] |
| Volkensiflavone | 88 | Bisflavonoid | Antioxidant and antioxidant | [45] |
| 4″-O-methyl-volkensiflavone | 89 | Bisflavonoid | Antioxidant, antibacterial and antituberculosis | [45] |
| Volkensiflavone-7-O-glucopyranoside | 90 | Bisflavonoid | Antioxidant | [45] |
| Morelloflavone | 91 | Bisflavonoid | Antioxidant and antibacterial | [45] |
| 3″-O-methyl-morelloflavone | 92 | Bisflavonoid | Antioxidant, antibacterial and antituberculosis | [45] |
| Morelloflavone-7-O-glucopyranoside | 93 | Bisflavonoid | Antioxidant | [45] |
| 4-hydroxybenzoic acid/p-hydroxybenzoic acid | 94 | Benzoic acid derivative | Antioxidant | [35] |
| p-hydroxycinnamate | 96 | Coumaric acid | Antioxidant and antibacterial | [35] |
4.1. Antioxidant activity
Oxidative stress and inflammation are the culprits in almost all diseases. Increased dietary intake of polyphenol-rich foods reported to significantly lower the prevalence of diseases owing to its antioxidant properties [51,52]. G. celebica were evaluated by different in vitro antioxidant assays like 2,2-diphenyl-1-picrylhydrazyl (DPPH), folin-Ciocalteu (FCA), lipoxygenase enzyme inhibition, ferric ion reducing antioxidant power (FRAP), ferrous ion chelating (FIC), ferric reducing power (FRP), and 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and free radical scavenging (FRS) capacity assays. Marlin et al. (2017) showed that ethyl acetate of G. celebica leaf extract has the most active antioxidant activity than n-hexane and methanol extract based on FRAP, lipoxygenase inhibition and total phenolic assays which were comparable to standard baicalein [13]. The EC50 values of FRAP recorded for hexane, ethyl acetate and methanol leaf extracts were consecutively 36.3, 2.97, and 7.42 μg/mL in comparison to baicalein (1.17 μg/mL). Similarly, ethyl acetate extract exhibited highest activity on lipoxygenase inhibition activity with IC50 of 2.05, 0.13 and 1.31 μg/mL consecutively for n-hexane, ethyl acetate and methanol with IC50 0.25 μg/mL recorded for baicalein. High total flavonoid content in the ethyl acetate extract (42.0 mg quercetin equivalents/g), presumably attributed to this good antioxidant capacity [13].
In another study, different part of the plant using leaf, pericarp and fruit pulp were investigated in G. celebica, G. mangostana and G. atroviridis [27]. Based on these three species investigated, G. celebica leaf and pericarp had the second highest activities on total phenolic and anthocyanin content, FRS, FRP and FIC after G. mangostana. G. celebica fruit pulp on the other hand possessed highest activities than G. atroviridis and G. mangostana on similar parameters tested but were relatively low compared to leaf and pericarp parts [27]. The fruit part also exhibited remarkably low percentage inhibition of low-density lipoprotein oxidation with IC50 value of 50.0 ± 4.8 than the positive control probucol of 0.3 ± 0.1 μg/mL suggested that fruit pulp had lowest antioxidant activities than leaf and pericarp [21,27]. Contrastingly in another study, the leaf extracts showed weak antioxidant activity suggesting that the difference in geographical location and climate conditions may affect in the antioxidant compound composition [40]. Their isolated compounds such as benzophenones (2, 4, 5, 7, 8 and 9), triterpenoid (31), xanthones (73 and 74), flavonoids (81, 82, 83 and 84), bisflavonoid (91), benzoic acid (94) and coumaric acid (96) showed remarkable radical scavenging capacities on DPPH, ABTS and FRAP assays comparable to their reference gallic acid, quercetin and ascorbic acid indicated that G. celebica could be a good source of antioxidants (Table 12). However to our knowledge, the antioxidant effects of G. celebica was only investigated in vitro and not yet studied in animal models. Hence detailed mechanistic insights into the plant's mitigative oxidative stress are still lacking and not fully understood remains to be further investigated. The bioactive compounds might confer antioxidant effects possibly by eradicating the formation of reactive oxygen species [17,45,31,35,42].
4.2. Cholinesterase inhibitory activity
Currently, no drug has yet emerged to cure for Alzheimer's disease. Anticholinesterase agents including rivastigmine, donepzil, and galanthamine can only temporarily slow the worsening of disease progression which warrants further research. Medicinal plants have emerged as a valuable resource as potential therapeutic agents that have been extensively studied [53,54]. Among the examined G. celebica bark extracts, ethyl acetate extract demonstrated the strongest inhibition against both acetylcholinesterase (AchE) and butyrylcholinesterase (BChE) enzymes with IC50 values of 13.7 ± 1.56 and 32.2 ± 0.36 μg/mL, compared to that of the reference drug physostigmine (IC50 values of 0.04 and 0.09 μg/mL) respectively. The dichloromethane extract on the other hand showed reasonable inhibition while the water and methanol extracts showed very weak inhibition [22]. Polyphenolic compounds (2, 10, 11, 12, 13, 14, 15, 26 and 30) isolated from G. celebica bark extract displayed good dual inhibition with more than 50 % inhibition on both enzymes. However, these compounds showed less inhibition effects than the standard physostigmine on both enzymes [32]. In contrast, 2β-hydroxy-3α-O-caffeoyltaraxar-14-en-28-oic acid (31), betulin (34) and betulinic acid (35) showed moderate inhibition on AChE with IC50 values of 13.5, 28.5 and 24.2 μM, respectively. Compound 31, taraxerol (32) and 35 were found to moderately inhibited BChE with IC50 values of 10.6, 17.8 and 19.1 μM, respectively with compounds 31 and 35 were more selective towards BChE with selectivity indices of 1.35 and 1.26, respectively [42]. The exact mechanism of how the plant acts to inhibit the enzymes is still unclear but a restoration of ACh function through elevation of its level ameliorating its deficiency.
