Abstract
Objectives
To estimate the incidence of anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis.
Methods
We conducted a retrospective cohort study of >10 million person-years of observation from members of Kaiser Permanente Southern California, 2011–2022. The electronic health record of individuals with text-string mention of NMDA and encephalitis were reviewed to identify persons who met diagnostic criteria for anti-NMDAR encephalitis. Age-standardized and sex-standardized incidences stratified by race and ethnicity were estimated according to the 2020 US Census population.
Results
We identified 70 patients who met diagnostic criteria for anti-NMDAR encephalitis. The median age at onset was 23.7 years (IQR = 14.2–31.0 years), and 45 (64%) were female patients. The age-standardized and sex-standardized incidence of anti-NMDAR encephalitis per 1 million person-years was significantly higher in Black (2.94, 95% CI 1.27–4.61), Hispanic (2.17, 95% CI 1.51–2.83), and Asian/Pacific Island persons (2.02, 95% CI 0.77–3.28) compared with White persons (0.40, 95% CI 0.08–0.72). Ovarian teratomas were found in 58.3% of Black female individuals and 10%–28.6% in other groups.
Discussion
Anti-NMDA receptor encephalitis disproportionately affected Black, Hispanic, or Asian/Pacific Island persons. Ovarian teratomas were a particularly common trigger in Black female individuals. Future research should seek to identify environmental and biological risk factors that disproportionately affect minoritized individuals residing in the United States.
Introduction
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is a rare subacute, rapidly progressive autoimmune encephalitis that can result in severe permanent neurologic deficits or death. Prior case-only, referral center–based studies1-3 describe a preponderance of anti-NMDAR encephalitis in female individuals, young adults, a strong association with ovarian teratomas, and rarely, with herpes simplex virus (HSV) encephalitis. The only multiethnic referral center–based cohort was composed of 32.1% White patients,2 suggesting racial and ethnic disparities in anti-NMDAR encephalitis, but this was not investigated further.
The crude incidence of anti-NMDAR encephalitis in the Netherlands is estimated at 1 per million.4 No prior studies have examined potential variation by race and ethnicity. The purpose of this study was to estimate the incidence of anti-NMDAR encephalitis in a large multiethnic, population-based cohort and determine whether it varies by race and ethnicity, sex, or age.
Methods
We conducted a retrospective cohort study using Kaiser Permanente Southern California's (KPSC) complete electronic health records (EHR), which include all inpatient and outpatient encounters, laboratory and imaging tests, diagnoses, medications, and demographic characteristics. KPSC provides comprehensive health care to >4.8 million members, representing approximately 20% of the general population in Southern California. The sociodemographic characteristics of KPSC members are representative of the underlying population.5,6
Potentially incident cases were identified using a text-string search for NMDA and encephalitis mentioned in the same encounter between January 1, 2011, and December 31, 2022 (n = 803, eFigure 1). The EHR was abstracted to confirm patients met diagnostic criteria for definite (positive CSF antibodies, n = 67) or probable (n = 3, missing CSF results) anti-NMDAR encephalitis.1,7
Race and ethnicity, surrogates for structural racism,8 were obtained from record review (cases only) and the EHR. We categorized race and ethnicity as White (non-Hispanic White), Hispanic (White, Hispanic), Black (regardless of ethnicity), Asian or Pacific Islander (PI), other or multiple races/ethnicities, and unknown because of missing information (0% of cases).
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review board at (KPSC) approved this study. Informed consent was waived because there was no direct patient contact.
Statistical Analyses
Incidence rates (IRs) were calculated by dividing the number of newly diagnosed cases in the group by the sum of person-years contributed by each member of that group. Confidence intervals (CIs) for IRs and incidence risk ratios (IRR) were calculated using Poisson regression. Age-adjusted and sex-adjusted (male, female) standardized incidence rates were calculated according to the 2020 US Census population. Annual variations in IRs were examined to minimize detection bias (2009–2022, eTable 1). The mean values and SDs of normally distributed variables were compared using 2-sample t tests; for binary or categorical variables, the χ2 test with Fisher exact test was used. All analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC). Sensitivity analyses excluding probable cases were performed.
