Abstract
Background.
Cervical screening has not effectively controlled cervical adenocarcinoma (AC). Human papillomavirus (HPV) testing is recommended for cervical screening but the optimal management of HPV-positive individuals to prevent AC remains a question. Cytology and HPV typing are two triage options to predict the risk of AC. We combined two potential biomarkers (atypical glandular cell, AGC, cytology and HPV-types 16, 18, or 45) to assess their joint effect on detecting AC.
Methods.
Kaiser Permanente Northern California (KPNC) used triennial co-testing with cytology and HPV testing (positive/negative) for routine cervical screening between 2003 and 2020. HPV typing of a sample of residual HPV test specimens was performed on a separate cohort selected from KPNC (Persistence and Progression, PaP, cohort). We compared risk of prevalent and incident histologic AC/AIS (adenocarcinoma in situ) associated with preceding combinations of cytologic results and HPV typing. Risk of squamous cell cancer (SCC)/cervical intraepithelial neoplasia grade 3 (CIN3) (SCC/CIN3) was also included for comparison.
Results.
Among HPV-positive individuals in PaP cohort, 99% of prevalent AC and 96% of AIS were linked to HPV-types 16, 18, or 45 (denoted HPV 16/18/45). Although rare (0.09% of screening population), the concurrent detection of HPV 16/18/45 with AGC cytology predicted a highly elevated relative risk of underlying histologic AC/AIS; the absolute risk of diagnosing AC/AIS was 12% and odds ratio (OR) was 1341 (95%CI:495–3630) compared to patients with other high-risk HPV types and normal cytology. Cumulatively (allowing non-concurrent results), approximately one-third of the AC/AIS cases ever had HPV 16/18/45 and AGC cytology (OR = 1785; 95%CI:872–3656). AGC was not as strongly associated with SCC/CIN3.
Conclusion.
Detection of HPV 16/18/45 positivity elevates risk of adenocarcinoma, particularly if AGC cytology is also found.
Keywords: Cervix, Screening, HPV, Adenocarcinoma, Cytology
1. Introduction
Well-established cervical cancer screening programs have greatly reduced rates of squamous cell carcinoma of the cervix (SCC) but unfortunately have not controlled adenocarcinoma of the cervix (AC) [1,2]. AC is the far less common histologic type (~10%) of cervical cancer globally but, in well screened populations, may comprise a quarter or more of new cases [2–11].
Cervical cancer of either histologic type arises from human papillomavirus (HPV) infection, which initially causes minor cellular changes but, when persistent, can lead to precancer. The goal of cervical cancer screening as secondary prevention for cervical cancer is to detect precancerous lesions that precede SCC or AC and treat them before they become cancer, while minimizing treatment of benign HPV infections destined to clear under cell-mediated immune control. High-quality cervical screening readily detects both minor and precancerous SCC precursors [11].
Compared with squamous precursors, AC tends to arise proximally often within the glandular epithelium of the endocervical canal, making detection of its precursors more difficult [13]. The immediate precursor to AC is known to be histologic adenocarcinoma in situ (AIS), which is itself uncommon and likely underdiagnosed. The earlier, more minor glandular precursors to adenocarcinoma are not clearly defined.
One way to identify the early precursors of AC is HPV typing. SCC is caused by a broad group of approximately a dozen carcinogenic high-risk HPV types, but AC is almost entirely caused by a subset including HPV 16, HPV 18, and HPV 45 [14,15]. AIS is known to be caused almost entirely by the same three types. This causal distinction was firmly established based on case series including tens of thousands of cervical cancer samples. Accordingly, it is logical to look for cytologic evidence of incipient AC/AIS among HPV 16/18/45-positive individuals.
The present investigation was designed to clarify possible cytologic precursors of AC, specifically targeting the diverse cytologic category of equivocal changes called Atypical Glandular Cells (AGC) [16]. A fraction of AGC appears to represent perimenopausal or other benign conditions with cytologic changes. Within the remainder, the relationship to AC has been assessed for each sub-type of AGC.
The two potential biomarkers, HPV 16/18/45 types and AGC cytology, can independently predict the AC risk. We hypothesized that the intersection of these biomarkers might define an even stronger AC precursor state worthy of particular consideration in cervical screening. With this aim, we assessed the joint effect of these biomarkers in comparison to other high-risk HPV types and other cytologic categories on predicting AC risk.
2. Methods
2.1. Cervical cancer screening at KPNC
This longitudinal cohort study included 1,907,323 individuals from Kaiser Permanente Northern California (KPNC), a large integrated healthcare system that based its routine cervical cancer screening program from 2003 until recently on cytology and HPV test (i.e., cotesting) [17]. Individuals (initially ≥30 years old but later including some 25–29 years old) were screened with triennial cotesting. During the study period (2003 to 2021), cervical cytology was conducted first using conventional smears, then using a liquid-based cytology method (BD Diagnostics, Burlington, NC, USA), and most recently switched to HPV testing with cytology triage. Until recently, HPV testing was performed mainly using Hybrid Capture 2 (HC2; Qiagen, Germantown, MD, USA) which tests for the pool of 13 carcinogenic, or “high-risk (HR)”, HPV types (alpha-9 species types HPV 16, 31, 33, 35, 52, and 58; alpha-7 types HPV 18, 39, 45, 59, and 68; alpha-5 type HPV51, and alpha-6 type HPV56). In practice, the assay is known also to cross-react with several genetically related types that are not classified as carcinogenic [18].
2.2. Screening biomarkers of interest: cytology and HPV testing
Cytology results were classified using the Bethesda System [19], which has separate grading scales for squamous and glandular abnormalities. The squamous pathway includes a very common equivocal category called atypical squamous cells of undetermined significance (ASC-US) and increasingly severe more definite abnormalities divided into low-grade squamous intraepithelial lesion (LSIL); atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); and squamous cell carcinoma (SCC). In contrast, the glandular pathway lacks a definite low-grade cytologic precursor category analogous to squamous LSIL. It includes equivocal atypical glandular cells (AGC), adenocarcinoma in situ (AIS) and adenocarcinoma (AC).
AGC, although rare, has defined Bethesda reporting recommendations: qualify glandular cells as endocervical or endometrial when possible, and further qualify the morphology as “not otherwise specified” or “favor neoplasia” for endocervical and glandular cells [19]. These sub-classifications were not routinely used at KPNC. However, the qualifiers in the pathology report could be grouped roughly into Bethesda System equivalents. Excluding use of AGC related to endometrial cells and other changes (i.e. tubal metaplasia, IUD, etc) not linked to HPV, AGC was classified as “endocervical” or “glandular”, and additional qualifiers of “not otherwise specified (NOS)” and “favor neoplasia” were also noted. We looked for combinations of HPV type and cytology at as fine a level as numbers permitted.