4.3. Cytotoxic properties
In vitro cytotoxicity of G. celebica was evaluated in human breast (MCF-7, MCF-7/TAMR-1 and MCF-10A), glioblastoma (DBTRG), hepatocellular carcinoma-derived (Huh7it-1), cervix adenocarcinoma (HeLa), lung carcinoma (A549) and murine melanoma (B16) cell lines. The acetone stembark extract exhibited high toxicity at concentrations of 2.5–20 μg/mL against Huh7it-1 cells with a CC50 value of 1.6 μg/mL while methanolic ethyl acetate bark extract (10–100 μg/mL) dose-dependently induced maximum cell death on MCF-7 and DBTRG cancer cell lines at 78.7 % and 64.3 %, respectively after 24 h treatment [25,46]. A similar outcome was also observed with their secondary bioactive compounds of 2,3′,4,5′-tetrahydroxy-6-methoxybenzophenone (2) and 18(13 → 17)-abeo-3β-acetoxy-9α,13β-lanost-24E-en-26-oic acid (36) at 20 μM that contained in bark extract, induced maximum cell death of 49 % and 24 % respectively on DBTRG cells after 72 h treatment. Both compounds 2 and 36 ranging from 10 to 75 μM up to 96 h treatment were found to cause a time- and concentration-dependent cell death on DBTRG cells with compound 36 exhibited higher cytotoxicity potential (EC50 value of 34 μM) than compound 2 (EC50 value of 48 μM) [17]. Apart from that, in another study, the essential oil derived G. celebica leaf extract was shown potently inhibited 50 % proliferation of the MCF-7 and MCF-7/TAMR-1 cells at 37.5 and 18.8 μg/mL respectively [43,47]. While in contrast, higher concentration (77.5 μg/mL) was needed to inhibit MCF-10A cells. The findings indicated that the essential oil had selective cytotoxic effects inducing apoptotic cell death in human breast cell lines [43,47]. In addition, xanthone (55 and 58) as well as triterpenoids (23, 24 and 25) displayed cytotoxic activities on MCF-7 cells [34]. Compound 55 had the strongest cytotoxicity activity with an IC50 value of 6.1 μM while compounds 23, 24, 25 and 58 exhibited weak cytotoxicity with IC50 values ranging from 37.0 to 61.9 μM in comparison with cisplatin and camptothecin at 12 and 0.03 μM, respectively. Compounds 19, 21, 48, 57, 60, 61 however were found inactive [34].
Similar effect was also observed with purified methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat (29) from the leaf extract that exhibited inhibitory activity in a time and dose dependently manner towards the MCF-7 cell proliferation with IC50 values of 82 and 70 μM in 24 and 48 h respectively [41]. Benzophenone (7, 8 and 9), on the other hand exhibited adequate cytotoxicity against four cancer cell lines (HeLa, MCF-7, A549, and B16) with IC50 values ranging of 10.2–16.9 μM, compared to cisplatin (18–43 μM) as positive control [31]. Collectively, this beneficial biological activity is mediated by their structure-activity relationship influenced by the presence of chemical structure such as higher number of hydroxyl groups, pyran ring, prenyls group and chromene system in their skeleton which responsible promoting apoptosis evidenced by inhibition of the oncogenic protein Akt, thereby increases the expression of poly (ADP-ribose) polymerase (PARP) apoptosis biomarker contributing to this cytotoxicity property. Of note, Akt is a provital survival signaling pathway involved in malignancy [31,34,41]. However, pro- or anti-apoptotic, estrogen receptor and phosphorylated Akt were not investigated that could be explored to understand the mechanistic effects providing directions for further research.
4.4. Antimicrobial activity
Antibacterial action of G. celebica extract and their compounds contained in aerial parts were investigated toward various bacteria strains [14,19,20,22,35,36,43,48]. So far, enormous reports were mainly restricted to in vitro studies, which do not guarantee the same results in animal and clinical setting. There is also very limited data reported for their antiviral and antifungal activity [20,46]. The bark extract was demonstrated to exhibits anti-hepatitis C virus activity against JFH1 strain genotype 2a hepatitis C virus at 20, 10, 5 and 2.5 μg/mL with an IC50 value less than 1.25 μg/mL [46]. Furthermore, the leaf extracts investigated on Candica albicans, Escherichia coli, Staphylococcus aureus and Shigella dysenteriae revealed that the extracts were effective in all tested organisms [20,48]. The chloroform and methanol extracts at 500 and 1000 μg/mL displayed inhibitory activity against Staphylococcus aureus and Shigella dysenteriae with a minimum inhibitory concentration (MIC) value of 0.5 mg/mL which were comparable to their positive control—tetracycline HCL and nystatin [20]. In another study, G. celebica, Dillenia excelsa and Kleinhovia hospita were found to possesed strongest inhibitory effect against Escherichia coli with MIC value of 31.3, 62.5 and 6.25 μg/mL, respectively among 34 plant other species tested. Further fractionation of the extracts showed that the methanol was more potent than the hexane and ethyl acetate fraction against Escherichia coli, suggesting that polar compounds contributed to antibacterial activity [48].
