ABSTRACT
Background
This study aimed to observe the efficacy and safety of tacrolimus in the treatment of refractory immunoglobulin A vasculitis nephritis (IgAVN).
Methods
Sixteen patients with IgAVN who had been previously treated with cyclophosphamide shock therapy at least five times, some of whom had also received mycophenolate but still had persistent proteinuria, were enrolled. The clinical and pathological data were collected and analysed.
Results
The average (mean ± standard deviation) age at the initial assessment for the group of 16 patients was 10 ± 2.7 years. Finally, at the end of their respective follow-up time point, 6 of the 16 patients achieved complete remission (37.5%), 5 achieved partial remission (31.2%), and 5 had no remission (31.2%). A significant difference was found in the median proteinuria before and after a 6-month course of tacrolimus treatment [19.2 (11.2, 31.9) vs 7.8 (4.3, 13.9) mg/kg/day] (P < .05). During the first 6 months of tacrolimus treatment, all patients’ estimated glomerular filtration rate levels remained normal. The mean tacrolimus blood concentration was 6.0 ± 2.6 ng/mL. The median prednisone dosage was decreased from 10 mg/day to 5 mg/day, and prednisone was eventually stopped in three individuals. No drug-related adverse effects were observed during treatment.
Conclusions
Tacrolimus has demonstrated efficacy in increasing remission rates, significantly lowering urinary protein levels, and reducing steroid use in children with refractory IgAVN. Further research is required to investigate its optimal blood concentrations, long-term effects and renoprotective properties.
Keywords: children, IgA vasculitis with nephritis, proteinuria, tacrolimus
KEY LEARNING POINTS.
What was known:
Immunoglobulin A vasculitis nephritis (IgAVN) has a generally favourable prognosis; however, a subgroup of individuals with refractory IgAVN may respond inadequately to routine therapy.
These people may benefit from different drug therapies focused on lowering urine proteins and reducing the risk of developing end-stage renal disease (ESRD).
This study adds:
Tacrolimus could be an effective treatment option for IgAVN children who are refractory to cyclophosphamide or combined with mycophenolate mofetil.
Tacrolimus can improve urinary protein in children with refractory IgAVN and reduce glucocorticoid use to some extent. Adverse effects of tacrolimus therapy were infrequent and minimal.
Potential impact:
For children with refractory IgAVN, the choice of tacrolimus therapy has the potential to increase the remission rate, improve prognosis and delay progression to ESRD.
INTRODUCTION
Immunoglobulin A vasculitis (IgAV), which is alternatively referred to as Henoch-Schönlein purpura, is widely recognized as the prevailing autoimmune vasculitis among children, with a reported annual incidence of 3–27 cases per 100 000 children [1]. It can involve the skin, joints, gastrointestinal tract and kidneys. The overall prognosis of IgAV is generally favourable, but renal damage is a major cause of mortality and a significant determinant of long-term prognosis [2]. When the kidneys are affected, the disease is termed IgA vasculitis with nephritis (IgAVN), which is one of the most serious complications of IgAV [3].
Massive proteinuria is an independent predictor of renal outcome in patients with IgAVN [4]. In the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines [5], children with proteinuria levels >1 g/day/1.73 m2 or mesangial proliferation are advised to take steroids either alone or in combination with cyclophosphamide (CTX). Steroids combined with CTX, mycophenolate mofetil (MMF) or azathioprine are mostly advised for children who have IgAVN with nephrotic proteinuria, haematuria with proteinuria, or crescent formation [6]. Among the cohort of pediatric patients diagnosed with IgAVN and subjected to renal biopsy at our establishment from 2011 to 2017, it was observed that a significant majority (61.8%) presented with nephrotic syndrome as the clinical manifestation. Furthermore, an overwhelming proportion (92.9%) of these individuals exhibited a favourable prognosis subsequent to the administration of glucocorticoids in conjunction with either CTX or MMF [7]. The treatment regimen recommended by the KDIGO guidelines is effective for most patients. However, some patients show resistance to or are unable to achieve complete remission using steroids and/or immunosuppressants, resulting in recurrent episodes of the disease, which is referred to as refractory IgAVN. These patients have a high risk of developing end-stage renal disease (ESRD).
Tacrolimus (TAC) is a potent immunosuppressant applied in the clinic. It has a strong inhibitory effect on T-cell proliferation and can be widely used in glomerular diseases, especially refractory glomerular diseases [8]. It has been reported in the literature that TAC can alleviate proteinuria in children with steroid-resistant nephrotic syndrome [9] and can also be used to treat refractory IgA nephropathy [10], which can significantly decrease proteinuria and haematuria in patients with fewer adverse effects. Additionally, TAC has been gradually employed to treat lupus nephritis, which can also swiftly and efficiently lower patients’ proteinuria [11].
According to the 2016 Chinese evidence-based guidelines [12], it is stated that steroids can be combined with TAC for the treatment of IgAVN, but there are few clinical reports. Some clinical studies have confirmed the efficacy of TAC in IgAVN [13, 14]. Nonetheless, a limited number of investigations have been conducted on the therapeutic application of TAC for refractory IgAVN.
Consequently, the objective of this study was to retrospectively analyse the clinical manifestations and laboratory tests of 16 paediatric patients with refractory IgAVN in our hospital and to evaluate the therapeutic efficacy of the patients and their prognosis after the use of TAC.