For either squamous or most glandular cervical cancers, the starting point of cervical carcinogenesis is HPV infection. Because we were looking for HPV-related AC precursor abnormalities, we excluded individuals who screened HR-HPV negative from analyses of the contributions of HPV16/18/45 to AIS/AC as they were considered “not on the HPV causal pathway”.
We performed HPV typing on only a subset of the study population, requiring reweighting as described below. From 2006 to 2010, we studied HPV typing within a subset of the KPNC cohort called the Persistence and Progression (PaP) population. This methodology and individual selection has been previously described in detail [22]. Briefly, we collected residual exfoliated cervical cell specimens left from the pooled HC2 HPV component of co-testing, unless individuals chose to opt out (<10%). In the PaP study, virtually all HPV-positive individuals with histologic diagnoses of cancer or precancer (n = 5179) and a large number (but small percentage) of <CIN2 (n = 13,635) were tested for specific HPV types. Over the years of study, several methods were used for HPV typing including MY09/M11 L1 degenerate primer PCR (MY09/11 PCR) [20], Linear Array HPV Genotyping System (Roche Molecular Diagnostics, Pleasanton, CA) and Onclarity (BD, Sparks, MD).
2.3. Histologic endpoints
With regard to histologic endpoints, we compared AC and AIS (denoted as AC/AIS) to SCC and its known immediate precursor Cervical Intraepithelial Neoplasia 3 (CIN3). Very rare adenosquamous cases were grouped with glandular, although excluding them would not have changed the conclusion. The histology of earlier, less severe intraepithelial cervical abnormalities is known to be non-specific and not always more informative than cytology [21]. Therefore, to categorize stages of the natural history of glandular vs. squamous abnormalities arising from HPV infection, we created four broad microscopic (histology combined with cytology) morphologic groups of increasing severity: normal cervix with HPV-negative result, minor HPV-related cytologic/histologic abnormalities, histologic precancer, cancer (Fig. 1). CIN2 was considered an equivocal diagnosis between minor and precancer; for clarity, it was excluded from the outcome categories. Including CIN 2 would have led to increased risk estimates in SCC/CIN3 pathway but no change in the pattern. Each individual was categorized according to the most severe result. First, all individuals ever diagnosed with cancer were categorized: AC (n = 399) or SCC (n = 511). Among the remaining individuals, those ever diagnosed with precancer were categorized: CIN3 (n = 11,145) or AIS (n = 1000). Among the remaining individuals, those diagnosed with cytology were categorized by decreasing order of severity: HSIL/ASC-H, then AGC, then ASCUS/LSIL, then HPV-positive NILM. The remaining individuals, all of whom had NILM cytology, negative HPV testing, and no histologic evidence of cancer or precancer, were classified as having a normal cervix.
Fig. 1.
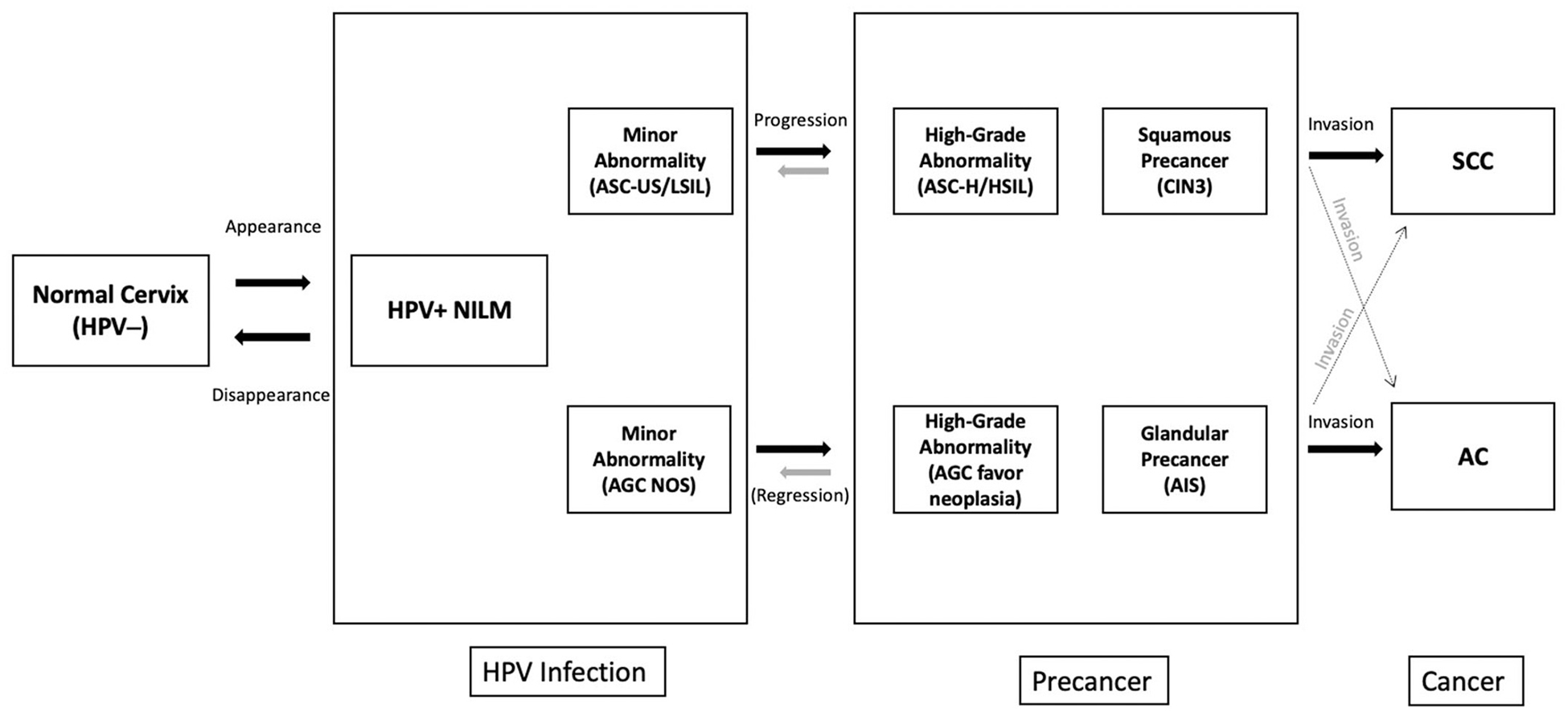
Natural History of Cervical Cancer for Squamous and Glandular Pathways, Distribution of Study Population at Baseline.