The essential oil collected from the leaf of G. celebica showed minimal inhibition activity on Gram-positive bacteria (Bacillus subtilis and methicillin-resistant Staphylococcus aureus) and Gram-negative bacteria (Proteus mirabilis) with MIC values ranging from 1.25 to 2.5 mg/mL [43]. The stem bark extract also showed lower inhibition growth activity on Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Salmonella enteritidis, Enterobacter sp., Salmonella typhosa, Shigella boydii, Alkaligenes sp. and Salmonella typhi, than their reference antibiotics (erythromycin and novobiosin) [19]. Meanwhile, the acetone of pericarp extract exhibited the strongest inhibition activity against Basillus subtilis with minimum bactericidal concentration (MBC) value of 225 μg/mL than other extracts [36]. Nevertheless, the acetone extract only showed good inhibition activity against Basillus subtilis but displayed slight or no activity towards other strains (Enterococcus faecalis, Klebsiella pneumonia and Escherichia coli). In contrast, the positive control streptomycin sulfate exerted the strongest inhibition activity toward all bacteria strains, with MIC value of 14.1 μg/mL recorded for all strains. Garcihombronane B (26) and garcihombronane C (27) together with a plant sterol, stigmasterol (76) were found in the pericarp, is believed to contribute to the antibacterial activity [36].
Jamila et al. (2014) evaluated (2R, 3S) volkensiflavone-7-O-rhamnopyranoside (87), volkensiflavone (88), 4″-O-methyl-volkensiflavone (89), volkensiflavone-7-O-glucopyranoside (90), morelloflavone (91) and 3″-O-methyl-morelloflavone (92) against Staphylococcus aureus, Basillus subtilis, Escherichia coli and Pseudomonas aeruginosa with streptomycin and gentamicin as the positive control [45]. Compounds 91 and 92 showed the highest activity with MIC values recorded at 62.5 μM against Staphylococcus aureus, Basillus subtilis, Escherichia coli, followed by compounds 88 and 89. Compounds 87 and 90 were found to be moderately active [45]. Additionally, leucodin (50), 1,3,6-trihydroxy-7-methoxy-2,8-(3-methyl-2-butenyl)xanthone (75) and p-hydroxycinnamate (96) were also observed to exhibits antibacterial activity against Staphylococcus aureus and Basillus subtilis with MIC values recorded 31.3, 250, 500 μM respectively for both strains [35].
Collectively, some of the extracts showed remarkable inhibitory activity against the organisms tested and some showed minimal antibacterial activity with unrealistically high inhibitory concentration which can be concluded that the effects depend on the parts of G. celebica plant as well as solvent (polar and polar). The possible mechanism of the antibacterial activity could be induction of lipid flippase activity, DNA gyrase and synthesis which enhances bacterial cellular membrane damage [55].
4.5. Anti-neuraminidase activity
Neuraminidase (NA) is a major target for influenza antivirals due to the fact that it has essential enzyme activity for virus replication in combating influenza viruses [56]. Muchtaridi et al. (2020) employed the 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) assay to evaluate the anti-neuraminidase activity of G. celebica using a bioassay-guided isolation method to obtain lead compounds [37]. The crude methanol leaf extract showed substantial inhibitory activity against Clostridium perfringens-NA with an IC50 value of 4.84 μg/mL. The fraction from the crude methanol extract, ethyl acetate, recorded IC50 values of 8.73 and 48.4 μg/mL, while 11.2 and 74 μg/mL were recorded for hexane, against Clostridium perfringens-NA and H1N1-NA, respectively. Compounds 26 (garcihombronane B/24E-3α,9,23-trihydroxy-17,14-friedolanostan-14,24-dien-26-oate), 28 (friedelin) and 29 (methyl-3α,23-dihydroxy-17,14-friedolanstan-8,14,24-trien-26-oat) were isolated from the hexane fraction, while compound 80 (catechin) was isolated from the ethyl acetate fraction. Compound 28 however was found to be not active against Clostridium perfringens-NA. Compounds 26 and 29 showed inhibition against C. perfringens-NA with a maximum inhibition of 79 % and 62 %, respectively, but did not show significant activity on H1N1-NA. This might be due to the fact that isolated compounds from the hexane fraction exhibit hydrophobic molecules. On the flip side, catechin showed good inhibition with IC50 values of 60.3 μM for C. perfringens-NA and 100 μM for H1N1-NA that shows promising potential as an anti-influenza agent [37]. Nevertheless, further study is needs to be executed to understand its protective mechanisms as well as modify its structure for better inhibitory activity.
4.6. Antiparasitic activity
Trypanosoma evansi was observed to have developed a certain degree of resistance toward commercially available drugs such as suramin, diminazene aceturate, melarsamine hydro-chloride, quinapyramine sulfate, and isometamidium chloride [[57], [58], [59]]. This gives rise to the need for a new treatment for the disease.
The aqueous G. celebica leaf extract ranging from 2 mg/mL to 2.27 μg/mL was found to exhibit a concentration-dependent effect on the growth of T. evansi with an IC50 value of 23.6 μg/mL at which a concentration of 222 μg/mL resulted in complete inhibition of T. evansi growth [23]. The beneficial effects of the extract were comparable to those of diminazene aceturate. The study also reported considerably high selective antitrypanosomal activity, with a selectivity index value of 616 in the treatment group and 2078 for diminazene aceturate [23]. In Sprague Dawley rats infected with T. evansi, oral administration of 300, 600 and 1200 mg/kg of G. celebica extract failed to cure rats infected with T. evansi [49]. Nonetheless, it resulted in longer post-infection survival in the treated animals compared to the untreated group. The positive control group treated with diminazene aceturate however, showed zero parasitemia one day after being treated and remained free of parasites until 50 days post-inoculation. The prolonging effect of the post-infection longevity by the extract in the treatment group implies the extract's potential to develop as a new antitrypanosomal drug. The possible mechanism behind the antitrypanosomal activity of the extract was associated with the reduction in kinetoplast DNA activity, leading to the inability of trypanosome cells to survive [49].