MATERIALS AND METHODS
Patient selection
A total of 16 patients, all aged 3–18 years and diagnosed with IgAVN, who had been previously treated with CTX shock therapy at least five times (each shock dose of 8–10 mg/kg/day for 2 consecutive days), along with some of the patients who took MMF (250 mg/dose twice daily for >3 months) but still had persistent proteinuria (≥150 mg/day) were included in the study. These patients were treated at the Department of Nephrology, Affiliated Children's Hospital of Chongqing Medical University, between 2012 and 2023. All patients clinically met the diagnostic criteria of the Chinese evidence-based guidelines for the diagnosis and treatment of Henoch-Schönlein purpura nephritis (2016) [12] and had undergone renal histopathologic biopsy before TAC treatment. The study was approved by the medical ethics committee of the hospital. The studies involving human participants were reviewed and approved by the Ethics Committee of the Children's Hospital of Chongqing Medical University (approval number: 2023-52). Patient consent was not required.
Methods
All demographic, clinical and laboratory data were collected. The demographic and clinical data included age at the time of IgAVN, sex, height, weight, blood pressure, medical history, type of immunosuppressive therapy used, duration of follow-up and adverse events during follow-up; laboratory data included routine blood counts (leukocytes, erythrocytes, platelets and neutrophil-to-lymphocyte ratio), urine parameters (24-h urinary protein, urine erythrocyte count, β2-microglobulin), biochemical parameters (albumin, serum creatinine, urea nitrogen, uric acid, total cholesterol), immune-related parameters [IgA, complement 3 (C3)], coagulation parameters (fibrinogen, D-dimer) and renal biopsy results, which were retrieved from medical records. Before TAC therapy, all patients had undergone renal histopathologic biopsy. One patient's renal biopsy material revealed no glomeruli, and his parents declined to have a second renal biopsy.
The primary outcomes were complete remission and partial remission [13]: complete remission was defined as a urinary protein test <150 mg/day. Patients achieving a reduction of >50% in 24-h urinary protein levels to <50 mg/kg after receiving TAC were categorized as experiencing partial remission. Those with 24-h urinary protein levels of ≥50 mg/kg, or whose reduction was <50% after receiving TAC, were considered to have shown no efficacy from the treatment. The evaluation of the safety of TAC was based on the analysis of adverse reactions after treatment, such as severe infection, sepsis, hyperlipidaemia, diabetes mellitus, gastrointestinal reactions, liver dysfunction, and the incidence of acute kidney injury (AKI) or chronic kidney disease (CKD).
After receiving CTX shock therapy more than five times or even partially combined with MMF for IgAVN, the patient's urine protein levels decreased statistically [77.3 (57.5, 171.6) vs 19.2 (11.2, 31.9) mg/kg/day, P < .001], but they did not yet fulfil the criteria for remission. At the same time, there was no statistically significant difference between the levels of urine erythrocyte count, serum creatinine or estimated glomerular filtration rate (eGFR) before and after CTX or and MMF therapy. This group of treatment refractory children received TAC (0.05–0.1 mg/kg/day) and glucocorticoids in combination with angiotensin-converting enzyme inhibitors (ACEIs) with the families’ permission. The dosage of TAC was then changed based on the blood concentration to keep the blood concentration at 5–10 ng/mL. Patients were monitored monthly until proteinuria became negative, after which they were followed up every 1–3 months.
Follow-up data for all patients were obtained for the initial 6-month period of TAC treatment. Seven patients were evaluated at the 12th and 18th month intervals, as well as five at the 24th and 36th months.
Clinical manifestations and pathological grading
Clinical manifestations refer to the evidence-based guidelines for the diagnosis and treatment of IgAVN developed by the Nephrology Group of the Chinese Medical Association's Pediatrics Branch, which are divided into seven types: [12] (i) isolated haematuria; (ii) isolated proteinuria; (iii) haematuria and proteinuria; (iv) nephritis syndrome; (v) nephrotic syndrome; (vi) rapidly progressive glomerulonephritis; and (vii) chronic nephritis (specific definitions of the seven types are detailed in Supplementary data, Table S1). In traditional pathological grading, glomerular pathological grading refers to International Study of Kidney Disease in Children (ISKDC) grading [15], which is divided into Grades I–VI based on the extent of crescentic lesions in the glomerulus. Detailed ISKDC grading is shown in Supplementary data, Table S2. Tubulointerstitial pathology was graded according to Bohle et al. [16] and was divided into grades (–) to (++++) based on the extent of interstitial fibrosis and tubular atrophy. Detailed tubulointerstitial injury grading is shown in Supplementary data, Table S3. The latest pathological assessment system of IgAVN proposed by Koskela et al. [17] and other scholars [18], namely the modified semiquantitative classification (SQC), was introduced to evaluate renal tissue based on four aspects: glomerular, tubular, interstitial and capillary changes, with a total score of 26. Supplementary data, Table S4 provides specific SQC scoring information. All pathological specimens were evaluated by our pathologists who are experienced in reading renal pathological specimens without informing the clinical information of the cases.
Statistical analysis
SPSS 25.0 software was used to process the data. The summary statistics of quantitative variables with normal distribution are expressed as the mean ± standard deviation. Nonnormally distributed variables are expressed as medians and interquartile ranges. Categorical data are expressed as ratios and percentages. One-way analysis of variance or Wilcoxon rank sum tests were used for continuous variables. Differences in proportions were compared using the χ2 test or Fisher's exact test. The Spearman rank correlation test was used in the analysis of the correlations. Values of P < .05 were considered statistically significant.