Fig. 1 depicts the natural history of cervical cancer in 2 separate pathways for squamous cell carcinoma (SCC) and adenocarcinoma (AC). The majority of the population (91%) has a normal cervix (no HPV infection) with negative HPV result in a screening population. The most common abnormality on the pathway to cervical cancer is HPV-positive NILM. Earlier more minor abnormality for SCC is ASC-US/LSIL which is more common than its equivalent on the AC path, AGC NOS (NOS: not otherwise specified). Even though AGC is a diverse cytologic category (therefore not being a direct counterpart of ASC-US/LSIL), it is the only known early minor precursor for AC and AGC NOS is more minor abnormality compared to AGC favor neoplasia (FN). At the precancer stage, cytologic high-grade abnormalities ASC-H/HSIL and histologic abnormalities, CIN3, are well-defined for the SCC pathway. However, AGC FN and adenocarcinoma in situ (AIS) are the only known immediate precursors for AC and these abnormalities are very uncommon and mostly underdiagnosed. Adenosquamous cases are grouped with AC in our study which is a very small percentage of the whole population. There is also crossover between the two pathways, which is represented with faded crossing arrows at the end of the fig. (10% of the AC/AIS cases are followed by HSIL cytology among HPV positives).
2.4. Statistical analysis
The study objective was to clarify the glandular versus squamous pathways with regard to risks of sequential transitions from normal cervix to HPV infection (with or without accompanying minor microscopic abnormalities), from infection to a precancer state (CIN3 or AIS), and from precancer to cancer (SCC or AC). For each stepwise transition on the squamous or glandular pathways, we studied absolute risk, relative risk as estimated by the odds ratio (OR) with 95% confidence intervals (95%CI), and selected attributable risks posed by the combinations of HPV type and cytology.
Cytologic results and HPV status (positive/negative) were available on practically all study participants at all screening visits in the KPNC cohort. The results presented without HPV genotyping are conducted on KPNC cohort (Fig. 1 and Fig. 2) while analyses presenting HPV genotyping results are conducted on PaP cohort (Fig. 3, Tables 1 and 2). Study populations of both cohorts are summarized in Supplemental Tables 1 and 2. Because HPV typing was available on only a fraction of the PaP subset (n = 19,416, Supplemental Table 1), inverse probability weighting was used to adjust for multistage sequential sampling (stages 1–3) in our study as described previously [22]. Briefly, sampling Stage 1 was the probability of having an HC2 test (HPV positive versus negative). Stage 2 was the probability of being selected, given the HC2 result, into the PaP study from 2007 to 2011. Stage 3 was the probability in the PaP study of being selected for HPV typing [22].
Fig. 2.
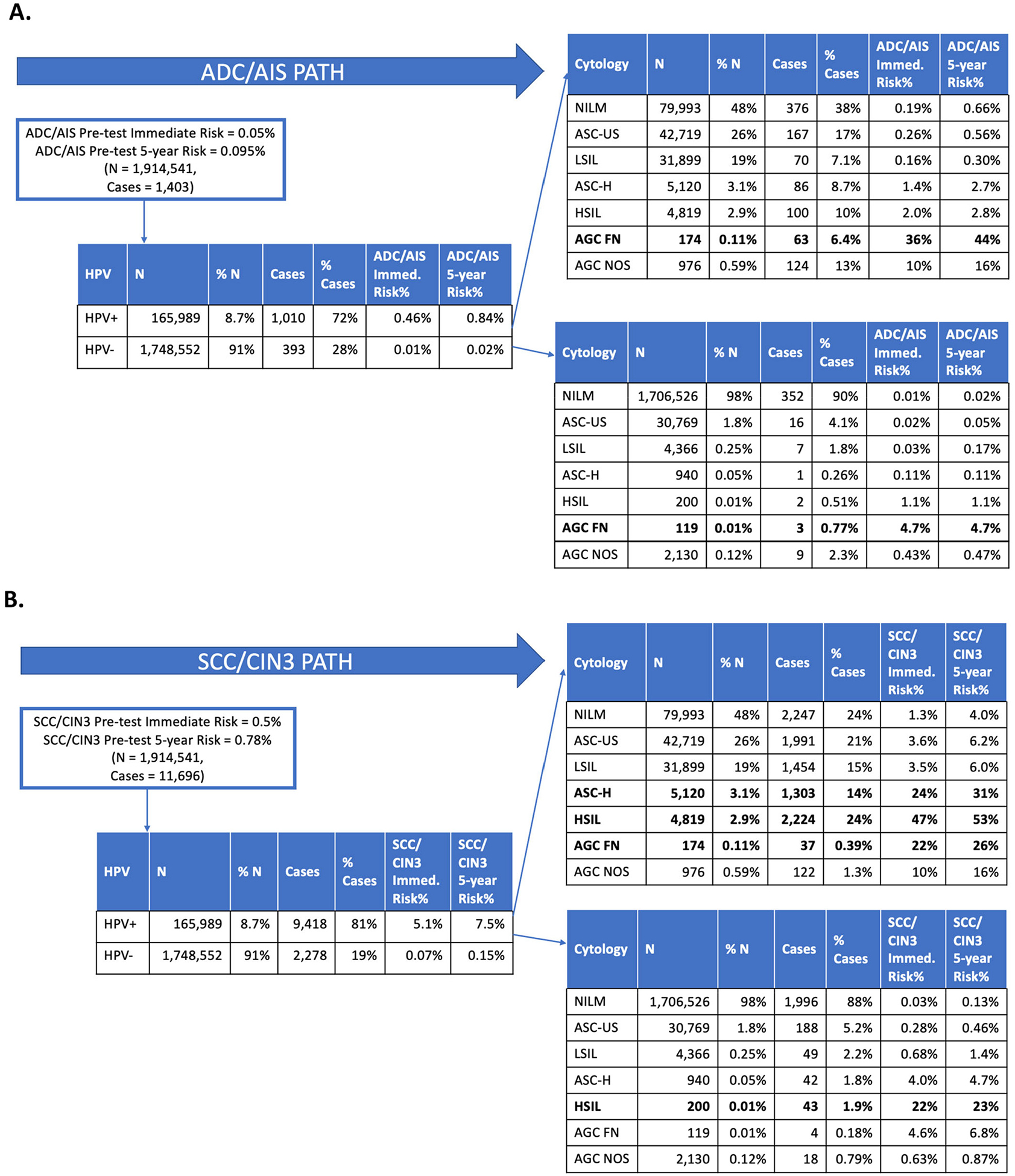
Step-by-step risk discrimination by HPV and cytology tests for AC/AIS and SCC/CIN3 endpoints.
Fig. 3.
