Abstract
Background
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare but serious disease characterised by the combination of small-to-medium vessel vasculitis, blood and tissue eosinophilia, and asthma and/or sinonasal disease. This study estimated the prevalence and incidence of diagnosed EGPA in the United Kingdom (UK), and described the demographics, clinical characteristics and healthcare resource utilisation (HCRU) of this population.
Methods
This retrospective longitudinal study of patients with newly diagnosed EGPA (index) (2005–2019) used the Clinical Practice Research Datalink AURUM and Hospital Episode Statistics databases. The primary outcomes were the annual prevalence (2005–2019) and incidence (2006–2019) of EGPA, and secondary outcomes included patient demographics and clinical characteristics, and HCRU in the year pre- and post-index (diagnosis).
Results
Populations of patients with EGPA comprised 940 prevalent cases and 502 incident cases, of which 377 were linked to Hospital Episode Statistics. EGPA prevalence increased from 22.7 to 45.6 cases per 1 000 000 (2005–2019), driven by patients aged ≥18 years. Incidence ranged from 2.3 to 4.0 per 1 000 000 person-years (2006–2019). Pre-index, the most common clinical symptoms were respiratory related, and the most common comorbidities were asthma (80.6%) and nasal polyps (32.1%). Post-index, 19.1% had an EGPA-related inpatient stay (median length of stay 11.0 days) and 38.7% had five or more oral corticosteroid (OCS) prescriptions with a mean OCS possession ratio per patient of 47.0%.
Conclusions
Although EGPA incidence in the UK remains relatively stable, prevalence is increasing, and HCRU and OCS use remain frequent, suggesting considerable healthcare burden for patients with EGPA.
Tweetable abstract
EGPA increased in prevalence from 2005 to 2019 in the UK; incidence remained stable. Post-diagnosis, 19% of patients had EGPA-related inpatient stays and 80% required oral corticosteroids, highlighting the high healthcare burden and severity of EGPA. https://bit.ly/3NOHoln
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare disease characterised by eosinophilic inflammation and necrotising vasculitis of small/medium-sized blood vessels [1–3]. EGPA is a type of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [2, 3], although ANCAs are only detected in ∼30–40% of cases [4, 5]. Elevated eosinophil counts in the blood and tissue, vasculitis and granuloma formation are all thought to contribute towards multiple organ injury and impairment [6]. EGPA is commonly characterised by asthma, elevated eosinophil counts, neuropathy and sinusitis [2, 3, 5, 7].
EGPA treatment, typically oral corticosteroids (OCS) and immunosuppressants, aims to induce remission and reduce disease relapses [8, 9]. However, OCS and immunosuppressants are associated with significant toxicity, particularly with chronic exposure [10, 11]. Additionally, not all patients achieve remission and others may experience exacerbations or relapses, especially when treatments are tapered [5, 12, 13], which, together with the wide range of organ systems involved, necessitates frequent healthcare resource utilisation (HCRU) [5, 9, 14, 15].
The prevalence and incidence of EGPA varies globally, with estimated prevalence of 2.0–38.0 per 1 000 000 people [14–17], and incidence of 1.2 per 1 000 000 person-years [14]. EGPA is a rare and challenging diagnosis, which is often misdiagnosed [18]. Therefore, local differences in awareness and recognition among healthcare providers may contribute to regional variations, as has been observed in other AAVs [18, 19]. Another contributing factor may be the changing classification criteria over time [2, 3, 20, 21]. Given the rarity of and difficulty diagnosing EGPA, limited information is available on the prevalence, incidence and associated burden of disease in the United Kingdom (UK) [17, 22].
This study aimed to estimate the prevalence and incidence of diagnosed EGPA in the UK, and to describe the demographics, clinical characteristics and HCRU of patients following EGPA diagnosis.
Materials and methods
Study design and data sources
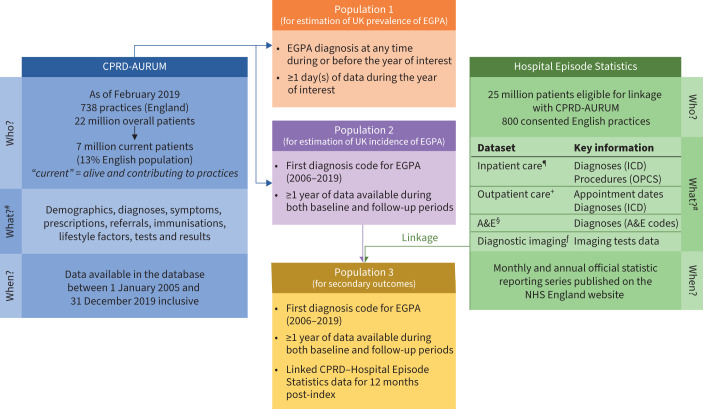
This was a retrospective, longitudinal study of patients newly diagnosed with EGPA (1 January 2005 to 31 December 2019) using the Clinical Practice Research Datalink (CPRD)-AURUM and Hospital Episode Statistics databases [23, 24]. The index date was the date of the first EGPA diagnosis during the study period based on the presence of a medcode identifier, Read code, Education Management Information System (EMIS) code or SNOMED identifier code for EGPA, allergic granulomatous angiitis or Churg-Strauss syndrome (supplementary table S1). The baseline and follow-up periods included the year pre- and post-index, respectively (supplementary figure S1). The CPRD-AURUM database (figure 1) consists of anonymised, longitudinal medical records of patients registered with contributing primary care practices across the UK (predominantly England and Northern Ireland) and contains data collected routinely from participating practices using the EMIS Web electronic patient record system software, including data on demographics, lifestyle factors, diagnoses, symptoms, prescriptions, referrals and medical tests [24]. As of February 2019, CPRD-AURUM contained data of >22 million patients from 738 general practitioner (GP) practices in England, of whom 7 million were active (still alive and registered with a GP practice), representing a coverage of ∼13% of the population of England. The CPRD-AURUM resource was launched in 2017, but the database includes a full historic collection of the coded part of each practice's electronic health records. Further characterisation of the data source has been published previously [24].