RESULTS
Basic clinical information
The average age of the 16 children at the time of the beginning of IgAVN was 10.4 ± 2.7 years, with 12 boys and 4 girls; the clinical typing was nephrotic in 8 cases, nephrotic combined with nephritic in 4 cases, haematuria and proteinuria in 2 cases and acute nephritic in 2 cases. All 16 children had a palpable purpuric eruption in addition to the renal manifestations, 8 patients had abdominal pain, 2 had joint pain and 1 had generalized oedema. At the start of the illness, the blood pressure of each patient was within the normal range. All patients had been treated with regular steroids in combination with ACEIs as well as immunosuppressants before TAC therapy: 3 patients received CTX and 13 received CTX + MMF. The median number of CTX shocks in all 16 patients was 8. The median interval between diagnosis and the start of TAC was 18.5 months. All patients had been taking TAC for >6 months and were also taking low to medium-dose glucocorticoids as well as ACEIs (which were tapered at follow-up depending on blood pressure and the degree of urinary protein and renal function).
Regarding laboratory data: 13 of these patients presented with massive proteinuria (>50 mg/kg/day) at the onset of the disease (median urinary protein was 77.7 mg/kg/day); all patients exhibited varying levels of urinary red blood cells in their urine tests; 1 patient had an eGFR of <90 mL/min/1.73 m2 at the time of diagnosis; and 3 patients had an increase in IgA, while 5 had a decrease in C3. The results of other tests such as routine blood tests, β2-microglobulin, urea nitrogen, uric acid, total cholesterol, fibrinogen and D-dimer were not found to be significantly abnormal.
Of the 15 patients with meaningful renal pathology biopsy results, 5 patients had a glomerular classification of Type IIIa, 7 patients had Type IIIb and 3 patients had Type IVb. Seven patients had tubulointerstitial grading of (–), five patients had (+) and three patients had (++). All of them had different degrees of glomerulosclerosis, glomerular necrosis, tubular atrophy and crescent formation. The average SQC score for these patients was 8.3 ± 2.1.
Their baseline clinical characteristics, laboratory data and renal biopsy data are presented in Table 1.
Table 1:
Clinical characteristics, baseline laboratory data and renal biopsy data in 16 patients.
| No. | Gender | Age, years | Clinical renal typing | Extra-renal symptoms | Immuno suppressants before TAC treatment suppressants before TAC treatment |
Duration of IgAVN before TAC, months | TAC treatment duration, months | Urinary protein, mg/kg/day | URBC, PCS/μL | Alb, g/L | SCr, μmol/L | eGFR, mL/min/1.73 m2 | IgA, g/L | C3, g/L | Glome rular sclerosis, % rular sclerosis, % |
Glome rular necrosis, % rular necrosis, % |
Tubular atrophy,% | Tubulo interstitial fibrosis,% interstitial fibrosis,% |
Crescents, % | ISKDC grade | Tubulo interstitial injury grade interstitial injury grade |
SQC scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 9.3 | Nephrotic syndrome | Skin purpuraAbdominal pain | CTX*13 | 37 | 36 | 68.9 | 29 | 27 | 44 | 149.16 | 1.49 | 0.98 | 15 | – | – | – | 8 | IIIa | (+) | 7 |
| 2 | M | 14.2 | Nephrotic syndromeNephritis syndrome | Skin purpura | CTX*8 + MMF | 11 | 12 | 74.8 | 310 | 30.3 | 102 | 101.92 | 1.43 | 0.9 | – | – | – | – | 60 | IV | (–) | 6 |
| 3 | M | 9.2 | Nephritis syndrome | Skin purpuraAbdominal pain | CTX*9 + MMF | 24 | 36 | 40.9 | 372 | 22.6 | 35.5 | 191.74 | 1.37 | 0.93 | – | – | – | – | 20 | IIIa | (+) | 5 |
| 4 | M | 13.6 | Nephrotic syndrome | Skin purpuraAbdominal pain | CTX*6 + MMF | 5 | 36 | 322.3 | 2720 | 17.8 | 89 | 104.29 | 1.31 | 0.58 | – | 33 | – | – | 33 | IIIb | (+) | 7 |
| 5 | M | 7.5 | Nephrotic syndromeNephritis syndrome | Skin purpuraAbdominal pain | CTX*9 + MMF | 35 | 36 | 160.8 | 561 | 19.9 | 45.2 | 140.91 | 2.74 | 0.82 | – | 67 | – | – | 44 | IIIb | (-) | 7 |
| 6 | M | 9.4 | Hematuria and proteinuria | Skin purpura | CTX*9 | 77 | 12 | 69.7 | 293 | 35.7 | 63 | 92.61 | 0.89 | 0.7 | 23 | – | 1 | 1 | – | IIIa | (++) | 12 |
| 7 | M | 6.5 | Hematuria and proteinuria | Skin purpuraAbdominal pain | CTX*9 + MMF | 32 | 36 | 46.5 | 871 | 43 | 34 | 180.18 | 1.44 | 0.7 | – | – | – | – | 9% | IIIa | (–) | 9 |