On the other hand, malaria is an acute, febrile and deadly illness caused by Plasmodium species that leads to 619 000 deaths in 2021 [60]. Both compound 8 (isoxanthochymol) and 30 (garcihombronane D) exhibited activity against chloroquine-sensitive P. falciparum strain with IC50 values of 7.71 and 2.99 μM, respectively [31,39]. While in another study, Abdullah et al. (2017) and Sofian et al. (2018) showed weak antiplasmodial activity of the leaf extract at concentration greater than 11 μg/mL [28,44]. Isoxanthochymol and garcihombronane D possibly confer the antimalarial effects by eradicating reactive oxygen species and hampering malarial life cycle by selectively inhibits the action of haemoglobin digestion in the blood stages as well as heme polymerase in malarial trophozoites [61].
4.7. Platelet aggregation inhibitory activity
Platelet aggregation may lead to serious conditions such as arterial thrombus formation event, which eventually cause ischaemic stroke and acute myocardial infarction [62]. The effectiveness of certain polyphenolics derived from plants has greatly linked in the prevention of cardiovascular diseases [52,63]. Anti-platelet aggregation activity was reported in G. celebica plant. The methanol extract of the leaf and twig were found to moderately inhibited the platelet aggregation activity induced by arachidonic acid (AA) with IC50 value of 25.6 and 29.5 μg/mL, respectively in comparison to reference standard acetyl salicylic acid at 4.6 μg/mL [21]. Bioassay-guided chemical investigation revealed that 3,5,3′,5′-tetrahydroxy-4-methoxybenzophenone (6), 1,7-dihydroxyxanthone (74), as well as triterpenoid—garcihombronane F (14) and garcihombronane D (30) were responsible for the antiaggregant property [30]. These effects could be attributable to its inhibitory capacity on release of adenosine diphosphate and thromboxane A2 that involved in process of sealing up injured endothelium and tissues [64].
4.8. Antituberculosis activity
An estimation of 1.3 million death attributed to tuberculosis (TB) has been reported in 2022 [65]. The medicines that currently used for improving tuberculosis have been associated with multidrug-resistant and various side efects. Thus, alternative of drug-resistant TB with fewer or no side efects remains a major challenge [66]. The anti-tuberculosis activity of G. celebica ethyl acetate bark extract was investigated against Mycobacterium tuberculosis H37Rv strain with gentamicin as a reference standard. The results demonstrated that the extract possessed a potent antituberculosis activity with a MIC value of 62.5 μg/mL which was compared with gentamicin (2.5 μg/mL) [24,45]. In another study, moderate inhibition was shown by 4″-O-methyl-volkensiflavone (89) and 3″-O-methyl-morelloflavone (92) with MIC values of 109 μM and 102 μM, respectively, against the M. tuberculosis H38Rv in comparison to their positive controls—streptomycin and gentamicin (MIC of 0.60 and 12.5 μM) indicated the effect was not as strong as the positive controls [45]. The mechanism of action this plant is not yet been identified which warrant future studies but most TB drugs targetted on cell wall, protein or nucleic acid synthesis of myobacterium tuberculosis.
4.9. Hepatoprotective activity
The hepatoprotective effect of G. celebica bark extract was demonstrated in carbon tetrachloride (CCl4)-induced hepatotoxicity in Sprague-Dawley rats [25]. In this model, oral administration of methanolic ethyl acetate extract (50, 250 and 500 mg/kg) for seven consecutive days prior to intoxication with CCl4 effectively decreased the elevation of serum liver enzymes—alkaline phosphatase (ALP), alanine transaminase (ALT) and aspartate transaminase (AST) at higher doses of 250 and 500 mg/kg. Apart from biochemical analysis, histopathology analysis also remarkably reduced the massive coagulation necrosis, hemorrhage and infiltration of inflammatory cells in the liver tissues of the treatment groups. The effect was comparable to that of the standard drug silymarin (500 mg/kg). The possible mechanism of this plant to exert hepatoprotective activity is believed to occur through regulation of the pathways such as mitogen activated protein kinase (MAPK) and nuclear factor erythroid 2–related factor 2 (Nrf2) which subsequently activates antioxidant enzymes to ameliorates the oxidative damage [67].
4.10. Antidiabetic activity
Diabetes mellitus is a severe chronic disease and management of the hydrolyzing enzymes α-glucosidase and α-amylase to control postprandial glucose levels is beneficial in type 2 diabetic and borderline patients [68]. The beneficial antidiabetic effect was displayed by G. celebica aqueous extract, which showed inhibition of α-glucosidase and α-amylase activity in in vitro study [24]. The IC50 values of α-glucosidase for the extract and standard reference acarbose were 392 μg/mL and 2562 μg/mL, while the IC50 values of α-amylase were 474 μg/mL and 11.7 μg/mL, respectively. The inhibition of the extract towards α-glucosidase was considerably higher than that of the control acarbose [24]. Another similar finding was also noted in a study conducted by Triadisti et al. (2017), where the ethyl acetate and methanol fractions exhibited good inhibitory activity toward α-glucosidase activity [50]. However, the ethyl acetate fraction (IC50 of 16.4 μg/mL) was shown to display stronger α-glucosidase inhibitory activity than the methanol fraction (IC50 of 59 μg/mL) and acarbose (39.5 μg/mL) [50]. To our knowledge, the availability of antidiabetic reports was very limited and not yet studied on cell lines and animal models. The underlying mechanism of the glucose-lowering properties suggested is via improvement in pancreatic islet function which abolishing insulin resistance that needed to be clarified in future study.