FIGURE 1.
Data sources and analysis populations. CPRD: Clinical Practice Research Datalink; EGPA: eosinophilic granulomatosis with polyangiitis; ICD: International Classification of Diseases; OPCS: Office of Population Censuses and Surveys's Classification of Surgical Operations and Procedures; A&E: accident and emergency; NHS: National Health Service. #: information from [24]; ¶: from 1997; +: from 2003; §: from 2007; ƒ: from 2012.
Anonymised data from CPRD-AURUM can be individually linked to secondary care and other health and area-based datasets, including the Hospital Episode Statistics database (figure 1). Linkage of CPRD-AURUM with Hospital Episode Statistics is possible for a subset of ∼25 million patients currently registered with 800 consented English practices that actively participate in the linkage scheme. The Hospital Episode Statistics database contains details of all inpatient episodes of care, outpatient appointments, and accident and emergency (A&E) attendances and diagnostic imaging at National Health Service (NHS) hospitals in England. These data are collected primarily for administrative purposes, although they are designed to enable secondary use. The inpatient data (Hospital Episode Statistics – Admitted Patient Care) includes coded diagnoses (using the International Classification of Diseases, tenth revision (ICD-10) codes), operations and procedures (Office of Population Censuses and Surveys, fourth revision (OPCS 4) codes), as well as patient demographic, admission and discharge information. Outpatient data contain appointment dates and times, and specialties, but limited clinical information [24].
Informed consent and ethics committee or institutional review board approval were not required as no direct patient contact or primary collection of patient data occurred. The CPRD obtains ethical research approval annually from the UK's Health Research Authority Research Ethics Committee to accumulate and distribute patient data.
Patient eligibility
Three patient populations were defined (figure 1). Populations 1 and 2 were based on CPRD-AURUM data only. To calculate the annual prevalence of EGPA (2005–2019) in the UK, population 1 was defined as patients with a diagnosis for EGPA at any time during or before the year of interest, ≥1 day(s) of CPRD-AURUM data during the year of interest. To calculate the incidence of EGPA in the UK, population 2 was defined as patients with a first diagnosis code for EGPA (2006–2019) and ≥1 calendar year of CPRD-AURUM data during both the baseline and follow-up periods. For the secondary outcomes, population 3 was defined as patients with a first diagnosis for EGPA (2006–2019) with at least one calendar year of CPRD-AURUM data records during baseline and follow-up periods, and linked to Hospital Episode Statistics data for 12 months post-index. To ensure only incident cases were captured accurately in populations 2 and 3, only patients with no diagnosis of EGPA during baseline were included.
Study outcomes
The primary outcome was the annual prevalence of diagnosed EGPA (population 1, overall, and stratified by age (0–17 and ≥18 years)) in 2005–2019 and the annual incidence rate of EGPA diagnosis in 2006–2019 (population 2).
Secondary outcomes included demographics at index, and clinical characteristics and Charlson Comorbidity Index (CCI) score during baseline, and HCRU (including OCS use) during the follow-up period (population 3). As Hospital Episode Statistics data are specific for England, the secondary outcomes data reflect an English rather than UK population. The CPRD-AURUM and Hospital Episode Statistics databases were used to identify clinical symptoms and comorbidity conditions using codes from a previous study (available upon request) [25].
Statistical analysis
This was a descriptive study, and neither hypothesis tests were conducted, nor formal power calculation performed. However, a feasibility assessment was performed, including widths calculations of the 95% confidence intervals for prevalence and incidence estimations, detailed in the supplementary methods and supplementary table S2.
EGPA prevalence was calculated as the number of patients with an EGPA diagnosis during and before a particular calendar year, divided by the number of patients with a calendar year of data in the CPRD-AURUM database on 31 December in each calendar year. EGPA incidence was calculated as the number of patients with an incident EGPA diagnosis from 1 January to 31 December in the calendar year of interest, divided by the total number of days at risk. For incidence rate, patients had at least 365 days after first registration in CPRD-AURUM prior to contributing to time at risk between 2006 and 2019. Time at risk started on day 366 after registration. This was to ensure that the incident cases were accurate and not an existing diagnosis that was recorded at time of registration.
All secondary outcomes were also analysed descriptively using mean±sd or median and interquartile range (IQR) for continuous variables and frequency (%) for categorical variables. CCI score was calculated using the Metcalfe adaptation [26]. HCRU assessments included the proportion of patients with at least one event and mean number of events including EGPA-related and all-cause inpatient stays, all-cause A&E visits, specialist outpatient visits, all-cause outpatient visits, all-cause procedures and all-cause primary care visits. For inpatient stays, the cumulative and median length of stays was also reported. OCS use was measured according to the number of prescriptions throughout the year and split into quartiles, total prescriptions, and average days per year of use. The OCS medication possession ratio (MPR) was calculated based on the total number of days covered by OCS prescriptions (derived using quantity/daily dose variables) during the follow-up period divided by duration.
Results
Patient populations
Population 1 and population 2 included 940 prevalent patients and 502 incident patients, respectively. There were 377 patients aged ≥18 years who were successfully linked to CPRD–Hospital Episode Statistics and eligible for inclusion in population 3.