| 8 | F | 9.8 | Nephrotic syndromeNephritis syndrome | Skin purpura | CTX*9 | 25 | 30 | 211.7 | 873 | 44.9 | 51 | 132.51 | 4.16 | 1.14 | – | 53 | – | – | 27% | IIIb | (–) | 9 |
| 9 | M | 14.3 | Nephrotic syndromeNephritis syndrome | Skin purpura | CTX*8 + MMF | 10 | 6 | 175.2 | 2099 | 28.8 | 62 | 164.68 | 3.57 | 0.64 | 7 | – | 10 | – | 29% | IIIb | (++) | 9 |
| 10 | M | 10.9 | Nephrotic syndrome | Skin purpura | CTX*10 + MMF | 38 | 18 | 89.3 | 4790 | 18.2 | 123 | 57.32 | 0.876 | 0.48 | / | / | / | / | / | / | / | / |
| 11 | M | 13 | Nephrotic syndrome | Skin purpuraAbdominal pain | CTX*9 + MMF | 6 | 6 | 79.8 | 732 | 36.2 | 54 | 183.34 | 2.06 | 0.67 | – | 27 | – | – | 36% | IIIb | (++) | 9 |
| 12 | M | 13.6 | Nephrotic syndrome | Skin purpuraAbdominal painArthralgia | CTX*7 + MMF | 9 | 6 | 89.7 | 154 | 34.7 | 54 | 178.76 | 1.9 | 1.06 | 24 | – | – | – | – | IIIb | (–) | 9 |
| 13 | F | 12.1 | Nephritis syndrome | Skin purpura | CTX*8 + MMF | 14 | 6 | 2.3 | 18 | 41.9 | 62 | 121.55 | 1.42 | 1.28 | – | – | 5 | 5 | 6 | IIIb | (+) | 8 |
| 14 | F | 7.1 | Nephrotic syndrome | Skin purpuraArthralgia | CTX*8 + MMF | 23 | 6 | 55.9 | 5 | 44.1 | 30 | 191.24 | 2.17 | 1.16 | – | – | – | – | 20 | IIIa | (+) | 6 |
| 15 | F | 7.2 | Nephrotic syndrome | Skin purpura | CTX*5 + MMF | 3 | 36 | 62.4 | 153 | 28.1 | 29 | 197.83 | 0.831 | 1.01 | 8 | – | 40 | 10 | 58 | IV | (++) | 12 |
| 16 | M | 8.8 | Nephrotic syndrome | Skin purpuraAbdominal painEdema | CTX*5 + MMF | 5 | 12 | 504.6 | 2342 | 17.2 | 48.7 | 122.80 | 2.09 | 0.6 | – | – | – | – | 59 | IV | (+) | 10 |
The renal biopsy material of patient number 10 showed the absence of glomeruli.
F, female; M, male; URBC, urine erythrocyte count; Alb, albumin; SCr, serum creatinine; –, not found; /, not involve.
Treatment effect
After 1 month of TAC treatment, the urinary protein started to show a downwards trend, with seven patients achieving partial remission (43.8%) and two achieving complete remission (12.5%). Significant reduction in urine protein levels was observed 3 months into TAC therapy, which continued up to 6 months, whereas urine erythrocyte count, serum creatinine and eGFR did not change over time. At Month 6, four patients were in partial remission (25%) and three were in complete remission (18.8%). The mean blood concentration of TAC at 1 month of treatment was 6.0 ± 2.6 (range 2.6–11.4) ng/mL. Spearman's rank correlation coefficient analysis revealed a non-significant correlation (r = 0.2, P = .446) between TAC blood concentrations and clinical efficacy classes (ineffective, partial remission and complete remission). Analysis of TAC blood concentrations at the 3rd and 6th month after treatment also did not show statistically significant correlations with efficacy levels.
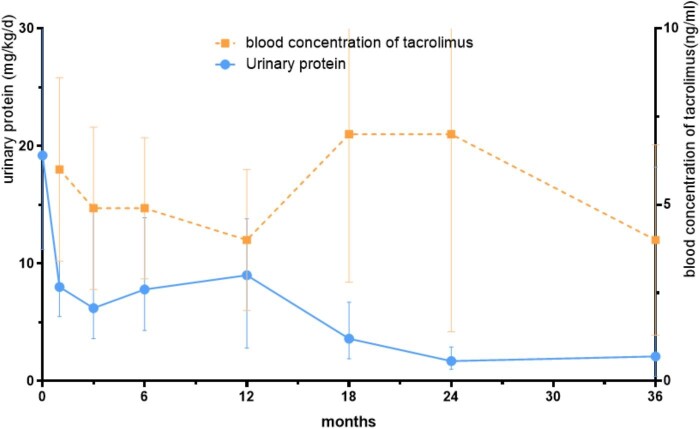
The changes in clinical data during the patient's use of CTX or and MMF are shown in Table 2, and those after the addition of TAC are shown in Table 3. The baseline was defined as the time when IgAVN was first diagnosed. Day 1 and Months 1–6 were defined as the first day of TAC addition and Months 1–6 of TAC addition. The remission rates at different follow-up time points are demonstrated in Table 4. The relationship between urine protein and TAC blood concentration from Day 1 to Month 36 following TAC addition in all participants is depicted in Fig. 1. Throughout the treatment, an observable decline in urinary protein levels was noted. Concurrently, the TAC blood levels were maintained at safe levels.
Table 2:
The change of clinical data before and after CTX or and MMF treatment.
| Baseline (n = 16) | Day 1 (n = 16) | P-value | |
|---|---|---|---|
| Urinary protein, mg/kg/day, median (IQR) | 77.3 (57.5, 171.6) | 19.2 (11.2, 31.9) | <.001 |
| URBC, /μL, median (IQR) | 467 (153, 1793) | 61 (5, 225) | .053 |
| SCr, μmmol/L, mean ± SD | 57.9 ± 26.3 | 51.5 ± 15.5 | .410 |
| eGFR, n (%) | |||
| >90 mL/min/1.73 m2 | 15 (93.7) | 16 (100) | |
| <90 mL/min/1.73 m2 | 1 (6.3) | 0 (0) | / |
The baseline was defined as the time when IgAVN was first diagnosed. Day 1 was defined as the first day of TAC addition.