5. Toxicology
G. celebica's leaf aqueous extract was reported for their toxicity in an acute toxicity study which each group orally administered with 300, 2000 and 5000 mg/kg of body weight of Sprague-Dawley rats for 14 days [69]. The finding revealed that a rat died in group administered with 5000 mg/kg thus categorized the leaf extract under class five compounds based on chemical categorisation set by Organization for Economic Co-operation and Development (OECD) representing lowest toxicity on laboratory rodents (LD50 value greater than 5000 mg/kg) [70]. Higher doses of 2000 and 5000 mg/kg showed mild vascular congestion with few necrotic cells observed in hepatic, spleenic, cardiac and renal tissues. Despite histopathological lesions were seen in the liver groups, there was no significant alterations to the liver's function based on the total bilirubin, ALT and AST levels than the control group suggests low toxic effects of this leaf extract. Nonetheless, the investigation on toxicity profiles of G. celebica are greatly lacking as this is the only study reported for their toxicity which required more toxicological studies to evaluate its safety profile, adverse effects and efficacy.
6. Conclusions and future perspectives
G. celebica covered in this review has displayed a vast range of phytochemical constituents with beneficial pharmacological effects. However, the research on this plant is scarcely reported and chiefly constitutes of in vitro studies which indicates its capacity as potential source in drug discovery remains questionable. Some studies also did not evaluate the activities of specific compounds but only discussed the activities of extracts and fractions. Of particular importance to note is the mechanism of action of the extract and compounds are still lacking and poorly understood emerges great lacunae. Achieving convincing results requires conducting in vivo studies and its possible protective molecular mechanisms which aim to aid its clinical applications as a modern medicine to ascertaine whether the pure isolates and extracts may be used as a lead in the drug development. In conclusion, a more evidence-based clinical profile needs to be established for G. celebica by conducting further investigation in the future.
Ethics statement
Review and/or approval by an ethics committee was not needed for this study because it reviewed primary data electronically.
Data availability statement
The data associated with this scoping review were not deposited into a publicly available repository because the review relies on open-source data. However, the dataset will be made available upon reasonable request from the corresponding author (juriyatijalil@ukm.edu.my).
CRediT authorship contribution statement
Nor Hidayah Mustafa: Writing – review & editing, Methodology, Investigation, Data curation. Juriyati Jalil: Writing – review & editing, Validation, Supervision, Project administration, Funding acquisition, Conceptualization. Kai En Leong: Writing – original draft, Methodology, Investigation, Data curation. Jamia Azdina Jamal: Validation. Khairana Husain: Validation.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and the funder had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Acknowledgments
This work was funded by the Universiti Kebangsaan Malaysia under grant number MUTIARA-A163258.
Footnotes
We understand that the corresponding author is the sole contact for the editorial process (including the editorial manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address that is accessible by the corresponding author.
References
- 1.The World Flora Online Garcinia hombroniana Pierre. http://www.worldfloraonline.org/taxon/wfo-0000694364 Available online:
- 2.Nazre M. Historical review and notes on the correct scientific name for seashore mangosteen. Genet. Resour. Crop Evol. 2010;57:1249–1259. doi: 10.1007/s10722-010-9588-y. [DOI] [Google Scholar]
- 3.Midin M., Goh H.H. In: Underutilised Crop Genomes. Compendium of Plant Genomes. Chapman M.A., editor. Springer; Cham: 2022. The mangosteen genome. [DOI] [Google Scholar]
- 4.Murthy H.N., Dandin V.S., Dalawai D., Park S.Y., Paek K.Y. Breeding of Garcinia spp. Advances in plant breeding strategies. Fruits. 2018;3:773–809. doi: 10.1007/978-3-319-91944-7_19. [DOI] [Google Scholar]
- 5.Stevens P.F. Springer; Berlin: 2007. Clusiaceae-Guttiferae. Flowering Plants· Eudicots; pp. 48–66. [DOI] [Google Scholar]
- 6.Hemshekhar M., Sunitha K., Santhosh M.S., Devaraja S., Kemparaju K., Vishwanath B.S., et al. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochem. Rev. 2011;10(3):325–351. doi: 10.1007/s11101-011-9207-3. [DOI] [Google Scholar]
- 7.Aravind A.P., Asha A.K., Rameshkumar K.B. Phytochemical analysis and antioxidant potential of the leaves of Garcinia travancorica Bedd. Nat. Prod. Res. 2016;30(2):232–236. doi: 10.1080/14786419.2015.1043551. [DOI] [PubMed] [Google Scholar]