Demographics and clinical characteristics
Patient demographics and clinical characteristics are shown in table 1. The mean±sd age at index was 57.4±14.2 years among 377 patients aged ≥18 years: 2% of patients were aged 18–25 years, 66% were aged 26–64 years and 32% were aged ≥65 years. Additionally, fewer than five patients were aged ≤17 years and were not included in population 3 for the secondary outcomes (for clinical characteristics/conditions with fewer than five patients, CPRD required data to be suppressed to minimise the risk of patient identification). In total, 51.2% of patients were female, and 84.6% had a CCI score ≥1. Blood eosinophil counts (BECs) at diagnosis were elevated, with a geometric mean±sd (95% CI) BEC of 1385.5±4.3 (1163.4–1649.9) cells·μL−1 (normal range 50–500 cells·μL−1) [27]. Only 13.8% of patients had BECs <400 cells·μL−1, while 38.2% had BECs ≥1000 cells·μL−1 (table 1). The most common clinical symptoms during baseline were cough/breathlessness (37.7%) and ear, nose and throat involvement (18.8%). The most common comorbidities pre-index were asthma (80.6%) and nasal polyps (32.1%).
TABLE 1.
Patient demographics# at index and clinical characteristics during the baseline period¶
| Patients | 377 |
| Age at index, years | |
| Mean±sd | 57.4±14.2 |
| Median (IQR) | 58 (48–68) |
| ≤17 | ƒ |
| 18–25 | 6 (1.6) |
| 26–64 | 249 (66.1) |
| ≥65 | 122 (32.4) |
| Females at index | 193 (51.2) |
| CCI score during baseline | |
| 0 | 58 (15.4) |
| 1 | 226 (60.0) |
| 2 | 56 (14.9) |
| ≥3 | 37 (9.8) |
| Blood eosinophil count during baseline+, cells·µL−1 | |
| Median (IQR) | 1170 (500–4800) |
| <400 | 52 (13.8) |
| ≥400–<1000 | 76 (20.2) |
| ≥1000 | 144 (38.2) |
| Missing | 105 (27.9) |
| Clinical symptoms during baseline § | |
| Cough or breathlessness | 142 (37.7) |
| ENT involvement | 71 (18.8) |
| Nonspecific chest symptoms | 37 (9.8) |
| Skin involvement | 30 (8.0) |
| Constitutional manifestations | 28 (7.4) |
| Musculoskeletal involvement | 15 (4.0) |
| Renal involvement | 16 (4.2) |
| Gastrointestinal involvement | 21 (5.6) |
| Eye involvement | 7 (1.9) |
| Chest pain | <5## |
| Comorbid conditions at any time prior to index § | |
| Asthma | 304 (80.6) |
| Nasal polyposis | 121 (32.1) |
| Chronic rhinosinusitis | 91 (24.1) |
| Allergic rhinitis | 61 (16.2) |
| Peripheral neuropathy | 43 (11.4) |
| Ischaemic stroke | 16 (4.2) |
| COPD | 15 (4.0) |
| Cardiomyopathy | 9 (2.4) |
| Hypereosinophilic syndrome | <5## |
| Heart failure | <5## |
Data are presented as n or n (%), unless otherwise stated. IQR: interquartile range; CCI: Charlson Comorbidity Index; ENT: ear, nose and throat. #: patients from population 3; ¶: baseline period defined as the year before index (inclusive); +: the maximum value was reported if multiple values were available; §: ≥1 code for characteristic of interest; ƒ: patients aged 0–17 years were not included due to the small number (fewer than five) of patients included in this age group; ##: for clinical characteristics/conditions with fewer than five patients, Clinical Practice Research Datalink required data to be suppressed to minimise the risk of patient identification.
Prevalence and incidence of EGPA
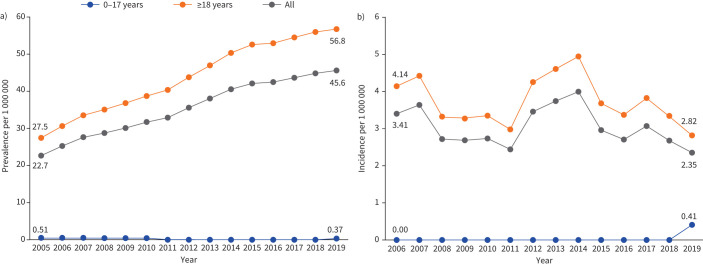
The overall annual prevalence of diagnosed EGPA increased from 22.7 (95% CI 20.0–25.7) to 45.6 (95% CI 42.1–49.4) cases per 1 000 000 people from 2005 to 2019 (figure 2a and supplementary table S3). The increase was driven by increased prevalence in patients aged ≥18 years. The prevalence in the paediatric population aged ≤17 years ranged between 0 and 0.51 (95% CI 0.01–2.84) per 1 000 000 people over the same period.
FIGURE 2.
a) Prevalence and b) incidence of eosinophilic granulomatosis with polyangiitis in the United Kingdom over time. Patients from population 1 (940 prevalent patients) and population 2 (502 incident patients).
Between 2006 and 2019, the overall incidence of EGPA diagnosis ranged between 2.3 (95% CI 1.6–3.4) and 4.0 (95% CI 2.9–5.4) cases per 1 000 000 person-years (figure 2b and supplementary table S3) and the incidence in patients aged ≥18 years ranged between 2.8 (95% CI 1.9–4.1) and 5.0 (95% CI 3.6–6.6) per 1 000 000 person-years. The incidence estimates in patients aged ≤17 years was 0 per 1 000 000 person-years for all years from 2006 to 2018 and 0.41 (95% CI 0.01–2.30) per 1 000 000 person-years in 2019.
HCRU and OCS use in the 12 months following EGPA diagnosis in England
In the first 12 months following EGPA diagnosis, 49.9% of patients had all-cause inpatient stays and 19.1% had EGPA-related inpatient stays (table 2). The mean±sd number of annual EGPA-related inpatient stays was 1.2±0.6 per patient, with a median (IQR) length of stay of 11 (6.0–17.0) days. 5% of patients required all-cause A&E visits, with a mean annual number of 1.8±1.7 visits per patient. Overall, 97.1% of patients had GP visits and 88.6% had outpatient visits (table 2). The most common specialist outpatient visits were with respiratory medicine (33.7% of patients, with an annual mean of 3.9±2.8 visits per patient), followed by general medicine (32.9% of patients, with an annual mean of 3.5±3.6 visits per patient) and rheumatology (31.8% of patients, with an annual mean of 2.8±2.6 visits per patient). The mean number of GP, nurse or allied health professional visits per patient per year was 16.0±11.1, 3.4±3.9 and 7.2±8.9, respectively.