URBC, urine erythrocyte count; SCr, serum creatinine; IQR, interquartile range; SD, standard deviation; /, not involve.
Table 3:
The change of clinical data before and after TAC therapy.
| Day 1 (n = 16) | Month 1 (n = 16) | Month 3 (n = 16) | Month 6 (n = 16) | P-value | |
|---|---|---|---|---|---|
| Urinary protein, mg/kg/day, median (IQR) | 19.2 (11.2, 31.9)a | 8 (5.5, 17.7) | 6.2 (3.6, 14.5)a | 7.8 (4.3, 13.9)a | <.05 |
| URBC, /μL, median (IQR) | 61 (5, 225) | 30 (4, 128) | 15 (1, 103) | 23 (6, 147) | .634 |
| SCr, μmmol/L, mean ± SD | 51.5 ± 15.5 | 53.1 ± 15.0 | 53.0 ± 14.2 | 53.8 ± 13.3 | .980 |
| eGFR, n (%) | |||||
| >90 mL/min/1.73 m2 | 16 (100) | 16 (100) | 16 (100) | 16 (100) | |
| <90 mL/min/1.73 m2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | / |
Day 1 and Months 1–6 were defined as the first day of TAC addition and Months 1–6 of TAC addition.
aPairwise comparisons were statistically significant.
URBC, urine erythrocyte count; SCr, serum creatinine; IQR, interquartile range; SD, standard deviation; /, not involve.
Table 4:
Remission rates at different follow-up time points.
| CR, n (%) | PR, n (%) | NR, n (%) | |
|---|---|---|---|
| Month 1 | 2 (12.5) | 7 (43.8) | 7 (43.7) |
| Month 3 | 4 (25) | 4 (25) | 8 (50) |
| Month 6 | 3 (18.8) | 4 (25) | 9 (56.2) |
| Month 12 | 1 (14.3) | 2 (28.6) | 4 (57.1) |
| Month 18 | 2 (28.6) | 3 (42.8) | 2 (28.6) |
| Month 24 | 3 (60) | 2 (40) | 0 |
| Month 36 | 3 (50) | 1 (16.7) | 2 (33.3) |
CR, complete remission; PR, partial remission; NR, no response.
Figure 1:
Urinary protein in relation to TAC blood concentrations at various study points (Day 1 to Month 36).
The clinical profile of the patients before and after the addition of TAC therapy is shown in Table 5. After therapy, just two children had an eGFR <90 mL/min/1.73 m2. Additionally, TAC decreases glucocorticoids use since three patients went off glucocorticoids throughout treatment, and patients’ prednisone doses were reduced from a median of 10 mg/day before to 5 mg/day after the inclusion of TAC. During treatment, 14 of the 16 children experienced remission, with a median response time of 1 month for those who attained complete or partial remission. Six of the patients who achieved remission relapsed due to upper respiratory tract infections. The median time to relapse was 3 months. Three of the six relapsed patients did not relapse again after achieving remission for the second time in the course of subsequent treatment, and the remaining three relapsed patients did not achieve remission at follow-up. In other words, at the last follow-up, 6 of the 16 patients were in complete remission (37.5%), 5 were in partial remission (31.2%) and 5 showed no response (31.2%). Of the five patients whose treatments were ultimately ineffective, two were consistently unresponsive to TAC whereas the remaining three failed to recover after relapsing.
Table 5:
Clinical profile of patients before and after the addition of TAC therapy.
| Urinary protein, mg/kg/day | Number of erythrocytes/μL | eGFR, mL/min/1.73 m2 | Prednisone, mg/day | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Treatment effect | Time required for remission months | Number of relapses, n |
| 1 | 17.4 | 2.7 | 6 | 0 | N | N | 10 | 0 | PR | 3 | 0 |
| 2 | 11.0 | 2.0 | 534 | 40 | N | N | 2.5 | 1.25 | CR | 1 | 0 |
| 3 | 7.2 | 0.3 | 0 | 0 | N | N | 7.5 | 0 | CR | 1 | 1 |
| 4 | 16.2 | 33.6 | 5 | 87 | N | N | 15 | 0 | NR | 18 | 1 |
| 5 | 19.1 | 0.5 | 10 | 0 | N | N | 10 | 6.25 | CR | 1 | 0 |
| 6 | 11.6 | 13.2 | 19 | 5 | N | N | 5 | 5 | NR | 1 | 1 |
| 7 | 19.2 | 2.1 | 44 | 6 | N | N | 7.5 | 5 | CR | 1 | 1 |
| 8 | 9.8 | 2.0 | 0 | 0 | N | N | 7.5 | 5 | PR | 24 | 0 |
| 9 | 25.1 | 64.7 | 0 | 344 | N | N | 10 | 5 | NR | 1 | 1 |
| 10 | 32.8 | 14.0 | 78 | 20 | N | N | 2.5 | 1.25 | PR | 3 | 0 |
| 11 | 29.3 | 1.5 | 102 | 149 | N | N | 15 | 12.5 | CR | 1 | 0 |
| 12 | 44.8 | 3.0 | 281 | 231 | N | N | 40 | 2.5 | PR | 1 | 0 |
| 13 | 8.3 | 13.6 | 159 | 222 | N | N | 10 | 7.5 | NR | – | – |
| 14 | 22.2 | 4.1 | 246 | 7 | N | N | 17.5 | 5 | CR | 1 | 0 |
| 15 | 62.4 | 30.9 | 153 | 75 | N | 4.2 | 5 | 2.5 | NR | – | – |
| 16 | 168.4 | 6.7 | 4075 | 39 | N | 88.7 | 25 | 5 | PR | 3 | 0 |
N, normal; CR, complete remission; PR, partial remission; NR, no response.