- 8.Triyasa K.S., Diantini A., Barliana M.I. A review of herbal medicine-based phytochemical of Garcinia as molecular therapy for breast cancer. Drug Des Devel Ther. 2022;16:3573–3588. doi: 10.2147/DDDT.S358229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins F.T., Doriguetto A.C., de Souza T.C., de Souza K.R.D., dos Santos M.H., Moreira M.E.C., et al. Composition, and anti-inflammatory and antioxidant activities of the volatile oil from the fruit peel of Garcinia brasiliensis. Chem. Biodivers. 2008;5(2):251–258. doi: 10.1002/cbdv.200890022. [DOI] [PubMed] [Google Scholar]
- 10.Pereira I.O., Marques M.J., Pavan A.L.R., Codonho B.S., Barbiéri C.L., Beijo L.A., et al. Leishmanicidal activity of benzophenones and extracts from Garcinia brasiliensis Mart. Fruits. Phytomedicine. 2010;17(5):339–345. doi: 10.1016/j.phymed.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Santa-Cecília F.V., Vilela F.C., Da Rocha C.Q., Dias D.F., Cavalcante G.P., Freitas L.A.S., et al. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J. Ethnopharmacol. 2011;133(2):467–473. doi: 10.1016/j.jep.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K., Akao Y., Ohguchi K., Ito T., Tanaka T., Iinuma M., et al. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorganic Med. Chem. 2005;13(21):6064–6069. doi: 10.1016/j.bmc.2005.06.065. https://doi:10.1016/j.bmc.2005.06.065 [DOI] [PubMed] [Google Scholar]
- 13.Marlin S., Elya B. Katrin. Antioxidant activity and lipoxygenase enzyme inhibition assay with total flavonoid content of Garcinia hombroniana Pierre leaves. Asian J. Pharm. Clin. Res. 2017;9(2):267–272. doi: 10.22159/ajpcr.2017.v10s5.23122. [DOI] [Google Scholar]
- 14.Klaiklay S., Sukpondma Y., Rukachaisirikul V., Phongpaichit S. Friedolanostanes and xanthones from the twigs of Garcinia hombroniana. Phytochemistry. 2013;85:161–166. doi: 10.1016/j.phytochem.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 15.John K.J., Kumar R.S., Suresh C.P., George J.K., Abraham Z. Occurrence, distribution and economic potential of seashore mangosteen (Garcinia hombroniana Pierre) in India. Genet. Resour. Crop Evol. 2008;55(2):183–186. doi: 10.1007/s10722-008-9306-1. [DOI] [Google Scholar]
- 16.Lim T.K. Garcinia hombroniana. Edible Med. Non-Medicinal Plants. 2012;2:56–58. doi: 10.1007/978-94-007-1764-0. [DOI] [Google Scholar]
- 17.Jamila N., Khairuddean M., Yaacob N.S., Kamal N.N.S.N.M., Osman H., Khan S.N., et al. Cytotoxic benzophenone and triterpene from Garcinia hombroniana. Bio-Organic Chem. 2014;54:60–67. doi: 10.1016/j.bioorg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Salleh W.M.N.H.W., On S., Ahmad F., Sirat H.M., Taher M., Sarker S.D., Nahar L.A. New xanthone and a new benzophenone from the roots of Garcinia hombroniana. Phytochem. Lett. 2020;35:216–219. https://doi:10.1016/j.phytol.2019.12.011 [Google Scholar]
- 19.Jamal Y., Praptiwi Y., Balit A.A. Phytochemical screening, toxicity and anti-bacterial assay from stem-bark extracts of Garcinia celebica and G. tetandra. Indonesian J. Pharm. 2001;12(2):97–102. [Google Scholar]
- 20.Widyowati R., Rahman A. Kandungan Kimia dan Aktivitas Antimikroba Ekstrak Garcinia celebica L. Terhadap Staphylococcus aureus, Shigella dysenteriae dan Candida albicans. Maj. Farm. Airlangga. 2010;8(2):23–27. [Google Scholar]
- 21.Jantan I., Jumuddin F.A., Saputri F.C., Rahman K. Inhibitory effects of the extracts of Garcinia species on human low-density lipoprotein peroxidation and platelet aggregation in relation to their total phenolic contents. J. Med. Plants Res. 2011;5(13):2699–2709. [Google Scholar]
- 22.Nargis J., Melati K., Lai C.S., Hasnah O., Wong K.C., Vikneswaran M., Khaw K.Y. Antioxidant, anti-cholinesterase and antibacterial activities of the bark extracts of Garcinia hombroniana. African J. Pharm. Pharmacol. 2013;7(8):454–459. [Google Scholar]
- 23.Dyary H.O., Arifah A.K., Sharma R.S., Rasedee A., Mohd-Aspollah M.S., Zakaria Z.A., et al. Antitrypanosomal screening and cytotoxic effects of selected medicinal plants. Trop. Biomed. 2014;31(1):89–96. [PubMed] [Google Scholar]
- 24.Jamila N., Khan N., Khan A.A., Khan S.N., Kim K.S. Phytochemical analysis, antioxidant, anti-hyperglycemic and antituberculosis activities of phylogenetically related Garcinia mangostana (mangosteen) and Garcinia hombroniana (seashore mangosteen) J. Chem. Soc. Pakistan. 2016;38(6):1181–1189. [Google Scholar]
- 25.Jamila N., Khan N., Khan A.A., Khan I., Khan S.N., Zakaria Z.A., et al. In vivo carbon tetrachloride-induced hepatoprotective and in vitro cytotoxic activities of Garcinia hombroniana (seashore mangosteen) African J. Tradit. Complement. Altern. Med. 2017;14(2):374–382. doi: 10.21010/ajtcam.v14i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Listiyani A., Elya B., Puspitasari N. Antioxidant activity and lipoxygenase enzyme inhibition assay with total flavonoids content from Garcinia hombroniana Pierre. Stem Bark Extract. Pharmacogn. J. 2017;9(2):267–272. doi: 10.5530/pj.2017.2.45. [DOI] [Google Scholar]
- 27.Chew Y.L., Lim Y.Y. Evaluation and comparison of antioxidant activity of leaves, pericarps and pulps of three Garcinia species in Malaysia. Free Radic. Antioxid. 2018;8(2):130–134. [Google Scholar]
- 28.Sofian F.F., Tjitraresmi A., Runadi D., Tanti G.A., Hamida A., Halimah E., et al. In vitro antiplasmodial activity of Dysoxylum caulostachyum (Miq) and Garcinia celebica (L) leaf extracts against Plasmodium falciparum. J. Pharm. Sci. Res. 2018;10(2):391–393. [Google Scholar]