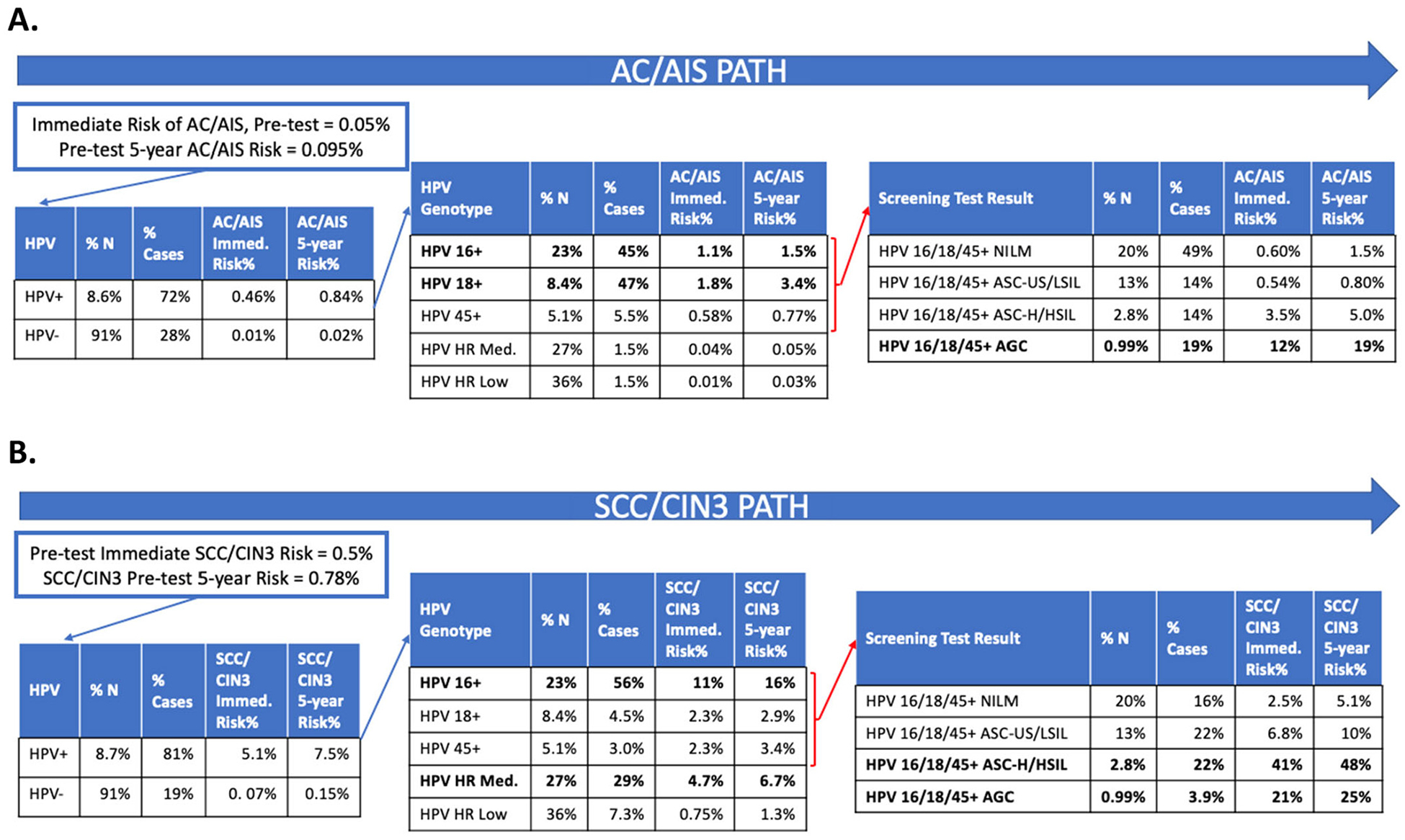
Step-by-step risk discrimination by HPV genotyping and cytology tests for AC/AIS and SCC/CIN3 endpoints.
Table 1.
Odds Ratios for HPV genotyping and cytology tests combinations.
| Cytology Result* | |||||
|---|---|---|---|---|---|
| HPV Genotype* | NILM | ASC-US/LSIL | ASC-H/HSIL | AGC | |
| OR for AC/AIS (95%CI) | HPV Other HR+ | 1 | 0.75 (0.18–3.2) | 4.6 (0.88–24) | 0 (0–0) |
| HPV 16/18/45+ | 69 (22–220) | 22 (8.3–56) | 152 (60–389) | 1341 (495–3630) | |
| OR for SCC/CIN3 (95%CI) | HPV Other HR+ | 1 | 1.6 (1.2–2.0) | 11 (8.6–14) | 2.1 (1.1–4.1) |
| HPV 16/18/45+ | 3.1 (2.1–4.7) | 4.6 (3.5–6.2) | 32 (23–44) | 38 (10–142) | |
NILM: negative for intraepithelial lesion or malignancy; ASC-US: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; ASC—H: atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; AGC: atypical glandular cells; AC: adenocarcinoma; AIS: adenocarcinoma in situ; SCC: squamous cell cancer; CIN3: cervical intraepithelial carcinoma grade 3.
All these test results are obtained at the enrolment of each individual in PaP cohort.
Table 2.
Comparison of Glandular and Squamous Precancer/Cancer Risks for women ever positive for HPV16/18/45 and/or cytological AGC.
| Ever Positive for HPV 16/18/45? | Ever Has AGC Cyto. Result? | N | % of N | AC/AIS Endpoint | SCC/CIN3 Endpoint | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC/AIS Cases | AC/AIS % of Cases | OR for AC/AIS (95%CI) | Attributable Risk for AC/AIS (%) | SCC/CIN3 Cases | SCC/CIN3% of Cases | OR for SCC/CIN3 (95% CI) | Attributable Risk for SCC/CIN3 (%) | ||||
| Yes | Yes | 1160 | 0.30 | 185 | 32 | 1785 (872–3656) | 32 | 228 | 4.9 | 18 (7–44) | 4.6 |
| Yes | No | 26,701 | 7.0 | 371 | 65 | 129 (55–305) | 64 | 2407 | 52 | 6.7 (5–9.1) | 44 |
| No | Yes | 4794 | 1.2 | 2 | 0.30 | 3.3 (0.4–28) | 0.21 | 66 | 1.4 | 1.0 (0.5–2.0) | 0.01 |
| No | No | 351,355 | 91 | 16 | 2.7 | 1.0 | – | 1965 | 42 | 1.0 | – |
Table 2 presents AC/AIS and SCC/CIN3 case distributions among patients ever/never positive for HPV 16/18/45 and ever/never had AGC cytology during the study period, 2006–2021 in PaP cohort. For odds ratio calculations, multinomial logistic regression is constructed with the worst histologic diagnosis being the response variable (levels are normal, glandular precancer/cancer, and squamous precancer/cancer) and ever being positive for HPV 16/18/45 and ever having AGC variables being the covariate as a combined single variable. CIN2 and CIN2/3 histologic diagnoses are grouped under the normal category of the worst histologic diagnosis variable in order to be stringent about case status. Patients that had ever tested positive for HPV 16/18/45 and ever had AGC cytology were 1785 times more likely (95%CI:872–3656) to have AC/AIS diagnosis in comparison to patients that never had HPV 16/18/45 and never had AGC cytology, which represented a greater than multiplicative effect modification. These results are from the PaP cohort and so the N’s presented are weighted sample sizes for each category. Individuals HPV-negative at enrollment were also included in this analysis. The sample sizes weight back to the HPV genotyped portion of the KPNC cohort.