The 11 patients who achieved final remission were classified as the effective group, while the 5 patients who did not were defined as the ineffective group. Despite no statistically significant difference in the initial clinical characteristics between the two groups, patients in the ineffective group tended to have higher mean SQC scores than those in the effective group. Additionally, the ineffective group showed more severe renal tubulointerstitial grading than the effective group, as indicated in Table 6.
Table 6:
Comparison of clinical characteristics at disease onset between remission and ineffective group.
| Clinical characteristics | Remission group (n = 11) | Ineffective group (n = 5) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 10.0 ± 2.6 | 11.3 ± 3.0 | .388 |
| Sex (female:male) | 9:2 | 3:2 | .547 |
| Duration of IgAVN before TAC, month, median (IQR) | 24 (9, 35) | 10 (4, 45) | .308 |
| IgA, g/L, mean ± SD | 1.98 ± 0.88 | 1.60 ± 1.13 | .486 |
| C3, g/L, mean ± SD | 0.89 ± 0.19 | 0.84 ± 0.30 | .671 |
| Urinary protein, mg/kg/day, median (IQR) | 76.8 (55.9, 160.8) | 69.7 (32.4, 248.8) | .913 |
| eGFR (n) | |||
| >90 mL/min/1.73 m2 | 11 | 5 | |
| <90 mL/min/1.73 m2 | 1 | 0 | .706 |
| SQC | 7.7 ± 1.7 | 9.6 ± 2.3 | .092 |
| Clinical manifestations (n) | |||
| Nephrotic syndrome | 7 | 3 | |
| Nephrotic syndrome and nephritis syndrome | 3 | 1 | |
| Nephritis syndrome | 0 | 1 | .643 |
| Haematuria and proteinuria | 1 | 0 | |
| ISKDC grade (n) | |||
| IIIa | 4 | 1 | |
| IIIb | 4 | 3 | .790 |
| IVb | 2 | 1 | |
| Tubulointerstitial injury grade (n) | |||
| Grade 1 (–) | 5 | 0 | |
| Grade 2 (+) | 4 | 2 | .096 |
| Grade 3 (++) | 1 | 3 |
IQR, interquartile range; SD, standard deviation.
Safety
One patient ultimately developed ESRD at Month 36 with an eGFR of 4.2 mL/min/1.73 m2, whereas the eGFR was normal at the beginning of the disease and for the first 18 months of TAC treatment. This child experienced persistent and severe proteinuria for a duration of 3 years, with a renal biopsy indicating an ISKDC grade of IV and a tubulointerstitial injury grade of (++). Consequently, the progression to Stage 5 CKD was attributed to the disease itself rather than the treatment with TAC. In another patient, eGFR levels also remained normal initially and during the first 6 months of follow-up with TAC treatment. However, at the end of the 12-month follow-up, a slight decline in eGFR to 88.7 mL/min/1.73 m2 was observed. Unfortunately, due to the lack of further follow-up, it was not feasible to monitor the long-term progression of this patient's renal function. The remaining 14 patients maintained their eGFR within normal ranges throughout the entire follow-up period with TAC.
Six of the 16 individuals receiving TAC experienced upper respiratory tract infections without the need for hospitalization. During treatment and subsequent follow-up, none of the patients had any serious drug toxicities such as severe infection, sepsis, hyperlipidaemia, gastrointestinal reactions, liver dysfunction, diabetes mellitus or AKI.
DISCUSSION
To further explore why the 16 children in this study were refractory, a series of other retrospective studies have reported that children with IgAVN who presented clinically with nephritic syndrome and nephrotic syndrome had a significantly higher risk of developing chronic renal failure and even progressing to ESRD than those who presented clinically with isolated haematuria and/or proteinuria, which represented the higher rate of poor prognosis for patients with more severe initial clinical symptoms [18–20]. The results of a cohort study of children diagnosed with IgAVN and who underwent a renal biopsy in our unit from 2011 to 2017 revealed that 14 of the 249 patients included had a poor prognosis at the end of the follow-up, while 11 of these (78.5%) had an initial clinical presentation of nephrotic syndrome [7]. This observation is further supported by our present study, which revealed that 12 out of 16 (75%) patients with an initial nephrotic syndrome presentation eventually progressed to a refractory state. Second, all patients in this study had severe proteinuria at presentation, which is in line with studies that have found that mean urinary protein during follow-up is an independent predictor of renal outcome in patients with IgAVN [4, 21]. Furthermore, all 15 children with renal biopsy results had an ISKDC classification of Grade III or higher, with most patients exhibiting tubulointerstitial damage. The fact that patients with tubulointerstitial pathology grading of (++) eventually did not achieve remission supports Wang et al.’s [18] and Fu et al.’s [22] assertion that the prognosis is worse the more severe the ISKDC grading and tubulointerstitial pathology grading. Additionally, some studies [7] have demonstrated that glomerulosclerosis, tubular atrophy and interstitial fibrosis strongly indicate a poor renal outcome. In the present study, the five patients whose treatments were ultimately ineffective had varying degrees of these changes in their renal biopsies. The SQC score is the latest system proposed in recent years to evaluate the pathology of IgAVN in children. Koskela et al. [17] concluded that patients with scores ≤10 have a good prognosis and that those with scores ≥11 are at greater risk of impaired renal function. The 15 patients with renal biopsy results in our subject group had an average SQC score of 8.3, and the 2 patients with a score higher than 11 (a score of 12) had a final follow-up result of no remission following TAC therapy. In conclusion, it is hypothesized that a more severe clinical manifestation at onset, massive proteinuria, higher ISKDC grading and tubulointerstitial pathology grading, and higher total SQC score may be factors in the poor prognosis and difficulty in achieving remission in patients with IgAVN.