- 29.Nargis J., Wong K.C., Khairuddin M., Chantrapromma S., Fun H.K. (2,4-Dihydroxy-6-Methoxyphenyl)(3,5-Dihydroxyphenyl)Methanone monohydrate. Acta Crystallogr., Sect. A E. 2011;67:2717–2718. doi: 10.1107/S1600536811037913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saputri F.C., Jantan I. Inhibitory activities of compounds from the twigs of Garcinia hombroniana Pierre on human low-density lipoprotein (LDL) oxidation and platelet aggregation. Phyther. Res. 2012:1845–1850. doi: 10.1002/ptr.4667. [DOI] [PubMed] [Google Scholar]
- 31.Pasaribu Y.P., Fadlan A., Fatmawati S., Ersam T. Biological activity evaluation and in silico studies of polyprenylated benzophenones from Garcinia celebica. Biomedicines. 2021;9(11):1654. doi: 10.3390/biomedicines9111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamila N., Khan N., Khan I., Khan A.A., Khan S.N.A. Bioactive cycloartane triterpene from Garcinia hombroniana. Nat. Prod. Res. 2015;30(12):1388–1397. doi: 10.1080/14786419.2015.1060594. [DOI] [PubMed] [Google Scholar]
- 33.Rukachaisirikul V., Saelim S., Karnsomchoke P., Phongpaichit S. Friedolanostanes and lanostanes from the leaves of Garcinia hombroniana. J. Nat. Prod. 2005;68(8):1222–1225. doi: 10.1021/np050131j. [DOI] [PubMed] [Google Scholar]
- 34.Bui T.Q., Bui A.T., Nguyen K.T., Nguyen V.T., Trinh B.T.D., Nguyen L.H.D.A. Depsidone and six triterpenoids from the bark of Garcinia celebica. Tetrahedron Lett. 2016;57(23):2524–2529. doi: 10.1016/j.tetlet.2016.04.104. [DOI] [Google Scholar]
- 35.Jamila N., Khairuddean M., Khan S.N., Khan N., Osman H. Phytochemicals from the bark of Garcinia hombroniana and their biological activities. Rec. Nat. Prod. 2014;8(3):312–316. [Google Scholar]
- 36.Muhammad N.A., Basar N., Jamil S. Antibacterial activity of phytochemicals from Garcinia parvifolia Miq. And Garcinia hombroniana Pierre. J. Sci. Math. Lett. 2019;7:44–51. [Google Scholar]
- 37.Muchtaridi M., Sugijanto M., Gazzali A.M., Wahab H.A. Anti-neuraminidase bioactives from manggis hutan (Garcinia celebica L.) leaves: partial purification and molecular characterization. Molecules. 2020;25(821):1–13. doi: 10.3390/molecules25040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rukachaisirikul V., Adair A., Dampawan P., Taylor W.C., Turner P.C. Lanostanes and friedolanostanes from the pericarp of Garcinia hombroniana. Phytochemistry. 2000;55(2):183–188. doi: 10.1016/s0031-9422(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 39.Elfita E., Muharni M., Latief M., Darwati D., Widiyantoro A., Supriyatna S., et al. Antiplasmodial and other constituents from four Indonesian Garcinia spp. Phytochemistry. 2009;70(7):907–912. doi: 10.1016/j.phytochem.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Rosli N., Khairuddean M., Jamain Z. Phytochemical and biological activity studies of the leaves of Garcinia hombroniana Pierre. MJChem. 2020;22(4):81–102. [Google Scholar]
- 41.Subarnas A., Diantini A., Abdulah R., Zuhrotun A., Nugraha P.A., Hadisaputri Y.E., et al. Apoptosis-mediated antiproliferative activity of friedolanostane triterpenoid isolated from the leaves of Garcinia celebica against MCF-7 human breast cancer cell lines. Biomed. Rep. 2016;4(1):79–82. doi: 10.3892/br.2015.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamila N., Khairuddean M., Yeong K.K., Osman H., Murugaiyah V. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzyme Inhib. Med. Chem. 2014;30(1):133–139. doi: 10.3109/14756366.2014.895720. [DOI] [PubMed] [Google Scholar]
- 43.Tan W.N., Tan Z.H., Zulkifli N.I., Nik Mohamed Kamal N.N.S., Rozman N.A.S., Tong W.Y., et al. Sesquiterpenes rich essential oil from Garcinia celebica L. and its cytotoxic and antimicrobial activities. Nat. Prod. Res. 2020;34(23):3404–3408. doi: 10.1080/14786419.2019.1569012. [DOI] [PubMed] [Google Scholar]
- 44.Abdulah R., Suradji E.W., Subarnas A., Supratman U., Sugijanto M., Diantini A., et al. Catechin isolated from Garcinia celebica leaves inhibit Plasmodium falciparum growth through the induction of oxidative stress. Pharmacogn. Mag. 2017;15(62):301–305. doi: 10.4103/pm.pm_571_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamila N., Khairuddean M., Khan S.N., Khan N. Complete NMR assignments of bioactive rotameric (3→8) biflavonoids from the bark of Garcinia hombroniana. Magn. Reson. Chem. 2014;52(7):345–352. doi: 10.1002/mrc.4071. [DOI] [PubMed] [Google Scholar]
- 46.Apriyanto D.R., Hartati S., Dewi B.E., Aoki-Utsubo C., Hotta H. In vitro study of Garcinia celebica L. Stem barks against hepatitis C virus and hepatocellular carcinoma. J. Phys. Conf. Ser. 2019;1360(1):1–4. doi: 10.1088/1742-6596/1360/1/012027. [DOI] [Google Scholar]
- 47.Kamal N.N.S.N.M., Alkanan A.T.Y., Muhamad M., Samad N.A., Wen-Nee T. Mechanistic Basis of Cytotoxic Action of Garcinia celebica ethereal oils in cultured breast cells. JUMMEC. 2023:1–8. doi: 10.22452/jummec.sp2023no1.1. [DOI] [Google Scholar]
- 48.Abdulah R., Milanda T., Sugijanto M., Barliana M.I., Diantini A., Supratman U., et al. Antibacterial properties of selected plants consumed by primates against Escherichia coli and Bacillus subtilis. Southeast Asian J. Trop. Med. Public Health. 2017;48(1):109–116. [PubMed] [Google Scholar]