Data Availability
Due to the KPSC institutional review board, data will be available upon reasonable request.
Results
Demographic and Clinical Characteristics
The clinical and demographic characteristics of individuals newly diagnosed with anti-NMDAR encephalitis stratified by race and ethnicity are summarized in Table 1. The median age at diagnosis was 23.8 years (range 2.5–68.2 years), and 45 (64%) were female patients. The median time from symptom onset to clinical diagnosis was 17 days (IQR 9–26 days) and was significantly longer in male patients compared with female individuals (23 vs 14 days, p = 0.006) but did not differ significantly between racial/ethnic or age groups (eTable 2). CSF pleocytosis (70%) and abnormal EEG (73%) were common, but abnormal brain MRI (n = 28, 40%) less so.
Table 1.
Clinical and Demographic Characteristics of Persons With Incident Anti-NMDAR Encephalitis Race/Ethnicity
| White | Hispanic | Black | Asian/PI | Total | p Value | |
| n = 6 | n = 42 | n = 12 | n = 10 | n = 70 | ||
| Age at diagnosis, y, median (IQR) | 31.2 (24.8–37.6) | 19.6 (11.6–28.1) | 28.1 (23.8–42.1) | 27.7 (11.7–29.5) | 23.8 (14.3–31.0) | 0.01 |
| <13, n (%) | 0 (0) | 13 (31.0) | 0 (0) | 3 (30.0) | 16 (22.9) | 0.07 |
| ≥13–40, n (%) | 5 (83.3) | 24 (57.1) | 8 (66.7) | 7 (70.0) | 44 (62.9) | |
| >40, n (%) | 1 (16.7) | 5 (11.9) | 4 (33.3) | 0 (0) | 10 (14.3) | |
| Time from symptom onset to diagnosis, d, median (IQR) | 30.5 (9.0–59.0) | 17.5 (11.0–25.0) | 15.5 (7.0–22.0) | 12.0 (6.0–23.0) | 17.0 (9.0–26.0) | 0.43 |
| Female, n (%) | 2 (33.3) | 25 (59.5) | 10 (83.3) | 8 (80.0) | 45 (64.3) | 0.13 |
| Presenting symptoms, n (%) | ||||||
| Seizures | 3 (50.0) | 22 (52.4) | 7 (58.3) | 4 (40.0) | 36 (51.4) | 0.89 |
| Psychobehavioral symptoms | 6 (100.0) | 41 (97.6) | 12 (100) | 9 (90.0) | 68 (97.1) | 0.44 |
| Movement disorders | 3 (50.0) | 16 (38.1) | 8 (66.7) | 9 (90.0) | 36 (51.4) | 0.01 |
| Sleep disturbances | 1 (16.7) | 21 (50.0) | 3 (25.0) | 3 (30.0) | 28 (40.0) | 0.25 |
| Clinical triggers, n (%) | ||||||
| None | 3 (50.0) | 29 (69.0) | 3 (25.0) | 8 (80.0) | 43 (61.4) | 0.002 |
| Teratoma/dermoid tumors | 1 (16.7) | 12 (28.6) | 8 (66.7) | 1 (10.0) | 22 (31.4) | |
| HSV infection | 2 (33.3) | 1 (2.4) | 0 (0) | 0 (0) | 3 (4.3) | |
| Other viral triggera | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 1 (1.4) | |
| Other tumorb | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 1 (1.4) | |
| CSF/serum antibody tests, n (%) | ||||||
| At least 1 positive | 5 (83.3) | 41 (97.6) | 12 (100) | 9 (90.0) | 67 (95.7) | 0.15 |
| CSF not done/serum negative | 1 (16.7) | 1 (2.4) | 0 (0.0) | 1 (10.0) | 3 (4.3) | |
| CSF pleocytosis presentc, n (%) | 3 (50.0) | 29 (69.0) | 9 (75.0) | 8 (80.0) | 49 (70.0) | 0.71 |
| MRI, n (%) | ||||||
| Normal | 3 (50.0) | 26 (61.9) | 5 (41.7) | 8 (80.0) | 42 (60.0) | 0.16 |
| Abnormal, unilateral | 0 (0) | 4 (9.5) | 2 (16.7) | 2 (20.0) | 8 (11.4) | |
| Abnormal, bilateral | 3 (50.00%) | 12 (28.6) | 5 (41.7) | 0 (0) | 20 (28.6) | |
| EEGd, n (%) | ||||||
| Normal | 4 (66.7) | 9 (21.4) | 2 (16.7) | 1 (10.0) | 16 (22.9) | 0.03 |
| Abnormal | 1 (16.7) | 31 (73.8) | 10 (83.3) | 9 (90.0) | 51 (72.9) |
Abbreviations: HSV = herpes simplex virus; IQR = interquartile range; NMDAR = N-methyl-d-aspartate receptor; PI = Pacific Islander.