IgAVN has become one of the most common secondary glomerular diseases in childhood [23, 24], and its long-term prognosis depends largely on renal involvement and its severity [25]. Most children respond well to active treatment, but some still have poor response or intolerance to conventional treatment, resulting in long-term renal involvement and increasing the chance of developing renal insufficiency. Therefore, it is particularly important to explore a more effective and safer immunosuppressant for refractory IgAVN patients.
In another study [14], 20 children with IgAVN with nephrotic proteinuria, nephrotic syndrome, acute nephritic syndrome or pathological Grade ≥IIIb were treated with TAC 0.05–0.1 mg//kg/day combined with low-dose glucocorticoids for 6 months. Finally, 12 children achieved complete remission and 8 children achieved partial remission. Transient elevation of alanine transaminase occurred in two patients and gastrointestinal symptoms occurred in three patients. All of them tolerated TAC well after symptomatic treatment. No severe adverse reactions such as nephrotoxicity occurred, suggesting that TAC may be an effective and well-tolerated drug for the treatment of IgAVN in children. In a patient with severe IgAVN and clinical manifestations of nephrotic syndrome, Andersen et al. [26] administered TAC 0.1 mg/kg/day orally and maintained blood concentrations between 5 and 10 μg/L. The patient eventually experienced clinical remission without experiencing any significant side effects after unsuccessful attempts with glucocorticoids combined with CTX, MMF and cyclosporine A. They proposed that TAC may be an effective long-term alternative to reduce proteinuria in patients with steroid-resistant nephrotic syndrome type IgAVN. The results of another controlled trial [13] also showed that TAC treatment of IgAVN in children was more effective in reducing proteinuria, and haematuria and improving renal function with relatively mild side effects. Each of the patients in our retrospective research developed refractory IgAVN despite the use of CTX, and some of them also received MMF. After the inclusion of TAC, the majority of patients experienced a decline in urinary protein levels. As a result, we believe that TAC offers refractory IgAVN alternative options: it has been shown to enhance remission rates within a safe blood concentration range, greatly reduce urinary protein levels and decrease reliance on glucocorticoids. Furthermore, we suggest that in future studies, more attention should be paid to the stability of TAC concentrations and the importance of individualized treatment. In clinical practice, the dosage and treatment regimen of TAC should be rationally adjusted according to the patient's specific situation and drug monitoring results to maximize the therapeutic effect and minimize the occurrence of adverse reactions.
The short duration, small sample size and retrospective observational strategy of this study were its primary limitations. The TAC or steroid dosages in the patient cohort were not standardized; rather, they were adjusted according to the clinical condition of the patients. Additionally, even though these individuals showed steroid resistance, we were unable to prove that concurrent steroid usage during TAC therapy had no impact on the results that followed. In addition, the patients took relevant doses of ACEIs concomitantly before and after treatment. The association between therapy and the results may have been masked by the confounding factors indicated above.
TAC, as a safe and potent immunosuppressant, may provide more options for the treatment of children with refractory IgAVN, but the specific efficacy, safety and individualized treatment of this drug require more prospective controlled trials with long-term follow-up.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the supportive role of the patients and support staff.
Contributor Information
Yueheng Gan, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
Jiahuan Chen, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
Mo Wang, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
Qiu Li, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
Anshuo Wang, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
Haiping Yang, Department of Nephrology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China.
FUNDING
We thank the National Key R&D Program of China (no. 2022YFC2705101), Chongqing Science and Health Joint Medical Research Project (grant no. 2023GGXM001), Program for Youth Innovation in Future Medicine, and Chongqing Medical University (no. W0098).