- 49.Dyary H.O., Arifah A.K., Sharma R.S.K., Rasedee A., Mohd Aspollah M.S., Zakaria Z.A., et al. In vivo antitrypanosomal activity of Garcinia hombroniana aqueous extract. Res. Vet. Sci. 2015:226–231. doi: 10.1016/j.rvsc.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Triadisti N., Sauriasari R., Elya B. Fractionation and α-glucosidase inhibitory activity of fractions from Garcinia hombroniana Pierre leaves extracts. Pharmacogn. J. 2017;9(4):488–492. doi: 10.5530/pj.2017.4.79. [DOI] [Google Scholar]
- 51.Mustafa N.H., Jalil J., Zainalabidin S., Saleh M.S.M., Asmadi A.Y., Kamisah Y. Molecular mechanisms of sacubitril/valsartan in cardiac remodeling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.892460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mustafa N.H., Jalil J., Zainalabidin S., Saleh M.S.M., Asmadi A.Y., Kamisah Y. Parkia speciosa hassk. Empty pod extract prevents cardiomyocyte hypertrophy by inhibiting MAPK and calcineurin-NFATC3 signaling pathways. Life. 2023;13(1) doi: 10.3390/life13010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lane C.A., Hardy J., Schott J.M. Alzheimer's disease. Eur. J. Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 54.Sharma K. Cholinesterase inhibitors as Alzheimer's therapeutics. Mol. Med. Rep. 2019;20(2):1479–1487. doi: 10.3892/mmr.2019.10374. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleh M.S.M., Jalil J., Zainalabidin S., Asmadi A.Y., Mustafa N.H., Kamisah Y. Genus Parkia: phytochemical, medicinal uses, and pharmacological properties. Int. J. Mol. Sci. 2021;22(2):618. doi: 10.3390/ijms22020618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrd-Leotis L., Cummings R.D., Steinhauer D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aregawi W.G., Agga G.E., Abdi R.D., Büscher P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites Vectors. 2019;12(1):67. doi: 10.1186/s13071-019-3311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rathore N.S., Manuja A., Manuja B.K., Choudhary S. Chemotherapeutic approaches against trypanosoma evansi: retrospective analysis, current status and future outlook. Curr. Top. Med. Chem. 2016;16(20):2316–2327. doi: 10.2174/1568026616666160413125802. [DOI] [PubMed] [Google Scholar]
- 59.Kasozi K.I., MacLeod E.T., Ntulume I., Welburn S.C. An update on African trypanocide pharmaceutics and resistance. Front. Vet. Sci. 2022;9(2022) doi: 10.3389/fvets.2022.828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization Malaria. 2023. https://www.who.int/news-room/fact-sheets/detail/malaria Available online:
- 61.Miguel-Blanco C., Murithi J.M., Benavente E.D., Angrisano F., Sala K.A., van Schalkwyk D.A., et al. The antimalarial efficacy and mechanism of resistance of the novel chemotype DDD01034957. Sci. Rep. 2021;11(1):1888. doi: 10.1038/s41598-021-81343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang S., Ninivaggi M., Chayoua W., de Laat B. VWF, platelets and the antiphospholipid syndrome. Int. J. Mol. Sci. 2021;22(8):4200. doi: 10.3390/ijms22084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jalil J., Jantan I., Ghani A.A., Murad S. Platelet-activating factor (PAF) antagonistic activity of a new biflavonoid from Garcinia nervosa var. Pubescens king. Molecules. 2012;17(9):10893–10901. doi: 10.3390/molecules170910893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prabhu A.N., Shivashankara A.R., Haniadka R., Palatty P.L., Prabhu D., Baliga M.S. Bioactive Food as Dietary Interventions for Cardiovascular Disease. Academic Press.; 2013. Antiatherogenic effects of ginger (Zingiber officinale Roscoe): scientific observations and ethnomedicinal validation; pp. 693–704. [Google Scholar]
- 65.World Health Organization Tuberculosis. 2021 https://www.who.int/health-topics/tuberculosis#tab=tab_1 Available online: [Google Scholar]
- 66.Yang T.W., Park H.O., Jang H.N., Yang J.H., Kim S.H., Moon S.H. Side effects associated with the treatment of multidrug-resistant tuberculosis at a tuberculosis referral hospital in South Korea: a retrospective study. Medicine. 2017;96(28):e7482. doi: 10.1097/MD.0000000000007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weng C.J., Yen G.C. Handbook of Antioxidants for Food Preservation. Woodhead Publishing; 2015. Natural plant extracts as antioxidants for food preservation; pp. 235–249. [Google Scholar]
- 68.Hossain U., Das A.K., Ghosh, S S., Sil P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dyary H.O., Arifah A.K., Sharma R.S.K., Rasedee A., Aspollah M.S.M., Zakaria Z.A., et al. Acute toxicological assessment of seashore mangosteen (Garcinia hombroniana) aqueous extract. J. Vet. Malaysia. 2016;28(2):4–11. [Google Scholar]
- 70.OECD . OECD (Organization for Economic Co-operation and Development); 2001. Guideline for Testing of Chemicals, 423: Acute Oral Toxicity-Acute Toxic Class Method; pp. 1–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with this scoping review were not deposited into a publicly available repository because the review relies on open-source data. However, the dataset will be made available upon reasonable request from the corresponding author (juriyatijalil@ukm.edu.my).