Patient had concomitant RSV infection at the same time of NMDAR onset.
Lung mass in smoker who died before biopsy could be obtained.
CSF testing was not performed in 1 White and 1 Black patient.
EEG was not performed/report not found for 1 White patient and 2 Hispanic patients.
Ovarian teratomas were found in 21 female patients (median age = 24.5 years, range 9.1–42.2) and a mediastinal teratoma in 1 Black male patient. Ovarian teratomas were significantly more common in Black female patients compared with other racial/ethnic groups, accounting for two-thirds of Black patients with anti-NMDAR encephalitis. HSV encephalitis preceded the onset of anti-NMDAR encephalitis in 3 patients by 1.3, 1.7, and 12.5 months. Most of the patients (n = 43, 61.4%) had no identifiable trigger, particularly those younger than 13 years (n = 12, 81.3%) compared with other age groups (n = 26, 59.1% ≥ 13–40 years; n = 4, 40.0% > 40 years, p = 0.012; eTable 2).
Incidence
The age-adjusted and sex-adjusted standardized incidence rates of anti-NMDAR encephalitis were higher in Black, Asian/PI, and Hispanic individuals than White persons (Figure). The risk of anti-NMDAR encephalitis was 7.01 (95% CI 2.63–18.70; p < 0.0001) higher in Black, 4.36 (95% CI 1.58–12.00; p = 0.0044) higher in Asian/PI, and 4.28 (95% CI 1.82–10.11, p = 0.0009) higher in Hispanic individuals compared with that in White persons (reference group). The age-standardized incidence rate was significantly higher among Black (IRR = 16.28, 95% CI 3.57–74.32, p = 0.0003), Asian/PI (IRR = 9.73, 95% CI 2.06–45.82, p = 0.0040), and Hispanic (IRR = 7.21, 95% CI 1.70–30.48, p = 0.0073) individuals compared with White female patients (reference group). Age-standardized incidence was similar between Black, Asian/PI, and White male patients but higher in Hispanic (IRR = 2.86, 95% CI 0.95–8.56, p = 0.0609) than White male patients (reference group), although this finding did not reach statistical significance. After considering the racial/ethnic distribution of the US population, we estimate that approximately 400 individuals will be newly diagnosed with anti-NMDAR encephalitis per year in the United States (Table 2). Analyses excluding probable cases yielded similar results (eTable 3).
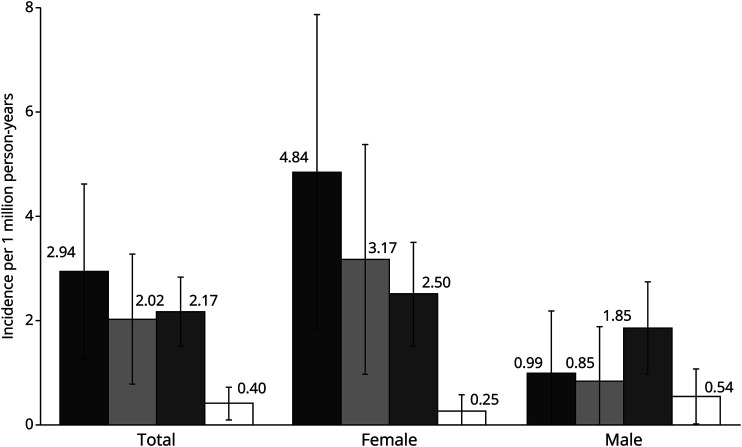
Figure. Incidence of Anti-NMDAR Encephalitis by Race/Ethnicity and Sex.