TABLE 2.
Healthcare resource utilisation in the year following eosinophilic granulomatosis with polyangiitis (EGPA) diagnosis in England
| Patients # | Events per patient per year | Length of stay, days | |||
| Mean±sd | Median (IQR) | Total | Median (IQR) | ||
| Patients ¶ | 377 | ||||
| Inpatient stays | |||||
| All-cause | 188 (49.9) | 1.7±1.3 | 2992 | 8.0 (3.0–17.0) | |
| EGPA-related | 72 (19.1) | 1.2±0.6 | 1283 | 11.0 (6.0–17.0) | |
| All-cause A&E visits | 19 (5.0) | 1.8±1.7 | 1.0 (1.0–2.0) | ||
| Outpatient visits to specialist + | |||||
| Respiratory medicine | 127 (33.7) | 3.9±2.8 | 3.0 (2.0–5.0) | ||
| General medicine | 124 (32.9) | 3.5±3.6 | 2.0 (1.0–4.0) | ||
| Rheumatology | 120 (31.8) | 2.8±2.6 | 2.0 (1.0–3.0) | ||
| ENT | 95 (25.2) | 2.8±1.9 | 2.0 (1.0–4.0) | ||
| Allied health professional episode | 70 (18.6) | 2.6±1.9 | 2.0 (1.0–3.0) | ||
| Ophthalmology | 54 (14.3) | 2.8±2.0 | 2.0 (1.0–4.0) | ||
| Nursing episode | 48 (12.7) | 2.5±2.6 | 1.0 (1.0–3.0) | ||
| General surgery | 47 (12.5) | 2.1±2.0 | 1.0 (1.0–3.0) | ||
| Dermatology | 46 (12.2) | 2.3±1.7 | 2.0 (1.0–3.0) | ||
| Nephrology | 37 (9.8) | 4.2±2.5 | 4.0 (2.0–6.0) | ||
| All-cause procedures | 196 (52.0) | 6.8±6.2 | 5.0 (2.0–8.0) | ||
| All-cause outpatient visits | 334 (88.6) | 9.8±7.4 | 8.0 (4.0–13.0) | ||
| All-cause primary care visits | |||||
| General practitioner | 366 (97.1) | 16.0±11.1 | 14.0 (8.0–22.0) | ||
| Nurse | 145 (38.5) | 3.4±3.9 | 2.0 (1.0–4.0) | ||
| Allied health professional | 251 (66.6) | 7.2±8.9 | 4.0 (2.0–9.0) | ||
Data are presented as n or n (%), unless otherwise stated. IQR: interquartile range; A&E: accident and emergency; ENT: ear, nose and throat. #: number of patients with one or more event; ¶: patients from population 3; +: top 10 most frequent specialty outpatient visits.
OCS use was high, with 38.7% of patients having five or more prescriptions for OCS during the 12-month follow-up period (figure 3). The proportion of patients with no OCS prescriptions increased as time from diagnosis lengthened, with 36.3% requiring no OCS 0–3 months post-index, increasing to 55.2% 9–12 months post-index (table 3). Patients had OCS prescriptions covering a mean of 47.0% of days in the year following diagnosis (MPR=0.47).
FIGURE 3.
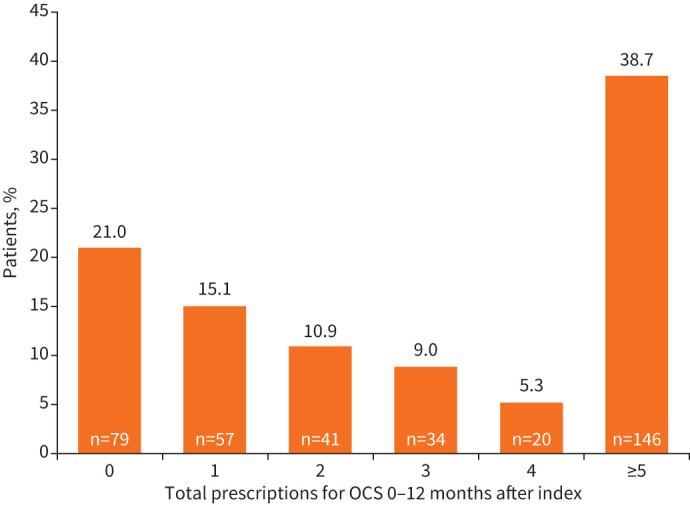
Prescriptions for oral corticosteroids (OCS) in the 12 months after index in England. Patients from population 3.
TABLE 3.
Oral corticosteroid (OCS) use in the year following eosinophilic granulomatosis with polyangiitis diagnosis in England#
| Patients ¶, n | 377 |
| OCS prescriptions | |
| 0–≤3 months post-index | |
| 0 | 137 (36.3) |
| 1 | 107 (28.4) |
| 2 | 58 (15.4) |
| 3 | 52 (13.8) |
| 4 | 11 (2.9) |
| ≥5 | 12 (3.2) |
| >3–≤6 months post-index | |
| 0 | 178 (47.2) |
| 1 | 77 (20.4) |
| 2 | 53 (14.1) |
| 3 | 40 (10.6) |
| 4 | 17 (4.5) |
| ≥5 | 12 (3.2) |
| >6–≤9 months post-index | |
| 0 | 201 (53.3) |
| 1 | 64 (17.0) |
| 2 | 52 (13.8) |
| 3 | 33 (8.8) |
| 4 | 20 (5.3) |
| ≥5 | 7 (1.9) |
| >9–≤12 months post-index | |
| 0 | 208 (55.2) |
| 1 | 66 (17.5) |
| 2 | 44 (11.7) |
| 3 | 36 (9.6) |
| 4 | 12 (3.2) |
| ≥5 | 11 (2.9) |
| Medication possession ratio, mean±sd | 0.47±0.47 |
Data are presented as n (%), unless otherwise stated. #: patients from population 3; ¶: patients with at least one event.