AGC: atypical glandular cells; AC: adenocarcinoma; AIS: adenocarcinoma in situ; SCC: squamous cell cancer; CIN3: cervical intraepithelial carcinoma grade 3.
We examined combinations of cytologic and HPV test results, in search of a combination strongly linked with high absolute, relative, and/or attributable risk of AC/AIS histology. As will be shown, each risk measure contributed differently to the inferences. With regard to calculations, absolute risk was computed by using the Prevalence-Incidence mixture model [23–26], which is a mixture of logistic regression for events present at the time of the baseline visit (prevalent disease at the time of the HPV genotyping test in the PaP study, defaulting to the first cotest in individuals not enrolled in the PaP study) and proportional hazards for events occurring after the baseline visit (incident disease). Relative risk as estimated by the OR was calculated by multinomial logistic regression. We used the multinomial logistic regression model to study the effect modification (“synergy”) between HPV type and the most severe previous and concurrent cytology results. In these models, HPV types (as HPV 16/18/45 and other high-risk (HR) types or ever and never HPV 16/18/45+) and cytology levels (as NILM, ASC-US/LSIL, ASC-H/HSIL, and AGC or ever and never AGC) are grouped together to create one covariate variable. To calculate ORs the lowest risk groups are chosen as the reference category. Specifically the reference category was HPV positivity in the “other HR” group and cytologic NILM for the concurrent results analysis. It was expanded longitudinally to include past history prior to the baseline visit for the most severe results ever analysis, to include never HPV 16/18/45 positive and never AGC. We calculated the attributable risk (AR) to estimate the proportion of individuals in a diagnostic group attributable to HPV types (HPV 16, HPV 18, and HPV 45 versus others), to AGC, and to their combination. AR was calculated as proportion of cases with that test result multiplied by (1-1/RR as estimated by1-1/OR) [27].
3. Results
3.1. Relative prevalence of glandular vs. squamous outcomes
Fig. 1 presents the cytology and histology of the individuals included in the KPNC cohort. Each pathway consisted of the following stages: normal cervix, minor to moderate (typically HPV infection-related) abnormalities, precancer, and cancer. Although the stages were analogous, the glandular pathway had relatively fewer individuals in the minor-to-moderate abnormality and precancer stages leading up to cancer compared to the squamous pathway. For the glandular pathway, 823 individuals had AGC, 1000 had AIS, and 399 had AC, giving ratios of minor cytologic abnormality (AGC) to precancer (AIS) to AC ratios of 8.5: 2.5: 1. For the squamous pathway, 77,162 had ASC-US/LSIL/ASC-H/HSIL, 11,145 had CIN3, and 511 had SCC, giving much larger ratios of minor-to-moderate cytologic abnormality to precancer, to SCC of 151 abnormal cytology and 22 CIN3 to 1 SCC.
3.2. Heterogeneity of AGC
The association of AGC with HPV infection varied by age. Among the 823 individuals with AGC cytology in KPNC cohort, HPV-positive individuals were younger than HPV-negative individuals (median age = 45, interquartile range (IQR) = 15, for HPV-negatives vs 35, IQR = 14, for HPV-positives). This difference is greatest for the AGC favor neoplasia subgroup (median age = 52, IQR = 17, for HPV-negatives vs 35, IQR = 16, for HPV-positives, p-value<0.0001 by Mann-Whitney-U test). AGC subtypes are not used routinely in KPNC; we reclassified them for this study by evaluating electronic health records (EHR) and found that the most important descriptor affecting risk was the AGC qualifier of risk “favor neoplasia”. We did not find any significant difference in risks across other subcategories of AGC (endocervical versus glandular), or the “NOS” qualifier.
3.3. Specificity and Strength of Association of HPV 18/45 more pronounced for AC than for SCC
A total of 19,416 individuals were included in the PaP cohort and had HPV typing information available (Supplemental Tables 1 and 2). Among HPV-positive individuals in this study, AC was caused almost entirely by HPV 16/18/45 (>98%), versus 63.5% of SCC caused by HPV 16/18/45 (Fig. 3). In the PaP cohort, we observed HPV 16 in 56.1% of AC and a similar percentage (61.7%) of SCC. (Supplemental Table 2) (Specific variants of HPV 16 were particularly linked to glandular lesions, and covered in another report [28].) In terms of relative risk, HPV 18 and HPV 45 were more strongly associated with glandular than squamous outcomes: HPV 18 was observed in 34.1% of AC versus 10.6% of SCC; HPV 45 was linked to 8.5% of AC versus 4.3% of SCC (for AC/AIS and SCC/CIN3 comparisons refer to Fig. 3).
Figs. 2 and 3 summarize the risk stratification yielded by HPV and cytology tests considered for both AC/AIS and SCC/CIN3 endpoints. Before considering screening test results, AC/AIS immediate risk in the KPNC population was 0.05% (Fig. 2 Panel A). Both AGC subtypes and HPV genotypes influenced risk. In the general KPNC cohort, among individuals who concurrently tested HPV positive and had a cytology result of AGC-favor-neoplasia, AC/AIS immediate risk was 36% (Fig. 2 Panel A). Notably, HPV+ and HSIL cytology predicted a high immediate absolute risk for the SCC/CIN3 endpoint (47% immediate CIN3+ risk; Fig. 2 Panel B), but not for AC/AIS (1.1% immediate CIN3+ risk; Fig. 2 Panel A). In the PaP cohort, individuals who concurrently tested HPV 16/18/45 positive and had a cytology result of AGC, AC/AIS risk was 12% (Fig. 3 Panel A); estimation by HPV type for AGC subcategory was not possible due to small numbers. The comparison with the squamous pathway showed strong differences.