AUTHORS’ CONTRIBUTIONS
Conceptualization: H.Y. and A.W. Data curation, formal analysis, visualization: Y.G. and J.C. Funding acquisition, supervision: H.Y., A.W., M.W. and Q.L. Investigation, methodology, resources: all authors. Project administration: H.Y. and A.W. Software: Y.G. and J.C. Writing–original draft: Y.G. and J.C. Writing—review and editing: H.Y., A.W., M.W. and Q.L. All authors read and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
REFERENCES
- 1. Watts RA, Hatemi G, Burns JC et al. Global epidemiology of vasculitis. Nat Rev Rheumatol 2022;18:22–34. 10.1038/s41584-021-00718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo F, Li Y, Zhang Y et al. Bibliometric analysis of IgA vasculitis nephritis in children from 2000 to 2022. Front Public Health 2022;10:1020231. 10.3389/fpubh.2022.1020231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim WK, Kim CJ, Yang EM. Risk factors for renal involvement in Henoch-Schönlein purpura. J Pediatr (Rio J) 2021;97:646–50. 10.1016/j.jped.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppo R, Andrulli S, Amore A et al. Predictors of outcome in Henoch-Schönlein nephritis in children and adults. Am J Kidney Dis 2006;47:993–1003. 10.1053/j.ajkd.2006.02.178 [DOI] [PubMed] [Google Scholar]
- 5. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–276. [DOI] [PubMed] [Google Scholar]
- 6. Ozen S, Marks SD, Brogan P et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis-the SHARE initiative. Rheumatology (Oxford) 2019;58:1607–16. 10.1093/rheumatology/kez041 [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Wang R, He X et al. Using MEST-C scores and the International Study of Kidney Disease in Children classification to predict outcomes of Henoch-Schönlein Purpura nephritis in children. Front Pediatr 2021;9:658845. 10.3389/fped.2021.658845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hao GX, Song LL, Zhang DF et al. Off-label use of tacrolimus in children with glomerular disease: effectiveness, safety and pharmacokinetics. Br J Clin Pharmacol 2020;86:274–84. 10.1111/bcp.14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen HX, Cheng Q, Li F et al. Efficacy and safety of tacrolimus and low-dose prednisone in Chinese children with steroid-resistant nephrotic syndrome. World J Pediatr 2020;16:159–67. 10.1007/s12519-019-00257-z [DOI] [PubMed] [Google Scholar]
- 10. Wan QJ, Hu HF, He YC et al. Tacrolimus combined with low-dose corticosteroids is an effective and safe therapeutic option for refractory IgA nephropathy. Exp Ther Med 2016;12:1934–8. 10.3892/etm.2016.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng Z, Zhang H, Peng X et al. Effect of Tacrolimus vs intravenous cyclophosphamide on complete or partial response in patients with lupus nephritis: a randomized clinical trial. JAMA Netw Open 2022;5:e224492. 10.1001/jamanetworkopen.2022.4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subspecialty Group of Renal Diseases, the Society of Pediatrics, Chinese Medical Association. Evidence-based guideline for diagnosis and treatment of Henoch-Schonlein purpura nephritis (2016). Chin J Pediatr 2017;55:647–51. [DOI] [PubMed] [Google Scholar]
- 13. Wu D, Ma R, Wang X et al. Efficacy and safety of Tacrolimus in the treatment of pediatric Henoch-Schönlein purpura nephritis. Paediatr Drugs 2022;24:389–401. 10.1007/s40272-022-00506-1 [DOI] [PubMed] [Google Scholar]
- 14. Zhang DF, Hao GX, Li CZ et al. Off-label use of tacrolimus in children with Henoch-Schönlein purpura nephritis: a pilot study. Arch Dis Child 2018;103:772–5. 10.1136/archdischild-2017-313788 [DOI] [PubMed] [Google Scholar]
- 15. Derham RJ. Prognosis of Henoch-Schönlein nephritis in children. Br Med J 1977;2:262. 10.1136/bmj.2.6081.262-d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohle A, Müller GA, Wehrmann M et al. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl 1996;54:S2–9. [PubMed] [Google Scholar]
- 17. Koskela M, Ylinen E, Ukonmaanaho EM et al. The ISKDC classification and a new semiquantitative classification for predicting outcomes of Henoch-Schönlein purpura nephritis. Pediatr Nephrol 2017;32:1201–9. 10.1007/s00467-017-3608-5 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Jiao J, Wan J et al. Value of the modified semiquantitative classification in predicting outcomes in children with Henoch-Schönlein purpura nephritis. Nephrology (Carlton) 2023;28:495–505. 10.1111/nep.14193 [DOI] [PubMed] [Google Scholar]
- 19. Davin JC, Coppo R. Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol 2014;10:563–73. 10.1038/nrneph.2014.126 [DOI] [PubMed] [Google Scholar]
- 20. Jelusic M, Sestan M, Cimaz R et al. Different histological classifications for Henoch-Schönlein purpura nephritis: which one should be used? Pediatr Rheumatol Online J 2019;17:10. 10.1186/s12969-019-0311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shrestha S, Sumingan N, Tan J et al. Henoch Schönlein purpura with nephritis in adults: adverse prognostic indicators in a UK population. QJM 2006;99:253–65. 10.1093/qjmed/hcl034 [DOI] [PubMed] [Google Scholar]
- 22. Fu HD, Mao JH, Huang L et al. Higher pathological grading is associated with unfavorable outcome of Henoch-Schonlein purpura nephritis in children. Int J Clin Exp Pathol 2016;9:4633–40. [Google Scholar]
- 23. Nie S, He W, Huang T et al. The spectrum of biopsy-proven glomerular diseases among children in China: a national, cross-sectional survey. Clin J Am Soc Nephrol 2018;13:1047–54. 10.2215/CJN.11461017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin XL, Zou MS, Zhang Y et al. Twenty-three-year review of disease patterns from renal biopsies: an experience from a pediatric renal center. J Nephrol 2013;26:699–707. 10.5301/jn.5000267 [DOI] [PubMed] [Google Scholar]
- 25. Stewart M, Savage JM, Bell B et al. Long term renal prognosis of Henoch-Schönlein purpura in an unselected childhood population. Eur J Pediatr 1988;147:113–5. 10.1007/BF00442205 [DOI] [PubMed] [Google Scholar]
- 26. Andersen RF, Rubak S, Jespersen B et al. Early high-dose immunosuppression in Henoch-Schönlein nephrotic syndrome may improve outcome. Scand J Urol Nephrol 2009;43:409–15. 10.3109/00365590903164480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.