Discussion
EGPA is a rare disease and previous estimates of the incidence and prevalence are limited [17, 22, 28, 29]. To our knowledge, this is the first study assessing the prevalence and incidence of diagnosed EGPA exclusively in the UK, together with the associated disease burden. This study reported prevalence and incidence estimates of EGPA in the UK population of 22.7–45.6 per 1 000 000 people and 2.3–4.0 per 1 000 000 person-years, respectively, which is higher than estimates reported for other European countries between 1992 and 2017 [14]. Furthermore, the annual EGPA prevalence increased over the study period, driven by increases in adult prevalence; whereas, overall, the incidence remained stable in all age-ranges. The results presented herein suggest a high healthcare burden for patients with EGPA in the UK, as well as a treatment burden suggested by the high OCS use in this population. This highlights an unmet clinical need that could potentially be addressed by optimised management and/or new optimised treatments. Recent published guidelines have highlighted the use of newer treatments such as biologics, including anti-interleukin (IL)-5 therapies, for the induction of remission or the maintenance of remission for patients with EGPA [18]. Clinical benefits of anti-IL-5 therapies for patients with EGPA include OCS-sparing effects [30–32].
Patient demographics and clinical characteristics were similar to those reported in previous retrospective database studies assessing the prevalence, incidence and burden of EGPA in other countries [5, 9, 15, 33]. Prior to diagnosis patients most commonly experienced respiratory-related symptoms and >80% had comorbid asthma. This is consistent with the commonly reported pattern of disease development leading to EGPA where the development of asthma typically pre-dates the development of hypereosinophilia and vasculitis by several years [5, 7].
Previously reported European and global pooled estimates from a meta-analysis of observational studies covering study periods from 1992 to 2017 indicated an EGPA prevalence of 12.1–15.3 per 1 000 000 and an incidence of 1.1–1.2 per 1 000 000 person-years, although individual studies varied substantially. However, the results of that meta-analysis, and the underlying original studies, have limitations, including the change of criteria for identifying patients with EGPA over time and their inconsistency across studies, and the estimates for prevalence were heavily influenced by the high patient sample of one particular study from claims databases in the United States of America (USA) [14]. In the UK, data from the early 2000s suggested a prevalence of 38.0 per 1 000 000 (2000) and an incidence of 4.2 per 1 000 000 person-years (2004), but with no clear explanation of the methods employed to obtain such estimates [22]. Similarly, a previous England-based study, which analysed Hospital Episode Statistics data, indicated a prevalence of 31.8 cases per 1 000 000 in 2016 [17]. By comparison, in the current study, the 2016 prevalence of EGPA was estimated to be 42.5 cases per 1 000 000. This discrepancy may be due to differences in data source, study methodology and reporting period. For example, the current study includes primary care data from across the UK (CPRD-AURUM database), whereas the England-based study only utilised Hospital Episode Statistics and therefore would not have captured patients seen in primary care but not treated in the hospital setting in that period, which may have not captured less severe cases of EGPA [17]. Finally, the previous study estimated the point prevalence on a given day in 2016, whereas our study estimated annual prevalence from 2005 to 2019.
In the current study, prevalence of EGPA increased two-fold from 2005 to 2019, while EGPA incidence varied, but had no overall increase. Similarly, a retrospective study of administrative claims from the Japan Medical Data Centre (JMDC) claims database (132 patients) found a nine-fold increase in EGPA prevalence from 4.2 to 38.0 per 1 000 000 from 2005 to 2017, where EGPA cases were diagnosed via ICD-10 code for EGPA (M30.1), plus an additional ICD-10 code for allergic rhinitis, asthma or chronic sinusitis prior to their EGPA diagnosis [15]. This trend of increasing EGPA prevalence in Japan has continued between 2017 and 2020 [34]. This is consistent with previous studies in Australia and France, which showed two- to three-fold increases in EGPA prevalence over 8–10 years from the late-1990s to mid-2000s, but with little change in incidence, although both studies were small with only eight and 31 EGPA cases identified, respectively [35, 36]. However, the previously mentioned systematic review and meta-analysis study reported no strong trends for increasing EGPA prevalence over time [14]. These apparent differences in prevalence highlight the difficulties in determining accurate prevalence estimates, and may reflect the impact of EGPA rarity, difficulty of diagnosis and disease under-recognition [37]. Nonetheless, EGPA prevalence may have increased over time due to changes to the diagnostic criteria, increased disease awareness and/or the combination of a stable incidence rate and high long-term survival rates [2, 14, 20, 21, 38]. The cumulative survival rate for patients with EGPA at 5 and 10 years from disease onset ranges between 89–97% and 79–89%, respectively [5, 13, 37, 39, 40]. Conventional therapy for EGPA allows for high overall survival rates, and patients with EGPA are living longer, despite living with a high disease burden [9, 41].