3.4. Strong effect modification of HPV 16/18/45 and AGC in risk of diagnosing glandular cancer and precancer
Overall, there was an unusually strong effect modification (in other words, a risk stratification) due to the combination of HPV 16/18/45 and AGC in the relative risk of developing glandular cancer and precancer. When both HPV 16/18/45 and AGC were found in the PaP cohort, joint effects were super-multiplicative (or “synergistic”, i.e., greater than the multiplicative product of the individual effects of HPV 16/18/45 and AGC) [29,30]. Compared to individuals with other high-risk HPV types and NILM cytology, HPV 16/18/45-positive AGC was 1341 times more likely to predict AC/AIS (OR = 1341; 95% CI:495–3630; Table 1). Risks conferred by other cytology/genotype combinations were elevated compared to NILM with other HPV types, but substantially lower than AGC with HPV 16/18/45 (Table 1). Looking at the full patient history, individuals that had ever tested positive for HPV16/18/45 and ever had AGC cytology were 1785 times more likely (95%CI:872–3656; AR 32%) to have AC/AIS diagnosis in comparison to individuals that never had HPV 16/18/45 and never had AGC cytology (Table 2). This represented a greater than multiplicative effect modification. The strong joint effects of HPV 16/18/45 and AGC were specific to the glandular pathway.
4. Discussion
We extended previous reports that individuals with HPV 16/18/45-positive AGC were at particularly high relative risk of diagnosis of AC [31–33]. This analysis added the novel observation that finding AGC increased by 20-fold the risk of AC, compared with individuals with any of the same three HPV types but normal cytology (NILM) (12% vs 0.6% immediate AC/AIS risk).
AC now comprises 1/4 of all cervical cancers in the US [34]. Current screening tests are highly effective at preventing SCC but less effective at preventing AC, such that in the well-screened KPNC cohort of nearly 2 million individuals, AC made up >40% of the cancers diagnosed (n = 399 AC compared with 511 SCC, Fig. 1). Adenocarcinoma precursor lesions are difficult to identify in cytology and even at colposcopy due to disease originating proximal to the squamocolumnar junction, in glandular epithelium characterized by cervical crypts. These data indicate that among patients testing positive for HPV 16/18/45 and AGC (even if not concurrent, and especially if the classification is AGC favor neoplasia), up to 1/3 will have a concurrent AIS or AC. These risks warrant consideration in the development of future guidelines. For individuals positive for HPV 16/18/45, the screening history could be informative because any AGC result, even if not concurrent, was linked to risk of AC/AIS.
Though the focus of this analysis is the glandular pathway, it is worth noting that in the 2019 guidelines, colposcopy is recommended for immediate CIN3+ risks of 4–24%, and either colposcopy or treatment is recommended for immediate CIN3+ risks of 25%–59%. The risk of 12% for HPV16/18/45+ AGC (NOS) falls within the risk range for which colposcopy is recommended, and the AGC algorithm in the 2019 guidelines does recommend colposcopy for these patients. However, the CIN3 + risk of 36% seen for HPV16/18/45+ AGC-favor neoplasia exceeds the threshold of offering the patient a choice between colposcopy or a diagnostic excisional procedure. As this option is not included in current management algorithms, adding risk-based recommendations for these results warrants consideration in future guideline updates.
Finding the very small proportion of cells yielding an AGC classification might have an element of chance during any screening round. Absent the detection of AGC, the individual would be called HPV positive and cytologically negative. Of note, NILM cytology was the most common (about half of the individuals) cytologic finding for individuals found to have AC/AIS. Also, finding HPV 16/18/45 confers an extremely high relative risk for a rare outcome. In absolute numbers, the squamous pathway is so much more common that absolute risk of CIN3 (but importantly not SCC) was also high with HPV 16/18/45. Finding these types raises concern regarding difficult-to-find glandular lesions, perhaps especially when squamous lesions are not found.
In the US, the 2019 consensus guidelines for management of screening abnormalities were revised to be risk-based, drawn from intensively studying the squamous pathway. The glandular pathway to AC is not quantified in the guidelines. Our findings from a risk-based perspective suggest that HPV-negative individuals are at very low risk of AC/AIS (0.01% immediate risk compared to the overall screening population in which immediate AC/AIS risk is only about 0.05%, Fig. 4). Among HPV positives, the immediate AC/AIS risk increases to 1.9% (a very large relative increase for a still uncommon outcome) if the individual has any of HPV 16/18/45 types. For other types or HPV negativity, the risk remains extremely low (0.04% immediate risk). The highest AC/AIS immediate risk is observed when AGC cytology is found with HPV 16/18/45 types (12%, OR = 1341, 95%CI = 495–3630), rising even higher if AGC is qualified as “favor neoplasia”. However, as mentioned above, NILM is very common preceding AC/AIS cases (49% of AC/AIS cases have HPV 16/18/45 NILM result); therefore, AGC is important when it is found but not necessary (Fig. 4).
Fig. 4.
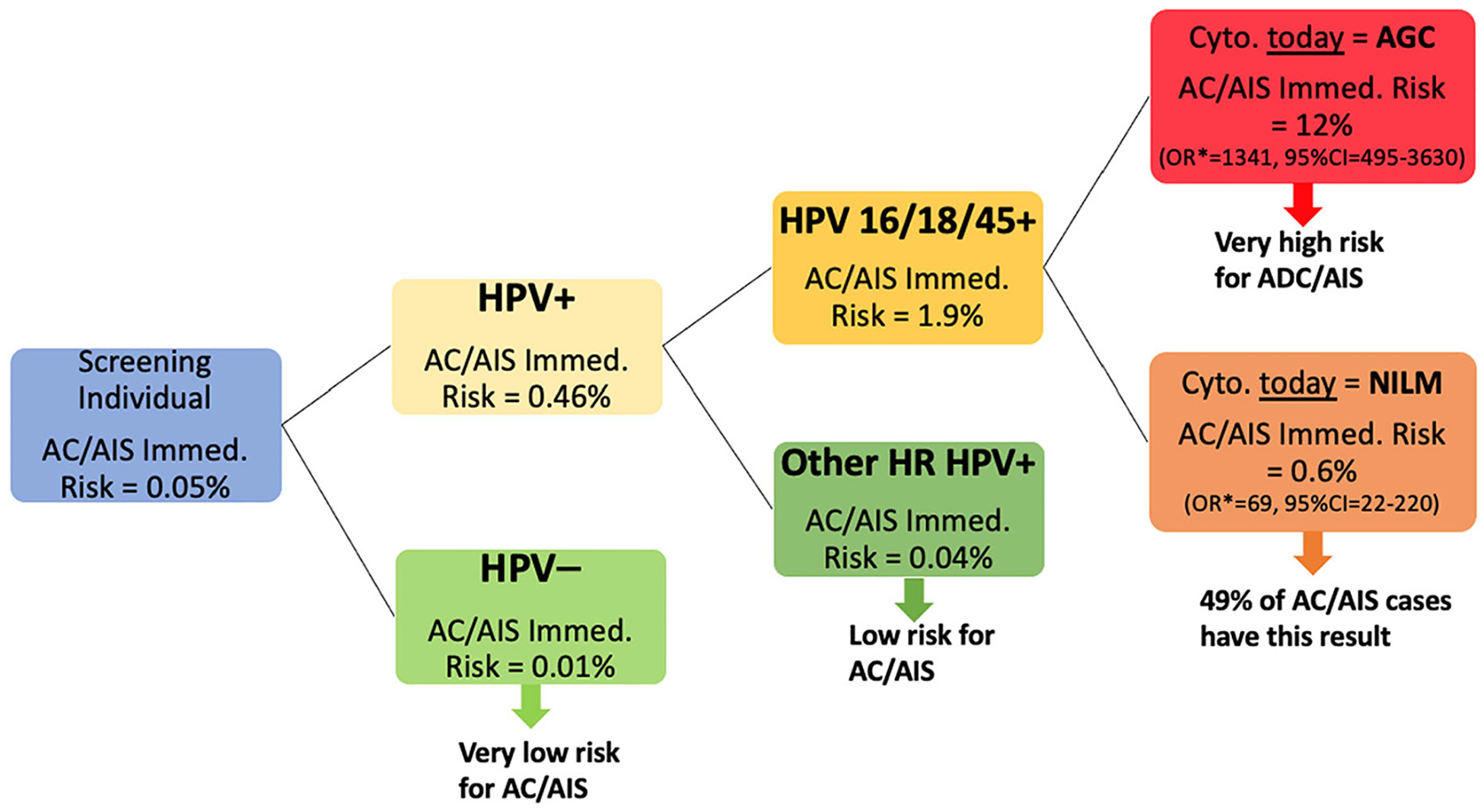
Clinical Implications.