This graph shows the annual incidence rates (indicated by the number above each bar) and 95% confidence intervals of anti-NMDAR encephalitis standardized by age and sex (total) or age alone (female, male) to the 2020 US Census in Black (in black), Asian/Pacific Islander (PI) (in gray), Hispanic (in dots), and White (in white) persons by sex. The overall increased incidence of NMDAR in Black, Asian/PI, and Hispanic individuals compared with White individuals is driven by a significantly higher incidence in Black (4.84, 95% CI 1.81–7.86), Asian/PI (3.17, 95% CI 0.97–5.37), and Hispanic (2.51, 95% CI 1.52–3.49) female individuals compared with White female individuals (0.25, 95% CI 0.00–0.60). The age-standardized incidence was highest in Hispanic male individuals (1.85, 95% CI 0.97–2.73) and lowest in White male individuals (0.94, 95% CI 0.01–1.06), but this difference did not reach statistical significance.
Table 2.
Standardized Incidence Rates and Estimated Number of Newly Diagnosed Individuals With Anti-NMDAR Encephalitis Annually in the United States by Race/Ethnicity
| Race/ethnicity | Cases with anti-NMDAR encephalitis | Active KPSC member person-years | Crude incidence per 1,000,000 person-years (95% CI) | Standardized incidence per 1,000,000 person-years (95% CI)a | US population in 2020b | Expected newly diagnosed patients with anti-NMDAR encephalitis annually |
| White | 6 | 15,838,182 | 0.38 (0.08–0.68) | 0.40 (0.08–0.72) | 191,722,195 | 77 |
| Hispanic | 42 | 20,126,744 | 2.09 (1.46–2.72) | 2.17 (1.51–2.83) | 65,329,087 | 142 |
| Black | 12 | 4,074,294 | 2.95 (1.28–4.61) | 2.94 (1.27–4.61) | 39,944,624 | 117 |
| Asian/Pacific Islander | 10 | 5,390,568 | 1.86 (0.71–3.01) | 2.02 (0.77–3.28) | 20,243,574 | 41 |
| Totalc | 70 | 45,429,789 | 1.54 (1.18–1.90) | — | 317,239,480 | 377 |
Abbreviations: KPSC = Kaiser Permanente Southern California; NMDAR = N-methyl-d-aspartate receptor.
Standardized to the 2020 US Census and adjusted for age and sex.
Per 2020 US Census.
Includes persons with other/missing/unknown race and ethnicity.
Discussion
That Black, Asian, and Hispanic individuals have 7- to 4-fold higher incidence of anti-NMDAR encephalitis than White individuals is a novel finding. Presence of antibodies against the NMDAR in CSF is pathologic and establishes the diagnosis in symptomatic patients.1 Yet, reliable estimates of incidence exist are challenging to generate because the disease is very rare, relatively new, and case identification in large populations is challenging without a specific ICD code. The only existing reliable estimate reported a crude estimate of 1.00/million although with confidence limits (0.62–1.59) that overlap with the rate in White people in our study. While another study attempted to estimate the incidence of autoimmune encephalitides in White individuals, it was limited by a small sample (∼3.3 million person-years), a timeframe (1995–2015) largely before the first description of anti-NMDAR encephalitis (2007), and identified only 1 patient with anti-NMDAR encephalitis.9
Consistent with prior studies, we found an association with ovarian teratomas2 highest in Black patients10 and rarely in young female patients2; rare association with HSV encephalitis; and high frequency of neuropsychiatric features, CSF pleocytosis, abnormal EEGs, and normal brain MRIs2 at anti-NMDAR encephalitis presentation. We found a lower female preponderance in Hispanic individuals compared with Black and Asian individuals in this study and compared with prior studies in non-Hispanic populations.2,4
This study is limited by a relatively small number of cases, particularly among White individuals and in the youngest and oldest age groups, variation in timing and type of EEGs and MRIs, lack of socioeconomic status data, and the inherent challenges in lumping large groups of people with diverse cultures and ancestral backgrounds into limited racial and ethnic categories. Whether findings from California can be extrapolated to other diverse populations is also unknown and should be addressed in future studies. Strengths include an exhaustive search strategy to identify cases, long study period improving precision, and decreasing the possibility of underdetection.