HCRU in the year after a EGPA diagnosis was common, with half of patients having an inpatient stay for any reason and almost one-fifth of patients having an EGPA-related inpatient stay. On average, patients had one EGPA-related inpatient visit per year, staying for a median of 11 days per visit. Given the cost of inpatient treatment and the high demand for hospital beds [33], the extended length of hospital stays for EGPA-related treatment demonstrates the sizeable per-patient disease burden for the UK health system. The high discrepancy between all-cause and EGPA-related inpatient stays may reflect an underestimation of the latter due to the challenge in attributing the varied clinical manifestations to EGPA [5, 12], and complications from OCS use [10, 18]. Additionally, many patients with EGPA experience asthma-related inpatients stays [9] and EGPA may therefore not be reported as the primary reason for such stays. The high HCRU burden of EGPA identified in this study is consistent with that demonstrated in other countries [9, 14, 15, 33]. For example, a previous systematic review and meta-analysis, which included studies from the USA, Europe, Australia and Japan, indicated that ∼42% of patients with EGPA required an unscheduled hospital visit [14]. Furthermore, the study found that patients with EGPA required a median of one (range 0–6) hospital visit and one (range 0–12) A&E visit annually [14], consistent with the data reported here.
The high OCS use observed in this study is broadly consistent with the OCS dependence demonstrated in previous studies [9, 15, 33]. Indeed, 38.7% of patients accumulated five or more OCS prescriptions over the year following diagnosis, although there is some evidence that these became less frequent with increasing time from diagnosis. Data on OCS dose were not available here, but previous studies have demonstrated a requirement for high-dose OCS among patients with EGPA. For example, a retrospective Japan-based study found that OCS dose reduced from baseline (mean of 39.1 mg·day−1) in the year following an EGPA diagnosis, but remained high in absolute terms (mean of 9.8 mg·day−1 and most patients had daily dose ≥15 mg·day−1) [15]. Combined with this study's results, these observations suggest that patients with EGPA remain dependent on OCS, increasing the potential for OCS-related toxicity [8, 42].
The burden of acute and chronic corticosteroid-related complications and associated HCRU in severe asthma and the risks increase with cumulative corticosteroid exposure are well documented [11, 42]. Treatment guidelines for EGPA highlight the importance of minimising OCS exposure [8], and novel OCS-sparing therapies that control symptoms while reducing treatment-related side-effects are needed. Given the role of eosinophils in the pathology of EGPA, biologics targeting IL-5, the major cytokine responsible for eosinophil differentiation, survival and activation [43–45], have been investigated for use in EGPA, and have shown benefit as OCS-sparing treatments [30, 46]. The anti-IL-5 monoclonal antibody mepolizumab is approved for the treatment of eosinophil-driven diseases including EGPA in multiple regions worldwide [47–49]; however, anti-IL-5 therapies are not currently approved by the National Institute for Health and Care Excellence in the UK for the treatment of EGPA.
A strength of this study was that it utilised the UK-wide CPRD-AURUM database to assess the prevalence and incidence of EGPA, as well as the England-specific Hospital Episode Statistics database which captures a patient's complete NHS HCRU profile. As of 2019, the CPRD-AURUM database included data from ∼13% of the population in England [24]. In another study with a similar approach, the use of ICD codes in the Hospital Episode Statistics database for the diagnosis of AAV was validated, as these codes were found to have an 86% positive predictive value [17]. Although diagnosis was obtained in CPRD-AURUM via different coding systems in the present study, this solidifies the Hospital Episode Statistics database as a promising data source for linkage to CPRD-AURUM for retrospective studies in EGPA. In terms of limitations, reasons for OCS use and the OCS dose were not captured in the Hospital Episode Statistics database, so it was not possible to distinguish whether OCS prescriptions were for EGPA or other comorbid conditions, or to calculate cumulative steroid exposure. EGPA diagnosis can be complicated by the heterogeneous nature of the disease, the need to exclude “vasculitis mimics” and other small/medium-vessel vasculitis, and overlap with other eosinophilic diseases, which can lead to delayed diagnosis or misdiagnosis [1–3]. Consequently, prevalence and incidence could have been underestimated. Furthermore, it is possible that a patient may have had a previous EGPA diagnosis from a non-CPRD-AURUM practice, which could have resulted in previously diagnosed patients being incorrectly included in the first diagnosis/incidence population. Moreover, the number of all-cause hospitalisations being nearly three-fold that of EGPA-related hospitalisations in this study might suggest under-coding. Additionally, the findings of the study may not be generalisable to practices and patients not enrolled in CPRD-AURUM, although a previous assessment of the database found that it was representative of the English population [24]. Finally, this study also shares limitations typical of retrospective database studies, such as potential inconsistencies and errors in the diagnostic codes used to identify EGPA and comorbidities.
Conclusion
In the UK, although the incidence of EGPA remains relatively stable, the prevalence of EGPA is increasing. This study adds to the currently limited UK-specific data on EGPA prevalence and incidence, and identifies for the first time the considerable healthcare burden for patients with EGPA in the UK, as indicated by frequent HCRU and OCS use. This study suggests a high level of remaining unmet need for patients with EGPA, and future studies are needed to understand the impact of new treatments on the patient and disease burden.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Table S1 00430-2023.table_S1 (623.6KB, pdf)
Supplementary methods and table S2 00430-2023.methods_and_table_S2 (652.5KB, pdf)
Table S3 00430-2023.table_S3 (641.5KB, pdf)
Figure S1 00430-2023.figure_S1 (360.1KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Acknowledgements: This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The data were provided by patients, and collected by the National Health Service as part of their care and support. The interpretations and conclusions contained in this study are those of the authors alone. Editorial support (in the form of collating and incorporating authors’ comments for each draft, grammatical editing and referencing) was provided by Frankie Wignall, Alice Rees and Benjamin Danet (Fishawack Indicia Ltd, UK, part of Avalere Health), and was funded by GSK.
Ethics statement: This study was conducted in accordance with the Declaration of Helsinki, and approved by the GSK Protocol Review Committee and CPRD Independent Scientific Advisory Committee (now replaced by the Research Data Governance process), which reviewed the protocol and approved access to CPRD data (approval no. 21_000352). Informed consent and ethics committee or institutional review board approval were not required as no direct patient contact or primary collection of patient data occurred. The CPRD obtains ethical research approval annually from the UK's Health Research Authority Research Ethics Committee to accumulate and distribute patient data.