Fig. 4 summarizes the clinical implementation of this study’s results. In the overall population, the immediate risk of AC/AIS is 0.05% which decreases to 1/5th when tested negative for HPV (0.01% immediate risk). Therefore, HPV-negative individuals can be confidently informed that they have a very low risk in terms of AC/AIS. The risk increases by 10-fold when HPV is positive (i.e., 0.46%). If the genotype of HPV is one of the high-risk types other than 16, 18, and 45, the immediate risk drops to less than the initial population immediate risk (0.04%), and individuals with this test result can be informed as having a low risk for AC/AIS. However, if HPV 16/18/45 is positive, the risk increases to 1.9%, and we looked at cytology results to see whether it is possible to obtain further risk stratification. When HPV 16/18/45 is positive, we obtained the highest immediate AC/AIS risk for AGC cytology (12% AC/AIS immediate risk; OR = 1341, 95%CI:495–3630; this OR is in comparison to the Other HR HPV+ and NILM cytology). However, if the cytology is NILM (and HPV 16/18/45+) then the risk is still high (0.6% AC/AIS immediate risk; OR = 69, 95%CI:22–220; this OR is in comparison to the Other HR HPV+ and NILM cytology) and 49% of the observed AC/AIS cases among HPV-positives have HPV 16/18/45+ and NILM cytology. This outcome might be caused by the underdiagnosis of an AGC result. We should also note that if the patients, who are currently HPV 16/18/45 positive, ever had AGC cytology in their past history, they are still at high risk of having AC/AIS (OR = 619, 95%CI:303–1264; this OR is in comparison to the Other HR HPV+ and never has AGC diagnosis).
* OR in comparison to the Other HR HPV+ and NILM (Negative for Intraepithelial Lesion or Malignancy) cytology.
AGC: atypical glandular cells; AC: adenocarcinoma; AIS: adenocarcinoma in situ.
4.1. Strengths and limitations
The KPNC dataset is one of the largest longitudinal studies of co-testing, with complete data collected on cytology, HPV, and histology results on nearly two million individuals over nearly two decades. However, limitations exist. All individuals are insured, therefore additional research in populations with limited insurance coverage is warranted, though prior comparisons with uninsured individuals show similar results [36]. The dataset lacked information on individual demographics including race and ethnicity, though race/ethnicity has not been associated with cervical cancer risk after controlling for screening [36]. HPV typing was not available on all individuals. In the PaP cohort, several typing studies were performed and the results were pooled [22]. HPV typing was performed on most cases of precancer/cancer. For the controls with <CIN2 outcomes, HPV typing was not performed as a single strictly random sample, although no biases that would affect the conclusions have been identified. Still, we acknowledge this limitation that precludes claiming exact estimation of risks. We also acknowledge that this analysis is relevant only to countries with robust cytology programs and may not be applicable to some lower resource settings.
5. Conclusions
The choice of cervical cancer screening method is moving towards primary HPV testing for individuals 25 years or older [23,35,37]. The argument for implementing primary HPV testing is that it is more sensitive than cytology and virtually as sensitive as HPV-cytology cotesting. We currently have limited ability to predict AC. Therefore, the importance of an uncommon but high-risk screening/triage combination supports the value of at least partial HPV typing and cytomorphologic assessment for triage of HPV-positive individuals. The identification of HPV 45 in the HPV test has merit in this context. Consideration of possible replacements for morphologic cytology as a triage should address how the information provided by AGC will be obtained [39–42]. Colposcopy guidelines recommend endocervical sampling for individuals with HPV 16/18 infections and AGC results, but the effectiveness of colposcopy with endocervical sampling to detect AIS remains limited, and more research is needed to optimize AC prevention in both screening and colposcopy.
Supplementary Material
HIGHLIGHTS.
Cervical screening has not controlled cervical adenocarcinoma.
We looked for early markers of adenocarcinoma that might be useful for early detection of adenocarcinoma precursors.
The combination of HPV 16/18/45 and cytologic AGC predicts high risk of adenocarcinoma and adenocarcinoma in situ.
Funding
This research has been supported by the Intramural Research Program of National Institutes of Health.
Declaration of Competing Interest
NW: Co-Chair of Enduring Guidelines Effort, TRB: Contract from NCI to Kaiser Permanente, RN: President of American Board of Pathology, Steering Committee member and Chair of ACS Primary HPV Screening Initiative, AR: Employee of Hologic but during this work was not employed by them, RBP: funding from NCI.
All other authors stated no conflict of interest.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2023.05.011.