Our study raises interesting questions about why minoritized individuals, particularly female individuals, in the United States may be more prone to aberrant humoral (and cell-mediated) immune responses. Current evidence suggests at best a weak association with certain human leukocyte antigen subtypes underscoring the importance of environmental factors in the development of anti-NMDAR encephalitis. Informed by the evidence in another autoimmune disease with a similar racial/ethnic distribution,11 we speculate that racial/ethnic disparity in anti-NMDAR encephalitis in the United States may at least partly be attributable to the embodiment of socioeconomic and environmental factors including poverty, racial discrimination, substandard health care,8,12 and earlier exposure and more vigorous responses to common childhood infections.13,14 These factors and their potential influence on immunologic dysregulation and epigenetic changes that could predispose individuals to anti-NMDAR encephalitis should be examined in future studies.
Appendix. Authors
| Name | Location | Contribution |
| Samir Alsalek, BS | Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Kathryn B. Schwarzmann, BA | Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Sakar Budhathoki, BA | Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Viridiana Hernandez-Lopez, BA | Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Jessica B. Smith | Department of Research and Evaluation, Southern California Permanente Medical Group, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Bonnie H. Li | Department of Research & Evaluation, Southern California Permanente Medical Group, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Annette Langer-Gould, MD, PhD | Department of Neurology, Los Angeles Medical Center, Southern California Permanente Medical Group; Department of Clinical Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
A. Langer-Gould has received grant support and awards from the Patient-Centered Outcomes Research Institute, the National MS Society, and Atara Biotherapeutics. She currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). S. Alsalek, K.B. Schwarzmann, S. Budhathoki, V. Hernandez-Lopez, J.B. Smith, and B.H. Li report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Dalmau J, Armangué T, Planagumà J, et al. . An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057. doi: 10.1016/S1474-4422(19)30244-3 [DOI] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. . Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. doi: 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaler FS, Zimmermann L, Kammermeier S, et al. . Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: real-world evidence from the GENERATE registry. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1088. doi: 10.1212/NXI.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastiaansen AEM, de Bruijn MAAM, Schuller SL, et al. . Anti-NMDAR encephalitis in The Netherlands, focusing on late-onset patients and antibody test accuracy. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1127. doi: 10.1212/NXI.0000000000001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koebnick C, Langer-Gould AM, Gould MK, et al. . Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41. doi: 10.7812/TPP/12-031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis AC, Voelkel JL, Remmers CL, Adams JL, McGlynn EA. Comparing kaiser permanente members to the general population: implications for generalizability of research. Perm J. 2023;27(2):87-98. doi: 10.7812/TPP/22.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graus F, Titulaer MJ, Balu R, et al. . A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936-944. doi: 10.2105/AJPH.2011.300544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey D, Pittock SJ, Kelly CR, et al. . Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177. doi: 10.1002/ana.25131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahrestani S, Brown NJ, Singh R, et al. . Evaluating the incidence and predictors of anti-NMDAR encephalitis in a contemporary cohort of patients diagnosed with dermoid tumors: a national inpatient sample analysis. J Clin Neurosci. 2022;102:109-113. doi: 10.1016/j.jocn.2022.06.018 [DOI] [PubMed] [Google Scholar]
- 11.Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford). 2017;56(suppl_1):i67-i77. doi: 10.1093/rheumatology/kew399 [DOI] [PubMed] [Google Scholar]
- 12.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav. 2010;51(Suppl):S15-S27. doi: 10.1177/0022146510383838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford JL, Stowe RP. Racial-ethnic differences in Epstein-Barr virus antibody titers among U.S. children and adolescents. Ann Epidemiol. 2013;23(5):275-280. doi: 10.1016/j.annepidem.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 14.Dowd JB, Palermo T, Chyu L, Adam E, McDade TW. Race/ethnic and socioeconomic differences in stress and immune function in the National Longitudinal Study of Adolescent Health. Soc Sci Med. 2014;115:49-55. doi: 10.1016/j.socscimed.2014.06.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the KPSC institutional review board, data will be available upon reasonable request.