Availability of data and materials: For requests for access to anonymised subject-level data, please contact the corresponding author.
Author contributions: J. Hwee, R.W. Jakes and Q. Fu were involved in the conception or design of the study, data acquisition, and analysis or interpretation of data. L. Harper, K. Nirantharakumar and G. Mu were involved in the conception or design of the study and the analysis or interpretation of data. All authors drafted the manuscript or revised it critically for important intellectual content and all authors approved the final version to be published.
Conflict of interest: J. Hwee, Q. Fu, G. Mu and R.W. Jakes are employees of GSK and own stocks/shares with GSK. L. Harper has received speaking fees, consulting fees or grant/research support from Viopharm, GSK, Roche and MSD. K. Nirantharakumar has been awarded academic institution research grants from NIHR, UKRI/MRC, Kennedy Trust for Rheumatology Research, Health Data Research UK, Wellcome Trust, European Regional Development Fund, Institute for Global Innovation, Boehringer Ingelheim, Action Against Macular Degeneration Charity, Midlands Neuroscience Teaching and Development Funds, South Asian Health Foundation, Vifor Pharma, College of Police, and CSL Behring; consulting fees from BI, Sanofi, CEGEDIM and MSD; and holds a leadership/fiduciary role with NICST, a charity, and OpenClinical, a social enterprise.
Support statement: This study was funded by GSK (207888). GSK was involved in the study design, data acquisition, data analysis and interpretation, and manuscript preparation. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int 2019; 68: 430–436. doi: 10.1016/j.alit.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11. doi: 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 3.Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis 2022; 81: 309–314. doi: 10.1136/annrheumdis-2021-221794 [DOI] [PubMed] [Google Scholar]
- 4.Chang HC, Chou PC, Lai CY, et al. Antineutrophil cytoplasmic antibodies and organ-specific manifestations in eosinophilic granulomatosis with polyangiitis: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2021; 9: 445–452. doi: 10.1016/j.jaip.2020.07.038 [DOI] [PubMed] [Google Scholar]
- 5.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheumatol 2013; 65: 270–281. doi: 10.1002/art.37721 [DOI] [PubMed] [Google Scholar]
- 6.Raffray L, Guillevin L. Treatment of eosinophilic granulomatosis with polyangiitis: a review. Drugs 2018; 78: 809–821. doi: 10.1007/s40265-018-0920-8 [DOI] [PubMed] [Google Scholar]
- 7.Doubelt I, Cuthbertson D, Carette S, et al. Clinical manifestations and long-term outcomes of eosinophilic granulomatosis with polyangiitis in North America. ACR Open Rheumatol 2021; 3: 404–412. doi: 10.1002/acr2.11263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2021; 73: 1366–1383. doi: 10.1002/art.41773 [DOI] [PubMed] [Google Scholar]
- 9.Gokhale M, Bell CF, Doyle S, et al. Prevalence of eosinophilic granulomatosis with polyangiitis and associated health care utilization among patients with concomitant asthma in US commercial claims database. J Clin Rheumatol 2021; 27: 107–113. doi: 10.1097/RHU.0000000000001198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson J, Doll H, Suppiah R, et al. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European Vasculitis Study Group trials. Rheumatology 2015; 54: 471–481. doi: 10.1093/rheumatology/keu366 [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre P, Duh MS, Lafeuille MH, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin 2017; 33: 57–65. doi: 10.1080/03007995.2016.1233101 [DOI] [PubMed] [Google Scholar]
- 12.Durel CA, Berthiller J, Caboni S, et al. Long-term followup of a multicenter cohort of 101 patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Arthritis Care Res 2016; 68: 374–387. doi: 10.1002/acr.22686 [DOI] [PubMed] [Google Scholar]
- 13.Saku A, Furuta S, Hiraguri M, et al. Longterm outcomes of 188 Japanese patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol 2018; 45: 1159–1166. doi: 10.3899/jrheum.171352 [DOI] [PubMed] [Google Scholar]
- 14.Jakes RW, Kwon N, Nordstrom B, et al. Burden of illness associated with eosinophilic granulomatosis with polyangiitis: a systematic literature review and meta-analysis. Clin Rheumatol 2021; 40: 4829–4836. doi: 10.1007/s10067-021-05783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sada KE, Kojo Y, Fairburn-Beech J, et al. The prevalence, burden of disease, and healthcare utilization of patients with eosinophilic granulomatosis with polyangiitis in Japan: a retrospective, descriptive cohort claims database study. Mod Rheumatol 2022; 32: 380–386. doi: 10.1093/mr/roab007 [DOI] [PubMed] [Google Scholar]
- 16.Mahr A, Guillevin L, Poissonnet M, et al. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg–Strauss syndrome in a French urban multiethnic population in 2000: a capture–recapture estimate. Arthritis Rheum 2004; 51: 92–99. doi: 10.1002/art.20077 [DOI] [PubMed] [Google Scholar]
- 17.Pearce FA, Griffiths B, Mukhtyar C, et al. Prevalence and mortality of ANCA-associated vasculitis in England. Rheumatology 2020; 59: Suppl. 2, ii85. doi: 10.1093/rheumatology/keaa206 [DOI] [Google Scholar]
- 18.Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis 2024; 83: 30–47. doi: 10.1136/ard-2022-223764 [DOI] [PubMed] [Google Scholar]
- 19.Watts RA, Hatemi G, Burns JC, et al. Global epidemiology of vasculitis. Nat Rev Rheumatol 2022; 18: 22–34. doi: 10.1038/s41584-021-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg–Strauss syndrome. Medicine 1984; 63: 65–81. doi: 10.1097/00005792-198403000-00001 [DOI] [PubMed] [Google Scholar]