References
- [1].Wang SS, Sherman ME, Hildesheim A, Lacey JV Jr., Devesa S, Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000, Cancer 100 (5) (2004) 1035–1044. [DOI] [PubMed] [Google Scholar]
- [2].Islami F, Fedewa SA, Jemal A, Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States, Prev. Med 123 (2019) 316–323. [DOI] [PubMed] [Google Scholar]
- [3].Adegoke O, Kulasingam S, Virnig B, Cervical cancer trends in the United States: a 35-year population-based analysis, J. Women’s Heal 21 (2012) 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pettersson BF, Hellman K, Vaziri R, Andersson S, Hellström AC, Cervical cancer in the screening era: who fell victim in spite of successful screening programs? J. Gynecol. Oncol 22 (2011) 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sherman ME, Wang SS, Carreon J, Devesa SS, Mortality trends for cervical squamous and adenocarcinoma in the United States: relation to incidence and survival, Cancer 103 (2005) 1258–1264. [DOI] [PubMed] [Google Scholar]
- [6].Van De Nieuwenhof HP, et al. , Significant decrease of adenocarcinoma in situ not reflected in cervical adenocarcinoma incidence in the Netherlands 1989–2003, Br. J. Cancer 98 (2008) 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Visioli CB, Zappa M, Ciatto S, Iossa A, Crocetti E, Increasing trends of cervical adenocarcinoma incidence in Central Italy despite extensive screening Programme, 1985–2000, Cancer Detect. Prev 28 (2004) 461–464. [DOI] [PubMed] [Google Scholar]
- [8].van der Horst J, Siebers AG, Bulten J, Massuger LF, de Kok IMCM, Increasing incidence of invasive and in situ cervical adenocarcinoma in the Netherlands during 2004–2013, Cancer Med 6 (2017) 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vinh-Hung V, et al. , Prognostic value of histopathology and trends in cervical cancer: a SEER population study, BMC Cancer 7 (2007) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S, Human papillomavirus and cervical cancer, Lancet 370 (2007) 890–907. [DOI] [PubMed] [Google Scholar]
- [11].Castle PE, et al. , Why does cervical cancer occur in a state-of-the-art screening program? Gynecol. Oncol 146 (2017) 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kjaer SK, Brinton LA, Adenocarcinomas of the uterine cervix: the epidemiology of an increasing problem, Epidemiol. Rev 15 (1993) 486–491. [DOI] [PubMed] [Google Scholar]
- [14].de Sanjose S, et al. , Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study, Lancet Oncol 11 (2010) 1048–1056. [DOI] [PubMed] [Google Scholar]
- [15].Guan P, Clifford GM, Franceschi S, Human papillomavirus types in glandular lesions of the cervix: a meta-analysis of published studies, Int. J. Cancer 132 (2013) 248–250. [DOI] [PubMed] [Google Scholar]
- [16].Solomon D, Nayar R, The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes, Springer, New York, NY, 2004. [Google Scholar]
- [17].Katki HA, et al. , Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice, Lancet Oncol 12 (2011) 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gravitt PE, et al. , A comparison of linear array and hybrid capture 2 for detection of carcinogenic human papillomavirus and cervical precancer in ASCUS-LSIL triage study, Cancer Epidemiol. Biomark. Prev 17 (2008) 1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nayar R, Wilbur DC, The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes, Springer, 2015. [Google Scholar]
- [20].Burk RD, et al. , Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women, J. Infect. Dis 174 (1996) 679–689. [DOI] [PubMed] [Google Scholar]
- [21].Stoler MH, Schiffman M, Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group, Interob-server reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study, JAMA 285 (11) (2001) 1500–1505. [DOI] [PubMed] [Google Scholar]
- [22].Demarco M, et al. , A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs, EClinicalMedicine 22 (2020), 100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheung LC, et al. , 2019 ASCCP risk-based management consensus guidelines: methods for risk estimation, recommended management, and validation, J Low Genit Tract Dis 24 (2020) 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheung LC, et al. , Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records, Stat. Med 36 (2017) 3583–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hyun N, et al. , Flexible risk prediction models for left or interval-censored data from electronic health records, Ann. Appl. Stat 11 (2017) 1063–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Landy R, et al. , Challenges in risk estimation using routinely collected clinical data: the example of estimating cervical cancer risks from electronic health-records, Prev. Med 111 (2018) 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schatzkin A, Freedman LS, Schiffman MH, Dawsey SM, Validation of intermediate end points in cancer research, J. Natl. Cancer Inst 82 (22) (1990) 1746–1752. [DOI] [PubMed] [Google Scholar]
- [28].Mirabello L, et al. , HPV 16 sublineage associations with histology-specific cancer risk using HPV whole-genome sequences in 3200 women, J. Natl. Cancer Inst 108 (2016) djw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kew MC, Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis, Liver Int 23 (2003) 405–409. [DOI] [PubMed] [Google Scholar]
- [30].Varela-Lema L, Ruano-Ravina A, Juiz Crespo MA, Barros-Dios JM, Tobacco consumption and oral and pharyngeal cancer in a Spanish male population, Cancer Lett 288 (2010) 28–35. [DOI] [PubMed] [Google Scholar]
- [31].Norman I, et al. , Atypical glandular cells and development of cervical cancer: population-based cohort study, Int. J. Cancer 151 (11) (2022) 2012–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bogani G, et al. , The impact of HPV-specific infection in women diagnosed with atypical glandular cells: results from the HPV-AGC study, Pathol. Res. Pract 216 (2020), 153184. [DOI] [PubMed] [Google Scholar]
- [33].Zuo T, et al. , High-risk human papillomavirus testing, genotyping, and histopathologic follow-up in women with abnormal glandular cells on Papanicolaou tests, Am. J. Clin. Pathol 156 (2021) 569–576. [DOI] [PubMed] [Google Scholar]
- [34].Cohen CM, et al. , Racial and ethnic disparities in cervical Cancer incidence, survival, and mortality by histologic subtype, J. Clin. Oncol 41 (5) (2023) 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Perkins RB, et al. , 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors, J. Low. Genit. Tract Dis 24 (2020) 102–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saraiya M, et al. , Risk of cervical precancer and cancer among uninsured and under-served women from 2009 to 2017, Am. J. Obstet. Gynecol 224 (4) (2021) 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schiffman M, Wentzensen N, Perkins RB, Guido RS, An introduction to the 2019 ASCCP risk-based management consensus guidelines, J. Low. Genit. Tract Dis 24 (2020) 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Clarke MA, et al. , Five-year risk of cervical Precancer following p16/Ki-67 dual-stain triage of HPV-positive women, JAMA Oncol 5 (2019) 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wentzensen N, et al. , Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population, Clin. Cancer Res 18 (2012) 4154–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wentzensen N, et al. , p16/Ki-67 dual stain cytology for detection of cervical Precancer in HPV-positive women, J. Natl. Cancer Inst 107 (2015) djv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wright TC, et al. , Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial, Gynecol. Oncol 144 (2017) 51–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