- 21.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007; 66: 222–227. doi: 10.1136/ard.2006.054593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts RA, Lane S, Scott DG. What is known about the epidemiology of the vasculitides? Best Pract Res Clin Rheumatol 2005; 19: 191–207. doi: 10.1016/j.berh.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 23.Thorn JC, Turner E, Hounsome L, et al. Validation of the hospital episode statistics outpatient dataset in England. Pharmacoeconomics 2016; 34: 161–168. doi: 10.1007/s40273-015-0326-3 [DOI] [PubMed] [Google Scholar]
- 24.Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol 2019; 48: 1740–1740g. doi: 10.1093/ije/dyz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce FA, Hubbard RB, Grainge MJ, et al. Can granulomatosis with polyangiitis be diagnosed earlier in primary care? A case–control study. QJM 2018; 111: 39–45. doi: 10.1093/qjmed/hcx194 [DOI] [PubMed] [Google Scholar]
- 26.Metcalfe D, Masters J, Delmestri A, et al. Coding algorithms for defining Charlson and Elixhauser co-morbidities in Read-coded databases. BMC Med Res Methodol 2019; 19: 115. doi: 10.1186/s12874-019-0753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130: 607–612. doi: 10.1016/j.jaci.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto S, Watts RA, Kobayashi S, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology 2011; 50: 1916–1920. doi: 10.1093/rheumatology/ker205 [DOI] [PubMed] [Google Scholar]
- 29.Pearce FA, Lanyon PC, Grainge MJ, et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology 2016; 55: 1656–1663. doi: 10.1093/rheumatology/kew232 [DOI] [PubMed] [Google Scholar]
- 30.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017; 376: 1921–1932. doi: 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019; 143: 2170–2177. doi: 10.1016/j.jaci.2018.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent BD, d'Ancona G, Fernandes M, et al. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00311-2019. doi: 10.1183/23120541.00311-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell CF, Blauer-Peterson C, Mao J. Burden of illness and costs associated with eosinophilic granulomatosis with polyangiitis: evidence from a managed care database in the United States. J Manag Care Spec Pharm 2021; 27: 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sada KE, Suzuki T, Joksaite S, et al. Trends in prevalence, treatment use, and disease burden in patients with eosinophilic granulomatosis with polyangiitis in Japan: real-world database analysis. Mod Rheumatol 2023; in press [ 10.1093/mr/road104]. doi: 10.1093/mr/road104 [DOI] [PubMed] [Google Scholar]
- 35.Ormerod AS, Cook MC. Epidemiology of primary systemic vasculitis in the Australian Capital Territory and south-eastern New South Wales. Intern Med J 2008; 38: 816–823. doi: 10.1111/j.1445-5994.2008.01672.x [DOI] [PubMed] [Google Scholar]
- 36.Vinit J, Muller G, Bielefeld P, et al. Churg–Strauss syndrome: retrospective study in Burgundian population in France in past 10 years. Rheumatol Int 2011; 31: 587–593. doi: 10.1007/s00296-009-1275-y [DOI] [PubMed] [Google Scholar]
- 37.Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013; 72: 1011–1017. doi: 10.1136/annrheumdis-2012-201531 [DOI] [PubMed] [Google Scholar]
- 38.Mun CH, Yoo J, Jung SM, et al. The initial predictors of death in 153 patients with ANCA-associated vasculitis in a single Korean centre. Clin Exp Rheumatol 2018; 36: Suppl. 111, 65–72. [PubMed] [Google Scholar]
- 39.Tsurikisawa N, Oshikata C, Kinoshita A, et al. Longterm prognosis of 121 patients with eosinophilic granulomatosis with polyangiitis in Japan. J Rheumatol 2017; 44: 1206–1215. doi: 10.3899/jrheum.161436 [DOI] [PubMed] [Google Scholar]
- 40.Samson M, Puéchal X, Devilliers H, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013; 43: 60–69. doi: 10.1016/j.jaut.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 41.Trivioli G, Terrier B, Vaglio A. Eosinophilic granulomatosis with polyangiitis: understanding the disease and its management. Rheumatology 2020; 59: Suppl. 3, iii84–iii94. doi: 10.1093/rheumatology/kez570 [DOI] [PubMed] [Google Scholar]
- 42.Dalal AA, Duh MS, Gozalo L, et al. Dose–response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm 2016; 22: 833–847. doi: 10.18553/jmcp.2016.22.7.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdala-Valencia H, Coden ME, Chiarella SE, et al. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J Leukoc Biol 2018; 104: 95–108. doi: 10.1002/JLB.1MR1117-442RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stirling RG, van Rensen EL, Barnes PJ, et al. Interleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med 2001; 164: 1403–1409. doi: 10.1164/ajrccm.164.8.2010002 [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi Y, Suda T, Ohta S, et al. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood 1991; 78: 2542–2547. doi: 10.1182/blood.V78.10.2542.2542 [DOI] [PubMed] [Google Scholar]
- 46.Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract 2021; 9: 1186–1193. doi: 10.1016/j.jaip.2020.09.054 [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency . Mepolizumab (Nucala) Summary of Product Characteristics. www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf. Date last updated: 13 November 2022. Date last accessed: 16 May 2023.
- 48.GSK . Mepolizumab (Nucala) prescribing information, Japan. https://gskpro.com/ja-jp/products-info/nucala/index/. Date last updated: March 2020. Date last accessed: 16 May 2023.
- 49.GSK . Mepolizumab (Nucala) prescribing information. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF. Date last updated: March 2023. Date last accessed: 16 May 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Table S1 00430-2023.table_S1 (623.6KB, pdf)
Supplementary methods and table S2 00430-2023.methods_and_table_S2 (652.5KB, pdf)
Table S3 00430-2023.table_S3 (641.5KB, pdf)
Figure S1 00430-2023.figure_S1 (360.1KB, pdf)